Published online May 21, 2020. doi: 10.3748/wjg.v26.i19.2374
Peer-review started: December 30, 2019
First decision: March 26, 2020
Revised: April 8, 2020
Accepted: April 24, 2020
Article in press: April 24, 2020
Published online: May 21, 2020
Processing time: 142 Days and 21.1 Hours
Post-transplant dyslipidemia (PTDL) is a common complication in liver recipients and can cause morbidity and threaten graft function. The crosstalk between metabolic inflammation and dyslipidemia has been recently revealed. However, the role of grafts’ and recipients’ metabolic status in the development of PTDL has not been evaluated.
To investigate the association of recipients’ metabolic inflammation status with PTDL and construct a predictive model.
A total of 396 adult patients who received primary liver transplantation between 2015 and 2017 were enrolled. Metabolomics and cytokines were analyzed using recipients’ pre-transplant peripheral blood in a training set (n = 72). An integrated prediction model was established according to the clinical risk factors and metabolic inflammation compounds and further verified in a validation set (n = 144).
The serum lipid profile took 3 mo to reach homeostasis after liver transplantation. A total of 278 (70.2%) liver recipients developed PTDL during a follow-up period of 1.78 (1.00, 2.97) years. The PTDL group showed a significantly lower tumor-free survival and overall survival than the non-PTDL group in patients with hepatocellular carcinoma (n = 169). The metabolomic analysis showed that metabolic features discriminating between the PTDL and non-PTDL groups were associated with lipid and glucose metabolism-associated pathways. Among metabolites and cytokines differentially expressed between the two groups, interleukin-12 (p70) showed the best diagnostic accuracy and significantly increased the predictive value when it was incorporated into the clinical model in both training and validation sets.
Recipients’ pre-transplant serum interleukin-12 (p70) level is associated with the risk of PTDL and has potential clinical value for predicting PTDL.
Core tip: Post-transplant dyslipidemia (PTDL) is a common complication in liver recipients and can cause morbidity and threaten graft function. The crosstalk between metabolic inflammation and dyslipidemia has been recently revealed, however, the role of recipients’ metabolic status in the development of PTDL has not been evaluated. Here, we conducted the first study to explore the association of recipients’ metabolic inflammation with PTDL and further evaluate the diagnostic efficacy of metabolic inflammation compounds. Interleukin-12 (p70) was found to be a valid predictor of PTDL and remarkably improve the predictive ability of the clinical model.
- Citation: Huang HT, Zhang XY, Zhang C, Ling Q, Zheng SS. Predicting dyslipidemia after liver transplantation: A significant role of recipient metabolic inflammation profile. World J Gastroenterol 2020; 26(19): 2374-2387
- URL: https://www.wjgnet.com/1007-9327/full/v26/i19/2374.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i19.2374
Dyslipidemia is a common complication after liver transplantation (LT)[1]. Due to the greatly prolonged patient survival after LT in recent years, the prevalence of post-transplant dyslipidemia (PTDL) has been reported to be up to 85%, higher than that in the general population[1,2]. PTDL was closely associated with organ allograft rejection, cardiovascular events, and graft dysfunction, which subsequently led to decreased patients’ survival[3-5]. Therefore, the early screening of patients at a high risk of developing PTDL will give the opportunity for prompt prevention and improving the long-term prognosis.
The underlying mechanism for developing PTDL is still unclear. Several clinical risk factors have been identified, including old age, high body mass index (BMI), renal dysfunction, and immunosuppressive agents[1,6]. Metabolic inflammation, which is initially mediated by innate immune signaling, is increasingly recognized as a driving force in the development of lipid disorders[7]. Of note, cytokines produced during metabolic inflammation have been proved to further worsen lipid disorders[8], which suggested that cytokines could serve as biomarkers for predicting PTDL. Currently, experimental and clinical studies have identified a number of cytokines associated with lipid-induced metabolic diseases, including interleukin (IL)-1, IL-18, IL-6, IL-8, and tumor necrosis factor-α[9,10]. In addition, cytokines can activate a series of metabolic pathways[11] that regulate metabolism and produce metabolites to support the precise changes of immune cell functions[12,13]. These studies further suggested that specific cytokines and metabolites may play roles in the development of PTDL.
In this study, we aimed to investigate the association of pre-transplant metabolite and cytokine profiles with PTDL and establish a predictive model using clinical parameters and metabolic inflammation compounds.
All adult (age ≥ 18 years) patients who received primary LT between January 2015 and December 2017 at the First Affiliated Hospital, Zhejiang University School of Medicine, China were enrolled in this study. The exclusion criteria were multi-organ transplantation, less than a 3-mo follow-up period, death within 3 mo post-LT, and incomplete data. Finally, a total of 396 patients were included. Patients’ characteristics are shown in Table 1. There was no living-donor LT. All patients received standard immunosuppressive protocol as described previously[14]. For hepatocellular carcinoma (HCC) patients, Hangzhou criteria were used as an indication of LT. Hangzhou criteria were defined as: (1) Total tumor diameter no more than 8 cm; and (2) Total tumor diameter more than 8 cm, with pathological grade I or II and preoperative alpha fetoprotein level no more than 400 ng/mL[15]. The diagnosis of recurrence was based on imaging appearance. Patients with hepatitis B virus (HBV) infection received standard anti-virus protocol post-LT (low-dose immunoglobulin and nucleoside analogs) as we described before[16]. The follow-up ended on January 1, 2019. Peripheral blood samples from 216 recipients were collected before LT. They were randomly divided (in a 1:2 ratio) into training (n = 72) and validation sets (n = 144). This study was approved by the Ethics Committee of our hospital according to the Regulations on Human Organ Transplant and national legal requirements. This study conformed to the guidelines of China Ethical Committee and the Declaration of Helsinki. No grafts from prisoners were obtained or used. Written informed consent was obtained from all patients.
