Published online Mar 7, 2019. doi: 10.3748/wjg.v25.i9.1158
Peer-review started: December 29, 2018
First decision: January 30, 2019
Revised: February 8, 2019
Accepted: February 15, 2019
Article in press: February 15, 2019
Published online: March 7, 2019
Processing time: 69 Days and 18 Hours
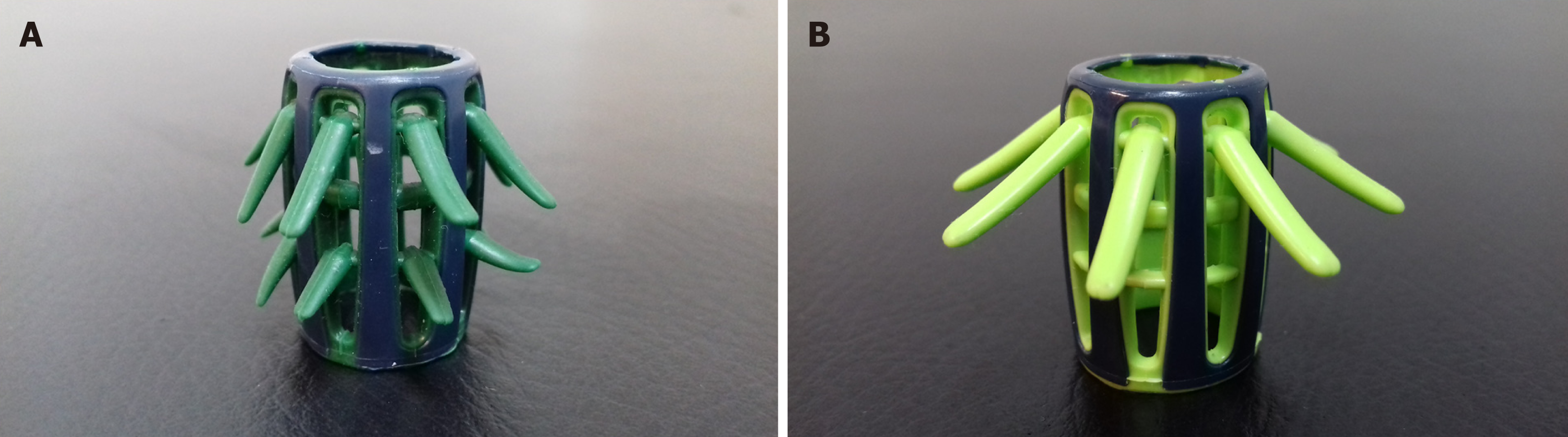
Endocuff - a plastic device with flexible projections - mounted on the distal tip of the colonoscope, promises improved colonic mucosa inspection.
To elucidate the effect of Endocuff on adenoma detection rate (ADR), advanced ADR (AADR) and mean number of adenomas per colonoscopy (MAC).
Literature searches identified randomized-controlled trials evaluating Endocuff-assisted colonoscopy (EAC) vs conventional colonoscopy (CC) in terms of ADR, AADR and MAC. The effect size on study outcomes was calculated using fixed or random effect model, as appropriate, and it is shown as relative risk (RR) [95% confidence interval (CI)] and mean difference (MD) (95%CI). The rate of device removal in EAC arms was also calculated.
We identified nine studies enrolling 6038 patients. All studies included mixed population (screening, surveillance and diagnostic examinations). Seven and two studies evaluated the first and the second-generation device, respectively. EAC was associated with increased ADR compared to CC [RR (95%CI): 1.18 (1.05-1.32); Ι2 = 71%]; EAC benefits more endoscopists with ADR ≤ 35% compared to those with ADR > 35% [RR (95%CI): 1.37 (1.08-1.74); Ι2 = 49% vs 1.10 (0.99-1.24); Ι2 = 71%]. In terms of AADR and MAC, no difference was detected between EAC and CC [RR (95%CI): 1.03 (0.85-1.25); Ι2 = 15% and MD (95%CI): 0.30 (-0.17-0.78); Ι2 = 99%]. Subgroup analysis did not show any difference between the two device generations regarding all three endpoints. In EAC arms, the device had to be removed in 3% (95%CI: 2%-5%) of the cases mainly due to tortuous sigmoid or presence of diverticula along it.
EAC increases ADR compared to CC, especially for endoscopists with lower ADR. On the other hand, no significant effect on AADR and MAC was detected.
Core tip: Colonoscopy is the optimal diagnostic modality for the detection and removal of colon adenomas. However, one fourth of them may remain undetected during conventional colonoscopy (CC). Endocuff - a single-use device mounted onto the tip of the scope - aims to improve lesion detection rate during colonoscopy. Our meta-analysis of nine randomized control studies including more than 6000 patients demonstrates the use of the Endocuff device significantly improves adenoma detection rate compared to CC, while endoscopists with lower adenoma detection rate may benefit at most from its use.
- Citation: Triantafyllou K, Gkolfakis P, Tziatzios G, Papanikolaou IS, Fuccio L, Hassan C. Effect of Endocuff use on colonoscopy outcomes: A systematic review and meta-analysis. World J Gastroenterol 2019; 25(9): 1158-1170
- URL: https://www.wjgnet.com/1007-9327/full/v25/i9/1158.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i9.1158
Colonoscopy is the preferred and most widely used method to screen for colorectal cancer (CRC) and it has been associated with decreased CRC incidence and deaths due to early detection and endoscopic resection of colorectal adenomas[1]. Adenoma detection rate (ADR) - the percentage of colonoscopies with at least one adenoma - is considered the cornerstone among quality indicators for colonoscopy since it is the only metric that can effectively predict post-colonoscopy CRC and is inversely associated with the risk of CRC cancer[2-4]. Nevertheless, colonoscopy remains an imperfect diagnostic modality, as it may fail to detect up to one fourth of existing colonic adenomas[5,6]. Accountable for this failure may be the poor bowel preparation, suboptimal maneuvering of the scope during withdrawal or difficulty to efficiently visualize the proximal aspects of the haustral folds, flexures or the ileocecal valve with conventional colonoscopy (CC). Various advanced technology endoscopes and add-on devices have been implemented in an effort to optimize the quality of colonoscopy by diminishing the procedures’ adenoma miss rate[7]. Endocuff (Arc Medical Design, Leeds, United Kingdom) and its descendant Endocuff-Vision (Norgine Pharmaceuticals Ltd, Uxbridge, United Kingdom) (Figure 1A and B) is a single-use device mounted on the tip of the scope that consists of a cylindrical core with one (Endocuff-Vision) or two (Endocuff) rows of flexible projections[8]. It is the add-on device with the most available literature, so far; still, its impact on ADR remains conflicting with data suggesting equivocal benefit from its use. Aside individual studies, three contemporary meta-analysis have tried to pool results of the aforementioned studies[9-11]. However, inclusion of different study designs, inconsistent endpoints among the studies analyzed and the high noted heterogeneity call for careful interpretation of their results. We therefore intended to re-evaluate the impact of Endocuff on ADR, through an updated systematic review with meta-analysis incorporating data from published randomized controlled trials (RCTs) and addressing limitations of previous meta-analysis.
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations[12] and the review’s protocol can be accessed at the International Prospective Register of Systematic Reviews (PROSPERO), under registration number CRD42018095779.
