Published online Jul 28, 2020. doi: 10.3748/wjg.v26.i28.4094
Peer-review started: January 30, 2020
First decision: April 22, 2020
Revised: May 21, 2020
Accepted: June 25, 2020
Article in press: June 25, 2020
Published online: July 28, 2020
Processing time: 180 Days and 1 Hours
Endoplasmic reticulum (ER) stress is an important mechanism in the progression of chronic and acute liver diseases, especially in the progression and recovery of liver fibrosis. Excessive and long-term ER stress induces apoptosis. ER stress-induced apoptosis is considered to be an important pathway in the development of liver fibrosis. Cyclooxygenase-2 (COX-2) induction is also closely related to ER stress. In our previous studies, we showed that celecoxib, a COX-2 inhibitor, improves liver fibrosis and portal hypertension. However, the role and mechanism of celecoxib in alleviating liver fibrosis remain unclear.
To investigate whether celecoxib alleviates liver fibrosis by inhibiting hepatocyte apoptosis via the ER stress response.
Cirrhosis was induced by intraperitoneal injections of thioacetamide (TAA) for 16 wk (injection dose is 200 mg/kg per 3 d for the first 8 wk and 100 mg /kg per 3 d after 8 wk). Thirty-six male Sprague-Dawley rats were randomly divided into three groups, namely, control group, TAA group, and TAA + celecoxib group. In the last 8 wk, TAA-induced cirrhotic rats received celecoxib (20 mg/kg/day) or the vehicle by gastric gavage. After 16 wk, the rats were sacrificed, and serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and albumin (ALB) were detected. The hepatic fibrosis areas were evaluated by Sirius red staining and the degree of fibrosis was assessed by measuring the level of hydroxyproline. ER stress levels were evaluated by detecting the marker proteins glucose-regulated protein 78 (GRP78), CCAAT/enhancer binding protein homologous protein (CHOP), PKR-like ER protein kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring enzyme 1 alpha (IRE1α). Apoptosis levels were evaluated by detecting caspase-12 and caspase-3.
The serum ALT and AST levels in the liver were significantly reduced by celecoxib; however, the serum ALB had no significant changes. Celecoxib significantly reduced the degree of liver fibrosis and the levels of hydroxyproline (-38% and -25.7%, respectively, P < 0.01). Celecoxib ameliorated ER stress by reducing the level of GRP78 compared to the TAA group (P < 0.05). Consistently, after celecoxib administration, the upregulation of TAA-induced hepatic apoptosis markers (caspase-12 and caspase-3) and CHOP were significantly inhibited. In addition, after celecoxib treatment, the expression of key molecules associated with ER stress (PERK, ATF6, and IRE1) was decreased (P < 0.05).
Therapeutic administration of celecoxib effectively reduces hepatic apoptosis in TAA-induced cirrhotic rats. The mechanism of action may be attributed to the suppression of CHOP expression, which subsequently inhibits ER stress.
Core tip: This study aimed to explore the important role of celecoxib in modulating hepatocyte apoptosis during the development of liver fibrosis, and to clarify that the mechanism of its regulation is mediated by endoplasmic reticulum stress, which in turn affects the progression of liver fibrosis. We concluded that therapeutic administration of celecoxib can effectively reduce hepatic apoptosis in thioacetamide-induced cirrhotic rats. The mechanism of action may be attributed to the suppression of CCAAT/enhancer binding protein homologous protein expression, which subsequently inhibits endoplasmic reticulum stress.
- Citation: Su W, Tai Y, Tang SH, Ye YT, Zhao C, Gao JH, Tuo BG, Tang CW. Celecoxib attenuates hepatocyte apoptosis by inhibiting endoplasmic reticulum stress in thioacetamide-induced cirrhotic rats. World J Gastroenterol 2020; 26(28): 4094-4107
- URL: https://www.wjgnet.com/1007-9327/full/v26/i28/4094.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i28.4094
Liver fibrosis is the outcome of hepatocellular injury-repair reactions caused by chronic viral hepatitis, alcoholism, autoimmune diseases, genetic and metabolic disorders, and repeated and sustained liver injury[1,2]. The pathological process of liver fibrosis is based on long-term, sustained action of various injuries, including hepatic parenchymal cell necrosis, apoptosis, and collagen synthesis, which leads to abnormal deposition of the extracellular matrix (ECM) in liver tissue, a pathological process that further triggers abnormal changes in liver structure and liver function. Hepatic stellate cells (HSCs) play a significant role in maintaining liver homeostasis, and they will undergo phenotypic conversion into myofibroblast cells under sustained liver injury, which express smooth muscle actin, cause ECM deposition, and promote liver fibrosis[3,4]. Additionally, the presence and exacerbation of inflammation are another important factor in the occurrence and development of liver fibrosis[5,6]. It is known that liver fibrosis can be reversed after removing the damage factors. Therefore, to explore a novel and targeted therapy is very useful for the treatment of liver fibrosis.
Recently, many studies have shown that hepatocyte apoptosis is the most basic central link that causes liver damage and liver disease[7]. There is plenty of endoplasmic reticulum (ER) in liver cells, and many liver diseases are related to ER stress and ER stress-mediated apoptosis, such as viral hepatitis, liver fibrosis, non-alcoholic fatty liver disease, alcoholic liver disease, acute liver failure, drug-induced liver disease, and liver cancer. A variety of metabolic, toxic, and inflammatory injuries can cause liver damage and disease. A common feature of these injuries is the activation of apoptosis and/or necrosis[8,9].
Therefore, inhibiting hepatocyte apoptosis and reducing liver tissue damage and the severity of inflammation are of great importance in delaying the progression of liver fibrosis. Recent studies have shown that ER stress is a critical event in the development of liver fibrosis[1,2,5,8]. However, the functional alteration in ER stress and its potential role in liver fibrosis are still unclear. The ER stress response is a self-protective mechanism for cell adaptation, but excessive or long-lasting ER stress causes apoptosis[10].
The ER has a high dynamic balance under normal circumstances. However, ER stress is triggered in response to various physiological or pathological stimuli, especially when the ability of accumulation of unfolded proteins within the ER exceeds the folding capacity of ER chaperones[11,12]. ER stress activates the adaptive unfolded protein response (UPR) and promotes the molecular chaperone protein glucose-regulated protein 78 (GRP78) dissociation[13]. ER stress or UPR transducers contain three transmembrane proteins: PKR-like ER protein kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring enzyme 1 alpha (IRE1α). When the ER stress response is started, the signaling proteins PERK, ATF6, and IRE1α are activated and dissociate from GRP78, thereby activating the UPR. Apoptosis occurs by increasing the activity of the proapoptotic transcription factor C/EBP homologous protein [CCAAT/enhancer binding protein homologous transcription factors (CHOP)] under prolonged ER stress, which rapidly increases through activation of the above-mentioned signaling proteins. Various injury factors activate the ER stress response, leading to liver fibrosis, atherosclerosis, ischemic heart disease, and myocardial injury[12,14,15]. Therefore, the search for drugs that effectively inhibit the ER stress response is of great significance for the treatment of liver diseases.
