Published online Aug 21, 2019. doi: 10.3748/wjg.v25.i31.4468
Peer-review started: March 29, 2019
First decision: June 10, 2019
Revised: June 25, 2019
Accepted: July 19, 2019
Article in press: July 19, 2019
Published online: August 21, 2019
Processing time: 145 Days and 17.4 Hours
Activation of hepatic stellate cells (HSCs) is a pivotal event in the onset and progression of liver fibrosis. Loss of microRNA-194 (miR-194) has been reported in activated HSCs, but the actual role of miR-194 in liver fibrosis remains uncertain.
To explore the role and potential mechanism of miR-194-mediated regulation of liver fibrosis in vitro and in vivo.
The expression of miR-194 was examined in human fibrotic liver tissues, activated HSCs, and a carbon tetrachloride (CCl4) mouse model by qPCR. The effects of AKT2 regulation by miR-194 on the activation and proliferation of HSCs were assessed in vitro. For in vivo experiments, we reintroduced miR-194 in mice using a miR-194 agomir to investigate the functions of miR-194 in liver fibrosis.
MiR-194 expression was notably lacking in activated HSCs from both humans and mice. Overexpression of miR-194 (OV-miR-194) inhibited α-smooth muscle actin (α-SMA) and type I collagen (Col I) expression and suppressed cell proliferation in HSCs by causing cell cycle arrest in G0/G1 phase. AKT2 was predicted to be a target of miR-194. Notably, the effects of miR-194 knockdown in HSCs were almost blocked by AKT2 deletion, indicating that miR-194 plays a role in HSCs via regulation of AKT2. Finally, miR-194 agomir treatment dramatically ameliorated liver fibrosis in CCl4-treated mice.
We revealed that miR-194 plays a protective role by inhibiting the activation and proliferation of HSCs via AKT2 suppression. Our results further propose miR-194 as a potential therapeutic target for liver fibrosis.
Core tip: The expression of miR-194 was significantly downregulated in activated primary hepatic stellate cells (HSCs) from CCl4-treated mice, TGF-β1-treated LX2 cells, and the liver of advanced fibrosis patients. MiR-194 significantly inhibited the expression of α-SMA, Col I, and cyclin D1 and suppressed the activation and proliferation of HSCs in vitro by repressing AKT2 signaling. MiR-194 could attenuate liver fibrosis progression to some extent in a mouse model. Reintroduction of miR-194 offered a possible therapeutic approach for ameliorating liver fibrosis.
- Citation: Wu JC, Chen R, Luo X, Li ZH, Luo SZ, Xu MY. MicroRNA-194 inactivates hepatic stellate cells and alleviates liver fibrosis by inhibiting AKT2. World J Gastroenterol 2019; 25(31): 4468-4480
- URL: https://www.wjgnet.com/1007-9327/full/v25/i31/4468.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i31.4468
Liver fibrosis, characterized by excessive accumulation of extracellular matrix (ECM) components, is a common stage in the progression of chronic liver diseases to cirrhosis[1]. Hepatic stellate cells (HSCs), the major mesenchymal cells in the liver, have been widely accepted to play a critical central role in liver fibrosis[2]. Liver fibrosis is characterized by the activation and proliferation of HSCs, which leads to scar formation. Upon stimulation by different damaging and/or inflammatory cytokines, HSCs are activated and transform to myofibroblast-like cells marked by the loss of lipid droplets, high expression of α-smooth muscle actin (α-SMA), and increased proliferation, contractility, and migration[3].
Previous studies suggested that overexpression of microRNA-194 (miR-194) significantly inhibited the activation and proliferation of HSCs[4]. However, the functions of miR-194 in liver fibrosis have not been fully elucidated. AKT, also known as protein kinase B, has three isoforms: AKT1/2/3. MiR-194 cooperates with AKT2 to regulate proliferation and the cell cycle in cancer[5]. Interestingly, the AKT pathway enhanced proliferation and ECM production in HSCs via TGF-β1[6,7]. The activation of HSCs is reversed by the deletion of AKT2[8].
Based on these previous findings, we examined the expression of miR-194 in experimental liver fibrosis models in vivo and in vitro, which showed that miR-194 was significantly downregulated in activated HSCs. Additionally, we investigated the role of miR-194 in HSCs. Our results showed that miR-194 attenuated the activation and proliferation of HSCs by suppressing AKT2. To explore the clinical implications of miR-194, we reintroduced miR-194 in a mouse model of CCl4-induced liver fibrosis by tail vein injection of a miR-194 agomir. In vivo application reduced the expression of AKT2 and ECM markers, and led to the recovery of fibrosis. Taken together, our results suggest that miR-194 can inhibit the activation and proliferation of HSCs by suppressing AKT2. Reintroduction of miR-194 might be a potential novel therapy for the treatment of liver fibrosis.
Human liver tissues were obtained through percutaneous liver biopsy from patients in our hospital with chronic hepatitis B (CHB), defined as those who had hepatitis B virus (HBV) infection and were positive for hepatitis B surface antigen for at least 6 months. Sixty human liver tissues [fibrosis stage S0 (no fibrosis, n = 12), S1 (mild fibrosis, n = 12), S2 (moderate fibrosis, n = 12), S3 (advanced fibrosis, n = 12), and S4 (cirrhosis, n = 12)] were included in the miRNA microarray and qPCR analyses. All patients provided written informed consent, and the study was approved by the ethics committee of our hospital. The fibrosis stage was determined by the Scheuer classification.
