Published online Jul 21, 2020. doi: 10.3748/wjg.v26.i27.3938
Peer-review started: March 2, 2020
First decision: April 25, 2020
Revised: May 9, 2020
Accepted: July 4, 2020
Article in press: July 4, 2020
Published online: July 21, 2020
Processing time: 141 Days and 10.3 Hours
Hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) differ in treatment and prognosis, warranting an effective differential diagnosis between them. The LR-M category in the contrast-enhanced ultrasound (CEUS) liver imaging reporting and data system (LI-RADS) was set up for lesions that are malignant but not specific to HCC. However, a substantial number of HCC cases in this category elevated the diagnostic challenge.
To investigate the possibility and efficacy of differentiating ICC from HCC classified in the LR-M category according to the CEUS LI-RADS.
Patients with complete CEUS records together with pathologically confirmed ICC and LR-M HCC (HCC classified in the CEUS LI-RADS LR-M category) between January 2015 and October 2018 were included in this retrospective study. Each ICC was assigned a category as per the CEUS LI-RADS. The enhancement pattern, washout timing, and washout degree between the ICC and LR-M HCC were compared using the χ2 test. Logistic regression analysis was used for prediction of ICC. Receiver operating characteristic (ROC) curve analysis was used to investigate the possibility of LR-M criteria and serum tumor markers in differentiating ICC from LR-M HCC.
A total of 228 nodules (99 ICCs and 129 LR-M HCCs) in 228 patients were included. The mean sizes of ICC and LR-M HCC were 6.3 ± 2.8 cm and 5.5 ± 3.5 cm, respectively (P = 0.03). Peripheral rim-like arterial phase hyperenhancement (APHE) was detected in 50.5% (50/99) of ICCs vs 16.3% (21/129) of LR-M HCCs (P < 0.001). Early washout was found in 93.4% (93/99) of ICCs vs 96.1% (124/129) of LR-M HCCs (P > 0.05). Marked washout was observed in 23.2% (23/99) of ICCs and 7.8% (10/129) of LR-M HCCs (P = 0.002), while this feature did not show up alone either in ICC or LR-M HCC. Homogeneous hyperenhancement was detected in 15.2% (15/99) of ICCs and 37.2% (48/129) of LR-M HCCs (P < 0.001). The logistic regression showed that rim APHE, carbohydrate antigen 19-9 (CA 19-9), and alpha fetoprotein (AFP) had significant correlations with ICC (r = 1.251, 3.074, and -2.767, respectively; P < 0.01). Rim APHE presented the best enhancement pattern for diagnosing ICC, with an area under the ROC curve (AUC) of 0.70, sensitivity of 70.4%, and specificity of 68.8%. When rim hyperenhancement was coupled with elevated CA 19-9 and normal AFP, the AUC and sensitivity improved to 0.82 and 100%, respectively, with specificity decreasing to 63.9%.
Rim APHE is a key predictor for differentiating ICC from LR-M HCC. Rim APHE plus elevated CA 19-9 and normal AFP is a strong predictor of ICC rather than LR-M HCC. Early washout and marked washout have limited value for the differentiation between the two entities.
Core tip: Hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) differ in treatment and prognosis, warranting an effective differential diagnosis between them. The LR-M category in the contrast-enhanced ultrasound liver imaging reporting and data system was set up for lesions that are malignant but not specific to HCC. Our study demonstrated that rim arterial phase hyperenhancement (APHE) is a key predictor for differentiating ICC from LR-M HCC, whereas early washout and marked washout have limited value for differentiating them. Rim APHE plus elevated carbohydrate antigen 19-9 and normal alpha fetoprotein is a strong predictor of ICC rather than LR-M HCC.
- Citation: Huang JY, Li JW, Ling WW, Li T, Luo Y, Liu JB, Lu Q. Can contrast enhanced ultrasound differentiate intrahepatic cholangiocarcinoma from hepatocellular carcinoma? World J Gastroenterol 2020; 26(27): 3938-3951
- URL: https://www.wjgnet.com/1007-9327/full/v26/i27/3938.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i27.3938
Liver cancer is the sixth most common cancer worldwide and the fourth leading cause of cancer-related death[1]. Hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) account for approximately 95% of all primary liver cancers[2,3]. However, ICC is more likely to result in a worse prognosis[4], and the treatment for ICC is quite different from that for HCC in specific cases. Therefore, it is of paramount importance to differentiate these two entities for appropriate intervention and better judgment of prognosis.
Over the past decade, contrast-enhanced ultrasound (CEUS) has been recommended as a useful tool for the characterization of focal liver lesions by several international professional societies in Europe and Asia[5-9]. However, CEUS was removed from the updated American Association for the Study of Liver Diseases 2011 guidelines as a diagnostic technique for HCC[10] because a single-center study with a limited sample size reported that CEUS may misdiagnose ICC as HCC in cirrhosis patients[11]. ICC is more likely to display peripheral rim arterial phase hyper- enhancement (APHE) followed by early and marked washout in CEUS images compared with HCC[12-16]. However, some studies showed that the aforementioned CEUS patterns may be detected in some HCC cases as well[12,13,17-19], which adds to the difficulty in the differential diagnosis between the two entities.
The America College of Radiology released CEUS liver imaging reporting and data system (LI-RADS) for standardizing CEUS diagnosis of liver nodules in patients at risk for HCC[19,20]. In this system, the LR-M category represents malignancies but is not specific for HCC[20]. However, previous studies revealed a high sensitivity of LR-M criteria for diagnosing non-HCC malignancy but a quite low positive predictive value (PPV) because of a high proportion of HCC in this category[15,17,21]. Until now, the diagnostic accuracy of LR-M criteria in differentiating ICC and LR-M HCC (defined as HCC, categorized as LR-M according to CEUS LI-RADS) has not been fully studied. Hence, this study focused on analyzing the CEUS features of ICC and LR-M HCC and further evaluating the possibility and efficacy of LR-M criteria in differentiation between them. We also associated CEUS patterns with tumor markers to investigate the potential diagnostic efficacy.
This retrospective study was approved by the institutional review board of West China Hospital of Sichuan University, and the requirement of written informed consent from patients was waived.
Patients with complete CEUS records together with pathologically confirmed ICC and LR-M HCC between January 2015 and October 2018 were included in this retrospective study. The patient selection flow chart is presented in Figure 1. In case of multiple lesions, the dominant tumor was chosen for analysis. Therefore, a total of 228 lesions were collected for analysis in this study.
All enrolled patients underwent conventional ultrasound and CEUS examinations using a Philips IU 22 system (Philips Medical Solutions; Mountain View, CA, United States) with a C5-1 MHz convex transducer. The CEUS study was performed after conventional ultrasound examination of the liver. A 1.2-2.4-mL bolus injection of sulfur hexafluoride-filled microbubble contrast agent (SonoVue; Bracco, Milan, Italy) was administered via a 20-gauge angiocatheter needle placed in the antecubital vein, followed by flushing with 5 mL of 0.9% sodium chloride solution. After the completion of the SonoVue injection, the imaging timer was initiated simultaneously. The still images and video clips of CEUS examination were digitally stored for further evaluation.