Extensive studies have confirmed that increased expression of cyclooxygenase-2 (COX-2) is related to the progression of fibrosis[16]. Therefore, we hypothesized that COX-2 plays an important role in the formation of liver fibrosis, and selective COX-2 inhibitors may have antifibrotic effects[17]. Our previous studies have shown that inhibition of COX-2 alleviates liver fibrosis and portal hypertension[18]. However, the mechanism by which inhibition of COX-2 alleviates liver fibrosis is still unclear. Celecoxib is a common selective COX-2 inhibitor, and due to its safety and effectiveness, it is currently widely used in the treatment of rheumatism and osteoarthritis[7]. Moreover, celecoxib has a pro-apoptotic effect on HSCs, the mechanism of which is to inactivate Akt in response to bile duct ligation and thioacetamide (TAA)[19,20]. Our previous study showed that long-term use of celecoxib effectively improves portal hypertension in a rat model of TAA-induced cirrhosis because it has dual inhibitory effects on intrahepatic angiogenesis and fibrosis[18]. In the present study, we explored the effects of celecoxib in reducing liver fibrosis by inhibiting the expression of biomarkers in the ER stress signaling pathway and reducing hepatic apoptosis in a rat model of TAA-induced cirrhosis.
Sprague–Dawley rats were provided by the Experimental Animal Center of Sichuan University, which were reared under conditions of constant temperature and humidity with a 12-h light-dark cycle. Animal experiments were approved by the Animal Use and Nursing Committee of Sichuan University and conducted according to regulations formulated by Sichuan University.
TAA (Sigma Chemical Company, St. Louis, Missouri, United States) was used to induce cirrhosis. The dose was 200 mg/kg per 3 d for 16 wk by intraperitoneal injection (i.p). Thirty-six male Sprague-Dawley rats weighing 180-230 g were randomly divided into three groups: Control, TAA, and TAA + celecoxib groups (12 rats per group). The administration of TAA + celecoxib (20 mg/kg per day, Pfizer, New York, NY, United States) began with TAA administration. The TAA group was injected with TAA + placebo, and the control group was injected with normal saline (1 mL, i.p., every 3 d). One week after the last treatment, all rats were anesthetized to avoid acute toxic effects. After the rats were sacrificed, the liver was cut into two parts. One part was fixed in 4% neutral buffered paraformaldehyde for histopathological and immunohistochemical analysis, and the other part was frozen at -80°C for further analysis. Serum samples were also collected and frozen at -80 °C for further analysis.
The general morphology of the liver at the time of sacrifice was observed, and the degree of cirrhosis was evaluated. The liver tissue was embedded in paraffin, sectioned (3 μm thick), and stained with hematoxylin-eosin (HE) for histological evaluation and with Sirius red for assessment of collagen deposition. The Ishak scoring system was used to randomly evaluate the fibrosis degree in three randomly selected fields (100 × magnification) per section. Liver tissue for transmission electron microscopy (TEM; H-600IV, Hitachi, Tokyo, Japan) was processed according to standard methods for observing morphological changes.
Paraffin sections were incubated with 3% H2O2 at 37°C for 10 min. After blocking for 20 min at room temperature, the sections were incubated overnight with primary antibody at 4°C, followed by incubation with a secondary antibody for 30 min at 37°C. Finally, a signal detection system (ZSGB-BIO) with diaminobenzidine was used as the substrate for coloration, with brown staining indicating positive staining. Five fields were randomly selected from each group, with six rats in each group. Antibodies to GRP78, CHOP, ATF6, ATF4, PERK, IRE1, and X-box binding protein-1 (XBP1) were purchased from Santa Cruz Biotechnology (CA, US) and Proteintech Group, Inc. (Wuhan, China). The secondary antibodies were purchased from Santa Cruz Biotechnology.
The Olympus AU2700 analyzer (Olympus, Hamburg, Germany) was used to detect serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, creatinine, and bilirubin levels according to the recommendations of the International Federation of Clinical Chemistry.
Hydroxyproline is the main component of collagen, and the content of which is an important indicator of the degree of collagen metabolism and fibrosis. In this study, the modified classical acid decomposition method was used to calculate the hydroxyproline content, and the specific steps were carried out according to the kit instructions.
The frozen liver tissue was homogenized in ice-cold RIPA buffer (Biosharp, Guangzhou, China) to prepare total protein extracts. Equal amounts (50 μg) of protein in each container were separated by 10% or 12% SDS-PAGE, and then transferred to PVDF membranes (Millipore, Billerica, MA, United States), followed by incubation with 2.5% skimmed milk powder in TBST Block (20 mmol/L Tris-HCl pH 7.5, 150 mmol/L NaCl and 0.1% Tween-20). Using glyceraldehyde 3-phosphate dehydrogenase as an internal reference, the expression of immunoreactive protein was detected using an ECL detection kit (Beyotime).
