Published online Jan 21, 2019. doi: 10.3748/wjg.v25.i3.346
Peer-review started: October 30, 2018
First decision: November 14, 2018
Revised: December 12, 2018
Accepted: December 21, 2018
Article in press: December 21, 2018
Published online: January 21, 2019
Processing time: 83 Days and 20.5 Hours
Exposure to high sustained +Gz (head-to-foot inertial load) is known to have harmful effects on pilots’ body in flight. Although clinical data have shown that liver dysfunction occurs in pilots, the precise cause has not been well defined.
To investigate rat liver function changes in response to repeated +Gz exposure.
Ninety male Wistar rats were randomly divided into a blank control group (BC group, n = 30), a +6 Gz/5 min stress group (6GS group, n = 30), and a +10 Gz/5min stress group (10GS group, n = 30). The 6GS and 10GS groups were exposed to +6 Gz and +10 Gz, respectively, in an animal centrifuge. The onset rate of +Gz was 0.5 G/s. The sustained time at peak +Gz was 5 min for each exposure (for 5 exposures, and 5-min intervals between exposures for a total exposure and non-exposure time of 50 min). We assessed liver injury by measuring the portal venous flow volume, serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST), liver tissue malondialdehyde (MDA), Na+-K+-ATPase, and changes in liver histology. These parameters were recorded at 0 h, 6 h, and 24 h after repeated +Gz exposures.
After repeated +Gz exposures in the 6GS and the 10GS groups, the velocity and flow signal in the portal vein (PV) were significantly decreased as compared to the BC group at 0 h after exposure. Meanwhile, we found that the PV diameter did not change significantly. However, rats in the 6GS group had a much higher portal venous flow volume than the 10GS group at 0 h after exposure. The 6GS group had significantly lower ALT, AST, and MDA values than the 10GS group 0 h and 6 h post exposure. The Na+-K+-ATPase activity in the 6GS group was significantly higher than that in the 10GS group 0 h and 6 h post exposure. Hepatocyte injury, determined pathologically, was significantly lower in the 6GS group than in the 10GS group.
Repeated +Gz exposures transiently cause hepatocyte injury and affect liver metabolism and morphological structure.
Core tip: Some clinical data showed that liver dysfunction was observed in pilots. However, the reason was not clear. We conducted this experimental study to investigate rat liver function changes in response to repeated +Gz exposures, and to observe the portal venous flow volume, liver function indexes, liver tissue malondialdehyde, Na+-K+-ATPase activity, and changes in liver histology. We found that repeated +Gz exposures transiently cause hepatocyte injury and affect liver metabolism and morphological structure.
- Citation: Shi B, Wang XQ, Duan WD, Tan GD, Gao HJ, Pan YW, Guo QJ, Zhang HY. Effects of positive acceleration (+Gz stress) on liver enzymes, energy metabolism, and liver histology in rats. World J Gastroenterol 2019; 25(3): 346-355
- URL: https://www.wjgnet.com/1007-9327/full/v25/i3/346.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i3.346
Exposure to high sustained +Gz (head-to-foot inertial load) is known to have harmful effects on pilots’ body in flight[1]. Along with fast developments in aviation and aerospace technologies, pilots are required to undertake sustained high G-acceleration stress. The characteristics of modern high performance aircraft flight in particular involve high acceleration (>9 Gz) that occurs repeatedly and is continued for 15-45 s, and may transcend the physiological tolerance of flight personnel, even resulting in pilot incapacitation and subsequent loss of life[2,3]. Repeated high-acceleration force exposures may result in cumulative adverse stress responses in the body[4]. Accordingly, safe flying is an issue that is attracting broad attention[5].
The vascular beds that ensure hepatic circulation include two vascular systems, the portal vein (PV) and hepatic artery (HA)[6]. Fighter pilots are frequently exposed to high Gz acceleration with the vector oriented in the foot-head direction. Under these conditions, blood and fluids are redistributed in the body and into the lower body[7]. There are some clinical reports in which liver dysfunction has been observed in pilots[8]. However, the reason for these abnormalities remains unclear. An important question to address is whether or not changes in the blood flow direction after +Gz exposure impairs liver function. Moreover, the manner in which portal venous hemodynamics changes after repeated +Gz exposures, and whether or not oxidative stress parameters increase the duration of these changes, are additional questions awaiting answers. To answer these questions, further studies on liver damage and the mechanisms of liver damage induced by high +Gz exposure are needed to provide evidence for effective preventative measures. In recent years, studies on the effects of high +Gz on the heart, brain, and lung have been addressed in considerable numbers of human and animal studies[9-11]. With the above in mind, we attempted to research the effects of acceleration on liver blood flow, function, and histology.
Healthy male Wistar rats, weighing 200-250 g, were raised in comfortable environment, and fed and water naturally. All animal experiments and procedures performed in this study were conducted in accordance with the Nursing Guidelines and Usage Specification.
