Published online Jul 14, 2020. doi: 10.3748/wjg.v26.i26.3834
Peer-review started: March 18, 2020
First decision: May 21, 2020
Revised: June 20, 2020
Accepted: June 30, 2020
Article in press: June 30, 2020
Published online: July 14, 2020
Processing time: 117 Days and 21.5 Hours
Helicobacter pylori (H. pylori) infection has been associated with a long-term risk of precancerous gastric conditions (PGC) even after H. pylori eradication.
To investigate the efficacy of High-Resolution White-Light Endoscopy with Narrow-Band Imaging in detecting PGC, before/after H. pylori eradication.
We studied 85 consecutive patients with H. pylori-related gastritis with/without PGC before and 6 mo after proven H. pylori eradication. Kimura-Takemoto modified and endoscopic grading of gastric intestinal metaplasia classifications, were applied to assess the endoscopic extension of atrophy and intestinal metaplasia. The histological result was considered to be the gold standard. The Sydney System, the Operative-Link on Gastritis-Assessment, and the Operative-Link on Gastric-Intestinal Metaplasia were used for defining histological gastritis, atrophy and intestinal metaplasia, whereas dysplasia was graded according to World Health Organization classification. Serum anti-parietal cell antibody and anti-intrinsic factor were measured when autoimmune atrophic gastritis was suspected.
After H. pylori eradication histological signs of mononuclear/polymorphonuclear cell infiltration and Mucosal Associated Lymphoid Tissue-hyperplasia, disappeared or decreased in 100% and 96.5% of patients respectively, whereas the Operative-Link on Gastritis-Assessment and Operative-Link on Gastric-Intestinal Metaplasia stages did not change. Low-Grade Dysplasia prevalence was similar on random biopsies before and after H. pylori eradication (17.6% vs 10.6%, P = 0.19), but increased in patients with visible lesions (0% vs 22.4%, P < 0.0001). At a multivariate analysis, the probability for detecting dysplasia after resolution of H. pylori-related active inflammation was higher in patients with regression or reduction of Mucosal Associated Lymphoid Tissue hyperplasia, greater alcohol consumption, and anti-parietal cell antibody and/or anti-intrinsic factor positivity [odds ratio (OR) = 3.88, 95% confidence interval (CI): 1.31-11.49, P = 0.01; OR = 3.10, 95%CI: 1.05-9.12, P = 0.04 and OR = 5.47, 95%CI: 1.33-22.39, P < 0.04, respectively].
High-Resolution White-Light Endoscopy with Narrow-Band Imaging allows an accurate diagnosis of Low-Grade Dysplasia on visible lesions after regression of H. pylori-induced chronic gastritis. Patients with an overlap between autoimmune/H. pylori-induced gastritis may require more extensive gastric mapping.
Core tip: Helicobacter pylori (H. pylori) infection is commonly responsible for precancerous gastric conditions. Heterogeneous long-term endoscopic follow-up studies (2-16 years), have shown conflicting results on the efficacy of H. pylori eradication in reducing the prevalence and histological progression of advanced precancerous gastric conditions. High-Resolution white-light endoscopy combined with narrow-band imaging allows for a more accurate diagnosis of gastric low-grade dysplasia when performed soon after H. pylori eradication. Subjects with an overlap between autoimmune and H. pylori-induced chronic gastritis should be considered to be at a higher risk for more severe gastric injury and they may require more extensive gastric mapping.
- Citation: Panarese A, Galatola G, Armentano R, Pimentel-Nunes P, Ierardi E, Caruso ML, Pesce F, Lenti MV, Palmitessa V, Coletta S, Shahini E. Helicobacter pylori-induced inflammation masks the underlying presence of low-grade dysplasia on gastric lesions. World J Gastroenterol 2020; 26(26): 3834-3850
- URL: https://www.wjgnet.com/1007-9327/full/v26/i26/3834.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i26.3834
Helicobacter pylori (H. pylori) infection has been associated with premalignant gastric conditions (PGC), such as chronic atrophic gastritis and intestinal metaplasia (IM), which are strongly associated with dysplasia and Lauren intestinal-type of gastric carcinoma (GC)[1-7]. Autoimmune atrophic gastritis (AAG) is responsible for progressive mucosal atrophy (antrum-sparing) with or without IM[8]. Many studies have attributed gastric carcinogenesis to both genetic predisposition and H. pylori-induced gastric inflammation[9-13]. H. pylori strains possessing higher cytotoxicity that infect genetically predisposed subjects have been considered responsible for more severe degrees of inflammation and rapid progression to intestinal-type GC[14-20]. Nowadays, PGC detection and surveillance are considered a cost-effective strategy for the prevention of high-grade dysplasia and GC only in intermediate or high-risk populations[7]. Heterogeneous long-term endoscopic follow-up studies, with a duration of between 2-16 years, have shown conflicting results on the efficacy of H. pylori eradication in reducing the prevalence and histological progression of advanced PGC, and in decreasing GC incidence[3,21-25]. The current European guidelines recommend H. pylori eradication in high-risk subjects[7,22,26,27]. Nevertheless, even after H. pylori eradication, the risk for PGC may remain or even increase in patients undergoing long-term surveillance[3,22].
A high-quality upper endoscopy should include at least five non-targeted biopsies at the lesser and greater curvatures of the antrum-corpus and at the incisura angularis for H. pylori infection diagnosis and the optimal detection and staging of advanced PGC, which are randomly distributed across the stomach[7,28,29]. Additional targeted biopsies of any visible lesions are recommended, since low-grade dysplasia (LGD) and high-grade dysplasia (HGD) may appear as endoscopically evident, depressed, flat, or raised lesions[7,28,29].
The Sydney System, the Operative-Link on Gastritis-Assessment (OLGA) and the Operative-Link on Gastric-Intestinal Metaplasia (OLGIM) classifications are commonly used to evaluate the histological inflammation and activity due to mononuclear and polymorphonuclear cells infiltration, as well as atrophy and IM, whereas dysplasia is commonly graded according to the World Health Organization classification[30,31]. During white-light endoscopy (WLE), the Kimura-Takemoto modified classification has been used to assess the extension of atrophy[32]. Several studies showed that magnification chromoendoscopy (CE) and narrow-band imaging (NBI) with or without magnification performed by expert endoscopists could be more accurate than WLE alone in diagnosing PGC, although random biopsies may detect PGC otherwise undetectable by NBI targeted biopsies. Thus, a combination of both random-WLE and targeted-NBI biopsies is suggested as the most accurate[29,33-37]. More recent some investigators created and validated a simplified NBI classification, with magnification [(endoscopic grading of gastric intestinal metaplasia (EGGIM)], using reproducible NBI features (based on endoscopic mucosal and vascular patterns)[29] of the whole gastric mucosa, with an accuracy (resulting from a multicentre study) of 73%, 87% and 92%, for H. pylori-infection, IM and dysplasia diagnosis, respectively[37].
For patients with an indefinite diagnosis for dysplasia, or with dysplasia resulting from random biopsies without endoscopic evidence of visible lesions, the current guidelines suggest an “immediate” endoscopic reassessment with high-resolution endoscopy with NBI to exclude LGD or HGD on missed visible lesions[7]. This procedure revises what was indicated in previous guidelines[28], which advised, in such an event, a delayed endoscopic follow-up within one year after the diagnosis[7,28].
On these premises, supposing that dysplastic lesions might be missed on the background of an active H. pylori related gastritis, we focused on the assessment of the combined diagnostic performance of high-resolution white-light endoscopy (HR-WLE) with NBI in detecting PGC, before and 6 mo after H. pylori eradication (primary end-point). As a secondary aim we considered some clinical factors, autoimmune laboratory markers, and histopathological features as potential co-factors for the development of dysplastic lesions.
