Published online Sep 7, 2019. doi: 10.3748/wjg.v25.i33.4796
Peer-review started: May 30, 2019
First decision: July 21, 2019
Revised: July 30, 2019
Accepted: August 7, 2019
Article in press: August 7, 2019
Published online: September 7, 2019
Processing time: 101 Days and 20.9 Hours
Inflammasomes are multiprotein intracellular complexes which are responsible for the activation of inflammatory responses. Among various subtypes of inflammasomes, NLRP3 has been a subject of intensive investigation. NLRP3 is considered to be a sensor of microbial and other danger signals and plays a crucial role in mucosal immune responses, promoting the maturation of proinflammatory cytokines interleukin 1β (IL-1β) and IL-18. NLRP3 inflammasome has been associated with a variety of inflammatory and autoimmune conditions, including inflammatory bowel diseases (IBD). The role of NLRP3 in IBD is not yet fully elucidated as it seems to demonstrate both pathogenic and protective effects. Studies have shown a relationship between genetic variants and mutations in NLRP3 gene with IBD pathogenesis. A complex interaction between the NLRP3 inflammasome and the mucosal immune response has been reported. Activation of the inflammasome is a key function mediated by the innate immune response and in parallel the signaling through IL-1β and IL-18 is implicated in adaptive immunity. Further research is needed to delineate the precise mechanisms of NLRP3 function in regulating immune responses. Targeting NLRP3 inflammasome and its downstream signaling will provide new insights into the development of future therapeutic strategies.
Core tip: NLRP3 inflammasome plays a major role in inflammatory bowel diseases (IBD) pathogenesis through its contribution to chronic inflammatory processes. Abnormal activation of NLRP3 inflammasome has been observed in inflamed tissue of IBD murine models and patients, highlighting its possible pathogenic role in the disease. However, protective effects of NLRP3 function have also been recorded. The pathogenic NLRP3 inflammasome activity in mucosal immune system may be implicated in the aberrant immune responses and in the disruption of intestinal homeostasis that characterizes IBD. Targeting NLRP3 inflammasome and its downstream signaling will provide new insights into the development of future therapeutic strategies.
- Citation: Tourkochristou E, Aggeletopoulou I, Konstantakis C, Triantos C. Role of NLRP3 inflammasome in inflammatory bowel diseases. World J Gastroenterol 2019; 25(33): 4796-4804
- URL: https://www.wjgnet.com/1007-9327/full/v25/i33/4796.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i33.4796
The innate immune system is the first-line host defense specified to recognize specific microbial pathogens, named pathogen-associated molecular patterns and damage-associated molecular patterns, and to sense microbial and other danger signals. These functions occur in macrophages, neutrophils, monocytes, dendritic cells (DCs), and epithelial cells through host pattern recognition receptors, such as toll-like receptors and nucleotide-binding domain leucine-rich repeat-containing receptors (NLRs)[1-4]. NLRs play a critical role in innate immune responses and intestinal tissue repair[1].
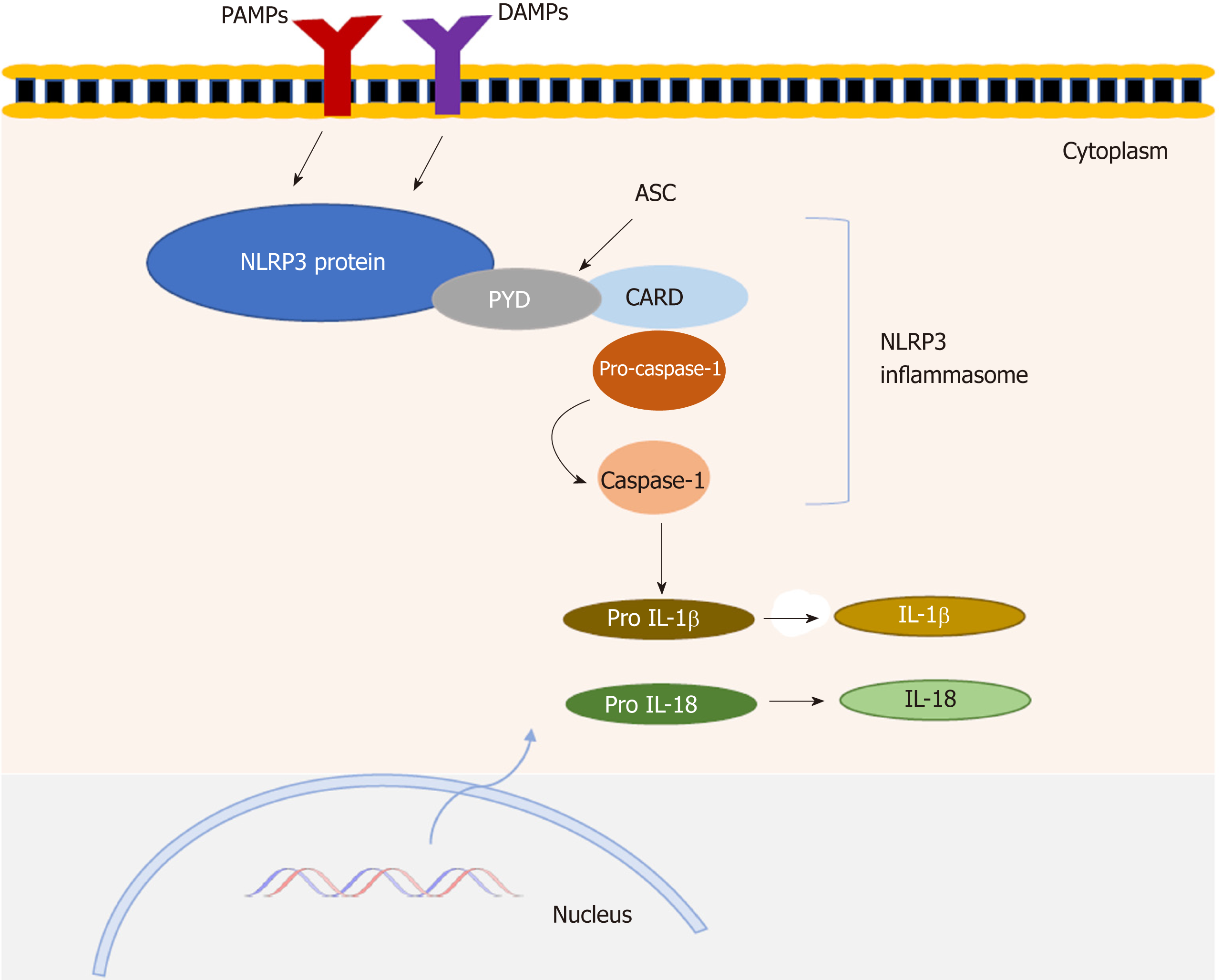
