Published online Apr 14, 2020. doi: 10.3748/wjg.v26.i14.1647
Peer-review started: December 19, 2019
First decision: January 19, 2020
Revised: March 14, 2020
Accepted: March 19, 2020
Article in press: March 19, 2020
Published online: April 14, 2020
Processing time: 117 Days and 2.8 Hours
Microvascular invasion (MVI) is an important prognostic factor affecting early recurrence and overall survival in hepatocellular carcinoma (HCC) patients after hepatectomy and liver transplantation, but it can be determined only in surgical specimens. Accurate preoperative prediction of MVI is conducive to clinical decisions.
To develop and validate a preoperative prediction model for MVI in patients with HCC.
Data from 454 patients with HCC who underwent hepatectomy at the First Affiliated Hospital of Nanjing Medical University between May 2016 and October 2019 were retrospectively collected. Then, the patients were nonrandomly split into a training cohort and a validation cohort. Logistic regression analysis was used to identify variables significantly associated with MVI that were then included in the nomogram. We evaluated the discrimination and calibration ability of the nomogram by using R software.
MVI was confirmed in 209 (46.0%) patients by a pathological examination. Multivariate logistic regression analysis identified four risk factors independently associated with MVI: Tumor size [odds ratio (OR) = 1.195; 95% confidence interval (CI): 1.107–1.290; P < 0.001], number of tumors (OR = 4.441; 95%CI: 2.112–9.341; P < 0.001), neutrophils (OR = 1.714; 95%CI: 1.036–2.836; P = 0.036), and serum α-fetoprotein (20–400 ng/mL, OR = 1.955; 95%CI: 1.055–3.624; P = 0.033; >400 ng/mL, OR = 3.476; 95%CI: 1.950–6.195; P < 0.001). The concordance index was 0.79 (95%CI: 0.74–0.84) and 0.81 (95%CI: 0.74–0.89) in the training and validation cohorts, respectively. The calibration curves showed good agreement between the predicted risk by the nomogram and real outcomes.
We have developed and validated a preoperative prediction model for MVI in patients with HCC. The model could aid physicians in clinical treatment decision making.
Core tip: Microvascular invasion (MVI) is an established risk factor for early recurrence and a poor prognosis in patients with hepatocellular carcinoma, but it can be confirmed only by postoperative pathology. Our study identified four predictors independently related to MVI based mainly on laboratory parameters and established a nomogram to predict the presence of MVI preoperatively. The model showed good performance in the evaluation of discrimination and calibration ability and could help optimize treatment options in the clinic.
- Citation: Wang L, Jin YX, Ji YZ, Mu Y, Zhang SC, Pan SY. Development and validation of a prediction model for microvascular invasion in hepatocellular carcinoma. World J Gastroenterol 2020; 26(14): 1647-1659
- URL: https://www.wjgnet.com/1007-9327/full/v26/i14/1647.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i14.1647
Hepatocellular carcinoma (HCC) represents one of the most common malignancies worldwide. It is the third leading cause of cancer-related deaths[1]. Hepatectomy and liver transplantation are considered the most effective treatments and provide a curative opportunity for selected patients. However, the prognosis of HCC is still poor, with a recurrence rate of more than 50% at 5 years after resection[2,3] due to frequent blood vessel invasion resulting in intrahepatic and extrahepatic metastases.
Vascular invasion is usually related to tumor metastasis, recurrence, and poor outcomes and is divided into macrovascular invasion and microvascular invasion (MVI) in HCC. Macrovascular invasion can be diagnosed by an imaging examination. Generally, patients with macrovascular invasion have no chance of radical resection or liver transplantation. In contrast, as a pathological concept, MVI can be confirmed only in surgical specimens. MVI is defined as a microscopic cancer cell nest in vessels lined with endothelial cells that is commonly observed in the small branches of the portal vein in adjacent liver tissues and occasionally in the hepatic artery, bile duct, and lymphatic vessels[4]. In the presence of MVI, tumor cells can spread and metastasize in the liver to form a portal vein tumor thrombus or multiple lesions or distant metastasis. It has been reported that the incidence of MVI in HCC patients ranges from 15% to 57%[5]. MVI is a definite factor leading to the early recurrence and poor long-term survival outcomes of HCC after resection and liver transplantation. The preoperative identification of MVI is beneficial to therapeutic decisions. Unfortunately, there is no effective and accurate prediction method before surgery.
Currently, a number of studies on the preoperative prediction of the risk of MVI in HCC and risk factors related to MVI have been carried out: The risk factors include tumor characteristics, serum tumor markers, imaging features, and gene tags. As a new strategy of combining multiple factors to predict MVI, a clinical prediction model has become a research focus. In particular, many radiomics models have been developed for diagnosing MVI preoperatively and noninvasively[6,7]. However, due to the lack of standardization in radiomics and overreliance on the subjective judgment of diagnostic radiologists, the accuracy and practicality of the radiomics model are still controversial[8]. Moreover, some radiological parameters are too specialized and thus cannot be understood and applied by clinicians. By contrast, routine laboratory tests are more common and easy to control and standardize, and data from different sources are accurate and comparable.
The purpose of this study was to identify clinical variables significantly associated with the risk of MVI and develop and validate a new clinical prediction model for the presence of MVI in patients with HCC before hepatectomy based on laboratory parameters.
