Published online Jan 28, 2019. doi: 10.3748/wjg.v25.i4.447
Peer-review started: November 12, 2018
First decision: December 20, 2018
Revised: December 23, 2018
Accepted: December 27, 2018
Article in press: December 28, 2018
Published online: January 28, 2019
Processing time: 79 Days and 13.7 Hours
Colonoscopy is considered a valid primary screening tool for colorectal cancer (CRC). The decreasing risk of CRC observed in patients undergoing colonoscopy is correlated with the adenoma detection rate (ADR). Due to the fact that screening programs usually start from the age of 50, very few data are available on the risk of adenoma between 40 and 49 years. However, the incidence of CRC is increasing in young populations and it is not uncommon in routine practice to detect adenomas or even advanced neoplasia during colonoscopy in patients under 50 years.
To compare the ADR and advanced neoplasia detection rate (ANDR) according to age in a large series of patients during routine colonoscopy.
All consecutive patients who were scheduled for colonoscopy were included. Exclusion criteria were as follows: patients scheduled for partial colonoscopy or interventional colonoscopy (for stent insertion or stenosis dilation). Colonoscopies were performed in our unit by a team of 30 gastroenterologists in 2016. We determined the ADR and ANDR in each age group in the whole population and in the population with an average risk of CRC (excluding patients with personal or family history of advanced adenoma or cancer).
6027 colonoscopies were performed in patients with a median age of 57 years (range, 15-96). The ADR and ANDR were 28.6% and 9.7%, respectively, in the whole population. When comparing patients aged 40-44 (n = 382) and 45-49 years (n = 515), a strong increase in all parameters from 45 years was observed, with the ADR rising from 9.7% in patients aged 40-44 to 21.2% between 45 and 49 (P < 0.001) and the ANDR increasing from 3.1% in patients aged 40-44 to 6.4% in those aged 45-49 years (P < 0.03). With regard to patients aged 50-54 (n = 849), a statistically significant increase in the ADR and ANDR was not observed between patients aged 45-49 and those aged 50-54 years. In the population with an average risk of CRC, the ADR and ANDR were still significantly higher in patients aged 45-49 compared with those aged 40-44 years.
This study shows a significant two-fold increase in the ADR and ANDR in patients aged 45 years and over.
Core tip: Despite the fact that the incidence of colorectal cancer (CRC) in individuals less than 50 years seems to have increased in the last decade, there are very few data on adenoma and advanced neoplasia in this age group. This is the first large study to evaluate the adenoma detection rate (ADR) and advanced neoplasia detection rate (ANDR) in patients under 50 years during routine colonoscopy in average-risk and high-risk CRC patients. This study showed a significant two-fold increase in the ADR and ANDR in patients aged 45 years and older, irrespective of a personal or family history of polyps or cancer. Such high rates in those aged 45 years and over should be taken into account in CRC screening campaigns.
- Citation: Karsenti D, Tharsis G, Burtin P, Venezia F, Tordjman G, Gillet A, Samama J, Nahon-Uzan K, Cattan P, Cavicchi M. Adenoma and advanced neoplasia detection rates increase from 45 years of age. World J Gastroenterol 2019; 25(4): 447-456
- URL: https://www.wjgnet.com/1007-9327/full/v25/i4/447.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i4.447
Colorectal cancer (CRC) is one of the most frequent cancers worldwide, and the second most common cause of cancer-related deaths[1,2]. It is now well established that screening programs can reduce CRC mortality through the detection of both precancerous lesions and early-stage cancer[3-8]. Different screening modalities are available, ranging from stool-based tests (guaiac test, immunochemical test or DNA assays) to endoscopy with varying sensitivity and specificity[9,10]. The choice of screening method usually depends on the national screening program policy. Irrespective of the method used, most scientific organizations recommend beginning screening at 50 years of age in average-risk populations[11]. Colonoscopy is considered a valid primary screening tool for CRC when performed every 10 years, usually from the age of 50 years[11,12]. Optimizing the quality of screening colonoscopy is necessary to improve CRC prevention[13]. One of the best indicators of the quality of colonoscopy is the adenoma detection rate (ADR) which is correlated with the polyp detection rate and the mean number of adenomas per colonoscopy[14-16].
It is worth discussing the age at which screening is initiated. It is not uncommon in routine practice to detect adenomas or advanced neoplasia during colonoscopy in patients aged less than 50 years. Moreover, the incidence of CRC is increasing in young populations (particularly in the United States)[17], and non-negligible rates of colonic adenomas and advanced neoplasia (13.3% and 3.4%, respectively) have already been reported in patients aged 40 to 49 with a family history of cancer[18]. However, due to the fact that screening programs frequently start from the age of 50, few recent data on the risk of adenoma in patients aged 40-44 and 45-49 years are available. The only way to estimate the incidence of adenomas is to determine the risk in a population referred for colonoscopy for indications other than screening. The aim of the current study was to determine, in routine practice, the adenoma detection rate (ADR) and advanced neoplasia detection rate (ANDR) according to age in a large population of consecutive patients admitted to our digestive endoscopy unit for colonoscopy over a period of one year.
