Published online May 28, 2020. doi: 10.3748/wjg.v26.i20.2618
Peer-review started: January 28, 2020
First decision: February 27, 2020
Revised: March 25, 2020
Accepted: May 14, 2020
Article in press: May 14, 2020
Published online: May 28, 2020
Processing time: 111 Days and 3.2 Hours
Persistent Helicobacter pylori (H. pylori) infection causes chronic inflammation, atrophy of the gastric mucosa, and a high risk of developing gastric cancer. In recent years, awareness of eradication therapy has increased in Japan. As H. pylori infections decrease, the proportion of gastric cancers arising from H. pylori uninfected gastric mucosa will increase. The emergence of gastric cancer arising in H. pylori uninfected patients though rarely reported, is a concern to be addressed and needs elucidation of its clinicopathological features.
To evaluate the clinicopathological features of early gastric cancer in H. pylori-uninfected patients.
A total of 2462 patients with 3375 instances of early gastric cancers that were treated by endoscopic submucosal dissection were enrolled in our study between May 2000 and September 2019. Of these, 30 lesions in 30 patients were diagnosed as H. pylori-uninfected gastric cancer (HpUIGC). We defined a patient as H. pylori-uninfected using the following three criteria: (1) The patient did not receive treatment for H. pylori, which was determined by investigating medical records and conducting patient interviews; (2) Lack of endoscopic atrophy; and (3) The patient was negative for H. pylori after being tested at least twice using various diagnostic methods, including serum anti-H. pylori-IgG antibody, urease breath test, rapid urease test, and microscopic examination.
The frequency of HpUIGC was 1.2% (30/2462) for the patients in our study. The study included 19 males and 11 females with a mean age of 59 years. The location of the stomach lesions was divided into three sections; upper third (U), middle third (M), lower third (L). Of the 30 lesions, 15 were U, 1 was M, and 14 were L. Morphologically, 17 lesions were protruded and flat elevated type (0-I, 0-IIa, 0-IIa + IIc), and 13 lesions were flat and depressed type (0-IIb, 0-IIc). The median tumor diameter was 8 mm (range 2-98 mm). Histological analysis revealed that 22 lesions (73.3%) were differentiated type.The HpUIGC lesions were classified into fundic gland type adenocarcinoma (7 cases), foveolar type well-differentiated adenocarcinoma (8 cases), intestinal phenotype adenocarcinoma (7 cases), and pure signet-ring cell carcinoma (8 cases). Among 30 HpUIGCs, 24 lesions (80%) were limited to the mucosa; wherein, the remaining 6 lesions showed submucosal invasion. One of the submucosal invasive lesions showed more than 500 μm invasion. The mucin phenotype analysis identified 7 HpUIGC with intestinal phenotype and 23 with gastric phenotype.
We elucidated the clinicopathological characteristics of HpUIGC, revealing recognition not only undifferentiated-type but also differentiated-type. In addition, intestinal phenotype tumors were also observed and could be an important tip.
Core tip: Chronic Helicobacter pylori (H. pylori) infection is a major risk factor for gastric cancer. Historically, gastric cancers in Japan were related to H. pylori infection, and the frequency of H. pylori uninfected gastric cancer (HpUIGC) was very rare. However, the rarity of gastric cancer in H. pylori negative patients may be partly owing to underreporting, and the mechanisms behind the development and progression of this type of gastric cancer must be elucidated. This study elucidated the clinicopathological features of H. pylori uninfected gastric cancer from 30 gastric cancer patients. Differentiated-type gastric cancers without submucosal invasion were most prominent.
- Citation: Sato C, Hirasawa K, Tateishi Y, Ozeki Y, Sawada A, Ikeda R, Fukuchi T, Nishio M, Kobayashi R, Makazu M, Kaneko H, Inayama Y, Maeda S. Clinicopathological features of early gastric cancers arising in Helicobacter pylori uninfected patients. World J Gastroenterol 2020; 26(20): 2618-2631
- URL: https://www.wjgnet.com/1007-9327/full/v26/i20/2618.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i20.2618
Most gastric cancers involve Helicobacter pylori (H. pylori) infection in their development, and in 1994 H. pylori was certified as a “definite carcinogen” for gastric cancer development[1,2]. H. pylori infection results in inflammation, atrophy of the gastric mucosa, and intestinal metaplasia; when H. pylori infection becomes chronic there is a high-risk gastric cancer[3].
In recent years, awareness of eradication therapy has increased in Japan, thus reducing the rate of H. pylori infection, especially in the young people due to the improvement of sanitary environment and expanding the indication of eradication[4]. As H. pylori infections decrease, the proportion of gastric cancers arising from H. pylori uninfected gastric mucosa will increase[5]. However, at the moment, H. pylori-uninfected gastric cancer (HpUIGC) is very rare as compared to H. pylori-positive gastric cancer (HpPGC). The definition is not yet well established, and its frequency is reported to differ from 0.4 to 5.4%[6-11]. Previous studies have reported that the undifferentiated-type of HpUIGC is more frequently observed than differentiated-type[7-9]. However, in recent years, differentiated-type gastric cancers such as oxyntic glands adenoma/adenocarcinoma and foveolar type adenoma/adenocarcinoma, even including HpUIGC, were reported[12,13]. Few studies have investigated HpUIGC and its clinicopathological features have not been sufficiently documented. Therefore, elucidation of characteristics of early-stage HpUIGC is essential. We evaluated the characteristics of HpUIGC treated with endoscopic submucosal dissection (ESD) focusing on pathological and endoscopic features.
Three criteria were used to determine whether a patient was H. pylori-uninfected: (1) No medical history of H. pylori eradication therapy, which was determined by investigating the patients’ medical records and conducting patient interviews; (2) Lack of endoscopic atrophy, patients with C-0 atrophy were selected as HpUIGC[14]. As a supplementary finding, we referenced the endoscopic findings of the Kyoto classification score, including RAC (regular arrangement of collecting venule)[15,16]. The endoscopic findings were subsequently verified by three skilled endoscopists (KH, CS, and SM). And (3) laboratory examination that included serum anti-H. pylori-IgG antibody, Urease breath test (UBT), Rapid urease test (RUT), and microscopic examination[17]. If a test was negative for H. pylori by two or more examinations this was considered H. pylori uninfected[18,19]. Among the HpUIGC patients, the presence or absence of pathological atrophy was evaluated using the updated Sydney system in the background mucosa of ESD specimens[20]. Tumors satisfying all the three conditions described above were identified as HpUIGC.
Between May 2000 and September 2019, a total of 2569 patients with 3477 gastric cancers were treated by endoscopic submucosal dissection (ESD) at Yokohama City University Medical Center. Of these patients, 2462 consecutive patients with 3370 gastric cancers were assessed for H. pylori status and enrolled in this study. The remaining 107 patients included 87 patients with cancer in their gastric remnants, 16 with cancer in their gastric tubes, 4 with neuroendocrine tumors, were excluded. Of the 3370 gastric cancers, 30 gastric cancers satisfied the three criteria outlined above and were classified as HpUIGCs.
We investigated the frequency and features of HpUIGC. Clinicopathological features including age, sex, location, macroscopic type, histological type, tumor size, depth of invasion, presence or absence of lymphovascular invasion, and treatment outcome were evaluated. The location of the gastric lesions was categorized based on stomach location: upper third (U), middle third (M), and lower third (L). The histological type was identified as differentiated or undifferentiated according to the 15th edition of the Japanese classification of gastric cancer[21]. The differentiated type was further classified into well-differentiated (tub1), moderately differentiated (tub2), or, papillary (pap) adenocarcinoma. The undifferentiated type was classified as poorly differentiated (por) or signet-ring cell (sig) adenocarcinoma. HpUIGC was further categorized into four types based on their histopathological features (1) Fundic gland adenocarcinoma, (2) Foveolar-type adenocarcinoma, (3) Intestinal phenotype adenocarcinoma, and (4) Pure signet-ring cell carcinoma. Finally, the 30 cases of HpUIGC were evaluated for their mucin phenotypes and endoscopic features.
