Published online Mar 14, 2020. doi: 10.3748/wjg.v26.i10.1020
Peer-review started: October 28, 2019
First decision: December 12, 2019
Revised: January 6, 2020
Accepted: March 9, 2020
Article in press: March 9, 2020
Published online: March 14, 2020
Processing time: 138 Days and 10 Hours
Rhabdomyolysis is a syndrome of skeletal muscle injury with release of cellular constituents such as potassium, phosphate, urate and intracellular proteins such as myoglobin into the circulation, which may cause complications including acute kidney injury, electrolyte disturbance and cardiac instability. Abnormal liver function tests are frequently observed in cases of severe rhabdomyolysis. Typically, there is an increase in serum aminotransferases, namely aspartate aminotransferase and alanine aminotransferase. This raises the question of liver injury and often triggers a pathway of investigation which may lead to a liver biopsy. However, muscle can also be a source of the increased aminotransferase activity. This review discusses the dilemma of finding abnormal liver function tests in the setting of muscle injury and the potential implications of such an association. It delves into some of the clinical and experimental evidence for correlating muscle injury to raised aminotransferases, and discusses pathophysiological mechanisms such as oxidative stress which may cause actual liver injury. Serum aminotransferases lack tissue specificity to allow clinicians to distinguish primary liver injury from muscle injury. This review also explores potential approaches to improve the accuracy of our diagnostic tools, so that excessive or unnecessary liver investigations can be avoided.
Core tip: There is observational and experimental data demonstrating that serum alanine and aspartate aminotransferases can be elevated in patients with rhabdomyolysis due to muscle release of these enzymes, and cause confusion with liver disease. Clinicians should firstly appreciate this association exists and secondly, understand the typical pattern and trajectory of the levels of creatine kinase and aminotransferases in the setting of rhabdomyolysis. An atypical trajectory, concurrently elevated bilirubin or γ-glutamyl transferase, or serum alanine aminotransferase levels above 800 U/L are inconsistent with isolated muscle injury as the cause of the elevated aminotransferases, and further investigation for liver disease may be warranted.
- Citation: Lim AK. Abnormal liver function tests associated with severe rhabdomyolysis. World J Gastroenterol 2020; 26(10): 1020-1028
- URL: https://www.wjgnet.com/1007-9327/full/v26/i10/1020.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i10.1020
Rhabdomyolysis is defined as muscle injury which is significant enough to result in release of potentially toxic cellular contents into the circulation. These cellular contents include metabolites such as potassium, phosphate and urate, enzymes such as creatine kinase (CK) and lactate dehydrogenase (LDH), and intracellular proteins such as myoglobin[1]. Serum CK is used to diagnose rhabdomyolysis and most studies use a cut-off of five times the upper limit of normal, or equivalent to 1000 U/L[1]. Symptoms of rhabdomyolysis are typically myalgias, weakness and dark urine due to myoglobinuria. Severe cases may result in compartment syndrome, electrolyte disturbance which may cause arrhythmia or cardiac instability, acute kidney injury (AKI) and disseminated intravascular coagulation[1-4]. Rhabdomyolysis was first recognised in war injuries and crush syndrome but many causes are now appreciated. The discussion of causes and treatment are beyond the scope of this review and are covered elsewhere[1-4]. The main aim of this review is to discuss the association between rhabdomyolysis and abnormal liver function tests.
The usual liver panel tests include bilirubin, alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT) and γ-glutamyl transferase (GGT). The aminotransferases (AST and ALT) are involved in liver gluconeogenesis and are good biomarkers for liver cell injury. AST is present in cytosolic and mitochondrial isoenzymes and is found in the liver, cardiac muscle, skeletal muscle, kidneys, brain, pancreas, lungs, leucocytes, and red cells. It is less sensitive and specific for the liver. On the other hand, ALT is a cytosolic enzyme which is more specific to the liver due to the high concentration in liver tissue. ALT is also found in skeletal muscle but in much lower concentrations[5].
