Published online Jul 28, 2020. doi: 10.3748/wjg.v26.i28.4126
Peer-review started: March 30, 2020
First decision: April 25, 2020
Revised: June 8, 2020
Accepted: July 15, 2020
Article in press: July 15, 2020
Published online: July 28, 2020
Processing time: 119 Days and 21.5 Hours
Primary sclerosing cholangitis (PSC) associated inflammatory bowel disease (IBD) is a unique form of IBD (PSC-IBD) with distinct clinical and histologic features from ulcerative colitis (UC) and Crohn disease (CD). In patients with PSC and IBD, the severity of the two disease processes may depend on each other.
To study the histologic and clinical features of PSC patients with and without IBD.
We assessed specimens from patients with UC (n = 28), CD (n = 10), PSC and UC (PSC-UC; n = 26); PSC and CD (PSC-CD; n = 6); and PSC and no IBD (PSC-no IBD; n = 4) between years 1999-2013. PSC-IBD patients were matched to IBD patients without PSC by age and colitis duration. Clinical data including age, gender, age at IBD and PSC diagnoses, IBD duration, treatment, follow-up, orthotopic liver transplantation (OLT) were noted.
PSC-UC patients had more isolated right-sided disease (P = 0.03), and less active inflammation in left colon, rectum (P = 0.03 and P = 0.0006), and overall (P = 0.0005) compared to UC. They required less steroids (P = 0.01) and fewer colectomies (P = 0.03) than UC patients. The PSC-CD patients had more ileitis and less rectal involvement compared to PSC-UC and CD. No PSC-CD patients required OLT compared to 38% of PSC-UC (P = 0.1). PSC-IBD (PSC-UC and PSC-CD) patients with OLT had severe disease in the left colon and rectum (P = 0.04).
PSC-UC represents a distinct form of IBD. The different disease phenotype in PSC-IBD patients with OLT may support liver-gut axis interaction, however warrants clinical attention and further research.
Core tip: This is a retrospective study evaluating biopsies and clinical features of primary sclerosing cholangitis (PSC) patients with and without inflammatory bowel disease (IBD) in comparison to subjects with ulcerative colitis (UC) or Crohn disease (CD). Patients with PSC-UC had a different disease distribution characterized by right sided colitis, a milder disease course with lower activity scores in biopsies, less need for colectomy, and less steroids compared to UC. PSC-CD patients were rare but had more ileal inflammation compared to PSC-UC. PSC-No IBD patients showed similar characteristics to PSC patients in general and only one patient received orthotopic liver transplantation (OLT) in this group. Ten PSC-UC patients received OLT in contrast to no patients with PSC-CD. The need for OLT in PSC-IBD (PSC-UC and PSC-CD) correlated with rectal involvement and higher activity scores in the left colon biopsies in comparison to patients without OLT. This may require clinical attention since the both the intestinal and liver disease seem to be “severe” in this group further supporting the importance of gut-liver interaction in these patients.
- Citation: Aranake-Chrisinger J, Dassopoulos T, Yan Y, Nalbantoglu I. Primary sclerosing cholangitis associated colitis: Characterization of clinical, histologic features, and their associations with liver transplantation. World J Gastroenterol 2020; 26(28): 4126-4139
- URL: https://www.wjgnet.com/1007-9327/full/v26/i28/4126.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i28.4126
Primary sclerosing cholangitis (PSC) is a progressive chronic cholestatic disease of the intrahepatic and extrahepatic biliary tree. Most patients will require orthotopic liver transplant (OLT) within 8 years[1]. PSC is associated with inflammatory bowel disease (IBD) in 70%-80% of patients[1]. Although ulcerative colitis (UC) is most often associated with PSC[2,3], a minority of patients with PSC and IBD will have Crohn disease (CD)[3,4]. The histopathologic and endoscopic features of IBD in patients with PSC-IBD have been reported to differ from patients with IBD only. There is generally less severe colitis and more pancolitis, or right-sided disease, in patients with PSC-UC compared to those with UC only[5-7]. Some studies have also suggested that there is increased rectal sparing and ileitis in PSC-UC[1], however, others have not confirmed these findings[5,6]. The histopathology of PSC-CD is not well characterized, though patients with PSC-CD are reported to have more colitis and less ileitis compared to those with CD without PSC[6,8]. Further, increased numbers of lamina propria IgG4 positive plasma cells in active and inactive PSC-UC compared to active and inactive CD and inactive UC have been reported suggesting immunophenotypic differences[9].
PSC-IBD may also have a distinct pathogenesis. Genetic studies have shown that HLA class II alleles associated with UC are different from those found in patients with PSC and patients with PSC-UC[10]. Further, known IBD-associated polymorphisms such as TLR-4, CARD15, CARD4, SLC22A4, SLC22A5, DLG5, and MDR1 are not found at increased frequency in patients with PSC, PSC-UC, or PSC-CD[10]. In addition patients with PSC and PSC-IBD have a similar gut microbial profile, which is different from patients with UC only and healthy controls, suggesting a microbiome connection[11,12].
There is also evidence that gut-liver cross talk may contribute to the pathogenesis of PSC-IBD both in human studies and animal models[13,14]. Hepatic disease severity in PSC is inversely associated with colitis in PSC-UC patients. PSC-UC patients who require OLT have been reported to have less severe colitis than those patients who do not[13-15]. Studies have also shown that severe IBD pre and post OLT is associated with recurrent disease in the allograft[18-20]. In patients with PSC-IBD, aberrant expression of gut-specific molecules in liver and liver-restricted molecules in gut likely provide a mechanism for T and B cell trafficking between the gut and the liver, and suggest the presence of several mechanisms for liver-gut cross talk[13].
Based on observed differences in distribution of colonic involvement, severity of inflammation, rate of colorectal neoplasia and clinical outcomes, PSC-IBD represents a unique form of IBD, distinct from UC or CD without PSC[6,8,21].
In light of the current literature findings, we studied PSC patients with and without IBD, who were referred to our center. Our study had two aims: (1) To describe the histopathologic features of PSC-UC and PSC-CD in comparison to UC and CD in the absence of PSC; and (2) To study the associations between clinical and histopathologic features of PSC-IBD with OLT.
This study was approved by the institutional review board. This study was exempt from patient consent since the data was analyzed in an anonymous fashion. The archives of the Lauren V. Ackerman Laboratory at Barnes-Jewish Hospital were searched for ileal and colonic biopsies and resections from patients with UC only, CD only, PSC-UC, PSC-CD, as well as patients with PSC without IBD. Colonic and ileal biopsies and resection specimens from well-characterized patients 18 years old and older between 1999 and 2013 were identified. In all cases of IBD, the diagnosis was confirmed by the treating gastroenterologist following established guidelines[22]. In all cases of PSC, the diagnosis was based on the presence of characteristic clinical and radiographic findings. In cases of PSC with normal colonic biopsies, the medical record was reviewed for subsequent development of inflammatory bowel disease. If IBD was later diagnosed, the cases were excluded. PSC-UC and UC patients, and PSC-CD and CD patients, were matched for age and IBD duration. Clinical data including gender, age, age at IBD diagnosis, age at PSC diagnosis, IBD duration, medical treatment, colectomy, OLT, development of dysplasia, and follow-up duration were collected from the electronic medical record. In addition, colonoscopic findings in PSC-UC and PSC-CD patients were also recorded.
