Published online Jun 7, 2020. doi: 10.3748/wjg.v26.i21.2768
Peer-review started: December 30, 2019
First decision: January 13, 2020
Revised: March 27, 2020
Accepted: May 26, 2020
Article in press: May 26, 2020
Published online: June 7, 2020
Processing time: 158 Days and 19.3 Hours
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease characterized by biliary inflammation and stricturing. Exploration of the pathogenesis of PSC in light of its association with inflammatory bowel disease (IBD) and the “gut-liver” axis is an emerging area of interest. A growing number of studies have begun to elucidate the role of the gut microbiota, its metabolites and its influence on host immune responses in the development of PSC and PSC-IBD. Studies of the fecal microbiota have highlighted enriched levels of certain species, including Veillonella, Streptococcus and Enterococcus, among others. A heightened immune response to enteric dysbiosis and bacterial translocation have also been implicated. For example, Klebsiella pneumoniae strains derived from gnotobiotic mice transplanted with PSC-IBD microbiota were found to induce pore formation in human intestinal epithelial cells and enhanced Th17 responses. Gut microbes have additionally been hypothesized to be implicated in PSC pathogenesis through their role in the synthesis of various metabolites, including bile acids (BAs), which function as signaling molecules with important gut and hepatic effects. An expanded knowledge of the gut microbiome as it relates to PSC offers critical insight into the development of microbe-altering therapeutic interventions, such as antibiotics, nutritional interventions and fecal microbial transplantation. Some of these have already shown some preliminary evidence of benefit. Despite exciting progress in the field, much work remains to be done; areas that are particularly lacking include functional characterization of the microbiome and examination of pediatric populations. In this review, we summarize studies that have investigated the microbiome in PSC and PSC-IBD as well as putative mechanisms, including the potential role of metabolites, such as BAs. We then briefly review the evidence for interventions with microbe-altering properties for treating PSC.
Core tip: The frequent coexistence of primary sclerosing cholangitis (PSC) and inflammatory bowel disease (IBD) points to the gut-liver axis as central to pathogenesis. The gut microbiome is hypothesized to be involved. A growing body of literature supports that PSC and PSC-IBD are associated with a distinct gut microbiome and more recent animal studies suggest a potential causal relationship. Microbial metabolites, such as bile acids, may mediate the effects of the gut microbiota in PSC. A sound understanding of the PSC microbiome has the potential to inform the development of microbe-altering therapeutic interventions.
- Citation: Little R, Wine E, Kamath BM, Griffiths AM, Ricciuto A. Gut microbiome in primary sclerosing cholangitis: A review. World J Gastroenterol 2020; 26(21): 2768-2780
- URL: https://www.wjgnet.com/1007-9327/full/v26/i21/2768.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i21.2768
Primary sclerosing cholangitis (PSC), a cholestatic liver disease that causes inflammation and fibrosis of the biliary tree, represents a pressing unmet need in the field of Hepatology[1]. Although a rare disease in the general population (prevalence 0-16 per 100000 adults[1], and 1.5 per 100000 children)[2], a striking association exists between PSC and inflammatory bowel disease (IBD); approximately 75% of adults and children with PSC have IBD[3,4], and conversely, up to 8% of IBD patients are found to have large duct PSC when screening magnetic resonance cholan-giopancreatography is applied[5]. The natural history of PSC is progression to end-stage biliary cirrhosis requiring liver transplantation (LT); in adults, the median LT-free survival after diagnosis is 14.5 years[6], and 30% of children require a LT by 10 years after diagnosis[4]. Furthermore, PSC is associated with a markedly increased risk of colorectal malignancy (20%-30% lifetime risk, four times higher than colitis without PSC) and hepatobiliary malignancy, particularly cholangiocarcinoma, which has a 20% lifetime risk and poor prognosis[7]. To date, no medical therapy has been shown to alter the natural history of PSC. The significance of PSC is underscored by the fact that it has been identified as the only predictor of premature mortality in IBD populations[8]. Even in the pediatric age range, it is a significant risk factor for cancer-related mortality[9].
There are now abundant data to support that IBD occurring in association with PSC (“PSC-IBD”) is distinct from conventional IBD not associated with PSC. A robust body of adult literature supports a distinct PSC-IBD clinical phenotype, characterized by a higher frequency of pancolitis (typically worse in the right colon, contrary to chronic ulcerative colitis (UC), which is most severe distally in the left colon), rectal sparing and backwash ileitis[3,10,11]. In addition, subclinical inflammation (mucosal disease in the presence of no or minimal symptoms) has been described and is hypothesized to contribute to the heightened neoplastic potential[12]. In a large retrospective review, we recently demonstrated that a similar phenotype is also observed in children[13]. In addition to the unique clinical phenotype of PSC-IBD, there is relatively little genetic overlap between PSC-IBD and conventional IBD, further supporting that PSC-IBD is a distinct entity[14]. This growing body of literature is important for several reasons; it highlights the need to conceptualize (and investigate) PSC-IBD as separate from IBD and the characteristic gut phenotype may provide insight into underlying pa-thogenesis.
Although the pathogenesis of PSC remains obscure, the striking association between PSC and IBD points to the gut-liver axis as integrally involved. While strong HLA associations and greater than 23 non-HLA susceptibility loci have been identified, known genetic risk factors currently account for less than 10% of PSC liability, highlighting the likely critical role of environmental factors[15], chief among them the gut microbiota. The microbiota can be considered an environmental factor on its own, but is also influenced by other environmental factors, such as diet, medications, hygiene and even mood[16]. A theory of PSC pathogenesis invoking the gut microbiota has long been postulated. The basic hypothesis is that gut microbes (or their products) translocate across a leaky gut barrier (due to inflammation) and access the liver via the portal circulation, where they trigger inflammatory and ultimately fibrotic processes. In the 1990s, Lichtman et al[17,18] provided the first compelling support for this hypothesis, showing that small bowel bacterial overgrowth, achieved using a blind jejunal loop, induced cholangiographic changes resembling PSC in rats. Moreover, these changes were reversible with antibiotics and peptidoglycan-degrading enzymes, but not prednisone or ursodiol. Since then, numerous additional findings have reinforced the role of the gut microbiota and its interactions with innate immune responses in PSC, including the identification of FUT2 (involved in handling translocated bacteria) as a PSC risk locus[14], and the observation that lipo-polysaccharide levels correlate with LT-free survival in PSC[19].
