Published online Dec 7, 2019. doi: 10.3748/wjg.v25.i45.6653
Peer-review started: September 17, 2019
First decision: November 4, 2019
Revised: November 8, 2019
Accepted: November 16, 2019
Article in press: November 16, 2019
Published online: December 7, 2019
Processing time: 79 Days and 23.5 Hours
Acute pancreatitis (AP) is often associated with intestinal injury, which in turn exaggerates the progression of AP. Our recent study has shown that a low level of serum irisin, a novel exercise-induced hormone, is associated with poor outcomes in patients with AP and irisin administration protects against experimental AP. However, the role of irisin in intestinal injury in AP has not been evaluated.
To investigate the effect of irisin administration on intestinal injury in experimental AP.
AP was induced in male adult mice by two hourly intraperitoneal injections of L-arginine. At 2 h after the last injection of L-arginine, irisin (50 or 250 μg/kg body weight) or 1 mL normal saline (vehicle) was administered through intraperitoneal injection. The animals were sacrificed at 72 h after the induction of AP. Intestinal injury, apoptosis, oxidative and endoplasmic reticulum (ER) stress were evaluated.
Administration of irisin significantly mitigated intestinal damage, reduced apoptosis, and attenuated oxidative and ER stress in AP mice. In addition, irisin treatment also effectively downregulated serum tumor necrosis factor-alpha and interleukin-6 levels and alleviated injury in the pancreas, liver and lung of AP mice.
Irisin-mediated multiple physiological events attenuate intestinal injury following an episode of AP. Irisin has a great potential to be further developed as an effective treatment for patients with AP.
Core tip: In this study, we reported for the first time that intraperitoneal injection of irisin could relieve intestinal injury in mice with acute pancreatitis (AP). AP was induced in male adult mice by two hourly intraperitoneal injections of L-arginine. At 2 h after the last injection of L-arginine, irisin (50 or 250 μg/kg body weight) or 1 mL normal saline (vehicle) was administered through intraperitoneal injection. Irisin alleviated intestinal and systemic inflammatory reactions by inhibiting intestinal endoplasmic reticulum stress and oxidative stress in mice with AP, reduced the degree of intestinal tissue apoptosis and injury and finally alleviated the condition of pancreatitis.
- Citation: Ren YF, Wang MZ, Bi JB, Zhang J, Zhang L, Liu WM, Wei SS, Lv Y, Wu Z, Wu RQ. Irisin attenuates intestinal injury, oxidative and endoplasmic reticulum stress in mice with L-arginine-induced acute pancreatitis. World J Gastroenterol 2019; 25(45): 6653-6667
- URL: https://www.wjgnet.com/1007-9327/full/v25/i45/6653.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i45.6653
Severe acute pancreatitis (SAP) often leads to the failure of multiple organs, including the lung, liver, kidney and intestines[1]. Intestinal injury results in massive cell death in the intestinal epithelium and basement membrane, which leads to the absorption of a large number of intestinal bacteria into the blood, aggravating the systemic inflammatory response and eventually causing systemic inflammatory response syndrome[2,3]. The resulting systemic inflammation adds to the burden on the organs and worsens the severity of acute pancreatitis (AP), delivering a second blow to the patient[4]. If left untreated, it can lead to multiple organ failure syndrome and ultimately death[5]. Therefore, in SAP, in addition to routine treatment of pancreatitis, the protection of intestinal function can also greatly improve the outcome of the disease and save the patient’s life. Currently, the treatment of SAP-associated intestinal injury is limited.
Oxidative stress refers to the imbalance of oxidants and antioxidants in the body, which leads to the infiltration of inflammatory cells, promoting the production and accumulation of reactive oxygen species (ROS) in cells and finally leading to the oxidative stress response[6]. When SAP occurs, inflammatory cells infiltrate the intestines and induce oxidative stress[7]. Oxidative stress further increases the infiltration of inflammatory cells in the intestine, aggravates the injury of intestinal cells, destroys the barrier function of the intestines and promotes the entry of intestinal bacteria into the blood[8-10].
Endoplasmic reticulum (ER) stress is another factor of intestinal injury in AP. ER stress can activate a large number of apoptosis-related proteins and induce apoptosis[11]. ER stress is often accompanied by oxidative stress (mutually reinforcing), which in coordination with oxidative stress promotes the infiltration and displacement of inflammatory cells, accelerates the apoptosis of intestinal cells and aggravates the intestinal injury in AP. Therefore, reducing oxidative stress and ER stress in the intestine has become an important approach to alleviate AP.
Irisin can act on white fat cells and induce their transformation into brown fat cells[12]. After exercise, muscles secrete a protein called PGC-1 alpha, which regulates a downstream factor to produce irisin through cleavage and modification[13]. Currently, scholars have reported that irisin reduces the severity of various diseases, including acute lung injury and heart ischemia-reperfusion injury by the anti-inflammatory response, antioxidative stress and anti-ER stress[14-16]. When the intestine is injured, oxidative stress and ER stress often occur in the intestine, aggravating the disorder of the cellular microenvironment. The inhibition of intestinal injury during AP can protect the intestinal cells from being damaged and reduce the entry of intestinal bacteria into the blood caused by the damaged intestinal epithelium. Therefore, our recent experiments mainly explored the effects of the intraperitoneal injection of exogenous irisin on intestinal oxidative stress and ER stress in AP mice and tested its protective function on damaged intestinal cells. Further studies were conducted to verify whether irisin can reduce the systemic inflammatory response in AP mice and protect a variety of extrapancreatic organs.