| PTDL (n = 278) | Non-PTDL (n = 118) | P value | |
| Recipient factors | |||
| Age (yr) | 48.2 ± 9.6 | 49.1 ± 9.1 | 0.411 |
| Male, n (%) | 237 (85.3) | 102 (86.4) | 0.758 |
| BMI (kg/m2) | 23.2 ± 3.2 | 22.3 ± 2.9 | 0.012 |
| MELD score | 20.9 ± 10.9 | 20.6 ± 11.7 | 0.777 |
| Laboratory value | |||
| Creatinine (μmol/L) | 65.0 (53.0-83.8) | 66.0 (55.0-78.0) | 0.925 |
| Albumin (g/L) | 34.7 (31.0-38.2) | 35.5 (32.2-39.6) | 0.218 |
| TB (μmol/L) | 63.5 (28.0-335.5) | 58.5 (22.3-205.8) | 0.066 |
| INR | 1.5 (1.3-1.9) | 1.4 (1.2-1.7) | 0.087 |
| AST (U/L) | 66.0 (37.0-128.8) | 71.5 (38.3-125.5) | 0.694 |
| ALT (U/L) | 43 (24.0-93.3) | 40.5 (26.0-125.0) | 0.621 |
| TC (mg/dL) | 100.5 (65.7-139.2) | 104.4 (73.5-135.3) | 0.947 |
| TG (mg/dL) | 88.6 (62.0-115.1) | 70.9 (53.1-97.4) | 0.017 |
| HDLC (mg/dL) | 23.2 (11.6-34.8) | 27.1 (15.5-42.5) | 0.024 |
| LDLC (mg/dL) | 47.6 (27.1-73.5) | 46.4 (30.9-69.6) | 0.676 |
| VLDLC (mg/dL) | 23.2 (15.5-38.7) | 19.3 (11.6-30.9) | 0.052 |
| FBG (mmol/L) | 6.3 (5.3-8.0) | 6.0 (5.3-7.4) | 0.328 |
| Cirrhosis, n (%) | 206 (74.1) | 94 (79.7) | 0.238 |
| HCC, n (%) | 108 (38.8) | 61 (51.7) | 0.018 |
| HBV status, n (%) | |||
| HBsAg positive | 215 (77.3) | 102 (86.4) | 0.038 |
| HBeAg positive | 81 (29.1) | 27 (22.9) | 0.201 |
| HBV DNA > 1000 copies/mL | 39 (14.0) | 18 (15.3) | 0.751 |
| Pre-LT AVT | 108 (38.8) | 52 (44.1) | 0.333 |
| Comorbidities, n (%) | |||
| Hepatic encephalopathy | 50 (18.0) | 25 (21.2) | 0.457 |
| Hepatorenal syndrome | 22 (7.9) | 10 (8.5) | 0.851 |
| Gastrointestinal bleeding | 57 (20.5) | 14 (11.9) | 0.040 |
| Ascites | 104 (37.4) | 31 (26.3) | 0.032 |
| Donor factors | |||
| Age (yr) | 38.8 ± 12.3 | 41.3 ± 12.8 | 0.071 |
| Male, n (%) | 235 (84.5) | 99 (83.9) | 0.874 |
| Cause of death, n (%) | |||
| Trauma | 171 (61.5) | 71 (60.2) | 0.802 |
| CVA | 87 (31.3) | 37 (31.4) | 0.934 |
| Other | 21 (7.6) | 10 (8.5) | 0.755 |
| DBD (vs DCD) | 49 (17.6) | 17 (14.4) | 0.432 |
| BMI (kg/m2) | 22.6 ± 2.6 | 23.0 ± 2.7 | 0.169 |
| Graft weight (kg) | 1.4 ± 0.3 | 1.4 ± 0.3 | 0.228 |
| Macrovesicular steatosis, n (%) | 50 (18.0) | 22 (18.6) | 0.877 |
| Creatinine (μmol/L) | 77.0 (56.5-118.5) | 82.2 (56.1-121.3) | 0.685 |
| Albumin (g/L) | 31.0 (28.0-36.5) | 30.9 (26.7-36.7) | 0.474 |
| TB (μmol/L) | 14.1 (9.1-23.4) | 16.5 (11.1-26.5) | 0.078 |
| AST (U/L) | 52.0 (33.0-87.2) | 51.5 (31.3-94.5) | 0.694 |
| ALT (U/L) | 34.0 (22.0-69.0) | 39.0 (22.5-76.3) | 0.345 |
| Operative factors | |||
| WIT (min) | 50.7 ± 14.3 | 47.0 ± 10.8 | 0.006 |
| CIT (h) | 8.4 ± 3.1 | 8.7 ± 3.4 | 0.359 |
| Blood loss (L) | 1.0 (0.8-2.0) | 1.0 (0.8-1.5) | 0.117 |
| Immunosuppressant, n (%) | |||
| IL2R antibody | 204 (73.4) | 93 (78.8) | 0.254 |
| Corticosteroid | 156 (56.1) | 58 (49.2) | 0.204 |
| Tacrolimus | 245 (88.1) | 103 (87.3) | 0.814 |
Dyslipidemia was defined as total cholesterol (TC) ≥ 240 mg/dL, or triglycerides (TG) ≥ 200 mg/dL, or high-density lipoprotein cholesterol (HDLC) < 40 mg/dL, or a need for using of medication for dyslipidemia[17].
An ultra-performance liquid chromatography-mass spectrometry-based metabo-lomics analysis was performed as described previously[18]. The raw data were processed using the Compound Discoverer 3.0 (Thermo Fisher) to perform peak alignment, peak picking, and quantization for each metabolite. Partial least squares-discriminant analysis was performed with R pls package. Peaks were then matched with the mzCloud (https://www.mzcloud.org/) and ChemSpider (http://www.chemspider.com/) databases to obtain the accurate qualitative and relative quantitative results. Mummichog enrichment analysis for differential metabolic features was performed using metaboanalyst R package. Mantel tests based on Bray-Curtis distance and Pearson’s correlation analysis were applied to evaluate the correlations between cytokines and metabolic profiles with R ggcor package.
Peripheral blood samples from 216 recipients were collected before LT. The cytokine immunoassays were carried out using blood samples collected under sterile conditions in BD vacutainer tubes containing EDTA-K2. Samples were examined for a panel of 37 serum cytokines using Bio-Plex Pro™ Human Inflammation Assays (No. 6625, Bio-Rad, Hercules, CA, United States) according to the standard protocol. The data were analyzed using Bio-Plex Data Pro™ software and Bio-Plex Manager software (Bio-Rad).
Data analyses were performed using SPSS for Windows version 11.0 (SPSS Inc., Chicago, IL, United States) and MedCalc for Windows version 11.4.2.0 (MedCalc Software, Mariakerke, Belgium). Quantitative variables were presented as the mean ± SD or median (interquartile range) and compared by Student’s t test or Mann-Whitney U test. Kaplan-Meier analysis was used for survival analysis. Categorical variables were presented as values (percentages) and compared by Pearson’s χ2 test or Fisher’s exact test. Risk factors for PTDL were evaluated by logistic regression analysis. Variables with statistical significance (P < 0.05) in univariate analysis were transferred to a subsequent stepwise multivariate regression analysis. The cut-off value was selected according to clinical value or median. The establishment of predictive models was described in our previous study[19]. The Hosmer and Lemeshow test was used to assess the models’ goodness-of-fit. The area under the receiver operating characteristic curve (AUC) was calculated to evaluate the predictive ability of PTDL models. The AUCs of different models were compared with MedCalc. A P value of < 0.05 was considered statistically significant.
