Published online Sep 21, 2019. doi: 10.3748/wjg.v25.i35.5376
Peer-review started: April 30, 2019
First decision: June 6, 2019
Revised: June 12, 2019
Accepted: July 19, 2019
Article in press: June 6, 2019
Published online: September 21, 2019
Processing time: 144 Days and 11.5 Hours
To date, the histopathological parameters predicting the risk of lymph node (LN) metastases and local recurrence, associated mortality and appropriateness of endoscopic or surgical resection in patients with gastric neuroendocrine neoplasms type 1 (GNENs1) have not been fully elucidated.
To determine the rate of LN metastases and its impact in survival in patients with GNEN1 in relation to certain clinico-pathological parameters.
The PubMed, EMBASE, Cochrane Library, Web of Science and Scopus databases were searched through January 2019. The quality of the included studies and risk of bias were assessed using the Newcastle-Ottawa Scale (NOS) in accordance with the Cochrane guidelines. A random effects model and pooled odds ratios (OR) with 95%CI were applied for the quantitative meta-analysis.
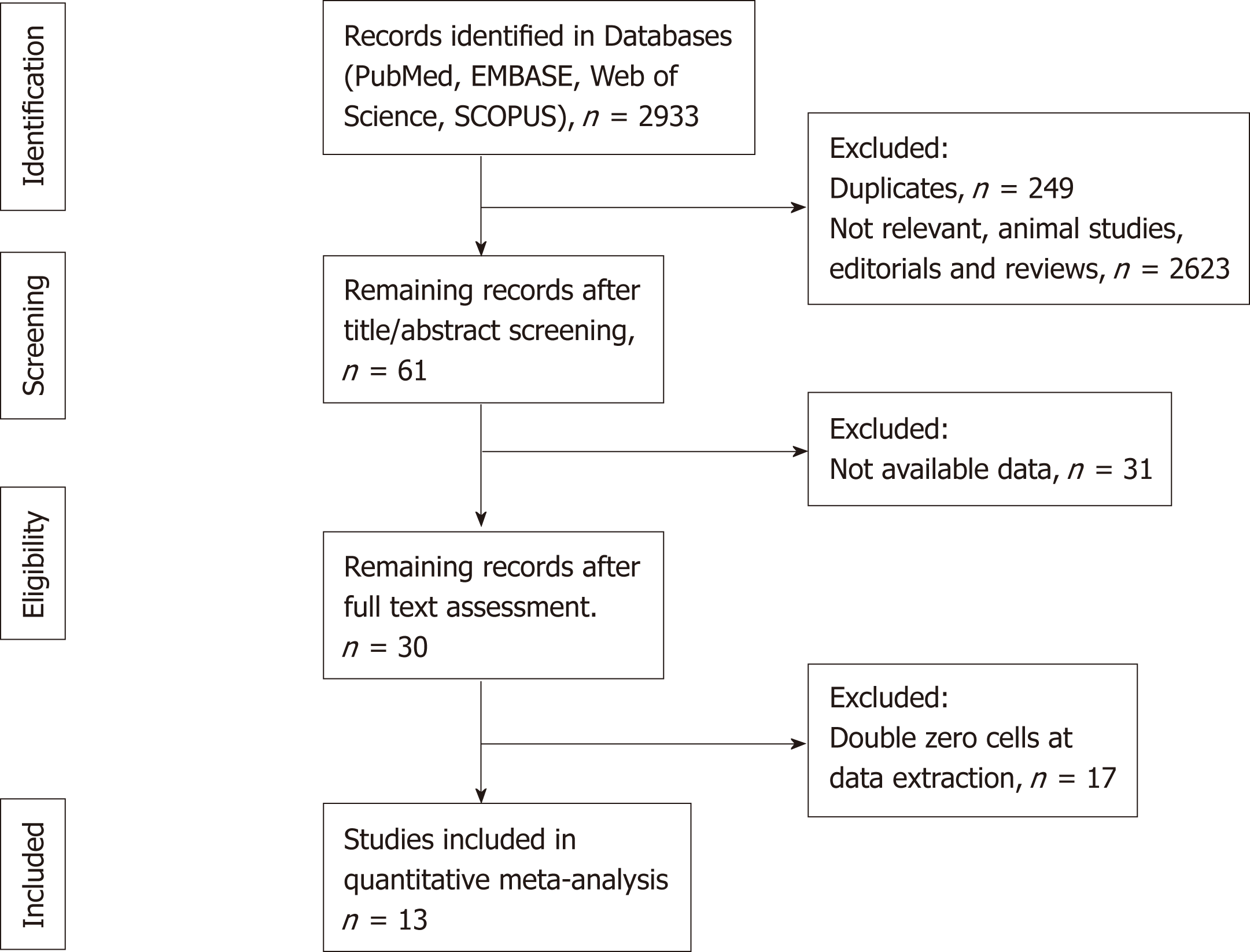
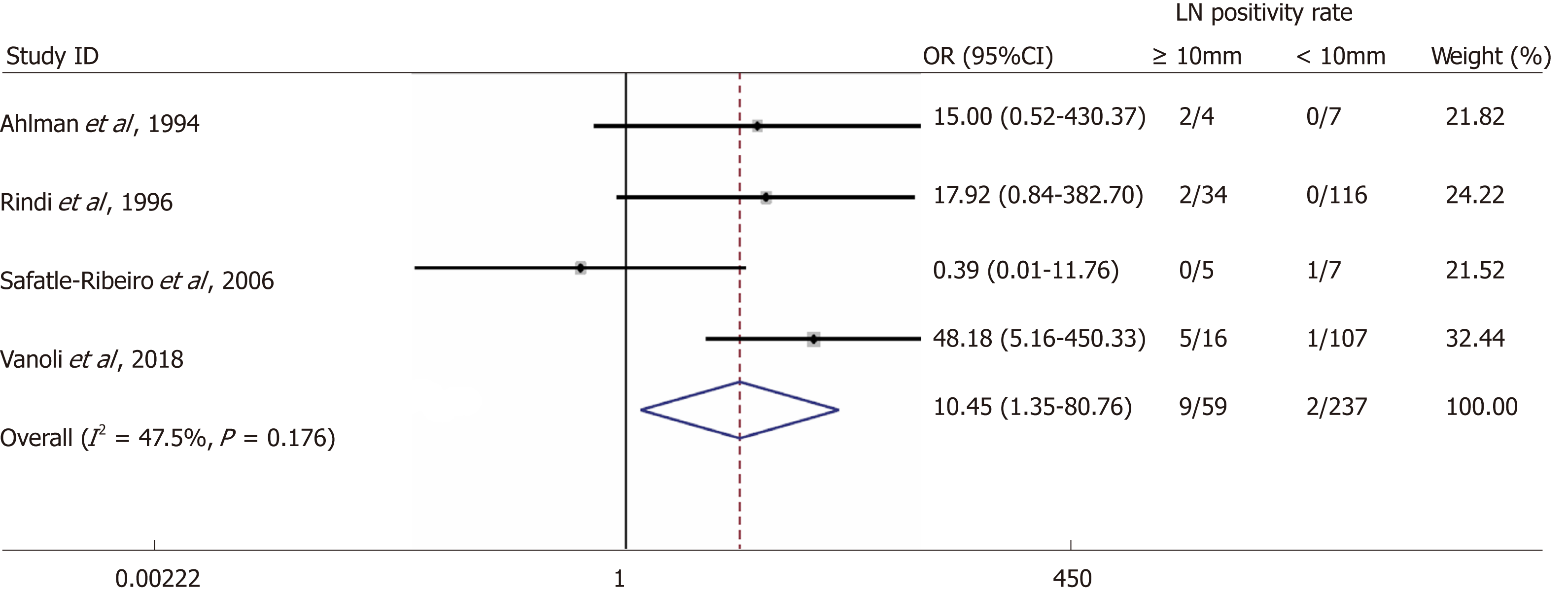
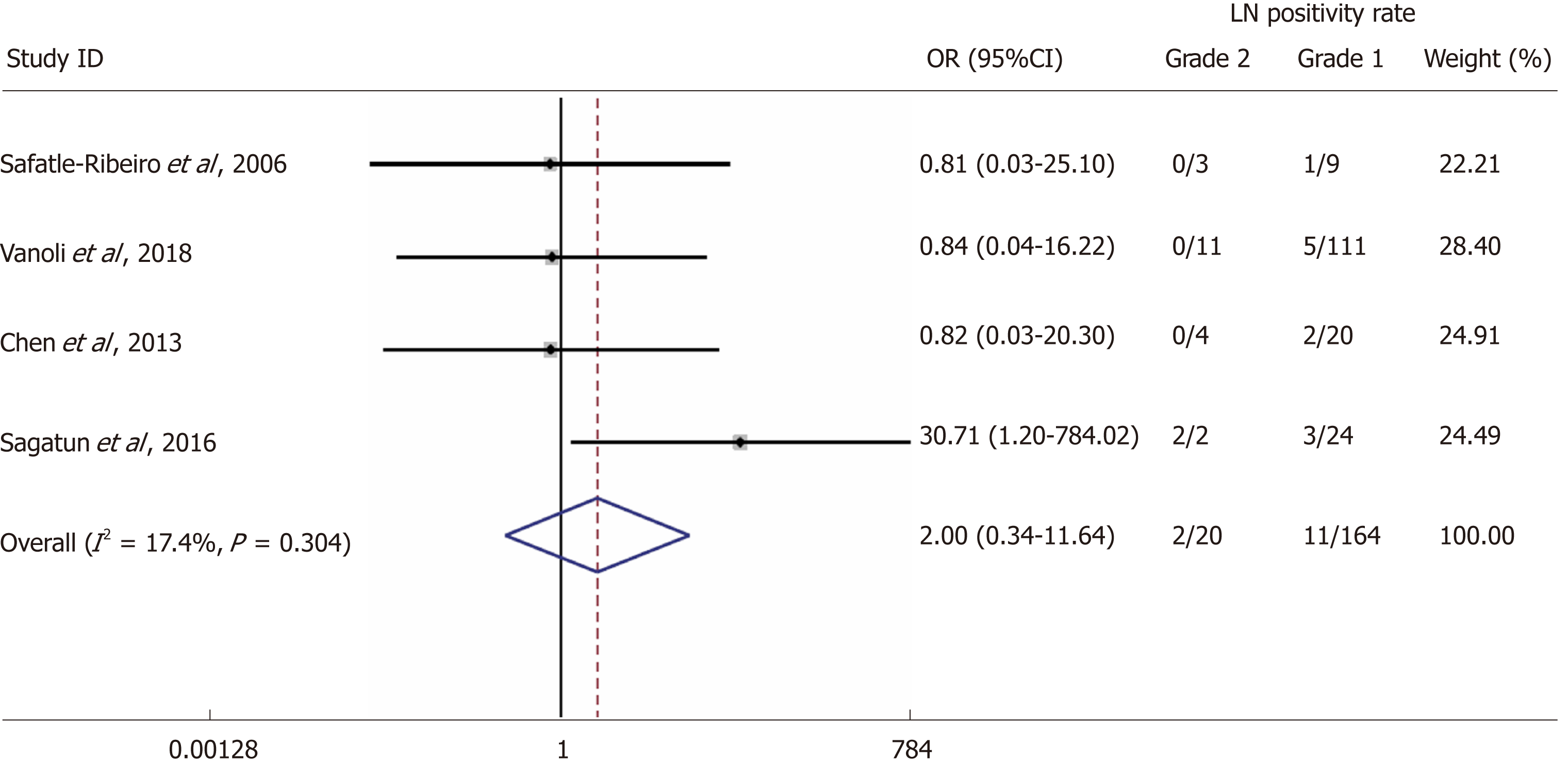
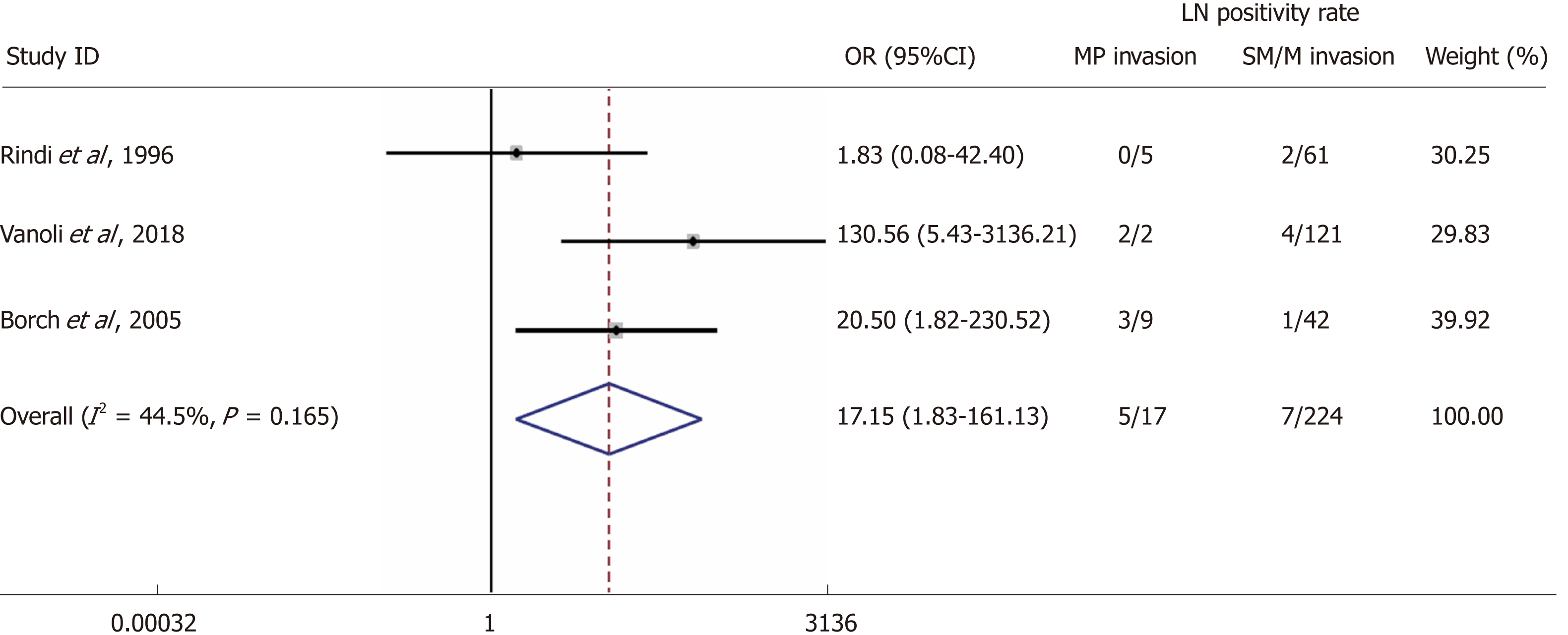
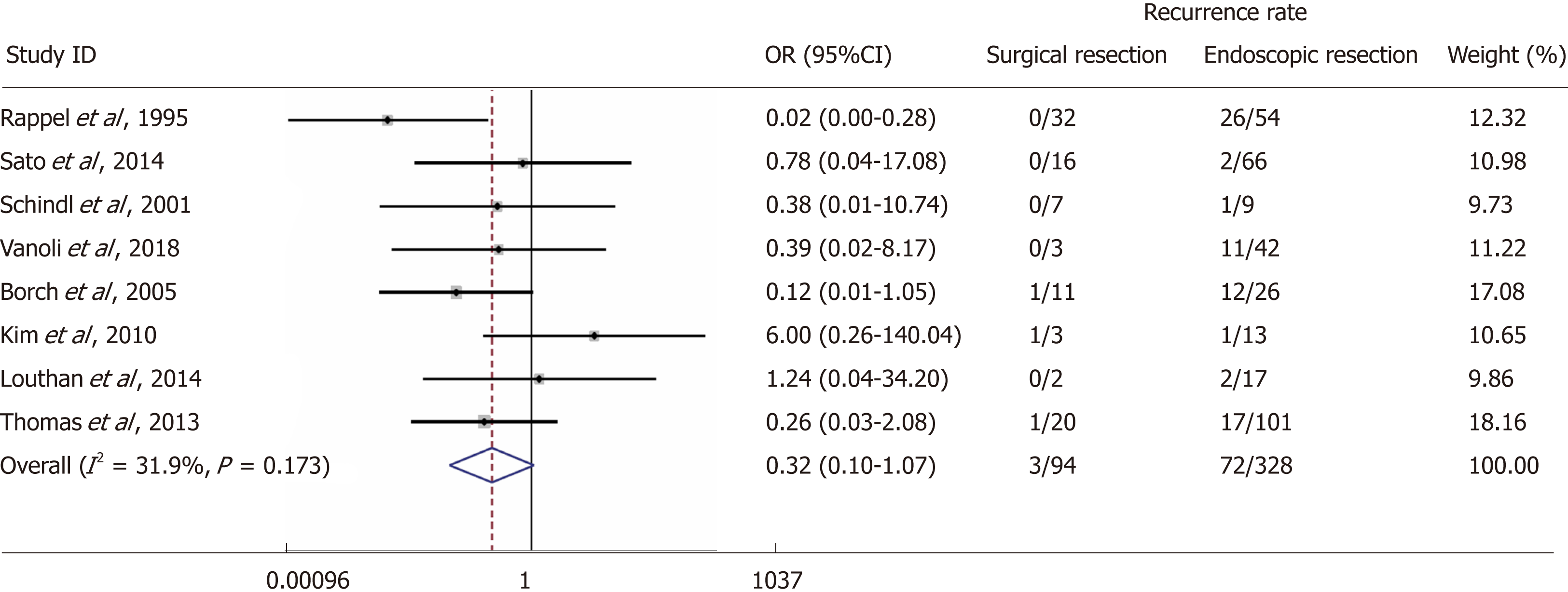
We screened 2933 articles. Thirteen studies with 769 unique patients with GNEN1 were included. Overall, the rate of metastasis to locoregional LNs was 3.3% (25/769). The rate of LN metastases with a cut-off size of 10 mm was 15.3% for lesions > 10 mm (vs 0.8% for lesions < 10 mm) with a random-effects OR of 10.5 (95%CI: 1.4 -80.8; heterogeneity: P = 0.126; I2 = 47.5%). Invasion of the muscularis propria was identified as a predictor for LN metastases (OR: 17.2; 95%CI: 1.8-161.1; heterogeneity: P = 0.165; I2 = 44.5%), whereas grade was not clearly associated with LN metastases (OR: 2; 95%CI: 0.3-11.6; heterogeneity: P = 0.304; I2 = 17.4%). With regard to GNEN1 local recurrence, scarce data were available. The 5-year disease-specific survival for patients with and without LN metastases was 100% in most available studies irrespective of the type of intervention. Surgical resection was linked to a lower risk of recurrence (OR: 0.3; 95%CI: 0.1-1.1; heterogeneity: P = 0.173; I2 = 31.9%). The reported complication rates of endoscopic and surgical intervention were 0.6 and 3.8%, respectively.
This meta-analysis confirms that tumor size ≥ 10 mm and invasion of the muscularis propria are linked to a higher risk of LN metastases in patients with GNEN1. Overall, the metastatic propensity of GNEN1 is low with favorable 5-year disease-specific survival rates reported; hence, no clear evidence of the prognostic value of LN positivity is available. Additionally, there is a lack of evidence supporting the prediction of local recurrence in GNEN1, even if surgery was more often a definitive treatment.
Core tip: Hitherto, risk parameters predicting metastatic disease and the appropriateness of endoscopic vs surgical management of patients with gastric neuroendocrine neoplasms type 1 (GNENs1) have not been thoroughly investigated. The present systematic review and meta-analysis prove that locoregional lymph node (LN) metastases in GNENs1 are relatively rare (3.3%). Furthermore, tumour size ≥ 10 mm and the presence of the muscularis propria invasion are associated with an increased risk for LN metastasis. The latter finding suggests that endoscopic ultrasound investigation is very valuable in the work up of these lesions. Finally, surgical resection is linked to a lower risk for recurrence.
- Citation: Tsolakis AV, Ragkousi A, Vujasinovic M, Kaltsas G, Daskalakis K. Gastric neuroendocrine neoplasms type 1: A systematic review and meta-analysis. World J Gastroenterol 2019; 25(35): 5376-5387
- URL: https://www.wjgnet.com/1007-9327/full/v25/i35/5376.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i35.5376
Gastric neuroendocrine neoplasms (GNENs) are rare and account for approximately 3% of all gastrointestinal neuroendocrine tumors and 0,3% of all gastric malignancies[1]. GNENs are divided into well-differentiated (WD) GNENs and neuroendocrine carcinomas (NECs). WD GNENs are mainly of enterochromaffin-like (ECL) cell origin, and three types are recognized[2-6]. Types 1 and 2 (GNEN1 and 2) are associated with hypergastrinaemia, the former because of autoimmune chronic atrophic gastritis and the latter due to Zollinger-Ellison syndrome in the context of multiple endocrine neoplasia type 1. Patients with type 3 GNENs have normal gastrin concentrations and a more aggressive clinical behavior mimicking that of gastric adenocarcinoma[7]. Finally, NECs are poorly-differentiated tumors that show a certain degree of neuroendocrine differentiation and the affected patients have normal circulating gastrin concentrations and a poor prognosis.
GNEN1s commonly exhibit a generally benign clinical course with a minority developing locoregional lymph node (LN) metastases and only a few cases of distant metastases have been reported[8]. In terms of the long-term disease-specific mortality, reports differ regarding its association with the presence of metastases in locoregional LNs and the selection of the type and the extent of intervention undertaken. This is mainly because in the majority of published GNEN series and existing NEN registries, GNEN1s are reported together with other types of GNENs. Importantly, compared with other types of GNENs, GNEN1s have distinct differences in tumour biology and patient outcomes. However, informative clinico-pathological features (size, grade, depth of invasion) are currently used indistinguishably for most GNEN types, even if GNEN are a heterogeneous group and the prognostic impact of such parameters may differ considerably among the types.
Due to the indolent course of GNEN1, endoscopic resection is considered the mainstay of treatment for these tumours ranging from simple polypectomy and endoscopic mucosal resection (EMR) to endoscopic submucosal resection (ESMR) and endoscopic submucosal dissection (ESD). Surgery has been considered for tumours not amenable to endoscopic treatment, i.e., locally advanced lesions > 10 mm invading deeper layers of the gastric wall. This approach in GNEN1 management is also in accordance with the current European Neuroendocrine Tumour Society guidelines[9]. However, risk stratification based on patient-related parameters to determine the risk of LN metastases and disease-specific mortality in patients with larger and more locally advanced GNEN1 remains to be defined. Therefore, there is a great need of a summary of the evidence regarding the risk of LN metastases and local recurrence in GNEN1s, as well as the appropriateness and safety of endoscopic versus surgical resection of larger lesions.
The aim of this systematic review and meta-analysis was to compare the rate of LN metastases and associated mortality, and recurrence in patients with GNEN1s undergoing endoscopic or surgical resection with respect to their clinico-pathological parameters, such as the size, grade and depth of invasion and to assess the rate of complications associated with the aforementioned interventions. Our hypothesis was that patients with particular clinico-pathological characteristics, i.e., larger lesions, grade 2 tumours and deeper invasion of the gastric wall, may be at a higher risk for locoregional LN metastases and/or local recurrence; thus, necessitating the implementation of a more patient-tailored management of GNEN1.
Retrospective cohort studies with GNEN and GNEN1 patients undergoing endoscopic or surgical resection were included in this systematic review and meta-analysis. The outcomes that were required for study selection included two or more of the following terms: tumour size, grade, depth of invasion, vascular invasion, LN metastases, local recurrence, disease-specific and overall survival and complications. For potentially eligible studies a sample size of at least ten patients with GNEN1 was required; hence case reports and small case series were excluded. Studies reporting data on GNEN type 2, type 3 and GNEC altogether with GNEN1 were also excluded. In particular, GNEN1 diagnosis was based on histopathological and biochemical criteria reported in the methods section of the included studies. Registry and institutional studies reporting data without specifying the diagnostic criteria of GNEN1 (histopathologic confirmation and hypergastrinaemia) were excluded from the present study. Among multiple reports from the same institution, only the latest eligible study was included. A study protocol for this meta-analysis was not registered before the study initiation. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed[10].
We conducted a systematic search to identify all potentially eligible studies in the PubMed, EMBASE, Cochrane Library, Web of Science and SCOPUS databases. Search terms included “Gastric Carcinoid”, “ECL cell carcinoid”, “Gastric Neuroendocrine Tumor”, ”Gastric Neuroendocrine Neoplasm”, “Neuroendocrine Tumor of the Stomach”, “Endoscopy”, "endoscopic resection”, “endoscopic mucosal resection”, “endoscopic submucosal dissection”, “polypectomy”, “polyp resection”, “mucosectomy”, “gastric resection”, “antrectomy”, “surgery”, “ surgical resection”, “partial resection”, “partial gastrectomy” and “gastrectomy”, and all the terms were used in combination with the Boolean operators AND OR. The search terms were input as free text. Two of the authors independently examined all potentially eligible titles and abstracts. Full articles were obtained for preliminary selected studies to finalize eligibility (Supplementary Table 1).
The hypothesis of the study was formulated prior to the data collection and extraction. Two reviewers independently extracted all available data. We defined the primary outcome as the rate of LN metastases after using different clinico-pathological data to stratify the patients. The secondary outcomes were recurrence rate, disease-specific mortality rate associated with LN metastases and type of intervention, and the complication rate in GNEN1 patients undergoing endoscopic and surgical resection. Potentially eligible studies with double zero cells in all strata and the investigated outcomes were excluded at the final stage of data extraction. Disagreements between the two reviewers were resolved by consensus.
Patients with distant stage disease were not included in the present meta-analysis. The staging methods applied in the included studies and according to the TNM classification varied greatly and was primarily based on available histopathology in all patients subjected to resective surgery. However, in patients subjected to endoscopic resection staging criteria were applied to data extracted from endoscopy and corresponding histopathology regarding T stage and endoscopic ultrasonography, as well as cross-sectional imaging regarding the presence of locoregional LN metastases (N stage).
The classification of observational studies described by Mathes et al[11] was applied in this meta-analysis. The quality of the included studies was measured by the Newcastle-Ottawa Scale (NOS) and independently assessed in accordance with the Cochrane guidelines by two reviewers The total NOS scores ranged from 0 to 9 for cohort studies; a score of 6 or higher indicated high quality[12].
Statistical analyses were conducted with STATA 14.0 software (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP). A random-effects model was adopted for summary statistics. Pooled odds ratios (OR) were reported for all investigated outcomes. Automatic correction set at 0.5 by default in the “metan” application of STATA in eligible data with single zero cells was applied as appropriate. Statistical heterogeneity was evaluated by the I2 method and the χ2 test was employed to provide P values; I2 values > 50% indicated a high degree of heterogeneity. Small study effects and the presence of publication bias were evaluated by Galbraith and funnel plots, respectively. The results were reported as OR with 95%CI and P values. The level of statistical significance was set at 5% (P < 0.05).
We screened 2933 potentially eligible articles. The 13 included studies had 769 unique patients with GNEN1. The results of the systematic literature search and the study selection process are shown in the PRISMA flow diagram (Figure 1). Table 1 summarizes the characteristics of the included studies.
| Included studies | Study design | No. of patients | Outcomes | Funding and conflict of interest statement | |
| Primary (positive LN status) | Secondary (R, DSS, complications) | ||||
| Ahlman et al[13], 1994 | Single-center restrospective cohort study | 11 | 2/11 (data available at the individual level) | Median follow-up 5 yr; 100% 5-yr DSS | Funding: Swedish Mekong River Commission, Swedish Cancer Society, Jubileumsklinikens Cancer Research Fund, Sahlgrenska Hospital Research Foundation, Göteborg Medical Society, Assar Gabrielsson Research Foundation, Östergötland County Council and AB Hässle. |
| Borch et al[19], 2005 | Multi-center prospective cohort study | 51 | 4/51 (data not available at the individual level) | 5-yr DSS reported for LN status and type of intervention strata. R also reported for different strata. | No funding or conflict of interest reported. |
| Chen et al[18], 2013 | Single-center retrospective cohort study | 56 | 2/56 (data not available at the individual level) | 100% 5- and 10 yr DSS reported. | Funding: National Center for Advancing Translational Studies. The authors report no conflicts of interest. |
| Kim et al[25], 2010 | Single-center retrospective cohort study | 22 | 1/22 (data not available at the individual level) | Mean follow-up 68months for GC cohort; 5-yr DSS 100%. R: 15/22 | No funding or conflict of interest reported |
| Louthan et al[24], 2014 | Single-center retrospective cohort study | 18 | 0/18 (data not available at the individual level) | Mean follow-up 47 months for GC cohort; 100% 5-yr DSS for type of intervention strata. | Funding: RVO VFN64165 and PRVOUK-P25/LF1/2. |
| Rappel et al[22], 1995 | Single-center retrospective cohort study | 88 | 0/88 (data not available at the individual level) | Median follow-up 72.2 mo; 100% 5-yr DSS; R: 26/54 | No funding or conflict of interest information mentioned in the article. |
| Rindi et al[14], 1996 | Multi-center retrospective cohort study | 152 | 2/152 (data not available at the individual level) | Mean follow up 58months; 5-yr DSS 100%; R: 77/119 | No funding or conflict of interest information mentioned in the article. |
| Safatle-Ribeiro et al[15], 2006 | Single-center restrospective cohort study | 13 | 1/13 (data available at the individual level) | Median follow-up 72.2 mo | No funding or conflict of interest information mentioned in the article. |
| Sagatun et al[17], 2016 | Single-center restrospective cohort study | 26 | 5/26 (data not available at the individual level) | Median follow-up not reported; 5-yr DSS not properly reported | No funding or conflict of interest reported. |
| Sato et al[20], 2014 | Multi-center retrospective cohort study | 82 | 0/82 (data not available at the individual level) | Median follow-up reported for different strata. 5-yr DSS not reported; R: 2/82. | No funding or conflict of interest reported. |
| Schindl et al[21], 2001 | Single-center retrospective cohort study | 16 | 0/16 (data not available at the individual level) | Median follow-up 70.3 mo; 5-yr DSS 100%. | No funding or conflict of interest information mentioned in article. |
| Thomas et al[23], 2013 | Multi-center retrospective cohort study | 111 | 2/111 (data not available at the individual level for all cases). | Mean follow up 76 mo; DSS not reported. R:8/111 | Funding: Selander foundation. No conflict of interest reported. |
| Vanoli et al[16], 2018 | Multi-center retrospective cohort study | 123 | 6/123 (data not available at the individual level) | Median follow up 87 mo. 5-yr DSS reported for different strata. | Funding: Internal university grants and the Associazione Italiana Ricerca sul Cancro; Fellowship from San Matteo Hospital Foundation. Conflict of interest: Novartis Pharma and Ipsen Pharma. |
The quality assessment of all the included studies is presented in Table 2 (NOS template). All studies were retrospective cohort studies based on the analysis of institutional multi- or single-centre data. We did not identify any controlled randomized trials or national registry studies eligible for inclusion in the meta-analysis. General factors accounting for poor quality were the lack of clarity regarding the diagnostic criteria of GNEN1, short duration of follow-up, ambiguity regarding the criteria for endoscopic or surgical intervention and failure to report the rate of complications for patients who underwent endoscopic or surgical treatment.
| Included studies | Selection | Comparability | Outcome |
| Ahlman et al[13], 1994 | *** | * | ** |
| Borch et al[19], 2005 | **** | ** | ** |
| Chen et al[18], 2015 | *** | * | ** |
| Kiim et al[25], 2010 | *** | * | ** |
| Louthan et al[24], 2014 | *** | * | ** |
| Rappel et al[22], 1995 | *** | * | ** |
| Rindi et al[14], 1996 | *** | ** | ** |
| Safatle-Ribeiro et al[15], 2006 | *** | ** | ** |
| Sagatun et al[17], 2016 | *** | * | * |
| Sato et al[20], 2014 | *** | * | * |
| Schindl et al[21], 2001 | *** | * | ** |
| Thomas et al[23], 2013 | *** | * | ** |
| Vanoli et al[16], 2018 | *** | ** | ** |
Reporting bias was visually assessed in funnel plots for each of the investigated parameters (Supplementary Figures 1A-4A). Complementary tests did not reveal small size effects (Supplementary Figures 1B-4B). The observed funnel plot asymmetry could be due to the few studies included in the analysis (< 10 studies in all meta-analyses performed) and also publication bias. Between studies heterogeneity was less than 50%, i.e., acceptable for all the meta-analyses in this study.
Overall, the rate of metastases to locoregional LNs was 3.3% (25/769). We identified four studies reporting LN status for primary tumours with a size cut-off of 10 mm[13-16]. The rate of LN metastases for a cut-off size of 10 mm were 15.3% vs 0.8% for lesions ≥ 10 mm and < 10 mm, respectively with a random-effects OR of 10.5 (95%CI: 1.4-80.8; heterogeneity: P = 0.126; I2 = 47.5%, Figure 2).
Four studies reporting LN status in connection to GNEN1 grade [Grade 1 (G1) vs Grade 2 (G1)] were included in this analysis[15-18]. The rates of LN metastases in G1 GNEN1 were 6.7% vs 10% for G2 lesions with a random-effects OR of 2(95%CI: 0.3 -11.6; heterogeneity, P = 0.304; I2 = 17.4%, Figure 3).
Three studies reported LN status in connection to muscularis propria invasion of the primary tumour and were included in the analysis[14,16,19]. The rates of LN metastases in patients demonstrating muscularis propria invasion were 29.4% vs 3.1% in patients without invasion with a random-effects OR of 17.2(95%CI: 1.8 -161.1; heterogeneity, P = 0.165; I2 = 44.5%, Figure 4).
Eight studies reporting local recurrence (n = 75/422) in connection to the type of intervention were included[16,19-25]. The rates of local recurrence in patients undergoing endoscopic resection were 22% (n = 72/328) vs 3.2% (n = 3/94) in patients subjected to surgical resection with a random-effects OR of 0.32 (95%CI: 0.1-1.1; heterogeneity, P = 0.173; I2 = 31.9%, Figure 5).
In seven studies, the reported 5-year disease-specific survival (DSS) rate was 100% in patients with (n = 17) and without locoregional LN metastases (n = 479)[13,14,18,23,26-28]. Only two studies reported 5-year DSS less than 100% stratified by LN status [5-year DSS: 80% (n = 8/10) vs 100% (n = 164/164)]; random-effects OR: 0.02; 95%CI: 0-0.21; heterogeneity: P = 0.850; I2 = 0%)[16,19].
In thirteen studies reporting 5-year DSS rates following endoscopic vs surgical resection, 100% 5-year DSS was evident in both arms [n = 456 (endoscopic intervention)and n=162 (surgical intervention)], irrespective of the type of intervention that was undertaken[13,18,20-27,29]. Two additional studies reported 5-year DSS rates less than 100% stratified by the type of intervention (5-year DSS: 99% for endoscopic vs 98% for surgical intervention; random-effects OR of 0.64; 95%CI: 0.1-8.5; heterogeneity: P = 0.270, I2 = 17.7%;)[15,16].
The complication rates attributed to endoscopic and surgical intervention were reported in six studies and were as low as 0.6% for the former and 3.8% for the latter[16,18,30-32]. The severity of complications ranged from mild to severe and even death in one operative case. However, the data were not sufficient to provide a more comprehensive classification of patients who underwent surgery (i.e., according to the Clavien-Dindo classification system).
Finally, scarce data were available or appropriately reported with respect to recurrence rates in relation to tumour size, grade and depth of invasion. In particular, one study only reported tumour size as a predictor of recurrence (OR: 1.7, 95%CI: 0.13-22)[32]. Similarly, another study addressed grade (OR: 0.2; 95%CI: 0.01-4.6)[33], and one study discussed depth of invasion (OR: 33 95%CI: 0.9-1220)[25].
Our systematic review and meta-analysis demonstrate that although the metastatic propensity of GNEN1 is low (3.3%), tumour size ≥ 10 mm and invasion of the muscularis propria in the gastric wall may be potential predictors of LN metastases in these patients. Foremost, the negative predictive value of tumour size for lesions < 10 mm and that of the absence of muscularis propria invasion with respect to the presence of locoregional LN metastases were as high as 99.2% and 96.9% respectively. Primary tumour grade was not clearly associated with the risk of LN metastases in GNEN1. Additionally, the disease course is indolent, and the overall prognosis is excellent, with a 5-year DSS of 100% in most studies, with only two patients reported with locoregional LN metastases who died within 5-years of diagnosis. Therefore, the presence of LN metastases does not seem to clearly affect survival in GNEN1 patients. Moreover, most studies reported 98%-100% 5-year DSS, irrespective of the type of intervention that was undertaken. However, studies reporting adequate follow-up i.e., of 10 years are lacking; hence, we were not able to provide evidence that prophylactic surgical resection exerts a survival benefit in the long-term. The complication rates of endoscopic vs surgical resection in the few studies reporting this information were 0.6 and 3.8%, respectively. Finally, scarce data were available with regard to GNEN1 local recurrence after endoscopic or surgical intervention; although the latter was associated with a lower recurrence rate [OR: 0.32 (95%CI: 0.1-1.1). Recurrence prediction stratified by patient-related parameters was not feasible in our study.
Larger sizes and the cut-off of 20 mm may be of particular interest in predicting metastatic disease to locoregional LN. Therefore, we scrutinized all available studies for this information, but a lack of data on larger sizes with double zero cells in the tables of the extracted data was evident in most studies; hence, meta-analysis at 20 mm size cut-off was not feasible. Regarding the presence of distant metastases in contemporary literature, GNEN1 is indeed a generally benign disease with very few metastatic cases reported, thus there was no sufficient material for a meta-analysis.
NOS-based quality assessment was undertaken and the included studies were generally assessed as being of moderate to high quality. Significant heterogeneity was not observed in the meta-analyses of clinico-pathological parameters investigated here, nor were small study effects. To avoid reporting bias, we also assessed and included non-English language studies, as well as unpublished data from conference papers. Various observational studies on GNEN1 have exhibited contradictory results regarding the association of certain clinico-pathological parameters with the risk of LN metastases, e.g., that of the Ki67 labeling index. This observation may be due to the inclusion of other GNEN types and GNECs, which are known for a more aggressive biological behavior compared to that of WD GNEN1[14,16]. Therefore, to control biases attributed to tumour heterogeneity, registry and institutional studies reporting data on all types of GNEN and GNECs together with GNEN1 were excluded from our study.
In the modern management of GNEN1, endoscopic ultrasonography is an important complementary diagnostic tool that can be utilized in the assessment of lesions that are potentially invading deeper layers of the gastric wall. Endoscopic ultrasonography helps to determine the feasibility of endoscopic resection and the possible presence of locoregional LN metastases.
The majority of GNEN1 lesions are small and have traditionally been treated endoscopically; thus, the presence of locoregional LN metastases may have been underestimated. This was evident in our meta-analysis as lesions < 10 mm accounted for the majority in studies reporting size in connection to patient outcomes [237/296 (80%) lesions < 10 mm; Figure 2]. Nevertheless, cross-sectional and functional imaging were not performed or reported in all patients in the included studies, as the sensitivity of these modalities for detecting LN metastases is low and their overall impact on GNEN1 management and clinical decision-making is rather limited[23]. This should of course be taken into consideration when interpreting the findings of this meta-analysis. Additionally, a few studies only reported patient data at the individual level and in most series, ambiguity regarding the criteria for surgical resection was noted. Thus, the rates and ORs of LN metastases in GNEN1s < 10 mm and ≥ 10 mm, has to be interpreted in light of this knowledge. Finally, patients subjected to endoscopic surveillance alone without any intervention, with or without somatostatin analogues treatment, were not included in the scope of the present systematic review and meta-analysis.
The prognostic significance of tumour grade was not confirmed in our meta-analysis, as no clear association with the presence of LN metastases was evident. This is indeed an important finding that contrasts with the existing evidence in GNEN3 and GNEC, in which Ki67 is of paramount importance in disease prognostication and patient management; thus, the implication is that the GNEN type may be the most significant factor affecting patient outcomes and that this factor should be separately addressed in future studies and national registry data. Another possible explanation is that the span of Ki67 in G2 tumours is wide (3-20) and cases with a higher level of Ki67 within G2 tumours may have a substantially different course. In particular, there are studies postulating that a higher Ki67 cut-off should be considered in the clinical praxis of NEN when G2 is determined[34-36]. Additionally, we cannot exclude the possibility that additive effects of other clinico-pathological parameters combined with the concomitant G2 status may trigger metastasis. Importantly, the GNEN1 clinical course seems to be mainly benign as most studies report a 5-year DSS of 100% in patients who had undergone resection, whether endoscopic or surgical. Thus, the tumour biology, the insufficient length of follow-up and the scarcity of LN metastases in these indolent neoplasms may be the reasons the present meta-analysis could not confirm a survival difference associated with the presence of LN metastases or the type of intervention in GNEN1 patients.
Our study has some limitations. Importantly, it represents an analysis of clinico-pathological parameters extracted from multiple retrospective studies on GNEN, particularly GNEN1; hence, the included studies lacked sufficient power and were often not designed to evaluate the end-points investigated in our meta-analysis. Further limitations include a diagnostic GNEN increment of more indolent lesions over time along with the wide-spread application of endoscopic screening and the clinical implementation of modern diagnostic and therapeutic modalities, such as endoscopic ultrasonography, EMR/ESMR and ESD, which may have confounded our results because different diagnostic and interventional techniques may have been potentially applied in the included studies. Additionally, a lack of data regarding larger primary tumour size cut-off values and the lack of a centralized pathology review may have caused the loss of valuable information and introduced certain biases. Another limitation was the lack of data on GNEN1 local recurrence and the lack of data at the individual level to evaluate potential additive effects of the investigated factors. Finally, heterogeneity of the included studies and the broad CIs of ORs encountered in the pooled analysis of the study estimates may be an important limitation, highlighting the need for further research in the field of GNEN1 to assess the outcomes investigated in our meta-analysis.