In this experiment, we chose +6 and +10 Gz as the acceleration test parameters, as reported previously[12,13]. Ninety rats were divided randomly into three groups: blank control group (BC group, n = 30), +6 Gz/5 min stress group (6GS group, n = 30), and +10 Gz/5 min stress group (10GS group, n = 30). The animal centrifuge used has a diameter of 2 m and has the capacity to generate a wide series of gravities, between +1 Gz and +15 Gz, with an initial rate of 0.1-6 Gz/s[14]. In the 6GS and 10GS groups, rats were repeatedly exposed to +6 Gz or +10 Gz stress (each time for 5 min, initial rate of approximately 1 G/s, 5 times with 0 Gz, at 5-min intervals), respectively. The rats in the blank control group were placed in the centrifuge arm and were subjected to the same process as the test groups, but were not exposed to acceleration. For sample collection, the combined exposure and interval time was 50 min. At 0, 6 h, or 24 h after the last centrifuge run, experimental rats were harvested and blood samples were obtained from the infra-hepatic vena cava to measure hepatic enzyme levels (n = 10 per measurement point). Half of liver lobe was instantly stored in liquid nitrogen and stored at -80 °C. The other half was fixed in 4% formalin, stained with hematoxylin and eosin, and analyzed by a microscopic examination. At the end of the observation period, all the rats were ultimately killed using chloral hydrate.
Using color Doppler ultrasound, we evaluated blood vessel diameter and the blood flow velocity in the PV. The blood flow volume of the PV was calculated with the flow equation: Q = π × (D/2)2 × Vmean × 60 (Q: flow volume per minute; Vmean: mean blood flow velocity; D: vessel diameter).
The extent of liver damage and activity changes in serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were quantified.
MDA is involved in the metabolism of lipid peroxidation products. The MDA content was determined using a standardized MDA assay kit (Nanjing Jiancheng Biotechnology Institute, Nanjing, China), in accordance with the operation manual. A peach red color is generated during the condensation reaction between thiobarbituric acid (TBA) and MDA. The results are expressed as nmol/mg protein.
Hepatic Na+-K+-ATPase activity was measured using an ATPase assay kit (Nanjing Jiancheng Biotechnology Institute, Nanjing, China). The measurement of the Na+-K+-ATPase activity was based on the quantification of inorganic phosphate that is formed by adenosine triphosphate decomposition[15].
Liver specimens were deparaffinized for morphological assessment. The histologic damage severity was stratified using Suzuki’s criteria[16]. Liver tissue slices were microscopically examined in a blinded method by an experienced histologist.
Statistical analyses were performed using SPSS version 13.0 statistical software (SPSS, Chicago, IL, United States). Experimental results are expressed as the mean ± standard deviation (SD). Differences were regarded as statistically significant at P < 0.05.
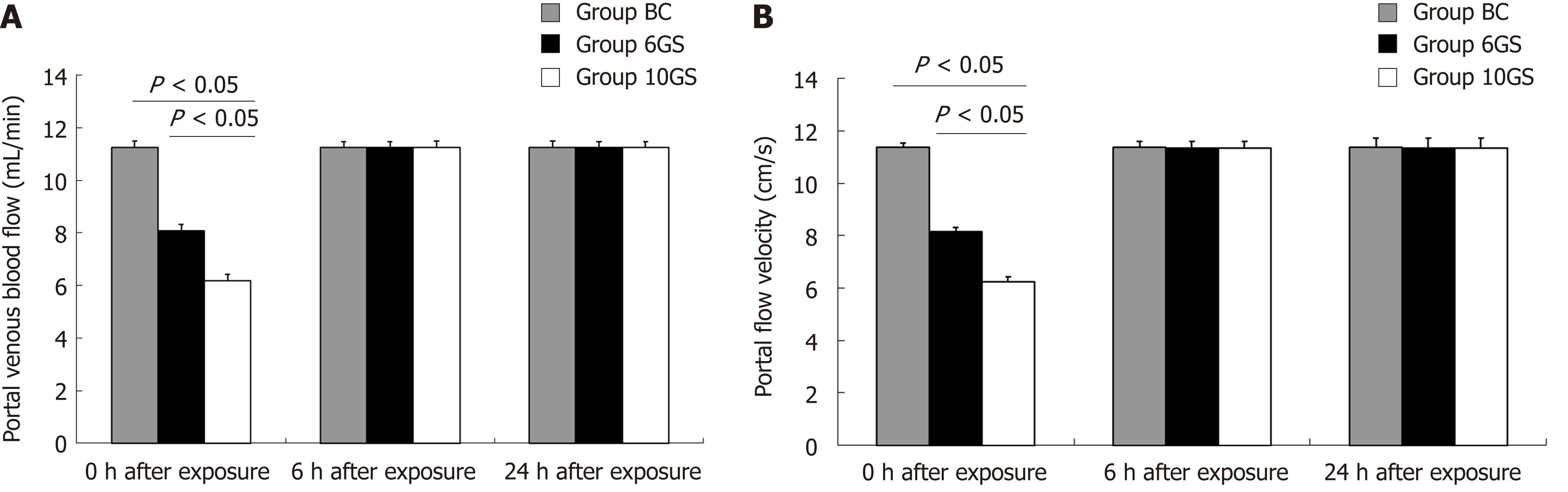
The normal portal venous flow in Wistar rats was 11.468 ± 0.237 mL/min. After repeated +Gz exposures in the 6GS and the 10GS groups, the velocity and flow signals in the PV were significantly reduced compared to the BC group (P < 0.01 at 0 h post exposure; Figure 1). Meanwhile, we found that the PV diameter did not change significantly. However, rats in the 6GS group had a much higher portal venous flow volume than those in the 10GS group (P < 0.01 at 0 h post exposure). All rats exhibited normal portal venous flow 6 h after repeated +Gz exposure. To summarize, as the G force increased, the portal venous blood flow decreased significantly, but transiently.
