Published online Feb 21, 2019. doi: 10.3748/wjg.v25.i7.824
Peer-review started: November 12, 2018
First decision: November 29, 2018
Revised: January 10, 2019
Accepted: January 18, 2019
Article in press: January 18, 2019
Published online: February 21, 2019
Processing time: 108 Days and 1.6 Hours
Intestinal ischemia reperfusion (I/R) injury is a serious but common pathophysiological process of many diseases, resulting in a high mortality rate in clinical practice. Ubiquitin-specific protease 22 (USP22) acts as regulator of cell cycle progression, proliferation, and tumor invasion. Depleted USP22 expression has been reported to contribute to arrested cell cycle and disrupted generation of differentiated cell types in crypts and villi. However, the role of USP22 in intestinal damage recovery has not been investigated. Therefore, elucidation of the underlying mechanism of USP22 in intestinal I/R injury may help to improve the tissue repair and patient prognosis in clinical practice.
To investigate the role of USP22 in intestinal cell proliferation and regeneration after intestinal I/R injury.
An animal model of intestinal I/R injury was generated in male Sprague-Dawley rats by occlusion of the superior mesenteric artery followed by reperfusion. Chiu’s scoring system was used to grade the damage to the intestinal mucosa. An in vitro model was developed by incubating rat intestinal epithelial IEC-6 cells in hypoxia/reoxygenation conditions in order to simulate I/R in vivo. siRNA and overexpression plasmid were used to regulate the expression of USP22. USP22, Cyclin D1, and proliferating cell nuclear antigen (PCNA) expression levels were measured by Western blot analysis and immunohistochemistry staining. Cell survival (viability) and cell cycle were evaluated using the Cell Counting Kit-8 and flow cytometry, respectively.
USP22 expression was positively correlated with the expression levels of PCNA and Cyclin D1 both in vivo and in vitro, which confirmed that USP22 was involved in cell proliferation and intestinal regeneration after intestinal I/R injury. Decreased levels of Cyclin D1 and cell cycle arrest were observed in the USP22 knockdown group (P < 0.05), while opposite results were observed in the USP22 overexpression group (P < 0.05). In addition, increased expression of USP22 was related to improved intestinal pathology or IEC-6 cell viability after I/R or hypoxia/reoxygenation. These results suggested that USP22 may exert a protective effect on intestinal I/R injury by regulating cell proliferation and facilitating tissue regeneration.
USP22 is correlated with promoting intestinal cell proliferation and accelerating intestinal tissue regeneration after intestinal I/R injury and may serve as a potential target for therapeutic development for tissue repair during intestinal I/R injury.
Core tip: Ubiquitin-specific protease 22 (USP22) belongs to the USPs family, which regulates cell cycle progression, proliferation, and tumor invasion. Depleted expression of USP22 has been linked to arrested cell cycle and disrupted distribution and generation of differentiated cell types in crypts and villi. However, its regulatory mechanism remains unclear. By generating models of ischemia reperfusion (I/R) injury and regulating USP22 expression levels, this study reveals that USP22 is correlated with promoting intestinal cell proliferation and accelerating intestinal tissue regeneration after intestinal I/R injury. USP22 might serve as a potential target for therapeutic development for tissue repair during intestinal I/R injury.
- Citation: Ji AL, Li T, Zu G, Feng DC, Li Y, Wang GZ, Yao JH, Tian XF. Ubiquitin-specific protease 22 enhances intestinal cell proliferation and tissue regeneration after intestinal ischemia reperfusion injury. World J Gastroenterol 2019; 25(7): 824-836
- URL: https://www.wjgnet.com/1007-9327/full/v25/i7/824.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i7.824
Intestinal ischemia reperfusion (I/R) injury is a serious pathophysiological process that occurs in many clinical conditions, including mesenteric arterial thrombotic or embolic diseases, shock, major surgery, and organ transplantation[1-3]. Intestinal I/R injury is a common pathogenesis of many diseases and also the initial factor of systemic inflammatory response syndrome and multiple organ dysfunction syndrome, resulting in a high mortality rate in clinical practice[4-6]. Intestinal I/R injury can result in serious damage to the mucosa and at the same time cause barrier dysfunction, which is a chief factor contributing to intestinal I/R injury. The dysfunction of the mucosal barrier and fragile immunization has been reported to be the leading cause of serious complications and death. Intestinal I/R injury is always followed by proliferation and subsequent differentiation of intestinal epithelial cells to rebuild the proper structure of the epithelium[3,7,8]. The intestinal barrier is an epithelial monolayer and serves as the first line of defense within the intestinal lumen against any unfavorable conditions[3,9]. It has been found that after the initiation of injury by intestinal I/R, the intestinal mucosal barrier undergoes regeneration through significant proliferation of undifferentiated progenitor cells[10]. However, insufficient proliferation and regeneration to fully rescue intestinal mucosal barrier function can be seen by the high mortality rate in clinical practice[11]. Therapeutic development of intestinal regeneration may serve as a good means to rescue those suffering from intestinal ischemia and improve patient prognosis. Currently, several studies have been focused on cell proliferation and tissue regeneration after I/R injury[12], but the underlying mechanisms remain largely unknown.
Ubiquitin-specific protease 22 (USP22) belongs to the largest subfamily of deubiquitinases, known as USPs, and is a conserved component of the hSAGA activating complex that takes ubiquitin from target proteins to regulate cell cycle progression, proliferation, and tumor invasion[13-16]. USP22 plays a pivotal role in stabilizing c-Myc, an oncogenic protein that controls the cells in the balance between proliferation and death[17,18]. The depletion of USP22 has been reported not only to induce arrest of the G1 cell cycle in colorectal cancer cells in vitro[18-20], but also to affect the distribution and proper generation of differentiated cell types in crypts and villi[21]. However, the role of USP22 in intestinal damage recovery has not been investigated. To date, studies regarding USP22 function have been mostly focused on its potential in facilitating and promoting stem cell-like characteristics in various tumor types. While the knowledge of USP22 in intestinal epithelial cell proliferation may provide evidence for clinical exploration of novel therapeutic targets against intestinal I/R, its function and physiological role during the intestinal I/R process still need to be elucidated.