Indications of gastric ESD were determined according to the gastric cancer treatment guidelines of the Japanese Gastric Cancer Association (JGCA). Briefly, the indication criteria were defined as differentiated-type mucosal gastric cancer lesions without ulcers [UL (-)] regardless of size, differentiated-type mucosal gastric cancer lesions ≤ 3 cm in size with ulcers [UL (+)], undifferentiated-type mucosal gastric cancer lesions ≤ 2 cm in size without ulceration [UL (-)], and confirming no evidence of lymph node metastasis (LNM), and distant metastasis by preoperative computed tomography[22].
All lesions were treated by ESD. The gastric ESDs were performed as previously described[23,24]. Briefly, after marking approximately 5 mm around the borders of the lesion, circumferential incision and submucosal dissection were made using an IT-knife2 (Olympus Medical Systems Corporation, Tokyo, Japan) or Dual knife (Olympus Medical Systems Corporation, Tokyo, Japan). Hyaluronic acid and/or glycerol were used as the submucosal injecting solution.
The resected specimens were fixed with 10% buffered formalin immediately after the procedure. To reliably evaluate the deepest part of the lesion and the horizontal margin, it was cut into thin sections (2-3 mm) parallel to the oral side to the anal side[21]. The resected specimens were embedded in paraffin and mounted on slides then subjected to hematoxylin and eosin staining and immunohistochemistry. Specimen size, tumor size, macroscopic type, and the depth of invasion were measured in accordance with the Japanese Gastric Cancer Treatment Guideline 2014 (Ver. 4)[22], describing the. Treatment was deemed curative when all of the following conditions were fulfilled: en bloc resection, negative horizontal margin (HM0), negative vertical margin (VM0), and no lymphovascular infiltration [ly(-), v(-)]. In histologically differentiated-type tumors with pT1a UL(-) regardless of tumor size, pT1a UL(+) with tumor size ≤ 3 cm, histologically of differentiated type, and pT1b (SM1, 500 microns from the muscularis mucosae) with tumor size ≤ 3 cm were judged curative. In histologically undifferentiated-type, pT1a, UL(-) with tumor size ≤ 2 cm was also considered as curative. Resections that does not satisfy any of the above criteria were considered non-curative.
Immunohistochemical staining of the 30 HpUIGCs was performed in representative sections taken from the tumor at its largest diameter. Mucin phenotype was evaluated using MUC2, MUC5AC, MUC6, CD10, CDX2, PG-I, and H+/K+ -ATPase markers. We used monoclonal antibodies against the following markers: Mucin 5AC (MUC5AC) as a marker for gastric foveolar cells, MUC6 as a marker for gastric mucous neck cells and pyloric glands, MUC2 as a marker for intestinal goblet cells, CD10 as a marker for small intestinal brush border, CDX2 as a marker for epithelial intestinal differentiation. The tumors that showed differentiation to the fundus gland were immunochemically stained for definitive diagnosis of the oxyntic tumor; PG-I was used as a marker for chief cells, and proton pump/H+/K+-ATPase alpha subunit as a marker for parietal cells. MUC2, MUC5AC, MUC6, CD10, PG-I, H+/K+-ATPase reactivity was considered significantly positive when > 10% of tumor cells were stained. Cases with < 10% positive cells were regarded as unaffected. The mucin phenotype was divided into (1) gastric phenotype, (2) intestinal phenotype, and (3) gastric and mixed intestinal phenotype. The gastric and mixed intestinal phenotype was subdivided into gastric phenotype dominant or intestinal phenotype dominant[25,26].
A total of 2462 consecutive patients with 3370 c gastric cancers (3132 early gastric cancer lesions and 238 adenomas) were enrolled. In total, 30 lesions form 30 patients (1.2%) were classified HpUIGC. The clinicopathological features of HpUIGC are shown in Table 1. The study included 19 males and 11 females with a mean age of 59 years. Of the 30 lesions, 15 were U, one was M and 14 were L. Morphologically, 17 lesions were protruded and flat elevated type (0-I, 0-IIa, 0-IIa+IIc), and 13 lesions were flat and depressed type (0-IIb, 0-IIc). Tumor diameter ranged from 2 mm to 98 mm, with a median diameter of 8 mm. Histopathologically, 22 lesions (73.3%) were identified as differentiated-type and eight lesions (26.7%) as undifferentiated-type. All of the undifferentiated lesions tested were signet-ring cell carcinomas. Tumor invasion in 24 lesions (80%) was limited to the mucosa, while the remaining 6 lesions showed submucosal invasion. One of the lesions invaded the submucosal layer to a depth of 500 μm (SM2). Outcomes for the HpUIGC patients were positive, all received successful en bloc resections, free from tumor margin. The curative resection rate was 96.3%. Details of 30 HpUNGCs are shown in Table 2.
| n = 30 | |
| mean age ± SD (yr) | 59 ± 9 |
| Gender, n | |
| Male | 19 (63.3%) |
| Female | 11 (46.7%) |
| Location, n | |
| Upper part | 15 (50%) |
| Middle part | 1 (3.3%) |
| Lower part | 14 (46.7%) |
| Morphology, n | |
| Protruded /flat | 17 (56.7%) |
| Depressed | 13 (43.3%) |
| Mean tumor diameter (range) | 8 (2-98 mm) |
| Depth of invasion, n | |
| M | 24 (80%) |
| SM1 | 5 (16.7%) |
| SM2 | 1 (3.3%) |
| Histological type, n | |
| Differentiated type | 22 (73.3%) |
| Undifferentiated type | 8 (26.7%) |
| Histological classification | |
| Fundic grand type adenocarcinoma | 7 (23.3%) |
| Foveolar type well differentiated adenocarcinoma | 8 (26.7%) |
| Intestinal phenotype adenocarcinoma | 7 (23.3%) |
| Pure signet ring call carcinoma | 8 (26.7%) |
| Ulcerative finding, n | |
| (-) | 30 (100%) |
| (+) | 0 (0%) |
| En-bloc resection, n | 96.7% |
| R0+curative resection (cura A) | 93.3% |
| Complication | |
| Perforation | 6.7% |
| Delayed perforation | 0% |
| Delayed bleeding | 0% |
| Case | Classification | Sex | Age | Location | Morphology | Size | Depth | Histology | v/ly | RUT | UBT | HP-IgG |
| 1 | Fundic | m | 55 | U | 0-IIc | 6 | sm1 | tub1 | 0 | + | + | |
| 2 | Fundic | m | 80 | U | 0-IIc | 12 | sm1 | tub2 > tub1 | 0 | + | + | |
| 3 | Fundic | f | 65 | U | 0-IIa | 6 | sm1 | tub1, | 0 | + | + | |
| 4 | Fundic | f | 65 | U | 0-IIa | 10 | m | tub1 | 0 | + | + | + |
| 5 | Fundic | m | 69 | U | 0-IIa + IIc | 8 | sm1 | tub1, | 0 | + | + | |
| 6 | Fundic | m | 62 | U | 0-IIa | 13 | sm2 | tub1 | 0 | + | + | |
| 7 | Fundic | m | 67 | U | 0-I | 6 | sm1 | tub1 | 0 | + | + | |
| 8 | Foveolar | f | 34 | U | 0-IIa | 35 | m | tub1 | 0 | + | + | |
| 9 | Foveolar | f | 66 | U | 0-IIa | 33 | m | pap | 0 | + | + | + |
| 10 | Foveolar | m | 63 | U | 0-IIa | 55 | m | tub1 | 0 | + | + | |
| 11 | Foveolar | f | 69 | U | 0-IIa | 98 | m | tub1 | 0 | + | + | |
| 12 | Foveolar | f | 51 | U | 0-IIa | 28 | m | tub1 | 0 | + | + | |
| 13 | Foveolar | m | 72 | U | 0-IIa | 63 | m | tub1 | 0 | + | + | |
| 14 | Foveolar | m | 64 | U | 0-IIa | 42 | m | tub1 > pap | 0 | + | + | |
| 15 | Foveolar | f | 50 | U | 0-IIa | 2 | m | tub1 | 0 | + | + | |
| 16 | Intestinal | m | 66 | L | 0-IIc | 9 | m | tub1 | 0 | + | + | |
| 17 | Intestinal | m | 49 | L | 0-IIc | 5 | m | tub1 | 0 | + | + | + |
| 18 | Intestinal | f | 65 | L | 0-IIa | 3 | m | tub1 | 0 | + | + | |
| 19 | Intestinal | f | 61 | L | 0-IIc | 5 | m | tub1 | 0 | + | + | |
| 20 | Intestinal | f | 43 | L | 0-IIc | 3 | m | tub1 | 0 | + | + | |
| 21 | Intestinal | m | 48 | L | 0-IIa | 7 | m | tub1 | 0 | + | + | |
| 22 | Intestinal | f | 52 | L | 0-IIa | 5 | m | tub1 | 0 | + | + | |
| 23 | Sig | m | 58 | L | 0-IIc | 4 | m | sig | 0 | + | + | |
| 24 | Sig | m | 55 | L | 0-IIc | 5 | m | sig | 0 | + | + | + |
| 25 | Sig | m | 65 | M | 0-IIc | 5 | m | sig | 0 | + | + | |
| 26 | Sig | m | 49 | L | 0-IIc | 13 | m | sig | 0 | + | + | |
| 27 | Sig | m | 53 | L | 0-IIc | 4 | m | sig | 0 | + | + | |
| 28 | Sig | m | 45 | L | 0-IIc | 8 | m | sig | 0 | + | + | |
| 29 | Sig | m | 46 | L | 0-IIb | 12 | m | sig | 0 | + | + | |
| 30 | Sig | m | 84 | L | 0-IIa | 2 | m | sig | 0 | + | + |
Histologically the HpUIGC lesions were classified into fundic gland type adenocarcinoma (7 cases), foveolar type well-differentiated adenocarcinoma (8 cases), intestinal phenotype adenocarcinoma (7 cases), and pure signet-ring cell carcinoma (8 cases). The histological and endoscopic findings of different types of lesions are explained below.
