Published online Nov 7, 2019. doi: 10.3748/wjg.v25.i41.6222
Peer-review started: August 27, 2019
First decision: September 19, 2019
Revised: October 11, 2019
Accepted: October 22, 2019
Article in press: October 22, 2019
Published online: November 7, 2019
Processing time: 71 Days and 15.8 Hours
Pediatric enteritis is one of the infectious diseases in the digestive system that causes a variety of digestive problems, including diarrhea, vomiting, and bellyache in children. Clinically, Helicobacter pylori (H. pylori) infection is one of the common factors to cause pediatric enteritis. It has been demonstrated that aberrant expression of microRNAs (miRNAs) is found in gastrointestinal diseases caused by H. pylori, and we discovered a significant increase of miR-32-5p in H. pylori-related pediatric enteritis. However, the exact role of miR-32-5p in it is still unknown.
To investigate the role of aberrant miR-32-5p in pediatric enteritis induced by H. pylori.
MiR-32-5p expression was detected by quantitative real time-polymerase chain reaction. The biological role of miR-32-5p in H. pylori-treated intestinal epithelial cells was evaluated by Cell Counting Kit-8 assay and flow cytometry. The potential target of miR-32-5p was predicted with TargetScanHuman and verified by luciferase assay. The downstream mechanism of miR-32-5p was explored by using molecular biology methods.
We found that miR-32-5p was overexpressed in serum of H. pylori-induced pediatric enteritis. Further investigation revealed that H. pylori infection promoted the death of intestinal epithelial cells, and increased miR-32-5p expression. Moreover, miR-32-5p mimic further facilitated apoptosis and inflammatory cytokine secretion of intestinal epithelial cells. Further exploration revealed that SMAD family member 6 (SMAD6) was the direct target of miR-32-5p, and SMAD6 overexpression partially rescued cell damage induced by H. pylori. The following experiments showed that miR-32-5p/SMAD6 participated in the apoptosis of intestinal epithelial cells induced by transforming growth factor-β-activated kinase 1 (TAK1)-p38 activation under H. pylori infection.
Our work uncovered the crucial role of aberrant expression of miR-32-5p in H. pylori–related pediatric enteritis, and suggested that the TAK1-p38 pathway is involved in it.
Core tip: Our study demonstrated the harmful role of aberrant miR-32-5p in Helicobacter pylori (H. pylori)-infected intestinal epithelial cells. Further investigation showed that SMAD family member 6 (SMAD6) was the downstream of miR-32-5p and exerted an opposite role in this process. What’s more, miR-32-5p/SMAD6 contributed to transforming growth factor-β-activated kinase 1-p38 cascade activation in intestinal epithelial cells under H. pylori infection. These findings provide a novel insight into the pathogenesis of pediatric enteritis caused by H. pylori.
- Citation: Feng J, Guo J, Wang JP, Chai BF. MiR-32-5p aggravates intestinal epithelial cell injury in pediatric enteritis induced by Helicobacter pylori. World J Gastroenterol 2019; 25(41): 6222-6237
- URL: https://www.wjgnet.com/1007-9327/full/v25/i41/6222.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i41.6222
Enteritis is a common disease of the digestive system in children among outpatients[1]. Etiologically, Helicobacter pylori (H. pylori) infection is one of the most important pathogenic factors to induce pediatric enteritis[2]. The stomach is the primary organ that is damaged by H. pylori. However, H. pylori-induced enteritis is seen with increasing incidence in recent years.
H. pylori infection is regarded as a class I carcinogen[3]. Normally, H. pylori-induced gastritis could lead to gastric ulcer, which is the major precancerous lesion if without treatment. Although mainly residing in stomach, H. pylori displays a strong ability of acid resistance. As a pathogen, H. pylori could attack and damage the mucosa of the digestive tract by recruiting and activating neutrophils[4], inducing abnormal expression of key proteins[5] and microRNAs (miRNAs)[6], and releasing cytotoxic substances[7]. A previous study showed that H. pylori infection accounted for 6% of children with duodenitis[8]. Moreover, Gimiga et al[9] found that gastritis and duodenitis contributed to half of children with upper gastrointestinal bleeding, and 36.89% of participants were diagnosed with H. pylori infection. These findings suggested a relatively high prevalence of children with H. pylori infection in the digestive system.
MicroRNAs (miRNAs) belong to non-coding RNA molecules that are abundant in eukaryotic organisms[10,11]. MiRNAs have no ability to encode proteins, but contribute to the modulation of gene expression[11,12]. A recent study revealed the upregulation of miR-146a and miR-155 in patients with gastritis induced by H. pylori infection[13], with the similar findings demonstrated by another research group[14]. Cortés-Márquez et al[14] further grouped the patients with gastritis by age, and found that both children and adults with H. pylori-induced gastritis displayed higher levels of miR-145a and miR-155. Moreover, the cancerization tendency of H. pylori-induced gastritis was correlated with the upregulation of miR-146a and miR-155[13,14]. In addition, H. pylori infection could result in the downregulation of miRNAs. The decreased expression of miR-24-3p was shown in H. pylori–induced gastritis tissue samples compared with the gastritis tissues without H. pylori infection[15]. Zou et al[16] demonstrated that gastric epithelial cells treated with miR-3178 mimic presented alleviated inflammation induced by H. pylori, and miR-3178 could block H. pylori–induced carcinogenesis by targeting the TRAF1-NF-κB pathway. Therefore, the relationship between miRNAs and H. pylori infection is not positively correlated. Except for causing gastric epithelial cell damage via abnormal expression of miRNAs, H. pylori infection-induced miRNAs might contribute to intestinal epithelial cell damage. However, little is currently known about miRNAs and H. pylori–related enteritis.
Herein, our research revealed significant upregulation of miR-32-5p by targeting SMAD family member 6 (SMAD6) in H. pylori-infected intestinal epithelial cells, which aggravated the damage of H. pylori to the cells and might contribute to the pathogenesis of pediatric enteritis induced by H. pylori.