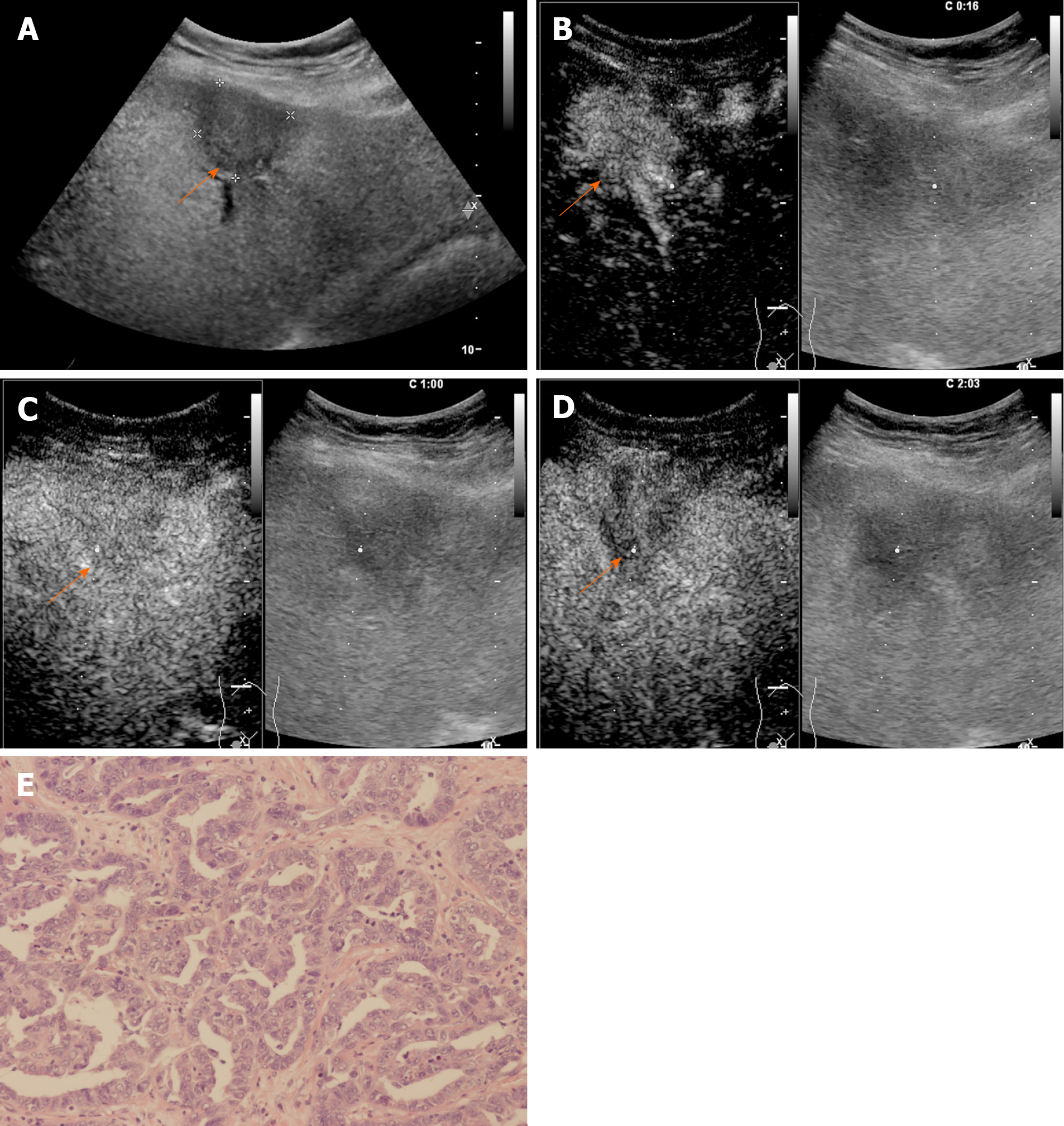
The CEUS images were numbered randomly after deidentification and then reviewed by two radiologists (WL and JL) with more than 5 years of experience in liver CEUS examination independently. Both radiologists were blinded to the clinical information of the patients. Arterial phase enhancement, presence or absence of early washout, and washout degree of the liver nodules were analyzed. The APHE pattern refers to lesions that manifest as hyperechoic when compared with the surrounding liver parenchyma in the arterial phase. Rim APHE is a sub-type of APHE, where the enhancement is most pronounced in the periphery of the lesion. Washout refers to a lesion that presents a reduction in enhancement either in whole or in part vs the surrounding liver parenchyma. Washout that occurs within 60 s is further termed “early washout”; otherwise, it is termed “late washout”. Marked washout is defined as a lesion that is virtually devoid of enhancement (so-called “punch-out”) within 120 s after contrast injection[22]. The enhancing feature of each lesion was analyzed, and the lesions were further classified into relevant categories according to the CEUS LI-RADS (2017 version) by both radiologists. If there was a discrepancy between the radiologists, arbitration from another senior radiologist (QL) with more than 10 years of experience in liver CEUS examination was performed. Meanwhile, the CEUS imaging features of lesions were recorded for further analysis.
Quantitative data are presented as the mean ± SD, and qualitative data are presented as absolute numbers and percentages. Enhancing patterns of the nodules in CEUS were compared by using the χ2 test, while quantitative variables were compared using student’s t test and the Mann-Whitney test. Logistic regression was used to predict the correlation between LR-M characteristics, serum tumor markers, and ICC or LR-M HCC. The diagnostic capability of CEUS and tumor markers in differentiating between ICC and LR-M HCC was analyzed by receiver operating characteristic (ROC) curve analysis. The cut-off values of 100 U/mL and 20 ng/mL were used for the elevation of carbohydrate antigen 19-9 (CA 19-9) and alpha fetoprotein (AFP), respectively, as recommended by previous studies[23-27]. Interobserver agreement was evaluated by the two radiologists by calculating the κ-value. A κ value < 0.2 indicates poor agreement, 0.21 to 0.40 indicates fair agreement, 0.41 to 0.60 indicates moderate agreement, 0.61 to 0.80 indicates good agreement, and 0.80 to 1 indicates almost perfect agreement. Significance was defined as P < 0.05. Statistical analyses were performed using a statistical software package (MedCalc10.4.7.0, Ostend, Belgium).
A total of 228 patients with 228 pathologically confirmed lesions, including 99 ICCs and 129 LR-M HCCs, were included in this study. The clinicopathological data of the patients, including age, gender, nodule size, etiology, tumor markers, fibrosis stage, and pathological results, are presented in Table 1.
