Published online Jun 21, 2020. doi: 10.3748/wjg.v26.i23.3260
Peer-review started: January 22, 2020
First decision: February 27, 2020
Revised: March 29, 2020
Accepted: May 15, 2020
Article in press: May 15, 2020
Published online: June 21, 2020
Processing time: 150 Days and 23.7 Hours
Pancreatic endocrine insufficiency after acute pancreatitis (AP) has drawn increasing attention in recent years.
To assess the impact of risk factors on the development of pancreatic endocrine insufficiency after AP.
This retrospective observational long-term follow-up study was conducted in a tertiary hospital. Endocrine function was evaluated by the oral glucose tolerance test. The data, including age, sex, body mass index, APACHE II score, history of smoking and drinking, organ failure, pancreatic necrosis, debridement of necrosis (minimally invasive and/or open surgery), and time interval, were collected from the record database.
A total of 361 patients were included in the study from January 1, 2012 to December 30, 2018. A total of 150 (41.6%) patients were diagnosed with dysglycemia (including diabetes mellitus and impaired glucose tolerance), while 211 (58.4%) patients had normal endocrine function. The time intervals (mo) of the above two groups were 18.73 ± 19.10 mo and 31.53 ± 27.27 mo, respectively (P = 0.001). The morbidity rates of pancreatic endocrine insufficiency were 46.7%, 28.0%, and 25.3%, respectively, in the groups with different follow-up times. The risk factors for pancreatic endocrine insufficiency after AP were severity (odds ratio [OR] = 3.489; 95% confidence interval [CI]: 1.501-8.111; P = 0.004) and pancreatic necrosis (OR = 4.152; 95%CI: 2.580-6.684; P = 0.001).
Pancreatic necrosis and severity are independent risk factors for pancreatic endocrine insufficiency after AP. The area of pancreatic necrosis can affect pancreatic endocrine function.
Core tip: This is the first research to explore the association between acute pancreatitis and pancreatic endocrine insufficiency in a longer time than before and we included patients who were followed for a long time from 3 mo to 7 years. Furthermore, we found that pancreatic necrosis and severity were independent risk factors for pancreatic endocrine insufficiency after AP. Debridement of necrosis (percutaneous catheter drainage and/or operative necrosectomy) was a protective factor on pancreatic endocrine insufficiency after AP. Our results will provide some guidance on the clinical practice in the future.
- Citation: Yu BJ, Li NS, He WH, He C, Wan JH, Zhu Y, Lu NH. Pancreatic necrosis and severity are independent risk factors for pancreatic endocrine insufficiency after acute pancreatitis: A long-term follow-up study. World J Gastroenterol 2020; 26(23): 3260-3270
- URL: https://www.wjgnet.com/1007-9327/full/v26/i23/3260.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i23.3260
Acute pancreatitis (AP) is an acute inflammation of the pancreas and the most common type of pancreatic disease. The incidence of AP has increased by at least 20% over the past 10 years[1]. Complications such as organ failure, systemic inflammatory response syndrome, and pancreatic necrosis, which are characterized by a high fatality rate, often occur after AP onset, especially in severe AP (SAP) patients. However, in recent years, we discovered another complication with an increasing frequency after AP onset, namely, pancreatic endocrine insufficiency, which is defined as post-pancreatitis diabetes mellitus (PPDM) or type 3c DM[2,3]. DM is associated with a heavy burden on the society due to related disabilities and high costs[2]. Therefore, it is important to determine the incidence rate, risk factors, treatment, and outcome of pancreatic endocrine insufficiency after AP onset, which remain unclear. The most controversial aspect is the risk factors for pancreatic endocrine insufficiency after AP. Previously published studies on risk factors for pancreatic endocrine insufficiency after AP showed conflicting results. In 2013, a study from New Zealand reported that hyperglycemia, obesity, and age were risk factors for pancreatic endocrine insufficiency after AP, but the severity of AP had a minimal effect[1]. However, another study[4] suggested that the severity of AP was a risk factor for DM after AP. Hence, in this study, we conducted a long-term follow-up investigation to assess the potential risk factors for and incidence of pancreatic endocrine insufficiency after AP onset.