For each patient with IBD, all available biopsy reports were screened, the most inflamed biopsy set with sufficient tissue, available in our files was selected for histologic and immunohistochemistry analysis. For patients with PSC-no IBD, the earliest biopsy was selected. Of note, the site of biopsies was obtained from endoscopy reports as designated by the submitting gastroenterologist.
The hematoxylin and eosin stained slides generated for routine clinical diagnosis were reviewed by two of the authors (IN and JAC). Histologic review was blinded to the clinical diagnosis, and disagreement as to interpretation of histologic features was resolved by consensus.
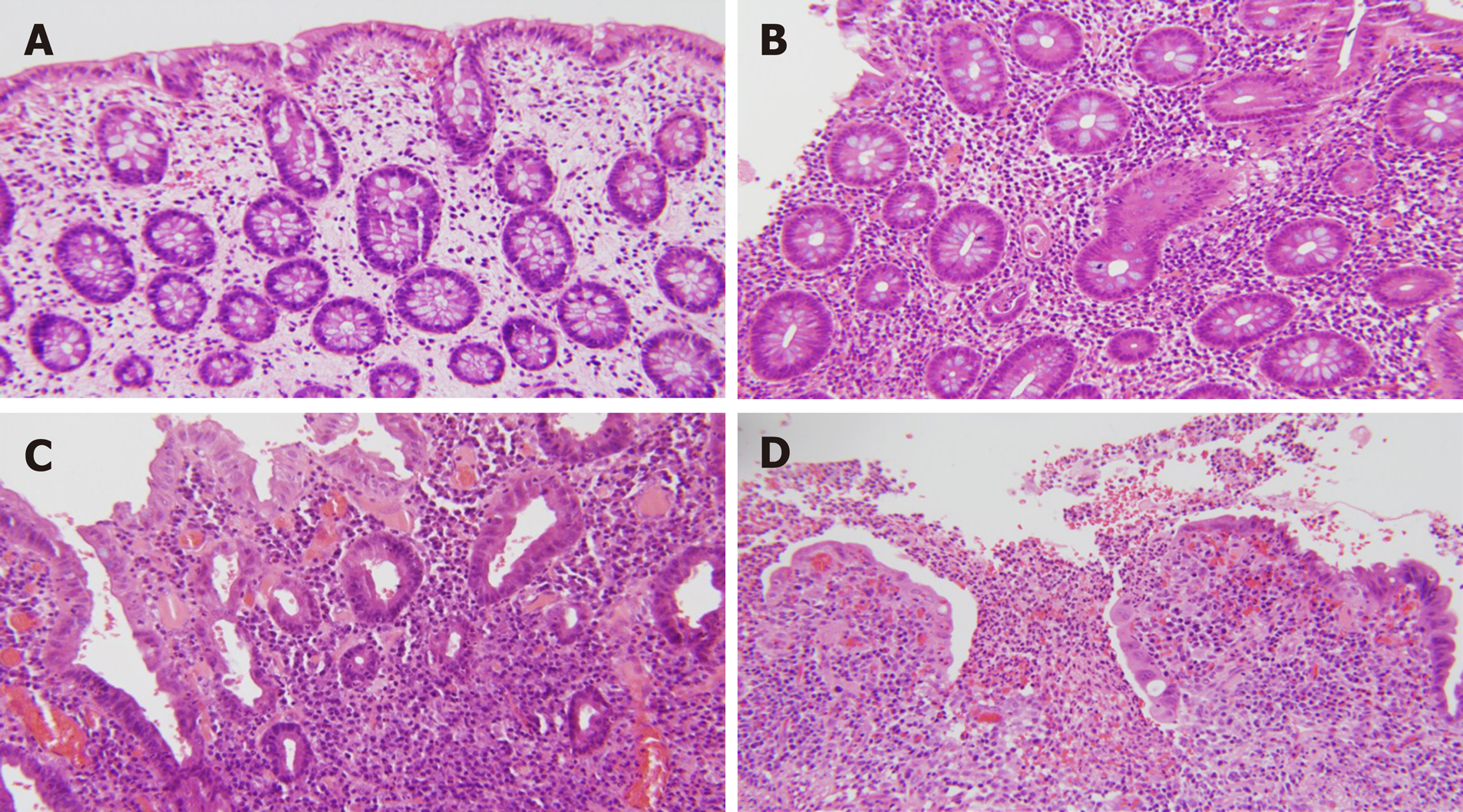
For each case the following features were noted at sites that were sampled: Distribution and presence of active inflammation, architectural distortion, basal plasmacytosis, Paneth cell metaplasia (left colon and rectum), and pyloric gland metaplasia. Pancolitis was defined as both right and left colonic disease, irrespective of rectal involvement. The presence of ileitis and granulomas was also noted. The degree (severity) of active inflammation at each site was also graded semi-quantitatively, and an activity score was assigned to each biopsy as described by Joo et al[5]. A score of 0 was assigned if there was no active inflammation, (1) If less than 50% of crypts showed neutrophilic cryptitis and/or crypt abscesses; (2) If greater than 50% showed neutrophilic cryptitis and/or crypt abscesses; and (3) If there was surface erosion or ulceration[5] (Figure 1).
The statistical methods for this work include summary descriptive statistics for continuous outcomes and categorical outcomes and were performed by a certified biostatistician (YY). For continuous outcomes, the descriptive statistics include minimum, maximum, mean, standard deviation, and median. For categorical outcomes, the proportions in each category are produced. These descriptive statistics are produced for entire patient sample and for subgroups of patients. Two-sample t test or Wilcoxon test is used to compare a continuous outcome between two groups and AVONA is used to compare a continuous outcome among three or more groups. For a binary outcome, we used Chi-square (χ2) test or Fisher's exact test to compare the proportion between two groups, and logistic regression model to compare the proportions among three or more groups. The P values are not adjusted for multiple comparisons since the study is exploratory in nature.
In addition, multivariable logistic regression is used to explore the relationship among liver transplantation (outcome variables) and a set of variables (independent variables) including disease distribution and severity, colectomy, and medical treatment in UC, CD, PSC-UC, and PSC-CD groups. Forward step-wise selection procedure was used for variable selection. The entry level of forward selection was set at 0.2 and 0.05 to compare the results. Calculations were performed using SAS® 9.4 software. P value ≤ 0.05 was considered statistically significant.
