Published online Aug 7, 2019. doi: 10.3748/wjg.v25.i29.3897
Peer-review started: March 25, 2019
First decision: April 11, 2019
Revised: June 4, 2019
Accepted: June 8, 2019
Article in press: June 6, 2019
Published online: August 7, 2019
Processing time: 138 Days and 1.1 Hours
Globally, 69.6 million individuals were infected with hepatitis C virus (HCV) infection in 2016. Of the six major HCV genotypes (GT), the most predominant one is GT1, worldwide. The prevalence of HCV in Central Asia, which includes most of the Commonwealth of Independent States (CIS), has been estimated to be 5.8% of the total global burden. The predominant genotype in the CIS and Ukraine regions has been reported to be GT1, followed by GT3. Inadequate HCV epidemiological data, multiple socio-economic barriers, and the lack of region-specific guidelines have impeded the optimal management of HCV infection in this region. In this regard, a panel of regional experts in the field of hepatology convened to discuss and provide recommendations on the diagnosis, treatment, and pre-, on-, and posttreatment assessment of chronic HCV infection and to ensure the optimal use of cost-effective antiviral regimens in the region. A comprehensive evaluation of the literature along with expert recommendations for the management of GT1-GT6 HCV infection with the antiviral agents available in the region has been provided in this review. This consensus document will help guide clinical decision-making during the management of HCV infection, further optimizing treatment outcomes in these regions.
Core tip: A high prevalence of hepatitis C virus (HCV) infection has been reported in Ukraine and most of the Commonwealth of Independent States regions. The scarcity of adequate epidemiological data, the lack of national guidelines, and multiple socio-economic barriers hinder the effective management of HCV infection in these regions. The current consensus document intends to guide clinicians and healthcare providers on the diagnosis, treatment, and pre-, on-, and posttreatment assessment of HCV infection and to help optimize the treatment outcomes in the region.
- Citation: Colombo MG, Musabaev EI, Ismailov UY, Zaytsev IA, Nersesov AV, Anastasiy IA, Karpov IA, Golubovska OA, Kaliaskarova KS, AC R, Hadigal S. Consensus on management of hepatitis C virus infection in resource-limited Ukraine and Commonwealth of Independent States regions. World J Gastroenterol 2019; 25(29): 3897-3919
- URL: https://www.wjgnet.com/1007-9327/full/v25/i29/3897.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i29.3897
Hepatitis C is a liver disease caused by hepatitis C virus (HCV), which manifests clinically as acute and chronic hepatitis[1,2]. There are six different genotypes of HCV (GT1-GT6)[1].
In the latest nationwide HCV disease burden estimation by the Polaris Observatory HCV collaborators in about 113 countries, the global prevalence of HCV infection was estimated to be about 1.0% in 2015 (71.1 million viremic HCV-infected individuals)[3]. In a separate analysis of the prevalence data from 109 countries estimated by the World Health Organization, the global epidemic size of HCV infection was found to be 69.6 million HCV-infected individuals in 2016[4]. In another recent, systematic review, the global genotype distribution pattern revealed the predominance of GT1 (49.1%), followed by GT3 (17.9%), GT4 (16.8%), GT2 (11.0%), GT5 (2%), mixed (1.8%), and GT6 (1.4%)[5]. In the same review, the prevalence of HCV infection in Central Asia, which included the Commonwealth of Independent States (CIS) regions of Armenia, Azerbaijan, Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan, and Uzbekistan, besides Mongolia and Georgia, was found to be 5.8%[5]. The predominant genotype in this region was reported to be GT1 (70.4%), followed by GT3 (19.6%) and GT2 (8.6%). The prevalence of mixed GTs was noted to be rare in this region, with a complete absence of cases of GT4, GT5, and GT6. In the Eastern European zone, which includes, among other countries, Ukraine and the three CIS regions of Belarus, Moldova, and Russia, the prevalence of HCV infection was found to be 3.1%. GT1 was the most predominant genotype (68.1%), followed by GT3 (26.6%), GT2 (4.3%), mixed GTs (0.5%), and GT4 (0.5%). No GT5 and GT6 cases were reported in this region[5]. The lack of robust epidemiological data at a national level and in some extended regions of Central Asia was cited as one of the major setbacks in this review[5].
Another survey was conducted by the Alliance for Public Health (Alliance, Ukraine) in collaboration with the Saint Petersburg-based International Treatment Preparedness Coalition in 11 Eastern Europe and Central Asian countries (including Armenia, Azerbaijan, Belarus, Georgia, Kazakhstan, Kyrgyzstan, Moldova, Russia, Tajikistan, Ukraine, and Uzbekistan)[6]. Among the CIS regions and Ukraine, the highest prevalence of HCV infection was reported in Uzbekistan (6.5%), followed by Ukraine (5%); Russia, Armenia, and Kyrgyzstan (4% each); Azerbaijan (3.2%), Tajikistan (2.3%), Belarus (2%-3%), Moldova (1.7%-4.0%), and Kazakhstan (1.5%-3.0%)[6]. Furthermore, the survey reported the lack of adequate HCV epidemiological data required to plan services and resources in the CIS region[6].
The dearth of data pertaining to HCV epidemiology, coupled with the disparity in the genotype distribution across Ukraine and the various CIS regions, highlights a clear unmet need in the optimal management of HCV infection in this region[5,6]. Several other unmet needs in the management of HCV infection in the CIS region have also been described in the literature. These include: (1) Lack of awareness on the disease and modes of transmission and weak epidemiological surveillance[7,8]; (2) Barriers in providing access to diagnostics and surveillance systems[7,9]; (3) Lack of adequate and updated national guidelines/strategies or other regulatory directives on the diagnosis and management of viral hepatitis and HCV infection[6-10]; (4) Fear of treatment side effects[8]; and (5) High treatment cost and lack of reimbursement coverage for treatment[8,9].
This primary aim of this consensus document is to guide physicians on the diagnosis and treatment of chronic HCV infection and to ensure the optimal use of cost-effective regimens in resource-limited settings in Ukraine and CIS countries.
On 9 April 2018, on the sidelines of the European Association for the Study of the Liver 2018 conference, a panel of experts in the field of hepatology from four countries in the Ukraine/CIS region (Uzbekistan, Ukraine, Belarus, and Kazakhstan) convened at Holiday Inn Paris-St. Germain des Près to review the updated literature on the management of HCV infection and to provide recommendations to optimize the: (1) Diagnosis of HCV infection; (2) Use of cost-effective treatment regimens for the management of HCV infection in resource-limited settings in Ukraine and CIS regions; and (3) Pre-, on-, and posttreatment assessments during HCV management.
The recommendations for the use of optimal treatment regimens in the management of HCV infection in Ukraine and the CIS region were graded by the expert panel as “Preferred,” “Alternative,” or “Not Recommended” (Table 1).
| Grading | Definition |
| Preferred | Treatment can be used in most patients and recommendation is based on optimal efficacy, favorable tolerability, toxicity profiles, treatment duration, and pill burden |
| Alternative | Treatment can be the one that is effective but with potential disadvantages/limitations in certain patient populations or with less supporting data as compared with the recommended regimens; in certain situations, an alternative regimen may be an optimal regimen for a specific patient population |
| Not recommended | Treatment is clearly inferior compared with the recommended or alternative regimens because of factors such as lower efficacy, unfavorable tolerability, toxicity, longer duration, and/or higher pill burden. Unless otherwise indicated, such regimens should not be administered in HCV-infected patients |
Consensus recommendations on the diagnosis of HCV infection: (1) Anti-HCV testing is recommended for the screening/initial testing of HCV infection. If the result is positive, the current infection should be confirmed with a sensitive HCV ribonucleic acid (RNA)/core antigen test; (2) Qualitative HCV RNA testing is a reasonable, good, and cost-effective method; it can replace quantitative testing in most patients; (3) It is important to consider quantitative viremia in immunocompromised patients; and (4) Genotyping is recommended to guide appropriate selection of the antiviral regimen.
Key international guidelines recommend initial HCV serological testing for the detection of anti-HCV antibodies and the diagnosis of HCV infection[11-13]. In case of a positive HCV test result, the diagnosis of chronic HCV infection may be established with a nucleic acid test or a sensitive nucleic acid diagnostic assay that detects HCV RNA[11-13]. In low- and middle-income countries, the use of a qualitative HCV RNA assay has been found to be feasible for providing broader access to HCV diagnosis and care[12]. A less sensitive alternative to the HCV RNA test for the diagnosis of HCV infection is the detection of the HCV core antigen[12]. The results of initial HCV serological testing may be negative in some HCV-infected cases (e.g., in case of early acute infection, in immunocompromised patients, or in patients on hemodialysis). In these patients, HCV RNA testing should be a part of the initial assessment[12].
Whenever the staging of hepatitis C is deemed necessary, the degree of liver fibrosis/cirrhosis should be assessed using liver biopsy or other noninvasive tests[1,13]. In resource-limited settings, however, the use of liver biopsy may be limited due to cost, invasiveness, and plausible complications, whereas the use of noninvasive tests, such as transient elastography, may be limited by cost and availability constraints. In these settings, serum noninvasive tests, such as the aminotransferase/platelet ratio index (APRI) or fibrosis-4 score, may be useful[11,13]. The APRI has been found to have sufficient sensitivity and specificity for predicting cirrhosis[14]. Besides the detection of liver fibrosis/cirrhosis, testing and detection of the HCV genotype should also be conducted to guide decisions on the choice of treatment[1,13].
Owing to the high prevalence of HCV infection in Ukraine and the CIS region, periodic screening programs should be conducted to detect infected individuals and to ensure a timely management of the disease. According to the Centers for Disease Control and Prevention, routine HCV screening is not recommended for the general population, pregnant women, healthcare workers, or nonsexual contacts of HCV-positive individuals[2]. Serological testing for HCV may be offered to adults born between 1945 and 1965, high-risk individuals, and those with a history of HCV risk exposure or behavior[2,11,13]. In individuals with a positive anti-HCV test result, further confirmation of the diagnosis of HCV infection should be made with an HCV RNA or HCV core antigen assay. Rapid diagnostic tests using serum, plasma, fingerstick whole blood, or saliva may be considered as alternatives to standard enzyme immu-noassays[12].
The treatment of HCV infection should focus on: (1) Achievement of sustained virologic response (SVR); (2) Education in liver-associated adverse effects, such as hepatic cirrhosis, end-stage liver disease, and hepatocellular carcinoma (HCC); (3) Management of extrahepatic manifestations; and (4) Reduction in mortality rate[11]. SVR is defined as the continued absence of detectable HCV RNA and/or HCV core antigen for at least 12 wk after the completion of therapy[11].
Consensus recommendations on pre-treatment assessments: (1) Liver fibrosis assessment: The use of liver biopsy and/or noninvasive markers is recommended for deciding on the regimen and the need for initiating additional measures for the management of cirrhosis (e.g., HCC screening); (2) Assessment for potential drug-drug interactions with concomitant medications is recommended; and (3) Recommended laboratory tests: Complete blood count; hepatic function tests [albumin, total and direct bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase, and alkaline phosphatase levels], international normalized ratio, calculated glomerular filtration rate (GFR), creatinine levels, hepatitis B surface antigen (HBsAg) test, tests for hepatitis B surface antibody (anti-HBs) and antibody to hepatitis B core antigen, additional test for PCR hepatitis B virus (HBV) DNA (quantitative, if the qualitative test yields positive results) in patients with HBsAg and/or antibody to hepatitis B core antigen positivity, and alpha-fetoprotein in patients with cirrhosis.
Pretreatment assessments for optimizing the choice of therapy should include the assessment of virologic parameters and the severity of liver disease. Other important parameters that must be assessed to guide treatment selection include alcohol intake, HBV/human immunodeficiency virus (HIV) co-infection, renal impairment, diabetes mellitus, autoimmunity, and cardiac diseases[12]. Alcohol consumption should be assessed, and, if needed, counseling should be provided to correct the same[12].
Treatment of HCV infection with direct-acting antivirals (DAAs) may result in reactivation of HBV infection in patients with HCV-HBV co-infection[13,15-18]. Patients with HCV-HBV co-infection have been noted to have accelerated progression of liver disease and an increased risk of HCC[11,13,19,20]. However, reactivation of HBV and subsequent hepatitis has been found to be rare in HCV-HBV co-infected patients who are HBsAg-negative or those who have baseline HBV DNA < 2000 IU/mL prior to DAA therapy[21-24]. Therefore, the expert panel recommended that all HCV patients with positive HBsAg/anti-HBs should be tested for HBV DNA (quantitative, if the qualitative test yields positive results). Patients who fulfil the standard treatment criteria for HBV should be initiated on HBV antiviral treatment. Other patients should be monitored periodically by the assessment of HBV DNA and ALT during HCV DAA therapy. Antiviral therapy for HBV infection should be initiated if patients develop HBV reactivation (presence of HBsAg and HBV DNA plus elevation in ALT more than the upper limit of normal[12]. A recent systematic review and meta-analysis has suggested that anti-HBV prophylaxis with tenofovir or entecavir may significantly reduce the risk of HBV reactivation in patients receiving DAA-based treatment[24].
Rapid progression of fibrosis has been noted in individuals with HCV-HIV co-infection. Persistent elevation of liver enzymes, especially aspartate aminotransferase, has been found to be a useful marker to predict the progression of fibrosis in these individuals[25]. Therefore, all individuals with HCV infection should be evaluated for HIV infection prior to deciding on the choice of therapy[11-13]. The plausibility of drug-drug interactions between DAAs and anti-retroviral therapy should be carefully considered in HCV-HIV-co-infected patients, and the choice and dose of DAAs should be optimized accordingly[11,12].
Several extrahepatic manifestations may occur in patients with HCV infection. Hence, these individuals should be assessed for plausible comorbidities, such as renal impairment, diabetes mellitus, autoimmunity, and cardiac diseases[12]. Additionally, assessment of HCV RNA or HCV core antigen and staging of fibrosis/cirrhosis are also important prior to the initiation of treatment for HCV infection. Furthermore, HCV genotype testing may be useful in guiding treatment selection and optimizing treatment outcomes[11,12].