Five-week-old male C57BL/6J mice were purchased from the Sino-British Sippr/BK Laboratory in Shanghai. All animals received humane care according to established standards and were maintained in an air-conditioned animal room at 25 °C with free access to water and food. All protocols conformed to the National Institute of Health (NIH) guidelines and all animals received care in compliance with the principles of laboratory animal care. After 1 wk of acclimation, mice were randomly divided into either a control (n = 5) or carbon tetrachloride (CCl4) group (n = 15). The CCl4 group received intraperitoneal injections of CCl4 (2 μL/g) mixed with olive oil (15% CCl4) three times weekly.
Then CCl4-treated mice were randomly divided into three groups after 4 wk of injection: CCl4 group (n = 5), agomir negative control (CCl4 + OV-NC) group (n = 5), and miR-194 agomir (CCl4 + OV-miR-194) group (n = 5). The CCl4 + OV-miR-194 and CCl4 + OV-NC groups received tail vein injections of miR-194 agomir or the corresponding controls at a dose of 2 nmol twice weekly for 2 wk. The control group was treated with olive oil. After 6 wk, all mice were sacrificed. Liver tissues were fixed with 10% formalin and embedded in paraffin. All sections were stained with hematoxylin and eosin (H&E) or Sirius Red. These procedures were approved by the ethics committee of our hospital.
Primary HSCs (pHSCs) from WT mice were isolated by pronase/collagenase perfusion followed by Nycodenz two-layer discontinuous density gradient centrifugation as reported[9]. Cell viability and purity (> 95%) were confirmed by trypan blue staining and autofluorescence. LX2 cells (a human HSC cell line) and pHSCs were cultured in DMEM supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco, United States). For treatment, HSCs were incubated with TGF-β1 (Sigma, United States) at a concentration of 10 ng/mL.
Chemically synthesized RNAs including negative control (OV-miR-NC), miR-194 mimic (OV-miR-194), control siRNA (si-NC), miR-194 inhibitor (si-miR-194), and siRNA against AKT2 (si-AKT2) were used (GenePharma, China). HSCs were transfected using Lipofectamine RNAi/MAX transfection reagent or Lipofectamine 2000 (Invitrogen, United States) according to the manufacturer’s instructions. Then, transfected cells were maintained at 37 °C in a 5% CO2 atmosphere for 48 h. All synthesized oligonucleotides in this study are listed in Supplementary Table 3.
QPCR was performed using a SYBR Green PCR Kit (Applied Biosystems, Foster City, CA, United States) and an ABI 7900HT Fast Real-Time PCR System (Applied Biosystems). The primers used in this study are listed in Supplementary Tables 1 and 2.
Western blot was performed using the following antibodies: anti-α-SMA, anti- type I collagen (Col I), anti-cyclin D1, anti-AKT2/p-AKT2 (1:1000, CST), and anti-GAPDH (1:10000, Abcam); the secondary antibody was HRP-conjugated IgG (1:10000, Santa Cruz).
LX2 cells were seeded in 96-well plates at a density of 5 × 103 cells per well. Cell proliferation was measured using a CCK8 assay (Dojindo, Kumamoto, Japan) with a 48 h incubation period at 37 °C, and the absorbance at 450 nm was read in a microplate reader (Epoch2, BioTek).
LX2 cells were seeded in 6-well plates at 3 × 105 cells per well. After transfection for 48 h, cells were trypsinized and fixed in 70% ethanol at -20°C for 24 h. Then, cells were stained using BD Pharmingen PI/ RNase staining buffer (BD Biosciences, United States). The cell cycle distribution was analyzed using an Accuri C6 flow cytometer (BD Biosciences, United States) and ModFit LT software.
Formaldehyde-fixed, paraffin-embedded liver sections were subjected to IHC following routine protocols as described. Anti-α-SMA (1:500) was used for IHC staining. For IF staining, cell slides were incubated with anti-α-SMA (1:200) primary antibody and with 4’,6-diamidino-2-phenylindole (DAPI) to stain nuclei. Representative images were obtained using an inverted microscope (Leica Microsystems, Germany).
All experiments were repeated three times. The bar and line graphs show the means and standard deviations. Data were analyzed using ANOVA. All statistical analyses were performed using SPSS 19.0 (SPSS Inc., Chicago). A P-value < 0.05 was considered statistically significant.