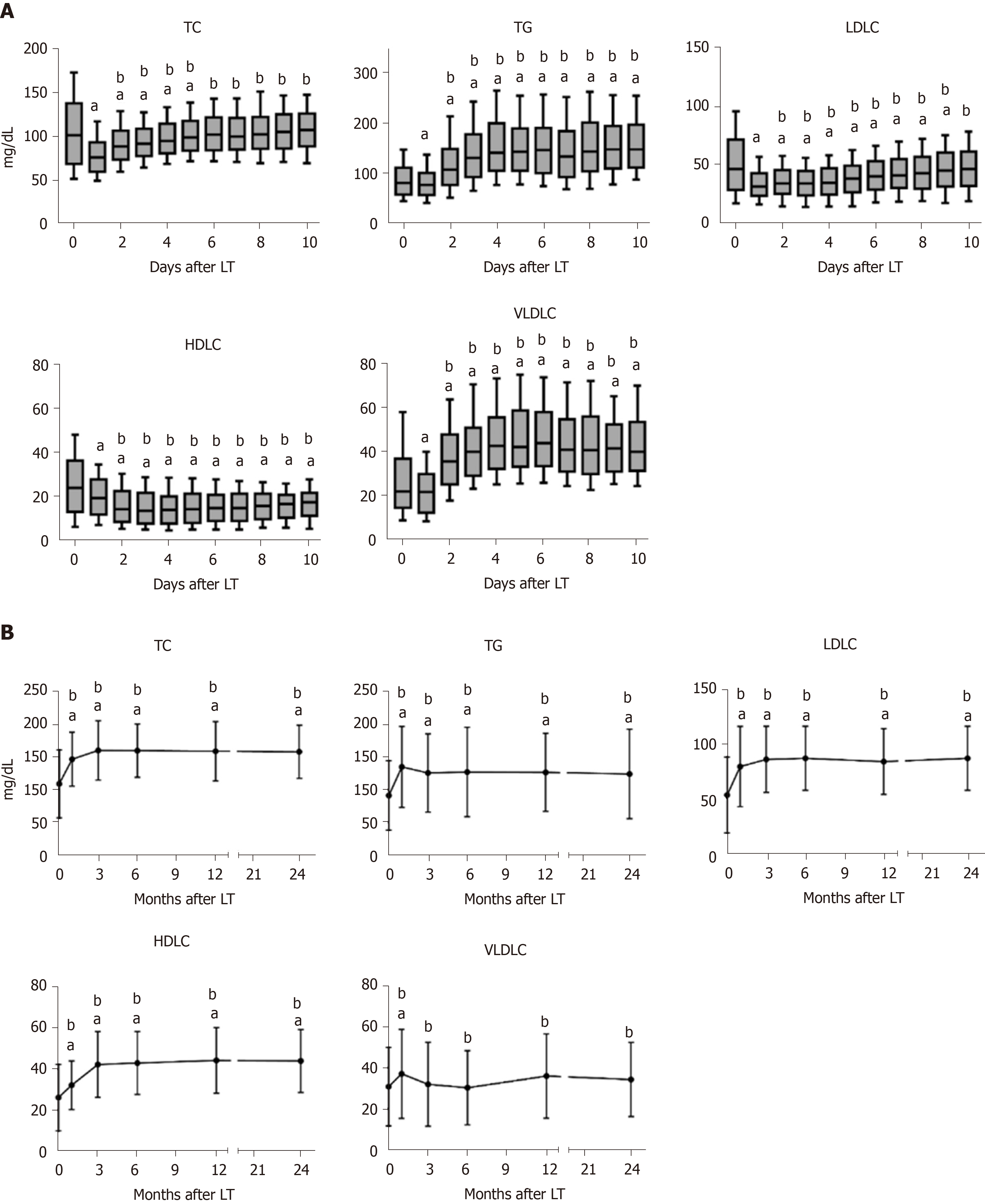
The dynamic changes of serum lipid profiles after LT are shown in Figure 1. TC, TG, LDLC, HDLC, and very low-density lipoprotein cholesterol sharply decreased in the first day following LT and gradually increased thereafter except HDLC (Figure 1A). At 3 mo post-LT, TC, TG, LDLC, and HDLC showed higher levels than preoperative levels, while very low-density lipoprotein cholesterol did not differ significantly compared to the preoperative levels. All lipids displayed relatively steady levels 3 mo post-LT (Figure 1B). The results suggested a 3-mo remodeling of lipid homeostasis in liver recipients.
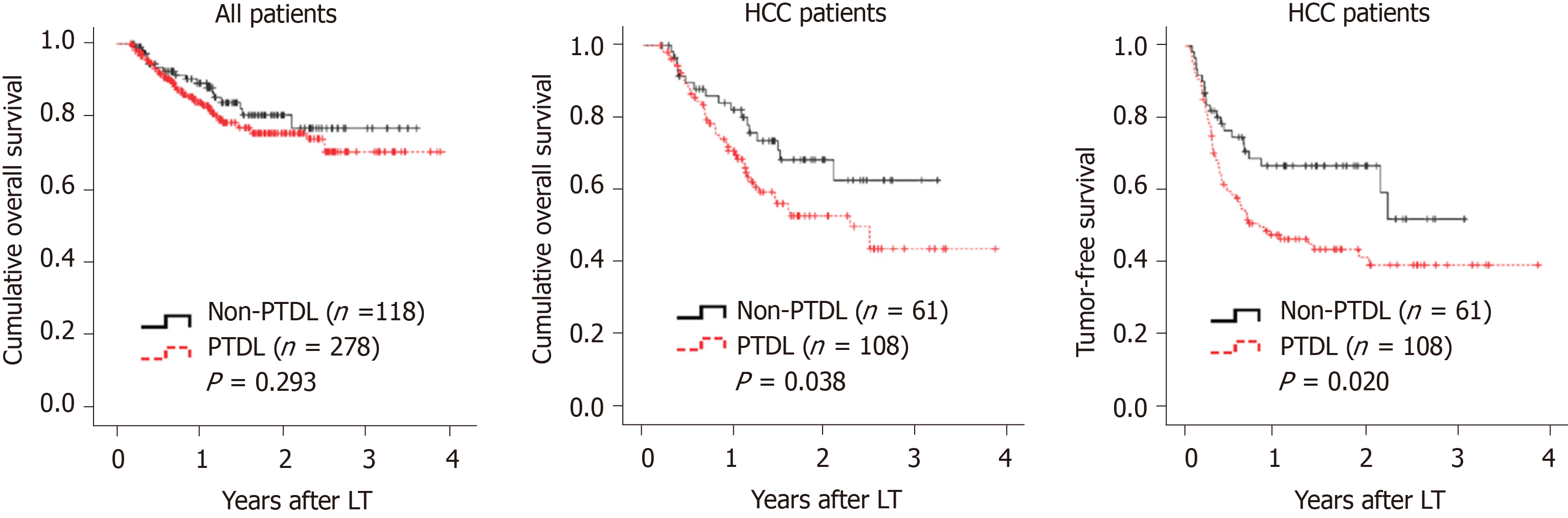
There were 278 (70.2%) patients who developed PTDL during a follow-up period of 1.78 (1.00, 2.97) years. The incidence of PTDL was 54.0% (214/396), 64.1% (238/369), and 69.9% (221/316) at 3 mo, 6 mo, and 1 year post-LT, respectively. The overall patient survival (Figure 2A) and post-transplant complications (Table 2) did not differ significantly between the two groups. However, among 169 HCC patients, the PTDL group showed a significantly lower overall patient survival (P = 0.038, Figure 2B) and tumor-free survival (P = 0.020, Figure 2C) than the non-PTDL group. There was no significant difference in HCC characteristics between the two groups (Supplementary Table 1). These results indicated that PTDL is associated with an adverse prognosis.