Treatment should be initiated in all individuals with chronic HCV infection, except in patients with a limited life expectancy that cannot be improved by treatment or transplantation. Patients with decompensated cirrhosis should be managed by an expert with relevant clinical experience[11].
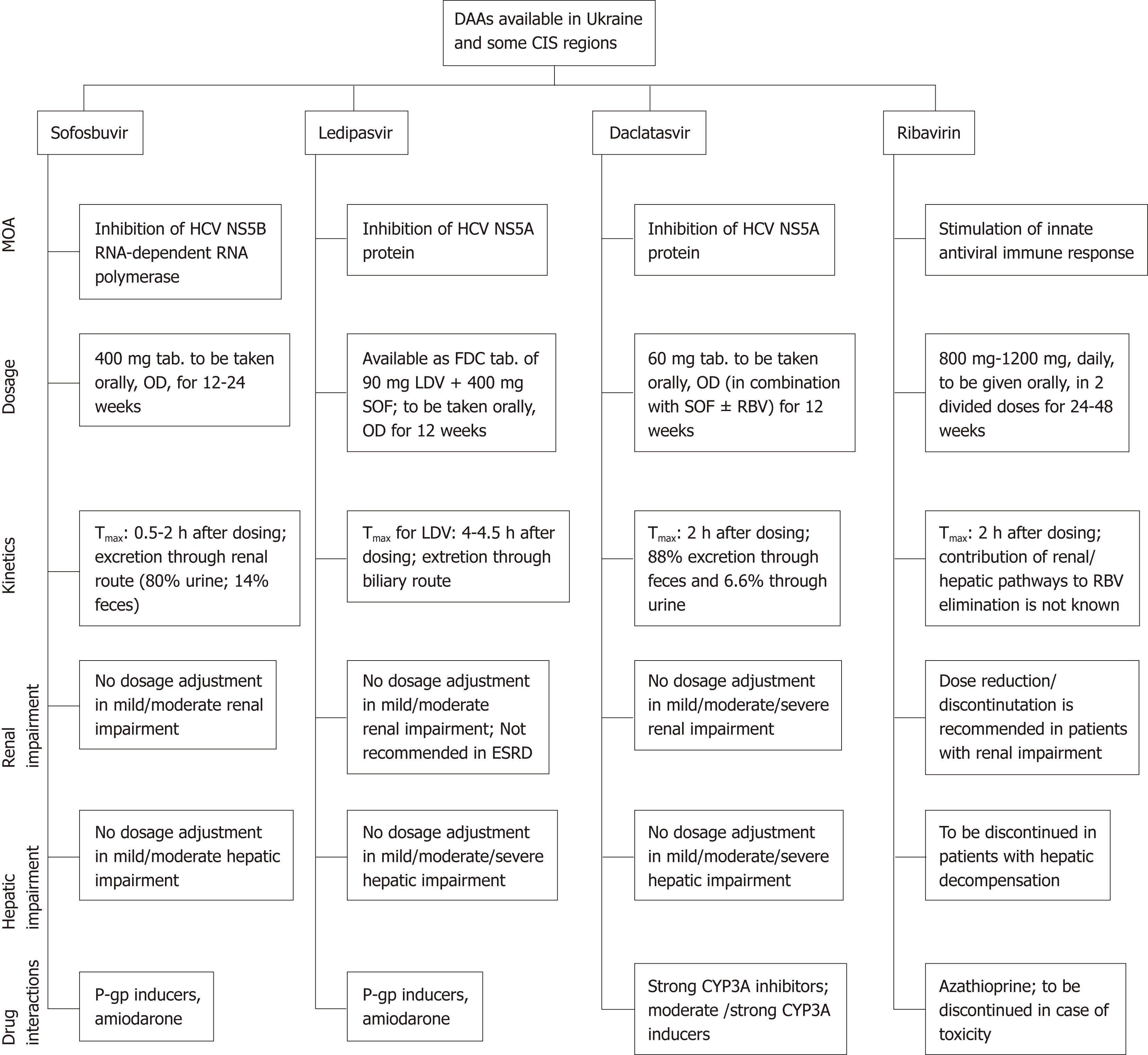
Pegylated interferon (peg-IFN) and ribavirin are still used and listed as first-line medications in Ukraine and some CIS countries. First-generation DAAs, such as boceprevir and telaprevir, that are no longer recommended are also registered in most CIS countries. One or more second-generation DAAs are available in Ukraine and in the majority of CIS regions[6]. A summary of the DAA regimens available in Ukraine and in some CIS regions, as compiled by the expert panel, is presented in Table 2. The pharmacological features of the DAAs available in this region have been described in Figure 1[26-29].
| Country | SOF | LDV/SOF | DCV |
| Uzbekistan | √ | √ | √ |
| Ukraine | √ | √ | |
| Belarus | √ | √ | √ |
| Kazakhstan | √ | √ | √ |
The regimens proposed for the treatment of patients with chronic HCV GT1 infection are listed in Table 3.
| Recommendation category | Treatment option/s | Treatment regimens |
| Preferred | LDV + SOF ± RBV | LDV + SOF for 12 wk |
| In treatment-naïve patients having HCV RNA < 6 million IU/mL in whom cirrhosis has been conclusively ruled out by transient elastography (FibroScan) or biopsy: LDV + SOF for 8 wk | ||
| In treatment-experienced cirrhotic patients/patients with decompensated liver disease/postliver transplant patients: LDV + SOF + RBV for 12 wk (or) LDV + SOF for 24 wk if RBV is ineligible | ||
| Alternative | SOF + DCV ± RBV | SOF + DCV for 12 wk (addition of RBV may be considered if cirrhosis has not been conclusively ruled out) |
| In patients with compensated cirrhosis: SOF + DCV ± weight-based RBV for 24 wk | ||
| In patients with decompensated cirrhosis: SOF + DCV + RBV for 12 wk (or) SOF + DCV for 24 wk if RBV is ineligible | ||
| Not recommended | Due to the advent of newer DAAs, pegylated interferon, boceprevir, and telaprevir-based regimens are not recommended. | |
Sofosbuvir + ledipasvir ± ribavirin: Sofosbuvir in combination with ledipasvir, with or without ribavirin, has been evaluated for the treatment of HCV GT1 infection in several clinical studies worldwide. The phase III ION-1 trial studied the efficacy of this regimen taken for 12 wk or 24 wk in previously untreated, chronic HCV GT1-infected patients (n = 865). About 67% of the patients had GT1a infection, and 16% had cirrhosis. Eligible patients were randomized in a 1:1:1:1 ratio to receive ledipasvir and sofosbuvir fixed-dose combination once daily for 12 wk or 24 wk, or ledipasvir-sofosbuvir + ribavirin for 12 wk or 24 wk. The primary endpoint of SVR at 12 wk after the end of treatment was 99%, 98%, 97%, and 99%, respectively, in the four treatment groups. In patients with cirrhosis, the rates of SVR ranged from 94% to 100% in the four treatment groups[30]. Several other clinical and real-world studies and meta-analyses have also reported the efficacy of this regimen in treating HCV GT1-infected patients, including: (1) Both treatment naïve and treatment-experienced patients[31-49]; (2) Patients with compensated cirrhosis or advanced liver disease[31,36,38,40,44-46,48,50-52]; and (3) Liver transplantation cases (the transplantation cases studied included treatment-naïve as well as treatment-experienced and those with cirrhosis and HCC prior to transplantation)[50,53-59]. The presence of fibrosis, cirrhosis, or HCC has been found to lower the SVR rates with sofosbuvir and ledipasvir combination in HCV GT1-infected patients in a few studies[56,58-62].
The phase III, open-label, randomized, ION-3 trial demonstrated that sofosbuvir in combination with ledipasvir given for a shorter duration of 8 wk to treatment- naïve HCV GT1-infected patients without cirrhosis achieved a 94% SVR rate, comparable to the same regimen given for 12 wk, or given in combination with ribavirin for 8 wk[63]. The effectiveness of the 8-wk regimen in the specified population has also been proven in other clinical and real-world studies[31,64-68].
The ION-4 trial was a multicenter, single-group, open-label study conducted to assess the effectiveness of sofosbuvir and ledipasvir fixed-dose combination in patients co-infected with HIV-1 and HCV GT1 or GT4 infection (n = 335; 55% were previously treated for HCV infection, and 20% had cirrhosis). The study demonstrated a 96% SVR rate at 12 wk after the treatment in patients with HCV GT1a and a 96% SVR rate in patients with HCV GT1b infection. The SVR rates were not affected by previous treatment or cirrhosis status[69]. High SVR rates have also been reported with this regimen in several other clinical and real-world studies in HCV GT1 individuals co-infected with HIV, including: (1) Both treatment-naïve and treatment-experienced patients[42,70-72] and (2) Those with cirrhosis[73,74].
The use of a ribavirin-free sofosbuvir and ledipasvir combination regimen has been found to be associated with a significant improvement in the quality of life in HCV GT1-infected patients, regardless of the treatment history or the presence of cirrhosis or HIV co-infection[75-78]. An increase in toxicity has been noted with the inclusion of ribavirin in the treatment regimen[75,79].
The efficacy and safety of sofosbuvir and ledipasvir combination has also been tested in HCV GT1-infected patients with severe renal insufficiency, including those undergoing dialysis and kidney transplantation with favorable tolerability and SVR rates[56,80-85]. Of note, the safety and SVR rates with this regimen have been noted to be better among noncirrhotic versus cirrhotic HCV GT1-infected patients with renal conditions, in a few studies[56,84].
Sofosbuvir + daclatasvir ± ribavirin: Sofosbuvir + daclatasvir with or without ribavirin has been evaluated in several clinical studies in varied HCV GT1-infected patient populations. This regimen, provided for 12 wk or 24 wk to treatment-naïve (n = 126) and for 24 wk to treatment-experienced (n = 41) HCV GT1-infected patients, has been found to result in high SVR rates (98%) in the open-label AI444040 trial[86]. Another open-label, phase III trial, viz. ALLY-1, included 76% HCV GT1-infected patients with: (1) Cirrhosis (compensated/decompensated) or (2) Postliver transplantation recurrence. The study evaluated the sofosbuvir, daclatasvir, and ribavirin combination regimen for 12 wk. In patients with cirrhosis interrupted by liver transplantation, treatment was extended for an additional period of 12 wk after transplantation. The SVR rates were 82% and 95% in patients with cirrhosis and liver transplant recipients, respectively. The regimen was well-tolerated, with no treatment-related serious adverse events[87]. In real-world settings and large-scale, multicentric studies, an optimal duration of 12 wk and 24 wk has been suggested with this regimen in noncirrhotic and cirrhotic HCV GT1-infected patients, respectively, for achieving favorable SVR rates[47,88]. The efficacy and safety of this regimen have also been proven in other clinical and real-world studies and meta-analyses that enrolled HCV GT1-infected patients, including treatment-experienced patients, patients with cirrhosis or advanced liver disease, and liver transplant recipients[46,49,57,89-95]. The SVR rates in a few studies were found to be lower in cirrhotic versus noncirrhotic HCV GT1-infected patients treated with this regimen[46,96].
The daclatasvir + sofosbuvir regimen has also been found to be effective, with high SVR rates in HCV GT1 patients co-infected with HIV, including treatment-experienced patients, patients with advanced liver disease, and patients undergoing liver transplantation[74,97-103].
Several studies have evaluated the use of this regimen in HCV GT1-infected patients with renal conditions. The combination of sofosbuvir and daclatasvir has been found to be well-tolerated and effective for the treatment of HCV GT1-infected patients with severe renal insufficiency, including those on dialysis or undergoing renal transplantation[80,85,104-107]. Furthermore, a pangenotypic regimen of daclatasvir and half-daily dose of sofosbuvir has been found to be effective for the treatment of HCV GT1-infected patients with an estimated GFR (eGFR) < 30 mL/min with favorable SVR rates (SVR12: 90%-100%)[105,108]. In pharmacokinetic studies, it has been noted that an impaired eGFR (30-60 mL/min) may not lead to the dose accumulation of sofosbuvir in HCV-positive kidney transplant recipients or hemodialysis patients[109,110]. Studies may be needed in future to understand further the kinetic profile of sofosbuvir-based treatment in HCV-positive end-stage kidney disease patients or renal transplant recipients.
The preferred regimens recommended by the expert panel for the treatment of patients with chronic HCV GT2 infection are given in Table 4.
| Recommendation category | Treatment option(s) | Treatment regimen |
| Preferred | SOF + DCV ± RBV | SOF + DCV for 12 wk in noncirrhotics |
| In decompensated cirrhosis and previous failures: | ||
| SOF + DCV + RBV for 12 wk | ||
| SOF + RBV | SOF + RBV for 12 wk in noncirrhotics | |
| To be extended to 24 wk in cirrhotics and treatment failures (Data are not available for patients with decompensated cirrhosis.) | ||
| Should be considered as an alternative regimen when DCV is not available | ||
| Not recommended | Due to the advent of newer DAAs, pegylated interferon, boceprevir, and telaprevir-based regimens are not recommended. | |
Sofosbuvir + daclatasvir ± ribavirin: The AI444040 trial (cited earlier) also included 26 treatment-naïve HCV GT2-infected patients who were treated with the sofosbuvir + daclatasvir regimen with or without ribavirin for 24 wk. SVR was attained in about 92% of these patients[86]. In the ALLY-1 trial, the SVR rate in HCV GT2-infected patients with cirrhosis treated with the sofosbuvir, daclatasvir, and ribavirin combination for 12 wk was 80%[87]. One hundred percent SVR rate was noted in another retrospective study conducted in HCV GT2-infected patients treated with the sofosbuvir and daclatasvir regimen (n = 13), regardless of the degree of baseline fibrosis. The treatment was also found to induce improvement in fibrosis in these patients[111]. The effectiveness of this regimen in treating HCV GT2-infected patients has been proven in routine clinical settings, with an SVR of 88.1%-100% and 94.5%-100% with daclatasvir and sofosbuvir combination with and without ribavirin, respectively[112,113]. Studies have also evaluated the efficacy and safety of this regimen in patients with recurrent HCV GT2 infection post liver transplantation and have reported favorable SVR rates, but the number of patients tested is very low to draw any clinically relevant conclusions in this setting[103].