Serum samples were obtained from children with H. pylori-induced enteritis (n = 15) and healthy controls (n = 15), and the participants were from Shanxi Provincial People’s Hospital. Procedures in this study were approved by the Ethics Committee of Shanxi University and Ethics Committee of Shanxi Provincial People’s Hospital, and complied with the guidelines of Declaration of Helsinki. Both guardians of the children and the participants were informed of the purpose of the study, and signed an informed consent form.
Human intestinal epithelial cell line HIEC-6 was cultured in RPMI 1640 medium with 10% fetal bovine serum (FBS). Human embryonic kidney cell line HEK-293T was cultured in DMEM medium with 10% FBS. The medium and FBS were purchased from ThermoFisher Scientific (United States). H. pylori strain T81213-NTB (ATCC 46396) was cultured as previously described[17]. The concentration of H. pylori was adjusted to 1 × 109 CFU/mL, and 1 × 106 CFU/mL was used in our experiments.
Serum miRNAs were extracted using a miRNeasy Serum/Plasma Kit (QIAGEN, Germany), miRNAs of cells were obtained with a miRNeasy Mini Kit (QIAGEN, Germany), and total RNA of cells was extracted using TRIzol (Takara, Japan). cDNA was acquired from the extracted RNA, and quantitative real time-polymerase chain reaction (qRT-PCR) was carried out by using a SYBR Premix Ex Taq II Kit (Takara, Japan). The expression level was calculated by using 2-ΔΔCT methods. Primers used in our study are displayed in Table 1.
| Gene | Sequence (5’-3’) |
| miR-32-5p | Forward: CGGTATTGCACATTACTAAGTTGCA |
| Reverse: CTCGCTTCGGCAGCACA | |
| U6 | Forward: AGGGGCCATCCACAGTCTTC |
| Reverse: AACGCTTCACGAATTTGCGT | |
| GAPDH | Forward: AAAAGGGCCCTGACAACTCTT |
| Reverse: ACCCTGTTGCTGTAGCCAAA | |
| SMAD6 | Forward: TTGTCATTTAGGGACCCTCAGC |
| Reverse: TTGGCAGGAAATGCAGGTTTG | |
| TNF-α | Forward: AAGATAGGGTGTCTGGCACA |
| Reverse: CCCTGAGGTGTCTGGTTTTCT | |
| IL-6 | Forward: AGCAGGCACCCCAGTTAAT |
| Reverse: AATCCTTTGCAGTGGAGGGA | |
| SMAD6 siRNA | Forward: CCACAUUGUCUUACACUGAAACGGA |
| Reverse: UCCGUUUCAGUGUAAGACAAUGUGG | |
| si-control | AAUUCUCCGAACGUGUCACGU |
| MiR-32-5p mimic | Forward: UAUUGCACAUUACUAAGUUGCA |
| Reverse: CAACUUAGUAAUGUGCAAUAUU | |
| Mimic NC | UUCUCCGAACGUGUCACUGUU |
| MiR-32-5p inhibitor | UGCAACUUAGUAAUGUGCAAUA |
| Inhibitor NC | CAGUACUUUUGUGUAGUACAA |
SiRNAs, miRNA mimic and inhibitor, and overexpression vectors were obtained from Sangon Biotech (China). Cell transfection was carried out with Lipofectamine 3000 (Invitrogen, United States) according to the manufacturer’s instructions. The sequences used are shown in Table 1.
Cell Counting Kit-8 (Beyotime, China) assay was utilized to evaluate cell viability according to the manufacturer’s instructions. Cells were seeded followed by cell transfection in the presence or absence of H. pylori. Ten microliters of the reagents were added into each well at 37 °C for 1.5 h. The value of optical density (OD) was detected with a microplate reader (Bio-Rad, United States) at 450 nm.
After cell transfection, cells were incubated with TAK1 inhibitor NG25 (5 μmol/L, APExBIO, United States) and p38 inhibitor (10 μmol/L, Cell Signaling Technoloby, United States) in the presence or absence of H. pylori, harvested, and stained using an Annexin V-FITC/PI apoptosis kit (Beyotime, China) according to the manufacturer’s instructions. The apoptotic rate is represented as the positive rate of cells labelled with Annexin V, and the results were analyzed by flow cytometry (Accuri C6, United States).
HEK-293T cells were used to validate the binding between miR-32-5p and SMAD6. Wild type (WT) and mutant (Mut) SMAD6 containing predicted sites with miR-32-5p were obtained from Sangon Biotech (China). After transfection for 48 h, the cells were tested using the Bio-Glo Luciferase Assay System (Promega, United States).
Western blot analysis was performed as previously described[16]. Primary antibodies (mouse monoclonal to SMAD6, Santa Cruz Biotechnology, United States; rabbit monoclonal to TAK1, Abcam, United States; rabbit monoclonal to TAK1, phosphor S439, Abcam; rabbit monoclonal to p38, Abcam; rabbit monoclonal to p38, phosphor T180, Abcam; mouse monoclonal to Actin, Beyotime, China) were used in our research. The ChemDoc XRS+ System (Bio-Rad, United States) was utilized to evaluate the protein bands.
The data are expressed as the mean ± standard deviation (SD) and analyzed by non-parametric t-tests using GraphPad Prism 6.0 software (GraphPad, United States). P < 0.05 was considered statistically significant.