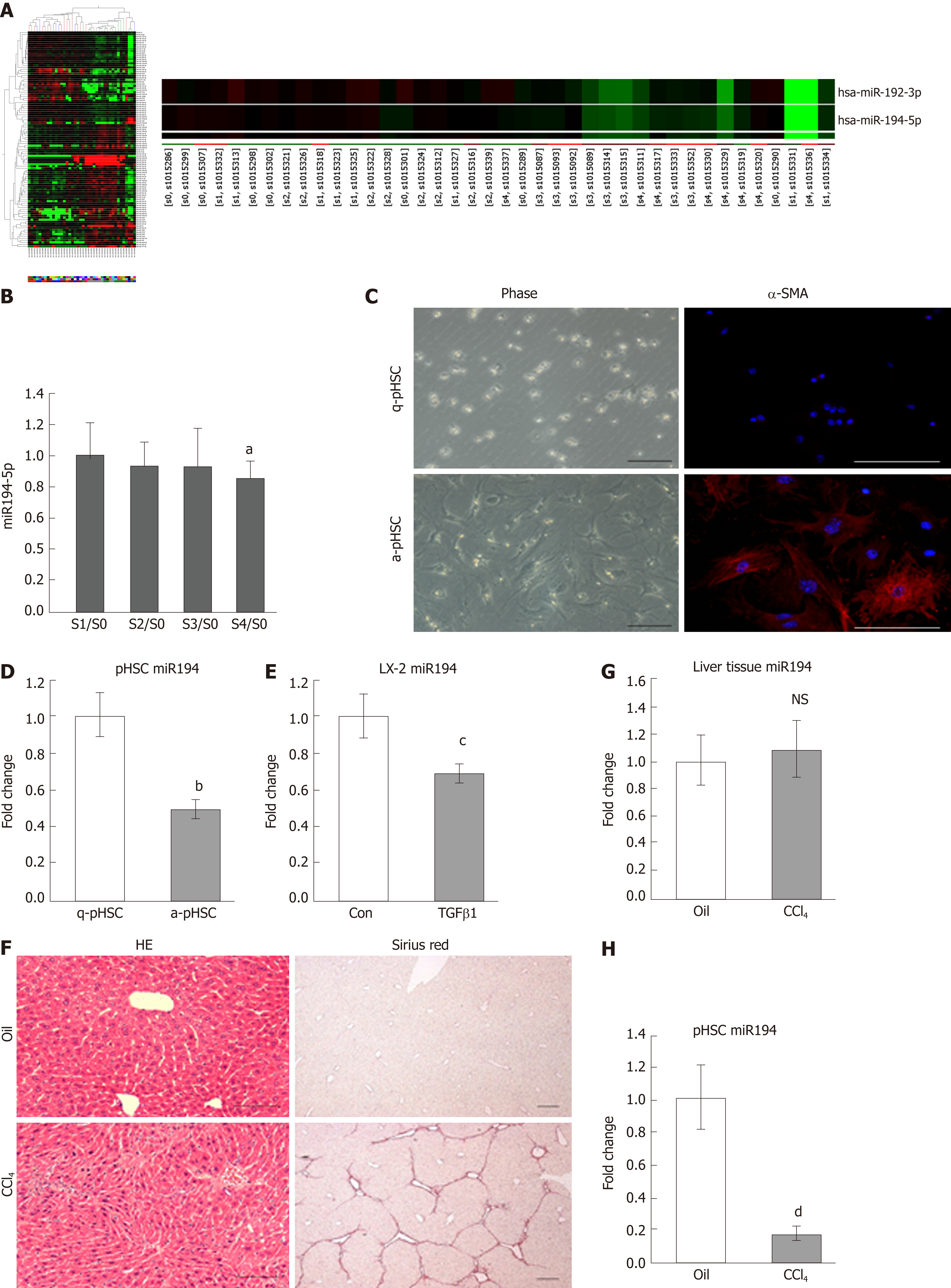
To explore liver fibrosis–related miRNAs, microarray analysis was performed with liver tissues from CHB patients (S0-4, n = 8 in each group). A total of 105 miRNAs were significantly differentially expressed in the fibrosis groups (S1-4) compared to the no-fibrosis group (S0, Figure 1A). We focused on miR-194, which was downregulated in fibrotic tissues (Figure 1A). Thus, we examined miR-194 expression in liver tissues of another CHB cohort by qPCR (S0-4, n = 4 in each group). The expression of miR-194 was significantly lower in the S4 group than in the S0 group (Figure 1B, P < 0.05). Freshly isolated primary HSCs (pHSCs) acquired a myofibroblastic phenotype and were fully activated, along with significant upregulation of α-SMA, after culture for 7 d (Figure 1C). The expression of miR-194 in activated pHSCs (a-pHSC, cultured for 7 d) was noticeably downregulated compared with that in quiescent pHSCs (q-pHSC, cultured for 0 d) (Figure 1D). This phenomenon was also observed in activated LX2 cells stimulated with TGF-β1 (10 ng/mL) for 48 h (Figure 1E). Then, CCl4 was used to induce liver fibrosis in mice. H&E staining showed that the number of apoptotic hepatocytes and infiltrated immune cells was increased; Sirius Red staining showed the deposition of excessive collagen fibers in CCl4-treated livers (Figure 1F). We did not observe significant changes in the expression of miR-194 between livers from the oil- and CCl4-treated mice (Figure 1G). Finally, pHSCs were isolated from both oil- and CCl4-treated mice. The expression of miR-194 was much lower in activated pHSCs isolated from CCl4-treated mice than in quiescent cells from oil-treated mice (Figure 1H). These data indicated that miR-194 is downregulated during HSC activation, an effect that may be associated with the progression of liver fibrosis.