This observational monocentric study was conducted in our unit from January 1, 2016 to December 31, 2016, by a team of 30 gastroenterologists. All patients were informed in writing of the use of their endoscopic procedure data for clinical research purposes and none expressed opposition. The data were retrospectively collected by extraction from our medical patient management software. Therefore, in accordance with French ethics law, this retrospective study did not require approval from an ethics committee. All authors declare that they have access to the study data and have reviewed and approved the final manuscript.
All consecutive patients who were scheduled for colonoscopy were included. Exclusion criteria were as follows: patients scheduled for partial colonoscopy or interventional colonoscopy (for stent insertion, stenosis dilation or hemostasis).
The following data were collected using dedicated software: Age, gender, indication for colonoscopy, preparation procedure and quality of preparation [assessed by the Boston Bowel Preparation Scale (BBPS)][19,20], cecal intubation, withdrawal time, number and size of polyps (< 1 cm or ≥ 1 cm) and polyp histopathology. Personal history of adenoma/cancer was defined as: a patient in whom a previous colonoscopy had found at least one adenoma or who was previously diagnosed with CRC. Family history of adenoma or cancer was defined as: a patient with at least one first-degree relative diagnosed with CRC, a patient with at least two second-degree relatives diagnosed with CRC or a patient with at least one first-degree relative with adenoma irrespective of the age of the relative. Patients with personal or family history of adenoma or cancer were considered high-risk patients for CRC while patients with other indications were considered average-risk patients for CRC. We determined the ADR (percentage of colonoscopies with at least one adenoma) and the ANDR (percentage of colonoscopies with at least one advanced neoplastic lesion as defined below).
The videocolonoscopes used were EVIS EXERA III CF-H190 (Olympus Co.) and more rarely EC-690 WM, and EC-600WM (Fujifilm Co.). Good preparation was defined as a BBPS score ≥ 6 with no sub-score < 2[14]. Withdrawal time was determined from the cecum to the anal verge, expressed in seconds and calculated on colonoscopies with no polyps.
An adenoma was defined as a tubular or tubulo-villous adenoma. Serrated polyps (SP) were defined as hyperplastic polyps, sessile serrated adenomas/polyps and traditional serrated adenomas. Hyperplastic polyps of the rectum and sigmoid colon were excluded, as they are not considered a risk for CRC[21]. Advanced neoplasia were defined as grade 4 or grade 5 of the Vienna classification (grade 4 corresponding to a non-invasive high-grade neoplasia, i.e., high-grade adenoma/dysplasia, non-invasive carcinoma and suspicion of invasive carcinoma; grade 5 corresponding to an invasive neoplasia, i.e., intramucosal carcinoma, submucosal carcinoma or beyond) or a polyp 1 cm or greater[22].
