Published online May 28, 2020. doi: 10.3748/wjg.v26.i20.2645
Peer-review started: February 03, 2020
First decision: February 29, 2020
Revised: March 27, 2020
Accepted: May 13, 2020
Article in press: May 13, 2020
Published online: May 28, 2020
Processing time: 122 Days and 6.9 Hours
Recent evidence has indicated the role of B cells and B cell-activating factor (BAFF) in the development of hepatocellular carcinoma (HCC).
To characterize circulating BAFF receptor expression and B cell subpopulations in patients with hepatitis B virus (HBV)-related HCC.
Peripheral blood samples collected from 41 patients with chronic HBV infection (25 patients without HCC and 16 patients with HCC) and 9 healthy controls were assessed for BAFF receptors [BAFF-R(B cell-activating factor receptor), transmembrane activator and cyclophilin ligand interactor, B-cell maturation antigen] and B cell subpopulations by multicolor flow cytometry.
The frequency of BAFF-R expressing B cells to total B cells was significantly lower in patients with HCC (3.39% ± 2.12%) compared with the non-HCC group (5.37% ± 1.90%) and healthy controls (6.23% ± 2.32%), whereas there was no difference in transmembrane activator and cyclophilin ligand interactor and B-cell maturation antigen. The frequencies of CD27+IgD+ memory B cells, CD27+IgD- class-switched memory B cells and plasmablasts were significantly lower in the patients with HCC compared to patients without HCC (1.23 ± 1.17 vs 3.09 ± 1.55, P = 0.001, 0.60 ± 0.44 vs 1.69 ± 0.86, P < 0.0001 and 0.16 ± 0.12 vs 0.37 ± 0.30, P = 0.014, respectively). However, the ratio of naïve and transitional B cell did not differ significantly between the three groups. In addition, decreased BAFF-R expression on B cells was significantly correlated with large tumor size and advanced tumor stage.
Our data demonstrated BAFF-R expression was reduced in B cells that involved with the frequencies of B cells maturation in patients with HCC. The depletion of BAFF-R might play an important role in the development of HCC in patients with chronic HBV infection.
Core tip: This study explored the expression of B cell-activating factor receptor (BAFF-R) and B cell subpopulations in patients with hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC). Our data showed that BAFF-R expression was significantly lower in patient with HCC compared with non-HCC and healthy controls. In addition, the frequencies of CD27+IgD+ memory B cells, CD27+IgD- class-switched memory B cells and plasmablasts were significantly lower in the patients with HCC compared to patients without HCC. In addition, decreased BAFF-R expression was significantly correlated with tumor size and tumor stage. This study is the first report suggesting that the depletion of BAFF-R in B cells might be responsible for B cell maturation in patients with HBV-related HCC.
- Citation: Khlaiphuengsin A, Chuaypen N, Sodsai P, Buranapraditkun S, Boonpiyathad T, Hirankarn N, Tangkijvanich P. Decreased of BAFF-R expression and B cells maturation in patients with hepatitis B virus-related hepatocellular carcinoma. World J Gastroenterol 2020; 26(20): 2645-2656
- URL: https://www.wjgnet.com/1007-9327/full/v26/i20/2645.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i20.2645
Hepatocellular carcinoma (HCC) represents one of the most common malignant tumors worldwide, especially in East and Southeast Asian countries, where hepatitis B virus (HBV) infection is highly prevalent[1]. In Thailand, the incidence of liver cancer is approximately 38.6 and 17.2 per 100000 person-years, respectively and at least 50%-60% of all HCC cases are attributable to chronic HBV infection[2]. Hepato-carcinogenesis is a complex multistep process involving the persistence of liver damage and inflammation, which results in a distinctive sequence of chronic hepatitis, cirrhosis and finally HCC development[3]. As chronic inflammation is a crucial driver of disease progression, accumulative evidence has indicated that immune-mediated host-virus interactions play an essential role in the development of HBV-related HCC[4]. While the importance of T-cell immunity has been well documented, the role of B cells in HCC progression is not yet completely understood and needs further investigation.
B cells are classically known to stimulate immune response via the activation of T cells and antibody production[5]. The activation of specific B cells results in the proliferation and development of memory B cells and antibody-producing plasma cells, which are beneficial to neutralize and control active viral replication. The survival of B cells depends on the expression of a functional B cell receptor and signals from B cell-activating factor (BAFF), a member of the tumor necrosis factor (TNF) superfamily[6]. This cytokine binds to three receptors expressed on B cells including BAFF receptor (BAFF-R), transmembrane activator and cyclophilin ligand interactor (TACI) and B-cell maturation antigen (BCMA). In general, BAFF-R activates downstream pathways that regulate survival and maturation of B cells, TACI induces immunoglobulin (Ig) class switching, whereas BCMA promotes plasma B cells survival[7]. Human B cells are comprised of distinct phenotypic and functional subpopulations characterized based on different developmental stages such as transitional, naïve, memory B cells and plasmablasts. Since BAFF receptors and B cell subsets play diverse but crucial roles in modulating B cell function, analysis of their expression and subpopulation frequencies could provide more insights into the immunological characteristics of B cell selection in patients with HCC.
Recent evidence has suggested that B cells exhibit dual biological effects in promoting and inhibiting the development and progression of several cancers[8]. Regarding HCC, a previous study showed that increased percentage of circulating B cells was found in individuals with advanced HCC compared with early tumor staging[9]. Recently, it was also demonstrated that tumor infiltrating B cells was associated with disease prognosis in patients with HBV-related HCC[10]. Moreover, the close proximity and interaction of tumor-infiltrating T cells and B cells suggested an increased immune activation that might contribute to better prognosis in patients with HCC[11]. In addition, we recently reported that plasma BAFF levels significantly increased in patients with HBV-related HCC compared with the non-HCC group and healthy controls[12]. Together, these data suggest that B cells and BAFF may play an important role in HCC development and progression in patients with chronic HBV infection. So far, the phenotypes of circulating B-cell subtypes in patients with HBV-related HCC have not been well characterized.
In the present study, we aimed to compare the expression of BAFF receptors and the distribution of B cell subsets in the peripheral blood of patients with HBV-related HCC compared to individuals without HCC and healthy controls. Our data showed that BAFF-R expression was significantly reduced in B cells that involved with the frequencies of B cells maturation in patients with HCC. Interestingly, decreased BAFF-R expression was significantly associated with progressive HCC including large tumor size and advanced cancer stage.