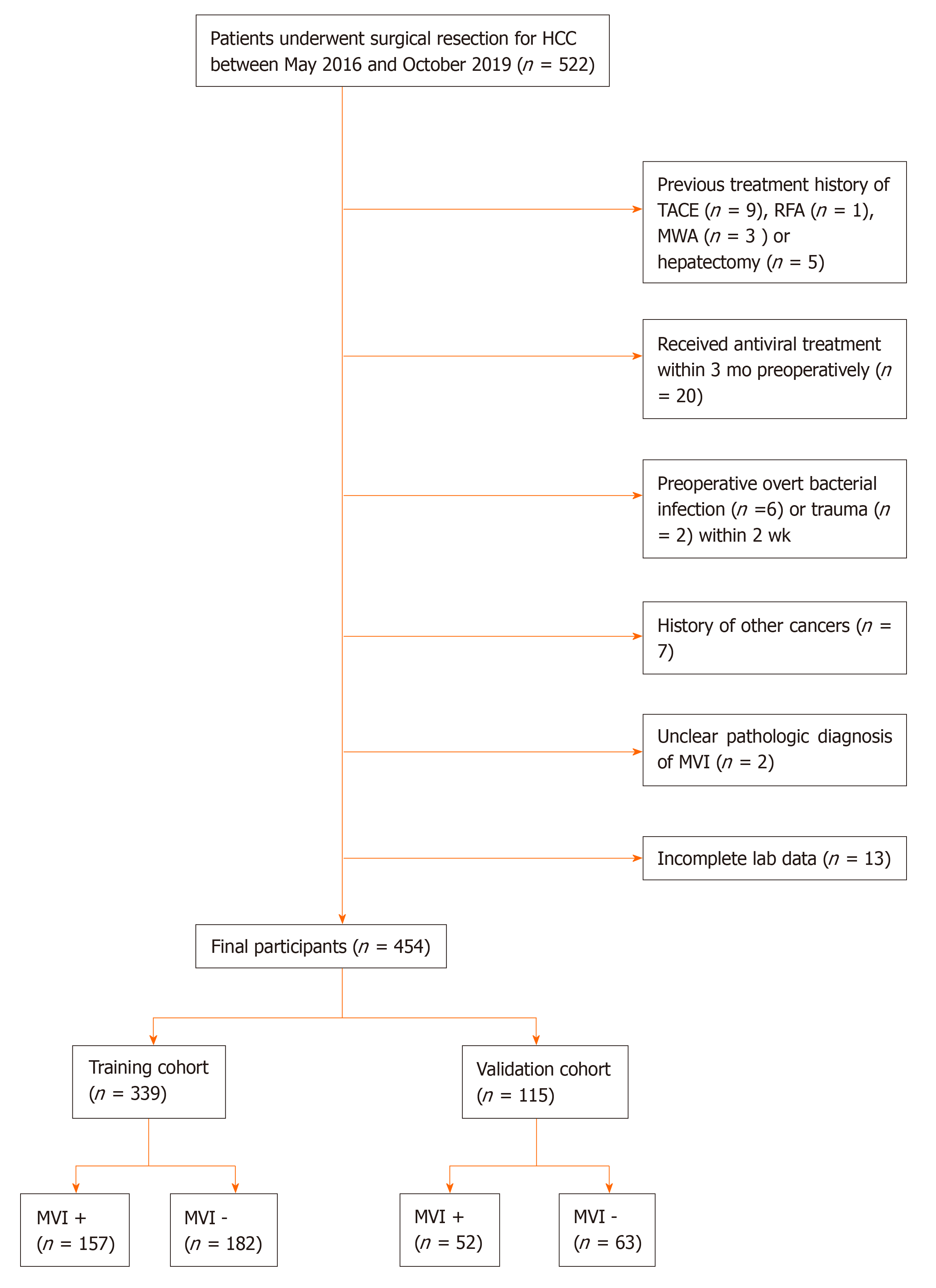
We retrospectively searched the hepatosurgical database of the First Affiliated Hospital of Nanjing Medical University from May 2016 to October 2019 to identify patients who were diagnosed with HCC histologically and underwent hepatic resection. The diagnosis of HCC followed the Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition)[4]. The inclusion criteria were as follows: (1) Age 18 years or older; (2) Underwent hepatectomy; and (3) Diagnosed with HCC confirmed by histology. The exclusion criteria were as follows: (1) History of HCC treatment; (2) Received antiviral treatment within 3 mo preoperatively; (3) Preoperative overt bacterial infection or trauma within 2 weeks; (4) History of other cancers; (5) Unclear pathologic diagnosis of MVI; and (6) Incomplete laboratory data. Finally, eligible patients were included in the study. Data on HCC patients collected from May 2016 to March 2019 were used as the training dataset, and data on HCC patients collected from April 2019 to October 2019 were used as the validation dataset. The current study was approved by the Institutional Ethics Committee of the First Affiliated Hospital of Nanjing Medical University.
All patients received a routine preoperative examination within 2 wk before hepatectomy that included whole blood count, blood biochemistry, coagulation function, hepatitis B immunology, and serum α-fetoprotein (AFP) tests and an imaging examination [abdominal ultrasonography, computed tomographic scan of the abdomen, and contrast-enhanced magnetic resonance imaging (MRI)]. Anatomic or nonanatomic resection was performed after the clinical evaluation, and all the obtained surgical specimens were histologically assessed to determine the presence of MVI and the Edmondson-Steiner grade by two pathologists. As previously described, MVI refers to the presence of tumor cell clusters in the blood vessels lined with endothelial cells only under microscopic observation. Imaging parameters mainly included the number of tumors and tumor size. For the derivative indicators involved, the neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio (PLR), and systemic immune-inflammation index (SII) were calculated as follows: NLR = neutrophil count/lymphocyte count, PLR = platelet count/lymphocyte count, and SII = platelet count × neutrophil count/lymphocyte count. The albumin-bilirubin (ALBI) grade was computed by the following formula: 0.66 × log10 (bilirubin μmol/L) − 0.085 × (albumin g/L). According to the previously described cut-off points, the patients were divided into three grades: ALBI grade 1 (≤ -2.60), ALBI grade 2 (> -2.60 to -1.39) and ALBI grade 3 (> -1.39)[9]. Serum AFP and hepatitis B immunology were measured by electrochemiluminescence immunoassays using a Cobas e602 automated analyzer (Roche, Germany). A Sysmex XN series automated hematology analyzer (Sysmex, Japan) and a Sysmex CS5100 automated blood coagulation analyzer (Sysmex, Japan) were used to determine the complete blood count and coagulation function, respectively. A Beckman Coulter AU5800 analyzer (Beckman Coulter, United States) was used to determine blood biochemistry.
