Published online Jan 21, 2019. doi: 10.3748/wjg.v25.i3.308
Peer-review started: October 10, 2018
First decision: November 17, 2018
Revised: November 27, 2018
Accepted: December 19, 2018
Article in press: December 19, 2018
Published online: January 21, 2019
Processing time: 103 Days and 7.7 Hours
Elastography-based liver stiffness measurement (LSM) is a non-invasive tool for estimating liver fibrosis but also provides an estimate for the severity of portal hypertension in patients with advanced chronic liver disease (ACLD). The presence of varices and especially of varices needing treatment (VNT) indicates distinct prognostic stages in patients with compensated ACLD (cACLD). The Baveno VI guidelines suggested a simple algorithm based on LSM < 20 kPa (by transient elastography, TE) and platelet count > 150 G/L for ruling-out VNT in patients with cACLD. These (and other) TE-based LSM cut-offs have been evaluated for VNT screening in different liver disease etiologies. Novel point shear-wave elastography (pSWE) and two-dimensional shear wave elastography (2D-SWE) methodologies for LSM have also been evaluated for their ability to screen for “any” varices and for VNT. Finally, the measurement of spleen stiffness (SSM) by elastography (mainly by pSWE and 2D-SWE) may represent another valuable screening tool for varices. Here, we summarize the current literature on elastography-based prediction of “any” varices and VNT. Finally, we have summarized the published LSM and SSM cut-offs in clinically useful scale cards.
Core tip: Elastography-based measurement of liver stiffness (LSM) and spleen stiffness (SSM) represent valuable non-invasive screening tools for esophageal varices (EVs). Transient elastography (TE) has been widely validated, and the combined TE-based LSM < 20 kPa and platelet count (PLT) > 150 G/L algorithm is able to rule-out varices-needing-treatment (VNTs). While LSM and SSM by novel shear wave elastography devices and magnetic resonance elastography (MRE) may be more accurate to reflect the risk of EV and VNT, their value in clinical practice remains to be established.
- Citation: Paternostro R, Reiberger T, Bucsics T. Elastography-based screening for esophageal varices in patients with advanced chronic liver disease. World J Gastroenterol 2019; 25(3): 308-329
- URL: https://www.wjgnet.com/1007-9327/full/v25/i3/308.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i3.308
In 2015, the Baveno VI consensus defined the term “compensated advanced chronic liver disease” (cACLD) in order to better define the spectrum of advanced fibrosis and cirrhosis in asymptomatic patients[1]. In patients with chronic liver diseases, transient elastography (TE) is recommended to screen for cACLD, with values between 10-15 kPa being suggestive and > 15 kPa being highly suggestive for cACLD[1]. Importantly, patients with cACLD should be evaluated for the presence of clinically significant portal hypertension (CSPH) and undergo regular surveillance for hepatocellular carcinoma (HCC). Detection of CSPH most commonly relies on endoscopic screening for esophageal varices (EV) but may be (earlier) identified by hepatic venous pressure gradient (HVPG) measurements showing HVPG ≥ 10 mmHg. Screening for EV is a cornerstone in the management of cirrhotic patients, since the presence of EV indicates a distinct inferior prognosis within cACLD patients. Moreover, varices-needing-treatment (VNT) are defined by large size (≥ 5 mm) but also include small varices (< 5 mm) in case of Child-C cirrhosis or if red spot signs are present (1,4). Patients with VNTs should receive primary prophylaxis of varices bleeding in order to prevent variceal bleeding, and reduce the risk of decompensation and death[2-4].
Fibrosis-induced increases of hepatic vascular resistance represents the main causative factor for CSPH. Consequently, CSPH leads to development of portosystemic collaterals, such as esophageal-, umbilical-, fundal- and/or rectal-varices. Thus, in cACLD patients the degree of hepatic fibrosis, i.e., LSM results are highly suggestive of CSPH and thus, for EV and VNT. Pathophysiologically, CSPH precludes the formation of EV and can be present in compensated patients that have not yet developed EV[5]. However, due to the limited availability of HVPG, CSPH is clinically most often diagnosed only after EV are detected by upper-gastrointestinal endoscopy[1,5]. Nevertheless, endoscopy is an invasive procedure, requiring training and specialized infrastructure and is not well perceived by patients. Therefore in recent years, several studies have investigated liver elastography as a non-invasive method for the diagnosis of EV and VNT. The Baveno VI consensus statement defined non-invasive criteria based on liver stiffness measurement (LSM) by transient elastography and platelet count by which patients can safely avoid screening endoscopy[1]. In this comprehensive literature review, we summarize current knowledge on non-invasive elastography-based methods for the detection of EV and VNT and its implications in daily clinical practice.
Referring to our national guidelines[5], EV should be graded as: absent, small (< 5mm of diameter) or large (> 5 mm of diameter), and the presence of red spots should be indicated for risk stratification[5]. While international guidelines also discriminate between small, medium and large varices[1,3] this discrimination is neither clinically useful, as the international recommendations for the management of medium-to-large varices are similar but only different to small varices and there is considerable inter-observer disagreement during endoscopical assessment of variceal size. Thus, we define for the purpose of this review the following categories: “any EV” as any EV detected on endoscopy (including small varices) and “varices-needing-treatment” (VNT) as all (medium)/large varices (≥ 5mm) and small varices found in patients with Child-Pugh C cirrhosis or small varices presenting red spot signs. Accordingly, the capability of non-invasive elastography methods to detect “any varices” and “VNT” was analyzed seperately.
Over the years, several methods to non-invasively stage fibrosis have been implemented: vibration-controlled or transient elastography (TE), point shear-wave-elastography (pSWE), two dimensional shear wave elastography (2D-SWE) and magnetic resonance elastography (MRE).
Elastography assesses tissue elasticity, which is defined as the tendency of tissue that resists deformation when force is applied and the return to its original form once the force is removed[6]. To calculate stiffness certain formulas are used that include variables for tissue elasticity, tissue density and shear wave velocity[7]. In SWE, dynamic stress is applied to the tissue via mechanical vibrations in TE, and acoustic radiation impulses in pSWE and 2D-SWE[6]. Shear waves generated by the device are propagated by the underlying tissue, and measured perpendicular to the acoustic radiation force or parallel to the one-dimensional TE impulse[6]. The shear wave propagation speed varies between tissue densities. This propagation velocity is then measured by the device and reflects tissue elasticity[6]. In MRE, a passive driver, stimulated by acoustic waves enacts physical pressure pulses on the right abdomen and thus, the liver, which is captured and visualized using specific magnetic resonance sequences and software algorithms. In contrast to TE and ultrasound-based SWE that evaluate liver stiffness only locally in small, defined areas limited to a few mm² (pSWE, TE) or up to 4 cm² (2D-SWE), MRE is able to assess stiffness within the entire volume of the liver (3D-SWE). MRE has been reported to be highly reproducible and very accurate in differentiating between low stages of liver fibrosis[8,9].
