Published online Jul 14, 2020. doi: 10.3748/wjg.v26.i26.3750
Peer-review started: December 27, 2019
First decision: February 14, 2020
Revised: May 14, 2020
Accepted: June 3, 2020
Article in press: June 3, 2020
Published online: July 14, 2020
Processing time: 200 Days and 9.5 Hours
Conventional Crohn’s disease (CD) treatments are supportive rather than curative and have serious side effects. Adipose-derived mesenchymal stem cells (ADSCs) have been gradually applied to treat various diseases. The therapeutic effect and underlying mechanism of ADSCs on CD are still not clear.
To investigate the effect of ADSC administration on CD and explore the potential mechanisms.
Wistar rats were administered with 2,4,6-trinitrobenzene sulfonic acid (TNBS) to establish a rat model of CD, followed by tail injections of green fluorescent protein (GFP)-modified ADSCs. Flow cytometry, qRT-PCR, and Western blot were used to detect changes in the Wnt signaling pathway, T cell subtypes, and their related cytokines.
The isolated cells showed the characteristics of ADSCs, including spindle-shaped morphology, high expression of CD29, CD44, and CD90, low expression of CD34 and CD45, and osteogenic/adipogenic ability. ADSC therapy markedly reduced disease activity index and ameliorated colitis severity in the TNBS-induced rat model of CD. Furthermore, serum anti-sacchromyces cerevisiae antibody and p-anti-neutrophil cytoplasmic antibody levels were significantly reduced in ADSC-treated rats. Mechanistically, the GFP-ADSCs were colocalized with intestinal epithelial cells (IECs) in the CD rat model. GFP-ADSC delivery significantly antagonized TNBS-induced increased canonical Wnt pathway expression, decreased noncanonical Wnt signaling pathway expression, and increased apoptosis rates and protein level of cleaved caspase-3 in rats. In addition, ADSCs attenuated TNBS-induced abnormal inflammatory cytokine production, disturbed T cell subtypes, and their related markers in rats.
Successfully isolated ADSCs show therapeutic effects in CD by regulating IEC proliferation, the Wnt signaling pathway, and T cell immunity.
Core tip: The prevalence and mortality of Crohn’s disease (CD) have been increasing globally, including in areas of Asia that previously had a low incidence. We aimed to investigate the effect and explore potential mechanisms of adipose-derived mesenchymal stem cells (ADSCs) in a 2,4,6-trinitrobenzene sulfonic acid-induced rat model of CD. Our study for the first time provided evidence that successfully isolated ADSCs show therapeutic effects in CD by regulating intestinal epithelial cell proliferation, the Wnt signaling pathway, and T cell immunity.
- Citation: Gao JG, Yu MS, Zhang MM, Gu XW, Ren Y, Zhou XX, Chen D, Yan TL, Li YM, Jin X. Adipose-derived mesenchymal stem cells alleviate TNBS-induced colitis in rats by influencing intestinal epithelial cell regeneration, Wnt signaling, and T cell immunity. World J Gastroenterol 2020; 26(26): 3750-3766
- URL: https://www.wjgnet.com/1007-9327/full/v26/i26/3750.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i26.3750
Crohn’s disease (CD), a major subtype of inflammatory bowel disease (IBD), is a chronic inflammatory disorder of the gastrointestinal tract. Patients with CD exhibit diverse symptoms and lesions ranging from abdominal pain, diarrhea, hematochezia, ulceration, and perforation of the gastrointestinal tract[1]. The prevalence and mortality of CD have been increasing globally, including in areas of Asia that previously had a low incidence[2]. Conventional CD treatments consist of aminosalicylates, corticoste-roids, antitumor necrosis factor agents, and immunomodulators[3]. Although the majority of patients benefit from these treatments, the treatments are supportive rather than curative and have serious side effects.
Mesenchymal stem cells (MSCs) are progenitor cells with self-renewal abilities, multiple-lineage differentiation potential, rapid growth, and immunomodulatory capabilities[4]. MSCs can be isolated from many tissues, including bone marrow (BM-MSC) and adipose tissue (ADSC). MSCs have been gradually applied to treat various diseases, mainly by transplantation to repair pathological cells and reconstruct normal cells[5]. The application of MSCs in treating IBD has become a hot research topic, but the underlying mechanisms are still vague[6]. The Wnt signaling (canonical and noncanonical) pathway is a major pathway in stem cell proliferation and differentiation[7]. The canonical Wnt pathway is activated by the binding of Wnt3a to the frizzled receptor (Fz) and its coreceptor complex[8]. This leads to stabilization of β-catenin, which translocates to the nucleus and interacts with T cell factor/lymphoid enhancer factor[9], further stimulating intestinal crypt cell proliferation and maintaining a stem cell state[10]. Noncanonical signaling is activated by Wnt5a and is implicated in the establishment of cell polarity and migration, inflammation, and cancer development. Ror2 acts as a receptor or coreceptor for Wnt5a and regulates Wnt5a-induced activation of planar cell polarity and Wnt-Ca2+ pathways, playing an important role in the maintenance of stemness[11]. A previous study showed the activation of Wnt3a in IBD rats, suggesting the potential involvement of the canonical Wnt pathway in CD[12]. However, the mechanisms of the noncanonical Wnt pathway in CD remain unclear and require further investigation.
Disturbances in the immune system also play an important role in the pathogenesis of CD. T cells play central roles in immune regulation and can be divided into T helper type 1 (Th1), Th2, Th17, and regulatory T (Treg) cells according to their different functions[13]. Previous studies showed that T cells are important mediators of the inflammatory response in CD[14], and the proportions of Th1 and Th17 cells was higher, while that of Treg cells was lower in CD patients compared to healthy controls. Moreover, patients with lower Treg/Th1 and Treg/Th17 ratios suffer a higher risk of CD recurrence, while Treg restoration prevented colitis in a mouse CD model[15]. These findings suggest that the imbalance in T cell subgroups is related to the activation and progression of CD. Since ADSCs have been shown to treat systemic lupus erythematosus by increasing the number of Treg cells and reducing Th17 cells[16], it is plausible that its therapeutic effect on CD may be through regulating T cell immunity. In addition to the Wnt pathway and T cell immunity, intestinal cell regeneration is also vital in CD progression, mainly by accelerating mucosal healing[17]. However, whether the therapeutic effect of ADSCs on CD occurs by influencing intestinal cell regeneration has not been reported.
The trinitrobenzene sulfonic acid (TNBS)-induced rat colitis model is one of the most commonly used experimental CD models[18]. Therefore, in this study, we explored the therapeutic effect of ADSCs by tail administration in a TNBS-induced rat CD model. We then traced the location of injected ADSCs to study its roles in repairing damaged intestinal epithelium, involving Wnt signaling pathways and T cell balance.
This study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal protocol was approved by the institutional review board of the First Affiliated Hospital of Zhejiang University and adhered to the standards articulated in animal research.
Inguinal fat, one of the major sources of MSCs, was obtained aseptically from rats and digested with collagenase type I in Dulbecco’s modified Eagle’s medium at 37°C for 45 min. Cell suspensions were sequentially filtered, centrifuged, resuspended, and cultured in DMEM-F12 medium [supplemented with 10% fetal bovine serum (FBS), 20 ng/μL transforming growth factor, and 1% penicillin/streptomycin] at 37°C with 5% CO2. When the adherent cells reached 80% to 90% confluence, the replication-defective recombinant lentiviral vector carrying green fluorescent protein (GFP) (LT88008, Vigene Biosciences) was added and cultured for 72 h, followed by phosphate buffered saline (PBS) washing and trypsinization. The final third-passage cells were used to evaluate the ADSC phenotype. The immunophenotyping of ADSCs was analyzed by flow cytometry. Thereafter, cell counting kit-8 was used to measure the number of live cells at days 0, 2, 4, 6, 8, and 10. ADSCs were separately cultured with osteogenic-inducing medium and adipogenic-inducing medium for 14 d. To assess the osteogenic capacity, alkaline phosphatase (ALP) activity was measured using an ELISA kit (C059-1, Nanjing Jiancheng, China) by detecting the absorbance at 520 nm. To assess the adipogenic capacity, routine red O staining was performed, and the dyed ADSCs were observed under a microscope.
