Published online Aug 7, 2019. doi: 10.3748/wjg.v25.i29.4019
Peer-review started: March 15, 2019
First decision: May 14, 2019
Revised: May 19, 2019
Accepted: July 1, 2019
Article in press: July 3, 2019
Published online: August 7, 2019
Processing time: 145 Days and 17.3 Hours
Post endoscopic retrograde cholangiopancreatography (ERCP) is comparatively complex application. Researchers has been investigated prevention of post-ERCP pancreatitis (PEP), since it has been considered to be the most common complication of ERCP. Although ERCP can lead various complications, it can also be avoided.
To study the published evidence and systematically review the literature on the prevention and treatment for PEP.
A systematic literature review on the prevention of PEP was conducted using the electronic databases of ISI Web of Science, PubMed and Cochrane Library for relevant articles. The electronic search for the review was performed by using the search terms “Post endoscopic retrograde cholangiopancreatography pancreatitis” AND “prevention” through different criteria. The search was restricted to randomized controlled trials (RCTs) performed between January 2009 and February 2019. Duplicate studies were detected by using EndNote and deleted by the author. PRISMA checklist and flow diagram were adopted for evaluation and reporting. The reference lists of the selected papers were also scanned to find other relevant studies.
726 studies meeting the search criteria and 4 relevant articles found in the edited books about ERCP were identified. Duplicates and irrelevant studies were excluded by screening titles and abstracts and assessing full texts. 54 studies were evaluated for full text review. Prevention methods were categorized into three groups as (1) assessment of patient related factors; (2) pharmacoprevention; and (3) procedural techniques for prevention. Most of studies in the literature showed that young age, female gender, absence of chronic pancreatitis, suspected Sphincter of Oddi dysfunction, recurrent pancreatitis and history of previous PEP played a crucial role in posing high risks for PEP. 37 studies designed to assess the impact of 24 different pharmacologic agents to reduce the development of PEP delivered through various administration methods were reviewed. Nonsteroidal anti-inflammatory drugs are widely used to reduce risks for PEP. Rectal administration of indomethacin immediately prior to or after ERCP in all patients is recommended by European Society for Gastrointestinal Endoscopy guidelines to prevent the development of PEP. The majority of the studies reviewed revealed that rectally administered indomethacin had efficacy to prevent PEP. Results of the other studies on the other pharmacological interventions had both controversial and promising results. Thirteen studies conducted to evaluate the efficacy of 4 distinct procedural techniques to prevent the development of PEP were reviewed. Pancreatic Stent Placement has been frequently used in this sense and has potent and promising benefits in the prevention of PEP. Studies on the other procedural techniques have had inconsistent results.
Prevention of PEP involves multifactorial aspects, including assessment of patients with high risk factors for alternative therapeutic and diagnostic techniques, administration of pharmacological agents and procedural techniques with highly precise results in the literature.
Core tip: This study systematically reviewed the literature on the prevention and treatment for post Endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis. PRISMA checklist and flow diagram were adopted for the evaluation and the reporting. Prevention methods were categorized in three groups as (1) assessment of patient related factors; (2) pharmacoprevention; and (3) procedural techniques for prevention. Patients with high risk factors should be carefully assessed, and alternative therapeutic and diagnostic techniques may be preferable for them instead of ERCP.
- Citation: Pekgöz M. Post-endoscopic retrograde cholangiopancreatography pancreatitis: A systematic review for prevention and treatment. World J Gastroenterol 2019; 25(29): 4019-4042
- URL: https://www.wjgnet.com/1007-9327/full/v25/i29/4019.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i29.4019
Endoscopic retrograde cholangiopancreatography (ERCP) has been a prominent technological innovation that has advanced in the field of gastrointestinal endoscopy[1] since its inception in 1968[2]. ERCP, a comparatively more complicated integral therapeutic modality among endoscopic techniques, is clinically the most common and specialized procedure used for the diagnosis and treatment of pancreatic and biliary system disorders[3-8]. Although it is superior to the traditional operation due to limited trauma, simplicity of the operation, and short recovery time in the treatment and diagnosis of duodenal and pancreatobiliary disorders[5,6], diagnostic and therapeutic ERCP can cause various complications such as pancreatitis, cholangitis, perforation, hemorrhage (especially postsphincterotomy), cholecystitis, cardio-pulmonary depression, asymptomatic hyperamylasemia, aspiration, hypoxia, bleeding, sepsis, adverse medication reactions, and death[9-16].
PEP is the most common complication of ERCP,[9,17-19] and itis a crucial factor in morbidity and mortality[20-25]. Chemical, mechanical, enzymatic, hydrostatic and thermal causes are considered as the pathophysiology of the PEP[22]. Although its determinants are unclear, development of PEP is thought to be based on a pro-inflammatory cascade caused by pancreatic acinar cell injury that induces to systemic cytokine release[3].
The incidence rates of PEP have been reported vary from less than 1% up to 40%, because of its dependence on patient factors, procedures, study definitions and methodology[9,23,26-30]. Incidence rates of the severe pancreatitis after ERCP changes between 0.1% and 0.5%[10,27,31-34].
The economic and the social impacts of PEP have been reported to be substantial[35]. The estimated annual cost of PEP in the USA is assessed to be around 200 million USD[36], while the overall mortality rate of PEP is found to be0,7%[37,38]. Furthermore, PEP has a crucial impact on endoscopist stress[39] and is considered as the most common determinant of malpractice lawsuits involving ERCP[40].
The standardized consensus definitions for PEP in the literature[3,9,28,41-43] were introduced by Cotton, Lehman[44] and Banks, Bollen[45]. The standard definition proposed by Cotton, Lehman[44] is as follows: “Pancreatitis after ERCP is a clinical illness with typical pain, associated with at least a threefold increase in serum amylase (or lipase) at 24 h, with symptoms impressive enough to require admission to hospital for treatment (or extension of an existing or planned admission).”The Atlanta criteria-based definition of PEP proposed by Banks, Bollen[45] is as follows: “The diagnosis of acute pancreatitis requires two of the following three features: (1) Abdominal pain consistent with acute pancreatitis (acute onset of a persistent, severe, epigastric pain often radiating to the back); (2) Serum lipase activity (or amylase activity) at least three times greater than the upper limit of normal; and (3) Characteristic findings of acute pancreatitis on contrast-enhanced computed tomography (CECT) and less commonly magnetic resonance imaging (MRI) or transabdominal ultrasonography”
PEP was viewed as an inevitable complication, with uncertain outcomes, and with no practicable strategy for its prevention in the past[35]. Research on the prevention of PEP has identified various approaches to reduce the occurrence and probability of PEP. Based on this research, three different strategies for prevention of the PEP including patient related, procedure related and pharmacological approaches were developed[3,9,30,35,46].
Development and improvement of efficient, safe, and cost-effective techniques for the prevention of PEP area crucial focus of endoscopic research[46] and will be reviewed and assessed in this study. In this context, risk factors and preventative measures extracted from the literature are identified and categorized to evaluate recent developments and approaches for the prevention of PEP.
The relevant studies in the literature were searched by the author using the databases of PubMed, ISI Web of was Science and Cochrane Library. The review was restricted covering the period between January 2009 and February 2019 in order to focus on the updates and the recent developments in the relevant field. The search terms for all databases consisted of the words [“Post endoscopic retrograde cholangio-pancreatography pancreatitis” [All Fields] AND “prevention” (All Fields)] OR “treatment” [(All Fields), ] “post-erpc pancreatitis” [(All Fields) AND “prevention” (All Fields) OR “treatment” (All Fields)], (“Post endoscopic retrograde cholangiopancreatography” (All Fields) AND “pancreatitis” (All Fields) AND “prevention” (All Fields) OR “treatment” (All Fields), (“post-erpc” [(All Fields)] AND “pancreatitis” (All Fields) AND “prevention” (All Fields) OR “treatment” [ (All Fields) ].
The relevance of the studies was determined by using the hierarchical approach of the PRISMA 2009 Statement. The assessment of the studies was based on title, abstract, and the full manuscript of the studies. The references of the selected studies were also scanned to find out further relevant studies. The inclusion criteria of the studies assessed in these reviews are as follows: (1) RCTs conducted to analyze prevention of PEP; (2) Publication in English; (3) Availability of the full text; and (4) Publication date between 2009 and February 2019.
Exclusion criteria of this review were determined as follows: (1) The article type as reviews, editorial letters, commentaries, clinical study protocols, retrospective studies and case reports; (2) Studies with insufficient information and descriptions; and (3) Duplicate studies in all databases were found by EndNote and excluded manually.
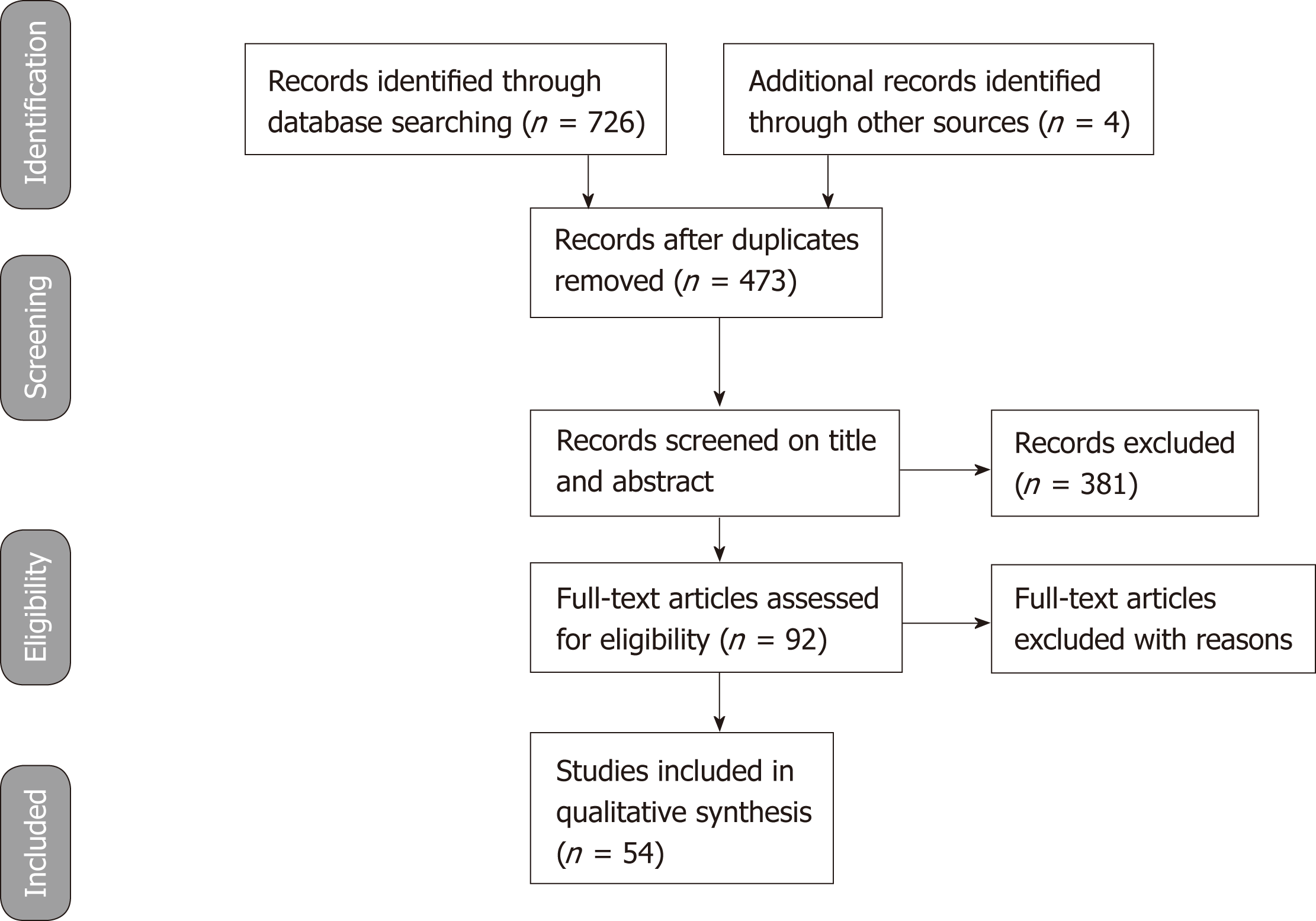
The stages of the literature review adopted from PRISMA 2009 are presented in Figure 1. The literature search through databases of PubMed, ISI Web of was Science and Cochrane Library identified 726 studies that met the search criteria. Additionally, 4 relevant articles found in the edited books on ERCP were included in the review. Search results were put together in EndNote to check for duplicate studies. 257 studies were found to be duplicates and these studies were removed from the list of search results. The eligibility of the 473 studies was evaluated by screening the titles and abstracts to see if they met the inclusion criteria. In this stage, the author only included RCTs and excluded all other publication types such as reviews, editorial letters, commentaries, clinical study protocols, retrospective studies and case reports. 381 studies were excluded due to not meeting the inclusion criteria. The full-text of the 92 remaining studies was reviewed. 38 of these studies were found to be irrelevant and excluded. The remaining 54 studies were included and assessed in this literature review.
The literature on the prevention of the PEP has mainly focused on the specific procedural techniques and pharmacological interventions to reduce the risk for PEP. Since the identification of risk factors increasing the probability of PEP is crucial for the prevention of PEP, the review has also focused on the risk factors related to patients. Therefore, the reviewed studies are categorized in these main topics.
Careful patient selection is considered to be the most significant and primary strategy for the prevention of PEP[26]. Alternative methods providing highly precise pancreaticobiliary imaging such as endoscopic ultrasound and magnetic resonance cholangiopancreatography can be preferred to prevent PEP for patients with high risk factors, particularly for the identification and exclusion of choledocholithiasis[47-49]. Therefore, identification of the patient-related risk factors is one of the most important aspects of prevention for PEP.