PSC-UC patients: Twenty-six PSC-UC patients and 28 age and IBD disease duration matched UC controls were identified (Table 1). Seventeen (65%) PSC-UC patients were male and had an average age of 37 years. The average age at IBD diagnosis was 27 years, and the average age at PSC diagnosis was 32 years. UC preceded PSC in 12 patients (46%), PSC preceded UC in 8 patients (31%), and in 5 patients (19%) the diagnoses were reached simultaneously. For one patient, the temporal relationship was not known (4%). The average duration of IBD was 10.8 years. Ten (38%) patients required OLT. Of these, UC preceded PSC in 6 patients, PSC proceeded UC in 2 patients, and 2 patients had the diagnoses made concurrently. The average duration of follow-up was 8.2 years; and only 4 patients (15%) developed low grade dysplasia during this time. No high-grade dysplasia or cancer were noted in PSC-UC group, whereas 1 patient developed cancer in UC controls and 4 patients were noted to have low grade dysplasia. Except one patient who died of sepsis, all PSC-UC patients were alive at the time of data collection.
| PSC-UC | UC | P value | PSC-CD | CD | P value | P value (PSC-UC vs PSC-CD) | |
| Male | 17 (65) | 18 (64) | 0.9 | 5 (83) | 2 (22) | 0.03a | 0.4 |
| Age at the time of biopsy (yr) (mean ± SD) | 37 ± 14.61 | 41 ± 15.55 | 0.4 | 38 ± 14.93 | 32 ± 10.21 | 0.2 | 0.8 |
| Age at IBD diagnosis (yr) (mean ± SD) | 27± 14.38 | 30 ± 10.61 | 0.3 | 33 ± 14.77 | 22 ± 10.48 | 0.09 | 0.2 |
| Age at PSC diagnosis (yr) (mean ± SD) | 32 ± 13.96 | NA | NA | 31± 12.87 | NA | NA | 0.8 |
| Interval between PSC and IBD diagnoses (yr) (mean ± SD) | 9 ± 10.45 | NA | NA | 1.4 ± 1.6733 | NA | NA | 0.1 |
| IBD duration at biopsy (yr) (mean ± SD) | 10.8 ± 10.84 | 9 ± 9.19 | 0.5 | 7.00 ± 5.18 | 9.8 ± 4.06 | 0.5 | 0.3 |
| PSC duration (yr) (mean) | 6 | NA | NA | 12.33 | NA | NA | 0.8 |
| Medical treatment | |||||||
| Ursodiol | 9 (35) | 0 | 0.0006a | 2 (33) | 0 | 0.1 | 1 |
| Steroids | 8 (31) | 19 (68) | 0.01a | 2 (33) | 2 (22) | 1 | 1 |
| ASA | 15 (58) | 17 (61) | 1 | 2 (33) | 6 (67) | 0.3 | 0.4 |
| Anti-TNF | 5 (19) | 7 (25) | 0.7 | 1 (17) | 7 (78) | 0.04a | 1 |
| Immunomodulators | 4 (15) | 8 (29) | 0.3 | 2 (33) | 3 (33) | 1 | 0.3 |
| Colectomy | 10 (38) | 19 (68) | 0.03a | 0 | 1 (11) | 0.9 | 0.9 |
| OLT | 10 (38) | NA | NA | 0 | NA | NA | 0.1 |
| Follow-up duration (yr) (mean ± SD) | 8.2 ± 4.61 | 8.2 ± 5.75 | 0.9 | 4.8 ± 2.23 | 5.9 ± 3.14 | 0.7 | 0.1 |
| Development of dysplasia/cancer | 4 (15) | 5 (18) | 0.8 | 0 | 0 | 1 | 0.9 |
| Resection used in histologic and IHC analysis | 6 (23) | 9 (32) | 0.4 | 0 | 3 (33) | 0.9 | 0.9 |
Between the PSC-UC and the UC group, there was no significant difference in gender (P = 0.9), age at IBD diagnosis (P = 0.3), duration of IBD (P = 0.5), duration of follow-up (P = 0.9), or development of dysplasia (P = 0.8). PSC-UC patients were less frequently treated with steroids (31%) compared to 68% of UC patients (P = 0.01), and patients with PSC-UC (38%) underwent colectomy less frequently than UC patients (68%) (P = 0.03).
Colonoscopy reports (not shown in tables) were available in 21 PSC-UC patients (1 flexible sigmoidoscopy). The five remaining patients, who did not have colonoscopy, had colectomy and the gross findings were noted. The colonoscopy was normal in three patients. Terminal ileum was noted to be normal in 57% of the cases (15 patients), whereas it was noted to be involved in 7% (2 patients), and data was not available in the rest. Pancolitis was the most commonly noted finding in 65% of all cases (17 patients). Isolated right-sided involvement was seen in only one patient (3%), and right side predominant disease was seen in four (15%). Rectal involvement and sparing were noted in four patients each (15%). Colonoscopic and gross examination correlated with the histologic findings in 50% of the patients.
A total of 181 biopsies and 15 resections from all patients were examined (Table 2). The PSC-UC group had more patients with disease confined to only the right side of colon compared to the UC group (29% vs 4%, P = 0.03). In contrast, isolated left sided disease was more commonly noted in UC patients (26% vs 4%, P = 0.06). Rectal involvement between the two groups was similar. No significant difference in pancolitis was observed between the two groups (67% vs 63%, P = 0.8).
