Published online Nov 7, 2019. doi: 10.3748/wjg.v25.i41.6190
Peer-review started: August 12, 2019
First decision: August 27, 2019
Revised: September 11, 2019
Accepted: October 17, 2019
Article in press: October 17, 2019
Published online: November 7, 2019
Processing time: 86 Days and 9.8 Hours
Acute liver failure (ALF) is a significant and complex hepatic insult that may rapidly progress to life-threatening conditions. Recently, menstrual blood stem cells (MenSCs) have been identified as a group of easily accessible mesenchymal stem cells with the advantages of non-invasive acquisition, low immunogenicity, a greater capacity of self-renewal and multi-lineage differentiation, making them promising candidates for stem cell-based therapy to revolutionize the treatment strategies for liver failure.
To investigate the therapeutic potential of MenSCs for treating ALF in pigs and to dynamically trace the biodistribution of transplanted cells.
MenSCs were labeled in vitro with PKH26, a lipophilic fluorescent dye. The treatment group received immediate transplantation of PKH26-labelled MenSCs (2.5 × 106/kg) via the portal vein after D-galactosamine injection, and the control group underwent sham operation. The survival time, liver function, and hepatic pathological changes were compared between the two groups. Three major organs (liver, lungs and spleen) were extracted from animals and imaged directly with the In vivo Imaging System (IVIS) at the predetermined time points. The regions of interest were drawn to quantify the cell uptake in different organs.
The labelling procedure did not affect the morphology, viability or multipotential differentiation of MenSCs. Biochemical analysis showed that the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL) and prothrombin time (PT) measured at selected time points 24 h after transplantation were significantly decreased in the treatment group (P < 0.05). The survival time of ALF animals was prolonged in the treatment group compared with the control group (75.75 ± 5.11 h vs 53.75 ± 2.37 h, log rank, P < 0.001). The liver pathological tissue in the MenSC treatment group showed obviously increased numbers of remaining hepatocytes and a comparatively slight necrotic degree and area. In addition, the IVIS imaging revealed that PKH26-positive MenSCs were clearly retained in the liver initially and then diffused through the systemic circulation. Interestingly, the signal intensity in the liver increased obviously at 36 h, which corresponded to the biochemical result that liver function deteriorated most rapidly at 24 - 36 h.
Our study demonstrates the therapeutic efficacy and homing ability of transplanted MenSCs in a large animal model of ALF and suggests that MenSC transplantation could be a promising strategy for treating ALF.
Core tip: In this study, we investigated for the first time the therapeutic potential of intraportally transplanted menstrual blood stem cells (MenSCs) in treating pigs with acute liver failure, and showed that MenSC treatment improved liver function and coagulation, alleviated the progression of liver injury, and prolonged the survival time of pigs. Additionally, ex vivo imaging also demonstrated the ability of MenSCs to home to pathological hepatic environments after transplantation. MenSC transplantation has the potential to be used as an available source for treating acute liver failure in future clinical therapy.
- Citation: Cen PP, Fan LX, Wang J, Chen JJ, Li LJ. Therapeutic potential of menstrual blood stem cells in treating acute liver failure. World J Gastroenterol 2019; 25(41): 6190-6204
- URL: https://www.wjgnet.com/1007-9327/full/v25/i41/6190.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i41.6190
Acute liver failure (ALF) is a significant and complex hepatic insult that may rapidly progress to life-threatening multiple organ failure. The survival prognosis of ALF is extremely poor with a high short-term mortality of 70% - 80%[1]. Liver transplantation is considered the ultimate therapeutic option for these patients, but its clinical use is hindered by organ shortage, high cost, surgical risk, and postoperative complications. Thus, stem cell transplantation, as the most cutting-edge medical technique, offers a new hope for revolutionizing the treatment strategies for liver failure[2,3].
Stem cells, with a capacity of self-renewal and multi-lineage differentiation, have become vital players in liver regeneration. In recent years, many studies have shown that stem cell-based therapy may alleviate fibrosis, reduce liver inflammation, promote hepatocyte regeneration, and subsequently improve the liver function of ALF patients[4]. Mesenchymal stem cells (MSCs) are defined as adherent, fibroblast-like adult stem cells with characteristic surface phenotypes and multipotential differentiation. Compared with embryonic stem cells and induced pluripotent stem cells, MSCs have less tumorigenicity and involve no ethical issues. They have been identified in a wide range of adult tissues, such as bone marrow, placenta, umbilical cord, adipose tissue, skeletal muscle, cornea, synovial membrane, etc[5]. However, the application potential of these cell lines is partly restricted by proliferative activity, limited sources, and the invasiveness of acquiring the cells.
In 2007, the Medistem group first isolated a highly proliferating adherent cell population from menstrual blood with non-haematopoietic markers, termed menstrual blood stem cells (MenSCs)[6]. Subsequent studies have demonstrated that MenSCs possess robust replicative capacity, multipotential differentiation, and angiogenesis and vascularization pathways[7]. Since they are isolated from menstrual blood, MenSCs have a high level of immune privilege and easy access for acquisition[8,9]. Moreover, MenSCs express pluripotency factors, making them easily reprogrammed into disease-specific pluripotent stem cells[10]. These characteristics make them an ideal tool for regenerative medicine. To date, MenSCs have shown promising results for diverse diseases, including liver fibrosis[11], muscular dystrophy[12], stroke[13], and glioma[14], in several animal models and pre-clinical studies. Recently, MenSCs in the treatment of an ALF mouse model have been evaluated[15]; however, little is known about the therapeutic effect of MenSCs in treating ALF in large animal models, and the potential mechanisms involved are not yet completely understood. Optimizing stem cell-based therapies would require a safe, sensitive, high-efficiency labeling method to dynamically monitor cellular viability, biodistribution, differentiation capacity and long-term fate after engraftment. PKH26, a lipophilic tracer dye, is an easy-to-use and good biocompatible fluorescent marker with high sensitivity, which can bond tightly to the cell membrane and be specific to original cells, thereby passing all progenies with division[16,17].
In this study, we aimed to evaluate the therapeutic potential of intraportal transplanted MenSCs in treating pigs with ALF and used a fluorescent dye (PKH26) to achieve real-time visualization for the biological distribution of infused stem cells.
The donors of menstrual blood signed written informed consent for the studies. MenSCs were isolated from the menstrual blood of healthy donors during the first day of menstruation as previously reported[18]. Briefly, mononuclear cells were collected by Ficoll-Paque (1.077 g/mL; Fisher Scientific, NH) density-gradient centrifugation according to the manufacturer’s instructions. The cells were suspended in menstrual stem cell culture medium (S-Evans Biosciences, China) overnight at 37 °C in 5% humidified CO2 until the medium was replaced to remove non-adherent cells by washing with 1x phosphate buffered saline (1x PBS). About one million primary cells were isolated from 5mL menstrual blood. When cells reached 80%-90% confluence, they were subcultured and used for experiments during passages 3 to 5.