The patient related risk factors for the development of the PEP in the literatureare summarized in the Table 1. Patient-related factors for developing the PEP found to be significant in the relevant studies include young age[23,50-53], female gender[23,51], suspected Sphincter of Oddi dysfunction (SOD)[50], history of previous PEP[51,52] and recurrent pancreatitis[51,52]. Although female gender has been found to have high risk for the PEP in the studies, it is not easy to distinguish the impact of SOD, mostly suspected in women with post-cholecystectomy abdominal pain[54]. On the contrary, PEP is less likely to occur in patients with chronic pancreatitis[9,30] indicating a partical loss of sensitivity to PEP stimulation[54], probably because of atrophy and decreased enzymatic activity[27].
| Definite | Possible | No risk |
| Young age | Absence of CBD stone | Normal/small CBD diameter |
| Female gender | Normal serum bilirubin | Pancreas divisum |
| Suspected SOD | Periampullary diverticulum | Allergy to contrast medium |
| Recurrent pancreatitis | ||
| Absence of chronic pancreatitis | ||
| History of previous PEP |
History of ERCP with sphincterotomy is also considered to decrease the risk of developing PEP, since prior sphincterotomy mostly separates the common bile from the main pancreatic duct, therefore decreasing the probability of pancreatic duct cannulation or injection, and enabling comparatively uncomplicated and efficient cannulation of the common bile duct (CBD)[26]. Regarding gland atrophy and calcification, chronic pancreatitis is also considered to reduce the risk of developing PEP[27].
While previous studies indicated that small CBD may be a risk factor for PEP, recent studies[23,51,52,55] found that it has no independent impact on the risk for PEP. Periampullary diverticulum, pancreas divisum and allergy to contrast medium are among the factors which have been found to have no risk on PEP[9,41]. Yet a recent study[23] analyzed data obtained from 3178 procedures administered on 2691 patients and concluded that periampullary diverticulum was one of the significant patient-related risk factors.
DiMagno et al[50] also found that chronic liver disease and smoking were among the predictors of prophylaxis for PEP.
More than 35 pharmacologic agents have been analyzed in terms of prevention for PEP in the literature[56]. These studies focused on the intervention of one or more hypothesized structures of injury within the framework of the main six fields as below (adapted from Cheon[57]): (1) The prevention of the inflammatory cascade; (2) The facilitation of cannulation; (3) The relief of a sphincter of Oddi spasm; (4) The inhibition of intra-acinartrypsinogen activation; and (5) The decrease of pancreatic enzyme secretion.
The reviewed articles studied the impact of the rectal indomethacin on the prevention of PEP are summarized in Table 2. The studies on other pharmacologic agents are summarized in Table 3.
| Authors | Year | Country | n | Intervention | Design | Incidence of PEP1 | P value | |||
| Study group | Control (compared) | |||||||||
| Elmunzer et al[60] | 2012 | United States | 602 | 2 × 50-mg indomethacin or 2 × placebo right after ERCP | Prospective, multicenter, placebo-controlled, double-blind | 27/295 (9.2%) [IND] | 52/307 (16.9%) [Placebo] | 0.005 | ||
| Döbrönte et al[65] | 2014 | Hungary | 665 | 100 mg indometha-cin or an placebo 10-15 min prior to ERCP | Prospective, multicenter, placebo- controlled | 20/347 (5.76%) [IND] | 22/318 (6.92%) [Placebo] | 0.541 | ||
| Patai et al[61] | 2015 | Hungary | 539 | 100 mg indomethacin or placebo 1 h prior to ERCP | Prospective, single center, placebo-controlled, double-blind | 18/270 (6.7%) [IND] | 37/269 (13.8%) [Placebo] | 0.406 | ||
| Andrade-Davila et al[56] | 2015 | Mexico | 166 | 100 mg of indomethacin or 2.6 g suppository of glycerin right after ERCP | Prospective, single center, placebo-controlled | 4/82 (4.87%) [IND] | 17/84 (20.23%) [GS] | 0.01 | ||
| Luo et al[62] | 2016 | China | 2600 | 100 mg indomethacin for unselected patients within 30 min prior to ERCP or 100 mg indomethacin just after ERCP for patients with high risks | Prospective, multicenter, single-blind | 47/1297 (4%) [Universal IND] | 100/1303 (8%) [Risk-stratified IND] | < 0.001 | ||
| Levenick et al[58] | 2016 | United States | 449 | 100 mg indomethacin or placebo during ERCP | Prospective, single center, double-blind, placebo-controlled | 16/223 (7.2%) [IND] | 11/226 (4.9%) [Placebo] | 0.33 | ||
| Hosseini et al[63] | 2016 | Iran | 406 | 100 mg indomethacin two hours before the ERCP or 1 L of ISP within 2 h before ERCP and 2 L within 16 h after ERCP or indomethacin and ISP or 2 g of glycerin in suppositories | Prospective, single center, blinded subject data | 11/100 (11%) [IND] | 10/100 (10%) [ISP] | 0/101 (0) [IND+ISP] | 17/105 (16%) [RG] | - |
| Mok et al[64] | 2017 | United States | 192 | LR + IND, NS + IND, LR + placebo or NS + placebo | Prospective, single center, double-blind, placebo-controlled | 3/48 (6%) [LR+IND] | 6/48 (13%) [NS+IND] | 9/48 (19%) [LR+Placebo] | 10/48 (21%) [NS+Placebo] | 0.04 |
| Agent | Authors | Year | Country | n | Design | Incidence of PEP1 | P value | ||
| Study group | Control (or compared) | ||||||||
| Intraduodenal Acetic Acid (IAA) | Fang et al[97] | 2018 | China | 210 | Prospective, single center, double-blind | 8/105 (7.6%) [IAA] | 11 /105 (10.5%) [Saline] | 0.47 | |
| Celecoxib | Kato et al[77] | 2017 | Japan | 170 | Prospective, single center | 10/85 (11.7%) [Celecoxib] | 13/85 (15.3%) [Saline] | 0.65 | |
| Raw Rhubarb Solution (RRS) | Wang et al[133] | 2017 | China | 500 | Prospective, single center | 5/250 (2%) [RRS] | 19/250 (7.6%) [Water] | 0.003 | |
| Nitroglycerin + Glucagon | Katsinelos et al[106] | 2017 | Greece | 455 | Prospective, single center, double–blind | 7/227 (3.08%) [Nitroglycerin + glucagon] | 17/228 (7.46%) [HBW] | 0.037 | |
| Ketoprofen | Onofrio et al[73] | 2017 | Brazil | 477 | Prospective, single center, double-blind, placebo-controlled | 5/224 (2.2 %) [Ketoprofen] | 5/253 (2 %) [Placebo] | 1.0 | |
| Vigorous IVFR | Choi et al[87] | 2017 | South Korea | 510 | Prospective, multi center, double-blind | 11/255 (4.3%) [Vigorous IVFR] | 25/255 (9.8%) [StandartIVFR] | 0.016 | |
| Aggressive Hydration with Lactated Ringer’s Solution (AHLRS) | Buxbaum et al[83] | 2014 | United States | 71 | Prospective, multicenter, controlled | 0/39 (0%) [AHLRS] | 4/23 (17%) [SHLRS] | 0.016 | |
| Udenafil+Aceclofenac | Lee et al[109] | 2015 | South Korea | 216 | Prospective, multicenter, double-blind, placebo-controlled | 17/107 (15.8%) [Udenafil+Aceclofenac] | 18/109 (16.5%) [Placebo] | 0.901 | |
| Somatostatin | Bai et al[127] | 2015 | China | 900 | Prospective, multicenter, open-label | 18/445 (4 %) [Somatostatin] | 34/455 (7.5 %) [No Somatostatin] | 0.03 | |
| Concepcion-Martin et al[129] | 2014 | Spain | 510 | Prospective, single-center, placebo-controlled, double-blind | 19/255 (7.5 %) [Somatostatin] | 17/255 (6.7 %) [Placebo] | 0.73 | ||
| Wang et al[130] | 2013 | China | 124 | Prospective, single-center, placebo-controlled | 6/36 (16.7%) [Pre-ERCP somatostatin] | 5/47 (10.6%) [Post-ERCP somatostatin] | 6/41 (14.6%) [Placebo] | 0.715 | |
| Intramuscular Diclofenac | Park et al[66] | 2015 | South Korea | 343 | Prospective, single center, double-blind, placebo-controlled | 22/173 (12.7 %) [Intramuscular Diclofenac] | 20/170 (11.8 %) [Placebo] | 0.87 | |
| Rectal Diclofenac | Otsuka et al[68] | 2012 | Japan | 104 | Prospective, multicenter, controlled | 2/51 (3.9%) [Diclofenac with Saline] | 10/53 (18.9%) [Saline] | 0.017 | |
| Nafamostat Mesilate (NM) | Kim et al[118] | 2016 | South Korea | 382 | Prospective, single center, comparative | 5/179 (2.8%) [NM - 24 hour infusion] | 4/192 (2.1%) [NM - 6 hour infusion] | 0.744 | |
| Ohuchida et al[114] | 2015 | Japan | 809 | Prospective, single center, double-blind | 14/405 (3.5) [NM] | 27/404 (6.7) [Glucose Solution] | 0.035 | ||
| Park et al[115] | 2011 | South Korea | 595 | Prospective, single-center, controlled | 8/198 (4%) [20 mg of NM] | 10/197 (5.1%) [50 mg of NM] | 26/200 (13%) [Dextrose] | < 0.0001 | |
| Yoo et al[117] | 2011 | South Korea | 286 | Prospective, single-center, double-blind, placebo controlled | 4/143 (2.8%) [NM] | 13/143 (9.1%) [Dextrose Solution] | 0.03 | ||
| Choi et al[116] | 2009 | South Korea | 704 | Prospective, single-center, double-blind, controlled | 12/354 (3.3%) [NM] | 26/350 (7.4%) [Dextrose Solution] | 0.018 | ||
| Ulinastatin + Nafamostat | Park et al[121] | 2014 | South Korea | 159 | Prospective, single center, placebo-controlled | 1/53 (1.9%) [Ulinastatin] | 2/53 (3.8%) [Nafamotat] | 7/53 (13.2) [Placebo] | 0.037 |
| Risperidone + Ulinastatin | Tsujino et al[125] | 2013 | Japan | 226 | Prospective, multicenter, placebo-controlled | 6/113 (5.3%) [Risperidone + Ulinastatin] | 10/113 (8.8 %,) [Ulinastatin] | 0.438 | |
| Udenafil | Oh et al[107] | 2011 | South Korea | 278 | Prospective, multicenter, double-blind, placebo-controlled | 11/137 (8.0%) [Udenafil] | 11/141 (7.8%) [Placebo] | 944 | |
| Allopurinol | Abbasinazari et al[135] | 2011 | Iran | 74 | Prospective, single-center, double-blind, placebo-controlled | 3/29 (10,4%) [Allopurinol] | 5/45 (11,1%) [Placebo] | 0.97 | |
| Neurokinin-1 receptor antagonist (Aprepitant) | Shah et al[136] | 2012 | United States | 73 | Prospective, single-center, double-blind, placebo-controlled | 7/34 (20.6%) [Aprepitant] | 7/39 (17.9%) [Placebo] | 1.0 | |
| Secretin | Jowell et al[96] | 2011 | United States | 869 | Prospective, single-center, double-blind, placebo controlled | 36/413 (8.7%) [Secretin] | 65/431 (15.1%) [Placebo] | 0.004 | |
| Epinephrine | Xu et al[143] | 2011 | China | 941 | Prospective, single-center, placebo controlled | 9/461 (1.95%) [Epinephrine] | 31/480 (6.45%) [Saline] | 0.0086 | |
| Valdecoxib and GlycerylTrinitrate (GTN) | Bhatia et al[70] | 2011 | India | 371 | Prospective, single-center, controlled | 12/121 (9.9%) [Valdecoxib] | 12/124 (9.7%) [GTN] | 13/126 (10.3%) [No intervention] | 0.986 |
| Glyceryl Nitrate | Nøjgaard et al[100] | 2009 | Multi Country | 806 | Prospective, multicenter, double-blind, placebo-controlled | 18/401 (4.5%) [Glyceryl Nitrate] | 29/405 (7.1%) [Placebo] | 0.11 | |
| Platelet Activating Factor (PAF) | Sherman et al[91] | 2009 | United States | 600 | Prospective, multicenter, double-blind, placebo-controlled | 35/200 (17.5%) [PAF 1 mg/kg] | 32/201 (15.9%) [PAF 5 mg/kg] | 39/199 (19.6%) [Placebo] | 0.59 |
| Interleukin-10 (IL-10) | Sherman et al[88] | 2009 | Multi Country | 305 | Prospective, multicenter, double-blind, placebo-controlled | 14/91 (15.4%) [IL-10 8 µg/kg] | 24/109 (22%) [IL-10 20 µg/kg] | 15/105 (14.3%) [Placebo] | 0.83 0.14 |
Nonsteroidal anti-inflammatory drugs: Nonsteroidal anti-inflammatory drugs (NSAIDs) are inexpensive, easily administered and very effective inhibitors of phospholipase A2, cyclooxygenase and neutrophil–endothelial interactions and are considered to have a significant impact on the pathogenesis of acute pancreatitis[56]. Given the findings of clinical trials in the literature, rectal indomethacin, an NSAID, is administered to patients with high risk factors undergoing ERCP to reduce risk for PEP[58]. Administration of rectal indomethacin right before and after ERCP has been recommended by European Society for Gastrointestinal Endoscopy guidelines for all patients without contraindication to prevent the development of PEP[59]. Only two of eight studies in this review concluded no supporting findings for indomethacin to prevent PEP (Table 2).
Andrade-Davila et al[56] conducted a controlled RCT between 2012 and 2013 in Mexico by comparing the administration of 100 mg of rectal indomethacin on 82 patients versus 2.6 g suppository of glycerin on the placebo group of 84 patients without placement of a pancreatic stent. Patients had at least one major and/or two minor risk factors for PEP. The PEP rate for the experimental group was 4.87% (4/82) and was 20.23% (17/84) for the placebo group (P = 0.01). Rectal indomethacin administered immediately after ERPC decreased the incidence of PEP among patients with high risk factors.