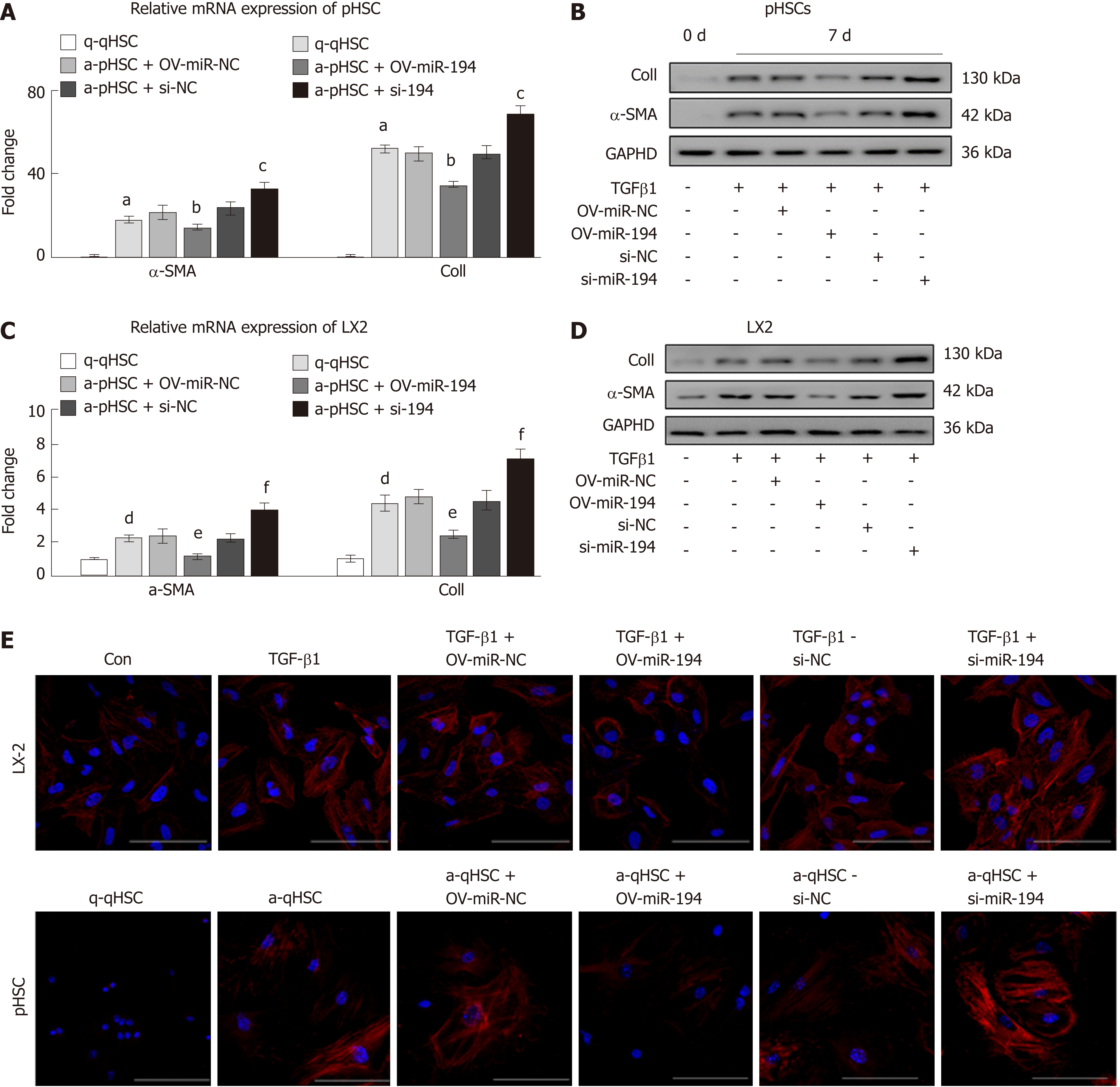
To investigate whether miR-194 could influence HSC activation, we isolated pHSCs from mice and transfected constructs into them to overexpress or knock down miR-194 in vitro (Suppl. Figure 1). As expected, both the mRNA and protein levels of Col I and α-SMA were significantly decreased in OV-miR-194 cells; in contrast, they were dramatically increased in si-miR-194 cells (Figure 2A-B). We also overexpressed or knocked down miR-194 in LX2 cells (Suppl. Figure 2), and a similar trend was observed. The enhanced mRNA and protein levels of Col I and α-SMA induced by TGF-β1 were significantly decreased with miR-194 overexpression but were also increased with miR-194 knockdown (Figure 2C-D). In addition, IF analysis of α-SMA further confirmed that OV-miR-194 markedly reduced the protein levels of α-SMA in both pHSCs and LX2 cells and that, conversely, si-miR-194 increased these levels (Figure 2E). Therefore, these results demonstrated that miR-194 suppresses the activation of HSCs.
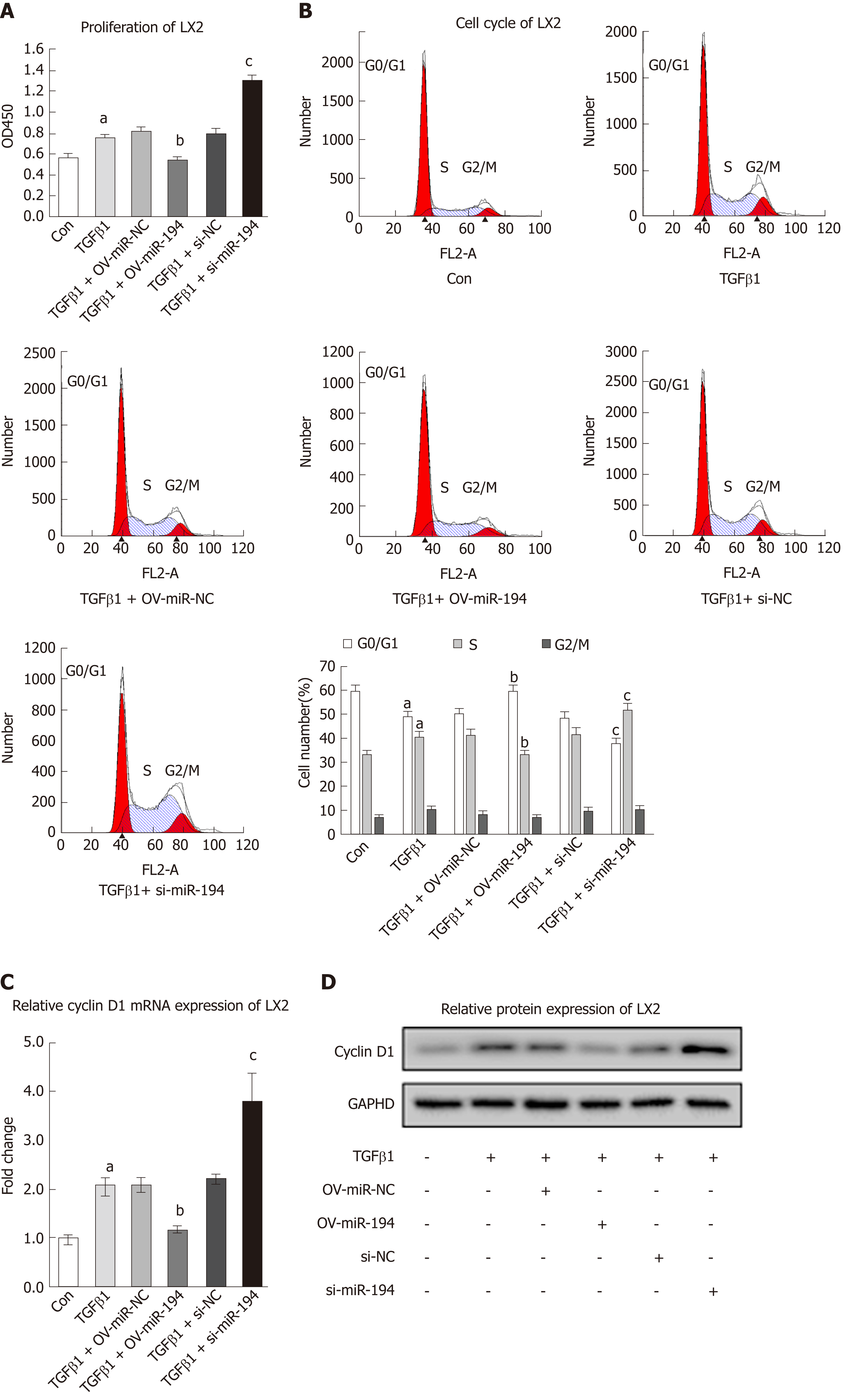
To ascertain whether miR-194 regulates the proliferation of HSCs, we examined its effects via a CCK8 assay. TGF-β1 treatment significantly promoted cell proliferation, while OV-miR-194 appreciably reversed this effect; in addition, si-miR-194 accelerated cell proliferation (Figure 3A). Generally, abnormal cell proliferation is related to apoptosis or alterations in the cell cycle. No significant changes were observed in the apoptosis rate between the OV-miR-194 and si-miR-194 groups (Suppl. Figure 3). However, cell cycle analysis revealed that the percentage of cells in G0/G1 phase was higher and the percentage of cells in S phase was lower in OV-miR-194 cells than in OV-miR-NC cells with TGF-β1 stimulation, while si-miR-194 had the opposite effect (Figure 3B). TGF-β1 treatment enhanced the expression of the G1-S transition promoter cyclin D1 at both the mRNA and protein levels; in addition, OV-miR-194 decreased cyclin D1 expression, and si-miR-194 increased cyclin D1 expression (Figure 3C-D). These findings further confirmed the effects of miR-194 on the suppression of proliferation via controlling the transition from G0/G1 to S phase. These data indicated that miR-194 suppresses cyclin D1 expression and subsequently inhibits HSC proliferation by causing cell cycle arrest in G0/G1 phase.
