Published online Aug 7, 2019. doi: 10.3748/wjg.v25.i29.3929
Peer-review started: March 28, 2019
First decision: April 16, 2019
Revised: May 29, 2019
Accepted: July 2, 2019
Article in press: July 3, 2019
Published online: August 7, 2019
Processing time: 133 Days and 12.1 Hours
Hepatocellular carcinoma (HCC) is a common and deadly malignancy. The disease usually develops on a background of chronic liver disease. Until recently, the most common etiology was infection with the hepatitis C virus (HCV). The advent of direct-acting antiviral (DAA) therapies has been a major breakthrough in HCV treatment. Sustained virologic response can now be achieved in almost all treated patients, even in patients with a high risk for the development of HCC, such as the elderly or those with significant fibrosis. Early reports raised concerns of a high risk for HCC occurrence after DAA therapy both in patients with previous resection of tumors and those without previous tumors. As the World Health Organization’s goals for eradication of HCV are being endorsed worldwide, the elimination of HCV seems feasible. Simultaneous to the decrease in the burden of cirrhosis from HCV, non-alcoholic fatty liver disease (NAFLD) incidence has been increasing dramatically including significant increased incidence of cirrhosis and HCC in these patients. Surprisingly, a substantial proportion of patients with NAFLD were shown to develop HCC even in the absence of cirrhosis. Furthermore, HCC treatment and potential complications are known to be influenced by liver steatosis. These changes in etiology and epidemiology of HCC suggest the beginning of a new era: The post–HCV era. Changes may eventually undermine current practices of early detection, surveillance and management of HCC. We focused on the risk of HCC occurrence and recurrence in the post–HCV era, the surveillance needed after DAA therapy and current studies in HCC patients with NAFLD.
Core tip: Hepatocellular carcinoma (HCC) is a common and deadly malignancy. One of the leading risk factors for HCC occurrence is liver cirrhosis secondary to hepatitis C virus (HCV) infection. Direct-acting antiviral therapy has revolutionized HCV eradication due to high sustained virologic response rates. However, early reports argued an increased risk of HCC occurrence and recurrence. Recently, non-alcoholic fatty liver disease has become the most common liver disorder in Western countries and a major cause of HCC. We aimed to review the changes in HCC management in the face of the changing epidemiology in the post-HCV era.
- Citation: Meringer H, Shibolet O, Deutsch L. Hepatocellular carcinoma in the post-hepatitis C virus era: Should we change the paradigm? World J Gastroenterol 2019; 25(29): 3929-3940
- URL: https://www.wjgnet.com/1007-9327/full/v25/i29/3929.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i29.3929
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the second most frequent cause of cancer-related death globally[1]. The incidence of HCC in all populations increases progressively with age, reaching a peak in the eight's decade[2]. Cirrhosis is a risk factor for tumor development regardless of its etiology[3]. One of the most common risk factors for HCC worldwide is cirrhosis secondary to chronic infection with either hepatitis C virus (HCV) or hepatitis B virus (HBV)[1]. The incidence of HCC shows high geographical divergence as most cases in Asia and Africa are attributable to HBV while HCV represents a major risk factor in western countries. The annual risk of HCC is as high as 3% in patients with cirrhosis and active HCV infection[4]. Direct-acting antiviral (DAA) therapy has revolutionized the treatment of HCV infection, because of its high efficacy and an excellent safety profile which enabled its use even in patients with decompensated liver disease, in whom interferon (IFN)-based regimens were not recommended[5]. The introduction of DAA agents has improved sustained virologic response (SVR) rates to more than 95% in all HCV genotypes and shortened treatment duration[6]. DAAs have shown high efficacy and safety even in special populations, as patients with human immunodeficiency virus coinfection, dialysis patients and patients with recurrent HCV infection after liver transplantation (LT)[5,7]. SVR reduces patients’ risk of developing liver cirrhosis and was shown to cause regression of fibrosis[8,9]. In patients with decompensated cirrhosis that achieved SVR, reduction in Model for End-Stage Liver Disease scores and hepatic venous pressure gradient were observed[10-12]. Recent data has also shown that SVR reduces liver specific and all-cause mortality[13]. The WHO’s goal to eradicate HCV might induce HCC risk reduction by preventing advancement of cirrhosis, allowing fibrosis regression and avoiding the carcinogenic effect of the virus[14,15]. However, early reports argued an increased risk of HCC occurrence and recurrence in patients achieving SVR[16-18]. In addition, financial resources needed for both simultaneously scaling up coverage of testing services and costs of therapy are major limitations, especially in resource limited countries[19].
Since 2014, the use of DAAs has decreased the burden of chronic HCV. Nonetheless, this decrease has been countered by a marked increase in the prevalence of nonalcoholic fatty liver disease (NAFLD)[20]. It is currently the second leading cause for LT and waitlist registration in males and females[21]. We aimed to review the changes in HCC management in the face of the changing epidemiology brought about by the advances in HCV therapy and the rise in incidence of NAFLD.
The introduction of DAA therapy led to short and long-term clinical benefits as a result of HCV elimination[14]. Previous prospective studies with IFN-based therapy concluded that treatment was strongly associated with a reduction in HCC risk[22-24]. A meta-analysis of 12 studies quantitatively evaluated the presumed benefit and showed that achieving an SVR with IFN was associated with a 76% reduction of HCC risk[22]. However, early studies of DAAs raised concerns that DAA-induced SVR didn't reduce occurrence of HCC and suggested a high risk of short-term recurrence in patients previously treated for HCC[16-18]. Conti et al[17] followed 344 consecutive cirrhotic patients, without HCC, who were treated with DAA for 24 wk and reported HCC occurrence in 9/285 patients (3.2%) and HCC recurrence in 17/59 patients (28.8%) previously treated for HCC. Child-Pugh class and a history of HCC were independently associated with HCC development but neither HCV genotype nor therapeutic DAA regimen correlated to HCC occurrence. Additional reports suggested an alarmingly high rates of HCC occurrence with de-novo HCC diagnosis in 6/66 patients (9%) within 6 months of DAA therapy[16] and 4/54 patients (7.4%) after a median follow-up of 12 mo[18]. These studies were small, single-centered, uncontrolled, retrospective cohorts without long term follow up period which precluded definite conclusions. In contrast, multiple large cohort studies have since demonstrated that DAA-induced SVR is associated with reduced risk of HCC occurrence[25-27]. Among 22500 patients treated with DAA in the national Veterans Health Administration system, there were 271 new cases of HCC