Elmunzer et al[60] investigated the impact of rectal indomethacin on 602 patients at high risk for PEP in a multicenter, randomized, placebo-controlled, double-blind RCT in United States. The rate of PEP was 9.2% among patients who received indome-thacin and was 16.9% among patients who received placebo (P = 0.005). Rectal indomethacin decreased the development of PEP among patients with high risk factors.
In their placebo-controlled, prospective RCT, Patai et al[61] also found positive impact of indomethacin on the prevention of PEP. Their study showed that rectally administered 100 mg indomethacin reduced development of PEP, especially in cases with patient and procedure-related risk factors and with difficult cannulation.
The administration timing of indomethacin and characteristics of patients can be significant impact on the clinical applications. Luo et al[62] compared impact of pre-procedural administration of 100 mg rectal indomethacin in 1297 patients (universal group) within 30 min before ERCP versus post-procedural administration of 100 mg rectal indomethacin in 1303 patients with high-risk factors (risk-stratified group) immediately after ERCP to prevent PEP. The rate of PEP was 4% in universal group and was 8% in the risk stratified group (P < 0.0001). Results showed that administration of rectal indomethacin prior to ERCP in universal group decreased PEP development in comparison of risk stratified group.
Hosseini et al[63] assessed rectal indomethacin with and without intravenous perfusion of normal saline to prevent PEP. In this RCT, 406 patients underwent ERCP and were randomized into four groups with different interventions. Interventions of (1) rectal indomethacin (100 mg); (2) intravenous (IV) saline perfusion; (3) both rectal indomethacin and IV saline; and (4) rectal glycerin were administered to groups before ERCP. The results indicated that intervention of rectal indomethacin and intravenous normal saline together before ERCP significantly reduced incidence rate of PEP.
Mok et al[64] performed a randomized, double-blinded, placebo-controlled RCT to analyze the effectiveness of indomethacin with or without bolus lactated Ringer’s solution (LR) in patients with high risk factors. Patients were randomized into four groups and received different interventions, including normal saline solution (NS) + placebo, LR + placebo, LR + IND NS + IND. Compared with NS + placebo, LR + IND decreased development of PEP and readmission rates.
There are also contradictory findings in the literature about the impact of indomethacin on the prevention of PEP. Döbrönte et al[65] conducted a prospective, randomized, placebo-controlled and multicentred study between 2012 and 2013 in order to compare 100 mg of rectally administered indomethacin on 347 patients vs an inert placebo on 318 patients, 10-15 min before ERCP. They found that rectally administered 100 mg of indomethacin prior to ERCP had no efficacy in preventing the development of PEP.
A prospective, double-blind, placebo-controlled RCT on the consecutive patients performed by Levenick et al[58] also found contradictory results about the impact of indomethacin on the prevention of PEP. 449 consecutive patients undergoing ERCP between 2013 and 2014 in the United States. 223 patients received a single dose of 100 mg dose of rectal indomethacin and 226 patients were received a placebo suppository during the ERCP. The incidence rate of PEP for these groups were 7.2% and 4.9%, respectively. The study revealed that rectally administered indomethacin did not have positive impact on the prevention of PEP.
The majority of clinical trials investigating impact of NSAIDs on the prevention of the PEP have been rectally administered[66]. Diclofenac is another NSAID and is often parenterally administrate because of its faster effect[67]. Park et al[66] administered either 90 mg of diclofenac or placebo to randomized 343 patients by intramuscular injection immediately after ERCP. PEP rate was 12.7% for the group that received diclofenac and 11.8% for the placebo group (P = 0.87). The results of the multivariate regression analysis also failed to demonstrate the prevention impact of diclofenac on the development of PEP. On the other hand, in their prospective, multicenter, controlled and RCT Otsuka et al[68] found contradictory results. Patients underwent ERCP were randomized into two groups and administered either 50 mg of rectal diclofenac with a saline infusion or only saline infusion 30 min before ERCP. The incidence of PEP was 3.9% (2/51) and 18.9% (10/53) (p = 0.017), respectively. They concluded that low-dose rectal diclofenac may have preventative impact on the development of PEP.
Aproinflammatory cascade with a little favorable circumstance for intervention will be induced after the injury of pancreatic acinar cell[69]. Cyclo-oxygenase (COX) enzymes are considered to have a crucial proinflammatory function in pancreatitis[70]. It was reported that the severity of experimental acute pancreatitis was alleviated when COX-2 was pharmacologically inhibited[71]. Bhatia et al[70] investigated the benefits of valdecoxib, a COX-2 inhibitor, and Glyceryltrinitrate (GTN) transdermal patch on PEP. 121 patients were administered 20 mg intravenous valdecoxib, 124 patients were administered GTN patch (10 mg/h) at the beginning of ERCP and 126 patients were assigned as control group. No significant difference was found in the frequency of PEP, indicating that valdecoxib and GTN had no beneficial impact on prophylaxis of PEP.
Ketoprofen, an effective NSAID, is an inhibitor of both COX1 and COX2, and can reach serum peak in minutes when received intravenously, while NSAIDS such as diclofenacor indomethacin can reach serum apex within 2–3 h when received rectally or orally[72]. Because of these advantages, Onofrio et al[73] tested intravenously administration of ketoprofen on consecutive patients with naïve papilla. Patients were randomly assigned to receive saline infusion with or without ketoprofenjust prior to ERCP. PEP rates were 2.2% in the ketoprofen group and 2 % in the control group (P = 1), indicating intravenously received ketoprofen just before ERCP did not reduced PEP incidence.
Prophylactic impact of rectal NSAIDs in PEP is considered to occur by inhibiting cyclooxygenase (COX) and phospholipase A2 enzymes, which are considered significant part of the primary inflammatory cascade of acute pancreatitis through regulation of proinflammatory mediators, i.e., platelet-activating factors and arachidonic acid products[74-76]. Kato et al[77] conducted a prospective, single center, controlled RCT to assess the prophylactic potential of celecoxib, a cyclooxygenase-2 inhibitor, on PEP. 85 patients received oral 400-mg celecoxib tablets 1 h prior to ERCP and saline infusion and another 85 patients received only saline infusion. The incidence of PEP for two groups was 15.3% (13/85) and 11.7% (10/85), respectively (P = 0.65). The difference between the frequency of PEP of groups was insignificant and demonstrated that orally administered of celecoxib did not reduced the rate of PEP.
Hydration: The basis of treatment for acute pancreatitis depends on hydration[78]. Animal studies concluded that pancreatic microvascular hypoperfusion developed necrosis[79]. Clinical researches on patients with acute pancreatitis testing fluid resuscitation indicated that hemoconcentration and reduced systemic perfusion can develop risk of pancreas necrosis and adverse results[80]. Wu et al[81] suggested that hydration with lactated Ringer’s solution (LRS) may reduce the risk for systemic inflammatory response syndrome. Trypsinogen activation and incidence of pancreatitis can be triggered by an acidic environment[82]. Buxbaum et al[83] performed a prospective, multicenter and controlled RCT to determine whether aggressive peri-procedural hydration with LRS diminish the incidence of PEP.
Thirty-nine patients received aggressive hydration with LRS (3 cc/kg/h during the ERCP, a 20 cc/kg bolus after the ERCP, and 3 cc/kg/h for 8 h after ERCP) and 23 patients received standard hydration with the same solution (1.5 cc/kg/hr during and for 8 h after ERCP). There was no PEP incidence in the first group and 17% of patients in the second group developed PEP (P = 0.016). Aggressive intravenous hydration with LRS was found to be effective in decreasing risk of PEP. Their findings also suggested that LRS is less risky than saline to lead metabolic acidosis, indicating protective impact of LRS.
The justification for hydration depends on the requirement for resolution of the hypovolemia[84] . Vigorous intravenous fluid resuscitation (IVFR) with LRS may lead a better acid-base balance and may induce an anti-inflammatory reaction, when compared with other crystalloid preparations[85,86]. In a prospective, multicenter, double-blind RCT Choi et al[87] tested the impact of periprocedural vigorous IVFR on the prevention of PEP. 510 patients with native papilla in Korea were randomized into two groups in a1:1 ratio. The first group received vigorous IVFR (LRS in an initial bolus of 10 mL/kg before the ERCP, 3 mL/kg/h during the ERCP, for 8 h after the ERCP, and a post-ERCPbolus of 10 mL/kg) and the second group received a standard IVFR (LRS at 1.5 mL/kg/h during and for 8 h after the ERCP). The incidence rate of PEP was 4.3% in the first group and 9.8% in the latter one (P = 0.016). The findings indicated that IVFR with LRS had preventative effect on PEP and reduced severity of PEP in both high-risk and average-risk cases.
Cytokines and mediators: Regardless of the trigger of pancreatitis, early intracellular events are followed by initial local and systemic inflammatory reactions which are increased by proinflammatory cytokines and chemokines. These are considered to contribute in the progress of pancreatic necrosis[88]. The administration of endogenous Interleukin-10 (IL-10), a potent inhibitor of cytokines, in animal models of pancreatitis with cerulein reduced the severity of acute pancreatitis[89], principally through inhibition of the development of acinar necrosis[90]. Sherman et al[88] investigated impact of IL-10 on PEP in patients with high risks. 91 patients received 8 µg/kg and 101 patients received 20 µg/kg of IL-10 and 105 patients received placebo through a single intravenous injection 15-30 min before ERCP. PEP incidences were 15%, 22%, and 14% in research groups respectively (P = 0.83). The study showed administration IL-10 failed to prevent PEP.
Platelet-activating factor (PAF), potent proinflammatory mediator, was reported to be related to acute pancreatitis, since its degradation or production is considered to be dysregulated, leading to inflammation via effector mechanisms that stimulate systemic or local tissue injury[91]. The release of amylase from isolated pancreatic acini was observed to increase due to the administration of exogenous PAF[92]. rPAF-AH, developed to prevent adverse implications of dysregulated PAF activity[91], alleviated pancreatic injury, cut down the lipase and amylase increment, and reduced pancreatitis-associated acute lung injury in an animal model of acute pancreatitis[93]. In their randomized, multicenter, double-blind, placebo-controlled RCT Sherman et al[91] analyzed prophylactic rPAF-AH administration in reduction of PEP incidence in high-risk patients. 200 patients received 1 mg/kg of rPAF-AH, 201 patients received 5 mg/kg ofrPAF-AH and another 199 patients received placebo intravenously. They concluded that rPAF-AH had no preventative impact on PEP.
Difficulties in cannulation of the CBD can cause papillary trauma and, thereby increasing the incidence of PEP[28,55]. Facilitation of cannulation may decrease risk for complications of ERCP, because difficult cannulation is reported to be a significant procedure-related risk factor of PEP[23,94]. Secretin, a gastrointestinal peptide endocrine hormone, can stimulate pancreatic bicarbonate excretion, thereby facilitating the cannulation[95]. Jowell et al[96] conducted a single center, prospective, double-blind, placebo-controlled RCT to evaluate effects of synthetic secretin in preventions of PEP. 426 patients were received secretin (16 µg) and 443 patients received placebo before ERCP. The incidence of PEP in the two groups were 8.7% and 15.1%, respectively (P = 0.004). Results showed that synthetic secretin was effective in prevention of PEP.
The limitations of secretin, such as its high price and limited availability, makes its use complicated in clinics[97]. Alternatively, intraduodenal acid infusion (ACI) was used in clinical trials, since it can physiologically stimulate secretin release in the body[98,99]. Fang et al[97] conducted a single center, double-blind RCT between 2016 and 2017 in China to investigate the impact of ACI on pancreatic duct cannulation during ERCP. Consecutive patients were randomized into two groups (105 in each group) and received 50 mL ACI infusion or 50 mL saline. The incidence rate of PEP for two groups was 7.6% and 10.5%, respectively (P = 0.470). Despite the statistically insignificant difference in the incidence of PEP in the two groups, ACI infusion significantly facilitated pancreatic duct cannulation and reduced radiation exposure.
Promoting efficient drainage of the pancreatic duct at the end of ERCP by administrating pharmacologic agents, instead of procedural techniques such as pancreatic stent placement, may be effective in ameliorating the adverse impacts of temporal blockage outflow of pancreatic juice induced by papillary edema and/or sphincter of Oddi spasm triggered by manipulation during ERCP and papillary trauma[70]. Glyceryltrinitrate (GTN), a nitric oxide donor, may prevent papillary edema through facilitating primary cannulation and may support pancreatic duct drainage after ERCP, ultimately leading relaxation of the sphincter of Oddi[70]. Glyceryl nitrate (GN), a nitrogen oxide donor, may stimulate dilation of the microvascular vessels and periampullar sphincter relief, therefore enhancing nutrition and circulation[100]. However, the results of RCTs conducted by et al[70] and Nøjgaard et al[100] showed that GTN and GN were not effective for the prevention of PEP.
Nitric oxide (NO) donor is another pharmacologic agent thought to facilitate CBD cannulation by decreasing the amplitude and baseline pressure caused by the sphincter of Oddi[101-103]. Additionally, intravenous glucagon, applied throughout the ERCP for prevention of duodenal motility, can be beneficial for relaxing the sphincter of Oddi[104,105], and therefore, can improve CBD cannulation[106]. The impact of the combination of sublingual nitroglycerin and intravenous glucagon administration on PEP was investigated by Katsinelos et al[106] between 2012 and 2015 in Greece through a prospective, single center, double–blind RCT study. 227 patients intravenously received 6 puffs (2.4 mg) sublingual nitroglycerin and glucagon 1mg and another 228 patients intravenously received 6 puffs sterile water and 20mg hyoscine-n-butyl bromide. PEP rates were significantly lower in the first group than the latter one. Administration of combined nitroglycerin and glucagon contributed a high selective CBD cannulation rates, thereby reducing of PEP incidence.