Based on the above background, we speculated that USP22 may participate in intestinal regeneration after I/R injury and may serve as a potential target in clinical practice. In this study, we observed for the first time the role of USP22 in intestinal regeneration during intestinal I/R injury and identified the advantages of having USP22 in intestinal epithelial cell proliferation after hypoxia/reoxygenation.
Thirty-five male Sprague-Dawley rats, weighing between 180 and 220 g, were randomly distributed into one sham group and four model groups with respect to the duration of reperfusion (n = 7 each) using a random number table. The sample size was determined by power analysis[22-24]. All animals were accommodated in different cages at the same proper and constant temperature and were acclimated for one week before the experiments. All animals were handled conforming to the approved protocol by the Animal Care and Use Committee of Dalian Medical University, Liaoning, China and in compliance with the National Institutes of Health guidelines. An animal model of intestinal I/R injury was developed through surgery as previously described by Megison et al[25]. Briefly, after identifying the superior mesenteric artery (SMA) in the midline laparotomy, the intestinal I/R injury was established by occluding the SMA with an atraumatic microvascular clamp for 60 min. Occlusion was confirmed after mesenteric pulsations ceased and the intestines became pale. Reperfusion was then performed for 3 h, 6 h, 12 h, or 24 h. The sham group was exposed to the same procedures without vascular occlusion. After being sacrificed, the ileum specimens in rats were excised by midline laparotomy.
After the rats were sacrificed, the specimens were excised, immediately fixed in 10% neutral buffered formalin, embedded in paraffin wax, and cut into consecutive 4-μm-thick slides. Hematoxylin and eosin (HE) staining was then performed. Chiu’s scoring system was used to quantitatively determine the histological scores of the intestine[26]. Immunohistochemical analysis was conducted according to the manufacture’s protocol. Briefly, the sections were incubated with an anti-PCNA monoclonal antibody overnight at 4 °C. While blind to the clinicopathological data of the patients, two experienced pathologists independently examined staining to determine the expression of PCNA. The number of positive cells that showed immune-reactivity in cell nuclei in the representative ten microscopic fields was counted and the percentage of positive cells was calculated.
IEC-6 cells (normal rat small intestinal epithelial cells) were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco BRL) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. All cells were cultured in an incubator maintained at 37 °C with 5% CO2. To imitate a hypoxic environment, we incubated the cells in a microaerophilic system (Thermo Fisher Scientific 8000, Marietta, GA, United States) containing 1% O2 and 5% CO2 balanced with 94% N2 gas for 6 h. Reoxygenation was achieved later by culturing the cells under a normoxic environment.
IEC-6 cells were transfected in a 6-well plate with USP22 siRNA (si-USP22, 50 nmol/L) or unspecific scrambled siRNA (GenePharma, Shanghai, China) using a Lipofectamine 3000 Reagent (Invitrogen L3000075, Shanghai, China). Target sequence for si-USP22 is as follows: Sense (5’-3’) GCUACCAAGAG UCCACAAA; antisense (5’-3’) UUUGUGGACUC UUGGUAGC. The negative control sequence is as follows: Sense (5’-3’) UUCUCCGAACG UGUCACGU; antisense (5’-3’) ACGUGACACGU UCGGAGAA. The ratio of siRNA and Lipofectamine 3000 was 100:3.75 (pmol:μL). For overexpression of USP22, the overexpression plasmid designed and synthesized by GenePharma was transfected into IEC-6 cells using a Lipofectamine 3000 Reagent. The cells were later cultured for 48 h post-transfection for further analysis.
Harvested cells and proteins from the intestinal samples were extracted according to the manufacturer’s instructions (KeyGEN Biotech, Nanjing, Jiangsu Province, China). Equal concentrations of protein were separated by SDS-PAGE and then transferred onto polyvinylidene fluoride membranes. Subsequently, the membranes were incubated at 4 °C overnight with a primary antibody against USP22 (1:1000; Proteintech 55110, Wuhan, Hubei Province, China), β-actin (1:1000; ZSGB-BIO PR-0255, Beijing, China), or Cyclin D1 (1:500; Proteintech 12363), followed by incubation with a horseradish peroxidase (HRP)-conjugated secondary antibody (1:1000; ZSGB-BIO ZDR-5306). Enhanced chemiluminescence was used to visualize and quantify the immunoreactive protein bands. The experiment was repeated in triplicate.
Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies CK04, Inc., Tokyo, Japan) was used to measure ratios of survival cells according to the manufacturer’s instructions. Briefly, approximately 3500 IEC-6 cells per well were seeded and received proper treatment in 96-well plates followed by addition of a CCK-8 solution to each well at a 1/10 dilution. After a one-hour incubation at 37 °C, the cells alive in each well are able to form formazan and cell viability could be evaluated by detecting the absorbance at 450 nm in each well using a microplate reader (Biotec, United States).
A Cell Cycle and Apoptosis Analysis Kit (Beyotime C1052, Shanghai, China) was used to analyze cell cycle distribution according to the manufacturer’s instructions. Briefly, transfected IEC-6 cells were seeded and fixed with 70% ethanol in 6-well plates overnight at 4 °C. The cells were washed twice and resuspended with a phosphate buffered saline (PBS) solution. Propidium iodide (GeneChem, Shanghai, China) and ribonuclease were added to PBS and cells were incubated for 30 min at 37 °C. A FACSCalibur flow cytometer (BD, United States) was used to detect cell cycle. The experiment was repeated in triplicate, and each experiment had two specimens.
All data are presented as the mean ± standard deviation (SD). SPSS 19.0 statistical software package (SPSS Inc., Chicago, IL, United States) was used to analyze the data. GPower 3.1 software package was used to determine the adequate sample size (significance level α = 0.05; desired statistic power 1-β = 0.8). Student’s t-test was used to determine significant differences between the two groups, and one-way ANOVA was adopted among multiple groups. Differences with a P-value less than 0.05 were considered statistically significant.