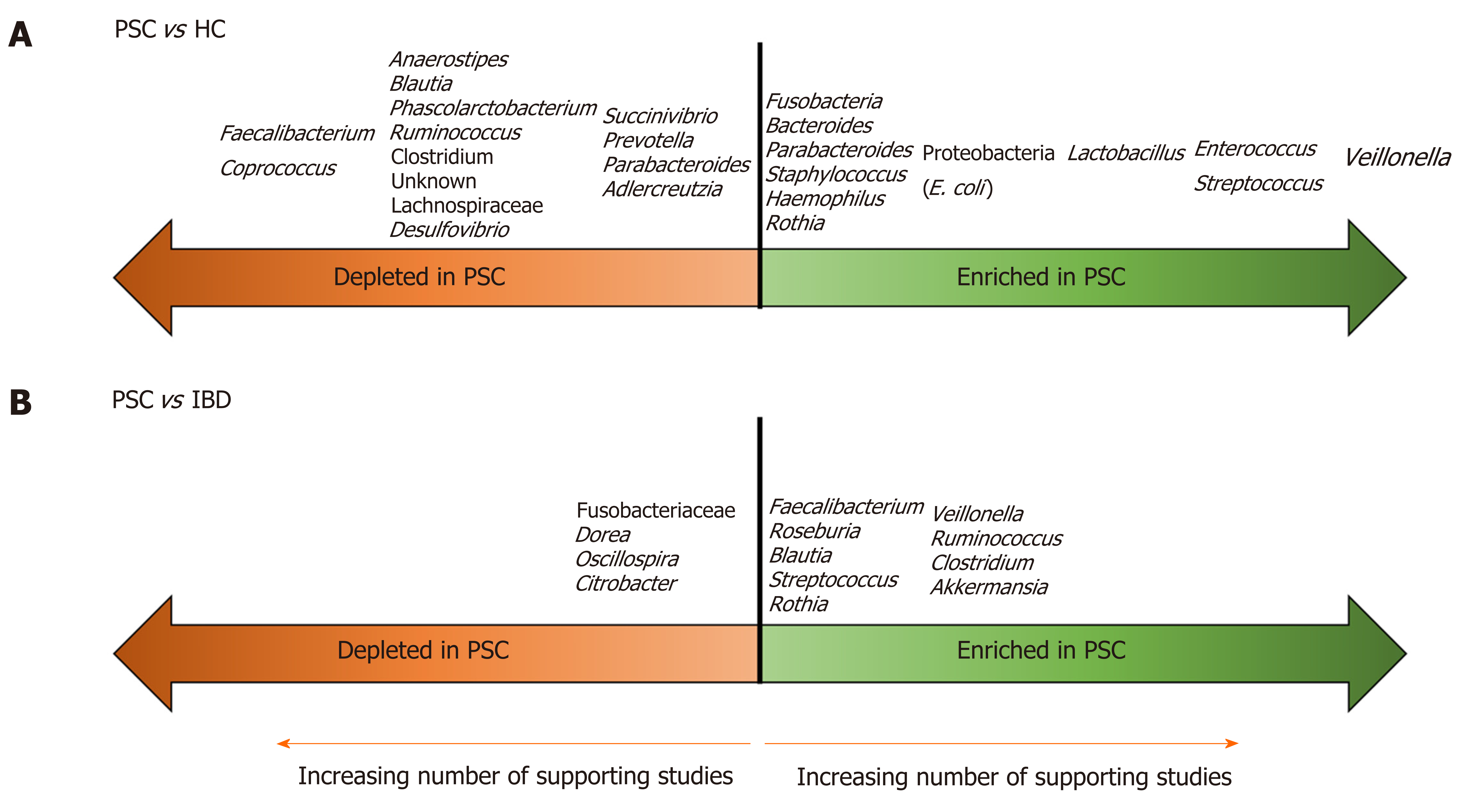
In the past five years, there has been a flurry of publications aimed at characterizing the gut microbiota in PSC/PSC-IBD compared to healthy controls (HCs) and IBD populations. There have been seven publications (four full-length papers[20-23], three letters[24-26]) on the fecal microbiota, and five (four full-length papers[27-30], one letter[31]) on the mucosal microbiota in PSC. To date, only one study, a letter investigating the fecal microbiota, examined a pediatric population (in Japan)[26]. All used 16S rRNA methods to establish microbial composition. These studies are summarized in Table 1. The findings are further summarized in Figure 1, which illustrates microbial taxa that are enriched and depleted in PSC compared to HCs and IBD patients.
| Ref. | Population | Sample source | Methods | Taxa depleted in PSC | Taxa enriched in PSC | Diversity in PSC |
| Adult | ||||||
| Mucosal | ||||||
| Quraishi et al[30], 2020 | 10 PSC-IBD 10 UC; 10 HC | Mucosal biopsies, sigmoid | 16S rRNA; V4 amplified Illumina MiSeq | vs HC: Lachnospiraceae | vs HC: Bacilli, Pseudomonas, Streptococcus, Haemophilus parainfluenzae | Alpha: No difference; Beta: All 3 groups significantly different from each other |
| vs UC: 26 taxa, including Lentisphaerae, Gammaproteobac-teria, Enterobacteriaceae, Prevotellaceae, Paraprevotellaceae, Coriobacteriaceae, Erysipelotrichaceae, Desulfovibrionaceae, Myxococcales, Streptococcus, Blautia | vs UC: 24 taxa, including Bacilli, Staphylococcus, Parvimonas sp., Bacteroides fragilis, Roseburia spp., Shewanella spp, Clostridium ramosum, Sphingomonas sp., Actinomyces, Rothia | |||||
| Quraishi et al[31], 2017 (Letter) | 11 PSC-IBD 10 IBD 9 HC | Mucosal biopsies, ascending, transverse, descending colon (results not different by site) | 16S rRNA; V3-V4 amplified; Illumina MiSeq | vs HC: Prevotella, Roseburia, Bacteroides | vs HC: Escherichia, Lachnospiraceae, Megasphera | Beta: Different from HC and IBD |
| Torres et al[27], 2016 | 13 PSC-UC; 6 PSC-CD; 1 PSC only; 13 UC; 2 CD; 9 HC | Mucosal biopsies from terminal ileum, right and left colon (results not different by site) | 16S rRNA; V3-V4 amplified; Illumina MiSeq | vs IBD | vs IBD: Barnesiellaceae, Blautia, Ruminococcus obeum | Alpha: Similar to HC and IBD; Beta: Similar to HC and IBD |
| Kevans et al[28], 2016 | 31 PSC-UC; 56 UC; 0 HC | Mucosal biopsies, left colon | 16S rRNA; V4 amplified; Illumina MiSeq | Nil after multiple testing adjustment | Nil after multiple testing adjustment | Alpha: ↓ vs UC (in 1 cohort only); Beta: No difference |
| Rossen et al[29], 2015 | 8 PSC-UC; 4 PSC-CD; 11 UC; 9 HC | Mucosal biopsies from ileocecum | 16S rRNA using HITChip | vs HC: Uncultured Clostridiales II | vs HC | Alpha: ↓ vs HC; Beta: Similar to HC and IBD |
| vs UC: Uncultured Clostridiales II | vs UC | |||||
| Fecal | ||||||
| Lemoinne et al[38], 2019 | 16 PSC-UC/IBD-U; 11 PSC-CD; 22 PSC only; 33 IBD; 30 HC | Stool | 16S rRNA; V3-V4 amplified; Illumina MiSeq | vs HC: Ruminococcus, Faecalibacterium, Lachnoclostridium, Blautia | vs HC: Veillonella, Sphingomonada-ceae, Alphaproteobac-teria, Rhizobiales | Alpha: ↓ vs HC and IBD; Beta: PSC-IBD different from HC and IBD, PSC-IBD similar to PSC only |
| Rühlemann et al[20], 2019 | 75 PSC-IBD; 62 PSC only; 118 UC; 133 HC | Stool | 16S rRNA; V1-V2 amplified MiSeq | vs HC: CoprococcusHoldemanella, Desulfovibrio, Faecalibacterium, Clostridium IV | vs HC: Veillonella, Streptococcus, Lactobacillus, Enterococcus; Proteobacteria (Gammaproteobacteria), Lactobacillales (Bacilli), Parabacteroides, Bacteroides spp. | Norwegian cohort; Alpha: ↓ vs HC, similar to UC; Beta: Different from HC and IBD; German cohort; Alpha: Similar to HC, ↑ vs UC; Beta: Different from HC and IBD, PSC-IBD similar to PSC only |
| vs UC | vs UC: Firmicutes | |||||
| PSC-IBD vs PSC only: Bilophila and Bacteroides OTU ↓ | PSC-IBD vs PSC only: Nil | |||||
| Rühlemann et al[25], 2017 (Letter) | 38 PSC-UC; 35 PSC only; 88 UC; 98 HC | Stool | 16S rRNA; V1-V2 | vs HC: Veillonella (no difference compared to UC) | Beta: Different from HC and IBD, PSC-IBD similar to PSC only | |
| Kummen et al[21], 2017 | 44 PSC-UC; 11 PSC-CD; 30 PSC only; 36 UC; 263 HC | Stool | 16S rRNA; V3-V4 amplified; Illumina MiSeq | vs HC: ML615J-28 unknown genus, Succinivibrio, Desulfovibrio, RF32 unknown genus, Phascolarctobacteri-um, Coprococcus, Lachnospiraceae unknown genus, Christensenellaceae unknown genus, Clostridiales unknown genus, YS2 unknown genus, S24.7 unknown genus | vs HC: Veillonella (V. dispar, V. parvula) | Alpha: ↓ vs HC, similar to IBD; Beta: Different from HC and IBD, PSC-IBD similar to PSC only |
| vs UC: Dorea, Oscillospira, Citrobacter | vs UC: Veillonella (V. dispar, V. parvula); Akkermansia, Clostridium, Ruminococcaceae | |||||
| PSC-IBD vs PSC only: Nil | PSC-IBD vs PSC only: Nil | |||||
| Bajer et al[22], 2017 | 32 PSC-IBD; 11 PSC only; 32 UC 31 HC | Stool | 16S rRNA; V4 amplified Illumina MiSeq | vs HC: Coprococcus (C. catus), unidentified Lachnospiraceae genera, Faecalibacterium prausnitzii, Ruminococcus gnavus, Prevotella copri (PSC only and PSC-IBD) Phascolarctobacterium (PSC-IBD); Adlercreutzia equolifaciens (PSC only) | vs HC: Rothia, Enterococcus, Streptococcus, Clostridium, Veillonella, Haemophilus (PSC only and PSC-IBD); Staphylococcus, Coprobacillus, Escherichia, Corynebacterium, Lactobacillus (PSC-IBD) | Alpha: Similar to HC and UC; Beta: Different from HC, PSC-IBD different from IBD, PSC-IBD similar to PSC only |
| vs UC: Fusobacteriaceae | vs UC: Rothia, Streptococcus, Veillonella, Blautia; Akkermansia muciniphila, Clostridium colinum | |||||
| Sabino et al[23], 2016 | 18 PSC only; 27 PSC-UC; 21 PSC-CD; 13 UC; 30 CD; 66 HC | Stool | 16S rRNA; V4 amplified; Illumina MiSeq | vs HC: Firmicutes Anaerostipes | vs HC: Bacteroidetes, Fusobacteria, Streptococcus, Enterococcus, Lactobacillus, Fusobacterium, Veillonella1 | Alpha: ↓ vs HC; Beta: Different from HC, PSC-UC different from UC, PSC-IBD similar to PSC only |
| PSC-IBD vs PSC only: Nil | PSC-IBD vs PSC only: Nil | |||||
| In a re-analysis by Vieira-Silva et al[24] in 2019, Fusobacterium was associated with intestinal inflammation and Enterococcus with cholangitis/biliary obstruction. | ||||||
| Pediatric | ||||||
| Iwasawa et al[26], 2017 (Letter) | 27 PSC; 16 UC; 23 HC | Stool | 16S rRNA; 1-V2 amplified | vs HC: Parabacteroides (P distasonis), Anaerostipes hadrus, Blautia obeum | vs HC: Enterococcus faecium, Enterococcus sp. NBRC 107345, Streptococcus parasanguinis, Veillonella sp. 3_1_44 | Alpha: ↓ vs HC, ↑ vs IBD; Beta: Different from HC and IBD |
| vs UC | vs UC: Faecalibacterium, Ruminococcus, Roseburia | |||||