A total of 50 participants including 41 patients with chronic HBV infection (25 without HCC and 16 with HCC) and 9 healthy individuals were recruited from King Chulalongkorn Memorial Hospital and blood donors at National Blood Centre Thai Red Cross Society, Bangkok, Thailand, respectively. The diagnosis of chronic HBV infection was confirmed by the presence of serum hepatitis B s antigen (HBsAg) at least 6 mo. Patients with co-infection with hepatitis C virus (HCV) and/or human immunodeficiency virus (HIV) were not included in this study. In addition, patients with evidence of other malignancies or autoimmune disorders were excluded.
Patients in the HCC group were diagnosed on the basis of typical imaging studies and/or histopathology (fine needle aspiration, core liver biopsy or surgical resection) according to the standard guideline[13]. Diagnostic criteria of HCC by imaging studies were based on findings of focal hepatic lesions with hyperattenuation at the arterial phase, hypoattenuation at the portal phase in dynamic CT or MRI. The clinical parameters of patients with HCC at initial diagnosis were collected, which included sex, age, liver function tests, serum alpha-fetoprotein (AFP) level and HCC staging classified by the Barcelona Clinic Liver Cancer (BCLC) system[14].
The study was approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (IRB no. 438/60) and participants had provided written informed consent. The study followed the Helsinki Declaration and Good Clinical Practice guidelines.
All blood samples were collected in sodium heparin tube and peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque density gradient centrifugation using Percoll PLUS density gradient media (GE Healthcare, Philadelphia, Pennsylvania, PA, USA) at 1500 rpm for 30 min at room temperature. PBMCs were washed twice with Hank’s balanced salt solution (HBSS) (GIBCO, New York, NY, USA) with 5% fetal bovine serum (FBS) and 100 U/mL penicillin and 100 μg/mL streptomycin. Total lymphocytes were counted and stored at liquid nitrogen before analysis.
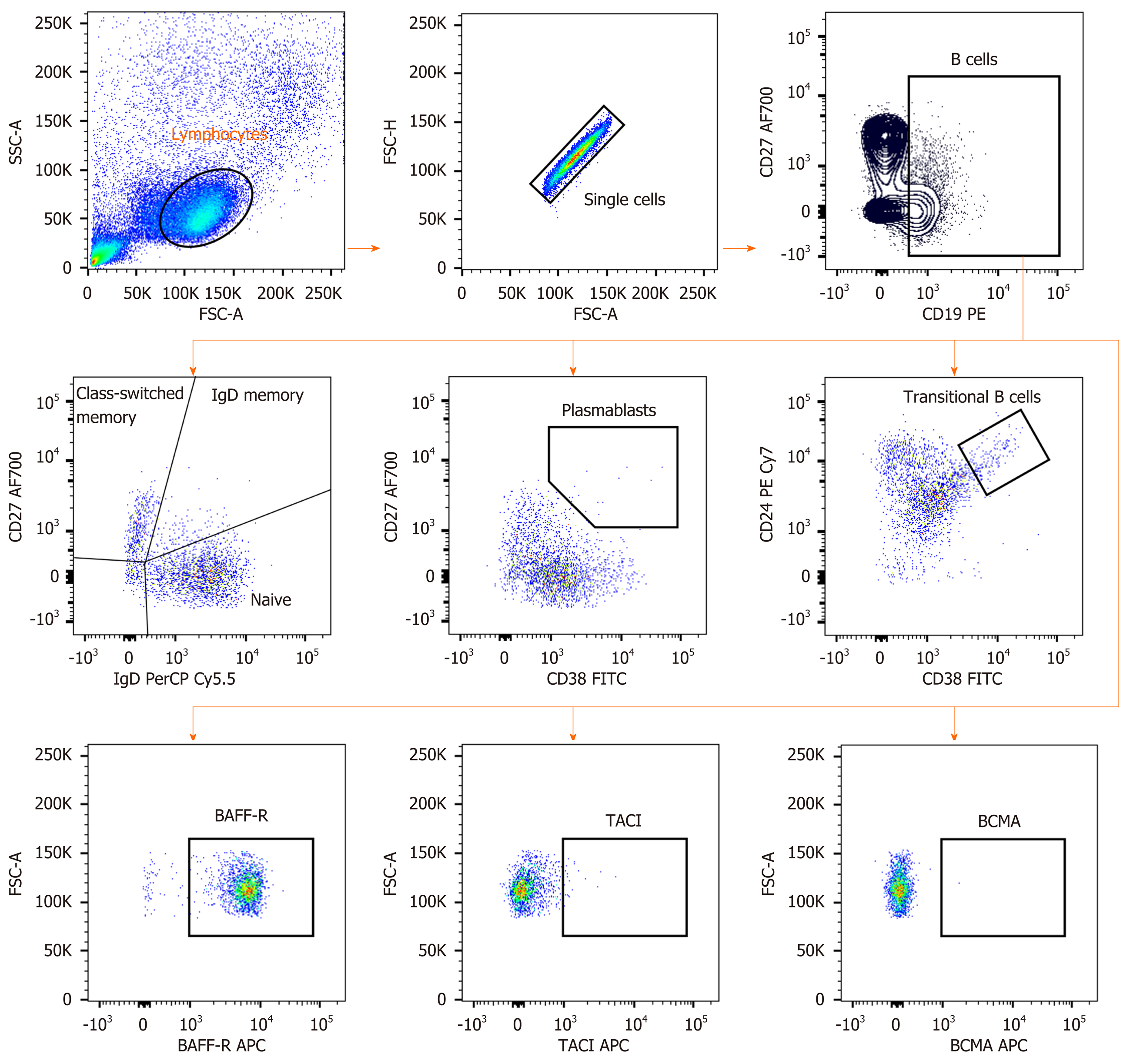
Cryopreserved PBMCs from each patient were defrosted and added in complete in RPMI1640 Medium (GIBCO, New York, NY, USA) supplement with 10% FBS. At least 200000 cells were stained with combinations of antibodies including anti-CD19-PE, anti-CD38-FITC, anti-IgD-PerCP-Cy5.5, anti-CD27 Alexa Fluor 700, anti-CD24 PE-CyTM7 (BD Biosciences, San Jose, CA, USA), anti-CD268 (BAFFR)-APC, anti-CD267 (TACI)-APC and anti-CD269 (BCMA)-APC (BioLegend, San Diego, CA, USA). The cells were re-suspended in the buffer before performed flow cytometry using BD LSR II Flow Cytometry Analyzer (BD Biosciences, San Jose, CA, USA). Flow cytometry data were analyzed with Flowjo software version 10 (Tree star, Ashland, Oregon, USA).
