Published online Nov 28, 2019. doi: 10.3748/wjg.v25.i44.6483
Peer-review started: August 18, 2019
First decision: October 14, 2019
Revised: October 22, 2019
Accepted: November 13, 2019
Article in press: November 13, 2019
Published online: November 28, 2019
Processing time: 101 Days and 20.3 Hours
The liver is a complex organ that performs several functions to maintain homeostasis. These functions are modulated by calcium, a second messenger that regulates several intracellular events. In hepatocytes and cholangiocytes, which are the epithelial cell types in the liver, inositol 1,4,5-trisphosphate (InsP3) receptors (ITPR) are the only intracellular calcium release channels. Three isoforms of the ITPR have been described, named type 1, type 2 and type 3. These ITPR isoforms are differentially expressed in liver cells where they regulate distinct physiological functions. Changes in the expression level of these receptors correlate with several liver diseases and hepatic dysfunctions. In this review, we highlight how the expression level, modulation, and localization of ITPR isoforms in hepatocytes and cholangiocytes play a role in hepatic homeostasis and liver pathology.
Core tip: Calcium regulates a variety of functions in our body. In the liver, inositol 1,4,5-trisphosphate receptors (ITPR) are the only expressed intracellular calcium release channels. ITPR regulates liver functions under healthy situation, but they can also be involved in liver diseases, depending for instance, in which isoform is expressed in a specific cell type, level of expression and where inside the cell each isoform is expressed. In this review, we discuss about ITPR roles in hepatic cells in physiological and pathological conditions.
- Citation: Lemos FO, Florentino RM, Lima Filho ACM, Santos MLD, Leite MF. Inositol 1,4,5-trisphosphate receptor in the liver: Expression and function. World J Gastroenterol 2019; 25(44): 6483-6494
- URL: https://www.wjgnet.com/1007-9327/full/v25/i44/6483.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i44.6483
The liver is an important and vital organ that regulates several functions, ranging from drug and macronutrient metabolism to immune system support[1-5]. Essentially all liver functions are at some point regulated by intracellular calcium (Ca2+). In hepatocytes and cholangiocytes, the principal epithelial cell types of the liver, inositol 1,4,5-trisphosphate (InsP3) receptors (ITPR) are the only intracellular Ca2+ release channels[6,7]. There are three types of ITPR: type 1 (ITPR1), type 2 (ITPR2) and type 3 (ITPR3)[8,9], and these receptors are expressed mainly along the endoplasmic and nucleoplasmic reticulum[10,11].
Dysregulation in the expression of ITPR can be a cause of several liver disorders, or can be involved in the development of diseases, such as cholestasis[12] and non-alcoholic fatty liver disease (NAFLD)[13]. In this review, we will discuss the expression and the physiological functions of each isoform of ITPR present in liver hepatocytes and cholangiocytes as well as their role in disease (Table 1).
| ITPR isoform | Cell type | Function | Ref. |
| ITPR1 | Hepatocytes | Glucose secretion | [64] |
| Lipid metabolism | [63,65] | ||
| Liver regeneration | [66,67] | ||
| Cholangiocytes | Bicarbonate secretion | [12,61] | |
| ITPR2 | Hepatocytes | Organic anion secretion | [6,62,69] |
| Liver regeneration | [13,71] | ||
| Cholangiocytes | bicarbonate secretion | [12,61] | |
| ITPR3 | Hepatocytes | Physiologically absent | [6] |
| Proliferation and survival of hepatocellular carcinoma | [74] | ||
| Cholangiocytes | Bicarbonate secretion | [12,61] | |
| Proliferation, migration, and survival of cholangiocarcinoma | [82] |
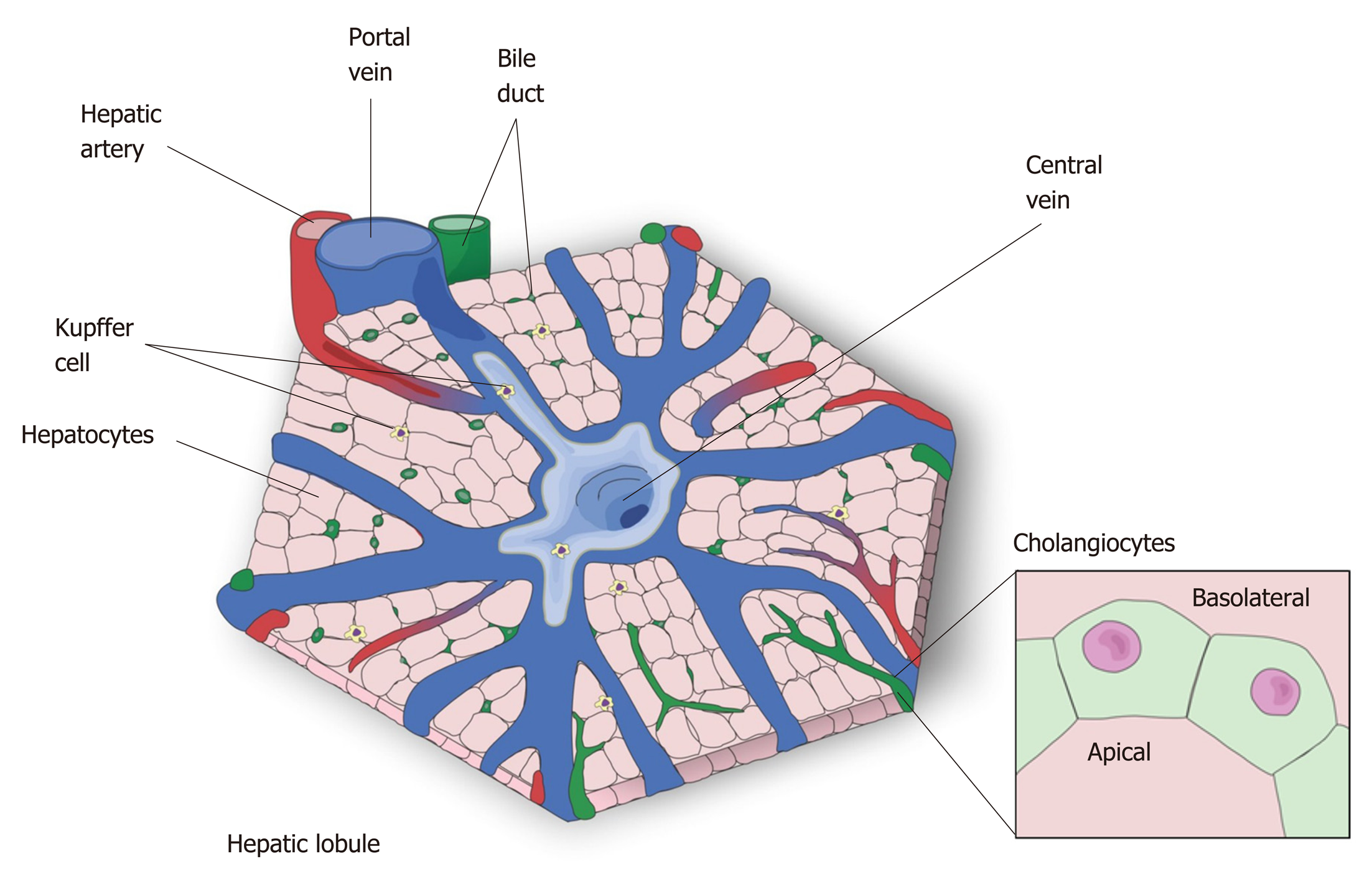
The liver is the largest internal organ[5] and is responsible for drug metabolism, albumin production, glycogen storage, cholesterol synthesis, bile secretion, and many other functions[14]. The liver is mostly composed of hepatocytes, which account for 80% of all cells in this organ[15]. The remaining 20% is composed mostly of cholangiocytes, Kupffer cells, stellate cells and liver sinusoidal endothelial cells[15]. Macroscopically, the liver is divided in four anatomic lobes, called the left, right, caudate and quadrate lobe[16,17]. In each lobe the cells are organized in a specific conformation, constituting a microscopic functional and structural unit, the lobule[14,18] (Figure 1). In the lobule, the hepatocytes are arranged in cords, connecting the portal triad to the central vein. In the space formed among the hepatocyte cords are the liver sinusoidal endothelial cells, the Kupffer cells, which are the resident macrophages in the liver, and the stellate cells, a cell type that stores vitamin A in its cytosol and secrets hepatocyte growth factor (HGF)[14,19,20].