The NLRP (NOD-like receptor family, pyrin domain-containing) subfamily comprises several subtypes and NLRP3 is one of the best-characterized. The multiprotein complex of NLRP3, called the NLRP3 “inflammasome”, consists of three major components—the sensor NLRP3 protein, the adaptor-apoptosis-associated speck-like protein containing a N-terminal PYRIN-PAAD-DAPIN domain and a C-terminal caspase recruitment domain (CARD) (ASC) and the effector protein-caspase-1[5,6]. Activation of NLRP3 occurs when the host is subjected to an exogenous or endogenous stimulus, resulting in the recruitment of ASC and caspase 1. The stimulated NLRP3 interacts with ASC and pro-caspase-1 binds to ASC via CARD to assemble into a large cytosolic complex, which triggers activation of caspase-1. Active caspase-1 cleaves the pro-inflammatory cytokines interleukin 1 β (IL-1β) and IL-18 from their precursors to their biologically active forms[7]. These cytokines induce inflammation by promoting the production of proinflammatory cytokines, chemokines and growth factors (Figure 1), as well as recruiting and activating other immune cells. NLRP3 inflammasome has been associated with a variety of inflammatory and autoimmune conditions including inflammatory bowel diseases (IBD)[8,9]. Crohn’s disease (CD) and ulcerative colitis (UC) are the main types of IBD. UC is usually limited to the colon and consists of diffuse mucosal inflammation, whereas CD can involve inflammation at any part of the gastrointestinal tract (from mouth to anus)[10,11]. Although the etiology of IBD pathogenesis is not fully elucidated, it has been widely suggested that a genetic-environmental mediated dysregulation of the mucosal immune response is implicated in these diseases. The NLRP3 inflammasome, acting as a sensor of microbial and other danger signals, plays a fundamental role in host defense[12-14]. Recent data have demonstrated the function of NLRP3 inflammasome, not only as a crucial mediator of host defense but also as a critical regulator of intestinal homeostasis[15]. However, the studies on the role of NLRP3 inflammasome in IBD have reported controversial findings.
The exact role of NLRP3 in IBD is not yet fully elucidated as it seems to demonstrate both pathogenic and protective effects. The study by Bauer et al[16] was conducted in two IBD models (dextran sulfate sodium and 2,4,6-trinitrobenzene sulfonic acid induced colitis) and showed that mice with NLRP3 deficiency [NLRP3(-/-)] exhibited attenuated colitis. This result was followed by increased numbers of immunosuppressive CD103+ tolerogenic DCs[16]. An abnormal NLRP3 activation has also been reported to play an important pathogenic role in IBD, in a study using a murine IBD model[17]. In this report, NLRP3 and ASC protein levels were significantly elevated in the colonic mucosa of deficient mice for anti-inflammatory cytokine IL-10 (IL-10-/-) before the onset of colitis compared to the wild type (WT) mice[17]. Other studies using spontaneous colitis mice showed that the inhibition of caspase-1 activity and the selected blockade of NLRP3 complex ameliorated colonic inflammation and were associated with decreased colitis[18,19]. In contrast, a protective role of NLRP3 inflammasome was recorded in studies presenting that mice with NLRP3, ASC or caspase-1 deficiency exhibited more severe experimental colitis and decreased intestinal epithelial integrity[20,21]. The association of NLRP3 deficiency with IBD severity was also highlighted in an oxazolone-induced colitis murine model, mediated by T helper 2 (Th2) cytokines (IL-4, IL-13)[22]. Th2 cytokines, such as IL-4 and IL-13, were increased at mRNA and protein level in NLRP3-/- mice compared to WT mice[22]. NLRP3-/- and caspase 1-/- mice exhibited severe colitis after oxazolone treatment compared to WT mice[22]. Administration of IL-1β or IL-18 prevented progression of colitis in NLRP3-/- mice, but did not affect the severity of colitis in WT mice[22].
