Published online May 7, 2020. doi: 10.3748/wjg.v26.i17.2040
Peer-review started: February 9, 2020
First decision: February 27, 2020
Revised: March 25, 2020
Accepted: April 15, 2020
Article in press: April 15, 2020
Published online: May 7, 2020
Processing time: 87 Days and 7.6 Hours
Hepatocellular carcinoma (HCC) is the most common primary liver tumor and has been considered a very immunogenic tumor. The treatment with radiofrequency ablation (RFA) has been established as the standard ablative therapy for early HCC, and is currently recognized as the main ablative tool for HCC tumors < 5 cm in size; however, progression and local recurrence remain the main disadvantages of this approach. To solve this clinical problem, recent efforts were concentrated on multimodal treatment, combining different strategies, including the combination of RFA and immunotherapy. This article reviewed the combination treatment of RFA with immunotherapy and found that this treatment strategy leads to an increased response of anti-tumor T cells, significantly reduces the risk of recurrence and improves survival rates compared to RFA alone. This review highlighted scientific evidence that supports the current recommendations for pre-clinical studies, and discuss the need for further research on this topic.
Core tip: Radiofrequency ablation (RFA) is a well-established surgical approach to treat hepatocellular carcinoma (HCC). However, it has the inconvenience of being related to tumor recurrence. The combination of RFA and immunotherapy has emerged as a promising approach to activate and potentialize local and systemic immune responses. It has potential to reinstate anti-tumor immunity in HCC through increase in antitumor T cell response. The approach seems quite pragmatic in limiting the recurrence and improving survival rates compared to RFA alone for HCC patients. Currently, there are only eight articles in the literature that studied the combination of RFA and immunotherapy.
- Citation: da Costa AC, Sodergren M, Jayant K, Santa Cruz F, Spalding D, Pai M, Habib N. Radiofrequency combined with immunomodulation for hepatocellular carcinoma: State of the art and innovations. World J Gastroenterol 2020; 26(17): 2040-2048
- URL: https://www.wjgnet.com/1007-9327/full/v26/i17/2040.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i17.2040
Hepatocellular carcinoma (HCC) is the most common primary liver tumor[1]. It currently represents the sixth most common cancer worldwide and second major cause of cancer-related deaths[2]. The incidence of HCC varies widely according to geographic location, and death rates are increasing in many parts of the world, including North America, Latin America, and central Europe[3,4]. The most common risk factors for HCC include chronic hepatitis B and C, alcohol consumption, metabolic liver disease (particularly nonalcoholic fatty liver disease) and exposure to dietary toxins, such as aflatoxins and aristolochic acid, indicating that persistent inflammation is involved in its carcinogenesis[5].
Radiofrequency ablation (RFA) is widely accepted as a first-line interventional oncology approach for HCC and has the advantages of high treatment efficacy and low risk of complications[6]. RFA causes destruction of the tumor through the induction of hyperthermia, and can be used to directly treat the liver tumors or to assist the liver resection[7,8]. The destruction of the tumor mediated by RFA may provide a way to the induction, in vivo, of the antitumoral immunity, through antigens presentation and maturation of the dendritic cells, having an action in resemblance to the vaccine[9]. The current evidence indicates that RFA leads to positive effects on the antitumoral immunity, not only at a local level, but also triggering systemic immune responses[9]. It has also been demonstrated an increase of CD4+ and CD8+ T cells in peritumoral area and on the peripheral blood[10]. Moreover, it is observed an increased number of pro-inflammatory agents, immunostimulators, such as IFNγ, and expression of BDCA-3+/B7-H3- dendritic cells[11]. Although this immunologic response is not enough to avoid recurrence by its own, it has potential to bring enhanced and synergistic immune response in combination with other immunomodulators, such as checkpoint inhibitors[10].
Recently, it has been observed that disease progression rates and local recurrence of HCC are high following RFA as monotherapy, particularly for larger tumors (> 3 cm)[6]. To solve this clinical problem, recent efforts have been made to improve the HCC multimodal treatment, combining different strategies, including the combination of RFA and immunotherapy. The intent of combination therapy is to achieve better outcomes in comparison to the RFA alone, in terms of improving survival rates and avoiding recurrence[6,12,13]. The association between RFA and immunomodulators induce a synergistic anticancer immune response as observed in pre-clinical studies, which seems quite promising to the future of oncological treatment[12].
This review sought to analyze the current recommendations of combined therapies for HCC with RFA and immunomodulation, and discuss the need for further research on this topic.