Phosphodiesterase type 5 (PDE-5) inhibitor, smooth-muscle relaxant, is considered to diminish basal sphincter of Oddi pressure[107]. It can reduce sphincter of Oddi tone, contribute easy cannulation and eventually decrease risks for PEP[108]. Oh et al[107] investigated administration of prophylactic udenafil, a phosphodiesterase-5 inhibitor, for the prevention of PEP. 280 patients were randomized in a 1:1 ratio and received udenafil (100 mg) or placebo. They found no significant difference between rates of PEP incidence of two groups, indicating udenafil had no prophylactic impact on PEP. Lee et al[109] performed a prospective, randomized, double-blind, placebo-controlled, multicenter RCT to investigate the efficacy of a combination of a high dose of udenafil (PDE-5 inhibitor) and aceclofenac (NSAID) on development of PEP in high-risk patients. Their rationale for this study depended on the potential of the combination to decrease the pressure of the sphincter of Oddi and inflammation in acute pancreatitis through modulation of the cytokine cascade. 216 patients were assigned into two groups in a 1:1 ratio and orally administered either PDE-5 inhibitor udenafil (200 mg.) and aceclofenac (100 mg.) or placebo. The incidence rate of PEP for two groups were 15.8% and 16.5%, respectively (P = 0.901). The statistically insignificant results indicated that administration of combined udenafil and aceclofenac had no impact to on the prevention of PEP.
The possible contribution of proteolytic enzymes on the development of PEP have made protease inhibitors (nafamostatmesilate, ulinastatin, gabexate) the focus of clinical trials[110]. Nafamostatmesilate (NM), a strong synthetic serine protease inhibitor, was developed by Fujii et al[111]. NM powerfully inhibits trypsin, a proteolytic enzyme which is thought to have a crucial role in triggering acute pancreatitis, as well as kallikrein, plasmin, and the complement components C1s and C1r[111-113]. Only one of five studies in our reviews showed no contribution of NM on the prevention of the PEP.
In a prospective, single center and double-blind RCT Ohuchida et al[114] administered either 20 mg of NM dissolved in 500 mL of 5% glucose solution to 405 patients or 500 mL of 5% glucose solution to 404 patients, over 2 h from the beginning of ERCP. The incidence of PEP was found to be 3.5% and 6.7% in the groups, respectively (P = 0.0349). The findings revealed that 20 mg NM administered in the short run can prevent PEP.
Park et al[115] conducted a prospective, single-center and controlled RCT to assess the administration of 50 mg NM for prevention of PEP. Enrolled patients underwent ERCP were assigned into three groups and intravenously administered 500 mL of 5% dextrose solution alone or with 20 mg or 50 mg of NM. Incidence of PEP was found 13.0%, 4.0% and 5.1%, respectively (P < 0.0001). They concluded that NM (20 or 50 mg) may effectively prevent PEP.
Choi et al[116] and Yoo et al[117] also found supportive evidence, indicating that prophylactic intravenous NM may decrease the risk for PEP. However, Kim et al[118] found contradictory results in their prospective, single center, comparative RCT investigating the impact of 24 and 6 h intravenous infusions of 20 mg NM. They randomized 382 patients undergoing ERCP into two groups and administered NM (20 mg) infusion prior to ERCP and continued for either 6 or 24. The incidence of PEP were 2.8% (5/179) and 2.1% (4/192), respectively (P = 0.744). They found that NM infusion had no benefit on the prevention of PEP, regardless of the duration.
Ulinastatin, another protease inhibitor, is obtained by purifying healthy human urine[118]. It can prevent the onset and development of pancreatitis through inhibition of the pancreatic enzyme activation pathway[119,120]. In a prospective, single center, placebo-controlled RCT Park et al[121] compared the impact of ulinastatin and nafamostat on the prevention of PEP. They assigned 159 patients into three groups and administered 150000 units of ulinastatin, 20 mg of nafamostat or placebo for a 2-4 h prior to ERCP to 6-8 h after ERCP. The incidence of PEP was 1.9%, 3.8% and 13.2, respectively (P = 0.037), indicating that both pharmacologic agents reduced the incidence of PEP.
Serotonin [5-hydroxytryptamine (5-HA)], a monoamine neurotransmitter found in the platelets, central nervous system and intestinal mucosa and can induce 11 subtypes of the 5-HA receptor which is considered to be related to acute pan-creatitis[122,123]. Some research has concluded that 5-HA2A antagonists may have amendatory effect on acute pancreatitis[124]. Risperidone, a potent 5-HA2A antagonist, is considered to prevent or decrease the primary events of acute pancreatitis[125]. It was reported that risperidone mitigated the increase of pancreatic enzymes and cellular infiltration into the pancreatic interstitial tissues in caerulein-induced acute pancreatitis[126]. Tsujino et al[125] performed a prospective, multicenter, placebo-controlled RCT in Japan to investigate the prophylaxis of risperidone combined with ulinastatin for PEP in high-risk patients.
Patients were randomized to receive either ulinastatin with or without risperidone. An oral risperidone tablet was administered 30–60 min prior to ERCP and ulinastatin was intravenously administered for 10 min just prior to ERCP. The incidence of PEP in these groups was 5.3% and 8.8 %, respectively (P = 0.438). They concluded that combination of oral risperidone with ulinastatindid did not decrease the rate of PEP in patients at high-risk.
Somatostatin is considered as a potent inhibitor of pancreatic enzyme secretion[127]. Somatostatin and its synthetic analog, octreotide, can influence exocrine function directly through decreasing the secretion of digestive enzymes, and indirectly through causing inhibition of secretin and cholecystokinin production[57]. In addition, somatostatin and octreotide may adjust the cytokine cascade and may have a cytoprotective impact on pancreatic cells, while the mechanisms of their cytoprotective effect are still unclear[128]. However, research investigating the pro-phylactic effect of somastatin on PEP have shown inconsistent results[127,129,130]. Only one of three studies reviewed showed that somatostatin was effective and beneficial for the prevention of PEP. Bai et al[127] conducted a multicenter, open-label RCT in China. 908 patients underwent ERCP were randomly assigned to administrate somatostatin 250 μg bolus injection before ERCP and 250 μg/h intravenous infusion for 11 h after ERCP or no somatostatin treatments. The results of this study showed that incidence of PEP for these groups were 7.5% and 4.0%, respectively (P = 0.03). Significant results indicated that somatostatin was effective in preventing PEP. The other two studies assessed the impact of somatostatin on the prevention of PEP conducted by Concepcion-Martin et al[129] and Wang et al[130] found contradictory results.
Concepcion-Martin et al[129] administered either an intravenous bolus of somatostatin followed by a 4-hourcontinuous infusion or a similar placebo to patients undergoing ERCP. Wang et al[130] administered 0.5 mg/h of somatostatin for 24 h starting from 1 h before ERCP to 36 patients in the first group, 0.5 mg/h of somatostatin for 24 h starting from 1 h after ERCP to 47 patients in the second group and saline for 24 hours starting from 1 hour before ERCP to 41 patients in the third group. Both of these studies did not find any supportive evidence of the preventive effect of somastation on PEP.
Raw rhubarb is a traditional Chinese medicine and considered to adequately relieve clinical symptoms, prevent the production of inflammatory mediators and cytokines and bacterial translocation, and mitigate abdominal compartment syndrome[131,132]. In a prospective, single center RCT Wang et al[133] assessed the efficiency of raw rhubarb for prevention of PEP. High risk patients were randomized into two groups. 250 patients drank a raw rhubarb soak solution per 3 h until defecation after ERCP and another 250 patients drank water after ERCP. PEP incidence was 2% (5/250) and 7.6% (19/250), respectively (P < 0.01). The results suggested that raw rhubarb solution is efficient for prevention of PEP in high-risk patients.
Oxygen derived free radicals may damage epithelial cells causing to capillary permeability and initiation of pancreatitis[134]. Allopurinol, one of inhibitors of xanthine oxidase, is thought to prevent or mitigate the initial complications caused by the cascade causing PEP by these agents[135]. In this context, Abbasinazari et al[135] performed a prospective, single-center, double-blind, placebo-controlled RCT to assess impact of allopurinol on prevention of PEP. Patients were divided in two groups and received 2 tablets of allopurinol (300 mg) or 2 tablets of placebo. One of the tablets was administered 3 h before ERCP and the other one just before the ERCP. PEP was developed in 3 of 29 patients (10.4%) in the allopurinol group and 5 of 45 patients in the control group (11.1%) (P = 0.97). The results of the study indicated that allopurinol was not effective in preventing PEP.
Neurogenic inflammation (pathologic activation of sensory neurons) is considered to contribute to the pathogenesis of acute pancreatitis[136]. Release of substance P, related to pancreatic vasodilation, edema, and cellular infiltration, can be stimulated by initiation of the capsaicin receptor (TRPV1) on sensory C and Aδ fibers[137,138]. Complications of neurogenic inflammation, such as pancreatitis, may be initiated through the attachment of Substance P to the neurokinin-1 receptor in the pancreas[139,140]. It was reported that intra-ductal administration of a neurokin1 antagonist diminished the severity of inflammation in a rat experiment of PEP[141]. Shah et al[136] conducted a prospective, single-center, double-blind, placebo-controlled RCT to evaluate the efficacy of aprepitant, a selective neurokinin-1 receptor antagonist, on the prevention of PEP in high risk patients. 39 patients received placebo and 34 patients received 125 mg oral aprepitant 4 h before ERCP, 80 mg 24 h after the first dose, and 80 mg 24 h after the second dose. 7 patients in each group developed PEP (P = 0.772). It was concluded that aprepitant was not efficient to reduce the incidence of PEP.
Papillary edema, triggered by manipulations during endoscopic treatment or cannulation, may temporarily prevent outflow of pancreatic juice[142], thereby raising ductal pressure, ultimately causing pancreatitis[143]. It was reported that the administration of epinephrine on the papilla may decrease papillary edema[144] and prevent acute pancreatitis after endoscopic balloon sphincteroplasty[142]. Application of epinephrine on the papilla is considered to mitigate edema, contribute the vascular permeability, relieve the muscles in the sphincter of Oddiand muscular layer of the duodenum, preventing increased pressure in the pancreatic duct by stopping the activation of pancreatic enzymes and the drainage of the pancreatic fluid[143]. In a hospital-based, prospective, controlled RCT, Xu et al[143] assessed to the impact of epinephrine sprayed on the papilla on reducing the development of PEP. 941 patients underwent ERCP were randomized to administer 20 mL of either 0.02% epinephrine or saline sprayed on the papilla after diagnostic ERCP. PEP occurred in 31 of 480 (6.45%) patients in the control and in 9 of 461 (1.95%) patients in the epinephrine group (P = 0.0086). They concluded that epinephrine administration on the papilla reduced the development of PEP.
Procedural techniques for prevention: The reviewed articles investigated the impact of the procedural techniques on the prevention of PEP are summarized on the Table 4.
| Proce-dure | Authors | Year | Country | n | Design | Incidence of PEP1 | P value | |||||
| Study group | Control (or compared) | |||||||||||
| Endosco-pic Nasobilia-ry Drainage (ENBD) | Huang et al[150] | 2018 | China | 155 | Prospec-tive, single center | 1/78 (1.28%) [ENBD] | 8/77 (10.4%) [No ENBD] | 0.018 | ||||
| Xu et al[145] | 2015 | China | 218 | Prospec-tive, single center | 0/41 (0%) [ENBD +EPBD] | 2/34 (5.9%) [ENBD +EST] | 5/38 (13.2%) [Only ENBD] | 6/36(16.7%) [EPBD] | 3/39 (7.7%) [EST] | 2/30(6.7%) [Neither] | - | |
| Wire-Guided Biliary Cannula-tion (WGC) | Kobayashi et al[158] | 2013 | Japan | 322 | Prospec-tive, multicen-ter, controlled | 10/163 (6.1%) [WGC] | 10/159 (6.3%) [CC] | 0.95 | ||||
| Lee et al[161] | 2009 | South Korea | 300 | Prospec-tive, single center | 3/150 (2%) [WGC] | 17/150 (11.3%)) [CC] | 0.001 | |||||
| Bassan et al[162] | 2018 | Asia Pacific Region | 707 | Prospec-tive, multicen-ter, single-blinded | NA/355 (9.3%) [0.035-inch wire] | NA/357 (7.8%) [0.025-inch wire] | 0.51 | |||||
| Pancrea-tic Stent Place-ment (PSP) | Fujisawa et al[156] | 2016 | Japan | 200 | Prospec-tive, single center | 2/98 (2%) [PSP 3 cm] | 9/102 (8.8%) [PSP 5 cm] | 0.035 | ||||
| Conigliaro et al[157] | 2013 | Italy | 40 | Prospec-tive, multicen-ter, blinded | 6/21 (29 %) [Immediate stent removal] | 0/19 [leaving the stent] | 0.021 | |||||
| Lee et al[151] | 2012 | South Korea | 101 | Prospec-tive, multicen-ter, | 6/50 (12%) [3F PSP] | 15/51 (29.4) [Nonstent] | 0.031 | |||||
| Sofuni et al[152] | 2011 | Japan | 407 | Prospec-tive, multicen-ter | 16/203 (7.9%) (PSP) | 31/204 (15.2%) [Nonstent] | 0.021 | |||||
| Pan et al[153] | 2011 | China | 40 | Prospec-tive, single center | 4/20 (20%) [PSP] | 14/20 (70%) [Nonstent] | < 0.01 | |||||
| Ito et al[154] | 2010 | Japan | 70 | Prospec-tive, single center | 0/35 (0%) [PSP] | 9/35 (24%) [Nonstent] | < 0.01 | |||||
| Kawagu-chi et al[155] | 2012 | Japan | 120 | Prospec-tive, single center | 1/60 (1.7%) [PSP] | 8/60 (13.3%) [Nonstent] | 0.0322 | |||||
| Needle Knife Sphincter-otomy (NKS) | Swan et al[164] | 2013 | Australia | 73 | Prospec-tive, single center, single blind | 8/39 (20.5%) [NKS] | 6/34 (17.6%) [CSC] | 1.0 | ||||
Endoscopic nasobiliary drainage (ENBD) placement ensures dependable biliary drainage and perfusion, as well as cholangiography[145]. ENBD decreases the necessity of instrumental stone extraction and repetitive endoscopy and cholangiography to evaluate whether the stones have been completely removed by transna-salcholangiograms[146]. It was reported that endoscopic sphincterotomy (EST) or endoscopic papillary balloon dilatation(EPBD) pursued by ENBD diminished development of PEP, especially in patients with infected bile, lasting stones, or blood clots in the biliary tree[147-149].