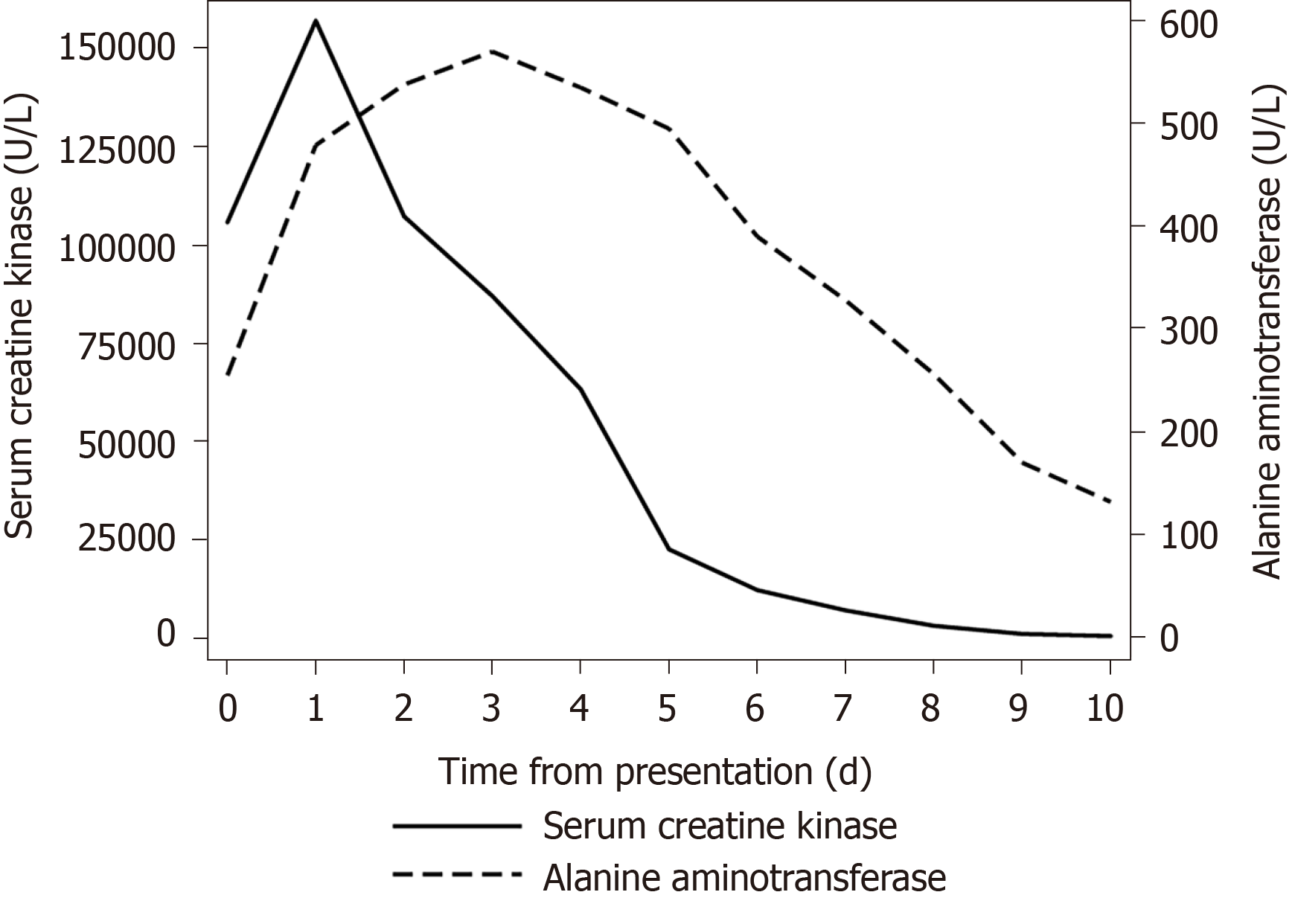
Abnormal liver function tests are frequently observed in patients with severe rhabdomyolysis but compared to electrolyte derangements, this is a much less appreciated phenomenon that is still shrouded in uncertainty. An example of such a case is shown in Figure 1.
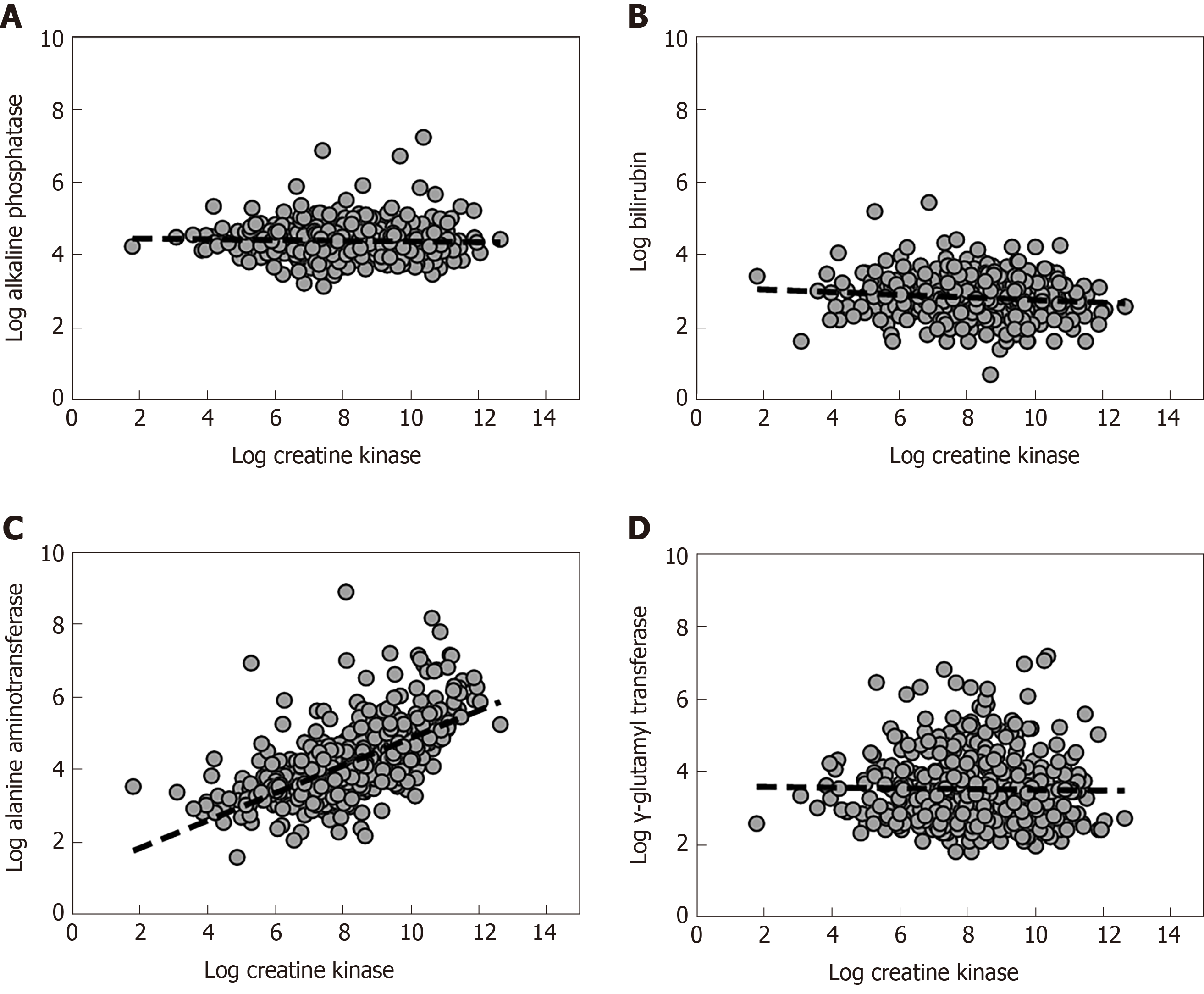
The quandary facing the clinician is determining the source of the elevated aminotransaminases in rhabdomyolysis, as the difference in aminotransferases between liver and muscle is quantitative rather than qualitative. At the first instance, the other liver markers may be useful for differentiating a muscle or liver source of AST or ALT[6]. GGT is not found in muscle and would suggest liver injury if elevated. Similarly, elevation in bilirubin would not be expected in isolated muscle injury. The association between serum CK and liver biochemistry is demonstrated in Figure 2. The uncertainty arises in patients with elevated CK and isolated increase in aminotransferases. Is it liver, muscle or both?
Why is the association between rhabdomyolysis and abnormal liver function tests important to recognise? Abnormalities of liver function tests are relatively common and liver injury is frequently associated with many commonly used medications. On the other hand, rhabdomyolysis is uncommon, and symptoms may be relatively mild.
When there is no history of muscle disease or injury, clinicians may erroneously attribute elevated aminotransferases to liver injury. Even with known rhabdomyolysis, additional tests for liver disease (biochemistry, serology and molecular) have mostly been negative, and 30%-50% of hepatobiliary imaging (sonography and computed tomography) were normal with most abnormalities consistent with hepatic steatosis[6]. Consequently, this