Therapeutic strategies targeting NLRP3 activity are used in IBD murine models, highlighting the potent clinical relevance of NLRP3 in the disease. A micro-RNA (miR-223) has been shown to be an important therapeutic target for IBD[23]. It regulates the NLRP3 inflammasome activity, by interfering and inhibiting mRNA expression of NLRP3 gene[23]. Treatment of experimental colitis mice with nanoparticles for overexpression of miR-223 ameliorated colitis symptoms and caused a decrease in protein levels of NLRP3 and IL-1β[23]. The therapeutic potential of the blockade of IL-1β and IL-18 cytokines has also been reported. Experimental colitis mice with genetic and pharmacological deficiency of IL-1β and IL-18 exhibited attenuated colitis[24]. Lastly, in a murine colitis model, suppression of pyroptosis signaling through Cholecalciterol Cholesterol Emulsion was associated with ameliorated disease[25].
Human data have demonstrated that the NLRP3 inflammasome activity plays a key part in IBD pathogenesis. Lazaridis et al[26], presented that ex vivo NLRP3 inflammasome was activated in CD patients whereas in UC patients NLRP3 activation occurred in late disease stage compared to controls. This finding was combined with an in vitro increase in IL-1β concentrations in peripheral blood mononuclear cells of CD patients compared to UC patients and controls[26]. A recent study has displayed an upregulation of NLRP3 components in both CD and UC patients as increased mRNA expression of NLRP3, IL-1β, ASC and Caspase-1 was observed in their colonic biopsies; this result was associated with increased disease activity[27]. Inhibition of NLRP3 inflammasome in CD patients resulted in suppressive response of proinflammatory cytokines and chemokines, emphasizing the pathogenic contribution of NLRP3 aberrant activation in the disease[17].
Moreover, IL-1β and IL-18 cytokines have been increased in plasma and colonic mucosa of IBD patients[28,29]. Increased IL-1β secretion from colonic tissues and macrophages in IBD patients has been correlated to the severity of the disease, by promoting chronic intestinal inflammation[27,28]. In a study encompassing children with IBD, the balance between IL-18 cytokine and its natural inhibitor IL-18-binding protein (IL-18BP) has been involved in IBD pathogenesis[30]. Specific microRNAs have been correlated to UC activity based on a high-throughput profiling of blood serum microRNAs of UC patients[31]. A major involvement of microRNAs in IBD development, through interfering NLRP3 activity, has been observed. NLRP3 deficiency in IBD patients, caused by a microRNA, called miR-223, which inhibits NLRP3 gene expression, was associated with active inflammation state in IBD, as increased miR-223 levels were observed in mucosal biopsies[23,32].
Clinical studies which target NLRP3 inflammasome activity are limited[33,34]. Curcumin, an NLRP3 inhibitor acting by interfering the inflammasome-mediated secretion of IL-1β and activation of caspase-1, has been proven to be a potential and safe agent for the treatment of UC[35]. The use of curcumin combined with mesalamine in UC patients was linked to clinical improvement and endoscopic remission[36,37].
The controversial data on the NLRP3 activity in IBD reveal the complicated and probably diverse role of NLRP3 inflammasome in IBD. The NLRP3 activation seems to be a major characteristic in inflamed tissue of IBD murine models and patients, as high expression levels of its components have been observed. Activation of NLRP3 inflammasome constitutes a crucial step in the initiation of inflammatory processes, which results in tissue damage and IBD clinical manifestations development. Thus, NLRP3 pathogenic effect may be due to increased or aberrant activity of the complex. The etiological factors of inflammasome abnormal activity remain to be clarified. Furthermore, the possibility of the NLRP3 inflammasome exerting a protective function during inflammation as a compensatory mechanism of maintaining intestinal homeostasis, should be investigated. Study on molecular regulation of NLRP3 inflammasome activity during inflammation will provide useful knowledge in development of therapeutic approaches in IBD. Activation of NLRP3 inflammasome as well as endpoints of this process (IL-1β, IL-18, pyroptosis) seem to be promising therapeutic options which need further research.
Numerous genetic studies have been performed to explain the association of genetic variants in NLRP3 inflammasome with IBD pathogenesis. These data suggest that NLRP3 inflammasome dysregulation may have a prominent role in the pathogenesis of IBD. Genetic variations could be responsible for NLRP3 enhanced or reduced activity, affecting the microenvironment balance and inflammatory state in pathological conditions such as IBD. Mutations or polymorphisms in NLRP3 have been associated with inflammatory diseases[38-40].
Specific single nucleotide polymorphisms (SNPs), which are located in a regulatory region downstream the NLRP3 gene, have been linked to CD susceptibility. These SNPs have been related to hypoproduction of IL-1β and decreased NLRP3 expression[41]. However, a panel study showed no significant associations among SNPs in the regulatory region of NLRP3 and CD pathogenesis[42]. Another SNP analysis in CD and UC patients of Chinese Han population demonstrated an association between two SNPs in NLRP3 gene, with susceptibility to UC but not to CD[43].