Huang et al[150] assessed whether the placement of an ENBD had any benefits on the prevention of PEP after endoscopic papillary large balloon dilation together with endoscopic biliary sphincterotomy. 155 patients with bile duct stones were randomized to an ENBD group or no-ENBD group. PEP incidences were 1.28% and 10.4%, respectively (P = 0.018). Results showed that administration of ENBD reduced and was safe for PEP.
Another study conducted by Xu et al[145], evaluated the efficacy of ENBD catheter placement after clearance of CBD stones, also showed that ENBD was beneficial for the prevention of PEP only with an accompanying EPBD procedure.
It is postulated that pancreatic stent placement (PSP) across the pancreatic sphincter may maintain flow of pancreatic secretions, which can be interrupted through papillary edema, and thereby contributing reduction of the PEP[9]. Five studies[151-155] in our review investigated efficacy of PSP in the prevention of PEP through a control group administered through non-stent procedure showed that PSP can reduce incidence rate of PEP.
The study with the largest number of participating patients in the reviewed articles was conducted by Sofuni et al[152] at 37 endoscopic units in Japan. They performed a prospective, multicenter and controlled RCT to investigate efficacy of a temporary-type PSP for the prevention of PEP through analyzing data obtained from 426 patients who underwent ERCP. 213 patients received stents and another 213 patients did not. PEP incidence was 7.9% and 15.2%, respectively (P = 0.021). The study concluded that PSP reduced the incidence of PEP.
Fujisawa et al[156] compared the impact of PSP between 3 cm and 5 cm pancreatic stents on prevention of PEP. 240 patients were randomized in a 1:1 ratio and underwent prophylactic insertion with 5-Fr unflanged 3 or 5-cm pancreatic stent. Per-protocol analysis showed that 3-cm stents are superior than 5-cm stents for prevention of PEP
In a prospective, multicenter, blinded RCT Conigliaro et al[157] compared the efficacy of duration of PSP in prevention of PEP using data obtained from patients receiving immediate 5-Fr unflanged pigtail pancreatic duct stenting after accidental wire-guided pancreatic duct cannulation during ERCP. After the ERCP process, stents were removed in 21 patients and were left in another 19 patients. PEP incidence was 29% in the first group and 0% in the second group (P = 0.021). They demonstrated that leaving pancreatic stents in place until spontaneous dislodgment occurs might reduce the development of PEP.
Wire-guided biliary cannulation (WGC) technique has been recommended for the reduction of PEP development and the facilitation of bile duct cannulation through using a radiopaque guidewire pierced the tip of a sphincterotome or a catheter[158]. Accession to bile duct using a guidewire is considered to decrease traumatic injury to the papilla and the pancreatic duct or prevent from hydrostatic pressure related to contrast injection, thereby contributing the prevention of PEP[159,160].
Kobayashi et al[158] and Lee et al[161] compared the effect of the WGC procedure versus conventional cannulation (CC) procedure on the prevention of PEP. Their findings were inconsistent. While Lee et al[161] found that WGC may be beneficial for the prevention of PEP, Kobayashi et al[158] concluded that the WGC technique did not decrease the risk of PEP.
In a single blinded, prospective, multicenter RCT, Bassan et al[162] compared 0.025-inch versus 0.035-inch guidewire on prevention of ERCP adverse events. 710 patients, with a healthy papilla and conventional anatomy, were randomized to either a 0.025-inch or 0.035-inch guidewire administration. The difference between the rate of PEP in these groups was found to be insignificant.
Needle knife sphincterotomy (NKS) is an advanced therapeutic measure used to facilitate deep cannulation in cases when traditional deep cannulation is insufficient[163]. Therefore, NKS is related to PEP, since it is often administered as a last resort after multiple and repeated failed cannulation attempts[164].
Swan et al[164] conducted a prospective, single center, single blind RCT to assess efficacy of early application of NKS during difficult cannulation on the prevention of PEP. 73 patients with an intact papilla underwent ERCP with difficult biliary cannulation were randomized to groups that administered either NKS or continued standard cannulation. The difference in rate of PEP between these groups was insignificant, revealing that early application of NKS during difficult cannulation was not effective in preventing the development of PEP.
PEP remains an important complication of ERCP and may have adverse impacts on the quality of patient life, morbidity, and mortality[165]. Its pathophysiology is still unclear and considered to be multifactorial. Clinical trials have analyzed different approaches for the prevention of PEP. Studies that investigate the prevention of PEP may be categorized into (1) assessment of patient related risk factors; (2) pharmacoprevention; and (3) procedural techniques for prevention.
Determination of patients with high risk factors for PEP is one of the most important aspects for the prevention of PEP. Patients with high risk factors should be carefully assessed, and alternative therapeutic and diagnostic techniques may be preferable for them instead of ERCP. EUS, MRCP and the other non-invasive techniques including percutaneous drain fluid analysis and radionucleotide-labeled scan, providing very accurate results in diagnosing pancreaticobiliary disorders and meet the need for diagnostic ERCP[26], can be preferable alternatives to reduce risks of PEP for these patients.
Pharmacological agents with highly precise results in the literature, such as NSAIDs, can be beneficial to attenuate development of PEP. Although many pharmacologic agents has been analyzed through data obtained from patients undergoing ERCP, NSAIDs (indomethacin and diclofenac) are in widespread use and the most promising option for the prevention of PEP[57]. NSAIDs should be rectally administered to all patients with high-risks and considered for patients with average-risks[3]. Other pharmacological agents, found consistently to have impact on the prevention of PEP in various studies, can be alternatively considered for the prevention of PEP (Table 3). Further studies are required for other pharmacological agents to identify their impacts more accurately.
Among the reviewed studies focused on the procedural techniques, PSP and ENBD are considered to have most efficacy in preventing PEP (Table 4). PSP and ENBD should be performed for to all patients with high-risks and considered for patients with average-risks. These techniques can facilitate the difficult and failed cannulation cases.
Due to the multifactorial mechanism of the introduction of PEP[57], prevention of PEP can fail through targeting only one causative factor[35]. Combination of multiple interventions may be more effective through proper patient selection, administration of prophylaxis pharmacologic agents and procedural techniques. However, further studies are needed to consolidate prophylaxis impacts of each of these interventional approaches on the prevention of PEP. Further researches should focus on performing meta-analysis to get pool effect and overcome heterogeneity, imprecision, and risk of publication bias. Thereby the assessment of the evidence quality obtained through the studies in the literature can be enhanced.
Endoscopic retrograde cholangiopancreatography (ERCP) has been used in the field of gastrointestinal endoscopy since its introduction as an important technological innovation. It is comparatively complex and can lead various complications. Post-ERCP pancreatitis (PEP) has been on the focus of the researches to investigate its prevention, since it has been considered to be the most common complication of ERCP.
Clinical trials have investigated different methods for the prevention of PEP. Each of these studies focused on a specific method. Our review gathered all preventative approaches for PEP investigated in the last ten years. Due to the conflicting data in the literature, advances in the reviewed field needed to be updated and supporting evidence needed review.
The objective of this study was to systematically review the literature on prevention of PEP with different preventive approaches.
We conducted an electronic search through databases of PubMed, ISI Web of Science and Cochrane Library for relevant articles performed via RCTs covering the time span of January 2009 and February 2019. The search was performed through terms “Post endoscopic retrograde cholangio-pancreatography pancreatitis” AND “prevention”. The reference lists of the identified papers were also scanned to find out further relevant studies.
54 studies were finally identified for full text review. The studies were categorized regarding prevention methods as (1) assessment of patient related factors, (2) pharmacoprevention and (3) procedural techniques for prevention. Female gender, young age, suspected Sphincter of Oddi dysfunction, absence of chronic pancreatitis, recurrent pancreatitis and history of previous PEP were the most common high risk factors for the patients to develop PEP. Rectally administered NSAIDs has been highly recommended for the prevention of PEP among the pharmacologic agents, while others had conflicting results and needed further research. Of the procedural techniques, Pancreatic Stent Placement and Endoscopic Nasobiliary Drainage can be beneficial in preventing PEP.
PEP is the most common complication in ERCP procedure and can be risky in patients with high risk factors. The pathophysiology of PEP is still in dispute. Due to its multifactorial pathophysiology, prevention of PEP should be assessed in multi aspects through evaluation of patient related risk factors, prophylaxis pharmacological agents and procedural techniques.
The multifactorial nature of PEP requires prophylaxis measures in multi facets. Due to its relation to a combination of various factors, multifactorial approach should be taken into account to prevent PEP through assessment of patient related risks and prophylaxis preventions of pharmacologic agents and procedural techniques.
| 1. | Baron TH, Kozarek RA, Carr-Locke DL. ERCP. Philadelphia: Elsevier 2019. . |
| 2. | McCune WS, Shorb PE, Moscovitz H. Endoscopic cannulation of the ampulla of vater: a preliminary report. Ann Surg. 1968;167:752-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 383] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 3. | Morales SJ, Sampath K, Gardner TB. A Review of Prevention of Post-ERCP Pancreatitis. Gastroenterol Hepatol (N Y). 2018;14:286-292. [PubMed] |
| 4. | Inamdar S, Han D, Passi M, Sejpal DV, Trindade AJ. Rectal indomethacin is protective against post-ERCP pancreatitis in high-risk patients but not average-risk patients: a systematic review and meta-analysis. Gastrointest Endosc. 2017;85:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Matsushita M, Koyabu M, Nishio A, Seki T, Okazaki K. Techniques of ERCP with a conventional endoscope in pancreatoduodenectomy anatomy. Gastrointest Endosc. 2017;86:747-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Li DP, Zhang QY, Cheng J, Li JZ, Wang L. The effect of somatostatin retained enema in the treatment of pancreatic ileus. Eur Rev Med Pharmacol Sci. 2017;21:3476-3481. [PubMed] |
| 7. | Tierney J, Bhutiani N, Stamp B, Richey JS, Bahr MH, Vitale GC. Predictive risk factors associated with cholangitis following ERCP. Surg Endosc. 2018;32:799-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Rodrigues-Pinto E, Macedo G, Baron TH. ERCP competence assessment: Miles to go before standardization. Endosc Int Open. 2017;5:E718-E721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Guda NM, Freeman ML. Overview of ERCP Complications: Prevention and Management. Lee LS, editor ERCP and EUS: A Case-Based Approach. New York: Springer 2015; 37-56. [DOI] [Full Text] |
| 10. | Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, Moore JP, Fennerty MB, Ryan ME, Shaw MJ, Lande JD, Pheley AM. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1716] [Cited by in RCA: 1703] [Article Influence: 56.8] [Reference Citation Analysis (2)] |
| 11. | Kahaleh M, Freeman M. Prevention and management of post-endoscopic retrograde cholangiopancreatography complications. Clin Endosc. 2012;45:305-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Zimmon DS, Falkenstein DB, Riccobono C, Aaron B. Complications of endoscopic retrograde cholangiopancreatography. Analysis of 300 consecutive cases. Gastroenterology. 1975;69:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 62] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Bilbao MK, Dotter CT, Lee TG, Katon RM. Complications of endoscopic retrograde cholangiopancreatography (ERCP). A study of 10,000 cases. Gastroenterology. 1976;70:314-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 354] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 14. | Skude G, Wehlin L, Maruyama T, Ariyama J. Hyperamylasaemia after duodenoscopy and retrograde cholangiopancreatography. Gut. 1976;17:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Deschamps JP, Allemand H, Janin Magnificat R, Camelot G, Gillet M, Carayon P. Acute pancreatitis following gastrointestinal endoscopy without ampullary cannulation. Endoscopy. 1982;14:105-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Odes HS, Novis BN, Barbezat GO, Bank S. Effect of calcitonin on the serum amylase levels after endoscopic retrograde cholangiopancreatography. Digestion. 1977;16:180-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Mine T, Morizane T, Kawaguchi Y, Akashi R, Hanada K, Ito T, Kanno A, Kida M, Miyagawa H, Yamaguchi T, Mayumi T, Takeyama Y, Shimosegawa T. Clinical practice guideline for post-ERCP pancreatitis. J Gastroenterol. 2017;52:1013-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 18. | Wang AY. Medications and Methods for the Prevention of Post-ERCP Pancreatitis. Gastroenterol Hepatol (N Y). 2017;13:188-191. [PubMed] |