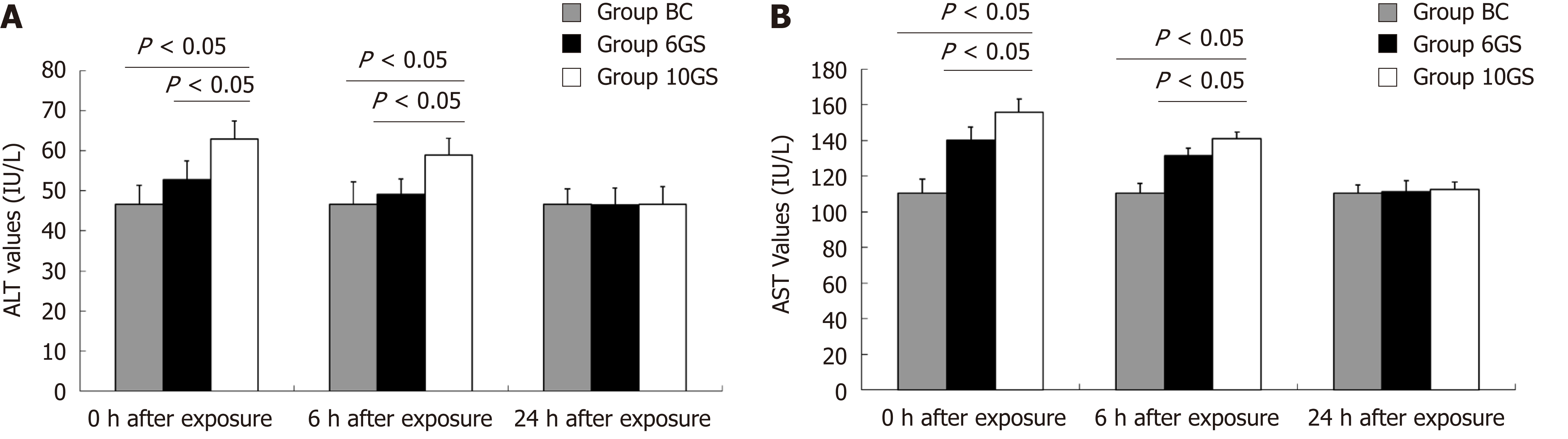
To assess liver cell damage in rats, plasma ALT and AST levels were determined 0, 6, and 24 h after repeated +Gz exposures. ALT and AST levels in the BC group were 46.6 ± 4.7 IU/L and 110.5 ± 7.6 IU/L, respectively.
After repeated +Gz exposures, ALT and AST values in the 6GS and the 10GS groups were higher than those in the BC group (P < 0.01 at 0 and 6 h time-points after exposure). However, rats in the 6GS group exhibited lower ALT and AST levels than those in the 10GS group at 0 and 6 h post exposure (P < 0.01, 6GS group vs 10GS group). The experimental groups showed normal ALT and AST levels 24 h after exposure (Figure 2). These consequences display that the degree of damage to the liver function positively correlated with the increase in G-value, but the abnormalities were transient.
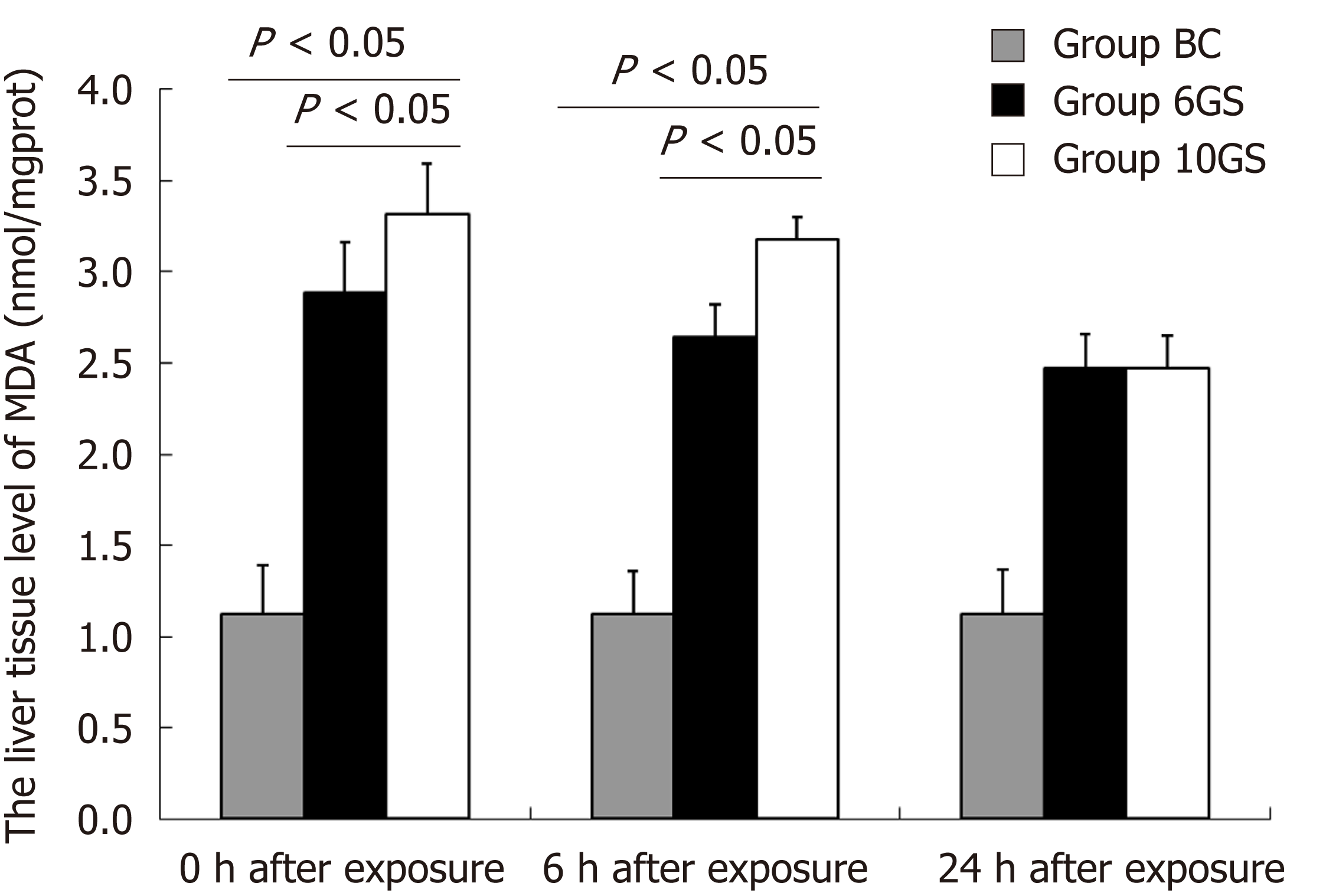
MDA concentration in both the 6GS and the 10GS groups had increased 0 h and 6 h after exposure. The MDA concentration in the 6GS group was lower than that in the 10GS group at 0 h (2.89 ± 