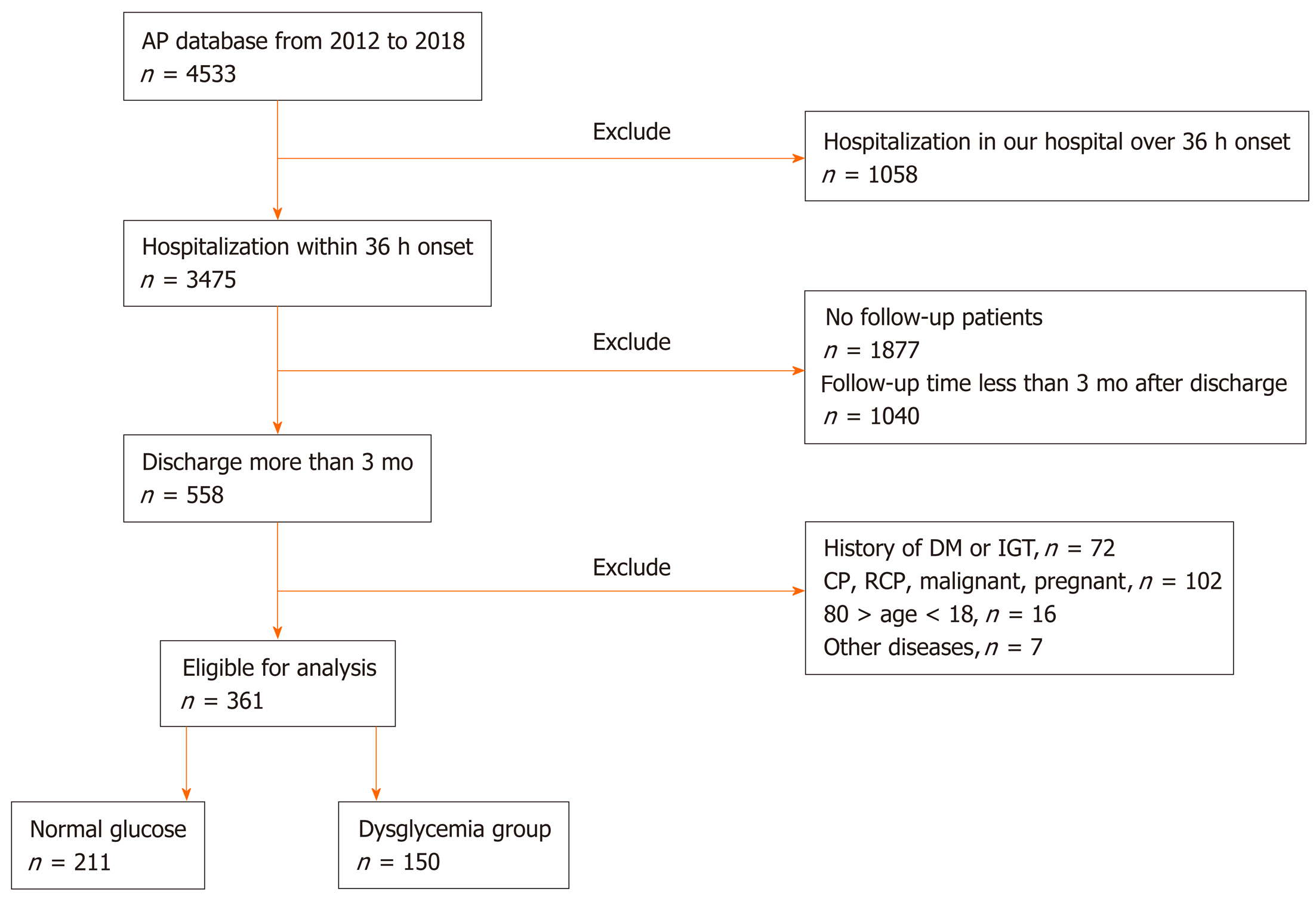
This was a long-term follow-up retrospective observational study with patient data collected from the AP database of the First Affiliated Hospital of Nanchang University; it was conducted from January 1, 2012 to December 30, 2018. A total of 361 AP patients agreed to be followed after discharge from the First Affiliated Hospital of Nanchang University in China. The diagnosis of pancreatic endocrine insufficiency in AP patients was performed at least 3 mo after discharge. Our study was approved by the ethics committee of our hospital (database approval number: 2011001).
Patients younger than 18 years or older than 80 years old were excluded from the study, as were those with the following conditions: (1) Diagnosed with DM before the onset of AP; (2) History of impaired glucose tolerance (IGT) or impaired fasting glucose (IFG) before the onset of AP; (3) Pancreatic insufficiency caused by other diseases, including cystic fibrosis, chronic pancreatitis (CP), pancreatic cancer, and Crohn’s disease, a history of gastrointestinal surgery, chronic kidney diseases, and liver diseases; (4) Recurrent pancreatitis or pre-AP portal hypertension; (5) Pregnant or lactating; (6) Malignant disease; and (7) Admission more than 48 h after the onset of AP. Patient demographics and clinical data, including age, sex, body mass index (BMI), etiology, complications, the severity of AP during hospitalization, treatment with minimally invasive or operative necrosectomy (ON), and death, were collected. Acute Physiology and Chronic Health Evaluation II (APACHE II) scores were calculated based on patient data.
The diagnosis of AP was based on the 2012 revised Atlanta classification criteria[5]. AP severity was classified into mild AP (MAP), moderate-severe AP (MSAP), and severe AP (SAP)[5]. Additionally, the etiologies of AP were classified into biliary[6], alcoholic[7,8], hypertriglyceridemic[7,8], and other types[8]. Organ failure was determined using the modified Marshall scoring system[5]. Pancreatic necrosis was indicated on contrast-enhanced computed tomography by diffuse enlargement of the pancreatic or peripancreatic volume, blurred edges, mild enhancement of the edema area after enhanced scanning, and no enhancement of necrotic areas. The mortality rate refers to death during hospitalization. The specific definition is shown in Supplementary Table 1.