Eligibility criteria were a priori determined according to the PICO statement as follows; P: patients undergoing colonoscopy for any indication; I: Endocuff-assisted colonoscopy (EAC); C: comparison of EAC with CC; O: colonoscopy outcomes (as defined in the Outcome measures section). We included studies only if they were prospective, randomized controlled in design, published as full text in the English language. Non-randomized prospective, retrospective, feasibility or pilot studies, meta-analysis, editorials, narrative reviews, case reports/series, conference abstracts, studies not reporting on ADR and duplicate publications were excluded.
A literature search of PubMed and the Cochrane Central Register of Controlled Trials electronic databases (from database inception to October 2018) was carried out to identify studies exploring the effect of EAC on adenoma detection. The search was performed independently by two investigators using the free text terms “adenoma*”, “random*” both as medical subject headings (MeSH) and free-text terms combined with the Boolean set operator ‘AND’ with the term: “Endocuff”, as medical subject heading and the free text term. The complete electronic search strategy is outlined in Supplementary Table 1. All references retrieved from the electronic databases were imported into reference management software (EndNote X7, Thomson Reuters, New York City, NY, United States). After duplicates’ removal, two masked reviewers first assessed the titles and abstracts of all results for inclusion; then, judged eligibility on the selected articles independently, using predesigned eligibility forms. Disagreements were resolved by consensus. Bibliographies of all eligible studies identified initially, were also hand-searched to identify any potentially studies missed during the initial search. Where data could not be retrieved from the published manuscript, the corresponding author was contacted for further information.
| Author (yr) | Country | Study period | Centres, n | Endoscopists, n | Device | Endoscopes | Patients, n | EAC/CC, n/n | Indication | Female EAC/CC, n (%) | Age EAC/CC, mean ± SD | Screening EAC/CC, n (%) | Bowel Preparation EAC/CC |
| Floer et al[15] | Germany | 02.2014-07.2014 | 4 | 10 | Endocuff | HD | 492 | 249/243 | Mixed | 127 (51)/134 (55.1) | 64 ± 3.2/63 ± 3.3 | NR | 11 (1-2)/1 (1-2) [median (IQR)] |
| Van Doorn et al[16] | Netherlands | 08.2013-10.2014 | 5 | 20 | Endocuff | HD | 1063 | 530/533 | Mixed | 266 (50.2)/248 (46.5) | 65 ± 2.2/65 ± 2.3 | 201 (37.9)/197 (36.9) | 29 (7-9)/8 (7-9) [median (IQR)] |
| Biecker et al[17] | Germany | 02.2013-08.2013 | 2 | 6 | Endocuff | HD | 498 | 245/253 | Mixed | 127 (51.8)/122 (48.2) | 65 ± 3.3/68 ± 3 | NR | 169% good/65% good |
| De Palma et al[18] | Italy | 02.2015 -03.2016 | 1 | 4 | Endocuff | HD | 274 | 137/137 | Mixed | 66 (48.2)/65 (47.4) | 55 ± 12.6/55.7 ± 12.3 | 32 (23.4)/29 (21.2) | 7.08 ± 1.06/7.18 ± 0.97 [mean ± SD] |
| Bhattacharyya et al[19] | United Kingdom | 09.2014-09.2015 | 1 | 4 | Endocuff Vision | HD | 531 | 266/265 | FOBT (+) screening, surveillance | 104 (39.1)/85 (32.1) | 68 ± 1.2/67 ± 1.2 | 180 (70.7)/186 (69.1) | 3Good/adequate 97.7% /Good adequate 97.7% |
| González-Fernández et al[20] | Mexico | 04.2014-11.2015 | 1 | 18 | Endocuff | Mixed | 337 | 174/163 | Screening | 124 (71)/124 (76) | 60 ± 1.8/62 ± 2.5 | 174 (100)/163 (100) | 27 (6-8)/7 (6-8) [median (IQR)] |
| Ngu et al[21] | United Kingdom | 11.2014-02.2016 | 7 | 48 | Endocuff Vision | Not reported | 1772 | 888/884 | FOBT (+) screening, surveillance | 381 (42.9)/ 382 (43.2) | 61.7 ± 11.7/62.1 ± 11.1 | 274 (30.9)/282 (32) | NR |
| Wada et al[22] | Japan | 04.2015-09.2015 | 1 | 1 | Endocuff | HD | 477 | 239/238 | Mixed | 117 (48.9)/123 (51.7) | 61.2 ± 3.3/62.2 ± 3.3 | 89 (37.2)/74 (31.1) | 27.91 ± 0.94/7.88 ± 1.03 [mean ± SD] |
| Rex et al[23] | United States, Italy | NR | 3 | 3 | Endocuff | HD | 594 | 299/295 | Mixed | 141 (47) /141 (47) | 63.2 ± 8.2/62.6 ± 8.3 | 126 (42)/127 (43) | 28.12 ± 1.33 overall, no differences between the 2 groups |
Two reviewers independently recorded data from all eligible studies onto a Microsoft Excel spreadsheet (XP professional edition; Microsoft, Redmond, WA, United States) using a predefined data extraction form. The following data were extracted from each trial: first author’s name, publication year, country of origin, number of centers, number of participating endoscopists, indication for colonoscopy among the population examined, generation of the device used, number of total participants enrolled, number of EAC examinations, mean age of participants and percentage of female among them. Additionally, we extracted the number of adenomas, advanced adenomas, mean number of adenomas per patient detected during EAC and the standard colonoscopy. Finally, for each EAC arm we extracted the number of colonoscopies where the device had to be removed to allow examination’s completion.