| PTDL (n = 278) | Non-PTDL (n = 118) | P value | |
| Acute kidney injury | 69 (24.8) | 27 (22.9) | 0.681 |
| Early allograft dysfunction | 84 (30.2) | 37 (31.4) | 0.822 |
| Portal vein stenosis | 5 (1.8) | 2 (1.7) | 0.943 |
| CMV infection | 4 (1.4) | 3 (2.5) | 0.730 |
| Bacterial infection | 28 (10.1) | 7 (5.9) | 0.184 |
| Diabetes mellitus | 21 (7.6) | 5 (4.2) | 0.233 |
| Hypertension | 13 (4.7) | 7 (5.9) | 0.602 |
| Cardiovascular diseases | 7 (2.5) | 1 (0.8) | 0.490 |
| Chronic kidney failure | 3 (1.1) | 0 (0) | 0.681 |
| Biliary complications | 40 (14.4) | 15 (12.7) | 0.659 |
| HCC recurrence1 | 60 (55.5) | 21 (34.4) | 0.008 |
Donors’ and recipients’ clinical parameters were compared between the PTDL and non-PTDL groups (Table 1). Compared to the non-PTDL group, the PTDL group showed significantly higher recipients’ BMI, higher TG, more gastrointestinal bleeding and ascites cases, but lower HDLC and fewer hepatitis B and HCC cases. In contrast, donor parameters did not differ significantly between the two groups. In logistic analysis, recipient overweight [odds ratio (OR) = 2.705, P = 0.002] and hypo-HDLC (OR = 3.090, P < 0.001) were independent risk factors for PTDL (Table 3).
| Univariate | Multivariate | |||
| OR (95% CI) | P value | OR (95%CI) | P value | |
| Overweight | 2.726 (1.494-4.973) | 0.001 | 2.705 (1.457-5.020) | 0.002 |
| Hyper-TG | 7.846 (1.032-59.679) | 0.047 | ||
| Hypo-HDLC | 3.116 (1.861-5.217) | < 0.001 | 3.090 (1.828-5.224) | < 0.001 |
| Hepatitis B | 0.489 (0.261-0.915) | 0.025 | ||
| Gastrointestinal bleeding | 1.916 (1.021-3.595) | 0.043 | ||
| Ascites | 1.677 (1.042-2.701) | 0.033 | ||
| HCC | 0.594 (0.385-0.916) | 0.019 | ||
| WIT | 2.286 (1.147-4.557) | 0.019 |
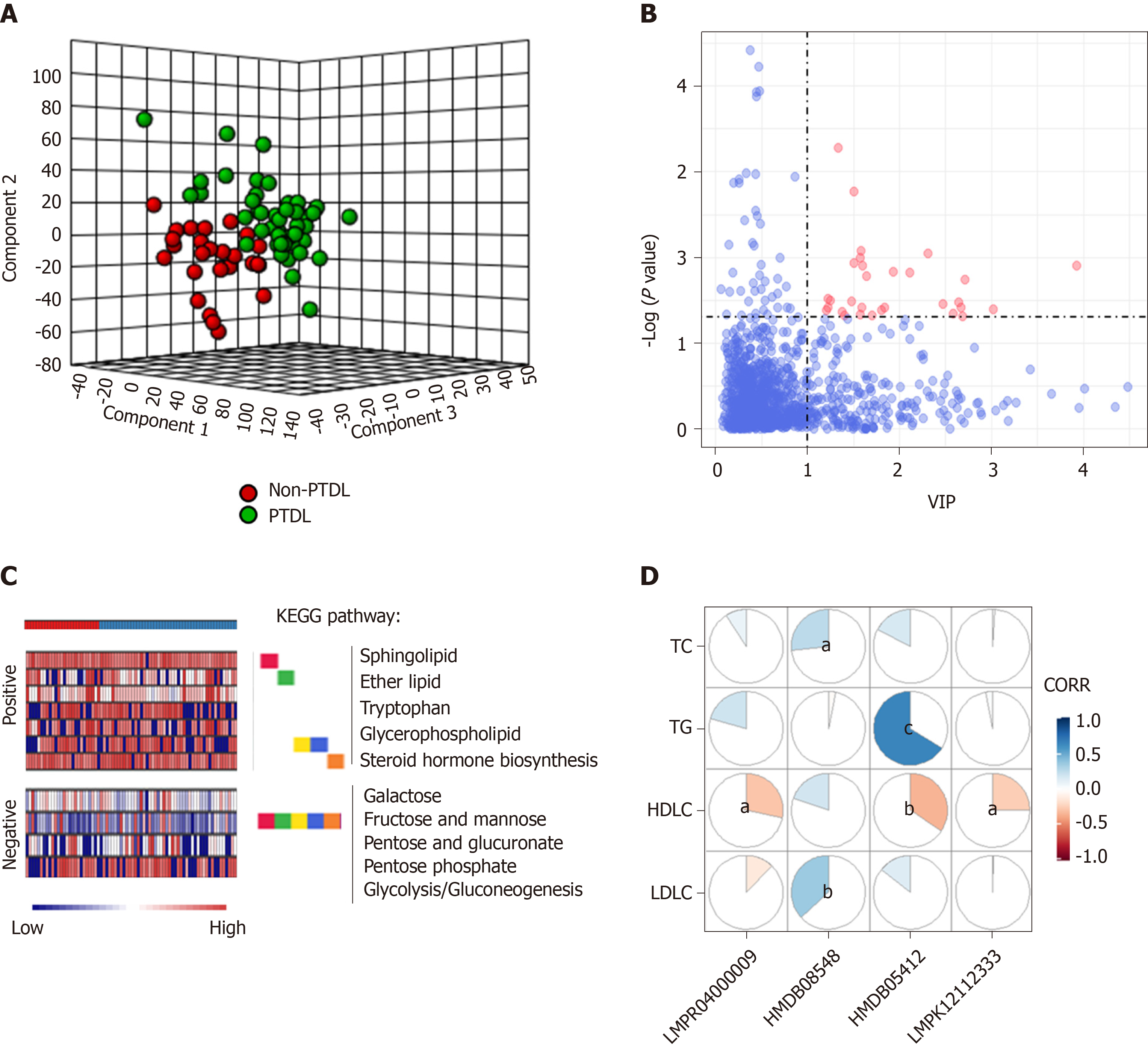
We performed an ultra-performance liquid chromatography-mass spectrometry based metabolomics approach to map the PTDL-associated metabolic feature. Partial least squares-discriminant analysis was used for quality control (Figure 3A). According to the selection criteria (Variable Importance in the Projection > 1 and P < 0.05), there were 30 significant differentially expressed metabolites in positive and negative ion modes between the PTDL (n = 45) and non-PTDL groups (n = 27) (Figure 3B). Mummichog analysis showed that the metabolic features discriminating between PTDL and non-PTDL group were associated with lipid metabolism-associated pathways (e.g., steroid hormone biosynthesis, glycerophospholipid, ether lipid, and sphingolipid) and glucose metabolism-associated pathways (e.g., glycolysis/ gluconeogenesis, pentose phosphate, pentose and glucuronate, fructose and mannose, and galactose) (Figure 3C). In addition, 4/30 metabolites were significantly related to the recipients’ lipid profile (Figure 3D). However, in predicting PTDL, these metabolites presented AUCs of < 0.65.