Categorical variables are displayed as the number and percentage, and continuous variables are presented as the median [interquartile range (IQR)]. Categorical variables were compared using the chi-square test or Fisher’s exact test. Comparisons of continuous variables between two different groups were conducted using the Mann-Whitney test. A univariate logistic regression analysis was used to assess the significance of each variable in the training cohort for the prediction of MVI. All variables with P < 0.05 in the univariate logistic regression analysis were incorporated into a multivariate logistic regression analysis. The nomogram for the prediction of MVI was established based on the results of the multivariate logistic regression analysis by using the rms package of R, version 3.6.1 (http://www.r-project.org/). To evaluate the prediction performance of the nomogram, we calculated the concordance index (C-index) with 1000 bootstrap samples to measure discrimination (the model's ability to distinguish between HCC patients with and without MVI) and generated calibration plots to measure calibration (consistency between the predicted probability and observed frequency of patients with MVI). The optimal cut-off value of the nomogram was determined by maximizing the Youden index. Additionally, we performed decision curve analysis (DCA) to evaluate the clinical usefulness and net benefits of the developed model. A P value less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS software, version 22 (SPSS, Inc., Chicago, IL) and R, version 3.6.1 (http://www.r-project.org/). This report followed the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis Or Diagnosis (TRIPOD) guidelines[10].
During our study period, a total of 522 patients were diagnosed with HCC histologically and underwent hepatectomy. Ultimately, 454 patients met the inclusion criteria. Among them, 339 patients whose data were collected between May 2016 and March 2019 formed the training cohort, and 115 patients whose data were collected between April and October 2019 formed the validation cohort (Figure 1). The clinicopathologic characteristics of the patients are summarized in Table 1. The median ages of patients in the training and validation cohorts were 57 and 59 years, respectively. The number of male patients was significantly higher than that of female patients. Approximately 80% of all patients had hepatitis B virus (HBV) infection. The histological examination confirmed MVI in 157 (46.3%) patients in the training cohort and 52 (45.2%) patients in the validation cohort. There was no significant difference in the distribution of variables between the training and validation cohorts except for the red blood cell distribution width (RDW), albumin (ALB), and ALBI grade.
| Characteristic | Training cohort (n = 339) | Validation cohort (n = 115) | P value |
| Median age (IQR), yr | 57 (49, 65) | 59 (51, 67) | 0.141 |
| Gender | |||
| Male | 284 (83.8) | 104 (90.4) | 0.080 |
| Female | 55 (16.2) | 11 (9.6) | |
| Tumor size, cm | 4.5 (3.0, 8.0) | 4.0 (2.5, 7.0) | 0.095 |
| Number of tumors | |||
| Single | 285 (84.1) | 89 (77.4) | 0.104 |
| Multiple | 54 (15.9) | 26 (22.6) | |
| Child-Pugh grade | |||
| A | 315 (92.9) | 104 (90.4) | 0.388 |