which developed after DAA treatment, including 183 in patients with SVR[25]. Additional 79/22579 (0.34%) cases developed during the course of DAA treatment and were excluded from primary analysis. The risk for HCC was higher in patients with cirrhosis than non-cirrhotics [adjusted hazard ratio (HR) = 4.73; 95% confidence interval (CI), 3.34-6.68] and SVR was associated with a 76% reduction in the risk of HCC compared with those who did not achieve SVR. Moreover, HCCs that were diagnosed during treatment were not more aggressive than those that occurred after the end of treatment. In a retrospective study by Ioannou et al[17] more than 60000 United States veterans with HCV that were treated with antiviral therapy between 1999-2015 including therapy with DAAs, IFN-based regimens or combined regimens were assessed[27]. HCC cases diagnosed within 6 months of treatment initiation were excluded. After adjustment to baseline characteristics, patients with DAA-induced SVR showed a 71% reduction in the risk for HCC compared to DAA-treatment failures. Furthermore, the reduction in HCC risk associated with SVR was similar irrespective of whether SVR was achieved by DAA-only, IFN alone or combined regimens, suggesting that eradication of HCV reduces the risk of HCC regardless of the antiviral regimen. A systematic meta-analysis of observational studies including 26 studies on HCC occurrence (IFN = 17, DAA = 9) reported higher HCC incidence in patients with DAA induced SVR than after IFN induced SVR (2.96/100 patient years and 1.14/100 patient years, respectively) but patients treated with DAAs were older and had a shorter follow-up[28]. In a meta-regression, after adjustment for study follow-up and age, DAA therapy was not associated with higher HCC occurrence compared to IFN. Additionally, a large national cohort of 17836 HCV-infected United States veterans (ERCHIVES database), compared DAA treated patients to IFN treated patients and untreated patients[26]. DAA-treated patients had a significantly higher HCC incidence rate than IFN treated patients but they also had a significantly higher rate of known risk factors for HCC, including cirrhosis, older age, and higher baseline Alfa-Feto protein level. A sub-analysis in cirrhotic patients (baseline FIB-4 score > 3.5) who achieved SVR, showed no significant difference in HCC incidence rate between the DAAs and IFN-treated groups (22.8 vs 21.2 cases per 1000 person-years, P = 0.7). Moreover, untreated cirrhotics had a twofold higher incidence rate than both treatment groups (45.31 cases per 1000 person-years, P = 0.03). Mariño et al[29] reported a 3.73% per 1000 person-years risk of developing HCC in 1123 cirrhotic patients treated with DAA during a median clinical follow-up of 19.6 mo. In agreement with results from the veterans' cohorts, the risk was higher in patients without SVR than those achieving SVR and with more severe disease (Child B or C, high liver stiffness measurement, the presence of clinically significant portal hypertension or decompensation). Moreover, Mariño et al[29] reported increased HCC risk (up to 3 times) with the presence of non-characterized nodules before DAAs treatment than in patients without or with well-defined benign nodules and concluded that a time-association to therapy is possible. It seems that the most important determinant of a lower HCC risk is HCV eradication, with a similar risk reduction irrespective of whether it is achieved by DAAs or IFN. However, greater absolute numbers of HCCs might be observed after DAA-based therapy because more patients are treated, and higher proportion are older with more advanced liver disease[14].
More controversial is whether there is a higher risk of tumor recurrence in patients with HCC treated with curative intent (either with resection or radio-frequency ablation) after achieving SVR. Unexpectedly high rates of HCC recurrence were reported in patients with complete radiologic response following DAA therapy[16,17]. HCC recurrence was detected in 17/59 (28.8%) patients in an Italian study[17] and in 16/58 (27.6%) patients in a Spanish population[16], during a median follow-up of 6 months. However, the small cohort size, lack of an untreated control arm, and short median duration of follow-up limited any definitive conclusions regarding the "pro"-malignant potential of anti-HCV treatment and the risk factors for recurrence[16,17]. Furthermore, the Spanish study also included patients treated with non-curative therapies such as chemoembolization, characterized by high early recurrence rates[16].
Two large controlled studies as well as one propensity-score–adjusted analysis reported no increase in HCC recurrence in patients with adequately treated HCC who received DAAs compared to untreated patients[30-32]. In the French CUPILT cohort (Compassionate use of Protease Inhibitors in viral C Liver Transplantation), 314 HCC-liver transplant recipients were treated with DAAs[30]. The mean time between LT and the initiation of DAA was 67 ± 60 mo. HCC recurrence was observed in only seven patients (2.2%). Most of these patients (5/7) had factors predictive of a recurrence based on histologic criteria in the native liver. Moreover, two patients experienced recurrence after LT but before the introduction of DAA. Hence, incomplete treatment or mistaken initial staging of tumor burden might induce interpretation biases in retrospective studies. This may lead to an erroneous attribution of DAAs being responsible for HCC recurrence[16,30].
In order to further assess the risk of HCC recurrence after DAA it was compared with the risk after IFN treatment[28,33,34]. The same meta-analysis and meta-regression of studies comparing HCC incidence evaluated 17 studies on HCC recurrence after DAA and IFN therapy[28], there was no difference in HCC recurrence after adjusting for study follow-up and age. Furthermore, a Propensity score analysis from Japan also showed no significant difference in HCC recurrence rates between patients treated with IFN-based regimens or DAAs[33]. Cumulative incidence of HCC recurrence in patients who achieved an SVR was significantly lower than in patients without an SVR in both arms of treatment. Another study from Japan reported recurrence rate after DAA therapy of 39% and 61% at 1 and 2 years, respectively, without significant difference from IFN based therapy including the patterns of recurrence between groups[34]. Achievement of an SVR was not significantly associated with the risk of early HCC recurrence in a multivariate analysis but tumor factors such as a history of multiple HCC treatments or short recurrence-free period were found as independent risk factors for recurrence after antiviral therapy.
More evidence against an association of DAA therapy with HCC recurrence arises from a propensity-score weighted analysis of 149 LT candidates with HCV and HCC with initial complete response to loco-regional therapies[35]. DAA use was not associated with increased risk of HCC recurrence but rather was associated with reduced risk of waitlist dropout due to tumor progression or death. In addition, DAA use was not associated with decreased probability of LT or overall survival. Thus, the data suggests a significant net-benefit ratio for DAA use even in this special population.