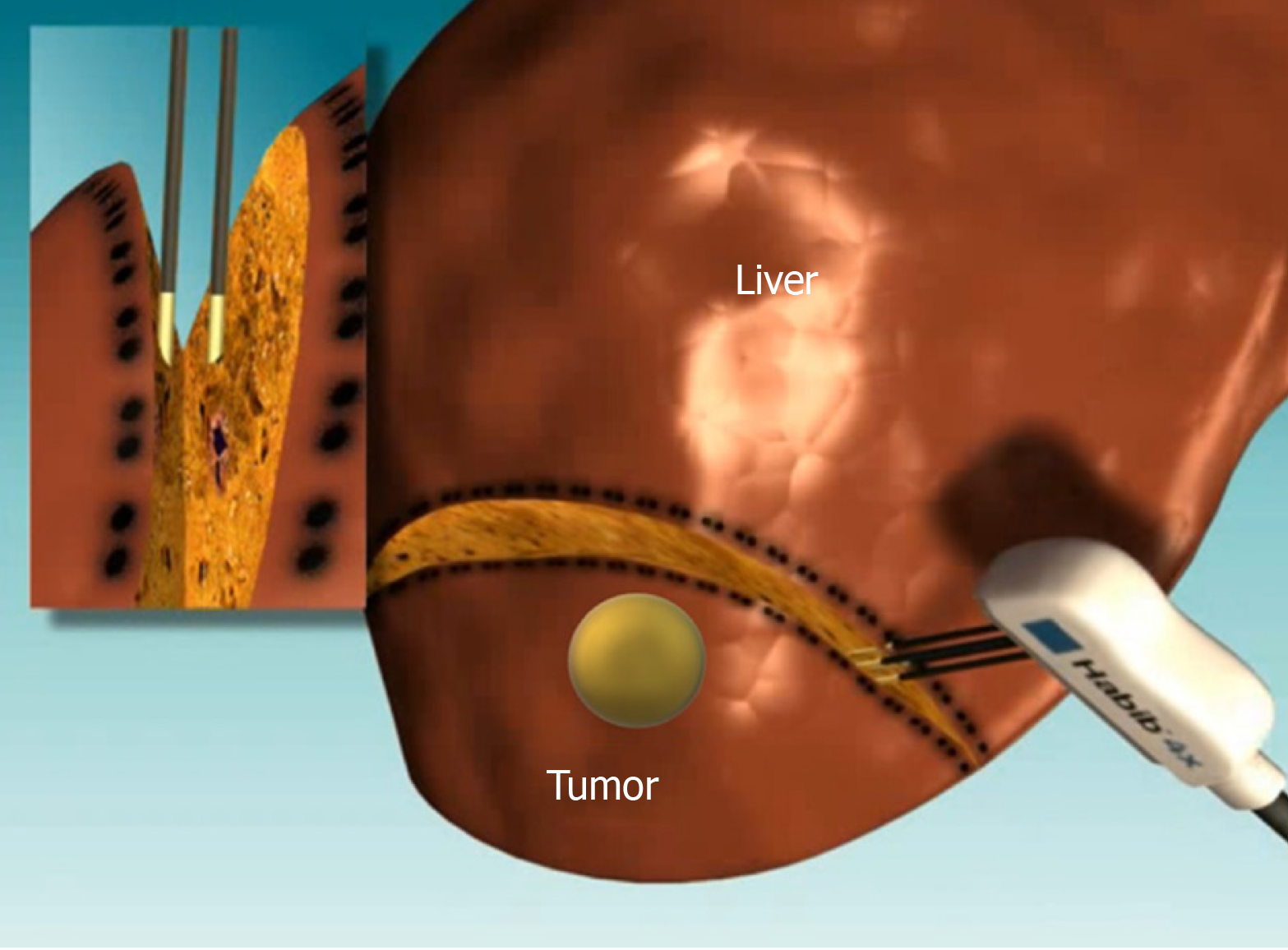
RFA technique involves application of high-frequency oscillating electrical currents to generate heat around the probe, which results in tissue hyperthermia (60-100 ºC) and coagulative necrosis (Figure 1)[2]. RFA has been established as standard ablative therapy for early stage HCC, being currently recognized as the main ablative tool for HCC tumors < 5 cm in size[1,6,14]. However, the recurrence and local progression rates of HCC increases after treatment with RFA in instances of bigger lesions; this remains as the biggest challenge of RFA[6]. Hence, RFA is not recommended to ablate tumors bigger than 5 cm[6,14]. Furthermore, more aggressive ablative approaches are associated with an increased risk of injuries because of unwanted thermal damage to important adjacent structures, such as the bile duct, gallbladder, diaphragm and intestinal tracts[15,16].
Recently, a multicenter study including 18296 patients with primary HCC (8211 undergoing RFA, 10085 undergoing hepatic resection), compared the effectiveness of RFA with surgical resection and outlined superior post-treatment outcomes following RFA[17]. However, several other randomized controlled clinical trials have compared RFA to surgical resection with ambiguous results[18-20]. While some studies reported comparable effectiveness regarding overall and recurrence-free survival[18], others have highlighted superior outcomes following surgical resection[19,20].