| B | 24 (7.1) | 11 (9.6) | |
| Clinical stage | |||
| I | 241 (71.1) | 80 (69.6) | 0.727 |
| II | 86 (25.4) | 29 (25.2) | |
| III | 12 (3.5) | 6 (5.2) | |
| Etiology | |||
| Hepatitis B | 253 (74.6) | 93 (80.9) | 0.175 |
| Non-hepatitis B | 86 (25.4) | 22 (19.1) | |
| AFP, ng/mL | |||
| ≤ 20 | 142 (41.9) | 46 (40.0) | 0.122 |
| 20–40 | 81 (23.9) | 38 (33.0) | |
| ≥ 400 L | 116 (34.2) | 31 (27.0) | |
| WBC, 109/L | |||
| ≤ 4.0 | 83 (24.5) | 33 (28.7) | 0.371 |
| > 4.0 | 256 (75.5) | 82 (71.3) | |
| Neutrophils, 109/L | |||
| ≤ 3.0 | 167 (49.3) | 65 (56.5) | 0.178 |
| > 3.0 | 172 (50.7) | 50 (43.5) | |
| PLT, 109/L | |||
| ≤ 125 | 128 (37.8) | 44 (38.3) | 0.923 |
| > 125 | 211 (62.2) | 71 (61.7) | |
| RDW | |||
| ≤ 13.0 | 119 (35.1) | 54 (47.0) | 0.024 |
| > 13.0 | 220 (64.9) | 61 (53.0) | |
| NLR | |||
| ≤ 2.0 | 150 (44.2) | 60 (52.2) | 0.141 |
| > 2.0 | 189 (55.8) | 55 (47.8) | |
| PLR | |||
| ≤ 100 | 166 (49.0) | 65 (56.5) | 0.161 |
| > 100 | 173 (51.0) | 50 (43.5) | |
| SII | |||
| ≤ 300 | 173 (51.0) | 66 (57.4) | 0.238 |
| > 300 | 166 (49.0) | 49 (42.6) | |
| PT, sec | |||
| ≤ 13.0 | 250 (73.7) | 92 (80.0) | 0.179 |
| > 13.0 | 89 (26.3) | 23 (20.0) | |
| FIB, g/L | |||
| ≤ 2.0 | 90 (26.5) | 24 (20.9) | 0.225 |
| > 2.0 | 249 (73.5) | 91 (79.1) | |
| ALB, g/L | |||
| ≤ 40 | 192 (56.6) | 50 (43.5) | 0.015 |
| > 40 | 147 (43.4) | 65 (56.5) | |
| ALT, U/L | |||
| ≤ 40 | 204 (60.2) | 77 (67.0) | 0.196 |
| > 40 | 135 (39.8) | 38 (33.0) | |
| AST, U/L | |||
| ≤ 35 | 160 (47.2) | 62 (53.9) | 0.213 |
| > 35 | 179 (52.8) | 53 (46.1) | |
| GGT, U/L | |||
| ≤ 45 | 120 (35.4) | 38 (33.0) | 0.647 |
| > 45 | 219 (64.6) | 77 (67.0) | |
| TB, μmol/L | |||
| ≤ 19 | 257 (75.8) | 85 (73.9) | 0.683 |
| > 19 | 82 (24.2) | 30 (26.1) | |
| ALP, g/L | |||
| ≤ 120 | 210 (61.9) | 71 (61.7) | 0.968 |
| > 120 | 129 (38.1) | 44 (38.3) | |
| GLU, mmol/L | |||
| ≤ 6.1 | 278 (82.0) | 97 (84.3) | 0.567 |
| > 6.1 | 61 (18.0) | 18 (15.7) | |
| ALBI grade | |||
| 1 | 164 (48.4) | 71 (61.7) | 0.017 |
| 2 | 171 (50.4) | 41 (35.7) | |
| 3 | 4 (1.2) | 3 (2.6) | |
| MVI | |||
| Absent | 182 (53.7) | 63 (54.8) | 0.839 |
| Present | 157 (46.3) | 52 (45.2) | |
| Edmondson-Steiner classification | |||
| I–II | 142 (41.9) | 43 (37.4) | 0.396 |
| III–IV | 197 (58.1) | 72 (62.6) |
The results of the univariate logistic regression analysis of the clinical features in the training cohort are shown in Table 2. Tumor size [odds ratio (OR) = 1.214; 95% confidence interval (CI): 1.155–1.332; P < 0.001], number of tumors (OR = 5.174; 95%CI: 2.611–10.252; P < 0.001), serum AFP (for 20–400 vs ≤ 20 ng/mL, OR = 1.936; 95%CI: 1.100–3.407; P = 0.022; for ≥400 vs ≤ 20 ng/mL, OR = 4.546; 95%CI: 2.687–7.691; P < 0.001), neutrophils (OR = 1.989; 95%CI: 1.289–3.069; P = 0.002), NLR (OR = 1.927; 95%CI: 1.244–2.983; P = 0.003), PLR (OR = 1.945; 95%CI: 1.261–3.000; P = 0.003), SII (OR = 2.170; 95%CI: 1.404–3.352; P < 0.001), and ALP (OR = 1.677; 95%CI: 1.078–2.610; P = 0.022) were significant preoperative risk factors associated with MVI in the univariate analysis, and all these predictors with a P value less than 0.05 were selected for the multivariate analysis. In the multivariate analysis, multiple tumors (OR = 4.441; 95%CI: 2.112–9.341; P < 0.001), large tumor size (OR = 1.195; 95%CI: 1.107–1.290; P < 0.001), high neutrophil level (OR = 1.714; 95%CI: 1.036–2.836; P = 0.036), and high serum AFP level (for 20–400 vs ≤ 20 ng/mL, OR = 1.955; 95%CI: 1.055–3.624; P = 0.033; for ≥400 vs ≤ 20 ng/mL, OR = 3.476; 95%CI: 1.950–6.195; P < 0.001) were independently associated with the presence of MVI (Table 3).
