Published online May 21, 2020. doi: 10.3748/wjg.v26.i19.2427
Peer-review started: January 21, 2020
First decision: March 6, 2020
Revised: April 23, 2020
Accepted: April 28, 2020
Article in press: April 28, 2020
Published online: May 21, 2020
Processing time: 121 Days and 9 Hours
Survival benefit of neoadjuvant chemotherapy (NAC) for advanced gastric cancer (AGC) is a debatable issue. Studies have shown that the survival benefit of NAC is dependent on the pathological response to chemotherapy drugs. For those who achieve pathological complete response (pCR), NAC significantly prolonged prolapsed-free survival and overall survival. For those with poor response, NAC yielded no survival benefit, only toxicity and increased risk for tumor progression during chemotherapy, which may hinder surgical resection. Thus, predicting pCR to NAC is of great clinical significance and can help achieve individualized treatment in AGC patients.
To establish a nomogram for predicting pCR to NAC for AGC patients.
Two-hundred and eight patients diagnosed with AGC who received NAC followed by resection surgery from March 2012 to July 2019 were enrolled in this study. Their clinical data were retrospectively analyzed by logistic regression analysis to determine the possible predictors for pCR. Based on these predictors, a nomogram model was developed and internally validated using the bootstrap method.
pCR was confirmed in 27 patients (27/208, 13.0%). Multivariate logistic regression analysis showed that higher carcinoembryonic antigen level, lymphocyte ratio, lower monocyte count and tumor differentiation grade were associated with higher pCR. Concordance statistic of the established nomogram was 0.767.
A nomogram predicting pCR to NAC was established. Since this nomogram exhibited satisfactory predictive power despite utilizing easily available pretreatment parameters, it can be inferred that this nomogram is practical for the development of personalized treatment strategy for AGC patients.
Core tip: Pathological complete response is an important prognosis factor for advanced gastric cancer patients who underwent neoadjuvant chemotherapy and tumor resection. In our study, we built a nomogram that predicted pathological complete response to neoadjuvant chemotherapy utilizing only easily available pretreatment parameters such as carcinoembryonic antigen level, lymphocyte ratio, monocyte count and tumor differentiation grade. It showed satisfactory predictive power with an area under the receiver operating characteristic curve of 0.823 and a concordance statistic of 0.767. It can be inferred that this nomogram is practical for the development of personalized treatment strategy for advanced gastric cancer patients.
- Citation: Chen YH, Xiao J, Chen XJ, Wang HS, Liu D, Xiang J, Peng JS. Nomogram for predicting pathological complete response to neoadjuvant chemotherapy in patients with advanced gastric cancer. World J Gastroenterol 2020; 26(19): 2427-2439
- URL: https://www.wjgnet.com/1007-9327/full/v26/i19/2427.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i19.2427
Gastric cancer is the fourth most common malignant tumor in the world, and it is one of the most common causes of cancer-related death[1]. In China, the diagnosis rate of early gastric cancer is low as a large proportion of patients are diagnosed only at an advanced stage[2].
For potentially curable advanced gastric cancer (AGC) patients, surgical resection with D2 lymphadenectomy followed by adjuvant chemotherapy was traditionally the standard clinical practice in Asia[3,4]. However, the survival outcome of this treatment modality remained poor with a 5-year relative survival rate of 17.9%-54.2%[5,6]. In recent years, neoadjuvant chemotherapy (NAC) was introduced into the treatment modality of AGC, in hopes to improve the overall survival of those with potentially curative tumor site[7]. NAC was found to bring forth downstaging of tumor and improve the chance for curative complete resection of tumor[8,9].
Nevertheless, survival benefit of NAC compared to traditional approach remains a debatable issue[7,10]. Multiple studies had shown that the survival benefit of NAC is dependent on the pathological response to chemotherapy drugs[11], which is defined by the proportion of residual tumor cells in the resected specimen after NAC. Pathological complete response (pCR) is the most preferable pathological response because it means no residual tumor cell is present in the resected specimen. Those with pCR after NAC are more inclined to have ideal prolapsed-free survival and overall survival[12,13]. Meanwhile, those who showed limited response, in which large amount of residual tumor cells are found to be present in the resected tumor, are still associated with poor prognosis[12,13]. For these non-responders, NAC yielded no survival benefit, only toxicity and increased risk of tumor progression during chemotherapy, which may hinder surgical resection. To achieve personalization in precision medicine, we should clearly identify responders and non-responders prior to providing individualized treatment modality to patients from either group.
Much effort had been made to identify the predictor of response to NAC[14-16]. However, these predictors were either not available in the routine clinical practice or their significance level was limited by the sample size. Furthermore, not much work has been done in this field.
Therefore, we sought to identify tumor biological characteristics and clinical parameter in this study that are associated with pCR after NAC. Secondly, we aimed to establish an individual nomogram for the prediction of pCR using only easily available pre-treatment clinical parameter, in an attempt to provide personalized treatment strategy to AGC patients.
A total of 208 patients were identified from the gastric cancer database of The Sixth Affiliated Hospital, Sun Yat-sen University from March 2012 to July 2019. Inclusion criteria were as follows: (1) Patients with histologically confirmed adenocarcinoma of the stomach or esophagogastric junction in a clinical stage of T3N+ or T4N0/+ as evaluated by abdominal-pelvic computed tomography; and (2) Patients who underwent NAC followed by gastrectomy with standardized D2 lymphadenectomy. Patients with insufficient information were excluded from the study.
After initial screening, a total of 208 patients were included in this study. All available pre-treatment clinical information was retrieved from the database, including gender, age, body mass index, biopsy pathological differentiation, tumor staging information according to the staging system of American Joint Committee on Cancer (AJCC) 8th edition, routine hematological and biochemical tests result and tumor markers. Patient information is listed in Table 1.