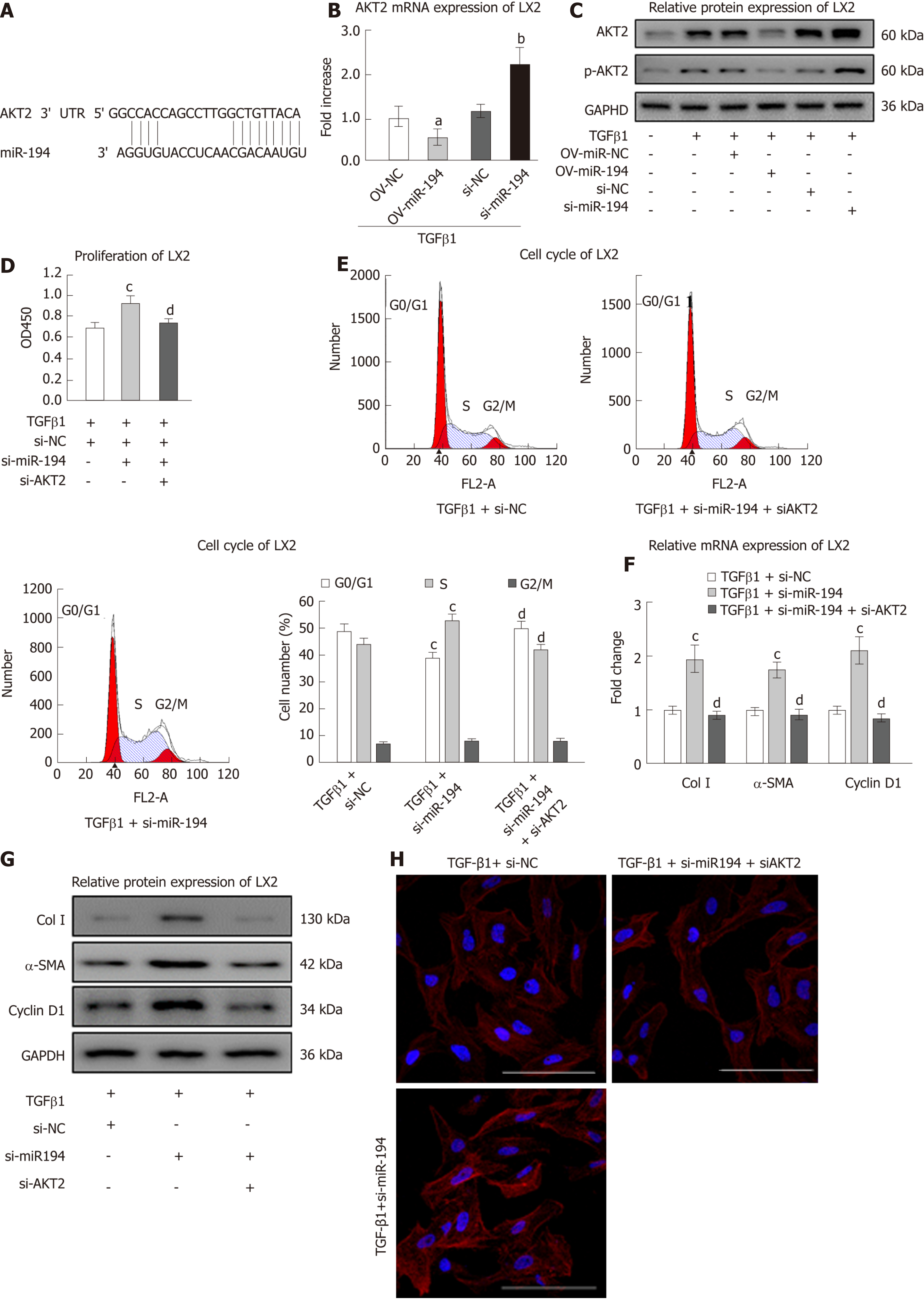
MiRNAs primarily negatively downregulate gene expression by binding to complementary sites in the 3’-untranslated region (3’-UTR) of mRNA sequences. To identify the relevant target genes of miR-194, we conducted bioinformatic analysis using TargetScan (http://www.targetscan.org/; Figure 4A). As expected, the mRNA expression of AKT2 was downregulated in OV-miR-194 cells and upregulated in si-miR-194 cells (Figure 4B). Western blot analysis revealed that OV-miR-194 decreased the protein levels of AKT2/p-AKT2 in TGF-β1-treated LX2 cells and that si-miR-194 had the opposite effects (Figure 4C). Then, we knocked down AKT2 in LX2 cells to assess the influence of its regulation by miR-194 (Supplementary Figure 4). The notable effect of si-miR-194 on increasing cell viability with TGF-β1 treatment was clearly reversed in the presence of si-AKT2 (Figure 4D). The decrease in the percentage of G0/G1 phase cells and increase in the percentage of S phase cells induced by si-miR-194 were also reversed by the addition of si-AKT2 (Figure 4E). Moreover, si-AKT2 restored the mRNA and protein levels of Col I, α-SMA, and cyclin D1, which were significantly increased by si-miR-194, in TGF-β1-treated LX2 cells (Figure 4F/G). Furthermore, the IF images showed that si-AKT2 could restore the levels of α-SMA proteins increased by si-miR-194 in TGF-β1-treated LX2 cells (Figure 4H). These results indicated that miR-194 suppresses the activation and proliferation of HSCs at least in part by suppressing AKT2 expression.
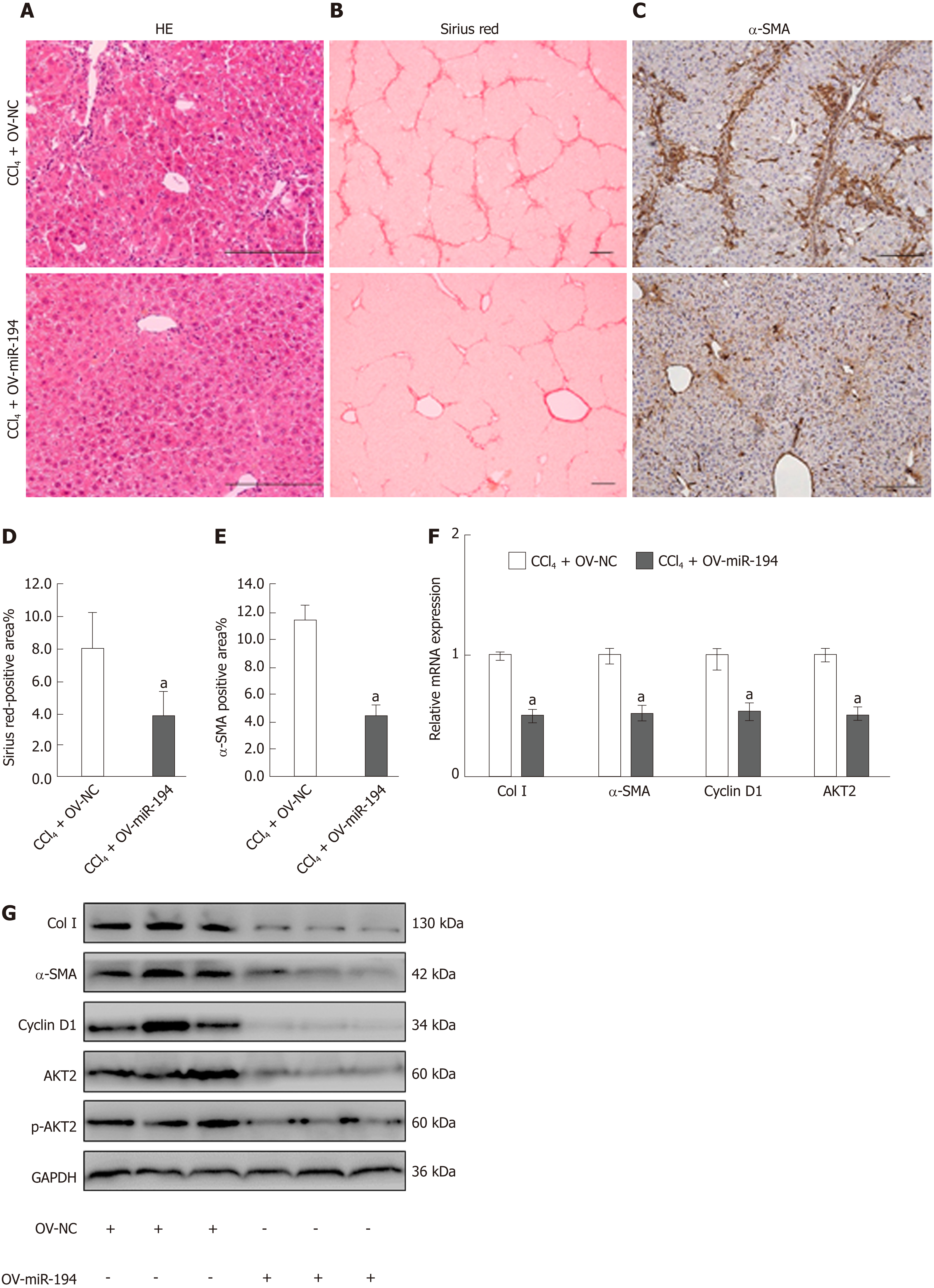