The natural immune system has potent antitumor action, which can be used to limit and treat hepatic tumors. The main purpose of immunotherapy is to enhance the expression of tumor-specific CD8+ effector-/CD4- helper T cells and the reinvigoration of exhausted T cells at the site of the tumor nodules[21].
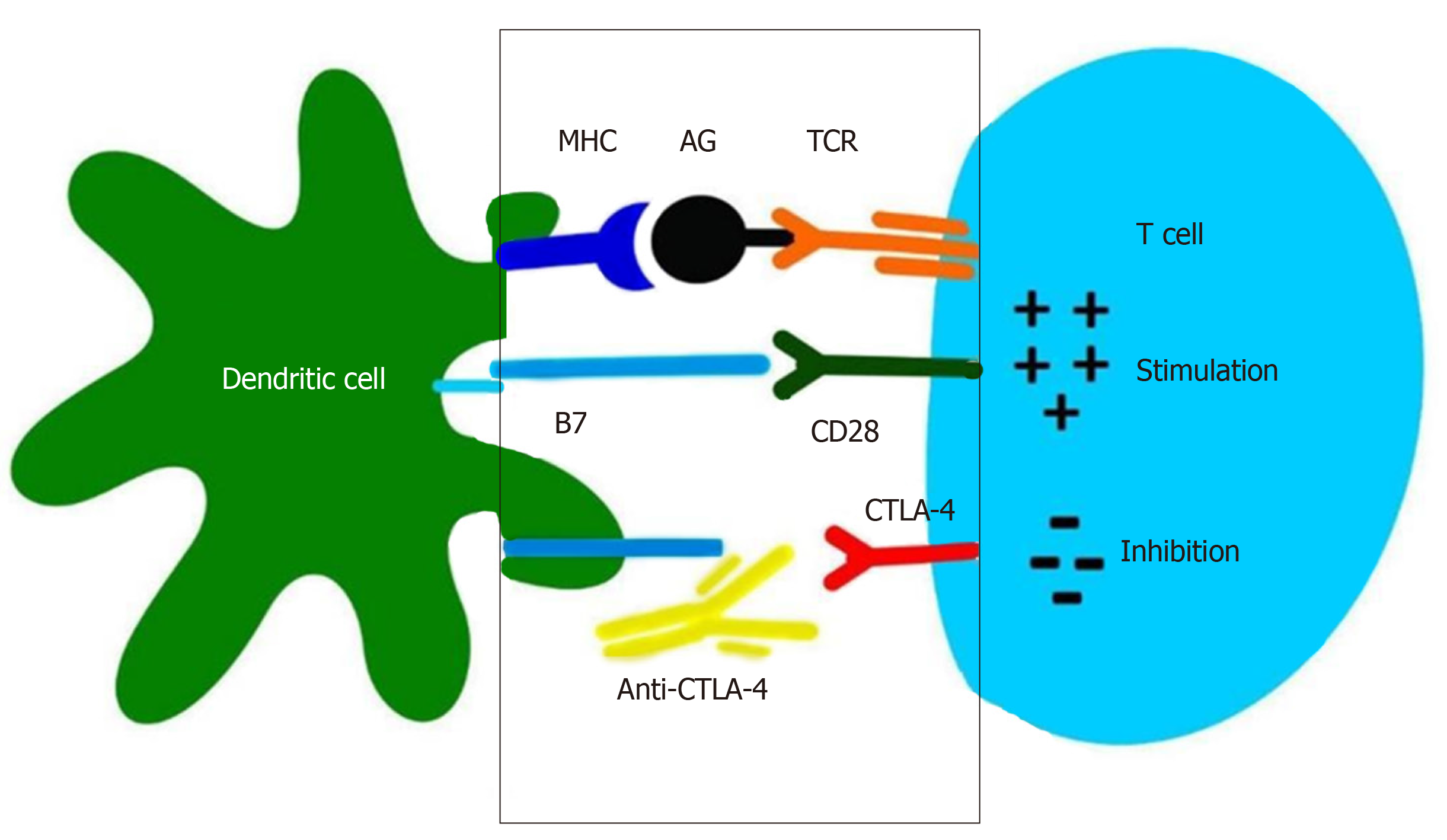
The main modalities of cancer immunotherapy are immune checkpoint inhibitors, therapy with CAR-T cells and vaccines[22]. Common immune checkpoint proteins include cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), programmed cell death protein-1 (PD-1), programmed cell death ligand 1 (PD-L1), VISTA, TIM-3, LAG-3, and OX40[23]. It is known that CTLA-4 and PD-1 inhibitors are negative regulators of T-cell immune function and they have the most important use in clinical research[24]. They have been well characterized and approved by the FDA for treating melanomas, and recent studies have outlined their role in management of HCC. CTLA-4 inhibitors, such as Tremelimumab and Ipilumimab, is predominantly expressed in activated T cells and natural killer (NK) cells[23]. CTLA-4 binds costimulatory B7 molecules (CD80/86) with a much higher affinity than CD28. Therefore, B7 binds CTLA-4 instead of CD28, what does not produce its usual stimulatory signal. When this protein is blocked, the inhibition on the immune system is released and the ability of T cells to kill cancer cells is increased[10]. Therefore, CTLA-4 functions to competitively inhibit T cell stimulation, and promote T cell anergy (Figure 2).