may result in unnecessary liver tests, including invasive tests such as a liver biopsy, which mostly reveals no abnormalities[7]. As a corollary of over-investigating for liver disease, there could be a failure to recognise and investigate muscle disease. For example, a 27-year old man endured seven years of investigations for abnormal aminotransferases and had two liver biopsies, before the identification of an elevated CK led to a final diagnosis of muscular dystrophy[8].
The finding of abnormal liver function tests is of significant concern in clinical trials of investigational products and may potentially see novel drug development falter. For example, in vaccine trials, vigorous physical activity have been known to confound results and interpretation of liver function tests, where increased CK and aminotransferases were noted[9,10]. Recognising the association could also prevent unnecessary avoidance of useful or critical treatments which are potentially hepatotoxic[11].
There are studies suggesting that abnormal liver function tests in patients with muscle injury is associated with higher mortality in some clinical contexts. In critically ill patients with rhabdomyolysis, patients who had a AST or ALT over 1000 U/L had a higher mortality than those with levels below 1000 U/L (61% vs 15%)[12]. In another rhabdomyolysis study, a higher CK/ALT ratio was associated with lower mortality, even after adjusting for age, AKI and sepsis[6]. In the general population, abnormal liver function is associated with increased all-cause mortality. Where ALT is concerned, there is geographical variation and Asian populations seem to have a higher risk than North American populations[13]. The reason for the association with mortality is not clear and should be investigated.