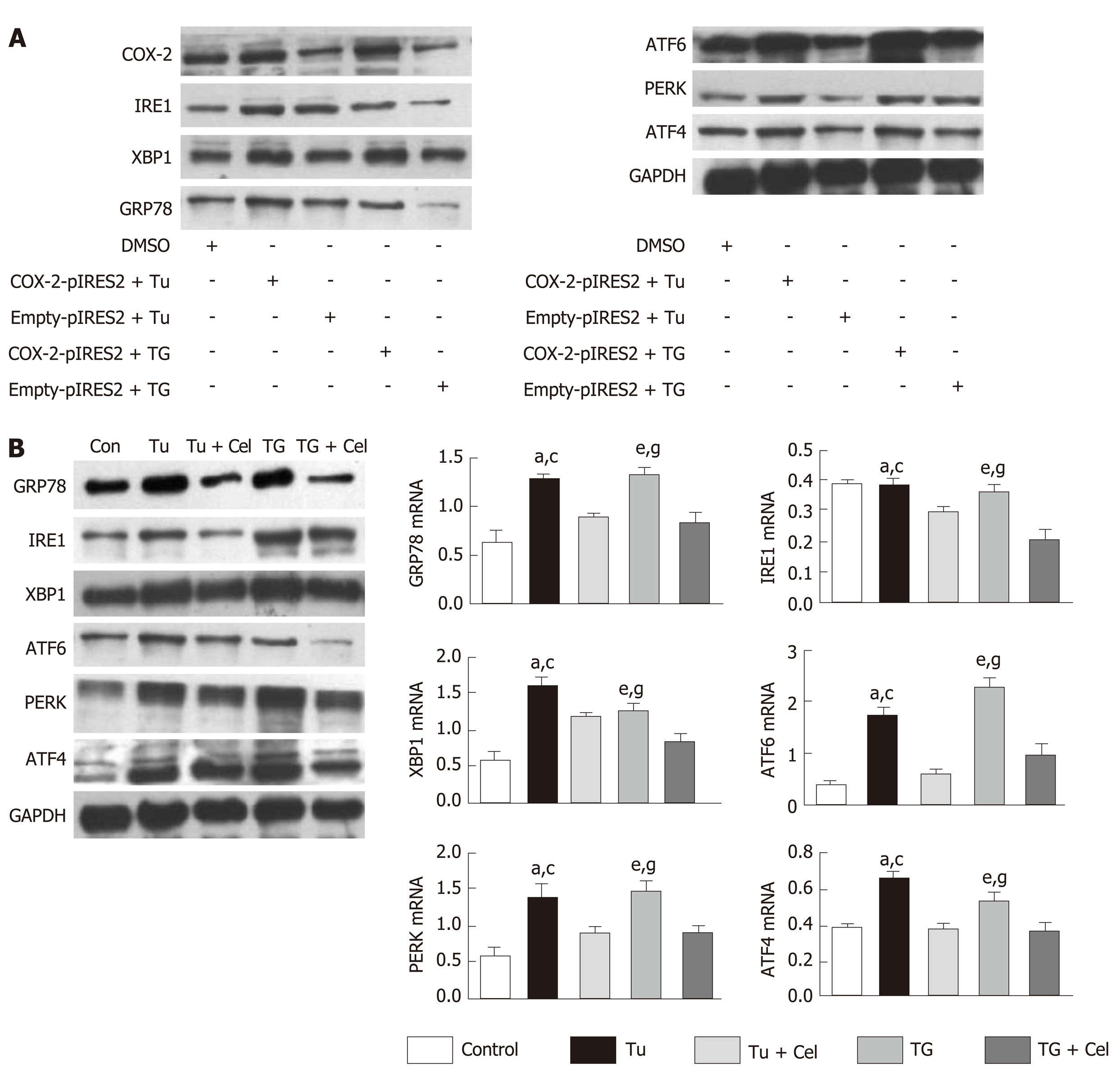
The hepatocyte cell line L02 was cultured, and the COX–2 plasmid and vacuolar plasmid (control) were added after the cells were attached to the wall. After 24 h of transfection, tunicamycin (Tu) and thapsigargin (TG) were separately added to induce the occurrence of ER stress, followed by determination of ER stress channel–associated proteins (GRP78, XBP1, ATF4, ATF6, PERK, and IRE1), thereby inferring the relationship between COX–2 and ER stress. Meanwhile, the expression of ER stress pathway–associated proteins was detected as well after intervention with celecoxib.
All results are expressed as the mean ± SD and analyzed with SPSS 20.0 software (SPSS, Chicago, IL, United States). One–way ANOVA was applied for comparisons among three or more groups. The Student-Newman-Keuls test was used to compare the difference between two groups. A P value < 0.05 was considered significant.
The effect of TAA on liver damage was evaluated by measuring the serum levels of AST and ALT. As shown in Figure 1A, the serum concentrations of AST and ALT in the TAA group were significantly increased compared with those in the control group, while the levels of both AST and ALT in the TAA group were higher than those in the TAA + celecoxib group, indicating that celecoxib significantly attenuated TAA-induced hepatocyte injury. In addition, the increased hydroxyproline concentration in the TAA group was significantly decreased in the TAA + celecoxib group. Severe pathological changes, such as structural rearrangement of hepatic lobules and formation of bridging fibrosis around cells, were shown by HE staining in the liver tissue of the TAA group, while these changes were significantly reduced in the TAA + celecoxib group. This result was also evidenced by damage to or the death of hepatocytes (Figure 1).
Liver sections of both the TAA group and TAA + celecoxib group showed a significant increase in collagen around the extracellular space, especially in the portal vein[18]. However, compared with the TAA group, the collagen concentration in the TAA + celecoxib group was significantly reduced (Figure 1B)
Compared with the control group, a large amount of ECM accumulated in the liver of animals in the TAA group, causing structural disruption and destruction, and hepatocytes were lost, forming continuous fibrous septa, central venous distortion, and regenerative nodules, although celecoxib treatment reduced the progression of liver fibrosis significantly. The Ishak score and percentage of fibrotic area in the TAA group were significantly higher than those of the TAA + celecoxib group (P < 0.05 and P < 0.01). The liver of animals in the normal control group was soft and red in color, while the liver of animals in the TAA group showed cirrhosis that was characterized by extensive nodular and continuous fibrotic septa. These cirrhotic lesions were significantly relieved after treatment with celecoxib, resulting in a nearly normal liver appearance in the TAA + celecoxib group.