To investigate the effects of miR-194 on liver fibrosis in vivo, we reintroduced miR-194 in the liver using a miR-194 agomir. Injection of the miR-194 agomir increased the miR-194 levels in liver tissue (Supplementary Figure 5). Histological examination showed that miR-194 ameliorated liver inflammation and fibrosis (Figure 5A-C). The Sirius Red-positive area in the CCl4 + OV-miR194 group was dramatically decreased compared to that in the CCl4 + OV-NC group (CCl4 + OV-NC: 8.02%, CCl4 + OV-miR194: 3.87%; P < 0.05; Figure 5B and D). In addition, as shown in the IHC images, the percentage of α-SMA-positive cells was markedly reduced in the CCl4 + OV-miR194 group (CCl4 + OV-NC: 11.45%, CCl4 + OV-miR194: 4.52%; P < 0.05; Figure 5C and E). Moreover, OV-miR-194 significantly reduced the mRNA and protein levels of Col I, α-SMA, cyclin D1, and AKT2/p-AKT2 in the liver of CCl4-treated mice compared with control mice (Figure 5F-G). Therefore, miR-194 attenuated liver fibrosis progression to some extent in this mouse model. Taken together, these findings indicate that miR-194 might alleviate liver fibrosis in vivo via the inhibition of AKT2 signaling.
Liver fibrosis is usually secondary to chronic hepatic injury and inflammation. However, once liver cirrhosis develops, the process is difficult to reverse[1]. It is essential to find an effective treatment. Increasing numbers of studies have focused on miRNA mechanisms in fibrotic diseases. MiR-194 usually inhibits tumor growth and invasiveness and has thus been considered a tumor suppressor. Moreover, miR-194 was downregulated in HSCs isolated from rats with BDL-induced liver fibrosis, reduced HSC proliferation, and inhibited the expression of α-SMA and Col I[4]. The role of miR-194 in the development of liver fibrosis remains unclear. First, we clarified that the expression of miR-194 was lacking in human livers with advanced fibrosis, activated HSCs in vitro, and activated pHSCs isolated from CCl4-treated mice. Activated HSCs are characterized by the upregulation of specific cytoskeletal stress fiber proteins, such as α-SMA, and the ECM proteins, such as Col I/III[10]. Second, we found that OV-miR-194 notably inactivated HSCs and dramatically suppressed Col I and α-SMA expression in HSCs in vitro. Generally, alterations in the cell cycle