Thirty male Wistar rats (150-200 g) aged 6 wk (Cavens Lab Animal, Suzhou, China) were randomly divided into three groups (n = 8 for each): Control, CD, and CD + GFP-ADSCs. All rats received food and water ad libitum and were maintained on a 12/12 h light/dark cycle. After 1 wk, rats in the CD and CD + GFP-ADSCs groups were administered with 1.0 mL of 20 mg TNBS in a 50% ethanol solution following a 24 h fast. Enemas were performed by inserting an 8 cm soft tube into the rat’s anus under inhalation anesthesia with 3% sodium phenobarbital. In the control group, the rats underwent with the same procedure and were administered with an equivalent amount of physiological saline. Subsequently, on day 8, the GFP-ADSCs were injected via the tail vein at a dose of 1 × 107 cells in 0.3 mL of PBS into the rats in the CD + GFP-ADSCs group. In the control and CD groups, the rats received 0.3 mL of PBS without ADSCs following the same protocol.
The body weight, stool consistency, and rectal bleeding of each rat were recorded on day 7 after model establishment and days 7, 14, 21, and 28 after ADSC treatment. A well-known formula to determine the serial disease activity index (DAI), ranging from 0 to 12, including aspects of weight loss, stool characteristics, and bloody stool, was used to assess the clinical severity of colitis. On day 28, all rats were sacrificed, and blood and tissue samples were collected. The colon was retrieved to observe morphological changes. A 0.5 cm length of colonic tissue from the area 6 cm above the anus was collected for hematoxylin and eosin (HE) staining, followed by Lgr5/CK-20 immunofluorescence detection by confocal microscopy, apoptosis analysis by the TUNEL method, and Western blot/qRT-PCR analysis for Wnt pathway/T cell immunity-related proteins and mRNA. Finally, the serum anti-sacchromyces cerevisiae antibody (ASCA) and p-antineutrophil cytoplasmic antibody (p-ANCA) levels were measured with ELISA kits (CK-EN34476, CK-EN35015, Yuanye Co. Ltd, Shanghai, China).
To test the effect of ADSCs on colonic epithelial cell regeneration, ADSCs were transfected with a lentiviral vector containing green fluorescent protein (LV-GFP). After 28 d of GFP-ADSC treatment, the rats were sacrificed, and the heart, liver, spleen, lung, kidney, and colon tissues were collected to detect the GFP-positive cell expression pattern throughout the body by fluorescence confocal microscopy. The colon section was additionally stained with antibodies against GFP, CD20, and Lgr5, followed by visualization using FITC-conjugated secondary antibodies under a confocal microscope. The number of positive cells was calculated and compared between different groups. For apoptosis analysis of the intestinal cells, colon tissue specimens were embedded in paraffin and sectioned at 5 μm for processing by the TUNEL method (Roche, Shanghai, China). The apoptotic cells were dyed and observed under an Olympus microscope. Ten visual fields were selected, 100 cells within each field were counted, and the following formula was applied: Apoptosis index = (apoptosis cell/total cell) × 100%[19].
Blood samples were collected in sterile vacutainer tubes containing heparin (100 U/mL). Peripheral blood mononuclear cells (PBMCs) were isolated by sequential centrifugation and suspended in RPMI-1640 with 10% FBS, followed by incubation at 37°C in a 5% CO2 incubator for 2-3 h. PBMCs with a viability greater than 95% as determined by the trypan blue dyeing method were chosen for further experiments. For Th1, Th2, and Th17 cell analysis, 200 mL of PBMC (1 × 107/mL) suspension was added with phorbol ester (50 ng/mL), ionomycin (1 μg/mL), and monensin (2 μmol/L) and incubated in a 5% CO2 incubator for 6 h. After triple washing with PBS, the resuspended PBMC suspension was separately added with CD4 monoclonal antibody and IFN-γ/IL-4/IL-17 monoclonal antibody. The mixture was cultured at 4°C for 30 min and analyzed by flow cytometry. For Treg cell analysis, the same amount of PBMC suspension was stained at 4 °C for 30 min with CD4 and CD25 monoclonal antibodies. Thereafter, 10 µL of Foxp3 monoclonal antibody was added and cultured for an additional 20 min, followed by flow cytometry analysis.
The transcription factor Foxp3 is a marker of Treg cells, GATA3 is a marker of T helper cells, RORγt is a transcription factor that is specific for Th17 lineage commitment, and T-bet is a biomarker for Th1 cells[13,20]. They were used as supplementary markers for different T cell types and routinely quantified by Western blot using an ECL chemiluminescence kit (Santa Cruz, United States). The levels of Wnt pathway/T cell immunity-related mRNA were detected by qRT-PCR. Total RNA was extracted from colon tissues with TRIzol reagent, and the concentration and quality were assessed. The RNA was then reverse-transcribed using a FastQuant RT kit according to the manufacturer’s protocol. qRT-PCR was conducted using SYBR Green SuperReal PreMix Plus and a 7500 Real-time PCR system. Gene expression levels were determined using the comparative threshold cycle (ΔΔCt) method. β-actin was used as an internal control for both Western blot and qRT-PCR. The detailed primer sequences for qRT-PCR are shown in Supplementary Table 1.
Statistical analyses were performed by using Statistical Package for the Social Sciences version 16.0. The data are presented as the mean ± standard deviation when they were normally distributed or as medians when the distribution was skewed. Unpaired t-test and Mann-Whitney U test for parametric and nonparametric analyses were used for comparisons between two groups. The Kruskal-Wallis test was used for comparisons among three groups. A P value < 0.05 was considered statistically significant.
