Published online Jun 14, 2020. doi: 10.3748/wjg.v26.i22.2931
Peer-review started: January 31, 2020
First decision: February 24, 2020
Revised: March 26, 2020
Accepted: May 30, 2020
Article in press: May 30, 2020
Published online: June 14, 2020
Processing time: 134 Days and 19.2 Hours
More than five years ago, the treatment of hepatitis C virus infection was revolutionized with the introduction of all-oral direct-acting antiviral (DAA) drugs. They proved highly efficient in curing patients with chronic hepatitis C (CHC), including patients with cirrhosis. The new DAA treatments were alleged to induce significant improvements in clinical outcome and prognosis, but the exact cause of the expected benefit was unclear. Further, little was known about how the underlying liver disease would be affected during and after viral clearance. In this review, we describe and discuss the liver-related effects of the new treatments in regards to both pathophysiological aspects, such as macrophage activation, and the time-dependent effects of therapy, with specific emphasis on inflammation, structural liver changes, and liver function, as these factors are all related to morbidity and mortality in CHC patients. It seems clear that antiviral therapy, especially the achievement of a sustained virologic response has several beneficial effects on liver-related parameters in CHC patients with advanced liver fibrosis or cirrhosis. There seems to be a time-dependent effect of DAA therapy with viral clearance and the resolution of liver inflammation followed by more discrete changes in structural liver lesions. These improvements lead to favorable effects on liver function, followed by an improvement in cognitive dysfunction and portal hypertension. Overall, the data provide knowledge on the several beneficial effects of DAA therapy on liver-related parameters in CHC patients suggesting short- and long-term improvements in the underlying disease with the promise of an improved long-term prognosis.
Core tip: Antiviral therapy of chronic hepatitis C, especially the achievement of a sustained virologic response, has several beneficial effects on liver-related parameters in chronic hepatitis C patients. There seems to be a time-dependent effect of therapy. Initially, liver inflammation ameliorates, followed by more discrete changes in structural liver lesions. These improvements are followed by beneficial effects on liver function, cognitive disturbances, and portal hypertension. Together, this suggests short- and long-term improvements in the underlying liver disease with the promise of an improved prognosis.
- Citation: Laursen TL, Sandahl TD, Kazankov K, George J, Grønbæk H. Liver-related effects of chronic hepatitis C antiviral treatment. World J Gastroenterol 2020; 26(22): 2931-2947
- URL: https://www.wjgnet.com/1007-9327/full/v26/i22/2931.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i22.2931
Hepatitis C virus (HCV) was isolated and named in 1989[1], and in 2015, more than 170 million people were infected worldwide with approximately 70 million chronically infected[2,3]. The primary mode of virus transmission in Western countries is via percutaneous exposure to blood, and the major infection route is unsafe drug injections[4-6]. At transmission, an acute HCV infection occurs, which is often asymptomatic[7]. In 60%-80% of patients, the infection becomes chronic[8,9], and chronic hepatitis C (CHC) is defined as positive HCV RNA for at least 6 mo. CHC holds the potential for inducing fibrosis and 10%-30% of those infected develop cirrhosis over decades, with the potential risk of complications and early death[10-12].
HCV is a hepatotropic positive single-stranded RNA virus that translates into a single polyprotein consisting of 3011 amino acids. The HCV genome is highly diverse and is separated into seven genotypes with several subtypes[13]. HCV circulates as a lipo-viro-particle consisting of the nucleocapsid surrounded by the envelope proteins E1 and E2, and several host lipoproteins[14]. HCV is cleaved by viral and host proteases into 10 proteins with diverse functions: 3 structural proteins (i.e., core, E1, and E2) and 7 nonstructural (NS) proteins (i.e., p7, NS2, NS3, NS4A, NS4B, NS5A, and NS5B)[15,16]. The two viral proteases, NS2 and NS3/4A, are involved in the process of producing nonstructural proteins. Replication itself is catalyzed by the RNA polymerase NS5B, while NS5A and NS3 are important regulatory proteins. NS4B participates in membrane rearrangements, leading to the formation of a membranous web that supports continued HCV replication[16,17].
HCV is a major challenge for the immune system, and host factors play important roles in the potential clearance of HCV and long-term disease progression[18,19]. An initial vigorous immune response is considered crucial, and both initial and consistent innate and adaptive immune responses are important determinants of whether the infection can be cleared or becomes chronic. The innate immune responses include the activation of proinflammatory pathways[20-23], and adaptive immune factors include weak or absent HCV-specific T-cell responses[24,25]. If HCV infection is not cleared, these mechanisms lead to sustained liver inflammation with the activation of macrophages, which subsequently activate or transdifferentiate hepatic stellate cells into myofibroblast-like cells with proinflammatory, contractile, and profibrogenic properties. This may initiate a vicious cycle where inflammatory and fibrogenic cells consistently stimulate each other[26], resulting in the accumulation of excessive extracellular matrix proteins and fibrosis progression[27].
The ultimate treatment goal of CHC is a sustained virologic response (SVR), defined as undetectable HCV RNA 12–24 wk after treatment cessation, corresponding to “cure” or, in other words, HCV eradication. The treatment has evolved drastically over the last decades. For years, CHC treatment was based on subcutaneous interferon treatments that had inadequate efficacy and severe adverse effects, leaving this type of treatment intolerable for many patients, especially those with cirrhosis[28-30]. Interferon treatment acts via a direct immune-stimulating mechanism and has several derivative effects on innate and adaptive immunity[31]. In 2001, pegylated interferon was introduced; it has a longer half-life and a more favorable pharmacokinetic profile, and it improved the SVR rate slightly, while the majority of the adverse effects remained[32-34]. In addition to interferon treatment, ribavirin was required. Ribavirin is a nucleoside analog of guanosine that is used to potentiate the effects of antiviral treatments[35] and is still used as a potential addition to some DAA regimens.
In 2011, the first DAA treatments were introduced, and as the name infers, the DAAs are specific inhibitors of the viral proteins. Boceprevir and telaprevir were the first generation of DAAs; they inhibit the NS3/4A protease, are effective against only genotype 1, and were approved as an add-on to the established pegylated interferon and ribavirin treatment. The treatment duration was between 24 and 48 wk, and the drugs improved the SVR rates[36-39] and held promise for a new era of all-oral DAA therapy.
In 2014, sofosbuvir was marketed in Europe. Sofosbuvir is a uridine nucleotide analog that inhibits NS5B RNA polymerase by competing with natural nucleotides and by inducing viral RNA chain termination. Sofosbuvir is orally administered for 12-24 wk, is pangenotypic, and may be combined with other DAAs that are genotype-specific[40]. With the introduction of sofosbuvir, high SVR rates, in general above 90%, were reached even in difficult-to-treat patient groups, e.g., patients with cirrhosis and nonresponders to previous therapy[41-44]. Several other DAAs were introduced starting in 2013, including ledipasvir (an NS5A inhibitor), daclatasvir (an NS5A inhibitor), and simeprevir (a second-generation NS3/4A inhibitor), and 3D combination [ombitasvir (an NS5A inhibitor), paritaprevir (an NS3/4A inhibitor), and ritonavir with the potential addition of dasabuvir (an NS5B inhibitor)], all of which contributed to increasing SVR rates, ultimately reaching 100%[43-47].
With the introduction of all-oral DAA therapy, a new era of CHC treatment commenced. Not only was it possible to successfully treat the “healthiest” patients, but treatment was suddenly a possibility for all patients with CHC. Rapidly, the scientific discussions changed focus to special groups such as cirrhosis patients and non-responders, and to the biological effects of HCV eradication.
Assessment of liver disease severity is crucial in CHC as it is related to morbidity and mortality[48-50]. Several components affect the prognosis of chronic liver disease and the three major factors are inflammation, fibrosis, and liver function[51]. As described above, the presence of inflammation drives the process of fibrogenesis, with structural changes leading to portal hypertension. In addition, liver function may be compromised, probably due to both ongoing hepatic inflammation and structural liver changes, with the loss of functional liver mass.
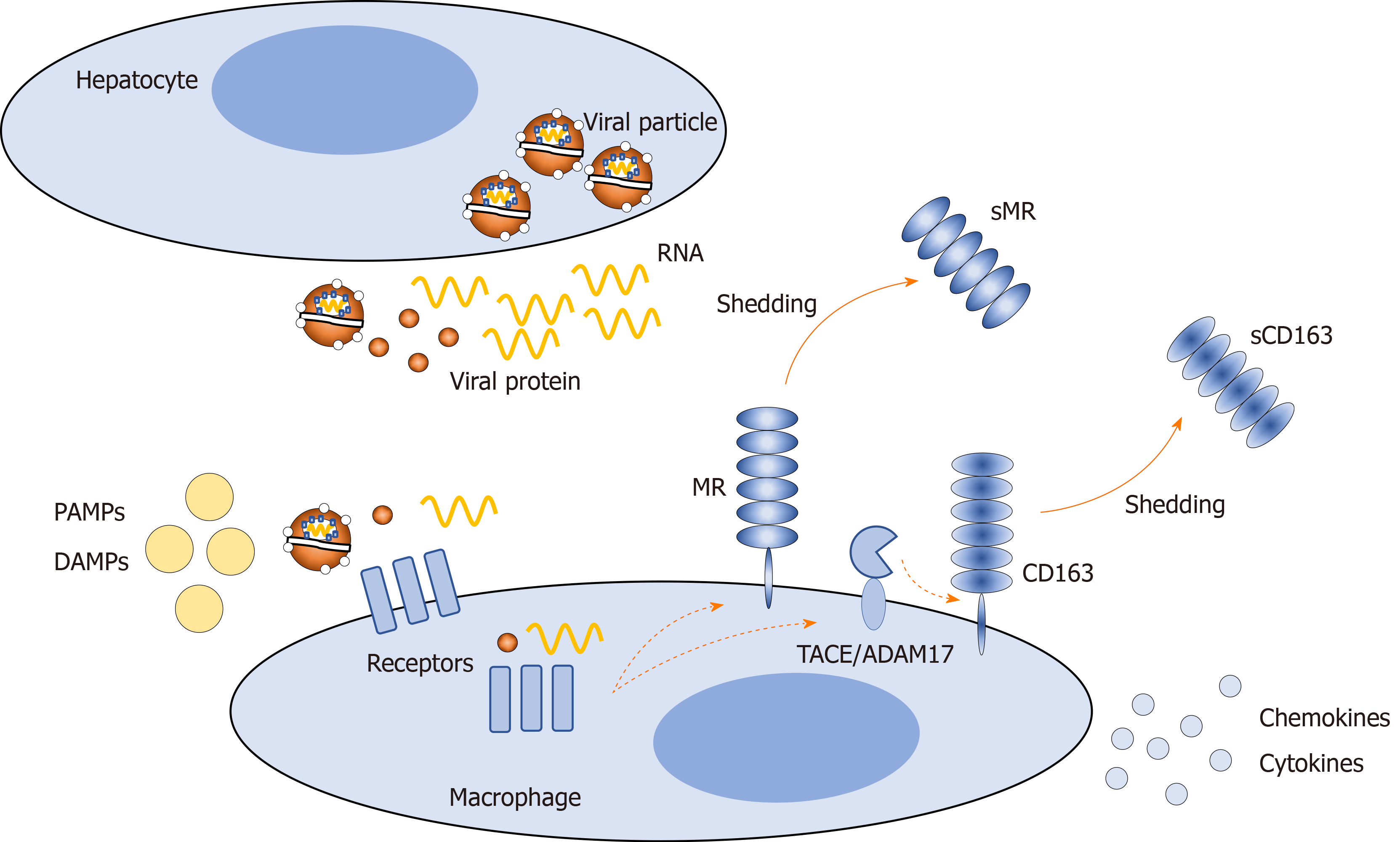
In HCV infection, hepatocytes release viral particles and components, which may bind directly to surface or endosomal macrophage receptors or interfere indirectly with these receptors[52]. In addition, other factors may act on macrophage receptors, such as pathogen-associated molecular patterns, including lipopolysaccharide, which may enter the circulation from the gut due to increased intestinal permeability, and damage-associated molecular patterns released from damaged hepatocytes. These factors activate macrophages, causing an altered proinflammatory phenotype and amplifying hepatic inflammation[53,54]. One way to assess hepatic inflammation noninvasively is to evaluate the presence of specific macrophage activation markers in the blood. Two such markers are soluble (s)CD163 and soluble mannose receptor (sMR)[55-57] (Figure 1). sCD163 and sMR are well-established markers of liver disease severity, portal hypertension, and prognosis in several liver and infectious diseases[58-68]. Furthermore, significant associations between macrophage activation and histological inflammatory activity and fibrosis have been observed in CHC[69-71]. After antiviral therapy of chronic hepatitis B and interferon treatment of CHC, macrophage activation is diminished in parallel with the amelioration of hepatic inflammation[72,73].