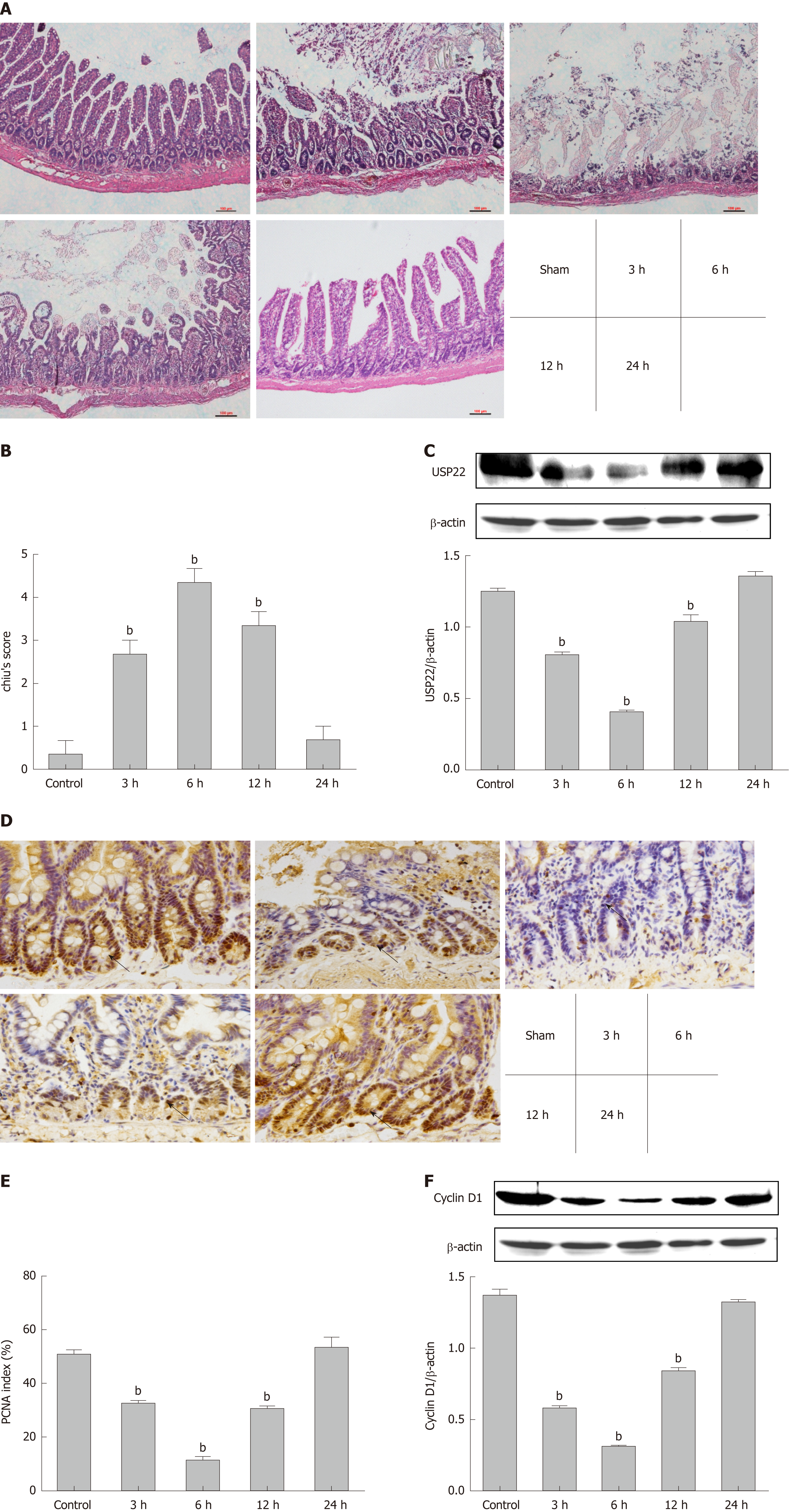
To evaluate the histopathological change of the intestinal mucosal barrier and its function after intestinal I/R injury in rats, HE staining and Chiu’s scoring were performed. It is evident that small intestinal villi were damaged during reperfusion (Figure 1A) and that I/R injury led to significant dysfunction of the small intestine with mucosal injury (Figure 1B). To investigate USP22 levels after intestinal I/R injury, we observed that USP22 was progressively downregulated from 0 h to 6 h in the early phase of reperfusion, whereas it reached its trough value at 6 h and recovered in the late phase of reperfusion (6-24 h). Changes in USP22 expression during the whole process were negatively correlated to corresponding Chiu’s scores.
Since PCNA is critically linked to DNA synthesis and plays a vital role in cell proliferation, the level of PCNA can, to some extent, mirror the level of proliferation. Thus, the proliferative activity of the intestinal mucosa during reperfusion at each given time point was assessed by immunohistochemical staining for PCNA (Figure 1D and E). It is intuitive that the level of PCNA was positively correlated with that of USP22. Furthermore, on account of being the main marker during the G1 phase of the cell cycle, Cyclin D1 expression level was also investigated. As shown in Figure 1F, Cyclin D1 levels were consistent with those of PCNA and USP22. Therefore, our results demonstrated that USP22 is positively correlated with intestinal regeneration and might play a vital role after I/R injury in vivo.