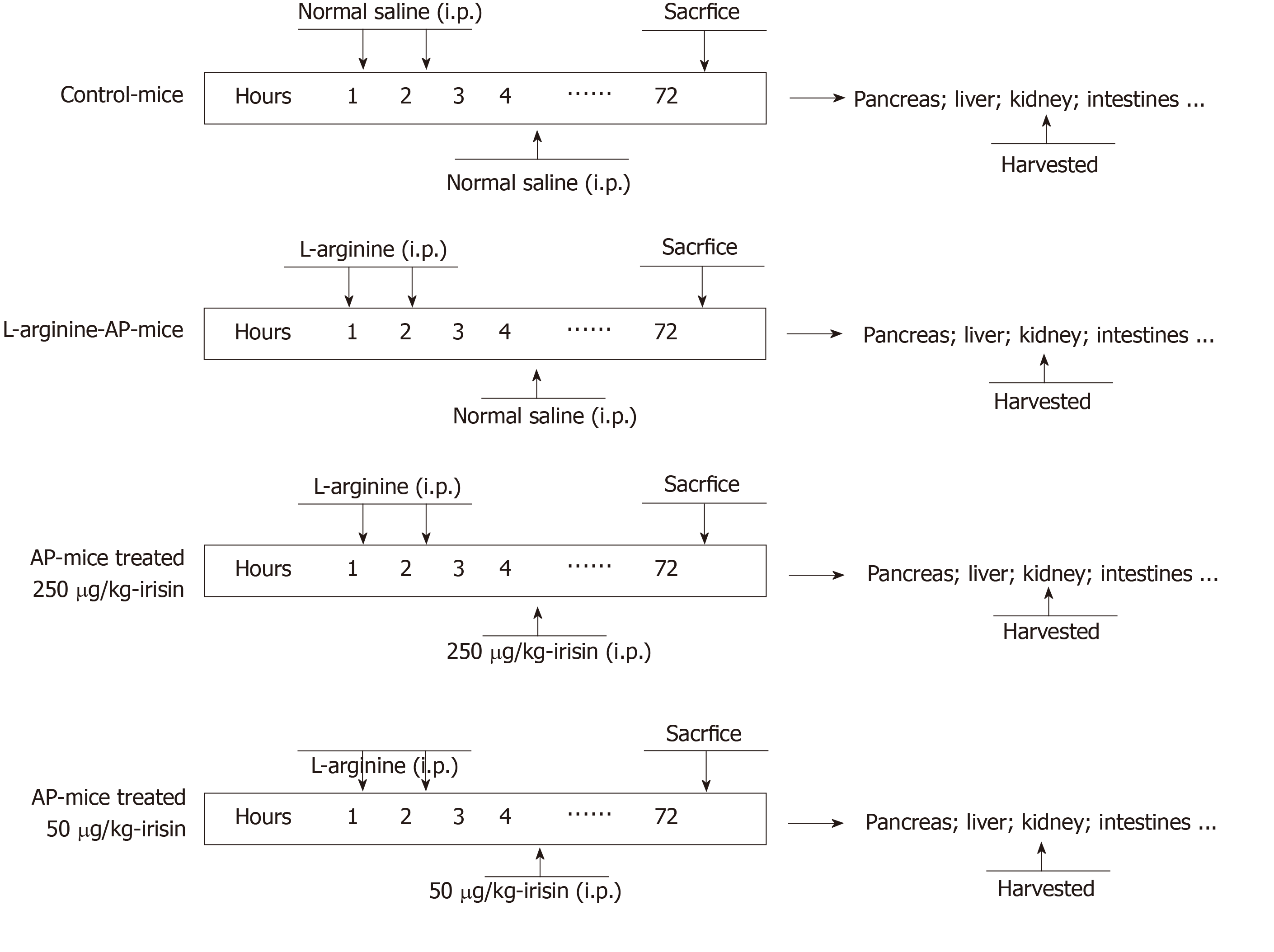
Male wild-type C57BL/6 J mice (aged 8-10 wk, weighing 20-22 g) were purchased from the Animal Experiment Center of Xi’an Jiaotong University Health Science Center. Prior to the experiments, all animals were housed for one week under standard conditions to acclimate to the surroundings. A total of 24 mice (n = 6/group) were used in this study. The mice were divided (six mice per group) as follows: (1) Control group (sham); (2) Vehicle group; (3) 50 μg/kg irisin group; and (4) 250 μg/kg irisin group. All experimental procedures were consistent with international guidelines for the care and use of laboratory animals and were approved by the Animal Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University.
Prior to the experiments, all animals were housed in Perspex cages, five mice per cage, at the animal facility of Xi’an Jiaotong University Health Science Center for one week under standard conditions (25 ± 2 °C, 12 h/12 h light/dark, 50% humidity) to acclimate to the surroundings. The mice were fed on a standard Purina mouse chow diet and allowed water (tap) ad libitum. Arginine-AP was induced by two hourly intraperitoneal injections of L-arginine[17,18] (0.2 mL, 4.0 g/kg L-arginine, A5006, Sigma-Aldrich, China). At 2 h after the last injection of L-arginine, normal saline (vehicle) or 50 μg/kg irisin or 250 μg/kg irisin (0.1 mL, 067-29A, Phoenix Pharmaceuticals, Inc., United States) was administered through intraperitoneal injection, as previously described[11,19]. The mice in the sham group were treated in the same way as the mice in the three experimental groups but injected with 0.9% NaCl instead of L-arginine and irisin (Figure 1).
The animals were anesthetized with isoflurane inhalation at 72 h after the first injection of L-arginine. After the collected pancreatic tissues were washed with PBS solution, they were placed into 4% formaldehyde for 48 h to prepare the embedded sections. Lung, liver and kidney tissues were obtained in the same way as the pancreas tissue. The small intestinal tissues were 6 cm in length and were collected 10 cm away from the ileum. The collected tissues were cut into two pieces. The 3 cm-long parts were inserted into Eppendorf tubes and stored at -80 °C for subsequent experiments. Another part was placed into 4% formaldehyde for 48 h to prepare the embedded sections. Blood from the arterial femoralis was obtained at 72 h after the first injection of L-arginine and placed at room temperature for one hour. After solidification, the supernatant was centrifuged for 15 min at 3000 rpm, and then the supernatant was collected and stored at -80 °C for subsequent experiments.
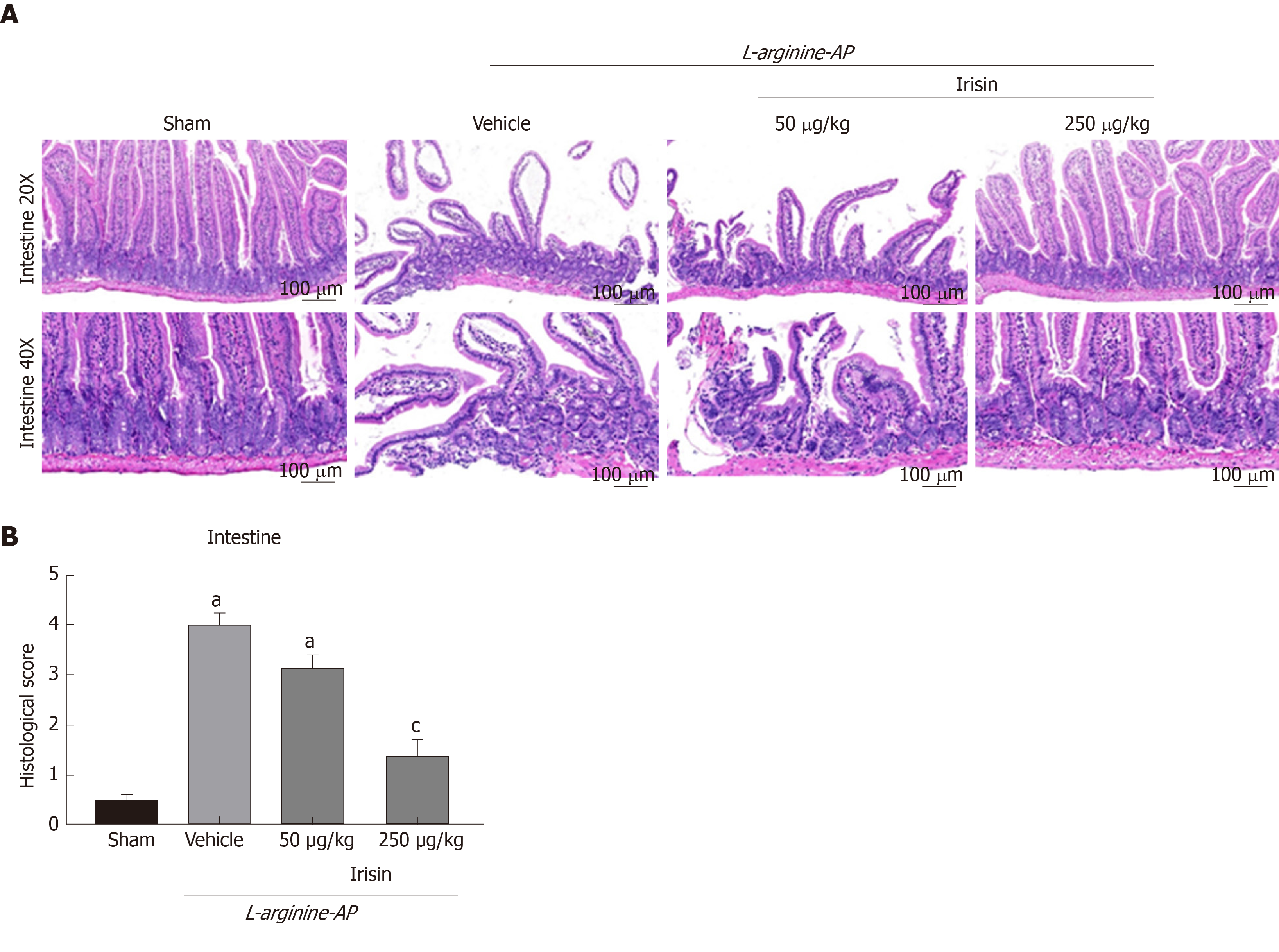
Hematoxylin and eosin (HE) staining was used to assess pancreatic, intestinal, pulmonary, renal and hepatic histology. As described previously, Zhang et al[20] recorded the histopathological scoring criteria of intestinal injury in detail. Histological score details are: No inflammation (0 point), mild (1 point) and severe (2 points). The infiltration depth was classified as follows: None (0 point), submucosal layer (1 point), muscular layer (2 points) and serous layer (3 points). Clearance loss was classified as follows: No (0 point), 1/3 crypt loss (1 point), 2/3 crypt loss (2 points), whole crypt loss/surface epithelial integrity (3 points) and loss of whole crypt and surface epithelial integrity (4 points). The percentage of organ involvement after injury was graded in the following order: 1% to 25% (1 point), 26% to 50% (2 points), 51% to 75% (3 points) and 76% to 100% (4 points). The pathological intestinal HE staining were scored according to their scoring rules[20]. All six sections from each group were evaluated, two fields were randomly photographed for each section, and pathological staining was quantified according to specific scoring criteria.