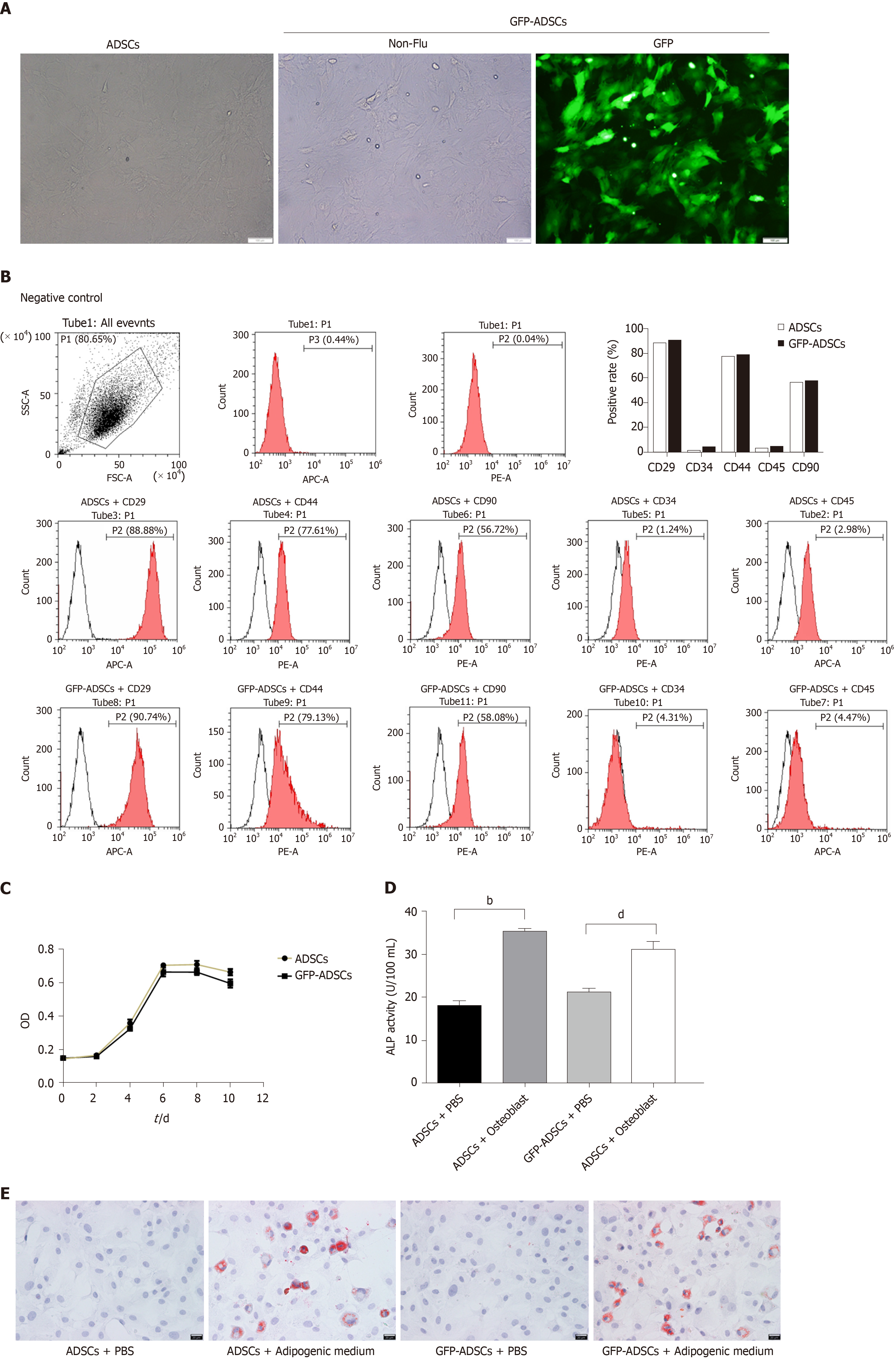
The isolated cells were evaluated based on morphology, molecular biomarkers, and stemness. As shown in Figure 1A, cells extracted from groin fat exhibited the characteristics of ADSCs, including spindle-shaped morphology and whirlpool/radial-arranged growth. The lentivirus-mediated construction of GFP-ADSCs was confirmed by green fluorescence detection. Flow cytometry showed that these cells had the typical marker pattern of ADSCs, including high expression of CD29, CD44, and CD90 but low levels of CD34 and CD45 (Figure 1B). The quality of the isolated cells was good, evidenced by a typical S-like proliferation curve (Figure 1C). The stemness of the isolated cells was evaluated by osteogenic and adipogenic induction. After osteogenic induction, ADSCs and GFP-ADSCs showed significantly higher ALP activity compared with that of controls (Figure 1D). After adipogenic induction, lipid droplets were observed in the cytoplasm of ADSCs and GFP-ADSCs, as shown by a brick-red color change after staining with Oil red O (Figure 1E). Taken together, these data suggested that these isolated cells presented a typical phenotype of ADSCs.
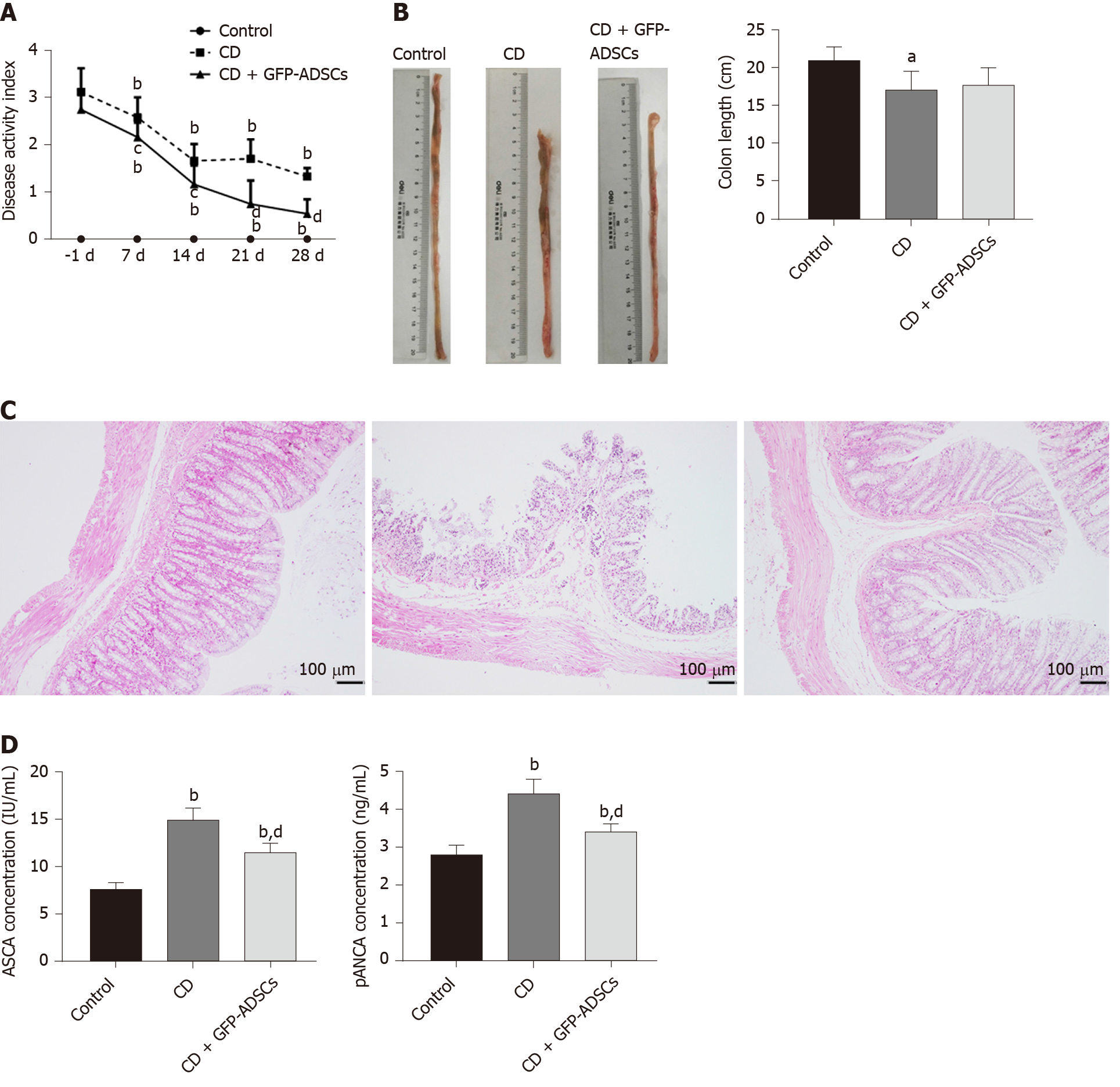
The therapeutic effect of ADSCs was evaluated in rats with TNBS-induced experimental colitis. To assess the severity of colitis, DAI and body weight changes were recorded every 7 d. The DAI was significantly increased, while the body weight was significantly decreased after TNBS administration, and ADSC therapy significantly reduced DAI in a time-dependent manner (Figure 2A). Although there was self-alleviation of DAI in the CD group after withdrawal of TNBS, a significantly lower DAI was observed in the CD + GFP-ADSCs group starting 7 d after TNBS delivery compared with that of the CD group. On day 28, the rat weight in the CD + GFP-ADSCs group became significantly higher than that in the CD group (Supplementary Table 2).