In HCV GT2-infected patients who cannot tolerate ribavirin, the use of sofosbuvir and daclatasvir for 12 wk in noncirrhotic patients, and for 24 wk in cirrhotic patients, including those with decompensated disease, has been found to achieve high SVR rates 12 wk after the treatment[114].The efficacy of the 12 wk sofosbuvir + daclatasvir regimen has also been proven in patients with HCV GT2 infection, co-infected with HIV-1[97,98].
One hundred percent SVR rate was achieved and no deterioration of renal function was noted in HCV GT2-infected patients with chronic kidney disease treated with sofosbuvir + daclatasvir ± ribavirin regimen[113], and 100% SVR rate was noted in HCV GT2-infected patients with end-stage renal disease (eGFR < 30 mL/min) with daclatasvir full dose plus low-dose sofosbuvir regimen[105]. However, the number of patients evaluated in these studies is too small, and the results need to be substantiated with larger, well-designed studies in future.
Sofosbuvir + ribavirin: The VALENCE trial enrolled HCV GT2- or GT3-infected patients (n = 419; 58% were previously treated with an IFN-based regimen and 21% had cirrhosis). Of the 419 patients, about 91 HCV GT2-infected patients were randomized in a 4:1 ratio to receive sofosbuvir + ribavirin or placebo for 12 wk. The primary endpoint was SVR at 12 wk after the therapy. The study findings revealed that the SVR rate was 93% in HCV GT2-infected patients treated with the sofosbuvir + ribavirin regimen[115]. Several other randomized and real-world studies have also reported high SVR rates with the sofosbuvir and ribavirin regimen (12 wk or 16 wk duration) in HCV GT2-infected patients, regardless of the treatment history or the presence of cirrhosis[96,116-120]. However, the presence of cirrhosis or a history of HCC was found to influence negatively the SVR rates in some real-world studies[121-124].
The efficacy of 48 wk of sofosbuvir and ribavirin combination regimen, given prior to liver transplantation (due to HCC), on the prevention of HCV recurrence post transplantation was assessed in an open-label study in 61 HCV-infected patients (GT2; n = 8). A total of 46 liver transplantations were done, of which 43 had HCV RNA level < 25 IU/mL at the time of transplantation (GT2; n = 6). The primary endpoint of HCV RNA level < 25 IU/mL at 12 wk after transplantation was achieved by all GT2-infected patients, with no evidence of HCV recurrence[125]. In a separate case study, a patient with liver transplant graft re-infected with HCV GT2 infection was safely and successfully treated with sofosbuvir and ribavirin combination regimen[126].
In randomized phase III studies, a treatment extension of about 24 wk with sofosbuvir and ribavirin regimen has been found to result in 100% SVR rates in treatment-experienced, cirrhotic HCV GT2-infected patients[118]. An extended-duration regimen has also been tested in real-world settings; treatment of treatment-naïve or treatment-experienced HCV GT2-infected patients with cirrhosis for up to 20 wk with this regimen resulted in 94.9% SVR rates[127].
The use of this regimen for the treatment of previously untreated and treatment-experienced HCV GT2-infected patients co-infected with HIV-1 for 12 wk and 12 wk or 24 wk, respectively, has been found to yield high SVR rates[128,129].
A recent study evaluated the efficacy and safety of sofosbuvir and ribavirin regimen in 231 HCV GT2-infected patients with renal dysfunction (82.8% and 17.2% with chronic kidney disease stage G1/2, and G3, respectively). While the overall SVR rate was 97%, the SVR rate in chronic kidney disease stages G1, G2, G3a, and G3b were 98.1%, 98.6%, 87.9%, and 100%, respectively. Multivariate analysis revealed that baseline renal dysfunction significantly and negatively influenced the SVR rates, thus suggesting the need for monitoring of baseline renal function in HCV GT2-infected patients treated with this regimen[130].
The preferred and alternative regimens for the treatment of HCV GT3 infection are listed in Table 5.
| Recommendation category | Treatment option(s) | Treatment regimen |
| Preferred | SOF + DCV ± RBV | SOF + DCV for 12 wk (addition of RBV may be considered if cirrhosis has not been conclusively ruled out) |
| In patients with compensated cirrhosis | ||
| Treatment-naïve patients: SOF + DCV + RBV for 24 wk if patients can tolerate ribavirin well, if not SOF+DCV for 24 wk | ||
| Treatment-experienced patients: SOF + DCV + RBV for 24 wk if patients tolerated ribavirin well, if not SOF + DCV for 24 wk | ||
| In patients with decompensated cirrhosis: | ||
| SOF + DCV + RBV for 12 wk | ||
| If RBV is ineligible: SOF + DCV for 24 wk | ||
| Alternative | SOF + RBV | SOF + RBV for 24 wk (should be considered only when preferred regimens are not available) |
| LDV + SOF + RBV | LDV + SOF + RBV for 12 wk (should be considered only when preferred regimens are not available) | |
| Not recommended | Due to the advent of newer DAAs, pegylated interferon, boceprevir, and telaprevir-based regimens are not recommended. | |
Sofosbuvir + daclatasvir ± ribavirin: While the AI444040 trial reported an 89% SVR rate in 18 treatment-naïve HCV GT3-infected patients treated with this regimen for 24 wk, the ALLY-1 trial that enrolled both treatment-naïve and treatment-experienced patients reported an 83% SVR rate in HCV GT3-infected patients with cirrhosis (n = 6) and a 91% SVR rate in liver transplant recipients with posttransplant recurrence of HCV GT3 infection (n = 11)[86,87]. The phase III ALLY-3 trial evaluated the once-daily, 12 wk sofosbuvir + daclatasvir regimen in HCV GT3-infected patients [previously untreated (n = 101) and treatment-experienced (n = 51)]. The SVR rates were 90% and 86% in treatment-naïve and treatment-experienced patients, respectively. About 19% of patients in the treatment-naïve and 25% of patients in the treatment-experienced groups had cirrhosis in this study. The SVR rates were 96% and 63% in patients without and with cirrhosis, respectively[131]. The ALLY-3+ trial was a randomized, phase III trial that included both treatment-naïve and treatment-experienced GT3-infected patients with advanced fibrosis or compensated cirrhosis. The efficacy and safety of daclatasvir + sofosbuvir with ribavirin given for either 12 wk or 16 wk were assessed in this trial. The SVR rates were 88% and 92% in the groups treated with the 12-wk regimen and the 16-wk regimen, respectively. In patients with cirrhosis, the corresponding SVR rates were 83% and 89%, respectively. Previous treatment had no influence on the SVR rates[132]. The ALLY-3C trial was a single-arm, phase III study that evaluated the efficacy and safety of sofosbuvir + daclatasvir + ribavirin regimen given for 24 wk in HCV GT3-infected patients with compensated cirrhosis. While the SVR12 rate was 87% in the primary analysis, the rates in treatment-naïve and treatment-experienced patients were 93% and 79%, receptively. The regimen was well-tolerated[133]. In real-world settings, treatment of HCV GT3-infected patients, including cirrhotic and treatment-experienced patients, and liver transplant recipients (with a history of HCC prior to transplantation) with recurrent cirrhosis, with this regimen has been found to result in high SVR rates[46,56,57,91,112,134-144].
The efficacy and safety of the daclatasvir + sofosbuvir regimen have also been proven in treatment-naïve and treatment-experienced HCV GT3-infected patients co-infected with HIV-1, including those with advanced liver disease and recurrent HCV after liver transplantation[73,97-99,101,103,140].
High SVR rates have been reported with the use of this regimen in HCV GT3-infected patients with advanced chronic kidney disease with an eGFR < 30 mL/min or those on dialysis[80-82,104]. Further, a regimen comprising low-dose sofosbuvir and full dose daclatasvir has been found to be safe and effective in achieving high SVR rates in HCV GT3-infected patients with eGFR < 30 mL/min or those on maintenance hemodialysis[105,108,145]. Full or half-dose sofosbuvir + daclatasvir regimen has also been evaluated in HCV GT3-infected renal transplant recipients with 100% SVR rates[85]. However, the number of patients included in all these studies are small, and large-scale studies may be needed to corroborate the significance of these findings.
Sofosbuvir + ribavirin: The VALENCE trial enrolled HCV GT2 (n = 91) or GT3 (n = 328)-infected patients and randomized them in a 4:1 ratio to receive sofosbuvir-ribavirin or placebo. The duration of treatment for GT3-infected patients was 24 wk. The study findings revealed high SVR rates (85%) in HCV GT3-infected patients[115]. The response rates were 91% and 68% in GT3-infected patients without and with cirrhosis, respectively[115]. Another prospective study reported an overall SVR rate of 99.2% in GT3-infected HCV patients who received sofosbuvir and ribavirin for 24 wk[146]. In a Russian phase IIIb study, treatment of HCV GT3-infected patients with sofosbuvir + ribavirin for 16 wk or 24 wk was found to be safe and associated with 87% and 90% SVR12 rates, respectively[147]. In a recent real-world study, treatment of HCV GT3-infected patients (n = 110) (51 with compensated and 59 with decompensated cirrhosis) with sofosbuvir + ribavirin for 24 wk resulted in achievement of SVR in 83.3% and 71.4% of treatment-naïve and treatment-experienced patients with compensated cirrhosis, respectively; and 77.8% and 75% of treatment-naïve and treatment-experienced patients with decompensated cirrhosis, respectively. The combination was well-tolerated; however, the outcomes in decompensated and treatment-experienced patients were noted to be suboptimal[148]. This combination regimen given for 24 wk was safe and effective in achieving 95% SVR12 and 94% SVR24 in HCV GT3-infected liver transplant recipients with recurrent HCV infection[149]. Administration of this regimen 48 wk before liver transplantation resulted in 80% of HCV GT3-infected patients achieving HCV RNA < 25 IU/mL 12 wk post transplantation[125].
In HCV GT3-infected patients co-infected with HIV, sofosbuvir and ribavirin regimen has been reported to be associated with significantly lower SVR12 when compared to daclatasvir and sofosbuvir regimen[150]. Literature is sparse on the safety and efficacy of this combination in HCV GT3-infected patients with renal conditions.
Sofosbuvir + ledipasvir + ribavirin: In an open-label trial, 12 wk of the sofosbuvir, ledipasvir, and ribavirin regimen administered to treatment-naïve (n = 26) and treatment-experienced (n = 50) HCV GT3-infected patients resulted in 100% and 82% SVR rates, respectively[151]. In another open-label trial, treatment-naïve HCV patients with and without compensated cirrhosis were treated with sofosbuvir, ledipasvir, and weight-based ribavirin for 12 wk. About 95% of the patients had GT3a infection. The overall SVR rate was 89%, with 79% and 94% SVR rates in patients with and without cirrhosis, respectively[152]. Real-world studies have reported ≥ 90% SVR12 rate with this regimen in HCV GT3-infected patients, including those with cirrhosis and advanced or compensated liver disease[46,134,140-142,153].
Evidence on the efficacy and safety of this regimen in HCV GT3-infected patients co-infected with HIV is limited to a few real-world studies[73,140,153], in which the SVR rates have been reported to be 100%, > 90%, and 80% in patients without and with compensated or decompensated cirrhosis, respectively[73].
Studies on the efficacy and safety of this regimen in HCV GT3-infected liver transplant recipients or patients with renal conditions are limited.
The treatment options for HCV GT4-infected patients listed in Table 6 have been recommended by the expert panel.
| Recommendation category | Treatment option(s) | Treatment regimen |
| Preferred | LDV + SOF ± RBV | LDV + SOF for 12 wk [Addition of RBV may be considered based on the physician’s discretion in treating difficult-to-treat patients (treatment-experienced patients, patients with cirrhosis)]. |
| In case of previous SOF treatment failure: LDV + SOF + RBV for 12 wk | ||
| Alternative | SOF + DCV ± RBV | SOF + DCV for 12 wk (Addition of RBV may be considered if cirrhosis has not been conclusively ruled out.) |
| Cirrhosis of any class: SOF + DCV + RBV for 12 wk | ||
| If RBV is ineligible, SOF + DCV for 24 wk | ||
| Not recommended | Due to the advent of newer DAAs, pegylated interferon, boceprevir, and telaprevir-based regimens are not recommended. | |
Sofosbuvir + ledipasvir + ribavirin: Several phase II studies have established the efficacy of the 12-wk sofosbuvir and ledipasvir regimen in the treatment of HCV GT4-infected patients, regardless of the treatment history or the presence of cirrhosis[154,155]. Administering the combination of this regimen with ribavirin for 12 wk or 24 wk also resulted in high SVR rates in phase II studies on HCV GT4-infected patients with advanced liver diseases[77]. Similarly, high SVR rates have been noted in a phase III study that used a 12-wk sofosbuvir and ledipasvir regimen with or without ribavirin for the treatment of HCV GT4-infected and cirrhotic patients (including both treatment-naïve and treatment-experienced patients)[156]. In another cohort study, the sofosbuvir and ledipasvir combination administered for 12 wk was associated with a 99% SVR rate in HCV GT4-infected patients[157]. In real-world studies and meta-analyses, favorable SVR rates have been noted with the sofosbuvir and ledipasvir regimen with or without ribavirin given for 12 wk or 24 wk in HCV GT4-infected patients, including treatment-naïve and treatment-experienced patients[49,158,159] and patients with advanced liver fibrosis and compensated and decompensated cirrhosis[158,159]. The addition of ribavirin has not been found to improve the efficacy of the combination regimen[158,159]. In a recent real-world study, an 8-wk regimen of ledipasvir + sofosbuvir was found to be well-tolerated and effective in treatment-naïve and noncirrhotic HCV GT4-infected patients (n = 45) (SVR12: 97.8%)[160]. Studies evaluating the efficacy of sofosbuvir + ledipasvir + ribavirin regimen in HCV GT4-infected liver transplant recipients are limited.