Intestinal tissue homogenate was obtained. A superoxide dismutase assay (SOD) kit (A001-3; Nanjing Jiancheng Bioengineering Institute, China), glutathione (GSH) assay kit (A006-2-1, Nanjing Jiancheng Bioengineering Institute, China) and malondialdehyde (MDA) assay kit (A003-1; Nanjing Jiancheng Bioengineering Institute, China) were used to measure the levels of SOD, GSH and MDA in the intestinal tissue according to the manufacturer’s instructions.
We used a TUNEL assay (11684795910, Roche, Switzerland) to identify apoptosis as reported previously[19]. After sectioning and fluorescence staining, the sections were observed by a fluorescence microscope with an excitation wavelength of 480 nm and an emission wavelength of 530 nm. Dihydroethidium (DHE) staining was used to identify ROS, and fresh frozen sections of the intestinal tissue were used. The sections were incubated with DHE dye (Sigma-Aldrich, United States), and the sections were observed by a fluorescence microscope. Three sections were randomly selected for each group, and two fields were randomly photographed for each section. Fluo-rescence staining was performed for quantitative determination using Image-Pro Plus 6.0 software.
The detailed Western blot steps were as previously described[19]. The membranes were incubated with primary antibodies (dilution: 1:1000-2000) (Supplemental Table 1) at 4 °C overnight, and then secondary IgG-HRP antibodies (dilution: 1:5000) (goat anti-mouse IgG or goat anti-rabbit IgG, Pioneer Biotechnology, China) were used. Bands were developed using a digital gel image analysis system (Bio-Rad, United States), and the expression levels of proteins were calculated by ImageJ software as the intensity relative to that of β-actin.
All measurement data are expressed as the means ± standard error of the mean. Because we were not assessing multiple dependent variables simultaneously in the current study, the differences between groups were compared by one-way ANOVA and Student-Newman-Keuls test. One-way ANOVA was used to analyze the differences between groups. All analyses were conducted with data statistics software GraphPad Prism version 8.0 (GraphPad Software, Inc., United States). P < 0.05 represented a significant difference.
Damage to the epithelial cells and basal cells of the small intestine leads to the absorption of a large amount of harmful substances into the blood and aggravates AP[2,21,22]. Therefore, we examined the intestinal lesions in AP mice in each group. As shown in Figure 2A and 2B, the number of intestinal villi decreased, and edema and dislodging were observed in the villi of the small intestine. The basal intestinal barrier was damaged, and numerous inflammatory cells infiltrated the intestine in vehicle-treated AP mice. The intestinal histological score of the vehicle-treated group was 7.3-times higher than that of the control group (P < 0.05). However, the administration of 250 μg/kg irisin improved the intestinal architecture, significantly reducing intestinal edema and decreasing the injury score (P < 0.05). However, 50 μg/kg irisin only slightly reduced the intestinal injury (P > 0.05, Figure 2A and B). These results suggest that irisin can reduce intestinal tissue damage in AP mice in a dose-dependent manner.
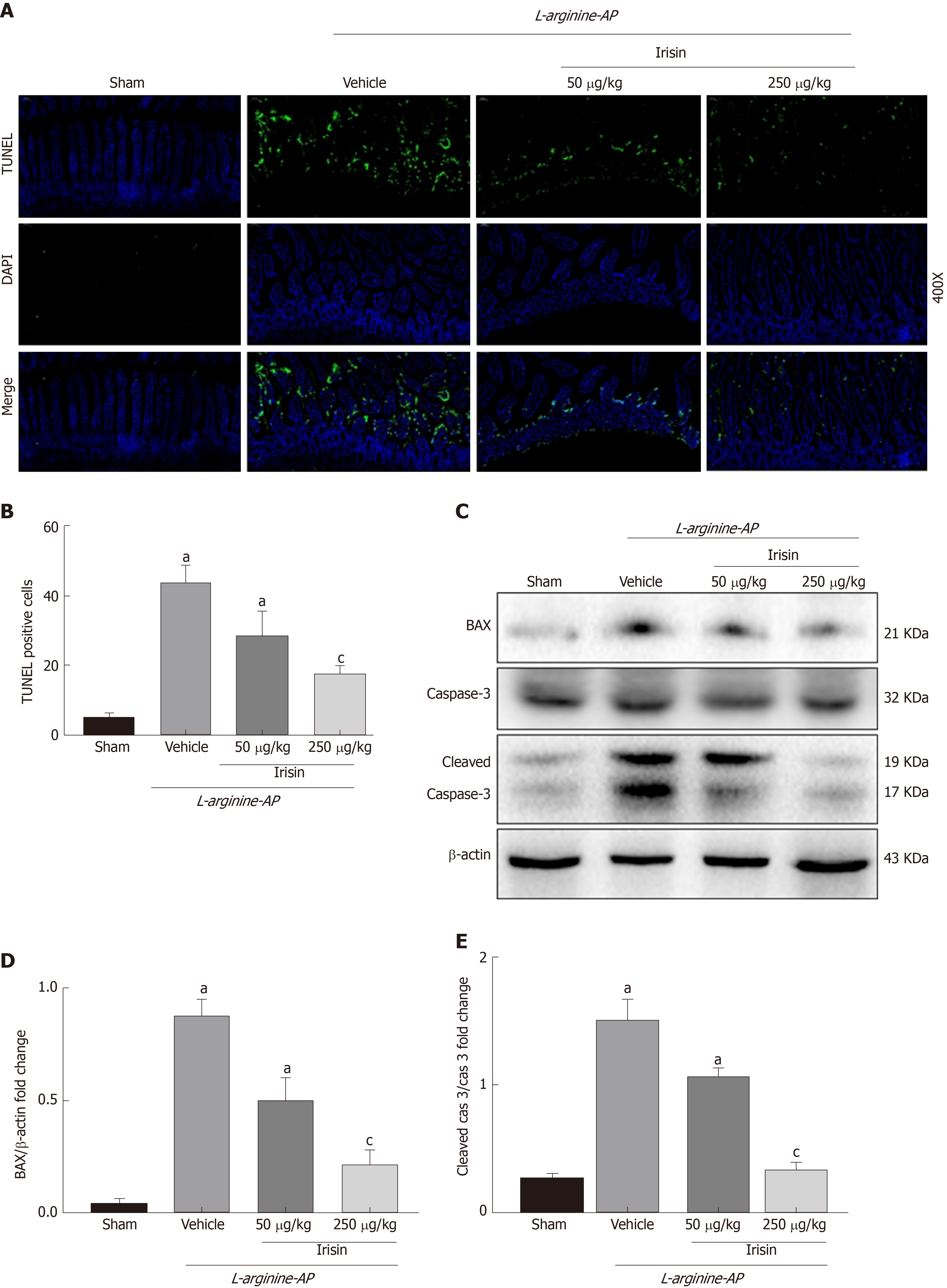
AP can lead to the apoptosis of intestinal cells in mice, which causes intestinal damage and affects the intestinal barrier function[23,24]. Therefore, we examined the apoptosis of intestinal cells to investigate the protective effect of irisin on intestinal tissue in AP mice. As shown in Figure 3A and 3B, TUNEL staining showed a significant increase in apoptosis in the basal and villous cells of the intestine in AP mice (P < 0.05). However, the administration of irisin effectively reduced intestinal apoptosis in AP mice in a dose-dependent manner (P < 0.05, Figure 3A and B).
BAX, a proapoptotic gene in the Bcl-2 gene family, antagonizes Bcl-2-mediated apoptosis inhibition and promotes apoptosis[25]. Consistent with the TUNEL staining, the expression of BAX was increased in the intestine of AP mice (P < 0.05). The increased expression of BAX promotes the conversion of inactive caspase-3 to active cleaved caspase-3, which is the main terminal cleavage enzyme in the process of apoptosis. Irisin treatment decreased the expression of BAX and level of cleaved caspase-3 in a dose-dependent manner (P < 0.05, Figure 3C-E), suggesting that irisin alleviates apoptosis.
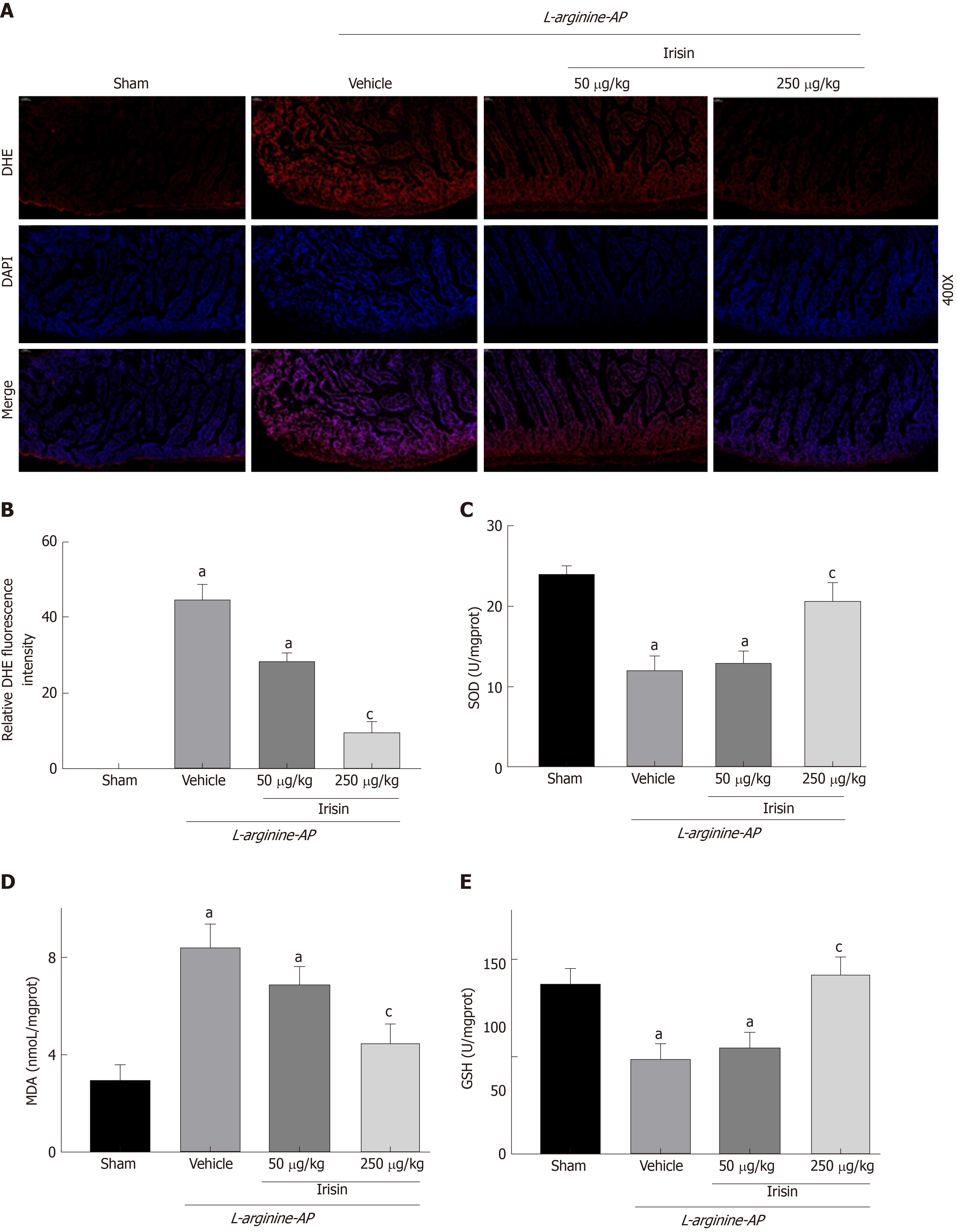
AP can lead to oxidative stress in the intestine, and the inflammatory response caused by oxidative stress can aggravate the damage of the intestine[26]. DHE fluorescence staining for ROS detection revealed that the fluorescence intensity of DHE in the intestinal tissues of AP mice was increased compared with that of the control mice (P < 0.05, Figure 4A and B). Administration of 250 μg/kg irisin effectively reduced the DHE fluorescence intensity in the intestinal tissues of AP mice, while the 50 μg/kg irisin treatment only slightly reduced the DHE fluorescence intensity in intestinal tissues of AP mice (P < 0.05, Figure 4A and B).
Consistently, the level of intestinal MDA, an indicator of oxidative stress, was increased in vehicle-treated AP mice (P < 0.05), and irisin treatment reduced intestinal MDA levels in AP mice in a dose-dependent manner (P < 0.05, Figure 4D). In contrast, the decreased levels of SOD and GSH (Figures 4C and E) in the intestines of AP mice were increased in a dose-dependent manner after irisin administration (P < 0.05, Figure 4C and E).