In interpreting studies of the microbiota, it is important to appreciate that microbial populations differ substantially between the gut lumen and mucosal surfaces. For example, in a large study of new-onset pediatric Crohn’s disease (CD), mucosal-associated dysbiosis was only weakly reflected in stool, and mucosal samples were superior to fecal samples for distinguishing CD from healthy controls[32]. It is also important to note that studies of the mucosal microbiota (compared to the fecal microbiota) may be susceptible to greater variation stemming from differences in sampling methods (biopsies, brushings, luminal washes, etc.) and other procedural factors.
Indeed, studies of the fecal microbiome in PSC are more consistent (than those of the mucosal microbiome) and they reveal a few themes. The first is that the gut bacterial alpha diversity is lower in PSC than HCs (in contrast, studies comparing alpha diversity between PSC and IBD cohorts have yielded variable findings). The second is that, although studies are not entirely consistent in terms of the specific microbes altered in PSC, they are consistent in demonstrating that the overall bacterial community is different in PSC/PSC-IBD vs HCs and IBD. The third is that, despite this heterogeneity, a few bacterial taxa are fairly consistently altered in the stool of PSC patients compared to HCs. Veillonella, in particular, was higher in the stool of PSC patients than HCs in all the studies in Table 1. Enterococcus, Streptococcus and Lactobacillus are also frequently enriched, as are members of the Proteobacteria phylum, such as E. coli. Several studies also support a relative depletion of short chain fatty acid (SCFA)-producing Firmicutes, such as Faecalibacterium and Coprococcus, in PSC.
In one study, Veillonella was 4.8-fold higher in individuals with PSC compared to HCs and displayed a positive correlation with the Mayo Risk Score (a PSC prognostic index). Moreover, Veillonella discriminated PSC from HCs with an area under the receiver operator characteristic curve (AUC) of 0.64[21]. This improved to 0.78 when other PSC-associated genera were added. Interestingly, Veillonella has been associated with pulmonary fibrotic conditions and CD recurrence post resection[33,34]. It is tempting to speculate that Veillonella directly contributes to fibrogenesis in PSC, but such evidence is lacking. Veillonella is not specific to PSC either; it is observed in a broad range of liver diseases, such as primary biliary cirrhosis, hepatitis B cirrhosis and hepatic encephalopathy, and may therefore reflect the common end-stage of multiple hepatic pathologies[35-37]. In another study by Sabino and colleagues, Enterococcus, Lactobacillus and Fusobacteria were overrepresented in PSC patients and a model including these three microbes correctly classified PSC 95% and 71% of the time in training and validation cohorts[23]. An Enterococcus operational taxonomic unit (OTU) correlated with alkaline phosphatase (ALP), a marker of PSC severity. More recently, this dataset was reanalyzed while accounting for stool moisture variation; Fusobacterium was found to be associated with intestinal inflammation severity, while Enterococcus was associated with biliary pathology[24]. High-Veillonella stool was observed across all disease groups in this study, whereas high-Fusobacteria stool was specific to CD or PSC-CD. In a recent study by Rühlemann et al[20], which investigated two geographically distinct cohorts (Germany and Norway), the fecal microbial composition identified PSC with AUC 0.88. These data, coupled with those presented above, highlight the possible utility of microbial signatures as prognostic and/or diagnostic markers in PSC. Rather than simply characterize the gut microbiota, Lemoinne and colleagues examined alterations in the network of correlations between bacteria in PSC patients; they found that compared to HCs and IBD patients, individuals with PSC had a decreased density of bacterial network correlations[38].
The 2019 study by Rühlemann et al[20] is important because it showed that the microbial alterations in PSC are independent from associated colitis; there were no significant differences between patients with PSC with or without concomitant IBD in beta diversity and taxonomic differences were few, limited to decreased Bilophila and a Bacteroides OTU in PSC-IBD. Similarly, several other studies from Table 1 support that the microbial findings in PSC are independent of IBD[21-23]. All of this ties in to an additional important theme, which is that the presence of IBD appears to have little effect on the composition of the gut microbiota in PSC patients, suggesting that it is PSC that drives the primary changes in microbial composition. Therefore, in addition to a distinct clinical phenotype and genetic architecture, PSC-IBD is characterized by a distinct gut microbiome compared to IBD without associated PSC.
Studies of the mucosal microbiome in PSC have been fewer in number and less consistent. Two studies found little to no changes between PSC patients and HCs[28,29], while others showed some features congruent with the fecal studies, including increased Bacilli, such as Streptococcus, and Proteobacteria (E. coli)[30,31]. A very recently published pilot study by Quraishi et al[30] warrants further discussion as it is the first to apply an integrative biology approach to the joint analysis of the gut microbiome, intestinal gene expression and immune cell signatures in PSC-IBD compared to UC and HCs. In this study, the microbial alterations in PSC-IBD vs UC (and inferred metagenomics) and the differentially expressed genes between these two groups implicated dysregulation of bile acid (BA) metabolism in PSC-IBD. Moreover, multi-omics integration revealed networks involved in bile acid homeostasis and cancer regulation in PSC-IBD. Other upregulated pathways were related to glucuronidation. Interestingly, amine oxidase-expressing bacteria, such as Sphingomonas sp., were also increased in PSC-IBD vs UC; this enzyme is associated with aberrant homing of gut lymphocytes to the liver, which is postulated to be involved in PSC pathogenesis.
While the focus has undoubtedly been on bacteria to date, investigators are beginning to examine the gut mycobiome in PSC as well. In the first such study, which examined fecal fungal profiles in 112 individuals, PSC patients were found to have fungal gut dysbiosis, characterized by altered composition and increased biodiversity (alpha diversity), compared to patients with IBD only and HCs[38]. In particular, an increase in the abundance of Exophiala genus and decrease of Saccharomyces cerevisiae were noted. In addition, the fungal microbiota of PSC patients displayed a disrupted correlation network with bacteria; a disrupted correlation network between bacteria and fungi has also been reported in patients with cirrhosis[39].