The colon length was significantly shorter in the CD group than in the control group, which partially recovered after ADSC therapy, although this difference was not statistically significant (Figure 2B). The intestinal ulceration and inflammation severity were further evaluated by HE staining. As illustrated in Figure 2C, the colon tissue structure of control rats was clear, the mucosa was complete, and the intestinal glands in the lamina propria were rich and closely arranged; in the CD group, the arrangement of intestinal glands was disordered, the mucosa was damaged, and there were a large number of infiltrated inflammatory cells; after ADSC treatment, the damaged mucosa and the disordered intestinal glands in the lamina propria were improved. Furthermore, we found a significant reduction in plasma ASCA and p-ANCA concentrations in GFP-ADSC-treated rats (Figure 2D).
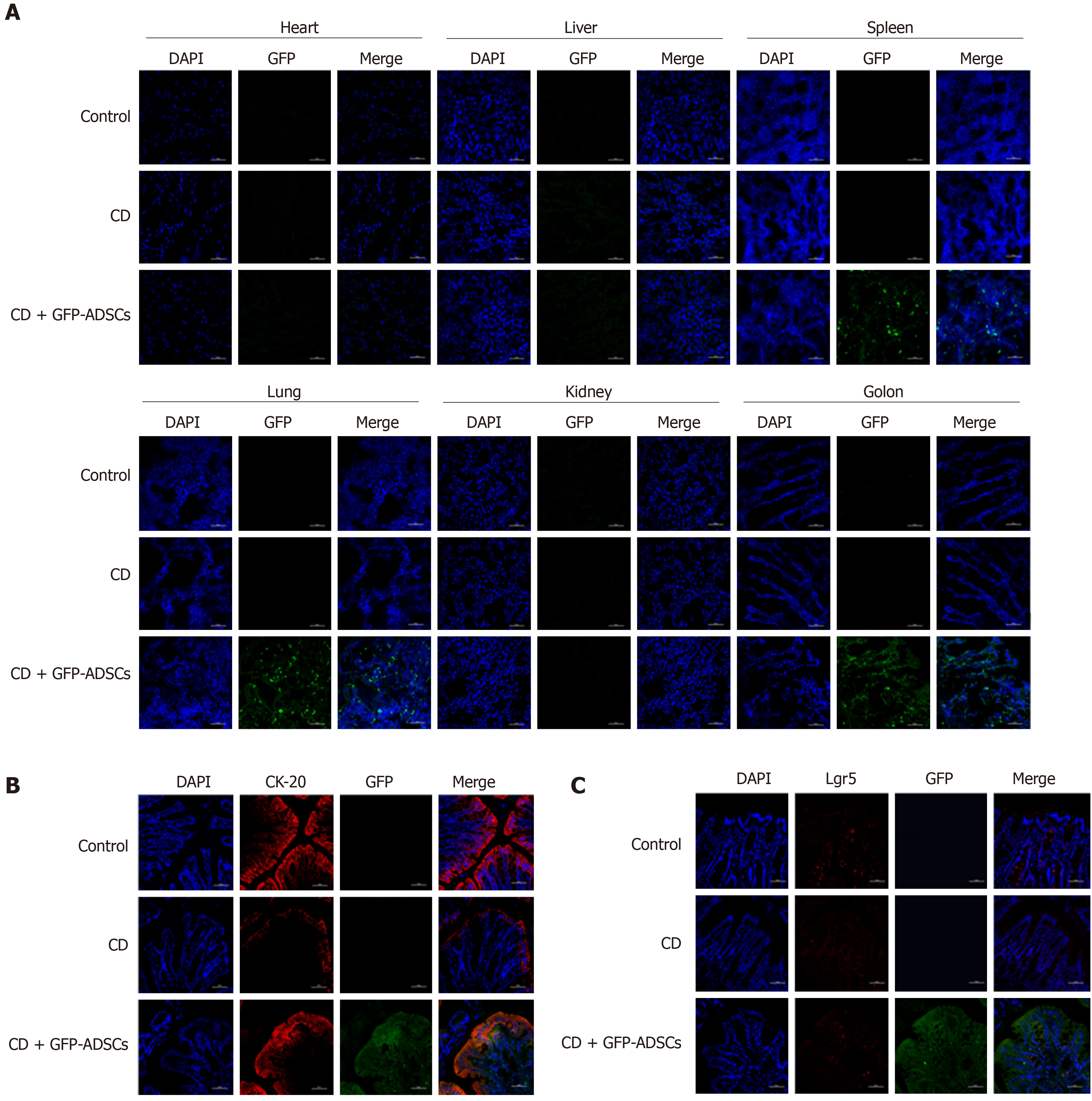
The distribution of ADSCs was detected by adding GFP to ADSCs and observed by using fluorescence confocal microscopy. GFP fluorescence was detected in the spleen, lung, and colon of the CD + GFP-ADSCs group, and the density was relatively higher in the colon. In contrast, GFP fluorescence was not observed in the heart, liver, and kidney (Figure 3A). CK-20 is a biomarker for intestinal epithelial cells (IECs) and is indicative of mucosal healing and proliferation. As shown in Figure 3B, more CK-20-positive cells were present in the bottom of the crypts in the ADSC-treated group than in the CD group, indicating an accelerated mucosal healing process. GFP green fluorescence merged images supported the specific proximity between epithelial cells and ADSCs, suggesting potential interplay. On the other hand, the typical biomarker of intestinal stem cells (ISCs), Lgr5, was weakly detected in the control and CD groups. The coexpression of GFP and Lgr5 was not observed in the CD + GFP-ADSCs group, indicating that ADSCs had no direct effect on ISCs (Figure 3C).
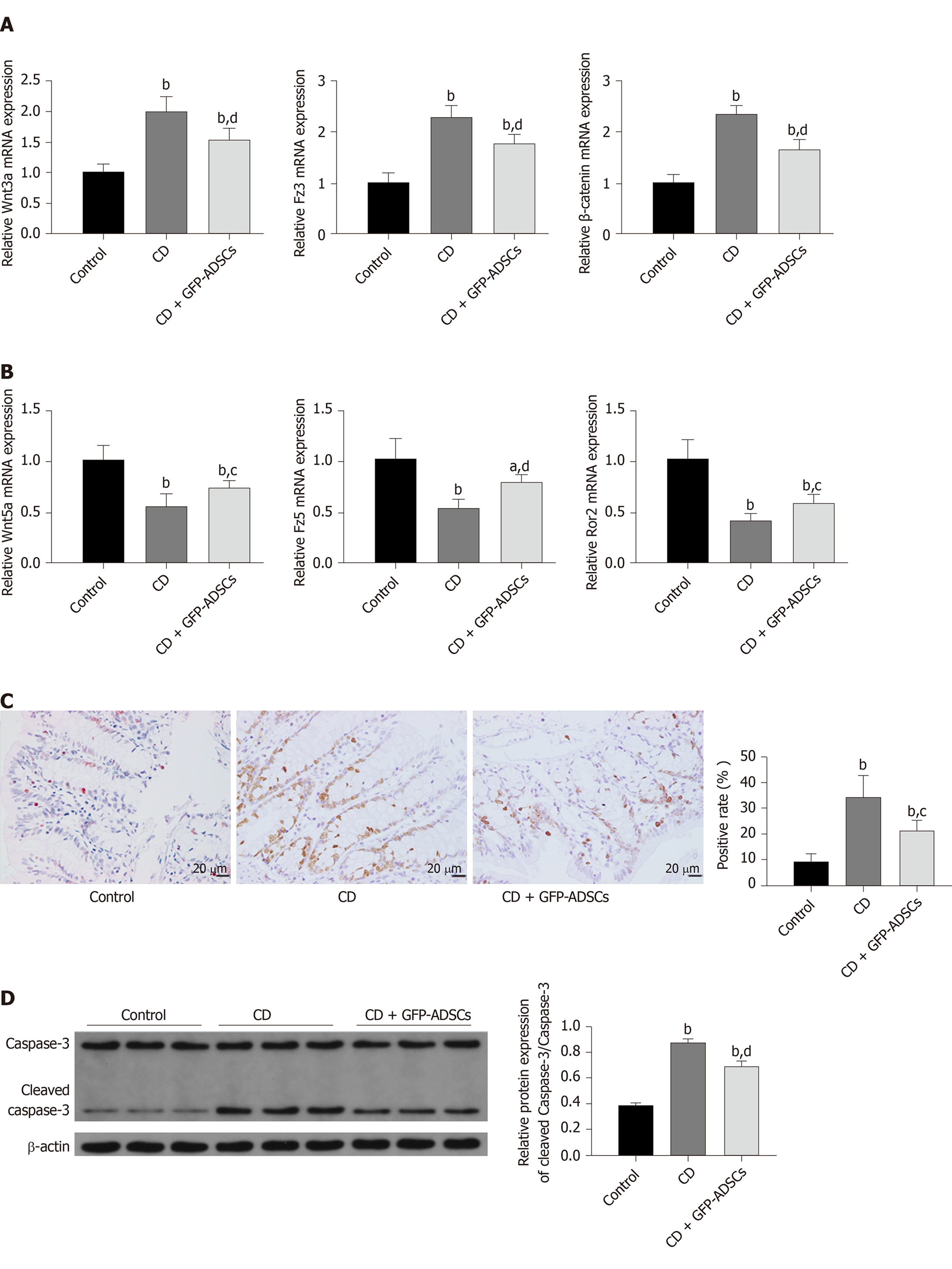
Expression of the Wnt signaling pathway was evaluated by qRT-PCR. The expression levels of Wnt3a, Fzd3, and β-catenin were significantly increased in the CD group compared with the control group, while GFP-ADSC treatment significantly antagonized such changes (Figure 4A). The expression levels of Wnt5a, Fzd5, and Ror2 were significantly decreased in the CD group compared with the control group, and GFP-ADSC delivery also antagonized such changes (Figure 4B). The apoptosis rate in colon tissue was assessed by a TUNEL assay (Figure 4C). Semiquantitative analysis showed that the number of TdT-positive cells was significantly increased in the rats administered with TNBS compared with the control group. GFP-ADSC administration significantly antagonized such change. Although the caspase-3 level was similar in the control, CD, and GFP-ADSC groups, Western blot analysis showed that the cleaved caspase-3 level was significantly increased in the CD group, and was significantly decreased in the GFP-ADSCs group compared with that in the CD group (Figure 4D).
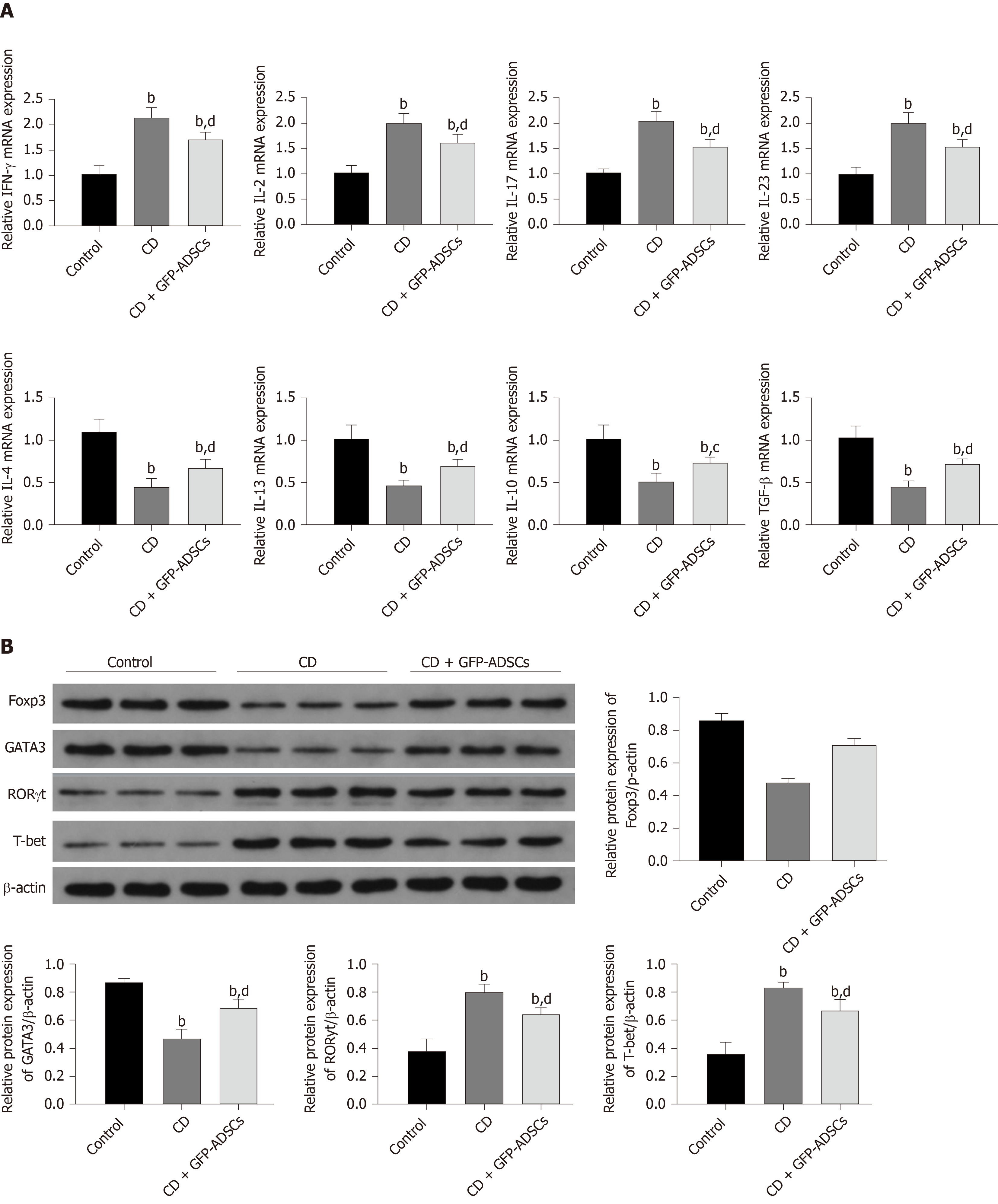
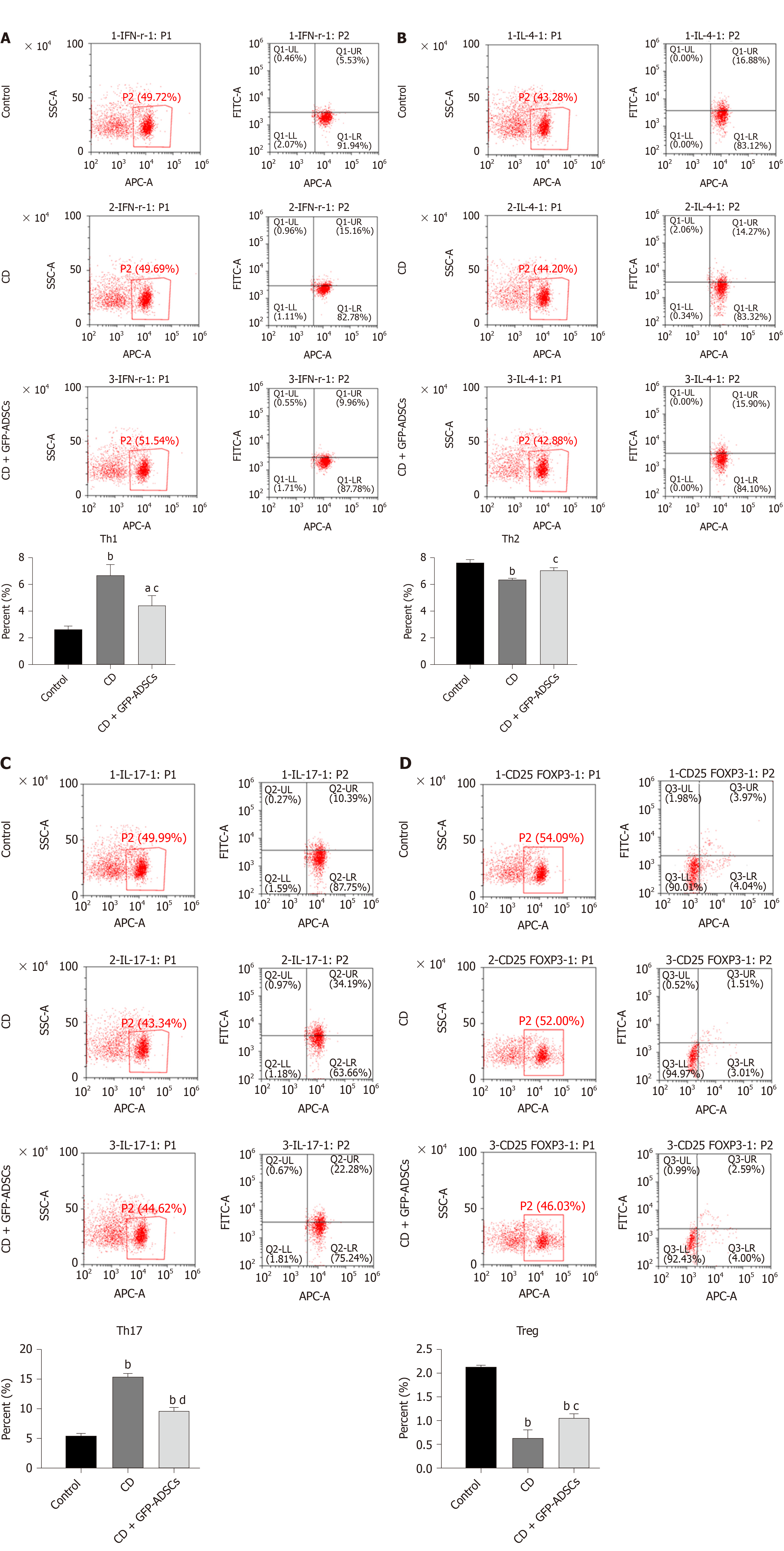