PD-1 is expressed in T cells, B cells, NK cells, mononuclear cells, and DCs. PD-1 inhibitors block the receptor binding of PD-L1 and PD-L2[25,26]. When PD-1 is activated by PD-L1, a ligand often found on tumor cells, it inhibits T cell functioning and induces apoptosis[24]. Some PD-1 inhibitors, such as Nivolumab, Pembrolizumab, and Pidilizumab, have been investigated for cancer treatment[27]. A phase I/II study has demonstrated the safety and antitumor effect of Nivolumab in patients with advanced HCC[28].
The basis of CAR-T cell therapy is to generate an immune-mediated antitumor response through gene transfer of a chimeric antigen receptor into T-cells[29]. Its benefits has been largely reported for hematological malignancies; however, for solid tumors, it is still incipient, with some challenges ahead, including the tumor microenvironment, which is more immunosuppressive, and the “on-target off-tumor” toxicity, since these lesions are generally expressed in other sites[29,30].
For vaccines, it consists of the generation of endogenous cancer-specific cytotoxic CD8+ T cells (CTLs), which present a paramount feature to kill cancer cells upon specific recognition through the T cell receptor of CTLs[31]. Therefore, it leads to cancer cell death through different mechanisms, including upregulation of FasL or TRAIL, leading to apoptosis, or through the induction of degranulation, releasing perforin and granzyme B[31,32].
A total of 37 articles were identified from the literature research for this review. However, only seven articles studied the combined therapy of RFA and immunotherapy[12,13,33-37]. Of these, 3 articles consisted of animal studies, and 4 consisted of human studies (Table 1).
| Ref. | Tumor | Intervention | Results | Level of evidence |
| Cui et al[12], 2014 | HCC from 2 to 8 cm | RFA with cellular immunotherapy | Avoid HCC recurrence | III |
| Tu et al[13], 2015 | Middle-advanced HCC | RFA and monoclonal antibody (131I-chTNT) | Increased circulating white blood cells; Increased overall survival; Improved progress-free survival | IV |
| Behm et al[33], 2016 | VX2 rabbit HCC | RFA and CpG B | Increased antitumor T cell response; prevented tumor spread; Improved survival | II |
| Ma et al[34], 2010 | HCC < 4 cm | RFA and autologous RAK cells | Increased intratumoral percentage of CD3+ CD8+ cells; avoided HCC recurrence | IV |
| Nakagawa et al[35], 2014 | C57B1/6 mice | RFA and OK-432 stimulated DCs | Decreased tumor volume; increased intratumoral CD8+ T cells | IV |
| Sodergren et al[36], 2019 | BALB/c mice | RFA, checkpoint blockade and MTL-CEBPA | Increase in CD8+ and CD49b+/CD45+ immune tumor response; abscopal effect | III |
| Bian et al[37], 2014 | Tumors < 3 cm vs > 3 cm | RFA and 131I metuximab | Prevention of tumor recurrence | II |
In a noncomparative clinical trial involving patients with advanced HCC, a combination therapy with Tremelimumab and RFA increased the number of intratumoral CD8+ T cells infiltration[38]. However, this study included a substantial number of patients treated with not only RFA, but also cryoablation and/or TACE.
A comparative study involving patients with middle-advanced stage HCC has investigated the effectiveness of monoclonal antibody (131I-chTNT) and RFA as a combination therapy[13]. This study showed that the survival time of patients who received combination therapy was significantly longer than that of the RFA alone group (P = 0.052). The number of white blood cells was significantly increased in the group that use monoclonal antibody treatment. In middle-advanced stage HCC patients, the combination of 131I- chTNT and RFA therapy was found to be significantly more effective than the RFA treatment alone for primary HCC[13].
Behm et al[33] studied the addition of CpG B oligonucleotides, toll like receptor (TLR) 9 agonists, to radiofrequency ablation in a VX-2 rabbit model of liver cancer, and observed that the combination significantly increased mean survival, cytolytic activity, and tumor-specific T cell activation compared to RFA alone. Additionally, the combined therapy demonstrated increased protection against pulmonary metastasis and peritoneum subjected to a re-challenge to injected malignant cells. Animals treated with combination RFA/CpG B survived longer than average in contrast to those treated with RFA or CpG B alone. In addition, they also observed that a smaller number of animals subjected to the combination therapy group showed residual malignant tissue after 120 days in comparison to monotherapy groups (P < 0.05), justifying the need for further exploration of the combination of RFA and TLR9 agonists in liver cancer.