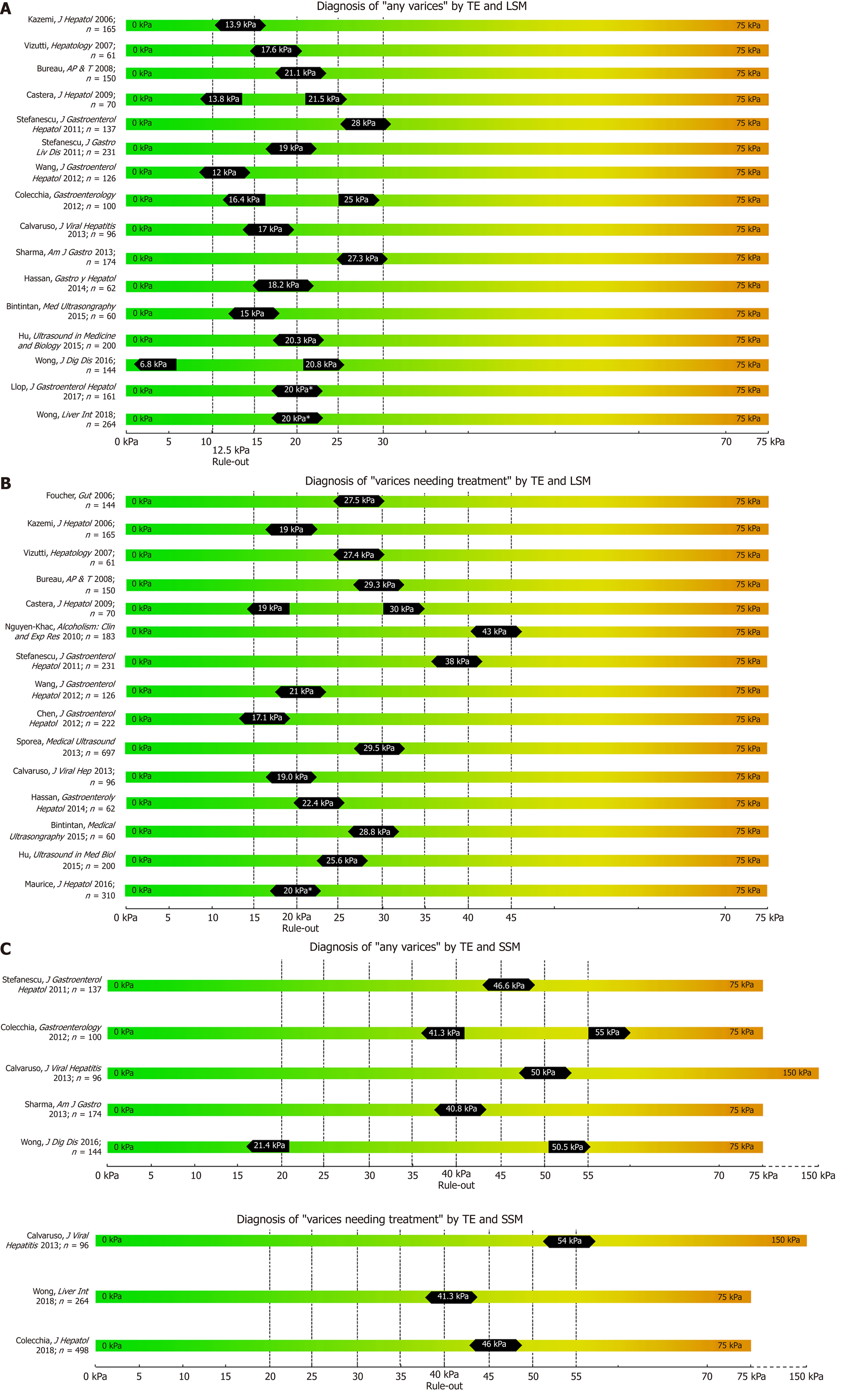
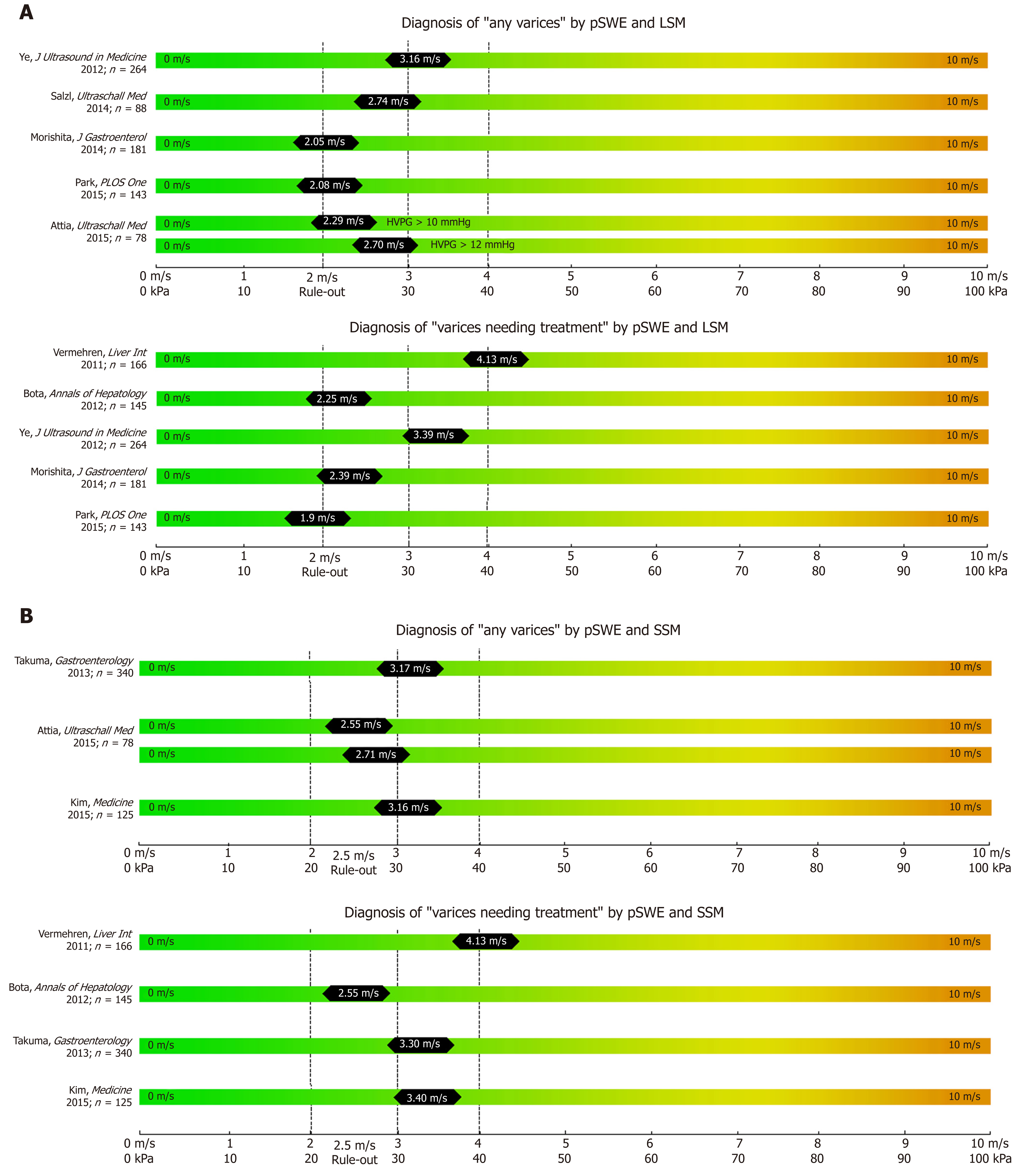
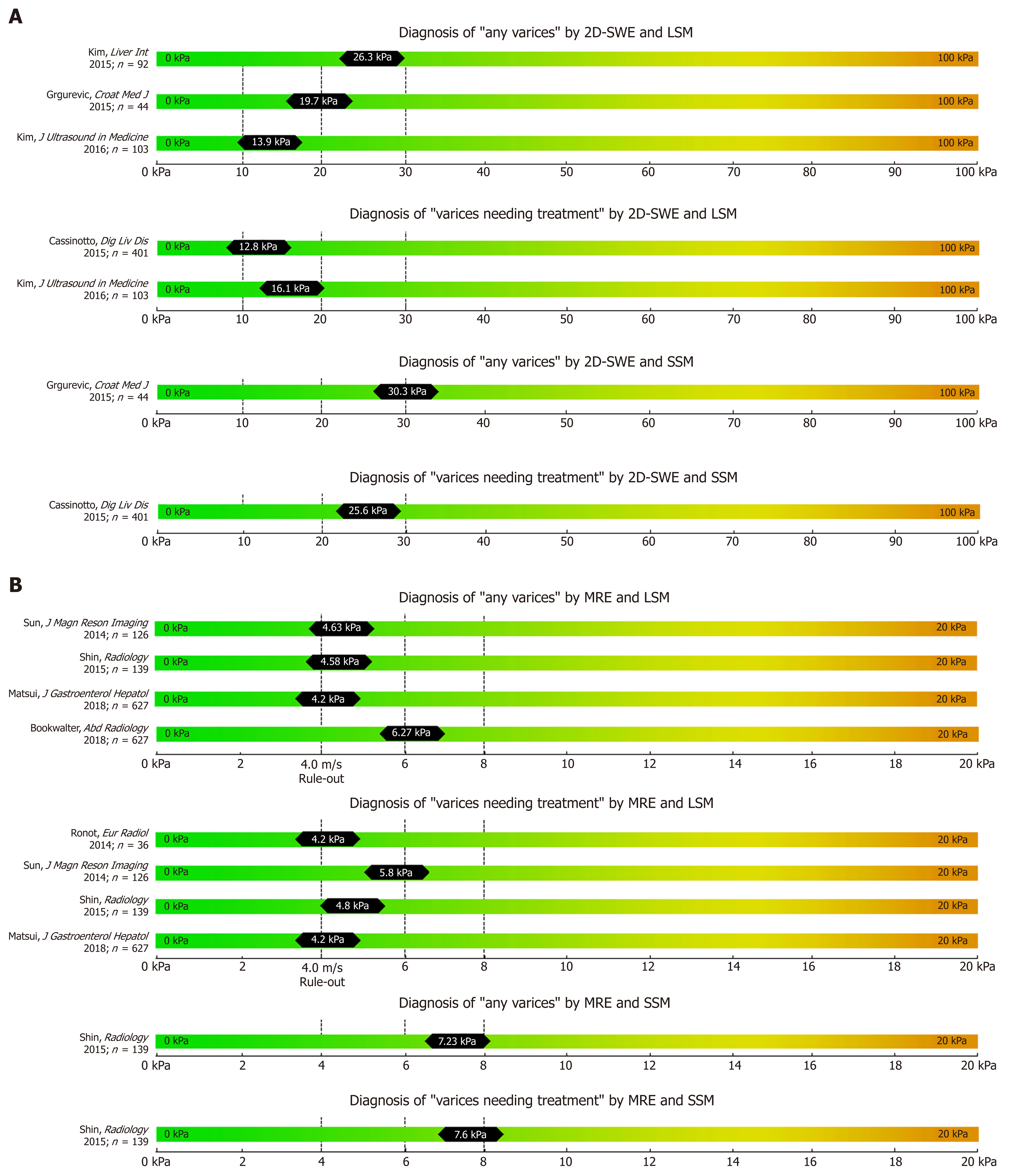
While most studies on TE, pSWE, 2D-SWE and MRE have focused on correlation with liver fibrosis on histology, several other studies have focused on their potential to non-invasively predict the presence of EV and VNT. We summarized the most important studies in Table 1 (TE), Table 2 (pSWE), Table 3 (2D-SWE) and Table 4 (MRE). We furthermore created graphical charts marking important cut-offs for each method, that can easily be implemented in daily clinical practice (see Figure 1 for TE-, Figure 2 for pSWE- , Figure 3A for 2D-SWE- and Figure 3B for MRE-derived cut-offs).
| Author, journal, year of publication | Country | Study design | Etiology | N Patients included % with EV % with VNT | TE-Cut-offs and AUC EV/VNT | Main conclusions (Specificity/Sensitivity, PPV/NPV) | |
| Foucher, Gut, 2006[16] | France | Prospective | Mixed | EV: VNT: | 144 42 (29%) 85 (59%) | LSM: n/a 27.5 kPa, AUC 0.73 | LSM: EV: n/a VNT: Sens. 88%, Spec. 53%, PPV: 45%, NPV: 90% |
| Kazemi, J Hepatol, 2006[15] | France | Prospective | Mixed | EV: VNT | Total: 165 74 (44.8%) 47 (28.5%) | LSM: 13.9 kPa, AUC: 0.84 19.0 kPa, AUC 0.83, | LSM: EV: Sens. 92%, Spec. 39%, PPV 55%, NPV 85% VNT: Sens. 89%, Spec. 59%, PPV 47%, NPV: 93% |
| Vizzutti, Hepatology, 2007[80] | Italy | Prospective | HCV | EV: VNT | Total: 61 30 (49.2%) 18 (38.2%) | LSM: 17.6 kPa, AUC 0.76 27.4 kPa, AUC 0.76 | LSM: EV: Sens. 90%, spec. 43%, PPV 77%, NPV 66% VNT: Sens. 70%, spec. 78%, PPV 90%, NPV 55% |
| Bureau, Aliment Pharmacol Ther, 2008[81] | France | Prospective | Mixed | EV: VNT | Total: 150 64 (42.6%) 43 (28.6%) | LSM: EV: 21.1 kPa, AUC 0.85 VNT/large: 29.3 kPa, AUC 0.76 | LSM: EV: sens. 84%, spec. 71% VNT: sens. 81%, spec. 61% |
| Castéra, J Hepatol, 2009[82] | France | Prospective | HCV | EV: VNT | Total: 70 25 (35.7%) 15 (21.4%) | LSM: 13.9 kPa, 17.6 kPa, 21.5 kP; AUC 0.96 19 kPa, 21.5 kPa; 30 kPa, AUC 0.87 | LSM: Cut-off 13.9 kPa: Sens. 96%, Spec. 39%, PPV 49%, NPV 94% Cut-off 17.6 kPa: Sens. 84%, Spec. 61%, PPV 57%, NPV 86% Cut-off 21.5 kPa: Sens. 76%, Spec. 78%, PPV 68%, NPV 84% Cut-off 19.0 kPa: Sens. 85%, Spec. 62%, PPV 35%, NPV 94% Cut-off 21.5 kPa: Sens. 85%, Spec. 68%, PPV 39%, NPV 95% Cut-off 30.5 kPa: Sens. 77%, Spec. 5%, PPV 56%, NPV 94%10 |
| Nguyen-Khac, Alcohol Clin Exp Res; 2010[20] | France | Prospective | Mixed | EV: VNT | Total: 183 NR 41 (22%) | LSM: 48 kPa; AUC 0.75 ALD: 47.2 kPa, AUC 0.77 Viral: 19.8 kPa, AUC 0.73 | LSM: Sens. 73%, Spec. 73%, PPV 44%, NPV 90% ALD: Sens. 85%, Spec. 64%, PPV 44%, NPV 93% Viral: Sens. 89%, Spec. 55%, PPV 27%, NPV 96% |
| Stefanescu, J Gastroenterol Hepatol, 2011[18] | Romania | Prospective | ALD and/or HCV or healthy controls | EV: VNT | Total: 137 116 (85%) 60 (44%) | LSM: EV: 28 kPa, AUC 0.75 SSM: EV: 46.4 kPa, AUC 0.78 LSM + SSM: EV: LSM 19 kPa, SSM 55 kPa NR | LSM: Sens. 74%, Spec. 64%, PPV 92%, NPV 31% SSM: Sens. 84%, Spec. 71%, PPV 94%, NPV 46% LSM (19 kPa) + SSM (55 kPa): Sens. 93%, Spec. 40%, PPV 95%, NPV 33% NR |
| Stefanescu, J Gastrointestin Liver Dis, 2011[83] | Romania | Prospective | ALD and/or HCV | EV: VNT | Total: 231 157 (68%) 68 (30%) | LSM: 19 kPa, AUC 0.66 38 kPa, AUC 0.69 | LSM: Sens. 84%, Spec. 32%, PPV 72%, NPV 49% Sens. 56%, Spec. 75%, PPV 47%, NPV 81% |
| Chen, J Gastroenterol Hepatol, 2012[84] | China | Prospective | HBV | EV: VNT | Total: 222 96 (43%) 82 (40%) | LSM: NR All 17.1 kPa, AUC 0.73 ALT >5 x ULN: 36.1 kPa, AUC 0.92 CPC A, rule-out EV : 7.9 kPa, AUC 0.79 CPC A, rule-in 34.6 kPa, AUC 0.79 | LSM: NR Cut-off 17.1 kPa: Sens. 90%, Spec. 44%, PPV 47%, NPV: 88% Cut-off 36.1 kPa: PPV 73%, NPV 100% Cut-off 7.9 kPa: Sens. 97%, NPV 95% Cut-off 34.6 kPa: Spec. 94%, PPV 73% LSPS: Cut-off 3.5: Sens. 78%, NPV 86% (exclusion) Cut-off 5.5: Spec. 90%, PPV 76% (inclusion) |
| Wang, J Gastroenterol Hepatol, 2012[85] | Taiwan | Prospective | HBV | EV: VNT | Total: 126 48 (38%) 13 (10%) | LSM: 12.0 kPa, AUC 0.79 21.0 kPa, AUC 0.87 | LSM: Sens. 67%, Spec. 77%, PPV 64%, NPV 79% Sens. 77%, Spec. 87%, PPV 40%, NPV 97% |
| Colecchia, Gastroenterology, 2012[32] | Italy | Prospective | HCV | EV: VNT: | Total: 100 53 (53%) 26 (26%) | LSM (AUC 0.90): cut-off 16.4 kPa cut-off 25.0 kPa SSM (AUC 0.94): cut-off 41.3 kPa cut-off 55.0 kPa NR | LSM: Sens. 96%, Spec. 60% (rule out) Sens. 57%, Spec. 98% (rule in) SSM: Sens. 98%, Spec. 66% (rule out) Sens. 72%, Spec. 96% (rule in) NR |
| Sporea, Med Ultrason, 2013[86] | Romania | Prospective | Viralalcoholic | EV: VNT: | Total: 697 387 (54.5%) 273 (39.1%) | NR All: 29.5 kPa (0.871) Alcohol: 32.5 kPa (0.836) Viral: 24.8 kPa (0.867) | NR Sens. 77.5%, Spec. 86.9% Sens. 85.0%, Spec. 74.6% Sens. 81.0%, Spec. 80.7% |
| Calvaruso, J Viral Hepat, 2013[33] | Italy | Prospective | HCV | EV: VNT: | Total: 96 54 (56.3%) 26 (27.1%) | LSM:17.0 kPa, AUC 0.71 Modified SSM (0-150kPa): 50.0kPa, AUC 0.70 LSM: 19.0 kPa, AUC 0.71 Modified SSM (0-150kPa): 54.0 kPa, AUC 0.82 | LSM: Sens. 71%, Spec. 57%, PPV 67%, NPV 62% Modified SSM: Sens. 65%, Spec. 61%, PPV: 69%, NPV: 57% LSM: Sens. 72% Spec. 55%, PPV 38%, NPV 84% Modified SSM: Sens. 80%, Spec. 70%, PPV: 47%, NPV: 90% |
| Shi, Liver Int, 2013[87] | China | Meta-analysis | Mixed Sub-analyses for viral etiologies | EV: VNT: | Total: 3644 1786 (49.0%) 1166 (32.0%) | LSM (pooled): 15.1-28.0 kPa; AUC 0.84 17.8-48.0 kPa; AUC 0.78 | LSM (pooled): Sens. 87%, Spec. 53%, PPV 79%, NPV 64% Sens. 86%, Spec. 59%,, PPV 79%, NPV 66% |
| Sharma, Am J Gastroenterol, 2013[30] | India | Prospective | Mixed | EV: VNT: | Total: 174 124 (71.3%) 78 (44.8%) | LSM: 27.3 kPa, AUC 0.91; SSM: 40.8 kPa, AUC 0.90 NR | LSM: Sens. 91%, Spec. 72%, PPV 89%, NPV 76%, SSM: Sens. 94%, Spec. 76%, PPV 91%, NPV 84% |
| Hassan, Gastroenterol Hepatol, 2014[88] | Egypt | Prospective | HCV | EV: VNT: | Total: 62 50 (81%) 32 (52%) | LSM: 18.2 kPa, AUC 0.79 22.4 kPa, AUC 0.80 | LSM: Sens. 82%, Spec. 73%, PPV 89%, NPV 49% Sens. 84%, Spec. 72%, PPV 84%, NPV 72% |
| Binţinţan, Med Ultrason, 2015[21] | Romania | Prospective | ViralALD | EV: VNT: | Total: 60 47 (78%) 32 (53%) | LSM: 15 kPa, AUC 0.96 28.8 kPa, AUC 0.90 | LSM: Sens. 95%, Spec. 100%, PPV 100%, NPV 86% Sens. 87%, Spec. 83%, PPV: 84%, NPV: 86% |
| Hu, Ultrasound Med Biol, 2015[89] | China | Prospective | Viral | EV: VNT: | Total: 200 110 (55%) 69 (35%) | LSM: 20.3 kPa, AUC 0.84 25.6 kPa, AUC 0.86 | LSM: Sens. 84%, Spec. 73%, PPV 72%, NPV: 91% Sens. 86%, Spec. 72%, PPV 79%, NPV: 81% |
| Li et al. Rev Esp Enferm Dig 2016[90] | International | Meta-analysis | Mixed | EV: VNT: | Total: 2,994 Studies: 20 NR NR | LSM NR 14.5-48.0 kPa (0.83) | LSM Sens 81%, Spec 71%, |