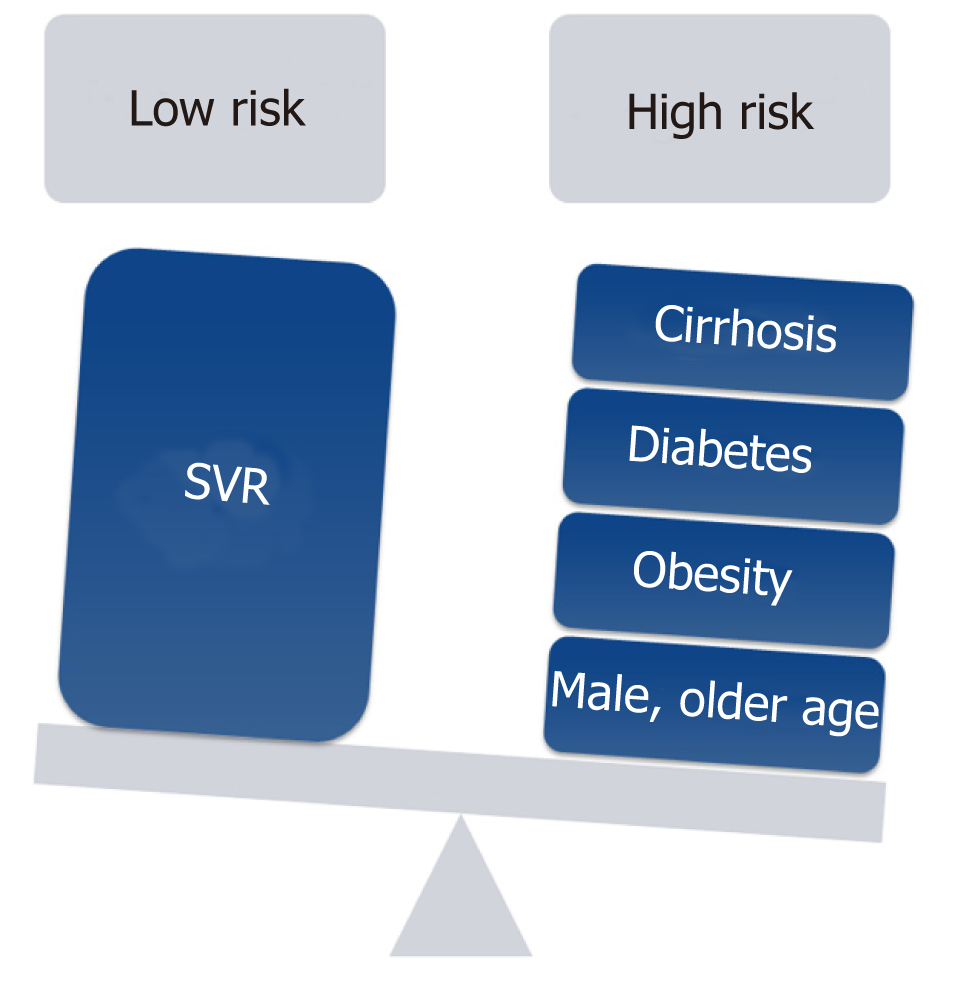
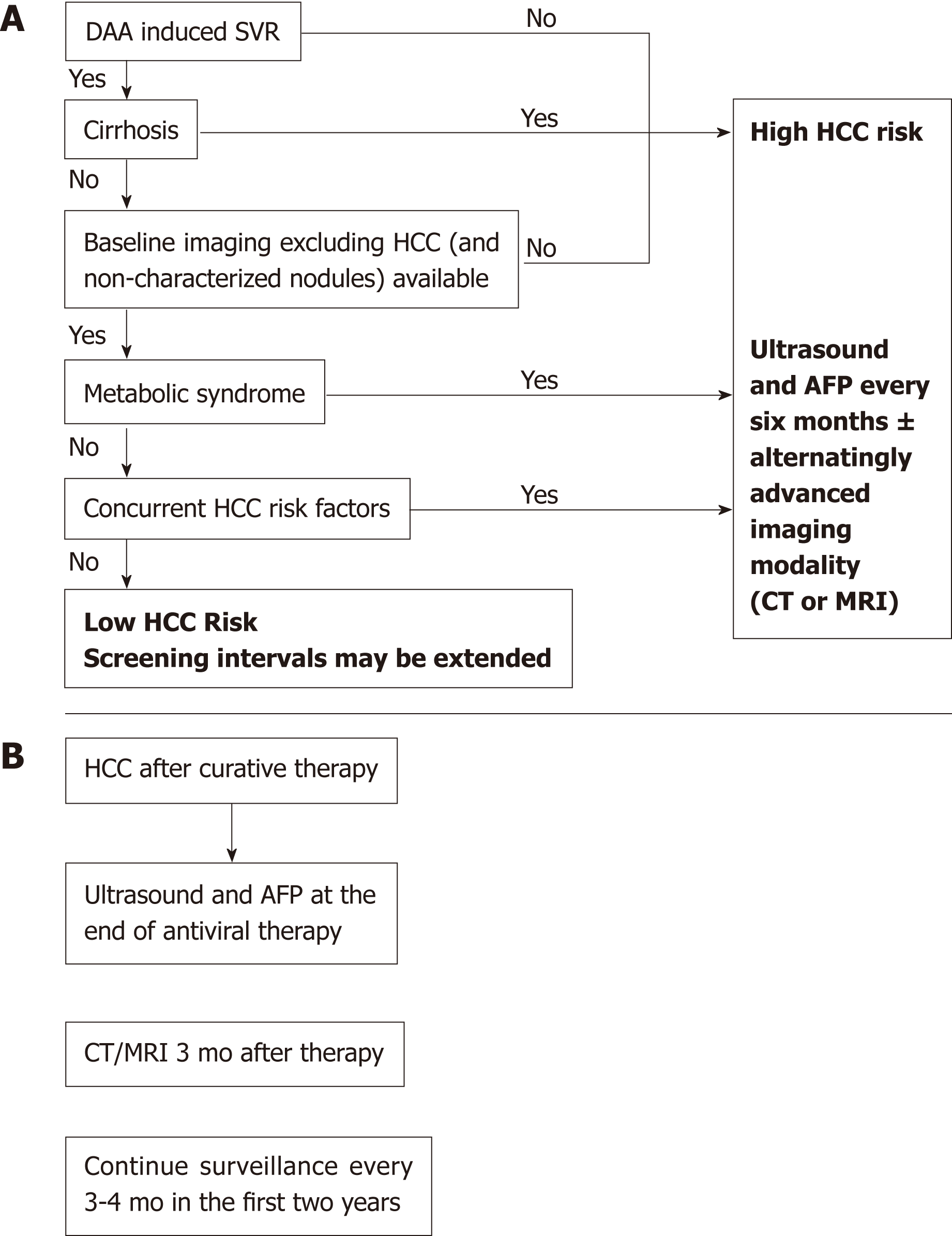
Nowadays, most patients with known chronic HCV have either received antiviral treatment or are expected to receive DAAs in the near future. Successful antiviral therapy leading to SVR in chronic HCV, decreases, but does not eliminate the risk of HCC[36]. Surveillance for HCC must therefore be continued following SVR for all HCV patients with advanced fibrosis (F3) and cirrhosis (F4)[37,38]. Despite this recommendation a cost-effectiveness analysis suggested that HCC surveillance is very unlikely to be cost-effective after achieving SVR in patients with advanced fibrosis, whereas both annual and biannual modalities were likely to be cost-effective for patients with cirrhosis[39]. Whether fibrosis regression translates into a reduced HCC risk beyond the benefit of achieving SVR is still unknown and further long term studies are needed to determine if patients who are proven to have marked reduction in fibrosis could discontinue surveillance[40]. D'Ambrosio et al[41] followed a small group of HCV patients treated with IFN-based regimens for almost 8 years after SVR but failed to prove any benefit of fibrosis regression on HCC occurrence. Furthermore, surveillance recommendations in HCV infected patients are currently based on a survival benefit for patients whose predicted HCC incidence exceed 1.5% per year but are based on older studies. With the advances in both antiviral therapy and current therapies of HCC, survival benefit may be seen with a lower threshold[36]. Recent studies tried to identify risk factors for HCC incidence after DAA therapy[26,42,43]. Lack of SVR was repeatedly found as the strongest predictor of HCC incidence after DAA therapy[26,36,42,43]. In a single center, longitudinal 3-years follow-up study, which included 565 cirrhotic patients, male gender, diabetes mellitus, and liver stiffness or FIB-4 score > 9 were found to be independent predictors of de-novo HCC. Nevertheless, diabetes mellitus was the only independent predictor of HCC recurrence[43]. Data from almost 2000 patients with 1-year follow-up suggested that age (> 50 years) and the presence of esophageal varices may predict HCC occurrence[44]. In contrast, patients within the “Extended Baveno Criteria” (Platelets > 110000/ µL and Liver Stiffness Measurement < 25 kPa), had a very low probability of developing HCC and could be candidates to a different surveillance program. Multivariate Cox regression analysis based on prospectively collected data from Italy showed that albumin level < 3.5 mg/dL and platelet count < 120 × 103/dL as well as absence of an SVR were independently associated with higher risk of HCC development[42]. Apparently, patients with a substantial risk for HCC after DAA induced SVR have other risk factors for HCC occurrence such as age, male sex or features of a severe liver disease[45]. Additionally, the metabolic syndrome showed an additive risk effect in patients with chronic viral hepatitis[38,46,47]. Patients with obesity, diabetes mellitus or the metabolic syndrome are probably still at risk for HCC, in spite of HCV eradication. Thus, it is important to estimate the risk of HCC occurrence in order to establish a proper and cost-effective screening strategy (Figure 1). Ioannou et al[27] developed and internally validated models for prediction of the risk for HCC by using baseline characteristics prior to antiviral treatment[36]. They identified four separate subgroups by cirrhosis and SVR status. HCC incidence was highest in the cirrhosis/no SVR subgroup and lowest in patients with no cirrhosis/SVR. Age, platelet count, aspartate aminotransferase/alanine aminotransferase ratio and albumin accounted for most of the prediction while other characteristics as sex, ethnicity, HCV genotype, body mass index (BMI), hemoglobin and INR had a smaller contribution. The risk model-based screening strategy showed superior net benefit than screening all cirrhotic patients or screening none of the non-cirrhotics. There is an intensive effort to validate sensitive and specific HCC blood-based biomarkers[48,49]. Potentially, these markers may be efficient in early HCC detection and may stratify patients according to their HCC risk. Thus, the strategy of one surveillance program fits all is being challenged as a result of the HCV revolution and stratifying patients according to risk factors seems reasonable but needs to be further validated. Figure 2 illustrates our suggested algorithms for HCC surveillance in HCV patients after DAA therapy according to HCC occurrence (Figure 2A) or recurrence (Figure 2B).