0.24 nmol/mg protein vs 3.32 ± 0.25 nmol/mg protein, P < 0.01) and 6 h (2.64 ± 0.18 vs 3.18 ± 0.19; P < 0.01) post exposure (Figure 3). Notably, the 6GS and 10GS group MDA concentrations did not recover to normal 24 h after exposure. Based on these data, it was concluded that repeated +Gz exposures may induce lipid peroxidation in the rat liver.
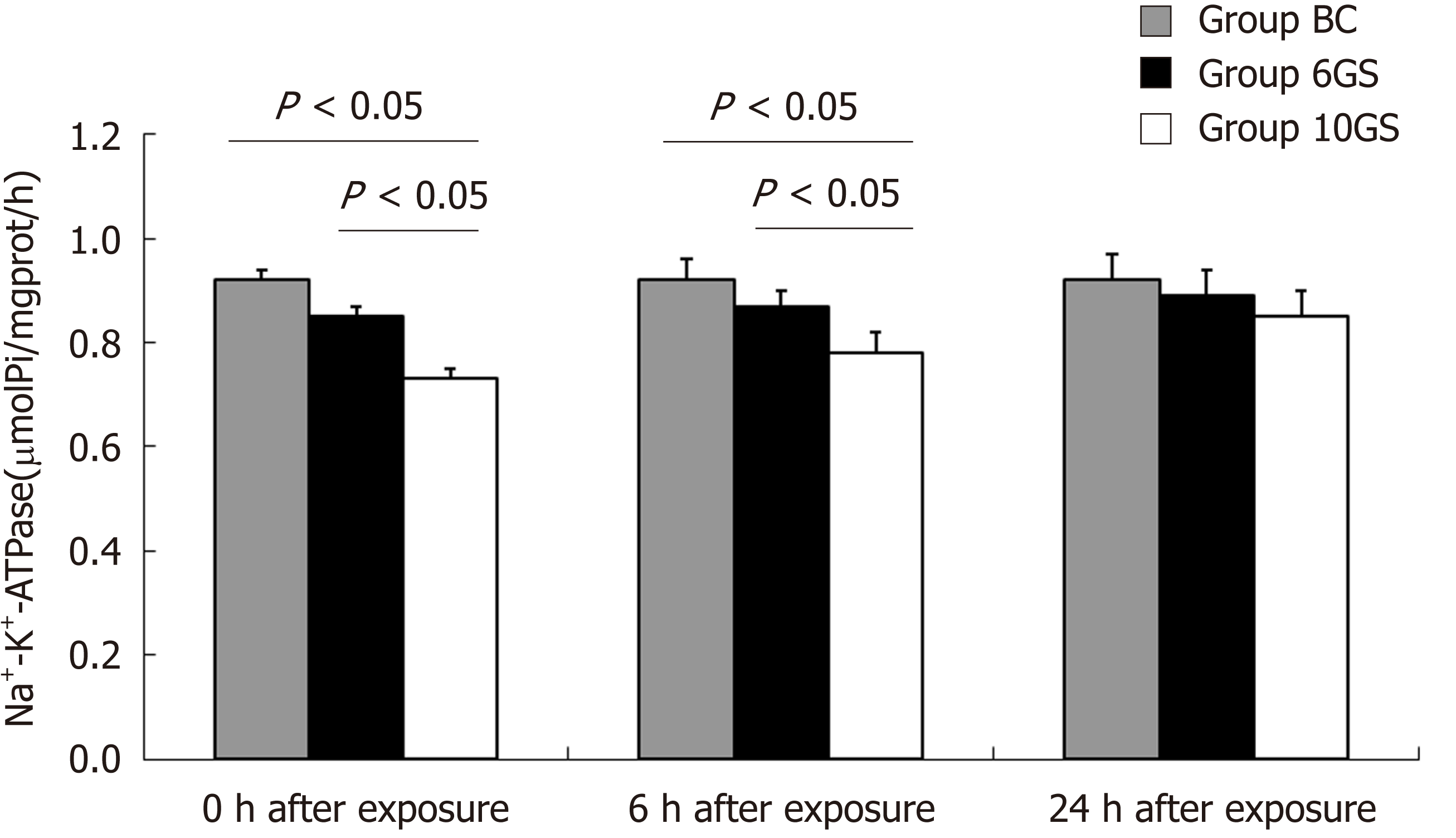
Na+-K+-ATPase is a cell membrane enzyme that is highly susceptible to lipid membrane peroxidation and free radical reactions[17]. Loss of its activity is a signal of indirect membrane damage. Na+-K+-ATPase activity decreased significantly after exposure in both the 6GS and the 10GS groups, compared to the BC group. The 6GS group had higher Na+-K+-ATPase activity than the 10GS group at 0 h (0.85 ± 0.04 μmolPi/mg protein/h vs 0.73 ± 0.05 μmolPi/mg protein/h, P < 0.01) and 6 h (0.87 ± 0.03 μmolPi/mg protein/h vs 0.78 ± 0.05 μmolPi/mg protein/h, P < 0.01) post exposure. There was no significant difference between the 6GS and the 10GS groups at 24 h after exposure (Figure 4).