The ADR and ANDR were analyzed in each age group in the whole population and in the population with an average risk of CRC (excluding patients with personal or family history of advanced adenoma or cancer). The NCSS v 10.0 was used to perform the statistical analysis. Quantitative variables were expressed as mean (SD) or as median and interquartile range (IQR). Qualitative variables were expressed as numbers and percentages. Continuous variables were compared using a Student’s t test or Wilcoxon-Mann-Whitney U test, as required. Categorical variables were compared using the chi-squared test or Fisher’s exact test, as required. Logistic regression analysis used a forward hierarchical stepwise method with switching to select independent variables related to the ADR. All significant variables in the univariate analysis were included in the model and were retained at each step if P > 0.05. Odds ratios (OR) and 95% confidence intervals (CI) are also provided.
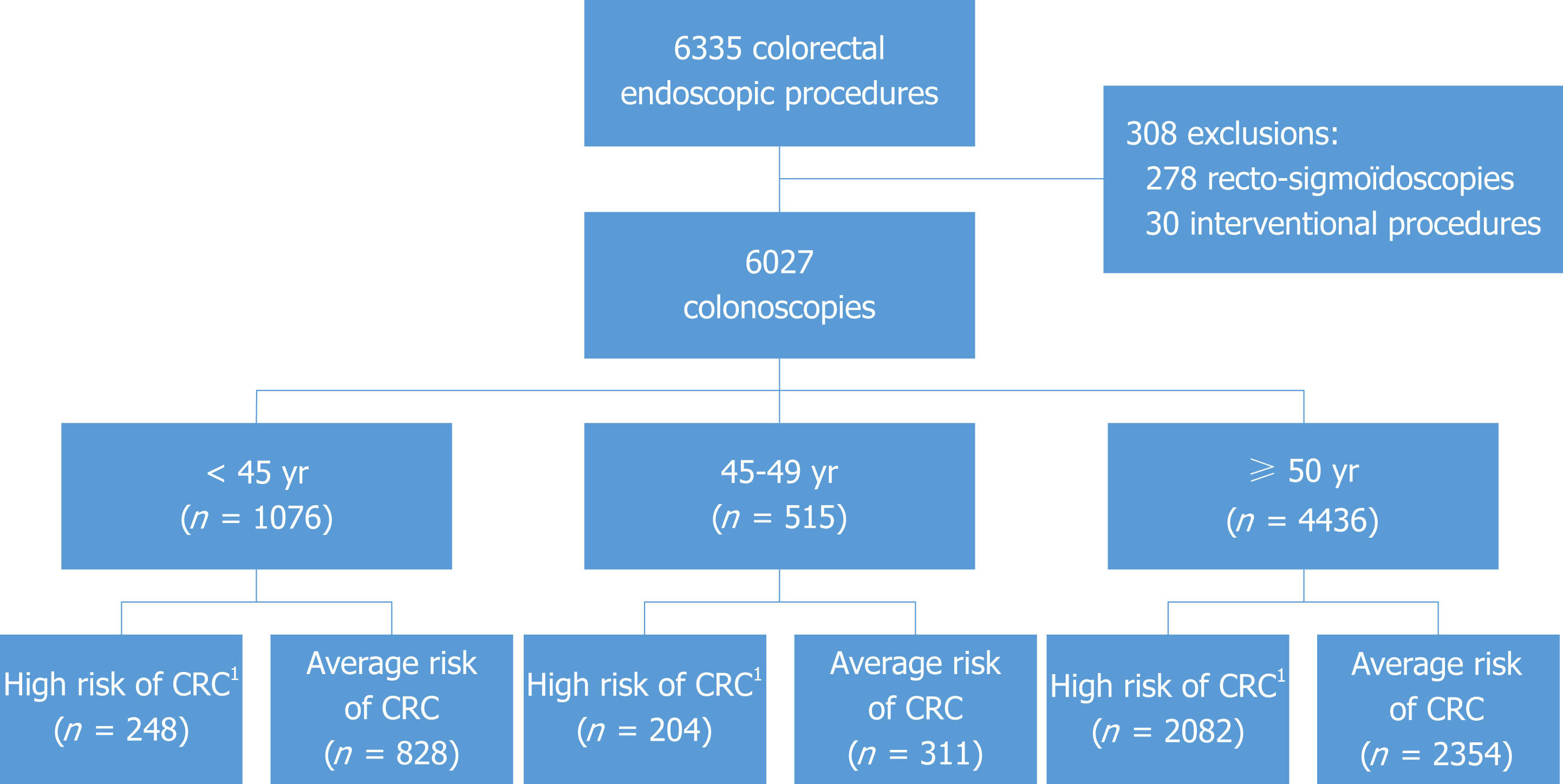
During the study period, 6335 colonoscopy procedures were performed. We excluded 278 sigmoidoscopies and 30 interventional procedures (Figure 1), leaving 6027 colonoscopies in 3308 women (54.9%) and 2719 men (45.1%) with a median age of 57 years (range, 15-96: IQR 18). The indication for colonoscopy was a personal history of adenoma or cancer in 1512 patients, family history of adenoma or advanced adenoma or cancer in 2534, a positive fecal immunochemical test in 391, digestive symptoms or hematochezia in 2306, screening colonoscopy in 320 and other causes in 476 (mainly inflammatory bowel disease in remission follow-up, suspected colonic lesions after computed tomography scan and post-diverticulitis colonoscopy). Sub-optimal preparation was noted in 6.2% of the patients. Cecal intubation was obtained in 99%. Median withdrawal time was 470 seconds (range 55-3840; IQR 240).
Of the 6027 colonoscopies, 2054 detected 3914 lesions or polyps with adenomas in 2914 (74.5%), SP in 788 (20.1%) and other polyps in 212 (5.4%). The ADR was 28.6% in this series. We found 690 advanced neoplasia in 584 patients leading to an ANDR of 9.7%. The serrated lesion detection rate (SDR) was 9.2%. In the multivariate analysis (Table 1), the variables associated with a higher ADR were: a personal history of polyps or cancer (OR 1.5), a positive fecal immunochemical test (OR 2.7), male gender (OR 1.7) and the age of the patient (≥ 45 years: OR 1.3). Colonoscopy for symptoms was associated with a lower risk of adenoma (OR 0.7).