| Patient characteristic | Pathology | P value | |
| ICC (n = 99) | LR-M HCC (n =129) | ||
| Age, mean ± SD, (range), yr | 59 ± 10.2 (57-61) | 52 ± 12.8 (50-54) | = 0.017 |
| Sex | |||
| Male | 51 (51.5) | 107 (82.9) | < 0.001 |
| Female | 48 (48.5) | 22 (17.1) | - |
| Nodule size, mean ± SD, (range), cm | 6.3 ± 2.8 (5.7-6.8) | 5.5 ± 3.5 (4.9-6.1) | 0.03 |
| Intrahepatic bile duct dilatation | 17 (17.2) | 3 (2.3) | 0.001 |
| CA 19-9 (U/mL) | 74.0 (41.9-136.5) | 18.8 (16.0-22.0) | < 0.001 |
| AFP (ng/mL) | 3.0 (2.7-3.5) | 67.3 (18.0-146.7) | < 0.001 |
| Etiology | |||
| HBV | 20 (20.2) | 114 (88.4) | < 0.001 |
| HCV | 1 (1) | 2 (1.5) | > 0.05 |
| Intrahepatic cholelithiasis | 4 (4) | 0 (0) | > 0.05 |
| Fatty liver | 0 | 5 (3.9) | > 0.05 |
| Unknown | 74 (74.7) | 2 (1.5) | < 0.001 |
| Fibrosis stage | |||
| S1 | 3 (3) | 4 (3.1) | > 0.05 |
| S2 | 7 (7.1) | 16 (12.4) | > 0.05 |
| S3 | 4 (4) | 20 (15.5) | 0.009 |
| S4 | 2 (2) | 60 (46.5) | < 0.001 |
| Unclassified | 83 (83.8) | 29 (22.5) | - |
| Tumor tissue differentiation | |||
| Well differentiated | 1 (1) | 2 (1.5) | - |
| Moderately differentiated | 21 (21.2) | 59 (45.7) | - |
| Poorly differentiated | 77 (77.8) | 68 (52.7) | - |
Interobserver agreement regarding the review of enhancing patterns in the arterial phase and portal/late phase showed good consistency, with κ values of 0.72 and 0.88, respectively. The tissue sample used for histological evaluation was obtained from surgical resection or percutaneous biopsy. Liver cirrhosis was found in 2% (2/99) of ICCs and 46.5% (60/129) of HCCs (P < 0.001). Chronic hepatitis B (CHB) was detected in 20.2% (20/99) of ICCs and 88.4% (114/129) of HCCs (P < 0.001), and intrahepatic duct dilatation was present in 17.2% (17/99) of ICCs vs 2.3% (3/129) of HCCs (P < 0.001). In terms of tumor differentiation, poor, moderate, and well differentiation was found in 52.7% (68/129), 45.7% (59/129), and 1.6% (2/129) of LR-M HCCs, respectively. Regarding the tumor markers, CA 19-9 was significantly higher in ICC than in LR-M HCC [74.0 (41.9-136.5) U/mL vs 18.8 (16.0-22.0) U/mL, P < 0.001], while AFP was significantly lower in ICC than in LR-M HCC [3.0 (2.7-3.5) ng/mL vs 67.3 (18.0-146.7) ng/mL, P < 0.001].