The global incidence of obesity has markedly increased in the last decades and so has the prevalence and incidence of NAFLD. It is estimated that in the United States, over 64 million people will be diagnosed with NAFLD, with annual direct medical costs of over $100 billion as a result of the high prevalence of the metabolic syndrome and its complications[50]. The definition of NAFLD is based on the evidence of hepatic steatosis (HS) and the absence of other known risk factors for hepatic fat accumulation (i.e., daily alcohol consumption, steatogenic medication usage, etc.)[38,51]. The liver histology differentiates between nonalcoholic fatty liver (NAFL) (less than 5% steatosis and no evidence of injury to hepatocytes) and nonalcoholic steatohepatitis (NASH) (steatosis is present in more than 5%, and so does hepatocellular injury, such as ballooning)[38,51]. Thus, the definitive diagnosis of NASH requires a liver biopsy.
NAFLD is the most common liver disorder in Western countries; Its prevalence is constantly rising from 15% in 2005 to 25% nowadays[52,53]. In 2016, a meta-analysis of 729 studies (a sample size of over 8 million subjects from 22 countries) estimated that the global prevalence of NAFLD is 25.24% (95%CI: 22.10-28.65) with the highest prevalence in the Middle East (31.79%, 95%CI: 13.48-58.23) and South America (30.45%, 95%CI: 22.74-39.440) while the lowest prevalence was reported from Africa (13.48%, 95%CI: 5.69-28.69)[54]. The prevalence of biopsy confirmed NASH among NAFLD patients ranged between 6.67%-29.85% in random biopsies to 60.64%-69.25% among patients with indicated biopsies. NAFLD is commonly referred to as the hepatic manifestation of the metabolic syndrome. It is associated with metabolic comorbidities such as obesity, diabetes mellitus type 2, and dyslipidemia[54,55]. Current recommendations by the European association for the study of the liver (EASL)[51] state that all individuals with steatosis should be screened for features of the metabolic syndrome and all individuals with metabolic features and persistently abnormal liver enzymes should be screened for NAFLD, because NAFLD is the main reason for unexpectedly elevated liver enzymes. Those metabolic comorbidities also correspond with the liver disease severity. In a Veterans Health Administration study of almost 400 patients, type 2 diabetes mellitus and BMI were the most significant predictors of advanced NAFLD [odds ratio (OR) 11.8, P < 0.001 and OR 1.4, P < 0.001, respectively][55]. The western diet is also a significant risk factor for NAFLD due to its high-calorie content, excess saturated fats, refined carbohydrates, sugar-sweetened beverages and high fructose intake[56,57]. Another risk factor is sedentary lifestyle which is more prevalent among NAFLD patients[58]. Several disease modifying genes have been investigated but only patatin like phospholipase domain containing 3 (PNPLA3) I148M variant at rs738409 was confirmed in multiple cohorts. It was initially identified from genome-wide association studies and was later correlated with disease severity, level of fibrosis and HCC development in patients with histologically proven NAFLD[59,60]. Recently, the transmembrane 6 superfamily member 2 (TM6SF2) gene has been reported as another disease modifier and the E167K variant was suggested to have clinical implication on progression to cirrhosis and HCC[61] and a possible protective effect regarding cardiovascular morbidity[62]. Moreover, a study by Koo et al[63] found that PNPLA3 and TM6SF2 risk variants have an additive effect on the risk for NASH (OR per risk allele, 2.03, 95%CI: 1.50-2.73, P < 0.001) and significant fibrosis (1.61, 95%CI: 1.19-2.17, P = 0.002) even when the model was adjusted for age, sex, CRP and insulin resistance. However, there are no current recommendations regarding HCC surveillance for carriers of these variants and genotyping in general is not yet recommended routinely[51].
Approximately 40% of NASH patients experience fibrosis progression[54]. According to a meta-analysis of 11 cohort and 411 patients with biopsy-proven NAFLD the fibrosis advancement rate is twofold higher in NASH compared to NAFL, corresponding to one fibrosis stage every 14.3 years in NAFL (95%CI: 9.1-50.0) and one every 7.1 years in NASH (95%CI: 4.8-14.3)[64]. In a study by Angulo et al[65] the stage of liver fibrosis and not the histologic features of steatosis was the determinant of overall mortality and liver-transplantation free-survival in patients with NAFLD. HCC occurrence significantly correlates with the degree of steatosis and stage of fibrosis[65]. In a recent large retrospective cohort study of nearly 600000 patients, the risk of HCC was 7-fold higher in patients with NAFLD than in matched controls (Adjusted HR 7.62 (5.76-10.09), P < 0.0001)[66]. In non-cirrhotic NAFLD patients HCC incidence rate per 1,000 PYs was 0.04 if FIB-4 was low (95%CI: 0.04-0.05) and 0.39 when FIB-4 was high (95%CI: 0.31-0.47). The presence of cirrhosis significantly increased the risk to 4.82 if FIB-4 was low (95%CI: 3.52-6.46), and 13.55 if FIB-4 was high (95%CI: 11.93-15.33)[66]. In a meta-analysis by Younossi et al[54] HCC incidence was 0.44 per 1000 person-years (95%CI: 0.29-0.66) in NAFLD patients and more than 12-fold higher in patients with NASH [5.29 per 1000 person-years (95%CI: 0.75-37.56)]. Although this incidence is significantly lower than that of chronic HBV or HCV[67], due to the high absolute number of patients with NAFLD and NASH worldwide this will obviously result in meaningful implications. Moreover, the incidence of NAFLD-related HCC has increased by 9% annually[68]. The incidence of HCC in patients with NAFLD is increased by associated features of the metabolic syndrome[69-71]. In terms of HCC-related mortality, in a large retrospective cohort study of the Surveillance, Epidemiology and End Results registries (2004-2009) which included approximately 5000 patients with HCC and 15000 matched-controls, NAFLD-HCC patients were older at diagnosis with shorter survival time than patients with viral hepatitis-associated HCC (1-year mortality: NAFLD-61.2%, HCV-51.3%, HBV-43.7%). NAFLD-HCC was found to be an independent risk factor for 1-year mortality with an OR of 1.21 (95%CI: 1.01-1.45)[68]. Some studies estimated that almost half of the cases of NASH-induced HCC arise in non-cirrhotic patients[72,73]. In a study by Mittal et al[74], around 13% of HCC reported in veterans did not have cirrhosis. Among other factors, having NAFLD was independently associated with HCC in the absence of cirrhosis. Nevertheless, according to the American association for the study of liver disease (AASLD) recommendations the risk of HCC is significantly lower in patients with NAFLD but without cirrhosis compared to NAFLD with cirrhosis, and surveillance is currently not recommended for these patients[67].