Ma et al[34] reported the combination of RFA and autologous RetroNectin activated killer (RAK) cells in the treatment of HCC with a tumor size less than 4 cm. Recently, T lymphocytes were found to be efficiently expanded by stimulation with a combination of immobilized RetroNectin and anti-CD3mAb in HCC. It was observed that the percentage of CD3+CD8+ cells displayed a trend of continuous increase during the study, with no severe adverse events along the follow-up period, with no recurrence nor deaths reported. These preliminary results suggest the feasibility and safety of the combined therapeutic regimen for HCC, and that the RAK cell adoptive immunotherapy might be helpful in preventing recurrence and metastasis rates in HCC patients after RFA[34].
Nakagawa et al[35] demonstrated that the administration of dendritic cells stimulated by OK-432 (a clinical bacterial product, which can induce DC maturation) after RFA conferred a significant decrease in mean tumor volume compared to RFA alone or RFA in combination with immature dendritic cells (P < 0.001). Additionally, they showed that combination therapy significantly increased the number of CD8+ T cells infiltrating untreated secondary tumors as compared to RFA alone (P < 0.001). On the basis of these findings, they believe that combination therapy for liver cancer consisting of OK-432-stimulated DCs combined with RFA can proceed to clinical trials, and it is anticipated to be markedly superior to RFA single therapy.
Cui et al[12] studied the efficiency and safety of the combined treatment, with radiofrequency ablation (RFA) with cellular immunotherapy (CIT), for HCC patients. In this study, 62 patients with HCC who were treated with radical RFA were divided into two groups: RFA alone (32 patients) and RFA + CIT (30 patients). Autologous mononuclear cells were collected from the peripheral blood and separated by apheresis, and then induced into NK cells, γδT cells and cytokine-induced killer (CIK) cells. The tumor recurrent status of these patients was evaluated with computed tomography or magnetic resonance imaging every 3 mo after RFA. Progression free survival (PFS), liver function, HCV viral load and adverse effects were examined. The results implied that PFS was higher in the RFA + CIT group than in the RFA alone group. In the RFA + CIT group, six courses had better survival prognosis than three courses. Viral load of hepatitis C was decreased in patients without antiviral therapy in the RFA + CIT group, but was increased in the RFA alone group. No significant adverse reaction was found in the patients to whom CIT was administered. In summary, these preliminary results suggest that the combination of sequential CIT with RFA for HCC is efficient and safe, and may be helpful in the prevention of recurrence. However, a multicenter, randomized controlled clinical trial is still needed to further verify its efficacy.
Sodergren et al[36] in a recent study to investigate any synergistic effect of MTL-CEBPA (enhancer-binding protein alpha) with RFA and immune checkpoint inhibition, initiated a reverse translation experiment, where syngeneic BNL hepatocellular carcinoma tumor cells were injected in the two opposite flanks of immunocompetent BALB/c mice (n = 8 in each group). Treatments for hepatoma bearing mice included: (1) RFA on one flank; (2) Anti-PD-1 inhibition immu-notherapy; and (3) MTL-CEBPA as well as combinations of all 3 interventions. They found that tumor control on the opposite flank was increased by addition of RFA, and it more prominent in the triple combination group (RFA + anti-PD1 + MTL-CEBPA), in which 2/8 of the animals showed a complete response and 5/8 a partial response. This was also the only group that showed a statistically significant increase in CD8+ (Cytotoxic) as well as CD49b+/CD45+ (Natural Killer) tumor infiltrating lymphocytes. These data suggest a clinical role for combination treatment with checkpoint blockade, RFA and MTL-CEBPA through the synergistic priming of the immune response, enabling RFA to have a pronounced anti-tumor abscopal effect.
A single-center randomized controlled trial of radioimmunoconjugate 131I metuximab in treatment of HCC after percutaneous RFA compared with RFA alone was conducted[37]. In this study, 127 patients received either RFA followed by 131I metuximab (n = 62) or RFA alone (n = 65), and the primary outcome assessed was overall tumor recurrence. The one- and two-year recurrence rates in the combination group were 31.8% and 58.5%, whereas those in the RFA alone group were 56.3% and 70.9%, respectively. The median time to overall tumor recurrence was 17 mo in the combination group and 10 mo in the RFA alone group (P = 0.03). The RFA-131I metuximab treatment showed a greater anti-recurrence benefit than RFA alone (P = 0.007). The 131I metuximab may yielded prevention of tumor recurrence after RFA[37].
The table below illustrates all the studies published so far in the literature and summarizes the findings regarding the combination of RFA and immunotherapy for the treatment of HCC (Table 1).