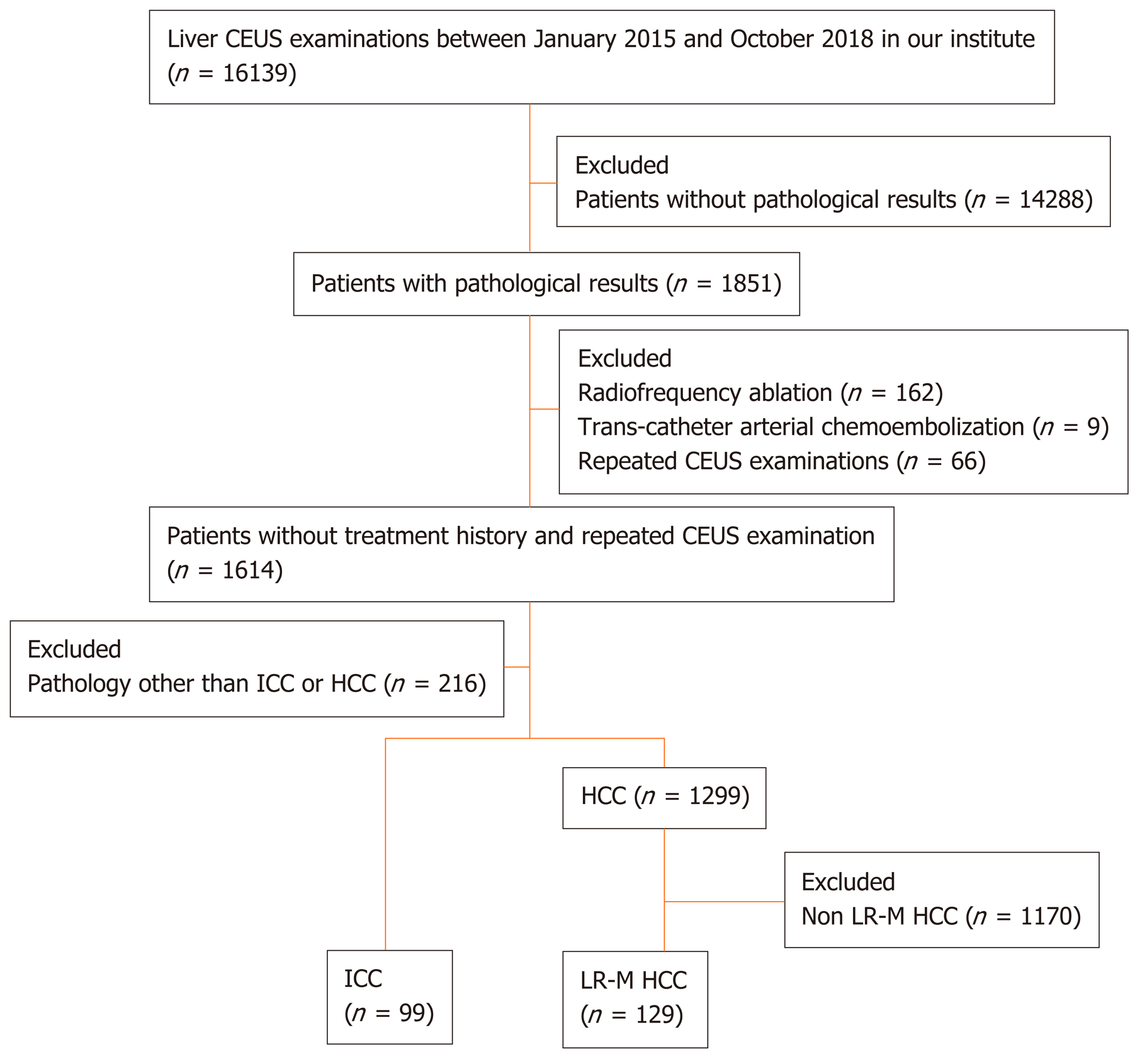
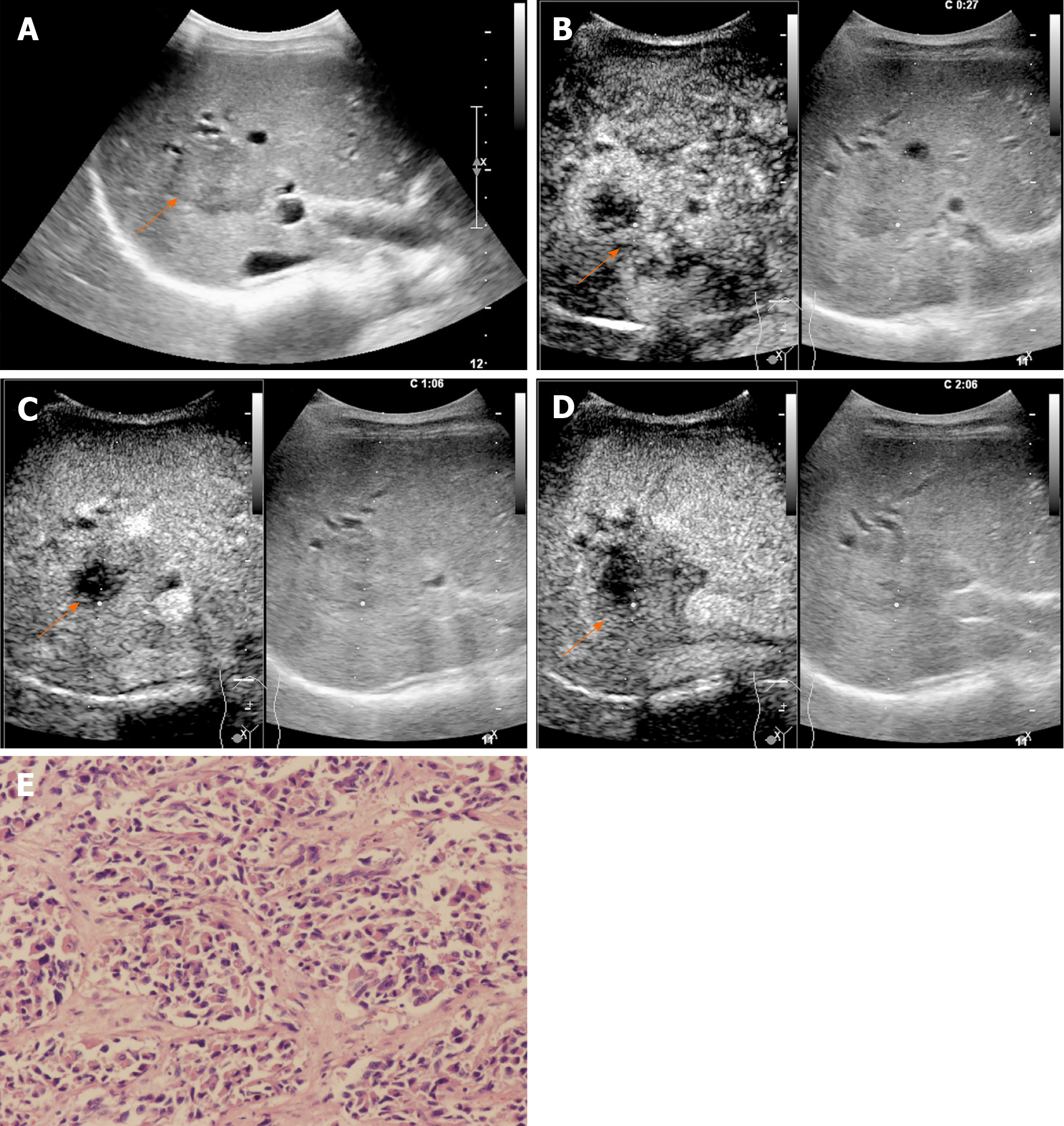
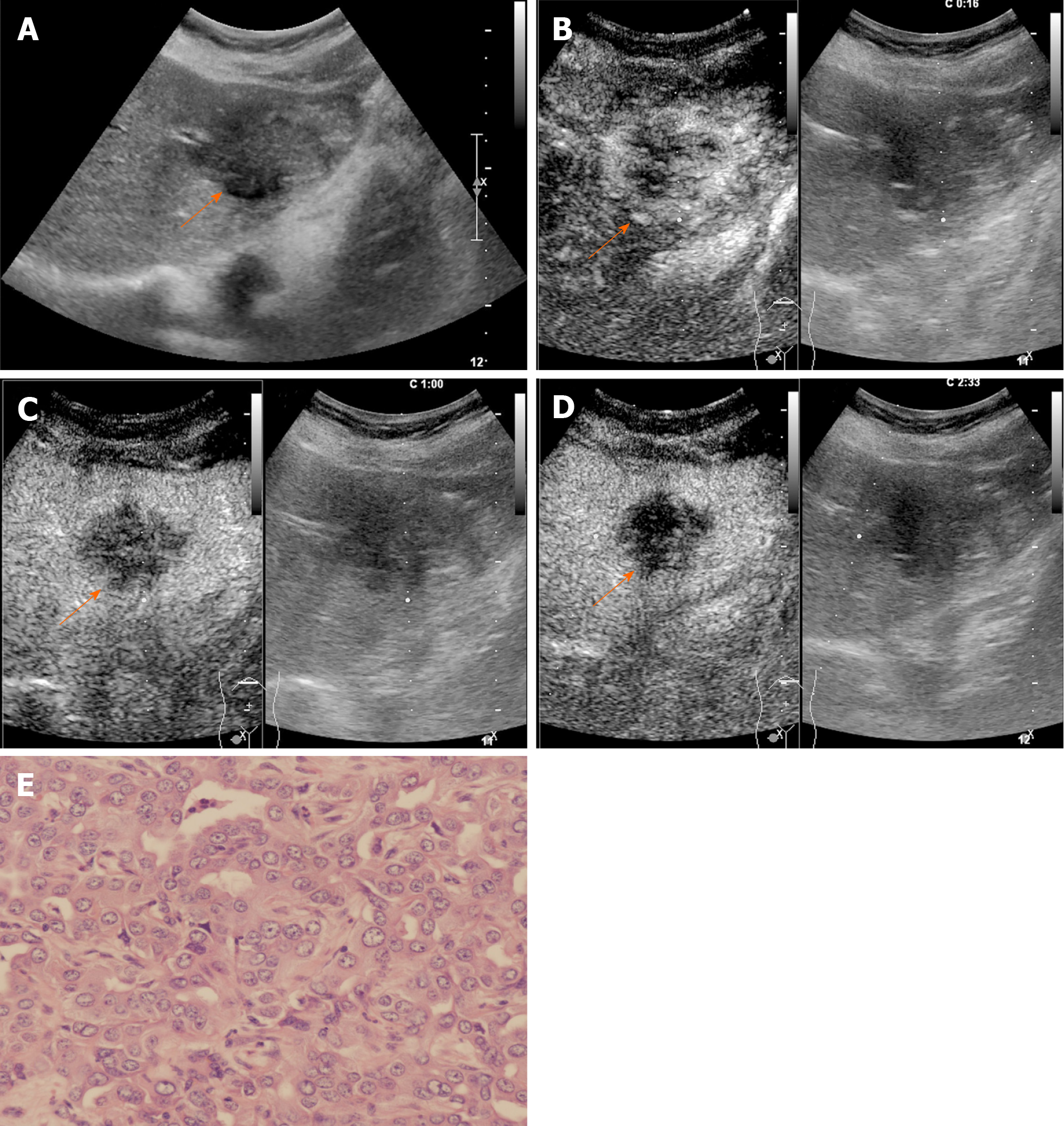
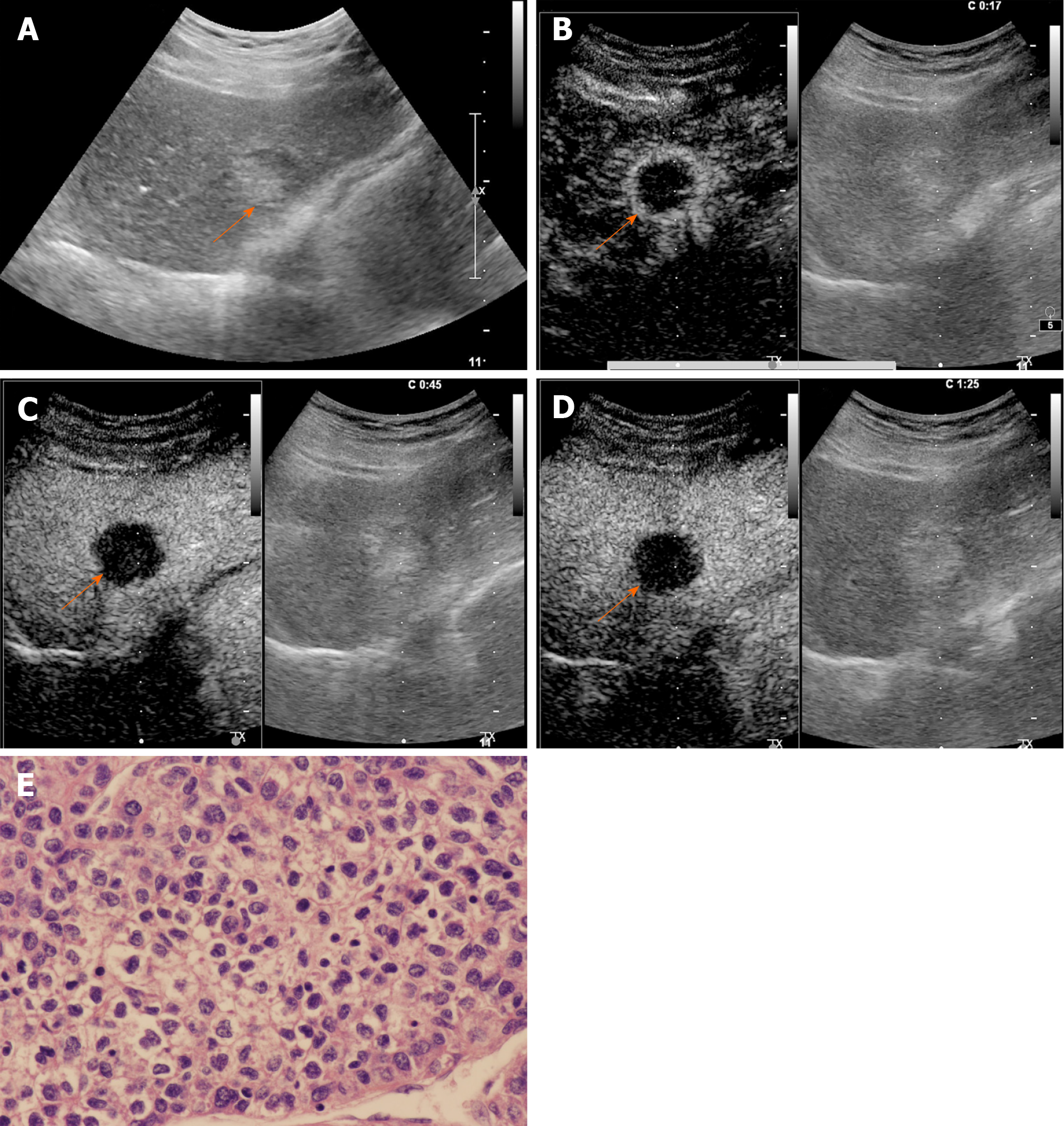
The CEUS image characteristics of ICC and LR-M HCC, including arterial phase enhancement pattern, washout onset timing, and washout degree are presented in Table 2. In the arterial phase, three types of enhancing patterns were illustrated: Homogeneous hyperenhancement, heterogeneous hyperenhancement, and rim hyperenhancement. Rim APHE was detected in 50.5% (50/99) of ICCs vs 16.3% (21/129) of LR-M HCCs (P < 0.0001) (Figure 2-4). Arterial homogeneous hyperenhancement was observed in 15.2% (15/99) of ICCs and 37.2% (48/129) of LR-M HCCs (P = 0.0004) (Figure 5). Early washout of contrast agent was illustrated in 93.4% (93/99) of ICCs vs 96.1% (124/129) of LR-M HCCs (P > 0.05). Marked washout of contrast agent within 120 s was shown in 23.2% (23/99) of ICCs vs 7.8% (10/129) of HCCs (P = 0.002). Of note, this feature did not show up alone in either of the two entities.
| Imaging characteristic | Pathology | P value | |
| ICC (n = 99) | LR-M HCC (n = 129) | ||
| Gray scale echogenicity | |||
| Hyperechoic | 4 (4) | 39 (30.0) | < 0.001 |
| Hypoechoic | 93 (93.9) | 82 (63.1) | < 0.001 |
| Mixed | 2 (2) | 8 (6.9) | > 0.05 |
| APHE | |||
| Homogeneous | 15 (15.2) | 48 (37.2) | < 0.001 |
| Heterogeneous | 34 (34.3) | 60 (46.5) | > 0.05 |
| Rim | 50 (50.5) | 21 (16.3) | < 0.001 |
| Early washout (onset < 60 s) | 93 (93.4) | 124 (96.1) | > 0.05 |
| Marked washout within 120 s | 23 (23.2) | 10 (7.8) | = 0.002 |
