Published online May 14, 2020. doi: 10.3748/wjg.v26.i18.2247
Peer-review started: February 13, 2020
First decision: March 15, 2020
Revised: March 19, 2020
Accepted: April 24, 2020
Article in press: April 24, 2020
Published online: May 14, 2020
Processing time: 90 Days and 23.1 Hours
Computed tomography (CT), liver stiffness measurement (LSM), and magnetic resonance imaging (MRI) are non-invasive diagnostic methods for esophageal varices (EV) and for the prediction of high-bleeding-risk EV (HREV) in cirrhotic patients. However, the clinical use of these methods is controversial.
To evaluate the accuracy of LSM, CT, and MRI in diagnosing EV and predicting HREV in cirrhotic patients.
We performed literature searches in multiple databases, including PubMed, Embase, Cochrane, CNKI, and Wanfang databases, for articles that evaluated the accuracy of LSM, CT, and MRI as candidates for the diagnosis of EV and prediction of HREV in cirrhotic patients. Summary sensitivity and specificity, positive likelihood ratio and negative likelihood ratio, diagnostic odds ratio, and the areas under the summary receiver operating characteristic curves were analyzed. The quality of the articles was assessed using the quality assessment of diagnostic accuracy studies-2 tool. Heterogeneity was examined by Q-statistic test and I2 index, and sources of heterogeneity were explored using meta-regression and subgroup analysis. Publication bias was evaluated using Deek’s funnel plot. All statistical analyses were conducted using Stata12.0, MetaDisc1.4, and RevMan5.3.
Overall, 18, 17, and 7 relevant articles on the accuracy of LSM, CT, and MRI in evaluating EV and HREV were retrieved. A significant heterogeneity was observed in all analyses (P < 0.05). The areas under the summary receiver operating characteristic curves of LSM, CT, and MRI in diagnosing EV and predicting HREV were 0.86 (95% confidence interval [CI]: 0.83-0.89), 0.91 (95%CI: 0.88-0.93), and 0.86 (95%CI: 0.83-0.89), and 0.85 (95%CI: 0.81-0.88), 0.94 (95%CI: 0.91-0.96), and 0.83 (95%CI: 0.79-0.86), respectively, with sensitivities of 0.84 (95%CI: 0.78-0.89), 0.91 (95%CI: 0.87-0.94), and 0.81 (95%CI: 0.76-0.86), and 0.81 (95%CI: 0.75-0.86), 0.88 (95%CI: 0.82-0.92), and 0.80 (95%CI: 0.72-0.86), and specificities of 0.71 (95%CI: 0.60-0.80), 0.75 (95%CI: 0.68-0.82), and 0.82 (95%CI: 0.70-0.89), and 0.73 (95%CI: 0.66-0.80), 0.87 (95%CI: 0.81-0.92), and 0.72 (95%CI: 0.62-0.80), respectively. The corresponding positive likelihood ratios were 2.91, 3.67, and 4.44, and 3.04, 6.90, and2.83; the negative likelihood ratios were 0.22, 0.12, and 0.23, and 0.26, 0.14, and 0.28; the diagnostic odds ratios were 13.01, 30.98, and 19.58, and 11.93, 49.99, and 10.00. CT scanner is the source of heterogeneity. There was no significant difference in diagnostic threshold effects (P > 0.05) or publication bias (P > 0.05).
Based on the meta-analysis of observational studies, it is suggested that CT imaging, a non-invasive diagnostic method, is the best choice for the diagnosis of EV and prediction of HREV in cirrhotic patients compared with LSM and MRI.
Core tip: To date, endoscopy is regarded as the “gold standard” for diagnosis of esophageal varices (EV) and prediction of high-bleeding-risk EV (HREV) in cirrhotic patients. This study came into the conclusion that computed tomography has higher accuracy in diagnosing EV and predicting HREV than liver stiffness measurement and magnetic resonance imaging in cirrhotic patients. It is suggested that computed tomography, a non-invasive diagnostic method, is the best choice for diagnosing EV and predicting HREV in cirrhotic patients compared with liver stiffness measurement and magnetic resonance imaging. However, in future, the head-to-head comparisons of these imaging tools in the same series of patients are required to confirm the predictive value, especially by using artificial intelligence technique.
- Citation: Li Y, Li L, Weng HL, Liebe R, Ding HG. Computed tomography vs liver stiffness measurement and magnetic resonance imaging in evaluating esophageal varices in cirrhotic patients: A systematic review and meta-analysis. World J Gastroenterol 2020; 26(18): 2247-2267
- URL: https://www.wjgnet.com/1007-9327/full/v26/i18/2247.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i18.2247
Globally, liver cirrhosis is the most common liver disease and the 11th leading cause of death. Approximately two million people die from liver disease every year and 50% of them die from complications of cirrhosis[1]. Portal hypertension (PH) with esophageal varices (EV) and the following lethal variceal hemorrhage is the most serious and common complication of cirrhosis. The incidence of EV in cirrhotic patients is 7% per year and the five-year cumulative incidence rate reaches 21%[2]. Although the treatment of variceal hemorrhage has been improved over the past two decades, the 6-wk mortality is 10%-20%[3]. The confirmation of varices and the most suitable treatment in the early phase is crucial in order to reduce the mortality. To date, endoscopy is regarded as the “gold standard” for diagnosing the presence of varices and predicting bleeding risk. Baveno VI recommends that compensated cirrhotic patients without varices whose etiological factor has been removed should receive endoscopy every 3 years[4]. Endoscopy, however, is invasive and un-comfortable. In addition to endoscopy, hepatic vein pressure gradient (HVPG) is considered as a “gold standard” in estimating PH and for risk stratification of liver cirrhosis. HVPG is superior to liver biopsy in predicting the occurrence of complications in cirrhotic patients, including EV and variceal hemorrhage[5]. It is promising that with the aid of HVPG-guided precise treatment, physicians can diagnose and treat PH similarly to “high blood pressure”[6]. However, HVPG measurement is also invasive and expensive. Therefore, non-invasive and easy-to-perform diagnostic techniques to predict complications in cirrhotic patients with PH are required in clinical practice.
So far, several models and parameters based on serum markers[7,8] have been proposed. However, poor reliability has prevented their use in clinical practice. Recently, multiple studies evaluated the accuracy of liver stiffness measurement (LSM), computed tomography (CT), and magnetic resonance imaging (MRI) in the diagnosis of EV and prediction of high-bleeding-risk EV (HREV) in cirrhotic patients. There have, however, been controversies regarding the use of LSM, CT, and MRI as non-invasive diagnostic methods for EV and prediction of HREV in cirrhotic patients. Therefore, the aim of this meta-analysis was to evaluate the value of the imaging methods for the diagnosis of EV and prediction of HREV in clinical practice.
This systematic review and meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines[9], and the protocol is registered at PROSPERO (CRD42019126278).
A systematic literature research based on PubMed, Embase, Cochrane, CNKI, and Wanfang databases using various combinations of Medical Subject Headings and non-Medical Subject Headings terms was performed independently by two reviewers. The search was limited to original full text articles published in English and Chinese.
The articles reporting the diagnostic value of LSM were searched using key words “LS,” “liver stiffness,” “FibroScan,” “esophageal varices”, and “cirrhosis”, and those reporting the diagnostic value of CT and MRI were searched based on key words “CT,” “computed tomography,” “esophageal varices”, and “cirrhosis” and “MR,” “magnetic resonance,” “esophageal varices”, and “cirrhosis”, respectively.
The last search was performed on April 26, 2019.
Eligibility criteria: The inclusion criteria were: (1) Patients were diagnosed with liver cirrhosis; (2) Endoscopy was performed to confirm the presence and/or grade of EV; (3) Relevant examinations, such as LSM, CT, or MRI, were performed; and (4) The diagnostic accuracy was compared between reference and LSM, CT, or MRI. The exclusion criteria were: (1) Duplicate articles; (2) Reviews; (3) Case reports; (4) Noncirrhotic patients; (5) Patients in whom the presence of varices evaluated was not evaluated by endoscopy; and (6) Lack of accuracy assessment.
Data extraction: The primary data were extracted by two reviewers independently. The study characteristics contained country, study design, age, gender, and etiology of liver cirrhosis. The data included patient number, cut-off value, and the sensitivity and specificity in the diagnosis of EV or HREV. The criteria for HREV based on endoscopy were any of the following[10-12]: (1) Varices diameter ≥ 5 mm and snakelike varices with red color signs; and (2) Large varices (diameter ≥ 10 mm) and nodular and tumor-shaped varices with or without red color signs.