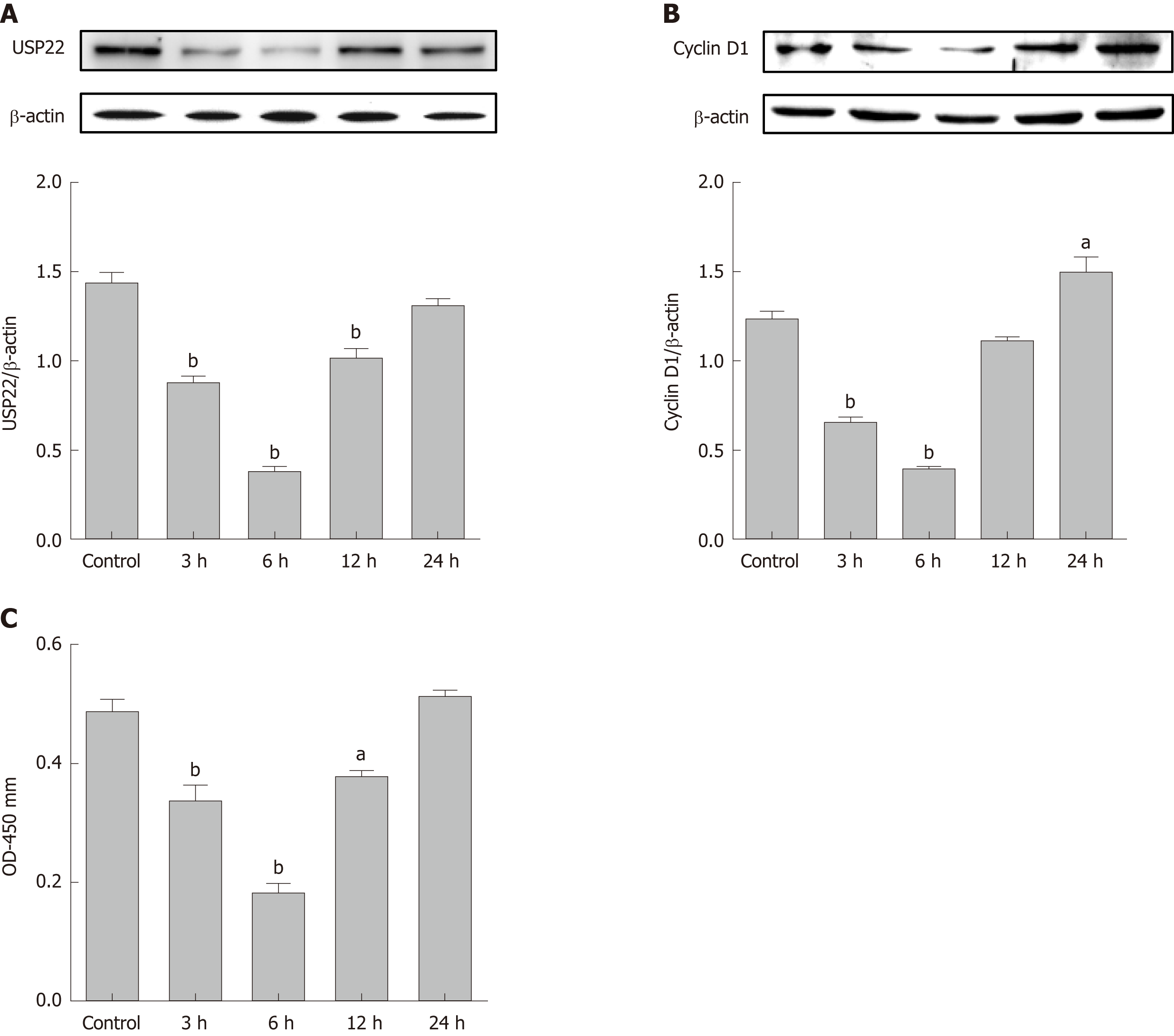
In line with the induction of USP22 during intestinal I/R in vivo, IEC-6 cells exposed to different time durations (3, 6, 12, or 24 h) of reoxygenation after 6-h hypoxia treatment were analyzed. The protein expression levels of USP22 were measured (Figure 2A) and IEC-6 cell proliferation was assessed (Figure 2B and C). USP22 expression was shown to be positively associated with Cyclin D1 and cell viability of IEC-6 cells in a time-dependent pattern. Thus, our results demonstrated that USP22 directly correlates to the proliferative and regenerative activity of IEC-6 cells after hypoxia/reoxygenation injury.
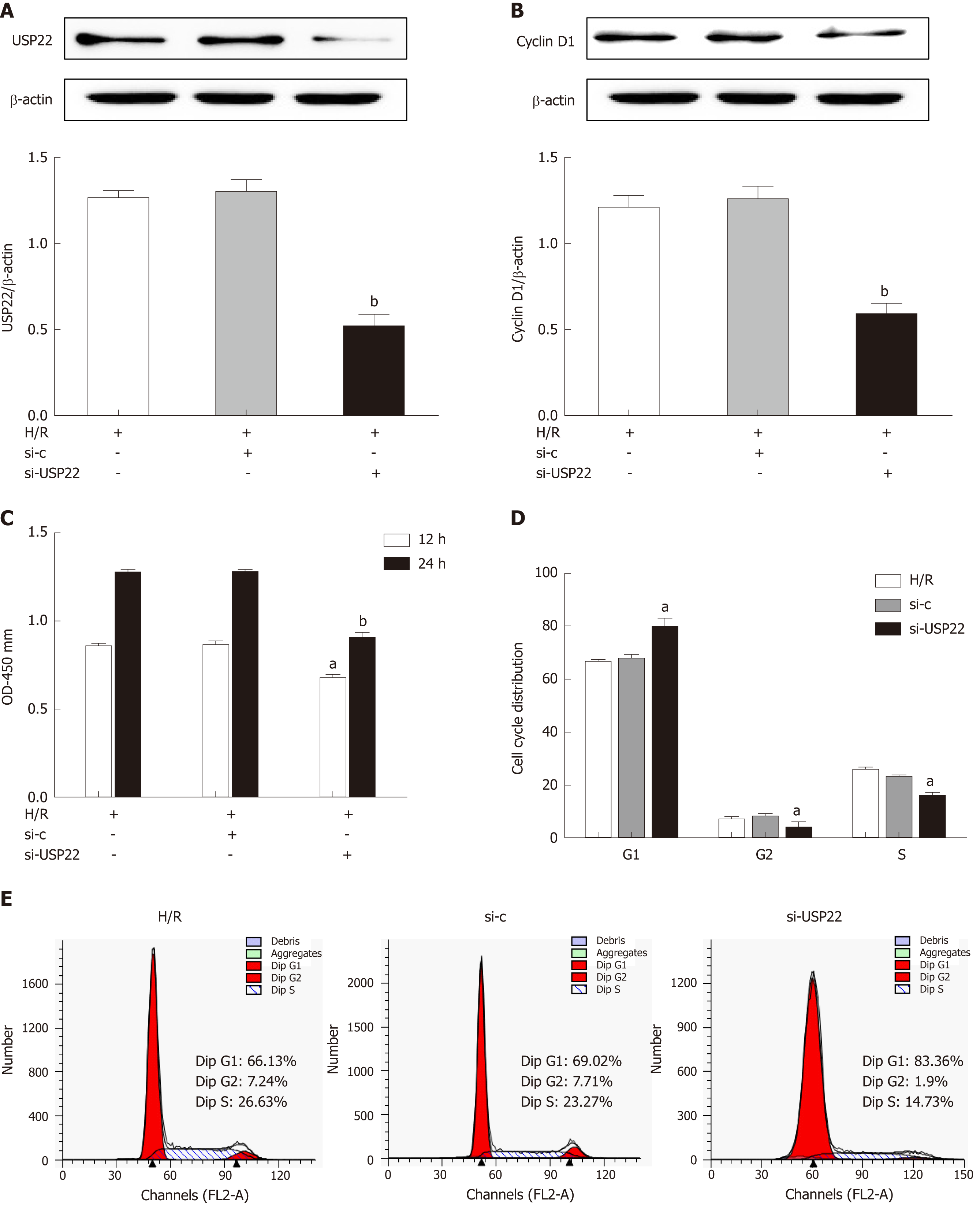
As the expression levels of USP22 changed during intestinal regeneration after I/R, the effect of silencing USP22 on the proliferation of IEC-6 cells was subsequently investigated. USP22 knockdown (Figure 3A) by using siRNA (si-USP22) dramatically decreased USP22 level and, in parallel, the levels of cyclin D1 and of cell vitality (Figure 3B) and viability (Figure 3C) compared with the negative control. Using flow cytometry, we elucidated how USP22 knockdown correlated with inhibited cell