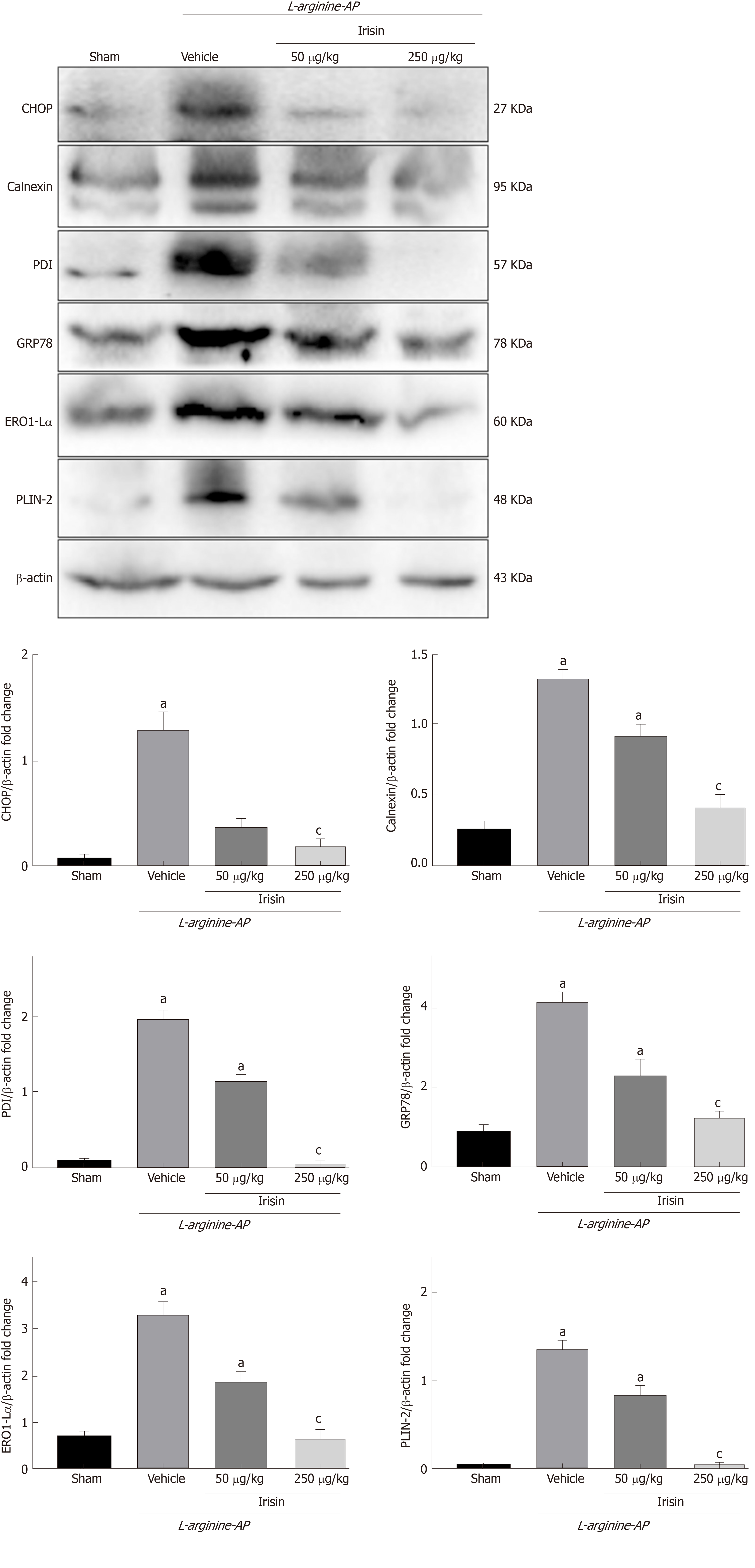
ER stress often accompanies oxidative stress and aggravates the damage of intestinal cells[27]. To investigate whether irisin could alleviate ER stress associated with AP in intestinal cells, we examined the expression of ER stress-related proteins in the intestine.
Western blot analysis showed that the level of intestinal GRP78, the most important initial factor in ER stress, increased in vehicle-treated AP mice, and irisin treatment reduced intestinal GRP78 expression in AP mice in a dose-dependent manner (P < 0.05, Figure 5). Consistently, the western blot analysis also showed that the levels of protein disulfide isomerase (PDI), Endoplasmic reticulum oxidoreductase 1-Lα (Ero1-Lα), calnexin, and Perilipin-2 (PLIN2), other ER stress-related proteins, were upregulated in vehicle-treated AP mice (P < 0.05, Figure 5). The administration of irisin dose-dependently decreased the protein expression of PDI, Ero1-Lα, calnexin and PLIN2 (P < 0.05, Figure 5).
Furthermore, when ER stress occurred in the intestinal cells of AP mice, the expression of the ER apoptosis-related protein C/EBP homologous protein (CHOP) was significantly increased compared with that in the control group (P < 0.05). The administration of irisin dose-dependently decreased the protein expression of CHOP (P < 0.05, Figure 5). Combined with the finding of reduced levels of apoptosis (Figure 3), this finding suggests that irisin alleviates ER stress.
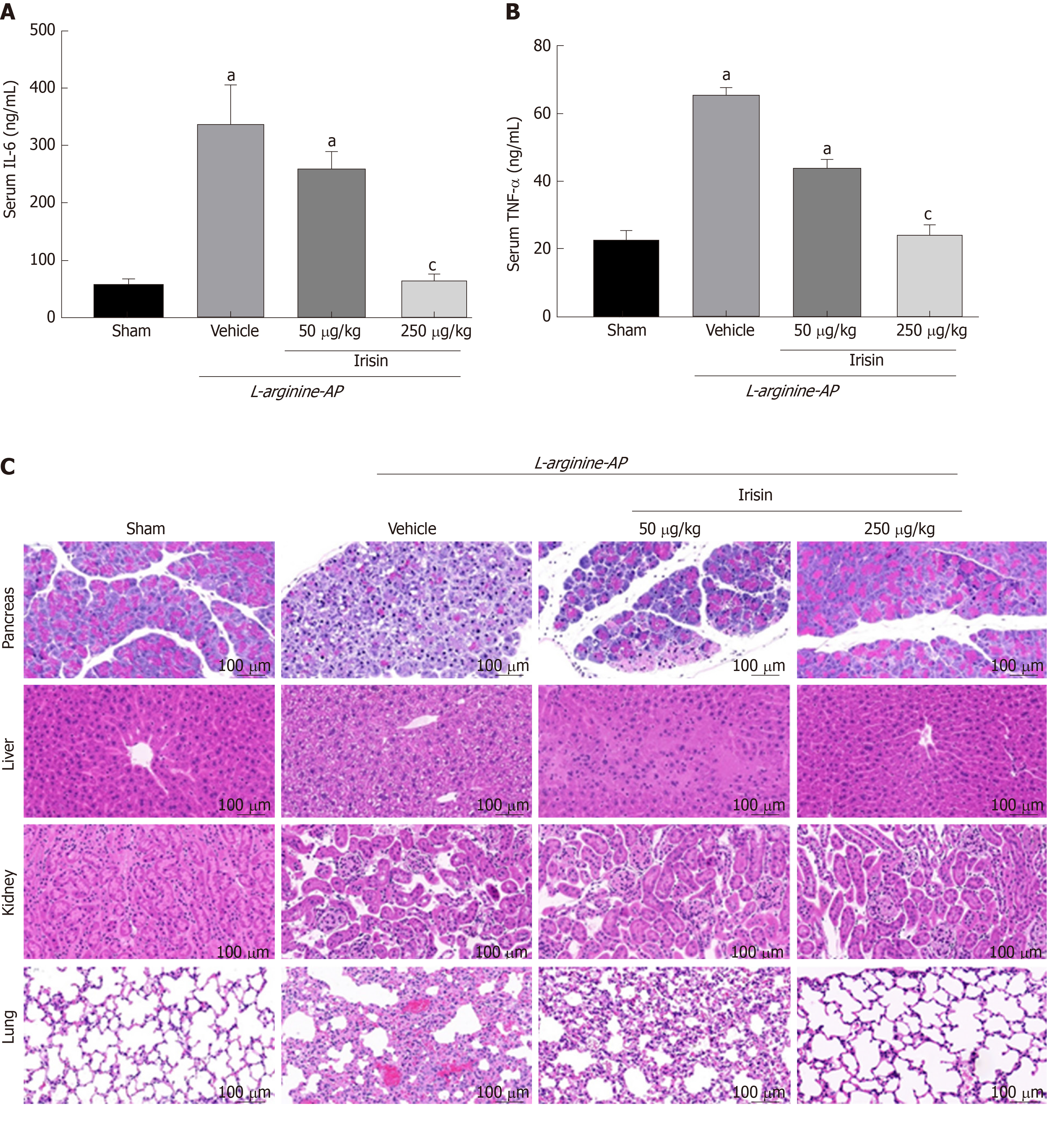
When SAP occurs, the release of a large amount of inflammatory mediators into the blood leads to SIRS, which is one of the causes of multiple organ function damage[5,28]. We therefore examined serum levels of inflammatory mediators such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α). As shown in Figure 6A and B, serum levels of IL-6 and TNF-α increased significantly in the AP group compared with the control group (P < 0.05). However, 250 µg/kg irisin treatment significantly reduced the serum levels of IL-6 and TNF-α, and 50 µg/kg irisin treatment only slightly reduced the serum levels of IL-6 and TNF-α (P < 0.05, Figure 6A and B). This finding suggests that irisin can relieve the systemic inflammatory response to AP.