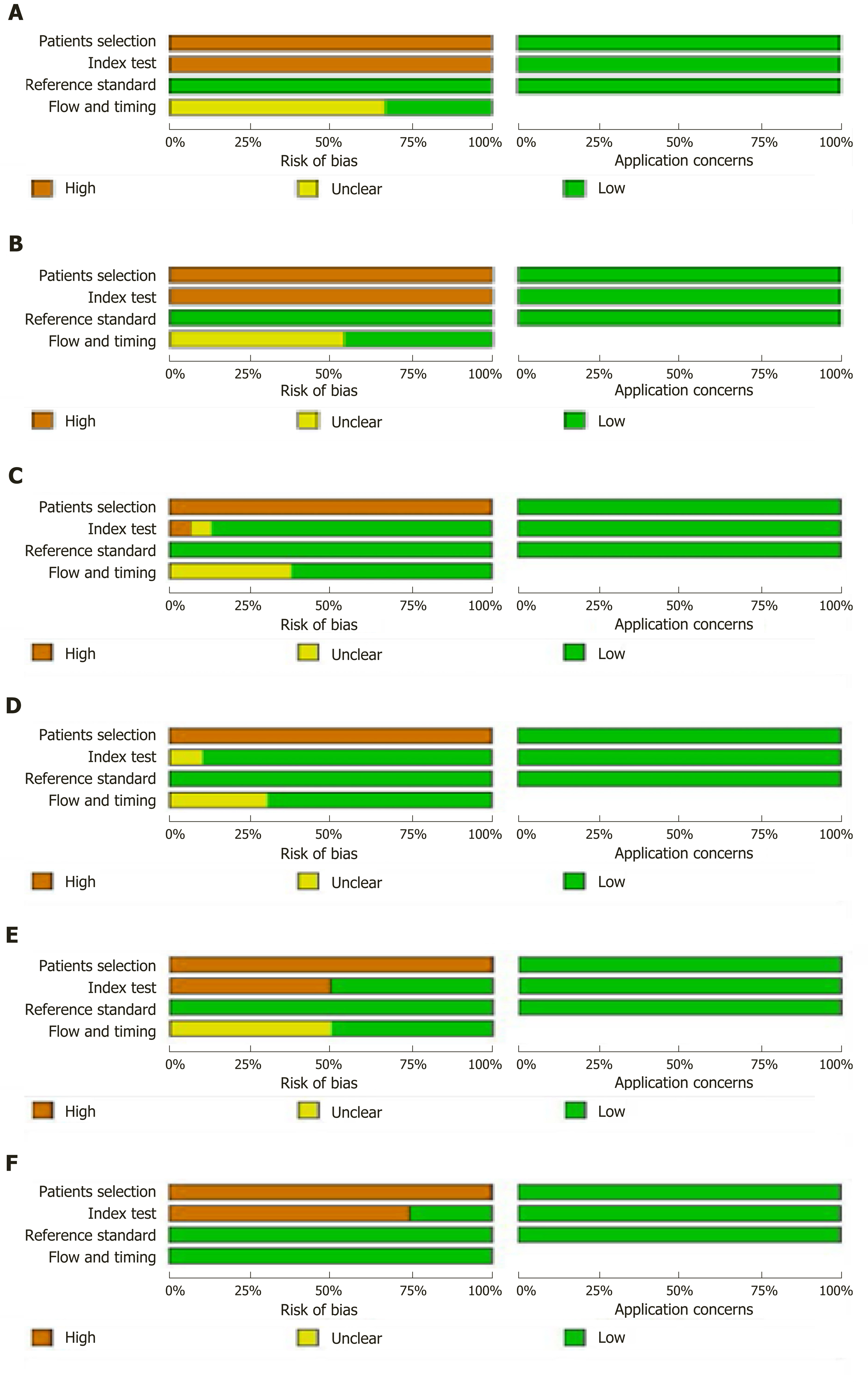
Two reviewers independently assessed the study quality using the Quality Assessment of Diagnostic Accuracy Studies-2 in RevMan5.3. They calculated the risk of bias as high, low, or unclear with regard to the following aspects: Patient selection, index test, reference standard, and flow and timing. Discrepancies were resolved by discussion between the two reviewers. Each question was judged as “yes”, “no”, or “unclear”.
First, true positive (TP) value, false positive (FP) value, false negative (FN) value, and true negative (TN) value were extracted from the original articles. Data analyses were conducted using Stata12.0, MetaDisc1.4, and RevMan5.3.
Second, the heterogeneity of all tested parameters was examined by Q-statistic test and I2 index. Heterogeneity was considered significant if P < 0.05 (Q-statistic test) or I2 ≥ 50%[13]. When heterogeneity was tested, we further evaluated the threshold effects by calculating the Spearman’s correlation coefficient. Threshold effects were considered significant if P < 0.05. If no threshold effects existed, sources of heterogeneity were analyzed by meta-regression according to study characteristics. Besides, we performed subgroup analysis according to the results of meta-regression.
The analysis was performed using the fixed-effects model or random-effects model if heterogeneity was considered significant. The diagnostic accuracy was evaluated by the area under the summary receiver operating characteristic curve (AUSROC) with 95% confidence interval (CI), summary sensitivity and specificity with 95%CI, summary positive likelihood ratio (PLR) and negative likelihood ratio (NLR) with 95%CI, and summary diagnostic odds ratio (DOR).
Finally, publication bias was evaluated using Deek’s funnel plot, with P < 0.05 as having significant publication bias[14].
All analyzed cirrhotic patients were diagnosed by histopathology and/or typical clinical symptoms and laboratory and imaging findings. The etiologies of liver cirrhosis included hepatitis B virus, hepatitis C virus, alcohol, autoimmune hepatitis, nonalcoholic steatohepatitis, and miscellaneous.
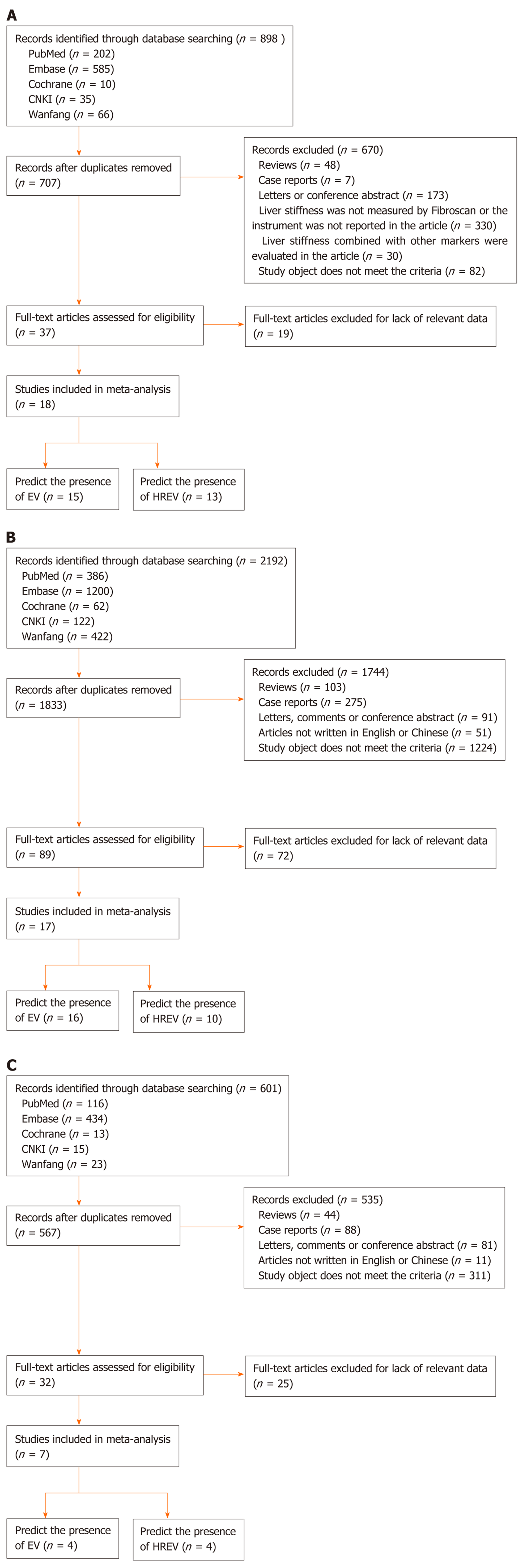
LSM: According to the aforementioned search strategy, 898 articles relevant to LSM and cirrhosis were identified. Eighteen best-matched articles were chosen for final meta-analysis[15-32]. The selection process is presented in Figure 1A. Fifteen out of eighteen selected publications[15-17,19-25,27-29,31,32] studied the diagnostic value for EV in 1836 patients. These studies were performed in Asia (n = 6), Europe (n = 7), and Africa (n = 2). In addition, 13[15-18,20-23,26,27,30-32] articles reported the predictive value of HREV in 2388 patients. These studies were performed in Asia (n = 5), Europe (n = 6), and Africa (n = 2), respectively.
CT: According to the search strategy, 17 out of 2192 articles relevant to CT imaging and cirrhosis were chosen for meta-analysis[33-49] (Figure 1B). Sixteen articles[33-38,40-49] enrolled 3327 patients (31 groups) and examined the diagnostic value of CT for EV. These studies were performed in Asia (n = 9), North America (n = 3), and Africa (n = 4) (Table 1). Besides, 10[34-36,39-43,45,47] articles reported the predictive value of HREV in 2686 patients (23 groups). These studies were performed in Asia (n = 5), North America (n = 3), and Africa (n = 2) (Table 2).