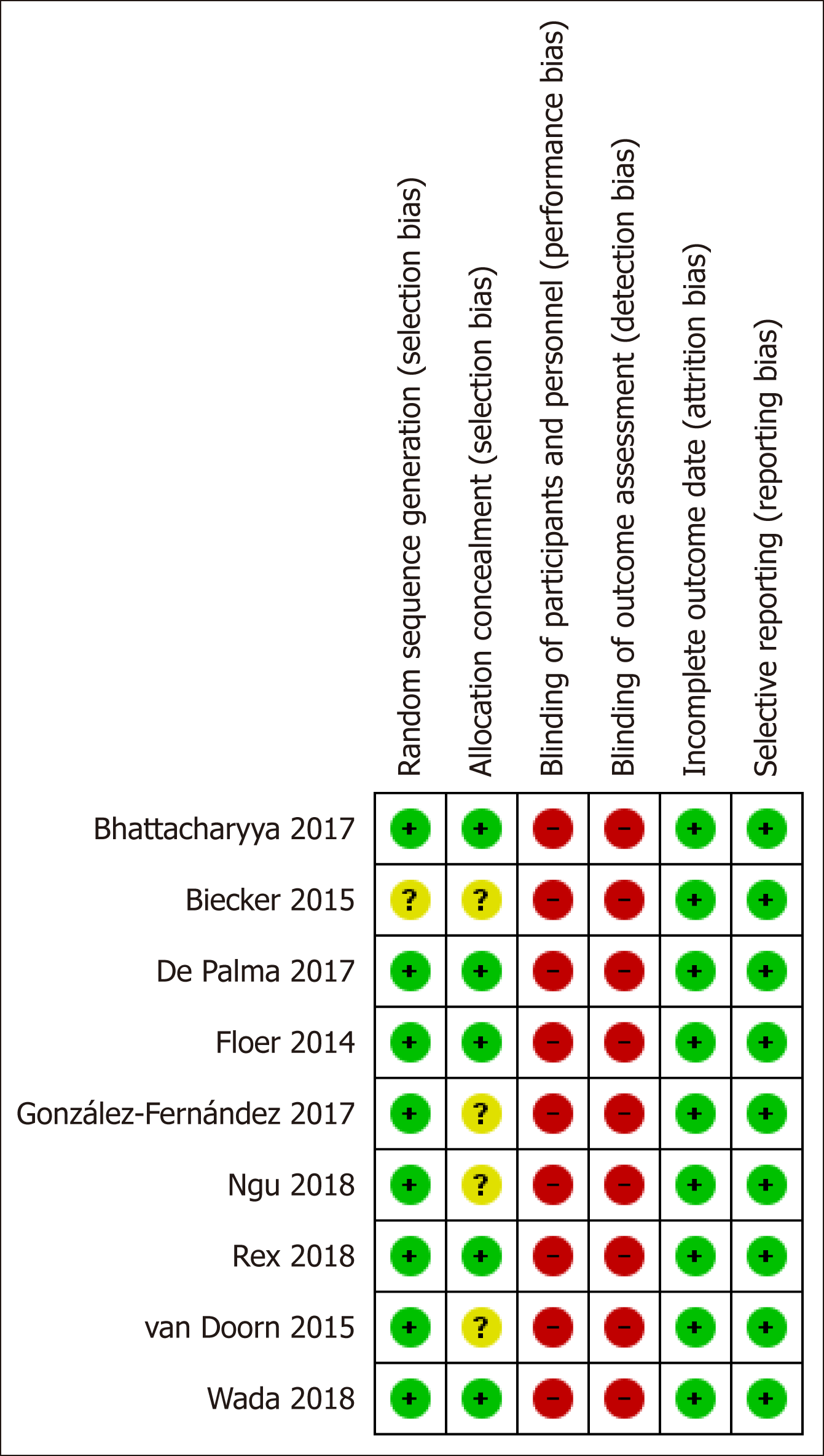
This was carried out independently by two investigators using the Cochrane collaboration’s risk of bias assessment tool[13]. Risk of bias was evaluated by recording the methods used to generate the randomization schedule and conceal treatment allocation (selection bias), whether blinding was implemented for participants, personnel (performance bias), and outcomes assessment (detection bias), what proportion of subjects completed follow-up (attrition bias) and whether there was evidence of selective reporting of outcomes (reporting bias). Each study included in the meta-analysis was classified as having a high, low or unclear risk of bias, with reference to each of the abovementioned domains, while disagreements were resolved by discussion.
The primary endpoint of this study was to examine the effect of EAC on ADR. Secondary endpoints encompass the effect of EAC on detection rate of advanced adenoma (AADR, referring to adenomas ≥ 10mm, with villous component, and/or high-grade dysplasia) and mean number of adenomas per colonoscopy (MAC). We also calculated the device removal rate in the EAC arms in order to reach the cecum.
Regarding the primary endpoint as well as AADR, relative risks (RRs) with 95% confidence intervals (95%CI) and respective number needed to treat (NNT) were calculated. For MAC, the inverse variance statistical method was used and mean difference (MD) with 95%CI was calculated. Device removal rate in the EAC arms was calculated using generic inverse variance analysis and is presented as percentage with respective 95%CI. All outcomes were meta-analyzed using either the fixed-effects model (Mantel and Haenszel method) or the random-effects model (DerSimonian and Laird method) in the absence or presence of significant heterogeneity, respectively. For all outcomes, the threshold for statistical significance was set to P < 0.05. Review Manager 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) and StatsDirect 3 (StatsDirect Ltd, Sale, Cheshire, England) software packages were used to meta-analyze all data and construct forest and funnel plots of all outcomes. Funnel plots were assessed for evidence of asymmetry, hence potential publication bias, using either the Egger test[14] in case of ≥ 10 available studies or visual inspection in case of less than 10 available studies.
Heterogeneity among studies was assessed using the I2 statistic with a cut-off of < 50% (or a P value < 0.1) as threshold to indicate statistically significant heterogeneity. In that case, multiple sensitivity analyses were carried out aiming to identify factors contributing to the detected heterogeneity. The predefined sensitivity analysis was performed by: (1) exclusion of one study at a time as proposed by the Cochrane collaboration; (2) per examinations’ indication rate (screening ≤ 50% vs > 50%); (3) per generation of device; and (4) per ADR (≤ 35% vs > 35%) of the CC group.
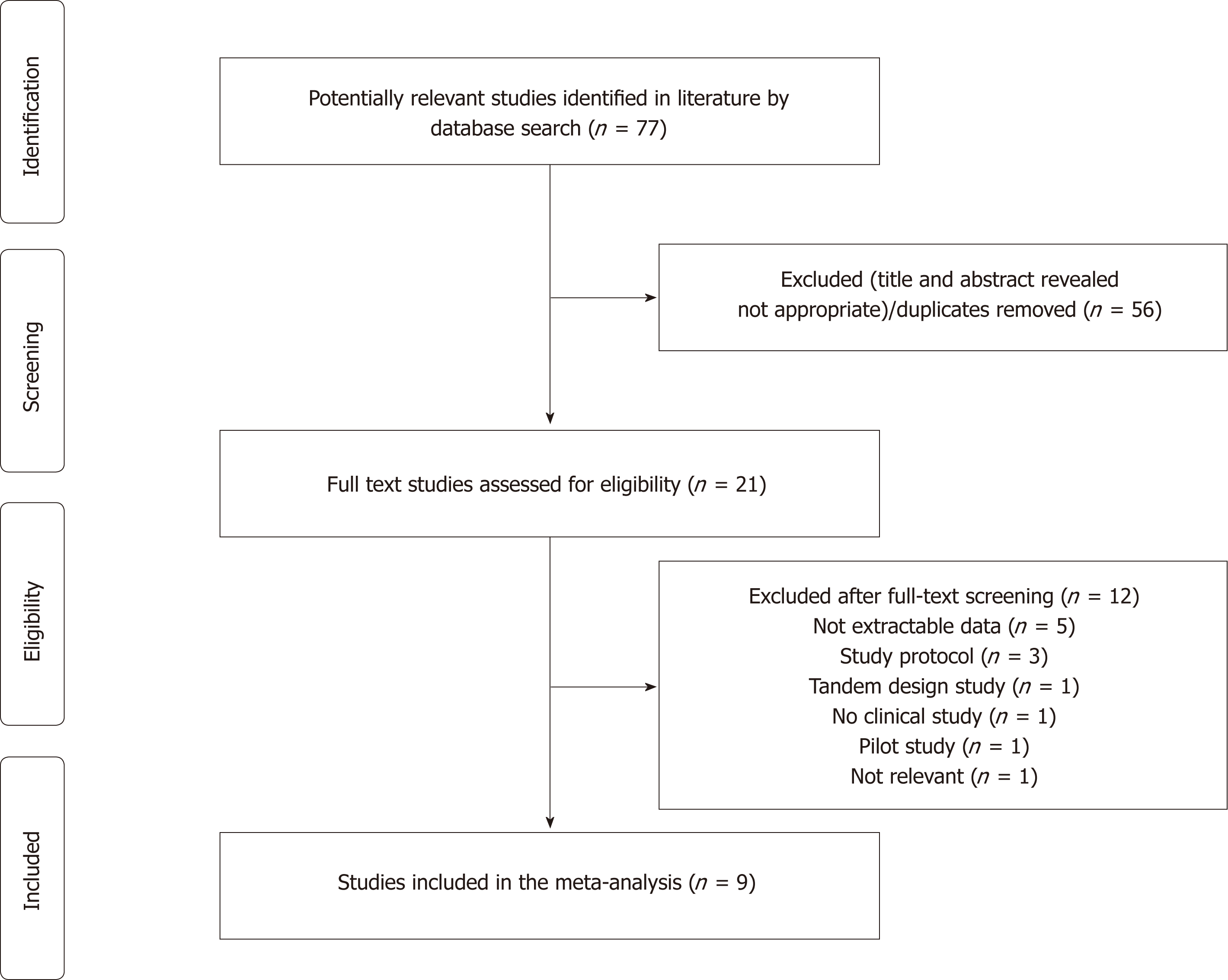
The initial search yielded 77 citations. After title and abstract review, 56 articles were excluded as irrelevant or duplicates; therefore 21 articles underwent a full-text assessment. Among these, 12 were further deemed to be ineligible for various reasons, which left 9 articles[15-23] meeting all inclusion criteria to be included in the systematic review and meta-analysis. Figure 2 depicts the exact search process.