Abdominal ultrasound is the first-line diagnostic procedure for HCC due to its relatively low-cost and absence of radiation exposure. The ultrasound sensitivity for HCC detection is 58%-89% and the specificity exceeds 90% in the general cirrhotic population[75]. However, its diagnostic ability is frequently limited in NAFLD patients due to excess weight and HS. The increased BMI leads to attenuation of the ultrasound beam by subcutaneous fat and the HS might attenuate the ultrasound pulse and reduce deep hepatic structures visualization[76]. In a study of 941 cirrhotic patients who underwent abdominal ultrasound, 20% of the ultrasound studies were considered inadequate for HCC exclusion[77]. NASH related cirrhosis (OR 2.87, 95%CI: 1.71-4.80), and BMI category (OR 1.67, 95%CI: 1.45-1.93), were found to be independent risk factors for an inadequate study. Other radiological methods, such as computed tomography scans or magnetic resonance imaging, can be utilized for HCC screening but since the population at risk is so large, they are not cost-effective and there is no current evidence to support the use of these modalities for initial HCC screening.
Once HCC is diagnosed, treatment options and potential complications are influenced by the liver steatosis as well. The risk profile of liver resection with curative intent in NAFLD patients with metabolic syndrome and no advanced fibrosis is similar to cirrhotic patients[78]. Co-morbidities such as dyslipidemia, hypertension, diabetes mellitus, obesity, heart and lung chronic dysfunction are commonly observed in these patients and play a significant and negative prognostic role. NASH is also the second leading etiology of HCC-related LT. Between 2002 to 2012, the prevalence of NASH related HCC as an indication for LT increased by nearly 4-fold, while the prevalence of LT due to HCV related HCC increased only by 2-fold[20].
These worrisome findings raise some questions regarding proper HCC screening in the NAFLD population and screening for NAFLD in the general population. As mentioned, in the western countries the average prevalence of NAFLD is reaching a quarter of the adult population, while our screening tools and appropriate treatment strategy are still inadequate. The EASL recommendations 2016[51] endorse screening for NAFLD in high-risk groups such as patients in diabetes mellitus or obesity clinics by liver enzymes and/or ultrasound as part of a routine work-up. On the other hand, the AASLD[38] recommendations recommend against routine screening for NAFLD because of the uncertain diagnostic accuracy and the limited treatment options alongside lack of cost-effectiveness of screening. A Markov model analysis suggested that screening for NASH in diabetic patients is currently not cost-effective due to a lack of an established effective treatment[79]. These data calls for establishment of a specific high-risk cohort within the NASH population which should undergo HCC surveillance.
DAA therapy is efficacious for HCV eradication with few side effects. The absolute risk of HCC occurrence or recurrence is mainly attributed to the more severe liver disease and older age of patients which can now be treated. There is no evidence that HCC occurrence or recurrence is different between patients treated with DAA or IFN therapy and the reduced risk is mainly associated with SVR. HCC surveillance is currently recommended after DAA therapy in all patients with cirrhosis albeit the risk might be reduced. Stratifying patients according to risk factors seems reasonable but needs further validation. In the last decades, NAFLD is becoming a major etiology of HCC in developed regions. The risk of HCC occurrence is increased by other features of the metabolic syndrome, evidence of NASH or advanced fibrosis. Moreover, NASH is an independent risk factor and can promote HCC development in non-cirrhotic patients. NAFLD associated fat depositions and inflammation can hinder HCC detection and treatment effectiveness. The HCC screening and surveillance protocols in the NAFLD population should be re-evaluated in this post-HCV era.
| 1. | Global Burden of Disease Liver Cancer Collaboration. Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, Artaman A, Ayele TA, Barac A, Bensenor I, Berhane A, Bhutta Z, Castillo-Rivas J, Chitheer A, Choi JY, Cowie B, Dandona L, Dandona R, Dey S, Dicker D, Phuc H, Ekwueme DU, Zaki MS, Fischer F, Fürst T, Hancock J, Hay SI, Hotez P, Jee SH, Kasaeian A, Khader Y, Khang YH, Kumar A, Kutz M, Larson H, Lopez A, Lunevicius R, Malekzadeh R, McAlinden C, Meier T, Mendoza W, Mokdad A, Moradi-Lakeh M, Nagel G, Nguyen Q, Nguyen G, Ogbo F, Patton G, Pereira DM, Pourmalek F, Qorbani M, Radfar A, Roshandel G, Salomon JA, Sanabria J, Sartorius B, Satpathy M, Sawhney M, Sepanlou S, Shackelford K, Shore H, Sun J, Mengistu DT, Topór-Mądry R, Tran B, Ukwaja KN, Vlassov V, Vollset SE, Vos T, Wakayo T, Weiderpass E, Werdecker A, Yonemoto N, Younis M, Yu C, Zaidi Z, Zhu L, Murray CJL, Naghavi M, Fitzmaurice C. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683-1691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1459] [Cited by in RCA: 1571] [Article Influence: 174.6] [Reference Citation Analysis (0)] |
| 2. | White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of Hepatocellular Carcinoma in All 50 United States, From 2000 Through 2012. Gastroenterology. 2017;152:812-820.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 339] [Article Influence: 37.7] [Reference Citation Analysis (1)] |
| 3. | West J, Card TR, Aithal GP, Fleming KM. Risk of hepatocellular carcinoma among individuals with different aetiologies of cirrhosis: A population-based cohort study. Aliment Pharmacol Ther. 2017;45:983-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 3126] [Article Influence: 208.4] [Reference Citation Analysis (0)] |
| 5. | European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol. 2018;69:461-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1281] [Cited by in RCA: 1234] [Article Influence: 154.3] [Reference Citation Analysis (0)] |
| 6. | Baumert TF, Berg T, Lim JK, Nelson DR. Status of Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection and Remaining Challenges. Gastroenterology. 2019;156:431-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 7. | AASLD-IDSA HCV Guidance Panel. Hepatitis C Guidance 2018 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin Infect Dis. 2018;67:1477-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 490] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 8. | Bachofner JA, Valli PV, Kröger A, Bergamin I, Künzler P, Baserga A, Braun D, Seifert B, Moncsek A, Fehr J, Semela D, Magenta L, Müllhaupt B, Terziroli Beretta-Piccoli B, Mertens JC. Direct antiviral agent treatment of chronic hepatitis C results in rapid regression of transient elastography and fibrosis markers fibrosis-4 score and aspartate aminotransferase-platelet ratio index. Liver Int. 2017;37:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 201] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 9. | Sporea I, Lupușoru R, Mare R, Popescu A, Gheorghe L, Iacob S, Șirli R. Dynamics of liver stiffness values by means of transient elastography in patients with HCV liver cirrhosis undergoing interferon free treatment. J Gastrointestin Liver Dis. 2017;26:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Mandorfer M, Kozbial K, Schwabl P, Freissmuth C, Schwarzer R, Stern R, Chromy D, Stättermayer AF, Reiberger T, Beinhardt S, Sieghart W, Trauner M, Hofer H, Ferlitsch A, Ferenci P, Peck-Radosavljevic M. Sustained virologic response to interferon-free therapies ameliorates HCV-induced portal hypertension. J Hepatol. 2016;65:692-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 249] [Article Influence: 24.9] [Reference Citation Analysis (5)] |