| Variable | Univariate analysis | Multivariate analysis | |||
| Odds ratio | P | Odds ratio | 95%CI | P value | |
| Male gender | 1.8 | 10-5 | 1.7 | 1.5-1.9 | 10-5 |
| Age > 45 yr | 1.5 | 10-5 | 1.3 | 1.1-1.6 | 0.0005 |
| Good prep | 1.3 | 0.02 | |||
| Screening | REF | REF | |||
| Family history | 0.60 | 0.0006 | 0.65 | 0.5-0.0 | 0.004 |
| Personal history | 1.5 | 0.001 | 1.53 | 1.2-2.0 | 0.002 |
| FIT + | 2.7 | 10-5 | 2.7 | 1.9-3.6 | 10-5 |
| Digestive symptoms | 0.6 | 0.0005 | 0.7 | 0.5-0.9 | 0.02 |
| Other indications | 0.36 | 10-5 | 0.4 | 0.3-0.5 | 10-5 |
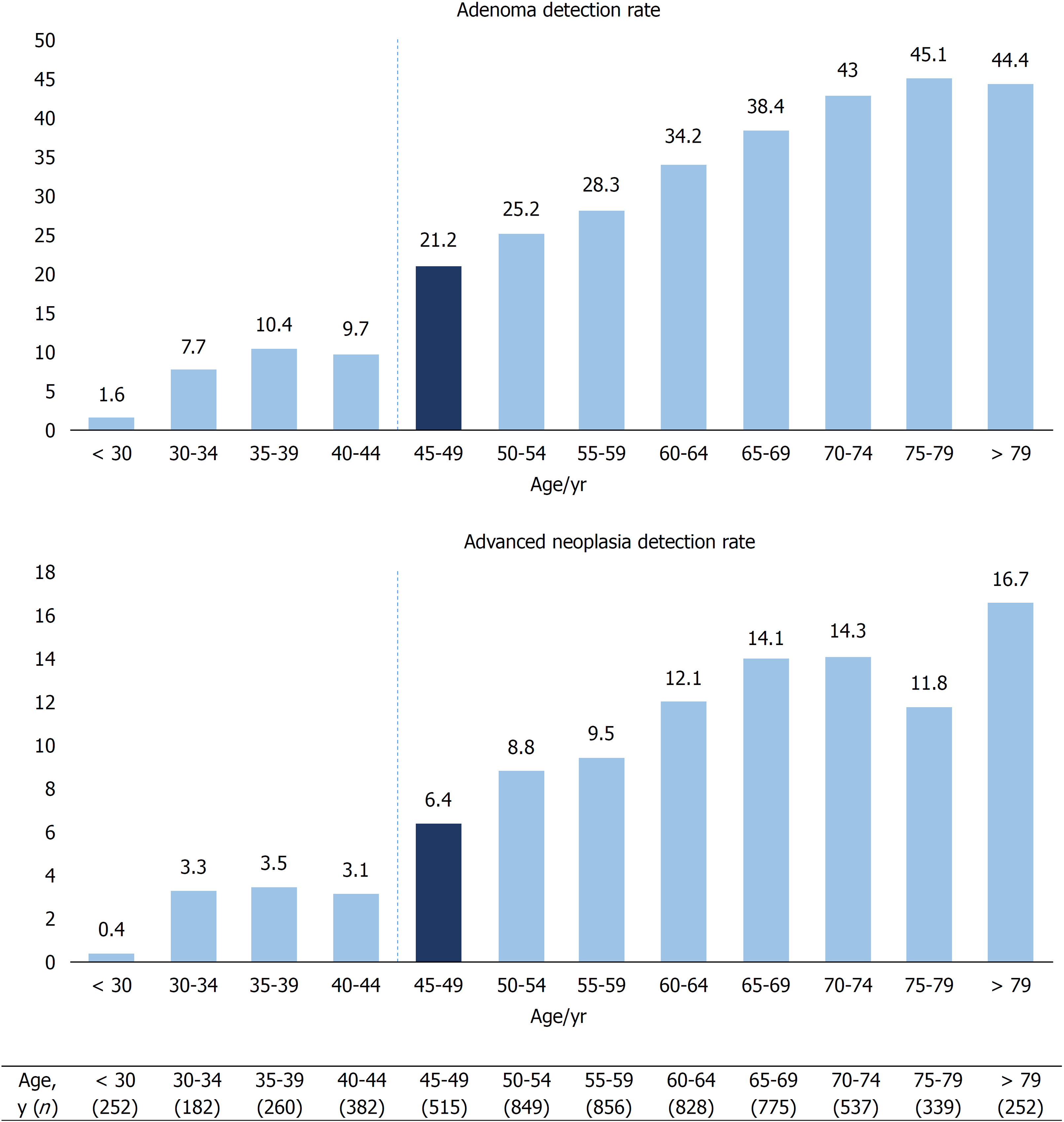
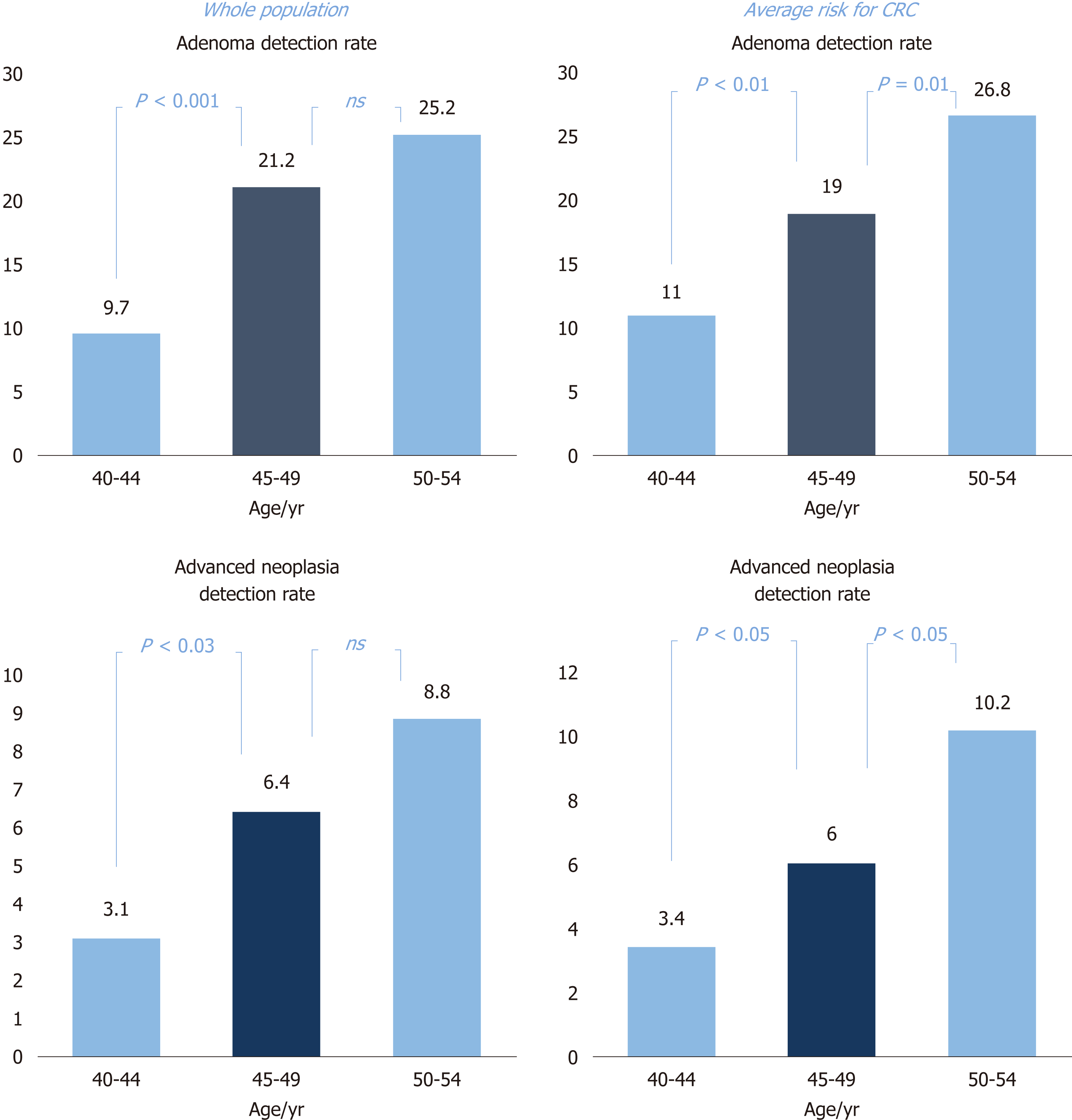
We examined the ADR and ANDR according to age using age intervals of 5 years. The results are presented in Figure 2. The ADR and ANDR markedly increased from 9.7% and 3.1% to 21.2 % (P < 0.001) and 6.4% (P < 0.03), respectively, in patients aged 40-44 and in those aged 45-49 years (Figure 2). The SDR also increased from 6% to 11.7% (P < 0.005) between patients aged 40-44 and those aged 45-49 years. When considering only asymptomatic patients (n = 3267), the ADR and ANDR also increased between patients aged 40-44 and those aged 45-49 years from 7.5% to 25.4% (P < 0.001) and 3.4% to 6% (P = 0.3), respectively.
A comparison of colonoscopy data and detection rates between patients aged 45-49 and those over 50 years is provided in Table 2. With regard to patients’ characteristics, the two groups were comparable, except for personal or family history of CRC or polyps and a higher proportion of patients over 50 years having no symptoms. Considering all patients over 50 years (n = 4436), the ADR and ANDR were significantly higher