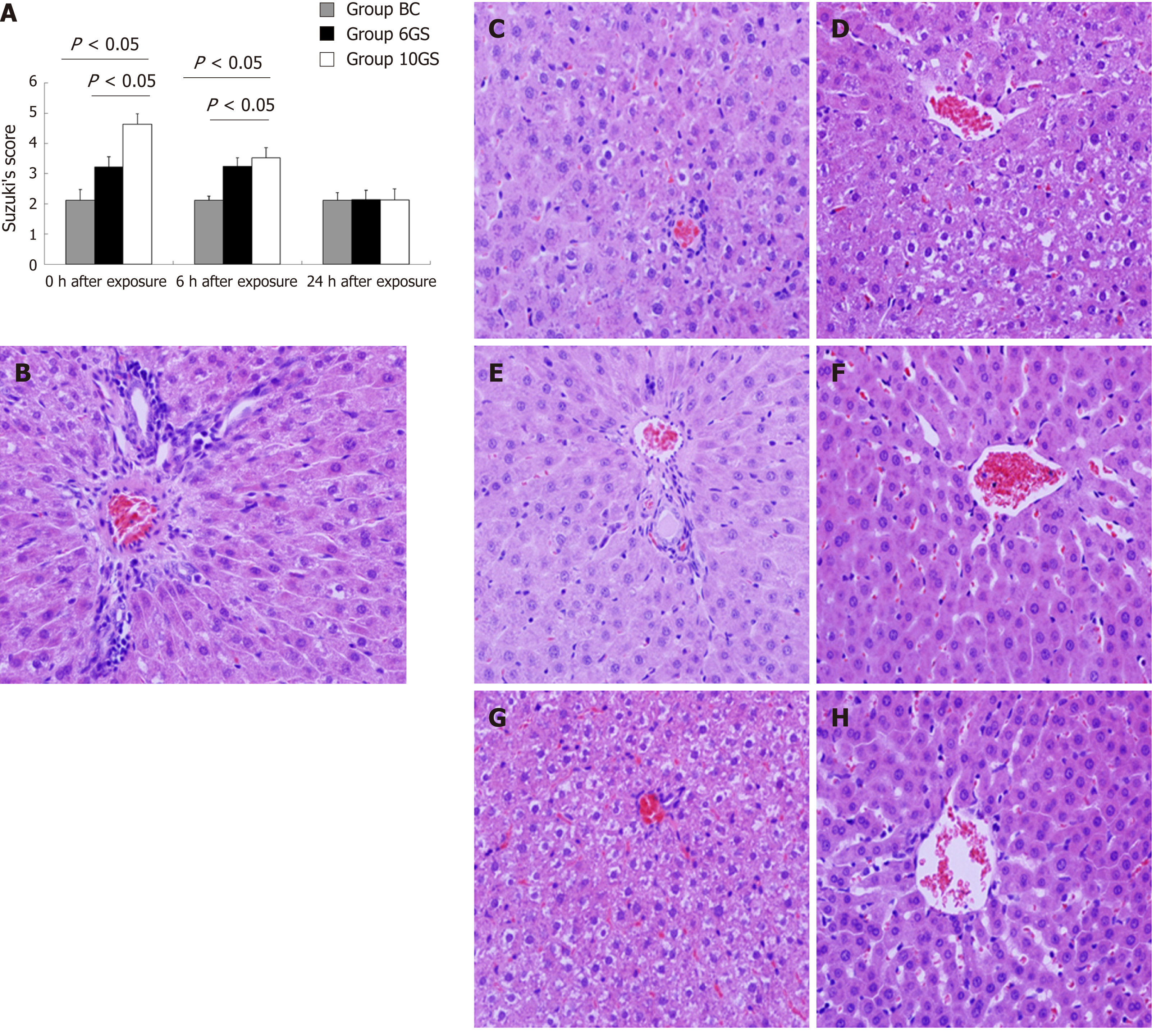
The hepatic pathological injury after repeated +Gz exposures was assessed and scored according to Suzuki’s criteria[16]. The structures of the hepatic lobules and liver antrum were clear, and cellular edema was not obvious in the BC group (Figure 5B, Suzuki’s score = 2.12 ± 0.35). At the 0 h time-point after exposure, the hepatic sinus cord-like structure was maintained in the 6GS group (Figure 5C, Suzuki’s score = 3.21 ± 0.13), whereas it was less well maintained in the 10GS group, which presented with hepatocyte edema (Figure 5D, Suzuki’s score = 4.63 ± 0.25). At the 6 h time-point post exposure, hepatocyte edema had been significantly relieved in the 10GS group (Figure 5F, Suzuki’s score = 3.53 ± 0.31, P < 0.01). There was no significant score difference between the 0 and 6 h time-points after exposure in the 6GS group (Figure 5C and E, Suzuki’s score = 3.21 ± 0.13 vs 3.24 ± 0.28, P < 0.01). The hepatic histology profiles in both the 6GS and the 10GS groups were nearly normal 24 h after exposure (Figure 5G, Suzuki’s score = 2.14 ± 0.33; Figure 5H, Suzuki’s score = 2.13 ± 0.36).