| 11. | Foster GR, Irving WL, Cheung MC, Walker AJ, Hudson BE, Verma S, McLauchlan J, Mutimer DJ, Brown A, Gelson WT, MacDonald DC, Agarwal K; HCV Research, UK. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;64:1224-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 364] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 12. | Lens S, Alvarado-Tapias E, Mariño Z, Londoño MC, LLop E, Martinez J, Fortea JI, Ibañez L, Ariza X, Baiges A, Gallego A, Bañares R, Puente A, Albillos A, Calleja JL, Torras X, Hernández-Gea V, Bosch J, Villanueva C, Forns X, García-Pagán JC. Effects of All-Oral Anti-Viral Therapy on HVPG and Systemic Hemodynamics in Patients With Hepatitis C Virus-Associated Cirrhosis. Gastroenterology. 2017;153:1273-1283.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 212] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 13. | Backus LI, Belperio PS, Shahoumian TA, Mole LA. Impact of Sustained Virologic Response with Direct-Acting Antiviral Treatment on Mortality in Patients with Advanced Liver Disease. Hepatology. 2019;69:487-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 169] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 14. | Ioannou GN, Feld JJ. What Are the Benefits of a Sustained Virologic Response to Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection? Gastroenterology. 2019;156:446-460.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 155] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 15. | Lazarus JV, Wiktor S, Colombo M, Thursz M; EASL International Liver Foundation. Micro-elimination - A path to global elimination of hepatitis C. J Hepatol. 2017;67:665-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 183] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 16. | Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, Lens S, Díaz A, Vilana R, Darnell A, Varela M, Sangro B, Calleja JL, Forns X, Bruix J. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 818] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 17. | Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, Foschi FG, Lenzi M, Mazzella G, Verucchi G, Andreone P, Brillanti S. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65:727-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 713] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 18. | Cardoso H, Vale AM, Rodrigues S, Gonçalves R, Albuquerque A, Pereira P, Lopes S, Silva M, Andrade P, Morais R, Coelho R, Macedo G. High incidence of hepatocellular carcinoma following successful interferon-free antiviral therapy for hepatitis C associated cirrhosis. J Hepatol. 2016;65:1070-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (4)] |
| 19. | Ward JW, Hinman AR. What Is Needed to Eliminate Hepatitis B Virus and Hepatitis C Virus as Global Health Threats. Gastroenterology. 2019;156:297-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 20. | Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188-2195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 596] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 21. | Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, Setiawan VW, Tran T, Ayoub WS, Lu SC, Klein AS, Sundaram V, Nissen NN. NASH Leading Cause of Liver Transplant in Women: Updated Analysis of Indications For Liver Transplant and Ethnic and Gender Variances. Am J Gastroenterol. 2018;113:1649-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 475] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 22. | Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: A meta-analysis of observational studies. Ann Intern Med. 2013;158:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 658] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 23. | Di Bisceglie AM, Shiffman ML, Everson GT, Lindsay KL, Everhart JE, Wright EC, Lee WM, Lok AS, Bonkovsky HL, Morgan TR, Ghany MG, Morishima C, Snow KK, Dienstag JL; HALT-C Trial Investigators. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N Engl J Med. 2008;359:2429-2441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 342] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 24. | Bruix J, Poynard T, Colombo M, Schiff E, Burak K, Heathcote EJ, Berg T, Poo JL, Mello CB, Guenther R, Niederau C, Terg R, Bedossa P, Boparai N, Griffel LH, Burroughs M, Brass CA, Albrecht JK; EPIC3 Study Group. Maintenance therapy with peginterferon alfa-2b does not prevent hepatocellular carcinoma in cirrhotic patients with chronic hepatitis C. Gastroenterology. 2011;140:1990-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology. 2017;153:996-1005.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 718] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 26. | Li DK, Ren Y, Fierer DS, Rutledge S, Shaikh OS, Lo Re V, Simon T, Abou-Samra AB, Chung RT, Butt AA. The short-term incidence of hepatocellular carcinoma is not increased after hepatitis C treatment with direct-acting antivirals: An ERCHIVES study. Hepatology. 2018;67:2244-2253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 27. | Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2017;pii:S0168-8278(17)32273-0. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 394] [Article Influence: 43.8] [Reference Citation Analysis (1)] |
| 28. | Waziry R, Hajarizadeh B, Grebely J, Amin J, Law M, Danta M, George J, Dore GJ. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J Hepatol. 2017;67:1204-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 392] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 29. | Mariño Z, Darnell A, Lens S, Sapena V, Díaz A, Belmonte E, Perelló C, Calleja JL, Varela M, Rodriguez M, Rodriguez de Lope C, Llerena S, Torras X, Gallego A, Sala M, Morillas RM, Minguez B, Llaneras J, Coll S, Carrion JA, Iñarrairaegui M, Sangro B, Vilana R, Sole M, Ayuso C, Ríos J, Forns X, Bruix J, Reig M. Time association between hepatitis C therapy and hepatocellular carcinoma emergence in cirrhosis: Relevance of non-characterized nodules. J Hepatol. 2019;70:874-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 30. | ANRS collaborative study group on hepatocellular carcinoma (ANRS CO22 HEPATHER, CO12 CirVir and CO23 CUPILT cohorts). Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts. J Hepatol. 2016;65:734-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 336] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 31. | Cheung MCM, Walker AJ, Hudson BE, Verma S, McLauchlan J, Mutimer DJ, Brown A, Gelson WTH, MacDonald DC, Agarwal K, Foster GR, Irving WL, HCV Research UK. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;65:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 324] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 32. | Singal AG, Rich NE, Mehta N, Branch A, Pillai A, Hoteit M, Volk M, Odewole M, Scaglione S, Guy J, Said A, Feld JJ, John BV, Frenette C, Mantry P, Rangnekar AS, Oloruntoba O, Leise M, Jou JH, Bhamidimarri KR, Kulik L, Tran T, Samant H, Dhanasekaran R, Duarte-Rojo A, Salgia R, Eswaran S, Jalal P, Flores A, Satapathy SK, Wong R, Huang A, Misra S, Schwartz M, Mitrani R, Nakka S, Noureddine W, Ho C, Konjeti VR, Dao A, Nelson K, Delarosa K, Rahim U, Mavuram M, Xie JJ, Murphy CC, Parikh ND. Direct-Acting Antiviral Therapy Not Associated With Recurrence of Hepatocellular Carcinoma in a Multicenter North American Cohort Study. Gastroenterology. 2019;156:1683-1692.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 33. | Nagata H, Nakagawa M, Asahina Y, Sato A, Asano Y, Tsunoda T, Miyoshi M, Kaneko S, Otani S, Kawai-Kitahata F, Murakawa M, Nitta S, Itsui Y, Azuma S, Kakinuma S, Nouchi T, Sakai H, Tomita M, Watanabe M; Ochanomizu Liver Conference Study Group. Effect of interferon-based and -free therapy on early occurrence and recurrence of hepatocellular carcinoma in chronic hepatitis C. J Hepatol. 2017;67:933-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 173] [Article Influence: 19.2] [Reference Citation Analysis (1)] |