| Ref. | Study design | Total patients | Mean age (yr) | Male (%) | Etiology (%) | Child-Pugh class (%) | CT scanner | Interval between CT and endoscopy | Presence of EV | |||
| TP | FP | FN | TN | |||||||||
| Cansu et al[33], 2014 | Prospective | 50 | 56.8 | 54 | HBV (40.0); HCV (30.0); Biliary (6.0); HBV + HCV (4.0); Alcohol (4.0%); Others (16.0) | A (52.0); B (36.0); C (12.0) | 16-slice | Within 4 wk | 33 | 2 | 0 | 15 |
| Prospective | 42 | 56.2 | 69 | HBV (45.2); HCV (23.8); Alcohol (4.8); HBV + HCV (2.4); Biliary (2.4); Others (21.4) | A (38.1); B (31.0); C (31.0) | 16-slice | Within 4 wk | 25 | 3 | 8 | 6 | |
| Deng et al[34], 2016 | Retrospective | 52 | 55.4 | 63.5 | Alcohol (30.8); HBV (25.0); HBV + Alcohol (9.6); HCV (3.8); HBV + HCV (1.9); Others (28.9) | A (49.0); B (39.2); C (11.8) | NR | NR | 43 | 2 | 2 | 5 |
| Desso-uky et al[35], 2013 | Prospective | 137 | 58.7 | 53.3 | HCV (67.9); HBV (19.7); HBV + HCV (10.2); Steatohepatitis (2.2) | A (55); B (31); C (14) | 16-slice | Within 24 h | 89 | 1 | 1 | 46 |
| Elalfy et al[36], 2016 | Retrospective | 124 | 56.5 | 52 | HCV (100) | A (62.9); B (37.1) | 16-slice | NR | 70 | 4 | 4 | 46 |
| Elkammash et al[37], 2015 | NR | 112 | 51.4 | 68.8 | HBV (46); HCV (44); Schisto-somiasis (10) | NR | 64-slice | Within 2 wk | 97 | 0 | 2 | 13 |
| 99 | 0 | 0 | 13 | |||||||||
| Jiang et al[38], 2015 | NR | 89 | 57 | 64 | HBV (71.9); Alcohol (11.2); HCV (7.9); Biliary (5.6); Drug (1.1); Unknown (2.3) | NR | 64-slice | NR | 58 | 3 | 8 | 20 |
| Kim et al[40], 2020 | Retrospective | 104 | 59 | 74 | HBV (72.1); HCV (12.5); Alcohol (6.7); Others (8.7) | A (41.3); B (30.8); C (27.9) | 16 or 64-slice | Within 4 wk | 180 | 9 | 8 | 11 |
| 169 | 9 | 19 | 11 | |||||||||
| 172 | 15 | 16 | 5 | |||||||||
| Kim et al[41], 2007 | Prospective | 90 | 54.8 | 72.2 | HBV (73.3); HCV (21.1); Alcohol (2.2); Others (3.3) | A (81.1); B (18.9) | 16-slice | Within 4 h | 50 | 15 | 3 | 22 |
| 47 | 12 | 6 | 25 | |||||||||
| 46 | 13 | 7 | 24 | |||||||||
| 46 | 8 | 7 | 29 | |||||||||
| Kim et al[42], 2007 | Retrospective | 67 | 56.2 | 58.2 | HCV (35.8); HBV (22.4); Alcohol (22.4); HBV + HCV (9.0); Others (10.4) | A (23.9); B (37.3); C (38.8) | NR | Within 4 wk | 29 | 6 | 13 | 19 |
| 27 | 3 | 15 | 22 | |||||||||
| Lipp et al[43], 2011 | Retrospective | 299 | 55.2 | 64.9 | NR | NR | 4 or 16 or 64-slice | Within 12 wk | 54 | 24 | 7 | 52 |
| 41 | 17 | 30 | 77 | |||||||||
| Moftah et al[44], 2014 | NR | 54 | 56.8 | 74.1 | NR | NR | 8-slice | NR | 48 | 0 | 2 | 4 |
| Perri et al[45], 2008 | Retrospective | 101 | NR | 63.4 | Viral (21.8); Alcohol (18.8); Biliary (17.8); NASH (14.9); Others (26.7) | A (44.6); B (39.6); C (15.8) | 4-slice or higher | 2 d1 | 73 | 10 | 6 | 12 |
| 68 | 12 | 11 | 10 | |||||||||
| Wu et al[46], 2009 | Prospective | 50 | 57.7 | 60 | HBV (76); Autoimmune (2); Others (22) | A (26.0); B (62.0); C (12.0) | 16-slice | Within 4 wk | 39 | 3 | 2 | 6 |
| 40 | 5 | 1 | 4 | |||||||||
| Yu et al[47], 2011 | Retrospective | 109 | 55.9 | 55 | HCV (46.8); Alcohol (17.4); HBV (6.4); Others (29.4) | NR | 16 or 64-slice | NR | 50 | 10 | 12 | 37 |
| 50 | 12 | 12 | 35 | |||||||||
| 49 | 24 | 13 | 23 | |||||||||
| 47 | 17 | 15 | 30 | |||||||||
| Zhao et al[48], 2016 | Retrospective | 143 | 52.39 | 67.1 | HBV (70.6); Alcohol (11.2); Autoimmune (4.9); HCV (3.5); Biliary (3.5); Others (6.3) | A (37.8); B (33.6); C (28.7) | 64-slice | 3.4 d2 | 11 | 3 | 1 | 27 |
| 2 | ||||||||||||
| Zhu et al[49], 2009 | Retrospective | 127 | 45.2 | 75.6 | HBV (74.8); Alcohol (10.2); HCV (4.7); Others (10.2) | A (37.8); B (37.0); C (25.2) | 4-slice | Within 4 wk | 72 | 15 | 14 | 26 |
| 67 | 11 | 19 | 30 | |||||||||
| Ref. | Study design | Total patients | Mean age (yr) | Male (%) | Etiology (%) | Child-Pugh class (%) | CT scanner | Cut-off value (mm) | Interval between CT and endoscopy | Presence of HREV | ||||
| TP | FP | FN | TN | |||||||||||
| Deng et al[34], 2016 | Retrospective | 52 | 55.4 | 64 | Alcohol (30.8); HBV (25.0); HBV + Alcohol (9.6); HCV (3.8); HBV + HCV (1.9); Others (28.9) | A (49.0); B (39.2); C (11.8) | NR | EVD 3.9 | NR | 35 | 4 | 4 | 9 | |
| Dessouky et al[35], 2013 | Prospective | 137 | 58.7 | 53 | HCV (67.9); HBV (19.7); HBV + HCV (10.2); Steato-hepatitis (2.2) | A (55); B (31); C (14) | 16-slice | EVD 3.0 | Within 24 h | 38 | 0 | 0 | 99 | |
| Elalfy et al[36], 2016 | Retrospective | 124 | 56.5 | 52 | HCV (100) | A (62.9); B (37.1) | 16-slice | PVD 12.5 | NR | 33 | 49 | 13 | 29 | |
| Kim et al[39], 2008 | Retrospective | 110 | 61 | 74 | HBV (60.9); HCV (29.1) Alcohol (6.4); HBV + HCV (1.8); Others (1.8) | A (63.6); B (26.4); C (10.0) | 16-slice | EVD 2.0 | 8.2 d1 | 123 | 15 | 9 | 61 | |
| 113 | 2 | 19 | 59 | |||||||||||
| 115 | 15 | 17 | 61 | |||||||||||
| Kim et al[40], 2020 | Retrospective | 104 | 59 | 74 | HBV (72.1); HCV (12.5); Alcohol (6.7); Others (8.7) | A (41.3); B (30.8); C (27.9) | 16 or 64-slice | EVD 2.0 | Within 4 wk | 34 | 3 | 3 | 70 | |
| 36 | 10 | 1 | 63 | |||||||||||
| 32 | 4 | 5 | 69 | |||||||||||
| Kim et al[41], 2007 | Prospective | 90 | 54.8 | 72 | HBV (73.3); HCV (21.1); Alcohol (2.2); Others (3.3) | A (81.1); B (18.9) | 16-slice | Grade 2 and Grade 33 | Within 4 h | 28 | 11 | 2 | 49 | |
| 28 | 5 | 2 | 55 | |||||||||||
| 27 | 9 | 3 | 51 | |||||||||||
| 27 | 2 | 3 | 58 | |||||||||||
| Kim et al[42], 2007 | Retrospective | 67 | 56.2 | 58 | HCV (35.8); HBV (22.4); Alcohol (22.4); HBV + HCV (9.0); Others (10.4) | A (23.9); B (37.3); C (38.8) | NR | EVD 3.0 | Within 4 wk | 11 | 9 | 1 | 46 | |
| 11 | 9 | 1 | 46 | |||||||||||
| Lipp et al[43], 2011 | Retrospective | 299 | 55.2 | 65 | NR | NR | 4 or 16 or 64-slice | EVD 4.0 | Within 12 wk | 18 | 11 | 12 | 96 | |
| 12 | 4 | 22 | 127 | |||||||||||
| Perri et al[45], 2008 | Prospective | 101 | NR | 63 | Viral (21.8); Alcohol (18.8); Biliary (17.8); NASH (14.9); Others (26.7) | A (44.6); B (39.6); C (15.8) | 4-slice or higher | EVD 5.0 | 2 d2 | 23 | 5 | 18 | 55 | |
| 27 | 8 | 14 | 52 | |||||||||||
| Yu et al[47], 2011 | Retrospective | 109 | 55.9 | 55 | HCV (46.8); Alcohol (17.4); HBV (6.4); Others (29.4) | NR | 16 or 64-slice | EVD 2.0 | NR | 25 | 24 | 1 | 59 | |
| 25 | 18 | 1 | 65 | |||||||||||
| 23 | 41 | 3 | 42 | |||||||||||
| 23 | 17 | 3 | 66 | |||||||||||
MRI: According to the search strategy, 7 out of 601 articles that evaluated MRI in liver cirrhosis were included in the meta-analysis[50-56] (Figure 1C). Four manuscripts reported the diagnostic value of MRI for EV, which included 750 patients (7 groups)[50-52,54]. These studies were performed in Asia (n = 3) and Africa (n = 1). Besides, 4 articles comprising 9 groups and 1053 patients studied the predictive value of HREV[53-56], which were performed in Asia (n = 3) and Europe (n = 1).
The quality of the eligible articles is shown in Figure 2.