A mutation affecting a component of NLRP3 inflammasome could also contribute to susceptibility to IBD. A study in CD patients, who carry a loss-of-function mutation of T60 CARD8, a negative regulator of inflammasome activation, has reported increased NLRP3 inflammasome activity and excessive production of IL-1β and IL-18 by monocytes[44]. This mutation resulted in decreased overall CARD8 function, which normally regulates negatively NLRP3 activation by inhibiting its oligomerization[44]. A combination of polymorphisms in NLRP3 and CARD8 genes has been linked to high risk of developing CD in men[45].
Nevertheless, hyperactivation of NLRP3 inflammasome has been suggested to be a protective mechanism against colitis in a murine model[46]. Particularly, genetically modified mice carrying the NLRP3 R258W mutation, which induces hyperactivation of NLRP3 inflammasome, were strongly resistant to experimental colitis[46]. This result was due to an excess of local IL-1β production, but not IL-18, which causes the intestinal microbiota to induce local regulatory T cells (Tregs) , maintaining intestinal homeostasis[46].
Mutations or polymorphisms related to NLRP3 inflammasome genes contribute to IBD susceptibility in various ways. It has been reported that an aberrant activity of NLRP3 inflammasome in IBD may be due to a specific genetic background. Further studies, which will elucidate the link among NLRP3 inflammasome associated genes, genetic susceptibility and the molecular function and mechanisms of NLRP3 inflammasome, will provide new insights into the field of IBD pathogenesis.
There is a complex interaction among the NLRP3 inflammasome, the mucosal immune response and the gut homeostasis. A disrupted inflammasome signaling may result in dysbiosis and increased colonization of pathobionts. Intestinal microbiota plays a crucial role in regulating gut homeostasis[47,48]. Alterations in the microbiota composition initiate aberrant innate immune responses[49]. Microbiota infiltrates into the lamina propria and recruits immune cells which secrete cytokines, chemokines and antimicrobial agents promoting inflammation[49]. NLRP3 inflammasome may also lead to death of innate cells such as macrophages and DCs by triggering a caspace-1-dependent form of cell death, called pyroptosis[50]. Thus, NLRP3 inflammasome can have a dual role in IBD pathogenesis, related to initiation and maintenance of inflammation. Firstly, a disrupted NLRP3 signaling may alter the colonization of intestinal microbiota, causing dysbiosis, a crucial condition for IBD development. Secondly, NLRP3 inflammasome through pyroptosis may promote a vicious circle of inflammation, leading to tissue destruction due to consecutive release of cellular debris, which will reactivate immune cells.
Aberrant Th cell responses play a major role in IBD pathogenesis. In particular, chronic inflammation in CD has been associated with Th1 immune responses. High levels of Th1 cytokines and high expression of transcription factors and cytokine receptors that promote Th1 cell development, have been reported[51]. Moreover, it has been noted that dysfunction of immunosuppressive Th cells, such as Tregs and Th3 cells, may constitute a pathogenic factor for CD. Th17 cells have an important role in IBD, and especially in CD, by stimulating intestinal inflammation and regulating the integrity of epithelial cell barrier[52]. By contrast, UC is considered to be a Th2 driven disease, as inflamed tissue in UC patients expresses high levels of Th2-associated cytokines[53,54].
NLRP3 protein, which is crucial for NLRP3 inflammasome formation, has been proven to be a key regulator in Th2 differentiation. Bruchard et al[55] supported that NLRP3 expression in naïve CD4+ T cells induced a Th2 immune profile in a mouse model. NLRP3 protein can act as a transcriptional factor, regulating the expression of genes associated with the Th2 cells, independently of the inflammasome[55]. IL-1β, which is produced as a result of activation of NLRP3 inflammasome, has been found to contribute to differentiation of Th lymphocytes, such as Th17 and Th1 derived from Th17 cells in vitro and in vivo[56], and to enhance the antigen-driven expansion of naive and memory T cells[57].
The mechanism, by which NLRP3 inflammasome links innate to adaptive immunity in IBD, has not been elucidated. Mak'Anyengo et al[58], using a T cell transfer murine colitis model, examined the role of the NLRP3 inflammasome in DCs’ differentiation, T cell polarization and intestinal inflammation. Intestinal DCs have a significant involvement in antigen presentation, T cell activity and Tregs differentiation. Specifically, intestinal CD103+ DCs have immunosuppressive function and promote Tregs activity[59,60]. Mak'Anyengo et al[58] showed that NLRP3 inflammasome-driven cytokine release of IL-1β led to the induction of Th17 inflammatory immune response[58]. NLRP3-deficient mice with decreased IL-1β levels were protected from colitis due to accumulation of CD103+ DCs. This study suggested that NLRP3 inflammasome acts as a checkpoint regulator of IL-1β and IL-18 in the intestine, controlling the secretion of DC-expanding cytokines by T cells in vitro and in vivo[58].