proliferation after hypoxia/reoxygenation by observing the cell cycle. We found that si-USP22-treated cells had a much higher percentage in G1 and significantly lower numbers in S phase than the counterparts of the negative control cells (Figure 3D and E). Thus, our results demonstrated that silencing USP22 closely correlates with IEC-6 cell proliferation potential after hypoxia/reoxygenation injury by stopping the cells entering S phase.
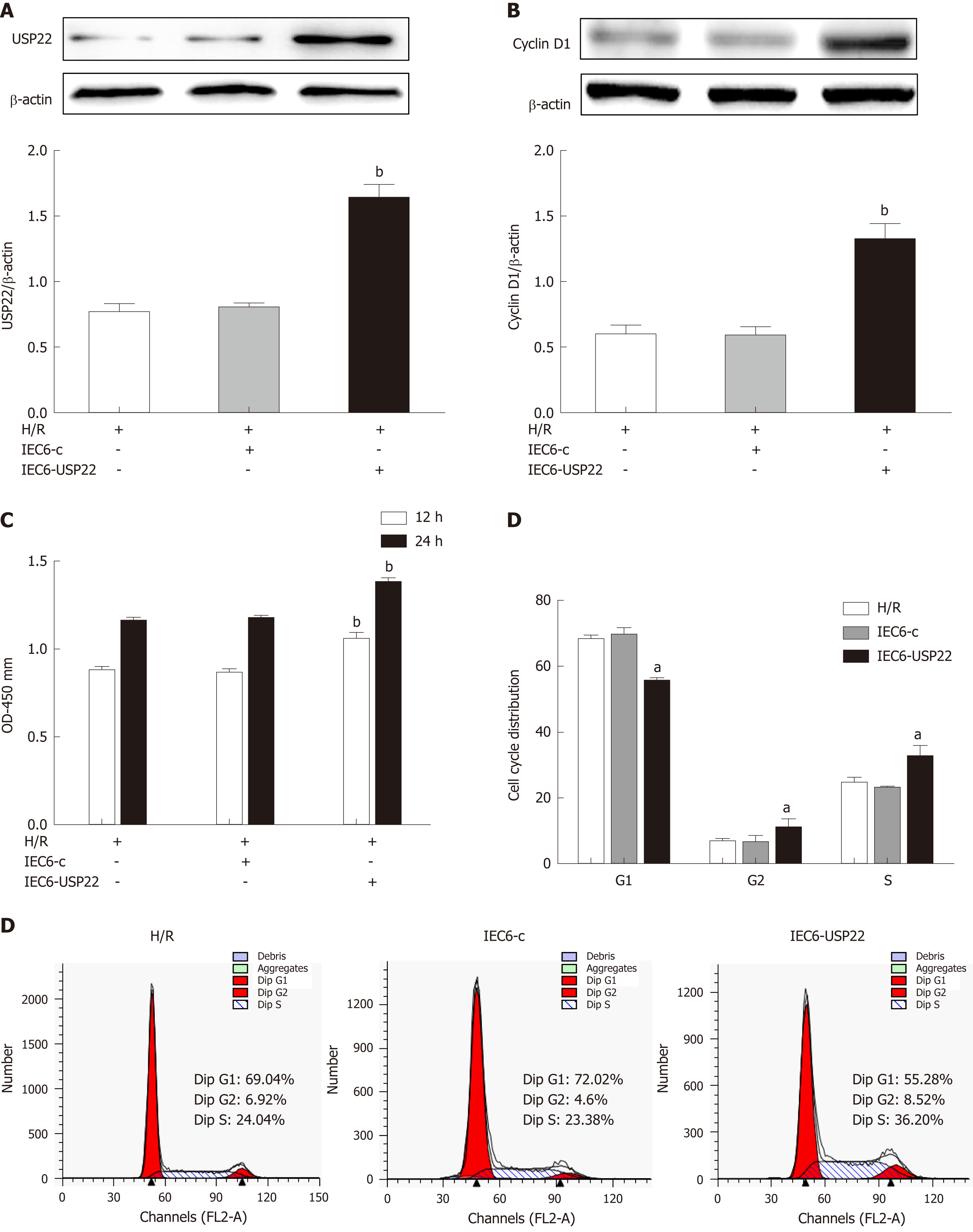
We further examined the effect of USP22 overexpression on intestinal cell proliferation by transfection with a USP22 expression plasmid. It can be seen in Figure 4A that USP22 was significantly overexpressed and that cell viability was dramatically increased (Figure 4B and C). By observing the cell cycle, we investigated how USP22 overexpression correlated with activated cell proliferation after hypoxia/regeneration. We found that USP22-overexpressing cells had a much lower number of cells in G1 phase and significantly more cells in S phase than the negative control cells (Figure 4D and E). Thus, our results demonstrated that USP22 overexpression may promote cell proliferation and viability in this process.