Immunotherapy is a relatively new strategy of treatment for HCC. It aims to improve the body’s immune system against tumor associated antigens and induces tumor cell death[6]. Immunotherapy has the benefit of eliminating residual microscopic lesions of the tumor and preventing recurrences after RFA, being clear that the combination of immunotherapy and RFA improves oncological outcomes in patients with HCC[34].
Immunosuppression in patients with HCC is an important factor leading to its recurrence and metastasis, justifying the need of improving the immune system with immunomodulatory drugs[12]. Cui and colleagues[12] demonstrated in their study that the progression-free survival curve in the group aged ≤ 60 years was significantly better than the group aged > 60 years (P > 0.05). This difference can be explained by the fact that the immune system changes with age progression, with a decrease in the performance of immune cells in the elderly, and a better immune function in young patients.
Kaseb et al[39] in a randomized, open-label, perioperative phase II study, assessed the efficacy and safety of anti-PD-1 and anti-CTLA-4 antibodies, evaluating nivolumab alone versus nivolumab plus ipilimumab in patients with resectable HCC. They reported that 37.5% of patients treated with perioperative immunotherapy had complete response, and this response was correlated with an increase in intratumoral infiltration of CD8+ T cells, making it clear that HCC is a very immunogenic tumor, and immunotherapy can be a useful tool as an effective neoadjuvant or adjuvant therapy. Based on this study, we can propose neoadjuvant treatment with PD1 and CTLA4 in order to reduce the size of the tumor, and then perform RFA in the residual lesion. Therefore, neoadjuvant immunotherapy reduces tumor size, making RFA more efficient, as well as having the benefit of preventing tumor recurrence and progression.
RFA treatment is increasingly being used for local ablation of solitary liver tumors up to 5 cm in size[1,6,14]. Unfortunately, many of these patients still experience recurrence and multiple metastases, suggesting that adding relevant systemic therapy with immunomodulators would be highly valuable[34].
The immune responses induced by ablation monotherapy are well documented, but independently they tend to be incapable of evoking a clinically significant antitumor response. By adding immunomodulators to traditional ablative techniques, several researchers have sought to amplify the induced immune response and trigger systemic antitumor activity[10]. Besides that, RFA is responsible for its ability to trigger a systemic antitumor immune response where surgical resection would not[40].
Tu et al[13] by analyzing the data from two groups, showed that in the RFA alone group, the lesion diameter of > 5 cm was significantly associated with poor prognosis, indicating that unless combined with immunotherapy, this model of treatment is insufficient to eliminate larger tumors. This study suggested that RFA combined with immunotherapy is superior to RFA alone in treating the primary HCC with diameter > 3 cm. However, for lesions with > 5 cm diameter, the doses of both therapies used in this study were relatively insufficient to control the local recurrence.
The combination of RFA and immunotherapy shows better results than isolated therapies on patients with HCC, suggesting that the combination is more beneficial than RFA alone. Furthermore, we observed that this treatment strategy leads to an increase in the response of antitumor T cells, significantly reduces the risk of recurrence and improves overall survival compared to RFA isolated. However, a limiting factor was the relatively small number of studies and the short follow-up periods[12,13,33-37]. Therefore, the long-term effects of these studies have yet to be validated. In addition, a multicenter randomized controlled clinical trial is utterly needed to further verify its effectiveness.
RFA is a safe and efficient treatment for HCC, however therapeutic outcomes are limited by recurrence and metastasis. In our review, we observed only seven articles that studied the combination of RFA and Immunotherapy. The combined treatment with RFA and immunotherapy has emerged as a promising approach to activate immune response. Here, the authors investigated the applicability of combined RFA and Immunotherapy and observed that this strategy has potential to reinstate anti-tumor immunity in HCC through increase in antitumor T cell response. The approach seems quite pragmatic in limiting the recurrence and improving survival compared to RFA alone for HCC patients. However further studies are of paramount importance to elucidate its true potential.