We investigated whether GFP-ADSC administration influences the production of inflammatory cytokines involved in intestinal inflammation. We observed an obvious increase in the expression of proinflammatory cytokine (IFN-γ, IL-2, IL-17, and IL-23) and a decrease in the expression of anti-inflammatory cytokine (IL-4, IL-13, IL-10, and TGF-β) in CD, which were further ameliorated after GFP-ADSC treatment (Figure 5A). Western blot analysis demonstrated that after TNBS delivery, the expression of Foxp3 and GATA3 was significantly decreased in the CD group, while the levels of RORγ and T-bet were significantly increased (Figure 5B). These changes were antagonized in the CD + GFP-ADSCs group. Th1/Th2/Th17/Treg subtype changes were detected by flow cytometry (Figure 6). We found that Th1/Th17 percentages were significantly increased and Th2/Treg percentage was markedly reduced in the CD group compared with the normal group. Finally, the administration of ADSCs significantly antagonized these changes.
MSCs were first identified as a marrow-derived clonal source of cells in the 1960s[21]. Despite different sources, MSCs have common features and behavioral traits, which not only provide cells for tissue reconstitution but also regulate inflammation and “educate” other cells to facilitate tissue repair[22]. Accumulating evidence suggests a therapeutic effect of MSCs in IBD[23,24] and ADSCs have been widely used because of their easy acquisition. In 2005, the first phase I clinical trial showed that 75% of fistulas were healed by ADSC therapy without any side effects[25], which opened the window to ADSC therapy for CD. Further studies showed that ADSC injection modulated the abnormal immune response in CD patients, resulting in clinical improvement[26]. However, the underlying mechanisms of ADSCs in CD are still vague. In this study, we demonstrated that ADSC administration alleviates TNBS-induced colitis by accelerating IEC regeneration, partially restoring the dysregulated Wnt signaling pathway and rebalancing T cell repertoire, which reinforces the effectiveness of ADSC therapy in CD and provides novel clues for further mechanism exploration.
Mucosal healing is associated with a more favorable prognosis for CD. Previous studies showed the therapeutic effects of ISCs in improving mucosal healing and functional engraftment of the derived colon epithelial cells after successful transplantation[27,28]. However, a lack of immunoregulation capacity may hamper further application of ISCs, especially compared with that of MSCs. Moreover, accumulating evidences suggest that systemically transplanted BM-MSCs may home to the injured gut and transdifferentiate into ISCs or interstitial lineage cells to repair the damaged tissue[29]. A recent study showed that intraperitoneally administered MSCs homing to inflamed tissues were a prerequisite to achieve their beneficial effect[30]. However, the effects of ADSC administration on CD and the mechanisms of mucosal healing have rarely been reported. Our results not only showed the therapeutic effect of ADSCs on the CD phenotype in TNBS-induced rats but also demonstrated that ADSCs engrafted into damaged colon and colocalized with IECs but not ISCs, partially clarifying the mechanisms of ADSCs in mucosal healing. This result enhances previous findings showing the therapeutic effect of local MSC administration in experimental colitis[31].