| PSC-UC1 | UC1 | P value | PSC-CD1 | CD1 | P value | P value (PSC-UC vs PSC-CD) | |
| Distribution of disease | |||||||
| Right colon only | 7 (29) | 1 (4) | 0.03a | 3 (50) | 2 (33) | 0.6 | 0.3 |
| Left colon only | 1 (4) | 7 (26) | 0.06 | 0 | 0 | 1 | 0.9 |
| Left and right colon | 16 (67) | 17 (63) | 0.8 | 3 (50) | 4 (67) | 0.6 | 0.4 |
| Rectum | 15 (68) | 18 (90) | 0.1 | 1 (20) | 5 (83) | 0.055 | 0.07 |
| Ileitis | 4 (31) | 1 (7) | 0.1 | 5 (100) | 4 (67) | 0.9 | 0.9 |
| Severity of disease (active inflammation) | |||||||
| Right colon (average activity score) | 1.25 | 0.96 | 0.2 | 1.5 | 1 | 0.3 | 0.5 |
| Left colon (average activity score) | 1.2 | 1.82 | 0.03a | 0.83 | 0.86 | 0.9 | 0.4 |
| Rectum (average activity score) | 0.96 | 2.05 | 0.0006a | 0.2 | 1.17 | 0.1 | 0.1 |
| Ileum (average activity score) | 0.31 | 0.07 | 0.2 | 1 | 1 | 1 | 0.01a |
| Max average activity score at any site | 1.46 | 2.29 | 0.0005a | 1.83 | 1.67 | 0.7 | 0.3 |
| Architectural distortion | |||||||
| Right colon | 24 (100) | 21 (78) | 0.9 | 6 (100) | 6 (100) | 1 | 1 |
| Left colon | 24 (96) | 26 (93) | 0.6 | 6 (100) | 7 (100) | 1 | 0.9 |
| Rectum | 18 (78) | 19 (95) | 0.1 | 5 (100) | 5 (83) | 0.9 | 0.9 |
| Any site | 26 (100) | 28(100) | 1 | 6 (100) | 9 (100) | 1 | 1 |
| Paneth cell metaplasia | |||||||
| Left colon | 11 (44) | 14 (50) | 0.7 | 1 (17) | 4 (57) | 0.1 | 0.2 |
| Rectum | 5 (22) | 6 (30) | 0.5 | 1 (20) | 0 | 0.9 | 0.9 |
| Basal plasmacytosis | |||||||
| Right colon | 14 (58) | 13 (48) | 0.5 | 2 (33) | 3 (50) | 0.5 | 0.3 |
| Left colon | 11 (44) | 22 (79) | 0.01a | 0 | 4 (57) | 0.9 | 0.9 |
| Rectum | 6 (26) | 19 (95) | 0.0004a | 1 (20) | 3 (50) | 0.3 | 0.7 |
| Any site | 16 (62) | 27 (96) | 0.01a | 3 (50) | 7 (78) | 0.2 | 0.6 |
| Pyloric gland metaplasia | |||||||
| Right colon | 2 (8) | 1 (4) | 0.7 | 1 (17) | 1 (17) | 1 | 0.6 |
| Left colon | 0 | 0 | 1 | 0 | 0 | 1 | 1 |
| Any site | 2 (8) | 1 (4) | 0.5 | 1 (17) | 1 (11) | 0.7 | 0.5 |
| Granulomas | |||||||
| Any site | 1 (4) | 0 | 0.9 | 1 (17) | 6 (67) | 0.08 | 0.3 |
The average activity score in right colon was higher in the PSC-UC group compared to UC (1.25 vs 0.96), however this did not reach statistical significance (P = 0.2). Left colon and rectum were less inflamed (lower activity scores) in the PSC-UC group (P = 0.03 and P = 0.0006). PSC-UC patients also had less active inflammation as an average of all sites examined compared to the UC group (P = 0.0005). Further, the PSC-UC patients showed less basal plasmacytosis in the left colon and rectum compared to the UC group (P = 0.01 and P = 0.0004, respectively), and similar frequencies of basal plasmacytosis in the right colon (P = 0.5). No difference was noted between the PSC-UC group and the UC group in distribution of architectural distortion, or Paneth cell or pyloric gland metaplasia.
PSC-CD patients: Six patients with PSC-CD and 10 age and IBD disease duration matched CD controls were identified (Table 1). Five of PSC-CD patients were male (83%) and they had an average age of 38 years, average age of IBD diagnosis of 33 years, and average age of PSC diagnosis of 31 years. The average duration of IBD was 7 years, and the average duration of follow-up was 4.8 years. None required OLT. In one patient the diagnosis of CD preceded PSC (17%), in two patients the diagnosis of PSC preceded CD (33%), in two patients the conditions were diagnosed simultaneously (33%), and in one patient the temporal relationship was unknown (17%). None of the patients within study or control groups developed dysplasia during follow-up. All PSC-CD patients were alive at the time of data collection.
The PSC-CD group had a male predominance compared to the CD group (P = 0.03). The PSC-CD and CD groups there were similar in terms of age at IBD diagnosis (P = 0.09), IBD duration (P = 0.5), need for colectomy (P = 0.9), duration of follow-up (P = 0.7), and development of dysplasia (P = 1.0). The CD controls received more anti-tumor necrosis factor (TNF) than the PSC-CD group (78% vs 17%, P = 0.04), but otherwise the treatment regimens were similar.
Endoscopic data was available (not shown in tables) in all PSC-CD patients. Inflammation or focal strictures within terminal ileum were noted in 50% of the patients. Isolated right-sided colitis was noted in one patient (16%), pancolitis in two (33%) and normal/inactive disease was noted in the remaining three (50%). Colonoscopy reports correlated with the histologic findings in four patients (66.6%).
A total of 68 biopsies and 3 resections were examined (Table 2). Ileal involvement was noted in all PSC-CD patients (sampled in five out of six) and in 67% of the CD group. Although PSC-CD patients had higher activity scores in right colon (1.5 vs 1), this did not reach statistical significance (P = 0.3). Rectum was mostly spared in PSC- CD (20% vs 83% involvement) compared to CD (P = 0.055). Six of the CD patients were noted to have granulomas, whereas this was noted in only one PSC-CD patient (67% vs 17%, P = 0.08). Other histologic parameters were similar between the two groups.
PSC-UC patients compared to PSC-CD: The PSC-UC and the PSC-CD groups showed similar characteristics in terms of gender, age at biopsy, age at IBD diagnosis, disease duration of IBD and PSC, type of medical treatment, follow-up duration, and development of dysplasia (Table 1 and 2). Further, there was no significant difference between the two groups in age at PSC diagnosis and interval between PSC and IBD diagnosis. It is noteworthy that no PSC-CD patients required OLT in comparison to 10 of 26 patients (38%) with PSC-UC, however this difference did not reach statistical significance (P = 0.1).
Histologically, ileal involvement was more common in PSC-CD compared to PSC-UC (100% vs 31%, P = 0.9), however this did not reach statistical significance. In addition, the activity score in terminal ileum in the PSC-CD group was higher compared to PSC-UC (1.0 vs 0.31, P = 0.01). PSC-UC patients had more rectal involvement compared to PSC-CD (68% vs 20%, P = 0.07), whereas right-sided colitis was more common in the PSC-CD group (50% vs 29%, P = 0.3). All other histologic characteristics were similar.
PSC-no IBD patients compared to PSC-UC and PSC-CD: Four patients with PSC-no IBD were identified (not shown in tables). One patient was male, and the average age was 53 years, which was older than those in the PSC-UC and PSC-CD groups, but the difference did not reach statistical significance (P = 0.1 and P = 0.2). The average age at PSC diagnosis in the PSC-no IBD group was 51.8 years, which was older compared PSC-UC or PSC-CD groups (P = 0.053 and P = 0.09). The average PSC duration was 1.25 years, which was shorter than the duration observed in the PSC-UC and PSC-CD groups, but the difference did not reach statistical significance (P = 0.1 and P = 0.2). The mean follow-up duration for the PSC-no IBD group was 7 years. Only one of the four patients in the PSC-no IBD group required OLT. This was not significantly different than the rate of transplantation observed in the PSC-UC or PSC-CD groups (P = 0.6 and P = 0.9). All PSC-no IBD biopsies were histologically normal. All PSC-no IBD patients were alive at the time of data collection (2016).