In this study, the effects of high +Gz acceleration on rat liver function was investigated. To this end, we devised an animal model of short-term repeated +Gz exposures. We chose Wistar rats as the experimental subjects because the human Glisson’s capsule is similar to that of rats and the model is simple and easy to control. Hepatic energy metabolism and an optimal intracellular environment rely on an adequate blood supply. Hepatocytes are very sensitive to ischemia and/or hypoxia in liver tissue[18,19]. Therefore, factors related to ischemia and/or hypoxia will definitely influence their metabolism[20]. Direct action and stress reaction caused by +Gz exposure can result in obvious hemodynamic changes between the upper and the lower body, in important organs, and on the body surface, which is similar to hepatic ischemia-reperfusion (I/R). Indeed, repeated +Gz exposures may cause hepatic I/R. Ischemia is defined as inadequate blood supply to an organ or part of an organ as a result of obstructed blood flow[21]. Our findings were consistent with those reported effects, with some significant differences between the acceleration exposed and the control rats observed.
Color Doppler ultrasound is a well-established method for assessing hemodynamic changes in liver circulation that occur under various physiological conditions[22,23]. As described by Kim et al[24], exposure to high +Gz accelerating force acting along the body axis from the head to the feet severely reduces blood supply to the internal organs.
Levels of ALT and AST can be used as measures of hepatic damage, and were used in this study to assess damage incurred due to repeated +Gz exposures. The 6GS group was associated with less cellular damage than the 10GS analogue; this was reflected by the lower serum ALT and AST levels. The results indicated that the degree of functional liver damage increased gradually with increasing G value. Zhang et al[25] reported that repeated +10 Gz stress had some impact on the oxygen radical metabolism in the rat liver. MDA is widely used as an indicator of oxidative stress, which is one of the end products of lipid peroxidation in the liver[26]. The results of our study displayed that rats in the 10GS group had more hepatic MDA than those in the 6GS group. After repeated +10 Gz exposures, the oxygen and nutrients supplied to the liver were reduced. After exposure, rats in the 6GS group presented with less oxidative stress-induced damage than rats in the 10GS group, as manifested by the lower MDA levels. In this study, changes in the MDA levels were in accordance with those caused by ischemia and/or hypoxia in rat livers[27], which also points toward ischemia or hypoxia as one of the main causes of high +Gz stress-induced liver injury.
Early research in this field found that positive acceleration affected the physiological indexes of the liver. Daligcon et al[28] reported that hyper-G stress increased levels of circulating catecholamines and glucagon, both effective stimulators of hepatic gluconeogenesis, and that continued hyperglycemia may be due, in part, to the control of the insulin-stimulated uptake by muscle tissues. They also found that hyper-G stress not only increased circulating and blood glucose levels, but also increased the content of liver glycogen. This was attributed to an increased rate of gluconeogenesis and the key role that epinephrine plays during the beginning of centrifugation exposure[29]. Later research reported that hypergravity exposure caused significant injury to the liver[30].
Our study has some limitations. First, we did not measure the blood flow changes in the HA due to technical limitations. This may be possible through technical advances in the future or the use of larger animal models, and we plan to actively pursue this research avenue in the near future. Additionally, other serum liver parameters such as alkaline phosphatase, gamma-glutamyl transferase, bilirubin, and serum lactate were not measured. We also plan to assess these parameters in future work. Moreover, physiological differences between rats and humans may render the data obtained herein non-transferable to human pilots under similar conditions of exposure.
In summary, the main findings of the study can be summarized as follows: first, short-term repeated exposures to either +6 Gz or +10 Gz temporarily reduced the portal venous flow. Second, ALT and AST levels only slightly increased in response to G exposure and soon reverted back to normal. An increase in G force resulted in additional liver damage. Third, evidence of oxidative damage was found, which may have been due to liver ischemia. Finally, repeated exposures were associated with a transient decrease of the liver energy, as indicated by the decrease in Na+-K+-ATPase activity. Although the rat data may not be directly extended to that of pilots, because of similar conditions of +Gz exposure, this model may be helpful in identifying more potential adverse effects of high +Gz stress on the human liver, and help develop practical effective protective measures. In the future, we will further expand our study to explore the effects of +Gz exposure over longer durations on liver function, with a view to elucidating the underlying pathophysiological mechanisms and proposing feasible protection to decrease adverse +Gz effects. This could be accomplished by applying an understanding of aviation medicine to aeronautical engineering technology development. This would be significant in aviation progress by ensuring flight safety, extending pilot flying-life, promoting good performance of combat aircrafts, and improving fighting capacity. These factors are essential for the development of a new generation of high-performance fighter aircrafts.
Some clinical data show that liver dysfunction was observed in pilots. However, there are many reasons for this: hepatitis virus, drug abuse, excessive drinking and so on. The objective of this study was to probe into whether positive acceleration affects rat liver function.