than those in the 45-49 years of age group with 34.6% vs 21.2% (P < 0.001) and 11.8% vs 6.4% (P < 0.001), respectively. In contrast, the SDR was not significantly different in those aged over 50 than in those aged 45-49 years, with 10.1% vs 11.7 % (P = 0.32), respectively.
| 45-49 yr (n = 515) | ≥ 50 yeas (n = 4436) | P value | ||
| Gender, male/female, n | 236/279 | 2028/2408 | 0.98 | |
| Indications for colonoscopy | ||||
| Patients without symptoms | 268 (52) | 3,006 (68) | < 0.001 | |
| High risk | ||||
| Personal or family history of polyps | 204 (39.6) | 2082 (46.9) | 0.002 | |
| Average risk | 311 (60.4) | 2354 (53) | < 0.001 | |
| Digestive symptoms | 247 (48) | 1430 (32.2) | ||
| Other | 64 (12.4) | 924 (20.8) | ||
| Colonoscopy data | ||||
| Sub-optimal preparation | 30 (5.8) | 275 (6.2) | 0.84 | |
| Mean number of polyps | 0.47 | 0.78 | - | |
| Median withdrawal time, s | 452 | 471 | 0.49 | |
| Histological data | ||||
| Polyp detection rate, % | 29.1 | 40 | < 0.001 | |
| Adenoma detection rate, % | 21.2 | 34.6 | < 0.001 | |
| Serrated polyp detection rate, % | 11.7 | 10.1 | 0.32 | |
| Advanced neoplasia detection rate, % | 6.4 | 11.8 | < 0.001 | |
From the 584 patients diagnosed with advanced adenoma during the study period, 71 underwent complementary treatment such as surgery, chemotherapy, radiotherapy or a combination of these treatments. The results showed that endoscopic resection was curative in 513 (88%) patients. When considering only patients aged under 50 years, 10 of 61 with advanced adenoma received additional treatment, resulting in a curative endoscopic resection rate of 51/61 (84%) (P = 0.7 compared with the whole population).