While the above studies represent an important first step along the path toward elucidating the role of the gut microbiome in PSC, they have important limitations. These include their cross-sectional nature and lack of functional data (through metagenomics, proteomics, metatranscriptomics and metabolomics investigations). Furthermore, there is a paucity of pediatric data. Children, particularly if sampled close to diagnosis, offer the important advantage of temporal proximity to disease origin and relatively fewer comorbidities, which may help to disentangle cause from consequence and minimize confounding variables.
Until recently, PSC microbiota studies have been largely associational. In the past few years, however, a handful of elegant animal experiments have yielded more persuasive support for a causal link, while simultaneously shedding light on the mechanisms by which pathobionts might interface with host immune responses to cause hepatobiliary injury in PSC[40]. Three of these recent studies have been very informative[41-43]. All three support the concepts of dysbiosis, bacterial translocation across the gut barrier, and heightened immune responses (adaptive or innate) in PSC pathogenesis. While the term “dysbiosis” is difficult to specifically define, in these studies, it generally denotes differences in the composition of the gut microbiota, as it relates to diversity and relative abundance of specific taxa, compared to a control group[44]. Importantly, two of these studies showed transferability of the PSC phenotype with fecal microbiota transplant (FMT)[41,42]. Specifically, Nakamoto et al[41] demonstrated that inoculation of germ-free (GF) mice with feces from patients with PSC-UC was associated with Th17 priming in the liver and increased susceptibility to hepatobiliary injury by diethyldithiocarbamate. Investigators isolated Klebsiella pneumoniae, Proteus mirabilis and Enterococcus gallinarum from the mesenteric lymph nodes of these animals. Specific K. pneumoniae strains were found to induce pore formation on human intestinal epithelial organoids, providing a putative mechanism for bacterial translocation, as further supported by the observation that transferring non-pore-inducing strains resulted in lower serum endotoxin levels and decreased Th17 responses. An imbalance between Th17 and T regulatory (Treg) responses has previously been implicated in PSC[45,46]. Tedesco et al[43] and Liao et al[42] both investigated MDR2-/- mice, an animal model of human PSC. Both groups confirmed dysbiosis and increased gut permeability in MDR2-/- mice. Tedesco et al[43] documented increased interleukin (IL)-17A levels, further supporting the role of Th17 responses, but extended previous findings by showing that this was mediated, at least in part, by expansion of unconventional T cells (γδ T cells). Lactobacillus gasseri, which was pH- and bile-resistant (suggesting selection of specific bacteria under PSC-induced conditions) was isolated from MDR2-/- livers and found to stimulate IL-17 production from MDR2-/--derived γδ T cells. Interestingly, γδ T cells from PSC patients, but not patients with other liver diseases like hepatitis C, produced IL-17 when stimulated. The gut dysbiosis in MDR2-/- mice reported by Liao et al[42] was characterized by significant alterations in Lachnospiraceae. In addition, Enterococcus was isolated from MDR2-/- livers. MDR2-/- mice displayed increased NLRP3 inflammasome activation in the liver and gut. Transferability of phenotype (NLRP3 activation, increased gut permeability, including suppressed zonulin-1 tight junction expression, elevated serum transaminases and an inflammatory infiltrate dominated by monocyte-derived macrophages) was achieved with FMT of MDR2-/- fecal samples into GF mice. In all three studies, inhibition of the postulated immunologic mechanism using (1) a small molecule antagonist of retinoid-related receptor-γt (a selective inhibitor of Th17 differentiation; (2) anti-γδ T-cell receptor; and (3) a pan-caspase inhibitor led to improvement of the phenotype.
While this initial support for a potential causal relationship between gut microbes and PSC is exciting and represents critical progress, it is important to recall that one cannot directly extrapolate the findings from these animal experiments to human PSC populations. Moreover, although these studies have begun to shed light on important implicated processes, none thus far has delineated a complete and coherent causal pathway between the microbiota and the clinical manifestations of PSC.
The gut microbiota may also contribute to PSC/PSC-IBD pathogenesis through synthesis of compounds or co-metabolism of molecules produced by the host. Bile acids (BAs) are one such example. The primary human BAs, cholic acid (CA) and chenodeoxycholic acid (CDCA), are synthesized from cholesterol in the liver and secreted into the gut. The majority (95%) are actively reabsorbed in their conjugated form in the terminal ileum via the apical sodium-dependent BA transporter (ASBT). This constitutes the BA enterohepatic circulation (EHC). Microbial deconjugation generates unconjugated BAs that escape active reuptake and reach the colon. Deconjugation of primary BAs is mediated by bile salt hydrolases, which are widely distributed among the gut microbiota, including members of the Firmicutes, Bacteroidetes and Actinobacteria phyla, such as Clostridium, Bacteroides, Lactobacillus, Bifidobacterium and Enterococcus[47-50]. About 5% of unabsorbed BAs enter the distal ileum and colon, where they undergo chemical diversification by the gut microbiota. The predominant secondary BAs are lithocholic acid (LCA) and deoxycholic acid (DCA), but over 50 different types of microbially derived secondary BAs can be detected in human feces[51]. Only a small fraction of gut microbes possess the functional ability to carry out the 7α-dehydroxylation step involved in generating secondary BAs, such as Clostridium spp[48,52-57]. DCA and LCA generated in this way can then be further microbially altered to produce other secondary BAs.
BAs function as critical signaling molecules via their interactions with several receptors, including the nuclear receptor Farnesoid X receptor (FXR) and the membrane-bound G protein-coupled bile acid receptor-1, also known as TGR5. These receptors have multiple effects on the liver and gut. The secondary BAs DCA and LCA are the most potent TGR5 ligands. TGR5 agonism is involved in cho-langioprotective and anti-inflammatory effects; on cholangiocytes, TGR5 stimulates chloride secretion via CFTR to maintain the bicarbonate umbrella; on macrophages, TGR5 activation leads to decreased NF-κβ transcriptional activity, thereby decreasing inflammatory cytokine production[58]. Stimulation of the TGR5-cAMP-protein kinase A axis by secondary BAs also inhibits NLRP3 inflammasome activation[59], which as described above, may be a mechanism involved in PSC. Tabibian et al[60] demonstrated that the GF state in MDR2-/- mice leads to more severe hepatobiliary injury, an observation that is hypothesized to be due to the absence of microbially derived secondary BAs. It is interesting to note that colesevelam administration has been shown to improve liver injury in MDR2-/- mice and that this benefit occurred in conjunction with a shift in the BA pool toward secondary BAs and enhanced TGR5 signaling[61,62]. TGR5 is relevant for the gut as well. TGR5 deficiency exacerbates injury-induced colitis in mice, while treatment of wild-type mice with oleanolic acid (which resembles LCA in shape and functions as a TGR5 agonist) attenuates colitis and monocyte infiltration[63]. FXR is another critical BA receptor. Activation in lamina propria monocytes and macrophages results in NF-κβ repression and downregulation of proinflammatory cytokines[64]. BA-dependent FXR activation also prevents gut barrier dysfunction and bacterial translocation in cholestatic rats[65]. Temporal fluctuations in DCA at the site of bowel injury, mediated by FXR and driven by regional shifts in the microbiota, have been shown to be integral for colonic repair processes[66]. In addition to the effects described above mediated by TGR5 and FXR, LCA derivatives have recently been demonstrated to control host immune responses by directly modulating Th17/Treg balance[67].
In summary, BAs, including secondary BAs, shaped by the gut microbiota, offer a potential link between the gut and liver in PSC-IBD. To date, there have been only two studies to report on fecal BA profiles in PSC-IBD (15 PSC-IBD/15 IBD patients in one[68], and 7 PSC-IBD/8 IBD/8 HCs in the other)[69]. Both were small and in several ways inconsistent, but some observations are noteworthy. In the first study, the total BA pool was significantly reduced in the PSC-IBD group and there was a trend toward more conjugated BAs in this group as well. The overall combination of fecal BAs allowed excellent separation of PSC-IBD from IBD, and the relative abundance of bacterial genera that correlated with BAs was 12% in PSC-IBD compared to only 0.4% in IBD. A negative correlation was seen between secondary BAs and endoscopic activity. In the second study, there were less secondary BAs in the PSC-IBD group with a lower DCA/CA ratio. Notably, there were two patients with colonic dysplasia, and they both had minimal fecal DCA. This line of investigation requires further exploration; pediatric data and mucosal BA characterization are lacking. Importantly, further understanding of the specific roles of BAs in PSC could lead to novel therapies, as discussed below.