Patients with OLT: A total of 11 patients received OLT (Table 3). Nine were men (89%) and 2 were women (19%). Ten of these patients had PSC-UC, and one had PSC-no IBD. Of note, none of the patients with PSC-CD required OLT. However, compared to PSC-UC, the difference did not reach statistical significance (P = 0.1). The demographic, clinical, and treatment modalities of patients with OLT did not differ from patients who did not require OLT (not shown in tables).
| Diagnosis | Gender | Age (yr) | Age at Dx of IBD (yr) | Age at Dx of PSC (yr) | Colitis duration (yr) | Colectomy | Time of sample | Distribution of IBD | Rectal involvement | Follow-up (yr) | Medical treatment for IBD |
| PSC UC | F | 45 | 19 | 40 | 26 | No | Post-OLTf | Right and left | Not involved | 8 | Ursodiol |
| PSC UC | F | 18 | 3 | 27 | 15 | Yes | Pre-OLT | Right and left | Involved | 15 | Azathioprine |
| PSC UC | M | 61 | 57 | 57 | 4 | No | Pre-OLT | Right and left | Involved | 10 | Mesalamine |
| PSC UC | M | 52 | 18 | 42 | 34 | Yes | Pre-OLT | Right and left | Involved | 16 | Ursodiol, azathioprine |
| PSC UC | M | 24 | 19 | 20 | 5 | Yes | Pre-OLT | Right and left | Involved | 7 | Steroids, mesalamine |
| PSC UC | M | 19 | 18 | 15 | 1 | No | Post-OLT | Right and left | Involved | 13 | Mesalamine |
| PSC UC | M | 22 | 20 | 20 | 2 | Yes | Post-OLT | Right and left | Involved | 4 | Mesalamine, azathioprine, infliximab, steroids |
| PSC UC | M | 55 | 52 | 40 | 3 | No | Post-OLT | Right only | Involved | 17 | Mesalamine |
| PSC UC | M | 60 | 34 | 58 | 26 | No | Pre-OLT | Left only | Involved | 4 | Mesalamine |
| PSC UC | M | 49 | 29 | 35 | 20 | Yes | Post-OLT | Right and left | Not involved | 10 | Steroids |
| PSC NIBD | M | 57 | NA | 57 | NA | NA | Pre-OLT | NA | NA | 14 | NA |
Five of eleven biopsies (45%) were obtained in the post-transplant period. Of these, three patients did not have material prior to OLT, and one developed IBD following OLT. The single patient who had a biopsy prior to OLT showed similar disease distribution compared to the biopsy that was included in the study.
Six biopsies were obtained pre-OLT. Four patients did not have material available for examination in the post-OLT period (Notably, one of these four patients had a recent biopsy, which showed normal colon throughout). Of the remaining two, one showed similar disease distribution, and the other one showed isolated right colon involvement (the biopsy included for analysis shows rectal involvement).
PSC-UC patients with OLT had more severe inflammation (higher activity scores) in the left colon compared with patients not requiring OLT (mean activity score of 1.7 vs 0.86) and a trend was observed (P = 0.06, not shown on tables).
Since none of the PSC-CD patients required OLT, analysis of histologic and clinical features in relation to OLT was not possible. Isolated right-sided disease and involvement of both right and left colon was noted in 3 patients each, whereas rectal involvement was seen in only one.
When PSC-UC and PSC-CD were combined as PSC-IBD, patients with PSC-IBD who had OLT showed a different phenotype characterized by having more rectal involvement (P = 0.04, Table 4). In addition, left colon and rectum were more inflamed (P = 0.04 for each respectively, Table 4). Multivariate analysis also confirmed that patients with OLT had severe colitis in their left colon (P = 0.04).
| PSC-IBD with OLT1 | PSC-IBD no OLT1 | P value (PSC-IBD with OLT vs PSC-IBD no OLT) | |
| Distribution | |||
| Right colon only | 1 (10) | 9 (30) | 0.1 |
| Left colon only | 1 (19) | 0 (0) | 0.3 |
| Right and left colon | 8 (80) | 11(37) | 0.2 |
| Rectum | 8 (80) | 8 (44) | 0.04a |
| Ileum | 2 (33) | 7 (58) | 0.6 |
| Dysplasia | 3 (30) | 1 (4.7) | 0.08 |
| Treatment | |||
| Ursodiol | 2 (20) | 9 (45) | 0.2 |
| Steroids | 3 (30) | 7 (33) | 1 |
| ASA | 6 (60) | 11 (52) | 1 |
| Anti-TNF | 1 (10) | 5 (24) | 0.6 |
| Immunomodulators | 3 (30) | 3 (14) | 0.3 |
| Colectomy | 5 (50) | 5 (22) | 0.2 |
| Severity of acute inflammation (average activity score) | |||
| Right colon | 1.5 | 1.2 | 0.4 |
| Left colon | 1.7 | 0.86 | 0.04a |
| Rectum | 1.3 | 0.6 | 0.04b |
| Ileum | 0.3 | 0.6 | 0.3 |
| IBD duration (yr) (mean ± SD) | 13.6 ± 12 | 8.5 ± 8.8 | 0.3 |
The clinical characteristics of PSC-UC compared to UC have been the topic of a number of studies. The majority of these describe this entity predominantly based on endoscopic, and clinical findings with little to no details of pathologic features[8,23]. However only a handful of investigators report some histologic features[5,15]. In our opinion, the first and most detailed histologic description of PSC-UC is provided by Joo et al[5]. Our group further extends some of their observations not only to PSC-UC patients but also to PSC-CD and PSC-no IBD groups with histologic characterization and studied their correlation with clinical outcomes and OLT.
Although considered as a different phenotype of IBD, some controversy remains as to the precise characterization of the histologic and endoscopic features of PSC-UC. Some authors have observed increased pancolitis in PSC-UC compared to UC[5,6], whereas others have noted an increased right-sided distribution in PSC-UC patients[7]. Loftus et al[8] found significantly more pancolitis in the PSC-IBD group compared to UC. However, their study group is heterogeneous and included both PSC-CD and PSC patients with indeterminate colitis. In addition, distribution of disease was determined by review of endoscopic and/or pathology reports, but a secondary review of the pathology material was not performed. Isolated right-sided disease (1 patient) or right colon predominant disease (4 patients) was noted in a small percentage of our PSC-UC patients by endoscopy. However, histologic examination showed that PSC-UC patients had more isolated right-sided disease (29% vs 4%, P = 0.03) compared to UC control patients further reiterating the value of pathologic examination. Ileal involvement was also more commonly noted in PSC-UC (31% vs 7%), although not statistically significant. No significant difference in pancolitis was observed (67% vs 63%) between the two groups.