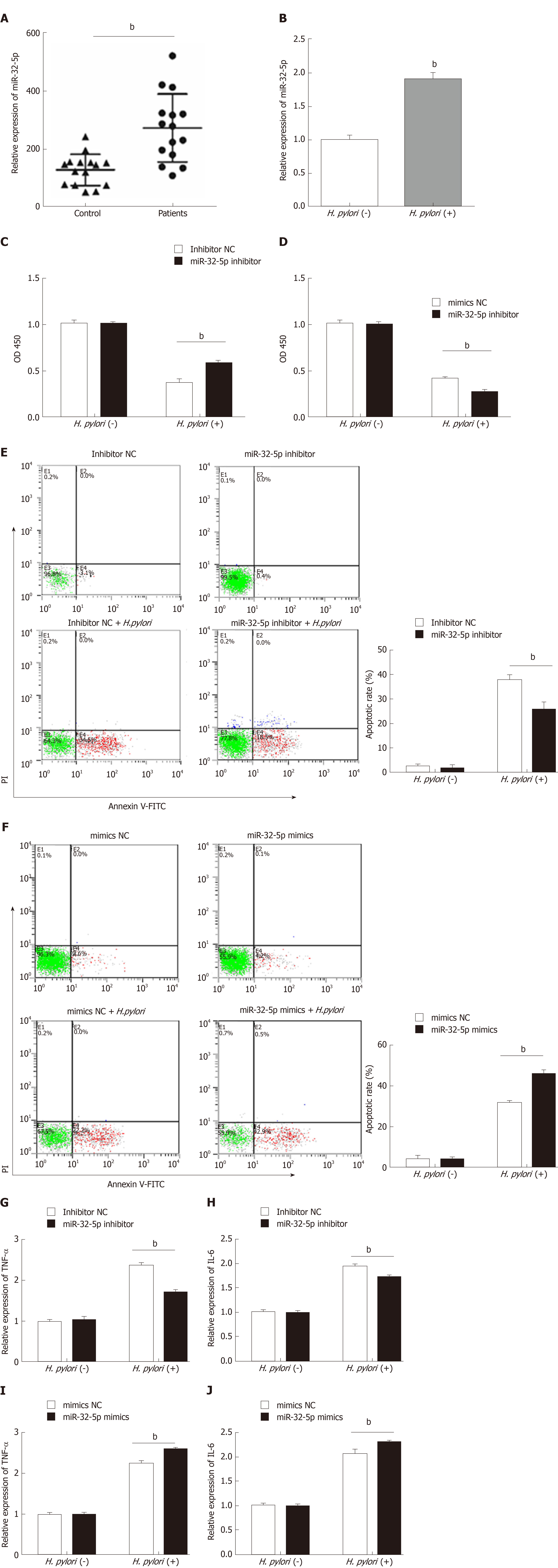
To explore the potential role of miR-32-5p in pediatric enteritis, we first monitored the expression of miR-32-5p. After separating the serum from children with enteritis induced by H. pylori and healthy controls, we found that miR-32-5p was upregulated in serum of children with H. pylori-induced pediatric enteritis (Figure 1A). Next, we treated intestinal epithelial cells with H. pylori. The qRT-PCR results displayed that H. pylori led to a significant increase of miR-32-5p in intestinal epithelial cells (Figure 1B). These findings suggested that miR-32-5p might play a crucial role in H. pylori-related pediatric enteritis.
Next, cell transfection with miR-32-5p inhibitor or miR-32-5p mimic was utilized to verify the role of miR-32-5p in cell viability in the presence or absence of H. pylori. We found that H. pylori impaired cell viability, and miR-32-5p inhibitor partially restored the viability of H. pylori-infected intestinal epithelial cells (Figure 1C). On the contrary, miR-32-5p mimic further caused the decrease of viability of H. pylori-infected intestinal epithelial cells (Figure 1D). In parallel, H. pylori-infected intestinal epithelial cells with miR-32-5p inhibitor displayed a lower apoptotic rate, and miR-32-5p mimic facilitated apoptosis of H. pylori-infected intestinal epithelial cells (Figure 1E and F). In addition, we found that TNF-α was upregulated in intestinal epithelial cells infected with H. pylori, which was decreased by miR-32-5p inhibitor transfection and further increased by miR-32-5p mimic (Figure 1G and I). IL-6 expression displayed the similar trend as TNF-α (Figure 1H and J).
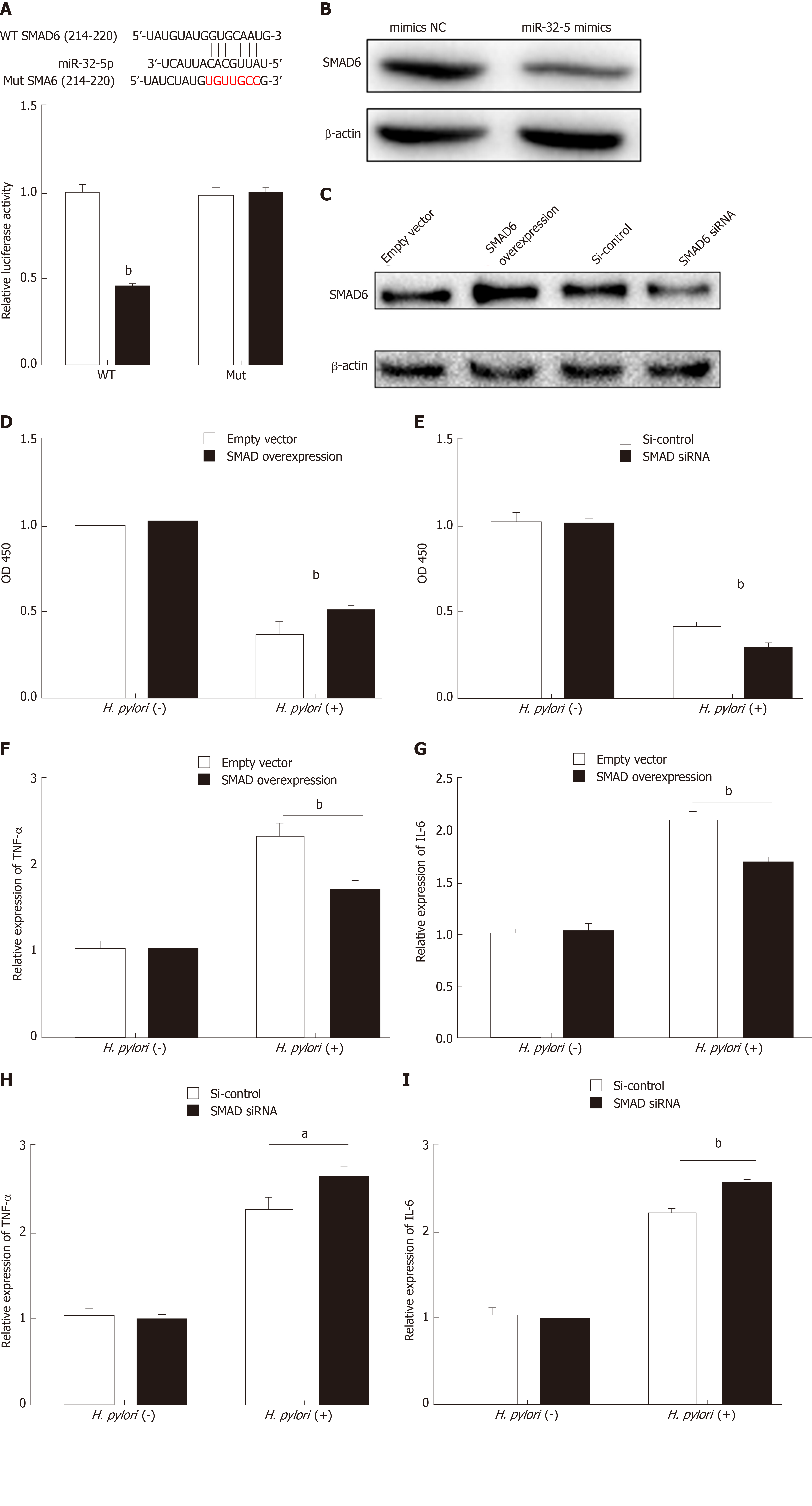
To further explore the downstream mechanism of miR-32-5p, we used TargetScanHuman 7.2 (http://www.targetscan.org/vert_72/) to identify SMAD6, acting as a mediator of anti-inflammatory activity, as the potential target of miR-32-5p. The luciferase assay showed that SMAD6 was the direct target of miR-32-5p (Figure 2A). After transfecting with miR-32-5p mimic into intestinal epithelial cells, we found that the protein level of SMAD6 was downregulated (Figure 2B). Next, we performed cell transfection assay to make SMAD6 overexpress and knock down (Figure 2C). We further found that SMAD6 overexpression ameliorated the