Fibrosis staging is important in CHC patients, as fibrosis stage is a strong predictor of complications and mortality[74,75]. Historically, liver biopsy has been the method of choice as it brings insight into the exact structure of the liver and yields information on inflammation and fibrosis as well as potential differential diagnoses. Liver histology from CHC patients covers a wide spectrum of abnormalities, from acute inflammation to lasting structural changes, often in combination[76,77]. Moreover, a liver biopsy may be used to assess fibrosis progression over time[11]. However, the biopsy represents a small portion of the liver, which may be heterogeneous. Some studies have shown sampling error in up to 30% of biopsies[78,79], with adequate biopsy length being of primary importance[80]. Liver biopsy may further be limited by inter- and intraobserver variation in histological assessment[81]; is invasive, with a small but significant risk of complications (e.g., pain, bleeding, and even mortality)[82]; and is disliked by many patients, which limits its use in follow-up studies. The lack of histological verification of structural liver changes is one of the major limitations in many studies.
Due to the limitations of liver biopsy, noninvasive methods for grading and staging liver inflammation and fibrosis have become increasingly sought and used. Noninvasive methods include biomarkers and imaging techniques, many of which were developed and validated in CHC patients. The biomarkers include scores such as the aspartate aminotransferase-to-platelet ratio index (APRI) based on aspartate transaminase (AST) and platelets[83]; the fibrosis-4 (FIB-4) index based on age, AST, alanine transaminase (ALT), and platelets[84]; the enhanced liver fibrosis test[85]; and the FibroTest[86], which have all been extensively investigated in CHC patients, yielding “good or decent” prediction of fibrosis and especially cirrhosis[85,87-92]. In addition, the APRI and FIB-4 predict HCV liver-related death[93].
The majority of the imaging techniques used for liver fibrosis assessment are based on the detection of the velocity of a propagating wave through liver tissue, where the velocity of the shear wave reflects tissue stiffness, with the highest velocity in the stiffest tissue. Many methods have been developed and tested, including transient elastography (TE) using the FibroScan® device (Echosens, Paris, France) and shear-wave elastographies (SWE), divided into point (p)-SWE, also known as acoustic radiation force impulse (ARFI) scans, and 2D-, and 3D-SWE. Last, magnetic resonance imaging elastography has been used in CHC patients[94]. FibroScan TE provides a measure of liver stiffness by Young’s modulus of elasticity and is expressed in kPa, whereas the ARFI scans yield the velocity of the shear wave reported in m/s.
The major quality of noninvasive fibrosis assessment inherently lies in the term noninvasive. In addition, the methods overall have practical advantages, including high applicability and good reproducibility and availability[94-97]. However, the methods also have several limitations. The performance of noninvasive methods to diagnose fibrosis or cirrhosis will depend on the prevalence of the disease stage in the cohort. This is known as spectrum bias and is especially relevant when comparing methods between cohorts. In addition, the use of noninvasive methods may be limited because most are not liver specific, and may be influenced by other factors[94,98-104].
Currently, the best validated method in CHC patients is TE using the FibroScan device. Several studies have shown good concordance between liver stiffness by FibroScan and histological stage[105,106], with good reproducibility, especially in patients with higher stages of fibrosis[107]. The ARFI method is becoming widely used and is in good agreement with liver histology in CHC patients[108,109].
The liver is an extraordinary organ with numerous and highly complex functions, several of which are exclusive to the liver. In routine clinical practice, standard blood parameters such as albumin and coagulation factors are often used to assess liver function. However, a more detailed analysis can be obtained by using other methods. Some metabolic liver functions are decreased in patients with chronic hepatitis, but data on improvements after interferon therapy are conflicting[110-113]. Assessment of these functions can provide insight into CHC by linking pathophysiological events such as inflammation and fibrosis with changes in liver function and can aid understanding of how prognosis is affected after antiviral treatment[114-117].
One exclusive liver function is galactose elimination, which depends on the enzyme galactokinase, primarily located in the hepatocyte cytoplasm[118]. The test evaluates the total functional capacity of the liver to eliminate galactose from the bloodstream, reflecting functional liver cell mass; and therefore, is decreased in patients with cirrhosis[114,119].
Another exclusive, and in addition vital liver function is ureagenesis, which covers the final and irreversible transformation of amino nitrogen to urea nitrogen[120]. Ureagenesis plays a key regulatory role in whole-body nitrogen homeostasis, and disturbances in ureagenesis are associated with hepatic encephalopathy due to reduced nitrogen clearance[121]. The ureagenesis capacity also depends on functional liver mass and is compromised in cirrhosis patients[122,123].
In addition, several other methods may be used to investigate diverse liver functions. The 13C-aminopyrine breath test assesses microsomal cytochrome P450 activity of the liver[124], whereas excretory liver function may be assessed through the measurement of plasma elimination of indocyanine green (ICG)[125].
Complications of CHC infection include cirrhosis development with esophageal varices that may bleed, ascites and hepatorenal syndrome, hepatic encephalopathy (HE), and hepatocellular carcinoma (HCC). Portal hypertension is a major risk factor for an unfavorable disease course[126]. Portal hypertension may be assessed by liver vein catheterization, where wedged hepatic venous pressure is obtained in a hepatic vein and free hepatic venous pressure is measured in the right hepatic vein close to the inferior vena cava. The hepatic venous pressure gradient (HVPG) is thus determined as the difference between wedged hepatic venous pressure and free hepatic venous pressure[127]. Portal hypertension is then defined as HVPG > 5 mmHg and clinically significant portal hypertension by HVPG > 10 mmHg, which comes with a significantly increased risk of varices[128].
The presence of even minimal HE affects the quality of life, poses an increased risk of developing clinically manifest HE and is associated with a more unfavorable prognosis[129,130]. It should be noted that CHC in itself may affect cognition irrespective of HE[131]. The detection of minimal HE relies on psychometric testing, and to evaluate cognitive function in a quantitative way, several methods may be applied. The major limitation of any test to assess HE is the fact that it is only one test. Minimal HE represents complex cognitive disturbances with no single manifestation, and one test will not be able to encompass the entire spectrum of deficits in patients with HE. This is mirrored in studies showing low concordance between the continuous reaction time test and the psychometric hepatic encephalopathy score or critical-flicker frequency[132,133].
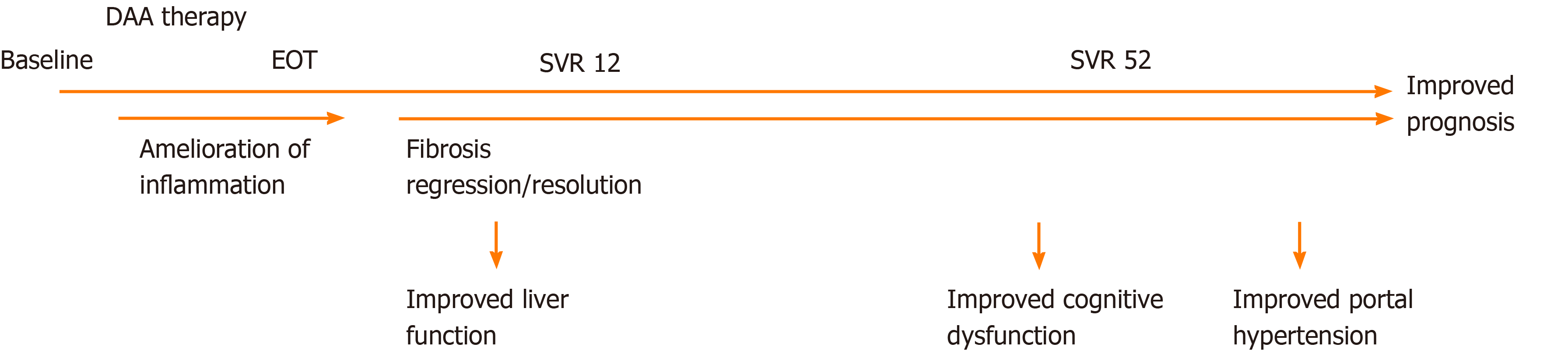
Amelioration of inflammation is the first critical step in stopping the vicious cycle of fibrosis progression in any chronic liver disease. With viral clearance, inflammation diminishes, and this is probably required before the reversal of fibrosis and improved function can occur. Currently, it is well established that antiviral therapies, interferon- or DAA-based resolve inflammation, as indicated by biomarkers[43,44,73] and liver biopsies[33,134-137]. In addition, liver damage diminishes with reductions in ALT levels following DAA therapy, but liver-specific enhanced macrophage activation resolves rapidly with treatment[138] (Figure 2). This is supported by results from chronic hepatitis B patients, where a treatment-induced decrease in circulating sCD163 parallels diminished CD163 expression in liver biopsies[72]. In addition, it is not the effect of the treatment per se or the different mechanisms of action of the respective treatments but the clearance of the virus that reduces inflammation, as only patients who achieve an SVR have a decrease in sCD163[139] and achieve inflammation resolution in liver biopsies[134,136].