A comparison of the LR-M features between ICC and LR-M HCC is presented in Table 3. Rim APHE followed by early washout was the most frequent combination of LR-M features, which was detected in 30.3% (30/99) of ICCs vs 10.1% (13/129) of LR-M HCCs (P = 0.0002). The presence of all three LR-M features in a nodule also showed a significant difference between the two entities (P = 0.0018).
| χ2 test | Rim APHE + late and mild washout | APHE + early and mild washout | APHE + late and marked washout | Rim APHE + early and marked washout | Rim APHE + early and mild washout | Rim APHE + late and marked washout | APHE + early and marked washout | |||||||
| ICC | LR-M HCC | ICC | LR-M HCC | ICC | LR-M HCC | ICC | LR-M HCC | ICC | LR-M HCC | ICC | LR-M HCC | ICC | LR-M HCC | |
| Positive | 4 | 5 | 42 | 101 | 0 | 0 | 14 | 3 | 30 | 13 | 2 | 0 | 7 | 7 |
| Negative | 95 | 124 | 57 | 28 | 99 | 129 | 8 | 126 | 9 | 116 | 97 | 129 | 92 | 122 |
| Proportion(%) | 4 | 3.9 | 42.4 | 78.3 | 0 | 0 | 14.1 | 2.3 | 30.3 | 10.1 | 2 | 0 | 7.1 | 5.4 |
| P value | > 0.05 | < 0.0001 | - | 0.0018 | 0.0002 | > 0.05 | > 0.05 | |||||||
| 95%CI | -5.6%-6.6% | 22.8%-47.8% | - | 4.3%-20.4% | 9.2%-31.3% | -1.3%-7.1% | -5.2%-9.4% | |||||||
Taking rim APHE, early washout, marked washout, homogeneous hyperenhancement, CA 19-9, and AFP as independent variables, the regression analysis showed that rim APHE, CA 19-9, and AFP had significant correlations with ICC (r = 1.251, 3.075, and -2.767, respectively; P < 0.01). ROC curve analysis for the diagnostic performance of LR-M characteristics in differentiating ICC from LR-M HCC is presented in Table 4. Rim APHE presented the best diagnostic performance for ICC, and the area under the ROC curve (AUC) was 0.70 [95% confidence interval (CI): 0.63-0.76], with a sensitivity of 70.4% (95%CI: 58.4%-80.7%) and specificity of 68.8% (95%CI: 60.9%-75.9%). When rim APHE was coupled with elevated CA 19-9 and normal AFP, the AUC and sensitivity improved to 0.82 (95% CI: 0.76-0.87) and 100% (95%CI: 86.8%-100%), respectively, with specificity decreasing to 63.9% (95% CI: 56.8%-70.5%).