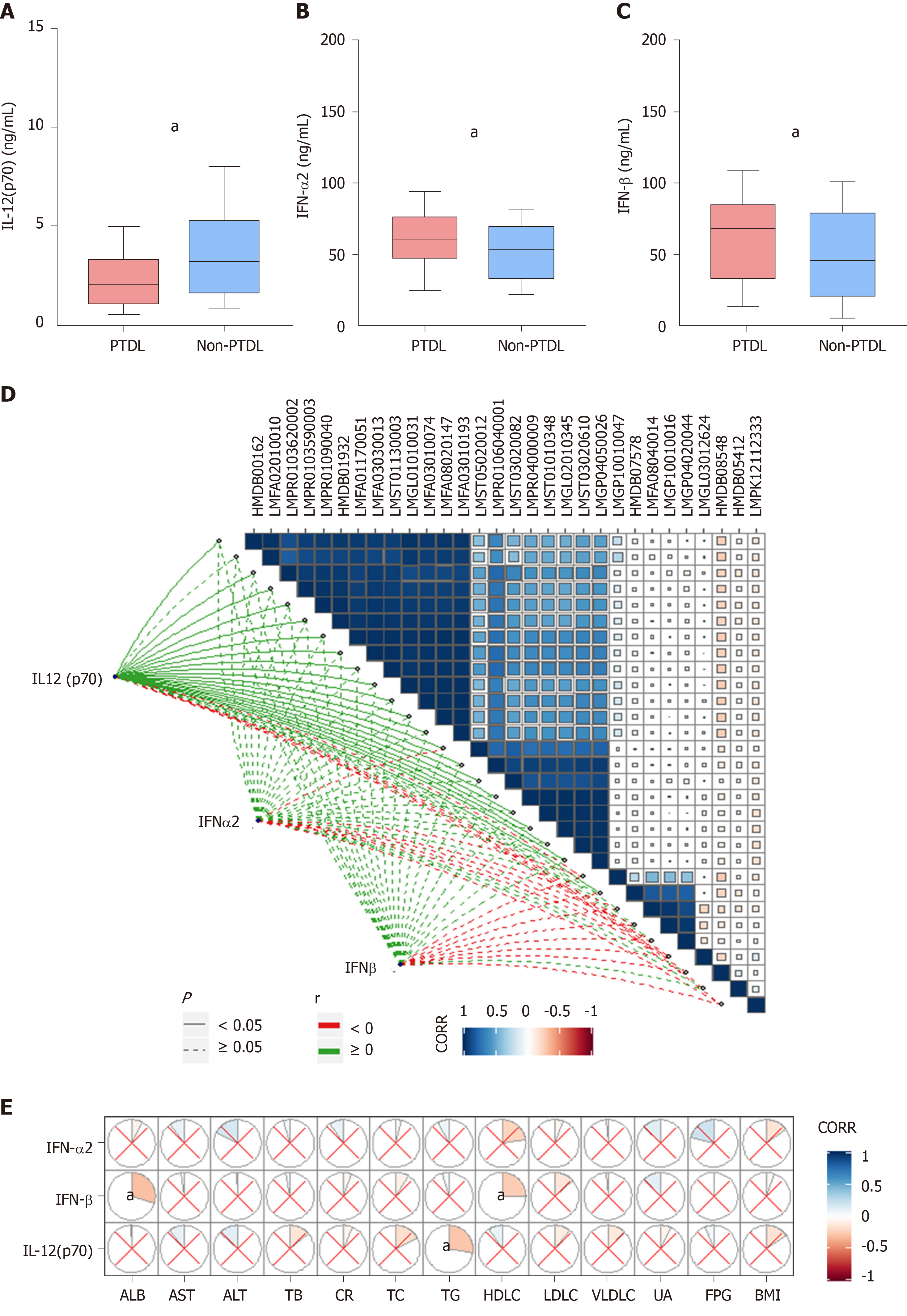
We further performed a cytokine immunoassay in the same cohort. Compared with the non-PTDL group, the PTDL group showed a significantly lower IL-12 (p70) level and higher IFN-α2 and IFN-β levels (Figure 4A-C). Mantel test suggested a significant correlation between IL-12 (p70) and most identified metabolites (21/30) (Figure 4D), which are enriched in lipid metabolism-associated pathways [e.g., diacylglycerol (20:5/20:5), 1-(11Z-docosenoyl)-glycero-3-phospho-(1'-sn-glycerol), 1-dodecanoyl-2-(5Z,8Z,11Z,14Z,17Z-eicosapentaenoyl)-sn-glycerol, and cholest-5,24-dien-3beta-ol 3-O-beta-D-glucopyranoside]. In contrast, these cytokines have no direct correlation with recipients’ pre-transplant metabolic parameters (Figure 4E). In predicting PTDL, IL-12 (p70) presented the best predictive ability among all metabolomic inflammation compounds in the training set (AUC = 0.706) and validation set (AUC = 0.762).
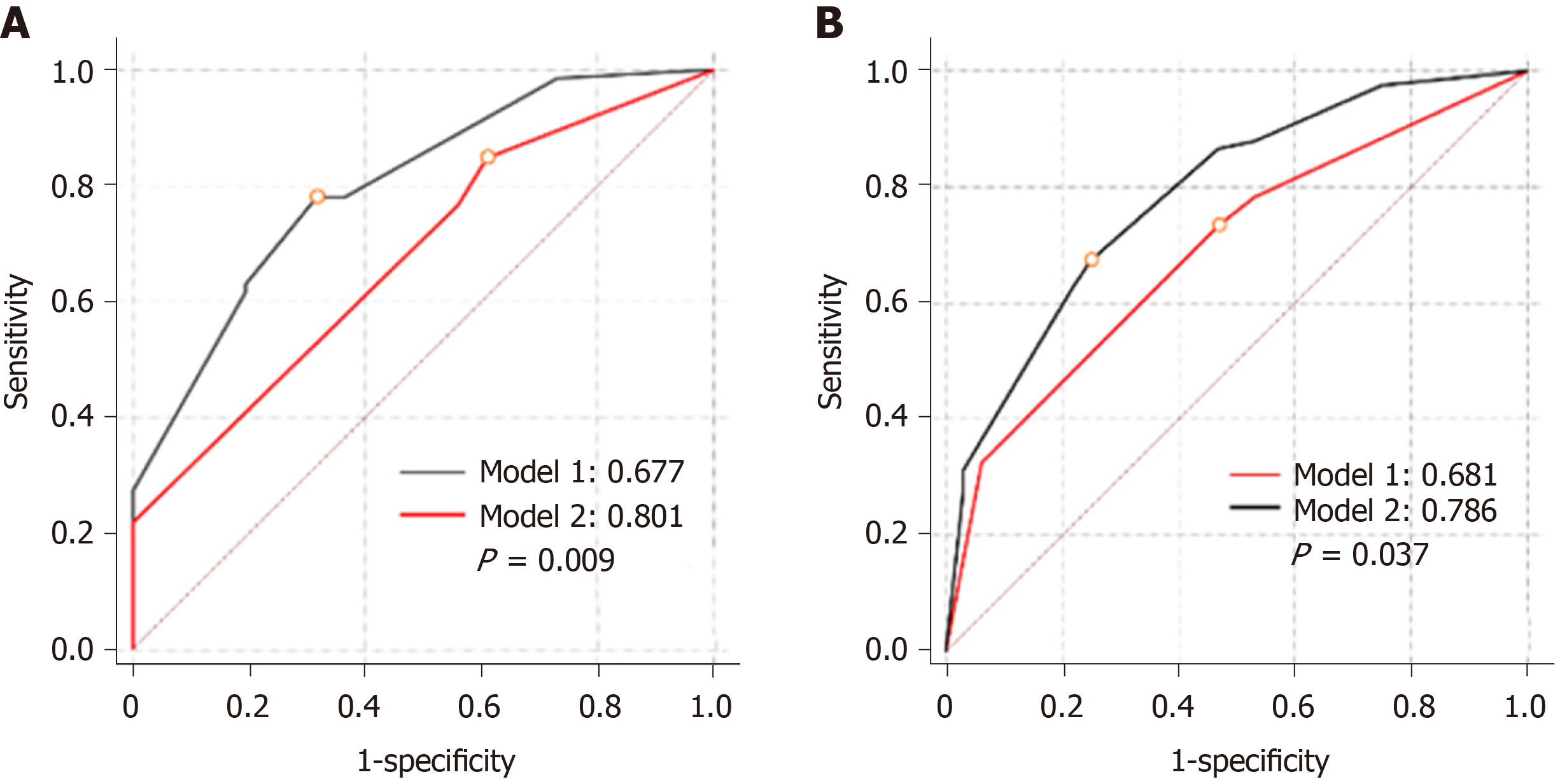
We constructed predictive models according to the logistic analysis (Table 4). Model 1 included only clinical parameters, whereas model 2 integrated clinical parameters with IL-12 (p70). Both models displayed good fits (P > 0.5 to reject model fit). Model 2 presented a significantly larger AUC than model 1 in both training (0.801 vs 0.677, P = 0.009) and validation sets (0.786 vs 0.691, P = 0.038) (Figure 5A and B).
| Multivariate | P value | ||
| β | OR (95%CI) | ||
| Model 1 | |||
| Recipient overweight | 0.991 | 2.693 (1.451-4.998) | 0.002 |
| Recipient hypo-HDLC | 1.134 | 3.107 (1.838-5.251) | < 0.001 |
| Constant | -0.249 | ||
| Model 2 | |||
| Recipient overweight | 1.625 | 3.425 (1.297-9.046) | 0.027 |
| Recipient hypo-HDLC | 1.231 | 5.077 (1.206-21.381) | 0.013 |
| Low IL-12 (p70) level | 1.846 | 6.336 (2.553-15.726) | < 0.001 |
| Constant | -1.494 | ||
In this study, we demonstrated a dynamic remodeling of lipid homeostasis in liver recipients. We found a longer remodeling time as compared with a previous study in living donor liver transplantation (3 mo vs 1 mo)[20], which indicates a longer period for close monitoring and prompt intervention. Furthermore, there was a very high incidence of PTDL in this study (> 70%) and previous ones[6]. We believed that it was due to not only the popularity of the disease but also a lack of proper screening and management of the disease. As a consequence, adverse outcomes such as HCC recurrence would occur. It should be an alarm bell for the transplant society, particularly in China, where there is the biggest HCC burden worldwide and HCC accounts for approximately 40% of LT indications. In addition, no formal guidelines have been developed to facilitate PTDL management up to now. Statins may be effective with a careful consideration of drug interactions and close patient monitoring[21]. Non-statin pharmacotherapy has also been evaluated in PTDL but only in a limited capacity[21]. Therefore, we appeal that more attention should be paid to PTDL.
We further evaluated the possible risk factors for PTDL and revealed that recipients’ but not grafts’ metabolic status determined the development of PTDL. It was not surprising to find a close correlation between PTDL and pre-transplant lipid profile as well as overweight[22,23]. It was important to determine a link between metabolic inflammation and PTDL. More importantly, most of the metabolites and cytokines that could distinguish PTDL from non-PTDL were not significantly correlated with serum lipid profile, indicating the