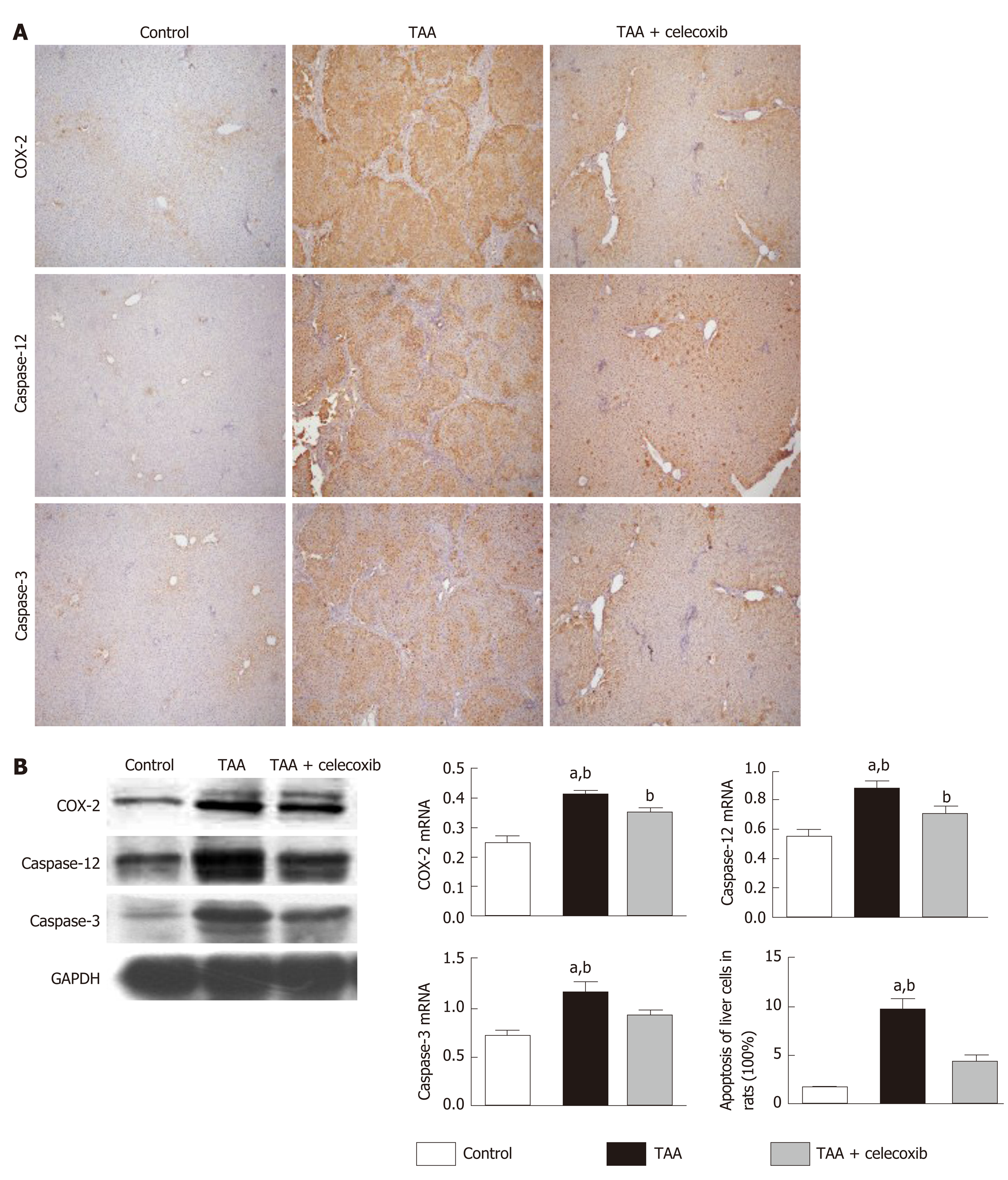
As shown in Figure 2A and B, compared with the control group, the protein level of COX-2 was significantly increased in the TAA group, while COX-2 expression was significantly reduced after celecoxib administration. In addition, the collagen III protein level was examined by immunohistochemical staining and Western blot analysis, which showed that the collagen III-positive area was significantly reduced by celecoxib treatment. After TAA treatment, the expression of caspase-12 and caspase-3 increased in liver tissue, while it decreased significantly in the TAA + celecoxib group. These results indicated that celecoxib inhibits hepatocyte apoptosis by inhibiting the expression of caspase-12 and caspase-3 in fibrotic liver tissue and further suppresses the progression of liver fibrosis.
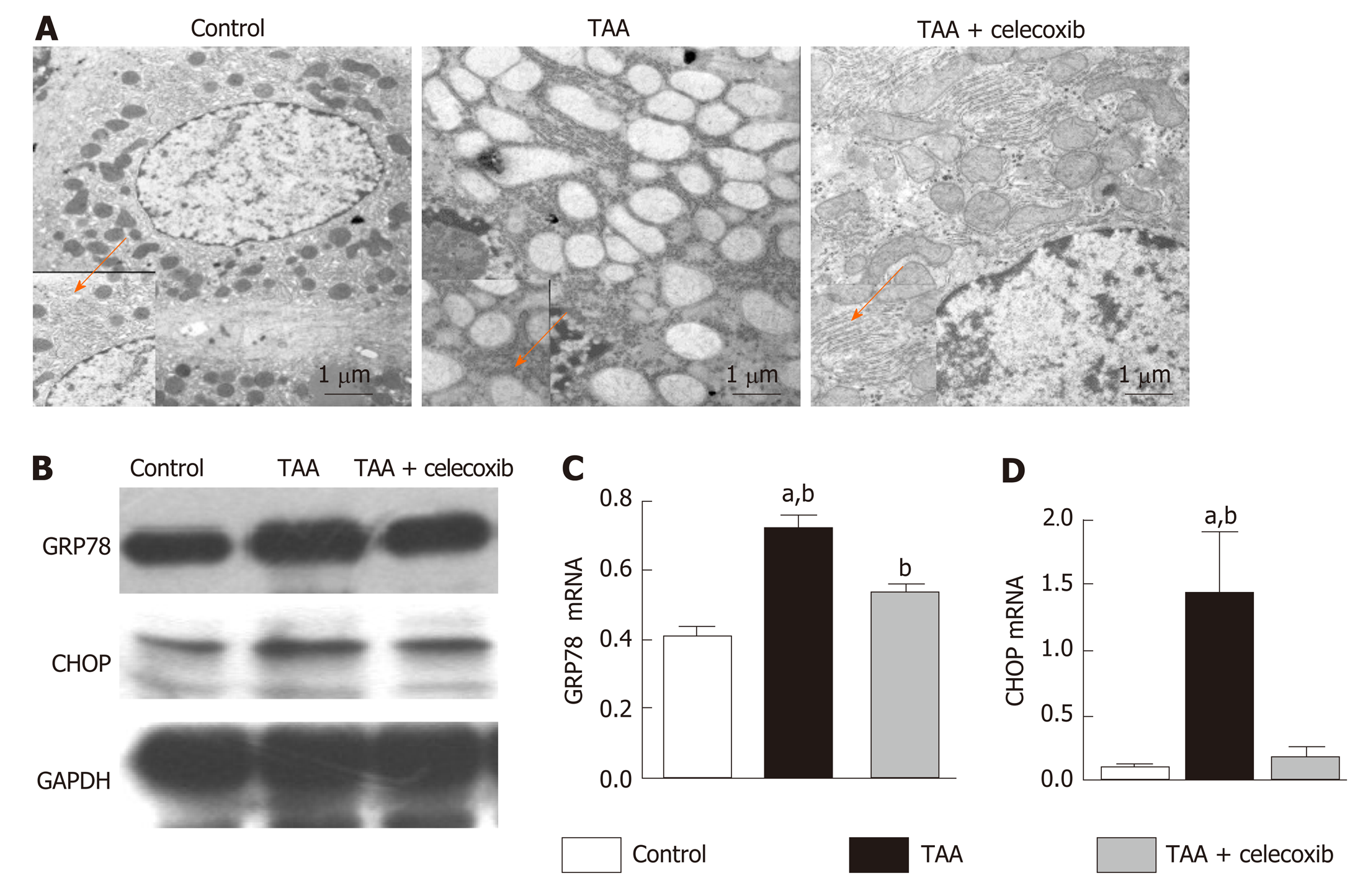
TEM analysis of liver sections showed severe damage to the mitochondria and ER in the TAA group, and the ER was swollen. In addition, broken ER and turbulent internal structures were observed in damaged hepatocytes (Figure 3A). After treatment with celecoxib, the mitochondria and ER of hepatocytes were protected, and the structure and morphology of the ER in hepatocytes were intact (Figure 3A). In addition, the marker proteins of ER stress were further examined by Western blot analysis, which showed that the expression levels of GRP78 and CHOP in the TAA group were obviously increased (Figure 3B), while their expression levels were obviously decreased after treatment with celecoxib. Collectively, these findings suggest that celecoxib inhibits ER stress in hepatocytes and further inhibits liver fibrosis by inhibiting the expression of GRP78 and CHOP.