| All patients (n = 361) | Normal glucose group (n = 211) | Dysglycemia group (n = 150) | P value | |
| Age | 48.63 ± 13.36 | 49.25 ± 13.83 | 47.75 ± 12.66 | 0.2921 |
| Sex, n (%) | ||||
| Male | 201 (55.7) | 117 (55.5) | 84 (56) | 0.9172 |
| Female | 160 (44.3) | 94 (44.5) | 66 (44) | |
| BMI | 23.36 ± 3.98 | 23.37 ± 4.039 | 23.36 ± 3.91 | 0.7711 |
| APACHE II score | 8.46 ± 3.80 | 8.18 ± 3.52 | 8.87 ± 4.14 | 0.5081 |
| Etiology, n (%) | ||||
| Biliary | 166 (46) | 103 (48.8) | 63 (42) | 0.4732 |
| HTG | 130 (36) | 69 (32.7) | 61 (40.7) | |
| Alcoholic | 52 (14.4) | 31 (14.7) | 21 (14) | |
| Others | 13 (3.6) | 8 (3.8) | 5 (3.3) | |
| Severity of AP | ||||
| Mild | 47 (13.0) | 41 (19.4) | 6 (4.0) | 0.0012 |
| Moderate | 199 (55.1) | 115 (54.5) | 84 (56.0) | |
| Severe | 115 (31.9) | 55 (26.1) | 60 (40.0) | |
| Organ failure | ||||
| Yes | 137 (38.0) | 53 (25.1) | 84 (56.0) | 0.0012 |
| No | 224 (62.0) | 158 (74.9) | 66 (44.0) | |
| Pancreatic necrosis | ||||
| Yes | 152 (42.1) | 34 (16.1) | 118 (78.7) | 0.0012 |
| No | 209 (57.9) | 177 (83.9) | 32 (21.3) | |
| Debridement of necrosis (minimally invasive and/or ON) | ||||
| Yes | 95 (26.3) | 25 (11.8) | 70 (46.7) | 0.0012 |
| No | 266 (73.7) | 186 (88.2) | 80 (53.3) | |
| History of smoking | ||||
| Yes | 81 (22.4) | 46 (21.8) | 35 (23.3) | 0.7312 |
| No | 280 (77.6) | 165 (78.2) | 115 (76.7) | |
| History of drinking | ||||
| Yes | 81 (22.4) | 68 (32.2) | 13 (8.6) | 0.7312 |
| No | 280 (77.6) | 143 (67.8) | 137 (91.4) | |
| Time interval (mo) | 24.04 ± 23.67 | 18.73 ± 19.10 | 31.53 ± 27.27 | 0.0011 |
Pancreatic endocrine function was assessed by the fasting blood glucose level and an oral glucose tolerance test (OGTT). All tests were performed at the First Affiliated Hospital of Nanchang University. We defined diabetes or IGT as pancreatic endocrine insufficiency or dysglycemia. DM and IGT were based on the diagnostic criteria for diabetes. The definition is shown in Supplementary Table 1. Symptoms such as abdominal pain and diarrhea, diet, exercise, and medication were assessed and recorded[2].
One-way analysis of variance was used to compare continuous variables, and the Kolmogorov-Smirnov test was employed to assess the normal distribution of the variables. Logarithmic transformation was adopted for variables that did not show a normal distribution. Subsequently, for analysis of the relationships between the values of possible risk factors and impaired pancreatic endocrine function, Cox regression was performed. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. P < 0.05 indicated a significant difference. Univariate Cox regression analysis was performed to explore potential confounders. Subsequently, variables with a P value below 0.05 in univariate analysis were selected for multivariable Cox regression to identify the independent predictors. Statistical analyses were performed using SPSS 24.0 for Windows.
A total of 4533 patients with AP were admitted to the First Affiliated Hospital of Nanchang University from 2012 to 2018, and 361 patients were ultimately enrolled in the study after the exclusion criteria were applied (Figure 1). The population included 201 (55.7%) males and 160 (44.3%) females. The mean age was 48.63 ± 13.36 years. The mean BMI was 23.62 ± 4.36. The shortest and longest follow-up times after AP were 3 mo and 7 years, respectively, with a mean ± SD follow-up time of 24.04 ± 23.67 mo. Seventy (46.7%) patients who were followed for 3 mo to 1 year, 42 (28.0%) who were followed for 1 year to 3 years, and 38 (25.3%) who were followed for 3 years to 7 years experienced dysglycemia. A total of 115 (31.9%) patients had SAP, 47 (13%) had MAP, and the remaining 199 (55.1%) had MSAP. The causes of disease included biliary causes (166, 46%), hyperlipidemia (130, 36%), alcohol (52, 14.4%), and other causes (13, 3.6%). There were 137 (38.0%) AP patients with organ failure. Pancreatic necrosis was present in 152 (42.1%) patients, and debridement of necrosis was performed in 95 (26.3%). A history of smoking and drinking was reported by 81 (22.4%) patients among all AP patients. The detailed data on patient conditions are listed in Table 1 and 2 and Supplemental Tables 2 and 3.