| Characteristics | Total | Non-pCR | pCR | P value |
| Number of patients | 208 | 181 | 27 | |
| Sex | ||||
| Male | 161 | 138 | 23 | 0.3 |
| Female | 47 | 43 | 4 | |
| Age | 59 (50-64) | 59 (50-64) | 59 (51-63) | 0.631 |
| BMI | 22 ± 2.9 | 22 ± 2.9 | 22 ± 3.4 | 0.226 |
| Location | ||||
| Esophago-gastric junction | 61 | 53 | 8 | 0.1 |
| Upper third | 25 | 20 | 5 | |
| Middle third | 43 | 42 | 1 | |
| Lower third | 79 | 66 | 13 | |
| Tumor differentiation | ||||
| Well differentiated | 8 | 5 | 3 | 0.025 |
| Moderately differentiated | 56 | 46 | 10 | |
| Moderately-poorly differentiated | 28 | 22 | 6 | |
| Poorly differentiated | 94 | 87 | 7 | |
| Signet ring cell | 22 | 21 | 1 | |
| Clinical T staging | ||||
| T3 | 103 | 88 | 15 | 0.725 |
| T4a | 84 | 75 | 9 | |
| T4b | 21 | 18 | 3 | |
| Clinical N staging | ||||
| N0 | 4 | 4 | 0 | 0.435 |
| N+ | 204 | 177 | 27 | |
| Regimen | ||||
| mFLOT | 122 | 102 | 20 | 0.217 |
| SOX/FOLFOX/XELOX | 75 | 69 | 6 | |
| other | 11 | 10 | 1 | |
| Cycles | 4 (4-5) | 4 (4-5) | 4 (4-4) | 0.766 |
| WBC, 109/L) | 6.47 ± 1.89 | 6.51 ± 1.93 | 5.80 ± 1.48 | 0.061 |
| RBC, 1012/L | 4.39 (3.84-4.72) | 4.42 (3.9-4.73) | 4.23 (3.69-4.55) | 0.263 |
| NEU, 109/L | 3.76 (2.82-4.9) | 3.86 (2.89-4.97) | 3.05 (2.61-3.58) | 0.589 |
| NEUR | 0.61 ± 0.1 | 0.62 ± 0.11 | 0.57 ± 0.07 | 0.2 |
| LYM, 109/L | 1.62 (1.26-2.07) | 1.6 (1.22-2.08) | 1.71 (1.42-2.07) | 0.107 |
| LYMR | 0.26 (0.2-0.33) | 0.24 (0.2-0.33) | 0.32 (0.28-0.35) | 0.001 |
| MONO, 109/L | 0.5 (0.4-0.64) | 0.51 (0.41-0.64) | 0.44 (0.31-0.57) | 0.017 |
| MONOR | 0.08 (0.07-0.1) | 0.08 (0.07-0.1) | 0.08 (0.06-0.09) | 0.211 |
| EOS, 109/L | 0.13 (0.08-0.21) | 0.13 (0.08-0.21) | 0.15 (0.08-0.22) | 0.428 |
| EOSR | 0.02 (0.01-0.03) | 0.02 (0.01-0.03) | 0.03 (0.02-0.05) | 0.071 |
| BASO, 109/L | 0.03 (0.02-0.04) | 0.03 (0.02-0.04) | 0.04 (0.01-0.05) | 0.513 |
| BASOR | 0.01 (0-0.01) | 0.01 (0-0.01) | 0.01 (0-0.01) | 0.299 |
| HGB, g/L | 123 (97.18-137) | 123 (99-137) | 118 (81-134.1) | 0.373 |
| PLT, 109/L | 262.5 (211.7-330) | 262 (212.8-328) | 263 (197-358) | 0.809 |
| UA, μmol/L | 348.7 (274-420.8) | 342 (263.9-413.3) | 407.3 (341-481.6) | 0.003 |
| Cr, μmol/L | 78.4 (66.4-89.1) | 77.3 (64.92-89) | 81.09 (75.5-89.5) | 0.176 |
| ALT, U/L | 13.8 (9.86-18.98) | 13.79 (9.91-19) | 14 (9.69-18) | 0.958 |
| AST, U/L | 18.2 (14.8-22.1) | 18.5 (14.84-22.11) | 17.02 (13.39-22.1) | 0.37 |
| r-GT, U/L | 18.8 (14.8-30.4) | 18.8 (14.61-30.28) | 18.51 (15-34.71) | 0.672 |
| TP, g/L | 68.2 ± 6.53 | 67.86 ± 6.52 | 70.37 ± 6.3 | 0.89 |
| Alb, g/L | 39.81 ± 4.39 | 39.59 ± 4.47 | 41.23 ± 3.53 | 0.1 |
| AKP, U/L | 84.6 (68.1-100.5) | 85.61 (70-101.55) | 77.44 (58.97-91) | 0.156 |
| PA, g/L | 0.2 ± 0.06 | 0.2 ± 0.06 | 0.21 ± 0.05 | 0.59 |
| TBIL, μmol/L | 11 (8.67-14.45) | 11.12 (8.85-14.39) | 10.48 (6.92-16.24) | 0.421 |
| DBIL, μmol/L | 2.21 (1.68-3.08) | 2.25 (1.68-3.08) | 2.05 (1.46-2.99) | 0.29 |
| LD, U/L | 167 (147.9-194.7) | 167 (147.8-191.4) | 168 (149.5-197.3) | 0.548 |
| CH, mmol/L | 4.79 ± 1.1 | 4.73 ± 1.1 | 5.15 ± 1 | 0.26 |
| TG, mmol/L | 1.06 (0.83-1.38) | 1.03 (0.83-1.38) | 1.15 (0.81-1.47) | 0.588 |
| HDL, mmol/L | 1.15 (0.95-1.32) | 1.14 (0.95-1.3) | 1.24 (1.06-1.41) | 0.088 |
| LDL, mmol/L | 3.06 (2.52-3.50) | 2.95 (2.47-3.51) | 3.25 (2.92-3.50) | 0.08 |
| CRP, mg/L | 2.36 (0.91-9.09) | 2.37 (0.91-9.78) | 2.17 (0.83-5.85) | 0.593 |
| CA125, U/mL | 14.95 (9.4-23.55) | 15.1 (9.5-24.4) | 12.5 (7.6-20) | 0.375 |
| CEA, ng/mL | 2.8 (1.43-5.92) | 2.51 (1.38-4.88) | 8.04 (2.36-26.2) | 0.002 |
| CA199, U/mL | 9.71 (2.86-40.35) | 8.87 (2.4-47.64) | 15.32 (4.46-34.15) | 0.303 |
| CA-153, U/mL | 7.5 (5.38-11) | 7.4 (5.3-10.8) | 8.3 (6.2-11.9) | 0.289 |
| AFP, ng/mL | 2.7 (1.97-4.28) | 2.67 (1.94-4.19) | 2.78 (2.06-10.96) | 0.551 |
| Blood type | ||||
| Type O | 75 | 72 | 3 | 0.005 |
| Type A | 61 | 46 | 15 | |
| Type B | 50 | 43 | 7 | |
| Type AB | 22 | 20 | 2 | |
This retrospective study was approved by the Institutional Review Board of The Sixth Affiliated Hospital, Sun Yat-sen University.
