Published online Feb 21, 2019. doi: 10.3748/wjg.v25.i7.777
Peer-review started: November 14, 2018
First decision: January 6, 2019
Revised: January 18, 2019
Accepted: January 26, 2019
Article in press: January 26, 2019
Published online: February 21, 2019
Processing time: 99 Days and 16.5 Hours
During the past decades, endoscopic resection techniques have gradually improved and gained more importance for the management of premalignant lesions and early cancers. These endoscopic resection techniques can be divided in 3 major groups: snare polipectomy, endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). The use of submucosal injection is essential for the majority of EMR techniques and is an integral part of ESD, whereas during polipectomy it is not crucial in most cases except to prevent bleeding in large polyps and/or those with large stalks as an alternative to mechanical methods. Injection provides a lifting up effect of the lesion separating it from the muscular layer, thereby reducing thermal injury and the risk of perforation and bleeding while also facilitating en-bloc resection by improving technical feasibility. With this work, we aim to review the most common endoscopic resection techniques and the importance of submucosal injection in each one of them. For that, we present some of the most commonly used submucosal injection solutions, taking into account their advantages and disadvantages. We also discuss, based on current recommendations and our own experience, how and when to preform submucosal injection, depending on lesions features and endoscopic resection technique that´s being used, to assure complete resection and to prevent associated adverse events. Finally, we also present and discuss some new proposed submucosal injection solutions, endoscopic resection techniques and devices that may have a major impact on the future of therapeutic endoscopy.
Core tip: In this work, we review the importance of submucosal injection and present some of the most commonly used solutions, comparing them taking into account their advantages and disadvantages. Unlike most of the previous papers about this subject, we organized this review in a more practical point of view. For that, we try to answer some essential questions like: what is the need for submucosal injection, when should we use it, what type of solution is more suitable for each endoscopic resection technique and how should we use them.
- Citation: Castro R, Libânio D, Pita I, Dinis-Ribeiro M. Solutions for submucosal injection: What to choose and how to do it. World J Gastroenterol 2019; 25(7): 777-788
- URL: https://www.wjgnet.com/1007-9327/full/v25/i7/777.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i7.777
During the past decades, endoscopic resection techniques have gradually improved and gained more importance in the management of premalignant lesions and early cancers of the digestive tract[1,2]. These endoscopic resection techniques can be divided in 3 major groups: snare polipectomy, endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). The use of submucosal injection is essential for the majority of EMR techniques and is an integral part of ESD, whereas during polipectomy it is not crucial in most cases except to prevent bleeding in large polyps and/or those with large stalks as an alternative to mechanical methods.
Injection lifts the lesion and separates it from the muscular layer, thereby reducing thermal injury and the risk of perforation and bleeding, while also facilitating en-bloc resection by improving technical feasibility[3,4]. An additional important aspect of injection is that if dyes are incorporated, lesion margins may become more clearly defined, especially in the colon. Several solutions have been used for submucosal injection, although there is still no consensus about which one is the best.
The ideal injection solution should provide a thick submucosal fluid cushion that remains in the submucosal space long enough (to avoid the need of multiple injections), should be inexpensive, widely available, improve outcomes, reduce adverse events, and should not damage tissue specimens in order to allow an accurate pathologic staging[5,6].
Taking into account the different types of solutions, normal saline (NS) has been the most widely used solution. It is simple to use and available at a low-cost, although the mucosal protrusion created by the submucosal injection of NS is only maintained for a short period of time. While this may not be a problem when removing small lesions, the need for repeated injections can increase procedure time when resecting larger and/or difficult lesion and in theory can also increase the risk of adverse events.
In order to overcome these drawbacks of NS and to improve the technical feasibility of EMR and ESD, several solutions have been developed. Submucosal injection of glucose solution, glycerol, sodium hyaluronate (SH), colloids, hydroxypropyl methylcellulose, fibrinogen solution and other alternatives have been investigated in different contexts. However, these solutions also have some disadvantages: they can be difficult to prepare or administer, may not readily available or only available at a high cost, and may induce tissue damage that can impair histological assessment or even be associated with toxicity.
The aim of this article was to review the indications of submucosal injection and to present some of the most commonly used solutions, comparing them taking into account their advantages and disadvantages. We organized this review to share information in a practical point of view, sharing also our own experience in this field. For that, we will try to answer some essential questions: what is the need for submucosal injection, when should we use it, what type of solution is more suitable for each endoscopic resection technique and how should we use them.
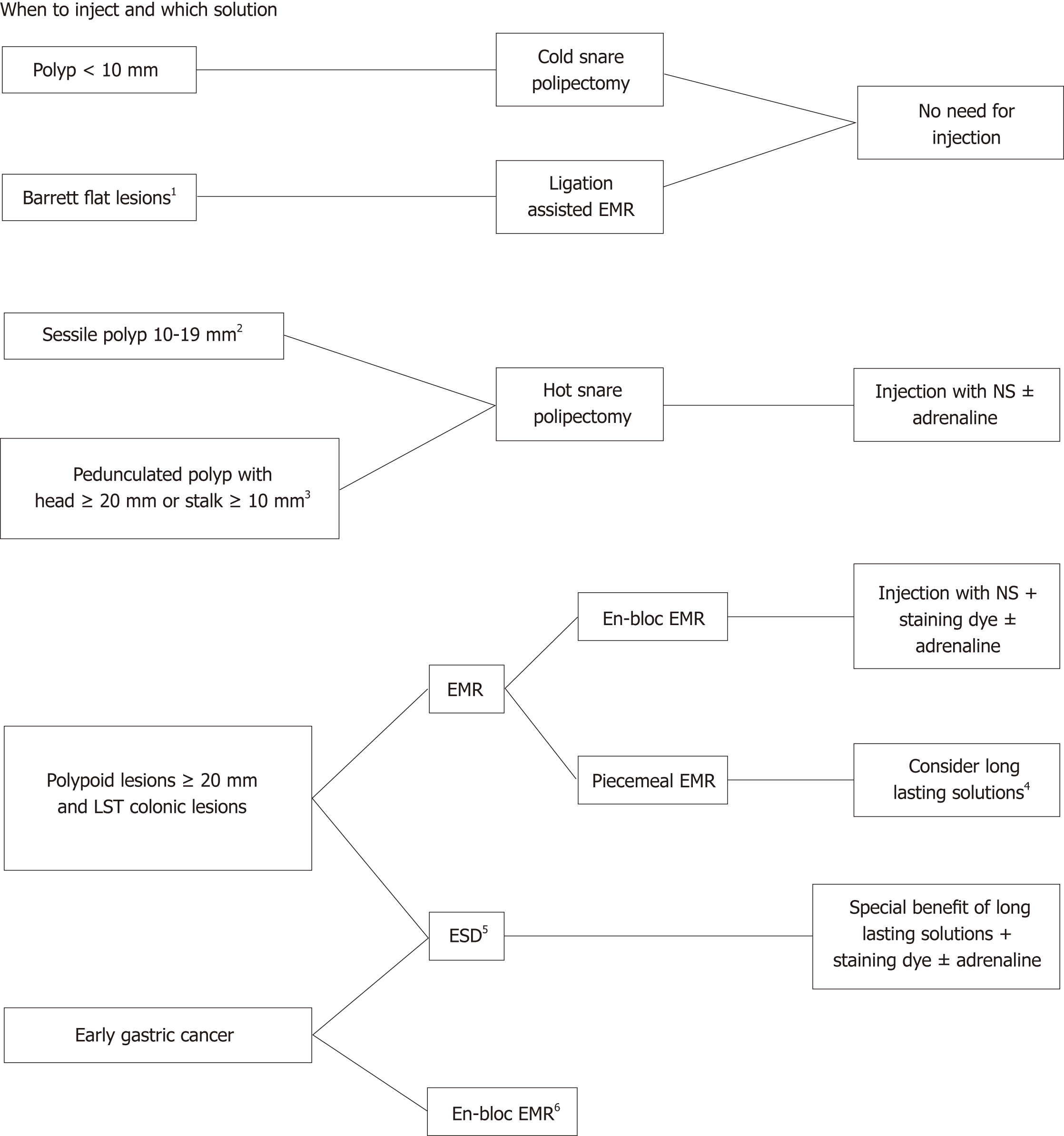
The main objective of submucosal injection is to separate the mucosal layer from the muscularis propria by filling the submucosal layer with fluid in order to decrease the risk of adverse events. This submucosal cushion reduces thermal injury and the risk of perforation and haemorrhage (by separating the mucosa from large submucosal vessels and also by vasoconstriction when adrenaline is part of the solution) while also facilitates en-bloc resection. In Figure 1, we present a decision algorithm that can be applicable in clinical practice.
The vast majority of colorectal polyps encountered during colonoscopy are < 5 mm, whereas only 10%-15% are ≥ 9 mm[7,8]. ESGE guidelines recommend cold snare polipectomy (CSP) as the preferred technique for removal of diminutive polyps (size ≤ 5 mm)[9]. This technique has high rates of complete resection, adequate tissue sampling for histology and low rates of adverse events. ESGE guidelines also suggest CSP for sessile polyps 6-9 mm in size because of its superior safety profile, although evidence comparing efficacy with hot snare polipectomy (HSP) is lacking. On the other hand, HSP is recommended for removal of sessile polyps 10-19 mm in size. In most cases, especially in the right colon, deep thermal injury with HSP is a potential risk and thus submucosal injection prior to HSP is generally advised. Regarding pedunculated polyps, ESGE guidelines suggest the use of HSP to decrease the risk of immediate bleeding, and injection of diluted adrenaline or clip placement should also be used in pedunculated polyps with a head ≥ 20 mm or a stalk ≥ 10 mm[9].
EMR is an endoscopic technique developed for the removal of sessile or flat neoplasms confined to the superficial layers (mucosa and submucosa) of the gastrointestinal tract by excising through the middle or deeper portion of the submucosa. Different EMR techniques are listed below and include: inject-and-cut EMR; Cap-assisted EMR and ligation-assisted EMR. In ligation-assisted EMR, a band ligation device is attached to the endoscope, and the banding cap is positioned over the target lesion. In this technique, although some endoscopists use submucosal injection prior to band placement, submucosal injection is not mandatory as the resection can be safely without this step[3,5,10,11].
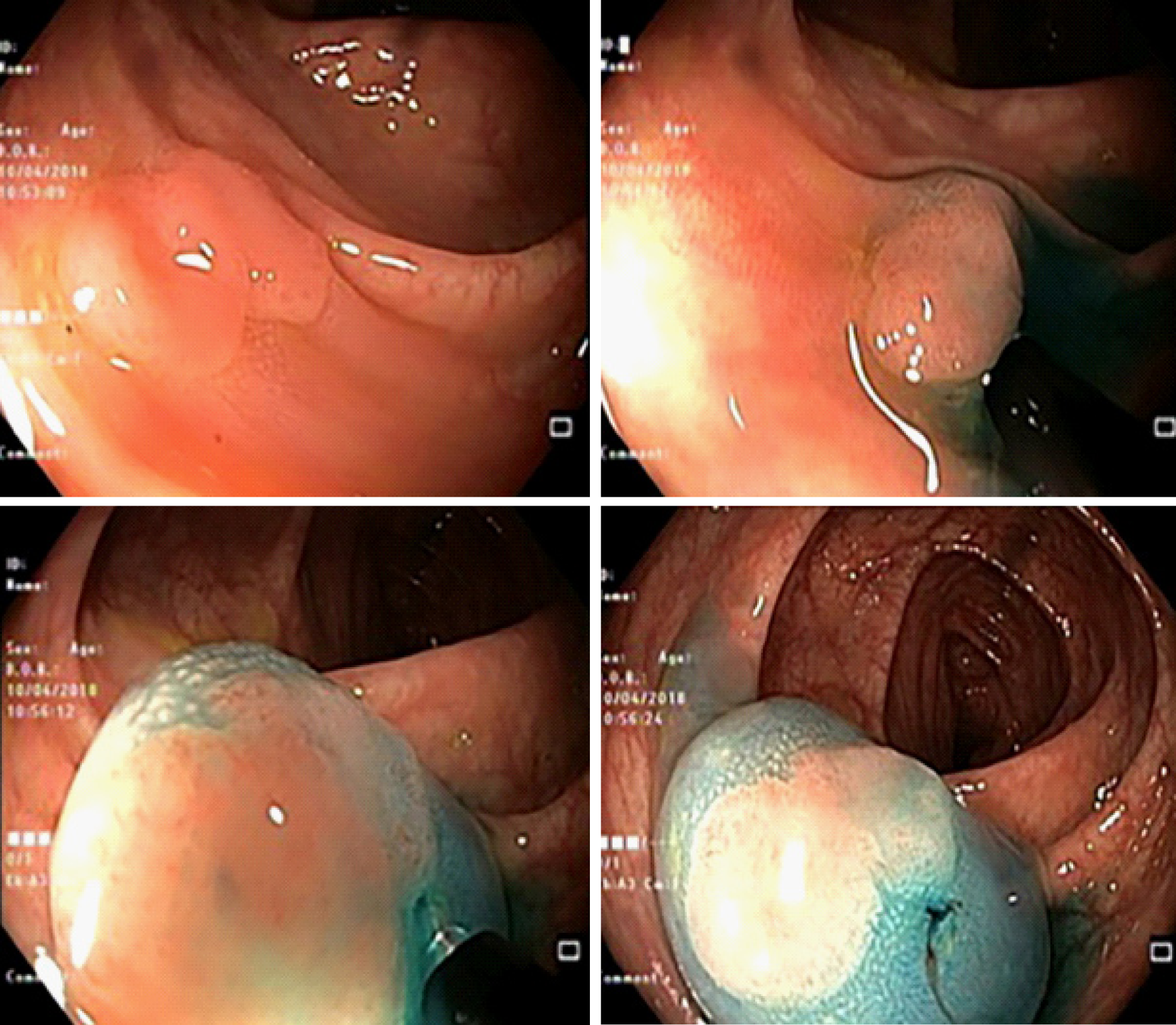
Inject-and-cut EMR is also often called saline solution lift-assisted polipectomy. The procedure starts with injection of a solution into the submucosal space under the lesion creating a safety cushion. The cushion lifts the lesion, facilitating capture and removal by using a snare while minimizing mechanical or electrocautery damage to the deeper layers of the gastrointestinal wall. The lesion may be removed in a single resection (Figure 2) or a piecemeal fashion (Figure 3). Recently, cold snare EMR was also described, and ESGE guidelines suggest that it can be an option in cases where the risk of deep thermal injury is high or unable to be tolerated, although evidence is still scarce. In this case, submucosal injection may still be needed.
Cap-assisted EMR also uses submucosal injection to lift the target mucosal lesion. Dedicated mucosectomy devices have been developed - a single-use cap affixed to the tip of the endoscope equipped with a specially designed crescent-shaped electrocautery snare that must be opened and positioned on the internal circumferential ridge at the tip of the cap. The endoscope is then positioned over the target lesion and suction is used to retract the mucosa into the cap, after which the snare is closed to capture the lesion (alternatively the lesion can be grasped with a forceps or endoscopic grasper). The lesion is then resected with standard hot snare excision technique.
The main drawback of standard EMR techniques is that size can preclude en-bloc resection, therefore resulting in piecemeal resections, leading to problems in correctly assessing the depth of tumour invasion and increasing the possibility of local recurrence. Consequently, en-bloc resection using this procedure is limited to lesions approximately 15-20 mm in size[12,13]. The choice of the technique between EMR and ESD is therefore especially important when there is suspicion of limited submucosal invasion, in which case adequate histopathological staging is of paramount importance. On the other hand, piecemeal EMR is accepted in Barrett’s dysplasia/adenocarcinoma and in colonic lesions without suspicion of submucosal invasion (since ESD is associated with higher risk if adverse events in these organs).
ESD, a relatively recent but widely accepted endoscopic resection procedure, was developed specifically for en-bloc resection of larger lesions[14-16]. Lesions are dissected directly along the submucosal layer using an electrosurgical knife, resulting in an en-bloc resection of even large lesions. Various submucosal injection solutions had previously been developed and shown to be satisfactory for use during EMR, but for the more time consuming ESD the use of a longer-lasting solution can be important to facilitate the procedure, to help identify the cutting line and maintaining a safe fluid cushion during dissection of the submucosal layer.
The majority of submucosal injection solutions is composed of a solvent (like water) and an osmotic agent (like sodium chloride). More complex solutions can also have a bulking and structuring agent, an oil component, an emulsifier and a contrast staining agent. A summary of the main features of some of the solutions discussed below is presented in Table 1.
| Solution | Cushion duration | Price | Advantages | Disadvantages |
| NS | + | Low | Widely available; Inexpensive; Non-toxic | Poor submucosal elevation |
| DW | ++ | Low | Widely available; Inexpensive | Moderate submucosal elevation; Significant tissue damage at high concentrations of dextrose |
| HPMC | +++ | Moderate | Great submucosal elevation; Widely available | Moderately expensive; Risk of antigenic reactions |
| HES | ++++ | Low/moderate | Excellent submucosal elevation; FDA-approved for submucosal injection; Reasonably priced | None |
| HA | ++++ | High | Excellent submucosal elevation | Expensive; Can stimulate the growth of residual tumour cells |
| Eleview® | ++++ | High | Excellent submucosal elevation; Non-toxic | Expensive |
As previously mentioned, NS is commonly used because it is safe, available at a low-cost, easy to use and with negligible/no potential toxic effect or damage to tissue specimen. The major limitation of this solution is its rapid absorption into the surrounding tissues, reducing the duration of a proper submucosal cushion. This limitation is not so important for endoscopic resection of small polypoid lesions (< 20 mm) in which a higher elevation and its maintenance for a longer period of time is not essential[5], but can theoretically hinder and increase procedure time in larger lesions or longer procedures. However, at the present time, there is no evidence of the superiority of other submucosal agents over NS in en-bloc resection rates or adverse events risk (perforation, bleeding and post-polipectomy coagulation syndrome). This lack of difference in en-bloc resection rates and adverse events risk between different submucosal injection solutions was shown in a recent systematic review and meta-analysis by Ferreira et al[17]. SH, one of the best studied solutions, was compared to NS in three randomized controlled trials (RCTs) (total of 423 patients submitted to gastric or colic EMR) and the pooled results failed to show a difference between SH and NS regarding complete resection with OR 1.09 [95% confidence interval (CI): 0.82-1.45]. In other RCTs, 50% dextrose (D50), succinylated gelatin (SG), fibrinogen and hydroxyethyl starch (HES) were also not superior to NS. Similarly, no single solution was shown to be more effective in decreasing post-polipectomy bleeding, but HES, SG, and fibrinogen have shown a non-significant favourable trend against NS with a pooled OR of 0.59 (95%CI: 0.3-1.01). For post-polipectomy coagulation syndrome, there is only one RCT for each solution and none for SH. These studies were underpowered to detect significant differences in this specific outcome but the pooled analyses suggest that NS may be effective in preventing perforations and coagulation syndrome with an OR = 0.27 (95%CI: 0.06-1.19), especially when compared to HES (OR = 0.15; 95%CI: 0.007-3.03) and D50 (OR = 0.16; 95%CI: 0.02-1.38)[17].
Glycerol is a hypertonic solution consisting of 10% glycerin and 5% fructose in an NS solution. Because of its hypertonic properties, glycerol produces a long-lasting submucosal elevation[18]. Changes in submucosal elevation immediately and 3, 5, and 7 min after injection of glycerol and NS were compared by Sumiyoshi et al[19]. The hemispheric shape produced by glycerol maintained the same height and configuration throughout the 7-min period while NS cushion began to decrease after 3 min, becoming unnoticeable at 7 min. One retrospective study compared en-bloc resection rates and complications for EMR of colorectal flat lesions like lateral spreading tumors (LST) using glycerol or NS[20]. For lesions between 10-19 mm, en-bloc resection was significantly higher when glycerol was used, but there were no differences for larger lesions. There were also no differences in complications such as perforations and delayed bleeding. Another advantage of the use of glycerol (over other solutions such as dextrose) is that this solution does not damage the resected specimen, allowing a correct histopathological analysis. Because glycerol is relatively inexpensive and readily available in Japan, it is considered superior to NS and widely used as submucosal injection solution in colorectal EMR.
Dextrose water (DW) is also a hypertonic solution. It is an inexpensive and readily available product that produces a longer submucosal elevation than NS solution. The main issue about this product is the potential histopathological tissue damage. In fact, considerable tissue damage and impaired ulcer healing after EMR can be expected with DW at concentrations ≥ 20%. For that reason, DW with concentrations ˃ 15% is not recommended as submucosal injection solution[6].
Hyaluronic acid (HA) is a type of glycosaminoglycan found in connective tissue that has a high viscosity and water retention capability. Moreover, it does not present toxicity or antigen-antibody reaction in humans. A classical HA use in submucosal injection is in the form of a 0.4% SH solution. Various studies reported that the use of HA provides the longest-lasting fluid cushion, higher successful en-bloc resection and lower perforation complication rates, particularly for colorectal ESD[21-24]. However, a recent systematic review and meta-analysis of solutions for submucosal injection and endoscopic resection concluded that the available evidence does not allow a robust conclusion to be drawn on the solutions´ effect on resection rate[17]. The main disadvantages of HA are its high cost and unavailability. It is also believed that this product can stimulate the growth of residual tumour cell due to enhancement of both tumour growth and CD44 expression of cancer cells at wound sites in murine models[25]. For all of these reasons, HA is considered a good option for ESD of larger lesions because of its long-lasting fluid cushion, however it cannot be recommended for endoscopic piecemeal resection procedures that have an increased risk of recurrence.
Hydroxypropyl methylcellulose (HPMC) is a cellulose derivative with viscoelastic properties that is primarily used in ophthalmology for creating artificial tears[26]. As HA, it also achieves a long-lasting submucosal elevation with minimal tissue reaction[27]. The major differences between these two solutions are that HPMC is less expensive than HA but, as synthetic product, HPCM can potentially give rise to an antigen-antibody reaction[18].
Fibrinogen mixture (FM) solution is available at a reasonable price and has a high viscosity that produces a long-lasting submucosal elevation. It also helps to keep a clear visual field during and after endoscopic resection by providing a microvascular hemostatic effect[28]. Like HA and HMPC, its main utility is the submucosal injection during ESD of larger lesions because it leads to fewer injections and shorter procedure times[29]. Because fibrinogen is produced from coagulation proteins of human serum, contamination with some viruses and the associated transmission risk is a possibility. Regardless of this disadvantage, FM can be considered a convenient option for submucosal injection during EMR and ESD due to its reasonable price compared with other viscous agents and to its hemostatic properties[5].
Succinylated gelatin (SG) is a widely available, inexpensive and safe colloidal solution that exerts an oncotic pressure similar to human albumin. The clinical efficacy of SG was evaluated by Moss et al[30] in a randomized double-blind trial, conducted to compare the performance of EMR with SG or NS for sessile colonic lesions ≥ 20 mm. The SG group registered fewer injections per lesion, lower injection volume and shorter procedure duration. There was also a trend towards higher en-bloc resection rate with the use of SG though without statistically significant difference[30].
Hydroxyethyl starch (HES) is a relatively safe and inexpensive solution, easily available as a colloidal volume expanding solution that is commonly used to treat hypovolemia. In the recent past, 6% HES has been tried out for submucosal lifting in EMRs in studies with porcine models with promising results. Compared to NS, 6% HES solution produced a more prolonged submucosal cushion and lower total procedure time for EMR[31]. Mehta et al[31] found a significant superiority of 6% HES compared with NS in the duration of submucosal lifting and the requirement for additional injected solution to maintain the LST elevated. Although use of 6% HES for fluid resuscitation in critically ill patients has been linked to increased mortality, acute kidney injury, and need for dialysis, the low doses used for submucosal injection are presumed to be safe for use in humans[31].
Eleview® is a synthetic solution that was specifically designed for colorectal endoscopic resection techniques procedures requiring submucosal injection. This product is supplied in five 10-mL ready-to-use ampules and is composed of water, sodium chloride, poloxamer 188 (bulking and structural agent), polyoxyl-15-hydroxystearate (emulsifier), medium-chain-triglycerides (oil component). This solution already includes methylene blue to improve visibility of the lesion and submucosal surface. By providing an immediate and long-lasting cushion beneath the polyp and improving the visibility of the lesion, Eleview® may help achieve a complete and safe removal of the lesion. When compared to SN, Eleview® has demonstrated better cushion-forming ability and a duration of lift of up to 45 min. As a ready to use, sterile, premixed composition, it is a convenient option for clinicians. A recent double-blind RCT comparing Eleview with NS showed that the mean injected volume was significantly lower in the Eleview group (16 mL vs 31mL, P < 0.001), and there was a trend towards shorter procedure and a lower number of resection pieces with this new solution[32]. Despite all these advantages, this solution is very expensive for routine use by most endoscopy centres[33]. ORISE gel, a similar solution from other manufacturer (Boston Scientific) is also available, showing comparable results with the former and recently received FDA approval for use as an injection solution throughout the gastrointestinal tract[34].
Some adjuvants may be added to the submucosal injection solution to aid endoscopic resection and to reduce the complications associated with it, such as bleeding and perforation. The most well-known and widely used adjuvants are diluted epinephrine and staining dye like diluted indigo carmine or methylene blue.
Immediate and delayed bleeding are the most frequent complications associated with endoscopic resections. Diluted epinephrine (1:50000-1:200000) is often added to the submucosal injection fluid because of the theoretical benefits of decreased bleeding and a sustained submucosal cushion (due to delayed absorption of fluid resulting from decreased vascular flow) and is generally considered to be safe.
However, submucosal injection of epinephrine can potentially result in systemic effects such as severe hypertension, ventricular tachycardia, and intestinal ischemia. However, the rare reports of these complications result mainly from hemostatic procedures that used higher concentrations of epinephrine (1:10000), rather than prophylactic injection during endoscopic resection. Because of its short-acting effect the main objective of diluted epinephrine injection is to decrease the risk of intra-procedural bleeding which also helps to maintain a clean resection field. The role of this agent in preventing delayed bleeding is however controversial. ESGE guidelines recommend the use of diluted epinephrine before hot-snare polipectomy of large pedunculated polyps (head size ≥ 20mm or stalk width ≥ 10mm), but there is no mention regarding the systematic need for its injection in other types of lesions. A recent meta-analysis concluded that the application of submucosal epinephrine injection before resecting larger polyps (≥ 20 mm) as a routine procedure is helpful to reduce the occurrence of early postpolipectomy bleeding[35]. However, in this meta-analysis injection of diluted epinephrine was not shown to significantly reduce the risk of delayed postpolipectomy bleeding.
The most commonly used staining agents are biologically inert blue colour dye like diluted indigo carmine and methylene blue. These are frequently added to the injection solution, identifying the area of submucosal injection and clearly distinguishing between the muscle layer and the submucosal layer. This also facilitates identification of the lateral and deep margins of the target lesion before and during the resection process. The staining dye may also help to evaluate the presence of residual lesion at the end of endoscopic resection and improve recognition of muscularis propria injury, which indicates intraprocedural perforation. For example, if muscularis propria is inadvertently resected, the transected surface will present a white central circular disk surrounded by blue-stained submucosal tissue giving it the appearance of a “target” (target sign). This is a very important aspect, because small perforations recognized during the procedure can be successfully sealed with endoscopic metal clips. For these reason, ESGE guidelines recommend that a biologically inert blue dye should be incorporated into submucosal injection solution to facilitate identification of fluid cushion extent, lesion margins and deep mural injury, when performing EMR of larger lesions (≥ 20 mm) or LST. The addition of staining dye in submucosal injection solution is mandatory when performing ESD.
As mentioned above, submucosal injection is essential in almost every EMR technique and it is indispensable when performing ESD. This next section is dedicated to some practical aspects and tips that should be taken into account when performing these two endoscopic resection techniques. The authors also present a brief summary of the main aspects discussed below in Figure 4.
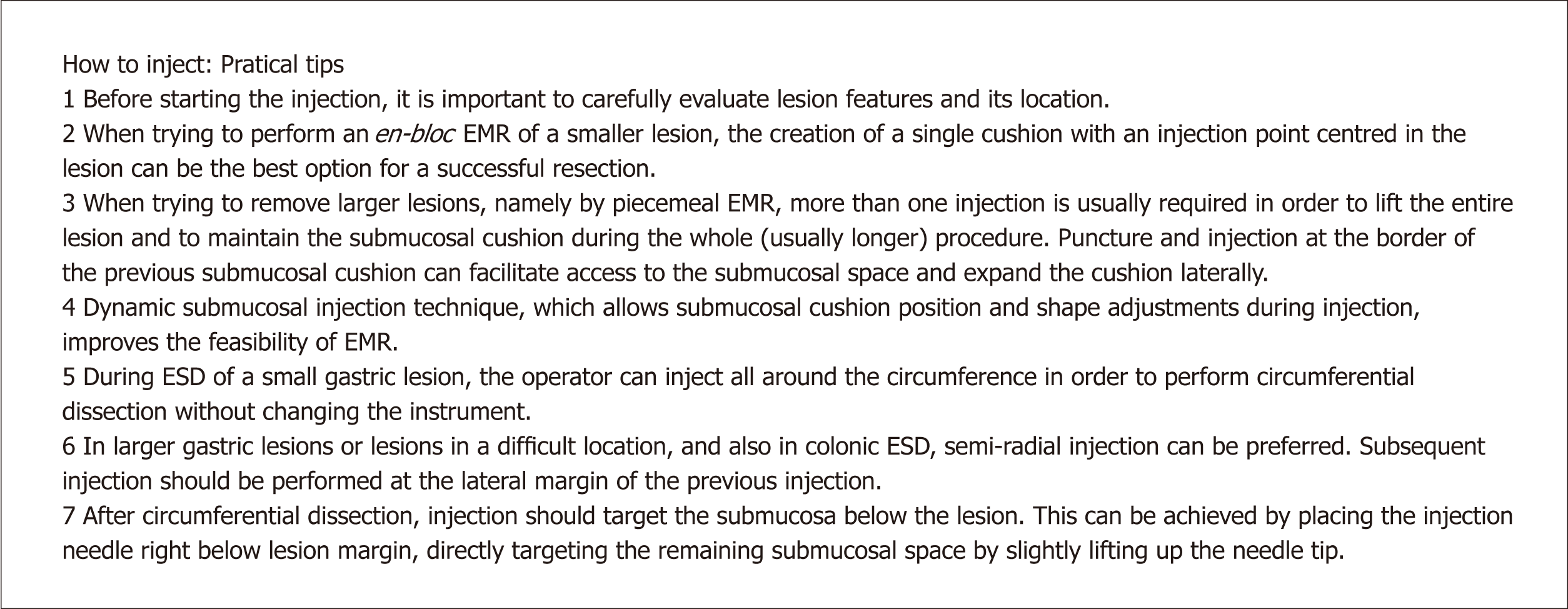
Submucosal injection is a key step of endoscopic resection techniques. This is normally made using an injector that goes through the working channel of the endoscope, which has a retractile needle that allows access to the submucosal space and injection of different types of solutions[36]. Before starting the injection, it is important to careful evaluate lesion morphology and localization. For instance, when trying to perform en-bloc resection of a smaller lesion, the creation of a single cushion with the injection point centered in the lesion may be the best option. However, when trying to remove larger lesions, namely by piecemeal EMR, more than one injection is usually needed to lift the entire lesion. In these instances, after the first puncture and injection into the submucosa, it can help to puncture and inject the border of the already formed submucosal cushion and expand it laterally. Soetkino et al[36] described this step either as static or dynamic submucosal injection. In the static technique, the needle punctures the submucosa and remains in a relatively fixed position during fluid injection and the lumen is inflated to visualize the position of the needle insertion point. In this case, many endoscopists report that the injected fluid rapidly dissipates, resulting in an insufficient submucosal cushion that hinders lesion snaring. On the other hand, dynamic submucosal injection helps produce a focal bulge under the lesion. First, keeping the catheter close to the endoscope tip, the needle is advanced into the submucosal plane and a small amount of solution is injected. Once the submucosal location is confirmed, subsequent injection is rapidly performed through the injector needle, while the needle position is slightly redirected within the injection site by slowly pulling back the catheter or slight deflections of the endoscope tip. In addition to these subtle movements, the lumen is gently aspirated to increase the size of the cushion. Many endoscopists prefer this last technique, which improves the feasibility of EMR[37].
Although sharing some common aspects with EMR, injection in ESD has some particularities. After delimitation, the first step consists in gaining access to the submucosal space through a mucosal incision with a sharp knife. The mucosal incision should be performed outside of the coagulation marks, and thus it is recommended to perform the first injections right outside of the marks, to reduce perforation risk when cutting into the mucosa/submucosa. In a small gastric lesion (< 20 mm), the operator can inject and lift the whole circumference in order to perform the other 3-4 mucosal incisions and circumferential dissection without changing the instrument. However, in larger gastric lesions or lesions located in a difficult location, and also in colonic ESD, semi-radial injection can be preferred. It is also important to recognize that subsequent injection should be performed at the lateral margin of the previous injection. After mucosal incision and circumferential/semi-circumferential dissection, injection should be performed as needed in the submucosal dissection plane. Injection should be precise and target the submucosa below the lesion and not the muscularis propria. This can be achieved by placing the injection catheter/needle right below lesion margin, directly targeting the remaining submucosal space by slightly lifting up the needle tip. While injecting, subtle movement in the endoscope shaft or wheels can be useful to direct the lesion to an adequate position, in order to provide a larger field of view and facilitate dissection. When using a cap, placing the tip of the endoscope below the lesion can also facilitate further injection. After complete dissection, coagulation of visible vessels should also be performed in gastric ESD, and sufficient submucosal lifting is generally advised in order to reduce thermal injury to the gastric wall, which could be accomplished with further injection or water jet elevation.
With the dissemination of endoscopic resection, recent focus has been put in optimizing and facilitating the procedures, with the aim of decreasing procedural time while maintaining high efficacy and minimizing adverse events. Devices have been developed that achieve submucosal lifting through ultrafine jet pressure, allowing ESD without the need for conventional injection (e.g., Hybrid-Knife®, Dual-Knife®). To combine the advantages of water jet lifting and of macromolecular solutions, a water-jet system with a bifunctional catheter (Nestis® Enki II®, Lyon, France) was developed and has been shown to be feasible, although it was not implemented in routine clinical practice, perhaps because the duration of elevation is not so important when high-pressure jets are used[38]. Devices that create submucosal blebs without needle injection were also developed for inject-and-cut EMR (e.g., ERBELift), with the theoretical advantage of easing the lifting of lesions and avoiding possible complications of needle manipulation, although it also demands device exchange between snares and the flexible “injector”/probe. Regarding solutions for lifting, recently there has been interest in the development of solutions with newer and useful properties, namely dissecting properties. In an animal study, a modified ESD technique using an endoscopic biocompatible gel (Cook Medical Inc) was shown to be feasible. In this technique, the gel with dissecting property was injected in a previously formed submucosal bleb after mucosal incision, and no further knife dissection was needed since auto-dissection was noted (the removal of the separated mucosa was accomplished with hot snare). Mean procedural time for 30-mm lesions was 7.5 min[39]. This gel was also found to be useful in peroral endoscopic miotomy (POEM), allowing the creation of a tunnel without dissection (“auto-tunneling”)[40]. A thiol compound called mesna that can chemically soften connective tissues and facilitate ESD was also evaluated in a human RCT and it was found that it can significantly reduce time-consuming cases (> 30 min, P = 0.049). Although mean dissecting time was not statistically significant lower in the mesna group (18.6 min vs 24.6 min, P = 0.128), mesna use was independently associated with lower dissecting time in multivariable analysis[41]. In conclusion, although there has been some development concerning injection solutions and devices, the progress has been slow since injection with NS and viscous solutions with conventional needles is highly efficacious and safe. However, further refinement of the technique is always welcome to improve its already good outcomes, to accelerate the learning curve and to facilitate the dissemination of endoscopic resection techniques.
According to current evidence, as pointed throughout this review, no solution has proven to be consistently superior in complete resection rate and in the reduction of adverse events incidence like post-polipectomy bleeding or coagulation syndrome/perforation. This is particularly evident in western countries where most of the injection solutions specifically developed for endoscopic resection in Asia are not commercially available or not approved by the Food and Drug Administration, leaving endoscopists to use a variety of injectable fluids off-label. We can conclude that for most of endoscopic resection, namely smaller lesions (about 20 mm), NS is still a good option because the height of the cushion and the duration of the elevation is not as preponderant a factor. For these lesions, the use of NS does not lead to a significant greater number of injections or to an increased procedure time. Despite the lack of proven superiority of a specific endoscopic submucosal injection solution in humans, in a recent randomized controlled trial of solutions currently available in the West, Eleview® and 6% hydroxyethyl starch were the best performing solutions for ESD in a porcine model. So, even though viscous solutions (namely starch or the new Eleview) can be relatively expensive, they can be particularly important in the resection of larger lesions, particularly during ESD, by decreasing the number of injections and the procedure time. In conclusion, when choosing the type of submucosal injection solution, we must take into account the lesion features and the endoscopic resection technique to be used, the local and own expertise, the availability and costs of the solution as well as the balance between its advantages and potential adverse effects.
| 1. | Isomoto H. Global dissemination of endoscopic submucosal dissection for early gastric cancer. Intern Med. 2010;49:251-252. [PubMed] |
| 2. | Choi KS, Jung HY, Choi KD, Lee GH, Song HJ, Kim DH, Lee JH, Kim MY, Kim BS, Oh ST, Yook JH, Jang SJ, Yun SC, Kim SO, Kim JH. EMR versus gastrectomy for intramucosal gastric cancer: comparison of long-term outcomes. Gastrointest Endosc. 2011;73:942-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (1)] |
| 3. | ASGE Technology Committee; Hwang JH, Konda V, Abu Dayyeh BK, Chauhan SS, Enestvedt BK, Fujii-Lau LL, Komanduri S, Maple JT, Murad FM, Pannala R, Thosani NC, Banerjee S. Endoscopic mucosal resection. Gastrointest Endosc. 2015;82:215-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 4. | Jung YS, Park DI. Submucosal injection solutions for endoscopic mucosal resection and endoscopic submucosal dissection of gastrointestinal neoplasms. Gastrointest Inte. 2013;2:73-77. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 5. | Uraoka T, Saito Y, Yamamoto K, Fujii T. Submucosal injection solution for gastrointestinal tract endoscopic mucosal resection and endoscopic submucosal dissection. Drug Des Devel Ther. 2009;2:131-138. [PubMed] |
| 6. | Fujishiro M, Yahagi N, Kashimura K, Matsuura T, Nakamura M, Kakushima N, Kodashima S, Ono S, Kobayashi K, Hashimoto T, Yamamichi N, Tateishi A, Shimizu Y, Oka M, Ichinose M, Omata M. Tissue damage of different submucosal injection solutions for EMR. Gastrointest Endosc. 2005;62:933-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 134] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 7. | Rastogi A. Optical diagnosis of small colorectal polyp histology with high-definition colonoscopy using narrow band imaging. Clin Endosc. 2013;46:120-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Rex DK. Narrow-band imaging without optical magnification for histologic analysis of colorectal polyps. Gastroenterology. 2009;136:1174-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 217] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 9. | Ferlitsch M, Moss A, Hassan C, Bhandari P, Dumonceau JM, Paspatis G, Jover R, Langner C, Bronzwaer M, Nalankilli K, Fockens P, Hazzan R, Gralnek IM, Gschwantler M, Waldmann E, Jeschek P, Penz D, Heresbach D, Moons L, Lemmers A, Paraskeva K, Pohl J, Ponchon T, Regula J, Repici A, Rutter MD, Burgess NG, Bourke MJ. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017;49:270-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 797] [Article Influence: 88.6] [Reference Citation Analysis (1)] |
| 10. | Khashab MA, Cummings OW, DeWitt JM. Ligation-assisted endoscopic mucosal resection of gastric heterotopic pancreas. World J Gastroenterol. 2009;15:2805-2808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | ASGE TECHNOLOGY COMMITTEE, Kantsevoy SV, Adler DG, Conway JD, Diehl DL, Farraye FA, Kwon R, Mamula P, Rodriguez S, Shah RJ, Wong Kee Song LM, Tierney WM. Endoscopic mucosal resection and endoscopic submucosal dissection. Gastrointest Endosc. 2008;68:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 221] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 12. | Tanaka S, Haruma K, Oka S, Takahashi R, Kunihiro M, Kitadai Y, Yoshihara M, Shimamoto F, Chayama K. Clinicopathologic features and endoscopic treatment of superficially spreading colorectal neoplasms larger than 20 mm. Gastrointest Endosc. 2001;54:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 232] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Uraoka T, Saito Y, Matsuda T, Ikehara H, Gotoda T, Saito D, Fujii T. Endoscopic indications for endoscopic mucosal resection of laterally spreading tumours in the colorectum. Gut. 2006;55:1592-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 309] [Article Influence: 15.5] [Reference Citation Analysis (1)] |
| 14. | Gotoda T. A large endoscopic resection by endoscopic submucosal dissection procedure for early gastric cancer. Clin Gastroenterol Hepatol. 2005;3:S71-S73. [PubMed] |
| 15. | Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, Amato A, Berr F, Bhandari P, Bialek A, Conio M, Haringsma J, Langner C, Meisner S, Messmann H, Morino M, Neuhaus H, Piessevaux H, Rugge M, Saunders BP, Robaszkiewicz M, Seewald S, Kashin S, Dumonceau JM, Hassan C, Deprez PH. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 958] [Article Influence: 87.1] [Reference Citation Analysis (0)] |
| 16. | Ohkuwa M, Hosokawa K, Boku N, Ohtu A, Tajiri H, Yoshida S. New endoscopic treatment for intramucosal gastric tumors using an insulated-tip diathermic knife. Endoscopy. 2001;33:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 306] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 17. | Ferreira AO, Moleiro J, Torres J, Dinis-Ribeiro M. Solutions for submucosal injection in endoscopic resection: a systematic review and meta-analysis. Endosc Int Open. 2016;4:E1-E16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Fujishiro M, Yahagi N, Kashimura K, Mizushima Y, Oka M, Enomoto S, Kakushima N, Kobayashi K, Hashimoto T, Iguchi M, Shimizu Y, Ichinose M, Omata M. Comparison of various submucosal injection solutions for maintaining mucosal elevation during endoscopic mucosal resection. Endoscopy. 2004;36:579-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 201] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | Sumiyoshi T, Fujii T, Sumiyoshi Y. Injected substances to the submucosa in endoscopic mucosal resection: glycerin solution versus normal saline solution. Gastrointest Endosc. 2002;55:AB110-AB110. |
| 20. | Uraoka T, Fujii T, Saito Y, Sumiyoshi T, Emura F, Bhandari P, Matsuda T, Fu KI, Saito D. Effectiveness of glycerol as a submucosal injection for EMR. Gastrointest Endosc. 2005;61:736-740. [PubMed] |
| 21. | Yamamoto H, Yube T, Isoda N, Sato Y, Sekine Y, Higashizawa T, Ido K, Kimura K, Kanai N. A novel method of endoscopic mucosal resection using sodium hyaluronate. Gastrointest Endosc. 1999;50:251-256. [PubMed] |
| 22. | Yamamoto H, Kawata H, Sunada K, Satoh K, Kaneko Y, Ido K, Sugano K. Success rate of curative endoscopic mucosal resection with circumferential mucosal incision assisted by submucosal injection of sodium hyaluronate. Gastrointest Endosc. 2002;56:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Yamamoto H, Kawata H, Sunada K, Sasaki A, Nakazawa K, Miyata T, Sekine Y, Yano T, Satoh K, Ido K, Sugano K. Successful en-bloc resection of large superficial tumors in the stomach and colon using sodium hyaluronate and small-caliber-tip transparent hood. Endoscopy. 2003;35:690-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 300] [Article Influence: 13.0] [Reference Citation Analysis (1)] |
| 24. | Fujishiro M, Yahagi N, Nakamura M, Kakushima N, Kodashima S, Ono S, Kobayashi K, Hashimoto T, Yamamichi N, Tateishi A, Shimizu Y, Oka M, Ogura K, Kawabe T, Ichinose M, Omata M. Successful outcomes of a novel endoscopic treatment for GI tumors: endoscopic submucosal dissection with a mixture of high-molecular-weight hyaluronic acid, glycerin, and sugar. Gastrointest Endosc. 2006;63:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 199] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 25. | Matsui Y, Inomata M, Izumi K, Sonoda K, Shiraishi N, Kitano S. Hyaluronic acid stimulates tumor-cell proliferation at wound sites. Gastrointest Endosc. 2004;60:539-543. [PubMed] |
| 26. | Ravalico G, Tognetto D, Baccara F, Lovisato A. Corneal endothelial protection by different viscoelastics during phacoemulsification. J Cataract Refract Surg. 1997;23:433-439. [PubMed] |
| 27. | Feitoza AB, Gostout CJ, Burgart LJ, Burkert A, Herman LJ, Rajan E. Hydroxypropyl methylcellulose: A better submucosal fluid cushion for endoscopic mucosal resection. Gastrointest Endosc. 2003;57:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Lee SH, Cho WY, Kim HJ, Kim HJ, Kim YH, Chung IK, Kim HS, Park SH, Kim SJ. A new method of EMR: submucosal injection of a fibrinogen mixture. Gastrointest Endosc. 2004;59:220-224. [PubMed] |
| 29. | Lee SH, Park JH, Park DH, Chung IK, Kim HS, Park SH, Kim SJ, Cho HD. Clinical efficacy of EMR with submucosal injection of a fibrinogen mixture: a prospective randomized trial. Gastrointest Endosc. 2006;64:691-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Moss A, Bourke MJ, Metz AJ. A randomized, double-blind trial of succinylated gelatin submucosal injection for endoscopic resection of large sessile polyps of the colon. Am J Gastroenterol. 2010;105:2375-2382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Mehta N, Strong AT, Franco M, Stevens T, Chahal P, Jang S, Lopez R, Patil D, Abe S, Saito Y, Uraoka T, Vargo J, Bhatt A. Optimal injection solution for endoscopic submucosal dissection: A randomized controlled trial of Western solutions in a porcine model. Dig Endosc. 2018;30:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Repici A, Wallace M, Sharma P, Bhandari P, Lollo G, Maselli R, Hassan C, Rex DK. A novel submucosal injection solution for endoscopic resection of large colorectal lesions: a randomized, double-blind trial. Gastrointest Endosc. 2018;88:527-535.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 33. | Huai ZY, Feng Xian W, Chang Jiang L, Xi Chen W. Submucosal injection solution for endoscopic resection in gastrointestinal tract: a traditional and network meta-analysis. Gastroenterol Res Pract. 2015;2015:702768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | US Food and Drug Administration. Available from: https://www.fda.gov. |
| 35. | Tullavardhana T, Akranurakkul P, Ungkitphaiboon W, Songtish D. Efficacy of submucosal epinephrine injection for the prevention of postpolypectomy bleeding: A meta-analysis of randomized controlled studies. Ann Med Surg (Lond). 2017;19:65-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 36. | Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc. 2003;57:567-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 378] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 37. | Soetikno R, Kaltenbach T. Dynamic submucosal injection technique. Gastrointest Endosc Clin N Am. 2010;20:497-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Pioche M, Lépilliez V, Déprez P, Giovannini M, Caillol F, Piessevaux H, Rivory J, Guillaud O, Ciocîrlan M, Salmon D, Lienhart I, Lafon C, Saurin JC, Ponchon T. High pressure jet injection of viscous solutions for endoscopic submucosal dissection (ESD): first clinical experience. Endosc Int Open. 2015;3:E368-E372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 39. | Khashab MA, Saxena P, Sharaiha RZ, Chavez YH, Zhang F, Kord Valeshabad A, Aguila G, Canto MI, Pasricha PJ, Kalloo AN. A novel submucosal gel permits simple and efficient gastric endoscopic submucosal dissection. Gastroenterology. 2013;144:505-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Khashab MA, Sharaiha RZ, Saxena P, Law JK, Singh VK, Lennon AM, Shin EJ, Canto MI, Aguila G, Okolo PI, Stavropoulos SN, Inoue H, Pasricha PJ, Kalloo AN. Novel technique of auto-tunneling during peroral endoscopic myotomy (with video). Gastrointest Endosc. 2013;77:119-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Sumiyama K, Toyoizumi H, Ohya TR, Dobashi A, Hino S, Kobayashi M, Goda K, Imazu H, Kawakita Y, Kato T, Tajiri H. A double-blind, block-randomized, placebo-controlled trial to identify the chemical assistance effect of mesna submucosal injection for gastric endoscopic submucosal dissection. Gastrointest Endosc. 2014;79:756-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Portugal
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P- Reviewer: Kobayashi N, Tabibian JH S- Editor: Ma RY L- Editor: A E- Editor: Huang Y