| Variable | All patients | Normal glucose group | Dysglycemna group |
| Pancreatic necrosis | 152 (100) | 34 (58.4) | 118 (41.6) |
| Part of pancreatic necrosis | |||
| Head of pancreas | 23 (15.1) | 3 (8.8) | 20 (16.9) |
| Body of pancreas | 29 (19.0) | 6 (17.6) | 23 (19.4) |
| Tail of pancreas | 79 (51.3) | 22 (64.8) | 57 (48.3) |
| Whole pancreas | 21 (13.8) | 3 (8.8) | 18 (15.2) |
| Area of pancreatic necrosis | |||
| 30% | 42 (27.6) | 27 (79.4) | 15 (12.7) |
| 30%-50% | 54 (35.6) | 3 (8.8) | 51 (43.2) |
| > 50% | 56 (36.8) | 4 (11.8) | 52 (44.1) |
| Variable | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age | 0.987 (0.966-1.008) | 0.784 | ||
| Sex | ||||
| Male | Reference | |||
| Female | 0.856 (0.618-1.184) | 0.348 | ||
| BMI | 1.026 (0.993-1.060) | 0.125 | ||
| APACHE II | 1.023 (0.980-1.068) | 0.296 | ||
| Etiology | ||||
| Biliary | Reference | |||
| HTG | 1.417 (0.995-2.017) | 0.053 | ||
| Alcoholic | 0.928 (0.565-1.527) | 0.770 | ||
| Others | 1.578 (0.626-3.975) | 0.333 | ||
| Severity of AP | ||||
| Mild | Reference | Reference | ||
| Moderate | 4.400 (1.920-10.088) | 0.001 | 3.489 (1.501-8.111) | 0.004 |
| Severe | 3.929 (1.695-9.103) | 0.001 | 2.529 (1.046-6.115) | 0.039 |
| Organ failure | ||||
| No | Reference | Reference | ||
| Yes | 1.163 (1.182-2.259) | 0.003 | 1.004 (0.695-1.452) | 0.982 |
| Pancreatic necrosis | ||||
| No | Reference | Reference | ||
| Yes | 3.287 (2.215-4.879) | 0.001 | 4.152 (2.580-6.684) | 0.001 |
| Part of pancreatic necrosis | ||||
| Head of pancreas | Reference | Reference | ||
| Body of pancreas | 4.905 (2.864-8.400) | 0.001 | 0.775 (0.388-1.550) | 0.470 |
| Tail of pancreas | 2.435 (1.561-3.801) | 0.001 | 6.433 (0.396-1.722) | 0.609 |
| Whole pancreas | 4.448 (2.486-7.959) | 0.001 | 0.675 (0.369-1.232) | 0.200 |
| Area of pancreatic necrosis | ||||
| 30% | Reference | Reference | ||
| 30%–50% | 3.988 (2.557-6.220) | 0.001 | 6.433 (2.919-14.180) | 0.001 |
| > 50% | 4,144 (2.661-6.456) | 0.001 | 8.093 (3.867-16.936) | 0.001 |
| Debridement of necrosis (minimally invasive and/or ON) | ||||
| No | Reference | Reference | ||
| Yes | 1.451 (1.045-2.014) | 0.026 | 0.683 (0.463-1.007) | 0.054 |
| History of smoking | ||||
| No | Reference | |||
| Yes | 1.276 (0.872-1.868) | 0.209 | ||
| History of drinking | ||||
| No | Reference | |||
| Yes | 1.276 (0.872-1.868) | 0.209 | ||
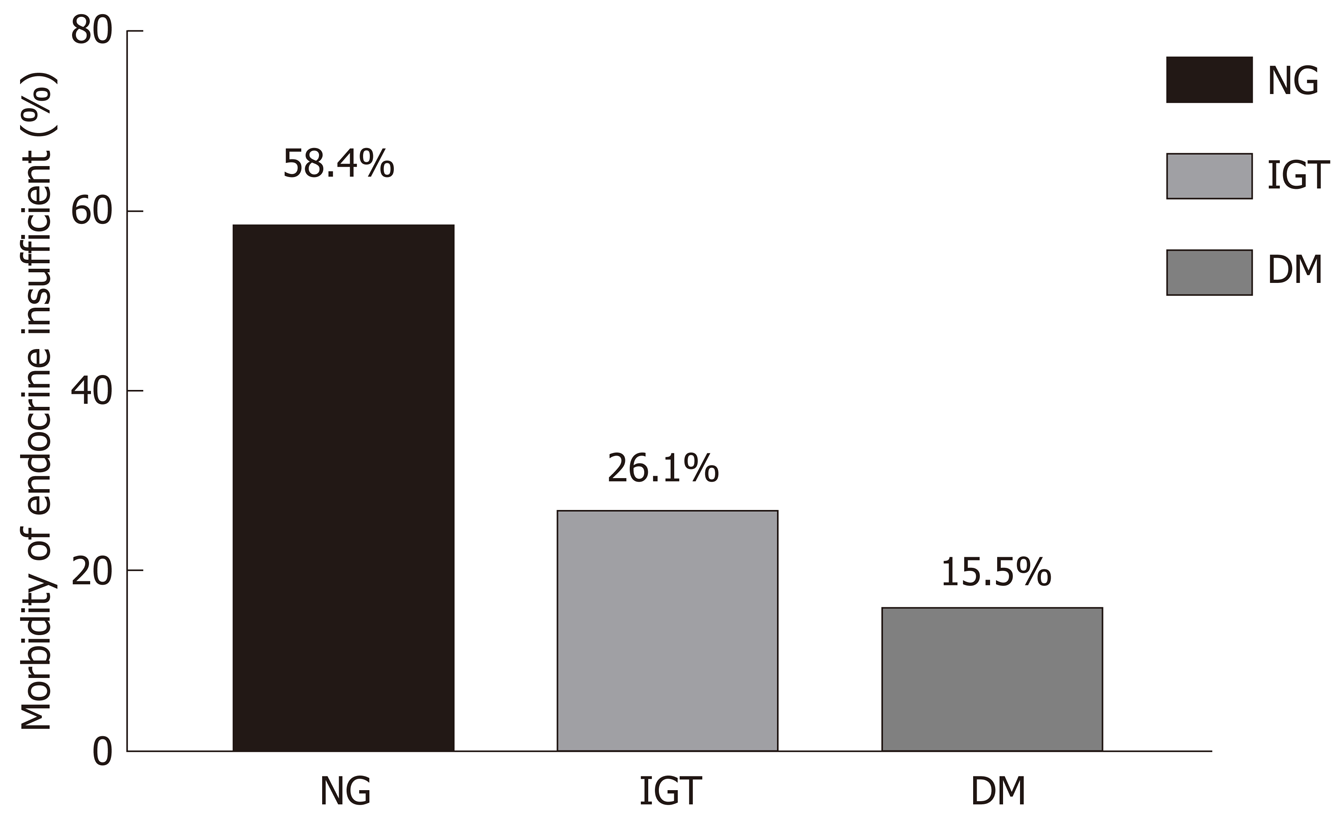
One hundred and fifty patients were diagnosed with dysglycemia. Ninety-four (26.1%) patients were diagnosed with IGT, 56 (15.5%) were diagnosed with DM, and 211 (58.4%) had normal glucose levels (as indicated in Table 1 and Figure 2).