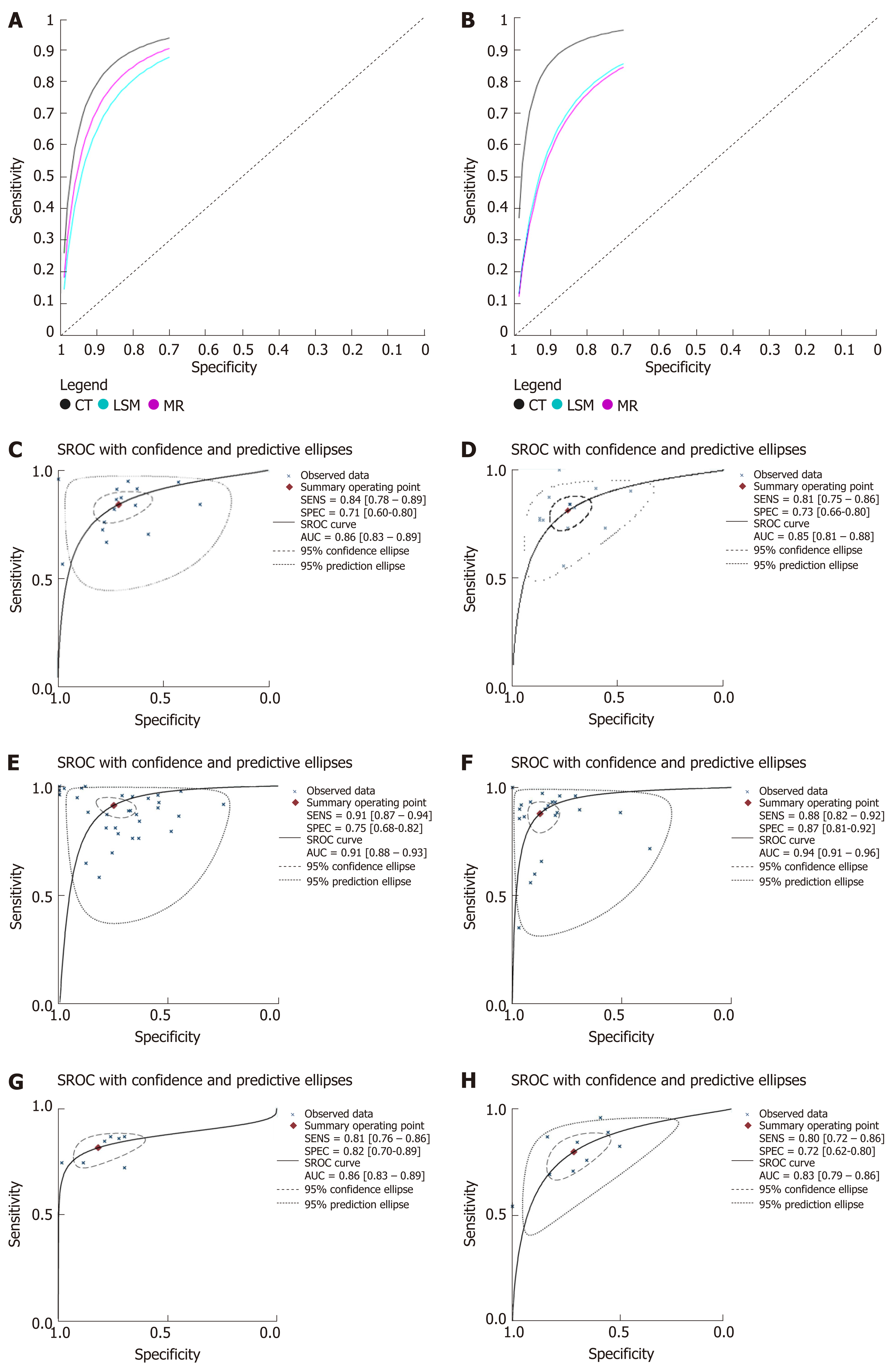
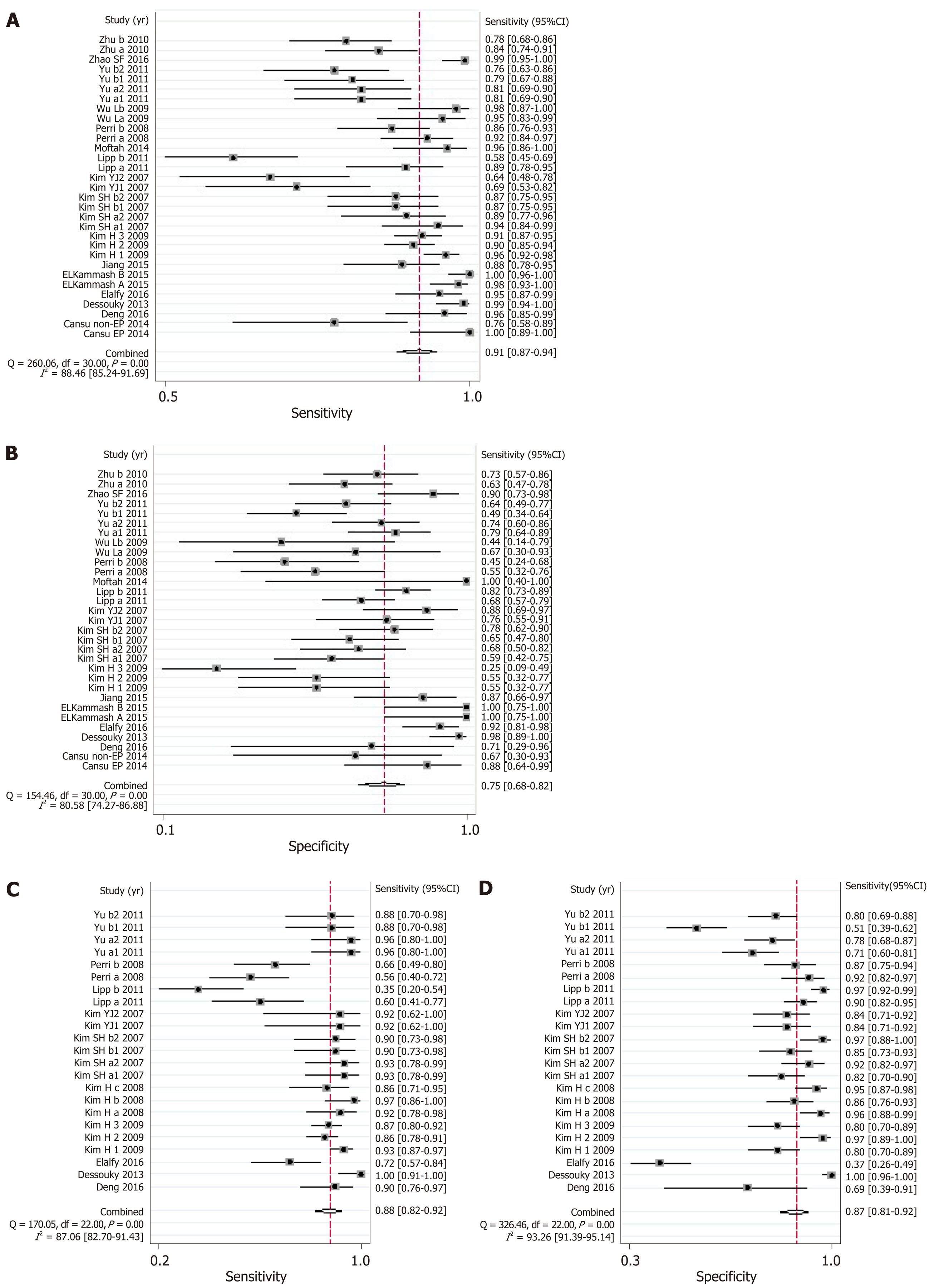
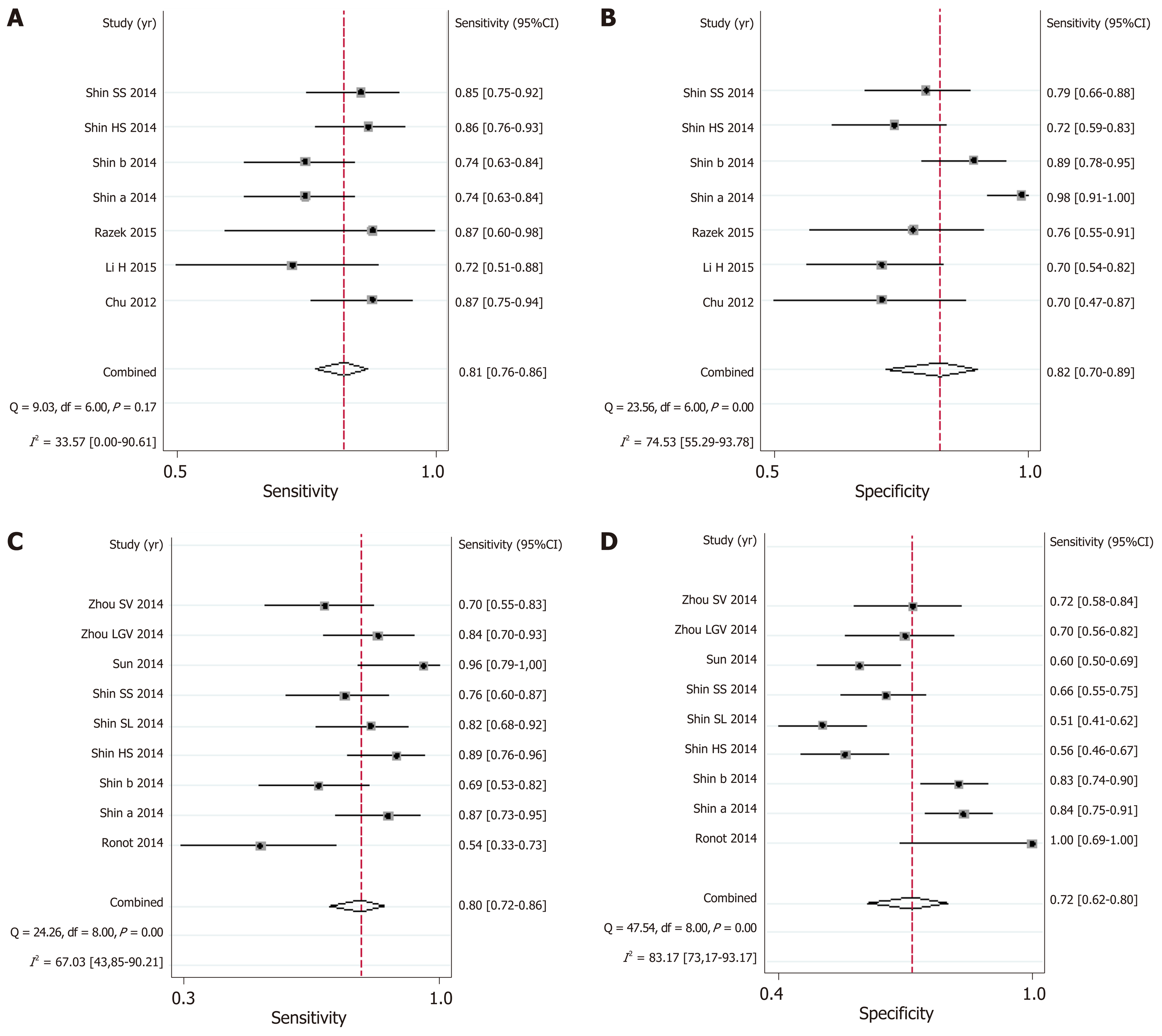
The results of meta-analysis are shown in Table 3. Significant heterogeneity was observed in all analyses (P < 0.05), except summary sensitivity in diagnosing EV and summary NLR in evaluating both EV and HREV using MRI (P > 0.05). Therefore, the random-effects model was used to combine effect quantity. Threshold effects were not found in all analyses (P > 0.05). CT had the highest AUSROC for the evaluation of EV and HREV (Figure 3A and B).