As an epithelial cell, the hepatocyte is polarized, with a basolateral membrane in contact with the sinusoids and an apical side forming the biliary canaliculus. The biliary canaliculus is a virtual space between two hepatocytes, into which hepatocytes secrete bile acids[4]. The biliary canaliculi join to form the bile duct, which is lined by cholangiocytes, specialized cells that secrete electrolytes and fluids into the bile, altering bile composition and viscosity. The bile duct conducts the bile to the gallbladder, where it is stored until its content are needed to help lipid digestion[21,22].
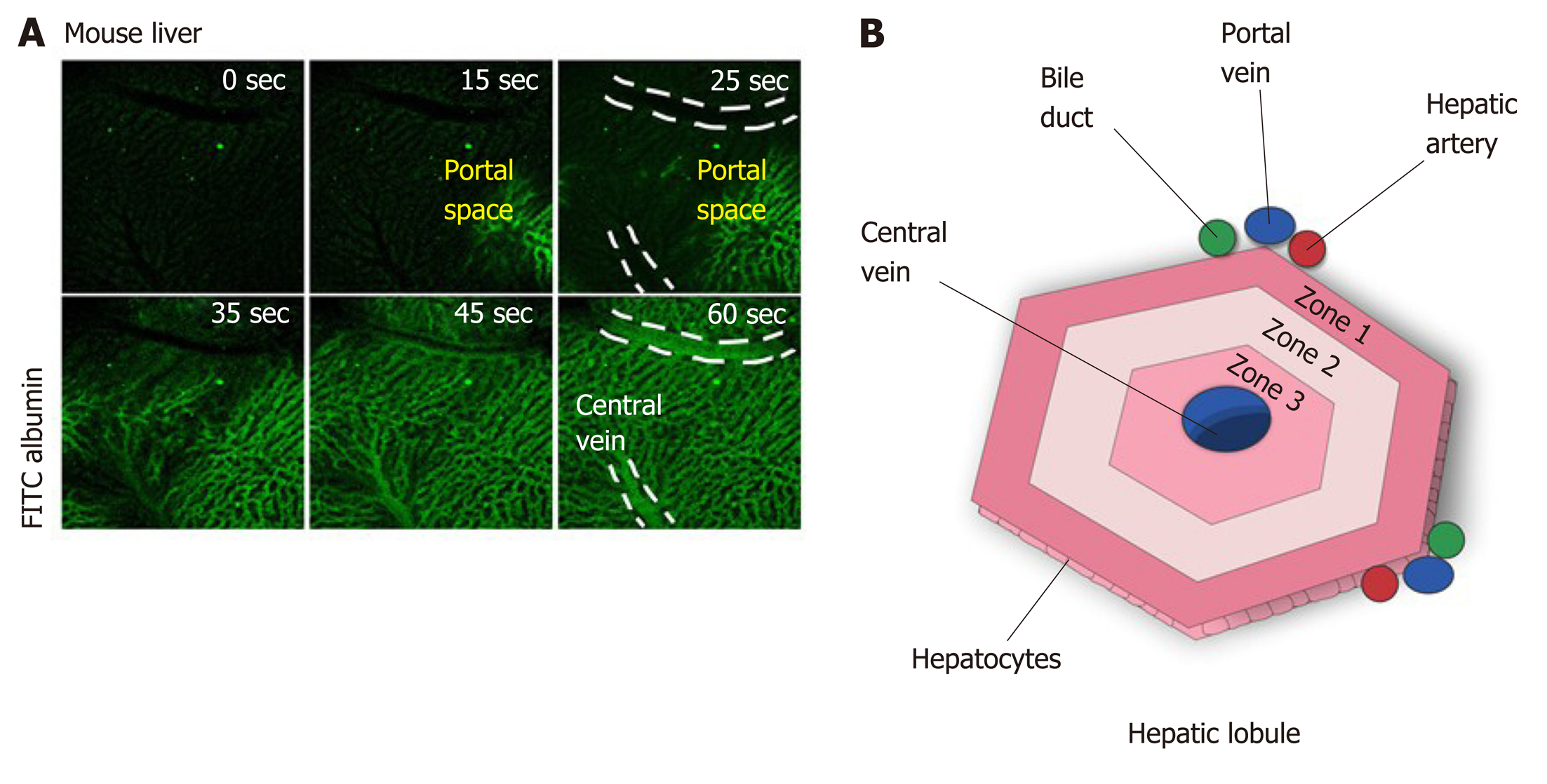
Blood from the portal vein passes throughout the sinusoids and drains into the central vein[14,23]. Near the portal vein there are two other important structures: the hepatic artery and the bile duct. Together, these structures form the portal triad. The hepatocytes around this area are more highly oxygenated than those that are closer to the central vein, because the blood reaches the portal triad first[14]. This region in the lobule is called zone 1, while zone 2 is the transitional zone, and zone 3 is the region near the central vein[24,25] (Figure 2). It has been shown that based on their zonal position, hepatocytes regulate specific liver functions. For example, hepatocytes in zone 1 are more involved in producing albumin and proteins of both the complement system and coagulation pathway, while hepatocytes from zone 3 are more important for drug metabolism and bile production[26].
Due to the key role of the liver in metabolism, hepatic tissue is continuously exposed to insults from xenobiotics, toxic metabolites and infectious agents[2]. As result of this, the liver has a remarkable capacity for regeneration. In mice, liver functions are restored within days of removing two-thirds of the organ. This capacity is also observed in humans for which liver function after partial hepatectomy is reestablished within a few weeks[27]. In many cases of liver disease, for which partial hepatectomy is indicated as a treatment, a small piece of healthy liver is implanted to drive hepatic tissue regeneration[27,28]. The path to regeneration depends on the extent of liver loss. When 1/3 of the liver is removed, the primary response is hepatocyte cellular hypertrophy, i.e., an increase in cell size. When liver loss reaches 2/3, hepatocyte hyperplasia, an increase of the number of hepatocytes occurs to reestablish liver function. When 80-90 % of the liver is removed, the biliary epithelial cells (BEC) turn into progenitor cells, which differentiate into hepatocytes or BEC[28] that are able to regenerate the tissue. Liver regeneration is a complex process and the mechanism by which the hepatocytes stop proliferating after reestablishment of liver function is poorly understood. It is important to highlight that Ca2+ signaling, and consequently the ITPR isoforms, play an essential role in liver regeneration, as discussed below.