| 1. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4363] [Article Influence: 545.4] [Reference Citation Analysis (6)] |
| 2. | Grandhi MS, Kim AK, Ronnekleiv-Kelly SM, Kamel IR, Ghasebeh MA, Pawlik TM. Hepatocellular carcinoma: From diagnosis to treatment. Surg Oncol. 2016;25:74-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 336] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 3. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21466] [Article Influence: 1951.5] [Reference Citation Analysis (6)] |
| 4. | Global Burden of Disease Liver Cancer Collaboration, Akinyemiju T. Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, Artaman A, Ayele TA, Barac A, Bensenor I, Berhane A, Bhutta Z, Castillo-Rivas J, Chitheer A, Choi JY, Cowie B, Dandona L, Dandona R, Dey S, Dicker D, Phuc H, Ekwueme DU, Zaki MS, Fischer F, Fürst T, Hancock J, Hay SI, Hotez P, Jee SH, Kasaeian A, Khader Y, Khang YH, Kumar A, Kutz M, Larson H, Lopez A, Lunevicius R, Malekzadeh R, McAlinden C, Meier T, Mendoza W, Mokdad A, Moradi-Lakeh M, Nagel G, Nguyen Q, Nguyen G, Ogbo F, Patton G, Pereira DM, Pourmalek F, Qorbani M, Radfar A, Roshandel G, Salomon JA, Sanabria J, Sartorius B, Satpathy M, Sawhney M, Sepanlou S, Shackelford K, Shore H, Sun J, Mengistu DT, Topór-Mądry R, Tran B, Ukwaja KN, Vlassov V, Vollset SE, Vos T, Wakayo T, Weiderpass E, Werdecker A, Yonemoto N, Younis M, Yu C, Zaidi Z, Zhu L, Murray CJL, Naghavi M, Fitzmaurice C. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683-1691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1459] [Cited by in RCA: 1574] [Article Influence: 174.9] [Reference Citation Analysis (0)] |
| 5. | Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2184] [Cited by in RCA: 3120] [Article Influence: 445.7] [Reference Citation Analysis (17)] |
| 6. | Chen L, Sun J, Yang X. Radiofrequency ablation-combined multimodel therapies for hepatocellular carcinoma: Current status. Cancer Lett. 2016;370:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Yao P, Morris DL. Radiofrequency ablation-assisted liver resection: review of the literature and our experience. HPB (Oxford). 2006;8:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Reccia I, Kumar J, Kusano T, Giakoustidis A, Zanellato A, Retsas P, Habib N, Jiao L, Spalding D, Pai M. Radiofrequency-assisted liver resection: Technique and results. Surg Oncol. 2018;27:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | den Brok MH, Sutmuller RP, Nierkens S, Bennink EJ, Frielink C, Toonen LW, Boerman OC, Figdor CG, Ruers TJ, Adema GJ. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer. 2006;95:896-905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 235] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 10. | Slovak R, Ludwig JM, Gettinger SN, Herbst RS, Kim HS. Immuno-thermal ablations - boosting the anticancer immune response. J Immunother Cancer. 2017;5:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 152] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 11. | Minami Y, Nishida N, Kudo M. Radiofrequency ablation of liver metastasis: potential impact on immune checkpoint inhibitor therapy. Eur Radiol. 2019;29:5045-5051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Cui J, Wang N, Zhao H, Jin H, Wang G, Niu C, Terunuma H, He H, Li W. Combination of radiofrequency ablation and sequential cellular immunotherapy improves progression-free survival for patients with hepatocellular carcinoma. Int J Cancer. 2014;134:342-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | Tu J, Ji J, Wu F, Wang Y, Zhang D, Zhao Z, Ying X. Effectiveness of combined (131)I-chTNT and radiofrequency ablation therapy in treating advanced hepatocellular carcinoma. Cell Biochem Biophys. 2015;71:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Yan K, Chen MH, Yang W, Wang YB, Gao W, Hao CY, Xing BC, Huang XF. Radiofrequency ablation of hepatocellular carcinoma: long-term outcome and prognostic factors. Eur J Radiol. 2008;67:336-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Park MJ, Kim YS, Rhim H, Lim HK, Lee MW, Choi D. A comparison of US-guided percutaneous radiofrequency ablation of medium-sized hepatocellular carcinoma with a cluster electrode or a single electrode with a multiple overlapping ablation technique. J Vasc Interv Radiol. 2011;22:771-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Woo S, Lee JM, Yoon JH, Joo I, Kim SH, Lee JY, Yoon JH, Kim YJ, Han JK, Choi BI. Small- and medium-sized hepatocellular carcinomas: monopolar radiofrequency ablation with a multiple-electrode switching system-mid-term results. Radiology. 2013;268:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Uhlig J, Sellers CM, Stein SM, Kim HS. Radiofrequency ablation versus surgical resection of hepatocellular carcinoma: contemporary treatment trends and outcomes from the United States National Cancer Database. Eur Radiol. 2019;29:2679-2689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Feng K, Yan J, Li X, Xia F, Ma K, Wang S, Bie P, Dong J. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 613] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 19. | Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, Zeng Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 653] [Article Influence: 40.8] [Reference Citation Analysis (1)] |