According to the 2012 Atlanta criteria, all AP patients were divided into MAP, MSAP, and SAP groups. Six (4%) MAP, 84 (56.0%) MSAP, and 60 (40.0%) SAP patients experienced IGT and DM, as listed in Table 1. Univariate and multivariate Cox regression analyses of severity in AP patients were performed, which revealed that there was a significant difference between the normal glucose group and the dysglycemia group (MSAP, χ2 = 3.489, P = 0.004; SAP, χ2 = 2.529, P = 0.039), as listed in Table 3.
Biliary factors, HTG, and alcohol are common causes of AP worldwide. In this study, 63 (42%) biliary AP patients and 61 (40.7%) HTG-AP patients experienced IGT and DM. The incidence of alcohol abuse was 14% and the incidence of other types was 3.3%, as listed in Table 1. There was no difference in the two groups regarding the etiology of AP (P > 0.05).
We found that 84 (56.0%) patients with AP and organ failure developed IGT and DM and 66 (44.0%) AP patients with organ failure did not. There were no differences between the normal glucose group and the dysglycemia group among AP patients with organ failure (P > 0.05), as listed in Table 1 and 3.
Pancreatic necrosis is a complication in AP patients and debridement of necrosis, such as percutaneous catheter drainage (PCD) and/or ON, can be used to treat this complication. According to their CT images, the patients were divided into pancreatic necrosis and non-pancreatic necrosis groups, and the morbidity of DM and IGT in patients with pancreatic necrosis was significantly higher than that in the non-pancreatic necrosis patients (χ2 = 4.152, P = 0.001). According to their CT images, the cases were divided into four subgroups: The head of the pancreas, the body of the pancreas, the tail of the pancreas, and the whole pancreas necrosis groups. The morbidity of DM and IGT showed no significant difference among the four subgroups, as listed in Table 2 and 3. According to the CT images, the area of pancreatic necrosis was also divided into three subgroups on the basis of the extent of pancreatic necrosis: Necrotic area < 30%, necrotic area 30%-50%, and necrotic area > 50%. The morbidity of DM and IGT showed a significant difference between patients with different extents of pancreatic necrosis (P = 0.001), as listed in Table 2 and 3.
Debridement of necrosis in AP patients was performed in 70 (46.7%) patients in the normal glucose group and 25 (11.8%) patients in the dysglycemia group. Next, univariate and multivariate Cox regression analyses were used, which revealed that there was no difference between the two groups classified by debridement of necrosis in AP patients (χ2 = 0.683, P = 0.054). The detailed data are listed in Table 1 and 3.
Factors such as age, sex, APACHE II score, etiology, severity of AP, organ failure, pancreatic necrosis, debridement of necrosis (PCD and/or ON), and history of smoking and drinking were included in the univariate and multivariate Cox regression analyses according to the abovementioned results and clinical characteristics. The results of multivariate Cox regression analysis showed that the severity of AP and pancreatic necrosis were independent risk factors for pancreatic endocrine insufficiency in AP patients and that the area of pancreatic necrosis was related to pancreatic endocrine function. The detailed data are listed in Table 2.
As an inflammatory response caused by the activation of pancreatic enzymes, AP can lead to dyspepsia, edema, bleeding, and necrosis of the pancreas. Increasing number of patients will develop pancreatic endocrine insufficiency after AP onset, which has attracted more attention recently. The traditional view is that transient high blood glucose after AP is related to pancreatic microcirculation disorder, acute stress, and excessive secretion of catecholamine, which cause carbohydrate metabolism disorder. Some previous studies have shown that high blood glucose recovers to normal levels soon as the disease improves. This opinion is similar to our finding that patients who were followed for 3 mo to 1 year after discharge experienced dysglycemia more frequently than patients who were followed for 1 year to 7 years after discharge. We speculated that the recovery of pancreatic function is associated with beta cell regeneration over time[5,9-11]. However, another study reported that patients could not fully recover from hyperglycemia and that hyperglycemia could occur again after a short recovery time[12,13], even leading to the development of DM and the need for lifelong treatment with drugs or insulin, but they could not confirm whether this effect resulted from the disease or the natural course[14]. Hence, larger sample sizes and higher-quality research should be used to verify this disputed opinion.