| Variable | OR (95%CI) | P value |
| Age, yr | 0.981 (0.962–1.001) | 0.062 |
| Gender, male vs female | 1.488 (0.823–2.689) | 0.188 |
| Number of tumors, multiple vs single | 5.174 (2.611–10.252) | < 0.001 |
| Tumor size, cm | 1.214 (1.155–1.332) | < 0.001 |
| Etiology, non-hepatitis B vs hepatitis | 0.837 (0.511–1.370) | 0.479 |
| AFP, ng/mL | ||
| 20–40 vs ≤ 20 | 1.936 (1.100–3.407) | 0.022 |
| ≥ 400 vs ≤ 20 | 4.546 (2.687–7.691) | < 0.001 |
| WBC, 109/L, > 4.0 vs ≤ 4.0 | 1.117 (0.711–1.927) | 0.537 |
| Neutrophils, 109/L, >3.0 vs ≤ 3.0 | 1.989 (1.289–3.069) | 0.002 |
| PLT, 109/L, > 125 vs ≤ 125 | 1.375 (0.883–2.143) | 0.159 |
| RDW, > 13.0 vs ≤ 13.0 | 1.116 (0.713–1.748) | 0.630 |
| NLR, > 2.0 vs ≤ 2.0 | 1.927 (1.244–2.983) | 0.003 |
| PLR, > 100 vs ≤ 100 | 1.945 (1.261–3.000) | 0.003 |
| SII, > 300 vs ≤ 300 | 2.170 (1.404–3.352) | < 0.001 |
| PT, sec, > 13 vs ≤ 13 | 1.514 (0.931–2.462) | 0.094 |
| ALB, g/L, > 40 vs ≤ 40 | 0.949 (0.617–1.460) | 0.812 |
| ALT, U/L, > 40 vs ≤ 40 | 0.882 (0.570–1.366) | 0.575 |
| AST, U/L, > 35 vs ≤ 35 | 1.275 (0.831–1.958) | 0.266 |
| GGT, U/L, > 45 vs ≤ 45 | 1.486 (0.947–2.334) | 0.085 |
| TB, μmol/L, > 19 vs ≤ 19 | 1.297 (0.788–2.133) | 0.307 |
| ALP, U/L, > 120 vs ≤ 120 | 1.677 (1.078–2.610) | 0.022 |
| FIB, g/L, > 2.0 vs ≤ 2.0 | 1.397 (0.852–2.290) | 0.185 |
| GLU, mmol/L, > 6.1 vs ≤ 6.1 | 0.904 (0.518–1.579) | 0.723 |
| ALBI grade, 1 vs 2 and 3 | 1.266 (0.825–1.942) | 0.281 |
| Variable | β | OR (95%CI) | P value |
| Number of tumors, multiple vs single | 1.491 | 4.441 (2.112–9.341) | < 0.001 |
| Tumor size, cm | 0.178 | 1.195 (1.107–1.290) | < 0.001 |
| Neutrophils, 109/L, > 3.0 vs ≤ 3.0 | 0.539 | 1.714 (1.036–2.836) | 0.036 |
| AFP, ng/mL | |||
| 20–400 vs ≤ 20 | 0.670 | 1.955 (1.055–3.624) | 0.033 |
| ≥ 400 vs ≤ 20 | 1.246 | 3.476 (1.950–6.195) | < 0.001 |
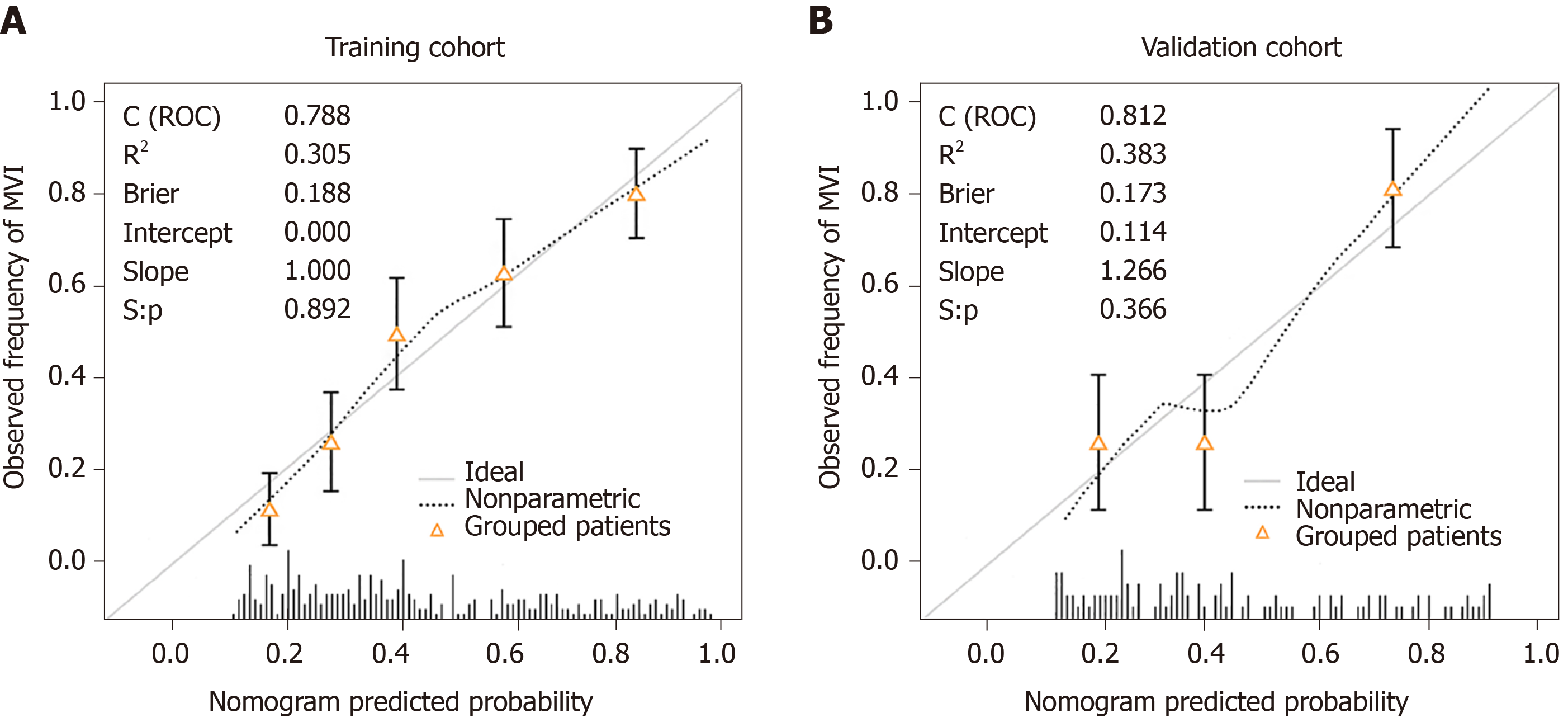
Based on the results of the multivariate analysis, we chose tumor size, number of tumors, neutrophils, and serum AFP for model development. The nomogram for predicting the presence of MVI in patients with HCC preoperatively is presented in Figure 2. The probability of MVI can be estimated by using this nomogram to calculate the total points for each patient. Further analysis indicated that the nomogram has excellent performance in distinguishing the absence or presence of MVI. In the training cohort, the C-index was 0.79 (95%CI: 0.74–0.84), and in the validation cohort, the C-index was 0.81 (95%CI: 0.74–0.89). According to the maximum Youden index, the optimal cut-off value for the prediction probability of the nomogram was 0.40. The sensitivity, specificity, negative predictive value, and positive predictive value when the model was used to differentiate between the presence and absence of MVI were 77.7%, 70.9%, 78.7%, and 69.7%, respectively, in the training cohort and 69.2%, 68.3%, 72.9%, and 64.3%, respectively, in the validation cohort (Table 4).