| Wong, J Dig Dis, 2016[17] | Hong Kong | Prospective | Chronic HBV | EV: VNT: | Total: 144 31 (21.5%) 2 (1.4%) | LSM: (0.690) Rule out: 6.8 kPa Rule in: 20.8 kPa SSM: (0.736) Rule out: 21.4 kPa Rule in: 50.5 kPa NR | Sens. 90.3%, Spec 29.2%, PPV 25.9%, NPV 91.7% Sens. 29.0%, Spec. 90.3%, PPV 45.0%, NPV 82.3% Sens. 90.3%, Spec 43.4%, PPV 30.4%, NPV 94.2% Sens. 45.2%, Spec. 90.3%, PPV 56.0%, NPV 85.7% NR |
| Maurice, J Hepatol, 2016 [22] | United Kingdom | Retrospective | Mixed | EV: VNT: | Total: 310 72 (23%) 15 (5%) | LSM: 20 kPa, AUC 0.686 LSM (20 kPa) and PLT (150 G/L): AUC 0.746 | Sens. 67%, Spec. 55%, PPV 7%, NPV 97% Sens. 87%, Spec. 34%, PPV 6%, NPV 98% |
| Abraldes, Hepatology, 2016 [19] | International | Retrospective | Mixed | EV: VNT: | Total: 518 217 (42%) 67 (13%) | NR (AUC 0.71) LSM: 14.0 kPa (AUC 0.67) LSM (20 kPa) and PLT (150 G/L): AUC 0.76 | NR NR NR |
| Marot, Liver Int, 2017[25] | International | Meta-analysis | Mixed | EV: VNT | Total: 3364 NR (49.0%) NR (32.0%) | LSM: 20 kPa: AUC: NR LSM (20 kPa) and PLT (150 G/L): AUC: NR | LSM and PLT (150 G/L): Sens. 89%, Spec. 38%, PPV: 43%, NPV: 86% Sens. 93%, Spec. 30%, PPV 14%, NPV 97% |
| Pu, World J Gastroenterol, 2017[26] | International | Meta-analysis | Mixed | EV: VNT: | Total: 2697 NR NR | LSM (pooled): 20 kPa, AUC 0.83 30 kPa, AUC: 0.83 | LSM: Pooled: Sens. 84%, Spec. 62%, Cut-off 20 kPa: Sens. 83%, Spec. 68%, PPV: n/a, NPV: n/a Pooled: Sens. 78%, Spec. 76%, Cut-off 30 kPa: Sens. 73%, Spec. 74%, PPV: n/a, NPV: n/a |
| Llop, J Gastroenterol Hepatol, 2017[23] | Spain | Retrospective analysis of prospective data | Mixed | EV: VNT: | Total: 161 25 (15.5%) NR | LSM: 20.0 kPa, AUC: NR LSM (20 kPa) and Plt (150 G/L), AUC: NR | LSM: Sens. 76%, Spec. 71%, PPV: 32%, NPV: 94% LSM+Plt: Sens. 88%, Spec. 38%, PPV 21%, NPV 94% |
| Wong, Liver Int, 2018[27] | Hong Kong | Prospective | Mixed | EV: VNT: | TE exam.: 264 51 (18.6%) 11 (4.0%) | LSM:20.0 kPa, AUC: NR LSM (20 kPa) and Plt (150 G/L): AUC: NR SSM: 41.3 kPa; AUC: NR | LSM: Sens: 96%, Spec.: 26%, PPV: 47%, NPV: 91% LSM+PLT: Sens. 91%, Spec.: 18.1%, PPV: 10%, NPV: 96% NR |
| Manatsathit, J Gastroenterol Hepatol, 2018[31] | USA | Meta-analysis | Mixed | EV: VNT: | LSM: 4,337 SSM: 1,1119 LSM: 1681 (56%) SSM: 968 (51%) LSM: 1466 (34%) SSM: 383 (34%) | LSM (pooled): cut-offs NR; AUC 0.82 SSM (pooled): cut-offs NR; AUC: 0.90 LSM (pooled): cut-offs NR; AUC 0.83 SSM (pooled): cut-offs NR; AUC 0.81 | LSM (pooled): Sens. 84%, Spec. 64% SSM (pooled): Sens. 91% Spec. 66% LSM: Sens. 85%, Spec. 64% SSM: Sens. 90%, Spec. 73% LSM: Sens. 85%, Spec. 63% SSM: Sens. 87%, Spec. 52% |
| Petta, J Hepatol, 2018[28] | Italy | Retrospective analysis of prospective data | NAFLD/NASH | EV: VNT: | Total: 790 249 (31.5%) 91 (11.5%) | NR LSM: 20 kPa + Plt 150 G/L: AUC LSM 25 kPa + Plt 110 G/L: AUC LSM 30 kPa + Plt 110 G&L: | NR Validation set: Sens. 37%, spec. 96%, PPV:18%, NPV:0.96 XL probe, Validation set: Sens. 93%, spec. 51%, PPV: 18%, NPV:98% M probe, validation set: Sens. 78%, spec. 68%, PPV:27%, NPV:96% |
| Colecchia, J Hepatol, 2018[34] | Italy | Prospective + retrospective validation cohort | Mixed | EV: VNT: | Total: 498 252 (50.6%) 100 (20.1%) | NR LSM: AUC: 0.768 LSM (20 kPa) + Plt (150 G/L): AUC 0.732 SSM: 46 kPa: AUC 0.847 LSM (20 kPa)+Plt (150 G/L)+SSM (46 kPa):AUC 0.787 | NR NR Sens. 98%, Spec. 26%, NPV 98% Sens. 98%, Spec. 44%, NPV 99% Sens. 96%, Spec. 53%, NPV 98% |
| Ref. | Country | Study design | N | Etiology | Device | Cut-off (AUC) for EV | Sens/Spec/+LR/-LR/p for EV | Cut-off (AUC) for VNT | Se/Sp/+LR/-LR/p for VNT | Comments |
| Vermehren, Liver International 2011[41] | Germany | Prospective | 166 | Mixed | Acuson S2000 | NR | NR | L-SWE: (0.58) S-SWE: (0.58) Youden: 4.13 m/s Highest NPV: 3.04 m/s | NR Youden: 35%/83%/2.06/0.78/PPV 54%/NPV 69% Highest NPV: 90%/25%/1.19/0,4/PPV 40%/NPV 81% | Cut-offs only calculated for S-SWE and TE, but not for L-SWE |
| Bota, Annals of Hepatology, 2012[39] | Romania | Prospective | 145 Cirrhosis: 24 | Mixed, healthy | Acuson S2000 | NR | NR | L-SWE: 2.25 m/s (0.596) S-SWE: 2.55 m/s (0.578) PRED: 0.395 (0.721) | LSM: 93.4%/28.9%/PPV48.5%/NPV:85.7% SSM: 96.7%/21%/PPV:47.6%/NPV:53.1% PRED: 75%/61.8%/PPV:61.4%/NPV:69.6%/p=0.0001 | VNT: Varices ≥grade 2 |
| Ye, Journal of Ultrasound in Medicine, 2012[91] | China | Prospective | 264, cirrhosis: 141 | Chronic HBV, healthy | Acuson S2000 | L-SWE: 3.16 m/s (0.83) | 84.1%/81.0%/NR | L-SWE: 3.39 m/s (0.83) | 78.9%/78.3% | Main focus on liver stiffness evaluation VNT: Varices ≥grade 3 |
| Takuma et al. Gastroenterology 2013[42] | Japan | Prospecttive | 340 | Mixed | Acuson S2000 | S-SWE: cirrhosis: 3.17 m/s (0.933) Comp.: 3.18 m/s (0.934) Decomp: 3.22 m/s (0.936) Viral: 3.18 m/s (0.937) Nonviral: 3.24 m/s (0.923) L-SWE: NR (0.746) | 98.4%/60.1%/2.468/0.025/PPV 61.0%/NPV 98.4%/acc. 75.0% 98.4%/63.4%/2.689/0.025/PPV: 50.4%/NPV: 99.0%/acc: 73.0% 98.6%/50.0%/1.971/0.029/PPV: 75.8%/NPV: 85.7%/acc: 79.8% 98.9%/59.9%/2.464/0.019/PPV: 57.5%/NPV: 99.0%/acc: 73.7% 97.7%/65.2%/2.808/0.036/PPV: 57.5%/NPV: 99.0%/acc: 73.7% | S-SWE: cirrhosis: 3.30 m/s (0.930) Comp: 3.30 m/s (0.921) Decomp: 3.45 m/s (0.934); S-Viral: 3.30 m/s (0.924); Nonviral: 3.41 m/s (0.944) | 98.9%/62.9%/2.661/0.018/PPV 47.8%/NPV 99.4%/acc. 72.1% 97.5%/66.7%/2.925/0.038/PPV: 38.6%/NPV: 99.2%/acc: 72.1% 97.9%/73.1%/3.643/0.029/PPV: 71.9%/NPV: 98.0%/acc: 83.3% 98.2%/63.8%/2.243/0.029/PPV: 43.2%/NPV: 99.2%/acc: 71.3% 96.9%/71.9%/2.267/0.043/PPV: 66.0%/NPV: 97.6%/acc: 80.9% | No cut-offs for L-SWE reported - significantly superior S-SWE results |
| Mori, BioMed Research International 2013[92] | Japan | Prospective | 33 cirrhosis: 24 | Mixed, including healthy | Acuson 2000 | NR | NR | NR | NR | Neither liver nor spleen stiffness correlated with presence of EV |
| Salzl, Ultraschall in der Medizin, 2014[36] | Austria | Prospective | 88 | Mixed | Acuson S2000 | L-SWE: 2.74 m/s (0.743) | 62.5%/89.5%/PPV: 91.5%/NPV: 56.9% | NR | NR | Size of EV was not defined |
| Morishita, Journal of Gastroenterology 2014[93] | Japan | Prospective | 181 | Chronic HCV | Acuson S2000 | L-SWE: 2.05 m/s (0.890) | Training set: 83%/76%/PPV: 78%/NPV: 81% Validation set: 83%/77%/PPV 59%/NPV: 92% | L-SWE: 2.39 m/s (0.868) | Training set: 81%/82%/PPV: 69%/NPV: 89% Validation set: 83%/77%/PPV: 59%/NPV: 92% | |
| Park, PloS ONE, 2015[94] | South Korea | Prospective | 143 | Mixed | Acuson S2000 | L-SWE: 2.08 m/s (0.769) ASPS: 1.67 (0.903) | 64.9%/81.1%/3.44/0.43/PPV 54.5%/NPV: 86.9% 81.1%/84.0%/5.06/0.23/PPV 63.8%/NPV: 92.7% | L-SWE: 1.90 (0.786) ASPS: 2.83 (0.946) | 85%/67.5%/2.61/0.22/PPV: 29.8%/NPV: 96.5% 90%/94.3%/15.81/0.11/PPV: 72.0%/NPV: 98.3% | High discriminative power of ASPS confirmed in validation cohort |
| Attia, Ultraschall in der Medizin, 2015[95] | Germany | Prospective | 78 | Mixed | Acuson S2000 | L-SWE (HVPG ≥10mmHg): 2.29 m/s (0.840) L-SWE (HVPG ≥12mmHg): 2.70 m/s (0.878) S-SWE (HVPG≥10mmHg):2.55 m/s(0.899) S-SWE (HVPG≥12mmHg):2.71 m/s (9.31) | 91%/85%/6.08/0.1/<0.001/PPV 95%/NPV 74% 93%/77%/400/0.09/<0.001/PPV 89%/NPV 85% 95%/90%/9.06/0.05/<0.001/PPV 97%/NPV 85% 95%/92%/11.86/0.06/<0.001/PPV97%/NPV 89% | NR | NR | SSM independently predicted presence of CSPH+EV |