The ION-4 trial was a large phase III trial that enrolled HCV GT1- or GT4-infected patients co-infected with HIV-1 who were treated with the sofosbuvir and ledipasvir regimen for 12 wk. About 55% of the patients were treatment-experienced, and 20% had cirrhosis. The SVR rate was noted to be 100% in the GT4-infected patients[69]. In real-world studies, treatment of HCV GT4-infected patients co-infected with HIV with ledipasvir and sofosbuvir regimen was associated with 96%, 94%, and 80% SVR rate in patients without cirrhosis and with compensated and decompensated cirrhosis, respectively[73].
Very few studies have evaluated the efficacy of sofosbuvir + ledipasvir ± ribavirin in HCV GT4-infected renal transplant recipients. One hundred percent SVR12 rates have been noted in these studies with good safety profile of the regimen[83,85]. However, the number of patients evaluated in these studies is too small, and results from studies in larger patient populations may be needed to translate these findings to clinical practice.
Sofosbuvir + daclatasvir ± ribavirin: In the ALLY-1 trial, the combination of sofosbuvir + daclatasvir with ribavirin for 12 wk or 24 wk was associated with a 100% SVR rate in GT4-infected patients with cirrhosis[87]. In another cohort study, the sofosbuvir and daclatasvir combination for 12 wk was associated with a 96% SVR in HCV GT4-infected patients[161]. A separate prospective study categorized HCV GT4-infected patients into two groups: Group 1 included treatment-naïve patients treated with sofosbuvir + daclatasvir for 12 wk; and group 2 included treatment-experienced patients treated with sofosbuvir + daclatasvir + ribavirin for 12 wk (sofosbuvir-experienced patients were treated for 24 wk). The SVR12 rate was 93.3% and 87.5% in groups 1 and 2, respectively. A significant improvement in liver fibrosis was also noted with the treatment in this study[162]. Real-world studies have also supported the efficacy of this combination regimen (with or without ribavirin) with high SVR rates in HCV GT4-infected patients[103,163,164], including those with decompensated cirrhosis and HCV recurrence after liver transplantation[103,163].
Daclatasvir + sofosbuvir has also been found to result in favorable SVR rates in HCV GT4-infected patients co-infected with HIV-1, including those with cirrhosis and advanced liver disease[73,97-101].
Studies evaluating the efficacy and safety of this regimen in HCV GT4-infected patients with renal conditions are limited.
The preferred and alternative regimens for the treatment of HCV GT5 or GT6 infections are listed in Table 7.
| Recommendation category | Treatment option(s) | Treatment regimen |
| Preferred | LDV + SOF ± RBV | LDV + SOF for 12 wk [Addition of RBV may be considered based on the physician’s discretion in treating difficult-to-treat patients (treatment-experienced patients, patients with cirrhosis)]. |
| In case of previous SOF treatment failure: LDV + SOF + RBV for 12 wk | ||
| Alternative | SOF + DCV ± RBV | SOF + DCV for 12 wk (Addition of RBV may be considered if cirrhosis has not been conclusively ruled out.) |
| Cirrhosis of any class: SOF + DCV + RBV for 12 wk | ||
| If RBV is ineligible, SOF + DCV for 24 wk | ||
| Not recommended | Due to the advent of newer DAAs, pegylated interferon, boceprevir, and telaprevir-based regimens are not recommended. | |
Sofosbuvir + ledipasvir ± ribavirin: The regimen of sofosbuvir and ledipasvir without ribavirin was evaluated in a single-arm, open-label, phase II trial, in GT5-infected treatment-naïve and treatment-experienced patients, including those with cirrhosis (n = 41). The treatment was provided for 12 wk. While the overall SVR rate with the combination was found to be 95%, the SVR rates in patients with and without cirrhosis were 89% and 97%, respectively[165]. Another prospective, open-label, multicentric study evaluated the efficacy and safety of ledipasvir and sofosbuvir combination given for 8 wk or 12 wk in 60 HCV GT6-infected patients. There were two patients with decompensation, three with liver cancer, and 14 with prior treatment exposure in the 12-wk group. The SVR12 rate was 95% in both the 8- wk and 12-wk treatment groups, and the regimen was found to be safe[166]. In another study conducted by Gane et al[151], the combination was evaluated with or without ribavirin for 12 wk in 126 treatment-naïve or treatment-experienced patients with HCV GT3 or GT6 infection (n = 25 for GT6). The SVR rate in patients with HCV GT6 infection was 96%. Recent real-world studies, systematic reviews, and meta-analyses have also reported high SVR rates (up to 100%) in HCV GT6-infected patients treated with this combination[47,49,167].
There is limited evidence on the efficacy and safety of this combination in HCV GT6-infected patients undergoing liver transplantation or with concomitant renal conditions.
Sofosbuvir + daclatasvir ± ribavirin: Data on the use of this regimen in the treatment of HCV GT5- or GT6-infected patients are limited. In the open-label ALLY-1 study, SVR was achieved in a single GT6-infected liver transplant recipient treated with the combination[87]. Real-world studies and systematic reviews have reported 94%-100% SVR rate with daclatasvir and sofosbuvir combination in HCV GT6-infected patients[47,167,168]. Data on the efficacy of this combination in HCV GT6-infected kidney transplant recipients are limited to studies with very limited patient population[169].
The expert panel recommended several on- and posttreatment assessments that should be conducted during the management of HCV infection (Table 8).
| Assessments | Expert recommendations |
| On-treatment | In patients with cirrhosis, CBC, creatinine level, estimated GFR, and hepatic function panel may be repeated after 4 wks |
| All patients on RBV should have CBC done at four and 8 wk to monitor for hemolysis | |
| HCV RNA testing (qualitative/quantitative) may not be required, as there are no current recommendations for response-guided therapy. Testing at the end of treatment is mandatory | |
| Assessment of potential drug-drug interactions with concomitant medications is recommended | |
| A periodic review of therapy compliance and the general condition of the patient is recommended | |
| Posttreatment | SVR should be assessed at 12 wk or 24 wk after the end of treatment |
| In patients who have failed therapy: | |
| Disease progression (hepatic function panel, CBC, and INR) should be assessed once in 6-12 mo | |
| In patients with advanced fibrosis (Metavir stages F3 or F4), screening for hepatocellular carcinoma with ultrasound is recommended every 6 mo | |
| Endoscopic screening for esophageal varices is recommended in cirrhotic patients | |
| In patients who achieve SVR: | |
| In patients with advanced fibrosis (Metavir stage F3 or F4), screening for hepatocellular carcinoma with ultrasound is recommended in every 6 mo | |
| Endoscopic screening for esophageal varices is recommended in cirrhotic patients with pretreatment varices or portal hypertensive gastropathy | |
| AFP as a screening test for HCC is recommended in cirrhotic patients |
On-treatment assessments help monitor treatment efficacy and safety, evaluate drug-drug interactions, and ensure medication adherence. In all HCV-infected patients receiving DAA-containing regimens (with or without ribavirin or peg-IFN), complete blood count, renal function tests, and hepatic function panel test should be conducted 4 wk after therapy initiation. Assessment for any side effects or drug-drug interactions, and treatment adherence, is also recommended in the fourth week of treatment[13]. In patients treated with ribavirin-containing regimens, complete blood count should be conducted at 4 wk and 8 wk of therapy to assess for any significant drop in hemoglobin levels[12,13]. Considering the resource-limited settings in the CIS and Ukraine regions, and the lack of any standard recommendations, the expert panel did not recommend HCV RNA testing during treatment[12,13]. However, the panel recommended HCV RNA testing at the end of therapy[11].
Posttreatment follow-up and assessment help confirm the elimination of the virus and prevent relapses. The expert panel recommended the assessment of HCV RNA at 12 wk or 24 wk after completion of therapy, for evaluation of SVR12 or SVR24, in line with the other international recommendations[11-13]. Furthermore, the panel provided posttreatment assessment recommendations for two categories of patients: (1) Those who have failed the therapy and (2) Those who achieved SVR.
Patients who fail the therapy not only remain carriers of the virus but also experience continued liver injury and fibrosis progression[170]. The incidence of death or liver transplantation can be as high as 12.2% in patients with advanced fibrosis and 31.5% among patients with cirrhosis[170]. Therefore, it is important to systematically assess reasons for failure of therapy in these patients. Such patients should also be followed up regularly to assess disease progression. Furthermore, patients with advanced fibrosis (Metavir stage F3 or F4) should be evaluated for HCC every 6 mo using ultrasound surveillance[12].
Among patients who have achieved SVR, those with advanced fibrosis (Metavir stage F3 or F4) should be evaluated for HCC every 6 mo using ultrasound surveillance. Furthermore, patients with cirrhosis should be screened endoscopically for esophageal varices and evaluated with alpha-fetoprotein test to screen for HCC[12,13].
The emergence of HCV variants with substitutions associated with resistance to DAAs is critical and is particularly noted with NS5A inhibitor-containing regimens. These substitutions are termed resistance-associated substitutions (RAS). Resistant HCV viruses that are enriched in patients with DAA therapy failure contain substitutions termed treatment-emergent RAS. Both baseline and treatment-selected RAS may negatively impact the response rate and treatment outcomes[11,13]. The RAS in the NS5A position for HCV genotypes 1a and 3 are currently considered clinically significant. Methods to detect RAS include population (Sanger) sequencing and deep sequencing [next generation sequencing], with 15% prevalence of RAS as the recommended cutoff[11].
Access to reliable HCV resistance testing techniques is limited in the resource constraint settings of Ukraine and CIS regions. Hence, no consensus could be formed on the methods of HCV resistance testing and reporting, and no recommendations could be made on the systematic testing for HCV resistance prior to DAA treatment or monitoring for HCV drug resistance during or after therapy. However, the following approaches were proposed to help overcome resistance: (1) Optimal risk stratification of patients, based on prior treatment, or degree of cirrhosis; (2) Determination of HCV genotype and subtype so as to help optimize the treatment approach; (3) Optimization of treatment duration and careful selection of patients for short-duration therapy; (4) Addition of ribavirin in selected populations, such as those with prior DAA failure or at risk of treatment failure, and those with baseline NS5A RAS; and (5) Optimal selection of DAA therapy combinations[11,13].
In Ukraine and the CIS regions, several challenges hinder the optimal management of HCV infection, including the lack of sufficient epidemiological data, the disparity in genotype distribution, barriers in access to diagnostics, lack of updated national guidelines, and financial constraints. The use of peg-IFN, ribavirin, and first-generation DAAs is still prevalent in these regions, with very few second-generation DAAs being available in most of the regions. There is a clear unmet need for the development of a guidance document for the optimal screening, diagnosis, monitoring, and management of HCV infection with the use of cost-effective, available DAA regimens. The current consensus document compiles the evidence-based recommendations on the diagnosis and management of HCV infection provided by key opinion leaders (from Ukraine and the CIS regions) in the field of hepatology. This document will help guide clinical decision-making on the diagnosis, treatment, and pre-, on-, and posttreatment assessments of HCV infection, further optimizing the treatment outcomes in these regions.