To rule out the possibility that our results were driven by patients at high risk for CRC, we excluded patients with personal or family history of polyps or cancer. In this average-risk population, we also observed a significant increase in both the ADR and ANDR in patients aged 40-44 and in those aged 45-49 years, from 11% to 19% (P < 0.01) and 2.7% to 6.4% (P < 0.05), respectively. The extent of this increase was therefore similar to that observed in the whole population (Figure 3).
This study demonstrated that adenoma and advanced neoplasia (i.e., a polyp greater than 1 cm in size or an adenoma with at least high-grade dysplasia) detection rates start to increase from 45 years of age, with a two-fold increase compared to those aged 40-44 years. These data were confirmed whether or not there was a personal or family history of polyps or cancer. Moreover, we did not observe any significant difference in the ADR and ANDR between patients aged 45-49 and those aged 50-54 years in the whole population. Of note, the increase in these detection rates from age 45 years also concerned SP.
This is one of the first large studies to evaluate adenoma and ANDR in patients under 50 years old during routine colonoscopy in average-risk and high-risk CRC patients. To our knowledge, the study by Regula et al[18] is the only published report to evaluate CRC screening in a young population aged 40 to 66 years. This very large Polish colonoscopy-based screening program on more than 50000 participants included patients aged 40 to 49 years, but only in cases with a family history of cancer of any type. These young patients constituted only 14.2% of the participants (vs 26.4% in our study). In the Polish study, the ANDR and ADR were 3.4% and 8.5%, respectively. The ANDR and ADR in our patients aged 45 to 49 were much higher at 9.7% and 21.2%, respectively. This discrepancy could be explained by the sharp rise in the ADR between 40-44 and 45-49 years and to a lesser degree by the high rate of completed colonoscopy (1% in our series vs 9% in the Polish study). However, the same difference was observed in those aged over 50 years, with an ADR of 13.1% in the Regula study vs 34.6% in our patients, the latter being much closer to other published data[14].
A reduction in the ADR and ANDR may have been expected by excluding patients with a personal or family history of polyps or CRC as previously described[2,18], but this was not the case. As familial syndromes account for no more than 20% of young-onset CRC[23], the high rates of detection in our series could have minimized the difference between high-risk and average-risk patients. This study raises questions regarding screening in patients less than 50 years. Most scientific organizations such as the French Society of Digestive Endoscopy or the American College of Gastroenterology agree that colonoscopy or other methods of CRC screening for average-risk patients must enroll patients aged 50 to 75 years[3,5,15], but little is known about the ADR and ANDR outwith this range. The incidence of CRC in individuals less than 50 years seems to have increased in the last decade[2,17,23]. In two recent studies involving approximately 600 patients in each study, young patients were diagnosed with significantly more advanced CRC in comparison to older patients[24,25]. Therefore, earlier screening may improve disease stage on presentation and the prognosis of CRC. Indeed, the United States multi-society task force on CRC recently recommended providing screening to African Americans as early as 45 years of age[26], thus confirming the validity of rethinking the “50-year-old barrier”.
If adenomas are detected as early as 45 years of age, they could be resected at 50 years of age. While this assertion may be acceptable for small and low-grade dysplastic adenomas, it is highly questionable for advanced adenomas. However, the medical benefit of performing colonoscopy as early as 45 years has to be balanced by the medico-economic feasibility of such a screening policy. Nevertheless, whatever the screening method, the high ANDR observed in our young patients has to be taken into account in order to improve the prevention of CRC and disease stage on presentation and prognosis.
Our study had some limitations. First, while this study has the advantage of describing “real-life” conditions, socio-economic level and environmental exposure as well as the way-of-life of a population of a major European capital could represent some biases compared to national screening campaigns which are more representative of the population of the entire country. Moreover, as 38% of our patients underwent colonoscopy due to symptoms, our population cannot be considered a screening population. However, such symptoms are not known to increase the risk of polyps and were not correlated with a high ADR in the multivariate analysis in our series (OR 0.7). A personal or family history of polyps or CRC (defined as high-risk patients) may also bias the results. For this reason, we have detailed the results obtained for high-risk and average-risk patients. However, the ADR and ANDR significantly increased in both populations (Figure 3). The number of patients in our study who underwent colonoscopy for screening was only 320 (approximately 5% of our population). Most of these patients were older than 50, as colonoscopy screening is not recommended for younger patients in France. The size of the screening population was too small to perform a reliable analysis. The conclusions of our study, as obtained on routinely explored patients, should therefore be transposed to screening with caution. In addition, it is well known that age is not the only risk factor for the development of adenomas. Therefore, it could be speculated that other confounding factors may be associated with our ADR and ANDR. We acknowledge that we did not consider ethnicity (which is not allowed in France), smoking or obesity, which are other known risk factors for CRC[27-30]. Nevertheless, we showed that age was an independent factor associated with a high ADR in the multivariate analysis (OR 1.3). Lastly, the colonoscopy quality criteria obtained in a single team such as ours with a long-standing awareness policy (sub-optimal preparation in only 6.2%, median withdrawal time of 490 s, cecal intubation rate of 99%) undoubtedly had a positive impact on detection rates and may thus moderate the reproducibility of these results.