| 19. | Testoni PA, Mariani A, Aabakken L, Arvanitakis M, Bories E, Costamagna G, Devière J, Dinis-Ribeiro M, Dumonceau JM, Giovannini M, Gyokeres T, Hafner M, Halttunen J, Hassan C, Lopes L, Papanikolaou IS, Tham TC, Tringali A, van Hooft J, Williams EJ. Papillary cannulation and sphincterotomy techniques at ERCP: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2016;48:657-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 431] [Article Influence: 43.1] [Reference Citation Analysis (1)] |
| 20. | Fogel EL, Eversman D, Jamidar P, Sherman S, Lehman GA. Sphincter of Oddi dysfunction: pancreaticobiliary sphincterotomy with pancreatic stent placement has a lower rate of pancreatitis than biliary sphincterotomy alone. Endoscopy. 2002;34:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 125] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Tarnasky PR, Palesch YY, Cunningham JT, Mauldin PD, Cotton PB, Hawes RH. Pancreatic stenting prevents pancreatitis after biliary sphincterotomy in patients with sphincter of Oddi dysfunction. Gastroenterology. 1998;115:1518-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 272] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 22. | Silviera ML, Seamon MJ, Porshinsky B, Prosciak MP, Doraiswamy VA, Wang CF, Lorenzo M, Truitt M, Biboa J, Jarvis AM, Narula VK, Steinberg SM, Stawicki SP. Complications related to endoscopic retrograde cholangiopancreatography: a comprehensive clinical review. J Gastrointestin Liver Dis. 2009;18:73-82. [PubMed] |
| 23. | Wang P, Li ZS, Liu F, Ren X, Lu NH, Fan ZN, Huang Q, Zhang X, He LP, Sun WS, Zhao Q, Shi RH, Tian ZB, Li YQ, Li W, Zhi FC. Risk factors for ERCP-related complications: a prospective multicenter study. Am J Gastroenterol. 2009;104:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 341] [Article Influence: 20.1] [Reference Citation Analysis (1)] |
| 24. | Cohen S. Bacon BR, Berlin JA, Fleischer D, Hecht GA, Loehrer PJ Sr, McNair AE Jr, Mulholland M, Norton NJ, Rabeneck L, Ransohoff DF, Sonnenberg A, Vannier MW. National Institutes of Health State-of-the-Science Conference Statement: ERCP for diagnosis and therapy, January 14-16, 2002. Gastrointest Endosc. 2002;56:803-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Brugge WR, Van Dam J. Pancreatic and biliary endoscopy. N Engl J Med. 1999;341:1808-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Elmunzer BJ. Reducing the risk of post-endoscopic retrograde cholangiopancreatography pancreatitis. Dig Endosc. 2017;29:749-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Freeman ML, DiSario JA, Nelson DB, Fennerty MB, Lee JG, Bjorkman DJ, Overby CS, Aas J, Ryan ME, Bochna GS, Shaw MJ, Snady HW, Erickson RV, Moore JP, Roel JP. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001;54:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 852] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 28. | Cheng CL, Sherman S, Watkins JL, Barnett J, Freeman M, Geenen J, Ryan M, Parker H, Frakes JT, Fogel EL, Silverman WB, Dua KS, Aliperti G, Yakshe P, Uzer M, Jones W, Goff J, Lazzell-Pannell L, Rashdan A, Temkit M, Lehman GA. Risk factors for post-ERCP pancreatitis: a prospective multicenter study. Am J Gastroenterol. 2006;101:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 438] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 29. | Zhou W, Li Y, Zhang Q, Li X, Meng W, Zhang L, Zhang H, Zhu K, Zhu X. Risk factors for postendoscopic retrograde cholangiopancreatography pancreatitis: a retrospective analysis of 7,168 cases. Pancreatology. 2011;11:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Cotton PB, Yaghoobi M, Cotton PB, Leung J. Adverse events: definitions, avoidance, and management. Cotton PB, Leung J. ERCP: The Fundamentals. Oxford: John Wiley Sons 2015; 371-396. |
| 31. | Freeman ML. Pancreatic stents for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis. Clin Gastroenterol Hepatol. 2007;5:1354-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 32. | Freeman ML. Adverse outcomes of ERCP. Gastrointest Endosc. 2002;56:S273-S282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 61] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Vandervoort J, Soetikno RM, Tham TC, Wong RC, Ferrari AP, Montes H, Roston AD, Slivka A, Lichtenstein DR, Ruymann FW, Van Dam J, Hughes M, Carr-Locke DL. Risk factors for complications after performance of ERCP. Gastrointest Endosc. 2002;56:652-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 146] [Article Influence: 6.1] [Reference Citation Analysis (4)] |
| 34. | Chen YK, Foliente RL, Santoro MJ, Walter MH, Collen MJ. Endoscopic sphincterotomy-induced pancreatitis: increased risk associated with nondilated bile ducts and sphincter of Oddi dysfunction. Am J Gastroenterol. 1994;89:327-333. [PubMed] |
| 35. | Freeman ML, Guda NM. Prevention of post-ERCP pancreatitis: a comprehensive review. Gastrointest Endosc. 2004;59:845-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 318] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 36. | Elmunzer BJ. Prevention of ERCP-induced Pancreatitis. The Pancreapedia: Exocrine Pancreas Knowledge Base 2015; . [DOI] [Full Text] |
| 37. | Elmunzer BJ, Waljee AK, Elta GH, Taylor JR, Fehmi SM, Higgins PD. A meta-analysis of rectal NSAIDs in the prevention of post-ERCP pancreatitis. Gut. 2008;57:1262-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 182] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 38. | Kochar B, Akshintala VS, Afghani E, Elmunzer BJ, Kim KJ, Lennon AM, Khashab MA, Kalloo AN, Singh VK. Incidence, severity, and mortality of post-ERCP pancreatitis: a systematic review by using randomized, controlled trials. Gastrointest Endosc. 2015;81:143-149.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 354] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 39. | Keswani RN, Taft TH, Coté GA, Keefer L. Increased levels of stress and burnout are related to decreased physician experience and to interventional gastroenterology career choice: findings from a US survey of endoscopists. Am J Gastroenterol. 2011;106:1734-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 40. | Cotton PB. Analysis of 59 ERCP lawsuits; mainly about indications. Gastrointest Endosc. 2006;63:378-82; quiz 464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 41. | Cotton PB, Cotton PB, Leung J. ERCP: Risks, Prevention, and Management. Cotton PB, Leung J. Advances Digestive Endoscopy: ERCP. Massachusetts: Blackwell Publishing 2005; 339-403. [DOI] [Full Text] |
| 42. | Parekh PJ, Majithia R, Sikka SK, Baron TH. The "Scope" of Post-ERCP Pancreatitis. Mayo Clin Proc. 2017;92:434-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 43. | Dumonceau JM, Andriulli A, Deviere J, Mariani A, Rigaux J, Baron TH, Testoni PA; European Society of Gastrointestinal Endoscopy. European Society of Gastrointestinal Endoscopy (ESGE) Guideline: prophylaxis of post-ERCP pancreatitis. Endoscopy. 2010;42:503-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 44. | Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1890] [Cited by in RCA: 2084] [Article Influence: 59.5] [Reference Citation Analysis (2)] |
| 45. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4699] [Article Influence: 361.5] [Reference Citation Analysis (48)] |
| 46. | Zhang H, Cho J, Buxbaum J. Update on the Prevention of Post-ERCP Pancreatitis. Curr Treat Options Gastroenterol. 2018;16:428-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Tse F, Liu L, Barkun AN, Armstrong D, Moayyedi P. EUS: a meta-analysis of test performance in suspected choledocholithiasis. Gastrointest Endosc. 2008;67:235-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 48. | Romagnuolo J, Bardou M, Rahme E, Joseph L, Reinhold C, Barkun AN. Magnetic resonance cholangiopancreatography: a meta-analysis of test performance in suspected biliary disease. Ann Intern Med. 2003;139:547-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 265] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 49. | Giljaca V, Gurusamy KS, Takwoingi Y, Higgie D, Poropat G, Štimac D, Davidson BR. Endoscopic ultrasound versus magnetic resonance cholangiopancreatography for common bile duct stones. Cochrane Database Syst Rev. 2015;CD011549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 50. | DiMagno MJ, Spaete JP, Ballard DD, Wamsteker EJ, Saini SD. Risk models for post-endoscopic retrograde cholangiopancreatography pancreatitis (PEP): smoking and chronic liver disease are predictors of protection against PEP. Pancreas. 2013;42:996-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Omar M, Ahmed A, Said O, El-Amin H. Risk factors for post-ERCP pancreatitis: a prospective multicenter study in upper Egypt. Egypt J Surg. 2015;34:1-10. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Testoni PA, Mariani A, Giussani A, Vailati C, Masci E, Macarri G, Ghezzo L, Familiari L, Giardullo N, Mutignani M, Lombardi G, Talamini G, Spadaccini A, Briglia R, Piazzi L; SEIFRED Group. Risk factors for post-ERCP pancreatitis in high- and low-volume centers and among expert and non-expert operators: a prospective multicenter study. Am J Gastroenterol. 2010;105:1753-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 210] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 53. | Lin Y, Liu X, Cao DQ, Tang JH, Wen JJ, Li TF, Liu PF, Xia TS. Analysis of risk factors and prevention strategies of post-ERCP pancreatitis. Eur Rev Med Pharmacol Sci. 2017;21:5185-5190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 54. | Srinivasan I, Freeman ML, Baron TH, Kozarek RA, Carr-Locke DL. Adverse Events of ERCP: Prediction, Prevention, and Management. Baron TH, Kozarek RA, Carr-Locke DL. ERCP. China: Elsevier 2019; 59-67. |
| 55. | Cotton PB, Garrow DA, Gallagher J, Romagnuolo J. Risk factors for complications after ERCP: a multivariate analysis of 11,497 procedures over 12 years. Gastrointest Endosc. 2009;70:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 485] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 56. | Andrade-Dávila VF, Chávez-Tostado M, Dávalos-Cobián C, García-Correa J, Montaño-Loza A, Fuentes-Orozco C, Macías-Amezcua MD, García-Rentería J, Rendón-Félix J, Cortés-Lares JA, Ambriz-González G, Cortés-Flores AO, Alvarez-Villaseñor Adel S, González-Ojeda A. Rectal indomethacin versus placebo to reduce the incidence of pancreatitis after endoscopic retrograde cholangiopancreatography: results of a controlled clinical trial. BMC Gastroenterol. 2015;15:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 57. | Cheon YK. Can postendoscopic retrograde cholangiopancreatography pancreatitis be prevented by a pharmacological approach? Korean J Intern Med. 2013;28:141-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 58. | Levenick JM, Gordon SR, Fadden LL, Levy LC, Rockacy MJ, Hyder SM, Lacy BE, Bensen SP, Parr DD, Gardner TB. Rectal Indomethacin Does Not Prevent Post-ERCP Pancreatitis in Consecutive Patients. Gastroenterology. 2016;150:911-7; quiz e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 153] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 59. | Dumonceau JM, Andriulli A, Elmunzer BJ, Mariani A, Meister T, Deviere J, Marek T, Baron TH, Hassan C, Testoni PA, Kapral C; European Society of Gastrointestinal Endoscopy. Prophylaxis of post-ERCP pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - updated June 2014. Endoscopy. 2014;46:799-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 418] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 60. | Elmunzer BJ, Scheiman JM, Lehman GA, Chak A, Mosler P, Higgins PD, Hayward RA, Romagnuolo J, Elta GH, Sherman S, Waljee AK, Repaka A, Atkinson MR, Cote GA, Kwon RS, McHenry L, Piraka CR, Wamsteker EJ, Watkins JL, Korsnes SJ, Schmidt SE, Turner SM, Nicholson S, Fogel EL; U. S. Cooperative for Outcomes Research in Endoscopy (USCORE). A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med. 2012;366:1414-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 529] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 61. | Patai Á. Solymosi N, Patai ÁV. Effect of rectal indomethacin for preventing post-ERCP pancreatitis depends on difficulties of cannulation: results from a randomized study with sequential biliary intubation. J Clin Gastroenterol. 2015;49:429-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 62. | Luo H, Zhao L, Leung J, Zhang R, Liu Z, Wang X, Wang B, Nie Z, Lei T, Li X, Zhou W, Zhang L, Wang Q, Li M, Zhou Y, Liu Q, Sun H, Wang Z, Liang S, Guo X, Tao Q, Wu K, Pan Y, Guo X, Fan D. Routine pre-procedural rectal indometacin versus selective post-procedural rectal indometacin to prevent pancreatitis in patients undergoing endoscopic retrograde cholangiopancreatography: a multicentre, single-blinded, randomised controlled trial. Lancet. 2016;387:2293-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 63. | Hosseini M, Shalchiantabrizi P, Yektaroudy K, Dadgarmoghaddam M, Salari M. Prophylactic Effect of Rectal Indomethacin Administration, with and without Intravenous Hydration, on Development of Endoscopic Retrograde Cholangiopancreatography Pancreatitis Episodes: A Randomized Clinical Trial. Arch Iran Med. 2016;19:538-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 64. | Mok SRS, Ho HC, Shah P, Patel M, Gaughan JP, Elfant AB. Lactated Ringer's solution in combination with rectal indomethacin for prevention of post-ERCP pancreatitis and readmission: a prospective randomized, double-blinded, placebo-controlled trial. Gastrointest Endosc. 2017;85:1005-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 65. | Döbrönte Z, Szepes Z, Izbéki F, Gervain J, Lakatos L, Pécsi G, Ihász M, Lakner L, Toldy E, Czakó L. Is rectal indomethacin effective in preventing of post-endoscopic retrograde cholangiopancreatography pancreatitis? World J Gastroenterol. 2014;20:10151-10157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 66. | Park SW, Chung MJ, Oh TG, Park JY, Bang S, Park SW, Song SY. Intramuscular diclofenac for the prevention of post-ERCP pancreatitis: a randomized trial. Endoscopy. 2015;47:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 67. | Zeitlinger M, Rusca A, Oraha AZ, Gugliotta B, Müller M, Ducharme MP. Pharmacokinetics of a new diclofenac sodium formulation developed for subcutaneous and intramuscular administration. Int J Clin Pharmacol Ther. 2012;50:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 68. | Otsuka T, Kawazoe S, Nakashita S, Kamachi S, Oeda S, Sumida C, Akiyama T, Ario K, Fujimoto M, Tabuchi M, Noda T. Low-dose rectal diclofenac for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a randomized controlled trial. J Gastroenterol. 2012;47:912-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 69. | Demols A, Deviere J. New frontiers in the pharmacological prevention of post-ERCP pancreatitis: the cytokines. JOP. 2003;4:49-57. [PubMed] |