| Variable | Value (95%CI) | |
| Training cohort | Validation cohort | |
| Sensitivity, % | 77.7 (71.1–84.3) | 69.2 (56.3–82.2) |
| Specificity, % | 70.9 (64.2–77.5) | 68.3 (56.4–80.1) |
| Positive predictive value, % | 69.7 (62.8–76.6) | 64.3 (51.3–77.2) |
| Negative predictive value, % | 78.7 (72.3–85.0) | 72.9 (61.2–84.6) |
| Positive likelihood ratio | 2.67 (2.10–3.40) | 2.18 (1.45–3.27) |
| Negative likelihood ratio | 0.31 (0.23–0.42) | 0.45 (0.30–0.69) |
| Concordance index | 0.79 (0.74–0.84) | 0.81 (0.74–0.89) |
| Predicted probability1 | 0.40 | 0.40 |
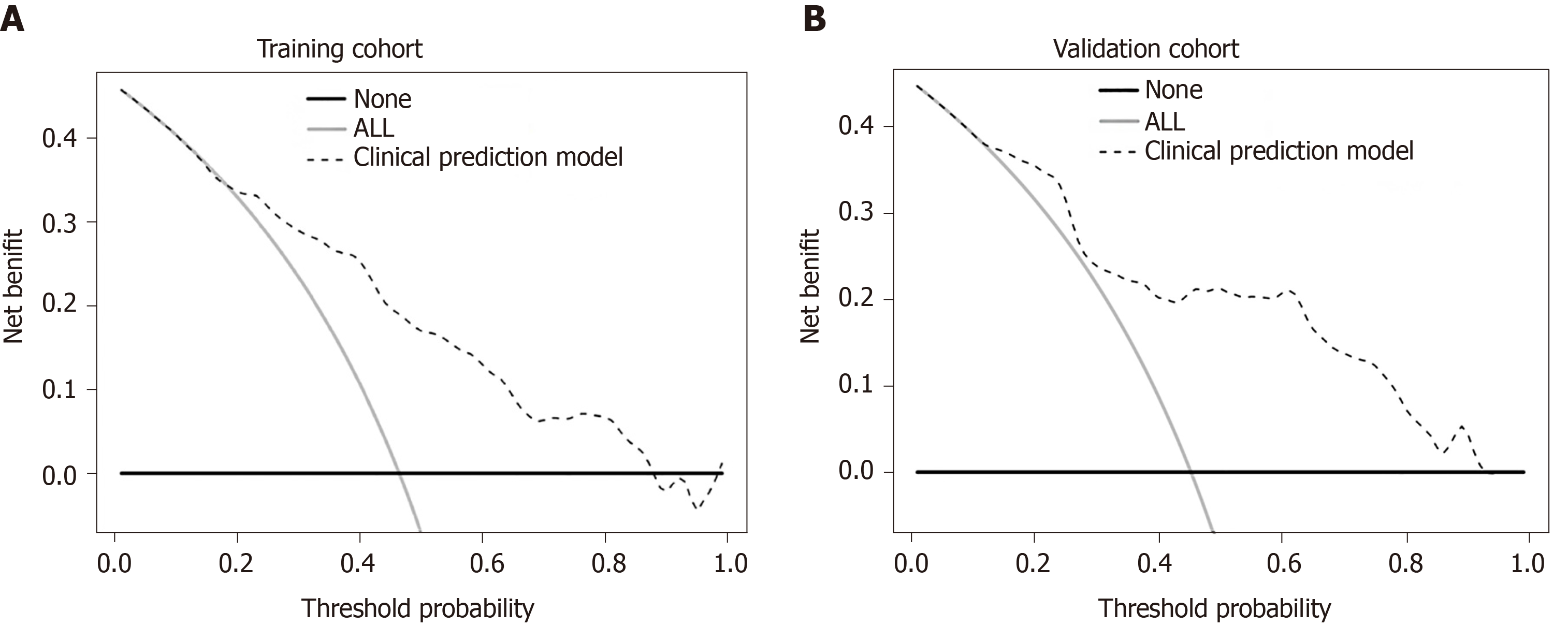
In addition, we generated calibration curves to evaluate the calibration of the prediction model. Calibration curves demonstrated acceptable model calibration, with good agreement between the observed frequency and predicted probability of patients with MVI in both datasets (Figure 3). Figure 4 illustrates the decision curves for the clinical model to predict the correct diagnosis of MVI in patients with HCC in both cohorts. DCA was used to evaluate the net benefit under different clinical decisions at a certain threshold probability. The model was useful between threshold probabilities of 48%–89%.
According to the statistical analysis of nonrandom split-data from a large retrospective cohort, we developed and validated a new preoperative prediction model for MVI in patients with HCC. The obtained nomogram could effectively distinguish between patients with and without MVI preoperatively and showed good agreement between the predicted probability and actual frequency of MVI.
MVI is common in HCC and reflects the high invasion and metastasis capacities of the tumor early. Even in small HCCs (< 3 cm), the incidence of MVI is still above 20%[11,12], and MVI is an important hidden danger of postoperative recurrence and poor outcomes. The Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition) emphasize that MVI is an important basis for assessing the risk of recurrence of HCC and the choice of treatment options and should be used as a routine pathological examination index[4]. In our study, the incidence of MVI was close to 46% in a total of 454 patients, and the incidence in small HCCs was 21.4%, consistent with the literature.
Additionally, the presence of MVI often affects the choice of clinical treatment options and postoperative efficacy. Cucchetti et al[13] reported that, compared with nonanatomical resection, anatomical resection can reduce the early recurrence rate after hepatic resection for early-stage HCC patients with poor differentiation or with MVI. Mazzaferro et al[14] demonstrated that preoperative assessment of the absence of MVI is crucial for selecting candidates for transplantation. For patients without MVI, the Milan criteria can be expanded to achieve the same expected survival outcomes as patients within the Milan criteria, whereas the presence of MVI doubles the hazard of recurrence and death. Vitale et al[15] reported that MVI has a strong negative impact on the benefit of liver transplantation and that hepatic resection should be preferred to liver transplantation in HCC patients within the Milan criteria who are predicted to be at high risk for MVI before surgery. Therefore, how to accurately predict MVI to optimize the treatment plan is the main problem faced by surgeons. However, there is no uniform scheme or standard for the preoperative prediction of MVI both in China and other countries.
Previous studies have confirmed that tumor diameter, number of tumors, AFP, protein induced by vitamin K absence or antagonist-II (PIVKA-II), inflammation-related indicators, etc. are independent risk factors for MVI, but the univariate analyses lack sensitivity and specificity for MVI prediction, resulting in limited clinical applications. Therefore, some clinical prediction models that combine clinical features, laboratory parameters, and imaging characteristics have been established to make accurate MVI predictions more likely. Lei et al[16] developed a nomogram that combines seven factors, namely, nodule number, tumor diameter, capsule, serum AFP, platelet count, hepatitis B virus DNA load, and typical dynamic pattern of tumors on contrast-enhanced MRI, for the preoperative prediction of MVI in HBV-related HCC within the Milan criteria. Xu et al[17] created a new algorithm based on large-scale clinico-radiologic and radiomic features, including AST, AFP, tumor margin, growth pattern, capsule, peritumoral enhance, radio-genomic venous invasion and, radiomic score, that showed good performance in predicting MVI for patients with HCC.