The Wnt signaling pathway was chosen as the candidate because of its capacity to regulate the self-renewal and differentiation of MSCs and to determine the fates of ISCs[32,33]. Therefore, this pathway acts as a bridge between MSCs and receptors. Previous studies showed activated canonical and suppressed noncanonical Wnt signaling in IBD[34]. In this study, when the intestinal epithelium was inflamed, canonical Wnt signaling was activated. After ADSC transplantation, the canonical Wnt signaling-related genes were downregulated. For the noncanonical Wnt pathway, the levels of relative genes were significantly decreased in the CD group but partially recovered after ADSC administration. Since the canonical Wnt pathway enhances MSC proliferation and the noncanonical Wnt pathway exerts the opposite effects, we speculated that the noncanonical Wnt pathway activated by ADSC transplantation may contribute to the transition of cell status from “proliferation” into “differentiation”[35].
Disturbed T cell immunity and changes in its associated cytokine network are actively involved in IBD[36]. It has been well accepted that the predominant inflammatory profile in CD involves activated Th1/Th17 but depressed Th2/Treg cell responses. In this study, we reconfirmed these findings in TNBS-induced rats with CD phenotype and further showed that the alleviation of colitis after ADSC administration was partially mediated by antagonizing those changes. Several molecular markers were selected, and the same trend was observed in the changes in T cell subtype. Our results were consistent with previous reports showing the contribution of T cells to the therapeutic effect of BM-MSCs on TNBS-induced colitis[37]. It is theoretically plausible that MSCs have the ability to suppress the proliferation and expansion of T helper cells while inducing the differentiation and activation of Treg cells, while the latter has the capacity to inhibit autoimmunity[38]. Additionally, a previous study showed that apoptosis was exacerbated in CD[39], which was also found in our study by the TUNEL method and the expression level of active caspase-3.
There are several limitations that should be acknowledged. First, it is well known that ISCs are located at the base of the intestinal crypts and renew the epithelium through differentiation to multiple epithelial lineages[40]. Although our results identified that ADSCs were colocalized with IECs but not ISCs, whether ADSCs could transdifferentiate into epithelial cells and the potential regulators are still unclear. Second, although we found a change in T cell immunity in CD and after ADSC treatment, the detailed mechanisms are still vague. Third, although we identified the change in the Wnt signaling pathway in CD and after ADSC therapy, further mechanism exploration has not been performed. Previous studies showed the effect of the Wnt signaling pathway on the balance between cell proliferation and its potential regulators[41]. Whether these factors are applicable in ADSC therapy requires further study. Fourth, studies are required to understand the potential risks of ADSC treatment, such as tumorigenicity and immune rejection[42]. Fifth, it may be not sufficient to use CK-20 as the marker of IECs and combination with Ki-67 should be considered in the future. Finally, the relationship between the Wnt signaling pathway and T cell distribution remains unclear. The mechanisms of ADSCs in modulating the interactions between them warrant further research.