| Kim, Medicine, 2015[96] | Korea | Prospective | 125 | Mixed | Acuson S2000 | L-SWE: cutoff NR (0.747) S-SWE: 3.16 m/s(0.785) | NR 87.0%/60.4%/PPV 77.9%/NPV 64.4% | L-SWE: cutoff NR (0.687) S-SWE: 3.40 m/s (0.762) | NR 78.9%/63.0%/PPV 60.3%/NPV 80.7% | VNT: medium-large varices |
| Park, Medical Ultrasound, 2016[97] | South Korea | Prospective | 366 | ALD or viral | iU22 | L-SWE: NR S-SWE: 29.9 kPa (0.859) | L-SWE:NR S-SWE: 58.1%/79.1%/<0.001/PPV:81.6%/NPV:82.8% | NR | NR | High rate of unreliable results (25%) No significant correlation with L-SWE |
| Wiles, Clinical Radiology, 2018[98] | UK | Prospective | 58 | Mixed | Acuson S2000 | NR | NR | NR | NR | ARFI not suitable to predict GOV (P = 0.15) |
| Lucchina et al. Ultrasound Med Biol 2018[38] | Italy | Prospective | 42 | Mixed | iU22 | L-SWE: 12.27 kPa (0.913)S-SWE: 23.87kPa (0.675) | 100%/66.67%/NR 73,81%/59.52%/NR | NR | NR | High rate of inconclusive results (22%) |
| Ref. | Country | Study design | Nr. | Etiology | Model | Cut-off (AUC) for EV | Sens/Spec/+LR/-LR/p for EV | Cut-off (AUC) for VNT | Se/Sp/+LR/-LR/p for VNT | Comment |
| Kim, Liver International, 2015[51] | Korea | Prospective | 92 | Mixed | Aixplorer | L-SWE: All patients: 26.3 kPa (0.683) Compensated cirrhosis: 14.2 kPa (0.925) | L-SWE: All patients: 61.4%/75.0%/PPV 89.6%/NPV 35.7%/P = 0.004 Compensated cirh.: 87.5%/90.0%/PPV 93.3%/NPV 81.8%/ P < 0.001 | NR | NR | Main focus on prediction of PHT/CSPH |
| Grgurevic, Croatian Medical Journal 2015[53] | Croatia | Retrospective | 44 | Mixed | Aixplorer | L-SWE: 19.7 kPa (0.796) S-SWE: 30.3 kPa (0.790) | L-SWE: 83.3%/66.6%/2.5/0.25/0.037 S-SWE: 79.6%/75.8%/3.3/0.27/0.009 | NR | NR | |
| Kasai Journal of Medical Ultrasonics, 2015[54] | Japan | Retrospective | 273 | Mixed | Aixplorer | Cut-off: NR AUC: (0.807) | NR | NR | NR | No cut-offs or sensitivity analyses reported; varices: ≥ grade 2 |
| Elkrief et al. Radiology 2015[56] | France | Prospective | 79 | Mixed | Aixplorer | NR | NR | NR | NR | EV not evaluated; Neither L-SWE, nor S-SWE, nor L-TE, nor LSPS predictive of VNT, but predictive of CSPH |
| Cassinotto, Digestive Liver Disease 2015[99] | France | Prospective | 401 | Mixed | Aixplorer | NR | NR | L-SWE: 12.8 (0.70) S-SWE: 25.6 (0.75) | L-SWE: 92%/36%/1.44/0.22/NR/PPV: 44%/NPV: 90% S-SWE: 94%/36%/1.47/0-17/NR/PPV:50%/NPV:90% | |
| Kim et al. Journal of Ultrasound in Medicine 2016[55] | Korea | Retrospective | 103 | Mixed | Aixplorer | L-SWE: 13.9 kPa (0.887) | 75%/88.9%/6.75/0.28/< 0.001 | 16.1 kPa (0.880) | 84.6%/85.6%/5.86/0.18/< 0.001 |
| Author, Journal, Year | Liver/Spleen | Patient N | Etiology | Cut-offs for EV (AUC) | EV: Sens/Spec/+LR/-LR/acc/p | Cut-offs for VNT (AUC) | VNT: Sens/Spec/+LR/-LR/acc/p | Prevalence of EV and VNT | Comment |
| Ronot et al. Eur Radiol 2014[63] | yes/yes | 36 | mixed | NR | NR | Gl, 84 Hz: 4.2 kPa (0.93) | 54%/100%/PPV: 33%/NPV: 79%/P = 0.001 | Any EV: 75%; VNT: 72% | Liver MRE not predictive of EV or VNT. Advantage of 3D multifrequency MRE |
| Sun et al. J Magn Reson Imaging 2014[62] | yes/no | 126 | mixed | L-MRE: 4.63 kPa (0.859) | NR | L-MRE: 5.803 kPa (0.810) | L-MRE: 96%/60%/n.r. | Any EV: 49%; VNT: 19% | Pearson correlation coefficient between liver stiffness and EV: 0.63 (P < 0.0001). |
| Shin et al. Radiology 2014[61] | yes/yes | 139 | mixed | L-MRE: 4.58 kPa (0.821) S-MRE: 7.23 kPa (0.833) | L-MRE: 87.4%/65.6%/n.r. S-MRE: 85.8%/65.4%/n.r. | L-MRE: 4.81 kPa (0.755) S-MRE: 7.60 kPa (0.750) | L-MRE: 84.4%/56.7%/n.r S-MRE: 68.3%/61.6%/n.r. | Any EV: 56%; VNT: 32% | Data cross-validated; several false-positive diagnoses of EV and VNT due to high liver stiffness and non-esophageal collaterals; 11 cases of false-negative diagnoses of EV: pre-hepatic PHT, iron deposition, large ascites. |
| Matsui, Journal of Gastroenterology and Hepatology, 2018[100] | yes/no | 627 | mixed | MRE: 4.2 kPa (0.85) PLT: 18*104 (0.77) | MRE: 85%/69%/PPV 32%/NPV 96% MRE + PLT: 93%/43%/PPV 22%/NPV 96% | MRE: 4.2 kPa (0.85) PLT: 18*104 (0.77) | MRE: 94%/65%/PPV 17%/ NPV 99% MRE + PLT: 100%/35%/PPV 10%/ NPV 100% | Any EV: 15.6% VNT: 4.5% | 3T device; also validated Baveno VI criteria (1) and modified Baveno VI criteria (30); excellent performance in NAFLD and viral hepatitis |
| Bookwalter, Abdominal Radiology, 2018[101] | yes/no | 55 | PSC | 6.27 kPa (NR) | Sens. 100%/Spec. 76.7% | NR | NR | NR | Several sequences, 2D and 3D modes, at 1,5T and 3T; Varices assessed by global LSM |
| Kim, European Radiologist, 2017[102] | yes/no | 84 | NR | GRE-MRE: (0.948) SE-EPI-MRE: (0.914) | NR | GRE-MRE: 4.493 kPa (0.752) SE-EPI-MRE: 5.880 kPa (0.839) | NR | Any EV: 17.9% VNT: 8.3% | Neither SE-EPI-, nor GRE-MRE reached significance regarding diagnostic performance |
TE is a one dimensional SWE method that measures liver (LSM) or spleen (SSM) stiffness at a depth of 2.5-6.5 cm beneath the skin with an exploration volume of 3 cm²[6]. The propagation velocity of the shear wave is directly proportional to the stiffness of the liver, which in other words means “the faster, the stiffer”[10]. Results measured by SWE are usually presented as either m/s (tissue velocity) or kPa (estimated tissue elasticity). To secure reliability and validity, at least ten measurements should be performed and results must fulfill established quality criteria (median/interquartile range ratio ≤ 30%)[10,11]. However, LSM results obtained in obese patients and patients with ascites have to be interpreted with caution; in some rare cases, valid measurements cannot be obtainable by TE-based LSM[10]. For obese patients, a separate probe (XL) has been developed that increases the proportion of (obese) patients in whom valid LSM can be obtained since measurements are read at high depth and recommendation to use the XL-probe are based on higher skin-to-liver-capsule distances. However, the LSM results obtained by the TE-XL probe may slightly deviate from the standard TE-M probe[12-14].
One of the first studies to report LSM cut-offs for predicting EV was Kazemi et al. in 2006[15]. In a prospective cohort with mixed etiologies, the authors identified 13.9 kPa for any varices (including small varices) and 19.0 kPa for VNT as the suitable cut-offs. In the same year, Foucher et al[16] published a cut-off of 27.5 kPa for ruling-out VNT, also in a mixed cohort of patients. Since then, several studies have been published (Table 1); unfortunately, results on optimal LSM cut-offs vary exceedingly. For the prediction of presence of varices of any size (“any varices”), cut-offs between 6.8 kPa[17] and 28.0 kPa[18] were reported. For the prediction of VNT, which should not be missed by non-invasive screening, values ranged between 14 kPa[19] and 43 kPa[20]. One of the main issues for the highly variable results are diverse aims and approaches of the authors: in studies with a focus on ruling in the presence of EV reporting positive-predictive values (PPV) > 90%, cut-offs range from 15 kPa[21] to 28kPa[18], whereas studies focusing on ruling out EV and reporting negative-predictive values (NPV) > 90%, results ranged between 19 kPa[15] and 48 kPa[20].