| 20. | Liu H, Wang ZG, Fu SY, Li AJ, Pan ZY, Zhou WP, Lau WY, Wu MC. Randomized clinical trial of chemoembolization plus radiofrequency ablation versus partial hepatectomy for hepatocellular carcinoma within the Milan criteria. Br J Surg. 2016;103:348-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 21. | Buettner N, Thimme R. Toward a Better Understanding of Hepatocellular Carcinoma Immune Infiltrates. Cell Mol Gastroenterol Hepatol. 2020;9:341-342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Lohmueller J, Finn OJ. Current modalities in cancer immunotherapy: Immunomodulatory antibodies, CARs and vaccines. Pharmacol Ther. 2017;178:31-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Xie Y, Xiang Y, Sheng J, Zhang D, Yao X, Yang Y, Zhang X. Immunotherapy for Hepatocellular Carcinoma: Current Advances and Future Expectations. J Immunol Res. 2018;2018:8740976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 24. | Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol. 2016;39:98-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1427] [Cited by in RCA: 1830] [Article Influence: 183.0] [Reference Citation Analysis (0)] |
| 25. | Shi L, Chen S, Yang L, Li Y. The role of PD-1 and PD-L1 in T-cell immune suppression in patients with hematological malignancies. J Hematol Oncol. 2013;6:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 249] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 26. | Dai S, Jia R, Zhang X, Fang Q, Huang L. The PD-1/PD-Ls pathway and autoimmune diseases. Cell Immunol. 2014;290:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 288] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 27. | Abdin SM, Zaher DM, Arafa EA, Omar HA. Tackling Cancer Resistance by Immunotherapy: Updated Clinical Impact and Safety of PD-1/PD-L1 Inhibitors. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3453] [Article Influence: 383.7] [Reference Citation Analysis (2)] |
| 29. | Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016;13:273-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 815] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 30. | Jackson HJ, Rafiq S, Brentjens RJ. Driving CAR T-cells forward. Nat Rev Clin Oncol. 2016;13:370-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 471] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 31. | Farhood B, Najafi M, Mortezaee K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review. J Cell Physiol. 2019;234:8509-8521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 1219] [Article Influence: 152.4] [Reference Citation Analysis (5)] |
| 32. | Briquez PS, Hauert S, de Titta A, Gray LT, Alpar AT, Swartz MA, Hubbell JA. Engineering Targeting Materials for Therapeutic Cancer Vaccines. Front Bioeng Biotechnol. 2020;8:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Behm B, Di Fazio P, Michl P, Neureiter D, Kemmerling R, Hahn EG, Strobel D, Gress T, Schuppan D, Wissniowski TT. Additive antitumour response to the rabbit VX2 hepatoma by combined radio frequency ablation and toll like receptor 9 stimulation. Gut. 2016;65:134-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Ma H, Zhang Y, Wang Q, Li Y, He J, Wang H, Sun J, Pan K, Chen M, Xia J. Therapeutic safety and effects of adjuvant autologous RetroNectin activated killer cell immunotherapy for patients with primary hepatocellular carcinoma after radiofrequency ablation. Cancer Biol Ther. 2010;9:903-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Nakagawa H, Mizukoshi E, Iida N, Terashima T, Kitahara M, Marukawa Y, Kitamura K, Nakamoto Y, Hiroishi K, Imawari M, Kaneko S. In vivo immunological antitumor effect of OK-432-stimulated dendritic cell transfer after radiofrequency ablation. Cancer Immunol Immunother. 2014;63:347-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Sodergren MH, Huang KW, Reebye V, Chee CE, Zacharoulis D, Habib R, Blakey D, Rossi J, Habib N. MTL-CEBPA combined with radiofrequency ablation and immunotherapy enhances immunological anti-tumour response in an HCC mouse model. Cancer Res. 2019;79. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 37. | Bian H, Zheng JS, Nan G, Li R, Chen C, Hu CX, Zhang Y, Sun B, Wang XL, Cui SC, Wu J, Xu J, Wei D, Zhang X, Liu H, Yang W, Ding Y, Li J, Chen ZN. Randomized trial of [131I] metuximab in treatment of hepatocellular carcinoma after percutaneous radiofrequency ablation. J Natl Cancer Inst. 2014;106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M, Uppala A, Korangy F, Kleiner DE, Figg WD, Venzon D, Steinberg SM, Venkatesan AM, Krishnasamy V, Abi-Jaoudeh N, Levy E, Wood BJ, Greten TF. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 661] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 39. | Kaseb A, Vence L, Blando J, Yadav S, Ikoma N, Pestana R, Vauthey J, Cao H, Chun Y, Sakamura D, Wolff R, Yao J, Allison J, Sharma P. Randomized, open-label, perioperative phase II study evaluating nivolumab alone versus nivolumab plus ipilimumab in patients with resectable HCC. Ann Oncol. 2019;30 Suppl 4:iv112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Mehta A, Oklu R, Sheth RA. Thermal Ablative Therapies and Immune Checkpoint Modulation: Can Locoregional Approaches Effect a Systemic Response? Gastroenterol Res Pract. 2016;2016:9251375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited Manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gorrell M, Mrzljak A, Suda T S-Editor: Wang YQ L-Editor: A E-Editor: Ma YJ