death of intestinal epithelial cells infected by H. pylori (Figure 2D). On the contrary, SMAD6 siRNA accelerated H. pylori–induced cell death (Figure 2E). In addition, SMAD6 could partially restrain the elevation of both TNF-α and IL-6 in intestinal epithelial cells infected by H. pylori (Figure 2F and G), while SMAD6 knockdown exerted an opposite role as SMAD6 overexpression did in the expression of TNF-α and IL-6 (Figure 2H and I). Therefore, these findings suggested that SMAD6 played a critical role in H. pylori–infected intestinal epithelial cells by acting as the downstream molecule of miR-32-5p.
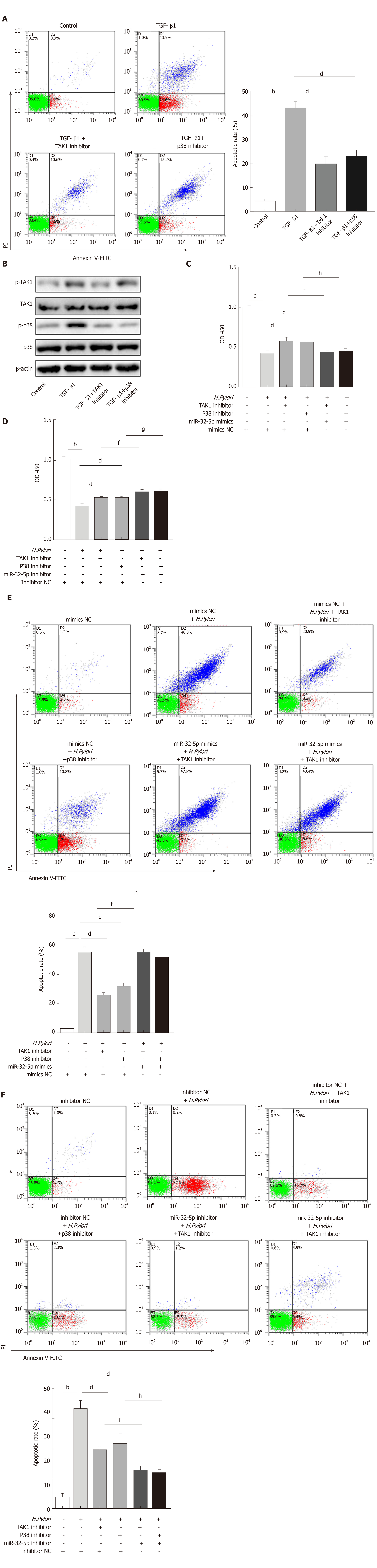
SMAD6 is a downstream molecule of transforming growth factor-β1 (TGF-β1), and transforming growth factor-β-activated kinase 1 (TAK1)-p38 cascade activation could be executed by TGF-β1[18,19]. We found that TGF-β1 treatment increased the apoptosis of intestinal epithelial cells, and both TAK1 inhibitor and p38 inhibitor could limit TGF-β1-induced apoptosis (Figure 3A). What’s more, TGF-β1 led to the upregulation of phosphorylated TAK1 (p-TAK1) and phosphorylated p38 (p-p38), which could be restrained by TAK1 inhibitor (Figure 3B). However, TGF-β1-mediated phosphorylation of TAK1 was not inhibited by p38 inhibitor (Figure 3B). As shown in Figure 3C and D, TAK1 inhibitor and p38 inhibitor could partially increase theviability of intestinal epithelial cells infected with H. pylori, while miR-32-5p mimic, combined with either TAK1 inhibitor or p38 inhibitor, led to a marked decrease in the cell viability (Figure 3C). On the contrary, miR-32-5p further increased cell viability with either TAK1 inhibitor or p38 inhibitor under H. pylori infection (Figure 3D). Consistent with the findings in the cell viability, we found that both TAK1 inhibitor and p38 inhibitor could partially restrained the apoptosis of H. pylori–infected intestinal epithelial cells (Figure 3E and F). When miR-32-5p mimic was transfected into the cells with either TAK1 inhibitor or p38 inhibitor under H. pylori infection, apoptosis increased significantly (Figure 3E). However, miR-32-5p inhibitor transfection played an opposite role as miR-32-5p mimic did (Figure 3F). Thus, these results suggested that TGF-β1-TAK1-p38 cascade contributed to intestinal epithelial cell damage under H. pylori infection.
