Published online Jun 14, 2020. doi: 10.3748/wjg.v26.i22.2916
Peer-review started: January 31, 2020
First decision: February 27, 2020
Revised: May 8, 2020
Accepted: May 21, 2020
Article in press: May 21, 2020
Published online: June 14, 2020
Processing time: 134 Days and 19.2 Hours
Malnutrition encompassing both macro- and micro-nutrient deficiency, remains one of the most frequent complications of alcohol-related liver disease (ArLD). Protein-energy malnutrition can cause significant complications including sarcopenia, frailty and immunodepression in cirrhotic patients. Malnutrition reduces patient’s survival and negatively affects the quality of life of individuals with ArLD. Moreover, nutritional deficit increases the likelihood of hepatic decompensation in cirrhosis. Prompt recognition of at-risk individuals, early diagnosis and treatment of malnutrition remains a key component of ArLD management. In this review, we describe the pathophysiology of malnutrition in ArLD, review the screening tools available for nutritional assessment and discuss nutritional management strategies relevant to the different stages of ArLD, ranging from acute alcoholic hepatitis through to decompensated end stage liver disease.
Core tip: Malnutrition is a common complication of alcohol-related liver disease (ArLD), which, if untreated, can adversely affect patient outcome and recovery. Prompt recognition of nutritional depletion may identify those patients who are at higher risk of clinical decompensation, but there are few guidelines to inform the clinical management of these complex patients. In this article, we discuss the pathophysiology and treatment of micro- and macro-nutrient deficiency in ArLD, and provide recommendations for the management of patients at different stages of their illness.
- Citation: Kamran U, Towey J, Khanna A, Chauhan A, Rajoriya N, Holt A. Nutrition in alcohol-related liver disease: Physiopathology and management. World J Gastroenterol 2020; 26(22): 2916-2930
- URL: https://www.wjgnet.com/1007-9327/full/v26/i22/2916.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i22.2916
The World Health Organization estimates that alcohol abuse accounts for approximately 3.3 million deaths every year[1], with a significant proportion due to liver disease[2]. Forty-one percent of liver deaths in Europe are related to harmful alcohol consumption[3]. Alcohol-related liver disease (ArLD) refers to a wide spectrum of liver pathologies, including steatosis (fatty liver), steatohepatitis (characterized by a combination of hepatic fat accumulation and inflammation), acute alcoholic hepatitis (AAH) and liver cirrhosis[4]. It is important to understand that whilst alcohol is the principle mediator of liver injury in many individuals with cirrhosis, it can play a significant contributory role in the progression of other liver diseases such as hereditary haemochromatosis and non-alcoholic steatohepatitis. The component of alcohol relating to conditions developing in such a setting are commonly described as alcohol-contributory liver disease (AcLD). Alcohol use disorders should be sought in all individuals presenting with chronic liver disease due to the prevalence of alcohol abuse across the diagnostic spectrum with both ArLD and AcLD requiring a common final pathway of management. Whilst targeted pharmaceutical interventions are lacking in patients with alcohol-related cirrhosis[5], sustained alcohol avoidance remains the cornerstone of ArLD and AcLD management and recovery[6].
Several studies have identified a strong relationship between poor nutrition and adverse outcomes in survival, quality of life and complications of alcohol-related cirrhosis, such as variceal bleeding, ascites, hepatic encephalopathy (HE), infection and hepato-renal syndrome[7-9]. Protein-energy malnutrition (PEM: Altered body composition due to an imbalance of energy, protein and micronutrients)[10,11] is one of the most frequent complications of harmful alcohol use and can occur at all stages of ArLD[12,13]. Studies have shown that up to half of outpatients with alcohol-related cirrhosis, and almost all hospitalized patients with AAH exhibit evidence of clinically significant nutritional depletion[13-15]. Early diagnosis of malnutrition allows clinicians to tailor therapeutic strategies to avoid potential adverse outcomes in chronic liver disease as well as predicting those patients at higher risk of hepatic decompensation and/or liver-related death[16]. A recent study of 363 patients admitted with AAH reported a one-year mortality of 14% and 76%, in individuals classified with mild or severe malnutrition respectively[17]. In contrast, nutritional supplementation has been shown to be an effective means of improving liver function and patient survival in AAH[18,19]. In a randomised multicentre trial of severe AAH patients, Cabré et al[20] compared short and long-term effects of steroids and total enteral nutrition via nasoduodenal tube (providing 2000 kcal/d for 4 wk). Although short-term mortality was no different, the study showed improved outcomes at 1 year follow-up for patients treated with total enteral nutrition (P = 0.04, intention-to-treat analysis), with 8% one-year mortality reported in the enterally fed group, compared to 37% in the prednisolone-only group during the follow-up period, with most deaths attributed to sepsis[20].
There can be little doubt that the lack of clinical practice guidelines aimed at assessing and grading ArLD-related malnutrition accounts for the poor recognition, diagnosis and treatment of this condition in clinical practice. The aim of this article is to define the relevant pathophysiology, summarise modes of assessment and discuss optimal nutritional management in different forms of ArLD.
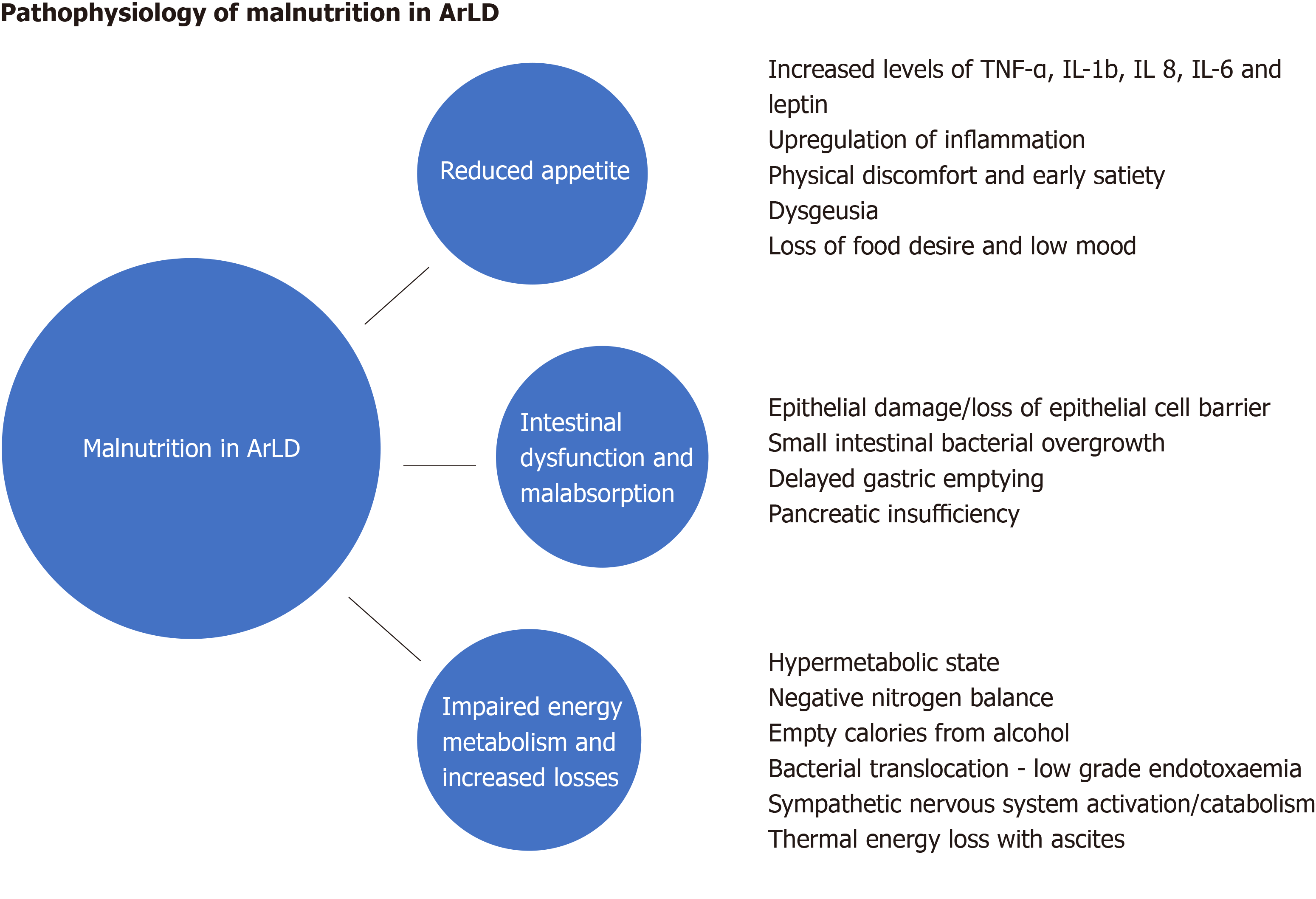
Malnutrition in ArLD and AcLD is multifarious and comprised of many interdependent elements, but simply increasing the availability of energy supplements is not enough to counteract the powerful forces that drive the catabolic state. Here we explore some of the elements that contribute to the condition (Figure 1).