Exposure to high sustained +Gz (head-to-foot inertial load) is conclusively known to have harmful effects on the human body during aviation activities. High-acceleration force exposures, particularly when occurring repeatedly, may result in accumulative adverse stress responses in the body, and safe flying is a social problem that has attracted broad attention. An important question to address is whether changes in the blood flow direction after +Gz exposure impair liver function in rats. Moreover, the manner in which the portal venous hemodynamics changes after repeated +Gz exposures, and whether oxidative stress parameters increase the duration of these changes are additional questions awaiting answers. To clarify these questions, further studies on liver damage and the mechanisms of liver damage induced by high +Gz exposures are needed, and this will provide evidence to take effective preventive measures.
In this article, an animal centrifuge model was used to study whether positive acceleration impairs liver function. This research may help find more potential adverse effects of high +Gz stress on the human liver, and help develop practical, effective protective measures. In the future, we will further expand our study to explore the effects of +Gz exposures of longer duration on the liver function, to illuminate the pathophysiological mechanisms that are involved.
Completely random grouping design and exploratory experimental research were performed based on the experimental animals. We adopted the method of acceleration exposure in rats. The difference of experimental data was detected by analysis of variance using SPSS version 13.0 statistical software (SPSS, Chicago, IL, United States). Experimental results are expressed as the mean ± standard deviation.
The main findings of the study showed that repeated +Gz exposures not only transiently impair liver function but also affect liver metabolism and morphological structure. Although there are some gaps between experimental animal model and real flight environment, this research may help find more potential adverse effects of high +Gz stress on the human liver, and help develop practical effective protective measures.
While fighter pilots are frequently exposed to high Gz acceleration with the vector in the foot-head direction, the blood and fluid of the body will be redistributed and flow along the direction of inertia force to the lower body. Many studies have demonstrated the harmful effects of repeated +Gz stress on the cardiac ultrastructure, metabolism, and function. It was, however, of scarcity that to investigate the effect of high +Gz exposure on the hepatobiliary system. Therefore, we propose some doubts: Do high +Gz exposures impair liver function of rats? How does the portal venous hemodynamics change after repeated +Gz exposures? Do oxidative stress parameters increase? Well then, should the changes of blood flow direction after +Gz exposure cause hepatic ischemia, so as to affect the liver function? We hypothesized that repeated +Gz exposures could transiently impair the liver function in rats.
The main findings of the study can be summarized as follows: First, short-term repeated exposures to either +6 Gz or +10 Gz reduced the portal venous flow. Blood redistribution between the liver and body surface or other organs is similar to liver ischemia reperfusion. Repeat +Gz exposures may result in liver ischemia-reperfusion injury. Second, alanine aminotransferase and aspartate aminotransferase levels were only slightly increased and could soon revert back to normal. With an increase in the G force, additional damage also occurred in the liver function of the rats. The results showed that this damage should be functional and reversible. Third, oxidative damage might be engaged in the pathophysiologic process during liver ischemia. After repeated +Gz exposures, the blood and nutrient substance supplied to the liver were reduced. The exposed group had oxidative stress injury, as reflected in the higher malondialdehyde (MDA) levels. In addition, the MDA levels increased as the G value increased. Fourth, repeated +Gz exposures had something to do with a temporary reduction of the liver metabolism, as indicated by a decrease in the Na+-K+-ATPase activity. The main role of the Na+-K+-ATPase is to maintain the structure and function of mitochondria. When the activities of the Na+-K+-ATPase decline, the structure of mitochondria is likely to change. Morphologically mitochondria became swelling and matrix density decreased.
Although trained pilots may not use the same way as the rats under similar conditions of exposure due to species difference, the research method in rats may help in investigating the pathophysiological mechanism of high +Gz stress in humans. In addition, this research may aid discovery of more potentially harmful effects of high +Gz stress on the human liver, and subsequently, help to prevent liver injury. Flight crews will be the research subject of the project in the future. Prospective study will be the best research method.