The treatment strategies for all patients were set by the multi-disciplinary team comprising of surgeons, medical oncologists and radiologists of The Sixth Affiliated Hospital, Sun Yat-sen University. The clinical staging was further confirmed by an experienced radiologist, and the eligibility for NAC was determined by both the medical oncologists and surgeons. Chemotherapy regimen adopted for NAC mainly consisted of mFLOT and FOLFOX (or its derived regimen such as SOX or XELOX). (1) mFLOT: Docetaxel 50-60 mg/m2 + Oxaliplatin 85 mg/m2 + Fluorouracil 2800 mg/m2 intravenous injection over 48 h; every 2 wk; (2) SOX: Oxaliplatin 130 mg/m2 intravenous injection + Tegafur Gimeracil Oteracil Potassium Capsule 40-60 mg bid D1-D14; every 3 wk; (3) XELOX: Oxaliplatin 130 mg/m2 + Capecitabine 1000 mg/m2 bid D1-D14; every 3 wk; and (4) FOLFOX: Oxaliplatin 85 mg/m2 + Fluorouracil 2800 mg/m2 continuous intravenous injection over 48 h; every 2 wk.
After the completion of NAC, the resectability of the primary tumor site was confirmed again by the multi-disciplinary team. All patients who were enrolled received curative tumor resection (total or subtotal gastrectomy) with D2 lymphadenectomy. Open or laparoscopic surgery was chosen according to the preference of the surgeon. A throughout examination of the abdominal cavity was routinely performed to determine the status of peritoneum metastasis, while peritoneal washing cytology test was not routinely conducted.
All resection specimens were sent to the Department of Pathology and were examined by the attending pathologist according to the same standards. pCR was defined as absence of residual cancer cell in the primary tumor and the dissected regional lymph node. Pathological tumor staging was also conducted according to the 8th AJCC Tumor, Node, Metastasis staging system[17].
Univariate analysis: The normality of data was assessed using the Kolmogorov-Smirnov test and normal probability plots. Parameters that were not normally distributed were expressed in the form of median (upper quartile to lower quartile) and were analyzed using a non-parametric test: Mann-Whitney test or Kruskal–Wallis test, as appropriate. Normally distributed parameters were expressed in the form of mean ± standard deviation and were analyzed by Student’s t-test. Categorical variable was analyzed by the chi-square test.
Multivariate analysis: Logistic regression was used to analyze variables related to the probability of pCR and to estimate regression coefficients and odds ratios with 95% confidence intervals for the parameters that had achieved a significance of P < 0.05 in the univariate analysis.
Parameters that achieved a significance of P < 0.05 in the logistic regression analysis were used to build a nomogram to predict the probability of pCR. Continuous variables enrolled in the model were transformed into categorical variables for clear comprehension. The concordance statistic was acquired for the nomogram, and internal validation using the bootstrap method was performed to determine the adjusted concordance statistic. Calibration curve of the nomogram was generated to show the relationship between the predicted and observed outcomes. All statistical analyses were performed using SPSS software ver. 22.0 (IBM, Armonk, NY, United States) and R version 3.6.1 software (The R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org).
A total of 208 patients diagnosed with adenocarcinoma of the stomach or esophagogastric junction from March 2012 and July 2019 were enrolled in the study. The majority of patients were male (161/208, 77.4%), and the median age of the study cohort was 59-years-old (range: 50–64). Overall, 45.2% of the tumors were poorly differentiated adenocarcinoma (91/208) and radiologically suspicious lymph node metastasis was detected in almost all patients (204/208, 98.1%).
All patients received treatments as depicted in Table 1. Patients received a median of four cycles of NAC before surgery, 58.7% of patients received mFLOT regimen (122/208) while 26.1% of patients received FOLFOX or its analogue (75/208). Four point eight percent of patients received a modified 2-drug regimen of docetaxel with fluorouracil (10/208), and one patient received docetaxel monotherapy. Detailed toxicity profile is listed in Table 2. The most common grade 3/4 hematological toxicities were anemia (92/208, 44.2%) and neutropenia (86, 41.3%). The incidence rates of grade 3/4 thrombocytopenia and febrile-neutropenia were 12.0% (25/208) and 3.7% (7/208), respectively. Grade 3/4 hematological toxicities were more common in docetaxel contained regimens than oxaliplatin-based doublet regimens in terms of anemia (48.9% vs 36%) and febrile-neutropenia (4.5% vs 1.3%), but the differences were not statistically significant.
| Items | mFLOT, n = 122 | FOLFOX, SOX/XELOX, n = 75 | Other1, n = 11 | |||
| Grade | 3 | 4 | 3 | 4 | 3 | 4 |
| Anemia | 41 (33.6) | 15 (12.3) | 18 (24) | 9 (12) | 6 (54.5) | 3 (27.3) |
| Neutropenia | 24 (19.7) | 26 (21.3) | 25 (33.3) | 5 (6.7) | 5 (45.5) | 1 (9.1) |
| Febrile-neutropenia | 6 (4.9) | 0 | 1 (1.3) | 0 | 0 | 0 |
| Thrombocytopenia | 8 (6.6) | 4 (3.3) | 8 (10.7) | 0 | 0 | 5 (45.5) |
All patients received subsequent radical resection surgery. Peritoneal washing cytology test was conducted in three patients, and the results were all negative.
Postoperative complications were observed in 44 patients (21.2%). The incidence rate was not statistically different between pCR (41/181, 22.7%) and Non-pCR group (3/27, 11.1%). As listed in Table 3, abdominal abscess was the most frequent complication in both groups, and all were resolved by non-surgical management, such as percutaneous centesis drainage, enteral nutrition support and antibiotic therapy. Two patients underwent reoperation due to intestinal obstruction. One patient died of progressive pneumonia 6 wk after surgery in the intensive care unit.