Overall, 9 studies with 9 sets of data enrolling 6038 patients were included in the analysis; 3027 patients underwent EAC and 3011 CC. Six studies were conducted in Europe[15-19,21], one in Mexico[20], one in Japan[22] and one in United States and Italy[23]. Five of the studies[15-17,21,23] were of multicenter design (2-7 recruiting sites) and in four studies[15,16,20,21] 10 or more endoscopists performed the examinations; in one study[20] fellows took also part in the examinations. The first generation Endocuff device was evaluated in 7[15-18,20,22,23] and Endocuff-Vision in 2 studies[19,21], respectively. In terms of colonoscopies’ indications most studies[15-18,22,23] evaluated patients of various indications, including screening, surveillance and diagnostic cases. In these studies, the percentage examinations for CRC screening per arm ranged from 21.1% to 43%; two studies[15,17] did not report the percentage of screening examinations. In two studies[19,21] fecal occult blood test (FOBT) (+) and surveillance cases were included, while a sole study[20] enrolled exclusively individuals undergoing screening colonoscopy. No difference in terms of participants’ gender (female 32.1%-76%) and mean age (55-68 years) was noted between EAC and CC group. Finally, different scales were used to estimate the quality of bowel preparation[24-26]; no differences were found between the two groups in any of the studies. Table 1 summarizes the basic characteristics of included studies.
The per study risk of bias according to Cochrane collaboration’s risk of bias assessment tool is summarized in Figure 3. Our study included well-designed RCTs with registered protocols and pre-specified endpoints that were reported adequately. However, concerns regarding potential selection bias were raised for one[17] and 4 studies[16,17,20,21] where authors did not report the exact methods of randomization and allocation, respectively. It is worthy to note that due to its nature - application of an additional device at the end of the scope - neither patients nor endoscopists could be blinded to the intervention, exposing all studies to inevitable, acknowledged by all authors, high risk of both performance and detection bias.
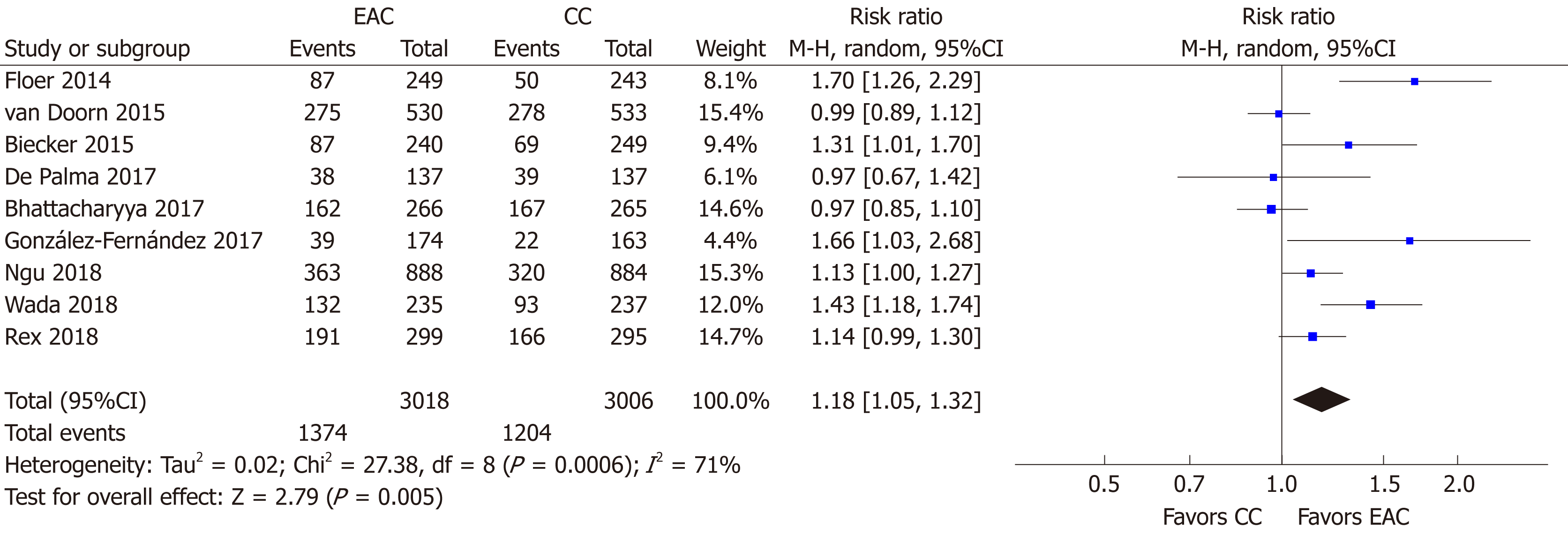
All 9 studies[15-23] (n = 6024) provided data regarding the primary endpoint. Overall, 1374/3018 individuals were detected with at least one adenoma in the EAC arm compared to 1204/3006 in the CC arm. Thus, the use of Endocuff devices was associated with a statistical significantly increased ADR [RR (95%CI): 1.18 (1.05-1.32), P = 0.005, NNT = 19; Figure 4] with substantial detected heterogeneity (I2 = 71%, P < 0.001). There was no evidence of publication bias (Supplementary Figure 1A).
We underwent a multiple sensitivity analysis in order to explore the aforementioned heterogeneity. As seen in Table 2, the step-by-step sensitivity analysis did not identify a sole study being responsible for the detected heterogeneity. Similarly, heterogeneity was not eliminated when studies were evaluated according to their density in screening examinations (≤ 50% or > 50% of the participants). Moreover, heterogeneity was further maintained independently of the device generation (Endocuff vs Endocuff-Vision) that was used. However, this was not the case when studies were assessed according to endoscopists’ ADR in the CC arm. In details, heterogeneity was eliminated (I2 = 49%, P = 0.12) for the group of studies[15,17,18,20] with low-to-moderate detectors (ADR in the CC arm ≤ 35%). Heterogeneity’s disappearance was accompanied with a further strengthen of the measure effect; RR (95%CI): 1.37 (1.08-1.74), P = 0.009, NNT = 11. On the contrary, heterogeneity was further present (I2 = 71%, P = 0.008) for studies[16,19,21-23] including high detectors (ADR in the CC arm > 35%) with marginal loss of statistical significance for the meta-analytic outcome [RR (95%CI): 1.10 (0.99-1.24), P = 0.08, NNT = 23].