This study identified progressive downregulation of USP22 that occurred at the early phase of reperfusion (0-6 h) in intestinal I/R or hypoxia/reoxygenation injury in vivo or in vitro. Whereas the expression of USP22 reached its trough value at 6 h, it recovered during 6-24 h in long-term reperfusion. The time-varying property of USP22 was in accordance with the histology and Chiu’s scores in different time point groups in intestinal I/R rats. Thus, it is evident that USP22 was involved in promoting crypt cell proliferation and in facilitating intestinal tissue regeneration after intestinal I/R injury. Downregulation of USP22 was closely correlated with reduced Cyclin D1 and cell viability with accumulation of cells in the G1 phase of the cell cycle and being stopped from entering S phase. Meanwhile, we observed increased levels of Cyclin D1 and cell viability and facilitated cell cycle in USP22-overexpressing intestinal cells. Our findings indicated that USP22 may play a pivotal role in intestinal cell proliferation and tissue repair after intestinal I/R injury.
USP22 serves as a key subunit of the hSAGA complex that can regulate proliferation-related gene expression, such as c-Myc and MAPK[15,19,27,28]. USP22 can enhance cell growth and promote cell cycle progression in some cell lines[14]. USP22 can also suppress apoptosis and promote cell proliferation by antagonizing p53 function through the regulation of SIRT1[29,30]. It was also confirmed in vivo by Kosinsky et al[21] that villous goblet cells are significantly increased in a global USP22 reduction mouse model, where goblet cells are crucial for intestinal barrier function, tissue repair and healing[31,32]. This evidence suggested that USP22 positively correlated with the level of regeneration; thus, it may play a pivotal role in promoting tissue repair. We observed PCNA levels during reperfusion after intestinal I/R since PCNA is the widely recognized mediator of proliferation levels[33]. With the time-dependent proliferative activity during intestinal I/R (Figure 1D-F), a powerful indication can be made that the severity of mucosal damage had a negative correlation to the corresponding proliferative activity (Figure 1C, E, and F), which is in accordance with our former research[7]. Interestingly, a positive relationship with proliferative levels was found when investigating the level of USP22 both in vivo and in vitro (Figures 1A, 2A). Thus, the assumption was made that USP22 is involved in the regeneration of intestinal I/R injury. Accordingly, we adopted a USP22 knockdown and overexpression transfection technique in intestinal cells to confirm this hypothesis.
Cyclin D1 is needed in the transition from G1 to S phase in the cell cycle and thus serves as a vital target for proliferative signals[34]. Being a crucial regulator of the cell cycle, Cyclin D1 is responsible for inducing the G1/S transition. It forms a complex that targets a transcriptional factor by binding with different cyclin-dependent kinases[35]. Therefore, downregulation of Cyclin D1 can cause cell cycle arrest in the G1/S phase[36]. We found that the alteration of Cyclin D1 shared the same pattern as USP22 expression levels and the proliferative activity as indicated by PCNA expression both in vivo and in vitro. As we investigated deeper and more precisely in vitro by means of flow cytometry, we observed the arrested cell cycle progression of IEC-6 cells in S phase in the USP22 knockdown group when compared to the hypoxia/reoxygenation group (P < 0.05). Moreover, the opposite tendency of Cyclin D1 and cell viability was also observed in the USP22 overexpression group. Thus, this finding led to the conclusion that USP22 is positively correlated with increased potential of cell proliferation after hypoxia/reoxygenation injury. In addition, the mechanism of USP22 on promoting cell cycle progression in colorectal cancer has been reported recently, which further confirmed our results[37].
In our present study, we focused on the effect of USP22 on intestinal damage repair in I/R injury and found that the regulatory effect of USP22 on I/R injury could be potentially utilized in the future. To the best of our knowledge, no prior study has investigated USP22 expression and its pro-regenerative properties in intestinal I/R injury. Some studies showed the association of USP22 with progression and therapeutic failure of colorectal cancer, which could be valuable for consultation in further study on intestinal I/R injury and cancer research[38,39].
There were limitations in our study. Although the in vitro experiments implied a direct link between USP22 and regeneration, the in vivo study should be better designed to imply causation and biological linkage in our further study. In addition, a great number of clinical samples are needed to prove that the conclusion is also applicable for human beings. Furthermore, observation has been made that USP22 was globally reduced in a mouse model and that goblet cells were abundant[21]. While goblet cells are known as mucus secreting cells, they also share gate-keeping roles with epithelial cells and are indispensable in regulating innate immune function[40,41]. Other studies also confirmed that antibodies were secreted within the mucus from intestinal epithelial goblet cells[31]. Thus, the role of USP22 in intestinal damage recovery needs further investigations and we would not only adopt USP22 knockout mouse models but also investigate more precisely the association of USP22 with immune barrier and the underlying mechanisms in intestinal I/R injury.
In conclusion, this study demonstrated that USP22 was involved in intestinal epithelial cell proliferation and tissue regeneration after hypoxia/reoxygenation or I/R injury. Our study revealed a novel role for USP22 in intestinal regeneration after I/R injury. Therefore, targeting USP22 signaling may increase the therapeutic potential for intestinal I/R injury. In our future research, we will try to elucidate the internal mechanisms of USP22 signaling in intestinal regeneration after I/R injury.
Ubiquitin-specific protease 22 (USP22) is a novel member of the USPs subfamily, acting as a regulator of cell cycle progression, proliferation, and tumor invasion. Decreased expression of USP22 has been identified to contribute to arrested cell cycle and disrupted generation of differentiated cell types in crypts and villi. However, the regulatory mechanism of USP22 remains unclear. Therefore, elucidation of the underlying mechanism may help to improve the tissue repair in intestinal ischemia reperfusion (I/R) injury.