NLRP3 inflammasome activation results in the maturation of proinflammatory cytokines IL-1β and IL-18. IL-1β has a multifunctional role in immune responses, inducing cytokine production, enhancing T cell activation and antigen recognition, and directing innate immune cells to the site of infection[61,62]. Increased levels of IL-1β have been recorded in IBD patients and mice models and have been associated with severity of disease[28]. IL-1β signaling is required for the development of acute inflammation in both T cell–independent and T cell–mediated colitis[28]. The pathogenic activity of IL-1β in IBD has been shown to induce the accumulation of IL-17A producing cells and Th17 inflammatory responses. However, the dominant role of IL-1β in IBD development has not been fully determined[28,63]. IL-1β signaling induces activation of nuclear factor kappa light chain enhancer of activated B cells (NF-κB) and mitogen-activated protein kinase signaling cascades which results in the transcriptional activation of genes encoding cytokines, chemokines and a variety of pro-inflammatory mediators[64,65].
IL-18 is another cytokine which belongs to the IL-1 family of cytokines. Constant expression of IL-18 has been proposed to be important for the maintenance of epithelial integrity. IL-18 can promote barrier function in the intestine, controlling the outgrowth of colitogenic bacteria[66]. The role of IL-18 in immune responses has also been noted. It induces interferon gamma (IFN-γ) production by natural killer and T cells in the presence of IL-12, whereas in the absence of IL-12, IL-18 promotes Th2 responses by inducing IL-4 production[67]. Although increased plasma levels of free IL-18 have been reported in CD patients[29], an immunomodulatory activity of this cytokine has been demonstrated in chronic inflammation in IBD[68]. An in vitro analysis of cells isolated from CD lesions showed that IL-18 affects IFN-γ and IL-10 production and apoptosis. T cells isolated from inflamed tissue of CD patients in the presence of IL-18 had increased IFN-γ and decreased IL-10 production compared to controls[68]. Inhibition of IL-18 with recombinant human IL-18 binding protein (rhIL-18BPa) in experimental colitis model was associated with reduced apoptosis of lamina propria CD4+ T cells[68]. Protective function of IL-18 in IBD has been suggested in a T-cell driven colitis model[69]. IL-18R1 receptor expression on CD4+ T cells seems to be crucial for suppression of IL-17 production and Th17 differentiation. In addition, during intestinal inflammation, IL-18/IL-18R1 signaling has been shown to play a key role in Tregs function, by promoting expression of their effector molecules[69].
NLRP3 inflammasome is probably a key point in inflammatory processes that characterize IBD. The differential role of the inflammasome in IBD is supported by controversial findings about its protective and pathogenic activity; NLRP3 inflammasome has either pathogenic activity, the etiological factors of which have not been elucidated, or it acquires a protective function during the disease, being a compensatory mechanism. Further animal and human studies are needed to examine these hypotheses. Specific genetic background may be responsible for the aberrant activity of NLRP3 inflammasome in IBD. Investigation of the link between genetic susceptibility of NLRP3 inflammasome associated genes and molecular regulation of NLRP3 inflammasome, is of particular importance. NLRP3 inflammasome acts as a potent regulator of mucosal immune responses and intestinal homeostasis due to its association with innate and adaptive immunity. Targeting activation of NLRP3 inflammasome and the related endpoints (IL-1β, IL-18, pyroptosis) will provide new insights into the development of novel therapeutic options in IBD.
| 1. | Prossomariti A, Sokol H, Ricciardiello L. Nucleotide-Binding Domain Leucine-Rich Repeat Containing Proteins and Intestinal Microbiota: Pivotal Players in Colitis and Colitis-Associated Cancer Development. Front Immunol. 2018;9:1039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | De Nardo D, De Nardo CM, Latz E. New insights into mechanisms controlling the NLRP3 inflammasome and its role in lung disease. Am J Pathol. 2014;184:42-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 181] [Article Influence: 13.9] [Reference Citation Analysis (9)] |
| 3. | Liston A, Masters SL. Homeostasis-altering molecular processes as mechanisms of inflammasome activation. Nat Rev Immunol. 2017;17:208-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 354] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 4. | Próchnicki T, Latz E. Inflammasomes on the Crossroads of Innate Immune Recognition and Metabolic Control. Cell Metab. 2017;26:71-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 241] [Article Influence: 26.8] [Reference Citation Analysis (2)] |
| 5. | Hauenstein AV, Zhang L, Wu H. The hierarchical structural architecture of inflammasomes, supramolecular inflammatory machines. Curr Opin Struct Biol. 2015;31:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Martinon F, Burns K, Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4047] [Cited by in RCA: 4841] [Article Influence: 201.7] [Reference Citation Analysis (4)] |