SCFAs (acetate, butyrate and propionate), derived from microbial fermentation of host-indigestible complex carbohydrates, represent another category of bacterial metabolites. As demonstrated in Table 1 and Figure 1, SCFA-producing Firmicutes are depleted in PSC/PSC-IBD relative to HCs. The potential role of SCFAs in PSC has been little explored. SCFAs influence both adaptive and innate immune responses, including the differentiation of regulatory T cells and production of proinflammatory cytokines[70], which may bear relevance to PSC disease processes.
Additional support for the role of the microbiota in PSC/PSC-IBD comes from preliminary interventional studies targeting or manipulating the gut microbiome. While large definitive randomized controlled trials (RCTs) are awaited, a handful of small RCTs and case series have shown biochemical improvement with antibiotic treatment (primarily vancomycin and metronidazole) of PSC patients[71]. More recently, in a pilot study of FMT in 10 PSC-IBD patients, 30% displayed an ALP reduction of at least 50%, without any adverse events[72]. An increase in bacterial diversity and engraftment correlated with a reduction in ALP[50]. Future study considerations include assessment of the efficacy of maintenance therapy and FMT via other, more practical routes, such as oral capsule.
Given the potential role of BAs in PSC, pharmacotherapies targeting BA pathways represent an active area of research for the treatment of PSC. Ursodeoxycholic acid (UDCA) is widely used. Its administration has been associated with biochemical benefits but not improvements in long-term clinical outcomes such as liver transplant or death. In fact, at higher doses of 28-30 mg/kg per day, UDA has been linked with an increased risk of serious adverse events[73]. In a small study of 15 adults with PSC that combined UDCA with all-trans retinoic acid, ALP levels fell but the change was not statistically significant[74]. As a result of the emerging evidence of potential harm at higher doses (without convincing evidence of clinically meaningful benefit), the most recent PSC guideline, put forth by the British Society of Gastroenterology in 2019, recommends against the routine use of UDCA for adults newly diagnosed with PSC[75]. A derivative of UCDA, termed norursodeoxycholic acid (norUDCA), has recently garnered attention. It showed biochemical benefit in a phase II RCT including 161 adult PSC patients. Twelve weeks of treatment with norUDCA resulted in significant reductions of serum ALP in a dose-dependent manner, with a similar safety profile to placebo[76]. Pharmacologic FXR agonists represent another category of BA-related therapies that are being explored in PSC. Obeticholic acid (OCA), an endogenous FXR ligand, was investigated in a phase II double-blind, placebo controlled RCT of 76 adult PSC patients. At week 24, OCA was associated with a significant decrease in ALP, satisfying the study’s primary outcome. There was, however, a higher rate of pruritus with OCA than placebo[77]. Cilofexor is a nonsteroidal FXR agonist. It too was recently tested in a phase II RCT and led to lower ALP levels at week 12. This improvement was seen regardless of UCDA treatment status and, importantly, cilofexor use was well tolerated[78]. Disruption of the EHC with inhibitors of ASBT (to prevent ileal BA reuptake) represents yet another potential therapeutic strategy for PSC. Studies of ASBT inhibitors are ongoing[73].
Further investigation is needed to definitively establish the utility of the above strategies and agents. Key limitations to existing data include their short-term (12 - 24 week) nature and the absence of good surrogate endpoints that have been shown to correlate with clinically meaningful outcomes. This latter limitation is a significant hindrance to the study of PSC in general.
In summary, PSC represents an urgent unmet need in the fields of IBD and Hepatology alike. The frequent coexistence of PSC and IBD points to the important role of the gut-liver axis and there is mounting evidence to suggest that the gut microbiota is implicated. Numerous studies demonstrate that the gut microbiome in individuals with PSC and PSC-IBD is distinct from that in healthy controls and individuals with IBD without liver disease, and a handful of animal experiments provide more compelling evidence of a causal role. Microbial metabolites, such as BAs and SCFAs, may represent an important intermediary between gut microbes and PSC disease processes. Despite tremendous progress in this field of study over the past several years, much work remains to be done including functional characterization of the gut microbiota and more careful examination of pediatric populations. The relationship between the microbiota and malignancy in PSC-IBD has largely been overlooked as well. The distinct PSC-IBD phenotype may offer important insight into pathophysiologic mechanisms. A better understanding of the gut microbiota in PSC and PSC-IBD may inform the development of useful microbe-altering interventions, with already some preliminary evidence of benefit, though longer-term and larger studies are needed.
| 1. | Dyson JK, Beuers U, Jones DEJ, Lohse AW, Hudson M. Primary sclerosing cholangitis. Lancet. 2018;391:2547-2559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 321] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 2. | Deneau M, Jensen MK, Holmen J, Williams MS, Book LS, Guthery SL. Primary sclerosing cholangitis, autoimmune hepatitis, and overlap in utah children: Epidemiology and natural history. Hepatology. 2013;58:1392-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 3. | Palmela C, Peerani F, Castaneda D, Torres J, Itzkowitz SH. Inflammatory Bowel Disease and Primary Sclerosing Cholangitis: A Review of the Phenotype and Associated Specific Features. Gut Liver. 2018;12:17-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 4. | Deneau MR, El-Matary W, Valentino PL, Abdou R, Alqoaer K, Amin M, Amir AZ, Auth M, Bazerbachi F, Broderick A, Chan A, Cotter J, Doan S, El-Youssef M, Ferrari F, Furuya KN, Gottrand M, Gottrand F, Gupta N, Homan M, Kamath BM, Kim KM, Kolho KL, Konidari A, Koot B, Iorio R, Ledder O, Mack C, Martinez M, Miloh T, Mohan P, O'Cathain N, Papadopoulou A, Ricciuto A, Saubermann L, Sathya P, Shteyer E, Smolka V, Tanaka A, Varier R, Venkat V, Vitola B, Vos MB, Woynarowski M, Yap J, Jensen MK. The natural history of primary sclerosing cholangitis in 781 children: A multicenter, international collaboration. Hepatology. 2017;66:518-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 155] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 5. | Lunder AK, Hov JR, Borthne A, Gleditsch J, Johannesen G, Tveit K, Viktil E, Henriksen M, Hovde Ø, Huppertz-Hauss G, Høie O, Høivik ML, Monstad I, Solberg IC, Jahnsen J, Karlsen TH, Moum B, Vatn M, Negård A. Prevalence of Sclerosing Cholangitis Detected by Magnetic Resonance Cholangiography in Patients With Long-term Inflammatory Bowel Disease. Gastroenterology. 2016;151:660-669.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 143] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 6. | Weismüller TJ, Trivedi PJ, Bergquist A, Imam M, Lenzen H, Ponsioen CY, Holm K, Gotthardt D, Färkkilä MA, Marschall HU, Thorburn D, Weersma RK, Fevery J, Mueller T, Chazouillères O, Schulze K, Lazaridis KN, Almer S, Pereira SP, Levy C, Mason A, Naess S, Bowlus CL, Floreani A, Halilbasic E, Yimam KK, Milkiewicz P, Beuers U, Huynh DK, Pares A, Manser CN, Dalekos GN, Eksteen B, Invernizzi P, Berg CP, Kirchner GI, Sarrazin C, Zimmer V, Fabris L, Braun F, Marzioni M, Juran BD, Said K, Rupp C, Jokelainen K, Benito de Valle M, Saffioti F, Cheung A, Trauner M, Schramm C, Chapman RW, Karlsen TH, Schrumpf E, Strassburg CP, Manns MP, Lindor KD, Hirschfield GM, Hansen BE, Boberg KM; International PSC Study Group. Patient Age, Sex, and Inflammatory Bowel Disease Phenotype Associate With Course of Primary Sclerosing Cholangitis. Gastroenterology. 2017;152:1975-1984.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 391] [Article Influence: 43.4] [Reference Citation Analysis (1)] |