The severity of disease also appears to be different in PSC-UC. Most literature reports a more severe disease in right colon compared to left and less severe disease overall[5,7,15]. Backwash ileitis and rectal sparing in this group of patients were also more commonly reported compared to UC patients without PSC[15,8].
Similar to the literature, PSC-UC patients in our cohort also had more inflammatory activity in right colon compared to UC patients (average activity score of 1.25 vs 0.96), although this did not reach statistical significance. PSC-UC patients also had milder colitis of the left colon (P = 0.03) and rectum (P = 0.0006), and a lower activity score overall (P = 0.0005) compared to UC patients, confirming the previously reported findings. Ileal inflammation was also more severe in PSC-UC but this did not reach statistical significance. In addition, basal plasmacytosis was less frequently noted in the left colon and rectum of PSC-UC patients compared to UC patients (P = 0.01 and P = 0.0004), suggesting less chronic damage in the distal colon and rectum in the former.
Clinically, PSC-UC also manifests itself as a “milder form of colitis” compared to UC with no PSC, requiring less steroid treatments, fewer hospitalization and even fewer colectomies[23,24]. Our results are also in keeping with this. In our study, PSC-UC patients required fewer courses of steroids (31% vs 68%, P = 0.01) and fewer colectomies (38% vs 68%, P = 0.03) compared to, age- and IBD duration-matched, patients with UC only. It has been noted that patients with PSC-UC have higher rates of dysplasia and colorectal malignancy compared to patients with UC only[25,26]. In our cohort, however, similar rates of dysplasia/cancer were observed in PSC-UC patients and UC patients (15% compared to 18%, P = 0.8).
Though PSC is much more frequently associated with UC, a minority of PSC patients will have CD. The number of patients with PSC-CD is very limited and histopathologic features are poorly characterized. To best of our knowledge, our study is the first to evaluate this difference on the basis of detailed microscopic evaluation. None of the PSC-CD patients had classic histologic features of CD and, they showed some differences compared to CD controls. First, a male predominance was noted (83% vs 22%, P = 0.03). Histologically involvement of ileum was more common (100% vs 67%, P = 0.9), and more cases had isolated right colon disease (50% vs 33%, P = 0.3). Rectal disease on the other hand was more common in the CD group (83% vs 20%, P = 0.055), which was also more inflamed (1.17 vs 0.2, P = 0.1). Granulomas were less frequently identified in PSC-CD compared to CD (17% vs 67%, P = 0.08). Although, terminal ileum strictures were noted in two PSC-CD patients, isolated small intestine disease was not noted in any of them. Clinically, CD patients received more anti-TNF compared to PSC-CD (78% vs 17%, P = 0.04). Loftus et al[8] describe similar features in their 5 PSC-CD patients and report that only 2 of these had diagnostic features of CD. In a Norwegian study, none of the 15 PSC-CD subjects were noted to have characteristic features of CD; in addition some of these were later classified as PSC-UC[15]. None of our PSC-CD subjects has been re-classified to PSC-UC at the time of data collection, and in general they have milder disease than CD patients.
Differences in disease distribution and severity were also noted between PSC-CD and PSC-UC. PSC-CD patients had more right sided (50% vs 29%, P = 0.3) and ileal disease (100% vs 31%, P = 0.9) compared to PSC-UC, whereas rectal involvement was more common in PSC-UC (68% vs 20%, P = 0.07). When compared to PSC-UC patients, the severity of inflammation in ileum (1 vs 0.31) was more profound in PSC-CD. PSC-CD did not significantly differ from PSC-UC in terms of patient characteristics, treatment modalities and clinical outcomes. It is likely that only the two patients with ileal strictures indeed represent PSC-CD in our cohort and the rest simply represents PSC-UC. However, the presence and severity of ileal disease compared to PSC-UC patients is compelling. Of note, distinction between PSC-CD vs PSC-UC was done by the treating gastroenterologist and it’s certainly possible that these patients had other clinical findings that were not explicitly discussed in the patient’s medical record, which led to the diagnosis. According to Jorgensen et al[15], “True PSC-CD” is a very rare entity and all cases of PSC-UC and PSC-CD should be classified as PSC-IBD. Such a conclusion however, will require detailed histologic analysis similar to our study but in a larger patient population.
In addition to the PSC-CD patients, we also describe the clinical features of a small but unique group of patients with PSC but no IBD for the first time in the literature. These four patients had a mean follow-up of 7 years and showed similar clinical characteristics to PSC-UC and PSC-CD. Although this is a very small group of patients, and they still have the potential to develop IBD later, further study of these patients may provide insights into mechanisms for developing IBD. Whether this can be explained based on the microbiome[11], liver-gut cross talk, undetected differences in inflammatory cell subtypes, genetic tendencies or a combination of these remains to be studied.
It has been noted that there is an increase in IgG4 positive plasma cells in patients with active and inactive PSC-UC compared to inactive UC, and active and inactive CD[9] in rectal biopsies, suggesting that IgG4 positive cells may play a role in pathogenesis. We investigated this in our study population and stained the most inflamed biopsy with CD3, CD20, and IgG4. Although PSC-UC patients had higher numbers of IgG4 positive plasma cells, no difference in IgG4 cell counts were observed between PSC-UC and UC, PSC-CD and CD or PSC-UC and PSC-CD groups (not shown on tables). It should be noted that increased numbers of IgG4 positive plasma cells could be observed in a variety of inflammatory conditions[27]. Typically, IgG4 related disorders display not only a lymphoplasmacytic infiltrate with increased numbers of tissue IgG4 plasma cells (or increased IgG4: IgG ratio), but also with accompanying storiform fibrosis, and obliterative phlebitis[28]. Therefore, in the absence of any of these findings, it is hard to make an argument that either PSC-UC or PSC-CD is an IgG4 driven processes.