| 7. | Próchnicki T, Mangan MS, Latz E. Recent insights into the molecular mechanisms of the NLRP3 inflammasome activation. F1000Res. 2016;5:pii: F1000 Faculty Rev-1469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 8. | Benetti E, Chiazza F, Patel NS, Collino M. The NLRP3 Inflammasome as a novel player of the intercellular crosstalk in metabolic disorders. Mediators Inflamm. 2013;2013:678627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Hutton HL, Ooi JD, Holdsworth SR, Kitching AR. The NLRP3 inflammasome in kidney disease and autoimmunity. Nephrology (Carlton). 2016;21:736-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 191] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 10. | Zhang YZ, Li YY. Inflammatory bowel disease: Pathogenesis. World J Gastroenterol. 2014;20:91-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 751] [Cited by in RCA: 1134] [Article Influence: 94.5] [Reference Citation Analysis (32)] |
| 11. | Ungar B, Kopylov U. Advances in the development of new biologics in inflammatory bowel disease. Ann Gastroenterol. 2016;29:243-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F, van Bruggen R, Tschopp J. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem. 2007;55:443-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 418] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 13. | Lei-Leston AC, Murphy AG, Maloy KJ. Epithelial Cell Inflammasomes in Intestinal Immunity and Inflammation. Front Immunol. 2017;8:1168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 14. | Zhen Y, Zhang H. NLRP3 Inflammasome and Inflammatory Bowel Disease. Front Immunol. 2019;10:276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 487] [Cited by in RCA: 521] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 15. | Zaki MH, Lamkanfi M, Kanneganti TD. The Nlrp3 inflammasome: contributions to intestinal homeostasis. Trends Immunol. 2011;32:171-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 241] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 16. | Bauer C, Duewell P, Lehr HA, Endres S, Schnurr M. Protective and aggravating effects of Nlrp3 inflammasome activation in IBD models: Influence of genetic and environmental factors. Dig Dis. 2012;30 Suppl 1:82-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 17. | Liu L, Dong Y, Ye M, Jin S, Yang J, Joosse ME, Sun Y, Zhang J, Lazarev M, Brant SR, Safar B, Marohn M, Mezey E, Li X. The Pathogenic Role of NLRP3 Inflammasome Activation in Inflammatory Bowel Diseases of Both Mice and Humans. J Crohns Colitis. 2017;11:737-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 18. | Zhang J, Fu S, Sun S, Li Z, Guo B. Inflammasome activation has an important role in the development of spontaneous colitis. Mucosal Immunol. 2014;7:1139-1150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 19. | Perera AP, Fernando R, Shinde T, Gundamaraju R, Southam B, Sohal SS, Robertson AAB, Schroder K, Kunde D, Eri R. MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci Rep. 2018;8:8618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 254] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 20. | Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 820] [Cited by in RCA: 840] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 21. | Dupaul-Chicoine J, Yeretssian G, Doiron K, Bergstrom KS, McIntire CR, LeBlanc PM, Meunier C, Turbide C, Gros P, Beauchemin N, Vallance BA, Saleh M. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32:367-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 432] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 22. | Itani S, Watanabe T, Nadatani Y, Sugimura N, Shimada S, Takeda S, Otani K, Hosomi S, Nagami Y, Tanaka F, Kamata N, Yamagami H, Tanigawa T, Shiba M, Tominaga K, Fujiwara Y, Arakawa T. NLRP3 inflammasome has a protective effect against oxazolone-induced colitis: A possible role in ulcerative colitis. Sci Rep. 2016;6:39075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 23. | Neudecker V, Haneklaus M, Jensen O, Khailova L, Masterson JC, Tye H, Biette K, Jedlicka P, Brodsky KS, Gerich ME, Mack M, Robertson AAB, Cooper MA, Furuta GT, Dinarello CA, O'Neill LA, Eltzschig HK, Masters SL, McNamee EN. Myeloid-derived miR-223 regulates intestinal inflammation via repression of the NLRP3 inflammasome. J Exp Med. 2017;214:1737-1752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 321] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 24. | Impellizzeri D, Siracusa R, Cordaro M, Peritore AF, Gugliandolo E, Mancuso G, Midiri A, Di Paola R, Cuzzocrea S. Therapeutic potential of dinitrobenzene sulfonic acid (DNBS)-induced colitis in mice by targeting IL-1β and IL-18. Biochem Pharmacol. 2018;155:150-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | Xiong Y, Lou Y, Su H, Fu Y, Kong J. Cholecalciterol cholesterol emulsion ameliorates experimental colitis via down-regulating the pyroptosis signaling pathway. Exp Mol Pathol. 2016;100:386-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Lazaridis LD, Pistiki A, Giamarellos-Bourboulis EJ, Georgitsi M, Damoraki G, Polymeros D, Dimitriadis GD, Triantafyllou K. Activation of NLRP3 Inflammasome in Inflammatory Bowel Disease: Differences Between Crohn's Disease and Ulcerative Colitis. Dig Dis Sci. 2017;62:2348-2356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 27. | Ranson N, Veldhuis M, Mitchell B, Fanning S, Cook AL, Kunde D, Eri R. NLRP3-Dependent and -Independent Processing of Interleukin (IL)-1β in Active Ulcerative Colitis. Int J Mol Sci. 2018;20:pii: E57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 28. | Coccia M, Harrison OJ, Schiering C, Asquith MJ, Becher B, Powrie F, Maloy KJ. IL-1β mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J Exp Med. 2012;209:1595-1609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 465] [Cited by in RCA: 496] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 29. | Ludwiczek O, Kaser A, Novick D, Dinarello CA, Rubinstein M, Tilg H. Elevated systemic levels of free interleukin-18 (IL-18) in patients with Crohn's disease. Eur Cytokine Netw. 2005;16:27-33. [PubMed] |