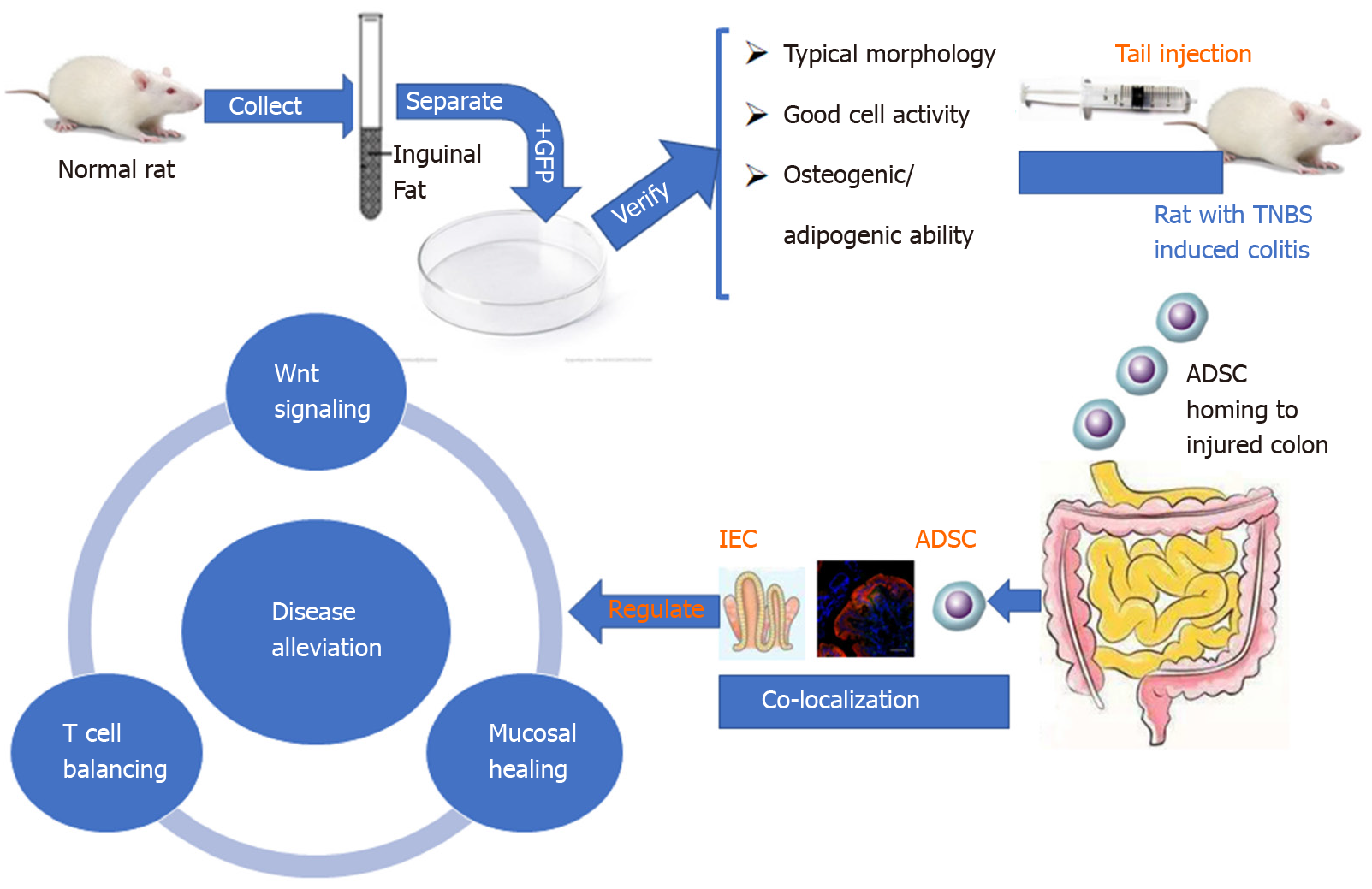
In conclusion, our findings not only confirmed the biological characteristics of ADSCs, such as localization and multilineage differentiation potential, but also suggested the effect of ADSC therapy in treating CD phenotype in a TNBS-induced rat model. We further investigated the potential underlining mechanisms, involving IEC proliferation, the Wnt signaling pathway, and T cell immunity, which provided novel clues for the pathogenesis and treatment of CD (Figure 7).
Crohn’s disease (CD) is a chronic relapsing inflammatory disorder of the gastrointestinal tract, especially involving the distal small intestine and the colonic mucosa. Conventional treatments are supportive rather than curative and have serious side effects.
Adipose-derived mesenchymal stem cells (ADSCs) have been gradually applied to treat various diseases. The therapeutic effect and underlying mechanism of ADSCs on CD are still not clear.
This study aimed to investigate the effect of ADSC administration on CD and explore potential mechanisms on intestinal epithelial cell regeneration, Wnt signaling, and T cell immunity.
Wistar rats were administered with 2,4,6-trinitrobenzene sulfonic acid (TNBS) to establish a rat model of CD, followed by tail injections of green fluorescent protein (GFP)-modified ADSCs. After tracing in vivo ADSC distribution, flow cytometry, qRT-PCR, and Western blot were used to detect changes in the Wnt signaling pathway, T cell subtypes, and their related cytokines.
The isolated cells showed the characteristics of ADSCs, including spindle-shaped morphology, high expression of CD29, CD44, and CD90, low expression of CD34 and CD45, and osteogenic/adipogenic ability. ADSC therapy markedly reduced disease activity index and ameliorated colitis severity in the TNBS-induced rat model of CD. Furthermore, serum anti-sacchromyces cerevisiae antibody and p-anti-neutrophil cytoplasmic antibody levels were significantly reduced in ADSC-treated rats. Mechanistically, the GFP-ADSCs were colocalized with intestinal epithelial cells in the CD rat model. GFP-ADSC delivery significantly antagonized TNBS-induced increased canonical Wnt pathway expression, decreased noncanonical Wnt signaling pathway expression, and increased apoptosis rates and protein level of cleaved caspase-3 in rats. In addition, ADSCs attenuated TNBS-induced abnormal inflammatory cytokine production, disturbed T cell subtypes, and their related markers in rats.
Successfully isolated ADSCs show co-location with IEC and therapeutic effects in CD by regulating IEC proliferation, the Wnt signaling pathway, and T cell immunity.