| Sensitivity analysis | ADR, I2% (P value)/RR (95%CI) | MAC, I2% (P value)/MD (95%CI) |
| None performed | 71 (< 0.001) | 99 (< 0.001) |
| 1.18 (1.05-1.32) | 0.30 (-0.17-0.78) | |
| By excluding one study at a time | ||
| Floer et al[15] | 61 (0.008)/1.13. (1.02-1.26) | 97 (< 0.001)/0.36 (-0.08-0.79) |
| Van Doorn et al[16] | 69 (0.002)/1.22 (1.07-1.38) | 99 (< 0.001)/0.32 (-0.20-0.85) |
| Biecker et al[17] | 73 (< 0.001)/1.17 (1.03-1.32) | 79 (< 0.001)/0.17 (0.01-0.33) |
| De Palma et al[18] | 74 (< 0.001)/1.19 (1.06-1.35) | ΝΑ |
| Bhattacharrya et al[19] | 68 (0.003)/1.22 (1.08-1.38) | 99 (< 0.001)/0.36 (-0.16-0.88) |
| González-Fernández et al[20] | 71 (< 0.001)/1.16 (1.03-1.30) | ΝΑ |
| Ngu et al[21] | 75 (< 0.001)/1.20 (1.04-.38) | 99 (< 0.001)/0.32 (-0.21-0.86) |
| Wada et al[22] | 66 (0.005)/1.14 (1.02-1.28) | 99 (< 0.001)/0.28 (-0.25-0.80) |
| Rex et al[23] | 74 (< 0.001)/1.19 (1.04-1.37) | 99 (<0.001)/0.30 (-0.21-0.82) |
| By indication of examinations | ||
| Screening ≤ 50%[16,18,21-23] | 63 (0.03)/1.31 (1.01-1.27) | 22 (0.28)/0.27 (0.15-0.40) |
| Screening > 50%[19,20] | 80 (0.02)/1.21 (0.70-2.09) | ΝΑ |
| By generation of device | ||
| First generation Endocuff[15-18,20,22,23] | 73 (0.001)/1.25 (1.07-1.46) | 100 (< 0.001)/0.39 (-0.20-0.98) |
| Endocuff Vision[19,21] | 68 (0.08)/1.05 (0.90-1.23) | 53 (0.14)/0.11 (-0.12-0.34) |
| By ADR of the conventional colonoscopy group | ||
| ≤ 35%[15,17,18,20] | 49 (0.12)/1.37 (1.08-1.74) | 100 (< 0.001)/0.50 (-0.48-1.48) |
| > 35%[16,19,21-23] | 71 (0.008)/1.10 (0.99-1.24) | 49 (0.10)/0.22 (0.08-0.37) |
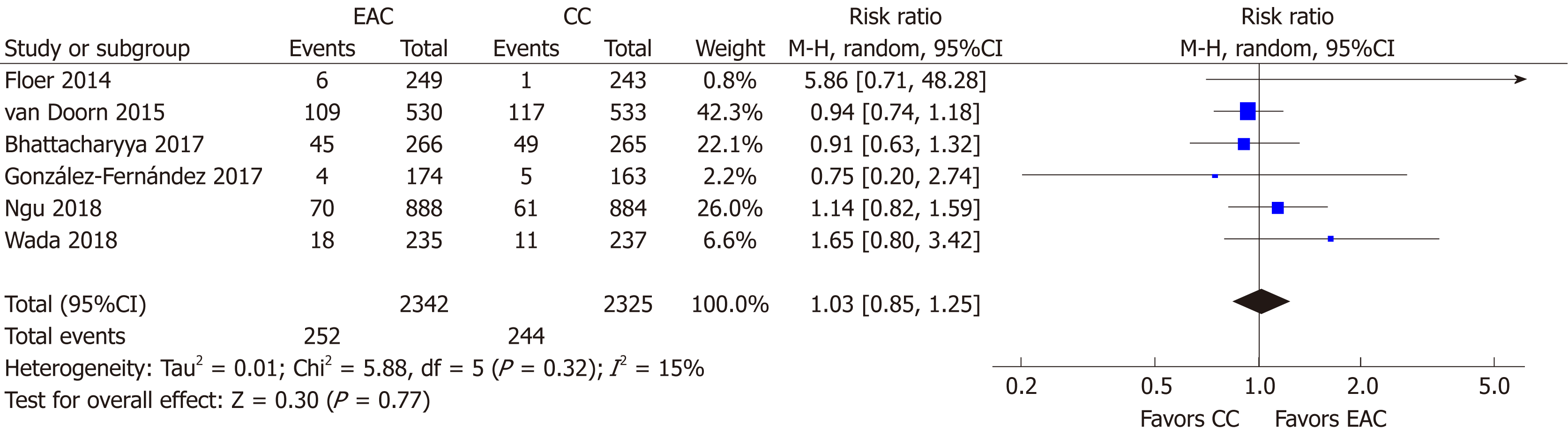
Advanced ADR: Advanced ADR was reported in 6 studies[15,16,19-22] (n = 4667). At least one advanced adenoma was detected in 252/2342 and in 244/2325 participants in the EAC and CC arm, respectively. Thus, detection of advanced adenomas was not profited when Endocuff devices were used; AADR [RR (95%CI): 1.03 (0.85-1.25)], P = 0.77, NNT = 333 (Figure 5). Neither heterogeneity (I2 = 15%, P = 0.32) nor publication bias was detected (Supplementary Figure 1B).
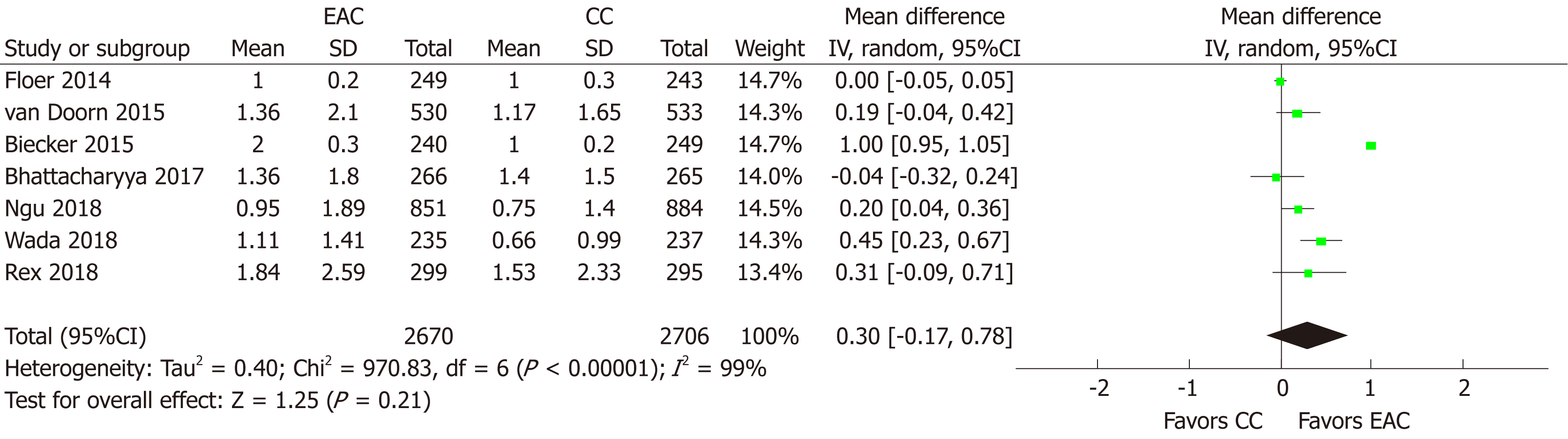
MAC: MAC was provided or it was calculated in 7 studies[15-17,19,21-23] (n = 4395). Using random effects model, MAC did not differ between EAC and CC [MD (95%CI): 0.30 (-0.17-0.78), P = 0.21] (Figure 6). There was no evidence of publication bias (Supplementary Figure 1C). However, substantial heterogeneity (I2 = 99%, P < 0.001) was present and multiple sensitivity analyses were performed to explore it (Table 2). Excluding one study at a time and rerunning the analysis failed to identify any single study that could contribute significantly to the detected heterogeneity. Evaluating separately studies with ≤ 50% of their participants[16,21-23] with screening indication led to elimination of the heterogeneity (I2 = 22%, P = 0.28) and altered significantly in favor of EAC the metanalytic outcome [MD (95%CI): 0.27 (0.15-0.40); P < 0.001]. Similarly, heterogeneity almost disappeared (I2 = 53%, P = 0.14) when only studies using the Endocuff Vision[19,21] were taken into account, but no effect on the measure effect was noted [MD (95%CI): 0.11 (-0.12-0.34), P = 0.35]. Finally, when looking separately at low-to-moderate and high detectors, heterogeneity was only eliminated (I2 = 49%, P = 0.10) for the group of high detectors[16,19,21-23]; elimination that was accompanied with an alteration in the meta-analytic outcome in favor of EAC [MD (95%CI): 0.22 (0.08-0.37), P = 0.003].