| 1. | World Health Organization. Hepatitis C. 2018. Available from: http://www.who.int/en/news-room/fact-sheets/detail/hepatitis-c. |
| 2. | Centers for Disease control and prevention. Hepatitis C. 2018. Accessed on: 01 August 2018; Available from: https://www.cdc.gov/hepatitis/hcv/index.htm. |
| 3. | Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1493] [Cited by in RCA: 1513] [Article Influence: 168.1] [Reference Citation Analysis (0)] |
| 4. | Hill AM, Nath S, Simmons B. The road to elimination of hepatitis C: analysis of cures versus new infections in 91 countries. J Virus Erad. 2017;3:117-123. [PubMed] |
| 5. | Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016;22:7824-7840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 514] [Cited by in RCA: 584] [Article Influence: 58.4] [Reference Citation Analysis (11)] |
| 6. | Maistat L, Kravchenko N, Reddy A. Hepatitis C in Eastern Europe and Central Asia: a survey of epidemiology, treatment access and civil society activity in eleven countries. Hepatol Med Policy. 2017;2:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | World Health Organization. Assessment of the viral hepatitis response in Ukraine. 2017; Available from: http://www.euro.who.int/data/assets/pdf_file/0007/372697/ukr-hepatitis-report-eng.PDF. |
| 8. | Bivol S, Sarang A. Hepatitis C in Russia: an epidemic of negligence. The Andrey Rylkov Foundation for Health and Social Justice. 2013.. Available from: http://en.rylkov-fond.org/wp-content/uploads/2014/07/ARF-HCV-report-2013-final_eng.pdf. |
| 9. | Leblebicioglu H, Arends JE, Ozaras R, Corti G, Santos L, Boesecke C, Ustianowski A, Duberg AS, Ruta S, Salkic NN, Husa P, Lazarevic I, Pineda JA, Pshenichnaya NY, Tsertswadze T, Matičič M, Puca E, Abuova G, Gervain J, Bayramli R, Ahmeti S, Koulentaki M, Kilani B, Vince A, Negro F, Sunbul M, Salmon D; ESGHV (part of ESCMID). Availability of hepatitis C diagnostics and therapeutics in European and Eurasia countries. Antiviral Res. 2018;150:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Buscarons AC. Strategies to Increase Access to Hepatitis C Treatment: A Question of Price or Public Health? Study based on the situation in the Eastern European selected countries Russia, Ukraine and Georgia. 2015. 2017; Available from: https://www.isglobal.org/documents/10179/3408669/Strategies+to+Increase+Access+to+Hepatitis+C+Treatment/2024ff58-b41a-41d1-9a96-7c19343ffe6c. |
| 11. | American Association for the Study of Liver Disease. Recommendations for Testing, Managing, and Treating Hepatitis C. 2018; Available from: https://www.hcvguidelines.org/sites/default/files/full-guidance-pdf/HCVGuidance _May_24_2018a.pdf. |
| 12. | European Association for the Study of the Liver. Recommendations on Treatment of Hepatitis C 2018. Available from: http://www.easl.eu/medias/cpg/2018/EASL%20Recommendations%20on%20Treatment%20of%20Hepatitis%20C%202018/English-report.pdf. |
| 13. | World Health Organization. Guidelines for the screening, care and treatment of persons with chronic hepatitis C infection Updated version, April 2016. Available from: http://apps.who.int/iris/bitstream/handle/10665/ 205035/9789241549615_eng.pdf;jsessionid=3CEAF78983CE7CDCC8CD39E2990145C7?sequence=1. |
| 14. | APRI calculator.. Available from: https://www.hepatitisc.uw.edu/page/clinical-calculators/apri. |
| 15. | Calvaruso V, Craxì A. HBV recurrence after HCV clearance on DAAs: Sometimes they come back. J Hepatol. 2017;67:898-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Aggeletopoulou I, Konstantakis C, Manolakopoulos S, Triantos C. Risk of hepatitis B reactivation in patients treated with direct-acting antivirals for hepatitis C. World J Gastroenterol. 2017;23:4317-4323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Liu CJ, Chuang WL, Sheen IS, Wang HY, Chen CY, Tseng KC, Chang TT, Massetto B, Yang JC, Yun C, Knox SJ, Osinusi A, Camus G, Jiang D, Brainard DM, McHutchison JG, Hu TH, Hsu YC, Lo GH, Chu CJ, Chen JJ, Peng CY, Chien RN, Chen PJ. Efficacy of Ledipasvir and Sofosbuvir Treatment of HCV Infection in Patients Coinfected With HBV. Gastroenterology. 2018;154:989-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 18. | Fabbri G, Mastrorosa I, Vergori A, Mazzotta V, Pinnetti C, Grisetti S, Zaccarelli M, Ammassari A, Antinori A. Reactivation of occult HBV infection in an HIV/HCV Co-infected patient successfully treated with sofosbuvir/ledipasvir: a case report and review of the literature. BMC Infect Dis. 2017;17:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Jamma S, Hussain G, Lau DT. Current Concepts of HBV/HCV Coinfection: Coexistence, but Not Necessarily in Harmony. Curr Hepat Rep. 2010;9:260-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Konstantinou D, Deutsch M. The spectrum of HBV/HCV coinfection: epidemiology, clinical characteristics, viralinteractions and management. Ann Gastroenterol. 2015;28:221-228. [PubMed] |
| 21. | Tamori A, Abiru S, Enomoto H, Kioka K, Korenaga M, Tani J, Enomoto M, Sugiyama M, Masaki T, Kawada N, Yatsuhashi H, Nishiguchi S, Mizokami M. Low incidence of hepatitis B virus reactivation and subsequent hepatitis in patients with chronic hepatitis C receiving direct-acting antiviral therapy. J Viral Hepat. 2018;25:608-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Ogawa E, Furusyo N, Murata M, Toyoda K, Hayashi T, Ura K. Potential risk of HBV reactivation in patients with resolved HBV infection undergoing direct-acting antiviral treatment for HCV. Liver Int. 2018;38:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Gane EJ, Hyland RH, An D, Svarovskaia ES, Brainard D, McHutchison JG. Ledipasvir and sofosbuvir for HCV infection in patients coinfected with HBV. Antivir Ther. 2016;21:605-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Jiang XW, Ye JZ, Li YT, Li LJ. Hepatitis B reactivation in patients receiving direct-acting antiviral therapy or interferon-based therapy for hepatitis C: A systematic review and meta-analysis. World J Gastroenterol. 2018;24:3181-3191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Konerman MA, Mehta SH, Sutcliffe CG, Vu T, Higgins Y, Torbenson MS, Moore RD, Thomas DL, Sulkowski MS. Fibrosis progression in human immunodeficiency virus/hepatitis C virus coinfected adults: prospective analysis of 435 liver biopsy pairs. Hepatology. 2014;59:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 26. | Sofosbuvir tablets for oral use. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/204671s002lbl.pdf. |
| 27. | Ledipasvir and sofosbuvir tablets for oral use. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205834s000lbl.pdf. |
| 28. | Daclatasvir tablets for oral use. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206843Orig1s000lbl.pdf. |
| 29. | Ribavirin tablets. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021511s023lbl.pdf. |
| 30. | Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P, Foster GR, Bräu N, Buti M, Jacobson IM, Subramanian GM, Ding X, Mo H, Yang JC, Pang PS, Symonds WT, McHutchison JG, Muir AJ, Mangia A, Marcellin P; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1357] [Cited by in RCA: 1376] [Article Influence: 114.7] [Reference Citation Analysis (1)] |
| 31. | Lawitz E, Poordad FF, Pang PS, Hyland RH, Ding X, Mo H, Symonds WT, McHutchison JG, Membreno FE. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2014;383:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 443] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 32. | Gane EJ, Stedman CA, Hyland RH, Ding X, Svarovskaia E, Subramanian GM, Symonds WT, McHutchison JG, Pang PS. Efficacy of nucleotide polymerase inhibitor sofosbuvir plus the NS5A inhibitor ledipasvir or the NS5B non-nucleoside inhibitor GS-9669 against HCV genotype 1 infection. Gastroenterology. 2014;146:736-743.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 33. | Mizokami M, Yokosuka O, Takehara T, Sakamoto N, Korenaga M, Mochizuki H, Nakane K, Enomoto H, Ikeda F, Yanase M, Toyoda H, Genda T, Umemura T, Yatsuhashi H, Ide T, Toda N, Nirei K, Ueno Y, Nishigaki Y, Betular J, Gao B, Ishizaki A, Omote M, Mo H, Garrison K, Pang PS, Knox SJ, Symonds WT, McHutchison JG, Izumi N, Omata M. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. Lancet Infect Dis. 2015;15:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 303] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 34. | Chuang WL, Chien RN, Peng CY, Chang TT, Lo GH, Sheen IS, Wang HY, Chen JJ, Yang JC, Knox SJ, Gao B, Garrison KL, Mo H, Pang PS, Hsu YC, Hu TH, Chu CJ, Kao JH. Ledipasvir/sofosbuvir fixed-dose combination tablet in Taiwanese patients with chronic genotype 1 hepatitis C virus. J Gastroenterol Hepatol. 2016;31:1323-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Lim YS, Ahn SH, Lee KS, Paik SW, Lee YJ, Jeong SH, Kim JH, Yoon SK, Yim HJ, Tak WY, Han SY, Yang JC, Mo H, Garrison KL, Gao B, Knox SJ, Pang PS, Kim YJ, Byun KS, Kim YS, Heo J, Han KH. A phase IIIb study of ledipasvir/sofosbuvir fixed-dose combination tablet in treatment-naïve and treatment-experienced Korean patients chronically infected with genotype 1 hepatitis C virus. Hepatol Int. 2016;10:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Wei L, Xie Q, Hou JL, Tang H, Ning Q, Cheng J, Nan Y, Zhang L, Li J, Jiang J, McNabb B, Zhang F, Camus G, Mo H, Osinusi A, Brainard DM, Gong G, Mou Z, Wu S, Wang G, Hu P, Gao Y, Jia J, Duan Z. Ledipasvir/sofosbuvir for treatment-naive and treatment-experienced Chinese patients with genotype 1 HCV: an open-label, phase 3b study. Hepatol Int. 2018;12:126-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, Nahass R, Ghalib R, Gitlin N, Herring R, Lalezari J, Younes ZH, Pockros PJ, Di Bisceglie AM, Arora S, Subramanian GM, Zhu Y, Dvory-Sobol H, Yang JC, Pang PS, Symonds WT, McHutchison JG, Muir AJ, Sulkowski M, Kwo P; ION-2 Investigators. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1065] [Cited by in RCA: 1066] [Article Influence: 88.8] [Reference Citation Analysis (1)] |
| 38. | Lim JK, Liapakis AM, Shiffman ML, Lok AS, Zeuzem S, Terrault NA, Park JS, Landis CS, Hassan M, Gallant J, Kuo A, Pockros PJ, Vainorius M, Akushevich L, Michael L, Fried MW, Nelson DR, Ben-Ari Z; HCV-TARGET Study Group. Safety and Effectiveness of Ledipasvir and Sofosbuvir, With or Without Ribavirin, in Treatment-Experienced Patients With Genotype 1 Hepatitis C Virus Infection and Cirrhosis. Clin Gastroenterol Hepatol. 2018;16:1811-1819.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Akuta N, Sezaki H, Suzuki F, Fujiyama S, Kawamura Y, Hosaka T, Kobayashi M, Kobayashi M, Saitoh S, Suzuki Y, Arase Y, Ikeda K, Kumada H. Ledipasvir plus sofosbuvir as salvage therapy for HCV genotype 1 failures to prior NS5A inhibitors regimens. J Med Virol. 2017;89:1248-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | Bourlière M, Bronowicki JP, de Ledinghen V, Hézode C, Zoulim F, Mathurin P, Tran A, Larrey DG, Ratziu V, Alric L, Hyland RH, Jiang D, Doehle B, Pang PS, Symonds WT, Subramanian GM, McHutchison JG, Marcellin P, Habersetzer F, Guyader D, Grangé JD, Loustaud-Ratti V, Serfaty L, Metivier S, Leroy V, Abergel A, Pol S. Ledipasvir-sofosbuvir with or without ribavirin to treat patients with HCV genotype 1 infection and cirrhosis non-responsive to previous protease-inhibitor therapy: a randomised, double-blind, phase 2 trial (SIRIUS). Lancet Infect Dis. 2015;15:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 228] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 41. | Aqel B, Leise M, Vargas HE, Watt KD, Keaveny AP, Zhang N, Zhang N, Pungpapong S. Multicenter Experience using Ledipasvir/Sofosbuvir ± RBV to Treat HCV GT 1 Relapsers after Simeprevir and Sofosbuvir Treatment. Ann Hepatol. 2018;17:815-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Tam E, Luetkemeyer AF, Mantry PS, Satapathy SK, Ghali P, Kang M, Haubrich R, Shen X, Ni L, Camus G, Copans A, Rossaro L, Guyer B, Brown RS; RESCUE and ACTG A5348 study investigators. Ledipasvir/sofosbuvir for treatment of hepatitis C virus in sofosbuvir-experienced, NS5A treatment-naïve patients: Findings from two randomized trials. Liver Int. 2018;38:1010-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Real-world effectiveness of ledipasvir/sofosbuvir in 4,365 treatment-naive, genotype 1 hepatitis C-infected patients. Hepatology. 2016;64:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 159] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 44. | Mehta V, Mahajan R, Midha V, Narang V, Kaur K, Singh A, Malhotra A, Parvez A, Sood A. Impact of Direct Acting Antiviral Therapy for Treatment of Hepatitis C Genotypes 1, 3 and 4: A Real Life Experience from India. J Clin Exp Hepatol. 2018;8:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 45. | Flisiak R, Łucejko M, Mazur W, Janczewska E, Berak H, Tomasiewicz K, Mozer-Lisewska I, Kozielewicz D, Gietka A, Sikorska K, Wawrzynowicz-Syczewska M, Nowak K, Zarębska-Michaluk D, Musialik J, Simon K, Garlicki A, Pleśniak R, Baka-Ćwierz B, Olszok I, Augustyniak K, Stolarz W, Białkowska J, Badurek A, Piekarska A. Effectiveness and safety of ledipasvir/sofosbuvir±ribavirin in the treatment of HCV infection: The real-world HARVEST study. Adv Med Sci. 2017;62:387-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 46. | Del Rio-Valencia JC, Asensi-Diez R, Villalobos-Torres L, Muñoz Castillo I. Direct-acting antiviral agents in patients with hepatitis C genotype 1-4 infections in a tertiary hospital. Rev Esp Quimioter. 2018;31:226-236. [PubMed] |
| 47. | Hu C, Yuan G, Liu J, Huang H, Ren Y, Li Y, Chen X, Li W, Wu T, Deng H, Peng Y, Zhang YY, Zhou Y. Sofosbuvir-Based Therapies for Patients with Hepatitis C Virus Infection: Real-World Experience in China. Can J Gastroenterol Hepatol. 2018;2018:3908767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | He QF, Zhang QF, Zhang DZ. Efficacy and Safety of Ribavirin with Sofosbuvir Plus Ledipasvir in Patients with Genotype 1 Hepatitis C: A Meta-Analysis. Dig Dis Sci. 2016;61:3108-3117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Ferreira VL, Leonart LP, Tonin FS, Borba HHL, Pontarolo R. Sustained Virological Response in Special Populations with Chronic Hepatitis C Using Interferon-Free Treatments: A Systematic Review and Meta-analysis of Observational Cohort Studies. Clin Drug Investig. 2018;38:389-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS, Fried MW, Terrault NA, O'Leary JG, Vargas HE, Kuo A, Schiff E, Sulkowski MS, Gilroy R, Watt KD, Brown K, Kwo P, Pungpapong S, Korenblat KM, Muir AJ, Teperman L, Fontana RJ, Denning J, Arterburn S, Dvory-Sobol H, Brandt-Sarif T, Pang PS, McHutchison JG, Reddy KR, Afdhal N; SOLAR-1 Investigators. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients With Advanced Liver Disease. Gastroenterology. 2015;149:649-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 638] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 51. | Tsuji K, Kurosaki M, Itakura J, Mori N, Takaki S, Hasebe C, Akahane T, Joko K, Yagisawa H, Takezawa J, Nakata R, Kusakabe A, Kojima Y, Kimura H, Tamada T, Kobashi H, Mitsuda A, Kondou M, Ogawa C, Uchida Y, Sohda T, Narita R, Izumi N. Real-world efficacy and safety of ledipasvir and sofosbuvir in patients with hepatitis C virus genotype 1 infection: a nationwide multicenter study by the Japanese Red Cross Liver Study Group. J Gastroenterol. 2018;53:1142-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 52. | Ahmed H, Elgebaly A, Abushouk AI, Hammad AM, Attia A, Negida A. Safety and efficacy of sofosbuvir plus ledipasvir with and without ribavirin for chronic HCV genotype-1 infection: a systematic review and meta-analysis. Antivir Ther. 2017;22:369-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Saab S, Rheem J, Jimenez MA, Fong TM, Mai MH, Kachadoorian CA, Esmailzadeh NL, Bau SN, Kang S, Ramirez SD, Grotts J, Choi G, Durazo FA, El-Kabany MM, Han SB, Busuttil RW. Effectiveness of Ledipasvir/Sofosbuvir with/without Ribavarin in Liver Transplant Recipients with Hepatitis C. J Clin Transl Hepatol. 2017;5:101-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Ueda Y, Ikegami T, Akamatsu N, Soyama A, Shinoda M, Goto R, Okajima H, Yoshizumi T, Taketomi A, Kitagawa Y, Eguchi S, Kokudo N, Uemoto S, Maehara Y. Treatment with sofosbuvir and ledipasvir without ribavirin for 12 weeks is highly effective for recurrent hepatitis C virus genotype 1b infection after living donor liver transplantation: a Japanese multicenter experience. J Gastroenterol. 2017;52:986-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 55. | Shoreibah M, Orr J, Jones D, Zhang J, Venkata K, Massoud O. Ledipasvir/sofosbuvir without ribavirin is effective in the treatment of recurrent hepatitis C virus infection post-liver transplant. Hepatol Int. 2017;11:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Saxena V, Khungar V, Verna EC, Levitsky J, Brown RS, Hassan MA, Sulkowski MS, O'Leary JG, Koraishy F, Galati JS, Kuo AA, Vainorius M, Akushevich L, Nelson DR, Fried MW, Terrault N, Reddy KR. Safety and efficacy of current direct-acting antiviral regimens in kidney and liver transplant recipients with hepatitis C: Results from the HCV-TARGET study. Hepatology. 2017;66:1090-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 57. | Rupp C, Hippchen T, Neuberger M, Sauer P, Pfeiffenberger J, Stremmel W, Gotthardt DN, Mehrabi A, Weiss KH. Successful combination of direct antiviral agents in liver-transplanted patients with recurrent hepatitis C virus. World J Gastroenterol. 2018;24:1353-1360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Liao HT, Tan P, Huang JW, Yuan KF. Ledipasvir + Sofosbuvir for Liver Transplant Recipients With Recurrent Hepatitis C: A Systematic Review and Meta-analysis. Transplant Proc. 2017;49:1855-1863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Liu J, Ma B, Cao W, Li M, Bramer WM, Peppelenbosch MP, Pan Q. Direct-acting antiviral agents for liver transplant recipients with recurrent genotype 1 hepatitis C virus infection: Systematic review and meta-analysis. Transpl Infect Dis. 2019;21:e13047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 60. | Ji F, Wei B, Yeo YH, Ogawa E, Zou B, Stave CD, Li Z, Dang S, Furusyo N, Cheung RC, Nguyen MH. Systematic review with meta-analysis: effectiveness and tolerability of interferon-free direct-acting antiviral regimens for chronic hepatitis C genotype 1 in routine clinical practice in Asia. Aliment Pharmacol Ther. 2018;47:550-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 61. | Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Real-world effectiveness and predictors of sustained virological response with all-oral therapy in 21,242 hepatitis C genotype-1 patients. Antivir Ther. 2017;22:481-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 62. | Itokawa N, Atsukawa M, Tsubota A, Ikegami T, Shimada N, Kato K, Abe H, Okubo T, Arai T, Iwashita AN, Kondo C, Mikami S, Asano T, Matsuzaki Y, Toyoda H, Kumada T, Iio E, Tanaka Y, Iwakiri K. Efficacy of direct-acting antiviral treatment in patients with compensated liver cirrhosis: A multicenter study. Hepatol Res. 2019;49:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 63. | Kowdley KV, Gordon SC, Reddy KR, Rossaro L. Ledipasvir and Sofosbuvir for 8 or 12 Weeks for Chronic HCV without Cirrhosis. N Engl J Med. 2014;370:1879-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 911] [Cited by in RCA: 929] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 64. | Kowdley KV, Sundaram V, Jeon CY, Qureshi K, Latt NL, Sahota A, Lott S, Curry MP, Tsai N, Chaiyakunapruk N, Lee Y, Petersen J, Buggisch P. Eight weeks of ledipasvir/sofosbuvir is effective for selected patients with genotype 1 hepatitis C virus infection. Hepatology. 2017;65:1094-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 65. | Lai JB, Witt MA, Pauly MP, Ready J, Allerton M, Seo S, Witt DJ. Eight- or 12-Week Treatment of Hepatitis C with Ledipasvir/Sofosbuvir: Real-World Experience in a Large Integrated Health System. Drugs. 2017;77:313-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Yanny B, Saab S, Durazo F, Latt N, Mitry A, Mikhail MM, Hanna RM, Aziz A, Sahota A. Eight-Week Hepatitis C Treatment with New Direct Acting Antivirals Has a Better Safety Profile While Being Effective in the Treatment-Naïve Geriatric Population Without Liver Cirrhosis and Hepatitis C Virus-RNA < 6 Million IU/mL. Dig Dis Sci. 2018;63:3480-3486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 67. | Buggisch P, Vermehren J, Mauss S, Günther R, Schott E, Pathil A, Boeker K, Zimmermann T, Teuber G, Vornkahl HP, Simon KG, Niederau C, Wedemeyer H, Zeuzem S. Real-world effectiveness of 8-week treatment with ledipasvir/sofosbuvir in chronic hepatitis C. J Hepatol. 2018;68:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 68. | Latt NL, Yanny BT, Gharibian D, Gevorkyan R, Sahota AK. Eight-week ledipasvir/sofosbuvir in non-cirrhotic, treatment-naïve hepatitis C genotype-1 patients with hepatitis C virus-RNA < 6 million: Single center, real world effectiveness and safety. World J Gastroenterol. 2017;23:4759-4766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 69. | Naggie S, Cooper C, Saag M, Workowski K. Ledipasvir and Sofosbuvir for HCV in Patients Coinfected with HIV-1. N Engl J Med. 2015;373:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 383] [Article Influence: 34.8] [Reference Citation Analysis (1)] |
| 70. | Townsend K. High Efficacy of Sofosbuvir/Ledipasvir for the Treatment of HCV Genotype 1 in Patients Coinfected With HIV on or off Antiretroviral therapy: Results from The NIAID ERADICATE Trial. AASLD LiverLearning®. 2014; 60133.. . |
| 71. | Osinusi A, Townsend K, Kohli A, Nelson A, Seamon C, Meissner EG, Bon D, Silk R, Gross C, Price A, Sajadi M, Sidharthan S, Sims Z, Herrmann E, Hogan J, Teferi G, Talwani R, Proschan M, Jenkins V, Kleiner DE, Wood BJ, Subramanian GM, Pang PS, McHutchison JG, Polis MA, Fauci AS, Masur H, Kottilil S. Virologic response following combined ledipasvir and sofosbuvir administration in patients with HCV genotype 1 and HIV co-infection. JAMA. 2015;313:1232-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 173] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 72. | Bhattacharya D, Belperio PS, Shahoumian TA, Loomis TP, Goetz MB, Mole LA, Backus LI. Effectiveness of All-Oral Antiviral Regimens in 996 Human Immunodeficiency Virus/Hepatitis C Virus Genotype 1-Coinfected Patients Treated in Routine Practice. Clin Infect Dis. 2017;64:1711-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 73. | Berenguer J. Gil-Martin Á, Jarrin I, Moreno A, Dominguez L, Montes M, Aldámiz-Echevarría T, Téllez MJ, Santos I, Benitez L, Sanz J, Ryan P, Gaspar G, Alvarez B, Losa JE, Torres-Perea R, Barros C, Martin JVS, Arponen S, de Guzmán MT, Monsalvo R, Vegas A, Garcia-Benayas MT, Serrano R, Gotuzzo L, Menendez MA, Belda LM, Malmierca E, Calvo MJ, Cruz-Martos E, González-García JJ. All-oral direct-acting antiviral therapy against hepatitis C virus (HCV) in human immunodeficiency virus/HCV-coinfected subjects in real-world practice: Madrid coinfection registry findings. Hepatology. 2018;68:32-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 74. | Navarro J, Laguno M, Vilchez HH, Guardiola JM, Carrion JA, Force L, Cairó M, Cifuentes C, Vilaró J, Cucurull J, Marco A, Roget M, Erice E, Crespo M; Catalano-Balear Study Group. Efficacy and safety of direct antiviral agents in a cohort of cirrhotic HCV/HIV-coinfected patients. J Antimicrob Chemother. 2017;72:2850-2856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 75. | Younossi ZM, Stepanova M, Omata M, Mizokami M, Walters M, Hunt S. Quality of life of Japanese patients with chronic hepatitis C treated with ledipasvir and sofosbuvir. Medicine (Baltimore). 2016;95:e4243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 76. | Younossi ZM, Stepanova M, Chan HL, Lee MH, Yu ML, Dan YY, Choi MS, Henry L. Patient-reported Outcomes in Asian Patients With Chronic Hepatitis C Treated With Ledipasvir and Sofosbuvir. Medicine (Baltimore). 2016;95:e2702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 77. | Manns M, Samuel D, Gane EJ, Mutimer D, McCaughan G, Buti M, Prieto M, Calleja JL, Peck-Radosavljevic M, Müllhaupt B, Agarwal K, Angus P, Yoshida EM, Colombo M, Rizzetto M, Dvory-Sobol H, Denning J, Arterburn S, Pang PS, Brainard D, McHutchison JG, Dufour JF, Van Vlierberghe H, van Hoek B, Forns X; SOLAR-2 investigators. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis. 2016;16:685-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 358] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 78. | Stokes W, Fenton C, Clement F, James M, Ronksley P, Tang KL. The Efficacy and Safety of 12 Weeks of Sofosbuvir and Ledipasvir versus Sofosbuvir, Ledipasvir, and Ribavirin in Patients with Chronic Hepatitis C, Genotype 1, Who Have Cirrhosis and Have Failed Prior Therapy: A Systematic Review and Meta-Analysis. Can J Gastroenterol Hepatol. 2017;2017:6468309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 79. | Tao T, Jiang X, Chen Y, Song Y. Efficacy and Safety of Ledipasvir/Sofosbuvir with and without Ribavirin in Patients with Chronic Hepatitis C Virus Genotype 1 Infection: a meta-analysis. Int J Infect Dis. 2017;55:56-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 80. | Singh A, Kumari S, Kumar P, De A, Singh V. Sofosbuvir with NS5A inhibitors in hepatitis C virus infection with severe renal insufficiency. J Viral Hepat. 2018;25:1501-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 81. | Akhil MS, Kirushnan B, Martin M, Arumugam K, Ganesh Prasad NK, Ravichandran R. Sofosbuvir-based treatment is safe and effective in Indian hepatitis C patients on maintenance haemodialysis: A retrospective study. Nephrology (Carlton). 2018;23:446-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 82. | Manoj Kumar. Nayak SL, Gupta E, Kataria A, Sarin SK. Generic sofosbuvir-based direct-acting antivirals in hepatitis C virus-infected patients with chronic kidney disease. Liver Int. 2018;38:2137-2148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 83. | Colombo M, Aghemo A, Liu H, Zhang J, Dvory-Sobol H, Hyland R, Yun C, Massetto B, Brainard DM, McHutchison JG, Bourlière M, Peck-Radosavljevic M, Manns M, Pol S. Treatment With Ledipasvir-Sofosbuvir for 12 or 24 Weeks in Kidney Transplant Recipients With Chronic Hepatitis C Virus Genotype 1 or 4 Infection: A Randomized Trial. Ann Intern Med. 2017;166:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 84. | Fernández I, Muñoz-Gómez R, Pascasio JM, Baliellas C, Polanco N, Esforzado N, Arias A, Prieto M, Castells L, Cuervas-Mons V, Hernández O, Crespo J, Calleja JL, Forns X, Londoño MC. Efficacy and tolerability of interferon-free antiviral therapy in kidney transplant recipients with chronic hepatitis C. J Hepatol. 2017;66:718-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 85. | Taneja S, Duseja A, De A, Kumar V, Ramachandran R, Sharma A, Dhiman RK, Gupta KL, Chawla Y. Successful treatment of chronic hepatitis C infection with directly acting antivirals in renal transplant recipients. Nephrology (Carlton). 2018;23:876-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 86. | Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy R. Daclatasvir plus Sofosbuvir for Previously Treated or Untreated Chronic HCV Infection. N Engl J Med. 2014;370:211-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 914] [Article Influence: 76.2] [Reference Citation Analysis (1)] |