It has been reported that the severity of IBD in patients with PSC-IBD can be related to the severity of the PSC. Marelli et al[17] found that PSC-UC patients that required OLT had a milder clinical course and less inflammation on histology compared to PSC-UC not requiring OLT. Similar observations have been noted by others[15,16]. However, others report that the severity of IBD in PSC correlates with liver disease both in humans and animal models, and patients with severe IBD tend to get recurrent PSC in the allograft[14,19,20]. In our PSC-UC cohort, the severity of colitis in left colon showed a trend with OLT (P = 0.06, not shown on tables). When PSC-UC and PSC-CD groups are combined as PSC-IBD, patients with OLT had a disease phenotype characterized by rectal involvement (P = 0.04) and higher left colonic and rectal activity scores (P = 0.04 for each respectively). Severe inflammation of the left colon was also noted to be statistically significant in OLT patients in multivariate analysis (P = 0.04). It is certainly possible that changes in treatment modalities following transplantation have played a role and caused recurrent disease[29] but this was not statistically significant in our cohort. Our results may be hindered by the heterogenous nature of their timeline (pre vs post-OLT), but we tried to overcome such biases by selecting the most inflamed set of samples, which likely represents the disease phenotype of patient's IBD. Nevertheless, severe inflammation of the left colon and rectum in PSC-IBD patients with OLT appears to be different than the “usual” pattern of disease in PSC-IBD, which is predominantly a right sided disease that manifests with milder inflammation in the left colon. Previous studies have supported the idea that the inflammatory state of the gut and the liver depend on each other. Although our statistical power is limited by our small cohort, based on our findings and other reports, it is not completely unreasonable to hypothesize that severe left sided colitis in our patients correlated with the need for OLT and alterations in liver-gut crosstalk may very likely have contributed to this potential phenotypic change[13,19,20,30]. Unfortunately, data for disease recurrence is not available in our cohort, therefore it’s unclear if severe left sided colitis also leads to recurrent PSC in the allograft.
In summary, our study confirms that PSC-UC is a distinct type of IBD based on the clinical and histologic findings in keeping with previous observations in the literature. PSC-CD, a cohort that has not been extensively studied before, showed more ileal and right colon involvement and rectal sparing compared to PSC-UC, but similar clinical features in general. These further supports the idea that PSC-CD and PSC-UC are part of a distinct PSC-IBD phenotype, but warrants further exploration. Similarly, PSC-no IBD patients may represent a distinct entity, and additional analysis of this unique but limited group of patients may provide insights into mechanisms and triggers for development of IBD in patients with PSC. PSC-IBD patients with OLT appear to have more frequent involvement of rectum and the severity of left sided colitis appears to be worse compared to patients who didn’t receive OLT. This is an interesting disease phenotype that likely deserves clinical attention.
Our study has some limitations including sample size, heterogeneity of the specimens and potential referral bias of the patients. Despite the fact that we are a major referral center and we collected data from over a 10-year period, the case number is limited by the rarity of this entity. The strengths of the study include detailed clinical, histologic characterization and description of distinct groups including PSC-no IBD and PSC-CD. In addition, histologic and clinical findings of patients with OLT are described. Our study makes interesting observations in these groups, which have not been reported before. Since the ultimate patient outcomes are related to the liver disease, it is important to identify the disease characteristics of IBD in PSC patients that correlate with the need for OLT regardless of the IBD type. We hope our observations can be extended to larger cohorts, which may ultimately change our understanding of this disease and also how we treat our patients.
Primary Sclerosing cholangitis (PSC) associated inflammatory bowel disease (IBD) is a unique form of IBD seen in patients with PSC. It has been characterized as to have right sided predominance in colon with less severe active inflammation. Most cases of PSC-IBD are classified as ulcerative colitis (UC), whereas rare cases exhibit features of Crohn disease. There is thought to be a link between the IBD severity and the progression of PSC.
The need for better characterization of the pathologic findings in PSC-IBD patients and its association with liver transplantation may further support the “gut-liver axis” theory and has potential clinical implications. In addition, little is known regarding PSC-Crohn disease and its clinical outcomes.
The primary aims in this study were to characterize the colon and ileal findings in PSC patients at a tertiary care center, better define the histologic features of PSC-IBD, and explore if there is any correlation between the intestinal disease and liver transplant status, since this can impact patient management.
This retrospective study was conducted in a single tertiary care center. Based on data search, cases with PSC and lower gastrointestinal biopsies were identified. Care was taken to examine the most inflamed biopsy. The hematoxylin and eosin slides were re-reviewed and several morphologic features were recorded. Pertinent clinical data was collected.
Our study confirmed the previously reported histologic findings in PSC-UC patients including a predominantly right sided involvement with overall less severity. We also described detailed histologic features of PSC-Crohn disease (CD) patients. None of the PSC-CD patients required liver transplantation, in contrast to ten PSC-UC patients. In our study, there was no correlation between the clinical parameters or treatment and orthotopic liver transplantation (OLT). When all PSC-IBD patients were analyzed together, severe left sided colitis correlated with the need for OLT.
In our cohort, PSC-IBD patients with severe left sided and rectal disease required OLT more commonly than other PSC-IBD patients. This is rather interesting, since it may indicate that these patients are at increased risk for progression of their liver disease and this has not been reported before.
The findings in this study further support the notion that gut and liver interact through several different mechanisms. Our results raise the possibility that an only a subset of patients with PSC-IBD (severe disease activity in left colon in our cohort) may be at increased risk for faster progression of liver disease, and eventually receive OLT. However, the contribution of other factors such as microbiome, genetic underpinnings, or others remain unanswered and should be further studied.
A portion of this work was presented at the 2015 United States and Canadian Academy of Pathology Annual Meeting in Boston, MA. The authors would like to acknowledge the support of Department of Pathology and Immunology, Lauren V. Ackerman Laboratory of Surgical Pathology, Washington University in St. Louis, School of Medicine for this study and Washington University in St. Louis School of Medicine Anatomic and Molecular Pathology (AMP) Laboratory for the technical support.