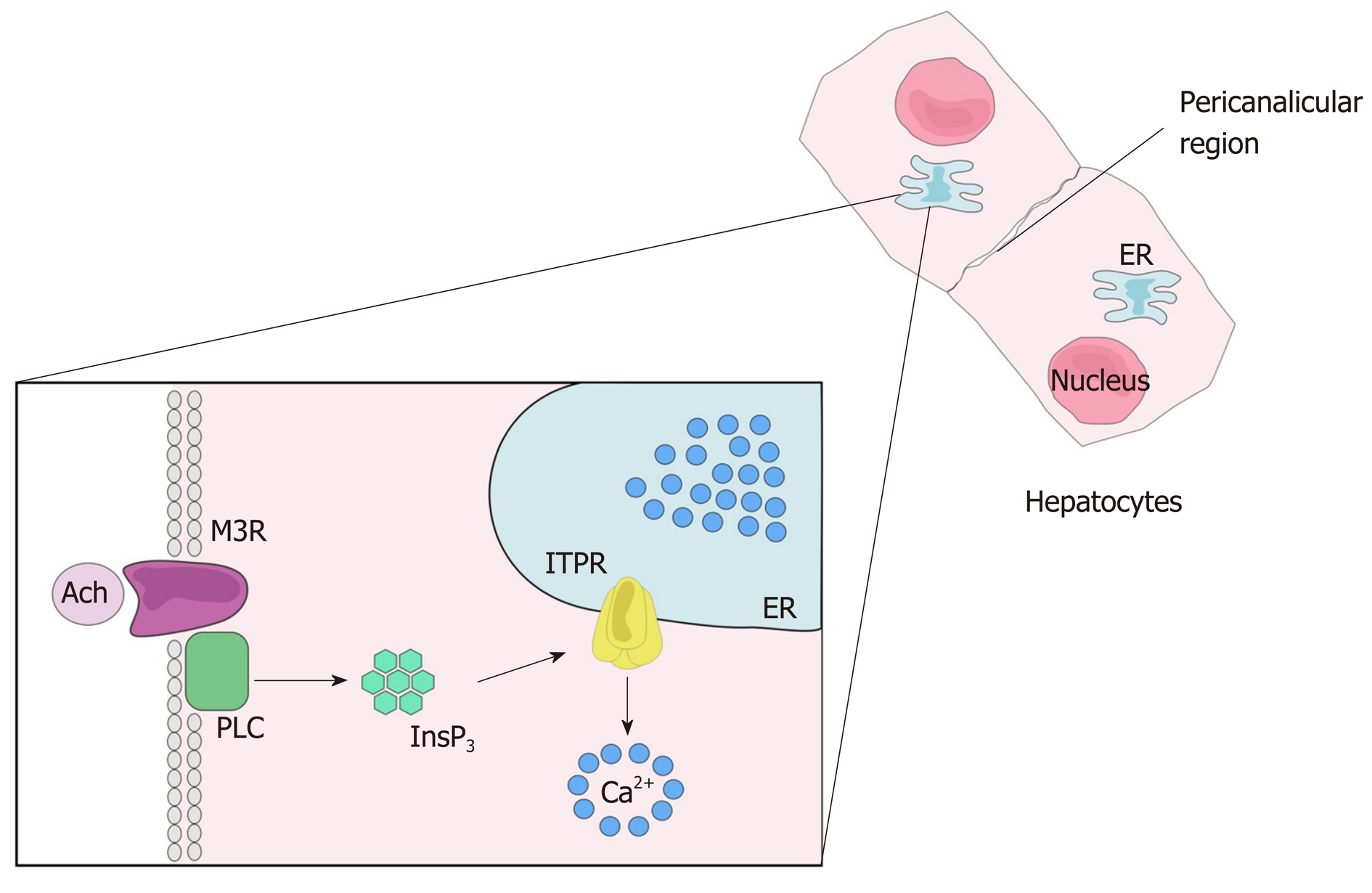
Many biological functions are regulated by intracellular Ca2+. These include cell proliferation, gene expression, secretion, motility and cell death, among others[29-33]. As in other tissues, Ca2+ signaling in the liver starts with the binding of an agonist to a receptor, which may be a G protein-coupled receptor (GPCR) (Figure 3) or a tyrosine kinase receptor (RTK). Upon agonist-receptor binding, phospholipase C (PLC) is activated (typically isoform PLCβ when GPCR is activated or isoform PLCγ after RTK activation), causing breakdown of the membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2), that generates diacylglycerol (DAG) and InsP3. DAG remains at the plasma membrane while InsP3 diffuses into the cytoplasm where it can bind to the InsP3 receptor (ITPR) localized along the endoplasmic reticulum membrane, nuclear envelope or nucleoplasmic reticulum. InsP3-ITPR binding causes a conformational change in the ITPR, leading to the release of internal Ca2+ stores[32,34]. InsP3 is inactivated either after conversion to inositol 1,2-bisphosphate (InsP2) by type I inositol polyphosphate 5-phosphatase or by InsP33-kinase mediated phosphorylation, forming inositol 1,3,4,5-tetrakisphosphate (InsP4)[35,36].
Because of the toxic effect of high concentrations of Ca2+ to the cells, this ion is promptly removed from the cytosol after its release. Different mechanisms are involved in this process, including the activation of plasma membrane Ca2+-ATPase or Na+/Ca2+ exchanger that exports Ca2+ out of the cell, while sarco-/endoplasmic Ca2+-ATPase (SERCA) and mitochondrial Ca2+ uptake 1 (MCU1) move Ca2+ into the endoplasmic reticulum and mitochondria, respectively[37,38].
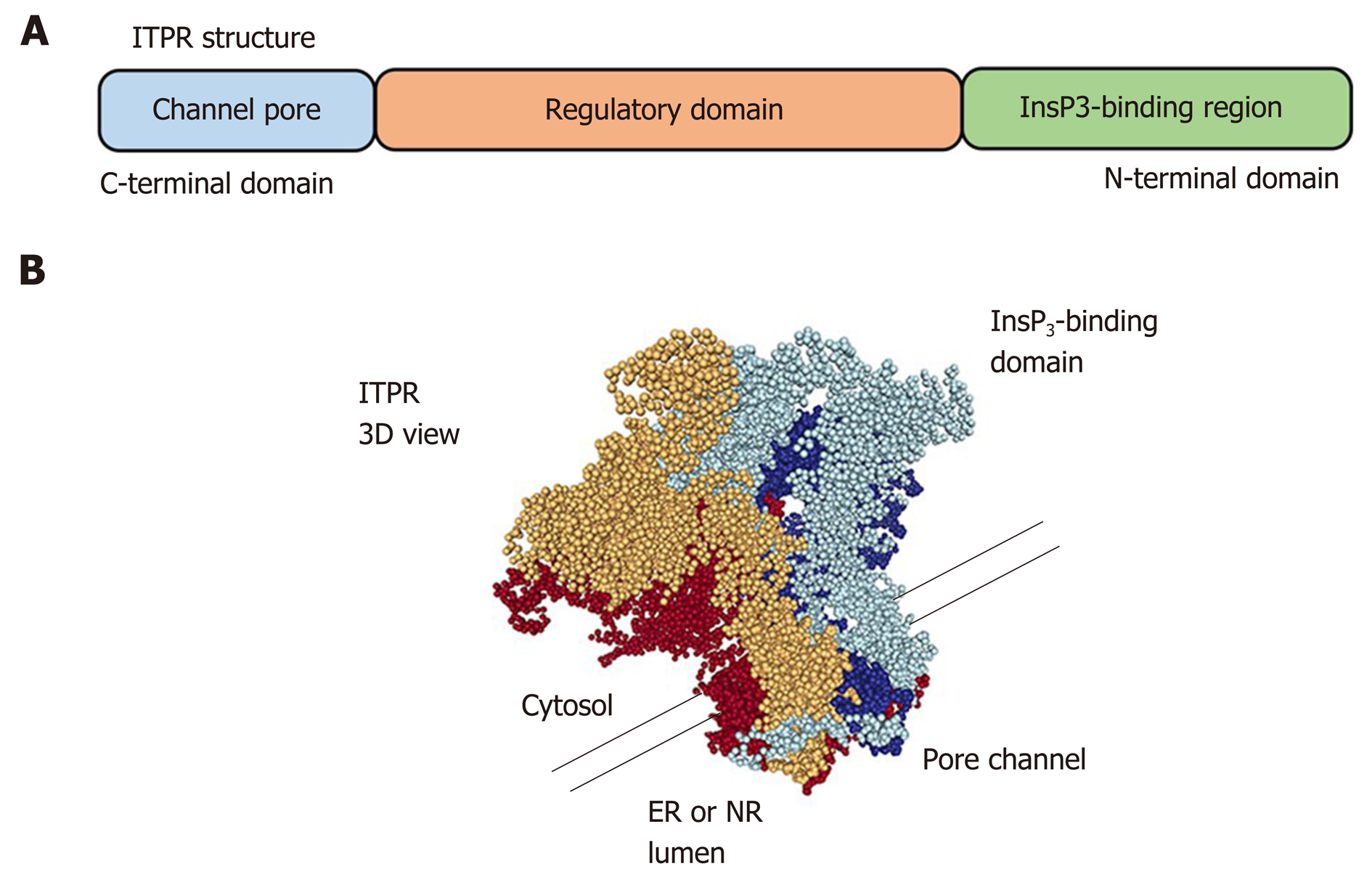
The ITPRs are formed by approximately 2700 amino acids[39,40] and are organized in three domains: a N-terminal domain, which includes the InsP3-binding region, a C-terminal domain, which forms the channel pore, and a regulatory domain between the other regions (Figure 4). ITPR is an intrinsic membrane protein, with 6 transmembrane segments[41,42]. There are some sites along the ITPR structure that regulate the activity of the receptor, or determine its localization by posttranslational modifications (phosphorylation, ubiquitination, oxidation, and