To summarize, in this large monocentric cohort of consecutive colonoscopies, we found a two-fold increase in the ADR and ANDR in patients aged 45 years and over, irrespective of a personal or family history of polyps or CRC.
Colonoscopy is considered a valid primary screening tool for colorectal cancer (CRC). The decreasing risk of CRC observed in patients undergoing colonoscopy is correlated with the adenoma detection rate (ADR). Due to the fact that screening programs usually start from the age of 50, very few data are available on the risk of adenoma between 40 and 49 years of age. However, the incidence of CRC is increasing in young populations and it is not uncommon in routine practice to detect adenomas or even advanced neoplasia during colonoscopy in patients under 50 years.
It is well known that early detection of adenomas reduces the incidence of CRC and allows the diagnosis and treatment of cancer at an earlier stage. As CRC is increasing in young populations, it is important to know at which age the increase in incidence of colonic adenomas and advanced colonic adenomas occurs.
The purpose of this study was to compare the ADR and advanced neoplasia detection rate (ANDR) according to age in a large series of patients during routine colonoscopy.
All consecutive patients who were scheduled for colonoscopy were included in this observational monocentric study conducted in our unit by a team of 30 gastroenterologists.
6027 colonoscopies were performed in patients with a median age of 57 years (range, 15-96). The ADR and ANDR were 28.6% and 9.7%, respectively, in the whole population. When comparing patients in the 40-44 (n = 382) and 45-49 year old groups (n = 515), a strong increase in all parameters from 45 years was observed, with the ADR rising from 9.7% in patients aged 40-44 to 21.2% in those aged 45-49 years (P < 0.001), and the ANDR increased from 3.1% in patients aged 40-44 to 6.4% in those aged 45-49 years (P < 0.03). In contrast, we did not observe a statistically significant increase in the ADR and ANDR between patients aged 45-49 and 50-54 years. When focusing on the population with an average risk for CRC, the ADR and ANDR were still significantly higher in patients aged 45 to 49 compared to patients aged 40 to 44 years.
This study showed a significant two-fold increase in the ADR and ANDR from 45 years of age, irrespective of a personal or family history of polyps or CRC.
This study raises questions regarding screening in patients less than 50 years of age. The medical benefit of performing colonoscopy as early as 45 years needs to be balanced by the medico-economic feasibility of such a screening policy. Nevertheless, whatever the screening method, the high ANDR observed in our young patients has to be taken into account in order to improve the prevention of CRC, disease stage on presentation and prognosis.
We would like to thank the following collaborators and nurses involved in this work: Dr Azria, Dr Bumsel, Dr Chemtob, Dr Chryssostalis, Dr Cohen, Mrs Cordier, Dr Debou, Dr Demont, Dr Etienney, Dr Evard, Dr Gillot, Dr Grateau, Dr Guigui, Dr Hagège, Dr Harboun, Mrs Hazoume, Dr Lab, Dr Lons, Dr Mehtari, Mrs Pattin, Dr Pecriaux, Dr Pellat, Mrs Pereira, Dr Petit, Mrs Ricq, Dr Roycourt, Mrs Tselikas, Dr Zago, Mrs Zanardo, Dr Zeitoun, Dr Zrihen, and and Dr Zylberberg.