| Items | Total, n = 208 | Non-pCR, n = 181 | pCR, n = 27 | P value |
| Any complication | 44 (21.2) | 41 (22.7) | 3 (11.1) | 0.25 |
| Abdominal abscess | 31 (14.9) | 28 (15.5) | 3 (11.1) | 0.60 |
| Anastomotic leakage | 10 (4.8) | 9 (5) | 1 (3.7) | 0.08 |
| Duodenal stump leakage | 2 (1) | 1 (0.6) | 1 (3.7) | 0.13 |
| Other leakage1 | 8 (3.8) | 7 (3.9) | 1 (3.7) | 0.97 |
| Bleeding | 4 (1.9) | 4 (2.2) | 0 | 0.44 |
| Intra-abdominal bleeding | 3 (1.4) | 3 (1.7) | 0 | 0.50 |
| Anastomotic bleeding | 1 (0.5) | 1 (0.6) | 0 | 0.70 |
| Pneumonia | 12 (5.8) | 10 (5.5) | 2 (7.4) | 0.13 |
| Pancreatic fistula | 3 (1.4) | 2 (1.1) | 1 (3.7) | 0.30 |
| Obstruction or ileus | 3 (1.4) | 2 (1.1) | 1 (3.7) | 0.30 |
| Diarrhea | 2 (1) | 2 (1.1) | 0 | 0.59 |
| Diabetes | 1 (0.5) | 0 | 1 (3.7) | 0.01 |
| Reoperation | 2 (1) | 1 (0.6) | 1 (3.7) | 0.13 |
| Death before discharge | 1 (0.5) | 1 (0.6) | 0 | 0.70 |
Ninety-two percent (191/208) of patients received postoperative adjuvant chemotherapy. Most patients (126/208, 60.6%) received platin-based doublet regimen, such as FOLFOX or its analog (SOX, XELOX). Although mFLOT was the mainstream regimen (122/208, 58.7%) in NAC before surgery, only a minority of patients (15/208, 7.2%) accepted mFLOT as adjuvant chemotherapy after surgery because of the rather intolerable toxicity. Other regimens include taxanes contained doublet regimen (27/208, 13.0%) and oral agents such as S-1 capsule or capecitabine (22/208, 10.6%), as listed in Table 4.
| Adjuvant chemotherapy | n = 208 | % |
| Platin-based doublet regimen | ||
| FOLFOX | 75 | 36.1 |
| SOX | 48 | 23.1 |
| XELOX | 3 | 1.4 |
| Taxanes contained regimen | ||
| mFLOT | 15 | 7.2 |
| Docetaxel plus fluorouracil | 25 | 12.0 |
| Docetaxel plus S-1 capsule | 2 | 1.0 |
| Monotherapy | ||
| S-1 capsule | 13 | 6.3 |
| Capecitabine | 9 | 4.3 |
| No adjuvant chemotherapy | 18 | 8.7 |
Based on the treatments above, only 13% (27/208) can be classified as achieving pCR.
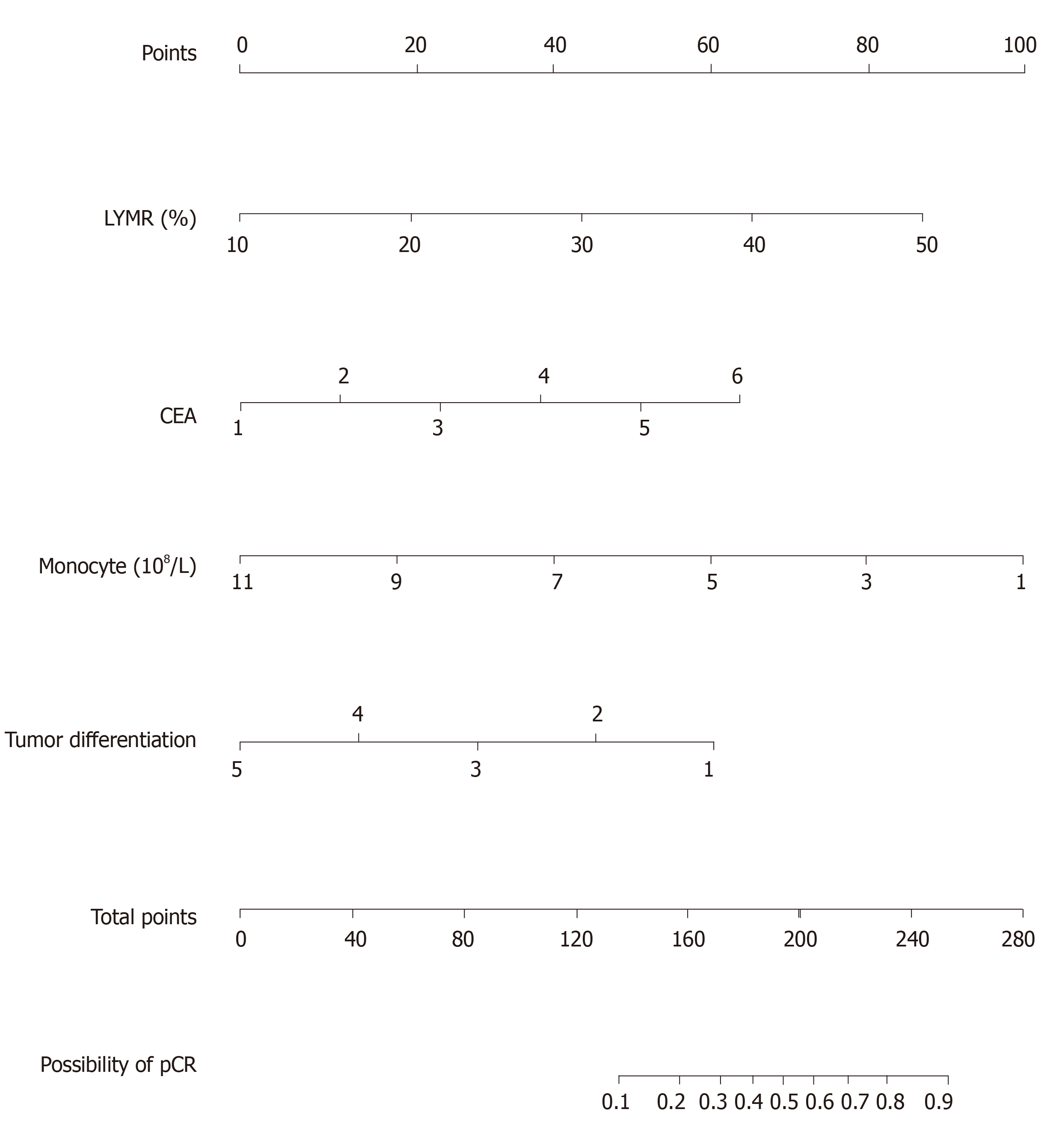
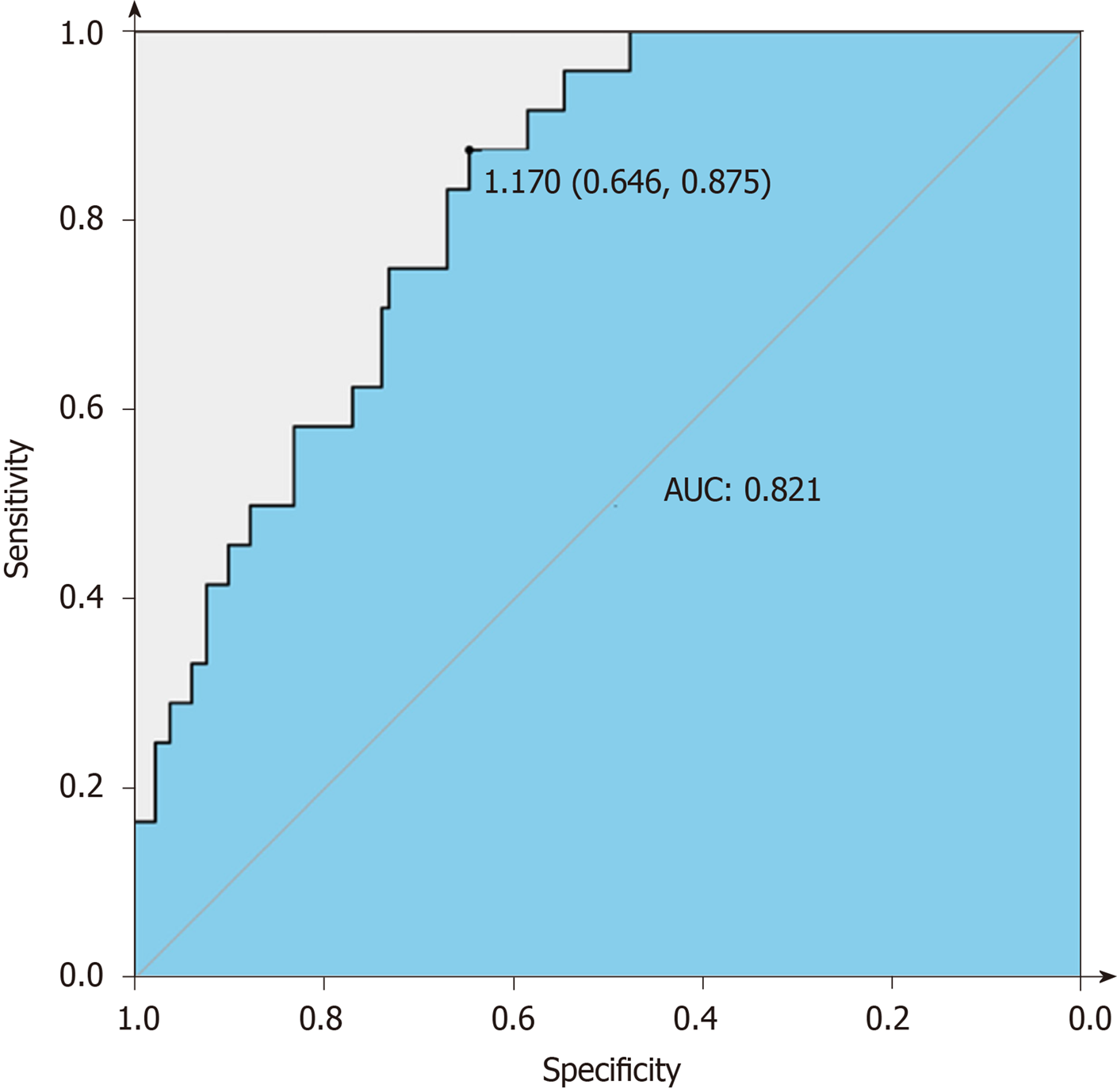
Univariable associations between the clinical parameters and pCR are shown in Table 1. Statistically significant factors (P < 0.05) include tumor differentiation, carcinoembryonic antigen (CEA) level, lymphocyte ratio (LYMR), monocyte (MONO) count, blood type and uric acid level. The multivariable analysis showed that higher CEA level and LYMR and lower MONO count and tumor differentiation grade are independent predictors of pCR