proteolytic frag-mentation) or by interaction with modulatory proteins, such as chromogranin A and B, neuronal Ca2+ sensor 1, cytochrome c, and antiapoptotic Bcl-2 family members[43-46]. There are three isoforms of ITPR: type 1 (ITPR1), type 2 (ITPR2) and type 3 (ITPR3)[8,9]. They share 70% homology[47], however each isoform of ITPR displays a distinct affinity to InsP3: ITPR2 has the highest affinity, ITPR1 has an intermediate affinity, and ITPR3 has the lowest affinity[48,49]. In order to open the Ca2+ channel, four InsP3 molecules need to bind to ITPR[50]. Moreover, Ca2+ ions directly modulate the open probability of the channel[51,52]. ITPR1 displays what is called a “bell shape” open probability curve, in other words, at lower concentrations of Ca2+ the ITPR1 releases Ca2+, while higher Ca2+ concentrations inhibit the channel[53,54]. For ITPR3, the open probability of the channel increases with increased Ca2+ concentration[52,55], and the ITPR2 dependence on Ca2+ concentration remains controversial. While single-channel studies show that ITPR2 displays the same configuration as observed for ITPR3, studies with whole cells exhibit similarity with ITPR1[51,56,57], suggesting an effect of the modulatory proteins on the ITPRs channel activity.
ITPRs are widely expressed, sometimes with the prevalence of a single ITPR isoform in a specific tissue. For example, in the central nervous the main ITPR isoform is ITPR1, regulating neurite formation among other functions[58]. ITPR2 is the isoform mainly expressed in cardiomyocytes, participating in heart rate and in the action potential duration[59]. In pancreatic tissue, ITPR2 and ITPR3 are involved in the exocytosis of zymogen granules[60].
In the liver, hepatocytes express ITPR1 and ITPR2[6], whereas all three isoforms are expressed in cholangiocytes[12]. Below, we discuss separately about the ITPR isoforms in hepatic cells, focusing on hepatocytes and cholangiocytes, while indicating their main function and expression pattern in normal condition and in liver disease.
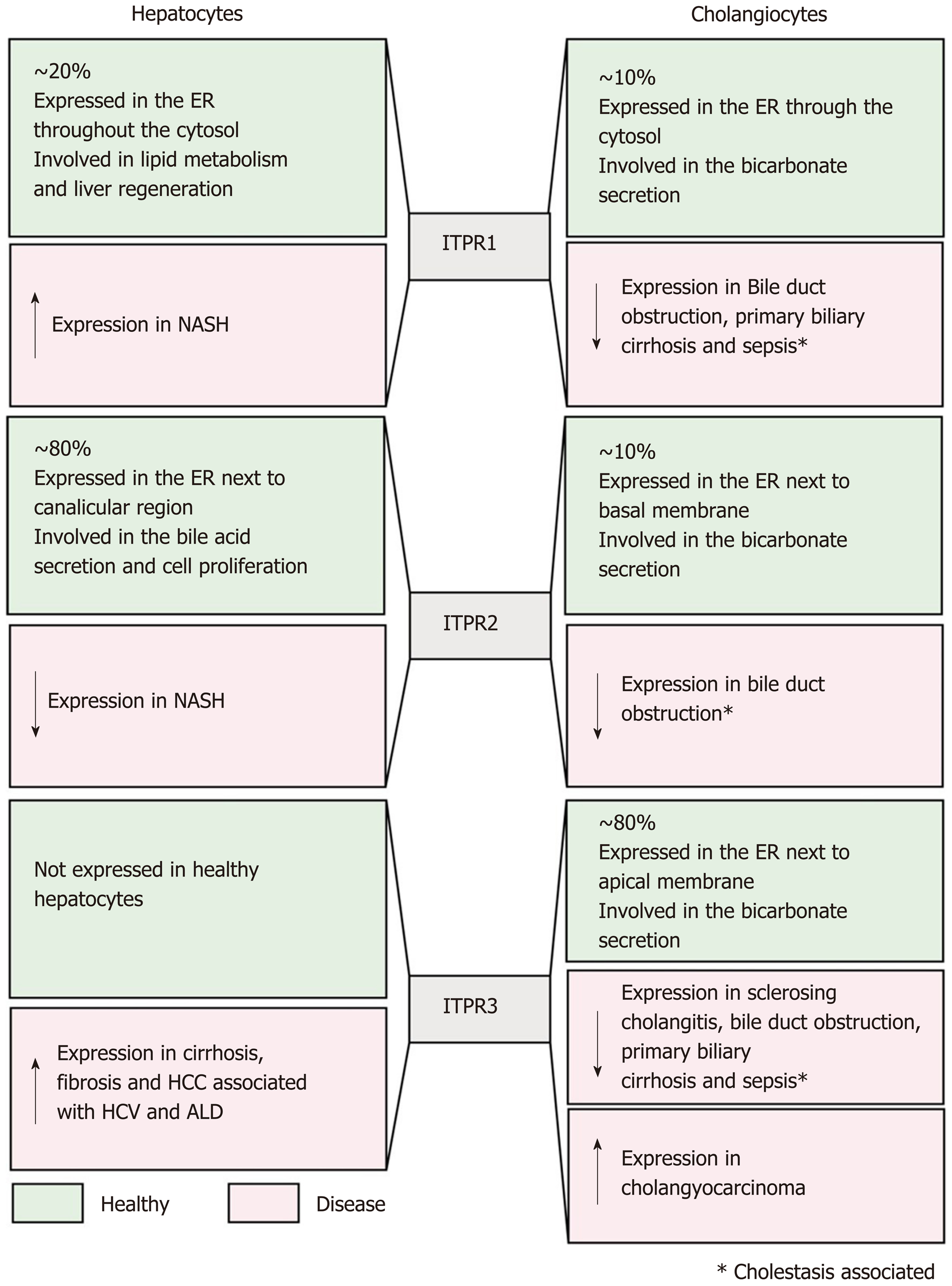
ITPR1 is expressed in both hepatocytes and cholangiocytes, corresponding to approximately 20% of the total ITPRs present in these cells. It is localized along the endoplasmic reticulum, throughout the cytoplasm and near the nucleus[6,61-63].