| 7. | Fung BM, Lindor KD, Tabibian JH. Cancer risk in primary sclerosing cholangitis: Epidemiology, prevention, and surveillance strategies. World J Gastroenterol. 2019;25:659-671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 88] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 8. | O'Toole A, Walsh P, Keegan D, Byrne K, Doherty G, O'Donoghue D, Mulcahy H. Mortality in inflammatory bowel disease patients under 65 years of age. Scand J Gastroenterol. 2014;49:814-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Joosse ME, Aardoom MA, Kemos P, Turner D, Wilson DC, Koletzko S, Martin-de-Carpi J, Fagerberg UL, Spray C, Tzivinikos C, Sladek M, Shaoul R, Roma-Giannikou E, Bronsky J, Serban DE, Ruemmele FM, Garnier-Lengline H, Veres G, Hojsak I, Kolho KL, Davies IH, Aloi M, Lionetti P, Hussey S, Veereman G, Braegger CP, Trindade E, Wewer AV, Hauer AC, de Vries ACH, Sigall Boneh R, Sarbagili Shabat C, Levine A, de Ridder L, Paediatric IBD Porto group of ESPGHAN. Malignancy and mortality in paediatric-onset inflammatory bowel disease: a 3-year prospective, multinational study from the paediatric IBD Porto group of ESPGHAN. Aliment Pharmacol Ther. 2018;48:523-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 10. | Loftus EV Jr, Harewood GC, Loftus CG, Tremaine WJ, Harmsen WS, Zinsmeister AR, Jewell DA, Sandborn WJ. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 529] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 11. | Ricciuto A, Kamath BM, Griffiths AM. The IBD and PSC Phenotypes of PSC-IBD. Curr Gastroenterol Rep. 2018;20:16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Krugliak Cleveland N, Rubin DT, Hart J, Weber CR, Meckel K, Tran AL, Aelvoet AS, Pan I, Gonsalves A, Gaetano JN, Williams KM, Wroblewski K, Jabri B, Pekow J. Patients With Ulcerative Colitis and Primary Sclerosing Cholangitis Frequently Have Subclinical Inflammation in the Proximal Colon. Clin Gastroenterol Hepatol. 2018;16:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Ricciuto A, Fish J, Carman N, Walters TD, Church PC, Hansen BE, Crowley E, Siddiqui I, Nguyen GC, Kamath BM, Griffiths AM. Symptoms Do Not Correlate With Findings From Colonoscopy in Children With Inflammatory Bowel Disease and Primary Sclerosing Cholangitis. Clin Gastroenterol Hepatol. 2018;16:1098-1105.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Jiang X, Karlsen TH. Genetics of primary sclerosing cholangitis and pathophysiological implications. Nat Rev Gastroenterol Hepatol. 2017;14:279-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (1)] |
| 15. | Chung BK, Hirschfield GM. Immunogenetics in primary sclerosing cholangitis. Curr Opin Gastroenterol. 2017;33:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Zuo T, Kamm MA, Colombel JF, Ng SC. Urbanization and the gut microbiota in health and inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2018;15:440-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 198] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 17. | Lichtman SN, Keku J, Clark RL, Schwab JH, Sartor RB. Biliary tract disease in rats with experimental small bowel bacterial overgrowth. Hepatology. 1991;13:766-772. [PubMed] |
| 18. | Lichtman SN, Sartor RB, Keku J, Schwab JH. Hepatic inflammation in rats with experimental small intestinal bacterial overgrowth. Gastroenterology. 1990;98:414-423. [PubMed] |
| 19. | Dhillon AK, Kummen M, Trøseid M, Åkra S, Liaskou E, Moum B, Vesterhus M, Karlsen TH, Seljeflot I, Hov JR. Circulating markers of gut barrier function associated with disease severity in primary sclerosing cholangitis. Liver Int. 2019;39:371-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 20. | Rühlemann M, Liwinski T, Heinsen FA, Bang C, Zenouzi R, Kummen M, Thingholm L, Tempel M, Lieb W, Karlsen T, Lohse A, Hov J, Denk G, Lammert F, Krawczyk M, Schramm C, Franke A. Consistent alterations in faecal microbiomes of patients with primary sclerosing cholangitis independent of associated colitis. Aliment Pharmacol Ther. 2019;50:580-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 21. | Kummen M, Holm K, Anmarkrud JA, Nygård S, Vesterhus M, Høivik ML, Trøseid M, Marschall HU, Schrumpf E, Moum B, Røsjø H, Aukrust P, Karlsen TH, Hov JR. The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut. 2017;66:611-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 326] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 22. | Bajer L, Kverka M, Kostovcik M, Macinga P, Dvorak J, Stehlikova Z, Brezina J, Wohl P, Spicak J, Drastich P. Distinct gut microbiota profiles in patients with primary sclerosing cholangitis and ulcerative colitis. World J Gastroenterol. 2017;23:4548-4558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 215] [Cited by in RCA: 279] [Article Influence: 31.0] [Reference Citation Analysis (4)] |
| 23. | Sabino J, Vieira-Silva S, Machiels K, Joossens M, Falony G, Ballet V, Ferrante M, Van Assche G, Van der Merwe S, Vermeire S, Raes J. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from IBD. Gut. 2016;65:1681-1689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 330] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 24. | Vieira-Silva S, Sabino J, Valles-Colomer M, Falony G, Kathagen G, Caenepeel C, Cleynen I, van der Merwe S, Vermeire S, Raes J. Quantitative microbiome profiling disentangles inflammation- and bile duct obstruction-associated microbiota alterations across PSC/IBD diagnoses. Nat Microbiol. 2019;4:1826-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 185] [Article Influence: 26.4] [Reference Citation Analysis (1)] |
| 25. | Rühlemann MC, Heinsen FA, Zenouzi R, Lieb W, Franke A, Schramm C. Faecal microbiota profiles as diagnostic biomarkers in primary sclerosing cholangitis. Gut. 2017;66:753-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Iwasawa K, Suda W, Tsunoda T, Oikawa-Kawamoto M, Umetsu S, Inui A, Fujisawa T, Morita H, Sogo T, Hattori M. Characterisation of the faecal microbiota in Japanese patients with paediatric-onset primary sclerosing cholangitis. Gut. 2017;66:1344-1346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Torres J, Bao X, Goel A, Colombel JF, Pekow J, Jabri B, Williams KM, Castillo A, Odin JA, Meckel K, Fasihuddin F, Peter I, Itzkowitz S, Hu J. The features of mucosa-associated microbiota in primary sclerosing cholangitis. Aliment Pharmacol Ther. 2016;43:790-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (1)] |
| 28. | Kevans D, Tyler AD, Holm K, Jørgensen KK, Vatn MH, Karlsen TH, Kaplan GG, Eksteen B, Gevers D, Hov JR, Silverberg MS. Characterization of Intestinal Microbiota in Ulcerative Colitis Patients with and without Primary Sclerosing Cholangitis. J Crohns Colitis. 2016;10:330-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 29. | Rossen NG, Fuentes S, Boonstra K, D'Haens GR, Heilig HG, Zoetendal EG, de Vos WM, Ponsioen CY. The mucosa-associated microbiota of PSC patients is characterized by low diversity and low abundance of uncultured Clostridiales II. J Crohns Colitis. 2015;9:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 30. | Quraishi MN, Acharjee A, Beggs AD, Horniblow R, Tselepis C, Gkoutus G, Ghosh S, Rossiter A, Loman N, van Schaik W, Withers D, Walters JRF, Hirschfield GM, Iqbal TH. A pilot integrative analysis of colonic gene expression, gut microbiota and immune infiltration in primary sclerosing cholangitis-inflammatory bowel disease: association of disease with bile acid pathways. J Crohns Colitis. 2020;jjaa02. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 31. | Quraishi MN, Sergeant M, Kay G, Iqbal T, Chan J, Constantinidou C, Trivedi P, Ferguson J, Adams DH, Pallen M, Hirschfield GM. The gut-adherent microbiota of PSC-IBD is distinct to that of IBD. Gut. 2017;66:386-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (1)] |