Loss of appetite and reduced food desire is related to the upregulation of inflammatory cytokines and appetite regulators in both acute and chronic liver disease. In patients with alcohol-related cirrhosis, tumour necrosis factor (TNF-α) and leptin (an appetite-regulating hormone secreted by adipose tissue) levels increase[21-23] which diminish appetite and cause early satiety. Increased TNF-α levels in AAH and alcohol-related cirrhosis upregulate secondary inflammatory cytokines such as interleukin (IL)-1b, IL-6 and IL-8, which increase appetite suppression and cause selective nutrient avoidance[24,25]. Whilst cytokines may act as a regulatory component of appetite in health, in disease states their dysregulation is a major contributor to the cachexia seen in all forms of acute and chronic disease[26]. Cytokines can also mediate their actions on appetite via neural and humoral effects and TNF-α further modulates metabolism by directly acting on the central nervous system to alter the release of neurotransmitters, which slow gut motility and gastric emptying[27]. Anorexia is worsened by physical symptoms of discomfort (nausea, bloating and fatigue), dysgeusia and the mechanical effects of large ascites[28]. These factors may impact upon the food choices of patients and affect both the quality and quantity of nutrition as a result.
Alcohol is absorbed by diffusion in the stomach and, to a lesser degree the duodenum and jejunum. Whilst acute and excessive alcohol consumption can cause gastric and duodenal erosions and villous-predominant epithelial loss in the upper jejunum[29], the effects of chronic alcohol consumption on the intestinal mucosa are poorly understood. They may include intestinal fibrosis and overgrowth of aerobic and anaerobic microorganisms which contribute to functional and morphological abnormalities of the small bowel[30]. Gerova et al[31] reported a higher frequency of small intestinal bacterial colonisation in patients with ArLD, with the changes occurring independently of the stage of liver dysfunction suggesting that the direct effect of alcohol on gut motility and immunity creates a permissive microenvironment for small bowel overgrowth at these sites.
In addition to changes in the gut microbiome, chronic alcohol ingestion can lead to a reduction in the adhesion of epithelial cell tight junctions[32] resulting in increased intestinal permeability, bacterial translocation and consequential increases in pro-inflammatory cytokines and lipopolysaccharides[33]. Chronic alcohol consumption impairs gut motility and alcohol-induced chemical gastritis delays gastric emptying, both of which significantly increase the oro-caecal transit time[34] leading to impaired absorption of nutrients. Furthermore, alcohol is an important risk factor for chronic pancreatitis and pancreatic exocrine insufficiency (PEI) which can exacerbate malabsorption[35].
Resting energy expenditure (REE) is the amount of energy an individual uses to perform vital organ functions free of activity and digestion. REE can be calculated using the predictive formula of Harris-Benedict[36] however its calculation can be unreliable in patients with altered body composition (by misconstruing the weight of extracellular fluid as dry body mass and overestimating the caloric requirements in cirrhotic patients with ascites). Indirect calorimetry is not subject to this limitation as it measures REE without reference to body composition by basing its calculation on oxygen consumption and carbon dioxide production[37]. Hypermetabolic states (REE > 110%) commonly occur in ArLD, where approximately 20% of patients exhibit features of hyper-metabolism[38] which accelerates calorific expenditure and promotes a negative nitrogen balance by increasing urinary and faecal nitrogen losses[39]. In heavy drinkers’, alternative alcohol-metabolic pathways are engaged following excessive alcohol consumption due to the zero-order kinetics of alcohol metabolism. The ensuing increase in acetaldehyde production (a toxic metabolite of alcohol) puts stress on microsomal re-oxidation pathways which utilise more oxygen and ATP[40] to recover nicotinamide adenine dinucleotide, thereby perpetuating the hyperdynamic metabolism by increasing energy utilisation.
AAH is a classical example of the alcohol-induced hypermetabolic state[41,42]. The accelerated catabolism typically seen in these patients is a composite of reduced oral energy intake with food as the individual becomes dependent on the calorific value of alcohol to provide their basal metabolic expenditure and subsequently becomes more protein-calorie deplete. Many patients reduce their alcohol intake before presenting with clinical manifestations of AAH[43] thus compounding the calorie debt and catalysing a chain of events leading to the establishment of a chemical and metabolic liver injury characterised by hepatitis and the sudden onset of jaundice and synthetic failure. It is pertinent that the proven treatments for AAH include alcohol cessation and nutritional therapy with high protein and calorie supplementation. Another driver of hyper-metabolism is systemic low grade endotoxaemia[44], driven by bacterial translocation, which can lead to upregulation of the sympathetic nervous outflow and worsening of the hypermetabolic state. This results in clinical features such as fever, tachycardia, hyperglycaemia and muscle wasting[45,46]. In such patients, the accumulation of ascites further increases REE under indirect colorimetry testing due to the energy expense required to maintain the large fluid volumes at body temperature. Improvements in energy expenditure are seen in patients after large volume paracentesis[47].
Excessive alcohol intake over a prolonged period results in impaired insulin resistance and increased cardiovascular morbidity and mortality[48,49]. In chronic alcohol consumption glycogen stores of the liver are depleted, whilst in acute episodes of heavy alcohol consumption (binge drinking) gluconeogenesis is inhibited and hepatic glycogenolysis stimulated to prevent hypoglycaemia. Therefore, whilst in a healthy individual acute alcohol consumption is unlikely to cause changes in the euglycemic state, in patients with chronic liver disease acute alcohol ingestion may precipitate hypoglycaemia[50,51].
Low to moderate doses of alcohol have little to no effect on muscle protein balance but acute ingestion of large doses of alcohol and chronic alcohol abuse causes changes to both whole-body and tissue-specific protein metabolism by increasing nitrogen excretion[52]. Myopathy is a common complication of chronic alcoholism and is the result of a prolonged imbalance between muscle protein growth and breakdown[53,54].
The liver plays a central role in lipid metabolism which follows a complex network of reactions and interplay of hormones, nuclear receptors, intracellular signalling pathways and transcription factors. Free fatty acids (FAs) are synthesised by the liver from glycolytic pathways and are directly mobilised from the gut and adipose tissue. Alcohol inhibits FA oxidation pathways (by decreasing expression of several PPARα-regulated genes)[55] and increases esterification of FAs resulting in an increased accumulation of intrahepatic triglyceride[56]. Alcohol also affects FA export from the liver by suppressing microsomal triglyceride transfer protein, as seen in livers of ethanol fed animals, which is required for the assembly of very low density lipoprotein prior to export[57]. The result is intrahepatic fat accumulation, which ultimately progresses to cirrhosis as a result of iterative cycles of injury and cell-death associated with sustained alcohol excess.
Thiamine (vitamin B1) serves as a cofactor for the enzymes involved in glucose metabolism. Thiamine deficiency results in decreased activities of these pathways which can result in reduced ATP synthesis leading to cell damage and cell death. Chronic alcoholism leads to thiamine deficiency as a result of inadequate nutritional intake and decreased absorption of thiamine from the gastrointestinal tract[58]. Careful reintroduction of diet may need to be considered if refeeding syndrome is a concern, as the sudden increase in carbohydrate consumption causes a shift from fats to carbohydrate for energy production, increasing the demand for thiamine and compounding any deficiency by further depleting stores[59]. Wernicke encephalopathy is an acute neurological crisis which results from exhausted thiamine stores and is characterised by the clinical triad of encephalopathy, oculomotor dysfunction, and gait ataxia. If left untreated individuals can develop permanent neuropsychiatric complications such as Korsakoff’s syndrome which is typified by a marked deficit in anterograde and retrograde memory, apathy, an intact sensorium, but relative preservation of long-term memory and other cognitive skills. Folate deficiency is also seen in these patients due to reduced dietary intake, intestinal malabsorption, reduced liver uptake, storage and increased urinary excretion[60]. Deficiencies in folate can cause defective DNA synthesis and repair which may manifest as macrocytic anaemia and muscle dysfunction.
Chronic alcohol consumption and jaundice cause vitamin A levels to fall[61]. The metabolism of vitamin A is similar to alcohol metabolism in the human body as they both involve oxidative pathways and are therefore vulnerable to alterations in the basal redox-state of the liver[62]. Alcohol dehydrogenase activity and cytochrome 2E1 negatively affect retinoid homeostasis[63] and chronic alcohol consumption leads to depletion of hepatic and plasma retinoid levels and retinoid binding proteins[64,65]. Alcohol is also believed to inhibit the cleavage of β-carotene, a dietary pro-vitamin A carotenoid[66]. Vitamin A deficiency can lead to the clinical presentation of night blindness.