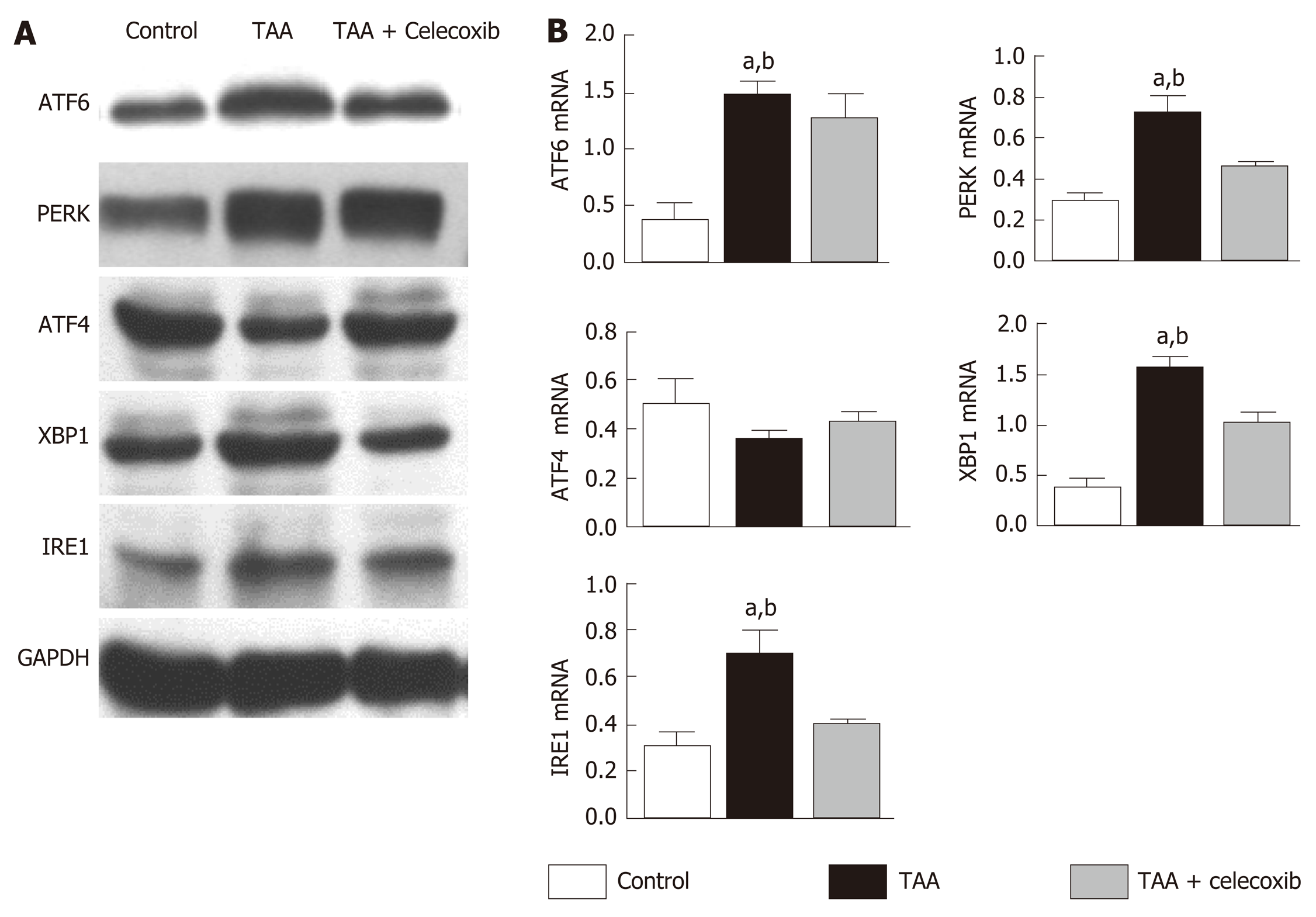
ER stress seems to exert its effects by activating the UPR signaling pathway. Therefore, the expression of UPR-related pathway proteins PERK, XBP1, ATF4, IRE1 and ATF6 was detected. The results showed that the expression levels of PERK, XBP1, ATF4, IRE1, and ATF6 were significantly increased in the TAA group than in the control group (Figure 4). After celecoxib treatment, the expression levels of these proteins were significantly reduced (Figure 4), suggesting that celecoxib inhibits hepatocyte apoptosis by inhibiting the expression of UPR-related pathway proteins in liver tissue.
Liver fibrosis is a chronic inflammatory process with significantly increased expression of COX-2. Celecoxib, a selective COX-2 inhibitor, significantly attenuates liver fibrosis. However, it is worth exploring whether celecoxib plays a therapeutic role by influencing ER stress-related signaling pathways at the cellular level. To verify the correlation between COX-2 and ER stress, we transfected a COX-2 expressing plasmid into a cell model and added the ER stress inducers chlamycin and carotene to induce ER stress. The results showed that the ER stress level and the expression of proteins of related signaling pathways were obviously increased in the COX-2-pIRES2 group compared with those of the DMSO group and Empty-pIRES2 group (Figure 5A). After celecoxib treatment, the expression of GRP78 was significantly decreased in the Tu + celecoxib and TG + celecoxib groups (Figure 5B). In addition, celecoxib significantly decreased the expression of ATF6, PERK, and IRE1, but did not affect the expression of ATF4 in the Tu + celecoxib and TG + celecoxib groups, which strongly suggested that celecoxib may have a significant effect on the expression of biomarkers in the ER stress signaling pathway.