To investigate the protective effect of irisin on multiple organ injury after relieving the systemic inflammatory response in AP, we further evaluated the pancreas, kidney, lung and liver injury in AP mice. As shown in Figure 6C, pancreas, kidney, lung and liver cells in AP mice were hyperemic, edematous, and necrotic with inflammatory cell infiltration. The pathological score showed that the pancreas, kidney, lung and liver injury indexes of AP mice were 4.4-times, 3.8-times, 3.6-times, and 4.2-times higher, respectively, compared with the control group (P < 0.05). When AP mice were given 50 μg/kg irisin and 250 μg/kg irisin, there was no significant improvement in renal injury. However, the pancreas, lung and liver injury decreased as the irisin concentration increased. This result suggests that irisin has no significant protective effect on the kidney injury in AP mice, while the protective effects on the pancreas, lung and liver injury in AP mice are dose-dependent (P < 0.05, Figure 6C).
In this study, we found that intraperitoneal injection of exogenous irisin effectively reduced intestinal injury caused by AP in mice. Irisin alleviated intestinal injury in AP mice by reducing oxidative stress and ER stress in intestinal cells. These effects reduced intestinal apoptosis in AP mice, and the protective effect of irisin on the intestines of AP mice was dose-dependent.
Irisin, a hormone secreted mainly by skeletal muscle, has been shown to protect against injury in multiple organs, including the liver, lungs, kidneys and heart[16,19,29-31]. In previous studies, we found that serum irisin levels in patients with AP are significantly lower than those in healthy people, and the degree of irisin decline is positively correlated with the severity of AP. Then, we constructed a variety of mouse AP models and confirmed that irisin could protect against the damage to pancreatic mitochondria in AP mice[19]. In this study, we found that intraperitoneal injection of irisin effectively reduced the levels of multiple inflammatory mediators such as IL-6 and TNF-α that are elevated in serum during AP and that irisin protected against multiple organ damage during AP in a dose-dependent manner. These results suggested that irisin reduced systemic inflammation during AP and protected damaged organs in a variety of ways.
Oxidative stress is a negative effect produced by free radicals and is considered to be an important factor leading to aging and disease[32]. Intestinal injury caused by various physical and chemical factors can lead to oxidative stress of mucosal cells and basal cells[33]. Pérez S et al[34] reported that oxidative stress in the intestine was mainly achieved by activating NOX1-ROS. Oxidative stress in the intestine can lead to inflammatory cell infiltration and intestinal villus necrosis and then cause the migration of bacteria into the blood, aggravating the systemic inflammation of AP[35]. Therefore, reducing oxidative stress can effectively alleviate the damage and necrosis of intestinal cells in AP, reducing the entry of intestinal bacteria into the blood and relieving the systemic inflammatory response in AP. In this study, we confirmed that injection of exogenous irisin can significantly alleviate the level of oxidative stress in the intestine of AP mice and reduce the injury and apoptosis rate of intestinal cells by detecting ROS-positive cells and measuring the levels of oxidative stress markers MDA, SOD and GSH. This finding suggested that antioxidant therapy is an important component in the treatment of AP-associated intestinal injury.
ER stress is characterized by the accumulation of misfolded and unfolded proteins in the ER lumen and disturbance of calcium ion balance[36]. ER stress can activate the unfolded protein response, ER overload response and activation of the caspase-12-mediated apoptosis signaling pathway. ER stress can not only induce the expression of GRP78, GRP94 and other ER chaperones but also induce apoptosis[37-39]. ER stress directly affects the outcome of cell stress, such as adaptation, injury or apoptosis. ER stress and oxidative stress often occur successively or concomitantly, and the two reactions cooperate to promote the damage of tissues and organs[40]. After ER stress, a large number of unfolded or misfolded proteins accumulate in the ER, leading to persistent aggravation of organelle damage. In AP, increased ER stress can activate the ER stress-related apoptotic proteins, CHOP, etc and induce the expression of apoptosis-related proteins BAX and caspase-3, eventually leading to increased apoptosis, aggravated intestinal mucosal epithelial injury and the progression of AP[41,42]. In our recent study, we showed that the expression level of ER stress proteins in the intestine of AP mice was significantly upregulated, suggesting that ER stress occurs in intestinal cells of AP mice. Apoptotic cells were labeled with fluorescent TUNEL, and the expression levels of apoptosis-related proteins BAX and caspase-3 were detected by Western blot analysis, showing that apoptosis of intestinal cells in AP mice was significantly increased. When AP mice were given exogenous irisin injections, the ER stress and apoptosis of intestinal cells were significantly alleviated, suggesting that irisin plays an important role in inhibiting intestinal ER stress of AP and that irisin could alleviate intestinal apoptosis induced by ER stress.
Physical exercise can favorably influence outcomes of patients with inflammatory bowel disease[43-46]. Voluntary exercise increased irisin levels and attenuated experimental colitis in high fat diet-fed mice[47]. However, forced treadmill exercise has been shown to exaggerate the severity of experimental colitis in mice fed a high fat diet[48]. This discrepancy may be related to differences in irisin release after voluntary and forced excise. Unfortunately, irisin levels were not measured in the forced exercise study. In our current study, we only assessed the effect of two different doses of irisin on intestinal injury in AP. It is possible higher doses of irisin may offer even better protection in this model. In this regard, the optimal dose of irisin in AP should be determined in the future.
In summary, irisin alleviates intestinal cell injury in AP mice by inhibiting oxidative stress and blocking ER stress. The present study represents an important step forward in the study of irisin in the treatment of AP-associated intestinal injury.
Acute pancreatitis (AP) is often associated with intestinal injury, which in turn exaggerates the progression of AP. Our recent study has shown that a low level of serum irisin, a novel exercise-induced hormone, is associated with poor outcomes in patients with AP, and irisin administration protects against experimental AP. However, the role of irisin in intestinal injury in AP has not been evaluated.
The main topic of this study was to investigate the effect of irisin administration on intestinal injury in experimental AP. We found that irisin alleviates intestinal cell injury in AP mice by inhibiting oxidative stress and blocking ER stress.
To investigate the effect of irisin administration on intestinal injury in experimental AP. Exogenous irisin can effectively reduce intestinal injury and systemic inflammation in mice with AP. In the future, irisin-related drugs can be developed for intestinal injury of AP.