Various mechanisms, in addition to dietary insufficiency, have been postulated to account for vitamin C deficiency in the context of chronic alcohol consumption[67]. Alcohol-induced enterocyte toxicity leads to intestinal malabsorption and hepatotoxicity which inhibit hepatic transformation of various vitamins (including vitamin C) to their active metabolites[68]. The imbalance in vitamin C is exacerbated by increased urinary ascorbic acid excretion following episodes of alcohol excess[69]. Some studies suggest that pre-treatment with vitamin C significantly enhances blood ethanol clearance, possibly as a result of its ability to supply peroxide and thus allowing catalase to contribute to ethanol oxidation[70]. Clinical manifestation of vitamin C deficiency is namely scurvy and can present as poor wound healing, gingival swelling, gum bleeding, loss of teeth and mucocutaneous petechiae; late disease may be life-threatening with anasarca, haemolysis and jaundice[71,72].
Zinc is absorbed via metal binding transcription factors and plays a key role in the regulation of gene expression. In alcohol-fed mice, alcohol disrupts gut permeability and increases oxidative stress, predominantly at the level of distal small bowel which interferes with zinc homeostasis and leads to reduced ileal zinc concentrations[73]. Animal studies have shown that zinc supplementation preserves intestinal integrity and prevents endotoxaemia, leading to inhibition of endotoxin-induced TNF-α production in the liver under both acute and chronic conditions of alcohol exposure[74]. In addition to reduced enteric absorption and increased urinary excretion of zinc, patients with alcohol-related cirrhosis often have diets lacking in protein and zinc, with zinc deficiency a common (and easily rectified) cause of dysgeusia. Zinc deficiency may manifest as acrodermatitis, anorexia, hypogonadism, altered immune function, poor wound healing, impaired night vision, diarrhoea, impaired mental function and portal systemic encephalopathy[75,76].
Magnesium is the second most abundant micronutrient in the human body and deficiency is almost universal in individuals with high levels of alcohol consumption and/or liver disease. It is a critical determinant of metabolism, acting as a co-factor in more than 300 enzymatic reactions involved in protein and nucleic acid synthesis and energy metabolism. Alcohol increases the urinary excretion of magnesium and total body stores of magnesium are depleted in nearly all patients with alcohol-related cirrhosis[77]. Further insensible losses occur as a result of alcohol-related diarrhoea, vomiting and concurrent use of drugs such as diuretics and aminoglycosides. Hypomagnesemia predisposes to metabolic bone disease, cardiovascular co-morbidities and is associated with seizure, depression and neuromuscular abnormalities[78,79] (Table 1).
| Nutrients | Effect of alcohol intake | Results | |
| Carbohydrate | Acute alcohol intake | Inhibits gluconeogenesis; stimulates hepatic glycogenolysis | Hypoglycaemic; hyperglycaemic |
| Chronic alcohol intake | Inhibits lactate stimulated gluconeogenesis; carbohydrate rich food taken with alcohol | Hyperlactatemia; delayed paradoxical hypoglycaemic state | |
| Proteins | Acute and chronic alcohol intake | Increases nitrogen excretion; imbalance between protein growth and breakdown | Muscle wasting and myopathy |
| Lipids | Acute and chronic alcohol intake | Inhibits β-oxidation and increases esterification of fatty acids | Increased accumulation of triglycerides in the hepatocytesFibrosis |
| Thiamine | Chronic alcohol intake | Inadequate nutritional intake Decreased absorption | Wernicke Korsakoff syndrome |
| Folate | Chronic alcohol intake | Reduced dietary intake; intestinal malabsorption; reduced liver uptake, storage; increased urinary excretion | Macrocytic anaemia; muscle dysfunction |
| Vitamin A | Chronic alcohol intake | Inhibit the cleavage of β-carotene, a dietary pro-vitamin A carotenoid | Xerophthalmia and night blindness |
| Vitamin C | Chronic alcohol intake | Intestinal malabsorption; hepatotoxicity inhibits hepatic transformation to their active metabolites | Scurvy and poor wound healing |
| Zinc | Chronic alcohol intake | Disrupts gut permeability; decreases ileal -zinc concentration; increased accumulation of reactive oxygen species and plasma endotoxin levels | Acrodermatitis; anorexia; hypogonadism; altered immune function; poor wound healing; impaired night vision; diarrhoea; impaired mental function and portal systemic encephalopathy |
| Magnesium | Chronic alcohol intake | Increases the urinary excretion of magnesium | Cardiovascular: Hypertension, stroke and myocardial infarction; Neurological: Seizure, depression and neuromuscular abnormalities |
The interactions of divalent cation deficiencies such as selenium and magnesium are poorly understood but seem to play a key role in the immune-paresis seen in alcohol-related cirrhosis. Selenium deficiency is common in alcohol-dependency[80,81] and proportionate to disease stage and increased levels of pro-inflammatory cytokines which play a role in liver injury and fibrosis. Current evidence suggests that micronutrient metabolism is impaired in decompensated liver disease and that by replacing these elemental deficiencies, clinicians may be able to counteract some of the immune-paresis and mood disorders commonly seen in these malnourished states[82,83].
Malnutrition and sarcopenia are important determinants of prognosis and survival in cirrhotic patients[84,85]. A South Korean study of patients with liver cirrhosis (62% with ArLD) showed that the presence of sarcopenia was associated with increased mortality [hazard ratio (HR) 2.27, 95% confidence interval (CI): 1.17-4.40, P = 0.015] and that accelerated loss of skeletal muscle was independently associated with poor outcome (HR 0.94, 95%CI: 0.90-0.99, P = 0.013)[86]. Poor nutrition increases the risk of complications and decompensation in liver disease patients[87]. Moreover, because muscle acts as an alternative site of ammonia detoxification[88] prospective studies in cirrhotic patients have shown that both overt and minimal HE are increased in patients with muscle depletion[89]. Nutrition has also been shown to have significant impact on ascites. Vidot et al[90] demonstrated that aggressive nutritional support in the form of supplemental tube feeding (for 7 ± 1 wk) significantly reduces ascites formation and the requirement for paracentesis (P < 0.001) in malnourished patients who fail to respond to standard oral nutrition.
Cirrhosis and malnutrition produce an acquired state of immune paresis which negatively impacts upon patient recovery and survival[91,92]. Protein malnutrition is an independent risk factor for infection and sepsis in hospitalized patients with cirrhosis, and septic episodes in these individuals are associated with higher in-hospital and post discharge mortality at six months (50% vs 11% respectively, P < 0.001)[93]. In patients with ArLD, the presence of sarcopenia (as recorded by the skeletal muscle index)[94] is independently associated with an increased likelihood of an individual being removed from the transplant waiting list due to clinical deterioration (HR 1.9, 95%CI: 1.2-3.1, P = 0.01) and a higher likelihood of waiting list death[95]. The impact of malnutrition and sarcopenia on post-transplant outcomes were reported by Kalafateli et al[96] using the Royal Free Hospital-Global Assessment (RFH‐GA) tool and the L3‐psoas muscle index (L3‐PMI) to assess nutritional status. Severe malnutrition, defined as RFH‐GA score 3, was associated with a prolonged intensive care stay i.e., > 5 d (odds ratio = 7.46, 95%CI: 1.57-35.43) whilst low L3‐PMI was an independent predictor for a hospital stay more than twenty days and higher 12-mo mortality[96]. The diagnosis and management of sarcopenia is therefore of paramount importance in the initial (and subsequent) assessment of liver-disease patients receiving clinical care.