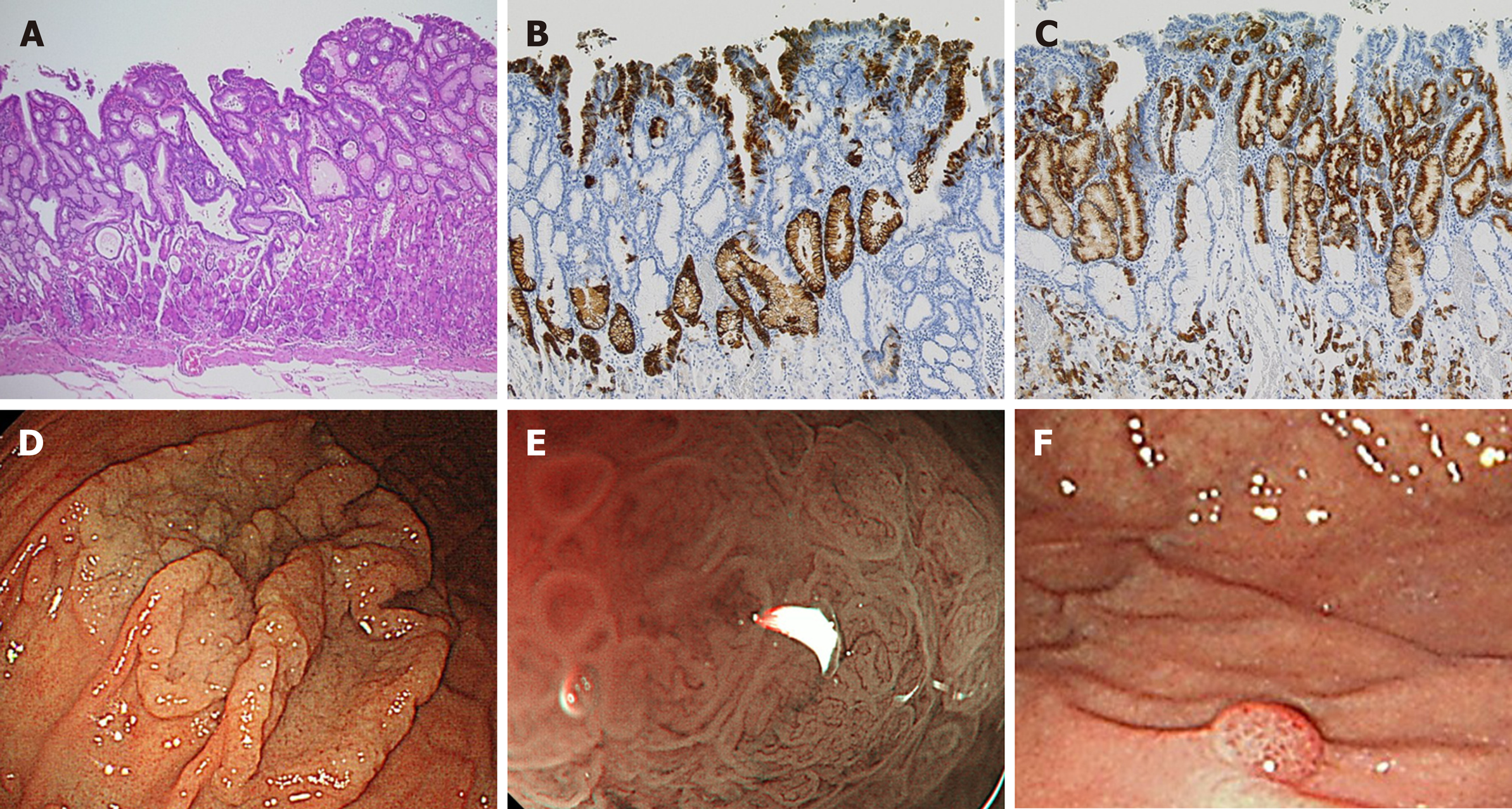
Fundic gland type adenocarcinoma (Figure 1): Histopathological finding: HE staining showed the presence of tumor cells mimicking fundic glands at the bottom of the mucosa, and the surface of the tumor was covered with non-cancerous epithelium. Immunohistologically, the fundic gland cancer cells were positive for PG-I and MUC6, and part of the tumor expressed H+/K+-ATPase. Six of the seven lesions showed submucosal invasion, while one of them showed SM2 invasion (distance from muscularis mucosae was 780 μm). None of the lesions revealed lymphovascular invasion. Endoscopic finding: All seven lesions were located in the upper part of the stomach and were recognized as small protrusions. With white light imaging, a yellowish-white tumor covered with non-cancerous epithelium was observed in submucosal tumors (SMTs). Magnified narrow-band imaging (ME-NBI) revealed dilated branched vessels and intervening part on the lesion’s surface.
Foveolar type well-differentiated adenocarcinoma (Figure 2): Histopathological finding: Seven of the foveolar type gastric cancers were composed of dysplastic columnar cells with clear cytoplasm and showed villous or papillary structures mimicking foveolar epithelium. On the surface of the mucosa, the expanded glands composed of non-tumor cells pushed up the cancerous epithelium. Tumor existed only on the surface, and atypia was recognized as low-grade well-differentiated adenocarcinoma. None of the lesions showed submucosal invasion, although the tumor size was large (mean diameter 37.3 ± 18.3 mm). All foveolar type gastric cancers were positive for MUC5AC, but negative for PG-I, MUC2, and CD10 were negative. MUC6 was positive for expanded non-cancerous glands in the middle to the bottom layer of the mucosa. No lymphovascular invasion was observed in any of the eight gastric cancers.Endoscopic finding: All lesions were located in the upper part of the stomach. Seven of eight foveolar-type well-differentiated adenocarcinomas were observed as laterally spreading elevated lesions with whitish color such as an intestinal-type adenoma, and one lesion showed raspberry-like appearance [13]. ME-NBI showed a papillary or villous shaped fine mucosal pattern with intra-structural irregular vessels in all lesions. One of the foveolar type well-differentiated adenocarcinomas was recognized as a small protrusion with a raspberry-like appearance in the greater curvature of the upper part of the stomach. Although this lesion resembled a hyperplastic polyp, tumor cell atypia revealed well-differentiated adenocarcinoma.