| 34. | Nishibatake Kinoshita M, Minami T, Tateishi R, Wake T, Nakagomi R, Fujiwara N, Sato M, Uchino K, Enooku K, Nakagawa H, Asaoka Y, Shiina S, Koike K. Impact of direct-acting antivirals on early recurrence of HCV-related HCC: Comparison with interferon-based therapy. J Hepatol. 2019;70:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 35. | Huang AC, Mehta N, Dodge JL, Yao FY, Terrault NA. Direct-acting antivirals do not increase the risk of hepatocellular carcinoma recurrence after local-regional therapy or liver transplant waitlist dropout. Hepatology. 2018;68:449-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 36. | Ioannou GN, Green PK, Beste LA, Mun EJ, Kerr KF, Berry K. Development of models estimating the risk of hepatocellular carcinoma after antiviral treatment for hepatitis C. J Hepatol. 2018;69:1088-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 37. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3143] [Article Influence: 392.9] [Reference Citation Analysis (3)] |
| 38. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 5209] [Article Influence: 651.1] [Reference Citation Analysis (9)] |
| 39. | Farhang Zangneh H, Wong WWL, Sander B, Bell CM, Mumtaz K, Kowgier M, van der Meer AJ, Cleary SP, Janssen HLA, Chan KKW, Feld JJ. Cost Effectiveness of Hepatocellular Carcinoma Surveillance After a Sustained Virologic Response to Therapy in Patients With Hepatitis C Virus Infection and Advanced Fibrosis. Clin Gastroenterol Hepatol. 2018;pii:S1542-3565(18)31394-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 40. | D'Ambrosio R, Colombo M. Should surveillance for liver cancer be modified in hepatitis C patients after treatment-related cirrhosis regression? Liver Int. 2016;36:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | D'Ambrosio R, Aghemo A, Rumi MG, Degasperi E, Sangiovanni A, Maggioni M, Fraquelli M, Perbellini R, Rosenberg W, Bedossa P, Colombo M, Lampertico P. Persistence of hepatocellular carcinoma risk in hepatitis C patients with a response to IFN and cirrhosis regression. Liver Int. 2018;38:1459-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Calvaruso V, Cabibbo G, Cacciola I, Petta S, Madonia S, Bellia A, Tinè F, Distefano M, Licata A, Giannitrapani L, Prestileo T, Mazzola G, Di Rosolini MA, Larocca L, Bertino G, Digiacomo A, Benanti F, Guarneri L, Averna A, Iacobello C, Magro A, Scalisi I, Cartabellotta F, Savalli F, Barbara M, Davì A, Russello M, Scifo G, Squadrito G, Cammà C, Raimondo G, Craxì A, Di Marco V; Rete Sicilia Selezione Terapia–HCV (RESIST-HCV). Incidence of Hepatocellular Carcinoma in Patients With HCV-Associated Cirrhosis Treated With Direct-Acting Antiviral Agents. Gastroenterology. 2018;155:411-421.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 275] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 43. | Degasperi E, D'Ambrosio R, Iavarone M, Sangiovanni A, Aghemo A, Soffredini R, Borghi M, Lunghi G, Colombo M, Lampertico P. Factors Associated With Increased Risk of De Novo or Recurrent Hepatocellular Carcinoma in Patients With Cirrhosis Treated With Direct-Acting Antivirals for HCV Infection. Clin Gastroenterol Hepatol. 2019;17:1183-1191.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 44. | Lleo A, Aglitti A, Aghemo A, Maisonneuve P, Bruno S, Persico M; collaborators. Predictors of hepatocellular carcinoma in HCV cirrhotic patients treated with direct acting antivirals. Dig Liver Dis. 2019;51:310-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 45. | van der Meer AJ, Feld JJ, Hofer H, Almasio PL, Calvaruso V, Fernández-Rodríguez CM, Aleman S, Ganne-Carrié N, D'Ambrosio R, Pol S, Trapero-Marugan M, Maan R, Moreno-Otero R, Mallet V, Hultcrantz R, Weiland O, Rutter K, Di Marco V, Alonso S, Bruno S, Colombo M, de Knegt RJ, Veldt BJ, Hansen BE, Janssen HLA. Risk of cirrhosis-related complications in patients with advanced fibrosis following hepatitis C virus eradication. J Hepatol. 2017;66:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 218] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 46. | Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, Wang LY, Sun CA, Lu SN, Chen DS, Chen CJ. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: A follow-up study in Taiwan. Gastroenterology. 2008;135:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 426] [Article Influence: 23.7] [Reference Citation Analysis (2)] |
| 47. | Yu MW, Lin CL, Liu CJ, Yang SH, Tseng YL, Wu CF. Influence of Metabolic Risk Factors on Risk of Hepatocellular Carcinoma and Liver-Related Death in Men With Chronic Hepatitis B: A Large Cohort Study. Gastroenterology. 2017;153:1006-1017.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 48. | Kisiel JB, Dukek BA, V S R Kanipakam R, Ghoz HM, Yab TC, Berger CK, Taylor WR, Foote PH, Giama NH, Onyirioha K, Abdallah MA, Burger KN, Slettedahl SW, Mahoney DW, Smyrk TC, Lewis JT, Giakoumopoulos M, Allawi HT, Lidgard GP, Roberts LR, Ahlquist DA. Hepatocellular Carcinoma Detection by Plasma Methylated DNA: Discovery, Phase I Pilot, and Phase II Clinical Validation. Hepatology. 2019;69:1180-1192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 49. | El-Emshaty HM, Entsar A, Gouida MS, ELSHAHAWY ZRJB. Associations between CD133, CK19 and G2/M in cirrhotic HCV (genotype-4) patients with or without accompanying tumor. Biocell. 2018;42:55-60. |
| 50. | Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, Racila A, Hunt S, Beckerman R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 955] [Article Influence: 95.5] [Reference Citation Analysis (1)] |
| 51. | European Association for the Study of the Liver (EASL). European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3279] [Article Influence: 327.9] [Reference Citation Analysis (6)] |
| 52. | Vernon G, Baranova A, Younossi ZM. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2325] [Article Influence: 155.0] [Reference Citation Analysis (1)] |
| 53. | Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol. 2019;70:531-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 943] [Cited by in RCA: 1565] [Article Influence: 223.6] [Reference Citation Analysis (3)] |
| 54. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7913] [Article Influence: 791.3] [Reference Citation Analysis (5)] |