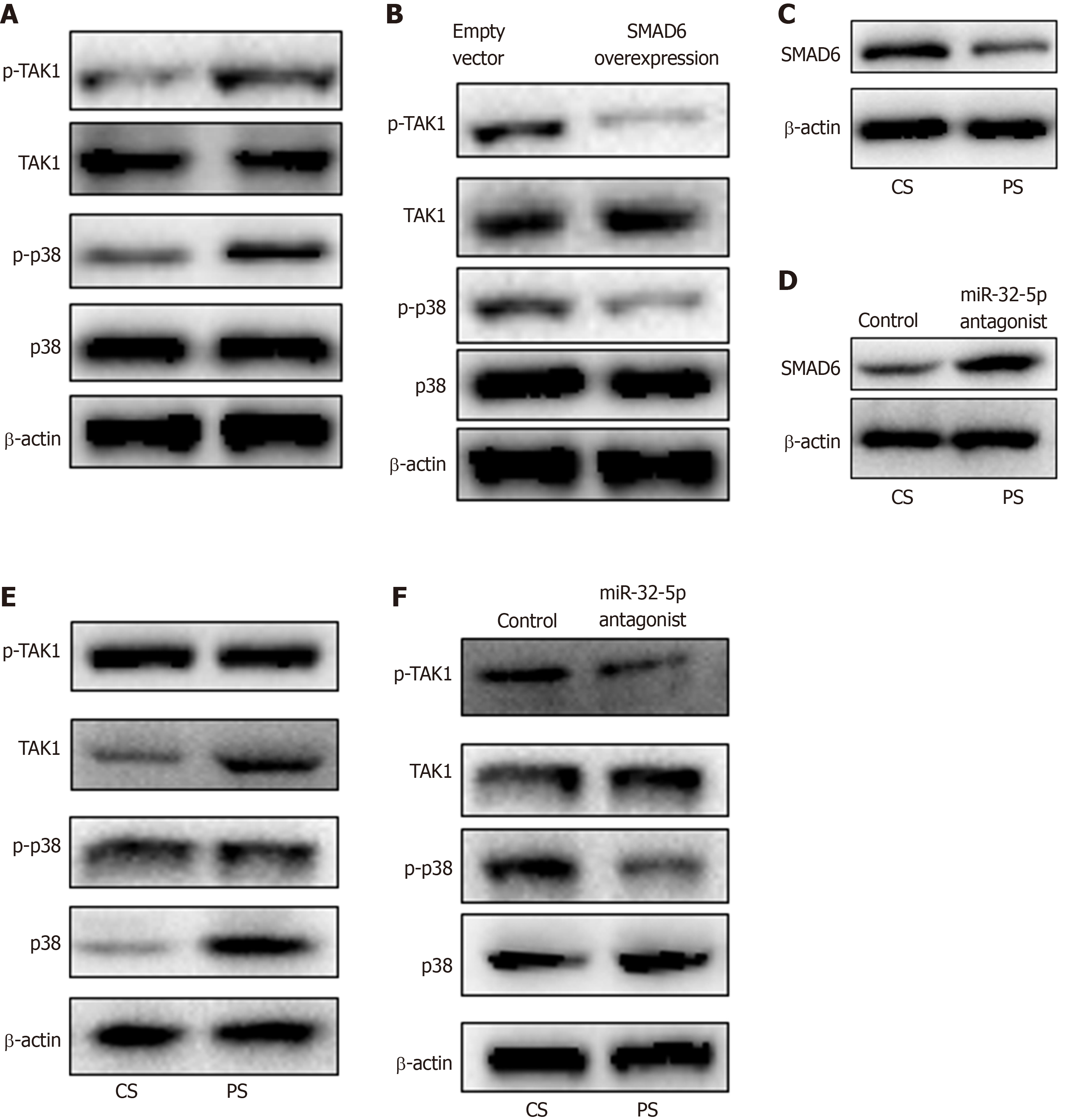
Next, we found the upregulation of p-TAK1 and p-p38 in intestinal epithelial cells with H. pylori infection (Figure 4A). SMAD6 is one of the inhibitory SMADs that could block TGF-β1 signaling[19]. Once SMAD6 overexpression plasmid was transfected into the cells, the activation of TAK1-p38 cascade was limited significantly (Figure 4B). Moreover, we stimulated intestinal epithelial cells with patient serum, and found that SMAD6 was downregulated (Figure 4C). When miR-32-5p antagonist was added into the patient serum, the downregulated expression of SMAD6 was reversed (Figure 4D). We also found that patient serum treatment activated TAK1 and p38 in intestinal epithelial cells compared with healthy control serum treatment (Figure 4E). However, miR-32-5p antagonist could result in downregulation of p-TAK1 and p-p38 in intestinal epithelial cells cultured in medium containing patient serum (Figure 4F).
As a common digestive disease in children, enteritis could cause abdominal pain, diarrhea, and dyspepsia. If chronic enteritis results from the nonstandard treatment, children might display malnutrition, even severely affecting health and growth of children. Virus infection is regarded as the leading cause of pediatric enteritis worldwide[20,21], which might lead to a local outbreak. However, what could not be ignored is that bacterial infection is also an important pathogenic factor resulting in pediatric enteritis. It has been reported that H. pylori infection displayed a remarkable correlation with pediatric enteritis[2,22]. However, there is a lack of systematic understanding about the correlation between H. pylori infection and the pathogenesis of pediatric enteritis.