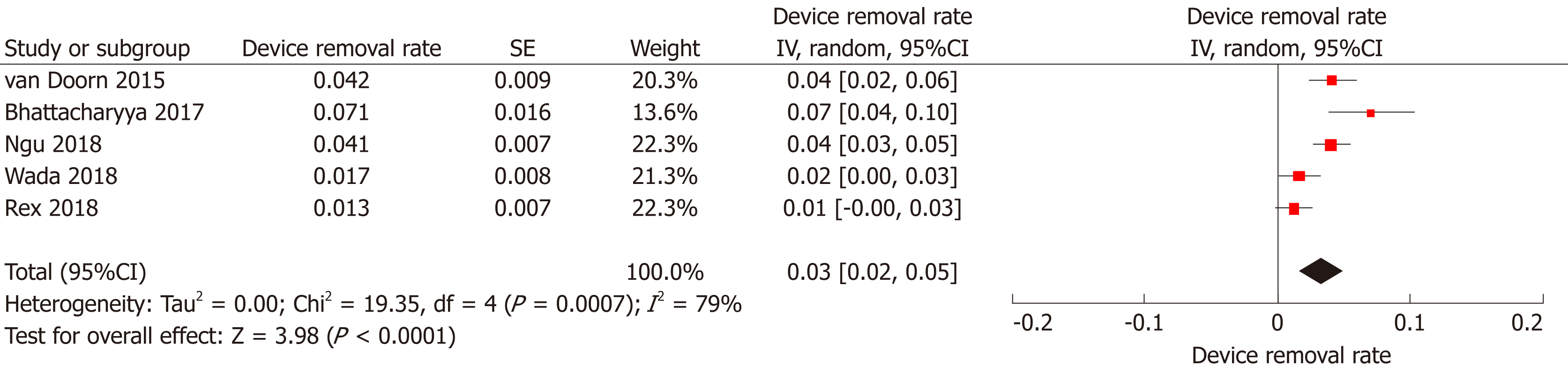
Device removal rate: In five studies[16,19,21-23], the authors reported the number of EAC examinations where the device had to be removed in order to allow its completion. Overall, the device was removed in 85 among 2219 examinations [Device removal rate (95%CI): 3% (2%-5%), P < 0.001, I2 = 79%, P < 0.001; Figure 7] with no detection of publication bias (Supplementary Figure 1D). In the vast majority of the cases (68/85) the Endocuff had to be removed due to either tortuous sigmoid or presence of sigmoid diverticular disease preventing performer to advance the scope beyond this level.
Realizing the inherit limitations of colonoscopy has led to adoption of various measures in order to improve its effectiveness; this includes advanced technology endoscopes, add-on devices, or adjustments in technique and preparation. Add-on devices have been widely accepted not only in clinical research, but also in everyday clinical practice, as they are accessories that are relatively cheap, easy to handle, safe, and single-use devices performance. Their impact on colonoscopy outcomes remains controversial, as published data are inhomogeneous. Thus, we undertook the task of evaluating the impact of the use of the most thoroughly investigated add-on device, namely Endocuff, primarily on ADR. This was done by means of an updated systematic review with meta-analysis, in which we incorporated data deriving exclusively from high-quality sources, i.e. only RCTs with registered protocols and pre-specified adequately reported endpoints, while simultaneously we attempted to tackle limitations of previously published relevant meta-analysis. In terms of our primary endpoint, our results display a statistically significant increase of ADR associated to EAC [RR (95%CI): 1.18 (1.05-1.32), NNT = 19]. The importance of this association is clear, as ADR is the most clinically relevant colonoscopy quality indicator, that inversely correlates with the risk of CRC cancer and interval CRC[2,3,27]. Moreover, this association was more profound for studies with low-to-moderate detectors (ADR in the CC arm ≤ 35%)[15,17,18,20].
So far, three relevant meta-analysis regarding EAC have been published[9-11]. However, our study has some significant differences. In the study by Chin et al[9], 9 studies involving 5624 patients were included and EAC was associated with increased ADR [odd ratio (OR) = 1.49, 95%CI: 1.23-1.80, P = 0.03] as well as detection of sessile serrated adenomas (OR = 2.34, 95%CI: 1.63-3.36, P < 0.001) compared to CC. The results are consistent to ours, but the quality of the studies included in this meta-analysis could be viewed as a limitation, as of the 9 studies that were analyzed, 4 were retrospective cohort studies, 1 was a prospective observational study and only 4 were RCTs; thus, its results could raise some skepticism. On the other hand, the meta-analysis by Facciorusso et al[10] had a different methodology and included studies with several distal add-on devices, that were compared either with each other or with CC. With regard to EAC performance, 9 studies assessed the impact of EAC compared to CC and found low-quality evidence suggesting that Endocuff increases ADR (RR = 1.21; 95%CI: 1.03-1.41). The results are in agreement to ours; however, the inclusion of various types of studies (including 4 abstracts) limits the quality of the evidence, as the authors themselves repeatedly acknowledge. Finally, the meta-analysis by Williet et al[11] demonstrated a significant increase of ADR in the EAC group compared to CC [41.3% vs 34.2%; risk ratio = 1.20, 95%CI: 1.06-1.36, P = 0.003], particularly for low-to-moderate ADR operators (< 35%): risk ratio = 1.51, 95%CI: 1.35-1.69, P < 0.001). These results are also in accordance to ours, but it should be pointed-out that they derive from the analysis of 12 papers - all RCTs - which however include 5 Abstracts among them.
Although ADR represents the most robust quality indicator of colonoscopy, it still has its own weaknesses[28-30] and thus a plethora of other quality indicators are also used in order to ameliorate the limitations of ADR. Among these metric quality indicators, adenoma miss rates (AMR) has been used extensively in tandem studies, as it is consistent to the back-to-back study design[31]. In a recently published meta-analysis[7], we showed that AMR was significantly lower when add-on devices were used, compared to CC. When restricting the analysis to EAC studies[18,32], Endocuff use significantly decreased AMR compared to CC (RR = 0.23, 95%CI: 0.14-0.39). ADR and AMR being in the same outcome direction -both in favor of devise use- has been recently proposed to enhance the validity of the results regarding ADR[33].