Is there data to demonstrate that muscle is the source of elevated aminotransferases? If we subscribe to this theory, a few basic facts need to be demonstrated. Firstly, aminotransferases can be localised to muscle at a cellular level. Secondly, we can increase serum aminotransferases by inducing muscle injury. Thirdly, we can show that the elevated aminotransferases normalise with resolution of muscle injury. Ideally, we should be able to demonstrate that no other evidence of liver injury exists.
In diverse animal species, ALT, AST and LDH can be found in multiple tissues, including kidney, liver and muscle[14,15]. Yang et al[16] used molecular methods to quantify the distribution of ALT1 and ALT2 mRNA in rats, and showed that ALT1 is mainly expressed (from high to low) in the intestines, liver, fat, colon, muscle and heart. ALT2 mRNA is more limited in distribution, to liver, muscle, brain and white adipose tissue. ALT1 is mostly intracytoplasmic while the more abundant ALT2 is localised to mitochondria. In humans, ALT is more specific for the liver than AST but it is known to exist in red blood cells, kidney, brain, heart and skeletal muscle[17]. However, there is paucity of data on the quantitative differences between tissues. Wroblewski[17] reported that ALT was 10 times more abundant in the liver than muscle. Apple and Rogers suggested that the ratio of the amount of ALT between an equivalent weight of muscle and liver was 1:4[18]. The differences between studies may reflect the method of measurement, and a more detailed analysis may be more revealing.
There are a few human studies which provided proof of concept for the association between muscle injury and elevated aminotransferases. Pettersson et al[19] subjected 15 fit young men aged 18-45 years to a one-hour weightlifting session. They demonstrated that AST, ALT, LDH, CK and myoglobin were significantly increased after the activity, and it took at least 7 d to normalise, while the bilirubin, ALP and GGT remained normal. In a study by Pal et al[20], 44 post-pubertal boys and girls underwent intensive treadmill exercise. They also demonstrated that serum ALT and AST increased significantly at 24 and 48 h in association with a raised CK and LDH. The effects were more pronounce in boys compared to girls. These prospective clinical studies provide better evidence than case reports because they were performed in otherwise healthy individuals with normal baseline biochemistry. There is also less risk of confounding by concurrent illness and medication use.
In a histological study, Apple and Rogers examined the serum and muscle activity of ALT in 30 marathon runners for acute changes. The investigators performed gastrocnemius muscle biopsies at 9 wk and 48 h prior to the marathon, and at 24 h after. They showed that serum ALT was significantly elevated at 24 h after the race compared to pre-race levels. ALT levels remained elevated at 96 h. The investigators noted that the muscle content of ALT did not significantly increase after the race and believed that the elevated serum ALT was more likely due to hepatic release[18]. It is difficult to support the conclusion of Apple and Rogers. They also showed that the ALT activity per wet tissue weight of muscle was around 20% that of the liver (12 U/g vs 50 U/g). The fallacy in their argument lies in the fact that absolute skeletal muscle mass far exceeds liver mass, being estimated at 21 kg in women and 33 kg in men, on average[21]. Trivial changes in one gram of muscle tissue may be significant when amplified 20000 times.
As shown in our case example, the temporal changes in CK and ALT is fairly typical in observational data of rhabdomyolysis as well as in human experimental data[19,22]. A significant rise in CK is usually detectable within 24 h after the inciting injury, peaks around 72 h, and declines over a period of 7 to 12 d. A significant rise in AST is usually detectable at 24 h when ALT may still be in the normal range until 48 h. The AST tends to peak around 3 to 4 d and the ALT peaks later at 4 to 5 d post injury. The AST tends to be higher than ALT such that the AST/ALT ratio is usually greater than one. In the case report of exertional rhabdomyolysis, the average AST/ALT ratio in the first seven days of admission was 3.0 (range, 1.24 to 4.72)[22]. The peak AST/ALT tends to occur at the same time as peak AST, on day 3 from onset of injury[19]. With severe rhabdomyolysis, the aminotransferases may remain abnormal for 2 to 3 wk. There may be a period when CK has normalised but aminotransferases remain elevated.
Mathur et al[11] conducted an observational study of inflammatory myopathy patients and demonstrated that abnormal serum aminotransferases follow the CK levels. In 85 patients with inflammatory myopathy and a mean CK of over 5000 U/L, the peak CK level was strongly correlated with the AST (r = 0.87) and ALT (r = 0.84). At the peak CK level, the mean ± SD for the AST was 215 ± 227 U/L, and the mean ± SD for ALT was 137 ± 137 U/L. More importantly, aminotransferases normalised in 85% of patients at the time of CK normalisation after treatment[11].