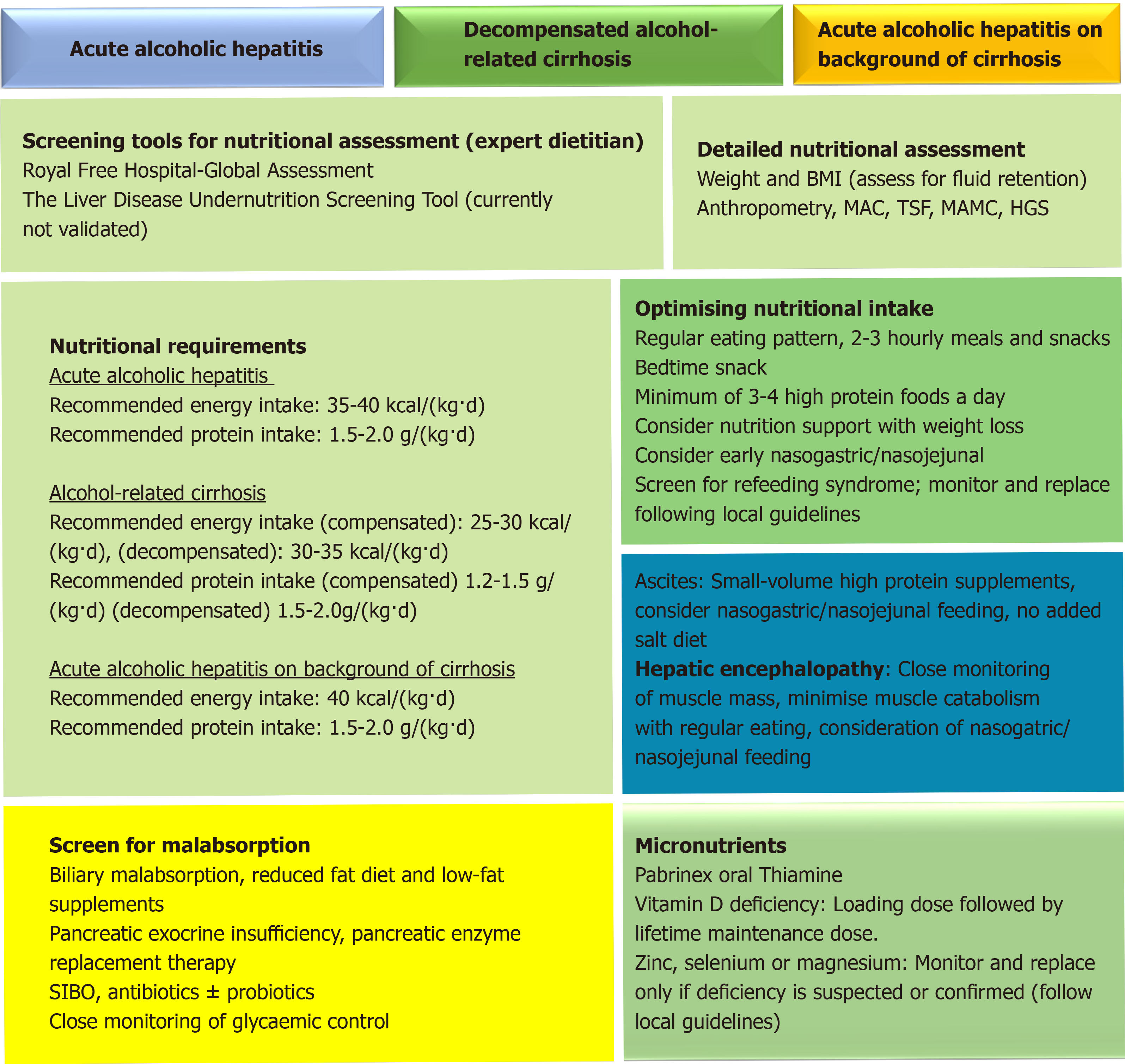
There is no gold standard for assessment of malnutrition in liver disease and none specifically designed for patients with ArLD, but there are a number of screening tools[97] that have been developed to assess malnutrition risk, although most lack external validation. The Liver Disease Undernutrition Screening Tool[98] is a 6-question nutrition screening tool which was found to accurately identify malnutrition (93%) in patients with liver cirrhosis although it has not been studied in longer-term outcomes. Whereas the Royal Free Hospital Nutritional Prioritisation Tool (RFH-NPT)[99] has been adapted to account for fluid overload. RFH-NPT is user friendly, quick to complete and is a good predictor of clinical deterioration. Given the high prevalence of malnutrition and sarcopenia in alcohol-related cirrhosis, all patients should undergo nutritional screening at the point of presentation, ideally using a standardised screening tool such as the RFH-NPT[100].
Body mass index (BMI) is often distorted in patients with chronic liver disease by fluid retention states like anasarca or ascites. Moreover, sarcopenic-obesity is another entity characterised by excessive fat and poor muscle mass and function[101]. In these settings, BMI proves to be an inadequate metric by which to predict complications and should be used in combination with objective measures of muscle mass and strength.
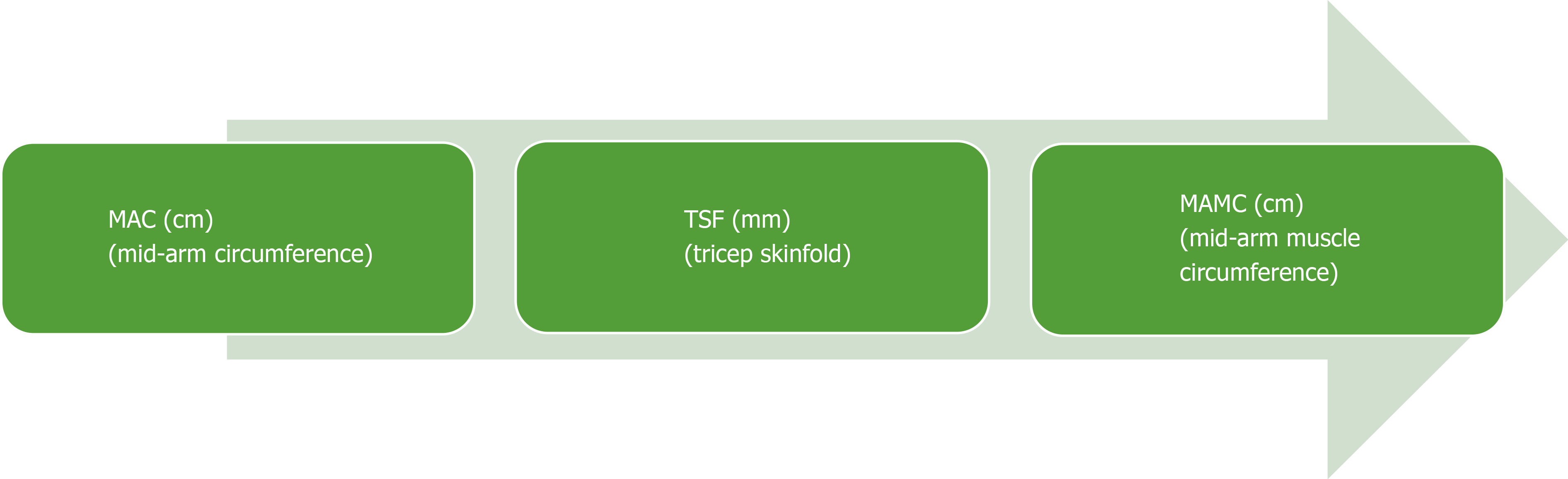
Muscle function tests are an important component of assessing nutrition risk. Hand-grip strength (HGS) has been well validated and is commonly used in clinical practice to record strength and muscle capacity[102]. A dynamometer measures the strength exerted by a patient’s non-dominant hand, the results of which are compared to tables of normal values based on sex and age of healthy volunteers. It is an inexpensive, easily replicated test and can be completed at the bedside or clinic. Observational studies have shown that HGS is strongly correlated with Child-Pugh score and can predict the risk of short-term morbidity in patients with alcohol-related cirrhosis[103]. Moreover, HGS operates as a predictive tool for complications of cirrhosis and muscle function testing can be used as a predictive determinant of HE[97]. Mid-arm circumference and triceps skinfold (TSF) are used to calculate skeletal muscle mass (mid-arm muscle circumference, MAMC) and it has been demonstrated that MAMC, TSF, HGS are accurate predictors of pre-transplant morbidity[97]. Both HGS and MAMC should be routinely monitored in clinic as they provide a good indication of nutritional state and are reliable predictors of clinical deterioration. Muscle strength (HGS) commonly falls before muscle mass depletes, and strength can reduce without a change to muscle mass, thus making HGS a useful dynamic predictor of nutritional decline[102] (Figure 2).
Early introduction of oral nutrition support improves survival for malnourished individuals with AAH although the data remains conflicted. A meta-analysis[104] of 7 randomized controlled trials demonstrated no mortality benefit with supplemental nutrition but the studies were under-powered. Cabré et al[20] found that 6-mo mortality increased in those whose overall calorie intake was lower than 21.5 kcal/kg per day, suggesting that additional oral nutritional supplementation in such individuals would improve survival. A daily energy target of 35-40 kcal/kg is recommended, but refeeding syndrome needs to be considered as it can be encountered in extreme cases. Intensive pre-supplementation of vitamins B and C with thiamine (e.g., Pabrinex®) is necessary to prevent acute depletion and the development of Wernicke’ syndrome. Refeeding syndrome can occur when there are shifts in fluid and electrolytes in patients who are malnourished after their nutritional intake increases and is more common with oral nutritional supplements or tube feeding as opposed to oral intake alone[105]. In advanced liver disease, PEM becomes more prevalent and the main challenge is to minimise muscle catabolism[106]. If AAH develops on the background of cirrhosis, energy and protein requirements are likely to increase. In practice a patient’s estimated energy requirements may increase to 40 kcal/kg per day if body weight is low and nutritional intake is negligible.
In patients with decompensated cirrhosis due to ArLD additional nutrition support is almost always indicated, particularly in patients with ascites. It is important to avoid prolonged fasting periods to minimise the breakdown of muscle and adipose stores for use as a metabolic fuel, and a regular 2-3 hourly eating pattern including a bed-time snack can support this. Whilst adjustments to the frequency of energy delivery are an effective means of preventing accelerated loss of skeletal fat mass by inhibiting gluconeogenesis; patients who graze constantly throughout the day protect muscle but may not consume enough calories to preserve adipose stores and additional calories may be required to prevent adipose wasting[106]. Energy requirements in compensated cirrhosis are therefore estimated at 25-30 kcal/kg per day and 30-35 kcal/kg per day in decompensated cirrhosis. For obese patients (BMI > 30 kg/m2) energy requirements are estimated at around 25 kcal/kg per day (Figure 3). All requirements should be based on estimated dry body weight and estimated BMI.
Naso-gastric (NG) or naso-jejunal (NJ) feeding is clinically indicated when energy and/or protein requirements cannot be met through oral intake alone. Other indications for initiating NG/NJ feeding in liver cirrhosis include early satiety from ascites, refractory ascites, optimisation of energy and protein requirements, or chronic vomiting. Kearns et al[107] assigned a control group with AAH with concomitant cirrhosis to receive standard oral intake whilst another group received enteral nutrition in addition to 40 kcal/kg a day and 1.5 g/kg per day protein orally. The enterally fed group received 200% more energy than the controls and showed an improvement in nitrogen balance, serum albumin and HE (P ≤ 0.02) after 3 wk. Whilst this study demonstrated a short-term improvement in nutritional status and reduction in liver-related adverse events, the small sample size and cross-sectional nature of this study limited assessment of longer-term outcomes. Other studies have highlighted the risks of intensive tube feeding in cirrhosis and retaining placement of short-term feeding tubes in situ can be a challenge, particularly in confused patients[18,108].
Protein requirements in the presence of ascites and/or oedema are particularly high due to the degree of protein loss encountered, particularly in those patients requiring frequent or large-volume paracentesis. A minimum protein intake of 1.2-1.5 g/kg of dry body weight/day is recommended for individuals with stable muscle mass[100] and in these individuals concentrated high protein supplements (60-125 mL containing 18-20 g protein) are used to support nutritional intake as they are often better tolerated, particularly in the presence of poor appetite, early satiety and fatigue. It is important to tailor sip feeds to individual needs (i.e., protein deficit, taste, early satiety and appetite) and when oral supplements are poorly tolerated, supplementary tube feeding can be initiated. Patients with high volume recurrent ascites with evidence of muscle loss commonly require 1.5-2 g/kg protein a day. Guideline recommendations for dietary salt intake are conflicting; some recommend strict reduction of sodium intake whilst others acknowledge that over-restriction can increase the risk of PEM due to food aversion. In practice, aggressive sodium restriction should be avoided wherever possible as the resulting diet is unpalatable and leads to avoidance of protein-rich foods. Patients should not be encouraged to restrict their salt intake below 60 mmol per day and we advocate a “no-added salt” diet with minimisation of pre-prepared foods such as crisps, tinned soups, microwave meals etc.[100,109].
HE has been observed to occur more frequently in the presence of sarcopenia and for this reason protein restriction is not recommended to support management of HE. There is a well-recognised association between muscle depletion and negative nitrogen balance with worsening liver decompensation and subsequent complications such as HE[109,110] and it is vitally important that clinical care plans limit the impact of PEM and muscle wasting by avoiding catabolism through encouraging small frequent meals, eating regularly and optimising protein intake (minimum of 1.2 g/kg per day) to support muscle mass[110]. Enteral (NG) tube feeding should be considered in the presence of advanced HE[111] particularly when sufficient oral intake is reduced or not feasible[100]. Nursing staff must be experienced in the management of tube-feed systems and aware of the increased risks of aspiration that can occur in encephalopathic patients. Great care should be taken to ensure that the tube is re-inserted correctly when it is displaced and the use of a bridle may be considered (if there is no risk of bleeding) to prevent the tube from being withdrawn inadvertently. Varices should not preclude enteral tube placement unless there are signs of active bleeding[112]. Despite the risks of tube-supported enteral feeding in liver patients, failure to implement adequate nutritional support in such cases will only lead to accelerated sarcopenia and a worsening of the patient’s clinical condition. The risk-benefit analysis in such patients needs to be carefully considered and patient choice always considered.
Steatorrhea (symptoms and signs including nausea, pale/yellow coloured, oily and foul-smelling stools) needs to be identified promptly to enable effective management. PEI should always be considered in patients with ArLD using appropriate testing such as faecal elastase measurement as there is a high prevalence amongst these individuals[113]. Treatment with pancreatic enzyme replacement therapies e.g., CREON™, PANCREX-V™ must be initiated at an early stage with education about dose titration to increase compliance. When jaundice is present, biliary malabsorption needs to be considered as patients can manifest symptoms indistinguishable from PEI. The choice of feed is crucial in cholestatic patients and low-fat feeds such as Meritene™ and Renapro Powder™ (orally) and Nutrison Peptisorb™ and Peptamen HN™ (enterally) should be chosen over high-lipid counterparts (Fortisip Compact Protein™ and Ensure Twocal™) to reduce the risk of exacerbating nutritional and trace element depletion. Management primarily involves reducing dietary fat intake although there is little consensus as to what constitutes a low-fat diet. Food frequencies should be assessed, and creamy or fried foods discouraged. Fat-soluble vitamins (vitamins A, D, E and K) must be supplemented. If steatorrhea is left untreated it can exacerbate malnutrition through reduced food intake (food aversion), dysgeusia and vitamin and mineral deficiencies.
The symptoms of small intestinal bacterial overgrowth (SIBO) include diarrhoea, steatorrhea, chronic abdominal pain, bloating and flatulence although some patients may be asymptomatic. It is commonly diagnosed via hydrogen or methane breath testing and treatment usually requires a course of non-absorbed antibiotics such as rifaximin or neomycin. One meta-analysis[114] identified a potential role for the use of probiotics, prebiotics and symbiotics - concluding that probiotics were better tolerated than lactulose, improved SIBO and the management of minimal HE [risk ratio (RR) 0.40, 95%CI: 0.32-0.50, P < 0.001] however lactulose remained the more effective treatment for overt HE (RR 0.34, 95%CI: 0.24-0.47, P < 0.0001). It is unlikely that the use of prebiotics could be sustained in decompensated patients, but in compensated disease this remains an area of interest. Moreover, since non-absorbed rifamycin-based therapies for HE has become widely available, it will be interesting to see how the use of antibiotic therapies for HE affects the prevalence of both overt and covert SIBO in cirrhosis.
Close monitoring of glycaemic control (particularly in patients with HE) is key to preventing hypo-and hyperglycaemia, especially in the presence of diabetes. Alongside prescribed oral hypoglycaemic medication or insulin therapies, foods and fluids high in sugar should be avoided but it is imperative not to remove dietary carbohydrates altogether as this can provoke further catabolic injury. Avoiding prolonged fasting with 2-3 hourly eating patterns, modifying the carbohydrate load and replacing it with higher protein sources is often effective. Tight glycaemic control can also reduce the risk of delayed-gastric emptying driven by hyperglycaemia which may cause nausea, vomiting, abdominal pain or discomfort. If suspected, this can be confirmed with gastric emptying scintigraphy. Diabetes should be routinely screened if PEI is present, particularly as pancreatic β cell damage in ArLD is common[115] and it should be noted that the use of haemoglobin as a direct marker of glycaemic control may be inaccurate in the context of anaemia or recent blood transfusions and must be interpreted with caution.
It is not known if replacing micronutrients prevents complications in decompensated cirrhosis or reduces sepsis in ArLD, but vitamins and trace elements must be corrected at presentation. If vitamin D deficiency is confirmed, this should be corrected with a vitamin D loading dose followed by maintenance therapy[116,117]. Suspected or confirmed deficiencies of vitamins A, E and/or K should be corrected using supplements, but in coagulopathic patients vitamin injections should not be given intramuscularly. Whilst there is no consensus regarding the replacement or supplementation of zinc, selenium or magnesium in cirrhosis we recommend that in stable outpatients trace elements are supplemented daily using an oral multi-vitamin such as forceval with additional folic acid, zinc, vitamin D and glutathione supplements provided as necessary[118]. In critically ill patients these elements should be supplemented parenterally where possible and enterally via an NG tube if possible[119]. Selenium should be given as a loading dose and then provided as a regular supplement whilst magnesium levels can be supplemented as the biochemical values demand. Zinc is commonly given as a zinc salt (e.g., zinc acetate) and ArLD patients with overt HE who are admitted to intensive care can be provided with a 3-5 d course of intravenous L-ornithine L-aspartate to optimise ammonia scavenging[120].