In normal liver tissue, ITPR1 regulates metabolism in hepatocytes[63-65]. After exposure to glucagon, mouse hepatocytes display an increase in ITPR1 phos-phorylation by the activity of protein kinase A (PKA), raising intracellular Ca2+ concentration that leads to glucose secretion[64]. More evidence of the ITPR1 function in liver metabolism was shown in obese (ob/ob) and high fat diet (HFD) mouse models. Ob/ob mice and mice maintained on a high-fat diet (HFD) overexpress ITPR1, increasing the amount of these Ca2+ channels in close proximity to the mitochondria[65]. In accordance with the increase in ITPR expression, cytoplasmic and mitochondrial Ca2+ concentration is increased in obese mice, causing mitochondrial dysfunction and impairment of metabolic homeostasis[65]. Conversely, the reduction of ITPR1 expression in the mouse liver, by short hairpin RNA technique, improved glucose tolerance and mitochondrial metabolism[65]. These results were validated in ITPR1 liver-specific knockout mice (ITPR1 LSKO). ITPR1 LSKO mice are leaner and display less hepatic steatosis after HFD, and also have reduced levels of triglycerides and lipogenic gene expression. These metabolic alterations are in accordance with the lower mitochondrial Ca2+ signal observed in isolated hepatocytes from ITPR1 LSKO mice[63]. Translational studies corroborate these findings by showing that liver specimens from non-alcoholic steatohepatitis (NASH) donors display increased hepatic ITPR1 expression which is concentrated closer to mitochondria. Based on these observations, it has been suggested that ITPR1 plays a role in steatosis in human fatty liver diseases[63].
Another function of ITPR1 in hepatocytes is related to the liver regeneration. Knocking down ITPR1 in rat with small interfering RNA (siRNA), attenuates Ca2+ signaling, and results in an impairment of hepatocytes proliferation after partial hepatectomy, measured by proliferating cell nuclear antigen staining positive cells. Consequently, the liver growth is diminished at the early phase (up to 48 h) of liver regeneration[66]. The involvement of ITPR1 in the beginning of the liver regeneration process is supported by the normal expression of this isoform immediately after the partial hepatectomy, followed by a downregulation of ITPR1 afterwards[67].
In cholangiocytes, ITPR1, together with the ITPR2, are responsible for releasing bicarbonate after the activation of type 3 muscarine receptor by acetylcholine. These findings were observed by using intrahepatic bile duct units isolated from rat liver tissue, previously transfected with ITPR1 and ITPR2 siRNA, and then by measuring the luminal pH after acetylcholine exposition. It was shown that the bicarbonate secretion was reduced in ITPR1 and ITPR2 knockdown cells[61]. Moreover, in cholestasis, which is a disorder that causes bile accumulation, the expression of ITPR1 is decreased, similarly to what occur to the other ITPR isoforms[12]. These observations suggest that the downregulation of ITPRs is an early event in the pathogenesis of cholestasis. As a consequence of the decrease of ITPR1 expression in a rat model of bile duct ligation, the Ca2+ signal is reduced and the biliary bicarbonate secretion is impaired in isolated cholangiocytes[12]. Together, these findings show that ITPR1 isoform plays a crucial role in hepatocyte metabolism and proliferation, as well as in cholangiocyte secretory activity, which are essential functions for normal liver.
ITPR2 is considered the principal intracellular Ca2+ release channel expressed in human and rodent hepatocytes[6,68]. This isoform is mostly concentrated in the canalicular membrane (apical region) of hepatocytes[62,69], and due to its localization and high affinity for InsP3[48,49], ITPR2 plays an essential role in bile formation[6,69]. ITPR2 modulates the multidrug resistance associated protein 2 (Mrp2), which is responsible for organic anion secretion into bile, such as bilirubin, glutathione S-conjugates, and oxidized glutathione. Impairment of intracellular Ca2+ signal inhibits the insertion of the Mrp2 into the apical plasma membrane in hepatocytes and reduces organic ion secretion. Similarly, hepatocytes isolated from ITPR2 knockout mice also presented decreased intracellular Ca2+ signaling, as well as impaired organic ion secretion[69].
Another bile salt transporter regulated by ITPR2 activity is the bile salt export pump (Bsep), an important protein normally positioned along the canalicular membrane of the hepatocyte. In a three-dimensional culture system of rat hepatocytes, siRNA against ITPR2 significantly reduced bile salt secretion, correlating with the downregulation and mislocalization of Bsep. Reduced bile secretion was also observed when the pericanalicular localization of ITPR2 was disrupted by methyl-β-cyclodextrin to disturb lipid rafts[62]. Confirming the importance of ITPR2 to the correct bile salt transporter localization and secretory activity, immunohistochemistry analysis of hepatocytes from lipopolysaccharides (LPS) and estrogen cholestasis rat models showed a reduction of ITPR2 expression level and its diffuse distribution, different from its normal localization to the apical membrane[62]. Conversely, fasting causes a physiological upregulation of ITPR2 expression level[70]. It was shown that overnight fasting raises the mRNA and protein levels of ITPR2 in rat hepatocytes. It happens by the activation of cAMP signaling caused by a fast-dependent increase of serum glucagon levels[70].
The correct expression level of ITPR2 is also important for hepatocyte proliferation. Downregulation of ITPR2 was observed in obese mice[71], a condition that compromises liver regeneration[72]. ITPR2 downregulation was also observed in human liver specimens of patients diagnosed with steatosis and NAFLD, common liver diseases associated with obesity[13]. Recently, the connection between lower expression of ITPR2 and impairment of liver regeneration in some liver diseases was clarified[13]. In both human biopsies of steatosis and NAFLD, as well as in a high fructose diet induced rat model of NAFLD, the transcriptional factor c-Jun activates a pro-inflammatory environment that negatively regulates ITPR2 expression in hepatocytes[13]. As consequence of downregulation of ITPR2, a delay of liver regeneration was observed. Similarly, ITPR2 knockout mice subjected to partial hepatectomy showed more liver damage and decreased proliferation of hepatocytes[13]. This was a consequence of decreased nuclear Ca2+ signaling, a fundamental event for cell proliferation[10,66]. ITPR2-knockout cells markedly reduced nucleoplasmic Ca2+ and proliferation rates compared to WT cells[13].
In cholangiocytes, ITPR2 represents about 10% of total ITPR, and is distributed diffusely throughout the endoplasmic reticulum membrane in the cytosol[12]. Functional studies showed that ITPR2 participates in the bicarbonate secretion by cholangiocytes. As discussed above, ITPR1 and ITPR2 knockdown cholangiocytes show a decrease in Ca2+ signal, and a reduction in Ca2+-dependent bicarbonate secretion when stimulated by acetylcholine[61]. Similar observations have been made in some cholestatic human diseases. The expression of ITPR2 is dramatically reduced in cholangiocytes from samples of patients with bile duct obstruction and primary biliary cirrhosis[12]. In summary, the ITPR2 displays an essential function in the liver, regulating bile formation and bicarbonate secretion, as well as regenerating hepatocytes.
In normal conditions, hepatocytes express ITPR1 and ITPR2 isoforms, but not the ITPR3[6]. However, ITPR3 is present in several hepatocellular carcinoma (HCC) cell lines[73,74], as well as in NASH-related HCC[75]. The mechanism of the “de novo” ITPR3 expression in hepatocytes in the context of HCC has been partially described and involves epigenetic modification[74], which represents changes in the genome structure that do not alter the nucleotide sequence. Examples include DNA methylation and histone modification[76]. Recently, bioinformatics analysis showed that the ITPR3 promoter region has a large number of CpG islands[74] that can be methylated by DNA methyltransferases, resulting in suppression of the gene[76,77]. Due to high level of DNA methylation at the ITPR3 promoter region, ITPR3 expression is repressed in hepatocytes under normal conditions. However, the referred methylation level is decreased in patients with HCC, allowing the expression of ITPR3 to be increased under hepatocellular disease conditions[74]. The expression of ITPR3 drives cell proliferation besides preventing the apoptotic cascade activation[74], events closely related to tumor development. Together, these findings put the ITPR3 Ca2+ channel as an essential factor that contributes to the pathogenesis of HCC.