| 30. | Leach ST, Messina I, Lemberg DA, Novick D, Rubenstein M, Day AS. Local and systemic interleukin-18 and interleukin-18-binding protein in children with inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Polytarchou C, Oikonomopoulos A, Mahurkar S, Touroutoglou A, Koukos G, Hommes DW, Iliopoulos D. Assessment of Circulating MicroRNAs for the Diagnosis and Disease Activity Evaluation in Patients with Ulcerative Colitis by Using the Nanostring Technology. Inflamm Bowel Dis. 2015;21:2533-2539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Bauernfeind F, Rieger A, Schildberg FA, Knolle PA, Schmid-Burgk JL, Hornung V. NLRP3 inflammasome activity is negatively controlled by miR-223. J Immunol. 2012;189:4175-4181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 395] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 33. | Perera AP, Kunde D, Eri R. NLRP3 Inhibitors as Potential Therapeutic Agents for Treatment of Inflammatory Bowel Disease. Curr Pharm Des. 2017;23:2321-2327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Shao BZ, Wang SL, Pan P, Yao J, Wu K, Li ZS, Bai Y, Linghu EQ. Targeting NLRP3 Inflammasome in Inflammatory Bowel Disease: Putting out the Fire of Inflammation. Inflammation. 2019;42:1147-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 35. | Gong Z, Zhou J, Li H, Gao Y, Xu C, Zhao S, Chen Y, Cai W, Wu J. Curcumin suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Mol Nutr Food Res. 2015;59:2132-2142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 36. | Iqbal U, Anwar H, Quadri AA. Use of Curcumin in Achieving Clinical and Endoscopic Remission in Ulcerative Colitis: A Systematic Review and Meta-analysis. Am J Med Sci. 2018;356:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 37. | Wang Y, Tang Q, Duan P, Yang L. Curcumin as a therapeutic agent for blocking NF-κB activation in ulcerative colitis. Immunopharmacol Immunotoxicol. 2018;40:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 38. | Jenko B, Praprotnik S, Tomšic M, Dolžan V. NLRP3 and CARD8 Polymorphisms Influence Higher Disease Activity in Rheumatoid Arthritis. J Med Biochem. 2016;35:319-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Lee YH, Bae SC. Association between functional NLRP3 polymorphisms and susceptibility to autoimmune and inflammatory diseases: A meta-analysis. Lupus. 2016;25:1558-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | von Herrmann KM, Salas LA, Martinez EM, Young AL, Howard JM, Feldman MS, Christensen BC, Wilkins OM, Lee SL, Hickey WF, Havrda MC. NLRP3 expression in mesencephalic neurons and characterization of a rare NLRP3 polymorphism associated with decreased risk of Parkinson's disease. NPJ Parkinsons Dis. 2018;4:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 41. | Villani AC, Lemire M, Fortin G, Louis E, Silverberg MS, Collette C, Baba N, Libioulle C, Belaiche J, Bitton A, Gaudet D, Cohen A, Langelier D, Fortin PR, Wither JE, Sarfati M, Rutgeerts P, Rioux JD, Vermeire S, Hudson TJ, Franchimont D. Common variants in the NLRP3 region contribute to Crohn's disease susceptibility. Nat Genet. 2009;41:71-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 423] [Cited by in RCA: 430] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 42. | Lewis GJ, Massey DC, Zhang H, Bredin F, Tremelling M, Lee JC, Berzuini C, Parkes M. Genetic association between NLRP3 variants and Crohn's disease does not replicate in a large UK panel. Inflamm Bowel Dis. 2011;17:1387-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. | Zhang HX, Wang ZT, Lu XX, Wang YG, Zhong J, Liu J. NLRP3 gene is associated with ulcerative colitis (UC), but not Crohn's disease (CD), in Chinese Han population. Inflamm Res. 2014;63:979-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 44. | Mao L, Kitani A, Similuk M, Oler AJ, Albenberg L, Kelsen J, Aktay A, Quezado M, Yao M, Montgomery-Recht K, Fuss IJ, Strober W. Loss-of-function CARD8 mutation causes NLRP3 inflammasome activation and Crohn's disease. J Clin Invest. 2018;128:1793-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 45. | Schoultz I, Verma D, Halfvarsson J, Törkvist L, Fredrikson M, Sjöqvist U, Lördal M, Tysk C, Lerm M, Söderkvist P, Söderholm JD. Combined polymorphisms in genes encoding the inflammasome components NALP3 and CARD8 confer susceptibility to Crohn's disease in Swedish men. Am J Gastroenterol. 2009;104:1180-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 46. | Yao X, Zhang C, Xing Y, Xue G, Zhang Q, Pan F, Wu G, Hu Y, Guo Q, Lu A, Zhang X, Zhou R, Tian Z, Zeng B, Wei H, Strober W, Zhao L, Meng G. Remodelling of the gut microbiota by hyperactive NLRP3 induces regulatory T cells to maintain homeostasis. Nat Commun. 2017;8:1896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 166] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 47. | Hirota SA, Ng J, Lueng A, Khajah M, Parhar K, Li Y, Lam V, Potentier MS, Ng K, Bawa M, McCafferty DM, Rioux KP, Ghosh S, Xavier RJ, Colgan SP, Tschopp J, Muruve D, MacDonald JA, Beck PL. NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm Bowel Dis. 2011;17:1359-1372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 357] [Cited by in RCA: 368] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 48. | Willing B, Halfvarson J, Dicksved J, Rosenquist M, Järnerot G, Engstrand L, Tysk C, Jansson JK. Twin studies reveal specific imbalances in the mucosa-associated microbiota of patients with ileal Crohn's disease. Inflamm Bowel Dis. 2009;15:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 369] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 49. | Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2488] [Cited by in RCA: 3699] [Article Influence: 308.3] [Reference Citation Analysis (1)] |