| 87. | Poordad F, Schiff ER, Vierling JM, Landis C, Fontana RJ, Yang R, McPhee F, Hughes EA, Noviello S, Swenson ES. Daclatasvir with sofosbuvir and ribavirin for hepatitis C virus infection with advanced cirrhosis or post-liver transplantation recurrence. Hepatology. 2016;63:1493-1505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 348] [Cited by in RCA: 349] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 88. | Pol S, Bourliere M, Lucier S, Hezode C, Dorival C, Larrey D, Bronowicki JP, Ledinghen VD, Zoulim F, Tran A, Metivier S, Zarski JP, Samuel D, Guyader D, Marcellin P, Minello A, Alric L, Thabut D, Chazouilleres O, Riachi G, Bourcier V, Mathurin P, Loustaud-Ratti V, D'Alteroche L, Fouchard-Hubert I, Habersetzer F, Causse X, Geist C, Rosa I, Gournay J, Saillard E, Billaud E, Petrov-Sanchez V, Diallo A, Fontaine H, Carrat F; ANRS/AFEF HEPATHER study group. Safety and efficacy of daclatasvir-sofosbuvir in HCV genotype 1-mono-infected patients. J Hepatol. 2017;66:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 89. | Welzel TM, Petersen J, Herzer K, Ferenci P, Gschwantler M, Wedemeyer H, Berg T, Spengler U, Weiland O, van der Valk M, Rockstroh J, Peck-Radosavljevic M, Zhao Y, Jimenez-Exposito MJ, Zeuzem S. Daclatasvir plus sofosbuvir, with or without ribavirin, achieved high sustained virological response rates in patients with HCV infection and advanced liver disease in a real-world cohort. Gut. 2016;65:1861-1870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 149] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 90. | Boglione L, Pinna SM, Cardellino CS, De Nicolò A, Cusato J, Carcieri C, Cariti G, Di Perri G, D'Avolio A. Treatment with daclatasvir and sofosbuvir for 24 weeks without ribavirin in cirrhotic patients who failed first-generation protease inhibitors. Infection. 2017;45:103-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 91. | Calvaruso V, Mazzarelli C, Milazzo L, Badia L, Pasulo L, Guaraldi G, Lionetti R, Villa E, Borghi V, Carrai P, Alberti A, Biolato M, Piai G, Persico M, Santantonio T, Felder M, Angelico M, Montalbano M, Mancusi RL, Grieco A, Angeli E, D'Offizi G, Fagiuoli S, Belli L, Verucchi G, Puoti M, Craxì A. Daclatasvir-based regimens in HCV cirrhosis: experience from the Italian early access program. Sci Rep. 2019;9:585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 92. | Fontana RJ, Brown RS, Moreno-Zamora A, Prieto M, Joshi S, Londoño MC, Herzer K, Chacko KR, Stauber RE, Knop V, Jafri SM, Castells L, Ferenci P, Torti C, Durand CM, Loiacono L, Lionetti R, Bahirwani R, Weiland O, Mubarak A, ElSharkawy AM, Stadler B, Montalbano M, Berg C, Pellicelli AM, Stenmark S, Vekeman F, Ionescu-Ittu R, Emond B, Reddy KR. Daclatasvir combined with sofosbuvir or simeprevir in liver transplant recipients with severe recurrent hepatitis C infection. Liver Transpl. 2016;22:446-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 93. | Liao H, Tan P, Zhu Z, Yan X, Huang J. Sofosbuvir in combination with daclatasvir in liver transplant recipients with HCV infection: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2017;41:262-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 94. | Lionetti R, Calvaruso V, Piccolo P, Mancusi RL, Mazzarelli C, Fagiuoli S, Montalbano M, Lenci I, Carrai P, Guaraldi G, Visco-Comandini U, Milana M, Biolato M, Loiacono L, Valente G, Craxì A, Angelico M, D'offizi G. Sofosbuvir plus daclatasvir with or without ribavirin is safe and effective for post-transplant hepatitis C recurrence and severe fibrosis and cirrhosis: A prospective study. Clin Transplant. 2018;32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 95. | Mucenic M, Bandeira de Mello Brandao A, Marroni CA, Medeiros Fleck A, Zanotelli ML, Kiss G, Meine MH, Leipnitz I, Soares Schlindwein E, Martini J, Costabeber AM, Sacco FKF, Cracco Cantisani GP. Daclatasvir and Sofosbuvir With or Without Ribavirin in Liver Transplant Recipients: A Single-Center Real-World Study. Transplant Proc. 2018;50:769-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 96. | Miotto N, Mendes LC, Zanaga LP, Lazarini MSK, Goncales ESL, Pedro MN, Goncales FL, Stucchi RSB, Vigani AG. All-oral direct antiviral treatment for hepatitis C chronic infection in a real-life cohort: The role of cirrhosis and comorbidities in treatment response. PLoS One. 2018;13:e0199941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 97. | Wyles DL, Ruane PJ, Sulkowski MS, Dieterich D, Luetkemeyer A, Morgan TR, Sherman KE, Dretler R, Fishbein D, Gathe JC, Henn S, Hinestrosa F, Huynh C, McDonald C, Mills A, Overton ET, Ramgopal M, Rashbaum B, Ray G, Scarsella A, Yozviak J, McPhee F, Liu Z, Hughes E, Yin PD, Noviello S, Ackerman P; ALLY-2 Investigators. Daclatasvir plus Sofosbuvir for HCV in Patients Coinfected with HIV-1. N Engl J Med. 2015;373:714-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 346] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 98. | Luetkemeyer AF, McDonald C, Ramgopal M, Noviello S, Bhore R, Ackerman P. 12 Weeks of Daclatasvir in Combination With Sofosbuvir for HIV-HCV Coinfection (ALLY-2 Study): Efficacy and Safety by HIV Combination Antiretroviral Regimens. Clin Infect Dis. 2016;62:1489-1496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 99. | Lacombe K, Fontaine H, Dhiver C, Metivier S, Rosenthal E, Antonini T, Valantin MA, Miailhes P, Harent S, Batisse D, Pageaux GP, Chas J, Aumaitre H, Dominguez S, Allegre T, Lafeuillade A, Billaud E, De Truchis P, Perre P, Leroy V, De Ledinghen V, Sogni P, Dabis F, Zhao Y, Filipovics A, Fedchuk L, Akremi R, Bennai Y, Salmon Ceron D. Real-World Efficacy of Daclatasvir and Sofosbuvir, With and Without Ribavirin, in HIV/HCV Coinfected Patients With Advanced Liver Disease in a French Early Access Cohort. J Acquir Immune Defic Syndr. 2017;75:97-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 100. | Mandorfer M, Schwabl P, Steiner S, Scheiner B, Chromy D, Bucsics T, Stättermayer AF, Aichelburg MC, Grabmeier-Pfistershammer K, Trauner M, Reiberger T, Peck-Radosavljevic M. Interferon-free treatment with sofosbuvir/daclatasvir achieves sustained virologic response in 100% of HIV/hepatitis C virus-coinfected patients with advanced liver disease. AIDS. 2016;30:1039-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 101. | Rockstroh JK, Ingiliz P, Petersen J, Peck-Radosavljevic M, Welzel TM, Van der Valk M, Zhao Y, Jimenez-Exposito MJ, Zeuzem S. Daclatasvir plus sofosbuvir, with or without ribavirin, in real-world patients with HIV-HCV coinfection and advanced liver disease. Antivir Ther. 2017;22:225-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 102. | Campos-Varela I, Moreno A, Morbey A, Guaraldi G, Hasson H, Bhamidimarri KR, Castells L, Grewal P, Baños I, Bellot P, Brainard DM, McHutchison JG, Terrault NA. Treatment of severe recurrent hepatitis C after liver transplantation in HIV infected patients using sofosbuvir-based therapy. Aliment Pharmacol Ther. 2016;43:1319-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 103. | Castells L, Llaneras J, Campos-Varela I, Bilbao I, Crespo M, Len O, Rodríguez-Frías F, Charco R, Salcedo T, Esteban JI, Esteban-Mur R. Sofosbuvir and daclatasvir in mono- and HIV-coinfected patients with recurrent hepatitis C after liver transplant. Ann Hepatol. 2017;16:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 104. | Gupta A, Arora P, Jain P. Sofosbuvir Based Regimen in Management of Hepatitis C for Patients With End Stage Renal Disease on Hemodialysis: A Single Center Experience from India. J Clin Exp Hepatol. 2018;8:116-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 105. | Taneja S, Duseja A, De A, Mehta M, Ramachandran R, Kumar V, Kohli HS, Gupta KL, Dhiman RK, Chawla Y. Low-Dose Sofosbuvir Is Safe and Effective in Treating Chronic Hepatitis C in Patients with Severe Renal Impairment or End-Stage Renal Disease. Dig Dis Sci. 2018;63:1334-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 106. | Duerr M, Schrezenmeier EV, Lehner LJ, Bergfeld L, Glander P, Marticorena Garcia SR, Althoff CE, Sack I, Brakemeier S, Eckardt KU, Budde K, Halleck F. A prospective study of daclatasvir and sofosbuvir in chronic HCV-infected kidney transplant recipients. BMC Nephrol. 2019;20:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 107. | Huang H, Tang H, Deng H, Shen J, Zhou Q, Xie W, Wu J, Chen J. Treatment of chronic hepatitis C viral infection with sofosbuvir and daclatasvir in kidney transplant recipients. Transpl Infect Dis. 2019;21:e13018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 108. | Goel A, Bhadauria DS, Kaul A, Verma P, Mehrotra M, Gupta A, Sharma RK, Rai P, Aggarwal R. Daclatasvir and reduced-dose sofosbuvir: An effective and pangenotypic treatment for hepatitis C in patients with estimated glomerular filtration rate <30 mL/min. Nephrology (Carlton). 2019;24:316-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 109. | Schrezenmeier E, Hoffmann F, Jaeger C, Schrezenmeier J, Lisec J, Glander P, Algharably E, Kreutz R, Budde K, Duerr M, Halleck F. Pharmacokinetics of Daclatasvir, Sofosbuvir, and GS-331007 in a Prospective Cohort of Hepatitis C Virus-Positive Kidney Transplant Recipients. Ther Drug Monit. 2019;41:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 110. | Desnoyer A, Pospai D, Lê MP, Gervais A, Heurgué-Berlot A, Laradi A, Harent S, Pinto A, Salmon D, Hillaire S, Fontaine H, Zucman D, Simonpoli AM, Muret P, Larrouy L, Bernard Chabert B, Descamps D, Yazdanpanah Y, Peytavin G. Pharmacokinetics, safety and efficacy of a full dose sofosbuvir-based regimen given daily in hemodialysis patients with chronic hepatitis C. J Hepatol. 2016;65:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 111. | Lv DD, Wang ML, Chen EQ, Wu DB, Tao YC, Zhang DM, Tang H. A retrospective study of the efficacy of sofosbuvir plus NS5A inhibitors for patients with hepatitis C virus genotype-2 chronic infection. Eur J Gastroenterol Hepatol. 2019;31:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 112. | Belperio PS, Shahoumian TA, Loomis TP, Mole LA, Backus LI. Real-world effectiveness of daclatasvir plus sofosbuvir and velpatasvir/sofosbuvir in hepatitis C genotype 2 and 3. J Hepatol. 2019;70:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 113. | Cheng PN, Chiu YC, Chien SC, Chiu HC. Real-world effectiveness and safety of sofosbuvir plus daclatasvir with or without ribavirin for genotype 2 chronic hepatitis C in Taiwan. J Formos Med Assoc. 2019;118:907-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 114. | Mangia A, Arleo A, Copetti M, Miscio M, Piazzolla V, Santoro R, Squillante MM. The combination of daclatasvir and sofosbuvir for curing genotype 2 patients who cannot tolerate ribavirin. Liver Int. 2016;36:971-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 115. | Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, Illeperuma A, Svarovskaia E, Brainard DM, Symonds WT, Subramanian GM, McHutchison JG, Weiland O, Reesink HW, Ferenci P, Hézode C, Esteban R; VALENCE Investigators. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370:1993-2001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 638] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 116. | Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR, Jacobson IM, Kowdley KV, Nyberg L, Subramanian GM, Hyland RH, Arterburn S, Jiang D, McNally J, Brainard D, Symonds WT, McHutchison JG, Sheikh AM, Younossi Z, Gane EJ. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1322] [Cited by in RCA: 1330] [Article Influence: 102.3] [Reference Citation Analysis (0)] |
| 117. | Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, Shiffman ML, Lawitz E, Everson G, Bennett M, Schiff E, Al-Assi MT, Subramanian GM, An D, Lin M, McNally J, Brainard D, Symonds WT, McHutchison JG, Patel K, Feld J, Pianko S, Nelson DR; POSITRON Study; FUSION Study. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 838] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 118. | Foster GR, Pianko S, Brown A, Forton D, Nahass RG, George J, Barnes E, Brainard DM, Massetto B, Lin M, Han B, McHutchison JG, Subramanian GM, Cooper C, Agarwal K; BOSON Study Group. Efficacy of sofosbuvir plus ribavirin with or without peginterferon-alfa in patients with hepatitis C virus genotype 3 infection and treatment-experienced patients with cirrhosis and hepatitis C virus genotype 2 infection. Gastroenterology. 2015;149:1462-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 185] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 119. | Welzel TM, Nelson DR, Morelli G, Di Bisceglie A, Reddy RK, Kuo A, Lim JK, Darling J, Pockros P, Galati JS, Frazier LM, Alqahtani S, Sulkowski MS, Vainorius M, Akushevich L, Fried MW, Zeuzem S; HCV-TARGET Study Group. Effectiveness and safety of sofosbuvir plus ribavirin for the treatment of HCV genotype 2 infection: results of the real-world, clinical practice HCV-TARGET study. Gut. 2017;66:1844-1852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 120. | Tacke F, Günther R, Buggisch P, Klinker H, Schober A, John C, Lutz T, Pfeiffer-Vornkahl H, Niederau C, Cornberg M, Sarrazin C, Mauss S. Treatment of HCV genotype 2 with sofosbuvir and ribavirin results in lower sustained virological response rates in real life than expected from clinical trials. Liver Int. 2017;37:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 121. | Ogawa E, Furusyo N, Nomura H, Takahashi K, Higashi N, Kawano A, Dohmen K, Satoh T, Azuma K, Nakamuta M, Koyanagi T, Kato M, Shimoda S, Kajiwara E, Hayashi J; Kyushu University Liver Disease Study (KULDS) Group. Effectiveness and safety of sofosbuvir plus ribavirin for HCV genotype 2 patients 65 and over with or without cirrhosis. Antiviral Res. 2016;136:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 122. | Akahane T, Kurosaki M, Itakura J, Tsuji K, Joko K, Kimura H, Nasu A, Ogawa C, Kojima Y, Hasebe C, Wada S, Uchida Y, Sohda T, Suzuki H, Yoshida H, Kusakabe A, Tamada T, Kobashi H, Mitsuda A, Kondo M, Shigeno M, Ide Y, Morita A, Kitamura T, Abe T, Izumi N. Real-world efficacy and safety of sofosbuvir + ribavirin for hepatitis C genotype 2: A nationwide multicenter study by the Japanese Red Cross Liver Study Group. Hepatol Res. 2019;49:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 123. | Han SY, Woo HY, Heo J, Park SG, Pyeon SI, Park YJ, Kim DU, Kim GH, Kim HH, Song GA, Cho M. The predictors of sustained virological response with sofosbuvir and ribavirin in patients with chronic hepatitis C genotype 2. Korean J Intern Med. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 124. | Yada M, Miyazaki M, Tanaka K, Masumoto A, Motomura K. Hepatocellular carcinoma or interferon-based therapy history attenuates sofosbuvir/ribavirin for Japanese genotype 2 hepatitis C virus. World J Gastroenterol. 2018;24:1478-1485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 125. | Curry MP, Forns X, Chung RT, Terrault NA, Brown R, Fenkel JM, Gordon F, O'Leary J, Kuo A, Schiano T, Everson G, Schiff E, Befeler A, Gane E, Saab S, McHutchison JG, Subramanian GM, Symonds WT, Denning J, McNair L, Arterburn S, Svarovskaia E, Moonka D, Afdhal N. Sofosbuvir and ribavirin prevent recurrence of HCV infection after liver transplantation: an open-label study. Gastroenterology. 2015;148:100-107.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 251] [Article Influence: 22.8] [Reference Citation Analysis (1)] |