Plasma BAFF levels were determined by ELISA (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's protocol. Qualitative hepatitis B s antigen (HBsAg) was measured by commercially available enzyme-linked immunosorbent assays (Abbott Laboratories, Chicago, IL). Serum HBV DNA levels were quantified by Abbott Real Time HBV assay (Abbott Laboratories).
Statistical analysis was performed by using SPSS version 22 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism v5.0 (GraphPad Software, San Diego, CA). Values were presented as mean ± standard deviation (SD), and percentages as appropriate. The statistical significance among groups was assessed by using the Kruskal-Wallis test, followed by multiple comparison test. The correlation data were analyzed by Spearman’s rank test. The P value less than 0.05 was considered statistically significant.
Baseline clinical characteristics of 9 healthy controls and 41 patients with chronic HBV infection (25 patients without HCC and 16 patients with HCC) are summarized in Table 1. Patients in each group were significantly older than healthy controls (P < 0.001). However, there was no difference in mean age distribution among groups of patients. Compared with the non-HCC group, patients with HCC had significantly lower mean hematocrit, platelet counts and serum albumin. However, there was no significant difference among groups in terms of other baseline clinical parameters.
| Healthy controls (n = 9) | Patients without HCC (n = 25) | Patients with HCC (n = 16) | P value | |
| Sex (males) | 4 (44.4%) | 12 (48.0%) | 13 (81.3%) | 0.072 |