In observational studies, the timing of the inciting injury relative to presentation or admission cannot always be accurately determined, and the biomarkers associated with rhabdomyolysis may be either rising or falling. Treatment with intravenous fluids often accelerates CK decline with little effect on aminotransferases, as CK is predominantly cleared by the kidneys but ALT is cleared by the liver itself.
So far, we assumed aminotransferases are released from injured muscle but there may be other mechanisms which cause liver injury. An earlier report suggested that proteases are released after rhabdomyolysis[23]. There have been other hypotheses but good evidence is not available. For an indirect mechanism to have a role, injured muscles should release mediators which exert a systemic effect. The best candidate is probably oxidative stress.
In rats, Plotnikov et al[24] found that myoglobin released from muscle causes lipid peroxidation of mitochondrial membranes, mitochondrial dysfunction and oxidative stress in renal tubular cells. Oxidative stress and lipid peroxidation of fatty acids may be responsible for increased F2-isoprostanes, which promote inflammation, endothelial dysfunction, vasoconstriction, and apoptosis[25]. In experimental rhabdomyolysis, Okubo et al[26] demonstrated that heme-activated platelets released from necrotic muscle promoted release of macrophage extracellular traps. The role of innate immune cells in inflammation and apoptosis is further supported by Kim et al[27], noting macrophage depletion is protective against AKI. In humans, Pereira et al[28] supported the concept of systemic inflammation in a case report of a soldier who presented with exertional rhabdomyolysis. Investigations showed elevated levels of proinflammatory cytokines (interleukin-1 and interleukin-6) and microvascular dysfunction which persisted for one week.
There is paucity of basic science research in the liver context, particularly for a direct role of myoglobin in inciting liver injury. There are limited studies implicating oxidative stress in the liver related to rhabdomyolysis. Pal et al[20] examined the effects of intense exercise in post-pubertal boys and girls, and demonstrated elevated serum CK and aminotransferases at 24 h and 48 h post-exertion. As evidence of oxidative stress, they showed that serum catalase activity and thiobarbituric acid-reactive substances (a marker of lipid peroxidation) were increased above baseline[20]. Georgakouli et al[29] also reported similar findings of increased aminotransferases, catalase and total antioxidant capacity in heavy alcohol drinkers after moderate intensity exercise.
In the setting of rhabdomyolysis, what is an expected level of ALT rise given the degree of muscle injury? In other words, is there a threshold for concern to justify further tests for liver disease? Alternatively, can we use other markers to help clinical decision making?
One simple idea is to adjust the aminotransferase level for other markers concurrently released by injured muscles but not liver. The most relevant in rhabdomyolysis is the CK/ALT ratio. Wang et al[30] examined the CK/ALT and CK/AST ratios in an experimental model of dystrophinopathy associated with acute liver injury, and in humans with dystrophinopathy. The authors reported that the CK/ALT ratio can differentiate between normal liver, acute liver injury and dystrophinopathy with or without liver injury, in their mouse model. In their patients, the CK/ALT ratio showed promise as it was less affected by age and other factors associated with muscle injury. Radke et al[31] demonstrated a significantly higher CK/ALT and CK/AST ratios in patients with rhabdomyolysis compared to patients who overdose on acetaminophen. The median CK/ALT ratio was 37.1 with rhabdomyolysis compared to 5.8 with acetaminophen overdose. At a cut-off CK/ALT ratio of 15, the sensitivity was 67% and specificity was 77%[31]. To differentiate liver from muscle injury, a high specificity is desirable, thus the CK/ALT ratio is far from ideal but an improvement on ALT alone. Given the non-linear correlation between CK and ALT, the utility of the ratio of the log-transformed CK to log-transformed ALT should be further studied[6].
Looking beyond CK and aminotransferases, plasma microRNAs (miRNAs) show promise. MicroRNAs are small, endogenous non-coding RNA molecules of around 22 nucleotides, which serves as gene regulators. Some microRNAs are cell and tissue-specific, and generally remain stable in plasma. They can be measured with sensitive molecular methods. In animal experiments, Laterza et al[32] examined miR-122 and miR-133a for liver and muscle injury, respectively. miR-122 was elevated in liver but not muscle injury, and miR-133a was elevated in muscle but not liver injury. Bailey et al[33] conducted an extensive evaluation of miRNAs with the goal of utilising them in clinical trials to distinguish liver from muscle injury. Among the promising candidates were miR-1, miR-133a, miR-133b and miR-206 for muscle, and miR-122 and miR-192 for liver. In animal experiments, these biomarkers showed superior specificity to CK and aminotransferases for muscle and liver injury respectively[33].