Nutritional assessment and management of patients with ArLD is made more complex by the number of pathogenic mechanisms involved in the clinical deterioration of patients. Nutritional and trace element depletion is commonly associated with ArLD and patients may rapidly develop features of severe PEM unless nutritional management strategies are initiated promptly. Moreover, complications such as nutritional immuno-paresis, sarcopenia and frailty can be difficult to reverse once they are established. Malnutrition and sarcopenia are strongly associated with the development of complications of cirrhosis and poor nutrition remains a strong predictor of both short and medium-term survival. Notwithstanding that, reversal of energy and protein deficits in both AAH and alcohol-related cirrhosis improve patient outcomes by improving function and physical condition and reducing mortality and morbidity. In that context it is important for clinicians managing such patients to have a good working knowledge of nutritional therapies specific for liver disease so treatments can be started swiftly and applied in a scientific manner.
| 1. | World Health Organization. Global status report on noncommunicable diseases 2014. 2014 [cited 20 November 2019]. Available from: https://apps.who.int/iris/bitstream/handle/10665/148114/9789241564854_eng.pdf. |
| 2. | Sassi F. Tackling Harmful Alcohol Use: Economics and Public Health Policy. Paris: OECD Publishing; 2015; . |
| 3. | Sheron N. Alcohol and liver disease in Europe--Simple measures have the potential to prevent tens of thousands of premature deaths. J Hepatol. 2016;64:957-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | MacSween RN, Burt AD. Histologic spectrum of alcoholic liver disease. Semin Liver Dis. 1986;6:221-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 165] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Jaurigue MM, Cappell MS. Therapy for alcoholic liver disease. World J Gastroenterol. 2014;20:2143-2158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Spengler EK, Dunkelberg J, Schey R. Alcoholic hepatitis: current management. Dig Dis Sci. 2014;59:2357-2366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Periyalwar P, Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis. 2012;16:95-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 199] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 8. | Dasarathy S. Consilience in sarcopenia of cirrhosis. J Cachexia Sarcopenia Muscle. 2012;3:225-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 212] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 9. | Saunders J, Brian A, Wright M, Stroud M. Malnutrition and nutrition support in patients with liver disease. Frontline Gastroenterol. 2010;1:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | European Association for the Study of the Liver;. European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 752] [Article Influence: 107.4] [Reference Citation Analysis (2)] |
| 11. | Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, Compher C, Correia I, Higashiguchi T, Holst M, Jensen GL, Malone A, Muscaritoli M, Nyulasi I, Pirlich M, Rothenberg E, Schindler K, Schneider SM, de van der Schueren MA, Sieber C, Valentini L, Yu JC, Van Gossum A, Singer P. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36:49-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 908] [Cited by in RCA: 1629] [Article Influence: 162.9] [Reference Citation Analysis (0)] |
| 12. | Mendenhall CL, Moritz TE, Roselle GA, Morgan TR, Nemchausky BA, Tamburro CH, Schiff ER, McClain CJ, Marsano LS, Allen JI. A study of oral nutritional support with oxandrolone in malnourished patients with alcoholic hepatitis: results of a Department of Veterans Affairs cooperative study. Hepatology. 1993;17:564-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 188] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Mendenhall CL, Moritz TE, Roselle GA, Morgan TR, Nemchausky BA, Tamburro CH, Schiff ER, McClain CJ, Marsano LS, Allen JI. Protein energy malnutrition in severe alcoholic hepatitis: diagnosis and response to treatment. The VA Cooperative Study Group #275. JPEN J Parenter Enteral Nutr. 1995;19:258-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 115] [Article Influence: 3.7] [Reference Citation Analysis (2)] |
| 14. | McClain CJ, Barve SS, Barve A, Marsano L. Alcoholic liver disease and malnutrition. Alcohol Clin Exp Res. 2011;35:815-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Cheung K, Lee SS, Raman M. Prevalence and mechanisms of malnutrition in patients with advanced liver disease, and nutrition management strategies. Clin Gastroenterol Hepatol. 2012;10:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 244] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 16. | Singal AK, Charlton MR. Nutrition in alcoholic liver disease. Clin Liver Dis. 2012;16:805-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Rossi RE, Conte D, Massironi S. Diagnosis and treatment of nutritional deficiencies in alcoholic liver disease: Overview of available evidence and open issues. Dig Liver Dis. 2015;47:819-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Foody W, Heuman DD, Mihas AA, Schubert ML. Nutritional therapy for alcoholic hepatitis: new life for an old idea. Gastroenterology. 2001;120:1053-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Stickel F, Hoehn B, Schuppan D, Seitz HK. Review article: Nutritional therapy in alcoholic liver disease. Aliment Pharmacol Ther. 2003;18:357-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Cabré E, Rodríguez-Iglesias P, Caballería J, Quer JC, Sánchez-Lombraña JL, Parés A, Papo M, Planas R, Gassull MA. Short- and long-term outcome of severe alcohol-induced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology. 2000;32:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 222] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 21. | Peng S, Plank LD, McCall JL, Gillanders LK, McIlroy K, Gane EJ. Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: a comprehensive study. Am J Clin Nutr. 2007;85:1257-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 186] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 22. | Nicolás JM, Fernández-Solà J, Fatjó F, Casamitjana R, Bataller R, Sacanella E, Tobías E, Badía E, Estruch R. Increased circulating leptin levels in chronic alcoholism. Alcohol Clin Exp Res. 2001;25:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 81] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Baskaran C, Eddy KT, Miller KK, Meenaghan E, Misra M, Lawson EA. Leptin secretory dynamics and associated disordered eating psychopathology across the weight spectrum. Eur J Endocrinol. 2016;174:503-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Kent S, Bret-Dibat JL, Kelley KW, Dantzer R. Mechanisms of sickness-induced decreases in food-motivated behavior. Neurosci Biobehav Rev. 1996;20:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 118] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Langhans W, Hrupka B. Interleukins and tumor necrosis factor as inhibitors of food intake. Neuropeptides. 1999;33:415-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 102] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Plata-Salamán CR. Anorexia during acute and chronic disease. Nutrition. 1996;12:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 161] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Grossberg AJ, Scarlett JM, Marks DL. Hypothalamic mechanisms in cachexia. Physiol Behav. 2010;100:478-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 28. | Aqel BA, Scolapio JS, Dickson RC, Burton DD, Bouras EP. Contribution of ascites to impaired gastric function and nutritional intake in patients with cirrhosis and ascites. Clin Gastroenterol Hepatol. 2005;3:1095-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Bode C, Bode JC. Effect of alcohol consumption on the gut. Best Pract Res Clin Gastroenterol. 2003;17:575-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 263] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 30. | Bode JC, Bode C, Heidelbach R, Dürr HK, Martini GA. Jejunal microflora in patients with chronic alcohol abuse. Hepatogastroenterology. 1984;31:30-34. [PubMed] |
| 31. | Gerova VA, Nakov VN, Stoynov SG, Nakov RV. Prevalence of Small Intestinal Bacterial Overgrowth in Patients with Liver Cirrhosis. J of GHR. 2013;2:479-482. [DOI] [Full Text] |
| 32. | Dunagan M, Chaudhry K, Samak G, Rao RK. Acetaldehyde disrupts tight junctions in Caco-2 cell monolayers by a protein phosphatase 2A-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1356-G1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 33. | Bala S, Marcos M, Gattu A, Catalano D, Szabo G. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS One. 2014;9:e96864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 254] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 34. | Di Ciaula A, Grattagliano I, Portincasa P. Chronic alcoholics retain dyspeptic symptoms, pan-enteric dysmotility, and autonomic neuropathy before and after abstinence. J Dig Dis. 2016;17:735-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Pezzilli R, Caputo F, Testino G, Patussi V, Greco G, Macciò L, Rossin MR, Mioni D, Balbinot P, Gandin C, Zanesini F, Frulloni L, Aricò S, Bottaro LC, Pellicano R, Scafato E; Italian Society of Alcohology (SIA). Alcohol-related chronic exocrine pancreatic insufficiency: diagnosis and therapeutic management. A proposal for treatment by the Italian Association for the Study of the Pancreas (AISP) and the Italian Society of Alcohology (SIA). Minerva Med. 2019;110:425-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Mahan LK, Escott-Stump S. Medical nutrition therapy for anemia: Krause’s food, nutrition, and diet therapy. Philadelphia: WB Saunders; 2000; 469. |