Regarding the incidence of pancreatic endocrine insufficiency after AP, Symersky[15] found that 32% of MAP patients and 42% of SAP patients developed pancreatic endocrine insufficiency and showed that a higher risk of glucose metabolism disorder often occurs in patients who received pancreas surgery. Another review reported that the occurrence of DM after AP was 23%, which was significantly higher than that in ordinary people (4%-9%)[1]. However, in our study, we found that DM and IGT occurred in 15.5% and 26.1% of all enrolled AP patients, respectively. Based on those studies, the incidence of pancreatic endocrine insufficiency after AP is heterogeneous[16-19], and we speculate that this is due to differences in inclusion criteria, diagnostic methods for DM (OGTT, HbA1c, or C-peptide), and follow-up times. Therefore, the incidence of pancreatic endocrine insufficiency in AP patients is still controversial and needs further investigation.
It is difficult to distinguish type 2 DM from AP-induced DM in clinical practice. The WHO and American DM Association consider diabetes caused by pancreatic injury to be T3c DM. In fact, 80% of T3c DM patients have had CP. AP, pancreatic cancer, and pancreatic surgery are the other common causes of T3c DM[2,20-22]. Research on the pathomechanism of T3c DM has mostly focused on CP rather than AP. Thus, the pathomechanism of T3c DM after AP onset is not well defined. Some studies speculated that this may occur for the following reasons. First, the pancreatic islets are damaged by pancreatic necrosis or pancreatic fibrosis after AP, which inhibits the secretion of insulin from pancreatic β-cells[23,24]. Second, α-cells in the pancreas release hyperglycemia-related factors that destroy the pancreatic tissue[23,24]. Therefore, we should pay more attention to the changes in blood glucose after AP.
Some researchers considered pancreatic endocrine insufficiency to be related to recurrent attacks of AP, alcohol abuse, sex, and age but not to the severity of AP[1,18,25]. Another study suggested that pancreatic endocrine insufficiency after AP was determined primarily by the severity of AP[16]. In our study, we found that the morbidity of pancreatic endocrine insufficiency in the MSAP and SAP groups was higher than that in the MAP group, which indicated that the severity of AP is correlated with pancreatic endocrine insufficiency. We hypothesized that the heterogeneity in those studies is partly due to the proportion of SAP cases; other causes may involve the occurrence and extent of pancreatic necrosis, which reflect the local circumstances of the pancreas.
Pancreatic necrosis, which is an important marker for disease severity, was found to be an independent risk factor for pancreatic endocrine insufficiency in the Cox logistic regression analysis. Large-scale pancreatic necrosis could lead to a great decline in β-cells in the pancreas and insulin secretion[26-29]. Many studies[30,31] have reported that a large extent of pancreatic necrosis in AP patients is associated with a higher risk of pancreatic endocrine insufficiency, which is similar to our study. Patients with an area of pancreatic necrosis greater than 30% (> 50% group and 30%-50% group) developed pancreatic endocrine insufficiency more easily than patients with an area of pancreatic necrosis < 30%, which indicates that the higher the degree of pancreatic necrosis, the higher the rate of pancreatic endocrine insufficiency. In addition, different locations of pancreatic necrosis, such as the head, body, tail, and whole pancreas, did not affect pancreatic endocrine insufficiency, which was evidenced in this study and a previous study by Lu et al[19]. In summary, we found that the severity of AP and pancreatic necrosis may be risk factors for pancreatic endocrine insufficiency.
Debridement of necrosis, such as PCD and/or ON treatment, is usually used to treat pancreatic necrosis to relieve symptoms in AP patients[16]. Chandrasekaran et al[32] assessed the outcomes in operative and nonoperative SAP patients and found that patients undergoing necrosectomy had a higher incidence of pancreatic endocrine insufficiency [61.9% in the operative group and 28.5% in the nonoperative group (P < 0.05)]. Another study reported[19] that PCD is a protective factor against insufficient endocrine function in AP patients[31,33]. However, in our study, we found that the debridement of necrosis in AP patients was not related to pancreatic endocrine insufficiency. At present, whether the debridement of necrosis affects pancreatic endocrine insufficiency is still unclear, and we hope that more research will be done to verify whether debridement of necrosis is a risk factor for pancreatic endocrine insufficiency after AP onset.
Previous studies revealed that gender, age, history of smoking, alcohol abuse, and hypertriglyceridemia were risk factors that contributed to pancreatic endocrine insufficiency after AP onset[17-19,23,34]. However, the results of our study showed that gender, age, history of smoking and drinking, and etiology did not affect pancreatic endocrine function, which may be related to the small sample size of this study. Therefore, future research should focus on the influence of the above risk factors on pancreatic endocrine function after AP.
Our study is the first to explore the association between AP and endocrine insufficiency with such a long follow-up time. We included patients who were followed for a long time, from 3 mo to 7 years. Our study included more AP patients than any previous study. This study not only assessed the effect of pancreatic necrosis on pancreatic endocrine function but also evaluated the effect of the area and location of pancreatic necrosis on pancreatic endocrine function. In this study, only the OGTT and fasting glucose were used to detect blood glucose. Pancreatic endocrine function should be further evaluated with HbA1c and C-peptide. This study found that pancreatic endocrine insufficiency recovers over time, but we did not determine which time point was associated with the highest incidence of pancreatic endocrine insufficiency.