with their respective odd ratios and corresponding 95% confidence intervals as shown in Table 5. The established logistic linear regression model was used to build a nomogram as shown in Figure 1, while the receiver operating characteristic curve of the nomogram is shown in Figure 2. Area under the curve is 0.823. The apparent concordance statistic is 0.767, indicating a strong discriminative ability in prediction. Calibration curves between predicted and actual observations were plotted for internal validation. The outcome demonstrated that this nomogram showed good statistical performance for predicting the probability of pCR, as shown in Figure 3.
| Items | OR | 95%CI | P value |
| UA, μmol/L | 1.48 | 1.48-2.2 | 0.052 |
| Blood type | 1.2 | 1.2-1.92 | 0.45 |
| Tumor differentiation | 0.65 | 0.65-1 | 0.048 |
| MONO, 109/L | 0.73 | 0.73-0.98 | 0.038 |
| CEA, ng/mL | 1.57 | 1.57-2.01 | < 0.01 |
| LYMR | 1.08 | 1.08-1.14 | < 0.01 |
NAC is emerging as a common perioperative treatment modality. However, there was lacking a prospective randomized controlled trial to prove that it can bring more survival benefit compared to adjuvant chemotherapy[7]. In a FLOT4 trial, researchers showed that NAC is able to elicit tumor downstaging, improve R0 resection rate and may eventually result in better survival in patients that are sensitive to chemotherapy drugs[8]. Nonetheless, NAC may result in tumor progression during chemotherapy for those who respond poorly to chemotherapy drugs, thus causing a probable hindrance to curative resection. This makes screening for sensitivity to NAC a crucial step before deciding the treatment modality for ACG patients. Henceforth, we set up an exploratory study to identify pre-treatment parameters that can predict sensitivity to NAC.
pCR was chosen as the marker for high sensitivity to NAC. Among all other potential markers, such as radiological response indicator (according to RECIST 1.1)[18] or Ryan’s classification system of tumor regression grading, pCR is more universal, objective and replicable. Many previous studies have proven that pCR is more closely related to prolonged survival after NAC and curative surgery[19-21]. pCR is also closely related to tumor downstaging (mostly to stage ypT0N0), and the 8th AJCC staging proved that downstaging to T0N0 showed favorable survival outcome with a 5-year relative survival rate of 89%. Therefore, we believe that predicting pCR alone is of great clinical significance.