This is an observational, prospective study performed at the tertiary care center of the National Institute of Gastroenterology “S De Bellis” (Castellana Grotte, Bari, Italy). Our research was carried out in compliance with the Declaration of Helsinki and with routine clinical practice (Clinical Trial Gov number NCT03917836). All the procedures received local ethics committee approval (Protocol 67/18/CT, 140/18/CE). All patients gave their written informed consent to take part in the study.
From June 2017 to April 2019, we enrolled 85 consecutive subjects, from a cohort study of 156 outpatients, who underwent high-resolution gastroscopies with/without NBI using the Olympus Evis Exera III processor® and Olympus GIF-HQ190® instruments (Olympus Tokyo, Japan). Figure 1 shows the inclusion and exclusion of enrolment steps.
For patients with suspected AAG, anti-parietal cell antibody (APCA) and anti-intrinsic factor (AIF) were measured in serum using indirect immunofluorescence method and immunoenzimatic assay respectively. APCA antibodies were detected by NOVA Lite® Stomach Kit (Inova Diagnostics, San Diego, CA, United States) and AIF antibodies were detected by an automated assay using a Beckman Coulter Unicel DXI 800 (Beckman Coulter Inc., Fullerton, CA, United States).
The endoscopic procedures were performed by two expert endoscopists (more than 200 HRE-NBI per year), one of whom (AP) was present during all procedures as either operator or supervisor. The statistical review of this study was executed by an experienced biomedical statistician. Based on previously published data[38], we calculated the sample size for the analysis of the primary outcome using the function ss2x2 implemented in the package exact2x2. We examined that 48 patients (24 controls and 24 treated) would yield a power of 0.82 at a significance level of 0.05. Therefore, we aimed at enrolling at least 48 patients. The study was completed when the 85 enrolled subjects had been endoscopically reassessed with HR-WLE and NBI, and with gastric biopsy samples according to the Sydney System[28], 6 mo after H. pylori eradication confirmed by 13C-urea breath-test (13C-UBT), in order to identify the diagnostic yield of HR-WLE with NBI in detecting PGC, during this short surveillance time.
13C urea breath test was performed under the following conditions: An 8-h fast, mouth washing before dosing, administration of 75 mg 13C urea in a water solution, collection of breath samples in two 10-mL glass-sample containers, (at baseline and 20 min) and subjects in a sitting position. The breath samples were analyzed by gas-chromatography-mass spectrometry (GC-MS; ABCA Sercon Gateway, United Kingdom). H. pylori infection was considered present if the difference in 13C/12C between baseline value and 20-min value exceeded 40/00.
Inclusion criteria were as follows: Patients ≥ 18 years undergoing upper gastrointestinal endoscopy for H. pylori-related chronic symptoms (i.e., recurrent abdominal pain, dyspepsia, or unexplained anemia), with positivity to 13C-UBT and with a histological diagnosis of H. pylori-related gastritis with/without PGC.
Exclusion criteria were as follows: Past GC or gastric related surgery, impossibility to perform at least five biopsies during endoscopy, relevant comorbidities (cardiac, respiratory, chronic renal insufficiency, chronic liver disease, and psychiatric conditions), anticoagulant therapy/coagulation disorders, non-steroidal anti-inflammatory drugs and/or long-term proton pump inhibitors users, and active smoking habit. Autoimmune co-morbidities were not considered as exclusion criteria.
A history of H. pylori infection was investigated on the basis of medical records or a face-to-face clinical examination. During each procedure the qualified endoscopists performed the biopsies according to the study protocol to ascertain or confirm H. pylori presence and its related histological alterations. All visible gastric lesions were initially evaluated by HR-WLE with NBI. In detail, we performed at least five random biopsies using HR-WLE with NBI, followed by targeted biopsies on endoscopically evident lesions, suggestive of IM or dysplasia[28]. Furthermore, at least five different images were acquired during gastroscopy by a single expert observer, who was blind to the final histology, and who predicted the diagnosis of normal mucosa, atrophy, IM, or dysplasia using the EGGIM classification according to Pimentel-Nunes et al[29].
The histological result was considered as the gold standard for the diagnosis of dysplasia and IM; all gastric biopsy specimens were independently assessed by two expert gastrointestinal pathologists (MLC and RA). The Sydney System, and the OLGA and OLGIM systems assessment were used for histological staging of gastritis[30,31,39]; the following diagnostic categories were considered for the evaluation of dysplasia according to the World Health Organization classification: Negative for intraepithelial neoplasia/dysplasia, indefinite for intraepithelial neoplasia/dysplasia, low-grade intraepithelial neoplasia/dysplasia, high-grade intraepithelial neoplasia/dysplasia and intra-mucosal invasive neoplasia/intra-mucosal car- cinoma[39]. In all patients, H. pylori eradication was assessed by a prior negative result of 13C-UBT and confirmed by histology.
Normal distribution of continuous variables was assessed with the Shapiro-Wilk test and data were expressed as mean and ± SD. Categorical variables were reported as percentages and compared using the χ2 test or Fisher’s exact test, when needed. The probability of detecting gastric dysplasia after H. pylori eradication was evaluated using univariate and multivariate logistic regression analyses. The association between each explanatory variable and the outcome (detection of dysplasia) was tested using the likelihood ratio test. For each variable included in the multivariate model, we calculated both unadjusted and adjusted odds ratios (OR), with their 95% confidence intervals (CI), and the level of significance (using the likelihood ratio test). Statistical significance was set at P < 0.05. All statistical analyses were performed using SPSS 23.0 software (SPSS, Chicago, IL, United States) and R version 3.4.3 (http://www.R-project.org/).