| 55. | Patel YA, Gifford EJ, Glass LM, McNeil R, Turner MJ, Han B, Provenzale D, Choi SS, Moylan CA, Hunt CM. Risk factors for biopsy-proven advanced non-alcoholic fatty liver disease in the Veterans Health Administration. Aliment Pharmacol Ther. 2018;47:268-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 56. | Barrera F, George J. The role of diet and nutritional intervention for the management of patients with NAFLD. Clin Liver Dis. 2014;18:91-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 57. | Chiu S, Sievenpiper JL, de Souza RJ, Cozma AI, Mirrahimi A, Carleton AJ, Ha V, Di Buono M, Jenkins AL, Leiter LA, Wolever TM, Don-Wauchope AC, Beyene J, Kendall CW, Jenkins DJ. Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of controlled feeding trials. Eur J Clin Nutr. 2014;68:416-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 219] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 58. | Gerber L, Otgonsuren M, Mishra A, Escheik C, Birerdinc A, Stepanova M, Younossi ZM. Non-alcoholic fatty liver disease (NAFLD) is associated with low level of physical activity: A population-based study. Aliment Pharmacol Ther. 2012;36:772-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 189] [Article Influence: 13.5] [Reference Citation Analysis (2)] |
| 59. | Valenti L, Al-Serri A, Daly AK, Galmozzi E, Rametta R, Dongiovanni P, Nobili V, Mozzi E, Roviaro G, Vanni E, Bugianesi E, Maggioni M, Fracanzani AL, Fargion S, Day CP. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1209-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 538] [Article Influence: 33.6] [Reference Citation Analysis (1)] |
| 60. | Liu YL, Patman GL, Leathart JB, Piguet AC, Burt AD, Dufour JF, Day CP, Daly AK, Reeves HL, Anstee QM. Carriage of the PNPLA3 rs738409 C >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol. 2014;61:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 435] [Article Influence: 36.3] [Reference Citation Analysis (1)] |
| 61. | Liu YL, Reeves HL, Burt AD, Tiniakos D, McPherson S, Leathart JB, Allison ME, Alexander GJ, Piguet AC, Anty R, Donaldson P, Aithal GP, Francque S, Van Gaal L, Clement K, Ratziu V, Dufour JF, Day CP, Daly AK, Anstee QM. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat Commun. 2014;5:4309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 480] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 62. | Dongiovanni P, Petta S, Maglio C, Fracanzani AL, Pipitone R, Mozzi E, Motta BM, Kaminska D, Rametta R, Grimaudo S, Pelusi S, Montalcini T, Alisi A, Maggioni M, Kärjä V, Borén J, Käkelä P, Di Marco V, Xing C, Nobili V, Dallapiccola B, Craxi A, Pihlajamäki J, Fargion S, Sjöström L, Carlsson LM, Romeo S, Valenti L. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology. 2015;61:506-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 430] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 63. | Koo BK, Joo SK, Kim D, Bae JM, Park JH, Kim JH, Kim W. Additive effects of PNPLA3 and TM6SF2 on the histological severity of non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2018;33:1277-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 64. | Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: A systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643-654.e1-9; quiz e39-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 932] [Cited by in RCA: 1300] [Article Influence: 118.2] [Reference Citation Analysis (1)] |
| 65. | Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389-397.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2304] [Cited by in RCA: 2326] [Article Influence: 211.5] [Reference Citation Analysis (2)] |
| 66. | Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, Li L, Desiderio R, Thrift AP, Asch SM, Chu J, El-Serag HB. Risk of Hepatocellular Cancer in Patients With Non-Alcoholic Fatty Liver Disease. Gastroenterology. 2018;155:1828-1837.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 613] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 67. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3434] [Article Influence: 429.3] [Reference Citation Analysis (3)] |
| 68. | Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, Hunt S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 628] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 69. | Schlesinger S, Aleksandrova K, Pischon T, Jenab M, Fedirko V, Trepo E, Overvad K, Roswall N, Tjønneland A, Boutron-Ruault MC, Fagherazzi G, Racine A, Kaaks R, Grote VA, Boeing H, Trichopoulou A, Pantzalis M, Kritikou M, Mattiello A, Sieri S, Sacerdote C, Palli D, Tumino R, Peeters PH, Bueno-de-Mesquita HB, Weiderpass E, Quirós JR, Zamora-Ros R, Sánchez MJ, Arriola L, Ardanaz E, Tormo MJ, Nilsson P, Lindkvist B, Sund M, Rolandsson O, Khaw KT, Wareham N, Travis RC, Riboli E, Nöthlings U. Diabetes mellitus, insulin treatment, diabetes duration, and risk of biliary tract cancer and hepatocellular carcinoma in a European cohort. Ann Oncol. 2013;24:2449-2455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 70. | Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: Umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 589] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 71. | Dyson J, Jaques B, Chattopadyhay D, Lochan R, Graham J, Das D, Aslam T, Patanwala I, Gaggar S, Cole M, Sumpter K, Stewart S, Rose J, Hudson M, Manas D, Reeves HL. Hepatocellular cancer: The impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol. 2014;60:110-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 456] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 72. | Kodama K, Kawaguchi T, Hyogo H, Nakajima T, Ono M, Seike M, Takahashi H, Nozaki Y, Kawanaka M, Tanaka S, Imajo K, Sumida Y, Kamada Y, Fujii H, Seko Y, Takehara T, Itoh Y, Nakajima A, Masaki N, Torimura T, Saibara T, Karino Y, Chayama K, Tokushige K. Clinical features of hepatocellular carcinoma in nonalcoholic fatty liver disease patients without advanced fibrosis. J Gastroenterol Hepatol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 73. | Mohamad B, Shah V, Onyshchenko M, Elshamy M, Aucejo F, Lopez R, Hanouneh IA, Alhaddad R, Alkhouri N. Characterization of hepatocellular carcinoma (HCC) in non-alcoholic fatty liver disease (NAFLD) patients without cirrhosis. Hepatol Int. 2016;10:632-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 74. | Mittal S, El-Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, May SB, Kramer JR, Richardson PA, Davila JA. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2016;14:124-131.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 512] [Article Influence: 51.2] [Reference Citation Analysis (1)] |
| 75. | Bolondi L. Screening for hepatocellular carcinoma in cirrhosis. J Hepatol. 2003;39:1076-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 136] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 76. | Uppot RN, Sahani DV, Hahn PF, Kalra MK, Saini SS, Mueller PR. Effect of obesity on image quality: Fifteen-year longitudinal study for evaluation of dictated radiology reports. Radiology. 2006;240:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 77. | Simmons O, Fetzer DT, Yokoo T, Marrero JA, Yopp A, Kono Y, Parikh ND, Browning T, Singal AG. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther. 2017;45:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 273] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 78. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6413] [Article Influence: 801.6] [Reference Citation Analysis (9)] |
| 79. | Corey KE, Klebanoff MJ, Tramontano AC, Chung RT, Hur C. Screening for Nonalcoholic Steatohepatitis in Individuals with Type 2 Diabetes: A Cost-Effectiveness Analysis. Dig Dis Sci. 2016;61:2108-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Israel
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aravinthan AD, Barone M, Bartosch B, Jin C, Wang L S-Editor: Yan JP L-Editor: A E-Editor: Ma YJ