It is necessary to explore whether USP22 is correlated with increased potential of cell proliferation and tissue regeneration in intestinal I/R injury. Recent studies have demonstrated that insufficient proliferation and regeneration of fully rescuing intestinal mucosal barrier function could be witnessed by the high mortality rate in clinical practice. Moreover, the potential role of USP22 in cell growth, cell cycle progression, and generation of differentiated cell types has also been reported in crypts and villi. These findings give us a good lead for further study regarding the mechanism of USP22 regulation during intestinal I/R injury.
In the previous study, we investigated the effect of USP22 on intestinal cell proliferation and regeneration after intestinal I/R injury both in vivo and in vitro by gain- and loss-of-function approaches. Our study provides significant insight into the signalling mechanism of USP22 during intestinal I/R injury that may contribute to the future investigation of more effective therapies in clinical practice.
Experiments using an animal model and in vitro model in rats and cells to better elucidate the pathophysiological process of intestinal I/R injury. Hematoxylin and eosin staining and Chiu’s scoring system were used to demonstrate the intestinal tissue injury histopathologically. Immunohistochemical staining for PCNA was carried out to display and observe positive nuclei of proliferating intestinal cells. Gene silencing or transfection was conducted to construct relatively stable USP22-depleted or -expressed cells to complete the following functional studies. A series of in vitro experiments, such as Western blot, Cell Counting Kit-8, and cell cycle analysis, were performed to explore the effect of USP22 on cell proliferation.
Experiments in vivo showed the correlation between USP22 and intestinal regenerative activity of intestinal cells after intestinal I/R injury. The results of in vitro experiments showed a direct positive correlation of USP22 with cell proliferation and cell cycle progression of intestinal cells after hypoxia/reoxygenation injury. This study could be valuable for consultation in further study on intestinal I/R injury and be potentially utilized in therapeutic enhancement in clinical practice. Limitations did exist that the in vivo study should be better designed to imply causation and biological linkage and USP22 knockout mouse models would be helpful. Clinical samples are also needed to better suit the application on human beings.
USP22 plays a positive role in intestinal epithelial cell proliferation and tissue regeneration in intestinal I/R injury. This study reveals a novel role for USP22 in intestinal regeneration after I/R injury. Targeting USP22 may increase the therapeutic potential for intestinal I/R injury in clinical practice.
Our study illuminates the role of USP22 in intestinal epithelial cell proliferation and tissue regeneration in intestinal I/R injury. Other researchers have reported the abundant mucus secreting goblet cells in a USP22 globally reduced mouse model. While goblet cells are also one of indispensable parts in regulating innate immune function, further investigation is needed.
| 1. | Collard CD, Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology. 2001;94:1133-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 458] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 2. | Tendler DA. Acute intestinal ischemia and infarction. Semin Gastrointest Dis. 2003;14:66-76. [PubMed] [DOI] [Full Text] |
| 3. | Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol Rev. 2007;87:545-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 433] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 4. | Zhou W, Yao J, Wang G, Chen Z, Li Z, Feng D, Li Y, Qasim W, Tan W, Ning S, Tian X. PKCζ phosphorylates TRAF2 to protect against intestinal ischemia-reperfusion-induced injury. Cell Death Dis. 2017;8:e2935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17:1391-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1886] [Cited by in RCA: 2624] [Article Influence: 174.9] [Reference Citation Analysis (0)] |
| 6. | Higuchi S, Wu R, Zhou M, Marini CP, Ravikumar TS, Wang P. Gut hyperpermiability after ischemia and reperfusion: Attenuation with adrenomedullin and its binding protein treatment. Int J Clin Exp Pathol. 2008;1:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Zu G, Yao J, Ji A, Ning S, Luo F, Li Z, Feng D, Rui Y, Li Y, Wang G, Tian X. Nurr1 promotes intestinal regeneration after ischemia/reperfusion injury by inhibiting the expression of p21 (Waf1/Cip1). J Mol Med (Berl). 2017;95:83-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Grootjans J, Thuijls G, Derikx JP, van Dam RM, Dejong CH, Buurman WA. Rapid lamina propria retraction and zipper-like constriction of the epithelium preserves the epithelial lining in human small intestine exposed to ischaemia-reperfusion. J Pathol. 2011;224:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Podolsky DK. Mucosal immunity and inflammation. V. Innate mechanisms of mucosal defense and repair: The best offense is a good defense. Am J Physiol. 1999;277:G495-G499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 86] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Itoh H, Yagi M, Hasebe K, Fushida S, Tani T, Hashimoto T, Shimizu K, Miwa K. Regeneration of small intestinal mucosa after acute ischemia-reperfusion injury. Dig Dis Sci. 2002;47:2704-2710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Mallick IH, Yang W, Winslet MC, Seifalian AM. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci. 2004;49:1359-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Hart ML, Grenz A, Gorzolla IC, Schittenhelm J, Dalton JH, Eltzschig HK. Hypoxia-inducible factor-1α-dependent protection from intestinal ischemia/reperfusion injury involves ecto-5'-nucleotidase (CD73) and the A2B adenosine receptor. J Immunol. 2011;186:4367-4374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Koutelou E, Hirsch CL, Dent SY. Multiple faces of the SAGA complex. Curr Opin Cell Biol. 2010;22:374-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 14. | Atanassov BS, Dent SY. USP22 regulates cell proliferation by deubiquitinating the transcriptional regulator FBP1. EMBO Rep. 2011;12:924-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 15. | Zhang XY, Pfeiffer HK, Thorne AW, McMahon SB. USP22, an hSAGA subunit and potential cancer stem cell marker, reverses the polycomb-catalyzed ubiquitylation of histone H2A. Cell Cycle. 2008;7:1522-1524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Wang L, Dent SY. Functions of SAGA in development and disease. Epigenomics. 2014;6:329-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 17. | Stine ZE, Walton ZE, Altman BJ, Hsieh AL, Dang CV. MYC, Metabolism, and Cancer. Cancer Discov. 2015;5:1024-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 1024] [Article Influence: 93.1] [Reference Citation Analysis (0)] |