| Group | LSM for presence of EV | LSM for presence of HREV | CT for presence of EV | CT for presence of HREV | MRI for presence of EV | MRI for presence of HREV |
| Diagnostic threshold | ||||||
| Spearman correlation coefficient | 0.36 | 0.21 | -0.2 | 0.12 | 0.27 | 0.57 |
| P value | 0.19 | 0.5 | 0.27 | 0.58 | 0.56 | 0.11 |
| SROC | ||||||
| AUSROC (95%CI) | 0.86 (0.83-0.89) | 0.85 (0.81-0.88) | 0.91 (0.88-0.93) | 0.94 (0.91-0.96) | 0.86 (0.83-0.89) | 0.83 (0.79-0.86) |
| I2 | 97.43% | 97.13% | 97.17% | 98.30% | 86.41% | 91.64% |
| P value | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 |
| Sensitivity | ||||||
| Summary sensitivity (95%CI) | 0.84 (0.78-0.89) | 0.81 (0.75-0.86) | 0.91 (0.87-0.94) | 0.88 (0.82-0.92) | 0.81 (0.76-0.86) | 0.80 (0.72-0.86) |
| I2 | 82.63% | 70.93% | 88.46% | 87.06% | 33.57% | 67.03% |
| P value | < 0.01 | < 0.01 | < 0.01 | < 0.01 | 0.17 | < 0.01 |
| Specificity | ||||||
| Summary specificity (95%CI) | 0.71 (0.60-0.80) | 0.73 (0.66-0.80) | 0.75 (0.68-0.82) | 0.87 (0.81-0.92) | 0.82 (0.70-0.89) | 0.72 (0.62-0.80) |
| I2 | 86.56% | 91.65% | 80.58% | 93.26% | 74.53% | 83.17% |
| P value | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 |
| PLR | ||||||
| Summary PLR (95%CI) | 2.91 (2.08-4.06) | 3.04 (2.38-3.89) | 3.67 (2.73-4.94) | 6.90 (4.54-10.49) | 4.44 (2.74-7.21) | 2.83 (2.11-3.80) |
| I2 | 82.66% | 85.63% | 83.81% | 91.04% | 31.66% | 51.94% |
| P value | < 0.01 | < 0.01 | < 0.01 | < 0.01 | 0.01 | < 0.01 |
| NLR | ||||||
| Summary NLR (95%CI) | 0.22 (0.16-0.30) | 0.26 (0.19-0.34) | 0.12 (0.08-0.18) | 0.14 (0.09-0.21) | 0.23 (0.18-0.28) | 0.28 (0.21-0.38) |
| I2 | 79.49% | 68.30% | 88.94% | 91.10% | <0.01% | 43.01% |
| P value | < 0.01 | < 0.01 | < 0.01 | < 0.01 | 0.53 | 0.08 |
| Summary DOR (95%CI) | 13.01 (7.83-21.64) | 11.93 (7.89-18.03) | 30.98 (16.02-59.91) | 49.99 (25.38-98.43) | 19.58 (11.39-33.66) | 10.00 (6.63-15.09) |
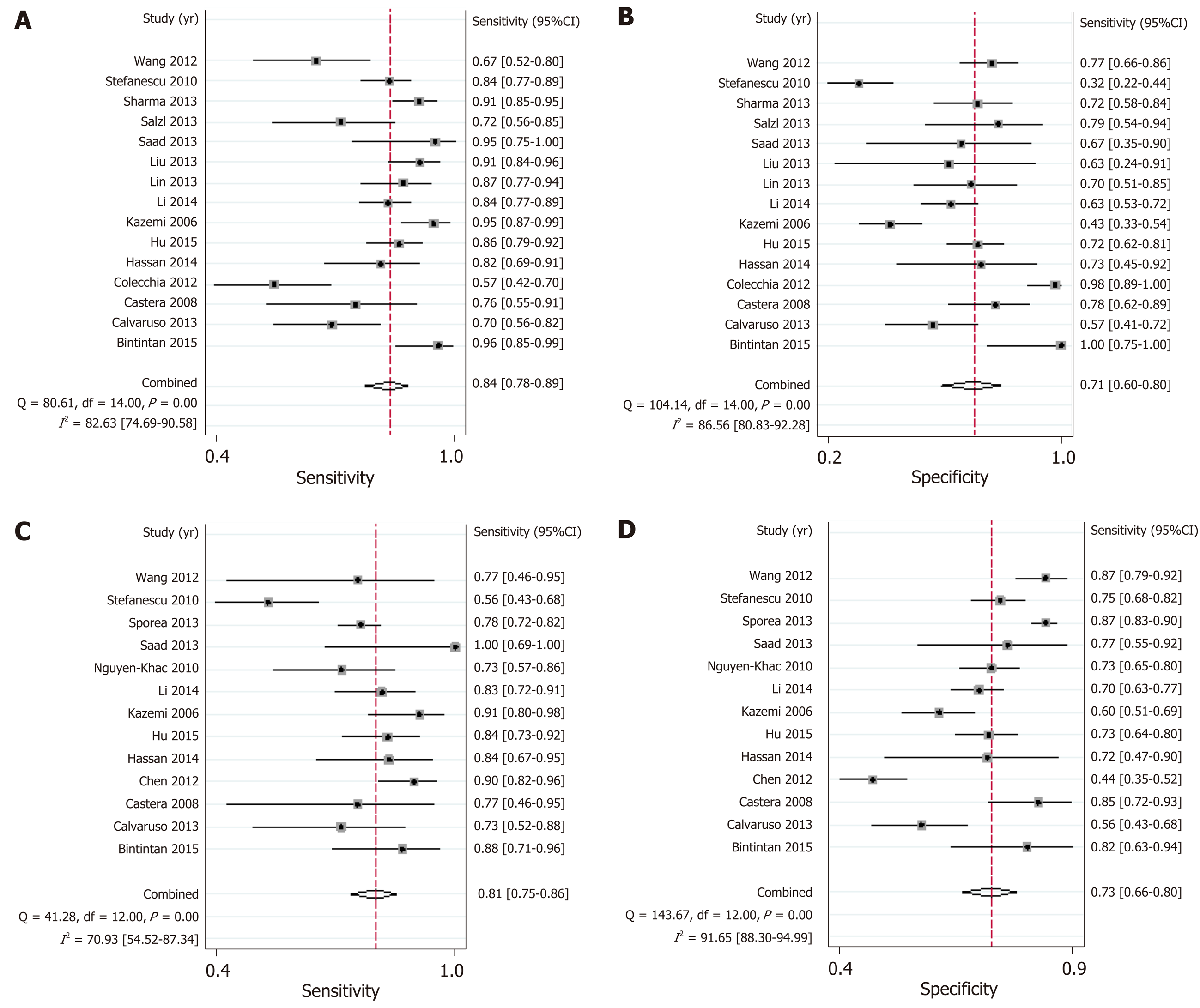
LSM: Using LSM to diagnose EV, the AUSROC was 0.86 (95%CI: 0.83-0.89, I2 = 97.43%, Figure 3C), with a summary sensitivity of 0.84 (95%CI: 0.78-0.89, I2 =82.63%; Figure 4A) and summary specificity was 0.71 (95%CI: 0.60-0.80, I2 = 86.56%; Figure 4B). The summary PLR, NLR, and DOR were 2.91 (95%CI: 2.08-4.06, I2 = 82.66%), 0.22 (95%CI: 0.16-0.30, I2 = 79.49%), and 13.01 (95%CI: 7.83-21.64; Table 3), respectively.
As for the predictive value of LSM for HREV, the AUSROC was 0.85 (95%CI: 0.81-0.88, I2 = 97.13%; Figure 3D), with a summary sensitivity of 0.81 (95%CI: 0.75-0.86, I2 = 70.93%; Figure 4C) and summary specificity of 0.73 (95%CI: 0.66-0.80, I2 = 91.65%; Figure 4D). The summary PLR, NLR, and DOR were 3.04 (95%CI: 2.38-3.89, I2 = 85.63%), 0.26 (95%CI: 0.19-0.34, I2 = 68.30%), and 11.93 (95%CI: 7.89-18.03; Table 3), respectively.
CT: The AUSROC of CT in the diagnosis of EV was 0.91 (95%CI: 0.88-0.93, I2 = 97.17%; Figure 3E), with a summary sensitivity of 0.91 (95%CI: 0.87-0.94, I2 = 88.46%) and specificity of 0.75 (95%CI: 0.68-0.82, I2 = 80.58%; Figure 5A and B). The summary PLR, NLR, and DOR were 3.67 (95%CI: 2.73-4.94, I2 = 83.81%), 0.12 (95%CI: 0.08-0.18, I2 = 88.94%), and 30.98 (95%CI: 16.02-59.91; Table 3), respectively.
The AUSROC of CT in the prediction of HREV was 0.94 (95%CI: 0.91-0.96, I2 = 98.30%; Figure 3F), with a summary sensitivity of 0.88 (95%CI: 0.82-0.92, I2 = 87.06%) and specificity of 0.87 (95%CI: 0.81-0.92, I2 = 93.26%; Figure 5C and D). The summary PLR, NLR, and DOR were 6.90 (95%CI: 4.54-10.49, I2 = 91.04%), 0.14 (95%CI: 0.09-0.21, I2 = 91.10%), and 49.99 (95%CI: 25.38-98.43; Table 3), respectively.