In 2015 the Baveno VI consensus report on the treatment of portal hypertension[1] was published. These guidelines proposed that in cACLD patients with LSM values < 20 kPa and platelet count (PLT) > 150 G/L, screening endoscopy for esophageal varices can be omitted, since these patients have a very low risk for VNT. Since then, multiple studies validating these criteria have been published[19,22-24]. Finally, in a meta-analysis by Marot[25] analyzing data of 3364 patients, the LSM cut-off of 20 kPa (alone) was found to predict the presence of EV with a PPV of 43% and a NPV of 86%. Importantly, another meta-analysis by Pu including 2697 patients reported similar results with a sensitivity of 84% and a specificity of 68% (PPV and NPV not reported)[26].
In summary, LSM is a very valuable non-invasive tool for non-invasive exclusion of VNT. However, due to the high variance of results (cut-offs, PPV and NPV values) reported in the literature, we cannot recommend to rely on TE as a single tool for the prediction of EV or VNT, but advise using combination algorithms instead.
Since the publication of the Baveno VI guidelines[1], most studies reported data on the combination algorithm of LSM + PLT (commonly at the cutoff 150G/L) to rule-out VNT. One of the first studies was the ’Anticipate study’[19]; however, with an AUC of 0.76, results for this algorithm were rather disappointing. Maurice et al[22] evaluated these criteria in 310 patients and reported a PPV of 6% and a NPV of 98%, indicating that these criteria be highly accurate for ruling out VNT as intended. Wong et al[27] prospectively analyzed 274 patients and found similar results with a PPV of 9.5% and a NPV of 95.5%. Most recently, the Baveno VI criteria were tested in a large cohort of NAFLD patients and performed very well, missing only 0.9% of large EV[28]. Moreover, a large meta-analysis by Marot including 3364 patients with mixed etiologies of liver disease reported an excellent NPV for ruling out VNT (98%) using the cut-offs proposed by the Baveno VI consensus[25].
However, according to a recent study by Augustín et al, 40% of all endoscopies performed when applying the Baveno VI criteria to rule out VNTs did not detect varices. The surprisingly low number of spared endoscopies could be increased by raising the cut-off for LSM to 25kPa and lowering the PLT threshold to 110 G/L (‘expanded Baveno VI criteria’)[24]. Also Jangouk et al. refined and redefined the Baveno VI criteria by applying the ’Meld-6’ rule, where patients that did not fulfill the Baveno VI criteria due to PLT < 150 G/L but had a MELD of six (i.e., normal/preserved liver function) were all not found with VNT, proposing that the number of spared endoscopies could be increased by this adaption of the Baveno VI criteria[29].
In 2013, Sharma et al[30] identified not only LSM, but also spleen stiffness measurement (SSM) as a non-invasive surrogate parameter for the prediction of EV. Indeed, several studies have subsequently investigated the predictive value of SSM using TE[27,30-34]. Importantly, SSM is also able to capture portal hypertension that is due to pre-sinusoidal or pre-hepatic causes that may not be detected by LSM. SSM has universally been shown to be at least equal, if not superior to LSM in regard to the detection of EV as well as of VNT[27,30-34]. This includes a very recent meta-analysis from 2018 by Manatsathit[31] including 4337 patients of mixed etiologies who calculated a pooled AUC of 0.90 for SSM for the detection of any EV as compared to a pooled AUC of 0.82 for LSM, and a pooled AUC of 0.81 vs 0.83 for the detection of VNT for LSM and SSM, respectively. Sharma et al[30] even concluded in their study that, in contrast to LSM, SSM can differentiate between small and large varices.
However, there is a limitation to SSM, as routine TE units (Fibroscan®, Echosens, France) are generally capped at a fibrosis value of 75 kPa. While LSM mostly remains within this range in cACLD patients, in severe portal hypertension SSM can exceed this threshold, especially in patients at highest risk of VNT. For this reason, in a study by Calvaruso et al[33] investigating SSM for the prediction of VNT, the authors used a modified TE unit with a maximum stiffness limit of 150 kPa, demonstrating superior ability of SSM to predict VNT with an AUC of 0.80 as compared to LSM with an AUC of 0.71, respectively. Accordingly, Stefanescu et al[18] combined LSM (cutoff 19 kPa) and SSM (cutoff: 55kPa) into a simple diagnostic algorithm to rule-in any EV with a sensibility of 93% and a PPV of 95%.
In conclusion, combining TE-based LSM with other non-invasive parameters such as PLT or SSM significantly increases the diagnostic accuracy for predicting the presence of EV. Given the convincing evidence, the combined Baveno VI criteria to rule out VNT at TE-based LSM < 20kPa and PLT > 150G/L have already been adapted into national guidelines[5].
In contrast to TE as a 1D-SWE that uses vibration-controlled dynamic stress, pSWE and 2D-SWE are based on the ultrasound-based acoustic radiation force impulse (ARFI) technology[6]. In pSWE, ARFI is used to generate a high-intensity short-duration acoustic pulse that slightly displaces liver tissue at one specific point[6,35]. As ARFI is usually implemented into modified ultrasound probes, one of the major advantages compared to TE is the availability to visualize liver tissue via B-Mode[6,10]. Furthermore, the tissue is being displaced directly in the liver with focus on the ROI rather than on the body surface, making the technique less prone to ascites or obesity[6,10]. However, pSWE is limited by the small region of interest (ROI) compared to other SWE modalities, which makes it more prone to “sample” bias and to artefacts, e.g., due to patient movements[10]. In 2014, Salzl et al[36] reported an AUC of 0.855 for the prediction of CSPH and an AUC of 0.743 for the prediction of EV using pSWE (as compared to EV prediction by TE with an AUC of 0.802). Similar results were found in a Japanese cohort of patients with mixed etiologies with an AUC 0.833 for the prediction of CSPH, an AUC of 0.789 for any varices and AUC an 0.788 for VNT respectively[37]. Most recently LSM via pSWE was found to predict presence of EV with an AUC of 0.913, as compared to pSWE-based SSM with an AUC of 0.675[38], however, only 21 patients with “low-grade EVs” and none with VNT have been included.
After the promising result of TE-based SSM, several studies have been published on the value of pSWE-based SSM for the diagnosis of CSPH and for EV[35]. The AUC of pSWE-based SSM ranged from 0.578[39] to 0.959[40] for any EV and between 0.580[41] to 0.955[37] for VNT. In a large cohort of 340 patients with mixed etiologies, SSM had the best diagnostic accuracy for the identification of patients with any EV (AUC 0.937 for viral, AUC 0.923 for non-viral etiologies) or VNT (AUC 0.924 for viral, 0.944 for non-viral etiologies) when compared to other non-invasive parameters (such as LSM, spleen diameter and PLT)[42].
Importantly, while TE-based elastography is a simple technique that can be performed by trained personal (e.g. nurses), while ultrasound-based pSWE (and 2D-SWE) is critically dependent on the experience of the ultrasound operator[43]. In addition, the accuracy of pSWE-based assessment of LSM and SSM can be improved by following quality criteria[44].
In synopsis, pSWE has been widely evaluated for the prediction of EV, mostly through SSM. Nevertheless this technique might not be available everywhere, AUC of SSM vary and too few studies have been published about pSWE-LSM to predict presence of EV.
In contrast to pSWE , 2D-SWE uses two-dimensional measurement of shear wave speed and multiple focal zones are measured[6]. Furthermore, real-time measurement - as displayed “on-screen” - is possible[10,35]. This enables the operator to analyze a larger amount of liver tissue in real-time and improves the applicability of elastography[10]. Nevertheless, 2D-SWE has not yet been introduced into clinical routine and are limited to specialized centers and should be only performed by experienced operators[35]. Importantly, there are not established/accepted quality criteria for 2D-SWE measurements for LSM and/or SSM[35].
2D-SWE-based LSM has been validated as a valid tool for non-invasive assessment of fibrosis in several studies[45-48]. The reliability of 2D-SWE-based LSM measurements was significantly higher as compared to the widely established TE-based LSM[49]: The AUC for 2D-SWE-based LSM for prediction of F ≥ 2 (cut-off 8.03 kPa) and F4 (cut-off 13.1 kPa) were 0.832 and 0.915, respectively. Indeed, most recently, two meta-analysis on 2D-SWE including 1134 patient[48] and 746 patients[50] have been published that both reported superior accuracy to detect significant fibrosis or cirrhosis using 2D-SWE as compared to TE.
Kim et al[51] evaluated 2D-SWE for the prediction of CSPH and reported an AUC of 0.819 at a 2D-SWE LSM cut-off of 15.2 kPa in 115 cirrhotic patients undergoing measurement of portal pressure by hepatic venous pressure gradient (HVPG). Importantly, a recent meta-analysis by Suh[50] showed an excellent diagnostic performance of 2D-SWE for predicting the presence of CSPH with sensitivity and specificity around 85%[50].
Studies reporting on the performance of 2D-SWE based non-invasive screening for EV are scarce. In a large prospective study investigating 2D-SWE-based liver (L-SWE) and spleen (S-SWE) for the diagnosis of CSPH yielded an AUC of 0.861 for L-SWE (24.6 kPa) and of 0.837 for S-SWE (26.3 kPa), respectively[52].
A Croatian study reported a cut-off of 19.7 for L-SWE (AUC 0.796) and 30.3 kPa for S-SWE (AUC 0.790) for the prediction of any EV in a cohort of 44 patients with cACLD[53]. Kasai et al[54] found significantly higher L-SWE values in 16 patients with EV than in 257 patients without EV, with similar AUC of 0.807. In a retrospective cohort of 103 cACLD patients 2D-SWE of L-SWE yielded an AUC of 0.887 (cut-off 13.9 kPa) for any EV and 0.880 (cut-off 16.1 kPa) for VNT[55]. Interestingly, a prospective study published in 2015 including 79 patients found no difference between LSM and SSM values (measured by 2D-SWE and by TE respectively) between patients with VNT and patients without VNT[56]. The resulting AUCs of 2D-SWE for detection of VNT were consequently unacceptably low with an AUC of 0.600 for L-SWE and an AUC of 0.580 for S-SWE, respectively[56].
In conclusion, while there is strong evidence for the value of 2D-SWE-based LSM for fibrosis assessment, studies on the value of 2D-SWE based LSM and SSM for the screening of EVs are limited. Larger studies using 2D-SWE-based liver and spleen stiffness measurement for the assessment of patients with cACLD are needed to establish its value for the prediction of any EV and of VNTs in daily clinical practice.
Several studies have been already been published on the correlation of MRE with liver fibrosis and found high diagnostic accuracies (> 90%) for the diagnosis of advanced fibrosis and cirrhosis[10,57,58]. Most recently, few studies on the value of MRE for the prediction of EV have been published with data on both liver MRE (L-MRE) and spleen MRE (S-MRE). Major advantages of MRE are its capability to evaluate the whole liver three-dimensionally (3D-SWE) and its excellent diagnostic accuracy for staging fibrosis[10]. Furthermore, failure rate is low and was reported to be mostly due to physical “non-fitting” into the MR device, claustrophobia or low hepatic signal related to iron overload[10,59]. Importantly, MRE shows excellent inter-observer and intra-observer agreement[60]. The main limitations of MRE include its high costs, limited availability and the need for specialized infrastructure and equipment. Shin et al[61] reported MRE data on 139 cirrhotic patients: Any EV were predicted by an L-MRE cut-off at 4.58 kPa (AUC 0.821) and by an S-MRE cut-off at 7.23 kPa (AUC 0.833). Furthermore, VNT were predicted by an L-MRE cut-off at 4.81 kPa (AUC 0.755) and a S-MRE cut-off at 7.6 kPa (AUC: 0.750).
In a South-Korean cohort of 126 patients[62], L-MRE cut offs were 4.63 kPa (AUC 0.859) for any EV, and 5.8 kPa (AUC 0.81) for VNT, respectively. Finally, Ronot et al[63] reported data on a small cohort of 36 patients and found 4.2 kPa (AUC 0.93) as an optimal cut-off for ruling out VNT (PPV 33%, NPV 79%).
Despite these promising results of MRE-based LSM and SSM for predicting any EV or VNT, more prospective studies are needed before implementing MRE for the non-invasive screening of EV/VNT in clinical practice. Furthermore, the feasibility of MRE is limited due to its inherent high costs, long examination times and considerable need for radiological expertise. Considering the limited data on MRE-based screening for EV/VNTs, as of now TE, pSWE and 2D-SWE are likely the first choice for screening of EV/VNTs in clinical practice – given their wider availability and lower costs.
In this review we summarized the current knowledge on elastography-based methods for the non-invasive prediction of any EVs and VNTs in adult patients with advanced chronic liver disease.