independent predictive values. Metabolic disorders have been proved to trigger the production of inflammatory compounds, which further worsened the lipid metabolism[7]. As the key medium in this vicious circle, metabolic inflammation compounds have potential predictive abilities for the development of dyslipidemia[24,25]. In this study, we proved that recipients’ initial (pre-transplant) metabolic status was associated with PTDL. Among metabolic inflammation compounds that were differentially expressed between the PTDL and non-PTDL groups, IL-12 (p70) had the best diagnostic ability and remarkably improved the predictive value of the clinical model. In addition, IL-12 (p70) has been suggested to trigger the pathophysiology cascade of metabolomics in our study, although the conclusion needed to be confirmed by further experiments. IL-12 (p70), a member of IL12 cytokine family, was composed of IL-12 (p40) and IL-12 (p35) subunits. Generally, IL-12 (p70) is produced by dendritic cells and influence the development of Th1 cells[26]. Metabolic disorders can directly interfere with dendritic cell responses and decrease the expression IL-12 (p70)[27]. On the other hand, inflammatory cytokines could also regulate lipid metabolism[28]. For instance, IL-12 cytokine family member IL-27 could reduce lipid accumulation and enhance cholesterol efflux[29]. In addition, several other inflammatory cytokines, including IL-6, IL-1β, and tumor necrosis factor-α, have been reported to be associated with dyslipidemia[24]. For instance, in a children cohort, serum levels of IL-10, IL-17, and IL-23 were differentially expressed between dyslipidemia children and healthy individuals[30]. In patients with olanzapine-induced dyslipidemia, IL-1ra was found to be a novel biomarker[31]. However, no significant relevance of these cytokines with PTDL was found in this study.
It was also noteworthy that PTDL might increase the risk of HCC recurrence after LT. In fact, dyslipidemia plays a critical role in the pathogenesis of human malignancies, such as colorectal cancer[32], pancreatic cancer[33], and HCC[34]. Previous studies have proven that, in dyslipidemia, enriched circulating cholesterol could provoke the growth of tumors possibly through its role in maintaining the fluidity and permeability of the plasma membrane[35]. In addition, cholesterol is essential for lipid rafts synthesis, which recruits tumor signal components and initiates different downstream signals[35,36]. Interestingly, IL-12 (p70) may perform a potent anti-cancer role through the recruitment and activation of immune cells, and secretion of other cytokines[37]. A previous study demonstrated that IL-12 (p70) could down-regulate the expression Stat3, which subsequently triggered the differentiation of M1 macrophages and finally inhibited HCC growth[38]. Therefore, low serum IL-12 (P70) levels might be an indicator of both PTDL and HCC recurrence.
In contrast to the recipient, graft characteristics did not significantly correlate with PTDL in this study. In fact, the graft would be the new metabolic center in liver recipients. The crosstalk between graft and recipient occurs immediately after organ implant[39] and both grafts’ and recipients’ phenotype and genotype could affect the development of post-transplant metabolic syndrome[14,19]. We proposed a “central (liver) regulation” of metabolism in some diseases such as post-transplant diabetes[40,41]. We herein hypothesized a “peripheral (e.g., fat, muscle, and the pancreas) regulation” of metabolism in PTDL.