MRI: The AUSROC of MRI in the diagnosis of EV was 0.86 (95%CI: 0.83-0.89, I2 = 86.41%; Figure 3G), with a summary sensitivity of 0.81 (95%CI: 0.76-0.86, I2 = 33.57%) and specificity of 0.82 (95%CI: 0.70-0.89, I2 = 74.53%; Figure 6A and B). The summary PLR, NLR, and DOR were 4.44 (95%CI: 2.74-7.21, I2 = 31.66%), 0.23 (95%CI: 0.18-0.28, I2 < 0.01%), and 19.58 (95%CI: 11.36-33.66; Table 3), respectively.
As for the prediction of HREV by MRI, the AUSROC was 0.83 (95%CI: 0.79-0.86, I2 = 91.64%; Figure 3H), with a summary sensitivity of 0.80 (95%CI: 0.72-0.86, I2 = 67.03%) and specificity of 0.72 (95%CI: 0.62-0.80, I2 = 83.17%; Figure 6C and D). The summary PLR, NLR, and DOR were 2.83 (95%CI: 2.11-3.80, I2 = 51.94%), 0.28 (95%CI: 0.21-0.38, I2 = 43.01%), and 10.00 (95%CI: 6.63-15.09; Table 3), respectively.
Based on this meta-analysis, CT had higher accuracy in evaluating the presence of both EV and HREV with an AUSROC of 0.91 and 0.94, respectively.
Based on the above results, we further focused on CT for diagnosis of EV and prediction of HREV. We performed meta-regression for CT to examine the source of heterogeneity and found that the accuracy of CT in the diagnosis EV was affected by CT scanner (P < 0.05).
64-slice scanner vs 16-slice scanner in diagnosis of EV: CT performed with a 64-slice scanner showed better accuracy in EV compared with imaging performed with a 16-slice scanner [AUSROC: 0.98 (95%CI: 0.97-0.99, I2 < 0.01%) vs 0.94 (95%CI: 0.92-0.96, I2 < 0.01%); summary sensitivity: 0.98 (95%CI: 0.91-1.00, I2 = 92.01%) vs 0.94 (95%CI: 0.88-0.97, I2 = 73.98%); summary specificity: 0.94 (95%CI: 0.82-0.98, I2 = 64.69%) vs 0.78 (95%CI: 0.65-0.87, I2 = 76.48%); and summary DOR: 904.11 (95%CI: 74.85-11000) vs 50.75 (95%CI: 16.21-158.911)]. I2-values decreased and indicated that there was no significant heterogeneity.
16-slice scanner in prediction of HREV: Based on the diameter of EV, the AUSROC for prediction of HREV using 16-slice CT scanner was 0.96 (95%CI: 0.93-0.97, I2 = 40.73%). The summary sensitivity, specificity, and DOR were 0.93 (95%CI: 0.89-0.96, I2 = 17.26%), 0.94 (95%CI: 0.87-0.97, I2 = 78.08%), and 192.47 (95%CI: 71.03-521.49), respectively. There was no statistically significant heterogeneity.
According to Deeks’ funnel plot, there was no evidence of significant publication bias (P > 0.05).
Esophageal variceal hemorrhage is a catastrophic and fatal complication of PH with cirrhosis. The current “gold standard” for the diagnosis of EV and HREV is endoscopy in clinical practice. However, periodic endoscopy is expensive and uncomfortable, and therefore not easily accepted by most patients. The advantages of non-invasive diagnostic tools for evaluating EV and HREV are repeatability and better patient acceptance. We therefore performed a meta-analysis to compare the accuracy of evaluating EV and HREV by three non-invasive diagnostic methods: CT, MRI, and LSM.
In this meta-analysis, we identified 18, 17, and 7 articles evaluating the accuracy of LSM, CT, and MRI for diagnosing EV and predicting HREV, respectively. The analysis showed that CT had the highest accuracy for both EV and HREV. The AUSROC was 0.91 and 0.94, and DOR was 30.98 and 49.99 for evaluating the presence of EV and HREV. Baveno VI consensus recommends that patients with a liver stiffness < 20 kPa on transient elastography and with a platelet count > 150 × 109/L have a very low risk of having varices requiring treatment, and can avoid screening endoscopy. In studies that validate the criteria, up to 100% of patients who met the criteria had an ultimately negative endoscopy, but it showed a relatively low specificity of 61.5%[57]. Rosman et al[58] investigated the utility of incorporating the CT or MR findings of portosystemic collateral vessels to predict HREV in patients who did not meet Baveno VI criteria. The presence of portosystemic collateral vessels to predict HREV yielded a sensitivity of 0.95 and specificity of 0.36 in these patients. Therefore, the use of additional portosystemic collateral vessels from CT or MRI can further help identify patients with compensatory cirrhosis who do not require endoscopy. The weakness of LSM using transient elastography is decreased applicability in obese patients and patients with ascites. Lipp et al[43] evaluated the ability of CT and MRI to detect EV and found that CT is a superior imaging modality to MRI. According to a meta-analysis performed by Deng et al[7], Lok score had the highest AUSROC of 0.79, followed by FIB-4, Forns, aspartate aminotransferase-to-alanine aminotransferase ratio, and aspartate aminotransferase-to-platelet ratio, for the diagnosis of EV. Aspartate aminotransferase-to-alanine aminotransferase ratio had the highest AUSROC of 0.74, followed by aspartate aminotransferase-to-platelet ratio, Lok, FIB-4, and Forns scores for the prediction of HREV. A significant heterogeneity (I2 ranged from 86.41% to 98.30%) was found in their meta-analysis. The CT scanner was significantly associated with heterogeneity in diagnosing EV. Subgroup analysis suggested that the accuracy of CT scanner with more slices was critical for diagnosing EV.
Compared with endoscopy, contrast-enhanced CT or MRI can clearly show the portal vein system and collateral circulation[59,60]. In addition to EV, they can be used for the diagnosis of other complications including hepatocellular carcinoma[61,62]. There is no doubt that endoscopy is irreplaceable. It can diagnose esophageal and gastric varices as well as other lesions that cause upper gastrointestinal bleeding, such as peptic ulcer. Combined with the ultrasound probe, it was applied to probe the blood vessels around the wall of the esophagus. Zheng et al[63] evaluated endoscopic ultrasound probe examinations for the prediction of recurrence of EV after endoscopic therapies by detecting peri-esophageal collateral veins, perforating veins, and para-esophageal collateral veins. The result showed that peri-esophageal collateral veins can predict 1-year variceal recurrence with a sensitivity of 45% and specificity of 86% when using a diameter of 3.5 mm as cut-off value.
There are several limitations of our analysis that should be taken into consideration. First, we searched the databases for articles only written in English and Chinese, which may miss some articles written in other languages. Second, though the Deek’s funnel plot asymmetry test showed no evidence of significant publication bias, there are probably studies of negative outcomes which have not been published. These research results may be missed. Third, the included articles had different definitions or cut-off values of HREV. Thus, no standard diagnostic thresholds for CT, MRI, and LSM were defined. Finally, we regarded endoscopy currently as the “gold standard” for diagnosing EV and HREV, nevertheless, there was no head-to-head controlled study of the above-mentioned non-invasive diagnostic methods in the same series of patients. This indirect comparison brought to a statistical bias, thus might attribute to study heterogeneity. Despite the limitations, new analysis techniques of radiomics are likely to improve diagnostic and predictive accuracy of many diseases. Choi et al[64] developed a deep learning system for accurate staging of liver fibrosis using CT. These promising results should initiate further studies on CT using artificial intelligence and machine learning technology to reduce the need for endoscopy.
In conclusion, based on this meta-analysis, CT has higher accuracy for evaluating both EV and HREV in cirrhotic patients. However, further head-to-head comparisons of these noninvasive diagnostic tools are required to confirm the predictive value in EV and HREV, particularly in view of the future use of artificial intelligence technology.
The non-invasive and easy-to-perform diagnostic techniques to predict complications in cirrhotic patients are required in clinical practice. Up to now, the clinical use of liver stiffness measurement (LSM), computed tomography (CT), and magnetic resonance imaging (MRI), as non-invasive diagnostic methods to diagnose esophageal varices (EV) and to predict high-bleeding-risk EV (HREV) in cirrhotic patients, is controversial.
The LSM, CT, and MRI for the diagnosis of EV and prediction of HREV, promising non-invasive diagnostic methods to predict complications in cirrhotic patients, are required in clinical practice. However, the accuracy, sensitivity, and specificity varied in different studies. The overall accuracy, sensitivity, and specificity of LSM, CT, and MRI in the diagnosis of EV and prediction of HREV in cirrhotic patients have not stated.
This is a very important and interesting systematic review and meta-analysis aimed to determine the overall accuracy and sensitivity of three non-invasive methods to diagnose EV and predict the risk of bleeding in patients with liver cirrhosis.
We performed literature searches by using selected keywords in PubMed, Embase, Cochrane, CNKI, and Wanfang databases for full-text articles published in English and Chinese. All statistical analyses were conducted using Stata12.0, MetaDisc1.4, and RevMan5.3. Summary sensitivity and specificity, positive likelihood ratio and negative likelihood ratio, diagnostic odds ratio, and the area under the summary receiver operating characteristic curves that evaluated the accuracy of LSM, CT, and MRI as candidates for diagnosing EV and predicting HREV in cirrhotic patients were analyzed. The random-effects model was used to combine effect quantity. The quality of the articles was assessed using the quality assessment of diagnostic accuracy studies-2 tool. Heterogeneity was examined by Q-statistic test and I2 index, and sources of heterogeneity were explored using meta-regression and subgroup analysis. Publication bias was evaluated using Deek’s funnel plot.