H. pylori is a common bacterium that could cause gastritis, gastric ulcer, and even gastric cancer[3,7,23]. A great deal of evidence has revealed that multiple factors contribute to H. pylori infection, including autophagy, miRNAs, and long non-coding RNAs (lncRNAs). H. pylori could promote autophagy of gastric adenocarcinoma epithelial cells, and increased antibiotic resistance of H. pylori-infected cells[24]. Yang et al[25] reported that miR-30d regulated autophagy-related proteins to facilitate H. pylori survival within cells. An increasing number of miRNAs and lncRNAs were identified by different research groups, which displayed a tight relationship with H. pylori infection[6,14,26,27]. In the current study, we found the upregulation of miR-32-5p in serum of patients with H. pylori-related pediatric enteritis and H. pylori-infected intestinal epithelial cells. In addition, miR-32-5p inhibition could rescue intestinal epithelial cells infected with H. pylori, reduce H. pylori-induced apoptosis, and restrain the increase of inflammatory cytokines of H. pylori-infected cells, which inferred that miR-32-5p contributed to intestinal epithelial cell injury caused by H. pylori infection.
Currently, only a few studies focus on miR-32-5p, and these research findings are largely about the relationship between miR-32-5p and the pathogenesis of tumors, including cervical cancer[28], prostate cancer[29], hepatocellular carcinoma[30], and pancreatic cancer[31]. Besides, Zhang and colleagues[32] discovered that Mycobacterium tuberculosis infection led to a significant increase of miR-32-5p in macrophages, which in turns promoted Mycobacterium tuberculosis survival in the infected cells by targeting the downstream molecules of miR-32-5p. We found that SMAD6 was a direct target of miR-32-5p to be involved in H. pylori-induced intestinal epithelial cell damage. It has been demonstrated that SMAD6 is one of the two inhibitory SMADs (e.g., SMAD6 and SMAD7) downstream of TGF-β, and could inhibit inflammation and cell apoptosis[19,33,34]. Our findings revealed that SMAD6 overexpression could increase resistance of intestinal epithelial cells to H. pylori, and reduce inflammatory cytokines caused by H. pylori. Next, we found that TGF-β1-activated TAK1-p38 cascade contributed to H. pylori-induced intestinal epithelial cell injury. Luo and colleagues reported that TGF-β1 mediated H. pylori-related gastritis, and blocking TGF-β1 could effectively alleviate gastritis[35]. As we verified, inhibition of TAK1 activation and p38 activation could partially protect epithelial cells from H. pylori infection. Moreover, miR-32-5p inhibition further improved the protective role of TAK1 inhibitor and p38 inhibitor in H. pylori-infected intestinal epithelial cells.
Recently, TGF-β was determined to be involved in H. pylori infection. A recent study found that H. pylori facilitated the expression of TGF-β, and induced downregulation of cystic fibrosis transmembrane conductance regulator (CFTR) and solute linked carrier 26 gene family A6 (SLC26A6) in the duodenum[36]. Patients with H. pylori-induced duodenal ulcers displayed significantly decreased expression of CFTR and SLC26A6, which displayed a remarkable correlation with the severity of the illness[37]. In addition to affecting the function of intestinal epithelial cells, H. pylori could modulate the role of immune cells in the intestinal tract. A previous study suggested that H. pylori led to the upregulation of heat shock protein 60 in macrophages to promote TGF-β production, which contributed to the infiltration of regulatory T cells (Treg) to cause persistent infection[38]. Clinical data showed an increased number of Treg in children with H. pylori infection, and TGF-β had a positive correlation with the increase of Treg[39]. In our study, we discovered a novel manner involving TGF-β1 in which H. pylori infection damaged intestinal epithelial cells. Induction of miR-32-5p by H. pylori infection led to decreased expression of SMAD6, which weakened the inhibitory role of SMAD6 in TGF-β1-TAK1-p38 cascade activation, as demonstrated by the treatment of serum samples in intestinal epithelial cells.
Based on the above findings, we concluded that H. pylori infection promotes miR-32-5p upregulation to aggravate apoptosis and inflammation in intestinal epithelial cells by targeting SMAD6 and activating the TAK1-p38 pathway, highlighting a crucial role of miR-32-5p in the pathogenesis of pediatric enteritis caused by H. pylori.
Helicobacter pylori (H. pylori) infection is a global issue that could cause a variety of diseases involving multiple organs. It is worth noting that the incidence of H. pylori-related enteritis in children increases, but the underlying mechanism is largely unknown. It has been reported that miR-32-5p is overexpressed in diseases associated with bacterial infection. However, the potential role of miR-32-5p in H. pylori-induced pediatric enteritis is not clear.
To investigate the exact role of miR-32-5p in the pathogenesis of pediatric enteritis with H. pylori infection, and to find a novel target for H. pylori-related enteritis in children.
To explore the aberrant expression and significance of miR-32-5p in children with H. pylori-related enteritis, especially in the damage of intestinal epithelial cells with H. pylori infection.
qRT-PCR was performed to detect the expression of miR-32-5p in clinical samples and H. pylori-infected intestinal epithelial cells. Cell Counting Kit-8 assay and flow cytometry were conducted to evaluate the role of miR-32-5p in H. pylori-infected intestinal epithelial cells. TargetScanHuman database and luciferase assay were utilized to verify the potential target of miR-32-5p. Western blot was employed to clarify the underlying mechanism of miR-32-5p in influencing H. pylori-infected intestinal epithelial cells.
The present study discovered the aberrant expression of miR-32-5p in pediatric enteritis with H. pylori infection and H. pylori-treated intestinal epithelial cells. The in vitro experiments showed the significance of miR-32-5p in regulating cell viability and apoptosis of H. pylori-treated intestinal epithelial cells. We identified that SMAD family member 6 (SMAD6) was the direct target of miR-32-5p, and SMAD6 partially counteracted the harmful role of miR-32-5p by inhibiting the activation of TAK1-p38 cascade. However, in vivo assays are needed to further verify our in vitro findings and significance of miR-32-5p in H. pylori-induced pediatric enteritis.