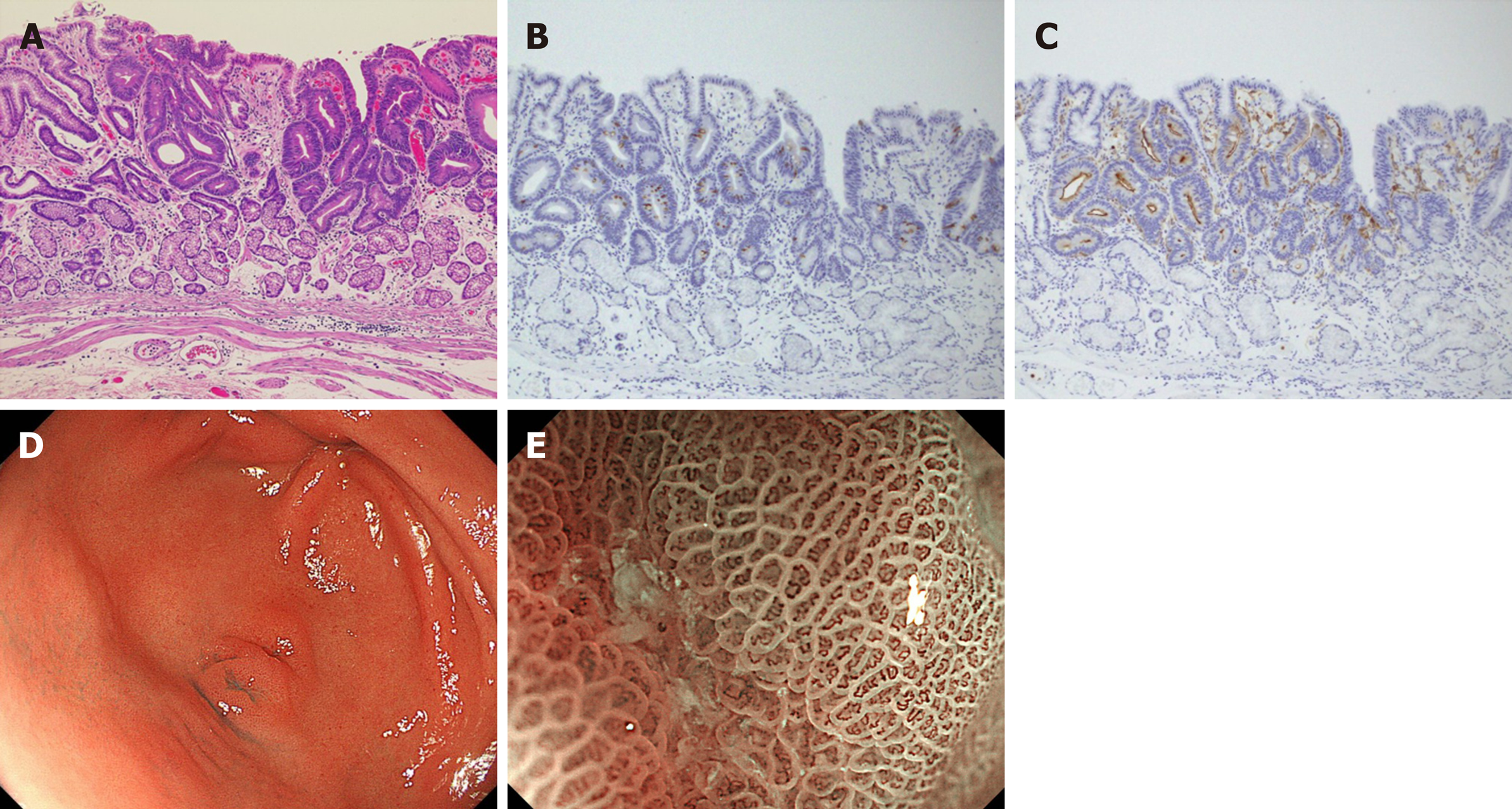
Intestinal phenotype adenocarcinoma (Figure 3): Histopathological finding: All tumors showed well-differentiated adenocarcinoma characterized by tubular structures lined by tall columnar cells with hyperchromatic, pencillate, and pseudostratified nuclei. Luminal borders were sharp with a brush border. Goblet cells were positive for MUC2 and the brush border of intestinal absorptive epithelial cells was positive for CD10. The surface epithelium was focally positive for MUC5AC, and deeper glands were focally positive for MUC6. These lesions were classified as the intestinal-type dominant mucin phenotype. Endoscopic finding: The white light image revealed a 0-IIa+IIc-type lesion mimicking verrucosa with a red tone, approximately 5 mm in size, and all the lesions were found in the gastric antrum. Unlike conventional verrucosa, which frequently occurs in the antrum, it was characterized by only one or two humps. An irregular microvascular pattern and irregular microsurface pattern with a demarcation line were observed in the recessed area with ME-NBI.
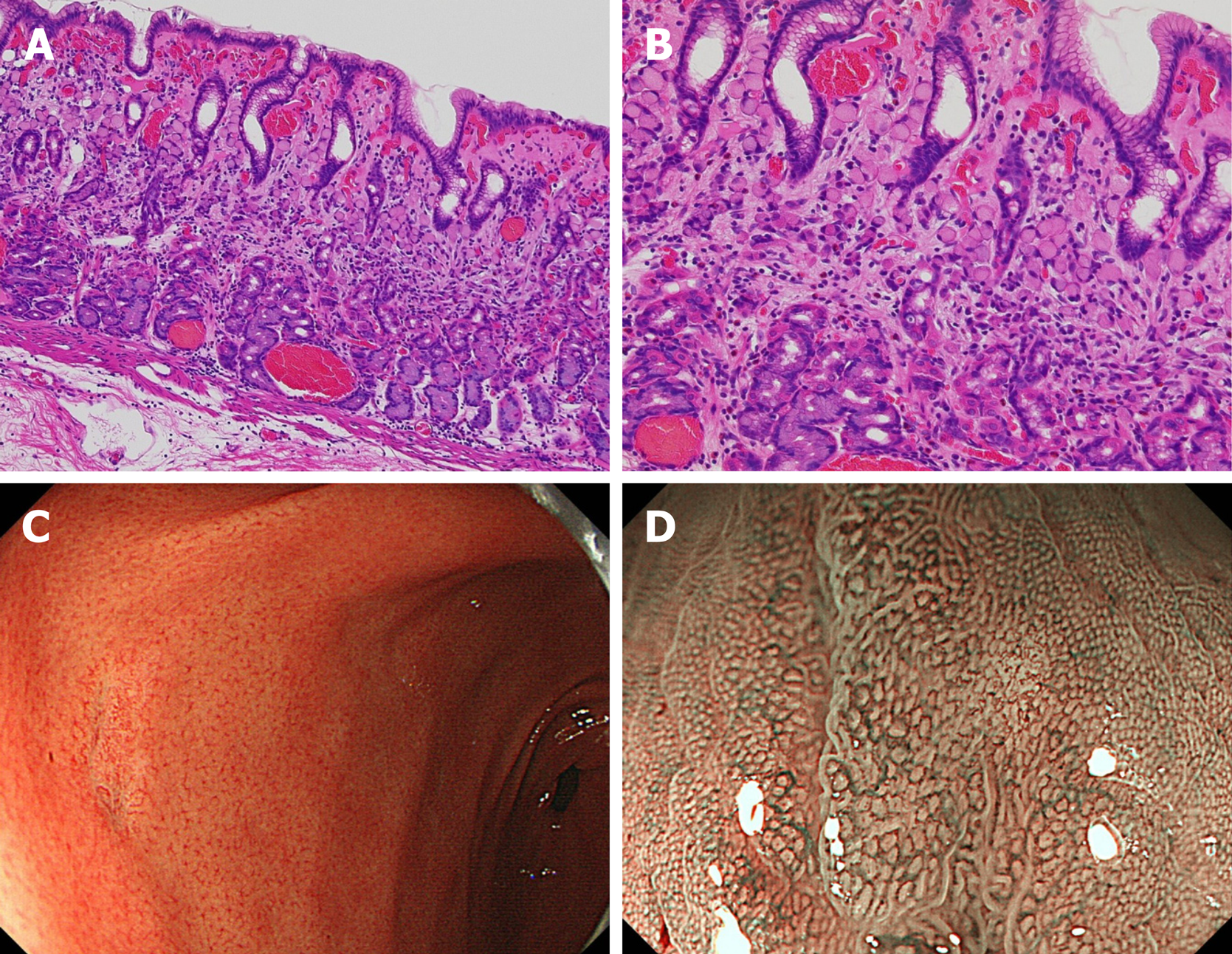
Pure signet-ring cell carcinoma (Figure 4): Histopathological finding: Pure signet-ring cell carcinoma existed in the proliferative zone to the surface layer of mucosa. Most of the lesions, cancer cells were limited to the proliferative zone, and the surface layer of mucosa was found to be covered with non-cancerous epithelium. Endoscopic finding: In the lower part of the stomach, mainly in the antrum, discolored and slightly depressed lesions were observed with a white light image. In six of these, the tumor size was less than 10 mm. Typical features such as corkscrew-like vessels[27], could not be observed.