| Age, yr | 27.6 (25-34) | 42.8 (20-77) | 60.8 (34-77) | < 0.001c |
| Hemoglobin (g/dL) | 13.3 ± 1.5 | 12.1 ± 1.9 | 0.082 | |
| Hematocrit (%) | 41.4 ± 4.1 | 36.7 ± 5.7 | 0.008b | |
| Platelets count (103/µL) | 246.4 ± 56.7 | 180.1 ± 80.8 | 0.007b | |
| White cell count (103/µL) | 6.5 ± 2.2 | 6.1 ± 2.0 | 0.59 | |
| INR | 1.0 ± 0.1 | 1.1 ± 0.2 | 0.34 | |
| AST (U/L) | 48.9 ± 42.2 | 80.0 ± 65.0 | 0.07 | |
| ALT (U/L) | 75.8 ± 83.1 | 56.9 ± 32.3 | 0.407 | |
| ALP (mg/L) | 59.3 ± 14.3 | 177.5 ± 118.0 | 0.066 | |
| Total bilirubin (mg/dL) | 0.7 ± 0.1 | 0.8 ± 0.4 | 0.757 | |
| Albumin (g/dL) | 4.5 ± 0.3 | 3.4 ± 0.6 | 0.001b | |
| AFP (IU/mL) | 2.4 ± 2.2 | 4810.2 ± 10236.1 | 0.531 | |
| log10 HBV DNA (IU/mL) | 5.4 ± 2.6 | 3.0 ± 1.9 | 0.066 | |
| BAFF (pg/mL) | 914.8 ± 159.3 | 1208.8 ± 386.1 | 0.042a | |
| BCLC stage | ||||
| A-B | 12 (75.0%) | |||
| C | 4 (25.0%) |
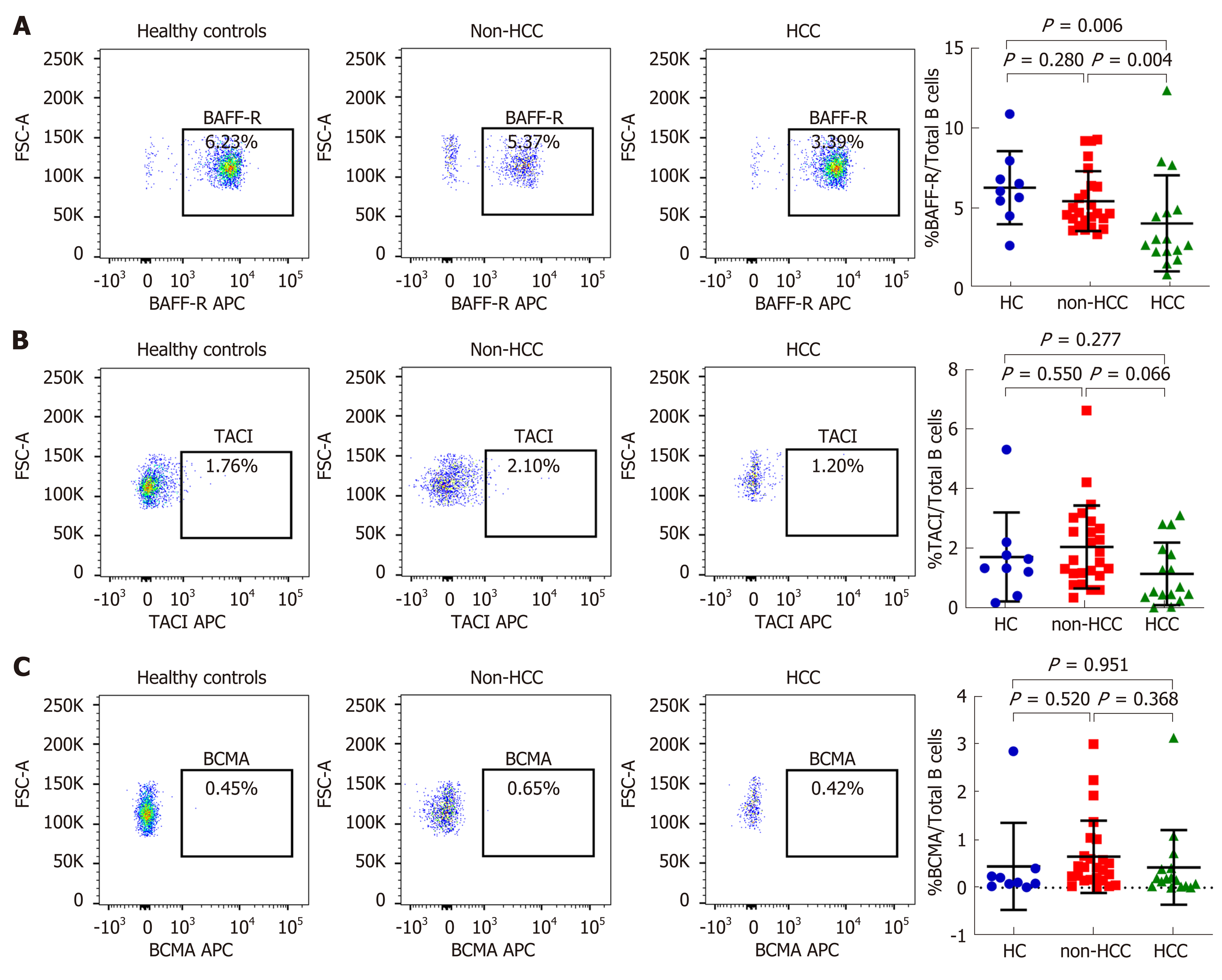
Patients with HCC had significantly higher mean plasma BAFF levels compared with those without HCC (1208.8 ± 386.1 vs 914.8 ± 159.3 pg/mL; P = 0.042) (Table 1), which confirmed our recent report[12]. Percentages of total B cells in peripheral blood among the studied groups were not different, although there was a trend of higher total B cells in the HCC group compared with the non-HCC group (20.09% ± 10.37% vs 15.03% ± 4.09%, P = 0.079, Supplement Figure S1). The expression of BAFF receptors including BAFF-R, TACI and BCMA were further investigated (Figure 1 and 2). We found that there was a significantly decreased in percentage of BAFF-R expressing B cells in the HCC group (3.39% ± 2.12%) compared with the non-HCC group (5.37% ± 1.90%; P = 0.004) and healthy controls (6.23% ± 2.32%; P = 0.006). However, the percentage of BAFF-R expressing B cells was similar between the non-HCC group and controls (P = 0.28). In addition, the frequencies of TACI and BCMA expressing B cells were not significantly altered among the studied groups.
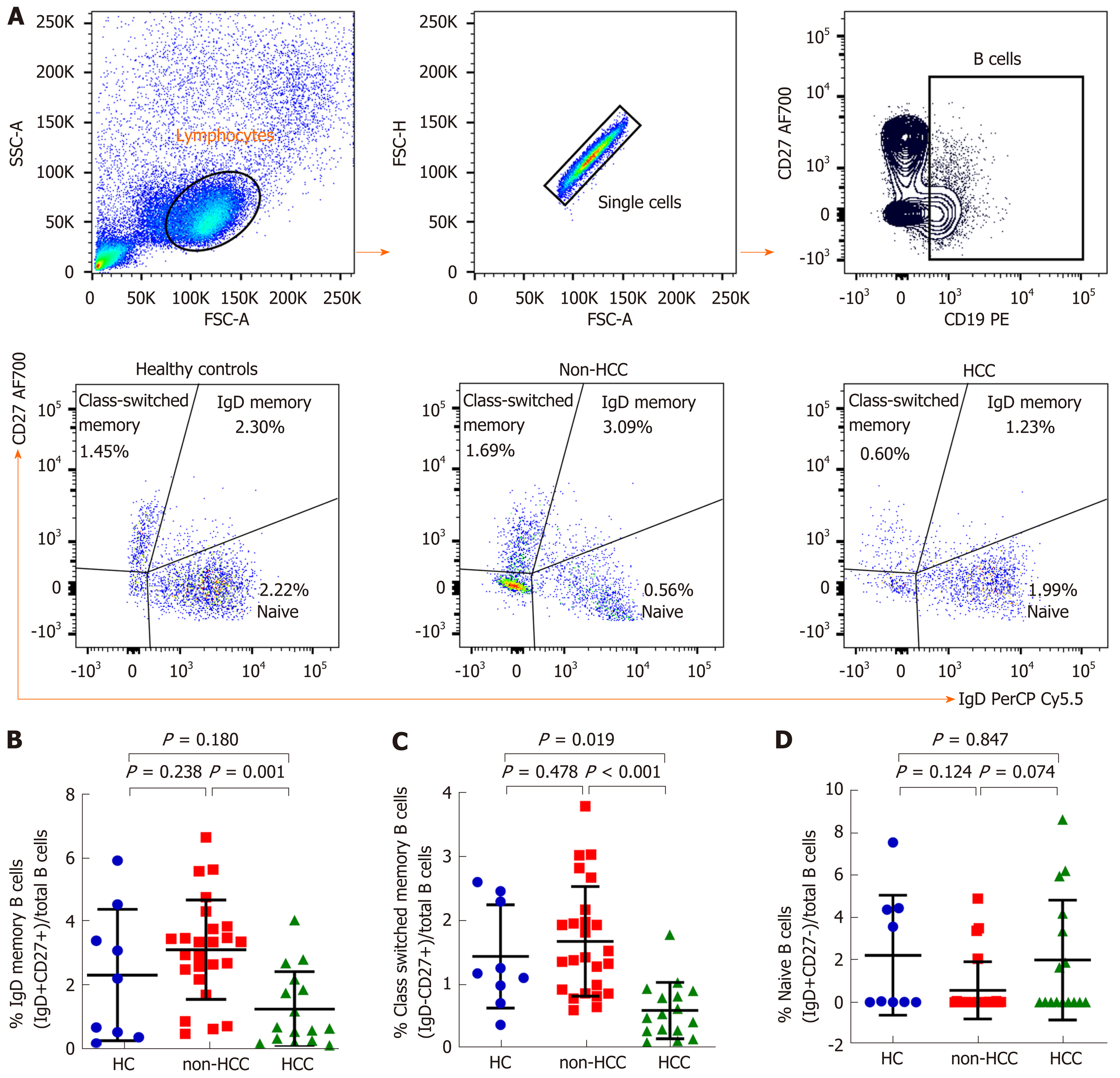
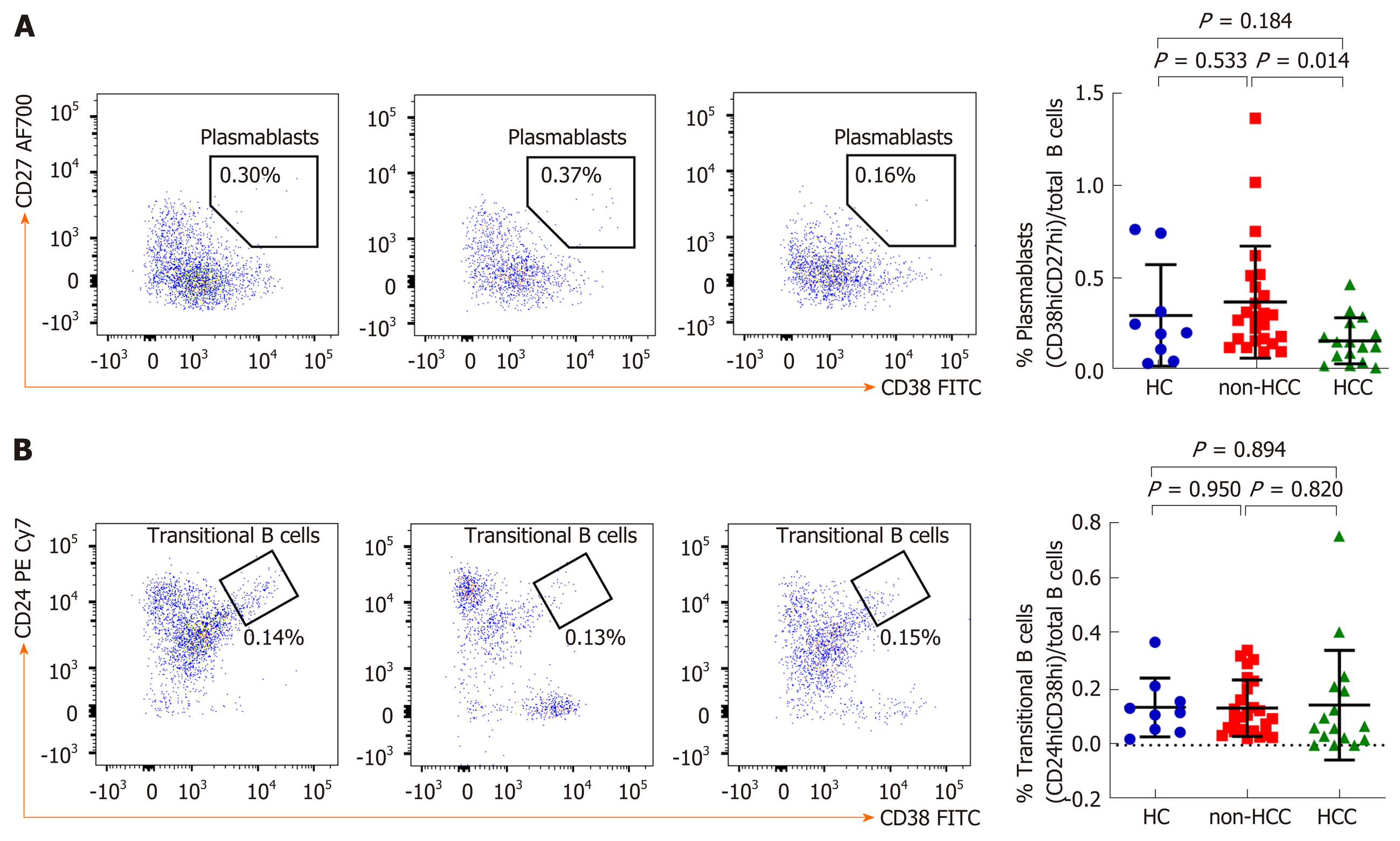
An in-depth analysis of B cell subpopulations was further analyzed in patients with or without HCC and in healthy controls. B cell population characteristics and gating strategy are shown in Figure 1. Overall, the absolute number of CD19+ B cells was not significantly different between groups. However, the frequency of IgD memory B cells (CD27+IgD+) were significant reduced in patients with HCC compared with the non-HCC individuals (1.23% ± 1.17% vs 3.09% ± 1.56%; P = 0.001) (Figure 3A and B). A significantly lower number of class-switched memory B cells (CD27+IgD-) in patients with HCC (0.60% ± 0.44%) was also observed when compared with patients without HCC (1.69% ± 0.86%; P < 0.001) and healthy controls (1.45% ± 0.81%; P = 0.019) (Figure 3A and C). Moreover, the frequency of plasmablasts was significantly decreased in the HCC group compared with the non-HCC group (0.16% ± 0.12% vs 0.37% ± 0.30%; P = 0.014) (Figure 4A). However, no significant difference between groups was found regarding the frequencies of naïve and transitional B cells (Figure 3A, D and 4B).
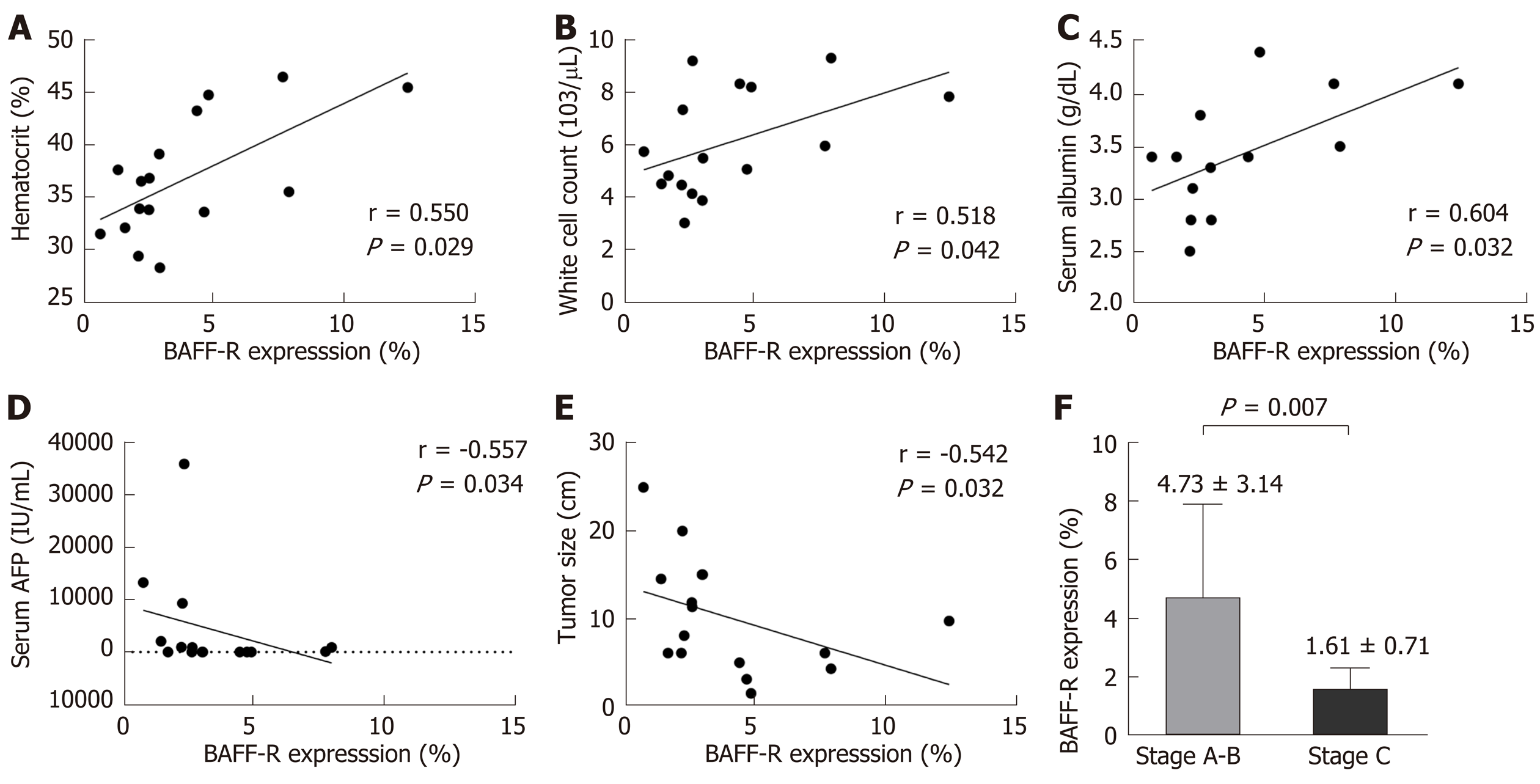
The clinical correlations of BAFF-R expression were further investigated in patients with HCC. Our results showed that BAFF-R had positive correlation with hematocrit (r = 0.550, P = 0.029), white cell count (r = 0.518, P = 0.042) and serum albumin (r = 0.604, P = 0.032). In contrast, BAFF-R exhibited negative correlation with patient’s age (r = -0.533, P = 0.033), serum AFP (r = -0.557, P = 0.034) and tumor size (r = -0.542, P = 0.032) (Figure 5A-E). BAFF-R did not significantly correlate with other clinical parameters including platelet counts, international normalized ratio (INR), total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and HBV DNA level. Regarding tumor staging, BAFF-R expression in patients in advanced BCLC stage (stage C) was significantly lower than that of patients with earlier tumor stages (stages A and B) (1.61% ± 0.71% vs 4.73% ± 3.14%, P = 0.007) (Figure 5F).
Chronic HBV infection is a complex infectious disorder characterized by immune-mediated liver inflammation, which results in repeated destruction and regeneration of hepatocytes, and ultimately leads to the development of HCC[3]. Overall, HBV-related HCC is a leading cause of cancer death in Thailand and other Asian countries, with a very poor prognosis because of its aggressiveness and high recurrence rates[15]. As a result, a better understanding regarding the molecular mechanisms and progression of HCC is highly needed. Among several immunological factors associated with hepatocarcinogenesis, it is likely that B cells and BAFF may contribute to disease progression and HCC development in patients with chronic HBV infection[10]. In this context, we previously demonstrated that higher expression of plasma BAFF was linked to disease severity and overall survival in HBV-related HCC[12]. However, the role of BAFF receptors and distribution of circulating B-cell subtypes in HCC remains unclear.
In the present study, we explored the expression of BAFF receptors including BAFF-R, TACI and BCMA, which are expressed almost exclusively on B cells[7]. Interestingly, we found that the percentage of B cells expressing BAFF-R was significantly decreased in patients with HBV-related HCC compared with HBV patients without HCC and healthy controls. However, frequencies of TACI and BCMA on B cells were not significantly altered among groups. Thus, these results might indicate that BAFF-R but not TACI and BCMA play an important role in HCC development in patients with chronic HBV infection. In fact, BAFF-R is clearly a key receptor involved in the successful survival and maturation of B cells[16]. Previous studies have demonstrated that BAFF-R is important not only in B cell development, but also is the major mediator of BAFF-dependent co-stimulatory responses in peripheral B and T cells[17]. Similarly to the study of BAFF-R-deficient mice, BAFF-R is crucial for the development of B cells up to the stage of IgM+ immature/transitional B cells but cannot complete maturation in the spleen[18]. Moreover, BAFF-R is considered to be the most important receptor due to its critical role in regulatory B cells (Bregs) survival[19]. As previously demonstrated, targeting of BAFF-R in patients with precursor B-lineage acute lymphoblastic leukemia (B-ALL) could significantly impact the survival and basal proliferation of leukemia B-cell precursors[20]. Moreover, downregulation of circulating BAFF-R was shown to be associated with disease activity in patients with in patients with autoimmune disorders[21]. In this study, we also demonstrated that decreased BAFF-R expression on B cells was significantly correlated with tumor size and more advanced BCLC stages, indicating a contribution of BAFF-R to the disease progression of HBV-related HCC.
Normal B cell development and survival can promote an effective immune response to clear pathogens, whereas abnormalities in B cells differentiation and activation leads to the disruption of B cell homeostasis[5]. Accordingly, circulating B cell subset frequencies were also determined in this study. We found that the frequencies of CD27+IgD+ memory B cells, CD27+IgD- class-switched memory B cells and plasmablasts were significantly lower in patients with HCC compared to the non-HCC group. In contrast, number of naïve B cells did not differ significantly among groups. These findings were consistent with a previous report demonstrating that decreased of CD27+ memory B cells was found in patients with HCC but no differences in CD27- naïve and total B cells[22]. Moreover, a similar result was observed in patients with HCV-related HCC in which CD27+ memory B cells, and more specifically CD27+IgM+ memory B cells, were markedly less frequent in patients with HCC independent of HCV infection[23]. Memory B cells are long-lived and could capture and present the antigen to MHC molecules and then modulate CD4+ and CD8+ T cells immune response[24]. They also could potentially activate other immune cells such as dendritic cells and macrophages by producing pro-inflammatory cytokines. Therefore, it is possible that the decrease in total memory B cells and plasmablast in HBV-related HCC might reflect the defect of generating protective immunity against HBV in these individuals. In a model of lung cancer, other cell types, such as myeloid-derived suppressor cells can al so impair B cells function through the secretion of IL-17, which is associated with decrease of antibody production[25]. In addition to anti-tumor capacity, B cells can also modulate T cell immune responses via the function of Bregs[26]. Here, we did not directly measure Bregs population but instead investigated the CD19+CD24hiCD38hi transitional B cells, which have predominant Breg in this subset[27]. In this context, we did not observe any significant difference of the transitional B cells among the studied groups.
Interestingly, different finding regarding the distribution of B cell subpopulations was observed in patients with colorectal cancer, in which higher percentages of memory and plasma B cells were detected in peripheral blood[28]. In addition, increased numbers of circulating switched memory cells and plasmablasts were observed in other solid tumors including urinary bladder cancer, malignant melanoma, pancreatic cancer and prostate cancer[29]. Moreover, there was evidence that antibodies produced by B cells play a role in the cancer progression by initiating chronic inflammation[30]. Taken together, it is likely that role of B cell substes might not be similar among different cancer types. Therefore, categorizing B cell subpopulations in each cancer type is important not only for a better understanding the mechanisms by which immune cell subsets affect tumor biology but also for designing a successful treatment using immunotherapeutic approaches.
Overall, our findings indicated that BAFF-R expressing B cells had a decreased dependency on the maturation of B cells in patients with HCC compared with the non-HCC groups. It is therefore possible that down-regulation of BAFF-R could constitute resistance to the biological actions of BAFF and as such might, in part, be responsible for the elevated plasma BAFF concentrations in patients with HCC. On the other hand, it might be possible that chronic elevated BAFF levels in patients with HCC could lead to a downregulation of BAFF-R expression, as previously observed in patients with autoimmune disorders[21]. At this point, however, the underlying mechanism by which BAFF and BAFF receptors involve in HCC development is not clear and needed to be further elucidated. In this perspective, the design of therapeutic target of BAFF or BAFF-R might also be important for patients with HCC in the future.
Despite our interesting findings on the role of B cells and their ligand/receptors in disease progression in patients with HBV-related HCC, there were some limitations in this study. First, the study was retrospective and there might have many possible confounding factors, such as age and sex. Second, the sample sizes of patients with or without HCC were relatively small and a replicate study with a larger number of patients is needed to verify these observations and would provide further insights into the role of BAFF, as well as BAFF receptors and B cell response in HBV-related HCC. Moreover, further studies also need to validate these observations in patients with HCC regardless of underlying etiologies and to elucidate the mechanistic roles of B cell-mediated immune response in hepatocarcinogenesis.
In summary, we found the expression of BAFF-R was reduced in B cells that might reflect the diferent frequency of B cells maturation in patients with HCC. The memory B cells especially CD27+IgD- class-switched memory B cells and plasmablasts were significantly decreased whereas naïve and transitional B cells were not different in patients with HCC as compared with the non-HCC group and healthy controls. Additionally, decreased BAFF-R expression on B cells was significantly correlated with tumor size and more advanced BCLC stages. Together, these data indicate that abnormalities of B cell development according to the expression of BAFF-R is contributable to the development and progression of HBV-related HCC.
Recently, B cell-activating factor (BAFF) receptors and B cell subsets play diverse but crucial roles in modulating B cell function. Therefore, analysis of their expression and subpopulation frequencies could provide more insights into the immunological characteristics of B cell selection in patients with hepatocellular carcinoma (HCC).
To evaluate the association of BAFF receptors on B cell subsets according to clinical outcome of patients with chronic HBV infection.
This study aimed to compare the expression of BAFF receptors and the distribution of B cell subsets in the peripheral blood of patients with HBV-related HCC compared to individuals without HCC and healthy controls.
Peripheral blood samples collected from chronic HBV infected patients with or without HCC and healthy controls were assessed for BAFF receptors [BAFF-R(B cell-activating factor receptor), transmembrane activator and cyclophilin ligand interactor, B-cell maturation antigen] and B cell subpopulations by multicolor flow cytometry.
The frequency of BAFF-R expression on B cells was significantly decreased in HBV-related HCC compared with HBV patients without HCC and healthy controls. In addition, the frequency of CD27+IgD+ memory B cells, CD27+IgD- class switched memory B cells and plasmablasts were significantly lower in patients with HCC compared to the non-HCC group. However, the frequency of naïve and transitional B cell did not differ significantly among groups. Moreover, decreased BAFF-R expression on B cells was significantly correlated with tumor size and more advanced Barcelona Clinic Liver Cancer stages.
The expression of BAFF-R on B cells was significantly decreased that involved in the different frequencies of B cells maturation in patients with HCC.
Larger samples and the mechanistic roles of B cell-mediated immune response in hepa-tocarcinogenesis are needed to further validate.
| 1. | El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264-1273.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2564] [Article Influence: 183.1] [Reference Citation Analysis (3)] |
| 2. | Tangkijvanich P, Mahachai V, Komolmit P, Fongsarun J, Theamboonlers A, Poovorawan Y. Hepatitis B virus genotypes and hepatocellular carcinoma in Thailand. World J Gastroenterol. 2005;11:2238-2243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Arzumanyan A, Reis HM, Feitelson MA. Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. Nat Rev Cancer. 2013;13:123-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 642] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 4. | Ringelhan M, Pfister D, O'Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 792] [Article Influence: 99.0] [Reference Citation Analysis (0)] |
| 5. | Gitlin AD, Nussenzweig MC. Immunology: Fifty years of B lymphocytes. Nature. 2015;517:139-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Lied GA, Berstad A. Functional and clinical aspects of the B-cell-activating factor (BAFF): a narrative review. Scand J Immunol. 2011;73:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 751] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 8. | Liu M, Sun Q, Wang J, Wei F, Yang L, Ren X. A new perspective: Exploring future therapeutic strategies for cancer by understanding the dual role of B lymphocytes in tumor immunity. Int J Cancer. 2019;144:2909-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Lin JC, Shih YL, Chien PJ, Liu CL, Lee JJ, Liu TP, Ko WC, Shih CM. Increased percentage of B cells in patients with more advanced hepatocellular carcinoma. Hum Immunol. 2010;71:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Zhang Z, Ma L, Goswami S, Ma J, Zheng B, Duan M, Liu L, Zhang L, Shi J, Dong L, Sun Y, Tian L, Gao Q, Zhang X. Landscape of infiltrating B cells and their clinical significance in human hepatocellular carcinoma. Oncoimmunology. 2019;8:e1571388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 11. | Garnelo M, Tan A, Her Z, Yeong J, Lim CJ, Chen J, Lim KH, Weber A, Chow P, Chung A, Ooi LL, Toh HC, Heikenwalder M, Ng IO, Nardin A, Chen Q, Abastado JP, Chew V. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut. 2017;66:342-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 379] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 12. | Khlaiphuengsin A, Chuaypen N, Pinjaroen N, Sirichindakul B, Hirankarn N, Tangkijvanich P. Plasma B-cell activating factor levels and polymorphisms in hepatitis B-related hepatocellular carcinoma: Clinical correlation and prognosis. Asian Pac J Allergy Immunol. 2019; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6630] [Article Influence: 442.0] [Reference Citation Analysis (1)] |
| 14. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3630] [Article Influence: 259.3] [Reference Citation Analysis (12)] |
| 15. | Ogunwobi OO, Harricharran T, Huaman J, Galuza A, Odumuwagun O, Tan Y, Ma GX, Nguyen MT. Mechanisms of hepatocellular carcinoma progression. World J Gastroenterol. 2019;25:2279-2293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 116] [Cited by in RCA: 179] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 16. | Smulski CR, Eibel H. BAFF and BAFF-Receptor in B Cell Selection and Survival. Front Immunol. 2018;9:2285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 272] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 17. | Ng LG, Sutherland AP, Newton R, Qian F, Cachero TG, Scott ML, Thompson JS, Wheway J, Chtanova T, Groom J, Sutton IJ, Xin C, Tangye SG, Kalled SL, Mackay F, Mackay CR. B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. J Immunol. 2004;173:807-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 401] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 18. | Batten M, Groom J, Cachero TG, Qian F, Schneider P, Tschopp J, Browning JL, Mackay F. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med. 2000;192:1453-1466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 545] [Cited by in RCA: 560] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 19. | Thompson JS, Bixler SA, Qian F, Vora K, Scott ML, Cachero TG, Hession C, Schneider P, Sizing ID, Mullen C, Strauch K, Zafari M, Benjamin CD, Tschopp J, Browning JL, Ambrose C. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293:2108-2111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 717] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 20. | Maia S, Pelletier M, Ding J, Hsu YM, Sallan SE, Rao SP, Nadler LM, Cardoso AA. Aberrant expression of functional BAFF-system receptors by malignant B-cell precursors impacts leukemia cell survival. PLoS One. 2011;6:e20787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Sellam J, Miceli-Richard C, Gottenberg JE, Ittah M, Lavie F, Lacabaratz C, Gestermann N, Proust A, Lambotte O, Mariette X. Decreased B cell activating factor receptor expression on peripheral lymphocytes associated with increased disease activity in primary Sjögren's syndrome and systemic lupus erythematosus. Ann Rheum Dis. 2007;66:790-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Wang XD, Wang L, Ji FJ, Zhu JM, Ayana DA, Fang XD. Decreased CD27 on B lymphocytes in patients with primary hepatocellular carcinoma. J Int Med Res. 2012;40:307-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Doi H, Iyer TK, Carpenter E, Li H, Chang KM, Vonderheide RH, Kaplan DE. Dysfunctional B-cell activation in cirrhosis resulting from hepatitis C infection associated with disappearance of CD27-positive B-cell population. Hepatology. 2012;55:709-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 24. | Milich DR, Chen M, Schödel F, Peterson DL, Jones JE, Hughes JL. Role of B cells in antigen presentation of the hepatitis B core. Proc Natl Acad Sci USA. 1997;94:14648-14653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 166] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Wang Y, Schafer CC, Hough KP, Tousif S, Duncan SR, Kearney JF, Ponnazhagan S, Hsu HC, Deshane JS. Myeloid-Derived Suppressor Cells Impair B Cell Responses in Lung Cancer through IL-7 and STAT5. J Immunol. 2018;201:278-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 26. | Balkwill F, Montfort A, Capasso M. B regulatory cells in cancer. Trends Immunol. 2013;34:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 27. | Blair PA, Noreña LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1274] [Article Influence: 79.6] [Reference Citation Analysis (0)] |
| 28. | Shimabukuro-Vornhagen A, Schlößer HA, Gryschok L, Malcher J, Wennhold K, Garcia-Marquez M, Herbold T, Neuhaus LS, Becker HJ, Fiedler A, Scherwitz P, Koslowsky T, Hake R, Stippel DL, Hölscher AH, Eidt S, Hallek M, Theurich S, von Bergwelt-Baildon MS. Characterization of tumor-associated B-cell subsets in patients with colorectal cancer. Oncotarget. 2014;5:4651-4664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 29. | Zirakzadeh AA, Marits P, Sherif A, Winqvist O. Multiplex B cell characterization in blood, lymph nodes, and tumors from patients with malignancies. J Immunol. 2013;190:5847-5855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | De Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 599] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Thailand
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Corresponding Author's Membership in Professional Societies: Center of Excellence in Hepatitis and Liver Cancer, Department of Biochemistry, Faculty of medicine, Chulalongkorn University
P-Reviewer: Hann HW, Kai K S-Editor: Zhang H L-Editor: A E-Editor: Zhang YL