Our research first identified the upregulation of miR-32-5p in H. pylori-related pediatric enteritis. Further exploration revealed that miR-32-5p inhibited SMAD6 to activate the TAK1-p38 signaling pathway, aggravating H. pylori-induced damage of intestinal epithelial cells. MiR-32-5p might be a potential target to overcome H. pylori-induced damage of intestinal epithelial cells in children.
Based on the clinical findings and in vitro experiments, miR-32-5p could be a novel therapeutic target for H. pylori-induced damage of intestinal epithelial cells in children. Further in vivo assays are of necessity to clarify the deleterious effects of miR-32-5p on H. pylori-infected intestinal epithelial cells, which is very meaningful and would contribute to clinical application.
| 1. | Chen J, Wan CM, Gong ST, Fang F, Sun M, Qian Y, Huang Y, Wang BX, Xu CD, Ye LY, Dong M, Jin Y, Huang ZH, Wu QB, Zhu CM, Fang YH, Zhu QR, Dong YS. Chinese clinical practice guidelines for acute infectious diarrhea in children. World J Pediatr. 2018;14:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Zhang SH, Zhu X. Advances in Helicobacter pylori in children. Linchuang Erke Zazhi. 2015;33:391-395. |
| 3. | Vogiatzi P, Cassone M, Luzzi I, Lucchetti C, Otvos L, Giordano A. Helicobacter pylori as a class I carcinogen: physiopathology and management strategies. J Cell Biochem. 2007;102:264-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Ren WK, Xu YF, Wei WH, Huang P, Lian DW, Fu LJ, Yang XF, Chen FJ, Wang J, Cao HY, Deng YH. Effect of patchouli alcohol on Helicobacter pylori-induced neutrophil recruitment and activation. Int Immunopharmacol. 2019;68:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Li FY, Weng IC, Lin CH, Kao MC, Wu MS, Chen HY, Liu FT. Helicobacter pylori induces intracellular galectin-8 aggregation around damaged lysosomes within gastric epithelial cells in a host O-glycan-dependent manner. Glycobiology. 2019;29:151-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Santos JC, Brianti MT, Almeida VR, Ortega MM, Fischer W, Haas R, Matheu A, Ribeiro ML. Helicobacter pylori infection modulates the expression of miRNAs associated with DNA mismatch repair pathway. Mol Carcinog. 2017;56:1372-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Mnich E, Kowalewicz-Kulbat M, Sicińska P, Hinc K, Obuchowski M, Gajewski A, Moran AP, Chmiela M. Impact of Helicobacter pylori on the healing process of the gastric barrier. World J Gastroenterol. 2016;22:7536-7558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Alper A, Hardee S, Rojas-Velasquez D, Escalera S, Morotti RA, Pashankar DS. Prevalence and Clinical, Endoscopic, and Pathological Features of Duodenitis in Children. J Pediatr Gastroenterol Nutr. 2016;62:314-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Gimiga N, Olaru C, Diaconescu S, Miron I, Burlea M. Upper gastrointestinal bleeding in children from a hospital center of Northeast Romania. Minerva Pediatr. 2016;68:189-195. [PubMed] |
| 10. | Bossi L, Figueroa-Bossi N. Competing endogenous RNAs: a target-centric view of small RNA regulation in bacteria. Nat Rev Microbiol. 2016;14:775-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 11. | Beermann J, Piccoli MT, Viereck J, Thum T. Non-coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol Rev. 2016;96:1297-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1378] [Cited by in RCA: 1364] [Article Influence: 136.4] [Reference Citation Analysis (2)] |
| 12. | Subramaniam S, Jeet V, Clements JA, Gunter JH, Batra J. Emergence of MicroRNAs as Key Players in Cancer Cell Metabolism. Clin Chem. 2019;65:1090-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Zabaglia LM, Sallas ML, Santos MPD, Orcini WA, Peruquetti RL, Constantino DH, Chen E, Smith MAC, Payão SM, Rasmussen LT. Expression of miRNA-146a, miRNA-155, IL-2, and TNF-α in inflammatory response to Helicobacter pylori infection associated with cancer progression. Ann Hum Genet. 2018;82:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Cortés-Márquez AC, Mendoza-Elizalde S, Arenas-Huertero F, Trillo-Tinoco J, Valencia-Mayoral P, Consuelo-Sánchez A, Zarate-Franco J, Dionicio-Avendaño AR, Herrera-Esquivel JJ, Recinos-Carrera EG, Colín-Valverde C, Rivera-Gutiérrez S, Reyes-López A, Vigueras-Galindo JC, Velázquez-Guadarrama N. Differential expression of miRNA-146a and miRNA-155 in gastritis induced by Helicobacter pylori infection in paediatric patients, adults, and an animal model. BMC Infect Dis. 2018;18:463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Li Q, Wang N, Wei H, Li C, Wu J, Yang G. miR-24-3p Regulates Progression of Gastric Mucosal Lesions and Suppresses Proliferation and Invasiveness of N87 Via Peroxiredoxin 6. Dig Dis Sci. 2016;61:3486-3497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Zou M, Wang F, Jiang A, Xia A, Kong S, Gong C, Zhu M, Zhou X, Zhu J, Zhu W, Cheng W. MicroRNA-3178 ameliorates inflammation and gastric carcinogenesis promoted by Helicobacter pylori new toxin, Tip-α, by targeting TRAF3. Helicobacter. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Beceiro S, Radin JN, Chatuvedi R, Piazuelo MB, Horvarth DJ, Cortado H, Gu Y, Dixon B, Gu C, Lange I, Koomoa DL, Wilson KT, Algood HM, Partida-Sánchez S. TRPM2 ion channels regulate macrophage polarization and gastric inflammation during Helicobacter pylori infection. Mucosal Immunol. 2017;10:493-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Liao SJ, Luo J, Li D, Zhou YH, Yan B, Wei JJ, Tu JC, Li YR, Zhang GM, Feng ZH. TGF-β1 and TNF-α synergistically induce epithelial to mesenchymal transition of breast cancer cells by enhancing TAK1 activation. J Cell Commun Signal. 