There were limitations in this study. First, this was an HBV- and male-dominant cohort. The results need to be verified in other cohorts with large samples. Second, the follow-up time was not long enough to observe the long-term outcomes such as cardiovascular events, which was reported to be associated with PTDL[42]. In addition, the role of PTDL in HCC recurrence needs to be further studied and the underlying mechanism needs to be explored.
In conclusion, PTDL is very common in liver recipients and associated with a poor prognosis. Recipients’ pre-transplant metabolic inflammation is correlated with the development of PTDL. Among the metabolic inflammation compounds, IL-12 (p70) could be a valid predictor of PTDL and remarkably improve the predictive ability of the clinical model. We recommend prophylactic regulation of metabolic inflammation for the prevention of the development of PTDL in LT candidates, particularly those with metabolic disorders.
Post-transplant dyslipidemia (PTDL) is a common complication in liver recipients and closely associated with organ allograft rejection, cardiovascular events, and graft dysfunction, which subsequently lead to decreased patient survival. The crosstalk between metabolic inflammation and dyslipidemia has been recently revealed.
The role of grafts’ and recipients’ metabolic status in the development of PTDL has not been evaluated.
The present study aimed to investigate the association of pre-transplant metabolite and cytokine profiles with PTDL and establish a predictive model using clinical parameters and metabolic inflammation compounds.
A total of 396 adult patients who received primary liver transplantation between 2015 and 2017 were enrolled. Metabolomics and cytokines were analyzed using recipients’ pre-transplant peripheral blood in a training set (n = 72). In addition, an integrated prediction model was established according to the clinical risk factors and metabolic inflammation compounds and further verified in a validation set (n = 144).
A 3-month remodeling of lipid homeostasis after liver transplantation was found in liver recipients. There were 278 (70.2%) patients who developed PTDL during a follow-up period of 1.78 (1.00, 2.97) years. The PTDL group showed a significantly lower tumor-free survival and overall survival than the non-PTDL group in patients with hepatocellular carcinoma (n = 169). In addition, the metabolomic analysis showed that metabolic features discriminating between the PTDL and non-PTDL groups were associated with lipid and glucose metabolism-associated pathways. Among metabolites and cytokines differentially expressed between the two groups, interleukin (IL)-12 (p70) showed the best diagnostic accuracy and significantly increased the predictive value when it was incorporated into the clinical model in both training and validation sets.
PTDL is very common in liver recipients and associated with a poor prognosis. Among the metabolic inflammation compounds, IL-12 (p70) could be a valid predictor of PTDL and remarkably improve the predictive ability of the clinical model.
Recipients’ pre-transplant serum IL-12 (p70) level is associated with the risk of PTDL and has potential clinical value for predicting PTDL. We recommend prophylactic regulation of metabolic inflammation for the prevention of the development of PTDL in liver transplantation candidates.
| 1. | Chu KKW, Chan SC, Sin SL, Chan ACY, Chok KSH, Cheng IKP, Lo CM. Lipid profiles of donors and recipients of liver transplant: like father like son. Hepatol Int. 2017;11:300-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Parekh J, Corley DA, Feng S. Diabetes, hypertension and hyperlipidemia: prevalence over time and impact on long-term survival after liver transplantation. Am J Transplant. 2012;12:2181-2187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Yuan J, Bagley J, Iacomini J. Hyperlipidemia Promotes Anti-Donor Th17 Responses That Accelerate Allograft Rejection. Am J Transplant. 2015;15:2336-2345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Hüsing A, Kabar I, Schmidt HH. Lipids in liver transplant recipients. World J Gastroenterol. 2016;22:3315-3324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (2)] |
| 5. | Raees-Jalali G, Eshraghian A, Faghihi A, Roozbeh J, Sagheb MM, Eshraghian H, Behzadi S. Hyperlipidemia after kidney transplantation: long-term graft outcome. Iran J Kidney Dis. 2012;6:49-55. [PubMed] |
| 6. | Ling Q, Wang K, Lu D, Guo HJ, Jiang WS, He XX, Xu X, Zheng SS. Major influence of renal function on hyperlipidemia after living donor liver transplantation. World J Gastroenterol. 2012;18:7033-7039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Chen Z, Yu Y, Cai J, Li H. Emerging Molecular Targets for Treatment of Nonalcoholic Fatty Liver Disease. Trends Endocrinol Metab. 2019;30:903-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 8. | Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 3201] [Article Influence: 400.1] [Reference Citation Analysis (2)] |
| 9. | Grebe A, Hoss F, Latz E. NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ Res. 2018;122:1722-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 664] [Article Influence: 94.9] [Reference Citation Analysis (0)] |
| 10. | Jin C, Henao-Mejia J, Flavell RA. Innate immune receptors: key regulators of metabolic disease progression. Cell Metab. 2013;17:873-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 11. | O'Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16:553-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1864] [Cited by in RCA: 2305] [Article Influence: 230.5] [Reference Citation Analysis (0)] |
| 12. | Chen M, Zhou K, Chen X, Qiao S, Hu Y, Xu B, Xu B, Han X, Tang R, Mao Z, Dong C, Wu D, Wang Y, Wang S, Zhou Z, Xia Y, Wang X. Metabolomic analysis reveals metabolic changes caused by bisphenol A in rats. Toxicol Sci. 2014;138:256-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Yang M, Qiu W, Chen B, Chen J, Liu S, Wu M, Wang KJ. The in vitro immune modulatory effect of bisphenol A on fish macrophages via estrogen receptor α and nuclear factor-κB signaling. Environ Sci Technol. 2015;49:1888-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 14. | Ling Q, Xie H, Li J, Liu J, Cao J, Yang F, Wang C, Hu Q, Xu X, Zheng S. Donor Graft MicroRNAs: A Newly Identified Player in the Development of New-onset Diabetes After Liver Transplantation. Am J Transplant. 2017;17:255-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Xu X, Lu D, Ling Q, Wei X, Wu J, Zhou L, Yan S, Wu L, Geng L, Ke Q, Gao F, Tu Z, Wang W, Zhang M, Shen Y, Xie H, Jiang W, Wang H, Zheng S. Liver transplantation for hepatocellular carcinoma beyond the Milan criteria. Gut. 2016;65:1035-1041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 202] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 16. | Lu D, Zhuo J, Yang M, Wang C, Pan L, Xie H, Xu X, Zheng S. The association between donor genetic variations in one-carbon metabolism pathway genes and hepatitis B recurrence after liver transplantation. Gene. 2018;663:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Kopin L, Lowenstein C. Dyslipidemia. Ann Intern Med. 2017;167:ITC81-ITC96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 440] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 18. | Gao J, Tarcea VG, Karnovsky A, Mirel BR, Weymouth TE, Beecher CW, Cavalcoli JD, Athey BD, Omenn GS, Burant CF, Jagadish HV. Metscape: a Cytoscape plug-in for visualizing and interpreting metabolomic data in the context of human metabolic networks. Bioinformatics. 