Table 1 shows that 13.0% of patients achieved pCR after NAC, which is similar to previous reports (8.4%-17.4%)[13,22-24]. This again indicated that only a portion of patients can benefit from NAC, signifying the importance of individualized treatment strategy. Based on this, we used only pretreatment parameters to create a nomogram for pCR prediction. After internal validation by bootstrapping, tumor differentiation, CEA, LYMR and MONO count were found to be useful pretreatment parameters in predicting pCR (concordance statistic of 0.767 after correction for optimism). Wang et al[25] showed that tumor differentiation is an independent predictive marker for histopathologic response to cytotoxic therapy and that poorly differentiated tumor is clearly less sensitive to NAC. Previous reports indicated that lymphocytes possess potent antibody-dependent anti-cancer activities, and thus a high LYMR is associated with stronger lymphocyte-mediated immune response to the tumor, leading to a higher probability of pCR[26-28]. It is known that monocytes promote tumor genesis and angiogenesis while suppressing the host immune response to cancer cells[29]. Besides, monocytes in the blood circulation were proven to be an important source of soluble mediators that may support the evolution of malignant cells[30,31].
Previous work showed that elevated monocyte count is associated with poor response to chemotherapy and confers a negative prognosis in cancer patients, which is in line with our findings[32,33]. Interestingly, as opposed to previous studies, our study indicated that higher CEA is associated with higher probability of pCR. Elevated CEA had always been associated with poor chemosensitivity[34-37], lower probability of pCR and a worse prognosis after perioperative chemotherapy[38,39]. A possible assumption to this phenomenon is that elevated CEA is associated with heavier tumor load and faster tumor growth rate[40,41], thus making it more susceptible to chemotherapy.
There were a few previous studies that aimed to find a predictor for response to NAC. It was reported that factors such as tumor blood supply, grade of differentiation and iodine uptake were associated with response to NAC[15,16]. Research also showed that certain radiomic features extracted from computed tomography image may be promising predictors for response to NAC[42-44]. Nevertheless, these studies require complicated additional examination, while some were limited by the small sample size. Besides, all these studies explored only one single factor or factors of a single dimension[15,16,42,43]. The major highlight of our study is the combination of four parameters to build a nomogram according to the weighting and importance of each parameter. Our nomogram is built upon CEA level, LYMR, MONO count and tumor differentiation grade, which are all easily assessable, making it practical for clinical use.
However, we acknowledge that there were some limitations to our study. Since most patients enrolled in the study underwent surgery in the recent 2 years, there were insufficient survival events to analyze the impact of the predictor and pCR on overall survival rate. Secondly, the chemotherapy regimen in our study was not unified, although we verified through chi square test that the different regimens were not associated with different responses (P = 0.217). Lastly, there was a lack of external validation in an independent cohort of patients.
A nomogram predicting pCR was built based on clinical parameters prior to the start of NAC. This nomogram showed satisfactory predicting power and can be useful in further development of an individualized treatment strategy for AGC patients.
Survival benefit of neoadjuvant chemotherapy (NAC) for advanced gastric cancer (AGC) is a debatable issue. Studies had shown that the survival benefit of NAC is dependent on the pathological response to chemotherapy drugs. For those who achieve pathological complete response (pCR), NAC significantly prolonged the prolapsed-free survival and overall survival. For those with poor response, NAC yielded no survival benefit, only toxicity and increased risk of tumor progression during chemotherapy, which may hinder surgical resection. Thus, predicting pCR to NAC is of great clinical significance and can help achieve individualized treatment in AGC patients.
Our goal was to establish a nomogram to assist with individualized therapy: To identify those who will have a positive response to NAC and advise them to adopted NAC strategy; to identify those who will have a poor response to NAC and avoid the NAC-related harm.
Our main goal was to establish a nomogram for the prediction of pCR using only easily available pre-treatment clinical parameters.
A total of 208 patients were identified from the gastric cancer database of The Sixth Affiliated Hospital, Sun Yat-sen University from March 2012 to July 2019. Included patients were diagnosed with AGC with a clinical stage of T3N+ or T4N0/+. All patients received NAC and subsequent gastrectomy with D2 lymphadenectomy. NAC regimen mainly consisted of mFLOT, Folfox6, SOX and XELOX. pCR was defined as absence of residual cancer cell in the primary tumor and the dissected regional lymph node.
A total of 208 patients diagnosed with adenocarcinoma of the stomach or esophagogastric junction were enrolled in the study. Patients characteristic and the treatments received are depicted in Table 1. Patients received a median of four cycles of NAC before surgery; 58.7% of patients received mFLOT regimen (122/208) while 26.1% of patients received FOLFOX or its analogue (75/208). The most common grade 3/4 hematological toxicities were anemia (92/208, 44.2 %) and neutropenia (86, 41.3 %). All treatments were followed by a radical resection surgery. Peritoneal washing cytology test was conducted in only three patients, and the results were all negative. Postoperative complications were observed in 44 patients (21.2%). The incidence rate does not differ between pCR (41/181, 22.7%) and Non-pCR group (3/27, 11.1%) statistically. Abdominal abscess was the most frequent complication in both groups, and all were resolved by non-surgical management, such as percutaneous centesis drainage, enteral nutrition support and antibiotic therapy. Based on the treatments above, only 13% (27/208) can be classified as achieving pCR. Univariable associations between the clinical parameters and pCR are shown in Table 1. Statistically significant factors (P < 0.05) include tumor differentiation, carcinoembryonic antigen level (CEA), lymphocyte ratio (LYMR), monocyte count (MONO), blood type and uric acid level. The multivariable analysis showed that higher CEA level and LYMR and lower MONO and tumor differentiation grade are independent predictors of pCR with their respective odd ratios and corresponding 95% confidence intervals, as shown in Table 5. The established logistic linear regression model was used to build a nomogram as shown in Figure 1, while the receiver operating characteristic curve of the nomogram is shown in Figure 2. Area under the curve was 0.823. The apparent concordance statistic was 0.767, indicating a strong discriminative ability in prediction. Calibration curves between predicted and actual observations were plotted for internal validation. The outcome demonstrated that this nomogram showed good statistical performance for predicting the probability of pCR, as shown in Figure 3.
A nomogram predicting pCR is built based on clinical parameters prior to the start of NAC. In this model, higher CEA level and LYMR and lower MONO and tumor differentiation grade are correlated with higher probability of pCR. Interestingly, the correlation between CEA level and pCR is opposite to the previous report, in which higher CEA level is often the predictor of poor response to NAC. The model was internally validated using bootstrap method and showed satisfactory predictive power. However, it should also be acknowledged that there is a lack of external validation in an independent cohort, and the survival impact of pCR was not elucidated owning to insufficient data.