| 37. | Feurer ID, Crosby LO, Mullen JL. Measured and predicted resting energy expenditure in clinically stable patients. Clin Nutrit. 1984;3:27-34. [RCA] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Müller MJ, Lautz HU, Plogmann B, Bürger M, Körber J, Schmidt FW. Energy expenditure and substrate oxidation in patients with cirrhosis: the impact of cause, clinical staging and nutritional state. Hepatology. 1992;15:782-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 184] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 39. | Reinus JF, Heymsfield SB, Wiskind R, Casper K, Galambos JT. Ethanol: relative fuel value and metabolic effects in vivo. Metabolism. 1989;38:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Lieber CS. The influence of alcohol on nutritional status. Nutr Rev. 1988;46:241-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 77] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Singal AK, Shah VH. Alcoholic hepatitis: prognostic models and treatment. Gastroenterol Clin North Am. 2011;40:611-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758-2769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 699] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 43. | Parker R, Neuberger JM. Alcohol, Diet and Drug Use Preceding Alcoholic Hepatitis. Dig Dis. 2018;36:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet. 1984;1:179-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 294] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 45. | McClain CJ, Barve S, Deaciuc I, Kugelmas M, Hill D. Cytokines in alcoholic liver disease. Semin Liver Dis. 1999;19:205-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 291] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 46. | Braillon A, Gaudin C, Poo JL, Moreau R, Debaene B, Lebrec D. Plasma catecholamine concentrations are a reliable index of sympathetic vascular tone in patients with cirrhosis. Hepatology. 1992;15:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Dolz C, Raurich JM, Ibáñez J, Obrador A, Marsé P, Gayá J. Ascites increases the resting energy expenditure in liver cirrhosis. Gastroenterology. 1991;100:738-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 95] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Friedman LA, Kimball AW. Coronary heart disease mortality and alcohol consumption in Framingham. Am J Epidemiol. 1986;124:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 211] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 49. | Longato L, Ripp K, Setshedi M, Dostalek M, Akhlaghi F, Branda M, Wands JR, de la Monte SM. Insulin resistance, ceramide accumulation, and endoplasmic reticulum stress in human chronic alcohol-related liver disease. Oxid Med Cell Longev. 2012;2012:479348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 50. | Freinkel N, Arky RA, Singer DL, Cohen AK, Bleicher SJ, Anderson JB, Silbert CK, Foster AE. Alcohol Hypoglycemia: IV: Current Concepts of Its Pathogenesis. Diabetes. 1965;14:350-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 64] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Williams HE. Alcoholic hypoglycemia and ketoacidosis. Med Clin North Am. 1984;68:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Steiner JL, Lang CH. Dysregulation of skeletal muscle protein metabolism by alcohol. Am J Physiol Endocrinol Metab. 2015;308:E699-E712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 53. | Duane P, Peters TJ. Nutritional status in alcoholics with and without chronic skeletal muscle myopathy. Alcohol Alcohol. 1988;23:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 54. | Ekbom K, Hed R, Kirstein L, Astrom Ke. Muscular Affections In Chronic Alcoholism. Arch Neurol. 1964;10:449-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 72] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 55. | Sozio M, Crabb DW. Alcohol and lipid metabolism. Am J Physiol Endocrinol Metab. 2008;295:E10-E16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 56. | Endo M, Masaki T, Seike M, Yoshimatsu H. TNF-alpha induces hepatic steatosis in mice by enhancing gene expression of sterol regulatory element binding protein-1c (SREBP-1c). Exp Biol Med (Maywood). 2007;232:614-621. [PubMed] |
| 57. | Sugimoto T, Yamashita S, Ishigami M, Sakai N, Hirano K, Tahara M, Matsumoto K, Nakamura T, Matsuzawa Y. Decreased microsomal triglyceride transfer protein activity contributes to initiation of alcoholic liver steatosis in rats. J Hepatol. 2002;36:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 58. | Hoyumpa AM Jr. Mechanisms of thiamin deficiency in chronic alcoholism. Am J Clin Nutr. 1980;33:2750-2761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 109] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 59. | Hershkowitz E, Reshef A, Munich O, Yosefi B, Markel A. Thiamine deficiency in self-induced refeeding syndrome, an undetected and potentially lethal condition. Case Rep Med. 2014;2014:605707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Medici V, Halsted CH. Folate, alcohol, and liver disease. Mol Nutr Food Res. 2013;57:596-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 61. | Lieber CS. Relationships between nutrition, alcohol use, and liver disease. Alcohol Res Health. 2003;27:220-231. [PubMed] |
| 62. | Shirakami Y, Lee SA, Clugston RD, Blaner WS. Hepatic metabolism of retinoids and disease associations. Biochim Biophys Acta. 2012;1821:124-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 63. | Koop DR, Tierney DJ. Multiple mechanisms in the regulation of ethanol-inducible cytochrome P450IIE1. Bioessays. 1990;12:429-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 147] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 64. | Sato M, Lieber CS. Hepatic vitamin A depletion after chronic ethanol consumption in baboons and rats. J Nutr. 1981;111:2015-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 120] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 65. | Leo MA, Sato M, Lieber CS. Effect of hepatic vitamin A depletion on the liver in humans and rats. Gastroenterology. 1983;84:562-572. [PubMed] |
| 66. | Leo MA, Lieber CS. Alcohol, vitamin A, and beta-carotene: adverse interactions, including hepatotoxicity and carcinogenicity. Am J Clin Nutr. 1999;69:1071-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 146] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 67. | Englard S, Seifter S. The biochemical functions of ascorbic acid. Annu Rev Nutr. 1986;6:365-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 356] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 68. | Majumdar SK, Patel S, Shaw GK, O'Gorman P, Thomson AD. Vitamin C utilization status in chronic alcoholic patients after short-term intravenous therapy. Int J Vitam Nutr Res. 1981;51:274-278. [PubMed] |
| 69. | Faizallah R, Morris AI, Krasner N, Walker RJ. Alcohol enhances vitamin C excretion in the urine. Alcohol Alcohol. 1986;21:81-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 70. | Susick RL Jr, Zannoni VG. Effect of ascorbic acid on the consequences of acute alcohol consumption in humans. Clin Pharmacol Ther. 1987;41:502-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 71. | Chen MF, Boyce HW Jr, Hsu JM. Effect of ascorbic acid on plasma alcohol clearance. J Am Coll Nutr. 1990;9:185-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 72. | Shaikh H, Faisal MS, Mewawalla P. Vitamin C deficiency: rare cause of severe anemia with hemolysis. Int J Hematol. 2019;109:618-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 73. | Zhong W, McClain CJ, Cave M, Kang YJ, Zhou Z. The role of zinc deficiency in alcohol-induced intestinal barrier dysfunction. Am J Physiol Gastrointest Liver Physiol. 2010;298:G625-G633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 74. | Kang YJ, Zhou Z. Zinc prevention and treatment of alcoholic liver disease. Mol Aspects Med. 2005;26:391-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 75. | Prasad AS. Clinical manifestations of zinc deficiency. Annu Rev Nutr. 1985;5:341-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 155] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 76. | Loomba V, Pawar G, Dhar KL, Setia MS. Serum zinc levels in hepatic encephalopathy. Indian J Gastroenterol. 1995;14:51-53. [PubMed] |
| 77. | Flink EB. Magnesium deficiency. Etiology and clinical spectrum. Acta Med Scand Suppl. 1981;647:125-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 78. | Altura BM, Altura BT, Carella A, Turlapaty PD. Hypomagnesemia and vasoconstriction: possible relationship to etiology of sudden death ischemic heart disease and hypertensive vascular diseases. Artery. 1981;9:212-231. [PubMed] |
| 79. | Touyz RM. Magnesium in clinical medicine. Front Biosci. 2004;9:1278-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 80. | Prystupa A, Kiciński P, Luchowska-Kocot D, Błażewicz A, Niedziałek J, Mizerski G, Jojczuk M, Ochal A, Sak JJ, Załuska W. Association between Serum Selenium Concentrations and Levels of Proinflammatory and Profibrotic Cytokines-Interleukin-6 and Growth Differentiation Factor-15, in Patients with Alcoholic Liver Cirrhosis. Int J Environ Res Public Health. 2017;14:437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 81. | Nangliya V, Sharma A, Yadav D, Sunder S, Nijhawan S, Mishra S. Study of trace elements in liver cirrhosis patients and their role in prognosis of disease. Biol Trace Elem Res. 2015;165:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 82. | Aaseth J, Thomassen Y, Alexander J, Norheim G. Decreased serum selenium in alcoholic cirrhosis. N Engl J Med. 1980;303:944-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 83. | Sher L. The link between alcohol abuse and suicide: possible role of selenium deficiency. Med Hypotheses. 2008;70:899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 84. | Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, Sawyer MB. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166-173, 173.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 632] [Article Influence: 45.1] [Reference Citation Analysis (1)] |