In conclusion, pancreatic necrosis and the severity of AP are independent risk factors for pancreatic endocrine insufficiency after AP. The area of pancreatic necrosis can affect pancreatic endocrine function after AP.
Pancreatic endocrine insufficiency after acute pancreatitis (AP) has drawn increasing attention in recent years.
In recent years, we discovered another complication with an increasing frequency after AP onset, namely, pancreatic endocrine insufficiency. DM is associated with a heavy burden on the society due to related disabilities and high costs. Therefore, it is important to determine the incidence rate, risk factors, treatment, and outcome of pancreatic endocrine insufficiency after AP onset, which remain unclear. Hence, in this study, we conducted a long-term follow-up investigation to assess the potential risk factors for and incidence of pancreatic endocrine insufficiency after AP onset.
The aim of the present study was to assess the impact of risk factors on the development of pancreatic endocrine insufficiency after AP.
This was a long-term follow-up retrospective observational study with patient data collected from the AP database of the First Affiliated Hospital of Nanchang University; it was conducted from January 1, 2012 to December 30, 2018. A total of 361 AP patients agreed to be followed after discharge. Pancreatic endocrine function was assessed by the fasting blood glucose level and an oral glucose tolerance test. All tests were performed at the First Affiliated Hospital of Nanchang University. One-way analysis of variance, Kolmogorov-Smirnov test, Cox regression, and univariate Cox regression analysis were used in this study.
The morbidity of DM and IGT in patients with pancreatic necrosis was significantly higher than that in the non-pancreatic necrosis patients (χ2 = 4.152, P = 0.001). According to the CT images, the area of pancreatic necrosis was divided into three subgroups on the basis of the extent of pancreatic necrosis: Necrotic area < 30%, necrotic area 30%-50%, and necrotic area > 50%. The morbidity of DM and IGT showed a significant difference between patients with different extents of pancreatic necrosis (P = 0.001).
The results of multivariate Cox regression analysis showed that the severity of AP and pancreatic necrosis were independent risk factors for pancreatic endocrine insufficiency in AP patients and that the area of pancreatic necrosis was related to pancreatic endocrine function.
This study is the first to explore the association between AP and endocrine insufficiency in such a long follow-up time from 3 mo to 7 years. We confirmed that pancreatic necrosis and the severity of AP are independent risk factors for pancreatic endocrine insufficiency after AP. Meanwhile, we proposed that the area of pancreatic necrosis can affect pancreatic endocrine function after AP and high blood glucose recovers to normal levels as the disease of AP improves in this study. Our result will provide some guidance on the clinical practice in the future.
In the future, pancreatic endocrine function should be further evaluated with HbA1c and C-peptide. This study found that pancreatic endocrine insufficiency recovers over time, but we did not determine which time point is associated with the highest incidence of pancreatic endocrine insufficiency. An RCT will be needed to evaluate the time point, and we should not only pay attention to the pancreatic endocrine insufficiency, but also the exocrine pancreatic insufficiency.
The authors would like to thank Professor Jian-Cheng Wang for help with the study.
| 1. | Das SL, Singh PP, Phillips AR, Murphy R, Windsor JA, Petrov MS. Newly diagnosed diabetes mellitus after acute pancreatitis: a systematic review and meta-analysis. Gut. 2014;63:818-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 295] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 2. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37 Suppl 1:S81-S90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2986] [Cited by in RCA: 3573] [Article Influence: 297.8] [Reference Citation Analysis (19)] |
| 3. | Pendharkar SA, Mathew J, Petrov MS. Age- and sex-specific prevalence of diabetes associated with diseases of the exocrine pancreas: A population-based study. Dig Liver Dis. 2017;49:540-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 4. | Vipperla K, Papachristou GI, Slivka A, Whitcomb DC, Yadav D. Risk of New-Onset Diabetes Is Determined by Severity of Acute Pancreatitis. Pancreas. 2016;45:e14-e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4706] [Article Influence: 362.0] [Reference Citation Analysis (48)] |
| 6. | van Santvoort HC, Bakker OJ, Besselink MG, Bollen TL, Fischer K, Nieuwenhuijs VB, Gooszen HG, Erpecum KJ; Dutch Pancreatitis Study Group. Prediction of common bile duct stones in the earliest stages of acute biliary pancreatitis. Endoscopy. 2011;43:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Scherer J, Singh VP, Pitchumoni CS, Yadav D. Issues in hypertriglyceridemic pancreatitis: an update. J Clin Gastroenterol. 2014;48:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 350] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 8. | Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400-15; 1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1428] [Article Influence: 109.8] [Reference Citation Analysis (3)] |
| 9. | Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet. 2015;386:85-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 880] [Cited by in RCA: 881] [Article Influence: 80.1] [Reference Citation Analysis (1)] |