Several potential therapies have been proposed in the study of the mechanism of fibrogenesis using in vitro and in vivo models[13,21,22]. Although treatments for underlying disease processes have been shown to be effective in reversing or reducing fibrosis, such as viral infections; however, no effective antifibrotic drugs have been found for humans[23].
Therefore, it is essential to update our understanding of the mechanism of fibrosis and translate these findings to the development of new treatment options. First, animal models, especially rodent models, are still used to determine the target correlation, and an antifibrotic drug with a curative effect is an important tool[24]. The animal models of liver fibrosis induced by chemicals such as carbon tetrachloride have been popular in previous studies[25]. However, that model has serious hepatocyte necrosis and is dependent on a large amount of oxidative stress, which has not been found to be so severe in human chronic liver disease. Another chemical, TAA, is a hepatotoxin that induces apoptosis of hepatocytes. It has been shown to be helpful in detecting the protective effects of drugs, and the pattern of fibrosis is very similar to that of substantial fibrosis in humans[26]. After 16 wk of intraperitoneal injection of TAA, the liver tissue of SD rats showed obvious inflammatory cell infiltration, disordered hepatocyte structure, hepatocyte degeneration and necrosis, and large amounts of collagen fiber deposition. The degree of liver fibrosis increased significantly, which indicated that the model of liver fibrosis was successfully established in this study. In addition, the expression levels of caspase-3 and caspase-12 in this liver fibrosis model increased significantly, suggesting that hepatocyte apoptosis occurs during liver fibrosis[27].
The hepatocyte ER is the main place for cells to process proteins and store Ca2+ and closely related to substance transport, exchange, and detoxification[28].Protein misfolding and the accumulation of unfolded proteins in the lumen as well as disorders in Ca2+ balance have been observed when ER stress occurs[29]. Several studies have demonstrated that ER stress is related to the progression of liver fibrosis and its recovery[30]. Although GRP78, XBP1, and CHOP are not unique, these are all known indicators of the activation of the ER stress pathways[31]. We revealed that the ER stress marker proteins GRP78 and CHOP increased significantly, indicating that an ER stress reaction occurred in the liver tissue of rats with liver fibrosis. ER stress exerts its physiological function mainly by activating the UPR signaling pathway. Our study also revealed that the expression of UPR signaling proteins in liver tissue of rats with liver fibrosis was significantly increased, which was consistent with the results reported by Volmer et al[32].
The expression of COX-2 is significantly increased in the fibrotic liver tissues of animals and humans, and its expression level is positively correlated with the degree of fibrosis[7]. Our previous studies confirmed that celecoxib can effectively alleviate liver fibrosis, but whether its mechanism is related to mediating the ER stress response has not been reported. Liver fibrosis is a chronic liver injury that is characterized by abnormal deposition of the ECM, which can progress to cirrhosis or even liver cancer[33]. Therefore, it is of great significance to find drugs that can effectively alleviate liver damage. Celecoxib is a highly selective COX-2 inhibitor that has been shown to have antiproliferative effects in synovial fibroblasts as well as in human tumor xenograft models of breast, colon, lung, and prostate cancers and hepatocellular carcinoma[34]. However, the principal mechanism by which celecoxib affects hepatocyte proliferation remains unclear. The results of this study indicated that the levels of ALT, AST, albumin, and hydroxyproline, the proportion of fibrotic liver tissue, Ishak score, and the expression levels of caspase-3 and caspase-12 were significantly decreased after celecoxib intervention, which strongly suggested that celecoxib attenuates liver fibrosis and inhibits hepatocyte apoptosis, thereby protecting hepatocytes.
CHOP is considered the major determinant of cell fate and ER stress-induced apoptosis[35]. It is a short-lived protein, and so under mild or transient ER stress, the expression of CHOP will not change, and cells adapt and recover[36]. But if the ER stress intensity is strong or duration is long, persistent CHOP expression and cell death will occur[37]. In the present study, we revealed for the first time that the expression of key ER stress molecules, such as CHOP, was obviously decreased after celecoxib treatment, indicating that celecoxib plays a role in inhibiting ER stress in rat liver fibrosis by inhibiting the expression of CHOP and the ER stress signaling pathway.
Liver fibrosis is an important sign of chronic liver diseases, which could be treated and even reversed at early stage[19]. At present, the gold standard method for diagnosing liver fibrosis is liver biopsy[38]. However, sampling error and potential complications are the limitations of this method. In recent years, non-invasive diagnostic techniques and molecular imaging quantitative detection technology have developed rapidly, and their application in quantitative detection of liver fibrosis has also received more and more attention[39]. It is well known that the apparent diffusion coefficient (ADC) decreases during liver fibrosis, and hepatic ADC value is a good predictor of fibrosis stage[40]. Recent studies showed that the ADC value is an effective non-invasive parameter for diagnosing and grading inflammation and liver fibrosis in children with chronic hepatitis, indicating that this method is of great significance in the diagnosis and classification of inflammation and liver fibrosis in children with chronic hepatitis[41,42]. Moreover, splenic ADC value can predict esophageal varices in patients with liver cirrhosis and has a good correlation with laboratory biomarkers and clinical features of esophageal varices[43], which provides new ideas for early diagnosis of esophageal varices in patients with chronic liver diseases.
Celecoxib is currently widely used in the clinical treatment of rheumatoid arthritis and osteoarthritis[17]. Some researchers have shown that celecoxib can effectively improve portal hypertension and fibrosis in animal models. However, whether celecoxib relieves liver fibrosis by inhibiting ER stress still needs further exploration. This study proposed for the first time that celecoxib affects the progression of liver fibrosis by inhibiting ER stress, and we hope that this result can provide new targets for the treatment of liver fibrosis. However, multiple complex signaling pathways are regulated by ER stress, and whether other signals are involved in this process still needs further investigation. Second, when studying ER stress, it is necessary not only to investigate the related biochemical indicators, but also study it from a physiological perspective. Third, the imaging technology for liver fibrosis is developing rapidly, which has the advantages of high accuracy and little invasiveness. However, such technology was not utilized in the diagnosis of liver fibrosis in our animal model, and we should be in full consideration of it in our future work.
In conclusion, celecoxib reduces the severity of liver damage, improves the pathological changes of liver tissue, and has a beneficial effect on experimental liver fibrosis. Celecoxib treatment effectively reduces hepatocyte apoptosis in TAA-induced cirrhotic rats, which may be achieved by inhibiting the expression of CHOP after ER stress induction.