| Criterion | AUC | 95%CI | Sensitivity(%) | 95%CI | Specificity (%) | 95%CI | +LR | 95%CI | -LR | 95%CI |
| Rim APHE | 0.7 | 0.63-0.76 | 70.4 | 58.4-80.7 | 68.8 | 60.9-75.9 | 2.3 | 1.9-2.7 | 0.4 | 0.3-0.7 |
| Early washout | 0.56 | 0.49-0.62 | 57.1 | 50.3-63.8 | 54.6 | 23.4-83.3 | 1.3 | 0.7-2.2 | 0.8 | 0.4-1.5 |
| Marked washout | 0.65 | 0.59-0.72 | 69.7 | 51.3-84.4 | 61 | 53.8-67.9 | 1.8 | 1.4-2.3 | 0.5 | 0.3-0.9 |
| Rim APHE + elevated CA 19-9 + normal AFP | 0.82 | 0.76-0.87 | 100 | 86.8-100 | 63.9 | 56.8-70.5 | 2.8 | 2.5-3.1 | - | - |
The LR-M category of CEUS LI-RADS was generated for lesions that are malignant but not specific to HCC[20]. There was a significantly low PPV of LR-M for the diagnosis of non-HCC malignancy due to a high proportion of HCC cases in this category, leading to the recommendation of biopsy for all CEUS LR-M lesions[28,29]. In this retrospective study, we focused on ICC and LR-M HCC, which composed the majority of LR-M lesions, expecting to achieve a better understanding of the differential diagnosis between the two entities. Our study demonstrated that rim APHE and marked washout were more frequently observed in ICCs than in LR-M HCCs (50.5% vs 16.3% and 23.2% vs 7.8%, respectively; P < 0.01). Although early washout was the most common feature in both ICCs and LR-M HCCs, the rate difference of this feature between the two entities was not significant. Marked washout did not show up alone either in ICC or in LR-M HCC. Of note, rim APHE was a key feature, which showed a significant positive correlation with ICCs in our study. The AUC, sensitivity, and specificity of rim APHE for the differential diagnosis was 0.70, 70.4%, and 68.8%, respectively. When rim APHE was coupled with elevated CA 19-9 and normal AFP, the AUC and sensitivity improved to 0.82 and 100%, with specificity decreasing to 63.9%.
Rim APHE was a symbolic wash-in pattern of ICC detected in 50.5% of ICC cases in the present study, which was in accordance with the rates of 43%-68.5% in previous reports[12-14,18]. Serum biomarkers, especially AFP and CA19-9, have been proven to be helpful for the diagnosis of HCC and ICC. In the study conducted by Chen et al[12], the investigators added CA 19-9 to their CEUS score nomogram to enhance the discriminatory power of the predictive model for the differentiation between ICC and HCC. We found that when using rim APHE plus CA 19-9 for the differential diagnosis, the AUC and sensitivity improved from 0.70 to 0.82 and 70.4% to 100%, respectively. However, rim APHE could be influenced by multiple factors, including tumor size, pathological constitution of a lesion, and liver background[18,30,31]. Small ICCs, especially those ≤ 2 cm, are rich in tumor cells with few fibrous tissues and no central necrosis[32], thus potentially mimicking the homogeneous hyperenhancement pattern of HCC[14,19,33,34]. Meanwhile, ICC showing rim APHE was more likely to be detected in livers without cirrhosis and chronic viral hepatitis [19,30,31,33]. In our study, chronic hepatitis B and cirrhosis were both more frequent in patients with LR-M HCCs than in those with ICCs (88.4% vs 20.2% and 46.5% vs 2%, respectively; P < 0.001). Similarly, in a recent study by Li et al[18], the authors proved that there was no significant difference in rim APHE, early washout, or marked washout between ICC patients with and without risk factors. All of these features were more frequent in ICCs than in HCCs, regardless of the risk factors].
In terms of washout pattern, previous studies indicated that ICC is prone to wash out earlier than HCC[12,13,15,34]. Although early washout was the most frequent feature of both ICCs and LR-M HCCs in this study, no significant difference was found in the rates of early washout between the two entities. This discrepancy may result from the difference in study subjects, as this study focused on LR-M HCC, which presented specific imaging features compared with typical HCC. The feature of washout within 60 s per LR-M criteria may be the primary reason why a substantial number of HCCs were classified as LR-M. In our study, 96.1% (124/129) of LR-M HCCs presented early washout, which is close to the results of 96% (214/224) in the study of Zheng et al[21]. Liu et al[13] found that the average washout time of ICCs was 27.5 s, compared with 70.1 s for HCCs (P < 0.05). Li et al[18] also reported that 90.7% and 92.7% of ICCs in patients with and without risk factors, respectively, presented washout within 45 s. Thus, the early washout setting in LR-M may need to be further modified to address a considerable number of misdiagnosed HCCs.
Marked washout of contrast agent within 120 s was found more frequently in ICCs than in LR-M HCCs (P = 0.002) in this study. At the time point of 2 min, only 23.2% of the ICCs in our study showed marked washout, which is close to the rate of 25% reported by Han et al[15]. Some studies also demonstrated that the efficacy of marked washout in differentiating ICC from HCC can only be slightly improved even by postponing the onset time of marked washout to 3 min[15,18]. Zheng et al[21] found 142 out of 153 LR-M nodules showing early washout within 60 s and without punch-out before 5 min were HCCs. The authors re-categorized lesions showing the aforementioned washout patterns into LR-5, and the specificity and PPV of LR-M as a predictor of non-HCC malignancy were remarkably improved from 88% to 96% and 36% to 58%, respectively (P < 0.001). In our study, marked washout within 2 min did not show up alone in both entities. Thus, this feature in LR-M criteria may need to be refined for better practical application.
There are several limitations of our study. First, due to the limited number of ICC cases, CEUS LI-RADS was applied in patients without risk factors for HCC. In clinical practice, chronic hepatitis or cirrhosis would not present in the majority of ICC patients. However, the LR-M features enabled the differentiation of ICC from LR-M HCC in our study, as also validated by Li et al[18]. Second, the scope of the study focused only on ICC and LR-M HCC. Other hepatic malignancies, such as combined hepatocellular-cholangiocarcinoma and metastasis, which also frequently present as LR-M tumors, were not enrolled in our study. Further studies are needed to validate the findings demonstrated in our study and determine, for example, how much referential value marked washout offers the LR-M category in the absence of arterial phase rim APHE and early washout and whether the onset time of early washout and marked washout should be adjusted to reduce the number of HCCs classified as LR-M tumors.