Overall, 18, 17, and 7 relevant articles on the accuracy of LSM, CT, and MRI in diagnosing EV and predicting HREV were retrieved. CT had higher accuracy than LSM and MRI in diagnosing EV and predicting HREV with areas under the summary receiver operating characteristic curves of 0.91 (95%CI: 0.88-0.93) and 0.94 (95%CI: 0.91-0.96), respectively. The sensitivities of LSM, CT, and MRI in diagnosing EV and predicting HREV were 0.84 (95%CI: 0.78-0.89), 0.91 (95%CI: 0.87-0.94), and 0.81 (95%CI: 0.76-0.86), and 0.81 (95%CI: 0.75-0.86), 0.88 (95%CI: 0.82-0.92), and 0.80 (95%CI: 0.72-0.86), respectively. The specificities were 0.71 (95%CI: 0.60-0.80), 0.75 (95%CI: 0.68-0.82), and 0.82 (95%CI: 0.70-0.89), and 0.73 (95%CI: 0.66-0.80), 0.87 (95%CI: 0.81-0.92), and 0.72 (95%CI: 0.62-0.80) , respectively. The positive likelihood ratios were 2.91, 3.67, and 4.44, and 3.04, 6.90, and 2.83, respectively. The negative likelihood ratios were 0.22, 0.12, and 0.23, and 0.26, 0.14, and 0.28, respectively. The diagnostic odds ratios were 13.01, 30.98, and 19.58, and 11.93, 49.99, and 10.00, respectively. A significant heterogeneity was observed in all analyses (P < 0.05). CT scanner was identified to be the source of heterogeneity. There was no significant difference in diagnostic threshold effects (P > 0.05) or publication bias (P > 0.05). To determine the risk for bleeding of EV using a non-invasive method might have important clinical applications in daily practice. The study gives an overall view of the problem, and for sure does give clinical details which could be useful in making decisions in everyday practice.
Based on the meta-analysis of observational studies, CT has higher accuracy in evaluating EV and HREV than LSM and MRI in cirrhotic patients. It is suggested that CT, a non-invasive diagnostic method, is the best choice for the diagnosis of EV and prediction of HREV in cirrhotic patients compared with LSM and MRI.
The results are very important with significant applications for clinicians in making decisions in daily practice for treatment of cirrhotic patients with portal hypertension. In future, the head-to-head or direct comparisons of these non-invasive methods in the same series of patients are required to confirm the predictive value, especially by using artificial intelligence technique.
We acknowledge the support in statistical methods review provided by Xiang-Yu Yan, PhD, Capital Medical University School of Public Health, Beijing, China.
| 1. | Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 2470] [Article Influence: 352.9] [Reference Citation Analysis (1)] |
| 2. | Pateu E, Oberti F, Calès P. The noninvasive diagnosis of esophageal varices and its application in clinical practice. Clin Res Hepatol Gastroenterol. 2018;42:6-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Buechter M, Kahraman A, Manka P, Gerken G, Jochum C, Canbay A, Dechêne A. Spleen and Liver Stiffness Is Positively Correlated with the Risk of Esophageal Variceal Bleeding. Digestion. 2016;94:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2609] [Cited by in RCA: 2359] [Article Influence: 214.5] [Reference Citation Analysis (4)] |
| 5. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1504] [Article Influence: 167.1] [Reference Citation Analysis (3)] |
| 6. | Li Y, Li P, Zhang YN, Ding HG. [Strategy for diagnosis and precise treatment of portal hypertension with hepatic venous pressure gradient: a dream or a reality?]. Zhonghua Gan Zang Bing Za Zhi. 2018;26:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Deng H, Qi X, Guo X. Diagnostic Accuracy of APRI, AAR, FIB-4, FI, King, Lok, Forns, and FibroIndex Scores in Predicting the Presence of Esophageal Varices in Liver Cirrhosis: A Systematic Review and Meta-Analysis. Medicine (Baltimore). 2015;94:e1795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Wasfy E, Elkassas G, Elnawasany S, Elkasrawy K, Abd-Elsalam S, Soliman S, Badawi R. Predicting Esophageal Varices in Cirrhotic Hepatitis C Virus Patients Using Noninvasive Measurement of Insulin Resistance Variables. Endocr Metab Immune Disord Drug Targets. 2018;18:573-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 48670] [Article Influence: 2862.9] [Reference Citation Analysis (3)] |
| 10. | Chinese Society of Gastroenterology; Chinese Society of Hepatology and Chinese Society of Endoscopy, Chinese Medical Association. Consensus on prevention and treatment for gastroesophageal varices and variceal hemorrhage in liver cirrhosis. Zhonghua Gan Zang Bing Za Zhi. 2008;16:564–570. [DOI] [Full Text] |
| 11. | Idezuki Y. General rules for recording endoscopic findings of esophagogastric varices (1991). Japanese Society for Portal Hypertension. World J Surg. 1995;19:420-2; discussion 423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 142] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1229] [Cited by in RCA: 1221] [Article Influence: 64.3] [Reference Citation Analysis (2)] |
| 13. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 48634] [Article Influence: 2114.5] [Reference Citation Analysis (4)] |
| 14. | Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1792] [Cited by in RCA: 2331] [Article Influence: 111.0] [Reference Citation Analysis (4)] |
| 15. | Bintintan A, Chira RI, Bintintan VV, Nagy GA, Manzat-Saplacan MR, Lupsor-Platon M, Stefanescu H, Duma MM, Valean SD, Mircea PA. Value of hepatic elastography and Doppler indexes for predictions of esophageal varices in liver cirrhosis. Med Ultrason. 2015;17:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Calvaruso V, Bronte F, Conte E, Simone F, Craxì A, Di Marco V. Modified spleen stiffness measurement by transient elastography is associated with presence of large oesophageal varices in patients with compensated hepatitis C virus cirrhosis. J Viral Hepat. 2013;20:867-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Castéra L, Le Bail B, Roudot-Thoraval F, Bernard PH, Foucher J, Merrouche W, Couzigou P, de Lédinghen V. Early detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: comparison of transient elastography (FibroScan) with standard laboratory tests and non-invasive scores. J Hepatol. 2009;50:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 273] [Article Influence: 16.1] [Reference Citation Analysis (1)] |
| 18. | Chen YP, Zhang Q, Dai L, Liang XE, Peng J, Hou JL. Is transient elastography valuable for high-risk esophageal varices prediction in patients with hepatitis-B-related cirrhosis? J Gastroenterol Hepatol. 2012;27:533-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Colecchia A, Montrone L, Scaioli E, Bacchi-Reggiani ML, Colli A, Casazza G, Schiumerini R, Turco L, Di Biase AR, Mazzella G, Marzi L, Arena U, Pinzani M, Festi D. Measurement of spleen stiffness to evaluate portal hypertension and the presence of esophageal varices in patients with HCV-related cirrhosis. Gastroenterology. 2012;143:646-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 391] [Article Influence: 27.9] [Reference Citation Analysis (1)] |
| 20. | Hassan EM, Omran DA, El Beshlawey ML, Abdo M, El Askary A. Can transient elastography, Fib-4, Forns Index, and Lok Score predict esophageal varices in HCV-related cirrhotic patients? Gastroenterol Hepatol. 2014;37:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Hu Z, Li Y, Li C, Huang C, Ou Z, Guo J, Luo H, Tang X. Using Ultrasonic Transient Elastometry (FibroScan) to Predict Esophageal Varices in Patients with Viral Liver Cirrhosis. Ultrasound Med Biol. 2015;41:1530-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Kazemi F, Kettaneh A, N'kontchou G, Pinto E, Ganne-Carrie N, Trinchet JC, Beaugrand M. Liver stiffness measurement selects patients with cirrhosis at risk of bearing large oesophageal varices. J Hepatol. 2006;45:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 269] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 23. | Li F, Yan T, Shao Q, Ji D, Li B, Li Z, Chen G. [Clinical study of FibroScan efficiency for diagnosing size of oesophageal varices in liver cirrhosis patients]. Zhonghua Gan Zang Bing Za Zhi. 2014;22:600-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 24. | Lin X, Shang G, Qi C, Chang X, Wei M, Chen W. Measurement of liver and spleen stiffness by transient elastography and its relationship with esophageal varices. Zhonghua Xiao Hua Za Zhi. 2018;38:129-131. [DOI] [Full Text] |
| 25. | Liu F, Li TH, Han T, Xiang HL, Zhang HS. [Non-invasive assessment of portal hypertension in patients with liver cirrhosis using FibroScan transient elastography]. Zhonghua Gan Zang Bing Za Zhi. 2013;21:840-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 26. | Nguyen-Khac E, Saint-Leger P, Tramier B, Coevoet H, Capron D, Dupas JL. Noninvasive diagnosis of large esophageal varices by FibroScan: strong influence of the cirrhosis etiology. Alcohol Clin Exp Res. 2010;34:1146-1153. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Saad Y, Said M, Idris MO, Rabee A, Zakaria S. Liver stiffness measurement by fibroscan predicts the presence and size of esophageal varices in egyptian patients with HCV related liver cirrhosis. J Clin Diagn Res. 2013;7:2253-2257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Salzl P, Reiberger T, Ferlitsch M, Payer BA, Schwengerer B, Trauner M, Peck-Radosavljevic M, Ferlitsch A. Evaluation of portal hypertension and varices by acoustic radiation force impulse imaging of the liver compared to transient elastography and AST to platelet ratio index. Ultraschall Med. 2014;35:528-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Sharma P, Kirnake V, Tyagi P, Bansal N, Singla V, Kumar A, Arora A. Spleen stiffness in patients with cirrhosis in predicting esophageal varices. Am J Gastroenterol. 2013;108:1101-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 30. | Sporea I, Raţiu I, Bota S, Şirli R, Jurchiş A. Are different cut-off values of liver stiffness assessed by transient elastography according to the etiology of liver cirrhosis for predicting significant esophageal varices? Med Ultrason. 2013;15:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Stefanescu H, Grigorescu M, Lupsor M, Maniu A, Crisan D, Procopet B, Feier D, Badea R. A new and simple algorithm for the noninvasive assessment of esophageal varices in cirrhotic patients using serum fibrosis markers and transient elastography. J Gastrointestin Liver Dis. 2011;20:57-64. [PubMed] |