As opposed to radiomic characteristics, our model was built from routine laboratory parameters and has potential advantages in standardization and popularization. In our report, tumor size, number of tumors, neutrophil count, and serum AFP were identified as independent risk factors significantly associated with MVI. Although PIVKA-II has been reported to be a predictor of MVI[18], it was eliminated as an initial variable in the data analysis, because PIVKA-II is not a routine laboratory test for HCC in our institution. HBV is the most important leading cause of HCC in China, whereas our results indicate that 20% of HCC is unrelated to HBV. The univariate analysis showed no significant difference between non-HBV- and HBV-related HCC. Compared to previous studies that limited the predicted population to patients with HBV-related HCC, our nomogram has a greater application scope.
Another notable predictor included was neutrophils. A number of circulating inflammatory markers from routine laboratory parameters, such as neutrophil, lymphocyte, and platelet counts and combined inflammatory scores, have been reported to have prognostic or clinically predictive value in patients with HCC[19]. As the most reported inflammatory score, NLR has been included in some nomograms to calculate the prediction probability of MVI in patients with HCC before surgery[20,21]. In our report, the neutrophil count was independently associated with MVI, and this is currently rarely reported. Hepatocarcinogenesis has been proven to be inextricably linked to inflammation. Most HCCs are accompanied by a background of chronic liver disease. Although the etiology and mechanisms vary, inflammation in HCC is uniform. The intricate interaction between the tumor itself and its microenvironment and the host immune response forms the basis for the progression of inflammation-driven HCC. A large multicenter cohort study demonstrated that a high level of neutrophils is the only significant and independent risk factor for driving progression and a poor prognosis in HCC compared to lymphocytes and platelets[22]. In recent years, the mechanism by which neutrophils exert protumoral activity has gradually been revealed. Neutrophils may be classified into several subtypes due to phenotypic switching mediated by the tumor microenvironment and show polarization, plasticity, and protumor/antitumor functions[23]. It has been proven that tumor-associated neutrophils can promote the development of HCC and therapeutic resistance by recruiting macrophages and Treg cells. Furthermore, neutrophils can form neutrophil extracellular traps (NETs) to capture circulating tumor cells and promote tumor metastasis[24-26]. The above evidence indicates the important role of neutrophils in the development of HCC, which requires attention and further research.
From a clinical point of view, surgeons often have to consider both risks and benefits when making treatment decisions. As mentioned above, MVI-positive patients who undergo anatomical resection to reduce the recurrence rate also face the risks of bleeding and liver failure due to the large resection range. Another issue to address is the allocation between the selection of suitable transplant candidates and the scarce liver resources in reality. Therefore, through clinical decision analysis, we provide the threshold probability range of the model with clinical net benefit that could help clinicians balance the risks and benefits under different conditions.
Undeniably, our study still had some limitations. First, all the data analyzed in this study were obtained from a single institution, and data from other centers are needed to further verify the reliability of the model. Second, the neutrophil count was considered an important predictor in our study. As a common inflammatory marker in peripheral blood, a rise in neutrophil levels usually occurs due to infections or injuries. However, "antiviral treatment", "infection", and "trauma" were set as the only three control conditions in our model. In fact, the neutrophil count can also fluctuate under the influence of various factors, such as time, eating, exercise, pain, and emotion. How to avoid or correct the effects of these factors on neutrophils is a challenge.
In conclusion, we have developed and validated a preoperative prediction model for MVI in patients with HCC. With the inclusion of two tumor features (number of tumors and tumor size) and two laboratory parameters (serum AFP and neutrophil count), our prediction model could effectively differentiate between HCC patients with and without MVI and provide a reliable basis for clinicians to optimize preoperative decisions.
Microvascular invasion (MVI) is a definite risk factor of early recurrence and poor surgical outcomes of hepatocellular carcinoma (HCC). Accurate preoperative prediction of MVI is helpful for the choice of clinical treatment options and evaluation of postoperative efficacy.
Histologic examination of the surgical specimens is the only reliable method to diagnose MVI. There is an urgent need for an effective tool to predict MVI preoperatively.
This study aimed to construct a new prediction model, mainly based on routine laboratory parameters, to achieve more accurate prediction for MVI in patients with HCC before surgery.
In this retrospective study, data from 454 patients with HCC who underwent hepatectomy were collected and nonrandomly split into a training cohort and a validation cohort. Univariate and multivariable logistic regression analyses were performed to identify variables significantly associated with MVI, and a new preoperative prediction model for MVI was established and further validated.
The incidence of MVI was 46.0% in patients with hepatectomy. Tumor size, number of tumors, neutrophils, and serum α-fetoprotein were identified as independent significant factors associated with MVI. A nomogram was established and showed good performance in the evaluation of discrimination and calibration.
This prediction model could effectively predict MVI with good discrimination and calibration ability.