To date, many studies have assessed changes in noninvasive measures after DAA therapy; improvements in several biomarker-based fibrosis panels have been shown, including APRI and FIB-4[140,141]. Several studies have also shown decreasing liver stiffness with DAA therapy[138,140,142,143]. In a 2018 review and meta-analysis of liver stiffness changes after interferon or DAA therapy, the authors showed a mean decrease of 2.4 kPa at the end of treatment, 3.1 kPa within the first 6 mo after the end of treatment, and 4.1 kPa more than 1 year after treatment. The authors speculate that the initial decrease is due partly to the resolution of inflammation and that the subsequent decrease pertains to fibrosis regression, which is supported by a larger decrease in patients with high baseline inflammation[144]. However, a consistent decrease from the end of treatment to the 1-year follow-up may represent fibrosis regression (Figure 2).
After the introduction of DAA therapy, there are only limited data from paired biopsies assessing fibrosis regression. In one study, cirrhosis resolved in seven of 14 patients[137]. In another study evaluating 10 patients with paired biopsies and liver stiffness measurements after DAA therapy, the results indicated that fibrosis regression occurs but is not as prominent as liver stiffness measurements might indicate[145]. Thus, data on changes in liver fibrosis after DAA therapy are scarce, and follow-up is still relatively short. Therefore, one can only speculate on the real effect based on the relevant literature from interferon-based studies and those using noninvasive measures. As shown by data from the interferon era, fibrosis regression or resolution takes place but is a slow process taking years[33,134-136,146], and the regression of cirrhosis is less common[136,147,148]. Additionally, it is known that fibrosis will progress without treatment[149], and progression holds an increased risk of clinical decompensation and HCC[150].
Data on the effects of interferon treatment on galactose metabolism are conflicting. In one study where disease severity at baseline was unclear, the galactose elimination capacity (GEC) improved 3 mo after the initiation of interferon therapy[151]. This was paralleled by findings in another study with GEC improvement after 3 mo of interferon treatment, although only in responders to treatment[110], whereas others have found no effect of treatment response on the GEC[112,152].
Data on the GEC after successful DAA therapy are limited but the GEC seems to improve 12 wk post-treatment[138] (Figure 2). In these patients, GEC reflects liver disease severity and fibrosis but not inflammation[111,153].
Conversely, the amelioration of inflammation seems to improve the capacity for ureagenesis, as has also been shown in acute alcoholic hepatitis[117], which also seems to be the case in CHC patients after DAA therapy (Authors’ unpublished data). Other data concerning treatment effects on functional hepatic nitrogen clearance (FHNC) are limited, but the acceleration of the urea cycle after successful interferon therapy was demonstrated in a metabolomics study[154].
Regarding the aminopyrine breath test and ICG, no data are available after DAA therapy. However, some studies have shown improvements after interferon therapy, while others could not detect any differences[110,151].
Treatment-naïve CHC patients have a higher risk of death compared with the background population[155,156], and the risk may be even higher in patients consuming moderate or excessive amounts of alcohol[157]. The achievement of an SVR with interferon-based treatments was associated with reduced mortality[158], in some studies, to a level comparable to the background population[159,160]; however, others find that it is still significantly higher[161]. Some have suggested that any potential outcome advantage wanes when patients are matched for liver function at baseline[162]. However, in one study in patients matched for liver function at baseline, the SVR was still associated with better survival[163]. After the development of cirrhosis, mortality is increased, and in one study of patients with CHC cirrhosis who achieved an SVR by interferon treatment, the authors showed a substantial remaining risk of HCC and clinical disease progression[164]. DAA therapy is still “new” on the market, and long-term follow-up studies are limited. Based on the first studies, it seems that DAA therapy leads to an improved prognosis with decreased mortality[165-167], potentially with a significant survival benefit as soon as 18 mo post-treatment[168]. Interestingly, patients without advanced liver disease also experience reduced mortality after DAA therapy[169], while potential issues including increases in MELD scores were observed after treatment of, e.g., decompensated cirrhosis patients[170]. From these studies, there seems to be a prognostic benefit in CHC patients following an SVR induced by DAA therapy, although the reasons for the benefit are not entirely clear. The available data do not provide causality; however, there are some indices suggesting that the beneficial effects of treatment on different aspects of liver disease and function may in fact lead to improved outcome.
First, low liver stiffness is a predictor of a good prognosis in patients with CHC, at least prior to therapy[171,172], but studies of noninvasive measures and associations with prognosis after DAA therapy are lacking.
Second, several studies have indicated that metabolic liver function is associated with prognosis. In one study, patients with a severely compromised GEC had a high risk of liver-related clinical outcomes[152]. Others have shown that the GEC is a strong predictor of mortality[114,173] and has prognostic value additive to the use of the Child-Pugh score[174]. Conversely, the GEC does not outperform established scores for the prediction of prognosis[175], even though the GEC was shown to be significantly higher in survivors of acute liver failure[176]. Disturbances in the urea cycle and related enzymes are associated with the severity of liver disease and potentially precede histological deterioration in chronic hepatitis[177-179]. On the basis of these results and the higher FHNC in survivors of alcoholic hepatitis[117], we speculate that improvements in metabolic liver functions may be succeeded by a better outcome in CHC patients.
Third, improvements of neurocognitive dysfunction are observed after interferon-based SVR[180]. This finding is corroborated by DAA-induced improvements in brain MR spectroscopy[181] and continuous reaction time (CRT)[138] but is not corroborated by the findings of others[182]. These discrepancies may reflect the different modalities used to assess cognitive dysfunction and the timing of the tests, e.g., the CRT was not significantly improved until 1 year after treatment cessation (Figure 2). Another factor in favor of improvement in cognitive functions is self-reported mood outcomes, which are indeed improved after antiviral therapy[183].
Fourth, one of the strong predictors of adverse outcomes in cirrhosis patients is portal hypertension, and thus, its reduction is warranted. In a small study of eight patients treated with pegylated interferon, ribavirin, and boceprevir, there was a significant improvement in HVPG at 24 wk of follow-up after treatment[184]. Whereas some did not observe an improvement in HVPG at the end of DAA treatment[185], other studies indicate that DAA therapies ameliorate portal hypertension at least in long-term follow-up[186,187] (Figure 2). In addition, follow-up HVPG measurements may predict the risk of hepatic decompensation[188].
Last, the development of HCC is a risk in CHC patients, especially in those with cirrhosis[189], and the risk increases in parallel with cirrhosis severity and portal hypertension[190]. In recent years, an intense debate regarding HCC risk after successful DAA therapy has flourished. In 2016, a study showed a greatly increased risk of HCC after DAA therapy, especially HCC recurrence[191]. This was followed by other studies with similar results[192,193]. However, over recent years, more data have appeared, and the general consensus is that DAA therapy does not increase the risk of HCC[194,195], but probably decreases the risk similar to interferon-based treatments[158]. At the same time, it seems clear that the risk of HCC does not disappear after treatment and that continued and probably life-long surveillance is needed at least in cirrhosis patients or until studies have defined which patients remain at risk[196,197].
Taken together, the evidence suggests a health benefit in patients who achieve an SVR. In our opinion, causality between the improvements and improved prognosis cannot be established from the available literature. However, as reviewed above, several studies indicate such associations. The magnitude and timing of the benefit, as well as its mechanisms, remain elusive, but the data indicate that there is an association between improvements in liver inflammation, fibrosis, and metabolic function and improved outcome after an SVR.
Several questions to be addressed in future studies can be raised. A major question discussed in this review is whether the beneficial effects of DAA therapy on the liver in fact lead to improvements in prognosis. We speculate that liver-related improvements precede clinical benefits and improve prognosis, but confirmation is needed. Such studies require long-term follow-up and large cohorts for high enough power in terms of achieving “enough” events.
Next, one could ask: how good does it get? It would be very interesting to evaluate metabolic liver function after longer follow-up to see, first, whether the improvements are sustained and second, whether further improvement occurs. Such a study would also be useful in terms of the associations between improvements in liver function and liver-related events/clinical benefit.
In addition, it is not entirely clear what happens with structural liver damage. A large study with liver biopsies after DAA therapy is warranted. In addition, such a study should include a noninvasive method to determine the degree of fibrosis. The results might enable clinicians to predict the severity of liver fibrosis after DAA therapy without the need for liver biopsies.
Another very interesting aspect is the determination of cirrhosis severity without the use of invasive methods. There is a large gap between the mere presence of cirrhosis (compensated cirrhosis) and the more severe decompensated cirrhosis, with the occurrence of clinical events. These two groups may respond differently to treatment in regard to the amelioration of liver-related effects. This needs further clarification in a study including decompensated cirrhosis patients.
Portal hypertension is a good predictor of liver-related events in cirrhosis, and a large study with liver vein catheterizations before and at long-term follow-up after DAA therapy to assess the timing and extent of improvement in portal hypertension is also in high demand.
Future studies should be designed with the usual major limitations in mind, thereby trying to minimize these limitations. They include lack of histological verification of the disease severity before treatment but especially after treatment, and in addition, the metabolic studies are often limited by sample size, as this study type is often logistically comprehensive and time-consuming.
From the literature, it seems clear that antiviral therapy, especially the achievement of an SVR, has several beneficial effects on liver-related parameters in CHC patients. There seems to be a time-dependent effect of DAA therapy. Initially, liver inflammation ameliorates, followed by more discrete changes in structural liver lesions. These improvements are followed by beneficial effects on metabolic liver function, cognitive disturbances, and portal hypertension (Figure 2).