In the future, we plan to add an external cohort for validation to strengthen the reliability of the nomogram. In addition, we plan to analyze the impact of pCR on survival.
The authors would like to thank Miss Pheier Saw for her careful language assistance.
| 1. | Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 743] [Cited by in RCA: 753] [Article Influence: 94.1] [Reference Citation Analysis (0)] |
| 2. | Zong L, Abe M, Seto Y, Ji J. The challenge of screening for early gastric cancer in China. Lancet. 2016;388:2606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 276] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 3. | Shen L, Shan YS, Hu HM, Price TJ, Sirohi B, Yeh KH, Yang YH, Sano T, Yang HK, Zhang X, Park SR, Fujii M, Kang YK, Chen LT. Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol. 2013;14:e535-e547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 391] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 4. | Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY, Mok YJ, Kim YH, Ji J, Yeh TS, Button P, Sirzén F, Noh SH; CLASSIC trial investigators. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1331] [Article Influence: 95.1] [Reference Citation Analysis (0)] |
| 5. | GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research International Collaboration) Group, Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M, Van Cutsem E, Buyse M. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 614] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 6. | Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387-4393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1119] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 7. | Coccolini F, Nardi M, Montori G, Ceresoli M, Celotti A, Cascinu S, Fugazzola P, Tomasoni M, Glehen O, Catena F, Yonemura Y, Ansaloni L. Neoadjuvant chemotherapy in advanced gastric and esophago-gastric cancer. Meta-analysis of randomized trials. Int J Surg. 2018;51:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 8. | Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, Kopp HG, Mayer F, Haag GM, Luley K, Lindig U, Schmiegel W, Pohl M, Stoehlmacher J, Folprecht G, Probst S, Prasnikar N, Fischbach W, Mahlberg R, Trojan J, Koenigsmann M, Martens UM, Thuss-Patience P, Egger M, Block A, Heinemann V, Illerhaus G, Moehler M, Schenk M, Kullmann F, Behringer DM, Heike M, Pink D, Teschendorf C, Löhr C, Bernhard H, Schuch G, Rethwisch V, von Weikersthal LF, Hartmann JT, Kneba M, Daum S, Schulmann K, Weniger J, Belle S, Gaiser T, Oduncu FS, Güntner M, Hozaeel W, Reichart A, Jäger E, Kraus T, Mönig S, Bechstein WO, Schuler M, Schmalenberg H, Hofheinz RD; FLOT4-AIO Investigators. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2086] [Cited by in RCA: 1809] [Article Influence: 258.4] [Reference Citation Analysis (0)] |
| 9. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4735] [Article Influence: 236.8] [Reference Citation Analysis (7)] |
| 10. | Reddavid R, Sofia S, Chiaro P, Colli F, Trapani R, Esposito L, Solej M, Degiuli M. Neoadjuvant chemotherapy for gastric cancer. Is it a must or a fake? World J Gastroenterol. 2018;24:274-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 11. | Lowy AM, Mansfield PF, Leach SD, Pazdur R, Dumas P, Ajani JA. Response to neoadjuvant chemotherapy best predicts survival after curative resection of gastric cancer. Ann Surg. 1999;229:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 190] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Achilli P, De Martini P, Ceresoli M, Mari GM, Costanzi A, Maggioni D, Pugliese R, Ferrari G. Tumor response evaluation after neoadjuvant chemotherapy in locally advanced gastric adenocarcinoma: a prospective, multi-center cohort study. J Gastrointest Oncol. 2017;8:1018-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Lorenzen S, Thuss-Patience P, Al-Batran SE, Lordick F, Haller B, Schuster T, Pauligk C, Luley K, Bichev D, Schumacher G, Homann N. Impact of pathologic complete response on disease-free survival in patients with esophagogastric adenocarcinoma receiving preoperative docetaxel-based chemotherapy. Ann Oncol. 2013;24:2068-2073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Sánchez de Molina ML, Díaz Del Arco C, Vorwald P, García-Olmo D, Estrada L, Fernández-Aceñero MJ. Histopathological factors predicting response to neoadjuvant therapy in gastric carcinoma. Clin Transl Oncol. 2018;20:253-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Ji X, Yang Q, Qin H, Zhou J, Liu W. Tumor blood supply may predict neoadjuvant chemotherapy response and survival in patients with gastric cancer. J Int Med Res. 2019;47:2524-2532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Gao X, Zhang Y, Yuan F, Ding B, Ma Q, Yang W, Yan J, Du L, Wang B, Yan F, Sedlmair M, Pan Z, Zhang H. Locally advanced gastric cancer: total iodine uptake to predict the response of primary lesion to neoadjuvant chemotherapy. J Cancer Res Clin Oncol. 2018;144:2207-2218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 17. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4679] [Article Influence: 519.9] [Reference Citation Analysis (4)] |
| 18. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 22755] [Article Influence: 1338.5] [Reference Citation Analysis (1)] |
| 19. | Becker K, Mueller JD, Schulmacher C, Ott K, Fink U, Busch R, Böttcher K, Siewert JR, Höfler H. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer. 2003;98:1521-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 625] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 20. | Rödel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R, Liersch T, Hohenberger W, Raab R, Sauer R, Wittekind C. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688-8696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 918] [Cited by in RCA: 954] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 21. | Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R, Haustermans K, Mohiuddin M, Pucciarelli S, Small W, Suárez J, Theodoropoulos G, Biondo S, Beets-Tan RG, Beets GL. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1507] [Article Influence: 94.2] [Reference Citation Analysis (0)] |