TE is an easy to use device with good applicability and well-trained nursing staff can usually perform LSM after training. Nevertheless the area of TE-based LSM is small and values can be significantly altered or even impossible to obtain in patients with obesity or ascites. Importantly, the combined TE-based LSM < 20 kPa plus PLT > 150 G/L has become a widely accepted non-invasive algorithm for ruling out VNTs[1,5]
Following TE-based LSM, pSWE and 2D-SWE have been developed as novel ultrasound-based elastography methods. Importantly, next to LSM also SSM - mostly by pSWE and 2D-SWE have been additionally introduced as a valuable screening tool for EV/VNTs. Both pSWE and 2D-SWE methods have the benefit of “seeing what you measure”, given the integration of the technique in standard ultrasound machines. However, the operator performing pSWE and 2D-SWE needs to be well-trained in ultrasound sonography and quality criteria for valid LSM and SSM have to be rigorously followed. Nevertheless there are some potential limiting factors that may hamper interpretation of results irrespective of operators experience and elastography method. It is known that in states of chronic inflammation such as viral hepatitis[64,65],autoimmune hepatitis[66] and alcoholic steatohepatitis[67] and in cases of acute liver damage[68] liver stiffness can be false positively increased. Furthermore increased LSM has also been described due to mechanical cholestasis[69]. Lastly hepatic congestion due congestive heart failure[70,71] and Budd-Chiari syndrome[72,73] thus generally speaking through increased venous pressure[74] is also known to increase elastography-based LSM values[75]. In a recent review by Lemmer et al[75] non-invasive methods to diagnose fibrosis in patients with congestive hepatopathy were discussed and conclusions were quite disillusioning since only very limited data exists. In a study that evaluated LSM in 32 patients with valvular heart disease that underwent valve operation, LSM was found to be consistently higher than in the control group, even though none of the participants were found with evidence of underlying chronic liver disease[76]. Furthermore LSM at baseline was significantly positively correlated with NT-proBNP, and central venous pressure during the operation and negatively with left ventricular ejection fraction[76]. Ninety days after surgery LSM values significantly decreased compared to 7 d after surgery (8.4 kPa vs 6.0 kPa, P = 0.026). On the other hand a study evaluating MRE-LSM and -SSM in congestive hepatopathy found promising results and reported significant correlation of LSM (r = 0.74, P = 0.02) and SSM (r = 0.97, P = 0.002) with fibrosis stage, although liver biopsy results were only available in 8 patients[77]. Therefore, given the potential pitfalls, we suggest that irrespective of the elastography method used, clinical signs of chronic liver disease, laboratory data and other co-morbidities should always be taken into account when performing LSM or SSM respectively.
Our extensive literature search revealed significant discrepancies between published LSM and SSM cut-offs using pSWE and 2D-SWE for EV/VNT screening. The absence of generally-accepted quality criteria for pSWE and 2D-SWE remains and validated cut-offs for ruling-in/out EVs/VNTs calls for further research on the clinical applicability of pSWE and 2D-SWE for the screening of EVs/VNTs. Moreover, presence of esophageal varices may be relied on cofounding factors, other than liver stiffness. Most recently, patients with large or even small portosystemic shunting were found to have an increased prevelance of esophageal varices[78], and although grade of portosystemic shunting was related to liver dysfunction, varices might be missed by transient elastography in those cases. Interestingly patients with preserved liver function (defined as MELD 6-9 or Child Pugh Stage A) and portosystemic shunting showed higher HVPG values and were found with significantly more portal hypertension related complications such as bleeding or ascites than in those without shunting[78] and this emphasizes even more that especially in those patients, where LSM might be low, esophageal varices might be missed. Furthermore, in the era of successful and highly efficient treatment of hepatitis C, nowadays quite a lot of cirrhotic patients present without the initial trigger for their underlying liver disease and it has been shown that directly acting antivirals significantly lower portal pressure[79]. Concerning this matter, no study has up to date evaluated applicability of elastography-based methods to predict esophageal varices in this cohort, and therefore it is not known whether published cut-offs work in this large subgroup of patients.
More recently, MRE-based LSM has been introduced as a very accurate method to stage liver fibrosis and with concomitant MRE-based LSM and SSM yielding excellent performance for non-invasive diagnosis of EV/VNTs. Consequently, MRE seems to be a highly accurate screening method for EVs/VNTs, however, studies are scarce and further evidence is needed.
In conclusion a vast amount of studies on the diagnostic performance of elastography-based methods for the presence of EV/VNTs have been published, mostly reporting data on TE. Both pSWE and 2D-SWE-based LSM and SSM represent promising tools for EV/VNT screening but further clinical studies and evaluation of specific cut-offs are required. MRE-based LSM and SSM-based screening of EV/VNT holds promise but is limited by its high costs. At the moment we strongly recommend to use the combined TE-LSM < 20 kPa and PLT > 150 G/L algorithm to rule-out VNTs. Considering the promising data on SSM and the ability of SSM to capture pre-sinusoidal/pre-hepatic components of CSPH, we strongly encourage further research on SSM for screening of CSPH and EV/VNTs. Finally, we have summarized the currently available data and published cut-offs for EV/VNT prediction by TE, pSWE, 2D-SWE and MRE on scale-cards for clinical practice.
| 1. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2609] [Cited by in RCA: 2357] [Article Influence: 214.3] [Reference Citation Analysis (4)] |
| 2. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1355] [Article Influence: 112.9] [Reference Citation Analysis (0)] |
| 3. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1500] [Article Influence: 166.7] [Reference Citation Analysis (3)] |
| 4. | Reiberger T, Ulbrich G, Ferlitsch A, Payer BA, Schwabl P, Pinter M, Heinisch BB, Trauner M, Kramer L, Peck-Radosavljevic M; Vienna Hepatic Hemodynamic Lab. Carvedilol for primary prophylaxis of variceal bleeding in cirrhotic patients with haemodynamic non-response to propranolol. Gut. 2013;62:1634-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 5. | Reiberger T, Püspök A, Schoder M, Baumann-Durchschein F, Bucsics T, Datz C, Dolak W, Ferlitsch A, Finkenstedt A, Graziadei I, Hametner S, Karnel F, Krones E, Maieron A, Mandorfer M, Peck-Radosavljevic M, Rainer F, Schwabl P, Stadlbauer V, Stauber R, Tilg H, Trauner M, Zoller H, Schöfl R, Fickert P. Austrian consensus guidelines on the management and treatment of portal hypertension (Billroth III). Wien Klin Wochenschr. 2017;129:135-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (3)] |
| 6. | Sigrist RMS, Liau J, Kaffas AE, Chammas MC, Willmann JK. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics. 2017;7:1303-1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 954] [Cited by in RCA: 1228] [Article Influence: 136.4] [Reference Citation Analysis (0)] |
| 7. | Xie LT, Yan CH, Zhao QY, He MN, Jiang TA. Quantitative and noninvasive assessment of chronic liver diseases using two-dimensional shear wave elastography. World J Gastroenterol. 2018;24:957-970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Venkatesh SK, Ehman RL. Magnetic resonance elastography of abdomen. Abdom Imaging. 2015;40:745-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (15)] |
| 9. | Horowitz JM, Venkatesh SK, Ehman RL, Jhaveri K, Kamath P, Ohliger MA, Samir AE, Silva AC, Taouli B, Torbenson MS, Wells ML, Yeh B, Miller FH. Evaluation of hepatic fibrosis: a review from the society of abdominal radiology disease focus panel. Abdom Radiol (NY). 2017;42:2037-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 10. | Friedrich-Rust M, Poynard T, Castera L. Critical comparison of elastography methods to assess chronic liver disease. Nat Rev Gastroenterol Hepatol. 2016;13:402-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 191] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 11. | Schwabl P, Bota S, Salzl P, Mandorfer M, Payer BA, Ferlitsch A, Stift J, Wrba F, Trauner M, Peck-Radosavljevic M, Reiberger T. New reliability criteria for transient elastography increase the number of accurate measurements for screening of cirrhosis and portal hypertension. Liver Int. 2015;35:381-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 12. | Şirli R, Sporea I, Deleanu A, Culcea L, Szilaski M, Popescu A, Dănilă M. Comparison between the M and XL probes for liver fibrosis assessment by transient elastography. Med Ultrason. 2014;16:119-122. [PubMed] |
| 13. | Sporea I, Șirli R, Mare R, Popescu A, Ivașcu SC. Feasibility of Transient Elastography with M and XL probes in real life. Med Ultrason. 2016;18:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Bazerbachi F, Haffar S, Wang Z, González JC, Arias-Loste MT, Crespo J, Darwish-Murad S, Ikram MA, Olynyk JK, Gan E, Petta S, Berzuini A, Prati D, de Lédinghen V, Wai-Sun Wong V, Del Poggio P, Chávez-Tapia NC, Chen YP, Cheng PN, Yuen MF, Das K, Chowdhury A, Caballeria L, Fabrellas N, Ginès P, Kumar M, Sarin SK, Conti F, Andreone P, Sirli R, Cortez-Pinto H, Carvalhana S, Sugihara T, Kim SU, Parikh P, Chayama K, Corpechot C, Kim KM, Papatheodoridis G, Alsebaey A, Kamath PS, Murad MH, Watt KD. Range of Normal Liver Stiffness and Factors Associated With Increased Stiffness Measurements in Apparently Healthy Individuals. Clin Gastroenterol Hepatol. 2018; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 15. | Kazemi F, Kettaneh A, N'kontchou G, Pinto E, Ganne-Carrie N, Trinchet JC, Beaugrand M. Liver stiffness measurement selects patients with cirrhosis at risk of bearing large oesophageal varices. J Hepatol. 2006;45:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 269] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 16. | Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Lédinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 966] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 17. | Wong GL, Kwok R, Chan HL, Tang SP, Lee E, Lam TC, Lau TW, Ma TM, Wong BC, Wong VW. Measuring spleen stiffness to predict varices in chronic hepatitis B cirrhotic patients with or without receiving non-selective beta-blockers. J Dig Dis. 2016;17:538-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Stefanescu H, Grigorescu M, Lupsor M, Procopet B, Maniu A, Badea R. Spleen stiffness measurement using Fibroscan for the noninvasive assessment of esophageal varices in liver cirrhosis patients. J Gastroenterol Hepatol. 2011;26:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 162] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 19. | Abraldes JG, Bureau C, Stefanescu H, Augustin S, Ney M, Blasco H, Procopet B, Bosch J, Genesca J, Berzigotti A; Anticipate Investigators. Noninvasive tools and risk of clinically significant portal hypertension and varices in compensated cirrhosis: The "Anticipate" study. Hepatology. 2016;64:2173-2184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 277] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 20. | Nguyen-Khac E, Saint-Leger P, Tramier B, Coevoet H, Capron D, Dupas JL. Noninvasive diagnosis of large esophageal varices by Fibroscan: strong influence of the cirrhosis etiology. Alcohol Clin Exp Res. 2010;34:1146-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Bintintan A, Chira RI, Bintintan VV, Nagy GA, Manzat-Saplacan MR, Lupsor-Platon M, Stefanescu H, Duma MM, Valean SD, Mircea PA. Value of hepatic elastography and Doppler indexes for predictions of esophageal varices in liver cirrhosis. Med Ultrason. 2015;17:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Maurice JB, Brodkin E, Arnold F, Navaratnam A, Paine H, Khawar S, Dhar A, Patch D, O'Beirne J, Mookerjee R, Pinzani M, Tsochatzis E, Westbrook RH. Validation of the Baveno VI criteria to identify low risk cirrhotic patients not requiring endoscopic surveillance for varices. J Hepatol. 2016;65:899-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 23. | Llop E, Lopez M, de la Revilla J, Fernandez N, Trapero M, Hernandez M, Fernández-Carrillo C, Pons F, Martinez JL, Calleja JL. Validation of noninvasive methods to predict the presence of gastroesophageal varices in a cohort of patients with compensated advanced chronic liver disease. J Gastroenterol Hepatol. 2017;32:1867-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Augustin S, Pons M, Maurice JB, Bureau C, Stefanescu H, Ney M, Blasco H, Procopet B, Tsochatzis E, Westbrook RH, Bosch J, Berzigotti A, Abraldes JG, Genescà J. Expanding the Baveno VI criteria for the screening of varices in patients with compensated advanced chronic liver disease. Hepatology. 2017;66:1980-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 208] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 25. | Marot A, Trépo E, Doerig C, Schoepfer A, Moreno C, Deltenre P. Liver stiffness and platelet count for identifying patients with compensated liver disease at low risk of variceal bleeding. Liver Int. 2017;37:707-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Pu K, Shi JH, Wang X, Tang Q, Wang XJ, Tang KL, Long ZQ, Hu XS. Diagnostic accuracy of transient elastography (FibroScan) in detection of esophageal varices in patients with cirrhosis: A meta-analysis. World J Gastroenterol. 2017;23:345-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Wong GLH, Kwok R, Hui AJ, Tse YK, Ho KT, Lo AOS, Lam KLY, Chan HCH, Lui RA, Au KHD, Chan HLY, Wong VWS. A new screening strategy for varices by liver and spleen stiffness measurement (LSSM) in cirrhotic patients: A randomized trial. Liver Int. 2018;38:636-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (2)] |