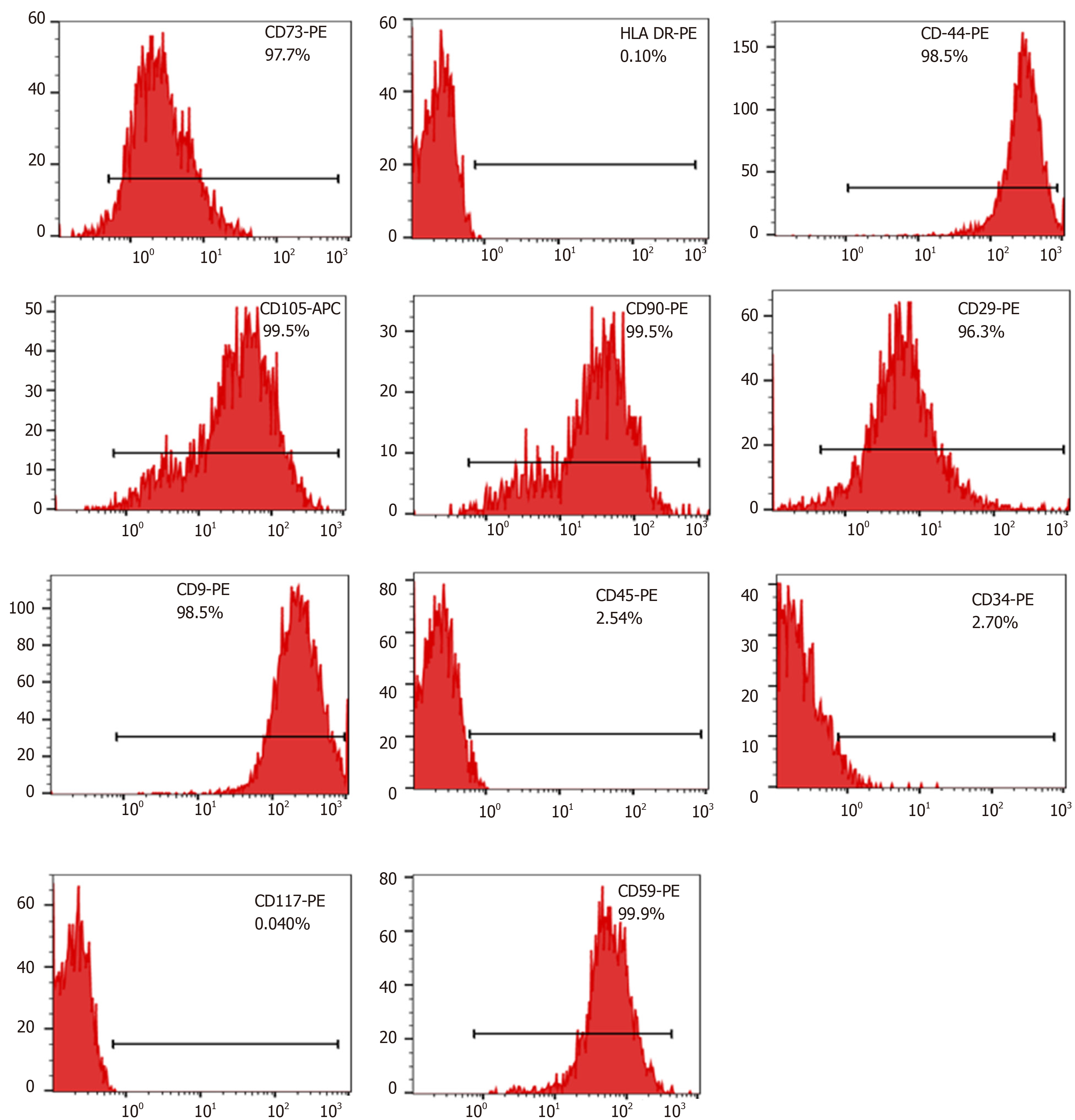
Surface markers of cultured MenSCs were analyzed using a flow cytometer (FC500MCL, Beckman Coulter, United States). The third and fifth passages of MenSCs ( 5 x 105 cells/100 μL) were incubated directly with phycoerythrin (PE) or allophycocyanin (APC)-conjugated mouse monoclonal antibodies against human CD9, CD29, CD41a, CD44, CD59, CD 73, CD90, CD105, CD14, CD34, CD45, CD117, and human leukocyte antigen (HLA)-DR (BD Biosciences, CA, United States) for 30 min in the dark at 4 °C, followed by washing and resuspension in PBS twice. Flow cytometry was conducted using a FACSCalibur system (FC500, Beckman Coulter, United States). Data were analyzed using FlowJo Version 10.05.
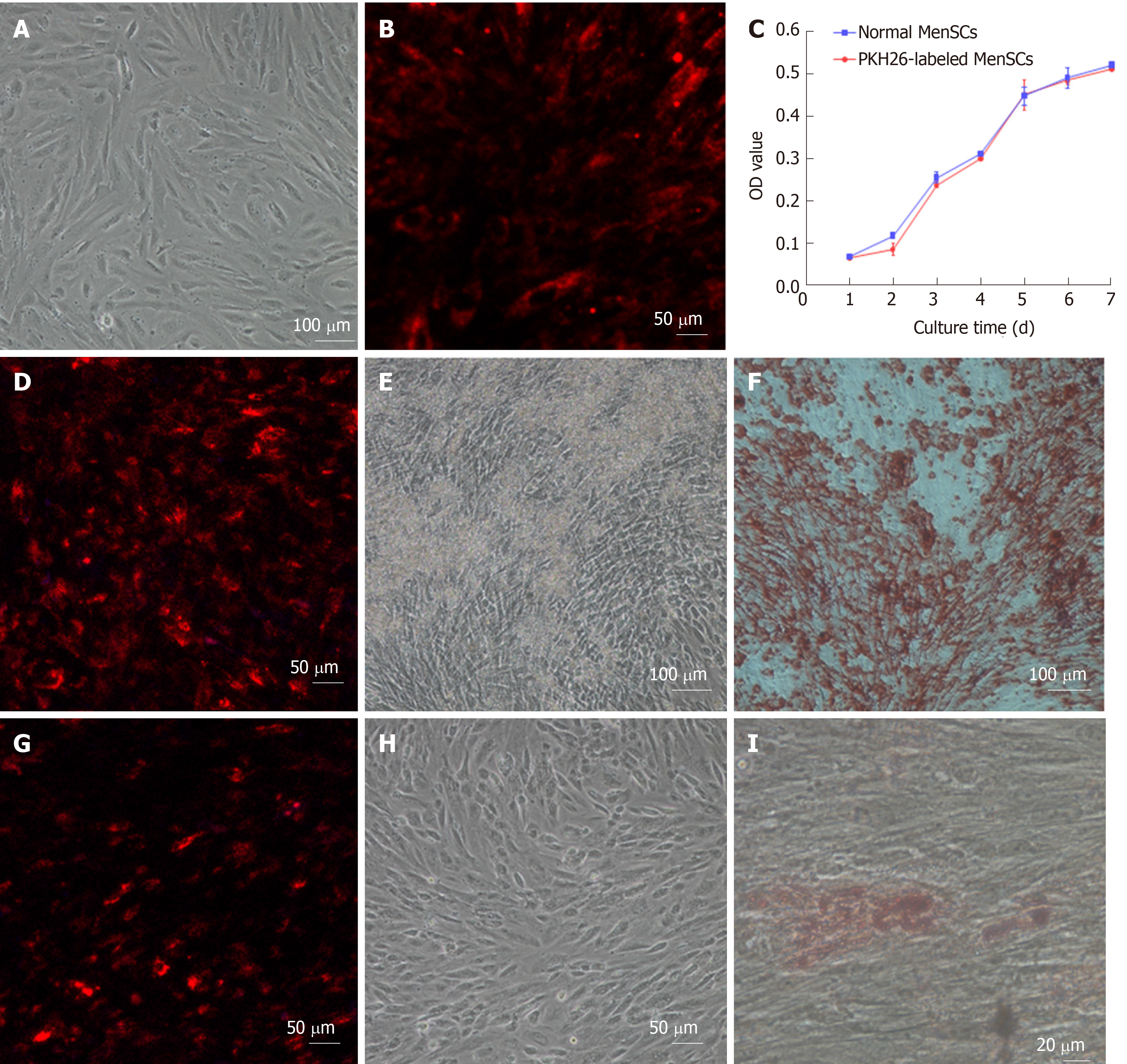
Cells were labeled with the fluorescent dye PKH26 (Sigma-Aldrich, United States) before transplantation according to the manufacturer’s protocol. MenSCs (2 x 107) were washed twice in serum-free medium after trypsinization and resuspended in 1mL of the dilution buffer. Before labeling, 4 μL stock solution of PKH26 was diluted with an equal volume of the dilution buffer and then immediately incubated with the cell suspension for 4 min at 25 °C. The labeling process was terminated by adding 2 mL fetal bovine serum. The cells were finally washed twice in complete culture medium, and the labelling ratio was counted under a confocal microscope (Carl Zeiss, Germany).