| 18. | Kim D, Hong A, Park HI, Shin WH, Yoo L, Jeon SJ, Chung KC. Deubiquitinating enzyme USP22 positively regulates c-Myc stability and tumorigenic activity in mammalian and breast cancer cells. J Cell Physiol. 2017;232:3664-3676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 19. | Zhang XY, Varthi M, Sykes SM, Phillips C, Warzecha C, Zhu W, Wyce A, Thorne AW, Berger SL, McMahon SB. The putative cancer stem cell marker USP22 is a subunit of the human SAGA complex required for activated transcription and cell-cycle progression. Mol Cell. 2008;29:102-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 355] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 20. | Benetatos L, Vartholomatos G, Hatzimichael E. Polycomb group proteins and MYC: The cancer connection. Cell Mol Life Sci. 2014;71:257-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Kosinsky RL, Wegwitz F, Hellbach N, Dobbelstein M, Mansouri A, Vogel T, Begus-Nahrmann Y, Johnsen SA. Usp22 deficiency impairs intestinal epithelial lineage specification in vivo. Oncotarget. 2015;6:37906-37918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Kim J, Seo BS. How to calculate sample size and why. Clin Orthop Surg. 2013;5:235-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13379] [Cited by in RCA: 18471] [Article Influence: 1154.4] [Reference Citation Analysis (0)] |
| 24. | Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175-191. [PubMed] |
| 25. | Megison SM, Horton JW, Chao H, Walker PB. A new model for intestinal ischemia in the rat. J Surg Res. 1990;49:168-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1258] [Cited by in RCA: 1452] [Article Influence: 25.9] [Reference Citation Analysis (6)] |
| 27. | Lee KK, Florens L, Swanson SK, Washburn MP, Workman JL. The deubiquitylation activity of Ubp8 is dependent upon Sgf11 and its association with the SAGA complex. Mol Cell Biol. 2005;25:1173-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 139] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 28. | Andika IB, Jamal A, Kondo H, Suzuki N. SAGA complex mediates the transcriptional up-regulation of antiviral RNA silencing. Proc Natl Acad Sci U S A. 2017;114:E3499-E3506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Li L, Osdal T, Ho Y, Chun S, McDonald T, Agarwal P, Lin A, Chu S, Qi J, Li L, Hsieh YT, Dos Santos C, Yuan H, Ha TQ, Popa M, Hovland R, Bruserud Ø, Gjertsen BT, Kuo YH, Chen W, Lain S, McCormack E, Bhatia R. SIRT1 activation by a c-MYC oncogenic network promotes the maintenance and drug resistance of human FLT3-ITD acute myeloid leukemia stem cells. Cell Stem Cell. 2014;15:431-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 180] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 30. | Lin Z, Yang H, Kong Q, Li J, Lee SM, Gao B, Dong H, Wei J, Song J, Zhang DD, Fang D. USP22 antagonizes p53 transcriptional activation by deubiquitinating Sirt1 to suppress cell apoptosis and is required for mouse embryonic development. Mol Cell. 2012;46:484-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 259] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 31. | Pelaseyed T, Bergström JH, Gustafsson JK, Ermund A, Birchenough GM, Schütte A, van der Post S, Svensson F, Rodríguez-Piñeiro AM, Nyström EE, Wising C, Johansson ME, Hansson GC. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev. 2014;260:8-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 974] [Article Influence: 88.5] [Reference Citation Analysis (0)] |
| 32. | Birchenough GM, Johansson ME, Gustafsson JK, Bergström JH, Hansson GC. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015;8:712-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 612] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 33. | Takasaki Y, Deng JS, Tan EM. A nuclear antigen associated with cell proliferation and blast transformation. J Exp Med. 1981;154:1899-1909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 285] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 34. | Baldin V, Lukas J, Marcote MJ, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1159] [Cited by in RCA: 1257] [Article Influence: 38.1] [Reference Citation Analysis (5)] |
| 35. | Tam SW, Theodoras AM, Shay JW, Draetta GF, Pagano M. Differential expression and regulation of Cyclin D1 protein in normal and tumor human cells: Association with Cdk4 is required for Cyclin D1 function in G1 progression. Oncogene. 1994;9:2663-2674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Liu L, Zhang H, Shi L, Zhang W, Yuan J, Chen X, Liu J, Zhang Y, Wang Z. Inhibition of Rac1 activity induces G1/S phase arrest through the GSK3/cyclin D1 pathway in human cancer cells. Oncol Rep. 2014;32:1395-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Gennaro VJ, Stanek TJ, Peck AR, Sun Y, Wang F, Qie S, Knudsen KE, Rui H, Butt T, Diehl JA, McMahon SB. Control of CCND1 ubiquitylation by the catalytic SAGA subunit USP22 is essential for cell cycle progression through G1 in cancer cells. Proc Natl Acad Sci U S A. 2018;115:E9298-E9307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 38. | Jiang S, Song C, Gu X, Wang M, Miao D, Lv J, Liu Y. Ubiquitin-Specific Peptidase 22 Contributes to Colorectal Cancer Stemness and Chemoresistance via Wnt/β-Catenin Pathway. Cell Physiol Biochem. 2018;46:1412-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 39. | Jiang S, Miao D, Wang M, Lv J, Wang Y, Tong J. MiR-30-5p suppresses cell chemoresistance and stemness in colorectal cancer through USP22/Wnt/β-catenin signaling axis. J Cell Mol Med. 2019;23:630-640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 40. | Ma J, Rubin BK, Voynow JA. Mucins, Mucus, and Goblet Cells. Chest. 2018;154:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 298] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 41. | Johansson ME, Hansson GC. Immunological aspects of intestinal mucus and mucins. Nat Rev Immunol. 2016;16:639-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 703] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P- Reviewer: Beales ILP, Corrales FJ, Grassi G, Vetvicka V S- Editor: Yan JP L- Editor: Wang TQ E- Editor: Huang Y