| 22. | Fields RC, Strong VE, Gönen M, Goodman KA, Rizk NP, Kelsen DP, Ilson DH, Tang LH, Brennan MF, Coit DG, Shah MA. Recurrence and survival after pathologic complete response to preoperative therapy followed by surgery for gastric or gastrooesophageal adenocarcinoma. Br J Cancer. 2011;104:1840-1847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 23. | Lorenzen S, Hentrich M, Haberl C, Heinemann V, Schuster T, Seroneit T, Roethling N, Peschel C, Lordick F. Split-dose docetaxel, cisplatin and leucovorin/fluorouracil as first-line therapy in advanced gastric cancer and adenocarcinoma of the gastroesophageal junction: results of a phase II trial. Ann Oncol. 2007;18:1673-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Stahl M, Walz MK, Stuschke M, Lehmann N, Meyer HJ, Riera-Knorrenschild J, Langer P, Engenhart-Cabillic R, Bitzer M, Königsrainer A, Budach W, Wilke H. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009;27:851-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 707] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 25. | Wang LB, Teng RY, Jiang ZN, Hu WX, Dong MJ, Yuan XM, Chen WJ, Jin M, Shen JG. Clinicopathologic variables predicting tumor response to neoadjuvant chemotherapy in patients with locally advanced gastric cancer. J Surg Oncol. 2012;105:293-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Shivakumar L, Ansell S. Targeting B-lymphocyte stimulator/B-cell activating factor and a proliferation-inducing ligand in hematologic malignancies. Clin Lymphoma Myeloma. 2006;7:106-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Sato H, Tsubosa Y, Kawano T. Correlation between the pretherapeutic neutrophil to lymphocyte ratio and the pathologic response to neoadjuvant chemotherapy in patients with advanced esophageal cancer. World J Surg. 2012;36:617-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 28. | Yao Y, Yuan D, Liu H, Gu X, Song Y. Pretreatment neutrophil to lymphocyte ratio is associated with response to therapy and prognosis of advanced non-small cell lung cancer patients treated with first-line platinum-based chemotherapy. Cancer Immunol Immunother. 2013;62:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 29. | Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5480] [Cited by in RCA: 5471] [Article Influence: 321.8] [Reference Citation Analysis (1)] |
| 30. | Wilcox RA, Wada DA, Ziesmer SC, Elsawa SF, Comfere NI, Dietz AB, Novak AJ, Witzig TE, Feldman AL, Pittelkow MR, Ansell SM. Monocytes promote tumor cell survival in T-cell lymphoproliferative disorders and are impaired in their ability to differentiate into mature dendritic cells. Blood. 2009;114:2936-2944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 31. | Wilcox RA, Ristow K, Habermann TM, Inwards DJ, Micallef IN, Johnston PB, Colgan JP, Nowakowski GS, Ansell SM, Witzig TE, Markovic SN, Porrata L. The absolute monocyte and lymphocyte prognostic score predicts survival and identifies high-risk patients in diffuse large-B-cell lymphoma. Leukemia. 2011;25:1502-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 32. | Donskov F, von der Maase H. Impact of immune parameters on long-term survival in metastatic renal cell carcinoma. J Clin Oncol. 2006;24:1997-2005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 247] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 33. | Sugimura K, Miyata H, Tanaka K, Takahashi T, Kurokawa Y, Yamasaki M, Nakajima K, Takiguchi S, Mori M, Doki Y. High infiltration of tumor-associated macrophages is associated with a poor response to chemotherapy and poor prognosis of patients undergoing neoadjuvant chemotherapy for esophageal cancer. J Surg Oncol. 2015;111:752-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 34. | Armstrong D, Raissouni S, Price Hiller J, Mercer J, Powell E, MacLean A, Jiang M, Doll C, Goodwin R, Batuyong E, Zhou K, Monzon JG, Tang PA, Heng DY, Cheung WY, Vickers MM. Predictors of Pathologic Complete Response After Neoadjuvant Treatment for Rectal Cancer: A Multicenter Study. Clin Colorectal Cancer. 2015;14:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 35. | Chen S, Chen YB, Li YF, Feng XY, Zhou ZW, Yuan XH, Qian CN. Normal carcinoembryonic antigen indicates benefit from perioperative chemotherapy to gastric carcinoma patients. World J Gastroenterol. 2012;18:3910-3916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Wallin U, Rothenberger D, Lowry A, Luepker R, Mellgren A. CEA - a predictor for pathologic complete response after neoadjuvant therapy for rectal cancer. Dis Colon Rectum. 2013;56:859-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 37. | Probst CP, Becerra AZ, Aquina CT, Tejani MA, Hensley BJ, González MG, Noyes K, Monson JR, Fleming FJ. Watch and Wait?--Elevated Pretreatment CEA Is Associated with Decreased Pathological Complete Response in Rectal Cancer. J Gastrointest Surg. 2016;20:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 38. | Sun Z, Zhang N. Clinical evaluation of CEA, CA19-9, CA72-4 and CA125 in gastric cancer patients with neoadjuvant chemotherapy. World J Surg Oncol. 2014;12:397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 39. | Deng K, Yang L, Hu B, Wu H, Zhu H, Tang C. The prognostic significance of pretreatment serum CEA levels in gastric cancer: a meta-analysis including 14651 patients. PLoS One. 2015;10:e0124151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 40. | Nakajima K, Ochiai T, Suzuki T, Shimada H, Hayashi H, Yasumoto A, Takeda A, Hishikawa E, Isono K. Impact of preoperative serum carcinoembryonic antigen, CA 19-9 and alpha fetoprotein levels in gastric cancer patients. Tumour Biol. 1998;19:464-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Ikeda Y, Oomori H, Koyanagi N, Mori M, Kamakura T, Minagawa S, Tateishi H, Sugimachi K. Prognostic value of combination assays for CEA and CA 19-9 in gastric cancer. Oncology. 1995;52:483-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 42. | Jiang Y, Yuan Q, Lv W, Xi S, Huang W, Sun Z, Chen H, Zhao L, Liu W, Hu Y, Lu L, Ma J, Li T, Yu J, Wang Q, Li G. Radiomic signature of 18F fluorodeoxyglucose PET/CT for prediction of gastric cancer survival and chemotherapeutic benefits. Theranostics. 2018;8:5915-5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 43. | Li Z, Zhang D, Dai Y, Dong J, Wu L, Li Y, Cheng Z, Ding Y, Liu Z. Computed tomography-based radiomics for prediction of neoadjuvant chemotherapy outcomes in locally advanced gastric cancer: A pilot study. Chin J Cancer Res. 2018;30:406-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 44. | Giganti F, Marra P, Ambrosi A, Salerno A, Antunes S, Chiari D, Orsenigo E, Esposito A, Mazza E, Albarello L, Nicoletti R, Staudacher C, Del Maschio A, De Cobelli F. Pre-treatment MDCT-based texture analysis for therapy response prediction in gastric cancer: Comparison with tumour regression grade at final histology. Eur J Radiol. 2017;90:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: International Gastric Cancer Association, (Fellow).
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hosoda K S-Editor: Tang JZ L-Editor: Filipodia E-Editor: Ma YJ