Cell viability was assessed by a cell counting kit-8 (CCK-8). Briefly, PKH26-labelled MenSCs (PKH26-MenSCs) and unlabelled MenSCs were separately seeded at a density of 1000 cells per well in 96-well plates. Ten microliters of CCK-8 reagent (Dojindo Laboratories, Japan) was added to 6 wells of each group every 24 h, and the cells were incubated for 1 h at 37 °C in 5% CO2. The absorbance at 450 nm was measured by a spectrophotometer (Beckman Coulter, United States), and the growth curve was drawn to compare cell proliferation ability between the two groups. To induce osteogenic differentiation, PKH26-MenSCs and unlabeled MenSCs were cultured in commercially available osteogenesis differentiation medium (Cyagen Biosciences, United States). On day 21, cells were stained with alizarin red S. To induce adipogenic differentiation, each group of cells was cultured in a commercially available adipogenesis differentiation medium purchased from Cyagen. On day 21, lipid droplets were visualized by oil red O.
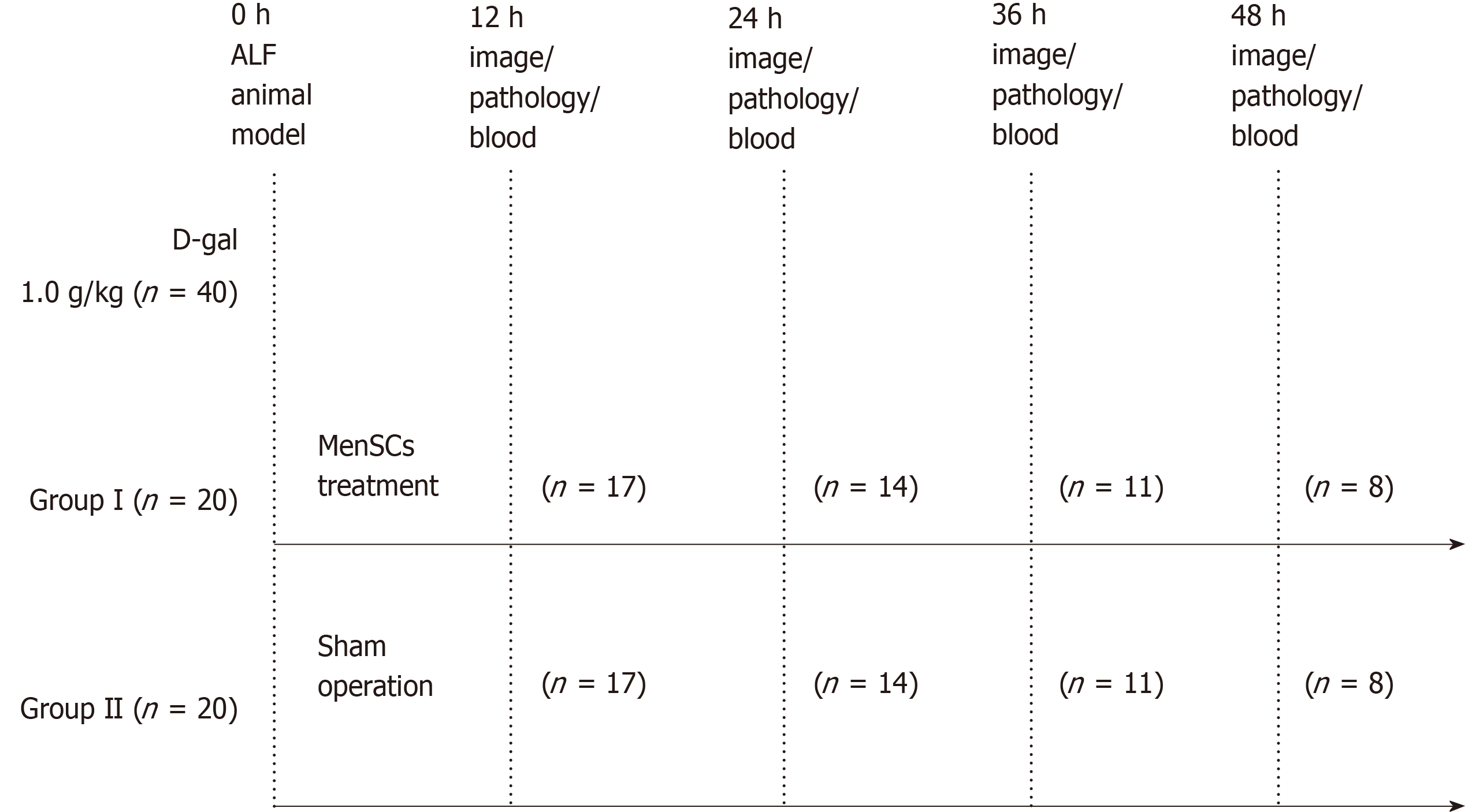
The experiments were approved by the Animal Care Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China on July 20th, 2015 (reference number: ZJU2015-511-09). A flow chart of the experimental design is presented in Figure 1. Forty male Bama experimental miniature pigs (Jiagan Biotechnology, China) with an average weight of 8 -12 kg were used in this study. All animals were fed in a single cage in a temperature-controlled environment (20-25 °C) with a 12 h light/12 h dark cycle and acclimatized for 7 d prior to the experiments. Sedation was induced with atropine (0.05 mg/kg), tramadol (2 mg/kg) and midazolam (0.1 mg/kg) by intramuscular injection. After anesthetized by intravenous injection of propofol (2.5 mg/kg/h), the animals were placed in the right lateral position and sterilized on their jugular skin. Then, an incision was made along the medial margin of the sternocleidomastoid followed by exposing and separating the external jugular vein. After the distal end of the vessel was ligated with sutures, the vessel was inserted into a double channel catheter (7 Fr). Finally, the wound was closed in layers, a sterile dressing was carried out. Twenty-four hours after jugular vein catheterization, ALF was induced with [D-galactosamine (D-gal), Hanhong Chemical, Shanghai, China] at a dose of 1.0 g/kg as previously described by our laboratory[19,20]. The animals were randomly allocated to two groups: Group I (n = 20), a treatment group, received an immediate intraportal transplantation of PKH26- MenSCs (2.5 × 106 /kg) suspended in 30 mL normal saline after D-gal injection. Specifically, after locating the portal vein, a puncture needle (18G) pierced the portal vein slowly under B-ultrasound guidance. When a free flow of blood appeared in the needle, PKH26- MenSCs were infused into the portal vein. Group II (n = 20), a sham operation group, received an equal volume of normal saline without MenSCs. No additional medical support was provided during the entire course of the experiment. Survival time was recorded until death without any human intervention.
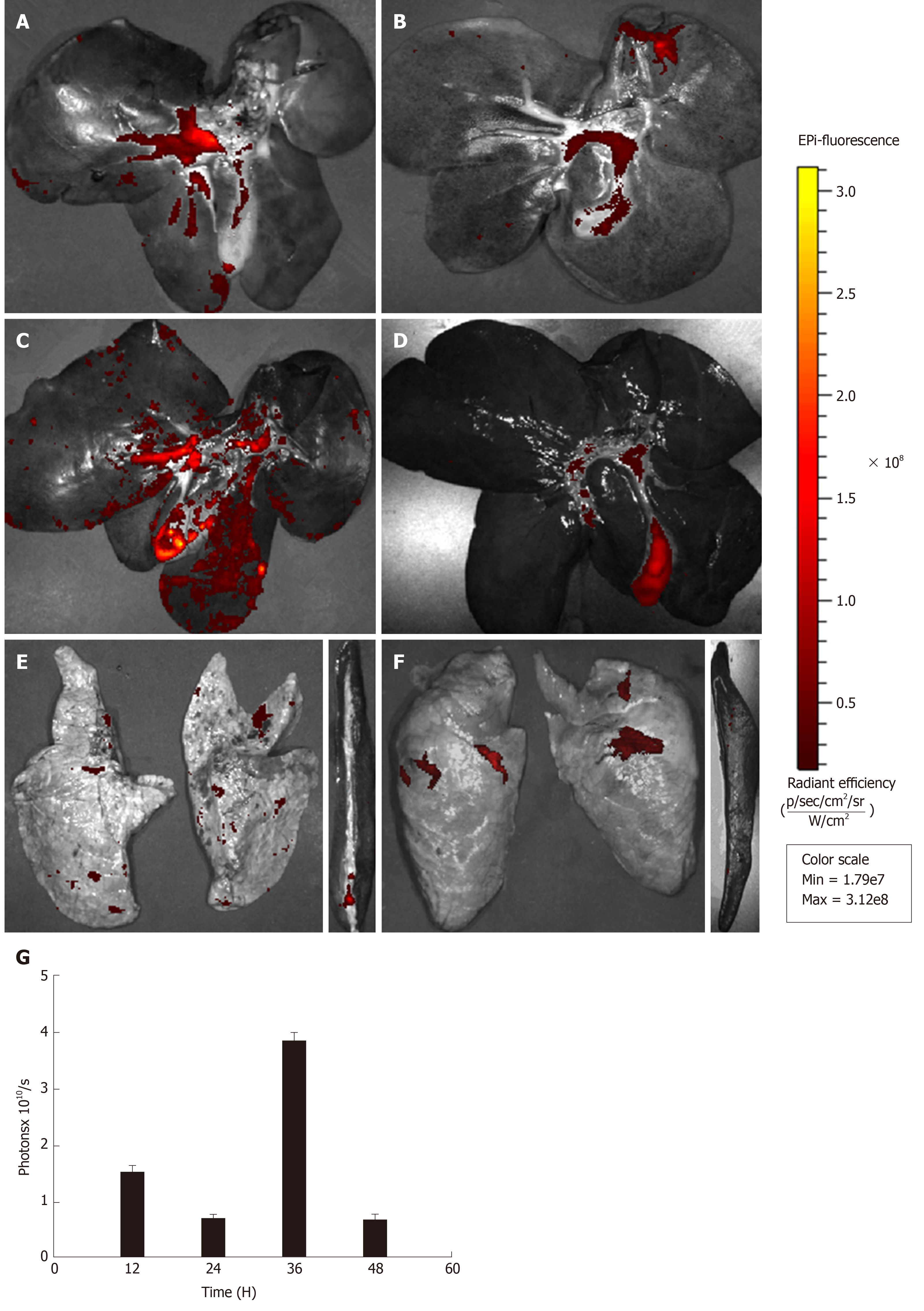
For dynamic tracing and imaging studies, immediately after the animals were sacrificed by propofol overdose, three major organs (the liver, spleen, and lungs) were isolated and placed into the In vivo Imaging System (IVIS) chamber (PerkinElmer, United States) and images were acquired using a CCD camera (Ex = 580 nm, Em = 535 nm, Exposure time = 5 s) at 12 h after transplantation, and then at 24 h, 36 h, and 48 h. The regions of interest (ROI) were drawn to quantify the cell uptake in different organs by calculating the total photon emission efficiency.
The mental status, appetite, and activity of the animals during the experiment were closely observed. Blood samples were collected using a jugular vein catheter after D-gal administration and every 12 h thereafter. At the predetermined time points, 2 mL whole blood was sampled in coagulation tubes, and prothrombin time (PT) was measured in the emergency laboratory of the First Affiliated Hospital of Zhejiang University. Another set of blood samples (3 mL whole blood) was centrifuged in BD Vacutainer SST tubes to collect serum for biochemical analysis with an automated biochemical analyzer (Abbott Aeroset, United States). Tissue samples of liver were collected to assess the effect of MenSC treatment on liver apoptosis and necrosis. At the set time points, following the imaging of the animals, sections of liver were preserved in 4% buffered formalin for 24 h, and then embedded in paraffin for haematoxylin and eosin (H&E) staining. Apoptosis of hepatocytes was assessed by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay (Roche, Germany).
All data were analyzed using SPSS for Windows version 19.0 (SPSS, Chicago, IL, United States) and GraphPad Prism 5 (GraphPad, San Diego, CA, United States). Continuous variables were compared with Student’s t test and expressed as the mean ± SE. The survival of animals was analyzed by a Kaplan-Meier graph and compared by log-rank tests. Statistical significance was taken as P < 0.05. A statistical review of the study was performed by a biomedical statistician.
Plastic-adherent MenSCs were successfully isolated from menstrual blood and expanded rapidly with a short doubling time of 24 - 36 h. They presented with a fibroblast-like spindle shape, formed a monolayer and grew adhering to the wall in a spiral or radial arrangement. The phenotypic analysis by flow cytometry showed that the third and fifth passages of MenSCs were positive for CD9, CD29, CD73, CD90, CD105, CD44, and CD59, but were negative for haematopoietic stem cell markers, such as CD34, CD45, and CD117, as well as the immune activation marker HLA-DR (Figure 2). These findings were consistent with the general characteristics of classic MenSCs.
The PKH26 labeled cell members of MenSCs emitted strong red fluorescence with a clear cell configuration under a confocal microscope, and nuclei were stained with DAPI. The labeling rate of PKH26 was more than 95%. After serial passages, the fluorescent signal intensity from the PKH26-MenSCs decreased with time but could also be detected (Figure 3).
PKH26-MenSCs presented with long, spindle-shaped, fibroblast-like adherent growth, and no remarkable morphological changes were found, which indicated that the labelling procedure did not impair cytomorphology. To assess the effect of PKH26 on the viability of MenSCs, CCK-8 assays were conducted and a cell growth curve generated. No significant difference between the PKH26-MenSCS and unlabeled MenSCs was observed on d 1 to d 7 (n = 6, P > 0.05), confirming that PKH26 dye had no effect on cell viability. After 3 wk of induction of adipogenic and osteogenic differentiation in vitro, bone nodules formed by osteogenically differentiated PKH26-MenSCs were visualized by alizarin red S staining. Adipogenic differentiation was identified by oil red O staining. Differentiated PKH26-MenSCs produced intracytoplasmic lipid droplets and stained positive (Figure 4). These results indicated that PKH26 had no adverse effect on the multipotential differentiation ability.