Goldstein[34] reported on work of the Predictive Safety Testing Consortium, which included a muscle injury biomarker panel. The biomarkers were CK (mass assay), fatty acid-binding protein 3, skeletal troponin I and myosin light chain 3. Experimentally, the accuracy of these biomarkers was assessed in tetramethyl-p-phenylenediamine induced skeletal muscle injury and acetaminophen-induced liver injury. AST levels could not be used to distinguish liver from muscle injury but the novel biomarkers showed higher specificity and correctly determined which tissue was injured histologically[34].
Some studies suggest that the relationship between serum CK and aminotransferases is approximately linear. In muscular dystrophy, Wang et al[30] showed a positive linear correlation between CK and ALT (r = 0.75), and between CK and AST (r = 0.79). The levels of CK and aminotransferases were dependent on age but this variability was diminished with the CK/ALT ratio. However, data from Weibrecht et al[35] suggested that neither ALT nor peak CK is normally distributed in rhabdomyolysis. Lim et al[6] confirmed this, and showed by linear and polynomial regression that the best functional form of this association is a linear relationship between the log-transformed CK and log-transformed ALT. The log-transformed CK, AKI stage, chronic liver disease and age together accounted for 46% of the observed variance in ALT. It is also possible to predict the ALT based on a regression model with these factors. In the worst case scenario, it was not common (< 5% chance) for the predicted serum ALT to exceed 500 U/L (for peak CK up to 160000 U/L), on average[6]. Even extrapolating to a peak CK of 400000 U/L, it was unlikely (< 1% chance) for it to exceed 800 U/L.
In such quantitative studies, consideration of confounding is important. One important factor is kidney function. Aminotransferases are much higher in patients with rhabdomyolysis and AKI compared to those without AKI[6,36]. On the other hand, there is an ordinal relationship between chronic kidney disease (CKD) and baseline aminotransferase levels. Compared to healthy individuals, patients with pre-dialysis CKD have lower aminotransferase levels, while patients on dialysis have the lowest levels[37,38]. Serum aminotransferases are also affected by age. In patients with rhabdomyolysis, Lau-Hing Yim et al[39] noted that peak CK showed a negative linear correlation with age (r = -0.42). However, AST tends to be more stable and as a result, the AST/ALT ratio increases with age[40,41]. Elinav et al[42] proposed an inverted U-shaped relationship between age and ALT. In their analysis, they included patients with a wide age range and showed that ALT peaked at 40-55 years. In multiple regression, age-squared and sex showed a statistically significant association with ALT activity[42]. Finally, there may be a sex difference as well, with some studies indicating that females have a lower baseline aminotransferase and CK levels than men[42,43], while the post-exercise rise in CK was lower in females[19]. In addition to age and sex, paediatric studies have also showed that body mass index and pubertal stage influenced ALT levels[44].
In patients with an isolated rise in aminotransferases, rhabdomyolysis should be considered in the differential diagnosis but current diagnostic tools do not have adequate specificity to differentiate liver injury from isolated muscle injury. Further research is required to meet this clinical need, and novel approaches and biomarkers may prove useful. The predicted ALT from regression modelling and the biochemical pattern and trajectory may be useful adjuncts to guide decision making whether more extensive or invasive tests for liver disease is warranted in patients with rhabdomyolysis.
I would like to thank Marcus Robertson, my gastroenterology colleague, for his insightful comments on this topic.
| 1. | Chavez LO, Leon M, Einav S, Varon J. Beyond muscle destruction: a systematic review of rhabdomyolysis for clinical practice. Crit Care. 2016;20:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 327] [Cited by in RCA: 271] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 2. | Zutt R, van der Kooi AJ, Linthorst GE, Wanders RJ, de Visser M. Rhabdomyolysis: review of the literature. Neuromuscul Disord. 2014;24:651-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 265] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 3. | Chatzizisis YS, Misirli G, Hatzitolios AI, Giannoglou GD. The syndrome of rhabdomyolysis: complications and treatment. Eur J Intern Med. 2008;19:568-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 173] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 4. | Giannoglou GD, Chatzizisis YS, Misirli G. The syndrome of rhabdomyolysis: Pathophysiology and diagnosis. Eur J Intern Med. 2007;18:90-100. [PubMed] |
| 5. | Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med. 2000;342:1266-1271. [PubMed] |
| 6. | Lim AKH, Arumugananthan C, Lau Hing Yim C, Jellie LJ, Wong EWW, Junckerstorff RK. A cross-sectional study of the relationship between serum creatine kinase and liver biochemistry in patients with rhabdomyolysis. J Clin Med. 2019;9:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Veropalumbo C, Del Giudice E, Esposito G, Maddaluno S, Ruggiero L, Vajro P. Aminotransferases and muscular diseases: a disregarded lesson. Case reports and review of the literature. J Paediatr Child Health. 2012;48:886-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Lash T, Kraemer RR. Elevated liver enzymes indicating a diagnosis of limb-girdle muscular dystrophy. J Gen Intern Med. 2014;29:813-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Johnson C, Monath TP, Kanesa-Thasan N, Mathis D, Miller C, Shapiro S, Nichols R, McCarthy K, Deary A, Bedford P. Exercise-induced serum enzyme elevations confounding the evaluation of investigational drug toxicity. Report of two cases in a vaccine trial. Hum Vaccin. 2005;1:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 10. | Malinoski FJ. Strenuous exercise simulating hepatic injury during vaccine trials. Vaccine. 1992;10:39-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Mathur T, Manadan AM, Thiagarajan S, Hota B, Block JA. Serum transaminases are frequently elevated at time of diagnosis of idiopathic inflammatory myopathy and normalize with creatine kinase. J Clin Rheumatol. 2014;20:130-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Raurich JM, Llompart-Pou JA, Rodríguez-Yago M, Ferreruela M, Royo C, Ayestarán I. Role of Elevated Aminotransferases in ICU Patients with Rhabdomyolysis. Am Surg. 2015;81:1209-1215. [PubMed] |
| 13. | Kunutsor SK, Apekey TA, Seddoh D, Walley J. Liver enzymes and risk of all-cause mortality in general populations: a systematic review and meta-analysis. Int J Epidemiol. 2014;43:187-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 14. | Boyd JW. The mechanisms relating to increases in plasma enzymes and isoenzymes in diseases of animals. Vet Clin Pathol. 1983;12:9-24. [PubMed] |
| 15. | Franson JC, Murray HC, Bunck C. Enzyme activities in plasma, kidney, liver, and muscle of five avian species. J Wildl Dis. 1985;21:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Yang RZ, Park S, Reagan WJ, Goldstein R, Zhong S, Lawton M, Rajamohan F, Qian K, Liu L, Gong DW. Alanine aminotransferase isoenzymes: molecular cloning and quantitative analysis of tissue expression in rats and serum elevation in liver toxicity. Hepatology. 2009;49:598-607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 17. | Wroblewski F. The clinical significance of alterations in transaminase activities of serum and other body fluids. Adv Clin Chem. 1958;1:313-351. [PubMed] |
| 18. | Apple FS, Rogers MA. Serum and muscle alanine aminotransferase activities in marathon runners. JAMA. 1984;252:626-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Pettersson J, Hindorf U, Persson P, Bengtsson T, Malmqvist U, Werkström V, Ekelund M. Muscular exercise can cause highly pathological liver function tests in healthy men. Br J Clin Pharmacol. 2008;65:253-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 221] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 20. | Pal S, Chaki B, Chattopadhyay S, Bandyopadhyay A. High-Intensity Exercise Induced Oxidative Stress and Skeletal Muscle Damage in Postpubertal Boys and Girls: A Comparative Study. J Strength Cond Res. 2018;32:1045-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol (1985). 2000;89:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1672] [Cited by in RCA: 2027] [Article Influence: 78.0] [Reference Citation Analysis (6)] |
| 22. | Paidoussis D, Dachs, RJ. Severe rhabdomyolysis associated with a popular high-intensity at-home exercise program. J Med Cases. 2013;4:12-14. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 23. | Hörl WH, Gantert C, Auer IO, Heidland A. In vitro inhibition of protein catabolism by alpha 2-macroglobulin in plasma from a patient with posttraumatic acute renal failure. Am J Nephrol. 1982;2:32-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Plotnikov EY, Chupyrkina AA, Pevzner IB, Isaev NK, Zorov DB. Myoglobin causes oxidative stress, increase of NO production and dysfunction of kidney's mitochondria. Biochim Biophys Acta. 2009;1792:796-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Panizo N, Rubio-Navarro A, Amaro-Villalobos JM, Egido J, Moreno JA. Molecular Mechanisms and Novel Therapeutic Approaches to Rhabdomyolysis-Induced Acute Kidney Injury. Kidney Blood Press Res. 2015;40:520-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 26. | Okubo K, Kurosawa M, Kamiya M, Urano Y, Suzuki A, Yamamoto K, Hase K, Homma K, Sasaki J, Miyauchi H, Hoshino T, Hayashi M, Mayadas TN, Hirahashi J. Macrophage extracellular trap formation promoted by platelet activation is a key mediator of rhabdomyolysis-induced acute kidney injury. Nat Med. 2018;24:232-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 180] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 27. | Kim JH, Lee DW, Jung MH, Cho HS, Jeon DH, Chang SH, Park DJ. Macrophage depletion ameliorates glycerol-induced acute kidney injury in mice. Nephron Exp Nephrol. 