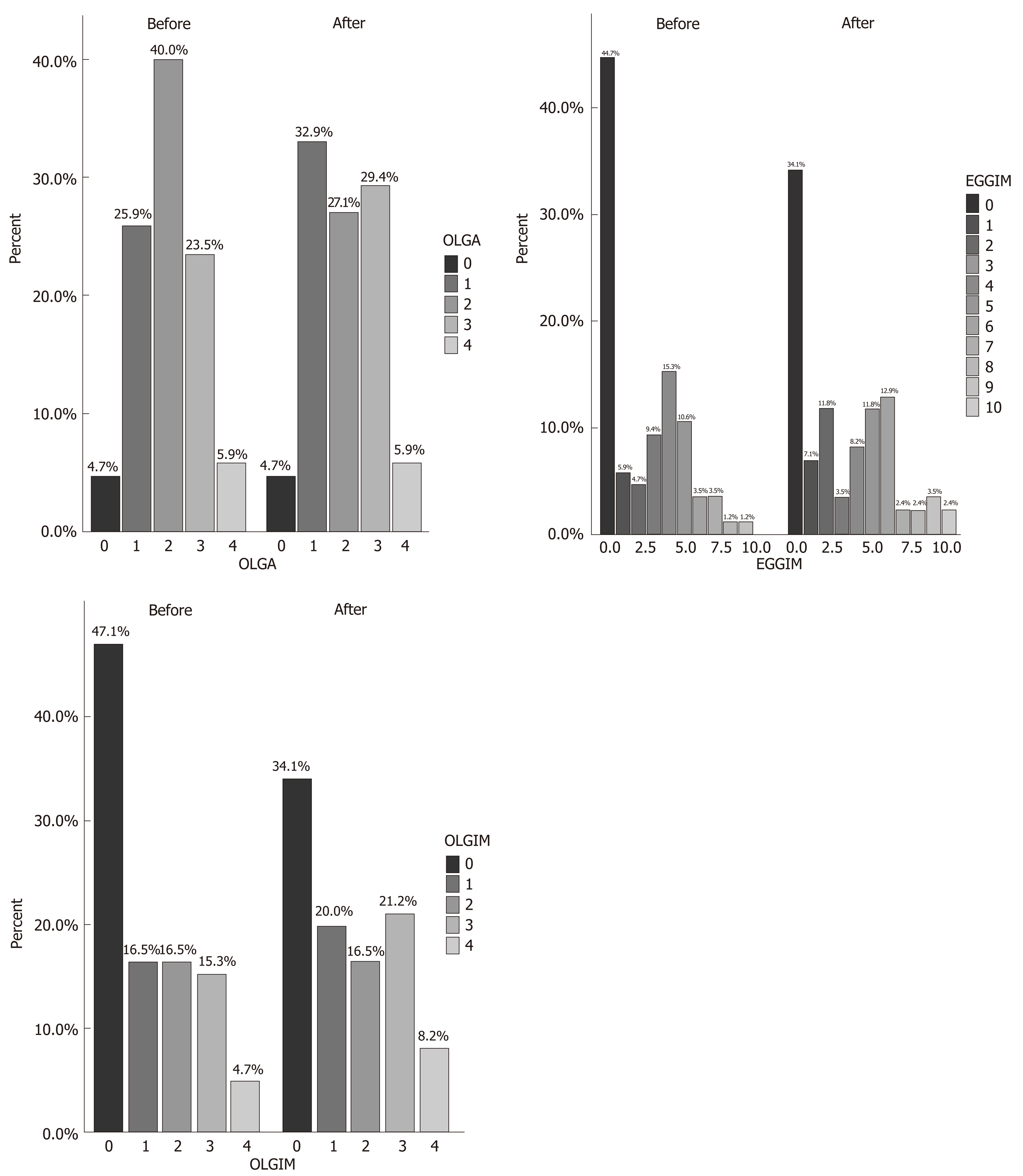
The clinical characteristics of the 85 enrolled patients are described in Table 1. At baseline, the majority of patients (71.8%) had an extensive endoscopic atrophic gastritis (pan-atrophy, corpus-predominant or antrum-predominant atrophy), and the minority (22.4%) had focal antrum atrophy (Table 2)[32]. The patients with a less advanced degree (0-2 and 3-4) of the EGGIM scale were 80%; histological gastritis was moderate/severe and mild in 77.6% and 22.4% of the patients respectively; 45.8% of patients also had mucosal associated lymphoid tissue (MALT) hyperplasia (Table 2). Fifteen patients (17.6%) had LGD on random biopsies. Among the 49 patients with histological pan-atrophy or corpus-predominant atrophy, 11 (22.4%) tested positive for APCA and/or for AIF antibodies. In 100% of the cases the histological mononuclear and polymorphonuclear cell infiltration disappeared or decreased after H. pylori eradication. Additionally, the proportion of patients with mild and moderate/severe grades of MALT hyperplasia significantly decreased after H. pylori eradication (29.4% vs 3.5%, P < 0.001 and 16.4% vs 0%, P < 0.001, respectively; Table 2). Nevertheless, the proportion of OLGA, OLGIM stages, and that of patients with histological diagnosis of LGD on random biopsies did not significantly change after H. pylori eradication (17.6% vs 10.6%, P = 0.19) (Table 2 and Figure 2). The detection of LGD on visible lesions significantly increased after H. pylori eradication (0% vs 22.3%, P < 0.001; Table 2). Among the 9 patients who showed LGD on random biopsies after H. pylori eradication, only 2 (22.2%) had already had a diagnosis of LGD on random biopsies before H. pylori eradication. Among the 6 patients with LGD on random and visible lesion biopsies after H. pylori eradication, two (33.3%) were negative for LGD before eradication. Furthermore, the proportion of the 13 patients with a new diagnosis of LGD only on visible lesions was significantly higher after H. pylori disappearance (0% vs 15.3%, P < 0.001) and in 6 of them (46.1%) LGD was not detected before H. pylori eradication.
| Parameters | Patients (n = 85) |
| Age, mean (SD), yr | 56.1 (12.3) |
| Gender, n (%) | |
| Female | 53 (62.4) |
| BMI, mean (SD), kg/m2 | 25.1 (2.2) |
| Alcohol users (12-24 g/dL /die), n (%) | 27 (31.8) |
| Previous smokers, n (%) | 12 (14.1) |
| Drug users, n (%) | 23 (27.1) |
| Family history of gastric cancer, n (%) | 4 (4.7) |
| Family history of other cancer, n (%) | 9 (10.6) |
| Autoimmune comorbidity | |
| Autoimmune atrophic gastritis | 12 (14.1) |
| Autoimmune thyroiditis | 6 (7.1) |
| Type-1/2 diabetes mellitus | 3 (3.5) |
| Skin psoriasis | 1 (1.2) |
| Rheumatoid arthritis | 1 (1.2) |
| Sjögren syndrome | 1 (1.2) |
| A. thyroiditis + vitiligo | 1 (1.2) |
| A. thyroiditis + Crohn’s disease | 1 (1.2) |
| A. thyroiditis + Sjögren syndrome | 1 (1.2) |
| APCA and/or AIF antibody positivity | 12 (14.1) |
| Endoscopy indication, n (%) | |
| Gastroesophageal reflux | 17 (20) |
| Recurrent abdominal pain | 12 (14.1) |
| Dyspepsia | 41 (48.2) |
| Unexplained anemia | 15 (17.7) |
| H. pylori eradication scheme, n (%) | |
| Quadruple1 | 53 (62.4) |
| Modified triple2 | 28 (32.9) |
| Triple3 | 3 (3.6) |
| Sequential4 | 1 (1.2) |
| H. pylori eradication cycles, n (%) | |
| One-cycle | 71 (83.5) |
| Two-cycles | 14 (16.5) |
| Parameters | Before H. pylori eradication (n = 85) | After H. pylori eradication (n = 85) | P value |
| Endoscopic atrophy1, n (%) | |||
| Absent | 5 (5.9) | 4 (4.7) | 1.0 |
| Antrum | 19 (22.4) | 19 (22.4) | 1.0 |
| Antrum-predominant | 18 (21.2) | 12 (14.1) | 0.23 |
| Corpus-predominant | 21 (24.7) | 23 (27.1) | 0.73 |
| Pan-atrophy | 22 (25.9) | 27 (31.8) | 0.40 |
| OLGA-scale, n (%) | |||
| Stage 0 | 4 (4.7) | 4 (4.7) | 1.0 |
| Stage 1 | 22 (25.9) | 28 (32.9) | 0.31 |
| Stage 2 | 34 (40) | 23 (27.1) | 0.07 |
| Stage 3 | 20 (23.5) | 25 (29.4) | 0.38 |
| Stage 4 | 5 (5.9) | 5 (5.9) | 1.0 |
| EGGIM-scale, n (%) | |||
| 0-2 | 47 (55.3) | 45 (52.9) | 0.76 |
| 3-4 | 21 (24.7) | 10 (11.8) | 0.03a |
| 5-6 | 12 (14.1) | 21 (24.7) | 0.08 |
| 7-8 | 4 (4.7) | 4 (4.7) | 1.0 |
| 9-10 | 1 (1.2) | 5 (5.9) | 0.21 |
| OLGIM-scale, n (%) | |||
| Stage 0 | 40 (47.1) | 29 (34.1) | 0.08 |
| Stage 1 | 14 (16.5) | 17 (20) | 0.55 |
| Stage 2 | 14 (16.5) | 14 (16.5) | 1.0 |
| Stage 3 | 13 (15.3) | 18 (21.2) | 0.32 |
| Stage 4 | 4 (4.7) | 7 (8.2) | 0.53 |
| Gastritis at histology2, n (%) | |||
| Quiescent | 0 | 81 (95.3) | < 0.0001b |
| Mild | 19 (22.4) | 4 (4.7) | 0.001b |
| Moderate | 46 (54.1) | 0 | < 0.0001b |
| Severe | 20 (23.5) | 0 | < 0.0001b |
| MALT-hyperplasia, n (%) | |||
| Absent | 46 (54.1) | 82 (96.5) | < 0.0001b |
| Mild | 25 (29.4) | 3 (3.5) | < 0.0001b |
| Moderate | 11 (12.9) | 0 | 0.007b |
| Severe | 3 (3.5) | 0 | 0.24 |
| Histological LGD3, n (%) | |||
| Absent | 70 (82.4) | 57 (67.1) | 0.02a |
| On random biopsies | 15 (17.6) | 9 (10.6) | 0.19 |
| On random + on lesions biopsies | 0 | 6 (7) | 0.03b |