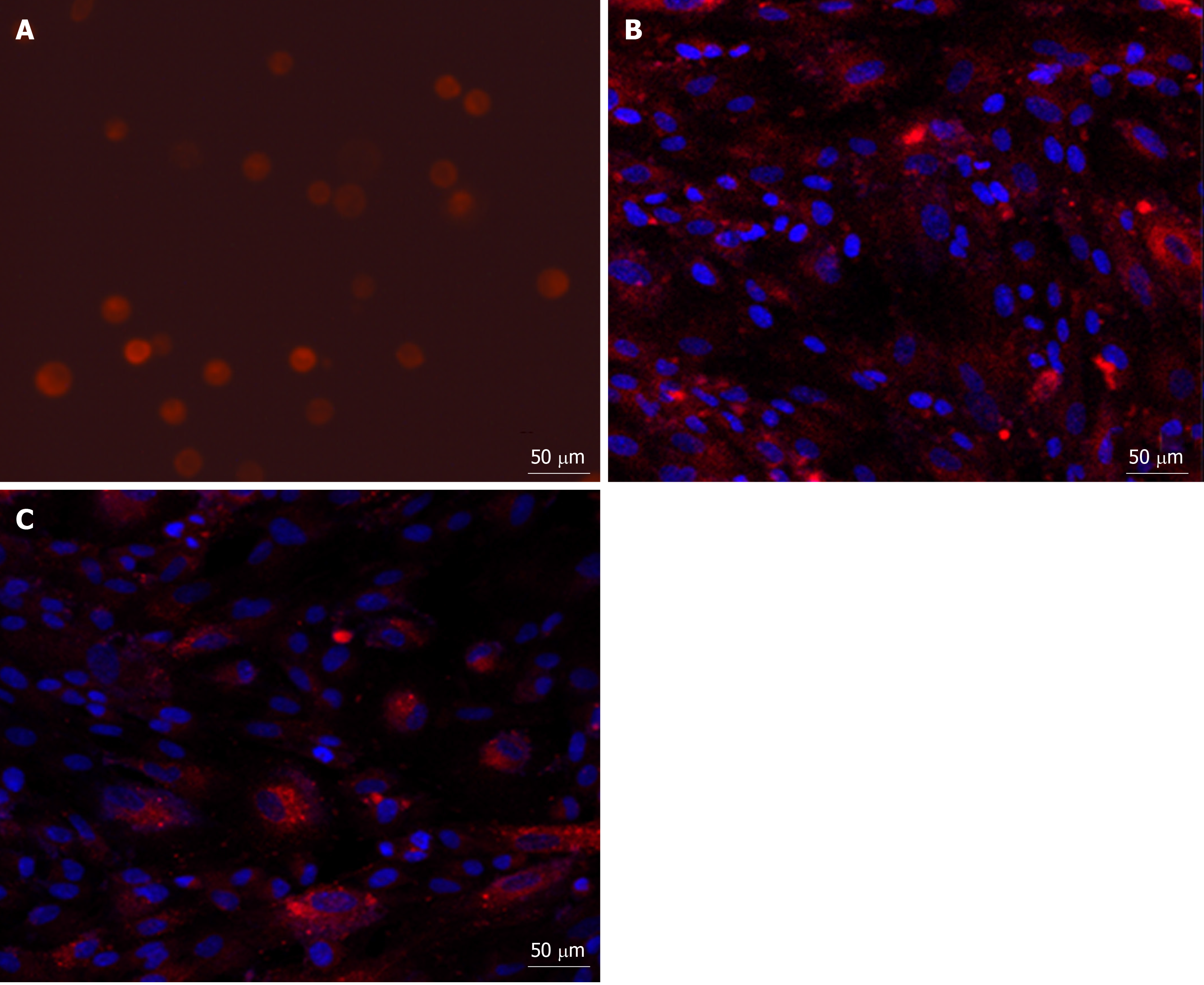
After MenSC transplantation, no serious adverse reaction was observed. Three major organs (the liver, spleen, and lungs), in which almost all transplanted MenSCs were predicted to be retained, were extracted from the animals at the predetermined time points and then imaged in the IVIS. The ex vivo imaging showed that most of the strong red fluorescence emitted from PKH26-MenSCs was distributed in the hepatic periportal area, and a small part was accumulated in the centrilobular regions. A weak signal was also detected in the lungs, and little signal was observed in the spleen (Figure 5A-F). At 12 h after transplantation, the ROI analysis revealed that the number of photons emitted from the liver was 1.55 × 1010 ± 2.97 × 108 p/s, and dropped to 7.74 × 109 ± 1.23 × 108 p/s at 24 h (P < 0.001). However, the signal intensity of the liver significantly increased to 3.65 × 1010 ± 1.38 × 108 p/s at 36 h (P < 0.001), and attenuated at 48 h after transplantation (Figure 5G).
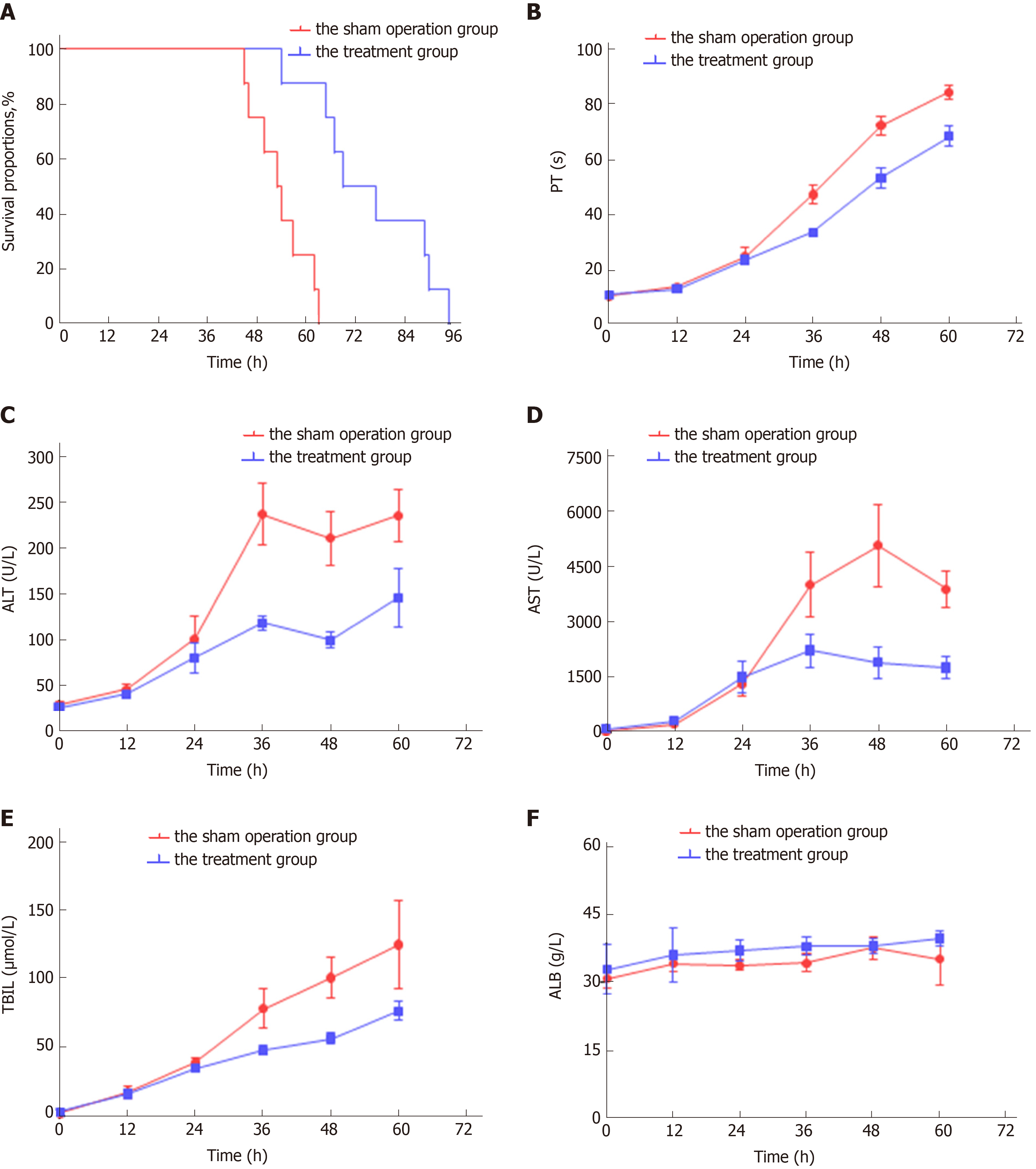
Portal vein puncture was well-tolerated in all experimental animals. Compared with the sham operation group, symptoms of hepatic encephalopathy, such as anorexia, dysphoria and limb tremors, in animals with MenSC treatment occurred obviously later. In the sham operation group, none of the animals survived more than 63 h (survival time, 53.75 ± 2.37 h). After the transplantation of 2.5 × 106/kg MenSCs via the intrahepatic portal vein in the treatment group, the survival time was significantly prolonged ( 75.75 ± 5.11 h, log rank, P < 0.001 ) (Figure 6A).
Continuous progression of ALF was observed in the sham operation group, manifesting as a dynamical increase in PT and the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (TBIL). The liver function of ALF animals without MenSC treatment deteriorated most rapidly at 24 h to 36 h after D-gal administration, and the phenomenon of enzyme-jaundice separation appeared at 48 h, indicating a wide range of hepatocyte necrosis. In the treatment group, biochemical parameters and coagulation function were similarly worsened as early as 24 h after ALF induction (P > 0.05). After 24 h, the deterioration trend was comparatively slowing down. The levels of ALT, AST, TBIL and PT measured at selected time points 24 h after transplantation were significantly lower than those in animals in the sham operation group (P < 0.05). No significant changes occurred in the serum albumin level in either group. Compared with the sham operation group, MenSC transplanted via the portal vein significantly improved the liver function of D-gal-induced ALF (Figure 6B-C).