| 70. | Bhatia V, Ahuja V, Acharya SK, Garg PK. A randomized controlled trial of valdecoxib and glyceryl trinitrate for the prevention of post-ERCP pancreatitis. J Clin Gastroenterol. 2011;45:170-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 71. | Ethridge RT, Chung DH, Slogoff M, Ehlers RA, Hellmich MR, Rajaraman S, Saito H, Uchida T, Evers BM. Cyclooxygenase-2 gene disruption attenuates the severity of acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 2002;123:1311-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 72. | Insel P, Hardman J, Limbird L. Analgesic-antipyretic and antiinflammatory agents and drugs employed in the treatment of gout. Hardman J, Limbird L. The pharmacological basis of therapeutics. New York: Mc-graw hill 1996; 617–657. |
| 73. | de Quadros Onófrio F, Lima JCP, Watte G, Lehmen RL, Oba D, Camargo G, Dos Santos CEO. Prophylaxis of pancreatitis with intravenous ketoprofen in a consecutive population of ERCP patients: a randomized double-blind placebo-controlled trial. Surg Endosc. 2017;31:2317-2324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 74. | Gross V, Leser HG, Heinisch A, Schölmerich J. Inflammatory mediators and cytokines--new aspects of the pathophysiology and assessment of severity of acute pancreatitis? Hepatogastroenterology. 1993;40:522-530. [PubMed] |
| 75. | Whitcomb DC. Acute pancreatitis: molecular biology update. J Gastrointest Surg. 2003;7:940-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 76. | Mäkelä A, Kuusi T, Schröder T. Inhibition of serum phospholipase-A2 in acute pancreatitis by pharmacological agents in vitro. Scand J Clin Lab Invest. 1997;57:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 112] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 77. | Kato K, Shiba M, Kakiya Y, Maruyama H, Ominami M, Fukunaga S, Sugimori S, Nagami Y, Watanabe T, Tominaga K, Fujiwara Y. Celecoxib Oral Administration for Prevention of Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis: A Randomized Prospective Trial. Pancreas. 2017;46:880-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 78. | Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1175] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 79. | Kusterer K, Enghofer M, Zendler S, Blöchle C, Usadel KH. Microcirculatory changes in sodium taurocholate-induced pancreatitis in rats. Am J Physiol. 1991;260:G346-G351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 80. | Muddana V, Whitcomb DC, Khalid A, Slivka A, Papachristou GI. Elevated serum creatinine as a marker of pancreatic necrosis in acute pancreatitis. Am J Gastroenterol. 2009;104:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 81. | Wu BU, Hwang JQ, Gardner TH, Repas K, Delee R, Yu S, Smith B, Banks PA, Conwell DL. Lactated Ringer's solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9:710-717.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 350] [Article Influence: 23.3] [Reference Citation Analysis (1)] |
| 82. | Seyama Y, Otani T, Matsukura A, Makuuchi M. The pH modulator chloroquine blocks trypsinogen activation peptide generation in cerulein-induced pancreatitis. Pancreas. 2003;26:15-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 83. | Buxbaum J, Yan A, Yeh K, Lane C, Nguyen N, Laine L. Aggressive hydration with lactated Ringer's solution reduces pancreatitis after endoscopic retrograde cholangiopancreatography. Clin Gastroenterol Hepatol. 2014;12:303-7.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 84. | Gardner TB, Vege SS, Chari ST, Petersen BT, Topazian MD, Clain JE, Pearson RK, Levy MJ, Sarr MG. Faster rate of initial fluid resuscitation in severe acute pancreatitis diminishes in-hospital mortality. Pancreatology. 2009;9:770-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 85. | Noble MD, Romac J, Vigna SR, Liddle RA. A pH-sensitive, neurogenic pathway mediates disease severity in a model of post-ERCP pancreatitis. Gut. 2008;57:1566-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 86. | Bhoomagoud M, Jung T, Atladottir J, Kolodecik TR, Shugrue C, Chaudhuri A, Thrower EC, Gorelick FS. Reducing extracellular pH sensitizes the acinar cell to secretagogue-induced pancreatitis responses in rats. Gastroenterology. 2009;137:1083-1092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 87. | Choi JH, Kim HJ, Lee BU, Kim TH, Song IH. Vigorous Periprocedural Hydration With Lactated Ringer's Solution Reduces the Risk of Pancreatitis After Retrograde Cholangiopancreatography in Hospitalized Patients. Clin Gastroenterol Hepatol. 2017;15:86-92.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 88. | Sherman S, Cheng CL, Costamagna G, Binmoeller KF, Puespoek A, Aithal GP, Kozarek RA, Chen YK, Van Steenbergen W, Tenner S, Freeman M, Monroe P, Geffner M, Deviere J; Interleukin-10 ERCP Study Group. Efficacy of recombinant human interleukin-10 in prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis in subjects with increased risk. Pancreas. 2009;38:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 89. | Rongione AJ, Kusske AM, Kwan K, Ashley SW, Reber HA, McFadden DW. Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology. 1997;112:960-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 229] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 90. | Van Laethem JL, Marchant A, Delvaux A, Goldman M, Robberecht P, Velu T, Devière J. Interleukin 10 prevents necrosis in murine experimental acute pancreatitis. Gastroenterology. 1995;108:1917-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 165] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 91. | Sherman S, Alazmi WM, Lehman GA, Geenen JE, Chuttani R, Kozarek RA, Welch WD, Souza S, Pribble J; rPAF-AH ERCP Study Group. Evaluation of recombinant platelet-activating factor acetylhydrolase for reducing the incidence and severity of post-ERCP acute pancreatitis. Gastrointest Endosc. 2009;69:462-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 92. | Jaworek J, Konturek SJ. Effects of prostaglandin of E, F and I series, leukotriene C and platelet activating factor on amylase release from isolated rat pancreatic acini. J Physiol Pharmacol. 1993;44:365-381. [PubMed] |
| 93. | Hofbauer B, Saluja AK, Bhatia M, Frossard JL, Lee HS, Bhagat L, Steer ML. Effect of recombinant platelet-activating factor acetylhydrolase on two models of experimental acute pancreatitis. Gastroenterology. 1998;115:1238-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 94. | Mariani A, Giussani A, Di Leo M, Testoni S, Testoni PA. Guidewire biliary cannulation does not reduce post-ERCP pancreatitis compared with the contrast injection technique in low-risk and high-risk patients. Gastrointest Endosc. 2012;75:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 95. | Devereaux BM, Fein S, Purich E, Trout JR, Lehman GA, Fogel EL, Phillips S, Etemad R, Jowell P, Toskes PP, Sherman S. A new synthetic porcine secretin for facilitation of cannulation of the dorsal pancreatic duct at ERCP in patients with pancreas divisum: a multicenter, randomized, double-blind comparative study. Gastrointest Endosc. 2003;57:643-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 96. | Jowell PS, Branch MS, Fein SH, Purich ED, Kilaru R, Robuck G, d'Almada P, Baillie J. Intravenous synthetic secretin reduces the incidence of pancreatitis induced by endoscopic retrograde cholangiopancreatography. Pancreas. 2011;40:533-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 97. | Fang J, Wang SL, Zhao SB, Hu LH, Yao J, Shen Z, Gu L, Xia T, Cai Q, Li ZS, Bai Y. Impact of intraduodenal acetic acid infusion on pancreatic duct cannulation during endoscopic retrograde cholangiopancreatography: A double-blind, randomized controlled trial. J Gastroenterol Hepatol. 2018;33:1804-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 98. | Brodows RG, Chey WY. Physiological doses of secretin do not stimulate acute insulin release. J Clin Endocrinol Metab. 1980;50:603-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 99. | Kilander AF, Hanssen LE, Gillberg RE. Secretin release in coeliac disease. Plasma secretin concentration and bicarbonate output to the duodenum after intraduodenal acid infusion in coeliac patients before and after treatment. Scand J Gastroenterol. 1983;18:765-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 100. | Nøjgaard C, Hornum M, Elkjaer M, Hjalmarsson C, Heyries L, Hauge T, Bakkevold K, Andersen PK, Matzen P; European Post-ERCP Pancreatitis Preventing Study Group. Does glyceryl nitrate prevent post-ERCP pancreatitis? A prospective, randomized, double-blind, placebo-controlled multicenter trial. Gastrointest Endosc. 2009;69:e31-e37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 101. | Staritz M, Poralla T, Ewe K, Meyer zum Büschenfelde KH. Effect of glyceryl trinitrate on the sphincter of Oddi motility and baseline pressure. Gut. 1985;26:194-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 102. | Kaufman HS, Shermak MA, May CA, Pitt HA, Lillemoe KD. Nitric oxide inhibits resting sphincter of Oddi activity. Am J Surg. 1993;165:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 103. | Luman W, Pryde A, Heading RC, Palmer KR. Topical glyceryl trinitrate relaxes the sphincter of Oddi. Gut. 1997;40:541-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 104. | Carr-Locke DL, Gregg JA, Aoki TT. Effects of exogenous glucagon on pancreatic and biliary ductal and sphincteric pressures in man demonstrated by endoscopic manometry and correlation with plasma glucagon. Dig Dis Sci. 1983;28:312-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 105. | Lahoti S, Catalano MF, Geenen JE, Hogan WJ. A prospective, double-blind trial of L-hyoscyamine versus glucagon for the inhibition of small intestinal motility during ERCP. Gastrointest Endosc. 1997;46:139-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 106. | Katsinelos P, Lazaraki G, Chatzimavroudis G, Katsinelos T, Georgakis N, Anastasiadou K, Gatopoulou A, Zeglinas C, Psarras K, Kountouras J. Impact of nitroglycerin and glucagon administration on selective common bile duct cannulation and prevention of post-ERCP pancreatitis. Scand J Gastroenterol. 2017;52:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 107. | Oh HC, Cheon YK, Cho YD, Do JH. Use of udenafil is not associated with a reduction in post-ERCP pancreatitis: results of a randomized, placebo-controlled, multicenter trial. Gastrointest Endosc. 2011;74:556-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 108. | Cheon YK, Cho YD, Moon JH, Im HH, Jung Y, Lee JS, Lee MS, Shim CS. Effects of vardenafil, a phosphodiesterase type-5 inhibitor, on sphincter of Oddi motility in patients with suspected biliary sphincter of Oddi dysfunction. Gastrointest Endosc. 2009;69:1111-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 109. | Lee TY, Choi JS, Oh HC, Song TJ, Do JH, Cheon YK. Oral udenafil and aceclofenac for the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis in high-risk patients: a randomized multicenter study. Korean J Intern Med. 2015;30:602-609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 110. | Kubiliun NM, Adams MA, Akshintala VS, Conte ML, Cote GA, Cotton PB, Dumonceau JM, Elta GH, Fogel EL, Freeman ML, Lehman GA, Naveed M, Romagnuolo J, Scheiman JM, Sherman S, Singh VK, Elmunzer BJ; United States Cooperative for Outcomes Research in Endoscopy (USCORE). Evaluation of Pharmacologic Prevention of Pancreatitis After Endoscopic Retrograde Cholangiopancreatography: A Systematic Review. Clin Gastroenterol Hepatol. 2015;13:1231-9; quiz e70-1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 111. | Fujii S, Hitomi Y. New synthetic inhibitors of C1r, C1 esterase, thrombin, plasmin, kallikrein and trypsin. Biochim Biophys Acta. 1981;661:342-345. [PubMed] |
| 112. | Keck T, Balcom JH, Antoniu BA, Lewandrowski K, Warshaw AL, Fernández-del Castillo CF. Regional effects of nafamostat, a novel potent protease and complement inhibitor, on severe necrotizing pancreatitis. Surgery. 2001;130:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 113. | Masci E, Cavallini G, Mariani A, Frulloni L, Testoni PA, Curioni S, Tittobello A, Uomo G, Costamagna G, Zambelli S, Macarri G, Innocenti P, Dragonetti C, Gabexate in Digestive Endoscopy-Italian Group II. Comparison of two dosing regimens of gabexate in the prophylaxis of post-ERCP pancreatitis. Am J Gastroenterol. 2003;98:2182-2186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 114. | Ohuchida J, Chijiiwa K, Imamura N, Nagano M, Hiyoshi M. Randomized controlled trial for efficacy of nafamostat mesilate in preventing post-endoscopic retrograde cholangiopancreatography pancreatitis. Pancreas. 2015;44:415-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 115. | Park KT, Kang DH, Choi CW, Cho M, Park SB, Kim HW, Kim DU, Chung CW, Yoon KT. Is high-dose nafamostat mesilate effective for the prevention of post-ERCP pancreatitis, especially in high-risk patients? Pancreas. 2011;40:1215-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 116. | Choi CW, Kang DH, Kim GH, Eum JS, Lee SM, Song GA, Kim DU, Kim ID, Cho M. Nafamostat mesylate in the prevention of post-ERCP pancreatitis and risk factors for post-ERCP pancreatitis. Gastrointest Endosc. 2009;69:e11-e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 117. | Yoo KS, Huh KR, Kim YJ, Kim KO, Park CH, Hahn T, Park SH, Kim JH, Park CK, Kwon YJ, Lehman GA. Nafamostat mesilate for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a prospective, randomized, double-blind, controlled trial. Pancreas. 2011;40:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 118. | Kim SJ, Kang DH, Kim HW, Choi CW, Park SB, Song BJ, Nam HS. A Randomized Comparative Study of 24- and 6-Hour Infusion of Nafamostat Mesilate for the Prevention of Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis: A Prospective Randomized Comparison Trial. Pancreas. 2016;45:1179-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 119. | Yoo JW, Ryu JK, Lee SH, Woo SM, Park JK, Yoon WJ, Lee JK, Lee KH, Hwang JH, Kim YT, Yoon YB. Preventive effects of ulinastatin on post-endoscopic retrograde cholangiopancreatography pancreatitis in high-risk patients: a prospective, randomized, placebo-controlled trial. Pancreas. 2008;37:366-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 120. | Chen CC, Wang SS, Lee FY. Action of antiproteases on the inflammatory response in acute pancreatitis. JOP. 2007;8:488-494. [PubMed] |