| Only on visible lesions | 0 | 13 (15.3) | 0.0001b |
The characteristics of the 19 subjects in whom LGD was detected on visible lesions after H. pylori eradication are shown in Supplementary Table 1. In details, 11 subjects (57.9%) were male, and in 8 patients (42.1%) LGD was missed before H. pylori eradication. Five patients (26.3%) had APCA and/or AIF positivity. Comparison of the patients with or without LGD (n = 28 vs n = 57 respectively) after H. pylori eradication is shown in Table 3. In patients with LGD, the age was older (60.9 ± 8.2 vs 53.7 ± 13.4 years, P = 0.01), there were more alcohol users or past smokers (53.6% vs 21.1%, P = 0.002 and 35.7% vs 3.5%, P < 0.001, respectively), there was a higher proportion of APCA and/or AIF antibody positivity (28.6% vs 7%, P = 0.02), there were more familial cases of GC (14.3% vs 0%, P < 0.001) and more advanced stages of OLGA and OLGIM, both at the baseline and after H. pylori eradication (P < 0.001 for both). When we compared the histological characteristics of the 28 patients with LGD after H. pylori eradication, we observed that the moderate/severe grades of gastritis and MALT hyperplasia significantly regressed during surveillance (P < 0.001 and P = 0.004, respectively), whereas OLGA stages were similar (P = 0.76; Supplementary Table 2). A higher prevalence of OLGIM stages 3-4 was observed after H. pylori eradication, but it did not reach the level of statistical significance (57.1% vs 75%, P = 0.16).
| Parameters | With LGD (n = 28) | Without LGD (n = 57) | P value |
| Age, mean (SD), yr | 60.9 (8.2) | 53.7 (13.4) | 0.01a |
| Gender, n (%) | |||
| Female | 15 (53.6) | 38 (66.7) | 0.24 |
| BMI, mean (SD), kg/m2 | 25 (2.4) | 25 (2.3) | 1.0 |
| Alcohol users (12-24 g/dL/die), n (%) | 15 (53.6) | 12 (21.1) | 0.002b |
| Previous smokers, n (%) | 10 (35.7) | 2 (3.5) | < 0.0001c |
| Family history of gastric cancer, n (%) | 4 (14.3) | 0 | 0.001c |
| Family history of other cancer, n (%) | 6 (21.4) | 3 (5.3) | 0.001c |
| Autoimmune comorbidity | |||
| Autoimmune atrophic gastritis | 8 (28.6) | 4 (7) | 0.02c |
| Autoimmune thyroiditis | 3 (10.7) | 3 (5.3) | 0.39 |
| A. thyroiditis + vitiligo | 1 (3.6) | 0 | 0.33 |
| A. thyroiditis + Crohn’s disease | 1 (3.6) | 0 | 0.33 |
| A. thyroiditis + Sjögren syndrome | 0 | 1 (1.7) | 1.0 |
| Sjögren syndrome | 0 | 1 (1.7) | 1.0 |
| Type-1/2 diabetes mellitus | 1 (3.6) | 2 (3.5) | 1.0 |
| Skin psoriasis | 1 (3.6) | 0 | 0.33 |
| Rheumatoid arthritis | 0 | 1 (1.7) | 1.0 |
| APCA and/or AIF antibody positivity | 8 (28.6) | 4 (7) | 0.02c |
| H. pylori eradication scheme, n (%) | |||
| Quadruple1 | 17 (60.7) | 36 (63.2) | 0.82 |
| Modified triple2 | 9 (32.1) | 19 (33.3) | 0.91 |
| Triple3 | 1 (3.6) | 2 (3.5) | 1.0 |
| Sequential4 | 1 (3.6) | 0 | 0.33 |
| H. pylori eradication cycles, n (%) | |||
| One cycle | 22 (78.6) | 46 (86) | 0.82 |
| Two cycles | 6 (21.4) | 8 (14) | 0.82 |
| OLGA scale before5 | |||
| Stage 0 | 0 | 4 (7) | 0.3 |
| Stage 1-2 | 8 (28.6) | 48 (84.2) | < 0.0001b |
| Stage 3-4 | 20 (71.4) | 5 (8.8) | < 0.0001c |
| OLGA scale after6 | |||
| Stage 0 | 0 | 4 (7) | 0.3 |
| Stage 1-2 | 7 (25) | 44 (77.2) | < 0.0001b |
| Stage 3-4 | 21 (75) | 9 (15.8) | < 0.0001b |
| OLGIM scale before | |||
| Stage 0 | 0 | 40 (70.1) | < 0.0001c |
| Stage 1-2 | 12 (42.8) | 16 (28.1) | 0.17 |
| Stage 3-4 | 16 (57.1) | 1 (1.8) | < 0.0001c |
| OLGIM scale after | |||
| Stage 0 | 0 | 29 (50.9) | < 0.0001c |
| Stage 1-2 | 7 (25) | 24 (42.1) | 0.12 |
| Stage 3-4 | 21 (75) | 4 (7) | < 0.0001c |
| Gastritis at histology before, n (%) | |||
| Quiescent | 0 | 0 | 1.0 |
| Mild | 3 (10.7) | 16 (28.1) | 0.10 |
| Moderate-severe | 25 (89.3) | 41 (71.9) | 0.10 |
| Gastritis at histology after, n (%) | |||
| Quiescent | 26 (92.9) | 55 (96.5) | 0.59 |
| Mild | 2 (7.1) | 2 (3.5) | 0.50 |
| Moderate-severe | 0 | 0 | 1.0 |
| MALT hyperplasia before, n (%) | |||
| Absent | 10 (35.7) | 36 (63.2) | 0.02b |
| Mild | 10 (35.7) | 15 (26.3) | 0.37 |
| Moderate | 6 (21.4) | 5 (8.8) | 0.17 |
| Severe | 2 (7.2) | 1 (1.8) | 0.25 |
| MALT hyperplasia after, n (%) | |||
| Absent | 10 (35.7) | 36 (63.2) | 0.02b |
| Mild | 18 (64.3) | 21 (36.8) | 0.02b |
| Moderate | 0 | 0 | 1.0 |
| Severe | 0 | 0 | 1.0 |
As shown in Supplementary Table 3, in patients without LGD after H. pylori eradication only gastritis significantly improved at the follow-up endoscopy (P < 0.001). Table 4 shows the results of the linear regression analysis. According to the multivariate analysis adjusted for age, alcohol use, MALT hyperplasia regression/reduction and for APCA and/or AIF antibody presence, the probability for detecting gastric LGD, randomly or on visible lesions after eradication of H. pylori, was significantly higher in patients with regression/reduction of MALT hyperplasia (OR = 3.10, 95%CI: 1.05-9.12, P = 0.04), alcohol consumption (OR = 3.88, 95%CI: 1.31-11.49, P = 0.01), and APCA and/or AIF antibody positivity (OR = 5.47, 95%CI: 1.33-22.39, P < 0.04).
| Univariate | Multivariate | |||
| Variable | OR (95% CI) | P value | OR1 (95%CI) | P value |
| Age, yr | 1.05 (1.01-1.11) | < 0.02 | 1.05 (1.00-1.10) | 0.07 |
| Gender | ||||
| Female | 1.002 | - | ||
| Male | 1.73 (0.69-4.37) | 0.24 | - | - |
| BMI, kg/m2 | 0.93 (0.75-1.15) | 0.49 | - | - |
| Alcohol use | ||||
| No | 1.002 | 1.002 | ||
| Yes | 4.33 (1.63-11.51) | < 0.01 | 3.88 (1.31-11.49) | 0.01 |
| Drug use | ||||
| No | 1.002 | - | ||
| Yes | 1.45 (0.54-3.94) | 0.46 | - | - |
| MALT hyperplasia regression/reduction | ||||
| No | 1.002 | - | ||
| Yes | 3.90 (1.20-7.91) | < 0.02 | 3.10 (1.05-9.12) | 0.04 |
| APCA and/or AIF antibody positivity | ||||
| No | 1.002 | |||
| Yes | 5.30 (1.44-19.56) | 0.01 | 5.47 (1.33-22.39) | < 0.02 |
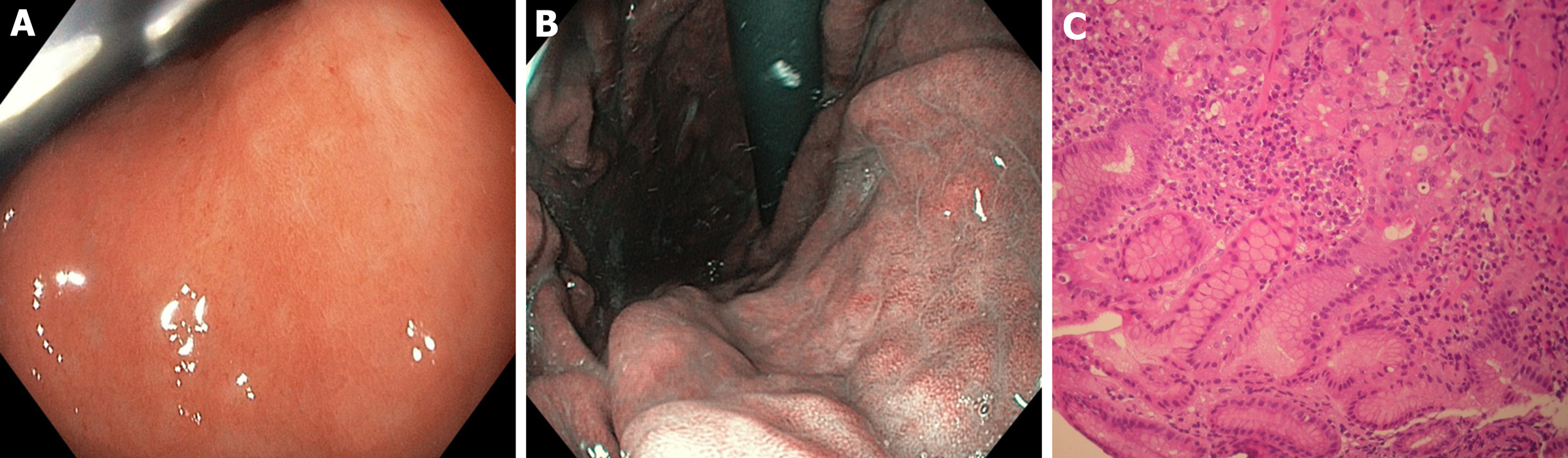
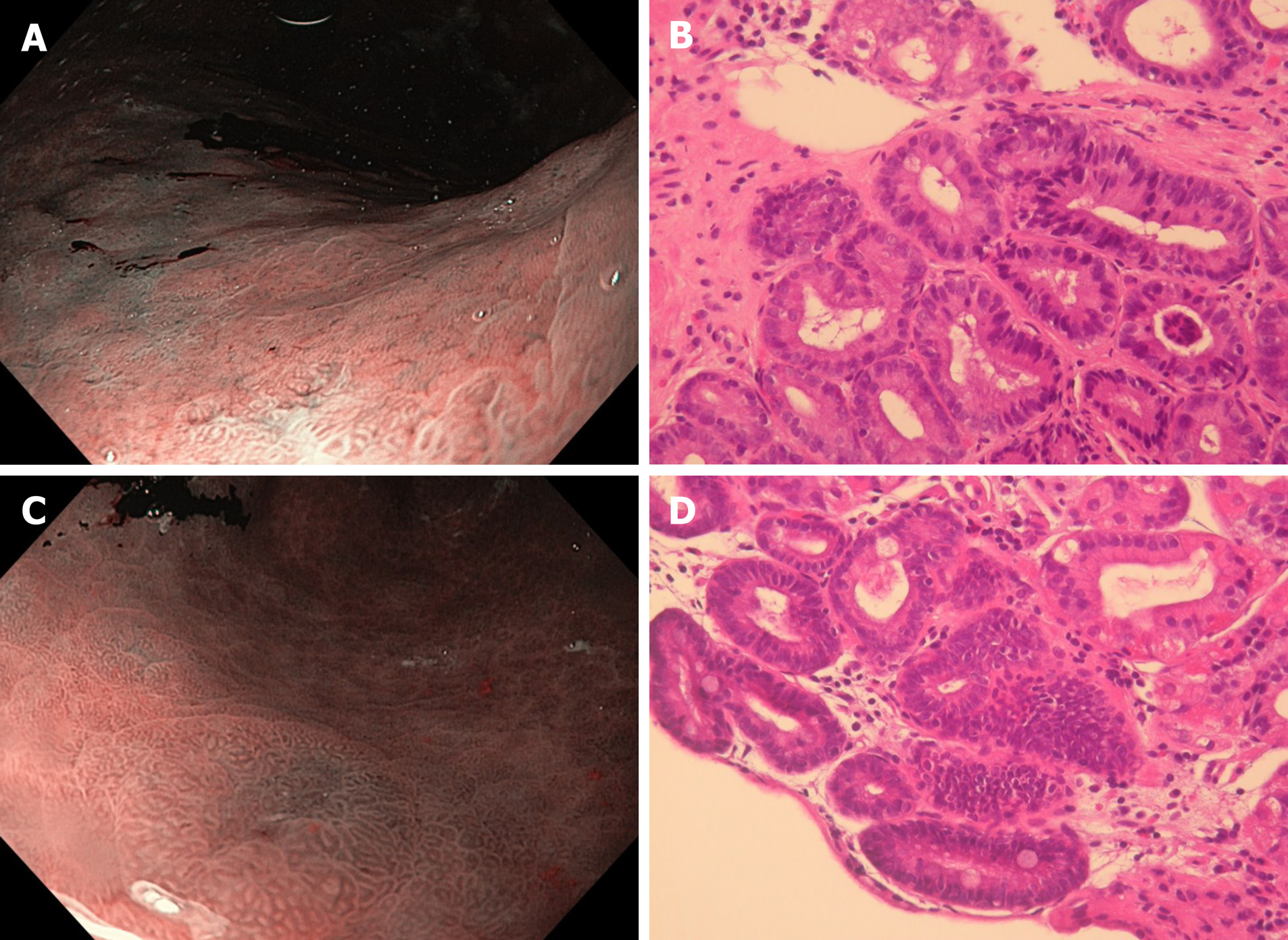
Our study shows that HR-WLE in combination with NBI can diagnose gastric LGD on visible lesions with the highest accuracy after regression of H. pylori-induced signs of active infection (Figure 3 and 4). This suggests that, in high-risk subjects without alarm features for malignancy, non-invasive tests should be used for prior H. pylori identification, and a high-quality upper endoscopy to identify dysplastic lesions should be postponed after H. pylori eradication has been achieved. This strategy could be useful for yielding a higher dysplasia detection rate and for better defining cancer risk and surveillance time.
Despite conflicting evidence, H. pylori eradication is presently advised in high-risk subjects for its potential to reduce GC incidence and induce regression of inflammation and atrophic gastritis[7,22-27,40]. Originally, the use of WLE alone provided a weak association between endoscopic and histological findings in diagnosing PGC, probably due to the inefficient technology of endoscopy imaging[41-43]; recent studies have instead shown that the use of HR-WLE allows for a high concordance accuracy for this purpose[7,36]. There is strong evidence that virtual chromoendoscopy should be used for the diagnosis of PGC because of its better performance compared to HR-WLE[34-37,44]. Capelle et al[33] studied high-risk patients with IM or dysplasia undergoing endoscopic surveillance 2.0 years (range 0.8–21.1) and 1.9 years (range 0.2–5.2) respectively after the initial diagnosis and showed a slightly better diagnostic yield for the detection of advanced PGC using HRE with NBI vs HR-WLE[33]. Conventional CE with the application of dyes has been associated with the highest PGC detection accuracy as compared to virtual chromoendoscopy although procedural time is considerably longer[45-47]. As a result of such evidence, the updated version of the European guidelines suggests that a high-quality endoscopy requires the use of NBI[7].
In our study, the histological signs of active gastritis and MALT hyperplasia disappeared or decreased in 100% and 96.5% of patients with or without PGC after H. pylori eradication, respectively. The overall prevalence of LGD on random biopsies at a 6-mo interval was similar (17.6% vs 10.6%). Nevertheless, among the patients with newly diagnosed LGD on visible lesions, the percentage of endoscopically missed dysplasia was 42.1% before H. pylori eradication, when active gastritis was present. Unexpectedly, at the baseline 26.3% of such patients (5/19) had an overlapping AAG with APCA and/or AIF positivity. This prevalence rose to 28.6% (8/28 patients) when we considered the total patients with dysplasia, and the percentage of this positivity did not change after H. pylori eradication (P = 1.0, data not shown). We observed an almost doubled prevalence of gastric LGD on biopsies performed randomly or on visible lesions before and after H. pylori disappearance (17.6% vs 32.9% respectively). A similar prevalence was found in another study from two referral centers using NBI endoscopy, in which dysplasia was detected in 28/85 (33%) and 38/85 H. pylori positive patients (45%)[37].
Patients with LGD were older and showed more advanced OLGA and OLGIM stages as compared to those without LGD, at baseline as well as after H. pylori eradication. The prevalence of OLGIM stages 3-4 showed a tendency to increase after H. pylori eradication only in patients with dysplasia, from 57.1% to 75%.
The higher prevalence of LGD after H. pylori eradication could depend on the presence of more severe and extensive mucosal atrophy and IM at baseline in our high-risk subgroup of patients rather than disease progression itself, considering the short interval of endoscopic surveillance of our study. In this scenario, the background of active H. pylori inflammation is likely to play a confounding role that may have hampered the accurate detection of gastric dysplasia. Nevertheless, the unexpected high baseline prevalence of dysplasia may be partially justified by the concomitant presence of AAG in a considerable number of our patients, in accordance with results from a recent study showing that in the presence of AAG the risk of developing more advanced stages on long-term follow-up is greater in patients with more severe gastric lesions[8]. The overlap between autoimmune and H. pylori-induced chronic gastritis may presumably be associated with a more severe gastric injury, especially in older subjects.
At a multivariate analysis, the probability for detecting gastric dysplasia after resolution of H. pylori-related active inflammation was significantly higher in older patients with regression or reduction of MALT hyperplasia, greater alcohol consumption, and APCA and/or AIF positivity. Therefore, MALT hyperplasia with active gastritis, whether induced or not by H. pylori, could be an additional confounding factor influencing the detection of PGC, and, particularly, LGD in high-risk patients (Table 4).
The prevalence of autoimmune diseases (24.7%) in our patients showed a tendency to increase among patients with LGD (35.7% vs 17.5% in those without LGD, P = 0.06 by χ2 test). Since autoimmune disorders are linked to the presence of DRB1 and DQB1 haplotypes our finding may suggest a reciprocal role between the genetics of immune response and H. pylori chronic infection.
Our results are in full accordance with the current European recommendations, which suggest to perform an endoscopic reassessment with HRE-NBI as soon as possible after dysplasia is diagnosed in the “apparent” absence of endoscopically visible lesions, to search for malignancy on misdiagnosed lesions[7,27,48]. Where the presence of H. pylori active gastritis without alarming features for malignancy is suspected, considering the present lack of clinical recommendations on the diagnosis and surveillance of PGC[7], our study suggests that the best diagnostic workout in symptomatic patients should entail performing prior H. pylori non-invasive tests, thus increasing the probability of detecting LGD on visible lesions at an endoscopy carried out soon after H. pylori eradication has been achieved.
There are some limitations to our study. The sample size is relatively small, although all endoscopies were performed in a standardized way by using the same biopsy protocol and technical procedures and with the presence of the same endoscopist throughout the study. The hypothesis that LGD could have progressed into visible lesions is unlikely due to our short 6-mo follow-up interval[7]: The risk of PGC progression has indeed been demonstrated to increase only on long-term follow-up (2-16 years), after eradication of H. pylori[22-25,40]. Our results may help explain the heterogeneous findings of a possible increased PGC prevalence after H. pylori eradication in long-term surveillance studies, as this might be due to a misdiagnosis at initial endoscopic examination.
In conclusion, HR-WLE with NBI can be more accurate in diagnosing LGD on visible lesions after H. pylori eradication has been achieved, probably due to the disappearance of the underlying confounding effects of inflammatory and mucosal lymphoproliferative changes induced by H. pylori chronic active infection. Elderly patients and those with autoimmune diseases could be at higher risk for H. pylori chronic infection. An effective and cost-effective strategy to diagnose LGD with the highest accuracy should entail a high-quality upper endoscopy performed soon after eradication of H. pylori infection, detected by prior non-invasive tests.
Helicobacter pylori (H. pylori) infection is frequently responsible for precancerous gastric conditions (PGC) and the long-term risk of PGC may even progress after H. pylori eradication.
Heterogeneous long-term endoscopic follow-up studies (2-16 years) have shown conflicting results on the efficacy of H. pylori eradication in reducing the prevalence and histological progression of advanced PGC. Moreover, High-Resolution White-Light Endoscopy (HR-WLE) in combination with narrow-band imaging (NBI) is effective in detecting PGC and determines the timing and mode of endoscopic surveillance.
To assess the efficacy of HR-WLE with NBI in detecting PGC, before and after H. pylori eradication at a short-term interval.
We evaluated 85 consecutive patients with H. pylori-related gastritis with/without PGC at baseline and 6 mo after proven H. pylori eradication. The Operative-Link on Gastritis-Assessment and Operative-Link on Gastric-Intestinal Metaplasia have been used as gold standards for histological definition of gastritis, atrophy and intestinal metaplasia. Serum anti-parietal cell antibody and anti-intrinsic factor were measured when autoimmune atrophic gastritis was suspected.
HR-WLE in combination with NBI allows for a more accurate diagnosis of gastric low-grade dysplasia (LGD) when performed soon after H. pylori eradication, due to regression of H. pylori-induced signs of inflammation. Furthermore, we observed an unexpected high prevalence of autoimmune disorders, suggesting an interaction between the genetics of immune response and H. pylori chronic infection, especially in relation to the risk of LGD development.
HR-WLE with NBI allows an accurate diagnosis of gastric LGD on visible lesions after regression of H. pylori-induced signs of active infection, especially in high-risk subjects. Patients with overlap between autoimmune and H. pylori-induced chronic gastritis may require more extensive gastric mapping.
Our findings will appeal to both clinical gastroenterologists and endoscopists, and stimulate the development of more accurate and cost-effective strategies for identifying patients with H. pylori infection who are at risk of gastric cancer. Subjects with an overlap between autoimmune and H. pylori-induced chronic gastritis should be considered to be at a higher risk of more severe gastric injury.
| 1. | Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735-6740. [PubMed] |
| 2. | Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M. A model for gastric cancer epidemiology. Lancet. 1975;2:58-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 746] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 3. | Mera RM, Bravo LE, Camargo MC, Bravo JC, Delgado AG, Romero-Gallo J, Yepez MC, Realpe JL, Schneider BG, Morgan DR, Peek RM, Correa P, Wilson KT, Piazuelo MB. Dynamics of Helicobacter pylori infection as a determinant of progression of gastric precancerous lesions: 16-year follow-up of an eradication trial. Gut. 2018;67:1239-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (1)] |
| 4. | Ihamäki T, Saukkonen M, Siurala M. Long-term observation of subjects with normal mucosa and with superficial gastritis: results of 23--27 years' follow-up examinations. Scand J Gastroenterol. 1978;13:771-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 42] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Carneiro F, Machado JC, David L, Reis C, Nogueira AM, Sobrinho-Simões M. Current thoughts on the histopathogenesis of gastric cancer. Eur J Cancer Prev. 2001;10:101-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | de Vries AC, van Grieken NC, Looman CW, Casparie MK, de Vries E, Meijer GA, Kuipers EJ. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 601] [Article Influence: 33.4] [Reference Citation Analysis (1)] |
| 7. | Pimentel-Nunes P, Libânio D, Marcos-Pinto R, Areia M, Leja M, Esposito G, Garrido M, Kikuste I, Megraud F, Matysiak-Budnik T, Annibale B, Dumonceau JM, Barros R, Fléjou JF, Carneiro F, van Hooft JE, Kuipers EJ, Dinis-Ribeiro M. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy. 2019;51:365-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 722] [Article Influence: 103.1] [Reference Citation Analysis (0)] |
| 8. | Miceli E, Vanoli A, Lenti MV, Klersy C, Di Stefano M, Luinetti O, Caccia Dominioni C, Pisati M, Staiani M, Gentile A, Capuano F, Arpa G, Paulli M, Corazza GR, Di Sabatino A. Natural history of autoimmune atrophic gastritis: a prospective, single centre, long-term experience. Aliment Pharmacol Ther. 2019;50:1172-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 9. | Wang P, Xia HH, Zhang JY, Dai LP, Xu XQ, Wang KJ. Association of interleukin-1 gene polymorphisms with gastric cancer: a meta-analysis. Int J Cancer. 2007;120:552-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Camargo MC, Mera R, Correa P, Peek RM, Fontham ET, Goodman KJ, Piazuelo MB, Sicinschi L, Zabaleta J, Schneider BG. Interleukin-1beta and interleukin-1 receptor antagonist gene polymorphisms and gastric cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1674-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 183] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 11. | Hnatyszyn A, Wielgus K, Kaczmarek-Rys M, Skrzypczak-Zielinska M, Szalata M, Mikolajczyk-Stecyna J, Stanczyk J, Dziuba I, Mikstacki A, Slomski R. Interleukin-1 gene polymorphisms in chronic gastritis patients infected with Helicobacter pylori as risk factors of gastric cancer development. Arch Immunol Ther Exp (Warsz). 2013;61:503-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Xue H, Lin B, Ni P, Xu H, Huang G. Interleukin-1B and interleukin-1 RN polymorphisms and gastric carcinoma risk: a meta-analysis. J Gastroenterol Hepatol. 2010;25:1604-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Loh M, Koh KX, Yeo BH, Song CM, Chia KS, Zhu F, Yeoh KG, Hill J, Iacopetta B, Soong R. Meta-analysis of genetic polymorphisms and gastric cancer risk: variability in associations according to race. Eur J Cancer. 2009;45:2562-2568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Figueiredo C, Machado JC, Pharoah P, Seruca R, Sousa S, Carvalho R, Capelinha AF, Quint W, Caldas C, van Doorn LJ, Carneiro F, Sobrinho-Simões M. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst. 2002;94:1680-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 474] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 15. | Amieva MR, El-Omar EM. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology. 2008;134:306-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 400] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 16. | Machado JC, Figueiredo C, Canedo P, Pharoah P, Carvalho R, Nabais S, Castro Alves C, Campos ML, Van Doorn LJ, Caldas C, Seruca R, Carneiro F, Sobrinho-Simões M. A proinflammatory genetic profile increases the risk for chronic atrophic gastritis and gastric carcinoma. Gastroenterology. 2003;125:364-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 361] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 17. | Huang JQ, Zheng GF, Sumanac K, Irvine EJ, Hunt RH. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology. 2003;125:1636-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 383] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 18. | Palli D, Masala G, Del Giudice G, Plebani M, Basso D, Berti D, Numans ME, Ceroti M, Peeters PH, Bueno de Mesquita HB, Buchner FL, Clavel-Chapelon F, Boutron-Ruault MC, Krogh V, Saieva C, Vineis P, Panico S, Tumino R, Nyrén O, Simán H, Berglund G, Hallmans G, Sanchez MJ, Larrãnaga N, Barricarte A, Navarro C, Quiros JR, Key T, Allen N, Bingham S, Khaw KT, Boeing H, Weikert C, Linseisen J, Nagel G, Overvad K, Thomsen RW, Tjonneland A, Olsen A, Trichoupoulou A, Trichopoulos D, Arvaniti A, Pera G, Kaaks R, Jenab M, Ferrari P, Nesi G, Carneiro F, Riboli E, Gonzalez CA. CagA+ Helicobacter pylori infection and gastric cancer risk in the EPIC-EURGAST study. Int J Cancer. 2007;120:859-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Basso D, Zambon CF, Letley DP, Stranges A, Marchet A, Rhead JL, Schiavon S, Guariso G, Ceroti M, Nitti D, Rugge M, Plebani M, Atherton JC. Clinical relevance of Helicobacter pylori cagA and vacA gene polymorphisms. Gastroenterology. 2008;135:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 295] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 20. | Rizzato C, Kato I, Plummer M, Muñoz N, Stein A, Jan van Doorn L, Franceschi S, Canzian F. Risk of advanced gastric precancerous lesions in Helicobacter pylori infected subjects is influenced by ABO blood group and cagA status. Int J Cancer. 2013;133:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Lee YC, Chen TH, Chiu HM, Shun CT, Chiang H, Liu TY, Wu MS, Lin JT. The benefit of mass eradication of Helicobacter pylori infection: a community-based study of gastric cancer prevention. Gut. 2013;62:676-682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 280] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 22. | Leung WK, Lin SR, Ching JY, To KF, Ng EK, Chan FK, Lau JY, Sung JJ. Factors predicting progression of gastric intestinal metaplasia: results of a randomised trial on Helicobacter pylori eradication. Gut. 2004;53:1244-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 323] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 23. | You WC, Brown LM, Zhang L, Li JY, Jin ML, Chang YS, Ma JL, Pan KF, Liu WD, Hu Y, Crystal-Mansour S, Pee D, Blot WJ, Fraumeni JF, Xu GW, Gail MH. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J Natl Cancer Inst. 2006;98:974-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 327] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 24. | Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY, Fong DY, Ho J, Ching CK, Chen JS; China Gastric Cancer Study Group. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1053] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 25. | Chen HN, Wang Z, Li X, Zhou ZG. Helicobacter pylori eradication cannot reduce the risk of gastric cancer in patients with intestinal metaplasia and dysplasia: evidence from a meta-analysis. Gastric Cancer. 2016;19:166-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 172] [Article Influence: 17.2] [Reference Citation Analysis (1)] |
| 26. | Chey WD, Wong BC; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 819] [Cited by in RCA: 838] [Article Influence: 44.1] [Reference Citation Analysis (3)] |
| 27. | Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T, Rugge M, Selgrad M, Suerbaum S, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2220] [Cited by in RCA: 2090] [Article Influence: 232.2] [Reference Citation Analysis (1)] |
| 28. | Dinis-Ribeiro M, Areia M, de Vries AC, Marcos-Pinto R, Monteiro-Soares M, O'Connor A, Pereira C, Pimentel-Nunes P, Correia R, Ensari A, Dumonceau JM, Machado JC, Macedo G, Malfertheiner P, Matysiak-Budnik T, Megraud F, Miki K, O'Morain C, Peek RM, Ponchon T, Ristimaki A, Rembacken B, Carneiro F, Kuipers EJ; European Society of Gastrointestinal Endoscopy; European Helicobacter Study Group; European Society of Pathology; Sociedade Portuguesa de Endoscopia Digestiva. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy. 2012;44:74-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 503] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 29. | Pimentel-Nunes P, Libânio D, Lage J, Abrantes D, Coimbra M, Esposito G, Hormozdi D, Pepper M, Drasovean S, White JR, Dobru D, Buxbaum J, Ragunath K, Annibale B, Dinis-Ribeiro M. A multicenter prospective study of the real-time use of narrow-band imaging in the diagnosis of premalignant gastric conditions and lesions. Endoscopy. 2016;48:723-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 195] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 30. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3625] [Article Influence: 120.8] [Reference Citation Analysis (6)] |
| 31. | Lahner E, Zagari RM, Zullo A, Di Sabatino A, Meggio A, Cesaro P, Lenti MV, Annibale B, Corazza GR. Chronic atrophic gastritis: Natural history, diagnosis and therapeutic management. A position paper by the Italian Society of Hospital Gastroenterologists and Digestive Endoscopists [AIGO], the Italian Society of Digestive Endoscopy [SIED], the Italian Society of Gastroenterology [SIGE], and the Italian Society of Internal Medicine [SIMI]. Dig Liver Dis. 2019;51:1621-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 143] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 32. | Kimura K, Takemoto T. An Endoscopic Recognition of the Atrophic Border and its Significance in Chronic Gastritis. Endoscopy. 1969;1:87-97. [RCA] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 780] [Article Influence: 43.3] [Reference Citation Analysis (5)] |
| 33. | Capelle LG, Haringsma J, de Vries AC, Steyerberg EW, Biermann K, van Dekken H, Kuipers EJ. Narrow band imaging for the detection of gastric intestinal metaplasia and dysplasia during surveillance endoscopy. Dig Dis Sci. 2010;55:3442-3448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (2)] |
| 34. | Panteris V, Nikolopoulou S, Lountou A, Triantafillidis JK. Diagnostic capabilities of high-definition white light endoscopy for the diagnosis of gastric intestinal metaplasia and correlation with histologic and clinical data. Eur J Gastroenterol Hepatol. 2014;26:594-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Ezoe Y, Muto M, Uedo N, Doyama H, Yao K, Oda I, Kaneko K, Kawahara Y, Yokoi C, Sugiura Y, Ishikawa H, Takeuchi Y, Kaneko Y, Saito Y. Magnifying narrowband imaging is more accurate than conventional white-light imaging in diagnosis of gastric mucosal cancer. Gastroenterology. 2011;141:2017-2025.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 290] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 36. | Ang TL, Pittayanon R, Lau JY, Rerknimitr R, Ho SH, Singh R, Kwek AB, Ang DS, Chiu PW, Luk S, Goh KL, Ong JP, Tan JY, Teo EK, Fock KM. A multicenter randomized comparison between high-definition white light endoscopy and narrow band imaging for detection of gastric lesions. Eur J Gastroenterol Hepatol. 2015;27:1473-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 37. | Pimentel-Nunes P, Dinis-Ribeiro M, Soares JB, Marcos-Pinto R, Santos C, Rolanda C, Bastos RP, Areia M, Afonso L, Bergman J, Sharma P, Gotoda T, Henrique R, Moreira-Dias L. A multicenter validation of an endoscopic classification with narrow band imaging for gastric precancerous and cancerous lesions. Endoscopy. 2012;44:236-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (1)] |
| 38. | Panarese A, Shahini E, Pesce F, Caruso ML. Detection of lesions in Helicobacter Pylori gastritis before and after eradication by expert endoscopists. UEG J. 2018;6:A734. |
| 39. | Yue H, Shan L, Bin L. The significance of OLGA and OLGIM staging systems in the risk assessment of gastric cancer: a systematic review and meta-analysis. Gastric Cancer. 2018;21:579-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (1)] |
| 40. | Kapadia CR. Gastric atrophy, metaplasia, and dysplasia: a clinical perspective. J Clin Gastroenterol. 2003;36:S29-36; discussion S61-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 41. | Carpenter HA, Talley NJ. Gastroscopy is incomplete without biopsy: clinical relevance of distinguishing gastropathy from gastritis. Gastroenterology. 1995;108:917-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 74] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Redéen S, Petersson F, Jönsson KA, Borch K. Relationship of gastroscopic features to histological findings in gastritis and Helicobacter pylori infection in a general population sample. Endoscopy. 2003;35:946-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 117] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 43. | Eshmuratov A, Nah JC, Kim N, Lee HS, Lee HE, Lee BH, Uhm MS, Park YS, Lee DH, Jung HC, Song IS. The correlation of endoscopic and histological diagnosis of gastric atrophy. Dig Dis Sci. 2010;55:1364-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 44. | Buxbaum JL, Hormozdi D, Dinis-Ribeiro M, Lane C, Dias-Silva D, Sahakian A, Jayaram P, Pimentel-Nunes P, Shue D, Pepper M, Cho D, Laine L. Narrow-band imaging versus white light versus mapping biopsy for gastric intestinal metaplasia: a prospective blinded trial. Gastrointest Endosc. 2017;86:857-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 45. | Areia M, Amaro P, Dinis-Ribeiro M, Cipriano MA, Marinho C, Costa-Pereira A, Lopes C, Moreira-Dias L, Romãozinho JM, Gouveia H, Freitas D, Leitão MC. External validation of a classification for methylene blue magnification chromoendoscopy in premalignant gastric lesions. Gastrointest Endosc. 2008;67:1011-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Tanaka K, Toyoda H, Kadowaki S, Hamada Y, Kosaka R, Matsuzaki S, Shiraishi T, Imoto I, Takei Y. Surface pattern classification by enhanced-magnification endoscopy for identifying early gastric cancers. Gastrointest Endosc. 2008;67:430-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Zhao Z, Yin Z, Wang S, Wang J, Bai B, Qiu Z, Zhao Q. Meta-analysis: The diagnostic efficacy of chromoendoscopy for early gastric cancer and premalignant gastric lesions. J Gastroenterol Hepatol. 2016;31:1539-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 48. | Lim H, Jung HY, Park YS, Na HK, Ahn JY, Choi JY, Lee JH, Kim MY, Choi KS, Kim DH, Choi KD, Song HJ, Lee GH, Kim JH. Discrepancy between endoscopic forceps biopsy and endoscopic resection in gastric epithelial neoplasia. Surg Endosc. 2014;28:1256-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Balaban DV, Inal V, Sintusek P S-Editor: Zhang L L-Editor: A E-Editor: Zhang YL