2019;13:369-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Jung SM, Lee JH, Park J, Oh YS, Lee SK, Park JS, Lee YS, Kim JH, Lee JY, Bae YS, Koo SH, Kim SJ, Park SH. Smad6 inhibits non-canonical TGF-β1 signalling by recruiting the deubiquitinase A20 to TRAF6. Nat Commun. 2013;4:2562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 20. | Mursalova N, Shugayev N, Suleymanova J, Daniels DS, Wasley A, Cohen AL, Aliabadi N. Rotavirus gastroenteritis surveillance in Azerbaijan, 2011-2016. Vaccine. 2018;36:7790-7793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Chen SY, Chiu CH. Worldwide molecular epidemiology of norovirus infection. Paediatr Int Child Health. 2012;32:128-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Bansal D, Patwari AK, Logani KB, Malhotra VL, Anand VK. Study of diagnostic modalities and pathology of Helicobacter pylori infection in children. Indian J Pathol Microbiol. 1999;42:311-315. [PubMed] |
| 23. | Reshetnyak VI, Reshetnyak TM. Significance of dormant forms of Helicobacter pylori in ulcerogenesis. World J Gastroenterol. 2017;23:4867-4878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (5)] |
| 24. | Chu YT, Wang YH, Wu JJ, Lei HY. Invasion and multiplication of Helicobacter pylori in gastric epithelial cells and implications for antibiotic resistance. Infect Immun. 2010;78:4157-4165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Yang XJ, Si RH, Liang YH, Ma BQ, Jiang ZB, Wang B, Gao P. Mir-30d increases intracellular survival of Helicobacter pylori through inhibition of autophagy pathway. World J Gastroenterol. 2016;22:3978-3991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Yang L, Long Y, Li C, Cao L, Gan H, Huang K, Jia Y. Genome-wide analysis of long noncoding RNA profile in human gastric epithelial cell response to Helicobacter pylori. Jpn J Infect Dis. 2015;68:63-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Sun F, Ni Y, Zhu H, Fang J, Wang H, Xia J, Ding F, Shen H, Shao S. microRNA-29a-3p, Up-Regulated in Human Gastric Cells and Tissues with H.Pylori Infection, Promotes the Migration of GES-1 Cells via A20-Mediated EMT Pathway. Cell Physiol Biochem. 2018;51:1250-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 28. | Liu YJ, Zhou HG, Chen LH, Qu DC, Wang CJ, Xia ZY, Zheng JH. MiR-32-5p regulates the proliferation and metastasis of cervical cancer cells by targeting HOXB8. Eur Rev Med Pharmacol Sci. 2019;23:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 29. | Zhang L, Li X, Chao Y, He R, Liu J, Yuan Y, Zhao W, Han C, Song X. KLF4, a miR-32-5p targeted gene, promotes cisplatin-induced apoptosis by upregulating BIK expression in prostate cancer. Cell Commun Signal. 2018;16:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 30. | Fu X, Liu M, Qu S, Ma J, Zhang Y, Shi T, Wen H, Yang Y, Wang S, Wang J, Nan K, Yao Y, Tian T. Exosomal miR-32-5p induces multidrug resistance in hepatocellular carcinoma via the PI3K/Akt pathway. J Exp Clin Cancer Res. 2018;37:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 193] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 31. | Gao ZQ, Wang JF, Chen DH, Ma XS, Wu Y, Tang Z, Dang XW. Long non-coding RNA GAS5 suppresses pancreatic cancer metastasis through modulating miR-32-5p/PTEN axis. Cell Biosci. 2017;7:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 32. | Zhang ZM, Zhang AR, Xu M, Lou J, Qiu WQ. TLR-4/miR-32-5p/FSTL1 signaling regulates mycobacterial survival and inflammatory responses in Mycobacterium tuberculosis-infected macrophages. Exp Cell Res. 2017;352:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 33. | Lee YS, Park JS, Jung SM, Kim SD, Kim JH, Lee JY, Jung KC, Mamura M, Lee S, Kim SJ, Bae YS, Park SH. Inhibition of lethal inflammatory responses through the targeting of membrane-associated Toll-like receptor 4 signaling complexes with a Smad6-derived peptide. EMBO Mol Med. 2015;7:577-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Zhang T, Wu J, Ungvijanpunya N, Jackson-Weaver O, Gou Y, Feng J, Ho TV, Shen Y, Liu J, Richard S, Jin J, Hajishengallis G, Chai Y, Xu J. Smad6 Methylation Represses NFκB Activation and Periodontal Inflammation. J Dent Res. 2018;97:810-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Luo J, Song J, Zhang H, Zhang F, Liu H, Li L, Zhang Z, Chen L, Zhang M, Lin D, Lin M, Zhou R. Melatonin mediated Foxp3-downregulation decreases cytokines production via the TLR2 and TLR4 pathways in H. pylori infected mice. Int Immunopharmacol. 2018;64:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Wen G, Deng S, Song W, Jin H, Xu J, Liu X, Xie R, Song P, Tuo B. Helicobacter pylori infection downregulates duodenal CFTR and SLC26A6 expressions through TGFβ signaling pathway. BMC Microbiol. 2018;18:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Wen G, Jin H, Deng S, Xu J, Liu X, Xie R, Tuo B. Effects of Helicobacter pylori Infection on the Expressions and Functional Activities of Human Duodenal Mucosal Bicarbonate Transport Proteins. Helicobacter. 2016;21:536-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Hsu WT, Ho SY, Jian TY, Huang HN, Lin YL, Chen CH, Lin TH, Wu MS, Wu CJ, Chan YL, Liao KW. Helicobacter pylori-derived heat shock protein 60 increases the induction of regulatory T-cells associated with persistent infection. Microb Pathog. 2018;119:152-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Yang YJ, Chuang CC, Yang HB, Lu CC, Sheu BS. Susceptibility to pediatric Helicobacter pylori infection correlates with the host responses of regulatory and effector T cells. Pediatr Infect Dis J. 2014;33:1277-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng TH, Rodrigo L, Stanciu C, Sipos F S-Editor: Wang J L-Editor: Wang TQ E-Editor: Ma YJ