| 10. | Garber AJ, Handelsman Y, Einhorn D, Bergman DA, Bloomgarden ZT, Fonseca V, Garvey WT, Gavin JR 3rd, Grunberger G, Horton ES, Jellinger PS, Jones KL, Lebovitz H, Levy P, McGuire DK, Moghissi ES, Nesto RW. Diagnosis and management of prediabetes in the continuum of hyperglycemia: when do the risks of diabetes begin? A consensus statement from the American College of Endocrinology and the American Association of Clinical Endocrinologists. Endocr Pract. 2008;14:933-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 11. | Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M; Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7955] [Cited by in RCA: 6690] [Article Influence: 267.6] [Reference Citation Analysis (5)] |
| 12. | Crandall JP, Knowler WC, Kahn SE, Marrero D, Florez JC, Bray GA, Haffner SM, Hoskin M, Nathan DM; Diabetes Prevention Program Research Group. The prevention of type 2 diabetes. Nat Clin Pract Endocrinol Metab. 2008;4:382-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 190] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 13. | Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B; American Diabetes Association. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30:753-759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Czakó L, Hegyi P, Rakonczay Z Jr, Wittmann T, Otsuki M. Interactions between the endocrine and exocrine pancreas and their clinical relevance. Pancreatology. 2009;9:351-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Symersky T, van Hoorn B, Masclee AA. The outcome of a long-term follow-up of pancreatic function after recovery from acute pancreatitis. JOP. 2006;7:447-453. [PubMed] |
| 16. | Tu J, Yang Y, Zhang J, Yang Q, Lu G, Li B, Tong Z, Ke L, Li W, Li J. Effect of the disease severity on the risk of developing new-onset diabetes after acute pancreatitis. Medicine (Baltimore). 2018;97:e10713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Malecka-Panas E, Gasiorowska A, Kropiwnicka A, Zlobinska A, Drzewoski J. Endocrine pancreatic function in patients after acute pancreatitis. Hepatogastroenterology. 2002;49:1707-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Winter Gasparoto RC, Racy Mde C, De Campos T. Long-term outcomes after acute necrotizing pancreatitis: what happens to the pancreas and to the patient? JOP. 2015;16:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 19. | Tu J, Zhang J, Ke L, Yang Y, Yang Q, Lu G, Li B, Tong Z, Li W, Li J. Endocrine and exocrine pancreatic insufficiency after acute pancreatitis: long-term follow-up study. BMC Gastroenterol. 2017;17:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Hardt PD, Brendel MD, Kloer HU, Bretzel RG. Is pancreatic diabetes (type 3c diabetes) underdiagnosed and misdiagnosed? Diabetes Care. 2008;31 Suppl 2:S165-S169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 134] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26 Suppl 1:S5-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1635] [Cited by in RCA: 2138] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 22. | Ewald N, Kaufmann C, Raspe A, Kloer HU, Bretzel RG, Hardt PD. Prevalence of diabetes mellitus secondary to pancreatic diseases (type 3c). Diabetes Metab Res Rev. 2012;28:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 193] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 23. | Wehmeyer MH, Dammermann W, Seiz O, Zinser ME, Galante A, Lohse AW, Sterneck M, Nashan B, Herden U, Lüth S. Chronic pancreatitis in patients with liver cirrhosis negatively affects graft survival after liver transplantation. Pancreatology. 2017;17:898-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Solomon SS, Duckworth WC, Jallepalli P, Bobal MA, Iyer R. The glucose intolerance of acute pancreatitis: hormonal response to arginine. Diabetes. 1980;29:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Shen HN, Yang CC, Chang YH, Lu CL, Li CY. Risk of Diabetes Mellitus after First-Attack Acute Pancreatitis: A National Population-Based Study. Am J Gastroenterol. 2015;110:1698-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 26. | Dugnani E, Gandolfi A, Balzano G, Scavini M, Pasquale V, Aleotti F, Liberati D, Di Terlizzi G, Petrella G, Reni M, Doglioni C, Bosi E, Falconi M, Piemonti L. Diabetes associated with pancreatic ductal adenocarcinoma is just diabetes: Results of a prospective observational study in surgical patients. Pancreatology. 2016;16:844-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Riveline JP, Boudou P, Blondeau B, Gautier JF. Glucagon-secretion inhibition using somatostatin: An old hormone for the treatment of diabetes-associated pancreatectomy. Diabetes Metab. 2017;43:269-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290:1884-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1068] [Cited by in RCA: 982] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 29. | Halter JB. Diabetes mellitus in an aging population: the challenge ahead. J Gerontol A Biol Sci Med Sci. 2012;67:1297-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Garip G, Sarandöl E, Kaya E. Effects of disease severity and necrosis on pancreatic dysfunction after acute pancreatitis. World J Gastroenterol. 2013;19:8065-8070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Boreham B, Ammori BJ. A prospective evaluation of pancreatic exocrine function in patients with acute pancreatitis: correlation with extent of necrosis and pancreatic endocrine insufficiency. Pancreatology. 2003;3:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Chandrasekaran P, Gupta R, Shenvi S, Kang M, Rana SS, Singh R, Bhasin DK. Prospective comparison of long term outcomes in patients with severe acute pancreatitis managed by operative and non operative measures. Pancreatology. 2015;15:478-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Gupta R, Wig JD, Bhasin DK, Singh P, Suri S, Kang M, Rana SS, Rana S. Severe acute pancreatitis: the life after. J Gastrointest Surg. 2009;13:1328-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Ibars EP, Sánchez de Rojas EA, Quereda LA, Ramis RF, Sanjuan VM, Peris RT. Pancreatic function after acute biliary pancreatitis: does it change? World J Surg. 2002;26:479-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Gonoi W, Kochhar R S-Editor: Ma YJ L-Editor: Wang TQ E-Editor: Zhang YL