Contrary to the normal hepatocytes, cholangiocytes constitutively express all three isoforms of ITPR[7], with the ITPR3 being the most widely expressed, constituting approximately 80% of ITPRs in this cell type. ITPR3 mainly localizes to the apical region of the cholangiocytes in rodents and humans[7]. This apical localization of ITPR3 in cholangiocytes is important for its physiological function of secreting bicarbonate[78]. It was shown that downregulation of ITPR3 selectively disturbs the cAMP-induced bicarbonate secretion[61]. Different from ITPR1 and ITPR2, in which the bicarbonate secretion is dependent on activation of muscarinic acetylcholine receptors, ITPR3 leads to bicarbonate secretion by a cAMP-dependent cascade, wherein activation of secretin receptor indirectly stimulates InsP3 production and Ca2+ release via ITPR3[61].
As described above to the other ITPR isoforms, the ITPR3 expression is pro-gressively decreased in bile duct ligation cholestasis rat model. Downregulation of ITPR3 was also observed after acute cholestasis, such as the endotoxin mouse model, as well as in chronic cholestatic disease in human, e.g., bile duct obstruction, biliary atresia, primary biliary cirrhosis, sclerosing cholangitis, and autoimmune cholestatic[12].
Several intracellular mechanisms have already been elucidated as being responsible for the loss of ITPR3 in cholangiocytes under pathological conditions. It was demonstrated for instance that LPS inoculation activates Toll like receptor 4 in cholangiocytes and, consequently, the transcription factor NF-κB. NF-κB then associates to the ITPR3 promoter region, inhibiting its expression in cholangiocytes. This mechanism is responsible for the loss of ITPR3 in patients affected by cholestasis due to sepsis or severe alcoholic hepatitis[79]. In cholangiopathies under oxidative stress conditions, including sclerosing cholangitis, primary biliary cholangitis, primary biliary obstruction and biliary atresia, the nuclear erythroid 2-like transcription factor 2 (Nrf2) is activated, acting negatively on ITPR3 expression[80]. Finally, the ITPR3 expression is also negatively regulated by the microRNA miR-506 in patients with primary biliary cholangitis[81].
Conversely to the downregulation of ITPR3 in cholangiopathies and cholestasis, this Ca2+ channel becomes over-expressed in cholangiocarcinoma[82]. ITPR3 accumulates in ER-mitochondrial junctions in cholangiocarcinoma cell lines, increasing mitochondrial Ca2+ signaling. Moreover, ITPR3 increases nuclear Ca2+ signaling in cholangiocarcinoma, which contributes to cell proliferation, migration, and survival[82].
Together, these findings show that ITPR3 is absent in healthy hepatocytes but is expressed in HCC and indicates that it may be a target to understand liver cancer and its clinical implications. On the other hand, in cholangiocytes, ITPR3 is crucial to bile formation and the decrease in its expression causes cholestasis, observed in many liver diseases, while it is over-expressed in cholangiocarcinoma, contributing to malignant features, such as cell proliferation, migration and survival.
In this review, we described several evidences of the role of the Ca2+ signaling, and consequently the activity of ITPRs, in normal liver functions. Mislocalization and/or change in expression level of these Ca2+ channels have been directly related to some liver disease (summarized in Figure 5). The alterations in ITPR expression and localization point these Ca2+ channels as a valuable biomarker for prediction and prognosis of hepatic disease. In addition to diagnosis for liver diseases, ITPR would be a rational target for these pathological conditions. Epigenetic modification, pro-inflammatory transcription factors and miRNA have already been associated to the modulation of ITPR expression in pathological conditions. However, this field remains to be better explored to elucidate the upstream cascade that drives ITPR expression alterations. Better understanding of this pathway could open the perspective of developing pharmacological strategies for liver diseases, specifically targeting each ITPR isoforms.
We thank Dr Christopher Kushmerick (Universidade Federal de Minas Gerais), Mr Sriram Amirneni (University of Pittsburgh) and Dr Michael Nathanson (Yale University) for generously providing many useful suggestions and comments on the manuscript. Fellowship and scholarships are acknowledged from Conselho Nacional de Desenvolvimento Científico e Tecnológico and Coordenação de Aperfeiçoamento de Pessoal.
| 1. | Han HS, Kang G, Kim JS, Choi BH, Koo SH. Regulation of glucose metabolism from a liver-centric perspective. Exp Mol Med. 2016;48:e218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 557] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 2. | Robinson MW, Harmon C, O'Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol. 2016;13:267-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 682] [Cited by in RCA: 841] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 3. | Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol. 2012;56:952-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 740] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 4. | Chiang JYL, Ferrell JM. Bile Acid Metabolism in Liver Pathobiology. Gene Expr. 2018;18:71-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 407] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 5. | Bhatia SN, Underhill GH, Zaret KS, Fox IJ. Cell and tissue engineering for liver disease. Sci Transl Med. 2014;6:245sr2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 233] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 6. | Hirata K, Pusl T, O'Neill AF, Dranoff JA, Nathanson MH. The type II inositol 1,4,5-trisphosphate receptor can trigger Ca2+ waves in rat hepatocytes. Gastroenterology. 2002;122:1088-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Hirata K, Dufour JF, Shibao K, Knickelbein R, O'Neill AF, Bode HP, Cassio D, St-Pierre MV, Larusso NF, Leite MF, Nathanson MH. Regulation of Ca(2+) signaling in rat bile duct epithelia by inositol 1,4,5-trisphosphate receptor isoforms. Hepatology. 2002;36:284-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Pantazaka E, Taylor CW. Differential distribution, clustering, and lateral diffusion of subtypes of the inositol 1,4,5-trisphosphate receptor. J Biol Chem. 2011;286:23378-23387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Tu H, Wang Z, Nosyreva E, De Smedt H, Bezprozvanny I. Functional characterization of mammalian inositol 1,4,5-trisphosphate receptor isoforms. Biophys J. 2005;88:1046-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Echevarría W, Leite MF, Guerra MT, Zipfel WR, Nathanson MH. Regulation of calcium signals in the nucleus by a nucleoplasmic reticulum. Nat Cell Biol. 2003;5:440-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 309] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 11. | Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983;306:67-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1962] [Cited by in RCA: 1932] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 12. | Shibao K, Hirata K, Robert ME, Nathanson MH. Loss of inositol 1,4,5-trisphosphate receptors from bile duct epithelia is a common event in cholestasis. Gastroenterology. 2003;125:1175-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Khamphaya T, Chukijrungroat N, Saengsirisuwan V, Mitchell-Richards KA, Robert ME, Mennone A, Ananthanarayanan M, Nathanson MH, Weerachayaphorn J. Nonalcoholic fatty liver disease impairs expression of the type II inositol 1,4,5-trisphosphate receptor. Hepatology. 2018;67:560-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Desmet VJ. Ductal plates in hepatic ductular reactions. Hypothesis and implications. II. Ontogenic liver growth in childhood. Virchows Arch. 2011;458:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Huppert SS, Iwafuchi-Doi M. Molecular regulation of mammalian hepatic architecture. Curr Top Dev Biol. 2019;132:91-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Dodds WJ, Erickson SJ, Taylor AJ, Lawson TL, Stewart ET. Caudate lobe of the liver: anatomy, embryology, and pathology. AJR Am J Roentgenol. 1990;154:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Sagoo MG, Aland RC, Gosden E. Morphology and morphometry of the caudate lobe of the liver in two populations. Anat Sci Int. 2018;93:48-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Saxena R, Theise ND, Crawford JM. Microanatomy of the human liver-exploring the hidden interfaces. Hepatology. 