contribute to abnormal cell proliferation. Cyclin D1 is important for the cell cycle G1/S transition[11]. Third, our data indicated that miR-194 inhibited cell proliferation and delayed cell cycle progression from G1 to S phase via downregulation of cyclin D1 in vitro. Moreover, we determined that miR-194 had no effect on HSC apoptosis. Therefore, miR-194 can suppress the activation and proliferation of HSCs and subsequently play a key role in liver fibrogenesis.
In colorectal and gallbladder cancers, miR-194 suppressed the proliferation of cancer cells via targeting the AKT2 pathway[5,12]. AKT2 is recognized as a prosurvival oncogene. Deletion of AKT2 plays a role in ameliorating liver fibrosis[8]. AKT2 was predicted as a target of miR-194, and this relationship was previously confirmed by dual luciferase reporter experiments[5,12,13]. Notably, the functions of si-miR-194, including the activation of and promoting the survival of HSCs, were effectively blocked by si-AKT2 in vitro. These results indicated that miR-194 inhibited the activation and proliferation of HSCs via targeting AKT2. However, there might be other pathways involved in the regulation of liver fibrosis by miR-194. Therefore, more studies are needed to investigate the underlying mechanism of miR-194 in liver fibrosis.
Since miR-194 inactivated HSCs in vitro, it is important to explore whether overexpression of miR-194 could ameliorate liver fibrosis in mice. Reintroduction of miR-194 via tail vein injection of a miR-194 agomir was used as a treatment in our research. H&E and Sirius Red staining showed that OV-miR-194 could visibly ameliorate liver inflammation and fibrosis. Similarly, restoration of miR-194 dramatically reduced the expression of α-SMA, Col I, and cyclin D1 associated with decreased levels of AKT2/p-AKT2. In contrast to previous studies, our study provided new evidence for the role of miR-194 in attenuating the progression of liver fibrogenesis, partly via targeting AKT2. Thus, reintroduction of miR-194 might be a potentially useful therapeutic approach for alleviating liver fibrosis.