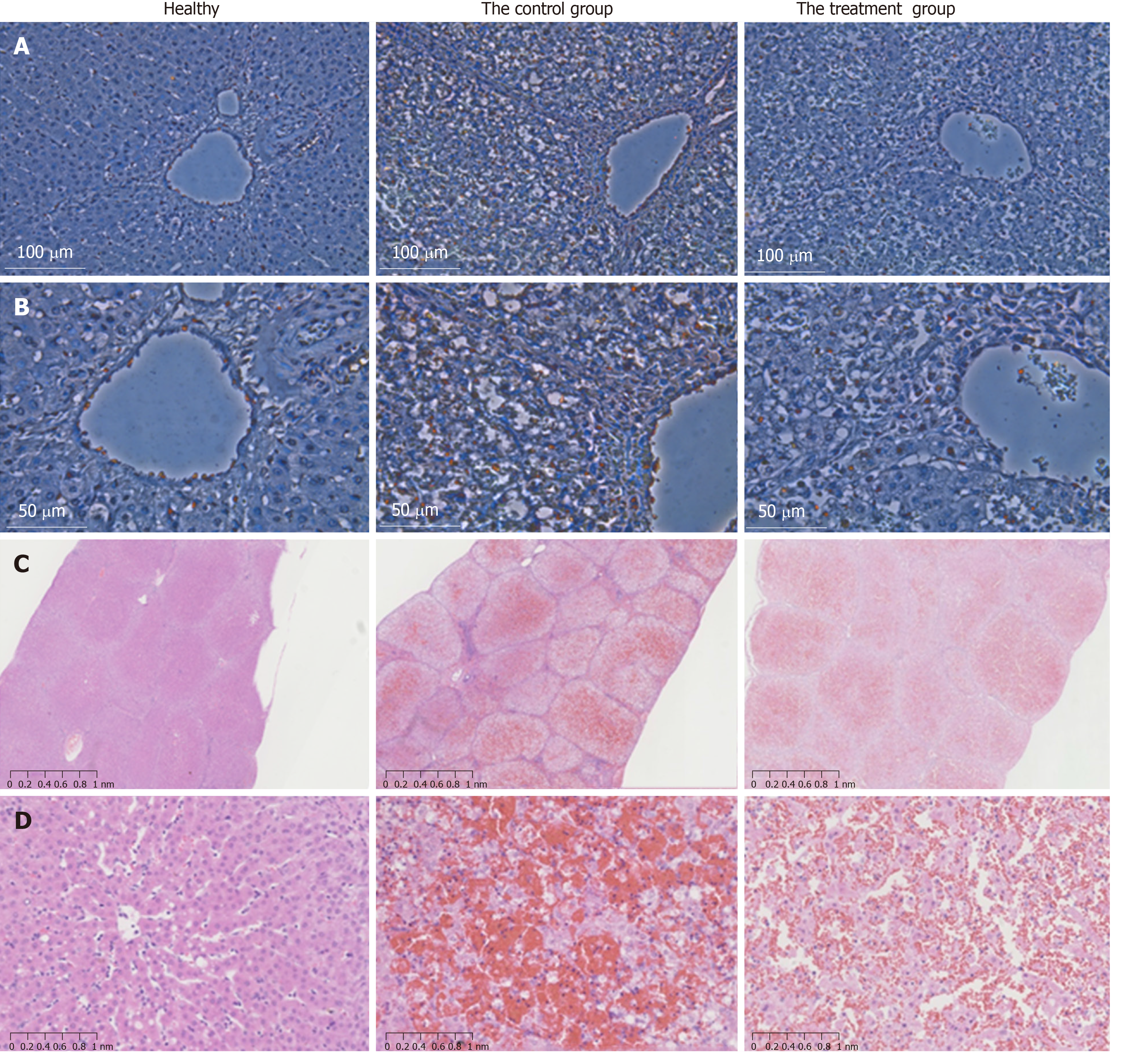
Liver tissues were collected for H&E staining to examine hepatic pathological changes. ALF was confirmed by the observation of massive necrosis, large areas of hemorrhage and inflammatory infiltration, hepatocyte apoptosis and vacuolar degeneration at 48 h after ALF induction in the liver tissues of the control group. Hepatic lobular structure was completely collapsed. In contrast, the liver pathological tissue in the MenSC treatment group showed obviously increased numbers of remaining hepatocytes and hepatic parenchymal cells and a comparatively slight necrotic degree and area. Positively labeled apoptotic hepatocytes were observed by TUNEL in the experimental groups at 36 h. However, the number of apoptotic cells was significantly lower in the post-mortem liver tissues of the MenSC treatment group than in the control group (Figure 7).
MSC transplantation has opened new frontiers in regenerative medicine with the possibility of repairing damaged tissues. There has been an increasing need for an ideal cell population that provides a high proliferation rate, easy access and sufficient doses of lower-passage MSCs. As a refreshing, non-invasive, and plentiful source of stem cells, MenSCs have shown promising effects in several pre-clinical models[21,22]. However, the therapeutic effect of MenSCs in ALF has not been well studied, although it is significant. Therefore, in this study, we investigated the efficacy of intraportally transplanted MenSCs in treating pigs with ALF, and a fluorescent dye (PKH26) was used to evaluate their homing ability in the injured liver and to dynamically monitor the biological distribution of infused stem cells.
Choosing an appropriate animal model is vital in estimating the efficacy of cell transplantation. In our study, D-gal was used to establish the ALF porcine model because the drug possesses a specific hepatotoxicity, controllable dose, and potential reversibility, and has been widely used for large animal studies[19,23]. Obviously, the pig model was more acceptable and suitable for further clinical research. The dose of D-gal for ALF induction was an issue of crucial importance. After D-gal (1.0 g/kg) administration, the biochemical indices of the sham operation group showed a stable toxic effect. The most rapid deterioration period of liver function and coagulation was established 24 - 36 h after modeling, and enzyme-jaundice separation appeared at 48 h, indicating a mass of hepatocyte necrosis. However, in the low-dose groups (< 1.0 g/kg), there was a high disparity in survival time, indicating that the ALF progressed differently in animals. The lower dose may not satisfy the process of ALF. In addition, it is a stable and exact ALF large animal model established by our laboratory, and some significant work has been published based on the ALF model[20,24]. Therefore, the D-gal-induced ALF porcine model in our study was supposed to be reliable and steady. The selection of transplantation methods is also vital. Several studies have demonstrated the feasibility and superiority of intraportal transplantation for cell survival compared to the peripheral vein route[24,25]. Thus, we used this approach to transplant MenSCs into the animals.
Our study explored the clinical potential of MenSCs in the treatment of ALF. The result revealed that the survival time was significantly prolonged, and the levels of ALT, AST, TBIL and PT were obviously decreased in the treatment group, which was also confirmed by the pathological improvements in pigs with MenSC transplant. These findings indicated that the immediate transplantation of MenSCs via the portal vein effectively improved liver function and coagulation, alleviated the progression of liver injury, and prolonged survival time. As a most commonly used type of MSC for cell therapy, human bone marrow mesenchymal stem cell (BMMSC) transplantation has been proven effective in preventing death from ALF in a porcine model[25]. Compared to bone marrow mesenchymal stem cells (BMMSCs), MenSCs presented a higher expression of ICM-1 and IL-8, which indicates that MenSCs may have superiority in anti-inflammatory actions[10,21]. Additionally, it was also observed that MenSCs proliferated faster and possessed a higher capacity of homing to injured sites than BMMSCs[26]. Nevertheless, whether MenSCs can ensure the long-term survival of individuals with ALF needs to be further studied. MenSC therapy may serve as a bridge to liver transplantation in ALF patients. Therefore, to achieve the optimal effectiveness of MenSC therapy, cell preconditioning, the timing of cell grafting, the therapeutic dose, and the delivery route need to be further investigated.