In our present meta-analysis, we examined the impact of EAC to the detection rate of advanced adenomas, which did not show an improvement from implementation of EAC as well as the MAC, which was associated with a benefit from performing EAC compared to CC for the group of high detectors; this is one of the few points where high detectors improve one of their metrics with implementation of EAC; usually the improvement in colonoscopy metrics addresses those endoscopists with lower performance[10,11]. Moreover, our analysis also showed that in terms of ADR, EAC benefits mostly those endoscopists with ADR ≤ 35% compared to those with ADR> 35% (i.e., low-to-moderate compared to high detectors). Similar results demonstrating that EAC mostly benefits low-to-moderate detectors instead of those with ADR > 45% were shown in the recent meta-analysis by Williet et al[11]. An interpretation of these results is difficult and rather speculative; a possible explanation could be that high detectors already have increased ADRs and have already reached the threshold of adenoma detection. On the other hand, even high detectors might have the opportunity to improve their metrics with EAC; as their ADR is already high, maybe the improvement that EAC can offer to these high detectors is limited in merely increasing the mean number of adenomas which they can detect in a given colonoscopy. Whether conceivable burden in terms of cost and local availability outweighs potential benefits of EAC in the high-detectors group remains to be answered in future trials.
Although we did not undertake formal cost-effectiveness analysis, this issue deserves our consideration. Taking into account both that the optimization by decrease of the post-polypectomy surveillance interval deriving from these modalities has been a target of skepticism lately[34] and that EAC was not associated with any benefit in terms of AADR and MAC, one could argue that with an overall NNT of 19 (the respective number escalates to 23 for high detectors) the true worth of EAC is questionable. Therefore, future studies on EAC or other devices and techniques that intend to improve ADR should perhaps focus more on their true cost-benefit, avoiding repetitions and analyses of the same studies, with similar outcomes, as is often occurs nowadays, not only in endoscopy but also in other medical fields[35].
Among the qualities of our meta-analysis we should highlight the use of a predefined protocol, the inclusion of studies only of the highest quality (full-text RCTs), as well as the fact that we performed multiple sensitivity analysis in order to ameliorate heterogeneity. This heterogeneity is one of the basic limitations of our study, besides our efforts to reduce it. Other limitations include those discussed in the previous paragraph, but -most important- the limitation shared by all studies that examine the value of add-on devices, i.e. that the device is not invisible and therefore the endoscopist cannot perform a truly blinded assessment.
To conclude, our study demonstrated that EAC increases ADR compared to CC. This benefit is more profound for endoscopists with a low-to moderate ADR (ADR ≤ 35%). It also seems to improve the MAC of high adenoma detectors (ADR > 35%); therefore, it can be used by all endoscopists to improve colonoscopy performance. However, its true value in terms of cost-benefit remains somewhat obscure and should be further investigated, especially in comparison to other low-cost techniques.
Although colonoscopy is the optimal diagnostic modality for colorectal cancer screening, it still remains imperfect since almost one fourth of colonic adenomas are not detected during conventional colonoscopy (CC). Endocuff is a single-use device mounted onto the tip of the scope devised to flatten mucosa folds; thus, promising to improve the detection of precancerous lesions.
To date, Endocuff is the add-on device with the most available literature. Despite that, whether it has beneficial impact on adenoma detection rate during colonoscopy still remains elusive. Data both from individual studies and contemporary meta-analysis remain conflicting, showing a small albeit incremental benefit of its use.
We aimed to systematically review the literature for published randomized controlled trials and re-evaluate the impact of Endocuff on adenoma detection rate (ADR), through a meta-analysis addressing the limitations of previous meta-analysis conducted on this matter.
We performed an electronic search in PubMed and the Cochrane Central Register of Controlled Trials electronic databases (from database inception to October 2018) using the free text terms “adenoma*”, “random*” both as medical subject headings and free-text terms combined with the Boolean set operator ‘AND’ with the term: “Endocuff”, as medical subject heading and the free text term. We restricted our search to prospective, randomized controlled in design, published as full text in the English language. To identify further relevant studies, we checked the reference lists of the selected articles.
We ultimately identified nine studies that matched the search criteria. enrolling 6038 patients. Of note, mixed population (screening, surveillance and diagnostic examinations) was included in all of them. Endocuff-assisted colonoscopy (EAC) was associated with increased ADR compared to CC and was of particular benefit for endoscopists with ADR lower than 35%. Regarding all other study outcomes (advanced ADR and mean number of adenomas per colonoscopy), no difference between the two modalities was evident. Similarly, multiples subgroup analysis did not show any difference between the two device generations regarding all three endpoints.
This meta-analysis of high-quality studies indicates that EAC improves ADR compared to CC and it is significantly more valuable for endoscopists with a low-to moderate ADR (ADR ≤ 35%). EAC is also a powerful tool in the hands of high adenoma detectors (ADR > 35%), as it seems to improve the mean number of adenomas per colonoscopy.
Although promising, more robust data are definitely warranted in order to systematically assess the performance of Endocuff. A significant issue that remains in future studies to be addressed is the efficacy of the device in terms of screening colonoscopy, since no study has been conducted in an exclusively screening population yet. Moreover, its true value in terms of cost-benefit is not clear yet, especially in comparison to other low-cost techniques.