Liver fibrosis is a significant sign of chronic liver diseases, which could be treated and even reversed in the early stage. Inhibition of hepatocyte apoptosis is an important cause of reversal of liver fibrosis. Endoplasmic reticulum (ER) stress-mediated apoptosis is one of the important mechanisms of liver fibrosis. The cyclooxygenase-2 inhibitor celecoxib can improve the thioacetamide (TAA)-induced liver fibrosis in rats, and thus reverse the development of liver fibrosis. Whether celecoxib can inhibit apoptosis by inhibiting ER stress and further reverse liver fibrosis remains to be further studied.
Celecoxib has been widely used in the clinical treatment of rheumatoid arthritis and osteoarthritis. However, whether celecoxib can suppress apoptosis by inhibiting ER stress and further reverse liver fibrosis remains to be further studied.
This study aimed to explore the important role of celecoxib in modulating hepatocyte apoptosis during the development of liver fibrosis, and to clarify whether its regulatory mechanism is mediated by ER stress.
Cirrhosis was induced by intraperitoneal injections of thioacetamide (TAA) for 16 wk (200 mg/kg per 3 d for the first 8 wk and 100 mg /kg per 3 d after 8 wk). Thirty-six male Sprague-Dawley rats were randomly divided into three groups: control, TAA, and TAA + celecoxib groups. In the last 8 wk, TAA-induced cirrhotic rats received celecoxib (20 mg/kg/day) or the vehicle by gastric gavage. After 16 wk, the rats were sacrificed, and serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and albumin (ALB) were detected. The hepatic fibrosis areas were evaluated by Sirius red staining and the degree of fibrosis was assessed by measuring the level of hydroxyproline. ER stress levels were evaluated by detecting the marker proteins glucose-regulated protein 78 (GRP78), CCAAT/enhancer binding protein homologous protein (CHOP), PKR-like ER protein kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring enzyme 1 alpha (IRE1α). Apoptosis levels were evaluated by detecting caspase-12 and caspase-3.
The serum ALT and AST levels in the liver were significantly reduced by celecoxib; however, the serum ALB had no significant changes. Celecoxib significantly reduced the degree of liver fibrosis and the levels of hydroxyproline (-38% and -25.7%, respectively, P < 0.01). Celecoxib ameliorated ER stress by reducing the level of GRP78 compared to the TAA group (P < 0.05). Consistently, after celecoxib administration, the upregulation of TAA-induced hepatic apoptosis markers (caspase-12 and caspase-3) and CHOP was significantly inhibited. In addition, after celecoxib treatment, the expression of key molecules associated with ER stress (PERK, ATF6, and IRE1) was decreased (P < 0.05).
Therapeutic administration of celecoxib effectively reduces hepatic apoptosis in TAA-induced cirrhotic rats. The mechanism of action may be attributed to the suppression of CHOP expression, which subsequently inhibits ER stress.
Our results indicate that celecoxib may play a role in inhibiting ER stress in rat liver fibrosis by inhibiting the expression of CHOP in the ER stress signaling pathway. Our data provide a new target for the treatment of liver fibrosis.
| 1. | Aydın MM, Akçalı KC. Liver fibrosis. Turk J Gastroenterol. 2018;29:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 311] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 2. | Hernández-Alvarez MI, Sebastián D, Vives S, Ivanova S, Bartoccioni P, Kakimoto P, Plana N, Veiga SR, Hernández V, Vasconcelos N, Peddinti G, Adrover A, Jové M, Pamplona R, Gordaliza-Alaguero I, Calvo E, Cabré N, Castro R, Kuzmanic A, Boutant M, Sala D, Hyotylainen T, Orešič M, Fort J, Errasti-Murugarren E, Rodrígues CMP, Orozco M, Joven J, Cantó C, Palacin M, Fernández-Veledo S, Vendrell J, Zorzano A. Deficient Endoplasmic Reticulum-Mitochondrial Phosphatidylserine Transfer Causes Liver Disease. Cell. 2019;177:881-895.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 309] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 3. | Burban A, Sharanek A, Guguen-Guillouzo C, Guillouzo A. Endoplasmic reticulum stress precedes oxidative stress in antibiotic-induced cholestasis and cytotoxicity in human hepatocytes. Free Radic Biol Med. 2018;115:166-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Chen H, He YW, Liu WQ, Zhang JH. Rosiglitazone prevents murine hepatic fibrosis induced by Schistosoma japonicum. World J Gastroenterol. 2008;14:2905-2911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Hézode C. Treatment of hepatitis C: Results in real life. Liver Int. 2018;38 Suppl 1:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut. 2015;64:830-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 722] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 7. | Choi W, Chu J. The characteristics of the expression of heat shock proteins and COX-2 in the liver of hamsters infected with Clonorchis sinensis, and the change of endocrine hormones and cytokines. Folia Parasitol (Praha). 2012;59:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Han CY, Rho HS, Kim A, Kim TH, Jang K, Jun DW, Kim JW, Kim B, Kim SG. FXR Inhibits Endoplasmic Reticulum Stress-Induced NLRP3 Inflammasome in Hepatocytes and Ameliorates Liver Injury. Cell Rep. 2018;24:2985-2999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 191] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 9. | Zhang H, Li J, Li L, Liu P, Wei Y, Qian Z. Ceramide enhances COX-2 expression and VSMC contractile hyperreactivity via ER stress signal activation. Vascul Pharmacol. 2017;96-98:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Maiers JL, Malhi H. Endoplasmic Reticulum Stress in Metabolic Liver Diseases and Hepatic Fibrosis. Semin Liver Dis. 2019;39:235-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 11. | Wang P, Li J, Tao J, Sha B. The luminal domain of the ER stress sensor protein PERK binds misfolded proteins and thereby triggers PERK oligomerization. J Biol Chem. 2018;293:4110-4121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 12. | Bocca C, Novo E, Miglietta A, Parola M. Angiogenesis and Fibrogenesis in Chronic Liver Diseases. Cell Mol Gastroenterol Hepatol. 2015;1:477-488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 13. | Chen RJ, Wu HH, Wang YJ. Strategies to prevent and reverse liver fibrosis in humans and laboratory animals. Arch Toxicol. 2015;89:1727-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Liu Z, Li C, Kang N, Malhi H, Shah VH, Maiers JL. Transforming growth factor β (TGFβ) cross-talk with the unfolded protein response is critical for hepatic stellate cell activation. J Biol Chem. 2019;294:3137-3151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Fang XS, Zhang MH, Guo JY, Jin Z. Effects of insulin-like growth factor-1 on endoplasmic reticulum stress and autophagy in rat gastric smooth muscle cells cultured at different glucose concentrations in vitro. Mol Cell Biochem. 