1999;30:1339-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 104] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Wisse E, Braet F, Luo D, De Zanger R, Jans D, Crabbé E, Vermoesen A. Structure and function of sinusoidal lining cells in the liver. Toxicol Pathol. 1996;24:100-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 177] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 20. | Maher JJ. Cell-specific expression of hepatocyte growth factor in liver. Upregulation in sinusoidal endothelial cells after carbon tetrachloride. J Clin Invest. 1993;91:2244-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 121] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Crawford JM. Development of the intrahepatic biliary tree. Semin Liver Dis. 2002;22:213-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 61] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Strazzabosco M, Fabris L. Functional anatomy of normal bile ducts. Anat Rec (Hoboken). 2008;291:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Krishna M. Microscopic anatomy of the liver. Clin Liver Dis (Hoboken). 2013;2:S4-S7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Torre C, Perret C, Colnot S. Transcription dynamics in a physiological process: β-catenin signaling directs liver metabolic zonation. Int J Biochem Cell Biol. 2011;43:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Kietzmann T. Metabolic zonation of the liver: The oxygen gradient revisited. Redox Biol. 2017;11:622-630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 322] [Cited by in RCA: 376] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 26. | Halpern KB, Shenhav R, Matcovitch-Natan O, Toth B, Lemze D, Golan M, Massasa EE, Baydatch S, Landen S, Moor AE, Brandis A, Giladi A, Avihail AS, David E, Amit I, Itzkovitz S. Single-cell spatial reconstruction reveals global division of labour in the mammalian liver. Nature. 2017;542:352-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 568] [Cited by in RCA: 795] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 27. | Hata S, Namae M, Nishina H. Liver development and regeneration: from laboratory study to clinical therapy. Dev Growth Differ. 2007;49:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Gilgenkrantz H, Collin de l'Hortet A. Understanding Liver Regeneration: From Mechanisms to Regenerative Medicine. Am J Pathol. 2018;188:1316-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 29. | Rodrigues MA, Gomes DA, Leite MF, Grant W, Zhang L, Lam W, Cheng YC, Bennett AM, Nathanson MH. Nucleoplasmic calcium is required for cell proliferation. J Biol Chem. 2007;282:17061-17068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 30. | Andrade V, Guerra M, Jardim C, Melo F, Silva W, Ortega JM, Robert M, Nathanson MH, Leite F. Nucleoplasmic calcium regulates cell proliferation through legumain. J Hepatol. 2011;55:626-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Guimarães E, Machado R, Fonseca MC, França A, Carvalho C, Araújo E Silva AC, Almeida B, Cassini P, Hissa B, Drumond L, Gonçalves C, Fernandes G, De Brot M, Moraes M, Barcelos L, Ortega JM, Oliveira A, Leite MF. Inositol 1, 4, 5-trisphosphate-dependent nuclear calcium signals regulate angiogenesis and cell motility in triple negative breast cancer. PLoS One. 2017;12:e0175041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Resende RR, Andrade LM, Oliveira AG, Guimarães ES, Guatimosim S, Leite MF. Nucleoplasmic calcium signaling and cell proliferation: calcium signaling in the nucleus. Cell Commun Signal. 2013;11:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3972] [Cited by in RCA: 4170] [Article Influence: 181.3] [Reference Citation Analysis (0)] |
| 34. | Oliveira AG, Guimarães ES, Andrade LM, Menezes GB, Fatima Leite M. Decoding calcium signaling across the nucleus. Physiology (Bethesda). 2014;29:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Irvine RF, Lloyd-Burton SM, Yu JC, Letcher AJ, Schell MJ. The regulation and function of inositol 1,4,5-trisphosphate 3-kinases. Adv Enzyme Regul. 2006;46:314-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Connolly TM, Bansal VS, Bross TE, Irvine RF, Majerus PW. The metabolism of tris- and tetraphosphates of inositol by 5-phosphomonoesterase and 3-kinase enzymes. J Biol Chem. 1987;262:2146-2149. [PubMed] |
| 37. | Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev. 1999;79:763-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1290] [Cited by in RCA: 1284] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 38. | Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK. MICU1 encodes a mitochondrial EF hand protein required for Ca2+. Nature. 2010;467:291-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 751] [Cited by in RCA: 731] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 39. | Jiang QX, Thrower EC, Chester DW, Ehrlich BE, Sigworth FJ. Three-dimensional structure of the type 1 inositol 1,4,5-trisphosphate receptor at 24 A resolution. EMBO J. 2002;21:3575-3581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 40. | Shah SZA, Zhao D, Khan SH, Yang L. Regulatory Mechanisms of Endoplasmic Reticulum Resident IP3 Receptors. J Mol Neurosci. 2015;56:938-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87:593-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1006] [Cited by in RCA: 991] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 42. | Baker MR, Fan G, Serysheva II. Structure of IP3R channel: high-resolution insights from cryo-EM. Curr Opin Struct Biol. 2017;46:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 43. | Yang J, Vais H, Gu W, Foskett JK. Biphasic regulation of InsP3 receptor gating by dual Ca2+ release channel BH3-like domains mediates Bcl-xL control of cell viability. Proc Natl Acad Sci USA. 2016;113:E1953-E1962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 44. | Choe CU, Ehrlich BE. The inositol 1,4,5-trisphosphate receptor (IP3R) and its regulators: sometimes good and sometimes bad teamwork. Sci STKE. 2006;2006:re15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 45. | Wang L, Wagner LE 2nd, Alzayady KJ, Yule DI. Region-specific proteolysis differentially regulates type 1 inositol 1,4,5-trisphosphate receptor activity. J Biol Chem. 2017;292:11714-11726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 46. | Khan MT, Wagner L 2nd, Yule DI, Bhanumathy C, Joseph SK. Akt kinase phosphorylation of inositol 1,4,5-trisphosphate receptors. J Biol Chem. 2006;281:3731-3737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 47. | Mikoshiba K. IP3 receptor/Ca2+ channel: from discovery to new signaling concepts. J Neurochem. 2007;102:1426-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 321] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 48. | Iwai M, Michikawa T, Bosanac I, Ikura M, Mikoshiba K. Molecular basis of the isoform-specific ligand-binding affinity of inositol 1,4,5-trisphosphate receptors. J Biol Chem. 2007;282:12755-12764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 49. | Newton CL, Mignery GA, Südhof TC. Co-expression in vertebrate tissues and cell lines of multiple inositol 1,4,5-trisphosphate (InsP3) receptors with distinct affinities for InsP3. J Biol Chem. 1994;269:28613-28619. [PubMed] |