Data from other centers are needed to further validate the clinical usability of this novel model.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56675] [Article Influence: 7084.4] [Reference Citation Analysis (135)] |
| 2. | Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 723] [Article Influence: 65.7] [Reference Citation Analysis (1)] |
| 3. | Roayaie S, Obeidat K, Sposito C, Mariani L, Bhoori S, Pellegrinelli A, Labow D, Llovet JM, Schwartz M, Mazzaferro V. Resection of hepatocellular cancer ≤ 2 cm: results from two Western centers. Hepatology. 2013;57:1426-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 326] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 4. | Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, Yang JM, Bie P, Liu LX, Wen TF, Han GH, Wang MQ, Liu RB, Lu LG, Ren ZG, Chen MS, Zeng ZC, Liang P, Liang CH, Chen M, Yan FH, Wang WP, Ji Y, Cheng WW, Dai CL, Jia WD, Li YM, Li YX, Liang J, Liu TS, Lv GY, Mao YL, Ren WX, Shi HC, Wang WT, Wang XY, Xing BC, Xu JM, Yang JY, Yang YF, Ye SL, Yin ZY, Zhang BH, Zhang SJ, Zhou WP, Zhu JY, Liu R, Shi YH, Xiao YS, Dai Z, Teng GJ, Cai JQ, Wang WL, Dong JH, Li Q, Shen F, Qin SK, Fan J. Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition). Liver Cancer. 2018;7:235-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 463] [Article Influence: 57.9] [Reference Citation Analysis (1)] |
| 5. | Rodríguez-Perálvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol. 2013;20:325-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 531] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 6. | Feng ST, Jia Y, Liao B, Huang B, Zhou Q, Li X, Wei K, Chen L, Li B, Wang W, Chen S, He X, Wang H, Peng S, Chen ZB, Tang M, Chen Z, Hou Y, Peng Z, Kuang M. Preoperative prediction of microvascular invasion in hepatocellular cancer: a radiomics model using Gd-EOB-DTPA-enhanced MRI. Eur Radiol. 2019;29:4648-4659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (1)] |
| 7. | Ma X, Wei J, Gu D, Zhu Y, Feng B, Liang M, Wang S, Zhao X, Tian J. Preoperative radiomics nomogram for microvascular invasion prediction in hepatocellular carcinoma using contrast-enhanced CT. Eur Radiol. 2019;29:3595-3605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 177] [Article Influence: 25.3] [Reference Citation Analysis (1)] |
| 8. | Ni M, Zhou X, Lv Q, Li Z, Gao Y, Tan Y, Liu J, Liu F, Yu H, Jiao L, Wang G. Radiomics models for diagnosing microvascular invasion in hepatocellular carcinoma: which model is the best model? Cancer Imaging. 2019;19:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (1)] |
| 9. | Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Iñarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1962] [Cited by in RCA: 2172] [Article Influence: 197.5] [Reference Citation Analysis (0)] |
| 10. | Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350:g7594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1604] [Cited by in RCA: 2641] [Article Influence: 240.1] [Reference Citation Analysis (0)] |
| 11. | Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, Yamaoka Y, Belghiti J, Lauwers GY, Poon RT, Abdalla EK. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 527] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 12. | Onaca N, Davis GL, Jennings LW, Goldstein RM, Klintmalm GB. Improved results of transplantation for hepatocellular carcinoma: a report from the International Registry of Hepatic Tumors in Liver Transplantation. Liver Transpl. 2009;15:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Cucchetti A, Qiao GL, Cescon M, Li J, Xia Y, Ercolani G, Shen F, Pinna AD. Anatomic versus nonanatomic resection in cirrhotic patients with early hepatocellular carcinoma. Surgery. 2014;155:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 14. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P; Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1619] [Article Influence: 89.9] [Reference Citation Analysis (1)] |
| 15. | Vitale A, Cucchetti A, Qiao GL, Cescon M, Li J, Ramirez Morales R, Frigo AC, Xia Y, Tuci F, Shen F, Cillo U, Pinna AD. Is resectable hepatocellular carcinoma a contraindication to liver transplantation? A novel decision model based on "number of patients needed to transplant" as measure of transplant benefit. J Hepatol. 2014;60:1165-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Lei Z, Li J, Wu D, Xia Y, Wang Q, Si A, Wang K, Wan X, Lau WY, Wu M, Shen F. Nomogram for Preoperative Estimation of Microvascular Invasion Risk in Hepatitis B Virus-Related Hepatocellular Carcinoma Within the Milan Criteria. JAMA Surg. 2016;151:356-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 476] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 17. | Xu X, Zhang HL, Liu QP, Sun SW, Zhang J, Zhu FP, Yang G, Yan X, Zhang YD, Liu XS. Radiomic analysis of contrast-enhanced CT predicts microvascular invasion and outcome in hepatocellular carcinoma. J Hepatol. 2019;70:1133-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 545] [Article Influence: 77.9] [Reference Citation Analysis (1)] |
| 18. | Poté N, Cauchy F, Albuquerque M, Voitot H, Belghiti J, Castera L, Puy H, Bedossa P, Paradis V. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol. 2015;62:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 266] [Article Influence: 24.2] [Reference Citation Analysis (4)] |
| 19. | Sanghera C, Teh JJ, Pinato DJ. The systemic inflammatory response as a source of biomarkers and therapeutic targets in hepatocellular carcinoma. Liver Int. 2019;39:2008-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 20. | Deng G, Yao L, Zeng F, Xiao L, Wang Z. Nomogram For Preoperative Prediction Of Microvascular Invasion Risk In Hepatocellular Carcinoma. Cancer Manag Res. 2019;11:9037-9045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Li P, Huang W, Wang F, Ke YF, Gao L, Shi KQ, Zhou MT, Chen BC. Nomograms based on inflammatory biomarkers for predicting tumor grade and micro-vascular invasion in stage I/II hepatocellular carcinoma. Biosci Rep. 2018;38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Margetts J, Ogle LF, Chan SL, Chan AWH, Chan KCA, Jamieson D, Willoughby CE, Mann DA, Wilson CL, Manas DM, Yeo W, Reeves HL. Neutrophils: driving progression and poor prognosis in hepatocellular carcinoma? Br J Cancer. 2018;118:248-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 23. | Giese MA, Hind LE, Huttenlocher A. Neutrophil plasticity in the tumor microenvironment. Blood. 2019;133:2159-2167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 562] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 24. | Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 1086] [Article Influence: 83.5] [Reference Citation Analysis (0)] |
| 25. | Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, Schott AF, Kinugasa-Katayama Y, Lee Y, Won NH, Nakasone ES, Hearn SA, Küttner V, Qiu J, Almeida AS, Perurena N, Kessenbrock K, Goldberg MS, Egeblad M. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med. 2016;8:361ra138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 761] [Article Influence: 84.6] [Reference Citation Analysis (0)] |
| 26. | van der Windt DJ, Sud V, Zhang H, Varley PR, Goswami J, Yazdani HO, Tohme S, Loughran P, O'Doherty RM, Minervini MI, Huang H, Simmons RL, Tsung A. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology. 2018;68:1347-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 395] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited Manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kang KJ, Hann HW S-Editor: Wang YQ L-Editor: Wang TQ E-Editor: Ma YJ