2019;451:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Jeong SW, Jang JY, Lee SH, Kim SG, Cheon YK, Kim YS, Cho YD, Kim HS, Lee JS, Jin SY, Shim CS, Kim BS. Increased expression of cyclooxygenase-2 is associated with the progression to cirrhosis. Korean J Intern Med. 2010;25:364-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Gao JH, Wen SL, Yang WJ, Lu YY, Tong H, Huang ZY, Liu ZX, Tang CW. Celecoxib ameliorates portal hypertension of the cirrhotic rats through the dual inhibitory effects on the intrahepatic fibrosis and angiogenesis. PLoS One. 2013;8:e69309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Wen SL, Gao JH, Yang WJ, Lu YY, Tong H, Huang ZY, Liu ZX, Tang CW. Celecoxib attenuates hepatic cirrhosis through inhibition of epithelial-to-mesenchymal transition of hepatocytes. J Gastroenterol Hepatol. 2014;29:1932-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Davies NM, McLachlan AJ, Day RO, Williams KM. Clinical pharmacokinetics and pharmacodynamics of celecoxib: a selective cyclo-oxygenase-2 inhibitor. Clin Pharmacokinet. 2000;38:225-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 319] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 20. | Paik YH, Kim JK, Lee JI, Kang SH, Kim DY, An SH, Lee SJ, Lee DK, Han KH, Chon CY, Lee SI, Lee KS, Brenner DA. Celecoxib induces hepatic stellate cell apoptosis through inhibition of Akt activation and suppresses hepatic fibrosis in rats. Gut. 2009;58:1517-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Takahara M, Shimomura I. Metabolic syndrome and lifestyle modification. Rev Endocr Metab Disord. 2014;15:317-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Wisse E, Braet F, Duimel H, Vreuls C, Koek G, Olde Damink SW, van den Broek MA, De Geest B, Dejong CH, Tateno C, Frederik P. Fixation methods for electron microscopy of human and other liver. World J Gastroenterol. 2010;16:2851-2866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (3)] |
| 23. | Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1987] [Cited by in RCA: 2221] [Article Influence: 85.4] [Reference Citation Analysis (10)] |
| 24. | Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1096] [Cited by in RCA: 1431] [Article Influence: 95.4] [Reference Citation Analysis (0)] |
| 25. | Popov Y, Schuppan D. Targeting liver fibrosis: strategies for development and validation of antifibrotic therapies. Hepatology. 2009;50:1294-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 256] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 26. | Iwaisako K, Brenner DA, Kisseleva T. What's new in liver fibrosis? The origin of myofibroblasts in liver fibrosis. J Gastroenterol Hepatol. 2012;27 Suppl 2:65-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 27. | Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2547] [Cited by in RCA: 2618] [Article Influence: 100.7] [Reference Citation Analysis (0)] |
| 28. | Lee AH, Glimcher LH. Intersection of the unfolded protein response and hepatic lipid metabolism. Cell Mol Life Sci. 2009;66:2835-2850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 29. | Xu D, Su C, Song X, Shi Q, Fu J, Hu L, Xia X, Song E, Song Y. Polychlorinated biphenyl quinone induces endoplasmic reticulum stress, unfolded protein response, and calcium release. Chem Res Toxicol. 2015;28:1326-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Yin XL, Zhang W, Yang Y, Shen H. Increasing expression of (CCAAT enhancer binding protein) homologous protein induced by endoplasmic reticulum stress in myocardium after cardiac arrest and resuscitation in rat. Resuscitation. 2012;83:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Senft D, Ronai ZA. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem Sci. 2015;40:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 888] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 32. | Volmer R, van der Ploeg K, Ron D. Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proc Natl Acad Sci USA. 2013;110:4628-4633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 521] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 33. | Thompson KJ, McKillop IH, Schrum LW. Targeting collagen expression in alcoholic liver disease. World J Gastroenterol. 2011;17:2473-2481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (2)] |
| 34. | Kusunoki N, Ito T, Sakurai N, Suguro T, Handa H, Kawai S. A novel celecoxib derivative potently induces apoptosis of human synovial fibroblasts. J Pharmacol Exp Ther. 2005;314:796-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Williams BL, Lipkin WI. Endoplasmic reticulum stress and neurodegeneration in rats neonatally infected with borna disease virus. J Virol. 2006;80:8613-8626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Wu T, Dong Z, Geng J, Sun Y, Liu G, Kang W, Zhang Y, Ge Z. Valsartan protects against ER stress-induced myocardial apoptosis via CHOP/Puma signaling pathway in streptozotocin-induced diabetic rats. Eur J Pharm Sci. 2011;42:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 37. | Magne L, Blanc E, Legrand B, Lucas D, Barouki R, Rouach H, Garlatti M. ATF4 and the integrated stress response are induced by ethanol and cytochrome P450 2E1 in human hepatocytes. J Hepatol. 2011;54:729-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 38. | Besheer T, Elalfy H, Abd El-Maksoud M, Abd El-Razek A, Taman S, Zalata K, Elkashef W, Zaghloul H, Elshahawy H, Raafat D, Elemshaty W, Elsayed E, El-Gilany AH, El-Bendary M. Diffusion-weighted magnetic resonance imaging and micro-RNA in the diagnosis of hepatic fibrosis in chronic hepatitis C virus. World J Gastroenterol. 2019;25:1366-1377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 39. | Petitclerc L, Sebastiani G, Gilbert G, Cloutier G, Tang A. Liver fibrosis: Review of current imaging and MRI quantification techniques. J Magn Reson Imaging. 2017;45:1276-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 40. | Berzigotti A, França M, Martí-Aguado D, Martí-Bonmatí L. Imaging biomarkers in liver fibrosis. Radiologia. 2018;60:74-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Razek AAKA, Khashaba M, Abdalla A, Bayomy M, Barakat T. Apparent diffusion coefficient value of hepatic fibrosis and inflammation in children with chronic hepatitis. Radiol Med. 2014;119:903-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Razek AA, Abdalla A, Omran E, Fathy A, Zalata K. Diagnosis and quantification of hepatic fibrosis in children with diffusion weighted MR imaging. Eur J Radiol. 2011;78:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 43. | Razek AA, Massoud SM, Azziz MR, El-Bendary MM, Zalata K, Motawea EM. Prediction of esophageal varices in cirrhotic patients with apparent diffusion coefficient of the spleen. Abdom Imaging. 2015;40:1465-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: El-Razek AA S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Zhang YL