| 28. | Petta S, Sebastiani G, Bugianesi E, Viganò M, Wong VW, Berzigotti A, Fracanzani AL, Anstee QM, Marra F, Barbara M, Calvaruso V, Cammà C, Di Marco V, Craxì A, de Ledinghen V. Non-invasive prediction of esophageal varices by stiffness and platelet in non-alcoholic fatty liver disease cirrhosis. J Hepatol. 2018;69:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 29. | Jangouk P, Turco L, De Oliveira A, Schepis F, Villa E, Garcia-Tsao G. Validating, deconstructing and refining Baveno criteria for ruling out high-risk varices in patients with compensated cirrhosis. Liver Int. 2017;37:1177-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 30. | Sharma P, Kirnake V, Tyagi P, Bansal N, Singla V, Kumar A, Arora A. Spleen stiffness in patients with cirrhosis in predicting esophageal varices. Am J Gastroenterol. 2013;108:1101-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 31. | Manatsathit W, Samant H, Kapur S, Ingviya T, Esmadi M, Wijarnpreecha K, McCashland T. Accuracy of liver stiffness, spleen stiffness, and LS-spleen diameter to platelet ratio score in detection of esophageal varices: Systemic review and meta-analysis. J Gastroenterol Hepatol. 2018;33:1696-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 32. | Colecchia A, Montrone L, Scaioli E, Bacchi-Reggiani ML, Colli A, Casazza G, Schiumerini R, Turco L, Di Biase AR, Mazzella G, Marzi L, Arena U, Pinzani M, Festi D. Measurement of spleen stiffness to evaluate portal hypertension and the presence of esophageal varices in patients with HCV-related cirrhosis. Gastroenterology. 2012;143:646-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 390] [Article Influence: 27.9] [Reference Citation Analysis (1)] |
| 33. | Calvaruso V, Bronte F, Conte E, Simone F, Craxì A, Di Marco V. Modified spleen stiffness measurement by transient elastography is associated with presence of large oesophageal varices in patients with compensated hepatitis C virus cirrhosis. J Viral Hepat. 2013;20:867-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 34. | Colecchia A, Ravaioli F, Marasco G, Colli A, Dajti E, Di Biase AR, Bacchi Reggiani ML, Berzigotti A, Pinzani M, Festi D. A combined model based on spleen stiffness measurement and Baveno VI criteria to rule out high-risk varices in advanced chronic liver disease. J Hepatol. 2018;69:308-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 35. | Berzigotti A. Non-invasive evaluation of portal hypertension using ultrasound elastography. J Hepatol. 2017;67:399-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 197] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 36. | Salzl P, Reiberger T, Ferlitsch M, Payer BA, Schwengerer B, Trauner M, Peck-Radosavljevic M, Ferlitsch A. Evaluation of portal hypertension and varices by acoustic radiation force impulse imaging of the liver compared to transient elastography and AST to platelet ratio index. Ultraschall Med. 2014;35:528-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 37. | Takuma Y, Nouso K, Morimoto Y, Tomokuni J, Sahara A, Takabatake H, Matsueda K, Yamamoto H. Portal Hypertension in Patients with Liver Cirrhosis: Diagnostic Accuracy of Spleen Stiffness. Radiology. 2016;279:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 38. | Lucchina N, Recaldini C, Macchi M, Molinelli V, Montanari M, Segato S, Novario R, Fugazzola C. Point Shear Wave Elastography of the Spleen: Its Role in Patients with Portal Hypertension. Ultrasound Med Biol. 2018;44:771-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Bota S, Sporea I, Sirli R, Focsa M, Popescu A, Danila M, Strain M. Can ARFI elastography predict the presence of significant esophageal varices in newly diagnosed cirrhotic patients? Ann Hepatol. 2012;11:519-525. [PubMed] |
| 40. | Rizzo L, Attanasio M, Pinzone MR, Berretta M, Malaguarnera M, Morra A, L'Abbate L, Balestreri L, Nunnari G, Cacopardo B. A new sampling method for spleen stiffness measurement based on quantitative acoustic radiation force impulse elastography for noninvasive assessment of esophageal varices in newly diagnosed HCV-related cirrhosis. Biomed Res Int. 2014;2014:365982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Vermehren J, Polta A, Zimmermann O, Herrmann E, Poynard T, Hofmann WP, Bojunga J, Sarrazin C, Zeuzem S, Friedrich-Rust M. Comparison of acoustic radiation force impulse imaging with transient elastography for the detection of complications in patients with cirrhosis. Liver Int. 2012;32:852-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 42. | Takuma Y, Nouso K, Morimoto Y, Tomokuni J, Sahara A, Toshikuni N, Takabatake H, Shimomura H, Doi A, Sakakibara I, Matsueda K, Yamamoto H. Measurement of spleen stiffness by acoustic radiation force impulse imaging identifies cirrhotic patients with esophageal varices. Gastroenterology. 2013;144:92-101.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 43. | Grădinaru-Taşcău O, Sporea I, Bota S, Jurchiş A, Popescu A, Popescu M, Şirli R, Szilaski M. Does experience play a role in the ability to perform liver stiffness measurements by means of supersonic shear imaging (SSI)? Med Ultrason. 2013;15:180-183. [PubMed] |
| 44. | Ferraioli G, De Silvestri A, Reiberger T, Taylor-Robinson SD, de Knegt RJ, Maiocchi L, Mare R, Bucsics T, Atzori S, Tinelli C, Sporea I. Adherence to quality criteria improves concordance between transient elastography and ElastPQ for liver stiffness assessment-A multicenter retrospective study. Dig Liver Dis. 2018;50:1056-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Zhang W, Zhu Y, Zhang C, Ran H. Diagnostic Accuracy of 2-Dimensional Shear Wave Elastography for the Staging of Liver Fibrosis: A Meta-analysis. J Ultrasound Med. 2018; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Zheng J, Guo H, Zeng J, Huang Z, Zheng B, Ren J, Xu E, Li K, Zheng R. Two-dimensional shear-wave elastography and conventional US: the optimal evaluation of liver fibrosis and cirrhosis. Radiology. 2015;275:290-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 47. | Gerber L, Kasper D, Fitting D, Knop V, Vermehren A, Sprinzl K, Hansmann ML, Herrmann E, Bojunga J, Albert J, Sarrazin C, Zeuzem S, Friedrich-Rust M. Assessment of liver fibrosis with 2-D shear wave elastography in comparison to transient elastography and acoustic radiation force impulse imaging in patients with chronic liver disease. Ultrasound Med Biol. 2015;41:2350-2359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 48. | Herrmann E, de Lédinghen V, Cassinotto C, Chu WC, Leung VY, Ferraioli G, Filice C, Castera L, Vilgrain V, Ronot M, Dumortier J, Guibal A, Pol S, Trebicka J, Jansen C, Strassburg C, Zheng R, Zheng J, Francque S, Vanwolleghem T, Vonghia L, Manesis EK, Zoumpoulis P, Sporea I, Thiele M, Krag A, Cohen-Bacrie C, Criton A, Gay J, Deffieux T, Friedrich-Rust M. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: An individual patient data-based meta-analysis. Hepatology. 2018;67:260-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 361] [Article Influence: 45.1] [Reference Citation Analysis (2)] |
| 49. | Bota S, Paternostro R, Etschmaier A, Schwarzer R, Salzl P, Mandorfer M, Kienbacher C, Ferlitsch M, Reiberger T, Trauner M, Peck-Radosavljevic M, Ferlitsch A. Performance of 2-D shear wave elastography in liver fibrosis assessment compared with serologic tests and transient elastography in clinical routine. Ultrasound Med Biol. 2015;41:2340-2349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 50. | Suh CH, Kim KW, Park SH, Lee SS, Kim HS, Tirumani SH, Lee JG, Pyo J. Shear Wave Elastography as a Quantitative Biomarker of Clinically Significant Portal Hypertension: A Systematic Review and Meta-Analysis. AJR Am J Roentgenol. 2018;210:W185-W195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 51. | Kim TY, Jeong WK, Sohn JH, Kim J, Kim MY, Kim Y. Evaluation of portal hypertension by real-time shear wave elastography in cirrhotic patients. Liver Int. 2015;35:2416-2424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 52. | Jansen C, Bogs C, Verlinden W, Thiele M, Möller P, Görtzen J, Lehmann J, Vanwolleghem T, Vonghia L, Praktiknjo M, Chang J, Krag A, Strassburg CP, Francque S, Trebicka J. Shear-wave elastography of the liver and spleen identifies clinically significant portal hypertension: A prospective multicentre study. Liver Int. 2017;37:396-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 53. | Grgurević I, Bokun T, Mustapić S, Hara T, Trkulja V, Heinzl R, Banić M, Puljiz Ž, Lukšić B, Kujundžić M. Real-time two-dimensional shear wave ultrasound elastography of the liver is a reliable predictor of clinical outcomes and the presence of esophageal varices in patients with compensated liver cirrhosis. Croat Med J. 2015;56:470-481. [PubMed] |
| 54. | Kasai Y, Moriyasu F, Saito K, Hara T, Kobayashi Y, Nakamura I, Sugimoto K. Value of shear wave elastography for predicting hepatocellular carcinoma and esophagogastric varices in patients with chronic liver disease. J Med Ultrason (2001). 2015;42:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 55. | Kim TY, Kim TY, Kim Y, Lim S, Jeong WK, Sohn JH. Diagnostic Performance of Shear Wave Elastography for Predicting Esophageal Varices in Patients With Compensated Liver Cirrhosis. J Ultrasound Med. 2016;35:1373-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 56. | Elkrief L, Rautou PE, Ronot M, Lambert S, Dioguardi Burgio M, Francoz C, Plessier A, Durand F, Valla D, Lebrec D, Vilgrain V, Castéra L. Prospective comparison of spleen and liver stiffness by using shear-wave and transient elastography for detection of portal hypertension in cirrhosis. Radiology. 2015;275:589-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 57. | Wang QB, Zhu H, Liu HL, Zhang B. Performance of magnetic resonance elastography and diffusion-weighted imaging for the staging of hepatic fibrosis: A meta-analysis. Hepatology. 2012;56:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 198] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 58. | Singh S, Venkatesh SK, Wang Z, Miller FH, Motosugi U, Low RN, Hassanein T, Asbach P, Godfrey EM, Yin M, Chen J, Keaveny AP, Bridges M, Bohte A, Murad MH, Lomas DJ, Talwalkar JA, Ehman RL. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol. 2015;13:440-451.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 437] [Article Influence: 39.7] [Reference Citation Analysis (1)] |
| 59. | Huwart L, Sempoux C, Vicaut E, Salameh N, Annet L, Danse E, Peeters F, ter Beek LC, Rahier J, Sinkus R, Horsmans Y, Van Beers BE. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 545] [Article Influence: 30.3] [Reference Citation Analysis (2)] |
| 60. | Runge JH, Bohte AE, Verheij J, Terpstra V, Nederveen AJ, van Nieuwkerk KM, de Knegt RJ, Baak BC, Jansen PL, Sinkus R, Stoker J. Comparison of interobserver agreement of magnetic resonance elastography with histopathological staging of liver fibrosis. Abdom Imaging. 2014;39:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 61. | Shin SU, Lee JM, Yu MH, Yoon JH, Han JK, Choi BI, Glaser KJ, Ehman RL. Prediction of esophageal varices in patients with cirrhosis: usefulness of three-dimensional MR elastography with echo-planar imaging technique. Radiology. 2014;272:143-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 62. | Sun HY, Lee JM, Han JK, Choi BI. Usefulness of MR elastography for predicting esophageal varices in cirrhotic patients. J Magn Reson Imaging. 2014;39:559-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 63. | Ronot M, Lambert S, Elkrief L, Doblas S, Rautou PE, Castera L, Vilgrain V, Sinkus R, Van Beers BE, Garteiser P. Assessment of portal hypertension and high-risk oesophageal varices with liver and spleen three-dimensional multifrequency MR elastography in liver cirrhosis. Eur Radiol. 2014;24:1394-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 64. | Vispo E, Barreiro P, Del Valle J, Maida I, de Ledinghen V, Quereda C, Moreno A, Macías J, Castera L, Pineda JA, Soriano V. Overestimation of liver fibrosis staging using transient elastography in patients with chronic hepatitis C and significant liver inflammation. Antivir Ther. 2009;14:187-193. [PubMed] |