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21466] [Article Influence: 1951.5] [Reference Citation Analysis (6)] |
| 2. | Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 2084] [Article Influence: 173.7] [Reference Citation Analysis (2)] |
| 3. | Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3107] [Cited by in RCA: 3179] [Article Influence: 96.3] [Reference Citation Analysis (1)] |
| 4. | Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, Ederer F. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2187] [Article Influence: 66.3] [Reference Citation Analysis (1)] |
| 5. | Faivre J, Dancourt V, Lejeune C, Tazi MA, Lamour J, Gerard D, Dassonville F, Bonithon-Kopp C. Reduction in colorectal cancer mortality by fecal occult blood screening in a French controlled study. Gastroenterology. 2004;126:1674-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 291] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 6. | Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF, Stewart ET, Waye JD. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1952] [Cited by in RCA: 2395] [Article Influence: 171.1] [Reference Citation Analysis (2)] |
| 7. | Shaukat A, Mongin SJ, Geisser MS, Lederle FA, Bond JH, Mandel JS, Church TR. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369:1106-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 666] [Article Influence: 51.2] [Reference Citation Analysis (2)] |
| 8. | Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ. 2014;348:g2467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 663] [Cited by in RCA: 635] [Article Influence: 52.9] [Reference Citation Analysis (2)] |
| 9. | Issa IA, Noureddine M. Colorectal cancer screening: An updated review of the available options. World J Gastroenterol. 2017;23:5086-5096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 434] [Cited by in RCA: 406] [Article Influence: 45.1] [Reference Citation Analysis (11)] |
| 10. | Navarro M, Nicolas A, Ferrandez A, Lanas A. Colorectal cancer population screening programs worldwide in 2016: An update. World J Gastroenterol. 2017;23:3632-3642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 374] [Cited by in RCA: 433] [Article Influence: 48.1] [Reference Citation Analysis (11)] |
| 11. | Winawer SJ. Screening sigmoidoscopy: Can the road to colonoscopy be less traveled? Ann Intern Med. 2003;139:1034-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Harewood GC, Lieberman DA. Colonoscopy practice patterns since introduction of medicare coverage for average-risk screening. Clin Gastroenterol Hepatol. 2004;2:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 163] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Waldmann E, Regula J, Ferlitsch M. How can screening colonoscopy be optimized? Dig Dis. 2015;33:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Kaminski MF, Thomas-Gibson S, Bugajski M, Bretthauer M, Rees CJ, Dekker E, Hoff G, Jover R, Suchanek S, Ferlitsch M, Anderson J, Roesch T, Hultcranz R, Racz I, Kuipers EJ, Garborg K, East JE, Rupinski M, Seip B, Bennett C, Senore C, Minozzi S, Bisschops R, Domagk D, Valori R, Spada C, Hassan C, Dinis-Ribeiro M, Rutter MD. Performance measures for lower gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy. 2017;49:378-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 528] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 15. | Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM; American College of Gastroenterology. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol. 2009;104:739-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 981] [Cited by in RCA: 1063] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 16. | Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger JE, Quinn VP, Ghai NR, Levin TR, Quesenberry CP. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1669] [Article Influence: 139.1] [Reference Citation Analysis (1)] |
| 17. | Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2526] [Cited by in RCA: 2937] [Article Influence: 326.3] [Reference Citation Analysis (7)] |
| 18. | Regula J, Rupinski M, Kraszewska E, Polkowski M, Pachlewski J, Orlowska J, Nowacki MP, Butruk E. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355:1863-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 546] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 19. | Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69:620-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 930] [Cited by in RCA: 988] [Article Influence: 58.1] [Reference Citation Analysis (1)] |
| 20. | Calderwood AH, Jacobson BC. Comprehensive validation of the Boston Bowel Preparation Scale. Gastrointest Endosc. 2010;72:686-692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 315] [Cited by in RCA: 326] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 21. | Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, Goldblum JR, Guillem JG, Kahi CJ, Kalady MF, O'Brien MJ, Odze RD, Ogino S, Parry S, Snover DC, Torlakovic EE, Wise PE, Young J, Church J. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315-29; quiz 1314, 1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 847] [Article Influence: 60.5] [Reference Citation Analysis (7)] |
| 22. | Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K, Hattori T, Hirota T, Itabashi M, Iwafuchi M, Iwashita A, Kim YI, Kirchner T, Klimpfinger M, Koike M, Lauwers GY, Lewin KJ, Oberhuber G, Offner F, Price AB, Rubio CA, Shimizu M, Shimoda T, Sipponen P, Solcia E, Stolte M, Watanabe H, Yamabe H. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Ahnen DJ, Wade SW, Jones WF, Sifri R, Mendoza Silveiras J, Greenamyer J, Guiffre S, Axilbund J, Spiegel A, You YN. The increasing incidence of young-onset colorectal cancer: a call to action. Mayo Clin Proc. 2014;89:216-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 357] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 24. | Ambe PC, Jansen S, Zirngibl H. New trend in colorectal cancer in Germany: are young patients at increased risk for advanced colorectal cancer? World J Surg Oncol. 2017;15:159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Dinaux AM, Leijssen LGJ, Bordeianou LG, Kunitake H, Berger DL. Rectal Cancer in Patients Under 50 Years of Age. J Gastrointest Surg. 2017;21:1898-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Levin TR, Lieberman D, Robertson DJ. Colorectal Cancer Screening: Recommendations for Physicians and Patients From the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153:307-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 539] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 27. | Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer. 2009;124:2406-2415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 371] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 28. | Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300:2765-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 590] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 29. | Ashktorab H, Vilmenay K, Brim H, Laiyemo AO, Kibreab A, Nouraie M. Colorectal Cancer in Young African Americans: Is It Time to Revisit Guidelines and Prevention? Dig Dis Sci. 2016;61:3026-3030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Robsahm TE, Aagnes B, Hjartåker A, Langseth H, Bray FI, Larsen IK. Body mass index, physical activity, and colorectal cancer by anatomical subsites: a systematic review and meta-analysis of cohort studies. Eur J Cancer Prev. 2013;22:492-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P- Reviewer: Cremers I, Fiorentini G, Gazouli M S- Editor: Yan JP L- Editor: Webster JR E- Editor: A