2014;128:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Pereira F, Moraes R, Bavel D, Lorenzo AR, Tibirica E. Exertional Rhabdomyolysis after Military Training Paralleled by Systemic Microvascular Dysfunction and Plasma Cytokine Increase: A Case Report. Arq Bras Cardiol. 2019;113:294-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Georgakouli K, Manthou E, Fatouros IG, Deli CK, Spandidos DA, Tsatsakis AM, Kouretas D, Koutedakis Y, Theodorakis Y, Jamurtas AZ. Effects of acute exercise on liver function and blood redox status in heavy drinkers. Exp Ther Med. 2015;10:2015-2022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Wang L, Chen M, Xu M, Li J, Feng P, He R, Zhu Y, Li H, Lin J, Zhang C. Ratio of Creatine Kinase to Alanine Aminotransferase as a Biomarker of Acute Liver Injury in Dystrophinopathy. Dis Markers. 2018;2018:6484610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Radke JB, Algren DA, Chenoweth JA, Owen KP, Ford JB, Albertson TE, Sutter ME. Transaminase and Creatine Kinase Ratios for Differentiating Delayed Acetaminophen Overdose from Rhabdomyolysis. West J Emerg Med. 2018;19:731-736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Laterza OF, Lim L, Garrett-Engele PW, Vlasakova K, Muniappa N, Tanaka WK, Johnson JM, Sina JF, Fare TL, Sistare FD, Glaab WE. Plasma MicroRNAs as sensitive and specific biomarkers of tissue injury. Clin Chem. 2009;55:1977-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 484] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 33. | Bailey WJ, Barnum JE, Erdos Z, LaFranco-Scheuch L, Lane P, Vlasakova K, Sistare FD, Glaab WE. A Performance Evaluation of Liver and Skeletal Muscle-Specific miRNAs in Rat Plasma to Detect Drug-Induced Injury. Toxicol Sci. 2019;168:110-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Goldstein RA. Skeletal Muscle Injury Biomarkers: Assay Qualification Efforts and Translation to the Clinic. Toxicol Pathol. 2017;45:943-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | Weibrecht K, Dayno M, Darling C, Bird SB. Liver aminotransferases are elevated with rhabdomyolysis in the absence of significant liver injury. J Med Toxicol. 2010;6:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 36. | Akmal M, Massry SG. Reversible hepatic dysfunction associated with rhabdomyolysis. Am J Nephrol. 1990;10:49-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Fabrizi F, Lunghi G, Finazzi S, Colucci P, Pagano A, Ponticelli C, Locatelli F. Decreased serum aminotransferase activity in patients with chronic renal failure: impact on the detection of viral hepatitis. Am J Kidney Dis. 2001;38:1009-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 38. | Ray L, Nanda SK, Chatterjee A, Sarangi R, Ganguly S. A comparative study of serum aminotransferases in chronic kidney disease with and without end-stage renal disease: Need for new reference ranges. Int J Appl Basic Med Res. 2015;5:31-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 39. | Lau Hing Yim C, Wong EWW, Jellie LJ, Lim AKH. Illicit drug use and acute kidney injury in patients admitted to hospital with rhabdomyolysis. Intern Med J. 2019;49:1285-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Goh GB, Pagadala MR, Dasarathy J, Unalp-Arida A, Pai RK, Yerian L, Khiyami A, Sourianarayanane A, Sargent R, Hawkins C, Dasarathy S, McCullough AJ. Age impacts ability of aspartate-alanine aminotransferase ratio to predict advanced fibrosis in nonalcoholic Fatty liver disease. Dig Dis Sci. 2015;60:1825-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | McPherson S, Hardy T, Dufour JF, Petta S, Romero-Gomez M, Allison M, Oliveira CP, Francque S, Van Gaal L, Schattenberg JM, Tiniakos D, Burt A, Bugianesi E, Ratziu V, Day CP, Anstee QM. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am J Gastroenterol. 2017;112:740-751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 644] [Cited by in RCA: 706] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 42. | Elinav E, Ben-Dov IZ, Ackerman E, Kiderman A, Glikberg F, Shapira Y, Ackerman Z. Correlation between serum alanine aminotransferase activity and age: an inverted U curve pattern. Am J Gastroenterol. 2005;100:2201-2204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 43. | Neal RC, Ferdinand KC, Ycas J, Miller E. Relationship of ethnic origin, gender, and age to blood creatine kinase levels. Am J Med. 2009;122:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Bussler S, Vogel M, Pietzner D, Harms K, Buzek T, Penke M, Händel N, Körner A, Baumann U, Kiess W, Flemming G. New pediatric percentiles of liver enzyme serum levels (alanine aminotransferase, aspartate aminotransferase, γ-glutamyltransferase): Effects of age, sex, body mass index, and pubertal stage. Hepatology. 2018;68:1319-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shimizu Y S-Editor: Wang JL L-Editor: A E-Editor: Ma YJ