| 1. | Quaglia A. Primary Sclerosing Cholangitis. In: Saxena R. Practical Hepatic Pathology: A Diagnostic Approach. Philadelphia, PA: Elsevier Saunders, 2011: 383-392. |
| 2. | Smith MP, Loe RH. Sclerosing cholangitis; review of recent case reports and associated diseases and four new cases. Am J Surg. 1965;110:239-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Takikawa H, Manabe T. Primary sclerosing cholangitis in Japan--analysis of 192 cases. J Gastroenterol. 1997;32:134-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Atkinson AJ, Carroll WW. Sclerosing cholangitis. Association with regional enteritis. JAMA. 1964;188:183-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 16] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Joo M, Abreu-e-Lima P, Farraye F, Smith T, Swaroop P, Gardner L, Lauwers GY, Odze RD. Pathologic features of ulcerative colitis in patients with primary sclerosing cholangitis: a case-control study. Am J Surg Pathol. 2009;33:854-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Boonstra K, van Erpecum KJ, van Nieuwkerk KM, Drenth JP, Poen AC, Witteman BJ, Tuynman HA, Beuers U, Ponsioen CY. Primary sclerosing cholangitis is associated with a distinct phenotype of inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2270-2276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 7. | Sano H, Nakazawa T, Ando T, Hayashi K, Naitoh I, Okumura F, Miyabe K, Yoshida M, Takahashi S, Ohara H, Joh T. Clinical characteristics of inflammatory bowel disease associated with primary sclerosing cholangitis. J Hepatobiliary Pancreat Sci. 2011;18:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Loftus EV, Harewood GC, Loftus CG, Tremaine WJ, Harmsen WS, Zinsmeister AR, Jewell DA, Sandborn WJ. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 529] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 9. | Raina A, Yadav D, Regueiro M, Krasinskas AM, Saul MI, Sapienza DA, Binion DG, Hartman DJ. Mucosal IgG4 cell infiltration in ulcerative colitis is linked to disease activity and primary sclerosing cholangitis. Inflamm Bowel Dis. 2013;19:1232-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Karlsen TH, Hampe J, Wiencke K, Schrumpf E, Thorsby E, Lie BA, Broomé U, Schreiber S, Boberg KM. Genetic polymorphisms associated with inflammatory bowel disease do not confer risk for primary sclerosing cholangitis. Am J Gastroenterol. 2007;102:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Kummen M, Holm K, Anmarkrud JA, Nygård S, Vesterhus M, Høivik ML, Trøseid M, Marschall HU, Schrumpf E, Moum B, Røsjø H, Aukrust P, Karlsen TH, Hov JR. The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut. 2017;66:611-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 327] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 12. | Quraishi MN, Acharjee A, Beggs AD, Horniblow R, Tselepis C, Gkoutos G, Ghosh S, Rossiter A, Loman N, van Schaik W, Withers D, Walters JRF, Hirschfield GM, Iqbal TH. A pilot integrative analysis of colonic gene expression, gut microbiota and immune infiltration in primary sclerosing cholangitis-inflammatory bowel disease: association of disease with bile acid pathways. J Crohns Colitis. 2020; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 13. | Eksteen B. The Gut-Liver Axis in Primary Sclerosing Cholangitis. Clin Liver Dis. 2016;20:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Mathies F, Steffens N, Kleinschmidt D, Stuhlmann F, Huber FJ, Roy U, Meyer T, Luetgehetmann M, von Petersdorff M, Seiz O, Herkel J, Schramm C, Flavell RA, Gagliani N, Krebs C, Panzer U, Abdullah Z, Strowig T, Bedke T, Huber S. Colitis Promotes a Pathological Condition of the Liver in the Absence of Foxp3+ Regulatory T Cells. J Immunol. 2018;201:3558-3568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Jørgensen KK, Grzyb K, Lundin KE, Clausen OP, Aamodt G, Schrumpf E, Vatn MH, Boberg KM. Inflammatory bowel disease in patients with primary sclerosing cholangitis: clinical characterization in liver transplanted and nontransplanted patients. Inflamm Bowel Dis. 2012;18:536-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Navaneethan U, Venkatesh PG, Mukewar S, Lashner BA, Remzi FH, McCullough AJ, Kiran RP, Shen B, Fung JJ. Progressive primary sclerosing cholangitis requiring liver transplantation is associated with reduced need for colectomy in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2012;10:540-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Marelli L, Xirouchakis E, Kalambokis G, Cholongitas E, Hamilton MI, Burroughs AK. Does the severity of primary sclerosing cholangitis influence the clinical course of associated ulcerative colitis? Gut. 2011;60:1224-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Joshi D, Bjarnason I, Belgaumkar A, O'Grady J, Suddle A, Heneghan MA, Aluvihare V, Rela M, Heaton N, Agarwal K. The impact of inflammatory bowel disease post-liver transplantation for primary sclerosing cholangitis. Liver Int. 2013;33:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Peverelle M, Paleri S, Hughes J, De Cruz P, Gow PJ. Activity of Inflammatory Bowel Disease After Liver Transplantation for Primary Sclerosing Cholangitis Predicts Poorer Clinical Outcomes. Inflamm Bowel Dis. 2020; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Buchholz BM, Lykoudis PM, Ravikumar R, Pollok JM, Fusai GK. Role of colectomy in preventing recurrent primary sclerosing cholangitis in liver transplant recipients. World J Gastroenterol. 2018;24:3171-3180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Broomé U, Bergquist A. Primary sclerosing cholangitis, inflammatory bowel disease, and colon cancer. Semin Liver Dis. 2006;26:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 158] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 22. | Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K, Jewell DP, Karban A, Loftus EV, Peña AS, Riddell RH, Sachar DB, Schreiber S, Steinhart AH, Targan SR, Vermeire S, Warren BF. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A-36A. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2148] [Cited by in RCA: 2429] [Article Influence: 202.4] [Reference Citation Analysis (1)] |
| 23. | Lundqvist K, Broomé U. Differences in colonic disease activity in patients with ulcerative colitis with and without primary sclerosing cholangitis: a case control study. Dis Colon Rectum. 1997;40:451-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 102] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Moayyeri A, Daryani NE, Bahrami H, Haghpanah B, Nayyer-Habibi A, Sadatsafavi M. Clinical course of ulcerative colitis in patients with and without primary sclerosing cholangitis. J Gastroenterol Hepatol. 2005;20:366-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Jayaram H, Satsangi J, Chapman RW. Increased colorectal neoplasia in chronic ulcerative colitis complicated by primary sclerosing cholangitis: fact or fiction? Gut. 2001;48:430-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Soetikno RM, Lin OS, Heidenreich PA, Young HS, Blackstone MO. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc. 2002;56:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 392] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 27. | Strehl JD, Hartmann A, Agaimy A. Numerous IgG4-positive plasma cells are ubiquitous in diverse localised non-specific chronic inflammatory conditions and need to be distinguished from IgG4-related systemic disorders. J Clin Pathol. 2011;64:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 250] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 28. | Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, Klöppel G, Heathcote JG, Khosroshahi A, Ferry JA, Aalberse RC, Bloch DB, Brugge WR, Bateman AC, Carruthers MN, Chari ST, Cheuk W, Cornell LD, Fernandez-Del Castillo C, Forcione DG, Hamilos DL, Kamisawa T, Kasashima S, Kawa S, Kawano M, Lauwers GY, Masaki Y, Nakanuma Y, Notohara K, Okazaki K, Ryu JK, Saeki T, Sahani DV, Smyrk TC, Stone JR, Takahira M, Webster GJ, Yamamoto M, Zamboni G, Umehara H, Stone JH. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25:1181-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1826] [Article Influence: 130.4] [Reference Citation Analysis (0)] |
| 29. | Papatheodoridis GV, Hamilton M, Mistry PK, Davidson B, Rolles K, Burroughs AK. Ulcerative colitis has an aggressive course after orthotopic liver transplantation for primary sclerosing cholangitis. Gut. 1998;43:639-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 104] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Trivedi PJ, Adams DH. Gut-liver immunity. J Hepatol. 2016;64:1187-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Kim J S-Editor: Liu M L-Editor: A E-Editor: Zhang YL