| 1. | Evans JM, Knapp CF, Goswami N. Artificial Gravity as a Countermeasure to the Cardiovascular Deconditioning of Spaceflight: Gender Perspectives. Front Physiol. 2018;9:716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Akparibo IY, Chumbley E. Aerospace, Gravitational Effects, High Performance. 2018; Epub ahead of print. [PubMed] |
| 3. | Öztürk C, İlbasmış MS, Akın A. Cardiac responses to long duration and high magnitude +Gz exposure in pilots: an observational study. Anadolu Kardiyol Derg. 2012;12:668-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Perrier E, Leduc PA, Manen O, Lerecouvreux M, Deroche J, Paris JF, Doireau P, Quiniou G, Geffroy S, Carlioz R. [The heart and aerobatics]. Arch Mal Coeur Vaiss. 2005;98:47-52. [PubMed] |
| 5. | Scott JM, Esch BT, Goodman LS, Bredin SS, Haykowsky MJ, Warburton DE. Cardiovascular consequences of high-performance aircraft maneuvers: implications for effective countermeasures and laboratory-based simulations. Appl Physiol Nutr Metab. 2007;32:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Greenway CV, Stark RD. Hepatic vascular bed. Physiol Rev. 1971;51:23-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 435] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Pattarini JM, Blue RS, Aikins LT, Law J, Walshe AD, Garbino A, Turney MW, Clark JB. Flat spin and negative Gz in high-altitude free fall: pathophysiology, prevention, and treatment. Aviat Space Environ Med. 2013;84:961-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Mitchell SJ, Evans AD. Flight safety and medical incapacitation risk of airline pilots. Aviat Space Environ Med. 2004;75:260-268. [PubMed] |
| 9. | Lu WH, Hsieh KS, Li MH, Ho CW, Wu YC, Ger LP, Wang JS, Chu H. Heart status following high G exposure in rats and the effect of brief preconditioning. Aviat Space Environ Med. 2008;79:1086-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Nishida Y, Maruyama S, Shouji I, Kemuriyama T, Tashiro A, Ohta H, Hagisawa K, Hiruma M, Yokoe H. Effects and biological limitations of +Gz acceleration on the autonomic functions-related circulation in rats. J Physiol Sci. 2016;66:447-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Lauritzsen LP, Pfitzner J. Pressure breathing in fighter aircraft for G accelerations and loss of cabin pressurization at altitude--a brief review. Can J Anaesth. 2003;50:415-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Cao XS, Sun XQ, Zhang S, Wang B, Wu YH, Liu TS, Wu XY. Acceleration after-effects on learning and memory in rats: +10 Gz or +6 Gz for 3 min. Neurosci Lett. 2007;413:245-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Zhou Y, Wang B, Wang YC, Wu YH, Zhang S, Geng J, Sun XQ. [Apoptosis in myocyte after repeated + Gz exposures in rats]. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2010;26:275-277. [PubMed] |
| 14. | Sun XQ, Zhang LF, Wu XY, Jiang SZ. Effect of repeated +Gz exposures on energy metabolism and some ion contents in brain tissues of rats. Aviat Space Environ Med. 2001;72:422-426. [PubMed] |
| 15. | Sousa L, Pessoa MTC, Costa TGF, Cortes VF, Santos HL, Barbosa LA. Iron overload impact on P-ATPases. Ann Hematol. 2018;97:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 730] [Article Influence: 22.1] [Reference Citation Analysis (5)] |
| 17. | Bonatsos V, Kappas I, Birbas K, Vlachodimitropoulos D, Toutouzas K, Karampela E, Syrmos N, Bonatsos G, Papalois AE. Effects of U-74389G (21-Lazaroid) and Ascorbic Acid on Liver Recovery After Acute Ischemia and Reperfusion in Rats. In Vivo. 2015;29:585-594. [PubMed] |
| 18. | Cannistrà M, Ruggiero M, Zullo A, Gallelli G, Serafini S, Maria M, Naso A, Grande R, Serra R, Nardo B. Hepatic ischemia reperfusion injury: A systematic review of literature and the role of current drugs and biomarkers. Int J Surg. 2016;33 Suppl 1:S57-S70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 291] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 19. | de Oliveira THC, Marques PE, Poosti F, Ruytinx P, Amaral FA, Brandolini L, Allegretti M, Proost P, Teixeira MM. Intravital Microscopic Evaluation of the Effects of a CXCR2 Antagonist in a Model of Liver Ischemia Reperfusion Injury in Mice. Front Immunol. 2018;8:1917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Peralta C, Jiménez-Castro MB, Gracia-Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. J Hepatol. 2013;59:1094-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 505] [Article Influence: 38.8] [Reference Citation Analysis (9)] |
| 21. | Chu MJ, Premkumar R, Hickey AJ, Jiang Y, Delahunt B, Phillips AR, Bartlett AS. Steatotic livers are susceptible to normothermic ischemia-reperfusion injury from mitochondrial Complex-I dysfunction. World J Gastroenterol. 2016;22:4673-4684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Heller MT, Tublin ME. The role of ultrasonography in the evaluation of diffuse liver disease. Radiol Clin North Am. 2014;52:1163-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Iranpour P, Lall C, Houshyar R, Helmy M, Yang A, Choi JI, Ward G, Goodwin SC. Altered Doppler flow patterns in cirrhosis patients: an overview. Ultrasonography. 2016;35:3-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Kim HS, Kim YW. Expression of phosphorylated extracellular signal-regulated kinase in rat kidneys exposed to high +Gz. Bosn J Basic Med Sci. 2012;12:269-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Chen LE, Wu F, Zhao A, Ge H, Zhan H. Protection Efficacy of the Extract of Ginkgo biloba against the Learning and Memory Damage of Rats under Repeated High Sustained +Gz Exposure. Evid Based Complement Alternat Med. 2016;2016:6320586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Luczaj W, Skrzydlewska E. [The present-day look at lipid peroxidation]. Postepy Biochem. 2006;52:173-179. [PubMed] |
| 27. | Nakagawa K, Kato S, Miyazawa T. Determination of Phosphatidylcholine Hydroperoxide (PCOOH) as a Marker of Membrane Lipid Peroxidation. J Nutr Sci Vitaminol (Tokyo). 2015;61 Suppl:S78-S80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Daligcon BC, Oyama J. Hyper-G stress-induced hyperglycemia in rats mediated by glucoregulatory hormones. Aviat Space Environ Med. 1985;56:37-42. [PubMed] |
| 29. | Daligcon BC, Oyama J, Hannak K. Increased gluconeogenesis in rats exposed to hyper-G stress. Life Sci. 1985;37:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Niu ZY, Zhang JZ, Shi SG, Wu B, Li D, Dang P, Kan GH. Effect of hypergravity on expression of c-fos gene in hepatocytes of Rhesus macaque. Shijie Huaren Xiaohua Zazhi. 2006;14:2793-2795. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P- Reviewer: Charco R, Tatsuya O, Tsegmed U, Yamamoto T S- Editor: Ma RY L- Editor: Wang TQ E- Editor: Huang Y