In, conclusion, our results have important implications for clinicians and researchers. The present study provides a systematic review and meta-analysis of GNEN1 confirming the indolent course of this neoplasm and providing patient-tailored parameters for disease prognostication and suggestions for future research. We demonstrated that locoregional LN metastases in GNEN1 are relatively rare (3.3%) and that tumour size and depth of invasion may be important predictive factors that can be used to assess the disease metastatic propensity. Generally, GNEN1 seems to have an overall excellent prognosis with very low disease-specific mortality rates reported. Additionally, survival does not seem to be negatively affected by either endoscopic or surgical resection. However, studies with long-term follow-up are scarce, and the true prognostic impacts of LN status and the type of intervention remain to be determined. Based on the findings of this meta-analysis, paying special attention to GNEN1 size and the depth of invasion and making use of diagnostic modalities such as endoscopic ultrasonography seem reasonable in clinical practice, and in future studies in the field of GNEN.
Gastric neuroendocrine neoplasms type 1 (GNENs1) exhibit a generally benign clinical course and have distinct differences in tumour biology and patient outcomes, as compared to other types of GNENs.
Informative clinico-pathological features (size, grade, depth of invasion) are currently used indistinguishably for most GNEN types and remain to be elucidated for GNEN1s in particular in order to determine the risk of lymph node (LN) metastases, disease-specific survival and local recurrence; and guide a more patient-tailored management.
The aim of our study was to compare the rate of LN metastases, disease-specific mortality, and recurrence rates post intervention in patients with GNEN1s undergoing endoscopic or surgical resection with respect to the aforementioned clinico-pathological parameters (size, grade and depth of invasion). Additionally, we aimed to evaluate the rate of procedural complications associated with endoscopic and surgical interventions.
The PubMed, EMBASE, Cochrane Library, Web of Science and SCOPUS databases were searched through January 2019. The quality of the included studies and risk of bias were assessed using the Newcastle-Ottawa Scale and in accordance with the Cochrane guidelines. A random effects model and pooled odds ratios with 95%CI were applied for the quantitative meta-analysis.
Although the metastatic propensity of GNEN1 is low (3.3%), tumour size ≥ 10 mm and invasion of the muscularis propria in the gastric wall may be utilized to predict the presence LN. The negative predictive value of tumour size for lesions < 10 mm and that of the absence of muscularis propria invasion with respect to the presence of locoregional LN metastases were as high as 99.2% and 96.9% respectively. Contrary to other GNEN types, tumour grade was not clearly associated with the risk of LN metastases in GNEN1. The disease prognosis is excellent, with a 5-year DSS of 100% in most studies; thus, the presence of LN metastases does not seem to clearly affect survival in GNEN1 patients. Moreover, most studies reported 98-100% 5-year DSS, irrespective of the type of intervention that was undertaken. However, studies reporting long-term follow-up (i.e., >10 years post-treatment surveillance) are lacking; hence, we were not able to provide evidence that prophylactic surgical resection exerts a survival benefit. The complication rates of endoscopic vs surgical resection in the few studies reporting this information were 0.6 and 3.8%, respectively. Finally, scarce data were available with regard to GNEN1 local recurrence after endoscopic or surgical intervention. Although surgery was associated with a lower recurrence rate, recurrence prediction stratified by patient-related parameters was not feasible in our study.
Herein, we have thoroughly investigated patient-related clinico-pathological risk parameters potentially predicting metastatic disease, recurrence following endoscopic or surgical management and disease-specific mortality rates. We confirmed that LN metastases in GNENs1 are relatively rare and that tumour size ≥ 10 mm, as well as the presence of the muscularis propria invasion are associated with an increased risk for LN metastasis. The latter finding suggests that endoscopic ultrasound investigation is very valuable in the work up of these lesions. Finally, surgical resection is linked to a lower risk for local recurrence.
The present study provides a systematic review and meta-analysis of GNEN1 confirming the indolent course of this neoplasm and providing suggestions for future research towards a stratified approach based on patient-tailored parameters in the era of personalized medicine. Foremost, based on our findings, special attention to GNEN1 size and the depth of invasion and making use of diagnostic modalities, such as endoscopic ultrasonography, seem reasonable in clinical practice, and in future studies with long-term follow up in the field of GNEN1.
| 1. | Grozinsky-Glasberg S, Alexandraki KI, Angelousi A, Chatzellis E, Sougioultzis S, Kaltsas G. Gastric Carcinoids. Endocrinol Metab Clin North Am. 2018;47:645-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 2. | Tsolakis AV, Portela-Gomes GM, Stridsberg M, Grimelius L, Sundin A, Eriksson BK, Oberg KE, Janson ET. Malignant gastric ghrelinoma with hyperghrelinemia. J Clin Endocrinol Metab. 2004;89:3739-3744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Tsolakis AV, Stridsberg M, Grimelius L, Portela-Gomes GM, Falkmer SE, Waldum HL, Janson ET. Ghrelin immunoreactive cells in gastric endocrine tumors and their relation to plasma ghrelin concentration. J Clin Gastroenterol. 2008;42:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Tsolakis AV, Grimelius L, Stridsberg M, Falkmer SE, Waldum HL, Saras J, Janson ET. Obestatin/ghrelin cells in normal mucosa and endocrine tumours of the stomach. Eur J Endocrinol. 2009;160:941-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Tsolakis AV, Grimelius L, Granerus G, Stridsberg M, Falkmer SE, Janson ET. Histidine decarboxylase and urinary methylimidazoleacetic acid in gastric neuroendocrine cells and tumours. World J Gastroenterol. 2015;21:13240-13249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Solcia E, Arnold R, Capella C, Klimstra DS, Klöppel G, Komminoth P, Rindi G. Neuroendocrine neoplasms of the stomach. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon World Health Organization 2010; 64-68. |
| 7. | Modlin IM, Lye KD, Kidd M. A 50-year analysis of 562 gastric carcinoids: small tumor or larger problem? Am J Gastroenterol. 2004;99:23-32. [PubMed] |
| 8. | Spampatti MP, Massironi S, Rossi RE, Conte D, Sciola V, Ciafardini C, Ferrero S, Lodi L, Peracchi M. Unusually aggressive type 1 gastric carcinoid: a case report with a review of the literature. Eur J Gastroenterol Hepatol. 2012;24:589-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Delle Fave G, O'Toole D, Sundin A, Taal B, Ferolla P, Ramage JK, Ferone D, Ito T, Weber W, Zheng-Pei Z, De Herder WW, Pascher A, Ruszniewski P; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for Gastroduodenal Neuroendocrine Neoplasms. Neuroendocrinology. 2016;103:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 366] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 10. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264-269, W64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21613] [Cited by in RCA: 18556] [Article Influence: 1091.5] [Reference Citation Analysis (0)] |
| 11. | Mathes T, Pieper D. Study design classification of registry-based studies in systematic reviews. J Clin Epidemiol. 2018;93:84-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 13618] [Article Influence: 851.1] [Reference Citation Analysis (1)] |
| 13. | Ahlman H, Kölby L, Lundell L, Olbe L, Wängberg B, Granérus G, Grimelius L, Nilsson O. Clinical management of gastric carcinoid tumors. Digestion. 1994;55 Suppl 3:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Rindi G, Bordi C, Rappel S, LaRosa S, Stolte M, Solcia E. Gastric carcinoids and neuroendocrine carcinomas: Pathogenesis, pathology, and behavior. I1996; 20: 168-172. . [PubMed] |
| 15. | Safatle-Ribeiro AV, Ribeiro U, Corbett CE, Iriya K, Kobata CH, Sakai P, Yagi OK, Pinto PE, Zilberstein B, Gama-Rodrigues J. Prognostic value of immunohistochemistry in gastric neuroendocrine (carcinoid) tumors. Eur J Gastroenterol Hepatol. 2007;19:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Vanoli A, La Rosa S, Miceli E, Klersy C, Maragliano R, Capuano F, Persichella A, Martino M, Inzani F, Luinetti O, Di Sabatino A, Sessa F, Paulli M, Corazza GR, Rindi G, Bordi C, Capella C, Solcia E. Prognostic Evaluations Tailored to Specific Gastric Neuroendocrine Neoplasms: Analysis Of 200 Cases with Extended Follow-Up. Neuroendocrinology. 2018;107:114-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 17. | Sagatun L, Fossmark R, Jianu CS, Qvigstad G, Nordrum IS, Mjønes P, Waldum HL. Follow-up of patients with ECL cell-derived tumours. Scand J Gastroenterol. 2016;51:1398-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Chen WC, Warner RRP, Harpaz N, Zhu H, Roayaie S, Kim MK. Gastric Neuroendocrine Tumor and Duodenal Gastrinoma With Chronic Autoimmune Atrophic Gastritis. Pancreas. 2019;48:131-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Borch K, Ahrén B, Ahlman H, Falkmer S, Granérus G, Grimelius L. Gastric carcinoids: biologic behavior and prognosis after differentiated treatment in relation to type. Ann Surg. 2005;242:64-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 193] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 20. | Sato Y, Imamura H, Kaizaki Y, Koizumi W, Ishido K, Kurahara K, Suzuki H, Fujisaki J, Hirakawa K, Hosokawa O, Ito M, Kaminishi M, Furuta T, Chiba T, Haruma K. Management and clinical outcomes of type I gastric carcinoid patients: retrospective, multicenter study in Japan. Dig Endosc. 2014;26:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Schindl M, Kaserer K, Niederle B. Treatment of gastric neuroendocrine tumors - The necessity of a type-adapted treatment. Arch Surg. 2001;136:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Rappel S, Altendorf-Hofmann A, Stolte M. Prognosis of gastric carcinoid tumours. Digestion. 1995;56:455-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 74] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Thomas D, Tsolakis AV, Grozinsky-Glasberg S, Fraenkel M, Alexandraki K, Sougioultzis S, Gross DJ, Kaltsas G. Long-term follow-up of a large series of patients with type 1 gastric carcinoid tumors: data from a multicenter study. Eur J Endocrinol. 2013;168:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Louthan O. Neuroendocrine neoplasms of the stomach. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Kim BS, Oh ST, Yook JH, Kim KC, Kim MG, Jeong JW, Kim BS. Typical carcinoids and neuroendocrine carcinomas of the stomach: differing clinical courses and prognoses. Am J Surg. 2010;200:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Manfredi S, Walter T, Baudin E, Coriat R, Ruszniewski P, Lecomte T, Laurenty AP, Goichot B, Rohmer V, Roquin G, Cojocarasu OZ, Lombard-Bohas C, Lepage C, Morcet J, Cadiot G. Management of gastric neuro-endocrine tumours in a large French national cohort (GTE). Endocrine. 2017;57:504-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Gladdy RA, Strong VE, Coit D, Allen PJ, Gerdes H, Shia J, Klimstra DS, Brennan MF, Tang LH. Defining surgical indications for type I gastric carcinoid tumor. Ann Surg Oncol. 2009;16:3154-3160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Lee HE, Mounajjed T, Erickson LA, Wu TT. Sporadic Gastric Well-Differentiated Neuroendocrine Tumors Have a Higher Ki-67 Proliferative Index. Endocr Pathol. 2016;27:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Grozinsky-Glasberg S, Thomas D, Strosberg JR, Pape UF, Felder S, Tsolakis AV, Alexandraki KI, Fraenkel M, Saiegh L, Reissman P, Kaltsas G, Gross DJ. Metastatic type 1 gastric carcinoid: a real threat or just a myth? World J Gastroenterol. 2013;19:8687-8695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 30. | Li QL, Zhang YQ, Chen WF, Xu MD, Zhong YS, Ma LL, Qin WZ, Hu JW, Cai MY, Yao LQ, Zhou PH. Endoscopic submucosal dissection for foregut neuroendocrine tumors: an initial study. World J Gastroenterol. 2012;18:5799-5806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Merola E, Sbrozzi-Vanni A, Panzuto F, D'Ambra G, Di Giulio E, Pilozzi E, Capurso G, Lahner E, Bordi C, Annibale B, Delle Fave G. Type I Gastric Carcinoids: A Prospective Study on Endoscopic Management and Recurrence Rate. Neuroendocrinology. 2012;95:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 32. | Postlewait LM, Baptiste GG, Ethun CG, Le N, Cardona K, Russell MC, Willingham FF, Kooby DA, Staley CA, Maithel SK. A 15-year experience with gastric neuroendocrine tumors: Does type make a difference? J Surg Oncol. 2016;114:576-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Uygun A, Kadayifci A, Polat Z, Yilmaz K, Gunal A, Demir H, Bagci S. Long-term results of endoscopic resection for type I gastric neuroendocrine tumors. J Surg Oncol. 2014;109:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Campana D, Ravizza D, Ferolla P, Faggiano A, Grimaldi F, Albertelli M, Ricci C, Santini D, Brighi N, Fazio N, Colao A, Ferone D, Tomassetti P. Risk factors of type 1 gastric neuroendocrine neoplasia in patients with chronic atrophic gastritis. A retrospective, multicentre study. Endocrine. 2017;56:633-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Klöppel G, La Rosa S. Ki67 labeling index: assessment and prognostic role in gastroenteropancreatic neuroendocrine neoplasms. Virchows Arch. 2018;472:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 36. | Hauck L, Bitzer M, Malek N, Plentz RR. Subgroup analysis of patients with G2 gastroenteropancreatic neuroendocrine tumors. Scand J Gastroenterol. 2016;51:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
Conflicts-of-interest statement: The authors state that they do not have any conflicts of interest to declare.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Sweden
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Krishna SG, Sato Y, Voutsadakis IA S-Editor: Cui LJ L-Editor: A E-Editor: Ma YJ