2010;26:971-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 183] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 19. | Ling Q, Xie H, Lu D, Wei X, Gao F, Zhou L, Xu X, Zheng S. Association between donor and recipient TCF7L2 gene polymorphisms and the risk of new-onset diabetes mellitus after liver transplantation in a Han Chinese population. J Hepatol. 2013;58:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Wolf JH, Holmes MV, Fouraschen S, Keating BJ, Baker T, Emond J, Rader DJ, Shaked A, Olthoff KM. Serum lipid expression correlates with function and regeneration following living donor liver transplantation. Liver Transpl. 2016;22:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Warden BA, Duell PB. Management of dyslipidemia in adult solid organ transplant recipients. J Clin Lipidol. 2019;13:231-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Thoefner LB, Rostved AA, Pommergaard HC, Rasmussen A. Risk factors for metabolic syndrome after liver transplantation: A systematic review and meta-analysis. Transplant Rev (Orlando). 2018;32:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Laish I, Braun M, Mor E, Sulkes J, Harif Y, Ben Ari Z. Metabolic syndrome in liver transplant recipients: prevalence, risk factors, and association with cardiovascular events. Liver Transpl. 2011;17:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 192] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 24. | Papapanagiotou A, Siasos G, Kassi E, Gargalionis AN, Papavassiliou AG. Novel Inflammatory Markers in Hyperlipidemia: Clinical Implications. Curr Med Chem. 2015;22:2727-2743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Ke C, Zhu X, Zhang Y, Shen Y. Metabolomic characterization of hypertension and dyslipidemia. Metabolomics. 2018;14:117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 26. | Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. 2012;13:722-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 1020] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 27. | Shamshiev AT, Ampenberger F, Ernst B, Rohrer L, Marsland BJ, Kopf M. Dyslipidemia inhibits Toll-like receptor-induced activation of CD8alpha-negative dendritic cells and protective Th1 type immunity. J Exp Med. 2007;204:441-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Yuan Y, Liu Q, Zhao F, Cao J, Shen X, Li C. Holothuria Leucospilota Polysaccharides Ameliorate Hyperlipidemia in High-Fat Diet-Induced Rats via Short-Chain Fatty Acids Production and Lipid Metabolism Regulation. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Fu H, Tang YY, Ouyang XP, Tang SL, Su H, Li X, Huang LP, He M, Lv YC, He PP, Yao F, Tan YL, Xie W, Zhang M, Wu J, Li Y, Chen K, Liu D, Lan G, Zeng MY, Zheng XL, Tang CK. Interleukin-27 inhibits foam cell formation by promoting macrophage ABCA1 expression through JAK2/STAT3 pathway. Biochem Biophys Res Commun. 2014;452:881-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Manti S, Leonardi S, Panasiti I, Arrigo T, Salpietro C, Cuppari C. Serum IL-10, IL-17 and IL-23 levels as "bioumoral bridges" between dyslipidemia and atopy. Cytokine. 2017;99:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Lin Y, Peng Y, He S, Xu J, Shi Y, Su Y, Zhu C, Zhang X, Zhou R, Cui D. Serum IL-1ra, a novel biomarker predicting olanzapine-induced hypercholesterolemia and hyperleptinemia in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2018;84:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Rodriguez-Broadbent H, Law PJ, Sud A, Palin K, Tuupanen S, Gylfe A, Hänninen UA, Cajuso T, Tanskanen T, Kondelin J, Kaasinen E, Sarin AP, Ripatti S, Eriksson JG, Rissanen H, Knekt P, Pukkala E, Jousilahti P, Salomaa V, Palotie A, Renkonen-Sinisalo L, Lepistö A, Böhm J, Mecklin JP, Al-Tassan NA, Palles C, Martin L, Barclay E, Farrington SM, Timofeeva MN, Meyer BF, Wakil SM, Campbell H, Smith CG, Idziaszczyk S, Maughan TS, Kaplan R, Kerr R, Kerr D, Passarelli MN, Figueiredo JC, Buchanan DD, Win AK, Hopper JL, Jenkins MA, Lindor NM, Newcomb PA, Gallinger S, Conti D, Schumacher F, Casey G, Aaltonen LA, Cheadle JP, Tomlinson IP, Dunlop MG, Houlston RS. Mendelian randomisation implicates hyperlipidaemia as a risk factor for colorectal cancer. Int J Cancer. 2017;140:2701-2708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 33. | La Torre G, Sferrazza A, Gualano MR, de Waure C, Clemente G, De Rose AM, Nicolotti N, Nuzzo G, Siliquini R, Boccia A, Ricciardi W. Investigating the synergistic interaction of diabetes, tobacco smoking, alcohol consumption, and hypercholesterolemia on the risk of pancreatic cancer: a case-control study in Italy. Biomed Res Int. 2014;2014:481019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Phan J, Ng V, Sheinbaum A, French S, Choi G, El Kabany M, Durazo F, Saab S, Tong M, Busuttil R, Han SH. Hyperlipidemia and Nonalcoholic Steatohepatitis Predispose to Hepatocellular Carcinoma Development Without Cirrhosis. J Clin Gastroenterol. 2019;53:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Alikhani N, Ferguson RD, Novosyadlyy R, Gallagher EJ, Scheinman EJ, Yakar S, LeRoith D. Mammary tumor growth and pulmonary metastasis are enhanced in a hyperlipidemic mouse model. Oncogene. 2013;32:961-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 36. | Huang J, Li L, Lian J, Schauer S, Vesely PW, Kratky D, Hoefler G, Lehner R. Tumor-Induced Hyperlipidemia Contributes to Tumor Growth. Cell Rep. 2016;15:336-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 37. | Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:155-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 530] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 38. | Wang Q, Cheng F, Ma TT, Xiong HY, Li ZW, Xie CL, Liu CY, Tu ZG. Interleukin-12 inhibits the hepatocellular carcinoma growth by inducing macrophage polarization to the M1-like phenotype through downregulation of Stat-3. Mol Cell Biochem. 2016;415:157-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Huang H, Zhang X, Zhang C, Chen H, Ling Q, Zheng S. The time-dependent shift in the hepatic graft and recipient macrophage pool following liver transplantation. Cell Mol Immunol. 2020;17:412-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Ling Q, Xu X, Wang B, Li L, Zheng S. The Origin of New-Onset Diabetes After Liver Transplantation: Liver, Islets, or Gut? Transplantation. 2016;100:808-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Ling Q, Huang H, Han Y, Zhang C, Zhang X, Chen K, Wu L, Tang R, Zheng Z, Zheng S, Li L, Wang B. The tacrolimus-induced glucose homeostasis imbalance in terms of the liver: From bench to bedside. Am J Transplant. 2020;20:701-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 42. | Ganda OP, Bhatt DL, Mason RP, Miller M, Boden WE. Unmet Need for Adjunctive Dyslipidemia Therapy in Hypertriglyceridemia Management. J Am Coll Cardiol. 2018;72:330-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 157] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Akbulut S, Chiu KW, Gassler N, Salvadori M, Tajiri K S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Ma YJ