| 121. | Park JY, Jeon TJ, Hwang MW, Sinn DH, Oh TH, Shin WC, Choi WC. Comparison between ulinastatin and nafamostat for prevention of post-endoscopic retrograde cholangiopancreatography complications: a prospective, randomized trial. Pancreatology. 2014;14:263-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 122. | Oguchi H, Terashima M, Koiwai T, Kawa S, Furuta S, Kobayashi M, Homma T. Effects of the S2-serotonergic receptor antagonist, ketanserin, on cerulein-induced pancreatitis in the rat. Life Sci. 1992;50:733-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 123. | Yoshino T, Yamaguchi I. Possible involvement of 5-HT2 receptor activation in aggravation of diet-induced acute pancreatitis in mice. J Pharmacol Exp Ther. 1997;283:1495-1502. [PubMed] |
| 124. | Ogawa T, Sugidachi A, Tanaka N, Fujimoto K, Fukushige J, Tani Y, Asai F. Effects of R-102444 and its active metabolite R-96544, selective 5-HT2A receptor antagonists, on experimental acute and chronic pancreatitis: Additional evidence for possible involvement of 5-HT2A receptors in the development of experimental pancreatitis. Eur J Pharmacol. 2005;521:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 125. | Tsujino T, Isayama H, Nakai Y, Ito Y, Togawa O, Toda N, Arizumi T, Kogure H, Yamamoto K, Mizuno S, Yashima Y, Yagioka H, Sasaki T, Matsubara S, Yamamoto N, Hirano K, Sasahira N, Tada M, Koike K. The results of the Tokyo trial of prevention of post-ERCP pancreatitis with risperidone (Tokyo P3R): a multicenter, randomized, phase II, non-placebo-controlled trial. J Gastroenterol. 2013;48:982-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 126. | Hamada K, Yoshida M, Isayama H, Yagi Y, Kanazashi S, Kashihara Y, Takeuchi K, Yamaguchi I. Possible involvement of endogenous 5-HT in aggravation of cerulein-induced acute pancreatitis in mice. J Pharmacol Sci. 2007;105:240-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 127. | Bai Y, Ren X, Zhang XF, Lv NH, Guo XG, Wan XJ, Nie ZG, Han ST, Bie P, Tian DA, Ji M, Li ZS. Prophylactic somatostatin can reduce incidence of post-ERCP pancreatitis: multicenter randomized controlled trial. Endoscopy. 2015;47:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 128. | Abdel Aziz AM, Lehman GA. Pancreatitis after endoscopic retrograde cholangio-pancreatography. World J Gastroenterol. 2007;13:2655-2668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 129. | Concepción-Martín M, Gómez-Oliva C, Juanes A, Díez X, Prieto-Alhambra D, Torras X, Sainz S, Villanueva C, Farre A, Guarner-Argente C, Guarner C. Somatostatin for prevention of post-ERCP pancreatitis: a randomized, double-blind trial. Endoscopy. 2014;46:851-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 130. | Wang ZK, Yang YS, Cai FC, Wang YH, Shi XL, Ding C, Li W. Is prophylactic somatostatin effective to prevent post-endoscopic retrograde cholangiopancreatography pancreatitis or hyperamylasemia? A randomized, placebo-controlled pilot trial. Chin Med J (Engl). 2013;126:2403-2408. [PubMed] |
| 131. | Li ZF, Xia XM, Huang C, Zhang S, Zhang J, Zhang AJ. Emodin and baicalein inhibit pancreatic stromal derived factor-1 expression in rats with acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2009;8:201-208. [PubMed] |
| 132. | Xia XM, Wang FY, Wang ZK, Wan HJ, Xu WA, Lu H. Emodin enhances alveolar epithelial barrier function in rats with experimental acute pancreatitis. World J Gastroenterol. 2010;16:2994-3001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 133. | Wang C, Li Q, Ye P, Zeng S, Li GH, Chen YX, Zhou XJ, Lv NH. Value of Raw Rhubarb Solution in the Precaution of Post-endoscopic Retrograde Cholangiopancreatography Pancreatitis in Patients with High-Risk Factors: A Predictive Random Compared Research in One Center. Dig Dis Sci. 2017;62:1043-1050. [PubMed] |
| 134. | Nordback IH, Cameron JL. The mechanism of conversion of xanthine dehydrogenase to xanthine oxidase in acute pancreatitis in the canine isolated pancreas preparation. Surgery. 1993;113:90-97. [PubMed] |
| 135. | Abbasinazari M, Mohammad Alizadeh AH, Moshiri K, Pourhoseingholi MA, Zali MR. Does allopurinol prevent post endoscopic retrograde cholangio- pancreatography pancreatitis? A randomized double blind trial. Acta Med Iran. 2011;49:579-583. [PubMed] |
| 136. | Shah TU, Liddle R, Branch MS, Jowell P, Obando J, Poleski M. Pilot study of aprepitant for prevention of post-ERCP pancreatitis in high risk patients: a phase II randomized, double-blind placebo controlled trial. JOP. 2012;13:514-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 137. | Nathan JD, Patel AA, McVey DC, Thomas JE, Prpic V, Vigna SR, Liddle RA. Capsaicin vanilloid receptor-1 mediates substance P release in experimental pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1322-G1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 138. | Nathan JD, Peng RY, Wang Y, McVey DC, Vigna SR, Liddle RA. Primary sensory neurons: a common final pathway for inflammation in experimental pancreatitis in rats. Am J Physiol Gastrointest Liver Physiol. 2002;283:G938-G946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 139. | Bhatia M, Saluja AK, Hofbauer B, Frossard JL, Lee HS, Castagliuolo I, Wang CC, Gerard N, Pothoulakis C, Steer ML. Role of substance P and the neurokinin 1 receptor in acute pancreatitis and pancreatitis-associated lung injury. Proc Natl Acad Sci U S A. 1998;95:4760-4765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 241] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 140. | Baluk P, Bertrand C, Geppetti P, McDonald DM, Nadel JA. NK1 receptors mediate leukocyte adhesion in neurogenic inflammation in the rat trachea. Am J Physiol. 1995;268:L263-L269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 141. | He ZJ, Winston JH, Yusuf TE, Micci MA, Elfert A, Xiao SY, Pasricha PJ. Intraductal administration of an NK1 receptor antagonist attenuates the inflammatory response to retrograde infusion of radiological contrast in rats: implications for the pathogenesis and prevention of ERCP-induced pancreatitis. Pancreas. 2003;27:e13-e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 142. | Ohashi A, Tamada K, Tomiyama T, Wada S, Higashizawa T, Gotoh Y, Satoh Y, Miyata T, Tano S, Ido K, Sugano K. Epinephrine irrigation for the prevention of pancreatic damage after endoscopic balloon sphincteroplasty. J Gastroenterol Hepatol. 2001;16:568-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 143. | Xu LH, Qian JB, Gu LG, Qiu JW, Ge ZM, Lu F, Wang YM, Li YM, Lu HS. Prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis by epinephrine sprayed on the papilla. J Gastroenterol Hepatol. 2011;26:1139-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 144. | Ohno T, Katori M, Nishiyama K, Saigenji K. Direct observation of microcirculation of the basal region of rat gastric mucosa. J Gastroenterol. 1995;30:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 145. | Xu XD, Dai JJ, Qian JQ, Wang WJ. Nasobiliary drainage after endoscopic papillary balloon dilatation may prevent postoperative pancreatitis. World J Gastroenterol. 2015;21:2443-2449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 146. | Ricci E, Conigliaro R, Bertoni G, Mortilla MG, Bedogni G, Contini S. Nasobiliary drainage following endoscopic sphincterotomy. A useful method of preventing and treating early complications. Surg Endosc. 1987;1:147-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 147. | Lee DW, Chan AC, Lam YH, Ng EK, Lau JY, Law BK, Lai CW, Sung JJ, Chung SC. Biliary decompression by nasobiliary catheter or biliary stent in acute suppurative cholangitis: a prospective randomized trial. Gastrointest Endosc. 2002;56:361-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 148. | Sharma BC, Kumar R, Agarwal N, Sarin SK. Endoscopic biliary drainage by nasobiliary drain or by stent placement in patients with acute cholangitis. Endoscopy. 2005;37:439-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 149. | Park SY, Park CH, Cho SB, Yoon KW, Lee WS, Kim HS, Choi SK, Rew JS. The safety and effectiveness of endoscopic biliary decompression by plastic stent placement in acute suppurative cholangitis compared with nasobiliary drainage. Gastrointest Endosc. 2008;68:1076-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 150. | Huang Q, Shao F, Wang C, Qi W, Qiu LJ, Liu Z. Nasobiliary drainage can reduce the incidence of post-ERCP pancreatitis after papillary large balloon dilation plus endoscopic biliary sphincterotomy: a randomized controlled trial. Scand J Gastroenterol. 2018;53:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 151. | Lee TH, Moon JH, Choi HJ, Han SH, Cheon YK, Cho YD, Park SH, Kim SJ. Prophylactic temporary 3F pancreatic duct stent to prevent post-ERCP pancreatitis in patients with a difficult biliary cannulation: a multicenter, prospective, randomized study. Gastrointest Endosc. 2012;76:578-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 152. | Sofuni A, Maguchi H, Mukai T, Kawakami H, Irisawa A, Kubota K, Okaniwa S, Kikuyama M, Kutsumi H, Hanada K, Ueki T, Itoi T. Endoscopic pancreatic duct stents reduce the incidence of post-endoscopic retrograde cholangiopancreatography pancreatitis in high-risk patients. Clin Gastroenterol Hepatol. 2011;9:851-8; quiz e110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 153. | Pan XP, Dang T, Meng XM, Xue KC, Chang ZH, Zhang YP. Clinical study on the prevention of post-ERCP pancreatitis by pancreatic duct stenting. Cell Biochem Biophys. 2011;61:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 154. | Ito K, Fujita N, Noda Y, Kobayashi G, Obana T, Horaguchi J, Takasawa O, Koshita S, Kanno Y, Ogawa T. Can pancreatic duct stenting prevent post-ERCP pancreatitis in patients who undergo pancreatic duct guidewire placement for achieving selective biliary cannulation? A prospective randomized controlled trial. J Gastroenterol. 2010;45:1183-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 155. | Kawaguchi Y, Ogawa M, Omata F, Ito H, Shimosegawa T, Mine T. Randomized controlled trial of pancreatic stenting to prevent pancreatitis after endoscopic retrograde cholangiopancreatography. World J Gastroenterol. 2012;18:1635-1641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 156. | Fujisawa T, Kagawa K, Ochiai K, Hisatomi K, Kubota K, Sato H, Nakajima A, Matsuhashi N. Prophylactic Efficacy of 3- or 5-cm Pancreatic Stents for Preventing Post-ERCP Pancreatitis: A Prospective, Randomized Trial. J Clin Gastroenterol. 2016;50:e30-e34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 157. | Conigliaro R, Manta R, Bertani H, Manno M, Barbera C, Caruso A, Olivetti G, Melotti G, Frazzoni M. Pancreatic duct stenting for the duration of ERCP only does not prevent pancreatitis after accidental pancreatic duct cannulation: a prospective randomized trial. Surg Endosc. 2013;27:569-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 158. | Kobayashi G, Fujita N, Imaizumi K, Irisawa A, Suzuki M, Murakami A, Oana S, Makino N, Komatsuda T, Yoneyama K. Wire-guided biliary cannulation technique does not reduce the risk of post-ERCP pancreatitis: multicenter randomized controlled trial. Dig Endosc. 2013;25:295-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 159. | Lella F, Bagnolo F, Colombo E, Bonassi U. A simple way of avoiding post-ERCP pancreatitis. Gastrointest Endosc. 2004;59:830-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 129] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 160. | Artifon EL, Sakai P, Cunha JE, Halwan B, Ishioka S, Kumar A. Guidewire cannulation reduces risk of post-ERCP pancreatitis and facilitates bile duct cannulation. Am J Gastroenterol. 2007;102:2147-2153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 161. | Lee TH, Park DH, Park JY, Kim EO, Lee YS, Park JH, Lee SH, Chung IK, Kim HS, Park SH, Kim SJ. Can wire-guided cannulation prevent post-ERCP pancreatitis? A prospective randomized trial. Gastrointest Endosc. 2009;69:444-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 162. | Bassan MS, Sundaralingam P, Fanning SB, Lau J, Menon J, Ong E, Rerknimitr R, Seo DW, Teo EK, Wang HP, Reddy DN, Goh KL, Bourke MJ. The impact of wire caliber on ERCP outcomes: a multicenter randomized controlled trial of 0.025-inch and 0.035-inch guidewires. Gastrointest Endosc. 2018;87:1454-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 163. | Lee A, Lee LS. Training in Endoscopic Retrograde Cholangiopancreatography. Lee LS, editor ERCP and EUS: A Case-Based Approach. New York: Springer 2015; 3-18. |
| 164. | Swan MP, Alexander S, Moss A, Williams SJ, Ruppin D, Hope R, Bourke MJ. Needle knife sphincterotomy does not increase the risk of pancreatitis in patients with difficult biliary cannulation. Clin Gastroenterol Hepatol. 2013;11:430-436.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 165. | Ilone S, Fauzi A. Diagnostic and Prevention Approach in Post Endoscopic Retrograde Cholangiopancreatography Pancreatitis. Indones J Gastroenterol Hepatol Digest Endosc. 2016;17:188-193. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
Conflict of interest statement: No potential conflict of interest.
PRISMA 2009 checklist statement: The author has read the PRISMA 2009 Checklist, and the paper was written and revised in accordance with the PRISMA 2009 Checklist.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Farhat S, Huan C, Manenti A, Yu XJ, Zhang ZH S-Editor: Gong ZM L-Editor: A E-Editor: Ma YJ