| 50. | Shi J, Gao W, Shao F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci. 2017;42:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1123] [Cited by in RCA: 2388] [Article Influence: 238.8] [Reference Citation Analysis (0)] |
| 51. | Matsuoka K, Inoue N, Sato T, Okamoto S, Hisamatsu T, Kishi Y, Sakuraba A, Hitotsumatsu O, Ogata H, Koganei K, Fukushima T, Kanai T, Watanabe M, Ishii H, Hibi T. T-bet upregulation and subsequent interleukin 12 stimulation are essential for induction of Th1 mediated immunopathology in Crohn's disease. Gut. 2004;53:1303-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 52. | Li N, Shi RH. Updated review on immune factors in pathogenesis of Crohn's disease. World J Gastroenterol. 2018;24:15-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (7)] |
| 53. | Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Bürgel N, Fromm M, Zeitz M, Fuss I, Strober W, Schulzke JD. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 806] [Cited by in RCA: 919] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 54. | Nemeth ZH, Bogdanovski DA, Barratt-Stopper P, Paglinco SR, Antonioli L, Rolandelli RH. Crohn's Disease and Ulcerative Colitis Show Unique Cytokine Profiles. Cureus. 2017;9:e1177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 55. | Bruchard M, Rebé C, Derangère V, Togbé D, Ryffel B, Boidot R, Humblin E, Hamman A, Chalmin F, Berger H, Chevriaux A, Limagne E, Apetoh L, Végran F, Ghiringhelli F. The receptor NLRP3 is a transcriptional regulator of TH2 differentiation. Nat Immunol. 2015;16:859-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 318] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 56. | Santarlasci V, Cosmi L, Maggi L, Liotta F, Annunziato F. IL-1 and T Helper Immune Responses. Front Immunol. 2013;4:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 57. | Vargas TR, Martin F, Apetoh L. Role of interleukin-1-family cytokines on effector CD4 T cell differentiation. World J Immunol. 2017;7:24-31. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (2)] |
| 58. | Mak'Anyengo R, Duewell P, Reichl C, Hörth C, Lehr HA, Fischer S, Clavel T, Denk G, Hohenester S, Kobold S, Endres S, Schnurr M, Bauer C. Nlrp3-dependent IL-1β inhibits CD103+ dendritic cell differentiation in the gut. JCI Insight. 2018;3:pii: 96322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 59. | Ruane DT, Lavelle EC. The role of CD103⁺ dendritic cells in the intestinal mucosal immune system. Front Immunol. 2011;2:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 60. | Lewis KL, Reizis B. Dendritic cells: Arbiters of immunity and immunological tolerance. Cold Spring Harb Perspect Biol. 2012;4:a007401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 61. | Dinarello CA. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur J Immunol. 2011;41:1203-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 636] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 62. | van de Veerdonk FL, Netea MG. New Insights in the Immunobiology of IL-1 Family Members. Front Immunol. 2013;4:167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 63. | Mao L, Kitani A, Strober W, Fuss IJ. The Role of NLRP3 and IL-1β in the Pathogenesis of Inflammatory Bowel Disease. Front Immunol. 2018;9:2566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 202] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 64. | Kanneganti TD, Lamkanfi M, Núñez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 797] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 65. | Palomo J, Dietrich D, Martin P, Palmer G, Gabay C. The interleukin (IL)-1 cytokine family--Balance between agonists and antagonists in inflammatory diseases. Cytokine. 2015;76:25-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 344] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 66. | Siegmund B. Interleukin-18 in intestinal inflammation: Friend and foe? Immunity. 2010;32:300-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 67. | Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 958] [Cited by in RCA: 1031] [Article Influence: 41.2] [Reference Citation Analysis (1)] |
| 68. | Maerten P, Shen C, Colpaert S, Liu Z, Bullens DA, van Assche G, Penninckx F, Geboes K, Vanham G, Rutgeerts P, Ceuppens JL. Involvement of interleukin 18 in Crohn's disease: Evidence from in vitro analysis of human gut inflammatory cells and from experimental colitis models. Clin Exp Immunol. 2004;135:310-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 69. | Harrison OJ, Srinivasan N, Pott J, Schiering C, Krausgruber T, Ilott NE, Maloy KJ. Epithelial-derived IL-18 regulates Th17 cell differentiation and Foxp3⁺ Treg cell function in the intestine. Mucosal Immunol. 2015;8:1226-1236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 196] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aldrich MB, Cheng TH, Rangel-Corona R, Schietroma M S-Editor: Yan JP L-Editor: A E-Editor: Ma YJ