| 32. | Gevers D, Kugathasan S, Denson LA, Vázquez-Baeza Y, Van Treuren W, Ren B, Schwager E, Knights D, Song SJ, Yassour M, Morgan XC, Kostic AD, Luo C, González A, McDonald D, Haberman Y, Walters T, Baker S, Rosh J, Stephens M, Heyman M, Markowitz J, Baldassano R, Griffiths A, Sylvester F, Mack D, Kim S, Crandall W, Hyams J, Huttenhower C, Knight R, Xavier RJ. The treatment-naive microbiome in new-onset Crohn's disease. Cell Host Microbe. 2014;15:382-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1945] [Cited by in RCA: 2468] [Article Influence: 205.7] [Reference Citation Analysis (1)] |
| 33. | De Cruz P, Kang S, Wagner J, Buckley M, Sim WH, Prideaux L, Lockett T, McSweeney C, Morrison M, Kirkwood CD, Kamm MA. Association between specific mucosa-associated microbiota in Crohn's disease at the time of resection and subsequent disease recurrence: A pilot study. J Gastroenterol Hepatol. 2015;30:268-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 34. | Molyneaux PL, Cox MJ, Willis-Owen SA, Mallia P, Russell KE, Russell AM, Murphy E, Johnston SL, Schwartz DA, Wells AU, Cookson WO, Maher TM, Moffatt MF. The role of bacteria in the pathogenesis and progression of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;190:906-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 426] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 35. | Deng YD, Peng XB, Zhao RR, Ma CQ, Li JN, Yao LQ. The intestinal microbial community dissimilarity in hepatitis B virus-related liver cirrhosis patients with and without at alcohol consumption. Gut Pathog. 2019;11:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Lv LX, Fang DQ, Shi D, Chen DY, Yan R, Zhu YX, Chen YF, Shao L, Guo FF, Wu WR, Li A, Shi HY, Jiang XW, Jiang HY, Xiao YH, Zheng SS, Li LJ. Alterations and correlations of the gut microbiome, metabolism and immunity in patients with primary biliary cirrhosis. Environ Microbiol. 2016;18:2272-2286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 190] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 37. | Sung CM, Lin YF, Chen KF, Ke HM, Huang HY, Gong YN, Tsai WS, You JF, Lu MJ, Cheng HT, Lin CY, Kuo CJ, Tsai IJ, Hsieh SY. Predicting Clinical Outcomes of Cirrhosis Patients With Hepatic Encephalopathy From the Fecal Microbiome. Cell Mol Gastroenterol Hepatol. 2019;8:301-318.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 38. | Lemoinne S, Kemgang A, Ben Belkacem K, Straube M, Jegou S, Corpechot C; Saint-Antoine IBD Network, Chazouillères O, Housset C, Sokol H. Fungi participate in the dysbiosis of gut microbiota in patients with primary sclerosing cholangitis. Gut. 2020;69:92-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 158] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 39. | Bajaj JS, Liu EJ, Kheradman R, Fagan A, Heuman DM, White M, Gavis EA, Hylemon P, Sikaroodi M, Gillevet PM. Fungal dysbiosis in cirrhosis. Gut. 2018;67:1146-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 40. | Kummen M, Hov JR. The gut microbial influence on cholestatic liver disease. Liver Int. 2019;39:1186-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 41. | Nakamoto N, Sasaki N, Aoki R, Miyamoto K, Suda W, Teratani T, Suzuki T, Koda Y, Chu PS, Taniki N, Yamaguchi A, Kanamori M, Kamada N, Hattori M, Ashida H, Sakamoto M, Atarashi K, Narushima S, Yoshimura A, Honda K, Sato T, Kanai T. Gut pathobionts underlie intestinal barrier dysfunction and liver T helper 17 cell immune response in primary sclerosing cholangitis. Nat Microbiol. 2019;4:492-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 334] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 42. | Liao L, Schneider KM, Galvez EJC, Frissen M, Marschall HU, Su H, Hatting M, Wahlström A, Haybaeck J, Puchas P, Mohs A, Peng J, Bergheim I, Nier A, Hennings J, Reißing J, Zimmermann HW, Longerich T, Strowig T, Liedtke C, Cubero FJ, Trautwein C. Intestinal dysbiosis augments liver disease progression via NLRP3 in a murine model of primary sclerosing cholangitis. Gut. 2019;68:1477-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 43. | Tedesco D, Thapa M, Chin CY, Ge Y, Gong M, Li J, Gumber S, Speck P, Elrod EJ, Burd EM, Kitchens WH, Magliocca JF, Adams AB, Weiss DS, Mohamadzadeh M, Grakoui A. Alterations in Intestinal Microbiota Lead to Production of Interleukin 17 by Intrahepatic γδ T-Cell Receptor-Positive Cells and Pathogenesis of Cholestatic Liver Disease. Gastroenterology. 2018;154:2178-2193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 44. | Ni J, Wu GD, Albenberg L, Tomov VT. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol. 2017;14:573-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1021] [Cited by in RCA: 1295] [Article Influence: 143.9] [Reference Citation Analysis (0)] |
| 45. | Katt J, Schwinge D, Schoknecht T, Quaas A, Sobottka I, Burandt E, Becker C, Neurath MF, Lohse AW, Herkel J, Schramm C. Increased T helper type 17 response to pathogen stimulation in patients with primary sclerosing cholangitis. Hepatology. 2013;58:1084-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 46. | Mathies F, Steffens N, Kleinschmidt D, Stuhlmann F, Huber FJ, Roy U, Meyer T, Luetgehetmann M, von Petersdorff M, Seiz O, Herkel J, Schramm C, Flavell RA, Gagliani N, Krebs C, Panzer U, Abdullah Z, Strowig T, Bedke T, Huber S. Colitis Promotes a Pathological Condition of the Liver in the Absence of Foxp3+ Regulatory T Cells. J Immunol. 2018;201:3558-3568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Begley M, Gahan CG, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev. 2005;29:625-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1071] [Cited by in RCA: 1265] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 48. | Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1644] [Cited by in RCA: 2134] [Article Influence: 101.6] [Reference Citation Analysis (0)] |
| 49. | Jones BV, Begley M, Hill C, Gahan CGM, Marchesi JR. Functional and Comparative Metagenomic Analysis of Bile Salt Hydrolase Activity in the Human Gut Microbiome. Natl Acad Sci USA. 2008;105:13580-13585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 813] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 50. | Winston JA, Theriot CM. Diversification of host bile acids by members of the gut microbiota. Gut Microbes. 2020;11:158-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 412] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 51. | Setchell KD, Lawson AM, Tanida N, Sjövall J. General methods for the analysis of metabolic profiles of bile acids and related compounds in feces. J Lipid Res. 1983;24:1085-1100. [PubMed] |
| 52. | Ridlon JM, Alves JM, Hylemon PB, Bajaj JS. Cirrhosis, bile acids and gut microbiota: unraveling a complex relationship. Gut Microbes. 2013;4:382-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 282] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 53. | Wells JE, Williams KB, Whitehead TR, Heuman DM, Hylemon PB. Development and Application of a Polymerase Chain Reaction Assay for the Detection and Enumeration of Bile Acid 7alpha-dehydroxylating Bacteria in Human Feces. Clin Chim Acta. 2003;331:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Kitahara M, Takamine F, Imamura T, Benno Y. Clostridium Hiranonis Sp. Nov., a Human Intestinal Bacterium With Bile Acid 7alpha-dehydroxylating Activity. Int J Syst Evol Microbiol. 2001;51:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 150] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 55. | Kitahara M, Takamine F, Imamura T, Benno Y. Assignment of Eubacterium Sp. VPI 12708 and Related Strains With High Bile Acid 7alpha-dehydroxylating Activity to Clostridium Scindens and Proposal of Clostridium Hylemonae Sp. Nov., Isolated From Human Faeces. Int J Syst Evol Microbiol. 2000;50:971-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 56. | Ridlon JM, Kang D-J, Hylemon PB. Isolation and Characterization of a Bile Acid Inducible 7alpha-dehydroxylating Operon in Clostridium Hylemonae TN271. Anaerobe. 2010;16:137-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 57. | Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, No D, Liu H, Kinnebrew M, Viale A, Littmann E, van den Brink MRM, Jenq RR, Taur Y, Sander C, Cross JR, Toussaint NC, Xavier JB, Pamer EG. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. 2015;517:205-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1172] [Cited by in RCA: 1411] [Article Influence: 117.6] [Reference Citation Analysis (0)] |