| 50. | Alzayady KJ, Wang L, Chandrasekhar R, Wagner LE 2nd, Van Petegem F, Yule DI. Defining the stoichiometry of inositol 1,4,5-trisphosphate binding required to initiate Ca2+ release. Sci Signal. 2016;9:ra35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 150] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 51. | Ramos-Franco J, Fill M, Mignery GA. Isoform-specific function of single inositol 1,4,5-trisphosphate receptor channels. Biophys J. 1998;75:834-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 136] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 52. | Hagar RE, Burgstahler AD, Nathanson MH, Ehrlich BE. Type III InsP3 receptor channel stays open in the presence of increased calcium. Nature. 1998;396:81-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 206] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 53. | Finch EA, Turner TJ, Goldin SM. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991;252:443-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 707] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 54. | De Young GW, Keizer J. A single-pool inositol 1,4,5-trisphosphate-receptor-based model for agonist-stimulated oscillations in Ca2+ concentration. Proc Natl Acad Sci USA. 1992;89:9895-9899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 472] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 55. | Hagar RE, Ehrlich BE. Regulation of the type III InsP(3) receptor by InsP(3) and ATP. Biophys J. 2000;79:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | Michikawa T, Hirota J, Kawano S, Hiraoka M, Yamada M, Furuichi T, Mikoshiba K. Calmodulin mediates calcium-dependent inactivation of the cerebellar type 1 inositol 1,4,5-trisphosphate receptor. Neuron. 1999;23:799-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 136] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 57. | Miyakawa T, Maeda A, Yamazawa T, Hirose K, Kurosaki T, Iino M. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO J. 1999;18:1303-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 329] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 58. | Fiedler MJ, Nathanson MH. The type I inositol 1,4,5-trisphosphate receptor interacts with protein 4.1N to mediate neurite formation through intracellular Ca waves. Neurosignals. 2011;19:75-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Guatimosim S, Amaya MJ, Guerra MT, Aguiar CJ, Goes AM, Gómez-Viquez NL, Rodrigues MA, Gomes DA, Martins-Cruz J, Lederer WJ, Leite MF. Nuclear Ca2+ regulates cardiomyocyte function. Cell Calcium. 2008;44:230-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 60. | Futatsugi A, Nakamura T, Yamada MK, Ebisui E, Nakamura K, Uchida K, Kitaguchi T, Takahashi-Iwanaga H, Noda T, Aruga J, Mikoshiba K. IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science. 2005;309:2232-2234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 267] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 61. | Minagawa N, Nagata J, Shibao K, Masyuk AI, Gomes DA, Rodrigues MA, Lesage G, Akiba Y, Kaunitz JD, Ehrlich BE, Larusso NF, Nathanson MH. Cyclic AMP regulates bicarbonate secretion in cholangiocytes through release of ATP into bile. Gastroenterology. 2007;133:1592-1602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 62. | Kruglov EA, Gautam S, Guerra MT, Nathanson MH. Type 2 inositol 1,4,5-trisphosphate receptor modulates bile salt export pump activity in rat hepatocytes. Hepatology. 2011;54:1790-1799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 63. | Feriod CN, Oliveira AG, Guerra MT, Nguyen L, Richards KM, Jurczak MJ, Ruan HB, Camporez JP, Yang X, Shulman GI, Bennett AM, Nathanson MH, Ehrlich BE. Hepatic Inositol 1,4,5 Trisphosphate Receptor Type 1 Mediates Fatty Liver. Hepatol Commun. 2017;1:23-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 64. | Wang Y, Li G, Goode J, Paz JC, Ouyang K, Screaton R, Fischer WH, Chen J, Tabas I, Montminy M. Inositol-1,4,5-trisphosphate receptor regulates hepatic gluconeogenesis in fasting and diabetes. Nature. 2012;485:128-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 174] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 65. | Arruda AP, Pers BM, Parlakgül G, Güney E, Inouye K, Hotamisligil GS. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat Med. 2014;20:1427-1435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 415] [Cited by in RCA: 583] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 66. | Oliveira AG, Andrade VA, Guimarães ES, Florentino RM, Sousa PA, Marques PE, Melo FM, Ortega MJ, Menezes GB, Leite MF. Calcium signalling from the type I inositol 1,4,5-trisphosphate receptor is required at early phase of liver regeneration. Liver Int. 2015;35:1162-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 67. | Nicou A, Serrière V, Hilly M, Prigent S, Combettes L, Guillon G, Tordjmann T. Remodelling of calcium signalling during liver regeneration in the rat. J Hepatol. 2007;46:247-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 68. | Nagata J, Guerra MT, Shugrue CA, Gomes DA, Nagata N, Nathanson MH. Lipid rafts establish calcium waves in hepatocytes. Gastroenterology. 2007;133:256-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 69. | Cruz LN, Guerra MT, Kruglov E, Mennone A, Garcia CR, Chen J, Nathanson MH. Regulation of multidrug resistance-associated protein 2 by calcium signaling in mouse liver. Hepatology. 2010;52:327-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 70. | Kruglov E, Ananthanarayanan M, Sousa P, Weerachayaphorn J, Guerra MT, Nathanson MH. Type 2 inositol trisphosphate receptor gene expression in hepatocytes is regulated by cyclic AMP. Biochem Biophys Res Commun. 2017;486:659-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 71. | Feriod CN, Nguyen L, Jurczak MJ, Kruglov EA, Nathanson MH, Shulman GI, Bennett AM, Ehrlich BE. Inositol 1,4,5-trisphosphate receptor type II (InsP3R-II) is reduced in obese mice, but metabolic homeostasis is preserved in mice lacking InsP3R-II. Am J Physiol Endocrinol Metab. 2014;307:E1057-E1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 72. | Kele PG, van der Jagt EJ, Gouw AS, Lisman T, Porte RJ, de Boer MT. The impact of hepatic steatosis on liver regeneration after partial hepatectomy. Liver Int. 2013;33:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 73. | Leite MF, Thrower EC, Echevarria W, Koulen P, Hirata K, Bennett AM, Ehrlich BE, Nathanson MH. Nuclear and cytosolic calcium are regulated independently. Proc Natl Acad Sci U S A. 2003;100:2975-2980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 167] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 74. | Guerra MT, Florentino RM, Franca A, Lima Filho AC, Dos Santos ML, Fonseca RC, Lemos FO, Fonseca MC, Kruglov E, Mennone A, Njei B, Gibson J, Guan F, Cheng YC, Ananthanarayanam M, Gu J, Jiang J, Zhao H, Lima CX, Vidigal PT, Oliveira AG, Nathanson MH, Leite MF. Expression of the type 3 InsP3 receptor is a final common event in the development of hepatocellular carcinoma. Gut. 2019;68:1676-1687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 75. | Liang JQ, Teoh N, Xu L, Pok S, Li X, Chu ESH, Chiu J, Dong L, Arfianti E, Haigh WG, Yeh MM, Ioannou GN, Sung JJY, Farrell G, Yu J. Dietary cholesterol promotes steatohepatitis related hepatocellular carcinoma through dysregulated metabolism and calcium signaling. Nat Commun. 2018;9:4490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 165] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 76. | Portela A, Esteller M. Epigenetic modifications and human disease. Nat Biotechnol. 2010;28:1057-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1865] [Cited by in RCA: 2031] [Article Influence: 135.4] [Reference Citation Analysis (0)] |
| 77. | Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293:1068-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1432] [Cited by in RCA: 1438] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 78. | Rodrigues MA, Gomes DA, Nathanson MH. Calcium Signaling in Cholangiocytes: Methods, Mechanisms, and Effects. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 79. | Franca A, Carlos Melo Lima Filho A, Guerra MT, Weerachayaphorn J, Loiola Dos Santos M, Njei B, Robert M, Xavier Lima C, Vieira Teixeira Vidigal P, Banales JM, Ananthanarayanam M, Leite MF, Nathanson MH. Effects of Endotoxin on Type 3 Inositol 1,4,5-Trisphosphate Receptor in Human Cholangiocytes. Hepatology. 2019;69:817-830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 80. | Weerachayaphorn J, Amaya MJ, Spirli C, Chansela P, Mitchell-Richards KA, Ananthanarayanan M, Nathanson MH. Nuclear Factor, Erythroid 2-Like 2 Regulates Expression of Type 3 Inositol 1,4,5-Trisphosphate Receptor and Calcium Signaling in Cholangiocytes. Gastroenterology. 2015;149:211-222.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 81. | Ananthanarayanan M, Banales JM, Guerra MT, Spirli C, Munoz-Garrido P, Mitchell-Richards K, Tafur D, Saez E, Nathanson MH. Post-translational regulation of the type III inositol 1,4,5-trisphosphate receptor by miRNA-506. J Biol Chem. 2015;290:184-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 82. | Ueasilamongkol P, Khamphaya T, Guerra MT, Rodrigues MA, Gomes DA, Kong Y, Wei W, Jain D, Trampert DC, Ananthanarayanan M, Banales JM, Roberts LR, Farshidfar F, Nathanson MH, Weerachayaphorn J. Type 3 Inositol 1,4,5-Trisphosphate Receptor Is Increased and Enhances Malignant Properties in Cholangiocarcinoma. Hepatology. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Morales-González JA, Sun XT S-Editor: Wang J L-Editor: A E-Editor: Zhang YL