In conclusion, rim APHE is a key predictor for differentiating ICC from LR-M HCC. Rim APHE plus elevated CA 19-9 and normal AFP is a strong predictor of ICC rather than LR-M HCC. Early washout and marked washout have limited value for the differentiation between the two entities.
Liver cancer is the sixth most common cancer worldwide and the fourth leading cause of cancer-related death. Hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) account for the majority of all primary liver cancers and differ in treatment and prognosis.
Contrast-enhanced ultrasound (CEUS) has been recommended and widely used for the characterization of focal liver lesions. However, the value of CEUS in differentiating between ICC and HCC remains controversial. The CEUS liver imaging reporting and data system (LI-RADS) released by the American College of Radiology has been developed for standardizing CEUS criteria for the diagnosis of focal liver lesions. In the criteria, the LR-M category represents malignancies but is not specific to HCC. Of note, the presence of a substantial number of HCCs in this category elevates the difficulty in the differential diagnosis between ICC and HCC, and the efficacy of LR-M features for the differentiation between them has not yet been fully evaluated.
The purpose of this study was to investigate the possibility and efficacy of differentiating ICC from HCC classified in the LR-M category according to the CEUS LI-RADS.
Patients with complete CEUS records together with pathologically confirmed ICC and LR-M HCC (HCC classified in the CEUS LI-RADS LR-M category) between January 2015 and October 2018 were included in this retrospective study. Each ICC was assigned a category as per the CEUS LI-RADS. The enhancement pattern, washout timing, and washout degree between the ICC and LR-M HCC were compared using the χ2 test. Logistic regression analysis was used for prediction of ICC. Receiver operating characteristic curve analysis was used to investigate the possibility of LR-M criteria and serum tumor markers in differentiating ICC from LR-M HCC.
A total of 228 nodules (99 ICCs and 129 LR-M HCCs) in 228 patients were included. The mean sizes of ICC and LR-M HCC were 6.3 ± 2.8 cm and 5.5 ± 3.5 cm, respectively (P = 0.03). Peripheral rim-like arterial phase hyperenhancement (rim APHE) was detected in 50.5% (50/99) of ICCs vs 16.3% (21/129) of LR-M HCCs (P < 0.001). Early washout was found in 93.4% (93/99) of ICCs vs 96.1% (124/129) of LR-M HCCs (P > 0.05). Marked washout was observed in 23.2% (23/99) of ICCs and 7.8% (10/129) of LR-M HCCs (P = 0.002), while this feature did not show up alone either in ICC or LR-M HCC. Homogeneous hyperenhancement was detected in 15.2% (15/99) of ICCs and 37.2% (48/129) of LR-M HCCs (P < 0.001). The logistic regression showed that rim APHE, carbohydrate antigen 19-9 (CA 19-9), and alpha fetoprotein (AFP) exhibited significant correlations with ICC (r = 1.251, 3.074, and -2.767, respectively; P < 0.01). Rim APHE presented the best enhancement pattern for diagnosing ICC, with an area under the receiver operating characteristic curve (AUC) of 0.70, sensitivity of 70.4%, and specificity of 68.8%. When rim hyperenhancement was coupled with elevated CA 19-9 and normal AFP, the AUC and sensitivity improved to 0.82 and 100%, respectively, with specificity decreasing to 63.9%.
This study illustrated that rim APHE is a key predictor for differentiating ICC from LR-M HCC. Rim APHE plus elevated CA 19-9 and normal AFP is a predictor of ICC rather than LR-M HCC. Early washout and marked washout have limited value for the differentiation between the two entities.
Rim APHE is a key predictor for differentiating ICC from LR-M HCC, and rim APHE plus elevated CA 19-9 and normal AFP is a predictor of ICC rather than LR-M HCC. The reference values of early washout (< 60 s) and marked washout within 120 s in the LR-M category are needed to further refine the CEUS LI-RADS criteria to avoid unnecessary biopsy.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56678] [Article Influence: 7084.8] [Reference Citation Analysis (135)] |
| 2. | Sia D, Villanueva A, Friedman SL, Llovet JM. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology. 2017;152:745-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 869] [Article Influence: 96.6] [Reference Citation Analysis (2)] |
| 3. | Massarweh NN, El-Serag HB. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control. 2017;24:1073274817729245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 454] [Article Influence: 50.4] [Reference Citation Analysis (1)] |
| 4. | Xue TC, Zhang BH, Ye SL, Ren ZG. Differentially expressed gene profiles of intrahepatic cholangiocarcinoma, hepatocellular carcinoma, and combined hepatocellular-cholangiocarcinoma by integrated microarray analysis. Tumour Biol. 2015;36:5891-5899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC, Chaubal NG, Chen MH, Clevert DA, Correas JM, Ding H, Forsberg F, Fowlkes JB, Gibson RN, Goldberg BB, Lassau N, Leen EL, Mattrey RF, Moriyasu F, Solbiati L, Weskott HP, Xu HX; World Federation for Ultrasound in Medicine; European Federation of Societies for Ultrasound. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver - update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol. 2013;39:187-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 506] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 6. | Claudon M, Cosgrove D, Albrecht T, Bolondi L, Bosio M, Calliada F, Correas JM, Darge K, Dietrich C, D'Onofrio M, Evans DH, Filice C, Greiner L, Jäger K, Jong Nd, Leen E, Lencioni R, Lindsell D, Martegani A, Meairs S, Nolsøe C, Piscaglia F, Ricci P, Seidel G, Skjoldbye B, Solbiati L, Thorelius L, Tranquart F, Weskott HP, Whittingham T. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) - update 2008. Ultraschall Med. 2008;29:28-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 503] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 7. | Italian Association for the Study of the Liver (AISF). AISF Expert Panel; AISF Coordinating Committee, Bolondi L, Cillo U, Colombo M, Craxì A, Farinati F, Giannini EG, Golfieri R, Levrero M, Pinna AD, Piscaglia F, Raimondo G, Trevisani F, Bruno R, Caraceni P, Ciancio A, Coco B, Fraquelli M, Rendina M, Squadrito G, Toniutto P. Position paper of the Italian Association for the Study of the Liver (AISF): the multidisciplinary clinical approach to hepatocellular carcinoma. Dig Liver Dis. 2013;45:712-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 8. | Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT, Shiina S, Cheng AL, Jia JD, Obi S, Han KH, Jafri W, Chow P, Lim SG, Chawla YK, Budihusodo U, Gani RA, Lesmana CR, Putranto TA, Liaw YF, Sarin SK. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 797] [Cited by in RCA: 844] [Article Influence: 52.8] [Reference Citation Analysis (2)] |
| 9. | Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y, Okusaka T, Miyayama S, Tsuchiya K, Ueshima K, Hiraoka A, Ikeda M, Ogasawara S, Yamashita T, Minami T, Yamakado K; Liver Cancer Study Group of Japan. JSH Consensus-Based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer. 2014;3:458-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 503] [Article Influence: 41.9] [Reference Citation Analysis (1)] |
| 10. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6631] [Article Influence: 442.1] [Reference Citation Analysis (1)] |