In conclusion, miR-194 deregulation is essential in the development of liver fibrosis. MiR-194 inhibits the activation and proliferation of HSCs by suppressing AKT2. In addition, reintroduction of miR-194 offers a possible therapeutic approach for ameliorating liver fibrosis.
Liver fibrosis is seriously endangering the safety of life. MiRNAs are reported as key regulators of cellular differentiation and of fibrosis-suppressive functions. Different classes of miRNAs have emerged as key regulators of important hepatic stellate cell (HSC) functions, such as activation, proliferation, and epigenetic gene regulation. Therefore, miRNAs represent novel mechanisms and targets for liver fibrosis. Loss of miR-194 has been reported in activated HSCs, but the actual role of miR-194 in liver fibrosis remains uncertain.
Our findings will provide a fundamental basis for the application of miR-194 in liver fibrosis therapy.
To measure the expression of miR-194 in fibrotic liver tissues and activated HSCs, and investigate biological functions and possible molecular mechanisms of miR-194 in liver fibrosis.
We detected the expression of miR-194 in human fibrotic liver tissues, activated HSCs, and a CCl4 mouse model by qPCR. The biological behavior of miR-194 in vitro was then assessed by overexpression and knockdown of miR-194 in HSCs. In further molecular mechanism studies, we reintroduced miR-194 in mice using a miR-194 agomir to investigate the functions of miR-194 in liver fibrosis in vivo.
In the current study, we found that miR-194 was lacking in activated HSCs from both humans and mice. MiR-194 had a role of inhibiting the activation and proliferation of HSCs by suppressing AKT2. Meanwhile, we confirmed that reintroduction of miR-194 agomir through the tail vein could attenuate liver fibrosis in CCl4-treated mice in association with the reduction of AKT2. However, the specific regulatory role of miR-194 in liver fibrosis patients remains unclear, which needs to be validated in clinical studies.
MiR-194 is downregulated in activated HSCs, which could inhibit the activation and proliferation of HSCs via suppressing AKT2. Reintroduction of miR-194 exerts a protective action against liver fibrosis.
This study provides insight into the role of miR-194 in alleviating liver fibrosis by decreasing AKT2. Reintroduction of miR-194 might be a therapeutic approach to prevent and cure liver fibrosis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pyrrho AD, Riccardi C, Suda T S-Editor: Ma YJ L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Popov Y, Schuppan D. Targeting liver fibrosis: strategies for development and validation of antifibrotic therapies. Hepatology. 2009;50:1294-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 256] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 2. | Reeves HL, Friedman SL. Activation of hepatic stellate cells--a key issue in liver fibrosis. Front Biosci. 2002;7:d808-d826. [PubMed] |
| 3. | Puche JE, Saiman Y, Friedman SL. Hepatic stellate cells and liver fibrosis. Compr Physiol. 2013;3:1473-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 572] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 4. | Venugopal SK, Jiang J, Kim TH, Li Y, Wang SS, Torok NJ, Wu J, Zern MA. Liver fibrosis causes downregulation of miRNA-150 and miRNA-194 in hepatic stellate cells, and their overexpression causes decreased stellate cell activation. Am J Physiol Gastrointest Liver Physiol. 2010;298:G101-G106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 183] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 5. | Wang SH, Wu XC, Zhang MD, Weng MZ, Zhou D, Quan ZW. Long noncoding RNA H19 contributes to gallbladder cancer cell proliferation by modulated miR-194-5p targeting AKT2. Tumour Biol. 2016;37:9721-9730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Kostopoulou F, Gkretsi V, Malizos KN, Iliopoulos D, Oikonomou P, Poultsides L, Tsezou A. Central role of SREBP-2 in the pathogenesis of osteoarthritis. PLoS One. 2012;7:e35753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Krycer JR, Sharpe LJ, Luu W, Brown AJ. The Akt-SREBP nexus: cell signaling meets lipid metabolism. Trends Endocrinol Metab. 2010;21:268-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 301] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 8. | He L, Gubbins J, Peng Z, Medina V, Fei F, Asahina K, Wang J, Kahn M, Rountree CB, Stiles BL. Activation of hepatic stellate cell in Pten null liver injury model. Fibrogenesis Tissue Repair. 2016;9:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Mederacke I, Dapito DH, Affò S, Uchinami H, Schwabe RF. High-yield and high-purity isolation of hepatic stellate cells from normal and fibrotic mouse livers. Nat Protoc. 2015;10:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 467] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 10. | Kumar P, Smith T, Rahman K, Mells JE, Thorn NE, Saxena NK, Anania FA. Adiponectin modulates focal adhesion disassembly in activated hepatic stellate cells: implication for reversing hepatic fibrosis. FASEB J. 2014;28:5172-5183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Baldin V, Lukas J, Marcote MJ, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812-821. [PubMed] |
| 12. | Zhao HJ, Ren LL, Wang ZH, Sun TT, Yu YN, Wang YC, Yan TT, Zou W, He J, Zhang Y, Hong J, Fang JY. MiR-194 deregulation contributes to colorectal carcinogenesis via targeting AKT2 pathway. Theranostics. 2014;4:1193-1208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Zhang Q, Wei T, Shim K, Wright K, Xu K, Palka-Hamblin HL, Jurkevich A, Khare S. Atypical role of sprouty in colorectal cancer: sprouty repression inhibits epithelial-mesenchymal transition. Oncogene. 2016;35:3151-3162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |