Published online Sep 14, 2019. doi: 10.3748/wjg.v25.i34.5082
Peer-review started: June 5, 2019
First decision: July 21, 2019
Revised: August 4, 2019
Accepted: August 24, 2019
Article in press: August 24, 2019
Published online: September 14, 2019
Processing time: 99 Days and 19.3 Hours
Managing familial pancreatic cancer (FPC) is challenging for gastroenterologists, surgeons and oncologists. High-risk individuals (HRI) for pancreatic cancer (PC) (FPC or with germline mutations) are a heterogeneous group of subjects with a theoretical lifetime cumulative risk of PC over 5%. Screening is mainly based on annual magnetic resonance imaging (MRI) and endoscopic ultrasound (EUS). The goal of screening is to identify early-stage operable cancers or high-risk precancerous lesions (pancreatic intraepithelial neoplasia or intraductal papillary mucinous neoplasms with high-grade dysplasia). In the literature, target lesions are identified in 2%-5% of HRI who undergo screening. EUS appears to provide better identification of small solid lesions (0%-46% of HRI) and chronic-pancreatitis-like parenchymal changes (14%-77% of HRI), while MRI is probably the best modality to identify small cystic lesions (13%-49% of HRI). There are no specific studies in HRI on the use of contrast-enhanced harmonic EUS. EUS can also be used to obtain tissue samples. Nevertheless, there is still limited evidence on the accuracy of imaging procedures used for screening or agreement on which patients to treat. The cost-effectiveness of screening is also unclear. Certain new EUS-related techniques, such as searching for DNA abnormalities or protein markers in pancreatic fluid, appear to be promising.
Core tip: High-risk individuals (HRI) for pancreatic cancer have a lifetime cumulative risk of this disorder of over 5%. The goal of screening is to identify operable cancers or precancerous lesions. Endoscopic ultrasound (EUS) appears to better identify solid lesions (0%-46% of HRI) and chronic-pancreatitis-like parenchymal changes (14%-77%), and magnetic resonance imaging to better identify small cysts (13%-49%). EUS is used to obtain tissue samples. There are no specific studies on contrast-enhanced harmonic EUS in HRI. There is limited evidence on the accuracy of imaging used for screening or agreement on which patients to treat. The cost-effectiveness of screening is also unclear. New EUS-related techniques (identifying DNA abnormalities or protein markers) appear to be promising.
- Citation: Lorenzo D, Rebours V, Maire F, Palazzo M, Gonzalez JM, Vullierme MP, Aubert A, Hammel P, Lévy P, Mestier L. Role of endoscopic ultrasound in the screening and follow-up of high-risk individuals for familial pancreatic cancer. World J Gastroenterol 2019; 25(34): 5082-5096
- URL: https://www.wjgnet.com/1007-9327/full/v25/i34/5082.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i34.5082
In the past few decades, the incidence of pancreatic cancer (PC) has continuously increased, while its prognosis remains poor, with a 5-year survival rate < 10% for all stages analysed together. PC is expected to become the second leading cause of cancer-related death in the United States in 2030[1]. Early-stage surgical resection is the only potentially curative treatment that increases survival. However, complete surgical resection can only be performed in a minority of patients, since 80% of patients have metastatic or locoregionally advanced disease at diagnosis[1-3].
Five to 10% of PCs are considered to be inherited[4,5]. While pathogenic germline mutations in specific genes have been associated with an increased risk of PC (from 4% to 40%), a causal germline mutation is identified in fewer than 20% of these families[6-10]. Several gene abnormalities and related hereditary syndromes have been associated with an increased risk of PC: BRCA1 and BRCA2 (hereditary breast ovarian cancer syndrome), PALB2 and the genes involved in the Fanconi pathway, CDKN2A (familial atypical multiple mole melanoma: FAMMM), the genes involved in Lynch syndrome (mainly MLH1, MSH2, MSH6, and PMS2), PRSS1 (hereditary pancreatitis), STK11 (Peutz-Jeghers syndrome), TP53 (Li-Fraumeni syndrome), APC (familial adenomatous polyposis), and ATM (ataxia telangiectasia)[4,9,11-16]. In the remaining 85% of families with no identified germline mutation, familial pancreatic cancer (FPC) is defined by the occurrence of PC in ≥ 2 first-degree relatives or in ≥ 3 relatives whatever the degree of relationship on the same side of the family[4,17].
Pancreatic screening is recommended in high-risk individuals (HRI) to identify early (pre)malignant pancreatic lesions and propose surgical resection with curative intent. The purpose of this screening is to reduce mortality related to PC. While numerous large and retrospective studies have reported on the short-term outcomes of pancreatic screening in HRI, follow-up was generally limited, and hence the magnitude of benefit of pancreatic screening in terms of curative potential remains currently unknown[11,12,17-19]. Additionally, although screening is usually based on yearly magnetic resonance imaging (MRI) and endoscopic ultrasound (EUS), the role of the latter has not been clearly defined. Our goal was to comprehensively review current data on pancreatic screening and follow-up of HRI, with an emphasis on the role of EUS.
The goal of screening in HRI (history of FPC or pathogenic germline mutations +/- family history of PC) is to reduce PC-related mortality by identifying morphological abnormalities that suggest the development of PC at an early and potentially curative stage[11,17-19]. In a meta-analysis from our group including all HRI treated by surgery reported in the literature, patients without invasive PC who underwent resection of premalignant lesions had no postoperative recurrence compared to those with invasive PC, and their 3-year overall survival was 100% vs 34.6%, respectively (P < 0.001)[11]. The lifetime risk of PC in HRI (with germline mutations or FPC) is estimated to be between 1% and 50% depending on the underlying predisposition and the number of affected relatives[12,17,20]. Table 1 presents the risk by mutations and their frequency in inherited cancers. Thus, HRI are theoretically good candidates for pancreatic screening.
| Condition | Gene | Relative risk of PC compared to the general population | Risk of pancreatic cancer at 70 yr (%) | % Among inherited cancers |
| No family history | 1 | 0.5-1 | ? | |
| 2 first degree relatives with PC | Unknown | 5-7 | 5-12 | 80-85 |
| 3 first degree relatives with PC | Unknown | 32 | 40 | |
| Hereditary breast ovarian cancer syndrome | BRCA1 | 2-4 | 3-4 | 1-5 |
| BRCA2 | 2-10 | 4-5 | 5-20 | |
| Genetic pancreatitis | PRSS1 | 50-80 | 40-55 | 1-4 |
| FAMMM | CDKN2A | 10-25 | 5-25 | 2-3 |
| Peutz-Jeghers syndrome | STK11 | 100-130 | 30-40 | 1-3 |
| Lynch syndrome | MLH1, MSH2, MSH6, PMS2 | 4-8 | 3-5 | 1-3 |
The first challenge of PC screening is the effective identification of good candidates, specifically, individuals with a theoretical risk threshold, arbitrarily set at 5%, of developing PC in their lifetime. Signoretti et al[18,21] have shown that the identification of (pre)malignant lesions varies depending on the genetic subgroup (3% in familial PC, 5% in FAMM syndrome, 6.3% in hereditary breast/ovarian cancer, and 12.2% in Peutz-Jeghers syndrome), while it was 42% in patients with hereditary pancreatitis who have the PRSS1 mutation. Corral et al[19] estimated that 135 patients needed to be screened to successfully identify 1 patient with a target lesion (high-risk lesion or PC) (95%CI: 88-303). This low rate was highly questionable, however, due to the very short follow-up period (3.3 years on average) reported in the studies[22]. Indeed, it contrasts with the delay that was estimated for a premalignant lesion to transform into invasive cancer (11 years) and does not enable the drawing of conclusions regarding the global yield of pancreatic screening in HRI.
Relevant imaging pancreatic abnormalities are identified at imaging in appro-ximately 50% of HRI, but this figure is difficult to interpret as there have been too few correlations of these imaging abnormalities with pathological examination due to the limited number of operated patients[11,18,23]. Another challenge of pancreatic screening is to identify and use the most appropriate screening techniques. Ideally, this would be the least invasive and reproducible technique that identifies the greatest number of premalignant lesions and that is the most acceptable for the patient.
The ultimate goal of this approach is to propose surgical resection of premalignant lesions [such as pancreatic intraepithelial neoplasia (PanIN) or intraductal papillary mucinous neoplasms (IPMN) with high-grade dysplasia (HGD)] or even early-stage invasive PC, which are found in approximately 3%-5% of HRI[11,19]. Finally, identifying lesions at high risk of (pre)malignancy and operating neither too early (low-grade dysplasia) nor too late (advanced PC) is challenging[17]. We recently found that an indication for prophylactic pancreatectomy was appropriate (based on identification of HGD or invasive PC) in 42.2% of surgically treated HRI[11]. The factors predicting surgical appropriateness were age > 50 years, presence of a germline mutation and the presence of high-risk radiological pancreatic abnormalities (the presence of “worrisome features”, “high-risk stigmata of malignancy”, or a solid pancreatic mass)[11].
In the past two decades, management of HRI has evolved and varies from one country to another. Screening should be performed in multidisciplinary teams in referral centres, which have more experience and expertise in screening methods (i.e., EUS and MRI) and in the treatment of invasive PC[17]. A recent meta-analysis estimated that the annual prevalence of high-risk lesions (early invasive PC, IPMN, or PanIN with HGD) detected in HRI was 3.3%, corresponding to 5/1000 person-years during follow-up and an individual probability of 0.5% per year[18].
The screening of HRI is mainly based on pancreatic morphological imaging [computed tomography (CT) scan, MRI and EUS][11,12,18,19]. For a long time, many studies have suggested that EUS might provide better detection of small solid lesions, while MRI can identify small cystic lesions[24-27]. In the study by Canto et al[25] in 216 HRI, EUS, MRI and CT scan detected pancreatic abnormalities (cysts, solid lesions or chronic pancreatitis) in 42.6%, 33.3% and 11% of patients, respectively. This corresponded to a sensitivity of 93% for EUS for the detection of solid lesions smaller than 2 cm compared to 53% and 67% for CT scan and MRI, respectively[25]. Harinck et al[27] performed a prospective comparison of EUS and MRI for the detection of clinically relevant pancreatic lesions at initial screening of 139 HRI. In this study, EUS and/or MRI detected pancreatic lesions in 6% of HRI: 2 solid tumours < 10 mm were only detected by EUS (1 invasive PC and 1 PanIN with low-grade dysplasia), and 25% of cysts were only detected by MRI[27]. Nevertheless, as all patients were not operated on, this study does not enable the evaluation of whether the lesions detected were all of pathological relevance. Table 2 reports the main characteristics of HRI screening techniques and imaging results in 16 published studies. Of note, MRI and CT scan protocols were not clearly described in most studies (e.g., matrix size, contrast enhancement, MRI sequences), and the results of EUS are well known to be operator-dependent as well as classical radiological procedures[28,29]. Indeed, Topazian et al[28] report a low interobserver agreement for the interpretation of pancreatic EUS in HRI (Kappa < 0.4 except for cysts). This is probably due to the lack of specific training for EUS, the lack of a standardized collection chart and a specific learning curve. Although all of the abovementioned studies included operated patients, the methods of detection of the pancreatic abnormalities that determined the surgical procedure were not described in detail. Thus, while the precise value of EUS compared to the other modalities is probably high (it may find more (pre)malignant lesions), this is difficult to determine in the absence of a large study correlating anatomopathological specimens to EUS findings.
| First author/yr | Setting | High risk condition | Patients included | Modalities of screening | Imaging results | Patients operated on | Operated lesions seen only by the EUS | Histological features with cancer or HGD |
| Brentnall et al[37] | Prospective, monocentric | FPC | 14 | EUS | CPis: 10 (77%) Cystic lesio: ? Solid lesio: 6 (46%) | 7 | 1NA | 7 dysplasia |
| Rulyak et al[38] | Prospective, monocentric | FPC | 35 | EUS, ERCP | 12 lesions | 12 | ? | 12 dysplasia |
| Kimmey et al[78] | Prospective, monocentric | FPC | 46 | EUS, ERCP | CPis: 24 (52%) Cystic lesion: ? Solid lesion: 12 (26%) | ? | ? | ? |
| Canto et al[12] | Prospective, monocentric | FPC, PJS, | 38 | EUS first, +/- ERCP, CT, FNA | CPis: 17 (45%) Cystic lesion: ? Solid lesion: 12 (31%) | 7 | 2/7 (1 PC, 1 PanIN3) | 1 PC 1 PanIN3 |
| Canto et al[23] | Prospective, monocentric | FPC, PJS, | 78 | EUS, CT +/- ERCP, FNA | CPis: 61 (78%) Cystic lesion: 9 (12%) Solid lesion: 8 (10%) | 7 | 3/7 (1 PanIN 3, 2 PanIN1-2) | 1 PC+IPMN 1 PanIN3 1PanIN3+IPMN |
| Poley et al[13] | Prospective, multicentric | FPC, PJS, FAMMM, FBOC, HP, LFS | 44 | EUS | CPis: 3 (7%) Cystic lesion: 7 (16%) Solid lesion: 3 (7%) | 10 | 1NA | 3 PC |
| Langer et al[15] | Prospective, multicentric | FPC, FAMMM | 76 | EUS + MRI | CPis: 17 (22%) Cystic lesion: 3 (4%) Solid lesion: 7 (9%) | 7 | 5/7 (9/21 non operated lesions seen only in EUS) | 0 PC or PanIN3 or IPMN with HGD |
| Verna et al[33] | Prospective, monocentric | FPC, FBOC | 51 | EUS n = 31 or MRI n = 33 +/- ERCP, FNA | CPis: 9 (29%) Cystic lesion: 12 (39%) Solid lesion: 2 (6%) | 5 | ? (2 solid lesions in EUS and MRI) | 1 PC 1 PanIN2 |
| Ludwig et al[34] | Prospective, monocentric | FPC | 109 | MRCP or CT EUS, FNA | 18 lesions | 6 | ? | 1 PC 2 MD IPMN 1 PanIN-3 1 PanIN-2 |
| Schneider et al[40] | Prospective, monocentric | FPC, FAMMM | 72 | EUS, MRI | 13 lesions | 9 | ? | 1 PC 1 PanIN-3 |
| Zubarik et al[16] | Prospective, monocentric | FPC, PJS, BRCA2 | 27 | CA19-9 +/- EUS (27/546) | 5 lesions | 5 | 1NA | 1 PC |
| Canto et al[25] | Prospective, multicentric | FPC, FBOC, PJS | 216 | CT, EUS, MRI/MRCP | CPis: 54 (25%) Cystic lesion: 79 (36%) Solid lesion: 3 (1.4%) | 5 | ? (20/216 non operated lesions seen only in EUS including 3 solid lesion) | 2 MD-IPMN 1 IPMN+PanIN3 |
| Al-Sukhni et al[32] | Prospective, monocentric | FPC, BRCA1/2, FAMMM, LKB1 | 252 | MRI, +/- CT, EUS, ERCP | CPis: ? Cystic lesion: 80 (32%) Solid lesion: 3 (1.2%) | 4 | 3 PC | |
| Sud et al[14] | Prospective, monocentric | FPC, BRCA1/2, MMR, CDKN2A, LKB1, PRSS1 | 30 | EUS +/- FNA | 3 lesions | 3 | 1NA | 2 PC |
| Mocci et al[10] | Prospective, multicentric | FPC, CDKN2A, MMR, PRSS1 | 41 | EUS, CT +/- MRI, FNA | CPis: 15 (37%) Cystic lesion:4 (10%) Solid lesion: 2 (5%) | 1 | 2NA | 1 NET 1 PanIN3 |
| Vasen et al[31] | Prospective, multicentric | FPC, CDKN2A, BRCA 1/2 | 411 | MRI, EUS, CT, FNA | CPis: ? Cystic lesion: 138 (34%) Solid lesion: 16 (4%) | 30 | 2/30 (866 MRIs and 106 EUS performed) | 16 PC 3 PanIN3 1 IPMN with high grade |
| Bartsch et al[4] | Prospective, multicentric | FPC, BRCA1/2, PALB2 | 234 | MRI, EUS, CT, FNA | CPis: ? Cystic lesion: 125 (49%) Solid lesion: 9 (4%) | 21 6/253 (2%) lesions (2 malignant and HGD) | ? | 2 PC 3 PanIN3 1 IPMN with HGD; multifocal PanIN2±BD-IPMN±AFL 1 NET 8 PanIN-2 |
| Harinck et al[27] | Prospective, multicentric | FPC, BRCA1/2, CDKN2A, LKB1, TP53, MMR | 139 | MRI +/- EUS | CPis: 41 (30%) Cystic lesion: 67 (48%) Solid lesion: 2 (1.4%) | 2 | 2/2 (1 PC, 1 PanIN-2) | 1 PC 1 PanIN-2 |
There are no approved biomarkers for the screening of PC in HRI. Only one study has reported the results of serum CA19-9 measurement for pancreatic screening in these patients. Twenty-seven out of 546 included patients (4.9%) had elevated CA19-9, and 5 (18.5%) of these had pancreatic lesions (1 PC, 2 IPMN, 1 PanIN, 1 neuroendocrine tumour). Nevertheless, the number of pancreatic lesions in the group with normal CA 19-9 levels was not reported[16]. CA19-9 is not recommended because of its low sensitivity and specificity[11,17-19,30].
The recent CAPS (International Cancer of the Pancreas Screening ) consensus has suggested that annual MRI [including magnetic resonance cholangio-pancreatography (MRCP)] and EUS are the best imaging modalities for the detection of significant PC precursor lesions[6]. In summary, and as recommended by the recent statement by the American Society of Clinical Oncology, pancreatic screening and follow-up in HRI should be based on MRI and EUS as complementary tests for the detection of pancreatic lesions[17].
While pancreatic screening usually begins when HRI are 40 years old, or 10 years before the youngest index case[23], this has recently been challenged because pancreatic screening rarely reveals relevant lesions before the age of 50[4]. We also recently reported that the risk of HGD or invasive cancer was 3 times higher in HRI > 50 years old operated on for pancreatic lesions than in HRI < 50 years old[11].
It is important to note that the majority of relevant pancreatic lesions (> 50% of cysts and solid tumours) are identified at the first screening rather than during follow-up[23,31,32]. The frequency of subsequent follow-up examinations varies depending on different studies and recommendations[4,30-34]. Several multicenter studies have compared different surveillance protocols (e.g., annual MRI and EUS or annual MRI with EUS every 3 years) and did not find any difference in terms of number of diagnosed lesions[4,31]. However, these studies compared the total number of diagnosed lesions, whereas only the number of (pre)malignant lesions would have had clinical relevance. Nevertheless, and unless demonstrated otherwise, it seems reasonable to perform one MRI and one EUS examination per year in HRI[11,19,31,32].
Life-long pancreatic follow-up is recommended in HRI, at least as long as patients remain fit for surgery, without severe comorbidities. In fact, there are no strong data to confirm when follow-up should be stopped[17,23]. Furthermore, the prognosis in another group at risk of PC (patients with IPMN) was poorer in patients who stopped surveillance after 5 years of surveillance, which advocates for prolonged surveillance[35].
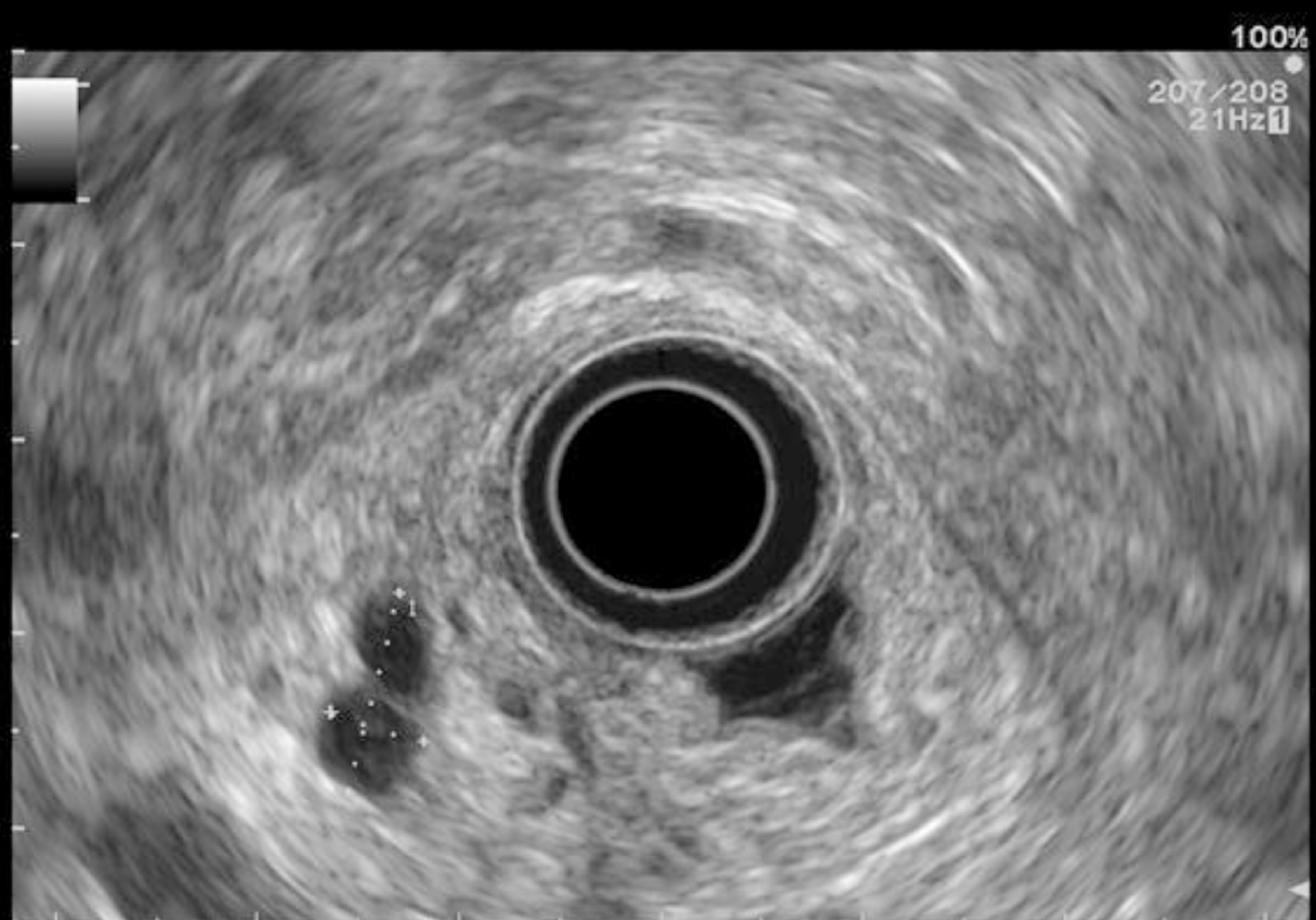
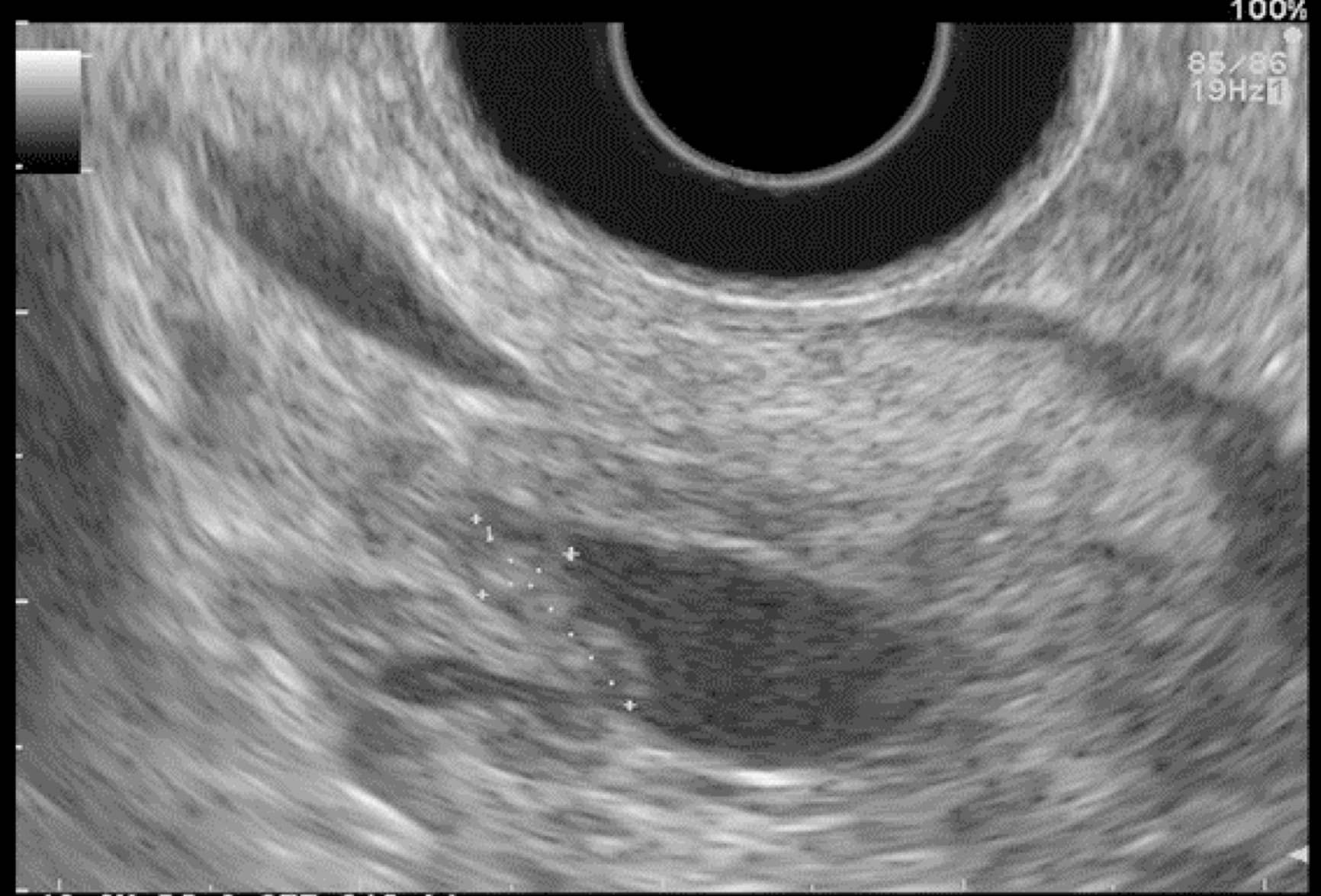
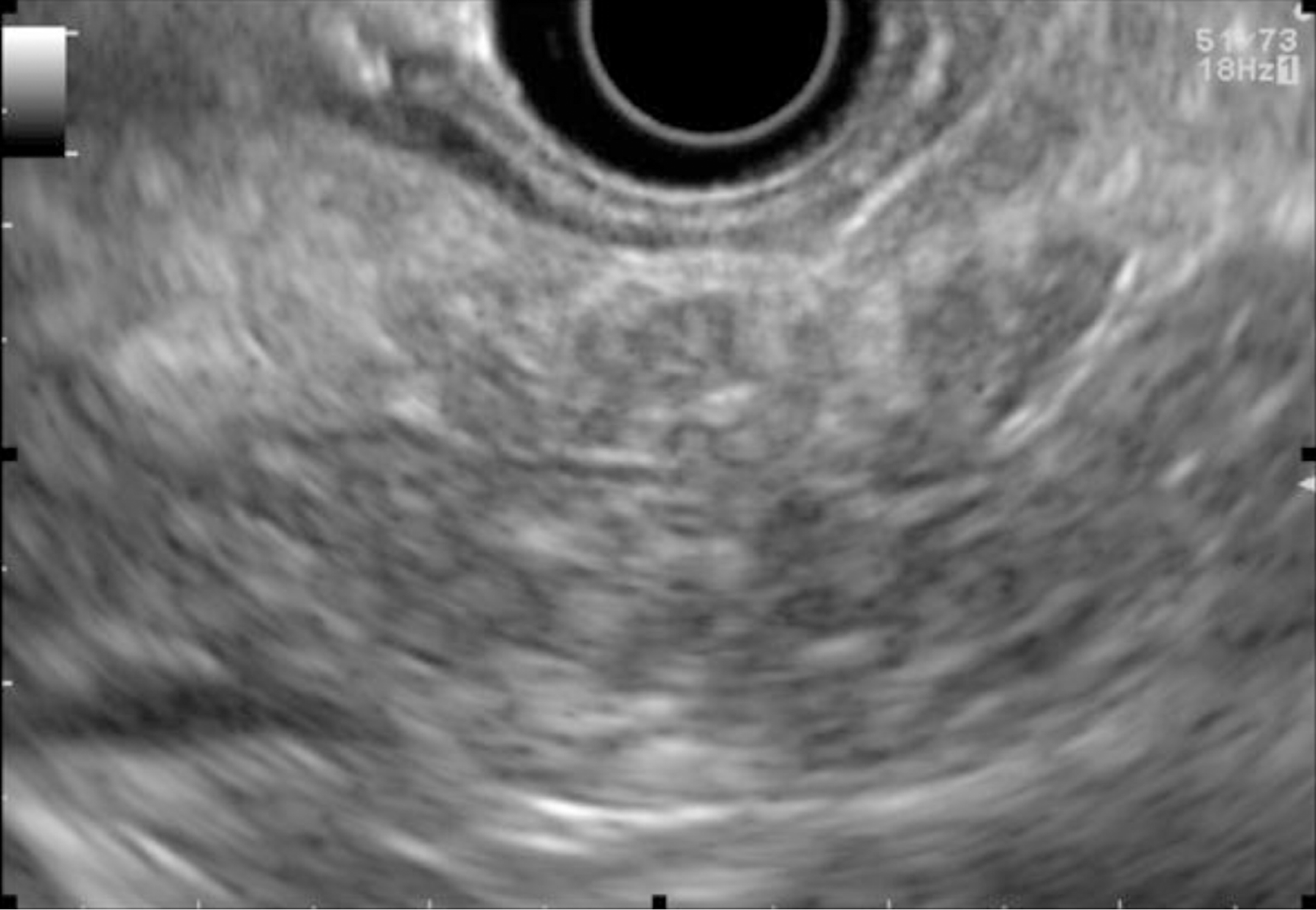
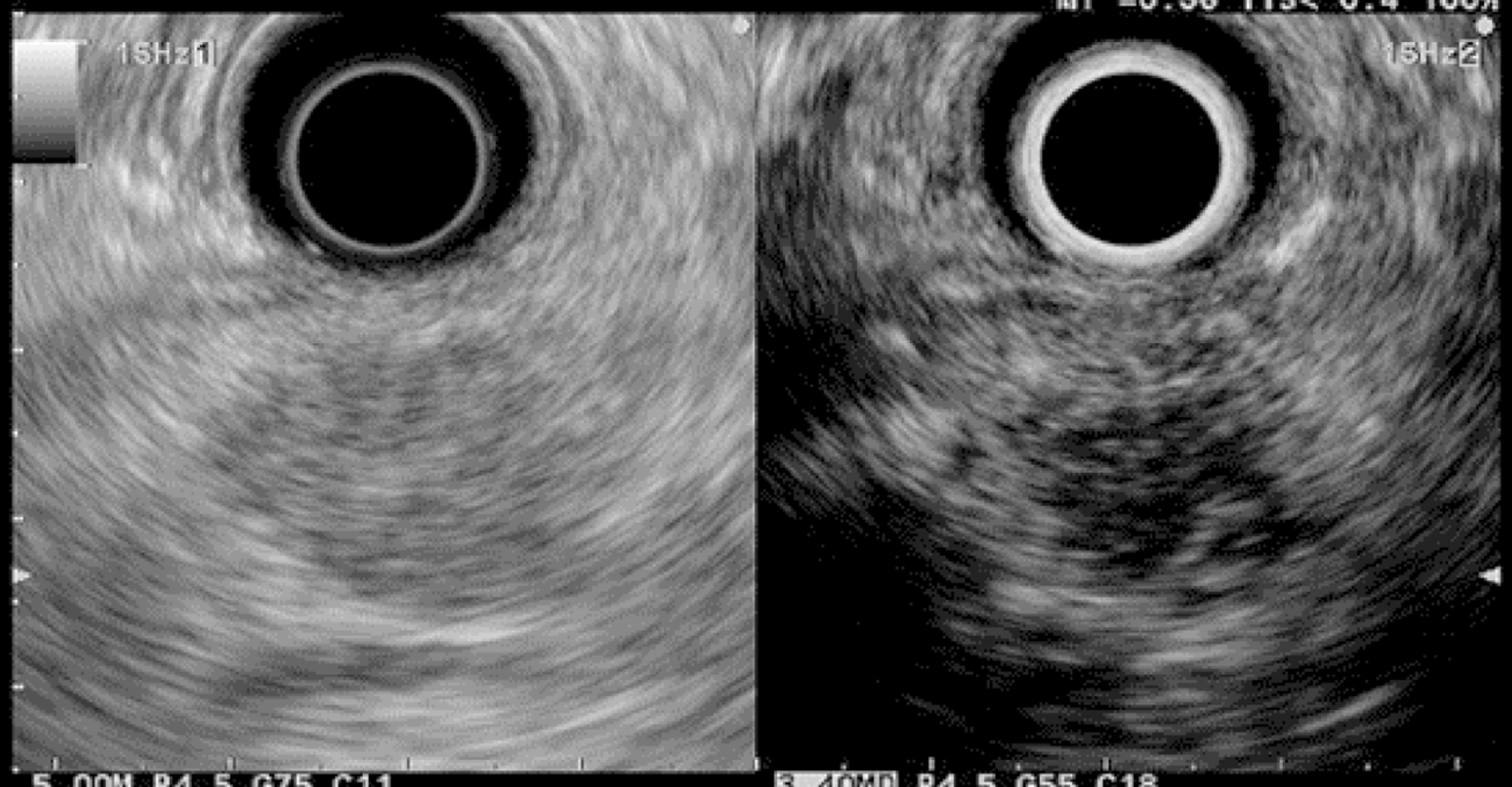
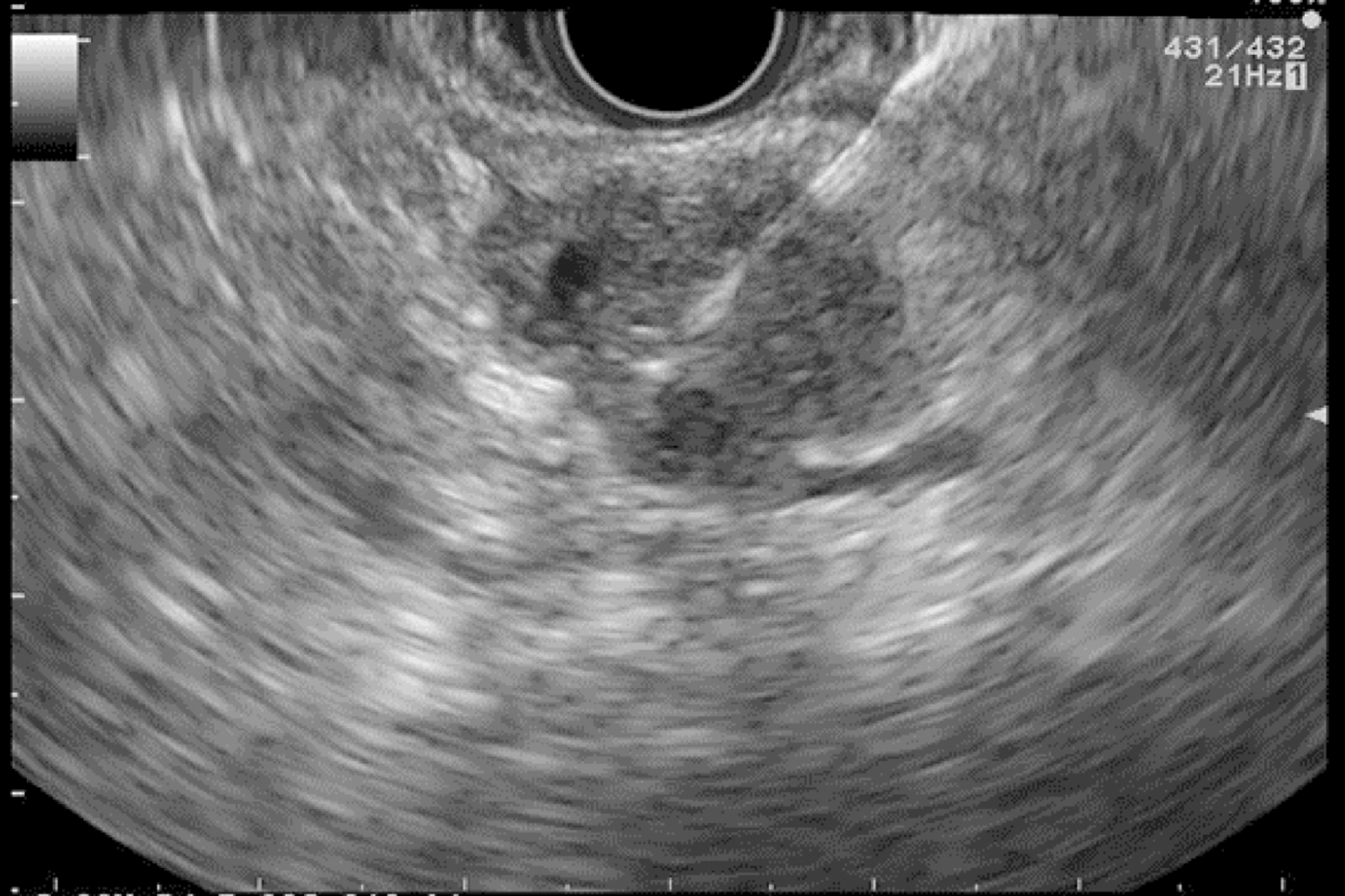
EUS pancreatic screening may identify pancreatic abnormalities in 19% to 79% of HRI (13–16, 25, 31, 34, 35, 37–40) (Table 2). These pancreatic “abnormalities” include cystic lesions (13%-49%) (Figures 1 and 2), chronic pancreatitis-like parenchymal changes (14%-77%) (Figure 3) and solid lesions (0%-46%) (Figure 4), which are always considered suspicious[4,10,18,23,26,27,39,40]. Of note, most studies that reported a low rate of EUS-detected abnormalities did not consider chronic pancreatitis-like parenchymal changes.
Pancreatic cystic lesions identified in HRI are mainly IPMN, which are more frequent in HRI (13%-20%) than in the general population (1%-5%)[18,26,41,42]. Whether IPMN in HRI have a different course than sporadic ones is unknown, and hence their monitoring could be similar by analogy with the general population. First, the initial characterization of IPMN should search for potential high-risk stigmata (obstructive jaundice associated with cystic lesions of the head of the pancreas, enhancing mural nodules > 5 mm, main pancreatic duct > 10 mm) and worrisome features (pancreatitis, cyst > 3 cm, enhancing mural nodule < 5 mm, thickened/enhancing cyst walls, main duct size 5-9 mm, abrupt change in calibre of pancreatic duct with distal pancreatic atrophy, lymphadenopathy, increased CA19-9 serum levels, and cyst growth rate > 5 mm per 2 years)[43,44]. These features are highly important for deciding on surgery because their presence is associated with an increased probability of finding invasive PC or HGD on the resected specimen[11]. EUS has been shown to be the best technique for the early detection of malignancy in patients with IPMN[45]. Nevertheless, the entire pancreatic parenchyma is at risk of PC, which can occur away from the cysts[46]. Thus, surveillance should not focus on pancreatic cysts alone. However, all cystic lesions found in HRI are not IPMN (but may be other benign cysts such as serous cystadenomas)[15,30,31]. Nevertheless, in the absence of specific worrisome criteria for non-IPMN cystic pancreatic lesions, it seems appropriate to use the abovementioned worrisome features for all cystic lesions, while it may lead to operating on benign cysts without potential pejorative evolution[11].
Chronic-pancreatitis-like parenchymal changes (CPis) are more common in HRI (up to 67%-80%) than in the general population (15%-17%)[12,23,47-49]. The diagnostic criteria for chronic pancreatitis with EUS (Rosemont Criteria) include major criteria (hyperechoic foci with shadowing and main pancreatic duct calcification, a lobularity with honeycombing) and minor criteria (small cysts, dilated ducts ≥ 3.5 mm, irregular pancreatic ducts, dilated side branches ≥ 1 mm, hyperechoic duct walls, strands, non-shadowing hyperechoic foci, and lobularity with noncontiguous lobules)[50]. Chronic-pancreatitis-like parenchymal atrophy and hyperechoic foci may correspond to multifocal PanIN[51-54]. Brune et al[51] reported a significant correlation between CPis on EUS and percentage of PanIN lesions on surgical specimens. The supposed explanation is that multifocal PanIN lesions produce obstructive lobular atrophy, which is probably the source of CPis[53]. Features of chronic pancreatitis during EUS-based surveillance of HRI are easily seen with good interobserver agreement[48]. One study showed that CPis generally have no or little progression over time, although the follow-up period was limited (3 years)[48]. A fatty pancreas has also been reported to be a risk factor for PC and should be noted on the EUS report[55].
Finally, EUS can identify solid pancreatic lesions in up to 20% of HRI during follow-up. These solid tumours are generally PC but may also be PanIN with HGD or neuroendocrine tumours[10,11,15,26,31,32,40]. In published studies, 14/53 (26%) of operated significant lesions (for which MRI and EUS data were available) were only seen on EUS (Table 1) [12,15,23,27,31]. However, as previously reported, 7/26 HRI operated on for a “pancreatic mass” (27%) had lesions with no/low malignant potential on the pathological specimen[11]. Nevertheless, it is difficult to consider not operating on an HRI with a “pancreatic mass” identified during EUS screening[11]. As discussed below, lesion sampling may be of special interest in this setting.
A study by Shin et al[29] suggested that compared to radial EUS, linear-array EUS improves the detection of pancreatic lesions in HRI, probably due to better visualization of the pancreatic tail. This same study reported a "second-pass effect" with additional lesions detected during a second EUS examination[29].
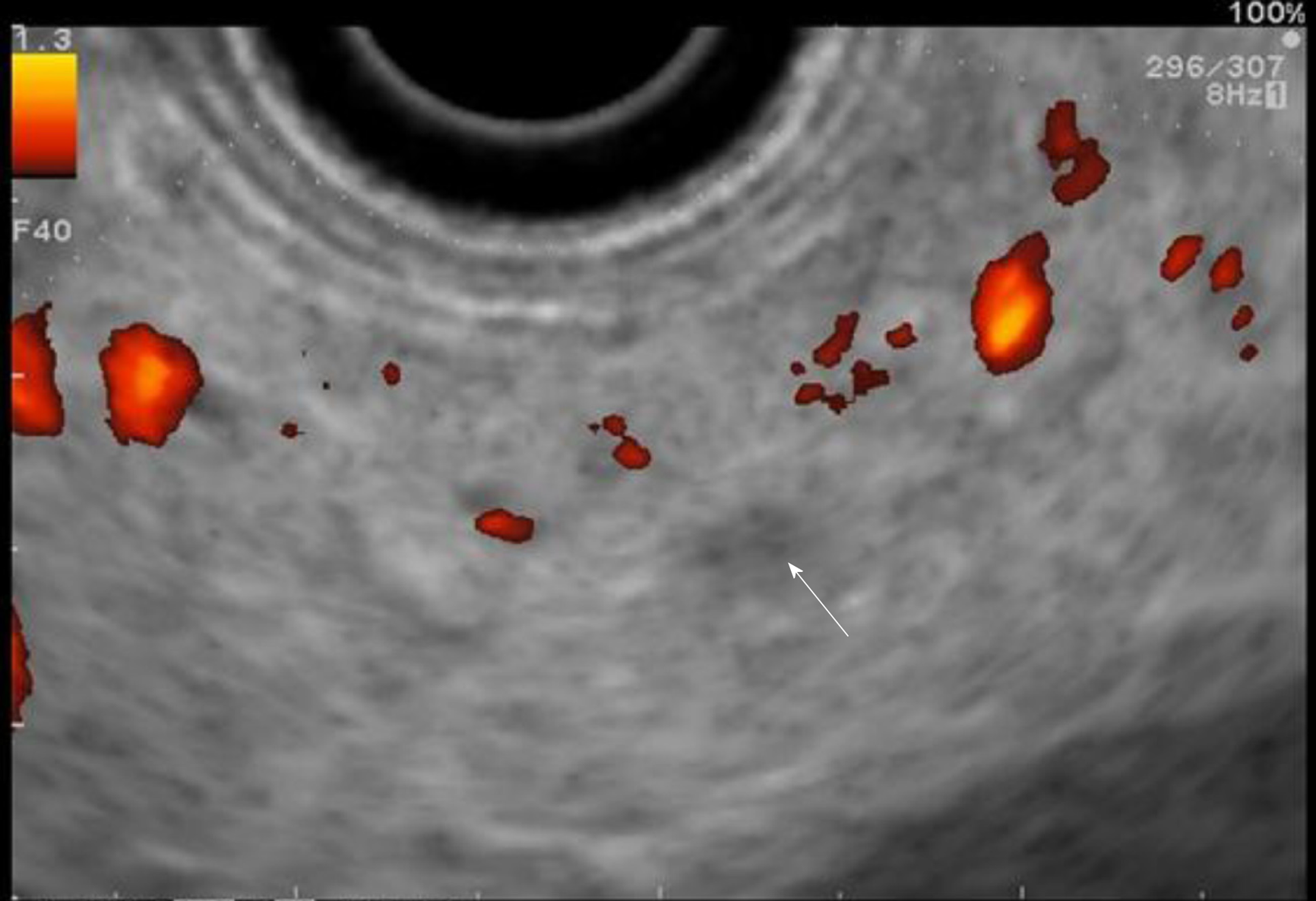
The use of contrast-enhanced harmonic EUS (CH-EUS) could improve its diagnostic performance. Indeed, it can identify solid neoplastic components in pancreatic cysts/IPMN as hyperenhanced lesions (Figure 5)[56]. The pooled sensitivity and specificity of CH-EUS for the diagnosis of PC are very high (91% and 87%, respectively) in the general population with solid pancreatic neoplasms[56,57]. Nevertheless, there is no specific study on the use of CH-EUS for pancreatic screening in HRI.
EUS elastography could also be useful for the characterization of solid pancreatic lesions in this population[58]. Iglesias-Garcia et al[59] reported a sensitivity, a specificity, and positive and negative predictive values of 100%, 85.5%, 90.7%, and 100%, respectively, for the diagnosis of sporadic PC.
The results of certain new techniques for the detection of (pre)malignant pancreatic lesions are especially promising in HRI, including the molecular imaging of cathepsin E in vivo using a confocal laser endomicroscopy miniprobe (overexpression of cathepsin E in PanIN) and the detection of DNA abnormalities or protein markers in pancreatic cyst fluid collected by fine needle aspiration (FNA) (mutations in genes such as TP53) (Figure 6)[57,60-63]. The role of these combined strategies (EUS with other new technological/biological techniques) as well as their impact on survival and cost-effectiveness must, however, still be defined[64]. Finally, another lead could also be the identification of molecular abnormalities (p53 mutations, KRAS mutations, DNA methylation markers) associated with the presence of PC and precursors of PC within pancreatic fluid collected by endoscopic retrograde cholangiopancreatography[65-69]. The role of these exams in FPC screening strategies must still be defined[68].
Another advantage of EUS is that FNA or fine needle biopsy (FNB) can be performed in solid pancreatic masses to obtain tissue samples for histopathological cha-racterization with a low risk of complications[11,14,23,33,70]. The complication rate of EUS-FNA is approximately 2%[70]. The sensitivity (84.3%), specificity (97%), and accuracy (84%) of EUS-FNA for solid pancreatic masses are high[71]. However, the negative predictive value is low (64%)[26,71], indicating that a negative FNA does not exclude the presence of a (pre)malignant lesion. Thus, in the presence of a solid pancreatic lesion with negative histological samples, FNA (or FNB) must be repeated in HRI, and prophylactic pancreatectomy should be discussed. The use of cutting needles for EUS-FNB should improve pancreatic histological samples[72]. In addition, EUS-FNA is associated with a non-negligible rate of false positives (2% in the literature, up to 33% in HRI, especially in patients with CPis). Thus, the diagnostic value of FNA may be limited in HRI screening[11].
The theoretical risk of hereditary transmission of PC-predisposing genetic abnormalities is up to 50%. Hence, in the setting of FPC without a known predisposing gene mutation, approximately 50% of HRI undergo screening theoretically without predisposing genetic abnormalities. Thus, the detection of any cystic lesions or CPis is important in FPC-related HRI without identified mutations because it probably indicates that this subject carries the unidentified mutation and is hence at risk of PC.
Most PC arise from PanIN lesions, with a delay of approximately ten years[22]. The challenge of screening is being able to propose surgery at the stage of high-grade PanINs. Some aspects of high-grade PanINs have been described with MRI and EUS, but these lesions lack specificity[23,51,73]. Our group and others previously reported that CPis (microcysts and/or hyperechoic foci of fibrosis) visualized at EUS were associated with PanIN (in up to 83% of cases)[51,54,74]. In an American study, the odds ratio for the association between intermediate-grade PanIN and hyperechoic foci without shadowing in the pancreas head was 8.5 (P = 0.05)[74]. This aspect would concern approximately 70% of HRI[12,23]. However, the assessment of such abnormalities might suffer from a lack of recognition, and the correlation between EUS aspect and pathology should be assessed in a large series of HRI[28,60].
Several studies have reported an increased risk of PC in patients with pancreatic cysts, whatever the etiology[73-75]. In some cases, and especially in HRI, MRI with MRCP and diffusion-weighted MR sequences can help identify very small cysts that do not communicate with the pancreatic ducts[73,74]. In a recent MRI study that included 100 patients, our group recently showed that the identification of non-communicating pancreatic microcysts had a 52.3% sensitivity, a 77.1% specificity, and a 61% accuracy for the diagnosis of PanIN[73]. In the same study, the association of global atrophy and non-communicating microcysts increased the predictive risk of PanIN. Interobserver agreement for the presence of microcysts was excellent with MRI (kappa = 0.92)[73].
The psychological burden in HRI of PC is significant because of the patient’s level of risk and his/her experience with close relatives suffering from this severe disease[15]. Patients must be informed that the aim of screening is to identify premalignant lesions or early invasive PC to propose a pancreatectomy with prophylactic and/or curative intent. Additionally, they must be informed of the potential benefits (avoiding the development of PC, being treated at a curable stage, or at least diagnosing PC at the earliest stage possible and prolonging survival) and risks (related to general anaesthesia and/or EUS and especially FNA, unnecessary pancreatic surgery with a risk of complications and diabetes) of pancreatic screening[17]. Moreover, because the sensitivity of the different imaging examinations is not 100%, patients must be informed of the risk of missing (pre)malignant lesions and developing advanced PC during follow-up intervals.
Several studies have investigated the psychological burden of pancreatic screening and repeated examinations. Overall, pancreatic screening may have a positive psychological impact on HRI[76]. Konings et al[48] reported a low psychological burden due to the examinations themselves, which were considered uncomfortable only in 10% of cases. Of note, although it is an invasive procedure, EUS was generally not considered to be more uncomfortable than MRI[48,76].
It is difficult to assess the cost-effectiveness of pancreatic screening in HRI because of the different screening practices in different countries and the results of screening in different studies[10,11,17,18,23,24,40]. A screening test can generally be considered acceptable if there is a positive benefit/cost ratio. Rulyak et al[38] compared one-time EUS-based screening to no screening in a hypothetical cohort of 100 HRI. They showed that screening increased life expectancy (38 years) in a cost-effective manner ($ 16885 per life-year saved). Latchford et al[77] created a model that showed that seven PCs would theoretically be detected in 250 patients who would undergo yearly EUS between the age of 40 and 55 years old. The cost would be $ 164.285 dollars per PC detected and $ 372708 per life saved. Overall, it is not clear whether EUS-based screening can be considered cost-effective[60].
PC is inherited in 5%-10% of cases. HRI of PC can benefit from pancreatic screening mainly based on annual MRI and EUS. Successful screening targets are early invasive PC and IPMN or PanIN with HGD, which may be treated surgically with curative intent. These lesions are identified in 2% to 5% of all HRI undergoing screening. EUS appears to be the best examination to identify small solid lesions and is complementary to MRI, whose performance may be higher for identifying small cystic lesions. Pancreatic abnormalities found by EUS are cystic lesions (13%-49% of HRI), solid lesions (0%-46%) and chronic pancreatitis-like parenchymal changes (> 50%). Of note, the latter frequently correspond to PanIN lesions. Finally, CH-EUS, EUS-elastography and other new techniques (including needle-based confocal laser endomicroscopy miniprobe and the detection of DNA abnormalities or protein markers by FNA) are being developed, but further studies are needed to evaluate their role in the management of HRI. There is still limited evidence on the accuracy, acceptability and cost of screening as it is currently recommended. HRI undergoing screening should continue to be included in large cohorts, such as the CAPS consortium (http://caps-registry.com), which is a major opportunity to improve PC screening.
Areas of currently unmet needs within the scope of hereditary PC include the following: (1) To constitute large cohorts of HRI undergoing long-term prospective follow-up; (2) To study the correlation between the pancreatic abnormalities identified at imaging (MRI and EUS) and the lesions identified at the pathological examination of surgically resected specimens; (3) To develop specific EUS and MRI training modules to improve the recognition of pancreatic lesions in HRI and interobserver agreement; (4) To develop new techniques such as biomarkers or new nuclear imaging techniques; and (5) To study more comprehensively the consequences of screening in terms of cost-effectiveness, psychological burden, and long-term surgical consequences in operated HRI.
| 1. | Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5379] [Cited by in RCA: 5369] [Article Influence: 447.4] [Reference Citation Analysis (0)] |
| 2. | Neuzillet C, Tijeras-Raballand A, Bourget P, Cros J, Couvelard A, Sauvanet A, Vullierme MP, Tournigand C, Hammel P. State of the art and future directions of pancreatic ductal adenocarcinoma therapy. Pharmacol Ther. 2015;155:80-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Harinck F, Poley JW, Kluijt I, Fockens P, Bruno MJ; Dutch Research Group of Pancreatic Cancer Surveillance in High-Risk Individuals. Is early diagnosis of pancreatic cancer fiction? Surveillance of individuals at high risk for pancreatic cancer. Dig Dis. 2010;28:670-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Bartsch DK, Slater EP, Carrato A, Ibrahim IS, Guillen-Ponce C, Vasen HF, Matthäi E, Earl J, Jendryschek FS, Figiel J, Steinkamp M, Ramaswamy A, Vázquez-Sequeiros E, Muñoz-Beltran M, Montans J, Mocci E, Bonsing BA, Wasser M, Klöppel G, Langer P, Fendrich V, Gress TM. Refinement of screening for familial pancreatic cancer. Gut. 2016;65:1314-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Stoffel EM. Screening in GI Cancers: The Role of Genetics. J Clin Oncol. 2015;33:1721-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Canto MI, Harinck F, Hruban RH, Offerhaus GJ, Poley JW, Kamel I, Nio Y, Schulick RS, Bassi C, Kluijt I, Levy MJ, Chak A, Fockens P, Goggins M, Bruno M; International Cancer of Pancreas Screening (CAPS) Consortium. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2013;62:339-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 546] [Cited by in RCA: 576] [Article Influence: 44.3] [Reference Citation Analysis (5)] |
| 7. | Kastrinos F, Mukherjee B, Tayob N, Wang F, Sparr J, Raymond VM, Bandipalliam P, Stoffel EM, Gruber SB, Syngal S. Risk of pancreatic cancer in families with Lynch syndrome. JAMA. 2009;302:1790-1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 389] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 8. | Giardiello FM, Brensinger JD, Tersmette AC, Goodman SN, Petersen GM, Booker SV, Cruz-Correa M, Offerhaus JA. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 961] [Cited by in RCA: 876] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 9. | Hu C, Hart SN, Polley EC, Gnanaolivu R, Shimelis H, Lee KY, Lilyquist J, Na J, Moore R, Antwi SO, Bamlet WR, Chaffee KG, DiCarlo J, Wu Z, Samara R, Kasi PM, McWilliams RR, Petersen GM, Couch FJ. Association Between Inherited Germline Mutations in Cancer Predisposition Genes and Risk of Pancreatic Cancer. JAMA. 2018;319:2401-2409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 432] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 10. | Mocci E, Guillen-Ponce C, Earl J, Marquez M, Solera J, Salazar-López MT, Calcedo-Arnáiz C, Vázquez-Sequeiros E, Montans J, Muñoz-Beltrán M, Vicente-Bártulos A, González-Gordaliza C, Sanjuanbenito A, Guerrero C, Mendía E, Lisa E, Lobo E, Martínez JC, Real FX, Malats N, Carrato A. PanGen-Fam: Spanish registry of hereditary pancreatic cancer. Eur J Cancer. 2015;51:1911-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | de Mestier L, Muller M, Cros J, Vullierme M-P, Vernerey D, Maire F, Dokmak S, Rebours V, Sauvanet A, Lévy P, Hammel P. Appropriateness of pancreatic resection in high-risk individuals for familial pancreatic ductal adenocarcinoma: a patient-level meta-analysis and proposition of the Beaujon score. United Eur Gastroenterol J. 2019;7:358-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Canto MI, Goggins M, Yeo CJ, Griffin C, Axilbund JE, Brune K, Ali SZ, Jagannath S, Petersen GM, Fishman EK, Piantadosi S, Giardiello FM, Hruban RH. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin Gastroenterol Hepatol. 2004;2:606-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 316] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 13. | Poley JW, Kluijt I, Gouma DJ, Harinck F, Wagner A, Aalfs C, van Eijck CH, Cats A, Kuipers EJ, Nio Y, Fockens P, Bruno MJ. The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol. 2009;104:2175-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 188] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 14. | Sud A, Wham D, Catalano M, Guda NM. Promising outcomes of screening for pancreatic cancer by genetic testing and endoscopic ultrasound. Pancreas. 2014;43:458-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Langer P, Kann PH, Fendrich V, Habbe N, Schneider M, Sina M, Slater EP, Heverhagen JT, Gress TM, Rothmund M, Bartsch DK. Five years of prospective screening of high-risk individuals from families with familial pancreatic cancer. Gut. 2009;58:1410-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 174] [Article Influence: 10.2] [Reference Citation Analysis (2)] |
| 16. | Zubarik R, Gordon SR, Lidofsky SD, Anderson SR, Pipas JM, Badger G, Ganguly E, Vecchio J. Screening for pancreatic cancer in a high-risk population with serum CA 19-9 and targeted EUS: a feasibility study. Gastrointest Endosc. 2011;74:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Stoffel EM, McKernin SE, Khorana AA. Evaluating Susceptibility to Pancreatic Cancer: ASCO Clinical Practice Provisional Clinical Opinion Summary. J Oncol Pract. 2019;15:108-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Signoretti M, Bruno MJ, Zerboni G, Poley JW, Delle Fave G, Capurso G. Results of surveillance in individuals at high-risk of pancreatic cancer: A systematic review and meta-analysis. United European Gastroenterol J. 2018;6:489-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Corral JE, Mareth KF, Riegert-Johnson DL, Das A, Wallace MB. Diagnostic Yield From Screening Asymptomatic Individuals at High Risk for Pancreatic Cancer: A Meta-analysis of Cohort Studies. Clin Gastroenterol Hepatol. 2019;17:41-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 20. | Das KK, Early D. Pancreatic Cancer Screening. Curr Treat Options Gastroenterol. 2017;15:562-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Rebours V, Lévy P, Mosnier JF, Scoazec JY, Soubeyrand MS, Fléjou JF, Turlin B, Hammel P, Ruszniewski P, Bedossa P, Couvelard A. Pathology analysis reveals that dysplastic pancreatic ductal lesions are frequent in patients with hereditary pancreatitis. Clin Gastroenterol Hepatol. 2010;8:206-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, Velculescu VE, Kinzler KW, Vogelstein B, Iacobuzio-Donahue CA. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114-1117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2041] [Cited by in RCA: 1983] [Article Influence: 123.9] [Reference Citation Analysis (0)] |
| 23. | Canto MI, Goggins M, Hruban RH, Petersen GM, Giardiello FM, Yeo C, Fishman EK, Brune K, Axilbund J, Griffin C, Ali S, Richman J, Jagannath S, Kantsevoy SV, Kalloo AN. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766-81; quiz 665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 369] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 24. | Yasuda I, Iwashita T, Doi S, Nakashima M, Moriwaki H. Role of EUS in the early detection of small pancreatic cancer. Dig Endosc. 2011;23 Suppl 1:S22-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, Zhang Z, Topazian M, Takahashi N, Fletcher J, Petersen G, Klein AP, Axilbund J, Griffin C, Syngal S, Saltzman JR, Mortele KJ, Lee J, Tamm E, Vikram R, Bhosale P, Margolis D, Farrell J, Goggins M; American Cancer of the Pancreas Screening (CAPS) Consortium. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 504] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 26. | Capurso G, Signoretti M, Valente R, Arnelo U, Lohr M, Poley JW, Delle Fave G, Del Chiaro M. Methods and outcomes of screening for pancreatic adenocarcinoma in high-risk individuals. World J Gastrointest Endosc. 2015;7:833-842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Harinck F, Konings IC, Kluijt I, Poley JW, van Hooft JE, van Dullemen HM, Nio CY, Krak NC, Hermans JJ, Aalfs CM, Wagner A, Sijmons RH, Biermann K, van Eijck CH, Gouma DJ, Dijkgraaf MG, Fockens P, Bruno MJ; Dutch research group on pancreatic cancer surveillance in high-risk individuals. A multicentre comparative prospective blinded analysis of EUS and MRI for screening of pancreatic cancer in high-risk individuals. Gut. 2016;65:1505-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 137] [Article Influence: 13.7] [Reference Citation Analysis (1)] |
| 28. | Topazian M, Enders F, Kimmey M, Brand R, Chak A, Clain J, Cunningham J, Eloubeidi M, Gerdes H, Gress F, Jagannath S, Kantsevoy S, LeBlanc JK, Levy M, Lightdale C, Romagnuolo J, Saltzman JR, Savides T, Wiersema M, Woodward T, Petersen G, Canto M. Interobserver agreement for EUS findings in familial pancreatic-cancer kindreds. Gastrointest Endosc. 2007;66:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Shin EJ, Topazian M, Goggins MG, Syngal S, Saltzman JR, Lee JH, Farrell JJ, Canto MI. Linear-array EUS improves detection of pancreatic lesions in high-risk individuals: a randomized tandem study. Gastrointest Endosc. 2015;82:812-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Canto MI, Almario JA, Schulick RD, Yeo CJ, Klein A, Blackford A, Shin EJ, Sanyal A, Yenokyan G, Lennon AM, Kamel IR, Fishman EK, Wolfgang C, Weiss M, Hruban RH, Goggins M. Risk of Neoplastic Progression in Individuals at High Risk for Pancreatic Cancer Undergoing Long-term Surveillance. Gastroenterology. 2018;155:740-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 327] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 31. | Vasen H, Ibrahim I, Ponce CG, Slater EP, Matthäi E, Carrato A, Earl J, Robbers K, van Mil AM, Potjer T, Bonsing BA, de Vos Tot Nederveen Cappel WH, Bergman W, Wasser M, Morreau H, Klöppel G, Schicker C, Steinkamp M, Figiel J, Esposito I, Mocci E, Vazquez-Sequeiros E, Sanjuanbenito A, Muñoz-Beltran M, Montans J, Langer P, Fendrich V, Bartsch DK. Benefit of Surveillance for Pancreatic Cancer in High-Risk Individuals: Outcome of Long-Term Prospective Follow-Up Studies From Three European Expert Centers. J Clin Oncol. 2016;34:2010-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 277] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 32. | Al-Sukhni W, Borgida A, Rothenmund H, Holter S, Semotiuk K, Grant R, Wilson S, Moore M, Narod S, Jhaveri K, Haider MA, Gallinger S. Screening for pancreatic cancer in a high-risk cohort: an eight-year experience. J Gastrointest Surg. 2012;16:771-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 33. | Verna EC, Hwang C, Stevens PD, Rotterdam H, Stavropoulos SN, Sy CD, Prince MA, Chung WK, Fine RL, Chabot JA, Frucht H. Pancreatic cancer screening in a prospective cohort of high-risk patients: a comprehensive strategy of imaging and genetics. Clin Cancer Res. 2010;16:5028-5037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 34. | Ludwig E, Olson SH, Bayuga S, Simon J, Schattner MA, Gerdes H, Allen PJ, Jarnagin WR, Kurtz RC. Feasibility and yield of screening in relatives from familial pancreatic cancer families. Am J Gastroenterol. 2011;106:946-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 35. | Imbe K, Nagata N, Hisada Y, Takasaki Y, Sekine K, Mishima S, Kawazoe A, Tajima T, Shimbo T, Yanase M, Akiyama J, Fujimoto K, Uemura N. Validation of the American Gastroenterological Association guidelines on management of intraductal papillary mucinous neoplasms: more than 5 years of follow-up. Eur Radiol. 2018;28:170-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Gangi A, Malafa M, Klapman J. Endoscopic Ultrasound-Based Pancreatic Cancer Screening of High-Risk Individuals: A Prospective Observational Trial. Pancreas. 2018;47:586-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Brentnall TA, Bronner MP, Byrd DR, Haggitt RC, Kimmey MB. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann Intern Med. 1999;131:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 274] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 38. | Rulyak SJ, Kimmey MB, Veenstra DL, Brentnall TA. Cost-effectiveness of pancreatic cancer screening in familial pancreatic cancer kindreds. Gastrointest Endosc. 2003;57:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | DaVee T, Coronel E, Papafragkakis C, Thaiudom S, Lanke G, Chakinala RC, Nogueras González GM, Bhutani MS, Ross WA, Weston BR, Lee JH. Pancreatic cancer screening in high-risk individuals with germline genetic mutations. Gastrointest Endosc. 2018;87:1443-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 40. | Schneider R, Slater EP, Sina M, Habbe N, Fendrich V, Matthäi E, Langer P, Bartsch DK. German national case collection for familial pancreatic cancer (FaPaCa): ten years experience. Fam Cancer. 2011;10:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 41. | Roch AM, Schneider J, Carr RA, Lancaster WP, House MG, Zyromski NJ, Nakeeb A, Schmidt CM, Ceppa EP. Are BRCA1 and BRCA2 gene mutation patients underscreened for pancreatic adenocarcinoma? J Surg Oncol. 2019;119:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 42. | Zanini N, Giordano M, Smerieri E, Cipolla d'Abruzzo G, Guidi M, Pazzaglini G, De Luca F, Chiaruzzi G, Vitullo G, Piva P, Lombardi R, Jovine E, Gatti M, Landolfo G. Estimation of the prevalence of asymptomatic pancreatic cysts in the population of San Marino. Pancreatology. 2015;15:417-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 1241] [Article Influence: 137.9] [Reference Citation Analysis (1)] |
| 44. | European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1144] [Cited by in RCA: 979] [Article Influence: 122.4] [Reference Citation Analysis (1)] |
| 45. | Kamata K, Kitano M, Kudo M, Sakamoto H, Kadosaka K, Miyata T, Imai H, Maekawa K, Chikugo T, Kumano M, Hyodo T, Murakami T, Chiba Y, Takeyama Y. Value of EUS in early detection of pancreatic ductal adenocarcinomas in patients with intraductal papillary mucinous neoplasms. Endoscopy. 2014;46:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Felsenstein M, Noë M, Masica DL, Hosoda W, Chianchiano P, Fischer CG, Lionheart G, Brosens LAA, Pea A, Yu J, Gemenetzis G, Groot VP, Makary MA, He J, Weiss MJ, Cameron JL, Wolfgang CL, Hruban RH, Roberts NJ, Karchin R, Goggins MG, Wood LD. IPMNs with co-occurring invasive cancers: neighbours but not always relatives. Gut. 2018;67:1652-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 47. | Petrone MC, Arcidiacono PG, Perri F, Carrara S, Boemo C, Testoni PA. Chronic pancreatitis-like changes detected by endoscopic ultrasound in subjects without signs of pancreatic disease: do these indicate age-related changes, effects of xenobiotics, or early chronic pancreatitis? Pancreatology. 2010;10:597-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 48. | Konings ICAW, Cahen DL, Harinck F, Fockens P, van Hooft JE, Poley JW, Bruno MJ. Evolution of features of chronic pancreatitis during endoscopic ultrasound-based surveillance of individuals at high risk for pancreatic cancer. Endosc Int Open. 2018;6:E541-E548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Thiruvengadam SS, Chuang J, Huang R, Girotra M, Park WG. Chronic pancreatitis changes in high-risk individuals for pancreatic ductal adenocarcinoma. Gastrointest Endosc. 2019;89:842-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 50. | Catalano MF, Sahai A, Levy M, Romagnuolo J, Wiersema M, Brugge W, Freeman M, Yamao K, Canto M, Hernandez LV. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc. 2009;69:1251-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 355] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 51. | Brune K, Abe T, Canto M, O'Malley L, Klein AP, Maitra A, Volkan Adsay N, Fishman EK, Cameron JL, Yeo CJ, Kern SE, Goggins M, Hruban RH. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am J Surg Pathol. 2006;30:1067-1076. [PubMed] [DOI] [Full Text] |
| 52. | Hruban RH, Canto MI, Goggins M, Schulick R, Klein AP. Update on familial pancreatic cancer. Adv Surg. 2010;44:293-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 190] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 53. | Aimoto T, Uchida E, Nakamura Y, Matsushita A, Katsuno A, Chou K, Kawamoto M, Naito Z, Tajiri T. Multicentric pancreatic intraepithelial neoplasias (PanINs) presenting with the clinical features of chronic pancreatitis. J Hepatobiliary Pancreat Surg. 2008;15:549-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 54. | Maire F, Couvelard A, Palazzo L, Aubert A, Vullierme MP, Rebours V, Hammel P, Sauvanet A, Levy P, Ruszniewski P. Pancreatic intraepithelial neoplasia in patients with intraductal papillary mucinous neoplasms: the interest of endoscopic ultrasonography. Pancreas. 2013;42:1262-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 55. | Lesmana CRA, Gani RA, Lesmana LA. Non-alcoholic fatty pancreas disease as a risk factor for pancreatic cancer based on endoscopic ultrasound examination among pancreatic cancer patients: A single-center experience. JGH Open. 2017;2:4-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 56. | Fusaroli P, Napoleon B, Gincul R, Lefort C, Palazzo L, Palazzo M, Kitano M, Minaga K, Caletti G, Lisotti A. The clinical impact of ultrasound contrast agents in EUS: a systematic review according to the levels of evidence. Gastrointest Endosc. 2016;84:587-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 57. | Li XZ, Song J, Sun ZX, Yang YY, Wang H. Diagnostic performance of contrast-enhanced ultrasound for pancreatic neoplasms: A systematic review and meta-analysis. Dig Liver Dis. 2018;50:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Kawada N, Tanaka S. Elastography for the pancreas: Current status and future perspective. World J Gastroenterol. 2016;22:3712-3724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 59. | Iglesias-Garcia J, Larino-Noia J, Abdulkader I, Forteza J, Dominguez-Munoz JE. EUS elastography for the characterization of solid pancreatic masses. Gastrointest Endosc. 2009;70:1101-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 60. | Lami G, Biagini MR, Galli A. Endoscopic ultrasonography for surveillance of individuals at high risk for pancreatic cancer. World J Gastrointest Endosc. 2014;6:272-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 61. | Bhutani MS, Koduru P, Joshi V, Saxena P, Suzuki R, Irisawa A, Yamao K. The role of endoscopic ultrasound in pancreatic cancer screening. Endosc Ultrasound. 2016;5:8-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 62. | Kanda M, Sadakari Y, Borges M, Topazian M, Farrell J, Syngal S, Lee J, Kamel I, Lennon AM, Knight S, Fujiwara S, Hruban RH, Canto MI, Goggins M. Mutant TP53 in duodenal samples of pancreatic juice from patients with pancreatic cancer or high-grade dysplasia. Clin Gastroenterol Hepatol. 2013;11:719-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 63. | Bournet B, Gayral M, Torrisani J, Selves J, Cordelier P, Buscail L. Role of endoscopic ultrasound in the molecular diagnosis of pancreatic cancer. World J Gastroenterol. 2014;20:10758-10768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Bhutani MS, Gupta V, Guha S, Gheonea DI, Saftoiu A. Pancreatic cyst fluid analysis--a review. J Gastrointestin Liver Dis. 2011;20:175-180. [PubMed] |
| 65. | Wong T, Howes N, Threadgold J, Smart HL, Lombard MG, Gilmore I, Sutton R, Greenhalf W, Ellis I, Neoptolemos JP. Molecular diagnosis of early pancreatic ductal adenocarcinoma in high-risk patients. Pancreatology. 2001;1:486-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 66. | Kisiel JB, Raimondo M, Taylor WR, Yab TC, Mahoney DW, Sun Z, Middha S, Baheti S, Zou H, Smyrk TC, Boardman LA, Petersen GM, Ahlquist DA. New DNA Methylation Markers for Pancreatic Cancer: Discovery, Tissue Validation, and Pilot Testing in Pancreatic Juice. Clin Cancer Res. 2015;21:4473-4481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 67. | Takano S, Fukasawa M, Kadokura M, Shindo H, Takahashi E, Hirose S, Maekawa S, Mochizuki K, Kawaida H, Itakura J, Katoh R, Fujii H, Sato T, Enomoto N. Next-Generation Sequencing Revealed TP53 Mutations to Be Malignant Marker for Intraductal Papillary Mucinous Neoplasms That Could Be Detected Using Pancreatic Juice. Pancreas. 2017;46:1281-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 68. | Yan L, McFaul C, Howes N, Leslie J, Lancaster G, Wong T, Threadgold J, Evans J, Gilmore I, Smart H, Lombard M, Neoptolemos J, Greenhalf W. Molecular analysis to detect pancreatic ductal adenocarcinoma in high-risk groups. Gastroenterology. 2005;128:2124-2130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 69. | Matsumoto K, Takeda Y, Harada K, Onoyama T, Kawata S, Horie Y, Sakamoto T, Ueki M, Miura N, Murawaki Y. Clinical Impact of the KL-6 Concentration of Pancreatic Juice for Diagnosing Pancreatic Masses. Biomed Res Int. 2015;2015:528304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 70. | Gonzalo-Marin J, Vila JJ, Perez-Miranda M. Role of endoscopic ultrasound in the diagnosis of pancreatic cancer. World J Gastrointest Oncol. 2014;6:360-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 71. | Eloubeidi MA, Chen VK, Eltoum IA, Jhala D, Chhieng DC, Jhala N, Vickers SM, Wilcox CM. Endoscopic ultrasound-guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: diagnostic accuracy and acute and 30-day complications. Am J Gastroenterol. 2003;98:2663-2668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 284] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 72. | Tian L, Tang AL, Zhang L, Liu XW, Li JB, Wang F, Shen SR, Wang XY. Evaluation of 22G fine-needle aspiration (FNA) versus fine-needle biopsy (FNB) for endoscopic ultrasound-guided sampling of pancreatic lesions: a prospective comparison study. Surg Endosc. 2018;32:3533-3539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 73. | Vullierme MP, Menassa L, Couvelard A, Rebours V, Maire F, Ibrahim T, Cros J, Ruszniewski P, Sauvanet A, Levy P, Soyer P, Vilgrain V. Non-branched microcysts of the pancreas on MR imaging of patients with pancreatic tumors who had pancreatectomy may predict the presence of pancreatic intraepithelial neoplasia (PanIN): a preliminary study. Eur Radiol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 74. | LeBlanc JK, Chen JH, Al-Haddad M, Luz L, McHenry L, Sherman S, Juan M, Dewitt J. Can endoscopic ultrasound predict pancreatic intraepithelial neoplasia lesions in chronic pancreatitis?: a retrospective study of pathologic correlation. Pancreas. 2014;43:849-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 75. | Munigala S, Gelrud A, Agarwal B. Risk of pancreatic cancer in patients with pancreatic cyst. Gastrointest Endosc. 2016;84:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 76. | Cazacu IM, Luzuriaga Chavez AA, Saftoiu A, Bhutani MS. Psychological impact of pancreatic cancer screening by EUS or magnetic resonance imaging in high-risk individuals: A systematic review. Endosc Ultrasound. 2019;8:17-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 77. | Latchford A, Greenhalf W, Vitone LJ, Neoptolemos JP, Lancaster GA, Phillips RK. Peutz-Jeghers syndrome and screening for pancreatic cancer. Br J Surg. 2006;93:1446-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 78. | Kimmey MB, Bronner MP, Byrd DR, Brentnall TA. Screening and surveillance for hereditary pancreatic cancer. Gastrointest Endosc. 2002;56:S82-S86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
Manuscript source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Friedel D, Vagholkar KR S-Editor: Tang JZ L-Editor: A E-Editor: Zhang YL