| 11. | Vilana R, Forner A, Bianchi L, García-Criado A, Rimola J, de Lope CR, Reig M, Ayuso C, Brú C, Bruix J. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology. 2010;51:2020-2029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 230] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 12. | Chen LD, Ruan SM, Liang JY, Yang Z, Shen SL, Huang Y, Li W, Wang Z, Xie XY, Lu MD, Kuang M, Wang W. Differentiation of intrahepatic cholangiocarcinoma from hepatocellular carcinoma in high-risk patients: A predictive model using contrast-enhanced ultrasound. World J Gastroenterol. 2018;24:3786-3798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Liu GJ, Wang W, Lu MD, Xie XY, Xu HX, Xu ZF, Chen LD, Wang Z, Liang JY, Huang Y, Li W, Liu JY. Contrast-Enhanced Ultrasound for the Characterization of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Liver Cancer. 2015;4:241-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Chen LD, Xu HX, Xie XY, Xie XH, Xu ZF, Liu GJ, Wang Z, Lin MX, Lu MD. Intrahepatic cholangiocarcinoma and hepatocellular carcinoma: differential diagnosis with contrast-enhanced ultrasound. Eur Radiol. 2010;20:743-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Han J, Liu Y, Han F, Li Q, Yan C, Zheng W, Wang J, Guo Z, Wang J, Li A, Zhou J. The Degree of Contrast Washout on Contrast-Enhanced Ultrasound in Distinguishing Intrahepatic Cholangiocarcinoma from Hepatocellular Carcinoma. Ultrasound Med Biol. 2015;41:3088-3095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Bohle W, Clemens PU, Heubach T, Zoller WG. Contrast-enhanced ultrasound (CEUS) for differentiating between hepatocellular and cholangiocellular carcinoma. Ultraschall Med. 2012;33:E191-E195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Terzi E, Iavarone M, Pompili M, Veronese L, Cabibbo G, Fraquelli M, Riccardi L, De Bonis L, Sangiovanni A, Leoni S, Zocco MA, Rossi S, Alessi N, Wilson SR, Piscaglia F; CEUS LI-RADS Italy study group collaborators:. Contrast ultrasound LI-RADS LR-5 identifies hepatocellular carcinoma in cirrhosis in a multicenter restropective study of 1,006 nodules. J Hepatol. 2018;68:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 223] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 18. | Li F, Li Q, Liu Y, Han J, Zheng W, Huang Y, Zheng X, Cao L, Zhou JH. Distinguishing intrahepatic cholangiocarcinoma from hepatocellular carcinoma in patients with and without risks: the evaluation of the LR-M criteria of contrast-enhanced ultrasound liver imaging reporting and data system version 2017. Eur Radiol. 2020;30:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 19. | Piscaglia F, Wilson SR, Lyshchik A, Cosgrove D, Dietrich CF, Jang HJ, Kim TK, Salvatore V, Willmann JK, Sirlin CB, Kono Y. American College of Radiology Contrast Enhanced Ultrasound Liver Imaging Reporting and Data System (CEUS LI-RADS) for the diagnosis of Hepatocellular Carcinoma: a pictorial essay. Ultraschall Med. 2017;38:320-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 20. | Huang JY, Li JW, Lu Q, Luo Y, Lin L, Shi YJ, Li T, Liu JB, Lyshchik A. Diagnostic Accuracy of CEUS LI-RADS for the Characterization of Liver Nodules 20 mm or Smaller in Patients at Risk for Hepatocellular Carcinoma. Radiology. 2020;294:329-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 21. | Zheng W, Li Q, Zou XB, Wang JW, Han F, Li F, Huang LS, Li AH, Zhou JH. Evaluation of Contrast-enhanced US LI-RADS version 2017: Application on 2020 Liver Nodules in Patients with Hepatitis B Infection. Radiology. 2020;294:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 22. | European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4562] [Article Influence: 325.9] [Reference Citation Analysis (5)] |
| 23. | Kassahun WT, Hauss J. Management of combined hepatocellular and cholangiocarcinoma. Int J Clin Pract. 2008;62:1271-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Patel AH, Harnois DM, Klee GG, LaRusso NF, Gores GJ. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:204-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 288] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 25. | Sherman M, Peltekian KM, Lee C. Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus: incidence and prevalence of hepatocellular carcinoma in a North American urban population. Hepatology. 1995;22:432-438. [PubMed] |
| 26. | Trevisani F, D'Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, Domenicali M, De Notariis S, Roda E, Bernardi M. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol. 2001;34:570-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 524] [Article Influence: 21.0] [Reference Citation Analysis (4)] |
| 27. | Trevisani F, D'Intino PE, Caraceni P, Pizzo M, Stefanini GF, Mazziotti A, Grazi GL, Gozzetti G, Gasbarrini G, Bernardi M. Etiologic factors and clinical presentation of hepatocellular carcinoma. Differences between cirrhotic and noncirrhotic Italian patients. Cancer. 1995;75:2220-2232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 28. | Wilson SR, Lyshchik A, Piscaglia F, Cosgrove D, Jang HJ, Sirlin C, Dietrich CF, Kim TK, Willmann JK, Kono Y. CEUS LI-RADS: algorithm, implementation, and key differences from CT/MRI. Abdom Radiol (NY). 2018;43:127-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 29. | Kim TK, Noh SY, Wilson SR, Kono Y, Piscaglia F, Jang HJ, Lyshchik A, Dietrich CF, Willmann JK, Vezeridis A, Sirlin CB. Contrast-enhanced ultrasound (CEUS) liver imaging reporting and data system (LI-RADS) 2017 - a review of important differences compared to the CT/MRI system. Clin Mol Hepatol. 2017;23:280-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 30. | Yuan MX, Li R, Zhang XH, Tang CL, Guo YL, Guo DY, Luo MK. Factors Affecting the Enhancement Patterns of Intrahepatic Cholangiocarcinoma (ICC) on Contrast-Enhanced Ultrasound (CEUS) and their Pathological Correlations in Patients with a Single Lesion. Ultraschall Med. 2016;37:609-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Yuan M, Li R, Zhang Y, Yang L, Zhang X, Tang C, Guo D. Enhancement Patterns of Intrahepatic Cholangiocarcinoma on Contrast-Enhanced Ultrasound: Correlation with Clinicopathologic Findings and Prognosis. Ultrasound Med Biol. 2019;45:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Yoshida Y, Imai Y, Murakami T, Nishikawa M, Kurokawa M, Yonezawa T, Tokunaga K, Fukushima Y, Wakasa K, Kim T, Nakamura H, Sakon M, Monden M. Intrahepatic cholangiocarcinoma with marked hypervascularity. Abdom Imaging. 1999;24:66-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Galassi M, Iavarone M, Rossi S, Bota S, Vavassori S, Rosa L, Leoni S, Venerandi L, Marinelli S, Sangiovanni A, Veronese L, Fraquelli M, Granito A, Golfieri R, Colombo M, Bolondi L, Piscaglia F. Patterns of appearance and risk of misdiagnosis of intrahepatic cholangiocarcinoma in cirrhosis at contrast enhanced ultrasound. Liver Int. 2013;33:771-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 34. | Wildner D, Bernatik T, Greis C, Seitz K, Neurath MF, Strobel D. CEUS in hepatocellular carcinoma and intrahepatic cholangiocellular carcinoma in 320 patients - early or late washout matters: a subanalysis of the DEGUM multicenter trial. Ultraschall Med. 2015;36:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: European Society of Radiology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Chen T S-Editor: Liu M L-Editor: Wang TQ E-Editor: Zhang YL