| 32. | Wang JH, Chuah SK, Lu SN, Hung CH, Chen CH, Kee KM, Chang KC, Tai WC, Hu TH. Transient elastography and simple blood markers in the diagnosis of esophageal varices for compensated patients with hepatitis B virus-related cirrhosis. J Gastroenterol Hepatol. 2012;27:1213-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Cansu A, Ahmetoglu A, Kul S, Yukunc G, Fidan S, Arslan M, Topbas M. Diagnostic performance of using effervescent powder for detection and grading of esophageal varices by multi-detector computed tomography. Eur J Radiol. 2014;83:497-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Deng H, Qi X, Zhang Y, Peng Y, Li J, Guo X. Diagnostic accuracy of contrast-enhanced computed tomography for esophageal varices in liver cirrhosis: a retrospective observational study. J Evid Based Med. 2017;10:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Dessouky BA, Abdel Aal el SM. Multidetector CT oesophagography: an alternative screening method for endoscopic diagnosis of oesophageal varices and bleeding risk. Arab J Gastroenterol. 2013;14:99-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Elalfy H, Elsherbiny W, Abdel Rahman A, Elhammady D, Shaltout SW, Elsamanoudy AZ, El Deek B. Diagnostic non-invasive model of large risky esophageal varices in cirrhotic hepatitis C virus patients. World J Hepatol. 2016;8:1028-1037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Elkammash T, Elfiky I, Zaiton F, Khorshed SE. Diagnostic performance of multidetector computed tomography in the evaluation of esoph ageal varices. Egypt J Radiol Nucl Med. 2016;47:43-51. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Jiang BT, Su Y, Li RH, Tao Y, Zhou Z, Xin GQ, Zhang P, Yan YT, Hu ZX. Study on gastroscopy and multi-slice spiral CT for the value of clinical diagnosis and treatment in cirrhotic esophageal and gastric varices. Zhonghua Xiao Hua Bing Yu Ying Xiang Za Zhi. 2015;5:25-27. |
| 39. | Kim H, Choi D, Gwak GY, Lee JH, Lee SJ, Kim SH, Lee JY, Park Y, Chang I, Lim HK. High-risk esophageal varices in patients treated with locoregional therapies for hepatocellular carcinoma: evaluation with regular follow-up liver CT. Dig Dis Sci. 2009;54:2247-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Kim H, Choi D, Gwak GY, Lee JH, Park MK, Lee HIe, Kim SH, Nam S, Yoo EY, Do YS. Evaluation of esophageal varices on liver computed tomography: receiver operating characteristic analyses of the performance of radiologists and endoscopists. J Gastroenterol Hepatol. 2009;24:1534-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Kim SH, Kim YJ, Lee JM, Choi KD, Chung YJ, Han JK, Lee JY, Lee MW, Han CJ, Choi JI, Shin KS, Choi BI. Esophageal varices in patients with cirrhosis: multidetector CT esophagography--comparison with endoscopy. Radiology. 2007;242:759-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Kim YJ, Raman SS, Yu NC, To'o KJ, Jutabha R, Lu DS. Esophageal varices in cirrhotic patients: evaluation with liver CT. AJR Am J Roentgenol. 2007;188:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 43. | Lipp MJ, Broder A, Hudesman D, Suwandhi P, Okon SA, Horowitz M, Clain DJ, Friedmann P, Min AD. Detection of esophageal varices using CT and MRI. Dig Dis Sci. 2011;56:2696-2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Moftah SG, Kamal S, Hanna ATK. CT esophagography: non invasive screening and grading of esophageal varices in cirrhosis. Egypt J Radiol Nucl Med. 2014;45:263-270. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Perri RE, Chiorean MV, Fidler JL, Fletcher JG, Talwalkar JA, Stadheim L, Shah ND, Kamath PS. A prospective evaluation of computerized tomographic (CT) scanning as a screening modality for esophageal varices. Hepatology. 2008;47:1587-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 46. | Wu L, Fan ZP, Gu HY, Feng Q. Value of multislice spiral CT in detecting and grading of esophageal varices in cirrhotic patients. Weichang Bing Xue. 2009;14:12-15. [DOI] [Full Text] |
| 47. | Yu NC, Margolis D, Hsu M, Raman SS, Lu DS. Detection and grading of esophageal varices on liver CT: comparison of standard and thin-section multiplanar reconstructions in diagnostic accuracy. AJR Am J Roentgenol. 2011;197:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (2)] |
| 48. | Zhao SF, Feng K, Qu QY, Cui M, Wang Y, Tan J, Song M. Application of CT portography in esophagogastric varices in cirrhotic patients. Weichang Bing Xue. 2016;21:615-619. [DOI] [Full Text] |
| 49. | Zhu K, Meng X, Pang P, Qian J, Shen M, Hu B, Shan H. Gastric varices in patients with portal hypertension: evaluation with multidetector row CT. J Clin Gastroenterol. 2010;44:e108-e115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Chu CQ, Li BC. Clinical value of enhanced T1 high resolution isotropic volume examination series of magnetic resonance imaging in collateral vessels of esophageal gastric varices. Zhonghua Xiao Hua Bing Yu Ying Xiang Za Zhi. 2012;2:14-17. [DOI] [Full Text] |
| 51. | Li H, Chen TW, Li ZL, Zhang XM, Li CJ, Chen XL, Chen GW, Hu JN, Ye YQ. Albumin and magnetic resonance imaging-liver volume to identify hepatitis B-related cirrhosis and esophageal varices. World J Gastroenterol. 2015;21:988-996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Razek AA, Massoud SM, Azziz MR, El-Bendary MM, Zalata K, Motawea EM. Prediction of esophageal varices in cirrhotic patients with apparent diffusion coefficient of the spleen. Abdom Imaging. 2015;40:1465-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 53. | Ronot M, Lambert S, Elkrief L, Doblas S, Rautou PE, Castera L, Vilgrain V, Sinkus R, Van Beers BE, Garteiser P. Assessment of portal hypertension and high-risk oesophageal varices with liver and spleen three-dimensional multifrequency MR elastography in liver cirrhosis. Eur Radiol. 2014;24:1394-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 54. | Shin SU, Lee JM, Yu MH, Yoon JH, Han JK, Choi BI, Glaser KJ, Ehman RL. Prediction of esophageal varices in patients with cirrhosis: usefulness of three-dimensional MR elastography with echo-planar imaging technique. Radiology. 2014;272:143-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 55. | Sun HY, Lee JM, Han JK, Choi BI. Usefulness of MR elastography for predicting esophageal varices in cirrhotic patients. J Magn Reson Imaging. 2014;39:559-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Zhou HY, Chen TW, Zhang XM, Zeng NL, Zhou L, Tang HJ, Wang D, Jian S, Liao J, Xiang JY, Hu J, Zhang Z. Diameters of left gastric vein and its originating vein on magnetic resonance imaging in liver cirrhosis patients with hepatitis B: Association with endoscopic grades of esophageal varices. Hepatol Res. 2014;44:E110-E117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Sousa M, Fernandes S, Proença L, Silva AP, Leite S, Silva J, Ponte A, Rodrigues J, Silva JC, Carvalho J. The Baveno VI criteria for predicting esophageal varices: validation in real life practice. Rev Esp Enferm Dig. 2017;109:704-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 58. | Rosman M, Aneke-Nash C, Whitsett M, Jacobson I M. The utility of radiographic evidence of portosystemic collateral vessels to improve the Baveno VI consensus recommendations in the non-invasive prediction of esophageal varices. Gastroenterology. 2019;156:1334. [DOI] [Full Text] |
| 59. | Palaniyappan N, Cox E, Bradley C, Scott R, Austin A, O'Neill R, Ramjas G, Travis S, White H, Singh R, Thurley P, Guha IN, Francis S, Aithal GP. Non-invasive assessment of portal hypertension using quantitative magnetic resonance imaging. J Hepatol. 2016;65:1131-1139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 60. | Januszewicz MM, Hałaburda-Rola M, Pruszyńska-Włodarczyk I, Czachór-Zielińska A, Rowiński O. Computed tomography evaluation of patent paraumbilical vein and its aneurysm in relation to other portosystemic collateral channels in patients with liver cirrhosis and portal hypertension. Pol J Radiol. 2019;84:e112-e117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Kim SY, An J, Lim YS, Han S, Lee JY, Byun JH, Won HJ, Lee SJ, Lee HC, Lee YS. MRI With Liver-Specific Contrast for Surveillance of Patients With Cirrhosis at High Risk of Hepatocellular Carcinoma. JAMA Oncol. 2017;3:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 284] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 62. | Li JP, Feng GL, Li DQ, Wang HB, Zhao DL, Wan Y, Jiang HJ. Detection and differentiation of early hepatocellular carcinoma from cirrhosis using CT perfusion in a rat liver model. Hepatobiliary Pancreat Dis Int. 2016;15:612-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 63. | Zheng J, Zhang Y, Li P, Zhang S, Li Y, Li L, Ding H. The endoscopic ultrasound probe findings in prediction of esophageal variceal recurrence after endoscopic variceal eradication therapies in cirrhotic patients: a cohort prospective study. BMC Gastroenterol. 2019;19:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 64. | Choi KJ, Jang JK, Lee SS, Sung YS, Shim WH, Kim HS, Yun J, Choi JY, Lee Y, Kang BK, Kim JH, Kim SY, Yu ES. Development and Validation of a Deep Learning System for Staging Liver Fibrosis by Using Contrast Agent-enhanced CT Images in the Liver. Radiology. 2018;289:688-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 163] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Manenti A, Sterpetti AV S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Zhang YL