All HpUIGC lesions were evaluated for their mucin phenotype (Table 3). The fundic gland adenocarcinoma, foveolar type well-differentiated adenocarcinoma, and pure signet-ring cell adenocarcinoma revealed gastric phenotype or gastric phenotype dominant, whereas intestinal phenotype adenocarcinoma showed intestinal phenotype dominant.
| MUC5AC | MUC6 | CD10 | MUC2 | |
| Fundic grand type adenocarcinoma | ++ | ++ | - | - |
| Foveolar type well differentiated adenocarcinoma | ++ | - | - | - |
| Intestinal phenotype adenocarcinoma | -~+ | -~+ | ++ | +~++ |
| Pure signet ring cell carcinoma | ++ | ++ | - | - |
We investigated long-term outcomes of 30 HpUIGC cases with a median 30-mo (ranged 10-138 mo) observation period. Neither gastric cancer mortality nor death from other diseases was observed; therefore, both overall survival and disease-free survival were 100%. Metachronous gastric cancer was not observed during patient follow-up.
H. pylori infection causes chronic inflammation and atrophy of the gastric mucosa and often results in gastric cancer. Since 1994, H. pylori has been recognized as a “definite carcinogen”, contributing to the development of gastric cancer[1-2]. A prospective study reported that H. pylori eradication therapy suppressed two-thirds of metachronous gastric cancer[28]. As a result, since 2010, the Japanese insurance system has allowed patients who have undergone endoscopic resection to receive H.pylori eradication therapy[17], and in the current Japan eradication therapy for H. pylori is insurance adaptation to all H. pylori infection patients. Improved sanitation has significantly reduced the rate of new H. pylori infections and H. pylori infection rate among young adults is reported to be decreasing yearly[4,5].
The frequency of H. pylori-negative gastric cancers is low[6-11]; however, this number is expected to increase, and the frequency of HpUIGC may increase proportionately. Currently, HpUIGC is still rarely reported so far, and the frequency varies considerably from 0.66% - 14% of gastric cancers[6-11,29-31]. The variation in this range may be owing to differences in the definition of H. pylori uninfected status in previous reports.
H. pylori detection methods possess high sensitivity and specificity and are usually divided into invasive (endoscopic based) and noninvasive methods. Invasive diagnostic tests include endoscopic imaging, histology, RUT, culture, and molecular methods. Non-invasive diagnostic tests include UBT, stool antigen test, serological, and molecular examinations. The accuracy of H. pylori infection diagnosis varies depending on the test. The sensitivity and specificity of UBT, serum anti-HP-IgG antibody, and RUT are 95% and 95%, 91%-100%, and 50%-91%, and 85%-95% and 95%-100%, respectively. However, some tests may produce false negatives owing to Proton Pomp Inhibitor (PPI) or patient factors, including past antibiotic use. To confirm H. pylori un-infected status, it is necessary to prove multiple tests[17,32-34]. In Japan, combination diagnostic testing showed the occurrence of gastric cancer ranged from 0.42% to 0.66% in patients without H. pylori infection[6,7,9]. Even if a patient is currently negative for H. pylori tests, there is a possibility of past infection; therefore, assessment of gastric atrophy is necessary to distinguish determine if there was a past infection. We emphasized endoscopic findings revealing C-0 atrophy The ESD specimen was confirmed to have no evidence of histological atrophy and inflammation in the background mucosa using the updated Sydney system[20]. In this study, the combination of two or more H. pylori tests (serum antibody, UBT, RUT, etc.) based on the Japanese society for H. pylori research guidelines in combination with no history of H. pylori eradication therapy were used to confirm H. pylori un-infection. As a result, HpUIGC was diagnosed in 30 of 2462 cases (1.2%). This was similar to previous reports. The average age of patients in our study was 59 years old; however, the males were, on average, older than the females[6-11].
Undifferentiated-type adenocarcinomas were more common than differentiated-type adenocarcinoma, and pure signet-ring cell carcinomas appeared at a rate similar to previous reports[5,7]. Unlike previous reports, most of the lesions (22/30 lesions) were the differentiated type. The eight undifferentiated-type adenocarcinomas were tiny pure signet-ring cell carcinomas that were confined to the proliferative zone and did not contain poorly differentiated type components. Signet-ring HpUIGCs were easily recognized owing to their lack of atrophy as minute discolored depressed lesions.
Unusual neoplastic changes in ME-NBI were owing to tumor cells only existing in the proliferative zone of the mucosa, and the surface layer being covered with non-cancerous epithelium. This is the reason why pathological findings do not show the typical corkscrew pattern[35]. Immunochemically, tumor cells showed a gastric phenotype. Reportedly, signet-ring cell carcinoma of the intestinal phenotype infiltrate from the proliferative zone of the mucosa into the deep mucosal layer, and infiltrate into the submucosa individually while maintaining the muscularis mucosa, and sometimes progressing to scirrhous gastric cancer. Gastric phenotype signet-ring cell carcinomas that progress from the proliferative zone to the surface layer of the mucosa, have a lower potential of being malignant potential than the intestinal phenotype[36,37].
Previous reports on the differentiated type of HpUIGC have mostly identified fundic gland type adenocarcinomas and gastric phenotype gastric cancer with low-grade atypia. However, with the small number of reports, it is difficult to identify the consistent clinicopathological features of HpUIGC. To the best of our knowledge, the present study reports the largest number of HpUIGC cases, 30, that had been evaluated for both endoscopic and pathological findings. It is notable that, in addition to pure signet-ring cell carcinoma and fundic gland type adenocarcinomas, which were often seen in previous reports, our study also included gastric phenotype low-grade adenocarcinoma, foveolar type adenocarcinoma, and intestinal-type adenocarcinoma. Additionally, our endoscopic findings and histopathological observations varied from those typically found in HpPGC.