| 1. | Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF, Stewart ET, Waye JD. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1952] [Cited by in RCA: 2386] [Article Influence: 170.4] [Reference Citation Analysis (2)] |
| 2. | Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger JE, Quinn VP, Ghai NR, Levin TR, Quesenberry CP. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1658] [Article Influence: 138.2] [Reference Citation Analysis (1)] |
| 3. | Kaminski MF, Wieszczy P, Rupinski M, Wojciechowska U, Didkowska J, Kraszewska E, Kobiela J, Franczyk R, Rupinska M, Kocot B, Chaber-Ciopinska A, Pachlewski J, Polkowski M, Regula J. Increased Rate of Adenoma Detection Associates With Reduced Risk of Colorectal Cancer and Death. Gastroenterology. 2017;153:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 395] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 4. | Kaminski MF, Thomas-Gibson S, Bugajski M, Bretthauer M, Rees CJ, Dekker E, Hoff G, Jover R, Suchanek S, Ferlitsch M, Anderson J, Roesch T, Hultcranz R, Racz I, Kuipers EJ, Garborg K, East JE, Rupinski M, Seip B, Bennett C, Senore C, Minozzi S, Bisschops R, Domagk D, Valori R, Spada C, Hassan C, Dinis-Ribeiro M, Rutter MD. Performance measures for lower gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy. 2017;49:378-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 523] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 5. | Heresbach D, Barrioz T, Lapalus MG, Coumaros D, Bauret P, Potier P, Sautereau D, Boustière C, Grimaud JC, Barthélémy C, Sée J, Serraj I, D'Halluin PN, Branger B, Ponchon T. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy. 2008;40:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 381] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 6. | van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 932] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 7. | Gkolfakis P, Tziatzios G, Facciorusso A, Muscatiello N, Triantafyllou K. Meta-analysis indicates that add-on devices and new endoscopes reduce colonoscopy adenoma miss rate. Eur J Gastroenterol Hepatol. 2018;30:1482-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Gkolfakis P, Tziatzios G, Spartalis E, Papanikolaou IS, Triantafyllou K. Colonoscopy attachments for the detection of precancerous lesions during colonoscopy: A review of the literature. World J Gastroenterol. 2018;24:4243-4253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Chin M, Karnes W, Jamal MM, Lee JG, Lee R, Samarasena J, Bechtold ML, Nguyen DL. Use of the Endocuff during routine colonoscopy examination improves adenoma detection: A meta-analysis. World J Gastroenterol. 2016;22:9642-9649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 10. | Facciorusso A, Del Prete V, Buccino RV, Della Valle N, Nacchiero MC, Monica F, Cannizzaro R, Muscatiello N. Comparative Efficacy of Colonoscope Distal Attachment Devices in Increasing Rates of Adenoma Detection: A Network Meta-analysis. Clin Gastroenterol Hepatol. 2018;16:1209-1219.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Williet N, Tournier Q, Vernet C, Dumas O, Rinaldi L, Roblin X, Phelip JM, Pioche M. Effect of Endocuff-assisted colonoscopy on adenoma detection rate: meta-analysis of randomized controlled trials. Endoscopy. 2018;50:846-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 12. | Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15040] [Cited by in RCA: 16680] [Article Influence: 1516.4] [Reference Citation Analysis (1)] |
| 13. | Higgins JPT GS. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011; Available from: http://handbook.cochrane.org. 2011. |
| 14. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [PubMed] |
| 15. | Floer M, Biecker E, Fitzlaff R, Röming H, Ameis D, Heinecke A, Kunsch S, Ellenrieder V, Ströbel P, Schepke M, Meister T. Higher adenoma detection rates with endocuff-assisted colonoscopy - a randomized controlled multicenter trial. PLoS One. 2014;9:e114267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 16. | van Doorn SC, van der Vlugt M, Depla A, Wientjes CA, Mallant-Hent RC, Siersema PD, Tytgat K, Tuynman H, Kuiken SD, Houben G, Stokkers P, Moons L, Bossuyt P, Fockens P, Mundt MW, Dekker E. Adenoma detection with Endocuff colonoscopy versus conventional colonoscopy: a multicentre randomised controlled trial. Gut. 2017;66:438-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 17. | Biecker E, Floer M, Heinecke A, Ströbel P, Böhme R, Schepke M, Meister T. Novel endocuff-assisted colonoscopy significantly increases the polyp detection rate: a randomized controlled trial. J Clin Gastroenterol. 2015;49:413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | De Palma GD, Giglio MC, Bruzzese D, Gennarelli N, Maione F, Siciliano S, Manzo B, Cassese G, Luglio G. Cap cuff-assisted colonoscopy versus standard colonoscopy for adenoma detection: a randomized back-to-back study. Gastrointest Endosc. 2018;87:232-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Bhattacharyya R, Chedgy F, Kandiah K, Fogg C, Higgins B, Haysom-Newport B, Gadeke L, Thursby-Pelham F, Ellis R, Goggin P, Longcroft-Wheaton G, Bhandari P. Endocuff-assisted vs. standard colonoscopy in the fecal occult blood test-based UK Bowel Cancer Screening Programme (E-cap study): a randomized trial. Endoscopy. 2017;49:1043-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | González-Fernández C, García-Rangel D, Aguilar-Olivos NE, Barreto-Zúñiga R, Romano-Munive AF, Grajales-Figueroa G, Zamora-Nava LE, Téllez-Avila FI. Higher adenoma detection rate with the endocuff: a randomized trial. Endoscopy. 2017;49:1061-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Ngu WS, Bevan R, Tsiamoulos ZP, Bassett P, Hoare Z, Rutter MD, Clifford G, Totton N, Lee TJ, Ramadas A, Silcock JG, Painter J, Neilson LJ, Saunders BP, Rees CJ. Improved adenoma detection with Endocuff Vision: the ADENOMA randomised controlled trial. Gut. 2019;68:280-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 22. | Wada Y, Fukuda M, Ohtsuka K, Watanabe M, Fukuma Y, Wada Y, Wada M. Efficacy of Endocuff-assisted colonoscopy in the detection of colorectal polyps. Endosc Int Open. 2018;6:E425-E431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Rex DK, Repici A, Gross SA, Hassan C, Ponugoti PL, Garcia JR, Broadley HM, Thygesen JC, Sullivan AW, Tippins WW, Main SA, Eckert GJ, Vemulapalli KC. High-definition colonoscopy versus Endocuff versus EndoRings versus full-spectrum endoscopy for adenoma detection at colonoscopy: a multicenter randomized trial. Gastrointest Endosc. 2018;88:335-344.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 24. | Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69:620-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 930] [Cited by in RCA: 987] [Article Influence: 58.1] [Reference Citation Analysis (1)] |
| 25. | Leighton JA, Rex DK. A grading scale to evaluate colon cleansing for the PillCam COLON capsule: a reliability study. Endoscopy. 2011;43:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 26. | Rees CJ, Thomas Gibson S, Rutter MD, Baragwanath P, Pullan R, Feeney M, Haslam N; British Society of Gastroenterology, the Joint Advisory Group on GI Endoscopy, the Association of Coloproctology of Great Britain and Ireland. UK key performance indicators and quality assurance standards for colonoscopy. Gut. 2016;65:1923-1929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 236] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 27. | Nally DM, Ballester AW, Valentelyte G, Kavanagh DO. The contribution of endoscopy quality measures to the development of interval colorectal cancers in the screening population: a systematic review. Int J Colorectal Dis. 2019;34:123-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Church J. Adenoma detection rate and the quality of colonoscopy: the sword has two edges. Dis Colon Rectum. 2008;51:520-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Rex DK, Ponugoti PL. Calculating the adenoma detection rate in screening colonoscopies only: Is it necessary? Can it be gamed? Endoscopy. 2017;49:1069-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 30. | Brand EC, Crook JE, Thomas CS, Siersema PD, Rex DK, Wallace MB. Development and validation of a prediction model for adenoma detection during screening and surveillance colonoscopy with comparison to actual adenoma detection rates. PLoS One. 2017;12:e0185560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Pohl H, Robertson DJ. Colorectal cancers detected after colonoscopy frequently result from missed lesions. Clin Gastroenterol Hepatol. 2010;8:858-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 213] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 32. | Triantafyllou K, Polymeros D, Apostolopoulos P, Lopes Brandao C, Gkolfakis P, Repici A, Papanikolaou IS, Dinis-Ribeiro M, Alexandrakis G, Hassan C. Endocuff-assisted colonoscopy is associated with a lower adenoma miss rate: a multicenter randomized tandem study. Endoscopy. 2017;49:1051-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Hassan C, Senore C, Manes G, Fuccio L, Iacopini F, Ricciardiello L, Anderloni A, Frazzoni L, Ballanti R, de Nucci G, Colussi D, Redaelli D, Lorenzetti R, Devani M, Arena I, Grossi C, Andrei F, Balestrazzi E, Sharma P, Rex DK, Repici A. Diagnostic yield and miss rate of EndoRings in an organized colorectal cancer screening program: the SMART (Study Methodology for ADR-Related Technology) trial. Gastrointest Endosc. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Hassan C, Repici A. Intensive post-polypectomy surveillance: Too much for too little? Dig Endosc. 2019;31:24-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Siontis KC, Ioannidis JPA. Replication, Duplication, and Waste in a Quarter Million Systematic Reviews and Meta-Analyses. Circ Cardiovasc Qual Outcomes. 2018;11:e005212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P- Reviewer: De Silva AP, Eleftheriadis NP S- Editor: Ma RY L- Editor: A E- Editor: Huang Y