| 58. | Högenauer K, Arista L, Schmiedeberg N, Werner G, Jaksche H, Bouhelal R, Nguyen DG, Bhat BG, Raad L, Rauld C, Carballido JM. G-protein-coupled bile acid receptor 1 (GPBAR1, TGR5) agonists reduce the production of proinflammatory cytokines and stabilize the alternative macrophage phenotype. J Med Chem. 2014;57:10343-10354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 59. | Guo C, Xie S, Chi Z, Zhang J, Liu Y, Zhang L, Zheng M, Zhang X, Xia D, Ke Y, Lu L, Wang D. Bile Acids Control Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome. Immunity. 2016;45:802-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 573] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 60. | Tabibian JH, O'Hara SP, Trussoni CE, Tietz PS, Splinter PL, Mounajjed T, Hagey LR, LaRusso NF. Absence of the intestinal microbiota exacerbates hepatobiliary disease in a murine model of primary sclerosing cholangitis. Hepatology. 2016;63:185-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 168] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 61. | Fuchs CD, Paumgartner G, Mlitz V, Kunczer V, Halilbasic E, Leditznig N, Wahlström A, Ståhlman M, Thüringer A, Kashofer K, Stojakovic T, Marschall HU, Trauner M. Colesevelam attenuates cholestatic liver and bile duct injury in Mdr2-/- mice by modulating composition, signalling and excretion of faecal bile acids. Gut. 2018;67:1683-1691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 62. | Biagioli M, Carino A, Fiorucci C, Marchianò S, Di Giorgio C, Roselli R, Magro M, Distrutti E, Bereshchenko O, Scarpelli P, Zampella A, Fiorucci S. GPBAR1 Functions as Gatekeeper for Liver NKT Cells and provides Counterregulatory Signals in Mouse Models of Immune-Mediated Hepatitis. Cell Mol Gastroenterol Hepatol. 2019;8:447-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 63. | Cipriani S, Mencarelli A, Chini MG, Distrutti E, Renga B, Bifulco G, Baldelli F, Donini A, Fiorucci S. The bile acid receptor GPBAR-1 (TGR5) modulates integrity of intestinal barrier and immune response to experimental colitis. PLoS One. 2011;6:e25637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 309] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 64. | Vavassori P, Mencarelli A, Renga B, Distrutti E, Fiorucci S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol. 2009;183:6251-6261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 513] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 65. | Verbeke L, Farre R, Verbinnen B, Covens K, Vanuytsel T, Verhaegen J, Komuta M, Roskams T, Chatterjee S, Annaert P, Elst IV, Windmolders P, Trebicka J, Nevens F, Laleman W. The FXR Agonist Obeticholic Acid Prevents Gut Barrier Dysfunction and Bacterial Translocation in Cholestatic Rats. Am J Pathol. 2015;185:409-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 66. | Jain U, Lai CW, Xiong S, Goodwin VM, Lu Q, Muegge BD, Christophi GP, VanDussen KL, Cummings BP, Young E, Hambor J, Stappenbeck TS. Temporal Regulation of the Bacterial Metabolite Deoxycholate during Colonic Repair Is Critical for Crypt Regeneration. Cell Host Microbe. 2018;24:353-363.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 67. | Hang S, Paik D, Yao L, Kim E, Trinath J, Lu J, Ha S, Nelson BN, Kelly SP, Wu L, Zheng Y, Longman RS, Rastinejad F, Devlin AS, Krout MR, Fischbach MA, Littman DR, Huh JR. Bile acid metabolites control TH17 and Treg cell differentiation. Nature. 2019;576:143-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 456] [Cited by in RCA: 1029] [Article Influence: 147.0] [Reference Citation Analysis (0)] |
| 68. | Torres J, Palmela C, Brito H, Bao X, Ruiqi H, Moura-Santos P, Pereira da Silva J, Oliveira A, Vieira C, Perez K, Itzkowitz SH, Colombel JF, Humbert L, Rainteau D, Cravo M, Rodrigues CM, Hu J. The gut microbiota, bile acids and their correlation in primary sclerosing cholangitis associated with inflammatory bowel disease. United European Gastroenterol J. 2018;6:112-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 69. | Vaughn BP, Kaiser T, Staley C, Hamilton MJ, Reich J, Graiziger C, Singroy S, Kabage AJ, Sadowsky MJ, Khoruts A. A pilot study of fecal bile acid and microbiota profiles in inflammatory bowel disease and primary sclerosing cholangitis. Clin Exp Gastroenterol. 2019;12:9-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 70. | Quraishi MN, Shaheen W, Oo YH, Iqbal TH. Immunological Mechanisms Underpinning Faecal Microbiota Transplantation for the Treatment of Inflammatory Bowel Disease. Clin Exp Immunol. 2019;199:24-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 71. | Shah A, Crawford D, Burger D, Martin N, Walker M, Talley NJ, Tallis C, Jones M, Stuart K, Keely S, Lewindon P, Macdonald GA, Morrison M, Holtmann GJ. Effects of Antibiotic Therapy in Primary Sclerosing Cholangitis with and without Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Semin Liver Dis. 2019;39:432-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 72. | Allegretti JR, Kassam Z, Carrellas M, Mullish BH, Marchesi JR, Pechlivanis A, Smith M, Gerardin Y, Timberlake S, Pratt DS, Korzenik JR. Fecal Microbiota Transplantation in Patients With Primary Sclerosing Cholangitis: A Pilot Clinical Trial. Am J Gastroenterol. 2019;114:1071-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 180] [Article Influence: 25.7] [Reference Citation Analysis (1)] |
| 73. | Suri J, Patwardhan V, Bonder A. Pharmacologic management of primary sclerosing cholangitis: what's in the pipeline? Expert Rev Gastroenterol Hepatol. 2019;13:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 74. | Assis DN, Abdelghany O, Cai SY, Gossard AA, Eaton JE, Keach JC, Deng Y, Setchell KD, Ciarleglio M, Lindor KD, Boyer JL. Combination Therapy of All-Trans Retinoic Acid With Ursodeoxycholic Acid in Patients With Primary Sclerosing Cholangitis: A Human Pilot Study. J Clin Gastroenterol. 2017;51:e11-e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 75. | Chapman MH, Thorburn D, Hirschfield GM, Webster GGJ, Rushbrook SM, Alexander G, Collier J, Dyson JK, Jones DE, Patanwala I, Thain C, Walmsley M, Pereira SP. British Society of Gastroenterology and UK-PSC guidelines for the diagnosis and management of primary sclerosing cholangitis. Gut. 2019;68:1356-1378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 200] [Article Influence: 28.6] [Reference Citation Analysis (1)] |
| 76. | Fickert P, Hirschfield GM, Denk G, Marschall HU, Altorjay I, Färkkilä M, Schramm C, Spengler U, Chapman R, Bergquist A, Schrumpf E, Nevens F, Trivedi P, Reiter FP, Tornai I, Halilbasic E, Greinwald R, Pröls M, Manns MP, Trauner M; European PSC norUDCA Study Group. norUrsodeoxycholic acid improves cholestasis in primary sclerosing cholangitis. J Hepatol. 2017;67:549-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 212] [Article Influence: 23.6] [Reference Citation Analysis (1)] |
| 77. | Larusso NF, Bowlus CL, Levy C, Vuppalanchi R, Floreani A, Andreone P, Srestha R, Trotter J, Goldberg D, Rushbrook S, Hirschfield GM, Van Biene C, Penceck R, Macconell L, David S. PC.01.8 The AESOP trial: A randomized, double-blind, placebo-controlled, phase 2 study of obeticholic acid in patients with primary sclerosing cholangitis. Dig Liver Dis. 2018;50:e67. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 78. | Trauner M, Gulamhusein A, Hameed B, Caldwell S, Shiffman ML, Landis C, Eksteen B, Agarwal K, Muir A, Rushbrook S, Lu X, Xu J, Chuang JC, Billin AN, Li G, Chung C, Subramanian GM, Myers RP, Bowlus CL, Kowdley KV. The Nonsteroidal Farnesoid X Receptor Agonist Cilofexor (GS-9674) Improves Markers of Cholestasis and Liver Injury in Patients With Primary Sclerosing Cholangitis. Hepatology. 2019;70:788-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 229] [Article Influence: 32.7] [Reference Citation Analysis (1)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Sitkin S, Wang T, Zhang XL S-Editor: Wang JL L-Editor: A E-Editor: Zhang YL