| 85. | Gunsar F, Raimondo ML, Jones S, Terreni N, Wong C, Patch D, Sabin C, Burroughs AK. Nutritional status and prognosis in cirrhotic patients. Aliment Pharmacol Ther. 2006;24:563-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 86. | Jeong JY, Lim S, Sohn JH, Lee JG, Jun DW, Kim Y. Presence of Sarcopenia and Its Rate of Change Are Independently Associated with Long-term Mortality in Patients with Liver Cirrhosis. J Korean Med Sci. 2018;33:e299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 87. | Huisman EJ, Trip EJ, Siersema PD, van Hoek B, van Erpecum KJ. Protein energy malnutrition predicts complications in liver cirrhosis. Eur J Gastroenterol Hepatol. 2011;23:982-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 88. | Lockwood AH, McDonald JM, Reiman RE, Gelbard AS, Laughlin JS, Duffy TE, Plum F. The dynamics of ammonia metabolism in man. Effects of liver disease and hyperammonemia. J Clin Invest. 1979;63:449-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 281] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 89. | Merli M, Giusto M, Lucidi C, Giannelli V, Pentassuglio I, Di Gregorio V, Lattanzi B, Riggio O. Muscle depletion increases the risk of overt and minimal hepatic encephalopathy: results of a prospective study. Metab Brain Dis. 2013;28:281-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 90. | Vidot H, Bowen DG, Carey S, McCaughan GW, Allman-Farinelli M, Shackel NA. Aggressive nutrition intervention reduces ascites and frequency of paracentesis in malnourished patients with cirrhosis and ascites. JGH Open. 2017;1:92-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 91. | Borzio M, Salerno F, Piantoni L, Cazzaniga M, Angeli P, Bissoli F, Boccia S, Colloredo-Mels G, Corigliano P, Fornaciari G, Marenco G, Pistarà R, Salvagnini M, Sangiovanni A. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Dig Liver Dis. 2001;33:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 301] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 92. | Christou L, Pappas G, Falagas ME. Bacterial infection-related morbidity and mortality in cirrhosis. Am J Gastroenterol. 2007;102:1510-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 93. | Merli M, Lucidi C, Giannelli V, Giusto M, Riggio O, Falcone M, Ridola L, Attili AF, Venditti M. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin Gastroenterol Hepatol. 2010;8:979-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 94. | Kim HY, Jang JW. Sarcopenia in the prognosis of cirrhosis: Going beyond the MELD score. World J Gastroenterol. 2015;21:7637-7647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 105] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (1)] |
| 95. | Bhanji RA, Narayanan P, Moynagh MR, Takahashi N, Angirekula M, Kennedy CC, Mara KC, Dierkhising RA, Watt KD. Differing Impact of Sarcopenia and Frailty in Nonalcoholic Steatohepatitis and Alcoholic Liver Disease. Liver Transpl. 2019;25:14-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 96. | Kalafateli M, Mantzoukis K, Choi Yau Y, Mohammad AO, Arora S, Rodrigues S, de Vos M, Papadimitriou K, Thorburn D, O'Beirne J, Patch D, Pinzani M, Morgan MY, Agarwal B, Yu D, Burroughs AK, Tsochatzis EA. Malnutrition and sarcopenia predict post-liver transplantation outcomes independently of the Model for End-stage Liver Disease score. J Cachexia Sarcopenia Muscle. 2017;8:113-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 239] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 97. | Ney M, Li S, Vandermeer B, Gramlich L, Ismond KP, Raman M, Tandon P. Systematic review with meta-analysis: Nutritional screening and assessment tools in cirrhosis. Liver Int. 2020;40:664-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 98. | McFarlane M, Hammond C, Roper T, Mukarati J, Ford R, Burrell J, Gordon V, Burch N. Comparing assessment tools for detecting undernutrition in patients with liver cirrhosis. Clin Nutr ESPEN. 2018;23:156-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 99. | Borhofen SM, Gerner C, Lehmann J, Fimmers R, Görtzen J, Hey B, Geiser F, Strassburg CP, Trebicka J. The Royal Free Hospital-Nutritional Prioritizing Tool Is an Independent Predictor of Deterioration of Liver Function and Survival in Cirrhosis. Dig Dis Sci. 2016;61:1735-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 100. | Plauth M, Bernal W, Dasarathy S, Merli M, Plank LD, Schütz T, Bischoff SC. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr. 2019;38:485-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 440] [Article Influence: 62.9] [Reference Citation Analysis (3)] |
| 101. | Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11:693-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 873] [Cited by in RCA: 794] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 102. | Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1744] [Cited by in RCA: 2157] [Article Influence: 107.9] [Reference Citation Analysis (0)] |
| 103. | Gaikwad NR, Gupta SJ, Samarth AR, Sankalecha TH. Handgrip dynamometry: a surrogate marker of malnutrition to predict the prognosis in alcoholic liver disease. Ann Gastroenterol. 2016;29:509-514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 104. | Antar R, Wong P, Ghali P. A meta-analysis of nutritional supplementation for management of hospitalized alcoholic hepatitis. Can J Gastroenterol. 2012;26:463-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 105. | Mehanna HM, Moledina J, Travis J. Refeeding syndrome: what it is, and how to prevent and treat it. BMJ. 2008;336:1495-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 378] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 106. | Ney M, Vandermeer B, van Zanten SJ, Ma MM, Gramlich L, Tandon P. Meta-analysis: oral or enteral nutritional supplementation in cirrhosis. Aliment Pharmacol Ther. 2013;37:672-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 107. | Kearns PJ, Young H, Garcia G, Blaschke T, O'Hanlon G, Rinki M, Sucher K, Gregory P. Accelerated improvement of alcoholic liver disease with enteral nutrition. Gastroenterology. 1992;102:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 164] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 108. | Moreno C, Deltenre P, Senterre C, Louvet A, Gustot T, Bastens B, Hittelet A, Piquet MA, Laleman W, Orlent H, Lasser L, Sersté T, Starkel P, De Koninck X, Negrin Dastis S, Delwaide J, Colle I, de Galocsy C, Francque S, Langlet P, Putzeys V, Reynaert H, Degré D, Trépo E. Intensive Enteral Nutrition Is Ineffective for Patients With Severe Alcoholic Hepatitis Treated With Corticosteroids. Gastroenterology. 2016;150:903-10.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 128] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 109. | Eghtesad S, Poustchi H, Malekzadeh R. Malnutrition in liver cirrhosis:the influence of protein and sodium. Middle East J Dig Dis. 2013;5:65-75. [PubMed] |
| 110. | Córdoba J, López-Hellín J, Planas M, Sabín P, Sanpedro F, Castro F, Esteban R, Guardia J. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 270] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 111. | Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1435] [Article Influence: 59.8] [Reference Citation Analysis (1)] |
| 112. | Al-Obaid LN, Bazarbashi AN, Cohen ME, Kim J, Lei Y, Axelrad JE, Fox A, Chandra S, Gordon FD. Enteric tube placement in patients with esophageal varices: Risks and predictors of postinsertion gastrointestinal bleeding. JGH Open. 2020;4:256-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 113. | Aoufi Rabih S, García Agudo R, Legaz Huidobro ML, Ynfante Ferrús M, González Carro P, Pérez Roldán F, Ruiz Carrillo F, Tenías Burillo JM. Exocrine pancreatic insufficiency and chronic pancreatitis in chronic alcoholic liver disease: coincidence or shared toxicity? Pancreas. 2014;43:730-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 114. | Shukla S, Shukla A, Mehboob S, Guha S. Meta-analysis: the effects of gut flora modulation using prebiotics, probiotics and synbiotics on minimal hepatic encephalopathy. Aliment Pharmacol Ther. 2011;33:662-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 115. | Blendea MC, Thompson MJ, Malkani S. Diabetes and Chronic Liver Disease: Etiology and Pitfalls in Monitoring. C. lin Diabetes. 2010;28:139-144. |
| 116. | The National Institute for Health and Care Excellence (NICE 2018). Vitamin D deficiency in adults-treatment and prevention. 2018 [cited 1 December 2019]. Available from: https://cks.nice.org.uk/vitamin-d-deficiency-in-adults-treatment-and-prevention. |
| 117. | Scientific Advisory Committee on Nutrition (SACN 2016). Vitamin D and Health. 2016 [cited 1 December 2019]. Available from: https://www.gov.uk/government/groups/scientific-advisory-committee-on-nutrition. |
| 118. | Richie JP Jr, Nichenametla S, Neidig W, Calcagnotto A, Haley JS, Schell TD, Muscat JE. Randomized controlled trial of oral glutathione supplementation on body stores of glutathione. Eur J Nutr. 2015;54:251-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 119. | Huang TS, Shyu YC, Chen HY, Lin LM, Lo CY, Yuan SS, Chen PJ. Effect of parenteral selenium supplementation in critically ill patients: a systematic review and meta-analysis. PLoS One. 2013;8:e54431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 120. | Butterworth RF, McPhail MJW. L-Ornithine L-Aspartate (LOLA) for Hepatic Encephalopathy in Cirrhosis: Results of Randomized Controlled Trials and Meta-Analyses. Drugs. 2019;79:31-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: the Member of the British Society of Gastroenterology, No. 1193.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barone M, Cravo M S-Editor: Yan JP L-Editor: A E-Editor: Liu MY