In conclusion, the published data suggest short- and long-term improvements in the underlying liver disease with the promise of an improved prognosis after DAA therapy in patients with CHC and advanced liver disease.
| 1. | Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4996] [Cited by in RCA: 4670] [Article Influence: 126.2] [Reference Citation Analysis (1)] |
| 2. | Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1493] [Cited by in RCA: 1514] [Article Influence: 168.2] [Reference Citation Analysis (0)] |
| 3. | Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1381] [Article Influence: 115.1] [Reference Citation Analysis (0)] |
| 4. | Dalgard O, Egeland A, Ervik R, Vilimas K, Skaug K, Steen TW. [Risk factors for hepatitis C among injecting drug users in Oslo]. Tidsskr Nor Laegeforen. 2009;129:101-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Duberg A, Janzon R, Bäck E, Ekdahl K, Blaxhult A. The epidemiology of hepatitis C virus infection in Sweden. Euro Surveill. 2008;13:18882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Christensen PB, Hay G, Jepsen P, Omland LH, Just SA, Krarup HB, Weis N, Obel N, Cowan S. Hepatitis C prevalence in Denmark -an estimate based on multiple national registers. BMC Infect Dis. 2012;12:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Wiese M, Grüngreiff K, Güthoff W, Lafrenz M, Oesen U, Porst H; East German Hepatitis C Study Group. Outcome in a hepatitis C (genotype 1b) single source outbreak in Germany--a 25-year multicenter study. J Hepatol. 2005;43:590-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 638] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 9. | Thomas DL, Astemborski J, Rai RM, Anania FA, Schaeffer M, Galai N, Nolt K, Nelson KE, Strathdee SA, Johnson L, Laeyendecker O, Boitnott J, Wilson LE, Vlahov D. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284:450-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 782] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 10. | Freeman AJ, Dore GJ, Law MG, Thorpe M, Von Overbeck J, Lloyd AR, Marinos G, Kaldor JM. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology. 2001;34:809-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 425] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 11. | Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2168] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 12. | Ikeda K, Saitoh S, Suzuki Y, Kobayashi M, Tsubota A, Koida I, Arase Y, Fukuda M, Chayama K, Murashima N, Kumada H. Disease progression and hepatocellular carcinogenesis in patients with chronic viral hepatitis: a prospective observation of 2215 patients. J Hepatol. 1998;28:930-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 315] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 13. | Simmonds P. The origin of hepatitis C virus. Curr Top Microbiol Immunol. 2013;369:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | André P, Komurian-Pradel F, Deforges S, Perret M, Berland JL, Sodoyer M, Pol S, Bréchot C, Paranhos-Baccalà G, Lotteau V. Characterization of low- and very-low-density hepatitis C virus RNA-containing particles. J Virol. 2002;76:6919-6928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 516] [Article Influence: 21.5] [Reference Citation Analysis (17)] |
| 15. | Penin F, Dubuisson J, Rey FA, Moradpour D, Pawlotsky JM. Structural biology of hepatitis C virus. Hepatology. 2004;39:5-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 466] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 16. | Moradpour D, Penin F. Hepatitis C virus proteins: from structure to function. Curr Top Microbiol Immunol. 2013;369:113-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 17. | Lohmann V. Hepatitis C virus RNA replication. Curr Top Microbiol Immunol. 2013;369:167-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Kim AY, Kuntzen T, Timm J, Nolan BE, Baca MA, Reyor LL, Berical AC, Feller AJ, Johnson KL, Schulze zur Wiesch J, Robbins GK, Chung RT, Walker BD, Carrington M, Allen TM, Lauer GM. Spontaneous control of HCV is associated with expression of HLA-B 57 and preservation of targeted epitopes. Gastroenterology. 2011;140:686-696.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Grebely J, Page K, Sacks-Davis R, van der Loeff MS, Rice TM, Bruneau J, Morris MD, Hajarizadeh B, Amin J, Cox AL, Kim AY, McGovern BH, Schinkel J, George J, Shoukry NH, Lauer GM, Maher L, Lloyd AR, Hellard M, Dore GJ, Prins M; InC3 Study Group. The effects of female sex, viral genotype, and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology. 2014;59:109-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 308] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 20. | Dolganiuc A, Chang S, Kodys K, Mandrekar P, Bakis G, Cormier M, Szabo G. Hepatitis C virus (HCV) core protein-induced, monocyte-mediated mechanisms of reduced IFN-alpha and plasmacytoid dendritic cell loss in chronic HCV infection. J Immunol. 2006;177:6758-6768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 168] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Dolganiuc A, Kodys K, Kopasz A, Marshall C, Do T, Romics L Jr, Mandrekar P, Zapp M, Szabo G. Hepatitis C virus core and nonstructural protein 3 proteins induce pro- and anti-inflammatory cytokines and inhibit dendritic cell differentiation. J Immunol. 2003;170:5615-5624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 192] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 22. | McGuinness PH, Painter D, Davies S, McCaughan GW. Increases in intrahepatic CD68 positive cells, MAC387 positive cells, and proinflammatory cytokines (particularly interleukin 18) in chronic hepatitis C infection. Gut. 2000;46:260-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 145] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Shrivastava S, Mukherjee A, Ray R, Ray RB. Hepatitis C virus induces interleukin-1β (IL-1β)/IL-18 in circulatory and resident liver macrophages. J Virol. 2013;87:12284-12290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 24. | Wedemeyer H, He XS, Nascimbeni M, Davis AR, Greenberg HB, Hoofnagle JH, Liang TJ, Alter H, Rehermann B. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol. 2002;169:3447-3458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 494] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 25. | Missale G, Bertoni R, Lamonaca V, Valli A, Massari M, Mori C, Rumi MG, Houghton M, Fiaccadori F, Ferrari C. Different clinical behaviors of acute hepatitis C virus infection are associated with different vigor of the anti-viral cell-mediated immune response. J Clin Invest. 1996;98:706-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 490] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 26. | Maher JJ. Interactions between hepatic stellate cells and the immune system. Semin Liver Dis. 2001;21:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 144] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3381] [Cited by in RCA: 4218] [Article Influence: 200.9] [Reference Citation Analysis (11)] |
| 28. | Poynard T, Marcellin P, Lee SS, Niederau C, Minuk GS, Ideo G, Bain V, Heathcote J, Zeuzem S, Trepo C, Albrecht J. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet. 1998;352:1426-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1667] [Cited by in RCA: 1640] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 29. | Shiffman ML, Hofmann CM, Luketic VA, Sanyal AJ, Contos MJ, Mills AS. Improved sustained response following treatment of chronic hepatitis C by gradual reduction in the interferon dose. Hepatology. 1996;24:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Idilman R, De Maria N, Colantoni A, Dokmeci A, Van Thiel DH. Interferon treatment of cirrhotic patients with chronic hepatitis C. J Viral Hepat. 1997;4:81-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436:967-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 740] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 32. | Glue P, Fang JW, Rouzier-Panis R, Raffanel C, Sabo R, Gupta SK, Salfi M, Jacobs S. Pegylated interferon-alpha2b: pharmacokinetics, pharmacodynamics, safety, and preliminary efficacy data. Hepatitis C Intervention Therapy Group. Clin Pharmacol Ther. 2000;68:556-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 347] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 33. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4736] [Cited by in RCA: 4559] [Article Influence: 182.4] [Reference Citation Analysis (4)] |
| 34. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL Jr, Häussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4847] [Cited by in RCA: 4753] [Article Influence: 198.0] [Reference Citation Analysis (1)] |
| 35. | Thomas E, Ghany MG, Liang TJ. The application and mechanism of action of ribavirin in therapy of hepatitis C. Antivir Chem Chemother. 2012;23:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 36. | Poordad F, McCone J Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N, DiNubile MJ, Sniukiene V, Brass CA, Albrecht JK, Bronowicki JP; SPRINT-2 Investigators. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1996] [Cited by in RCA: 1984] [Article Influence: 132.3] [Reference Citation Analysis (1)] |
| 37. | Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N, Burroughs M, Brass CA, Albrecht JK, Esteban R; HCV RESPOND-2 Investigators. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1306] [Cited by in RCA: 1311] [Article Influence: 87.4] [Reference Citation Analysis (2)] |
| 38. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R, George J, Rizzetto M, Shouval D, Sola R, Terg RA, Yoshida EM, Adda N, Bengtsson L, Sankoh AJ, Kieffer TL, George S, Kauffman RS, Zeuzem S; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1905] [Cited by in RCA: 1865] [Article Influence: 124.3] [Reference Citation Analysis (4)] |
| 39. | Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, Focaccia R, Younossi Z, Foster GR, Horban A, Ferenci P, Nevens F, Müllhaupt B, Pockros P, Terg R, Shouval D, van Hoek B, Weiland O, Van Heeswijk R, De Meyer S, Luo D, Boogaerts G, Polo R, Picchio G, Beumont M; REALIZE Study Team. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1214] [Article Influence: 80.9] [Reference Citation Analysis (4)] |
| 40. | Gentile I, Maraolo AE, Buonomo AR, Zappulo E, Borgia G. The discovery of sofosbuvir: a revolution for therapy of chronic hepatitis C. Expert Opin Drug Discov. 2015;10:1363-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Reddy KR, Lim JK, Kuo A, Di Bisceglie AM, Galati JS, Morelli G, Everson GT, Kwo PY, Brown RS Jr, Sulkowski MS, Akuschevich L, Lok AS, Pockros PJ, Vainorius M, Terrault NA, Nelson DR, Fried MW, Manns MP; HCV-TARGET Study Group. All-oral direct-acting antiviral therapy in HCV-advanced liver disease is effective in real-world practice: observations through HCV-TARGET database. Aliment Pharmacol Ther. 2017;45:115-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 42. | Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, Nahass R, Ghalib R, Gitlin N, Herring R, Lalezari J, Younes ZH, Pockros PJ, Di Bisceglie AM, Arora S, Subramanian GM, Zhu Y, Dvory-Sobol H, Yang JC, Pang PS, Symonds WT, McHutchison JG, Muir AJ, Sulkowski M, Kwo P; ION-2 Investigators. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1065] [Cited by in RCA: 1066] [Article Influence: 88.8] [Reference Citation Analysis (1)] |
| 43. | Reddy KR, Bourlière M, Sulkowski M, Omata M, Zeuzem S, Feld JJ, Lawitz E, Marcellin P, Welzel TM, Hyland R, Ding X, Yang J, Knox S, Pang P, Dvory-Sobol H, Subramanian GM, Symonds W, McHutchison JG, Mangia A, Gane E, Mizokami M, Pol S, Afdhal N. Ledipasvir and sofosbuvir in patients with genotype 1 hepatitis C virus infection and compensated cirrhosis: An integrated safety and efficacy analysis. Hepatology. 2015;62:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 201] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 44. | Poordad F, Schiff ER, Vierling JM, Landis C, Fontana RJ, Yang R, McPhee F, Hughes EA, Noviello S, Swenson ES. Daclatasvir with sofosbuvir and ribavirin for hepatitis C virus infection with advanced cirrhosis or post-liver transplantation recurrence. Hepatology. 2016;63:1493-1505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 348] [Cited by in RCA: 348] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 45. | Aqel BA, Pungpapong S, Leise M, Werner KT, Chervenak AE, Watt KD, Murphy JL, Ryland K, Keaveny AP, McLemore R, Vargas HE. Multicenter experience using simeprevir and sofosbuvir with or without ribavirin to treat hepatitis C genotype 1 in patients with cirrhosis. Hepatology. 2015;62:1004-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 46. | Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, Shiffman ML, Wedemeyer H, Berg T, Yoshida EM, Forns X, Lovell SS, Da Silva-Tillmann B, Collins CA, Campbell AL, Podsadecki T, Bernstein B. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 685] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 47. | Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, Tam E, Marinho RT, Tsai N, Nyberg A, Box TD, Younes Z, Enayati P, Green S, Baruch Y, Bhandari BR, Caruntu FA, Sepe T, Chulanov V, Janczewska E, Rizzardini G, Gervain J, Planas R, Moreno C, Hassanein T, Xie W, King M, Podsadecki T, Reddy KR; PEARL-III Study; PEARL-IV Study. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 548] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 48. | D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1892] [Cited by in RCA: 2215] [Article Influence: 110.8] [Reference Citation Analysis (3)] |
| 49. | Niederau C, Lange S, Heintges T, Erhardt A, Buschkamp M, Hürter D, Nawrocki M, Kruska L, Hensel F, Petry W, Häussinger D. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology. 1998;28:1687-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 440] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 50. | Bruno S, Zuin M, Crosignani A, Rossi S, Zadra F, Roffi L, Borzio M, Redaelli A, Chiesa A, Silini EM, Almasio PL, Maisonneuve P. Predicting mortality risk in patients with compensated HCV-induced cirrhosis: a long-term prospective study. Am J Gastroenterol. 2009;104:1147-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 51. | Marcellin P, Asselah T, Boyer N. Fibrosis and disease progression in hepatitis C. Hepatology. 2002;36:S47-S56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 52. | Heydtmann M. Macrophages in hepatitis B and hepatitis C virus infections. J Virol. 2009;83:2796-2802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 53. | Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146:1513-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 777] [Article Influence: 64.8] [Reference Citation Analysis (2)] |
| 54. | Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143:1158-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 530] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 55. | Møller HJ, Hald K, Moestrup SK. Characterization of an enzyme-linked immunosorbent assay for soluble CD163. Scand J Clin Lab Invest. 2002;62:293-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 146] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 56. | Rødgaard-Hansen S, Rafique A, Christensen PA, Maniecki MB, Sandahl TD, Nexø E, Møller HJ. A soluble form of the macrophage-related mannose receptor (MR/CD206) is present in human serum and elevated in critical illness. Clin Chem Lab Med. 2014;52:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 57. | Martinez-Pomares L. The mannose receptor. J Leukoc Biol. 2012;92:1177-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 423] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 58. | Rødgaard-Hansen S, Rafique A, Weis N, Wejse C, Nielsen H, Pedersen SS, Møller HJ, Kronborg G. Increased concentrations of the soluble mannose receptor in serum from patients with pneumococcal bacteraemia, and prediction of survival. Infect Dis (Lond). 2015;47:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 59. | Holland-Fischer P, Grønbæk H, Sandahl TD, Moestrup SK, Riggio O, Ridola L, Aagaard NK, Møller HJ, Vilstrup H. Kupffer cells are activated in cirrhotic portal hypertension and not normalised by TIPS. Gut. 2011;60:1389-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 60. | Grønbæk H, Rødgaard-Hansen S, Aagaard NK, Arroyo V, Moestrup SK, Garcia E, Solà E, Domenicali M, Piano S, Vilstrup H, Møller HJ; CANONIC study investigators of the EASL-CLIF Consortium. Macrophage activation markers predict mortality in patients with liver cirrhosis without or with acute-on-chronic liver failure (ACLF). J Hepatol. 2016;64:813-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 61. | Grønbaek H, Sandahl TD, Mortensen C, Vilstrup H, Møller HJ, Møller S. Soluble CD163, a marker of Kupffer cell activation, is related to portal hypertension in patients with liver cirrhosis. Aliment Pharmacol Ther. 2012;36:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 62. | Laursen TL, Rødgaard-Hansen S, Møller HJ, Mortensen C, Karlsen S, Nielsen DT, Frevert S, Clemmesen JO, Møller S, Jensen JS, Bendtsen F, Grønbaek H. The soluble mannose receptor is released from the liver in cirrhotic patients, but is not associated with bacterial translocation. Liver Int. 2017;37:569-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 63. | Sandahl TD, Støy SH, Laursen TL, Rødgaard-Hansen S, Møller HJ, Møller S, Vilstrup H, Grønbæk H. The soluble mannose receptor (sMR) is elevated in alcoholic liver disease and associated with disease severity, portal hypertension, and mortality in cirrhosis patients. PLoS One. 2017;12:e0189345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 64. | Rode A, Nicoll A, Møller HJ, Lim L, Angus PW, Kronborg I, Arachchi N, Gorelik A, Liew D, Kazankov K, Vilstrup H, Grønbæk H. Hepatic macrophage activation predicts clinical decompensation in chronic liver disease. Gut. 2013;62:1231-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 65. | Waidmann O, Köberle V, Bettinger D, Trojan J, Zeuzem S, Schultheiß M, Kronenberger B, Piiper A. Diagnostic and prognostic significance of cell death and macrophage activation markers in patients with hepatocellular carcinoma. J Hepatol. 2013;59:769-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 66. | Waidmann O, Brunner F, Herrmann E, Zeuzem S, Piiper A, Kronenberger B. Macrophage activation is a prognostic parameter for variceal bleeding and overall survival in patients with liver cirrhosis. J Hepatol. 2013;58:956-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 67. | Kazankov K, Rode A, Simonsen K, Villadsen GE, Nicoll A, Møller HJ, Lim L, Angus P, Kronborg I, Arachchi N, Gorelik A, Liew D, Vilstrup H, Frystyk J, Grønbæk H. Macrophage activation marker soluble CD163 may predict disease progression in hepatocellular carcinoma. Scand J Clin Lab Invest. 2016;76:64-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 68. | Kazankov K, Tordjman J, Møller HJ, Vilstrup H, Poitou C, Bedossa P, Bouillot JL, Clement K, Grønbaek H. Macrophage activation marker soluble CD163 and non-alcoholic fatty liver disease in morbidly obese patients undergoing bariatric surgery. J Gastroenterol Hepatol. 2015;30:1293-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 69. | Kazankov K, Barrera F, Møller HJ, Bibby BM, Vilstrup H, George J, Grønbaek H. Soluble CD163, a macrophage activation marker, is independently associated with fibrosis in patients with chronic viral hepatitis B and C. Hepatology. 2014;60:521-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 70. | Dultz G, Gerber L, Farnik H, Berger A, Vermehren J, Pleli T, Zeuzem S, Piiper A, Kronenberger B, Waidmann O. Soluble CD163 is an indicator of liver inflammation and fibrosis in patients chronically infected with the hepatitis B virus. J Viral Hepat. 2015;22:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 71. | Andersen ES, Rødgaard-Hansen S, Moessner B, Christensen PB, Møller HJ, Weis N. Macrophage-related serum biomarkers soluble CD163 (sCD163) and soluble mannose receptor (sMR) to differentiate mild liver fibrosis from cirrhosis in patients with chronic hepatitis C: a pilot study. Eur J Clin Microbiol Infect Dis. 2014;33:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 72. | Laursen TL, Wong GL, Kazankov K, Sandahl T, Møller HJ, Hamilton-Dutoit S, George J, Chan HL, Grønbaek H. Soluble CD163 and mannose receptor associate with chronic hepatitis B activity and fibrosis and decline with treatment. J Gastroenterol Hepatol. 2018;33:484-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 73. | Dultz G, Gerber L, Zeuzem S, Sarrazin C, Waidmann O. The macrophage activation marker CD163 is associated with IL28B genotype and hepatic inflammation in chronic hepatitis C virus infected patients. J Viral Hepat. 2016;23:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 74. | Xu F, Moorman AC, Tong X, Gordon SC, Rupp LB, Lu M, Teshale EH, Spradling PR, Boscarino JA, Trinacty CM, Schmidt MA, Holmberg SD; Chronic Hepatitis Cohort Study (CHeCS) Investigators, Holmberg SD, Teshale EH, Spradling PR, Moorman AC, Xing J, Tong X, Xu F, Gordon SC, Nerenz DR, Lu M, Lamerato L, Wang Y, Rupp LB, Akkerman N, Oja-Tebbe N, Zhang T, Li J, Sitarik A, Larkin D, Boscarino JA, Daar ZS, Curry PJ, Smith RE, Vijayadeva V, Parker JV, Schmidt MA, Donald JL, Keast EM. All-Cause Mortality and Progression Risks to Hepatic Decompensation and Hepatocellular Carcinoma in Patients Infected With Hepatitis C Virus. Clin Infect Dis. 2016;62:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 75. | Bruden DJT, McMahon BJ, Townshend-Bulson L, Gounder P, Gove J, Plotnik J, Homan C, Hewitt A, Barbour Y, Spradling PR, Simons BC, McArdle S, Bruce M. Risk of end-stage liver disease, hepatocellular carcinoma, and liver-related death by fibrosis stage in the hepatitis C Alaska Cohort. Hepatology. 2017;66:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 76. | Scheuer PJ, Ashrafzadeh P, Sherlock S, Brown D, Dusheiko GM. The pathology of hepatitis C. Hepatology. 1992;15:567-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 344] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 77. | Dienes HP, Popper H, Arnold W, Lobeck H. Histologic observations in human hepatitis non-A, non-B. Hepatology. 1982;2:562-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 110] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 78. | Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1581] [Article Influence: 65.9] [Reference Citation Analysis (0)] |
| 79. | Maharaj B, Maharaj RJ, Leary WP, Cooppan RM, Naran AD, Pirie D, Pudifin DJ. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet. 1986;1:523-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 464] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 80. | Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1408] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 81. | Cholongitas E, Senzolo M, Standish R, Marelli L, Quaglia A, Patch D, Dhillon AP, Burroughs AK. A systematic review of the quality of liver biopsy specimens. Am J Clin Pathol. 2006;125:710-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 82. | Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1761] [Article Influence: 70.4] [Reference Citation Analysis (1)] |
| 83. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3344] [Article Influence: 145.4] [Reference Citation Analysis (0)] |
| 84. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3814] [Article Influence: 190.7] [Reference Citation Analysis (0)] |
| 85. | Lichtinghagen R, Pietsch D, Bantel H, Manns MP, Brand K, Bahr MJ. The Enhanced Liver Fibrosis (ELF) score: normal values, influence factors and proposed cut-off values. J Hepatol. 2013;59:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 277] [Article Influence: 21.3] [Reference Citation Analysis (10)] |
| 86. | Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T; MULTIVIRC Group. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1038] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 87. | Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review. Hepatology. 2007;46:912-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 293] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 88. | Poynard T, Morra R, Halfon P, Castera L, Ratziu V, Imbert-Bismut F, Naveau S, Thabut D, Lebrec D, Zoulim F, Bourliere M, Cacoub P, Messous D, Munteanu M, de Ledinghen V. Meta-analyses of FibroTest diagnostic value in chronic liver disease. BMC Gastroenterol. 2007;7:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 229] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 89. | Ragazzo TG, Paranagua-Vezozzo D, Lima FR, de Campos Mazo DF, Pessoa MG, Oliveira CP, Alves VAF, Carrilho FJ. Accuracy of transient elastography-FibroScan®, acoustic radiation force impulse (ARFI) imaging, the enhanced liver fibrosis (ELF) test, APRI, and the FIB-4 index compared with liver biopsy in patients with chronic hepatitis C. Clinics (Sao Paulo). 2017;72:516-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 90. | Yen YH, Kuo FY, Kee KM, Chang KC, Tsai MC, Hu TH, Lu SN, Wang JH, Hung CH, Chen CH. APRI and FIB-4 in the evaluation of liver fibrosis in chronic hepatitis C patients stratified by AST level. PLoS One. 2018;13:e0199760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 91. | Petersen JR, Stevenson HL, Kasturi KS, Naniwadekar A, Parkes J, Cross R, Rosenberg WM, Xiao SY, Snyder N. Evaluation of the aspartate aminotransferase/platelet ratio index and enhanced liver fibrosis tests to detect significant fibrosis due to chronic hepatitis C. J Clin Gastroenterol. 2014;48:370-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 92. | Martinez SM, Fernández-Varo G, González P, Sampson E, Bruguera M, Navasa M, Jiménez W, Sánchez-Tapias JM, Forns X. Assessment of liver fibrosis before and after antiviral therapy by different serum marker panels in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2011;33:138-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 93. | Nunes D, Fleming C, Offner G, Craven D, Fix O, Heeren T, Koziel MJ, Graham C, Tumilty S, Skolnik P, Stuver S, Horsburgh CR Jr, Cotton D. Noninvasive markers of liver fibrosis are highly predictive of liver-related death in a cohort of HCV-infected individuals with and without HIV infection. Am J Gastroenterol. 2010;105:1346-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 94. | European Association for Study of Liver. Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1177] [Cited by in RCA: 1381] [Article Influence: 125.5] [Reference Citation Analysis (1)] |
| 95. | Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 1100] [Article Influence: 61.1] [Reference Citation Analysis (1)] |
| 96. | Bota S, Sporea I, Sirli R, Popescu A, Danila M, Costachescu D. Intra- and interoperator reproducibility of acoustic radiation force impulse (ARFI) elastography--preliminary results. Ultrasound Med Biol. 2012;38:1103-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 97. | Boursier J, Isselin G, Fouchard-Hubert I, Oberti F, Dib N, Lebigot J, Bertrais S, Gallois Y, Calès P, Aubé C. Acoustic radiation force impulse: a new ultrasonographic technology for the widespread noninvasive diagnosis of liver fibrosis. Eur J Gastroenterol Hepatol. 2010;22:1074-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 98. | Castéra L, Foucher J, Bernard PH, Carvalho F, Allaix D, Merrouche W, Couzigou P, de Lédinghen V. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51:828-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 426] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 99. | Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, Bonino F, Brunetto MR. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007;14:360-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 512] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 100. | Arena U, Vizzutti F, Corti G, Ambu S, Stasi C, Bresci S, Moscarella S, Boddi V, Petrarca A, Laffi G, Marra F, Pinzani M. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 582] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 101. | Millonig G, Reimann FM, Friedrich S, Fonouni H, Mehrabi A, Büchler MW, Seitz HK, Mueller S. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 471] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 102. | Kjærgaard M, Thiele M, Jansen C, Stæhr Madsen B, Görtzen J, Strassburg C, Trebicka J, Krag A. High risk of misinterpreting liver and spleen stiffness using 2D shear-wave and transient elastography after a moderate or high calorie meal. PLoS One. 2017;12:e0173992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 103. | Popescu A, Bota S, Sporea I, Sirli R, Danila M, Racean S, Suseanu D, Gradinaru O, Ivascu Siegfried C. The influence of food intake on liver stiffness values assessed by acoustic radiation force impulse elastography-preliminary results. Ultrasound Med Biol. 2013;39:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 104. | Bota S, Sporea I, Peck-Radosavljevic M, Sirli R, Tanaka H, Iijima H, Saito H, Ebinuma H, Lupsor M, Badea R, Fierbinteanu-Braticevici C, Petrisor A, Friedrich-Rust M, Sarrazin C, Takahashi H, Ono N, Piscaglia F, Marinelli S, D'Onofrio M, Gallotti A, Salzl P, Popescu A, Danila M. The influence of aminotransferase levels on liver stiffness assessed by Acoustic Radiation Force Impulse Elastography: a retrospective multicentre study. Dig Liver Dis. 2013;45:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 105. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1796] [Cited by in RCA: 1861] [Article Influence: 88.6] [Reference Citation Analysis (2)] |
| 106. | Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC, Beaugrand M. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1090] [Cited by in RCA: 1097] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 107. | Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, Colombo M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 661] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 108. | Fierbinteanu-Braticevici C, Andronescu D, Usvat R, Cretoiu D, Baicus C, Marinoschi G. Acoustic radiation force imaging sonoelastography for noninvasive staging of liver fibrosis. World J Gastroenterol. 2009;15:5525-5532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 133] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (2)] |
| 109. | Friedrich-Rust M, Wunder K, Kriener S, Sotoudeh F, Richter S, Bojunga J, Herrmann E, Poynard T, Dietrich CF, Vermehren J, Zeuzem S, Sarrazin C. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology. 2009;252:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 468] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 110. | Ocker M, Ganslmayer M, Zopf S, Gahr S, Janson C, Hahn EG, Herold C. Improvement of quantitative testing of liver function in patients with chronic hepatitis C after installment of antiviral therapy. World J Gastroenterol. 2005;11:5521-5524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 111. | Herold C, Heinz R, Radespiel-Tröger M, Schneider HT, Schuppan D, Hahn EG. Quantitative testing of liver function in patients with cirrhosis due to chronic hepatitis C to assess disease severity. Liver. 2001;21:26-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 112. | Reichen J, Solioz M, Bühler H, Gonvers JJ, Knoblauch M, Lavanchy D, Malé PJ, Meyer B, Schmid M, Bianchi L. Low-dose interferon in chronic hepatitis non-A/non-B: effects on quantitative liver function and structure in a randomized, controlled multicenter trial. Clin Investig. 1993;71:888-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 113. | Bianchi G, Marchesini G, Vilstrup H, Fabbri A, De Mitri MS, Zoli M, Pisi E. Hepatic amino-nitrogen clearance to urea-nitrogen in control subjects and in patients with cirrhosis: a simplified method. Hepatology. 1991;13:460-466. [PubMed] |
| 114. | Jepsen P, Vilstrup H, Ott P, Keiding S, Andersen PK, Tygstrup N. The galactose elimination capacity and mortality in 781 Danish patients with newly-diagnosed liver cirrhosis: a cohort study. BMC Gastroenterol. 2009;9:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 115. | Merkel C, Marchesini G, Fabbri A, Bianco S, Bianchi G, Enzo E, Sacerdoti D, Zoli M, Gatta A. The course of galactose elimination capacity in patients with alcoholic cirrhosis: possible use as a surrogate marker for death. Hepatology. 1996;24:820-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 116. | Schmidt LE, Ott P, Tygstrup N. Galactose elimination capacity as a prognostic marker in patients with severe acetaminophen-induced hepatotoxicity: 10 years' experience. Clin Gastroenterol Hepatol. 2004;2:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 117. | Glavind E, Aagaard NK, Gronbaek H, Orntoft NW, Vilstrup H, Thomsen KL. Time course of compromised urea synthesis in patients with alcoholic hepatitis. Scand J Gastroenterol. 2018;53:592-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 118. | Frey PA. The Leloir pathway: a mechanistic imperative for three enzymes to change the stereochemical configuration of a single carbon in galactose. FASEB J. 1996;10:461-470. [PubMed] |
| 119. | Tygstrup N. The galactose elimination capacity in control subjects and in patients with cirrhosis of the liver. Acta Med Scand. 1964;175:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 85] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 120. | Vilstrup H. Synthesis of urea after stimulation with amino acids: relation to liver function. Gut. 1980;21:990-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 99] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 121. | Meijer AJ, Lamers WH, Chamuleau RA. Nitrogen metabolism and ornithine cycle function. Physiol Rev. 1990;70:701-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 396] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 122. | Rafoth RJ, Onstad GR. Urea synthesis after oral protein ingestion in man. J Clin Invest. 1975;56:1170-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 51] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 123. | Sandahl TD, Aagaard NK, Thomsen KL, Grøfte T, Greisen J, Christiansen JS, Vilstrup H. Effects of insulin-like growth factor-I administration on in vivo regulation of urea synthesis in normal subjects and patients with cirrhosis. Liver Int. 2011;31:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 124. | Armuzzi A, Candelli M, Zocco MA, Andreoli A, De Lorenzo A, Nista EC, Miele L, Cremonini F, Cazzato IA, Grieco A, Gasbarrini G, Gasbarrini A. Review article: breath testing for human liver function assessment. Aliment Pharmacol Ther. 2002;16:1977-1996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 125. | Nielsen NC. Spectrophotometric determination of indocyanine green in plasma especially with a view to an improved correction for blank density. Scand J Clin Lab Invest. 1963;15:613-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 126. | D'Amico G, Garcia-Pagan JC, Luca A, Bosch J. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: a systematic review. Gastroenterology. 2006;131:1611-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 354] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 127. | Keiding S, Vilstrup H. Intrahepatic heterogeneity of hepatic venous pressure gradient in human cirrhosis. Scand J Gastroenterol. 2002;37:960-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 128. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2609] [Cited by in RCA: 2358] [Article Influence: 214.4] [Reference Citation Analysis (4)] |