| 65. | Arena U, Vizzutti F, Corti G, Ambu S, Stasi C, Bresci S, Moscarella S, Boddi V, Petrarca A, Laffi G, Marra F, Pinzani M. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 581] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 66. | Hartl J, Denzer U, Ehlken H, Zenouzi R, Peiseler M, Sebode M, Hübener S, Pannicke N, Weiler-Normann C, Quaas A, Lohse AW, Schramm C. Transient elastography in autoimmune hepatitis: Timing determines the impact of inflammation and fibrosis. J Hepatol. 2016;65:769-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 67. | Mueller S, Millonig G, Sarovska L, Friedrich S, Reimann FM, Pritsch M, Eisele S, Stickel F, Longerich T, Schirmacher P, Seitz HK. Increased liver stiffness in alcoholic liver disease: differentiating fibrosis from steatohepatitis. World J Gastroenterol. 2010;16:966-972. [PubMed] |
| 68. | Sagir A, Erhardt A, Schmitt M, Häussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology. 2008;47:592-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 390] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 69. | Millonig G, Reimann FM, Friedrich S, Fonouni H, Mehrabi A, Büchler MW, Seitz HK, Mueller S. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 471] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 70. | Lebray P, Varnous S, Charlotte F, Varaut A, Poynard T, Ratziu V. Liver stiffness is an unreliable marker of liver fibrosis in patients with cardiac insufficiency. Hepatology. 2008;48:2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 71. | Frulio N, Laumonier H, Balabaud C, Trillaud H, Bioulac-Sage P. Hepatic congestion plays a role in liver stiffness. Hepatology. 2009;50:1674-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 72. | Mukund A, Pargewar SS, Desai SN, Rajesh S, Sarin SK. Changes in Liver Congestion in Patients with Budd-Chiari Syndrome following Endovascular Interventions: Assessment with Transient Elastography. J Vasc Interv Radiol. 2017;28:683-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 73. | Wang HW, Shi HN, Cheng J, Xie F, Luo YK, Tang J. Real-time shear wave elastography (SWE) assessment of short- and long-term treatment outcome in Budd-Chiari syndrome: A pilot study. PLoS One. 2018;13:e0197550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 74. | Millonig G, Friedrich S, Adolf S, Fonouni H, Golriz M, Mehrabi A, Stiefel P, Pöschl G, Büchler MW, Seitz HK, Mueller S. Liver stiffness is directly influenced by central venous pressure. J Hepatol. 2010;52:206-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 411] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 75. | Lemmer A, VanWagner LB, Ganger D. Assessment of Advanced Liver Fibrosis and the Risk for Hepatic Decompensation in Patients With Congestive Hepatopathy. Hepatology. 2018;68:1633-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 76. | Chon YE, Kim SU, Park JY, Kim DY, Ahn SH, Han KH, Chon CY, Lee S. Dynamics of the liver stiffness value using transient elastography during the perioperative period in patients with valvular heart disease. PLoS One. 2014;9:e92795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 77. | Poterucha JT, Johnson JN, Qureshi MY, O'Leary PW, Kamath PS, Lennon RJ, Bonnichsen CR, Young PM, Venkatesh SK, Ehman RL, Gupta S, Smyrk TC, Dearani JA, Warnes CA, Cetta F. Magnetic Resonance Elastography: A Novel Technique for the Detection of Hepatic Fibrosis and Hepatocellular Carcinoma After the Fontan Operation. Mayo Clin Proc. 2015;90:882-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 78. | Simón-Talero M, Roccarina D, Martínez J, Lampichler K, Baiges A, Low G, Llop E, Praktiknjo M, Maurer MH, Zipprich A, Triolo M, Vangrinsven G, Garcia-Martinez R, Dam A, Majumdar A, Picón C, Toth D, Darnell A, Abraldes JG, Lopez M, Kukuk G, Krag A, Bañares R, Laleman W, La Mura V, Ripoll C, Berzigotti A, Trebicka J, Calleja JL, Tandon P, Hernandez-Gea V, Reiberger T, Albillos A, Tsochatzis EA, Augustin S, Genescà J; Baveno VI-SPSS group from the Baveno Cooperation. Association Between Portosystemic Shunts and Increased Complications and Mortality in Patients With Cirrhosis. Gastroenterology. 2018;154:1694-1705.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 181] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 79. | Mandorfer M, Kozbial K, Schwabl P, Freissmuth C, Schwarzer R, Stern R, Chromy D, Stättermayer AF, Reiberger T, Beinhardt S, Sieghart W, Trauner M, Hofer H, Ferlitsch A, Ferenci P, Peck-Radosavljevic M. Sustained virologic response to interferon-free therapies ameliorates HCV-induced portal hypertension. J Hepatol. 2016;65:692-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 250] [Article Influence: 25.0] [Reference Citation Analysis (5)] |
| 80. | Vizzutti F, Arena U, Romanelli RG, Rega L, Foschi M, Colagrande S, Petrarca A, Moscarella S, Belli G, Zignego AL, Marra F, Laffi G, Pinzani M. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology. 2007;45:1290-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 532] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 81. | Bureau C, Metivier S, Peron JM, Selves J, Robic MA, Gourraud PA, Rouquet O, Dupuis E, Alric L, Vinel JP. Transient elastography accurately predicts presence of significant portal hypertension in patients with chronic liver disease. Aliment Pharmacol Ther. 2008;27:1261-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 274] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 82. | Castéra L, Le Bail B, Roudot-Thoraval F, Bernard PH, Foucher J, Merrouche W, Couzigou P, de Lédinghen V. Early detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: comparison of transient elastography (FibroScan) with standard laboratory tests and non-invasive scores. J Hepatol. 2009;50:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 273] [Article Influence: 16.1] [Reference Citation Analysis (1)] |
| 83. | Stefanescu H, Grigorescu M, Lupsor M, Maniu A, Crisan D, Procopet B, Feier D, Badea R. A new and simple algorithm for the noninvasive assessment of esophageal varices in cirrhotic patients using serum fibrosis markers and transient elastography. J Gastrointestin Liver Dis. 2011;20:57-64. [PubMed] |
| 84. | Chen YP, Zhang Q, Dai L, Liang XE, Peng J, Hou JL. Is transient elastography valuable for high-risk esophageal varices prediction in patients with hepatitis-B-related cirrhosis? J Gastroenterol Hepatol. 2012;27:533-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 85. | Wang JH, Chuah SK, Lu SN, Hung CH, Chen CH, Kee KM, Chang KC, Tai WC, Hu TH. Transient elastography and simple blood markers in the diagnosis of esophageal varices for compensated patients with hepatitis B virus-related cirrhosis. J Gastroenterol Hepatol. 2012;27:1213-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 86. | Sporea I, Grădinaru-Taşcău O, Bota S, Popescu A, Şirli R, Jurchiş A, Popescu M, Dănilă M. How many measurements are needed for liver stiffness assessment by 2D-Shear Wave Elastography (2D-SWE) and which value should be used: the mean or median? Med Ultrason. 2013;15:268-272. [PubMed] |
| 87. | Shi KQ, Fan YC, Pan ZZ, Lin XF, Liu WY, Chen YP, Zheng MH. Transient elastography: a meta-analysis of diagnostic accuracy in evaluation of portal hypertension in chronic liver disease. Liver Int. 2013;33:62-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 88. | Hassan EM, Omran DA, El Beshlawey ML, Abdo M, El Askary A. Can transient elastography, Fib-4, Forns Index, and Lok Score predict esophageal varices in HCV-related cirrhotic patients? Gastroenterol Hepatol. 2014;37:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 89. | Hu Z, Li Y, Li C, Huang C, Ou Z, Guo J, Luo H, Tang X. Using Ultrasonic Transient Elastometry (FibroScan) to Predict Esophageal Varices in Patients with Viral Liver Cirrhosis. Ultrasound Med Biol. 2015;41:1530-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 90. | Li T, Qu Y, Yang B, Xue Y, Wang L. Evaluation of large esophageal varices in cirrhotic patients by transient elastography: a meta-analysis. Rev Esp Enferm Dig. 2016;108:464-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 91. | Ye XP, Ran HT, Cheng J, Zhu YF, Zhang DZ, Zhang P, Zheng YY. Liver and spleen stiffness measured by acoustic radiation force impulse elastography for noninvasive assessment of liver fibrosis and esophageal varices in patients with chronic hepatitis B. J Ultrasound Med. 2012;31:1245-1253. [PubMed] |
| 92. | Mori K, Arai H, Abe T, Takayama H, Toyoda M, Ueno T, Sato K. Spleen stiffness correlates with the presence of ascites but not esophageal varices in chronic hepatitis C patients. Biomed Res Int. 2013;2013:857862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 93. | Morishita N, Hiramatsu N, Oze T, Harada N, Yamada R, Miyazaki M, Yakushijin T, Miyagi T, Yoshida Y, Tatsumi T, Kanto T, Takehara T. Liver stiffness measurement by acoustic radiation force impulse is useful in predicting the presence of esophageal varices or high-risk esophageal varices among patients with HCV-related cirrhosis. J Gastroenterol. 2014;49:1175-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 94. | Park Y, Kim SU, Park SY, Kim BK, Park JY, Kim DY, Ahn SH, Tak WY, Kweon YO, Han KH. A novel model to predict esophageal varices in patients with compensated cirrhosis using acoustic radiation force impulse elastography. PLoS One. 2015;10:e0121009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 95. | Attia D, Schoenemeier B, Rodt T, Negm AA, Lenzen H, Lankisch TO, Manns M, Gebel M, Potthoff A. Evaluation of Liver and Spleen Stiffness with Acoustic Radiation Force Impulse Quantification Elastography for Diagnosing Clinically Significant Portal Hypertension. Ultraschall Med. 2015;36:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 96. | Kim HY, Jin EH, Kim W, Lee JY, Woo H, Oh S, Seo JY, Oh HS, Chung KH, Jung YJ, Kim D, Kim BG, Lee KL. The Role of Spleen Stiffness in Determining the Severity and Bleeding Risk of Esophageal Varices in Cirrhotic Patients. Medicine (Baltimore). 2015;94:e1031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 97. | Park J, Kwon H, Cho J, Oh J, Lee S, Han S, Lee SW, Baek Y. Is the spleen stiffness value acquired using acoustic radiation force impulse (ARFI) technology predictive of the presence of esophageal varices in patients with cirrhosis of various etiologies? Med Ultrason. 2016;18:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 98. | Wiles R, Patanwala I, Hankinson B, Healey P, Farrell C, Griffin C, Bonnett L, Richardson P. Can acoustic radiation force imaging of the liver and spleen predict the presence of gastroesophageal varices? Clin Radiol. 2018;73:1046-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 99. | Cassinotto C, Charrie A, Mouries A, Lapuyade B, Hiriart JB, Vergniol J, Gaye D, Hocquelet A, Charbonnier M, Foucher J, Laurent F, Chermak F, Montaudon M, de Ledinghen V. Liver and spleen elastography using supersonic shear imaging for the non-invasive diagnosis of cirrhosis severity and oesophageal varices. Dig Liver Dis. 2015;47:695-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 100. | Matsui N, Imajo K, Yoneda M, Kessoku T, Honda Y, Ogawa Y, Tomeno W, Fujisawa N, Misumi T, Kazumi K, Saito S, Nakajima A. Magnetic resonance elastography increases usefulness and safety of non-invasive screening for esophageal varices. J Gastroenterol Hepatol. 2018;33:2022-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 101. | Bookwalter CA, Venkatesh SK, Eaton JE, Smyrk TD, Ehman RL. MR elastography in primary sclerosing cholangitis: correlating liver stiffness with bile duct strictures and parenchymal changes. Abdom Radiol (NY). 2018;43:3260-3270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 102. | Kim YS, Song JS, Kannengiesser S, Seo SY. Comparison of spin-echo echoplanar imaging and gradient recalled echo-based MR elastography at 3 Tesla with and without gadoxetic acid administration. Eur Radiol. 2017;27:4120-4128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Austria
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P- Reviewer: Gali-Muhtasib H, Lim SC, Peparini N, Tanabe S S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y