The occurrence of fundic gland type adenocarcinomas has been reported next to the undifferentiated type in previous reports of HpUIGC[38,39]. This tumor has a gastric phenotype and low-grade adenocarcinoma occurring in the fundic gland on the middle layer of mucosa or just above the muscularis mucosae. The tumor is covered with non-cancerous epithelium; therefore, it demonstrates a submucosal tumor-like (SMT-like) morphology and sometimes infiltrates the submucosa. Similar to previous research, all cases in the present study showed SMT-like morphology, of which six cases (85.7%) showed frequent submucosal invasion (SM1: 5, SM2: 1) without lymphovascular invasion[12,39,40]. The only case where a tumor invaded into SM2 received additional surgical treatment as per the guidelines[22]. Proximal gastrectomy was selected, and no lymph node metastasis was observed in the resected specimen.
The foveolar type adenocarcinoma mainly showed low-grade atypia ade-nocarcinoma with a tendency to differentiate into the foveolar epithelium. Since there are few reports on HpUIGC differentiated cancer other than fundic gland type adenocarcinoma[11], the characteristics have not been clarified. This tumor has been classified as dysplasia/adenoma in the West[41]; however, in Japan, only the typical pyloric gland adenoma is classified as adenoma, and other fundic gland types and foveolar type are often treated as an adenocarcinoma even if it is non-invasive. Therefore, we classified them as foveolar-type adenocarcinomas. This type of tumor had unique histological findings such as MUC6 positive cell proliferation beneath the superficial dysplasia/well-differentiated adenocarcinoma in situ components[42]. We found MUC5AC positive tumor cells derived from the foveolar epithelium and a MUC6 positive cystic expanded gland in the middle layer of the mucosa, so flat or protruded macroscopic type was defined as a characteristic.
Whether this MUC6-positive cell was cancerous or noncancerous is still controversial. However, we determined that MUC6 positive cells were non-cancerous since the junction between MUC5AC positive cancer cells and MUC6 positive cells was clear, and no cell atypia was found in MUC6 positive cells with low KI-67 index. This tumor showed discolored flat elevation with a granular structure similar to intestinal adenoma as a colonic lateral spreading tumor (LST)[43]. However, this tumor was seen in the greater curvature of the fornix in the upper third of the stomach, not in the lower part of the stomach where intestinal-type adenomas occur frequently.
Although the intestinal phenotype of HpUIGC is rare and only a few cases were seen in case reports[44-47], it is essential to recognize that there are not a few intestinal phenotype adenocarcinomas among HpUIGCs. In this study, 7 verrucous-like tumors found in the antrum predominantly showed the intestinal phenotype, and to the best of our knowledge, this is the first report that revealed the endoscopic and the pathological features of this kind of tumor. In intestinal phenotype cancers, as the tumor grows, the gastric phenotype of the background mucosa gradually changes to the intestinal phenotype of the tumor and is eventually replaced or considered to be null. In this study, the tumor showed mixed gastrointestinal phenotype as the intestinal phenotype adenocarcinoma was very small, and the gastric phenotype in the background remained. Intestinal phenotype adenocarcinoma is characterized by a macroscopic type resembling a single verrucous found in the antrum. It is desirable to perform the endoscopy screening with ME-NBI in H. pylori uninfected patients to identify this tumor. It is reported that gastric epithelial cells might be generated by stem cells and progenitor cells located in the isthmus[48]. The types of stem cells in the isthmus are different between corpus and antrum, and the only antrum contains intestinal stem cells. Considering these observations, the intestinal-type aden-ocarcinoma we observed in the current study could be generated from the intestinal stem cells and, thus, detected only in the antrum.
In recent years, NBI diagnosis has become indispensable in the diagnosis of gastric cancer. In Japan, Yao's VS (microvascular architecture and microsurface structure) classification system is widely cited[49]. Further, the classification of Yokoyama et al[35] is useful because it can aid in identifying early gastric cancers in NBI images. However, in this HpUIGC series, there are many cases that cannot be characterized by the conventional NBI classification of gastric cancer. For example, in fundic gland type adenocarcinoma, the demarcation line is not clear because the tumor is covered with non-neoplastic foveolar epithelium so that the surface microstructure pattern is regular and typical NBI findings of differentiated type adenocarcinoma such as fine network pattern or intralobular loop pattern could not be confirmed. In addition, pure signet-ring cell carcinoma does not exhibit a typical corkscrew pattern as mentioned above. These cases classified in accordance with the NBI classification system. Therefore, HpUIGC may not conform to the conventional NBI classification system.
The mucin phenotype of gastric cancer is related to the growth, and biological malignancy of the tumor, and especially the differentiated-type gastric cancer with gastric phenotype may mix undifferentiated comportment as growth. Therefore, the biological malignancy of the differentiated-type gastric cancer with the gastric phenotype was considered higher than the intestinal-type[50-52]. However, in recent years, among the differentiated-type gastric cancer with the gastric phenotype, the existence of tumors with low-grade atypia such as extremely well-differentiated adenocarcinoma has been clarified. The gastric phenotype of differentiated adenocarcinoma is often difficult to distinguish from normal mucosa or regenerative epithelium because of its low-grade atypia[53]. Among HpUIGCs, the gastric phenotype is predominant in most of the undifferentiated and differentiated adenocarcinomas developed from the fundic gland area[9,29].
The etiology of gastric cancers, excluding H. pylori infection, is known to be associated with several factors including lifestyle, viral infection, autoimmune disorders, and germline mutations, but the main causal factor of HpUIGC remains unclear[54,55]. Bile acid reflux into the remnant stomach after gastrectomy is considered a risk factor for carcinogenesis, particularly, after Billroth II reconstruction[56]. Similarly, intestinal metaplasia caused by bile acid exposure might pose a risk of carcinogenesis in the stomach without H. pylori infection. In order to definitively determine the gastric cancer risk factors, further case studies and genetic analyses are needed. Although long-term outcomes of the present study were favorable, the observation period was short.
The study design and small sample number may be limitations to our study. A single-center retrospective cohort study and a small number of H. pylori-uninfected gastric cancer patients may not be representative of the broader population. In addition, although two or more tests were used to confirm H. pylori infection status the following the guidelines confirmed negative, but because the study period is long, the second limitation is that the types of tests are not unified.
The present study elucidated the clinicopathological features of HpUIGC, which is very rare. Herein, we classified HpUIGC into four categories according to histopathology and studied their endoscopic and pathological characteristics. To the best of our knowledge, we are the first to report that differentiated-type gastric cancers can possess the gastric and intestinal phenotype. HpUIGC malignancy is low; however, because the carcinogenic mechanism is unclear and further studies are required.
In recent years, awareness of eradication therapy has increased in Japan. As Helicobacter pylori(H. pylori) infections decrease, the proportion of gastric cancers arising from H. pylori uninfected gastric mucosa will increase. The emergence of gastric cancer arising in H. pylori uninfected patients though rarely reported, is a concern to be addressed and needs elucidation of its clinicopathological features.
Previously, H. pylori-uninfected gastric cancer including case report such as undifferentiated gastric cancer or fundic gland-type gastric cancer was reported. However, due to the rare frequency, there was very few reports. In the future, H.pylori-uninfected gastric cancer may increase relatively; therefore, importance of clarifying the clinicopathological features of those is desired. In this study, we experienced 30 cases of H. pylori-uninfected early gastric cancer and could classify histopathological features of these.
To clarify clinicopathological feature of H. pylori-uninfected gastric cancer (HpUNGC) treated by endoscopic submucosal dissection (ESD).
This study is retrospective study. A total of 2462 patients with 3375 instances of early gastric cancers that were treated by ESD were enrolled in our study between May 2000 and September 2019. We defined a patient as H. pylori-uninfected using the following three criteria; i) the patient did not receive treatment for H. pylori, which was determined by investigating medical records and conducting patient interviews, ii) lack of endoscopic atrophy, and iii) the patient was negative for H. pylori after being tested at least twice using various diagnostic methods, including serum anti-H. pylori-IgG antibody, urease breath test, rapid urease test, and microscopic examination.
Of these, 30 lesions in 30 patients were diagnosed as HpUIGC. Histologically 30 HpUIGC lesions were classified into 4 types (fundic gland type adenocarcinoma, foveolar type well-differentiated adenocarcinoma, intestinal phenotype adenocarcinoma, and pure signet-ring cell carcinoma). Unlike previous reports, most of the lesions (22/30 lesions) were the differentiated type.
In this study, we classified 30 HpUIGCs into 4 types histologically. Unlike previous reports, there were more differentiated cancers than undifferentiated cancers. Although the most of HpUIGC showed gastric phenotype, it is essential to recognize that there are not a few intestinal phenotype adenocarcinomas among HpUIGCs. HpUIGC is very rare, among which, histologically high incidence of undifferentiated adenocarcinoma. Besides undifferentiated adenocarcinoma and gastric fundic gland type adenocarcinoma, there is another HpUIGC having different histopathological features. HpUIGC may show various type of his-topathological features. Histologically, HpUIGC is classified into at least 4 types (fundic gland type adenocarcinoma, foveolar type well-differentiated adenocarcinoma, intestinal phenotype adenocarcinoma, and pure signet-ring cell carcinoma). To the best of our knowledge, the present study reports the largest number of HpUIGC cases that had been evaluated for both endoscopic and pathological findings. To recognize clinicopathological feature of HpUIGC will be helpful for early detection of HpUIGC in the future clinical practice.
To recognize the various clinicopathological features of HpUIGC is useful for clinical diagnosis in the future. Because HpUIGC is rare frequency, we consider multicenter clinical trial for case collection to elucidate more detail of the clinicopathological characteristics of HpUIGC. Multicenter observational trial is the best method for the future research.
| 1. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3248] [Article Influence: 129.9] [Reference Citation Analysis (1)] |
| 2. |
Schistosomes, liver flukes and Helicobacter pylori.
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. |
| 3. | Parsonnet J, Vandersteen D, Goates J, Sibley RK, Pritikin J, Chang Y. Helicobacter pylori infection in intestinal- and diffuse-type gastric adenocarcinomas. J Natl Cancer Inst. 1991;83:640-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 248] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | Ueda J, Gosho M, Inui Y, Matsuda T, Sakakibara M, Mabe K, Nakajima S, Shimoyama T, Yasuda M, Kawai T, Murakami K, Kamada T, Mizuno M, Kikuchi S, Lin Y, Kato M. Prevalence of Helicobacter pylori infection by birth year and geographic area in Japan. Helicobacter. 2014;19:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Yamamoto Y, Fujisaki J, Omae M, Hirasawa T, Igarashi M. Helicobacter pylori-negative gastric cancer: characteristics and endoscopic findings. Dig Endosc. 2015;27:551-561. [PubMed] [DOI] [Full Text] |
| 6. | Ono S, Kato M, Suzuki M, Ishigaki S, Takahashi M, Haneda M, Mabe K, Shimizu Y. Frequency of Helicobacter pylori -negative gastric cancer and gastric mucosal atrophy in a Japanese endoscopic submucosal dissection series including histological, endoscopic and serological atrophy. Digestion. 2012;86:59-65. [PubMed] [DOI] [Full Text] |
| 7. | Matsuo T, Ito M, Takata S, Tanaka S, Yoshihara M, Chayama K. Low prevalence of Helicobacter pylori-negative gastric cancer among Japanese. Helicobacter. 2011;16:415-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 179] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 8. | Horiuchi Y, Fujisaki J, Yamamoto N, Shimizu T, Miyamoto Y, Tomida H, Taniguchi C, Morishige K, Omae M, Ishiyama A, Yoshio T, Hirasawa T, Yamamoto Y, Tsuchida T, Igarashi M, Nakajima T, Takahashi H. Biological behavior of the intramucosal Helicobacter pylori-negative undifferentiated-type early gastric cancer: comparison with Helicobacter pylori-positive early gastric cancer. Gastric Cancer. 2016;19:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Kato S, Matsukura N, Tsukada K, Matsuda N, Mizoshita T, Tsukamoto T, Tatematsu M, Sugisaki Y, Naito Z, Tajiri T. Helicobacter pylori infection-negative gastric cancer in Japanese hospital patients: incidence and pathological characteristics. Cancer Sci. 2007;98:790-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Yoon H, Kim N, Lee HS, Shin CM, Park YS, Lee DH, Jung HC, Song IS. Helicobacter pylori-negative gastric cancer in South Korea: incidence and clinicopathologic characteristics. Helicobacter. 2011;16:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Yamada A, Kaise M, Inoshita N, Toba T, Nomura K, Kuribayashi Y, Yamashita S, Furuhata T, Kikuchi D, Matsui A, Mitani T, Ogawa O, Iizuka T, Hoteya S. Characterization of Helicobacter pylori-Naïve Early Gastric Cancers. Digestion. 2018;98:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Ueyama H, Matsumoto K, Nagahara A, Hayashi T, Yao T, Watanabe S. Gastric adenocarcinoma of the fundic gland type (chief cell predominant type). Endoscopy. 2014;46:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Shibagaki K, Fukuyama C, Mikami H, Izumi D, Yamashita N, Mishiro T, Oshima N, Ishimura N, Sato S, Ishihara S, Nagase M, Araki A, Ishikawa N, Maruyama R, Kushima R, Kinoshita Y. Gastric foveolar-type adenomas endoscopically showing a raspberry-like appearance in the Helicobacter pylori -uninfected stomach. Endosc Int Open. 2019;7:E784-E791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Toyoshima O, Yamaji Y, Yoshida S, Matsumoto S, Yamashita H, Kanazawa T, Hata K. Endoscopic gastric atrophy is strongly associated with gastric cancer development after Helicobacter pylori eradication. Surg Endosc. 2017;31:2140-2148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 779] [Article Influence: 43.3] [Reference Citation Analysis (5)] |
| 15. | Yagi K, Nakamura A, Sekine A. Characteristic endoscopic and magnified endoscopic findings in the normal stomach without Helicobacter pylori infection. J Gastroenterol Hepatol. 2002;17:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 134] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Haruma K. Kyoto Classification of Gastritis. Editors: Mototsugu Kato, Kazuhiko Inoue, Kazunari Murakami, Tomoari Kamada. Shinryo Bunko publisher. 2017. |
| 17. | Kato M, Ota H, Okuda M, Kikuchi S, Satoh K, Shimoyama T, Suzuki H, Handa O, Furuta T, Mabe K, Murakami K, Sugiyama T, Uemura N, Takahashi S. Guidelines for the management of Helicobacter pylori infection in Japan: 2016 Revised Edition. Helicobacter. 2019;24:e12597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 232] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 18. | Chey WD. Proton pump inhibitors and the urea breath test: how long is long enough? Am J Gastroenterol. 1997;92:720-721. [PubMed] |
| 19. | Graham DY, Opekun AR, Hammoud F, Yamaoka Y, Reddy R, Osato MS, El-Zimaity HM. Studies regarding the mechanism of false negative urea breath tests with proton pump inhibitors. Am J Gastroenterol. 2003;98:1005-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3624] [Article Influence: 120.8] [Reference Citation Analysis (6)] |
| 21. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2950] [Article Influence: 196.7] [Reference Citation Analysis (1)] |
| 22. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1954] [Article Influence: 217.1] [Reference Citation Analysis (1)] |
| 23. | Hirasawa K, Kokawa A, Oka H, Yahara S, Sasaki T, Nozawa A, Morimoto M, Numata K, Taguri M, Morita S, Maeda S, Tanaka K. Risk assessment chart for curability of early gastric cancer with endoscopic submucosal dissection. Gastrointest Endosc. 2011;74:1268-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Koh R, Hirasawa K, Yahara S, Oka H, Sugimori K, Morimoto M, Numata K, Kokawa A, Sasaki T, Nozawa A, Taguri M, Morita S, Maeda S, Tanaka K. Antithrombotic drugs are risk factors for delayed postoperative bleeding after endoscopic submucosal dissection for gastric neoplasms. Gastrointest Endosc. 2013;78:476-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 25. | Shiroshita H, Watanabe H, Ajioka Y, Watanabe G, Nishikura K, Kitano S. Re-evaluation of mucin phenotypes of gastric minute well-differentiated-type adenocarcinomas using a series of HGM, MUC5AC, MUC6, M-GGMC, MUC2 and CD10 stains. Pathol Int. 2004;54:311-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Tajima Y, Yamazaki K, Makino R, Nishino N, Aoki S, Kato M, Morohara K, Kaetsu T, Kusano M. Gastric and intestinal phenotypic marker expression in early differentiated-type tumors of the stomach: clinicopathologic significance and genetic background. Clin Cancer Res. 2006;12:6469-6479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video). Endoscopy. 2004;36:1080-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 338] [Article Influence: 15.4] [Reference Citation Analysis (3)] |
| 28. | Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M; Japan Gast Study Group. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 952] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 29. | Kakinoki R, Kushima R, Matsubara A, Saito Y, Okabe H, Fujiyama Y, Hattori T. Re-evaluation of histogenesis of gastric carcinomas: a comparative histopathological study between Helicobacter pylori-negative and H. pylori-positive cases. Dig Dis Sci. 2009;54:614-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3302] [Cited by in RCA: 3313] [Article Influence: 78.9] [Reference Citation Analysis (2)] |
| 31. | Kwak HW, Choi IJ, Cho SJ, Lee JY, Kim CG, Kook MC, Ryu KW, Kim YW. Characteristics of gastric cancer according to Helicobacter pylori infection status. J Gastroenterol Hepatol. 2014;29:1671-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Vaira D, Holton J, Menegatti M, Ricci C, Gatta L, Geminiani A, Miglioli M. Review article:invasive and non-invasive tests for Helicobacter pylori infection. Aliment Pharmacol Ther. 2000;14 Suppl 3:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Ricci C, Holton J, Vaira D. Diagnosis of Helicobacter pylori: invasive and non-invasive tests. Best Pract Res Clin Gastroenterol. 2007;21:299-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 34. | Wang YK, Kuo FC, Liu CJ, Wu MC, Shih HY, Wang SS, Wu JY, Kuo CH, Huang YK, Wu DC. Diagnosis of Helicobacter pylori infection: Current options and developments. World J Gastroenterol. 2015;21:11221-11235. [PubMed] [DOI] [Full Text] |
| 35. | Yokoyama A, Inoue H, Minami H, Wada Y, Sato Y, Satodate H, Hamatani S, Kudo SE. Novel narrow-band imaging magnifying endoscopic classification for early gastric cancer. Dig Liver Dis. 2010;42:704-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Tian MM, Zhao AL, Li ZW, Li JY. Phenotypic classification of gastric signet ring cell carcinoma and its relationship with clinicopathologic parameters and prognosis. World J Gastroenterol. 2007;13:3189-3198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Aihara R, Mochiki E, Kamiyama Y, Kamimura H, Asao T, Kuwano H. Mucin phenotypic expression in early signet ring cell carcinoma of the stomach: its relationship with the clinicopathologic factors. Dig Dis Sci. 2004;49:417-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Komeda Y, Watanabe T, Matsui S, Kashida H, Sakurai T, Kono M, Minaga K, Nagai T, Hagiwara S, Enoki E, Kudo M. A case of small invasive gastric cancer arising from Helicobacter pylori-negative gastric mucosa: Fundic gland-type adenocarcinoma. JGH Open. 2017;1:74-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Miyazawa M, Matsuda M, Yano M, Hara Y, Arihara F, Horita Y, Matsuda K, Sakai A, Noda Y. Gastric adenocarcinoma of the fundic gland (chief cell-predominant type): A review of endoscopic and clinicopathological features. World J Gastroenterol. 2016;22:10523-10531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | Benedict MA, Lauwers GY, Jain D. Gastric Adenocarcinoma of the Fundic Gland Type: Update and Literature Review. Am J Clin Pathol. 2018;149:461-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 41. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2759] [Article Influence: 459.8] [Reference Citation Analysis (3)] |
| 42. | Ichihara S, Kawamura M, Hirasawa K, Yagi K. MUC6-positive cell proliferation in the glandular neck zone of low-grade well-differentiated carcinoma. Pathol Int. 2018;68:624-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 43. | Kudo S. Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy. 1993;25:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 562] [Article Influence: 17.0] [Reference Citation Analysis (11)] |
| 44. | Yoshii S, Hayashi Y, Takehara T. Helicobacter pylori-negative early gastric adenocarcinoma with complete intestinal mucus phenotype mimicking verrucous gastritis. Dig Endosc. 2017;29:235-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Ozaki Y, Suto H, Nosaka T, Saito Y, Naito T, Takahashi K, Ofuji K, Matsuda H, Ohtani M, Hiramatsu K, Nemoto T, Imamura Y, Nakamoto Y. A case of Helicobacter pylori-negative intramucosal well-differentiated gastric adenocarcinoma with intestinal phenotype. Clin J Gastroenterol. 2015;8:18-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Kotani S, Miyaoka Y, Fujiwara A, Tsukano K, Ogawa S, Yamanouchi S, Kusunoki R, Fujishiro H, Kohge N, Ohnuma H, Kinoshita Y. Intestinal-type gastric adenocarcinoma without Helicobacter pylori infection successfully treated with endoscopic submucosal dissection. Clin J Gastroenterol. 2016;9:228-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Kobayashi Y, Komazawa Y, Nagaoka M, Takahashi Y, Yuki M, Shizuki T, Nabika T. Helicobacter pylori-negative intestinal-type gastric adenoma successfully treated by endoscopic submucosal dissection: a case report. Endosc Int Open. 2016;4:E986-E989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 48. | Kim TH, Shivdasani RA. Stomach development, stem cells and disease. Development. 2016;143:554-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 49. | Yao K. Clinical Application of Magnifying Endoscopy with Narrow-Band Imaging in the Stomach. Clin Endosc. 2015;48:481-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Shimoda T, Fujisaki J, Kashimura H, Ikegami M, Ishii T, Matsui T. Histological Type of Gastric Carcinoma in Relation to the Mode of Intramural Spreading of Cancer Cells. Stomach Intestine. 1991;26:1125-1134. |
| 51. | Nishikura K, Watanabe H, Ajioka Y. Differentiated Gastric Adenocarcinoma with Gastric Phenotype: Its New Classification and Histopathological Characteristics. Stomach Intestine. 1999;34:495-506. |
| 52. | Ushiku T, Arnason T, Ban S, Hishima T, Shimizu M, Fukayama M, Lauwers GY. Very well-differentiated gastric carcinoma of intestinal type: analysis of diagnostic criteria. Mod Pathol. 2013;26:1620-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 53. | Yao T, Utsunomiya T, Oya M, Nishiyama K, Tsuneyoshi M. Extremely well-differentiated adenocarcinoma of the stomach: clinicopathological and immunohistochemical features. World J Gastroenterol. 2006;12:2510-2516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 54. | Zamcheck N, Grable E, Ley A, Norman L. Occurrence of gastric cancer among patients with pernicious anemia at the Boston City Hospital. N Engl J Med. 1955;252:1103-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 82] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 55. | Cisco RM, Ford JM, Norton JA. Hereditary diffuse gastric cancer: implications of genetic testing for screening and prophylactic surgery. Cancer. 2008;113:1850-1856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 56. | Offerhaus GJ, Tersmette AC, Huibregtse K, van de Stadt J, Tersmette KW, Stijnen T, Hoedemaeker PJ, Vandenbroucke JP, Tytgat GN. Mortality caused by stomach cancer after remote partial gastrectomy for benign conditions: 40 years of follow up of an Amsterdam cohort of 2633 postgastrectomy patients. Gut. 1988;29:1588-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Corresponding Author's Membership in Professional Societies: Japan gastroenterological endoscopy society, 37620893.
P-Reviewer: Eleftheriadis N, Ziogas DE S-Editor: Zhang H L-Editor: A E-Editor: Zhang YL