| 129. | Das A, Dhiman RK, Saraswat VA, Verma M, Naik SR. Prevalence and natural history of subclinical hepatic encephalopathy in cirrhosis. J Gastroenterol Hepatol. 2001;16:531-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 205] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 130. | Amodio P, Del Piccolo F, Marchetti P, Angeli P, Iemmolo R, Caregaro L, Merkel C, Gerunda G, Gatta A. Clinical features and survivial of cirrhotic patients with subclinical cognitive alterations detected by the number connection test and computerized psychometric tests. Hepatology. 1999;29:1662-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 177] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 131. | Campagna F, Montagnese S, Schiff S, Ruzzoli M, Biancardi A, Iannizzi P, Pujatti PL, Angeli P, Gatta A, Merkel C, Leandro G, Mapelli D, Amodio P. Confounders in the detection of minimal hepatic encephalopathy: a neuropsychological and quantified EEG study. Liver Int. 2015;35:1524-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 132. | Lauridsen MM, Jepsen P, Vilstrup H. Critical flicker frequency and continuous reaction times for the diagnosis of minimal hepatic encephalopathy: a comparative study of 154 patients with liver disease. Metab Brain Dis. 2011;26:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 133. | Lauridsen MM, Schaffalitzky de Muckadell OB, Vilstrup H. Minimal hepatic encephalopathy characterized by parallel use of the continuous reaction time and portosystemic encephalopathy tests. Metab Brain Dis. 2015;30:1187-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 134. | Shiratori Y, Imazeki F, Moriyama M, Yano M, Arakawa Y, Yokosuka O, Kuroki T, Nishiguchi S, Sata M, Yamada G, Fujiyama S, Yoshida H, Omata M. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000;132:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 541] [Article Influence: 20.8] [Reference Citation Analysis (1)] |
| 135. | Toccaceli F, Laghi V, Capurso L, Koch M, Sereno S, Scuderi M; Italian Hepanet Group. Long-term liver histology improvement in patients with chronic hepatitis C and sustained response to interferon. J Viral Hepat. 2003;10:126-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 136. | Shiffman ML, Sterling RK, Contos M, Hubbard S, Long A, Luketic VA, Stravitz RT, Fuchs M, Sanyal AJ. Long term changes in liver histology following treatment of chronic hepatitis C virus. Ann Hepatol. 2014;13:340-349. [PubMed] |
| 137. | Schwabl P, Mandorfer M, Steiner S, Scheiner B, Chromy D, Herac M, Bucsics T, Hayden H, Grabmeier-Pfistershammer K, Ferlitsch A, Oberhuber G, Trauner M, Peck-Radosavljevic M, Reiberger T. Interferon-free regimens improve portal hypertension and histological necroinflammation in HIV/HCV patients with advanced liver disease. Aliment Pharmacol Ther. 2017;45:139-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 138. | Laursen TL, Siggaard CB, Kazankov K, Sandahl TD, Møller HJ, Tarp B, Kristensen LH, Laursen AL, Leutscher P, Grønbaek H. Time-dependent improvement of liver inflammation, fibrosis and metabolic liver function after successful direct-acting antiviral therapy of chronic hepatitis C. J Viral Hepat. 2020;27:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 139. | Lund Laursen T, Brøckner Siggard C, Kazankov K, Damgaard Sandahl T, Møller HJ, Ong A, Douglas MW, George J, Tarp B, Hagelskjaer Kristensen L, Lund Laursen A, Hiramatsu A, Nakahara T, Chayama K, Grønbaek H. Rapid and persistent decline in soluble CD163 with successful direct-acting antiviral therapy and associations with chronic hepatitis C histology. Scand J Gastroenterol. 2018;53:986-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 140. | Knop V, Hoppe D, Welzel T, Vermehren J, Herrmann E, Vermehren A, Friedrich-Rust M, Sarrazin C, Zeuzem S, Welker MW. Regression of fibrosis and portal hypertension in HCV-associated cirrhosis and sustained virologic response after interferon-free antiviral therapy. J Viral Hepat. 2016;23:994-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 128] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 141. | Elsharkawy A, Alem SA, Fouad R, El Raziky M, El Akel W, Abdo M, Tantawi O, AbdAllah M, Bourliere M, Esmat G. Changes in liver stiffness measurements and fibrosis scores following sofosbuvir based treatment regimens without interferon. J Gastroenterol Hepatol. 2017;32:1624-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 142. | Kobayashi N, Iijima H, Tada T, Kumada T, Yoshida M, Aoki T, Nishimura T, Nakano C, Takata R, Yoh K, Ishii A, Takashima T, Sakai Y, Aizawa N, Nishikawa H, Ikeda N, Iwata Y, Enomoto H, Hirota S, Fujimoto J, Nishiguchi S. Changes in liver stiffness and steatosis among patients with hepatitis C virus infection who received direct-acting antiviral therapy and achieved sustained virological response. Eur J Gastroenterol Hepatol. 2018;30:546-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 143. | Lens S, Alvarado-Tapias E, Mariño Z, Londoño MC, LLop E, Martinez J, Fortea JI, Ibañez L, Ariza X, Baiges A, Gallego A, Bañares R, Puente A, Albillos A, Calleja JL, Torras X, Hernández-Gea V, Bosch J, Villanueva C, Forns X, García-Pagán JC. Effects of All-Oral Anti-Viral Therapy on HVPG and Systemic Hemodynamics in Patients With Hepatitis C Virus-Associated Cirrhosis. Gastroenterology. 2017;153:1273-1283.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 212] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 144. | Singh S, Facciorusso A, Loomba R, Falck-Ytter YT. Magnitude and Kinetics of Decrease in Liver Stiffness After Antiviral Therapy in Patients With Chronic Hepatitis C: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;16:27-38.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 145. | Pockros P, Crissien-Martinez AM, Frenette C, Skillin C, Bao F, Du E, Pan JJ, Waalen J. Degree of liver fibrosis regression predicted by transient elastography after cure of chronic hepatitis C with direct acting antivirals is overestimated but confirmed by liver biopsy. J Hepatol. 2017;66:S108. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 146. | Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, Ling MH, Albrecht J. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 807] [Article Influence: 33.6] [Reference Citation Analysis (1)] |
| 147. | D'Ambrosio R, Aghemo A, Rumi MG, Ronchi G, Donato MF, Paradis V, Colombo M, Bedossa P. A morphometric and immunohistochemical study to assess the benefit of a sustained virological response in hepatitis C virus patients with cirrhosis. Hepatology. 2012;56:532-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 325] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 148. | Manne V, Akhtar E, Saab S. Cirrhosis regression in patients with viral hepatitis B and C: a systematic review. J Clin Gastroenterol. 2014;48:e76-e84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 149. | Ghany MG, Kleiner DE, Alter H, Doo E, Khokar F, Promrat K, Herion D, Park Y, Liang TJ, Hoofnagle JH. Progression of fibrosis in chronic hepatitis C. Gastroenterology. 2003;124:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 272] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 150. | Hoefs JC, Shiffman ML, Goodman ZD, Kleiner DE, Dienstag JL, Stoddard AM; HALT-C Trial Group. Rate of progression of hepatic fibrosis in patients with chronic hepatitis C: results from the HALT-C Trial. Gastroenterology. 2011;141:900-908.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 151. | Stintzing S, Schmitt C, Ocker M, Ganslmayer M, Zopf S, Gahr S, Hahn EG, Herold C. Liver function under interferon/ribavirin therapy of chronic hepatitis C. Hepatogastroenterology. 2009;56:462-465. [PubMed] |
| 152. | Everson GT, Shiffman ML, Hoefs JC, Morgan TR, Sterling RK, Wagner DA, Lauriski S, Curto TM, Stoddard A, Wright EC; HALT-C Trial Group. Quantitative liver function tests improve the prediction of clinical outcomes in chronic hepatitis C: results from the Hepatitis C Antiviral Long-term Treatment Against Cirrhosis Trial. Hepatology. 2012;55:1019-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 153. | Herold C, Heinz R, Niedobitek G, Schneider T, Hahn EG, Schuppan D. Quantitative testing of liver function in relation to fibrosis in patients with chronic hepatitis B and C. Liver. 2001;21:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 154. | Chang ML, Cheng ML, Chang SW, Tang HY, Chiu CT, Yeh CT, Shiao MS. Recovery of pan-genotypic and genotype-specific amino acid alterations in chronic hepatitis C after viral clearance: transition at the crossroad of metabolism and immunity. Amino Acids. 2017;49:291-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 155. | El-Kamary SS, Jhaveri R, Shardell MD. All-cause, liver-related, and non-liver-related mortality among HCV-infected individuals in the general US population. Clin Infect Dis. 2011;53:150-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 156. | Omland LH, Krarup H, Jepsen P, Georgsen J, Harritshøj LH, Riisom K, Jacobsen SE, Schouenborg P, Christensen PB, Sørensen HT, Obel N; DANVIR Cohort Study. Mortality in patients with chronic and cleared hepatitis C viral infection: a nationwide cohort study. J Hepatol. 2010;53:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 157. | Younossi ZM, Zheng L, Stepanova M, Venkatesan C, Mir HM. Moderate, excessive or heavy alcohol consumption: each is significantly associated with increased mortality in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2013;37:703-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 158. | van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, Duarte-Rojo A, Heathcote EJ, Manns MP, Kuske L, Zeuzem S, Hofmann WP, de Knegt RJ, Hansen BE, Janssen HL. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584-2593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1165] [Cited by in RCA: 1176] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 159. | Bruno S, Di Marco V, Iavarone M, Roffi L, Crosignani A, Calvaruso V, Aghemo A, Cabibbo G, Viganò M, Boccaccio V, Craxí A, Colombo M, Maisonneuve P. Survival of patients with HCV cirrhosis and sustained virologic response is similar to the general population. J Hepatol. 2016;64:1217-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 160. | Bruno S, Maisonneuve P. Reply to: "Methodological considerations when calculating person-time at risk for patients with chronic hepatitis C undergoing antiviral treatment". J Hepatol. 2017;67:428-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 161. | Innes H, McDonald S, Hayes P, Dillon JF, Allen S, Goldberg D, Mills PR, Barclay ST, Wilks D, Valerio H, Fox R, Bhattacharyya D, Kennedy N, Morris J, Fraser A, Stanley A, Bramley P, Hutchinson SJ. Mortality in hepatitis C patients who achieve a sustained viral response compared to the general population. J Hepatol. 2017;66:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 162. | Fattovich G, Giustina G, Degos F, Diodati G, Tremolada F, Nevens F, Almasio P, Solinas A, Brouwer JT, Thomas H, Realdi G, Corrocher R, Schalm SW. Effectiveness of interferon alfa on incidence of hepatocellular carcinoma and decompensation in cirrhosis type C. European Concerted Action on Viral Hepatitis (EUROHEP). J Hepatol. 1997;27:201-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 144] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 163. | Iacobellis A, Siciliano M, Perri F, Annicchiarico BE, Leandro G, Caruso N, Accadia L, Bombardieri G, Andriulli A. Peginterferon alfa-2b and ribavirin in patients with hepatitis C virus and decompensated cirrhosis: a controlled study. J Hepatol. 2007;46:206-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 164. | van der Meer AJ, Feld JJ, Hofer H, Almasio PL, Calvaruso V, Fernández-Rodríguez CM, Aleman S, Ganne-Carrié N, D'Ambrosio R, Pol S, Trapero-Marugan M, Maan R, Moreno-Otero R, Mallet V, Hultcrantz R, Weiland O, Rutter K, Di Marco V, Alonso S, Bruno S, Colombo M, de Knegt RJ, Veldt BJ, Hansen BE, Janssen HLA. Risk of cirrhosis-related complications in patients with advanced fibrosis following hepatitis C virus eradication. J Hepatol. 