To date, near-infrared dyes have been utilized for dynamically tracing stem cells in vivo with improved signal detection capacity[27-30]. To achieve real-time monitoring of infused stem cells, we labeled the cells with PKH26. The advantage of the labeling method in our study mainly lies in its simplicity and high efficiency, with no need for additional genetic manipulation. Some studies have demonstrated the feasibility of PKH26 for the in vivo imaging of grafted stem cells in rat liver[31,32]. Therefore, PKH26 is a suitable cell dye to be used for tracing. The IVIS imaging revealed that the transplanted MenSCs were initially clearly retained in the liver and then diffused through the systemic circulation, leading to a decrease in the signal at 24 h after transplantation. Interestingly, the ability of MenSCs to home to pathological hepatic environments was confirmed by the notably increased signal intensity of the ROI in the liver at 36 h, which corresponded to the biochemical result that liver function deteriorated most rapidly at 24 - 36 h, supporting the concept that the damaged liver may release some chemotactic factors to mobilize stem cells migration to the injured position[33,34]. The homing mechanisms of MenSCs in the pathological hepatic environments have remained unclear and need to be explored. So far, MenSCs have been reported to function via antiapoptotic effects[35], anti-inflammatory effects[36], immunomodulation[37], angiogenesis[14], and the mobilization of endogenous stem cells[38]. Some groups have demonstrated that the mechanisms of direct differentiation and cell replacement could not explain the function compensation[39,40]. The paracrine effects of MenSCs may play a pivotal role in tissue regeneration. Additionally, cell fusion also contributes to the therapeutic effect[37]. To better translate from bench to bedside, we should furnish deeper insights into the therapeutic mechanism and optimize stem-cell-based therapies.
ALF is a life-threatening liver disease with an extremely high mortality due to lack of effective treatment methods. However, few preclinical studies have reported the therapeutic effect of MenSCs in treating ALF in large animal models, although MenSCs have shown promising properties in tissue regeneration. Hence, our research provided feasibility and preliminary basis for future exploration and clinical application. Since the clinical trials of MenSCs are a drop in the bucket, there is still a long way to go before they are applied in clinical practice and translate to the medicine. We need to take into account their in-vivo survival time, long-term safety, standard collection procedure, and heterogeneity derived from diverse donors before MenSC transplantation becomes a clinical strategy for treating ALF[41].
The current study has some limitations. First, we established the ALF porcine model by D-gal. The model focused on drug-induced liver failure and cannot reflect the diversity of causes for liver failure in human patients. Furthermore, the survival time in the animals was short in our study, so we did not carry out further experiments to confirm the in-vivo differentiation of MenSCs. The therapeutic dose, the quality of MenSCs, the delivery route and the timing of grafting may lead to the result.
In summary, the effectiveness and dynamic biodistribution of immediate intraportal transplantation of MenSCs were investigated in a large animal model of ALF for the first time. These findings suggest that MenSC transplantation may be considered an alternative approach for treating ALF.
Acute liver failure (ALF) is a rapidly progressing liver disorder with extremely poor survival prognosis due to lack of effective treatment methods. In recent years, stem cell-based therapy has emerged as a new hope for revolutionizing the treatment of ALF. Thus, seeking for an ideal cell line for transplantation is of great importance.
Menstrual blood stem cells (MenSCs) are a group of easily accessible mesenchymal stem cells with advantages of a high level of immune privilege, robust replicative capacity and multipotential differentiation. However, little is known about the therapeutic effect of MenSCs in treating ALF in large animal models. MenSC transplantation could be a promising strategy for treating ALF.
The aim of this study was to investigate the efficacy of MenSCs for treating ALF in pigs and to dynamically trace the biodistribution of transplanted cells.
MenSCs were labelled in vitro with the fluorescent dye PKH26. Phenotypic analysis of MenSCs was performed using a flow cytometer. Cell viability was assessed by CCK-8. Cell multipotential differentiation was assessed by osteogentic and adipogenic differentiation. ALF porcine model was induced with D-galactosamine at a dose of 1.0 g/kg. The treatment group received transplantation of PKH26-labelled MenSCs via the portal vein under B-ultrasound guidance. The liver, lungs and spleen of sacrificed animals were imaged with the In vivo Imaging System (IVIS) using a CCD camera.
The labelling procedure did not affect the morphology, viability or multipotential differentiation of MenSCs. The survival time was significantly prolonged, and the levels of ALT, AST, TBIL and PT were obviously decreased in the treatment group compared with the control group. The liver pathological tissue in MenSC treatment group showed obviously increased numbers of remaining hepatocytes and a comparatively slight necrotic degree and area. The IVIS imaging revealed that PKH26-positive MenSCs were clearly retained in the liver initially and then diffused through the systemic circulation. The homing ability of MenSCs was confirmed by the markedly increased signal intensity in the liver at 36 h after transplantation. However, to achieve the optimal effectiveness of MenSC therapy, the therapeutic dose, cell preconditioning, the timing of cell grafting and the delivery route need to be further investigated.
This study showed that the immediate transplantation of MenSCs via the portal vein effectively improved liver function and coagulation, alleviated the progression of liver injury, and prolonged survival time. The study also demonstrated the ability of MenSCs to home to pathological hepatic environments after transplantation.
The therapeutic effect and homing ability of intraportally transplanted MenSCs in a porcine ALF model have been confirmed. MenSC transplantation may be a promising strategy for treating ALF.
| 1. | Cardoso FS, Marcelino P, Bagulho L, Karvellas CJ. Acute liver failure: An up-to-date approach. J Crit Care. 2017;39:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Peng L, Xie DY, Lin BL, Liu J, Zhu HP, Xie C, Zheng YB, Gao ZL. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 2011;54:820-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 280] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 3. | Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1005] [Cited by in RCA: 902] [Article Influence: 39.2] [Reference Citation Analysis (1)] |
| 4. | Forbes SJ, Newsome PN. New horizons for stem cell therapy in liver disease. J Hepatol. 2012;56:496-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Hu C, Li L. In Vitro and In Vivo Hepatic Differentiation of Adult Somatic Stem Cells and Extraembryonic Stem Cells for Treating End Stage Liver Diseases. Stem Cells Int. 2015;2015:871972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Meng X, Ichim TE, Zhong J, Rogers A, Yin Z, Jackson J, Wang H, Ge W, Bogin V, Chan KW, Thébaud B, Riordan NH. Endometrial regenerative cells: a novel stem cell population. J Transl Med. 2007;5:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 387] [Cited by in RCA: 434] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 7. | Bockeria L, Bogin V, Bockeria O, Le T, Alekyan B, Woods EJ, Brown AA, Ichim TE, Patel AN. Endometrial regenerative cells for treatment of heart failure: a new stem cell enters the clinic. J Transl Med. 2013;11:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Gargett CE, Schwab KE, Zillwood RM, Nguyen HP, Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80:1136-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 368] [Article Influence: 21.6] [Reference Citation Analysis (1)] |