Systemic ADSC infusion may be a potential choice for CD therapy.
| 1. | Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2256] [Article Influence: 132.7] [Reference Citation Analysis (10)] |
| 2. | Engel MA, Khalil M, Neurath MF. Highlights in inflammatory bowel disease--from bench to bedside. Clin Chem Lab Med. 2012;50:1229-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Shi HY, Ng SC. The state of the art on treatment of Crohn's disease. J Gastroenterol. 2018;53:989-998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Zaher W, Harkness L, Jafari A, Kassem M. An update of human mesenchymal stem cell biology and their clinical uses. Arch Toxicol. 2014;88:1069-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Casteilla L, Planat-Benard V, Laharrague P, Cousin B. Adipose-derived stromal cells: Their identity and uses in clinical trials, an update. World J Stem Cells. 2011;3:25-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 410] [Article Influence: 27.3] [Reference Citation Analysis (3)] |
| 6. | Dave M, Mehta K, Luther J, Baruah A, Dietz AB, Faubion WA. Mesenchymal Stem Cell Therapy for Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Inflamm Bowel Dis. 2015;21:2696-2707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Nusse R, Clevers H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell. 2017;169:985-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2031] [Cited by in RCA: 3350] [Article Influence: 372.2] [Reference Citation Analysis (0)] |
| 8. | Steinhart Z, Angers S. Wnt signaling in development and tissue homeostasis. Development. 2018;145:dev146589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 594] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 9. | Koch S. Extrinsic control of Wnt signaling in the intestine. Differentiation. 2017;97:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 10. | Shi J, Chi S, Xue J, Yang J, Li F, Liu X. Emerging Role and Therapeutic Implication of Wnt Signaling Pathways in Autoimmune Diseases. J Immunol Res. 2016;2016:9392132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 11. | Endo M, Nishita M, Fujii M, Minami Y. Insight into the role of Wnt5a-induced signaling in normal and cancer cells. Int Rev Cell Mol Biol. 2015;314:117-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Cosín-Roger J, Ortiz-Masiá D, Calatayud S, Hernández C, Esplugues JV, Barrachina MD. The activation of Wnt signaling by a STAT6-dependent macrophage phenotype promotes mucosal repair in murine IBD. Mucosal Immunol. 2016;9:986-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 150] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 13. | Xiao J, Zhu F, Liu X, Xiong J. Th1/Th2/Th17/Treg expression in cultured PBMCs with antiphospholipid antibodies. Mol Med Rep. 2012;6:1035-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Brand S. Crohn's disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn's disease. Gut. 2009;58:1152-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 527] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 15. | Chao K, Zhang S, Yao J, He Y, Chen B, Zeng Z, Zhong B, Chen M. Imbalances of CD4(+) T-cell subgroups in Crohn's disease and their relationship with disease activity and prognosis. J Gastroenterol Hepatol. 2014;29:1808-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Zhang W, Feng YL, Pang CY, Lu FA, Wang YF. Transplantation of adipose tissue-derived stem cells ameliorates autoimmune pathogenesis in MRL/lpr mice : Modulation of the balance between Th17 and Treg. Z Rheumatol. 2019;78:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Carulli AJ, Keeley TM, Demitrack ES, Chung J, Maillard I, Samuelson LC. Notch receptor regulation of intestinal stem cell homeostasis and crypt regeneration. Dev Biol. 2015;402:98-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Antoniou E, Margonis GA, Angelou A, Pikouli A, Argiri P, Karavokyros I, Papalois A, Pikoulis E. The TNBS-induced colitis animal model: An overview. Ann Med Surg (Lond). 2016;11:9-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 258] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 19. | Jin X, Chen D, Zheng RH, Zhang H, Chen YP, Xiang Z. miRNA-133a-UCP2 pathway regulates inflammatory bowel disease progress by influencing inflammation, oxidative stress and energy metabolism. World J Gastroenterol. 2017;23:76-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Mirlekar B, Ghorai S, Khetmalas M, Bopanna R, Chattopadhyay S. Nuclear matrix protein SMAR1 control regulatory T-cell fate during inflammatory bowel disease (IBD). Mucosal Immunol. 2015;8:1184-1200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | BECKER AJ, McCULLOCH EA, TILL JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 930] [Cited by in RCA: 809] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 22. | Ke C, Biao H, Qianqian L, Yunwei S, Xiaohua J. Mesenchymal stem cell therapy for inflammatory bowel diseases: promise and challenge. Curr Stem Cell Res Ther. 2015;10:499-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Fu ZW, Zhang ZY, Ge HY. Mesenteric injection of adipose-derived mesenchymal stem cells relieves experimentally-induced colitis in rats by regulating Th17/Treg cell balance. Am J Transl Res. 2018;10:54-66. [PubMed] |
| 24. | Hoffman JM, Sideri A, Ruiz JJ, Stavrakis D, Shih DQ, Turner JR, Pothoulakis C, Karagiannides I. Mesenteric Adipose-derived Stromal Cells From Crohn's Disease Patients Induce Protective Effects in Colonic Epithelial Cells and Mice With Colitis. Cell Mol Gastroenterol Hepatol. 2018;6:1-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | García-Olmo D, García-Arranz M, Herreros D, Pascual I, Peiro C, Rodríguez-Montes JA. A phase I clinical trial of the treatment of Crohn's fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 576] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 26. | González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 489] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 27. | Fukuda M, Mizutani T, Mochizuki W, Matsumoto T, Nozaki K, Sakamaki Y, Ichinose S, Okada Y, Tanaka T, Watanabe M, Nakamura T. Small intestinal stem cell identity is maintained with functional Paneth cells in heterotopically grafted epithelium onto the colon. Genes Dev. 2014;28:1752-1757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 28. | Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, Ichinose S, Nagaishi T, Okamoto R, Tsuchiya K, Clevers H, Watanabe M. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med. 2012;18:618-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 640] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 29. | Qu B, Xin GR, Zhao LX, Xing H, Lian LY, Jiang HY, Tong JZ, Wang BB, Jin SZ. Testing stem cell therapy in a rat model of inflammatory bowel disease: role of bone marrow stem cells and stem cell factor in mucosal regeneration. PLoS One. 2014;9:e107891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Lopez-Santalla M, Mancheño-Corvo P, Escolano A, Menta R, Delarosa O, Redondo JM, Bueren JA, Dalemans W, Lombardo E, Garin MI. Comparative Analysis between the In Vivo Biodistribution and Therapeutic Efficacy of Adipose-Derived Mesenchymal Stromal Cells Administered Intraperitoneally in Experimental Colitis. Int J Mol Sci. 2018;19:1853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | de la Portilla F, Yuste Y, Pereira S, Olano C, Maestre MV, Padillo FJ. Local Mesenchymal Stem Cell Therapy in Experimentally Induced Colitis in the Rat. Int J Stem Cells. 2018;11:39-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Neth P, Ciccarella M, Egea V, Hoelters J, Jochum M, Ries C. Wnt signaling regulates the invasion capacity of human mesenchymal stem cells. Stem Cells. 2006;24:1892-1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 33. | Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology. 2008;134:849-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 318] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 34. | Xing Y, Chen X, Cao Y, Huang J, Xie X, Wei Y. Expression of Wnt and Notch signaling pathways in inflammatory bowel disease treated with mesenchymal stem cell transplantation: evaluation in a rat model. Stem Cell Res Ther. 2015;6:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Boland GM, Perkins G, Hall DJ, Tuan RS. Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem. 2004;93:1210-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 446] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 36. | Chen ML, Sundrud MS. Cytokine Networks and T-Cell Subsets in Inflammatory Bowel Diseases. Inflamm Bowel Dis. 2016;22:1157-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 37. | Zuo D, Liu X, Shou Z, Fan H, Tang Q, Duan X, Cao D, Zou Z, Zhang L. Study on the interactions between transplanted bone marrow-derived mesenchymal stem cells and regulatory T cells for the treatment of experimental colitis. Int J Mol Med. 2013;32:1337-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Corridoni D, Arseneau KO, Cominelli F. Inflammatory bowel disease. Immunol Lett. 2014;161:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 39. | Sturm A, de Souza HS, Fiocchi C. Mucosal T cell proliferation and apoptosis in inflammatory bowel disease. Curr Drug Targets. 2008;9:381-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Bjerknes M, Cheng H. Intestinal epithelial stem cells and progenitors. Methods Enzymol. 2006;419:337-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Vanuytsel T, Senger S, Fasano A, Shea-Donohue T. Major signaling pathways in intestinal stem cells. Biochim Biophys Acta. 2013;1830:2410-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 42. | Liu Y, Niu R, Yang F, Yan Y, Liang S, Sun Y, Shen P, Lin J. Biological characteristics of human menstrual blood-derived endometrial stem cells. J Cell Mol Med. 2018;22:1627-1639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Scaringi S S-Editor: Liu M L-Editor: Wang TQ E-Editor: Ma YJ