2017;66:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 218] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 165. | Carrat F, Fontaine H, Dorival C, Simony M, Diallo A, Hezode C, De Ledinghen V, Larrey D, Haour G, Bronowicki JP, Zoulim F, Asselah T, Marcellin P, Thabut D, Leroy V, Tran A, Habersetzer F, Samuel D, Guyader D, Chazouilleres O, Mathurin P, Metivier S, Alric L, Riachi G, Gournay J, Abergel A, Cales P, Ganne N, Loustaud-Ratti V, D'Alteroche L, Causse X, Geist C, Minello A, Rosa I, Gelu-Simeon M, Portal I, Raffi F, Bourliere M, Pol S; French ANRS CO22 Hepather cohort. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet. 2019;393:1453-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 483] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 166. | Backus LI, Belperio PS, Shahoumian TA, Mole LA. Impact of Sustained Virologic Response with Direct-Acting Antiviral Treatment on Mortality in Patients with Advanced Liver Disease. Hepatology. 2019;69:487-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 169] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 167. | Kozbial K, Moser S, Al-Zoairy R, Schwarzer R, Datz C, Stauber R, Laferl H, Strasser M, Beinhardt S, Stättermayer AF, Gschwantler M, Zoller H, Maieron A, Graziadei I, Trauner M, Steindl-Munda P, Hofer H, Ferenci P. Follow-up of sustained virological responders with hepatitis C and advanced liver disease after interferon/ribavirin-free treatment. Liver Int. 2018;38:1028-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 168. | Butt AA, Yan P, Simon TG, Abou-Samra AB. Effect of Paritaprevir/Ritonavir/Ombitasvir/Dasabuvir and Ledipasvir/Sofosbuvir Regimens on Survival Compared With Untreated Hepatitis C Virus-Infected Persons: Results From ERCHIVES. Clin Infect Dis. 2017;65:1006-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 169. | Backus LI, Belperio PS, Shahoumian TA, Mole LA. Direct-acting antiviral sustained virologic response: Impact on mortality in patients without advanced liver disease. Hepatology. 2018;68:827-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 170. | Fernández Carrillo C, Lens S, Llop E, Pascasio JM, Crespo J, Arenas J, Fernández I, Baliellas C, Carrión JA, de la Mata M, Buti M, Castells L, Albillos A, Romero M, Turnes J, Pons C, Moreno-Planas JM, Moreno-Palomares JJ, Fernández-Rodriguez C, García-Samaniego J, Prieto M, Fernández Bermejo M, Salmerón J, Badia E, Salcedo M, Herrero JI, Granados R, Blé M, Mariño Z, Calleja JL. Treatment of hepatitis C virus infection in patients with cirrhosis and predictive value of model for end-stage liver disease: Analysis of data from the Hepa-C registry. Hepatology. 2017;65:1810-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 171. | Vergniol J, Foucher J, Terrebonne E, Bernard PH, le Bail B, Merrouche W, Couzigou P, de Ledinghen V. Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology. 2011;140:1970-1979, 1979.e1-1979.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 312] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 172. | Cepeda JA, Thomas DL, Astemborski J, Sulkowski MS, Kirk GD, Mehta SH. Increased Mortality Among Persons With Chronic Hepatitis C With Moderate or Severe Liver Disease: A Cohort Study. Clin Infect Dis. 2017;65:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 173. | Salerno F, Borroni G, Moser P, Sangiovanni A, Almasio P, Budillon G, Capuano G, Muraca M, Marchesini G, Bernardi M, Marenco G, Molino G, Rossaro L, Solinas A, Ascione A. Prognostic value of the galactose test in predicting survival of patients with cirrhosis evaluated for liver transplantation. A prospective multicenter Italian study. AISF Group for the Study of Liver Transplantation. Associazione Italiana per lo Studio del Fegato. J Hepatol. 1996;25:474-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 174. | Merkel C, Gatta A, Zoli M, Bolognesi M, Angeli P, Iervese T, Marchesini G, Ruol A. Prognostic value of galactose elimination capacity, aminopyrine breath test, and ICG clearance in patients with cirrhosis. Comparison with the Pugh score. Dig Dis Sci. 1991;36:1197-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 175. | Albers I, Hartmann H, Bircher J, Creutzfeldt W. Superiority of the Child-Pugh classification to quantitative liver function tests for assessing prognosis of liver cirrhosis. Scand J Gastroenterol. 1989;24:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 184] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 176. | Addario L, Scaglione G, Tritto G, Di Costanzo GG, De Luca M, Lampasi F, Galeota Lanza A, Picciotto FP, Tartaglione MT, Utech W, Macr M, Giannelli E, Ascione A. Prognostic value of quantitative liver function tests in viral cirrhosis: a prospective study. Eur J Gastroenterol Hepatol. 2006;18:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 177. | Ohnishi M, Higuchi A, Matsumura H, Arakawa Y, Nakamura H, Nirei K, Yamamoto T, Yamagami H, Ogawa M, Gotoda T, Matsuoka S, Nakajima N, Sugitani M, Moriyama M, Murayama H. Involvement of Ornithine Carbamoyltransferase in the Progression of Chronic Hepatitis C and Liver Cirrhosis. Int J Med Sci. 2017;14:629-638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 178. | Maier KP, Talke H, Gerok W. Activities of urea-cycle enzymes in chronic liver disease. Klin Wochenschr. 1979;57:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 179. | El-Sheikh RM, Mansy SS, Nessim IG, Hosni HN, El Hindawi A, Hassanein MH, AbdelFattah AS. Carbamoyl phosphate synthetase 1 (CPS1) as a prognostic marker in chronic hepatitis C infection. APMIS. 2019;127:93-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 180. | Byrnes V, Miller A, Lowry D, Hill E, Weinstein C, Alsop D, Lenkinski R, Afdhal NH. Effects of anti-viral therapy and HCV clearance on cerebral metabolism and cognition. J Hepatol. 2012;56:549-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 181. | Alsop D, Younossi Z, Stepanova M, Afdhal NH. Cerebral MR spectroscopy and patient-reported mental health outcomes in hepatitis C genotype 1 naive patients treated with ledipasvir and sofosbuvir. Hepatology. 2014;60:221a-221a. |
| 182. | Curry MP, Moczynski NP, Liu L, Stamm L, Yun C, Brainard DM, McHutchison JG, Alsop D, Afdhal NH. The Effect of Sustained Virologic Response on Cerebral Metabolism and Neurocognition in Patients with Chronic Genotype 1 Hcv Infection. J Hepatol. 2016;64:S797-S797. |
| 183. | Dusheiko G. The impact of antiviral therapy for hepatitis C on the quality of life: a perspective. Liver Int. 2017;37 Suppl 1:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 184. | Puente Á, Cabezas J, López Arias MJ, Fortea JI, Arias MT, Estébanez Á, Casafont F, Fábrega E, Crespo J. Influence of sustained viral response on the regression of fibrosis and portal hypertension in cirrhotic HCV patients treated with antiviral triple therapy. Rev Esp Enferm Dig. 2017;109:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 185. | Afdhal N, Everson GT, Calleja JL, McCaughan G, Bosch J, Denning J, Brainard DM, McHutchison JG, Brandt-Sarif T, An D, Charlton M, Reddy KR, Asselah T, Gane E, Forns X. Effect of long term viral suppression with sofosbuvir plus ribavirin on hepatic venous pressure gradient in HCV-infected patients with cirrhosis and portal hypertension. J Hepatol. 2015;62:S269-S270. [DOI] [Full Text] |
| 186. | Mandorfer M, Kozbial K, Schwabl P, Freissmuth C, Schwarzer R, Stern R, Chromy D, Stättermayer AF, Reiberger T, Beinhardt S, Sieghart W, Trauner M, Hofer H, Ferlitsch A, Ferenci P, Peck-Radosavljevic M. Sustained virologic response to interferon-free therapies ameliorates HCV-induced portal hypertension. J Hepatol. 2016;65:692-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 250] [Article Influence: 25.0] [Reference Citation Analysis (5)] |
| 187. | Mauro E, Crespo G, Montironi C, Londoño MC, Hernández-Gea V, Ruiz P, Sastre L, Lombardo J, Mariño Z, Díaz A, Colmenero J, Rimola A, Garcia-Pagán JC, Brunet M, Forns X, Navasa M. Portal pressure and liver stiffness measurements in the prediction of fibrosis regression after sustained virological response in recurrent hepatitis C. Hepatology. 2018;67:1683-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 188. | Mandorfer M, Kozbial K, Schwabl P, Chromy D, Semmler G, Stättermayer AF, Pinter M, Hernández-Gea V, Fritzer-Szekeres M, Steindl-Munda P, Trauner M, Peck-Radosavljevic M, García-Pagán JC, Ferenci P, Reiberger T. Changes in Hepatic Venous Pressure Gradient Predict Hepatic Decompensation in Patients Who Achieved Sustained Virologic Response to Interferon-Free Therapy. Hepatology. 2020;71:1023-1036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 189. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1832] [Article Influence: 83.3] [Reference Citation Analysis (3)] |
| 190. | Ganne-Carrié N, Chastang C, Chapel F, Munz C, Pateron D, Sibony M, Dény P, Trinchet JC, Callard P, Guettier C, Beaugrand M. Predictive score for the development of hepatocellular carcinoma and additional value of liver large cell dysplasia in Western patients with cirrhosis. Hepatology. 1996;23:1112-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 129] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 191. | Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, Lens S, Díaz A, Vilana R, Darnell A, Varela M, Sangro B, Calleja JL, Forns X, Bruix J. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 818] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 192. | Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, Foschi FG, Lenzi M, Mazzella G, Verucchi G, Andreone P, Brillanti S. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65:727-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 713] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 193. | Kozbial K, Moser S, Schwarzer R, Laferl H, Al-Zoairy R, Stauber R, Stättermayer AF, Beinhardt S, Graziadei I, Freissmuth C, Maieron A, Gschwantler M, Strasser M, Peck-Radosalvjevic M, Trauner M, Hofer H, Ferenci P. Unexpected high incidence of hepatocellular carcinoma in cirrhotic patients with sustained virologic response following interferon-free direct-acting antiviral treatment. J Hepatol. 2016;65:856-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 178] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 194. | Cheung MCM, Walker AJ, Hudson BE, Verma S, McLauchlan J, Mutimer DJ, Brown A, Gelson WTH, MacDonald DC, Agarwal K, Foster GR, Irving WL, HCV Research UK. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;65:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 324] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 195. | ANRS collaborative study group on hepatocellular carcinoma (ANRS CO22 HEPATHER, CO12 CirVir and CO23 CUPILT cohorts). Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts. J Hepatol. 2016;65:734-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 336] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 196. | D'Ambrosio R, Colombo M. Should surveillance for liver cancer be modified in hepatitis C patients after treatment-related cirrhosis regression? Liver Int. 2016;36:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 197. | Ioannou GN, Beste LA, Green PK, Singal AG, Tapper EB, Waljee AK, Sterling RK, Feld JJ, Kaplan DE, Taddei TH, Berry K. Increased Risk for Hepatocellular Carcinoma Persists Up to 10 Years After HCV Eradication in Patients With Baseline Cirrhosis or High FIB-4 Scores. Gastroenterology. 2019;157:1264-1278.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 309] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Denmark
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Shabraw MHF, Yang SS S-Editor: Wang JL L-Editor: A E-Editor: Liu MY