Arginine-AP was induced by two hourly intraperitoneal injections of 4.0 g/kg L-arginine. At 2 h after the last injection of L-arginine, normal saline (vehicle) or 50 μg/kg or 250 μg/kg irisin was administered through intraperitoneal injection. The animals were sacrificed 69 h after irisin treatment (i.e. 72 h after the first injection of L-arginine). Blood and tissue samples were collected. Intestinal injury, apoptosis, oxidative stress, endoplasmic reticulum stress levels as well as systemic inflammation were measured.
Administration of irisin significantly mitigated intestinal damage, reduced apoptosis, and attenuated oxidative and ER stress in AP mice. In addition, irisin treatment also effectively downregulated serum TNF-α and IL-6 levels and alleviated injury in the pancreas, liver and lung of AP mice. Until now, the exact molecular mechanism by which irisin alleviates intestinal injury in AP remains unknown.
In this study we report for the first time that irisin-mediated multiple physiological events attenuate intestinal injury following an episode of AP. Irisin has a great potential to be further developed as an effective treatment for patients with AP. In summary, irisin alleviates intestinal cell injury in AP mice by inhibiting oxidative stress and blocking ER stress. We believe that future studies can further explore the specific mechanism of irisin relieving intestinal injury in AP. The present study represents an important step forward in the study of irisin in the treatment of AP-associated intestinal injury.
In this study, we can learn that the protective effect of exercise on multiple organs can be revealed by modern science. In the future, our research direction should be to further explore the specific mechanism of irisin’s protective effect on intestinal injury of AP and even multiple organs. Future research should start from animal experiments and carry out more clinical studies so that basic science can make contributions to human health as soon as possible.
| 1. | Cheng Z, Abrams ST, Alhamdi Y, Toh J, Yu W, Wang G, Toh CH. Circulating Histones Are Major Mediators of Multiple Organ Dysfunction Syndrome in Acute Critical Illnesses. Crit Care Med. 2019;47:e677-e684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 2. | Cen ME, Wang F, Su Y, Zhang WJ, Sun B, Wang G. Gastrointestinal microecology: a crucial and potential target in acute pancreatitis. Apoptosis. 2018;23:377-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Tan C, Yang L, Shi F, Hu J, Zhang X, Wang Y, Deng Z, Li J, Yuan H, Shi T, Li C, Xiao Y, Peng Y, Xu W, Huang Y. Early Systemic Inflammatory Response Syndrome Duration Predicts Infected Pancreatic Necrosis. J Gastrointest Surg. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Pasari LP, Khurana A, Anchi P, Aslam Saifi M, Annaldas S, Godugu C. Visnagin attenuates acute pancreatitis via Nrf2/NFκB pathway and abrogates associated multiple organ dysfunction. Biomed Pharmacother. 2019;112:108629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Garg PK, Singh VP. Organ Failure Due to Systemic Injury in Acute Pancreatitis. Gastroenterology. 2019;156:2008-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 438] [Article Influence: 62.6] [Reference Citation Analysis (1)] |
| 6. | Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Aspects Med. 2011;32:234-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 767] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 7. | Yao L, Cheng C, Yang X, Han C, Du D, Liu T, Chvanov M, Windsor J, Sutton R, Huang W, Xia Q. Ethyl pyruvate and analogs as potential treatments for acute pancreatitis: A review of in vitro and in vivo studies. Pancreatology. 2019;19:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Tsai K, Wang SS, Chen TS, Kong CW, Chang FY, Lee SD, Lu FJ. Oxidative stress: an important phenomenon with pathogenetic significance in the progression of acute pancreatitis. Gut. 1998;42:850-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 125] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Tian R, Tan JT, Wang RL, Xie H, Qian YB, Yu KL. The role of intestinal mucosa oxidative stress in gut barrier dysfunction of severe acute pancreatitis. Eur Rev Med Pharmacol Sci. 2013;17:349-355. [PubMed] |
| 10. | Siriwardena AK, Mason JM, Balachandra S, Bagul A, Galloway S, Formela L, Hardman JG, Jamdar S. Randomised, double blind, placebo controlled trial of intravenous antioxidant (n-acetylcysteine, selenium, vitamin C) therapy in severe acute pancreatitis. Gut. 2007;56:1439-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Biczo G, Vegh ET, Shalbueva N, Mareninova OA, Elperin J, Lotshaw E, Gretler S, Lugea A, Malla SR, Dawson D, Ruchala P, Whitelegge J, French SW, Wen L, Husain SZ, Gorelick FS, Hegyi P, Rakonczay Z, Gukovsky I, Gukovskaya AS. Mitochondrial Dysfunction, Through Impaired Autophagy, Leads to Endoplasmic Reticulum Stress, Deregulated Lipid Metabolism, and Pancreatitis in Animal Models. Gastroenterology. 2018;154:689-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 304] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 12. | Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3682] [Cited by in RCA: 3677] [Article Influence: 262.6] [Reference Citation Analysis (0)] |
| 13. | Yang Z, Chen X, Chen Y, Zhao Q. PGC-1 mediates the regulation of metformin in muscle irisin expression and function. Am J Transl Res. 2015;7:1850-1859. [PubMed] |
| 14. | Chen RR, Fan XH, Chen G, Zeng GW, Xue YG, Liu XT, Wang CY. Irisin attenuates angiotensin II-induced cardiac fibrosis via Nrf2 mediated inhibition of ROS/ TGFβ1/Smad2/3 signaling axis. Chem Biol Interact. 2019;302:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Askari H, Rajani SF, Poorebrahim M, Haghi-Aminjan H, Raeis-Abdollahi E, Abdollahi M. A glance at the therapeutic potential of irisin against diseases involving inflammation, oxidative stress, and apoptosis: An introductory review. Pharmacol Res. 2018;129:44-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 135] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 16. | Chen K, Xu Z, Liu Y, Wang Z, Li Y, Xu X, Chen C, Xia T, Liao Q, Yao Y, Zeng C, He D, Yang Y, Tan T, Yi J, Zhou J, Zhu H, Ma J, Zeng C. Irisin protects mitochondria function during pulmonary ischemia/reperfusion injury. Sci Transl Med. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 161] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 17. | Kui B, Balla Z, Vasas B, Végh ET, Pallagi P, Kormányos ES, Venglovecz V, Iványi B, Takács T, Hegyi P, Rakonczay Z. New insights into the methodology of L-arginine-induced acute pancreatitis. PLoS One. 2015;10:e0117588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Kui B, Balla Z, Végh ET, Pallagi P, Venglovecz V, Iványi B, Takács T, Hegyi P, Rakonczay Z. Recent advances in the investigation of pancreatic inflammation induced by large doses of basic amino acids in rodents. Lab Invest. 2014;94:138-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Ren Y, Qiu M, Zhang J, Bi J, Wang M, Hu L, Du Z, Li T, Zhang L, Wang Y, Lv Y, Wu Z, Wu R. Low Serum Irisin Concentration Is Associated with Poor Outcomes in Patients with Acute Pancreatitis, and Irisin Administration Protects Against Experimental Acute Pancreatitis. Antioxid Redox Signal. 2019;31:771-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Zhang M, Wu YQ, Xie L, Wu J, Xu K, Xiao J, Chen DQ. Isoliquiritigenin Protects Against Pancreatic Injury and Intestinal Dysfunction After Severe Acute Pancreatitis via Nrf2 Signaling. Front Pharmacol. 2018;9:936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Fishman JE, Levy G, Alli V, Zheng X, Mole DJ, Deitch EA. The intestinal mucus layer is a critical component of the gut barrier that is damaged during acute pancreatitis. Shock. 2014;42:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Miao YF, Kang HX, Li J, Zhang YM, Ren HY, Zhu L, Chen H, Yuan L, Su H, Wan MH, Tang WF. Effect of Sheng-jiang powder on multiple-organ inflammatory injury in acute pancreatitis in rats fed a high-fat diet. World J Gastroenterol. 2019;25:683-695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Ye C, Liu L, Ma X, Tong H, Gao J, Tai Y, Huang L, Tang C, Wang R. Obesity Aggravates Acute Pancreatitis via Damaging Intestinal Mucosal Barrier and Changing Microbiota Composition in Rats. Sci Rep. 2019;9:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Xiong Y, Chen L, Fan L, Wang L, Zhou Y, Qin D, Sun Q, Wu J, Cao S. Free Total Rhubarb Anthraquinones Protect Intestinal Injury via Regulation of the Intestinal Immune Response in a Rat Model of Severe Acute Pancreatitis. Front Pharmacol. 2018;9:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Walensky LD. Targeting BAX to drug death directly. Nat Chem Biol. 2019;15:657-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 26. | Huang L, Jiang Y, Sun Z, Gao Z, Wang J, Zhang D. Autophagy Strengthens Intestinal Mucosal Barrier by Attenuating Oxidative Stress in Severe Acute Pancreatitis. Dig Dis Sci. 2018;63:910-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Yin P, Xu J, He S, Liu F, Yin J, Wan C, Mei C, Yin Y, Xu X, Xia Z. Endoplasmic Reticulum Stress in Heat- and Shake-Induced Injury in the Rat Small Intestine. PLoS One. 2015;10:e0143922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Johnson CD, Kingsnorth AN, Imrie CW, McMahon MJ, Neoptolemos JP, McKay C, Toh SK, Skaife P, Leeder PC, Wilson P, Larvin M, Curtis LD. Double blind, randomised, placebo controlled study of a platelet activating factor antagonist, lexipafant, in the treatment and prevention of organ failure in predicted severe acute pancreatitis. Gut. 2001;48:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 232] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 29. | Metwally M, Bayoumi A, Romero-Gomez M, Thabet K, John M, Adams LA, Huo X, Aller R, García-Monzón C, Teresa Arias-Loste M, Bugianesi E, Miele L, Gallego-Durán R, Fischer J, Berg T, Liddle C, Qiao L, George J, Eslam M. A polymorphism in the Irisin-encoding gene (FNDC5) associates with hepatic steatosis by differential miRNA binding to the 3’UTR. J Hepatol. 2019;70:494-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 30. | Zhao YT, Wang J, Yano N, Zhang LX, Wang H, Zhang S, Qin G, Dubielecka PM, Zhuang S, Liu PY, Chin YE, Zhao TC. Irisin promotes cardiac progenitor cell-induced myocardial repair and functional improvement in infarcted heart. J Cell Physiol. 2019;234:1671-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Peng H, Wang Q, Lou T, Qin J, Jung S, Shetty V, Li F, Wang Y, Feng XH, Mitch WE, Graham BH, Hu Z. Myokine mediated muscle-kidney crosstalk suppresses metabolic reprogramming and fibrosis in damaged kidneys. Nat Commun. 2017;8:1493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 158] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 32. | Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1535] [Cited by in RCA: 2003] [Article Influence: 182.1] [Reference Citation Analysis (7)] |
| 33. | Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94:329-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1089] [Cited by in RCA: 1669] [Article Influence: 139.1] [Reference Citation Analysis (0)] |
| 34. | Pérez S, Taléns-Visconti R, Rius-Pérez S, Finamor I, Sastre J. Redox signaling in the gastrointestinal tract. Free Radic Biol Med. 2017;104:75-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 212] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 35. | Bopanna S, Nayak B, Prakash S, Shalimar, Mahapatra SJ, Garg PK. Increased oxidative stress and deficient antioxidant levels may be involved in the pathogenesis of idiopathic recurrent acute pancreatitis. Pancreatology. 2017;17:529-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Gonzalez-Teuber V, Albert-Gasco H, Auyeung VC, Papa FR, Mallucci GR, Hetz C. Small Molecules to Improve ER Proteostasis in Disease. Trends Pharmacol Sci. 2019;40:684-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 37. | Cao Y, Long J, Liu L, He T, Jiang L, Zhao C, Li Z. A review of endoplasmic reticulum (ER) stress and nanoparticle (NP) exposure. Life Sci. 2017;186:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 38. | Hetz C, Axten JM, Patterson JB. Pharmacological targeting of the unfolded protein response for disease intervention. Nat Chem Biol. 2019;15:764-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 211] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 39. | Lin Y, Jiang M, Chen W, Zhao T, Wei Y. Cancer and ER stress: Mutual crosstalk between autophagy, oxidative stress and inflammatory response. Biomed Pharmacother. 2019;118:109249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 355] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 40. | Tang Q, Zheng G, Feng Z, Chen Y, Lou Y, Wang C, Zhang X, Zhang Y, Xu H, Shang P, Liu H. Trehalose ameliorates oxidative stress-mediated mitochondrial dysfunction and ER stress via selective autophagy stimulation and autophagic flux restoration in osteoarthritis development. Cell Death Dis. 2017;8:e3081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 207] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 41. | Li Y, Guo Y, Tang J, Jiang J, Chen Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim Biophys Sin (Shanghai). 2014;46:629-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 382] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 42. | Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2364] [Cited by in RCA: 3193] [Article Influence: 228.1] [Reference Citation Analysis (0)] |
| 43. | Bilski J, Mazur-Bialy A, Brzozowski B, Magierowski M, Zahradnik-Bilska J, Wójcik D, Magierowska K, Kwiecien S, Mach T, Brzozowski T. Can exercise affect the course of inflammatory bowel disease? Experimental and clinical evidence. Pharmacol Rep. 2016;68:827-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 44. | Legeret C, Mählmann L, Gerber M, Kalak N, Köhler H, Holsboer-Trachsler E, Brand S, Furlano R. Favorable impact of long-term exercise on disease symptoms in pediatric patients with inflammatory bowel disease. BMC Pediatr. 2019;19:297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Vanhelst J, Vidal F, Turck D, Drumez E, Djeddi D, Devouge E, Spyckerelle C, Zandzou SG, Legrand C, Michaud L, Béghin L, Gottrand F, Coopman S, Ley D. Physical activity is associated with improved bone health in children with inflammatory bowel disease. Clin Nutr. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 46. | Eckert KG, Abbasi-Neureither I, Köppel M, Huber G. Structured physical activity interventions as a complementary therapy for patients with inflammatory bowel disease - a scoping review and practical implications. BMC Gastroenterol. 2019;19:115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 47. | Mazur-Bialy AI, Bilski J, Wojcik D, Brzozowski B, Surmiak M, Hubalewska-Mazgaj M, Chmura A, Magierowski M, Magierowska K, Mach T, Brzozowski T. Beneficial Effect of Voluntary Exercise on Experimental Colitis in Mice Fed a High-Fat Diet: The Role of Irisin, Adiponectin and Proinflammatory Biomarkers. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 48. | Bilski J, Mazur-Bialy A, Wojcik D, Magierowski M, Surmiak M, Kwiecien S, Magierowska K, Hubalewska-Mazgaj M, Sliwowski Z, Brzozowski T. Effect of Forced Physical Activity on the Severity of Experimental Colitis in Normal Weight and Obese Mice. Involvement of Oxidative Stress and Proinflammatory Biomarkers. Nutrients. 2019;11:pii: E1127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Barreto SG, Magierowski M, Perse M S-Editor: Tang JZ L-Editor: Filipodia E-Editor: Ma YJ