| 9. | Mutlu L, Hufnagel D, Taylor HS. The endometrium as a source of mesenchymal stem cells for regenerative medicine. Biol Reprod. 2015;92:138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Verdi J, Tan A, Shoae-Hassani A, Seifalian AM. Endometrial stem cells in regenerative medicine. J Biol Eng. 2014;8:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Chen L, Zhang C, Chen L, Wang X, Xiang B, Wu X, Guo Y, Mou X, Yuan L, Chen B, Wang J, Xiang C. Human Menstrual Blood-Derived Stem Cells Ameliorate Liver Fibrosis in Mice by Targeting Hepatic Stellate Cells via Paracrine Mediators. Stem Cells Transl Med. 2017;6:272-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 12. | Cui CH, Uyama T, Miyado K, Terai M, Kyo S, Kiyono T, Umezawa A. Menstrual blood-derived cells confer human dystrophin expression in the murine model of Duchenne muscular dystrophy via cell fusion and myogenic transdifferentiation. Mol Biol Cell. 2007;18:1586-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 13. | Borlongan CV, Kaneko Y, Maki M, Yu SJ, Ali M, Allickson JG, Sanberg CD, Kuzmin-Nichols N, Sanberg PR. Menstrual blood cells display stem cell-like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem Cells Dev. 2010;19:439-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 158] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 14. | Han X, Meng X, Yin Z, Rogers A, Zhong J, Rillema P, Jackson JA, Ichim TE, Minev B, Carrier E, Patel AN, Murphy MP, Min WP, Riordan NH. Inhibition of intracranial glioma growth by endometrial regenerative cells. Cell Cycle. 2009;8:606-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Fathi-Kazerooni M, Tavoosidana G, Taghizadeh-Jahed M, Khanjani S, Golshahi H, Gargett CE, Edalatkhah H, Kazemnejad S. Comparative restoration of acute liver failure by menstrual blood stem cells compared with bone marrow stem cells in mice model. Cytotherapy. 2017;19:1474-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Rosen AB, Kelly DJ, Schuldt AJ, Lu J, Potapova IA, Doronin SV, Robichaud KJ, Robinson RB, Rosen MR, Brink PR, Gaudette GR, Cohen IS. Finding fluorescent needles in the cardiac haystack: tracking human mesenchymal stem cells labeled with quantum dots for quantitative in vivo three-dimensional fluorescence analysis. Stem Cells. 2007;25:2128-2138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 147] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 17. | Shao-Fang Z, Hong-Tian Z, Zhi-Nian Z, Yuan-Li H. PKH26 as a fluorescent label for live human umbilical mesenchymal stem cells. In Vitro Cell Dev Biol Anim. 2011;47:516-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Mou XZ, Lin J, Chen JY, Li YF, Wu XX, Xiang BY, Li CY, Ma JM, Xiang C. Menstrual blood-derived mesenchymal stem cells differentiate into functional hepatocyte-like cells. J Zhejiang Univ Sci B. 2013;14:961-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Li LJ, Du WB, Zhang YM, Li J, Pan XP, Chen JJ, Cao HC, Chen Y, Chen YM. Evaluation of a bioartificial liver based on a nonwoven fabric bioreactor with porcine hepatocytes in pigs. J Hepatol. 2006;44:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Zhou N, Li J, Zhang Y, Lu J, Chen E, Du W, Wang J, Pan X, Zhu D, Yang Y, Chen Y, Cao H, Li L. Efficacy of coupled low-volume plasma exchange with plasma filtration adsorption in treating pigs with acute liver failure: A randomised study. J Hepatol. 2015;63:378-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Ulrich D, Muralitharan R, Gargett CE. Toward the use of endometrial and menstrual blood mesenchymal stem cells for cell-based therapies. Expert Opin Biol Ther. 2013;13:1387-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Rodrigues MC, Lippert T, Nguyen H, Kaelber S, Sanberg PR, Borlongan CV. Menstrual Blood-Derived Stem Cells: In Vitro and In Vivo Characterization of Functional Effects. Adv Exp Med Biol. 2016;951:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 23. | Lv G, Zhao L, Zhang A, Du W, Chen Y, Yu C, Pan X, Zhang Y, Song T, Xu J, Chen Y, Li L. Bioartificial liver system based on choanoid fluidized bed bioreactor improve the survival time of fulminant hepatic failure pigs. Biotechnol Bioeng. 2011;108:2229-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Cao H, Yang J, Yu J, Pan Q, Li J, Zhou P, Li Y, Pan X, Li J, Wang Y, Li L. Therapeutic potential of transplanted placental mesenchymal stem cells in treating Chinese miniature pigs with acute liver failure. BMC Med. 2012;10:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Li J, Zhang L, Xin J, Jiang L, Li J, Zhang T, Jin L, Li J, Zhou P, Hao S, Cao H, Li L. Immediate intraportal transplantation of human bone marrow mesenchymal stem cells prevents death from fulminant hepatic failure in pigs. Hepatology. 2012;56:1044-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Luz-Crawford P, Torres MJ, Noël D, Fernandez A, Toupet K, Alcayaga-Miranda F, Tejedor G, Jorgensen C, Illanes SE, Figueroa FE, Djouad F, Khoury M. The immunosuppressive signature of menstrual blood mesenchymal stem cells entails opposite effects on experimental arthritis and graft versus host diseases. Stem Cells. 2016;34:456-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Ma HC, Shi XL, Ren HZ, Yuan XW, Ding YT. Targeted migration of mesenchymal stem cells modified with CXCR4 to acute failing liver improves liver regeneration. World J Gastroenterol. 2014;20:14884-14894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Ezzat T, Dhar DK, Malago M, Olde Damink SW. Dynamic tracking of stem cells in an acute liver failure model. World J Gastroenterol. 2012;18:507-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Jiang H, Cheng Z, Tian M, Zhang H. In vivo imaging of embryonic stem cell therapy. Eur J Nucl Med Mol Imaging. 2011;38:774-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Chen J, Wang C, Lü S, Wu J, Guo X, Duan C, Dong L, Song Y, Zhang J, Jing D, Wu L, Ding J, Li D. In vivo chondrogenesis of adult bone-marrow-derived autologous mesenchymal stem cells. Cell Tissue Res. 2005;319:429-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 31. | Ikeda E, Yagi K, Kojima M, Yagyuu T, Ohshima A, Sobajima S, Tadokoro M, Katsube Y, Isoda K, Kondoh M, Kawase M, Go MJ, Adachi H, Yokota Y, Kirita T, Ohgushi H. Multipotent cells from the human third molar: feasibility of cell-based therapy for liver disease. Differentiation. 2008;76:495-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 32. | Zhan Y, Wang Y, Wei L, Chen H, Cong X, Fei R, Gao Y, Liu F. Differentiation of hematopoietic stem cells into hepatocytes in liver fibrosis in rats. Transplant Proc. 2006;38:3082-3085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N, Yarmush ML. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 2010;19:667-679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 539] [Cited by in RCA: 538] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 34. | Cho JW, Lee CY, Ko Y. Therapeutic potential of mesenchymal stem cells overexpressing human forkhead box A2 gene in the regeneration of damaged liver tissues. J Gastroenterol Hepatol. 2012;27:1362-1370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Chen L, Xiang B, Wang X, Xiang C. Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure. Stem Cell Res Ther. 2017;8:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 160] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 36. | Xiang B, Chen L, Wang X, Zhao Y, Wang Y, Xiang C. Transplantation of Menstrual Blood-Derived Mesenchymal Stem Cells Promotes the Repair of LPS-Induced Acute Lung Injury. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 37. | Lv Y, Xu X, Zhang B, Zhou G, Li H, Du C, Han H, Wang H. Endometrial regenerative cells as a novel cell therapy attenuate experimental colitis in mice. J Transl Med. 2014;12:344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Drago H, Marín GH, Sturla F, Roque G, Mártire K, Díaz Aquino V, Lamonega R, Gardiner C, Ichim T, Riordan N, Raimondi JC, Bossi S, Samadikuchaksaraei A, van Leeuwen M, Tau JM, Núñez L, Larsen G, Spretz R, Mansilla E. The next generation of burns treatment: intelligent films and matrix, controlled enzymatic debridement, and adult stem cells. Transplant Proc. 2010;42:345-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Shi D, Zhang J, Zhou Q, Xin J, Jiang J, Jiang L, Wu T, Li J, Ding W, Li J, Sun S, Li J, Zhou N, Zhang L, Jin L, Hao S, Chen P, Cao H, Li M, Li L, Chen X, Li J. Quantitative evaluation of human bone mesenchymal stem cells rescuing fulminant hepatic failure in pigs. Gut. 2017;66:955-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 40. | Wu X, Luo Y, Chen J, Pan R, Xiang B, Du X, Xiang L, Shao J, Xiang C. Transplantation of human menstrual blood progenitor cells improves hyperglycemia by promoting endogenous progenitor differentiation in type 1 diabetic mice. Stem Cells Dev. 2014;23:1245-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 41. | Chen L, Qu J, Xiang C. The multi-functional roles of menstrual blood-derived stem cells in regenerative medicine. Stem Cell Res Ther. 2019;10:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 350] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nacif LS, Shimizu Y S-Editor: Wang J L-Editor: Ma JY E-Editor: Ma YJ