| 126. | Sasaki R, Kanda T, Ohtsuka M, Yasui S, Haga Y, Nakamura M, Yokoyama M, Wu S, Nakamoto S, Arai M, Maruyama H, Miyazaki M, Yokosuka O. Successful Management of Graft Reinfection of HCV Genotype 2 in Living Donor Liver Transplantation from a Hepatitis B Core Antibody-Positive Donor with Sofosbuvir and Ribavirin. Case Rep Gastroenterol. 2016;10:366-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 127. | Mangia A, Susser S, Piazzolla V, Agostinacchio E, De Stefano G, Palmieri V, Spinzi G, Carraturo I, Potenza D, Losappio R, Arleo A, Miscio M, Santoro R, Sarrazin C, Copetti M. Sofosbuvir and ribavirin for genotype 2 HCV infected patients with cirrhosis: A real life experience. J Hepatol. 2017;66:711-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 128. | Sulkowski MS, Naggie S, Lalezari J, Fessel WJ, Mounzer K, Shuhart M, Luetkemeyer AF, Asmuth D, Gaggar A, Ni L, Svarovskaia E, Brainard DM, Symonds WT, Subramanian GM, McHutchison JG, Rodriguez-Torres M, Dieterich D; PHOTON-1 Investigators. Sofosbuvir and ribavirin for hepatitis C in patients with HIV coinfection. JAMA. 2014;312:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 203] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 129. | Molina JM, Orkin C, Iser DM, Zamora FX, Nelson M, Stephan C, Massetto B, Gaggar A, Ni L, Svarovskaia E, Brainard D, Subramanian GM, McHutchison JG, Puoti M, Rockstroh JK; PHOTON-2 study team. Sofosbuvir plus ribavirin for treatment of hepatitis C virus in patients co-infected with HIV (PHOTON-2): a multicentre, open-label, non-randomised, phase 3 study. Lancet. 2015;385:1098-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 146] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 130. | Sho T, Suda G, Nagasaka A, Yamamoto Y, Furuya K, Kumagai K, Uebayashi M, Terashita K, Kobayashi T, Tsunematsu I, Onodera M, Meguro T, Kimura M, Ito J, Umemura M, Izumi T, Kawagishi N, Ohara M, Ono Y, Nakai M, Natsuizaka M, Morikawa K, Ogawa K, Sakamoto N; NORTE Study Group. Safety and efficacy of sofosbuvir and ribavirin for genotype 2 hepatitis C Japanese patients with renal dysfunction. Hepatol Res. 2018;48:529-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 131. | Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N, Freilich BF, Younes ZH, Harlan W, Ghalib R, Oguchi G, Thuluvath PJ, Ortiz-Lasanta G, Rabinovitz M, Bernstein D, Bennett M, Hawkins T, Ravendhran N, Sheikh AM, Varunok P, Kowdley KV, Hennicken D, McPhee F, Rana K, Hughes EA; ALLY-3 Study Team. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61:1127-1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 504] [Cited by in RCA: 513] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 132. | Leroy V, Angus P, Bronowicki JP, Dore GJ, Hezode C, Pianko S, Pol S, Stuart K, Tse E, McPhee F, Bhore R, Jimenez-Exposito MJ, Thompson AJ. Daclatasvir, sofosbuvir, and ribavirin for hepatitis C virus genotype 3 and advanced liver disease: A randomized phase III study (ALLY-3+). Hepatology. 2016;63:1430-1441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 211] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 133. | Poordad F, Shiffman ML, Ghesquiere W, Wong A, Huhn GD, Wong F, Ramji A, Shafran SD, McPhee F, Yang R, Noviello S, Linaberry M; ALLY-3C study team. Daclatasvir and sofosbuvir with ribavirin for 24 weeks in chronic hepatitis C genotype-3-infected patients with cirrhosis: a Phase III study (ALLY-3C). Antivir Ther. 2019;24:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 134. | Del Rio-Valencia JC, Asensi-Diez R, Madera-Pajin R, Yunquera-Romero L, Muñoz-Castillo I. Interferon-free treatments in patients with hepatitis C genotype 3 infection in a tertiary hospital. Rev Esp Quimioter. 2018;31:35-42. [PubMed] |
| 135. | Hézode C, De Ledinghen V, Fontaine H, Zoulim F, Lebray P, Boyer N, Larrey D, Silvain C, Botta-Fridlund D, Leroy V, Bourliere M, D’Alteroche L, Hubert-Fouchard I, Guyader D, Rosa I, Nguyen-Khac E, Di Martino V, Carrat F, Fedchuk L, Akremi R, Bennai Y, Bronowicki JP. Daclatasvir plus sofosbuvir with or without ribavirin in patients with HCV genotype 3 infection: Interim analysis of a French multicenter compassionate use program. J Hepatol. 2015;62 Suppl 2:S265-266. [DOI] [Full Text] |
| 136. | Leroy V, Hezode C, Metivier S, Tateo M, Conti F, Nguyen-Khac E, Lacoste D, Vergniol J, Truchi R, Guyader D, Riachi G, Michau C, Blaison D, Oberti F, Fontaine H, Di Martino V, Bronowicki JP, Akremi R, Bennai Y, Filipovics A, Pageaux GP. Daclatasvir plus sofosbuvir with or without ribavirin in patients with HCV infection and decompensated cirrhosis: Interim analysis of a French multicentres, compassionate use programme. J Hepatol. 2016;64:S829. |
| 137. | Hézode C, Lebray P, De Ledinghen V, Zoulim F, Di Martino V, Boyer N, Larrey D, Botta-Fridlund D, Silvain C, Fontaine H, D'Alteroche L, Leroy V, Bourliere M, Hubert-Fouchard I, Guyader D, Rosa I, Nguyen-Khac E, Fedchuk L, Akremi R, Bennai Y, Filipovics A, Zhao Y, Bronowicki JP. Daclatasvir plus sofosbuvir, with or without ribavirin, for hepatitis C virus genotype 3 in a French early access programme. Liver Int. 2017;37:1314-1324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 138. | Petersen J, Welzel TM, Herzer K, Ferenci P, Gschwantler M, Cornberg M, Ingiliz P, Berg T, Spengler U, Weiland O, van der Valk M, Klinker H, Rockstroh J, Peck-Radosavljevic M, Zhao Y, Jimenez-Exposito MJ, Zeuzem S. Daclatasvir plus sofosbuvir with or without ribavirin for the treatment of chronic HCV infection in patients with decompensated cirrhosis: Results of a European multicentre compassionate use programme. J Hepatol. 2016;64:S781-S782. [DOI] [Full Text] |
| 139. | Tao YC, Deng R, Wang ML, Lv DD, Yuan M, Wang YH, Chen EQ, Tang H. Satisfactory virological response and fibrosis improvement of sofosbuvir-based regimens for Chinese patients with hepatitis C virus genotype 3 infection: results of a real-world cohort study. Virol J. 2018;15:150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 140. | Wehmeyer MH, Ingiliz P, Christensen S, Hueppe D, Lutz T, Simon KG, Schewe K, Boesecke C, Baumgarten A, Busch H, Rockstroh J, Schmutz G, Kimhofer T, Berger F, Mauss S, Schulze Zur Wiesch J. Real-world effectiveness of sofosbuvir-based treatment regimens for chronic hepatitis C genotype 3 infection: Results from the multicenter German hepatitis C cohort (GECCO-03). J Med Virol. 2018;90:304-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 141. | Alonso S. Riveiro-Barciela M, Fernandez I, Rincón D, Real Y, Llerena S, Gea F, Olveira A, Fernandez-Carrillo C, Polo B, Carrión JA, Gómez A, Devesa MJ, Baliellas C, Castro Á, Ampuero J, Granados R, Pascasio JM, Rubín A, Salmeron J, Badia E, Planas JM, Lens S, Turnes J, Montero JL, Buti M, Esteban R, Fernández-Rodríguez CM. Effectiveness and safety of sofosbuvir-based regimens plus an NS5A inhibitor for patients with HCV genotype 3 infection and cirrhosis. Results of a multicenter real-life cohort. J Viral Hepat. 2017;24:304-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 142. | Dalgard O, Weiland O, Noraberg G, Karlsen L, Heggelund L, Färkkilâ M, Balslev U, Belard E, Øvrehus A, Skalshøi Kjær M, Krarup H, Thorup Røge B, Hallager S, Madsen LG, Lund Laursen A, Lagging M, Weis N. Sofosbuvir based treatment of chronic hepatitis C genotype 3 infections-A Scandinavian real-life study. PLoS One. 2017;12:e0179764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 143. | Pellicelli A, Messina V, Giannelli V, Distefano M, Palitti VP, Vignally P, Tarquini P, Izzi A, Moretti A, Babudieri S, Dell'Isola S, Marignani M, Scifo G, Iovinella V, Cariti G, Pompili M, Candilo FD, Fontanella L, Ettorre GM, Vennarecci G, Ippolito AM, Barbarini G. High efficacy and safety of flat-dose ribavirin plus sofosbuvir/daclatasvir in genotype 3 cirrhotic patients. Gut Liver. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 144. | Teegen EM, Globke B, Schott E, Pratschke J, Eurich D. A Closing Chapter: Hepatitis C Genotype 3 Elimination in Liver Transplant; Sofosbuvir/Daclatasvir in a Hard-to-Treat Population. Exp Clin Transplant. 2018;16:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 145. | Sperl J, Frankova S, Kreidlova M, Merta D, Tothova M, Spicak J. Combination of sofosbuvir and daclatasvir in the treatment of genotype 3 chronic hepatitis C virus infection in patients on maintenance hemodialysis. Ther Clin Risk Manag. 2017;13:733-738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 146. | Iqbal S, Yousuf MH, Yousaf MI. Dramatic response of hepatitis C patients chronically infected with hepatitis C virus genotype 3 to sofosbuvir-based therapies in Punjab, Pakistan: A prospective study. World J Gastroenterol. 2017;23:7899-7905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 147. | Isakov V, Zhdanov K, Kersey K, Svarovskaia E, Massetto B, Zhu Y, Knox SJ, Bakulin I, Chulanov V. Efficacy of sofosbuvir plus ribavirin in treatment-naive patients with genotype-1 and -3 HCVinfection: results from a Russian Phase IIIb study. Antivir Ther. 2016;21:671-678. |
| 148. | Butt N, Akbar A, Abbasi A, Reema S, Baqar JB, Shaikh QH. Safety and Efficacy of Sofosbuvir with Ribavirin® in Hepatitis C, Genotype 3 Patients with Cirrhosis: A Real-world Experience. Cureus. 2019;11:e4012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 149. | Anand AC, Agarwal SK, Garg HK, Khanna S, Gupta S. Sofosbuvir and Ribavirin for 24 Weeks Is An Effective Treatment Option for Recurrent Hepatitis C Infection After Living Donor Liver Transplantation. J Clin Exp Hepatol. 2017;7:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 150. | Swallow E, Song J, Yuan Y, Kalsekar A, Kelley C, Peeples M, Mu F, Ackerman P, Signorovitch J. Daclatasvir and Sofosbuvir Versus Sofosbuvir and Ribavirin in Patients with Chronic Hepatitis C Coinfected with HIV: A Matching-adjusted Indirect Comparison. Clin Ther. 2016;38:404-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 151. | Gane EJ, Hyland RH, An D, Svarovskaia E, Pang PS, Brainard D, Stedman CA. Efficacy of ledipasvir and sofosbuvir, with or without ribavirin, for 12 weeks in patients with HCV genotype 3 or 6 infection. Gastroenterology. 2015;149:1454-1461.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 152. | Feld JJ, Ramji A, Shafran SD, Willems B, Marotta P, Huchet E, Vachon ML, Svarovskaia ES, Huang KC, Hyland RH, Yun C, Massetto B, Brainard DM, McHutchison JG, Tam E, Bailey R, Cooper C, Yoshida EM, Greenbloom S, Elkhashab M, Borgia S, Swain MG. Ledipasvir-Sofosbuvir Plus Ribavirin in Treatment-Naive Patients With Hepatitis C Virus Genotype 3 Infection: An Open-Label Study. Clin Infect Dis. 2017;65:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 153. | Moser S, Kozbial K, Laferl H, Schütz A, Reiberger T, Schwabl P, Gutic E, Schwanke C, Schubert R, Luhn J, Lang T, Schleicher M, Steindl-Munda P, Haltmayer H, Ferenci P, Gschwantler M. Efficacy of ledipasvir/sofosbuvir plus ribavirin for 12 weeks in patients with chronic hepatitis C genotype 3 and compensated liver disease. Eur J Gastroenterol Hepatol. 2018;30:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 154. | Kohli A, Kapoor R, Sims Z, Nelson A, Sidharthan S, Lam B, Silk R, Kotb C, Gross C, Teferi G, Sugarman K, Pang PS, Osinusi A, Polis MA, Rustgi V, Masur H, Kottilil S. Ledipasvir and sofosbuvir for hepatitis C genotype 4: a proof-of-concept, single-centre, open-label phase 2a cohort study. Lancet Infect Dis. 2015;15:1049-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 122] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 155. | Abergel A, Metivier S, Samuel D, Jiang D, Kersey K, Pang PS, Svarovskaia E, Knox SJ, Loustaud-Ratti V, Asselah T. Ledipasvir plus sofosbuvir for 12 weeks in patients with hepatitis C genotype 4 infection. Hepatology. 2016;64:1049-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 156. | Shiha G, Esmat G, Hassany M, Soliman R, Elbasiony M, Fouad R, Elsharkawy A, Hammad R, Abdel-Razek W, Zakareya T, Kersey K, Massetto B, Osinusi A, Lu S, Brainard DM, McHutchison JG, Waked I, Doss W. Ledipasvir/sofosbuvir with or without ribavirin for 8 or 12 weeks for the treatment of HCV genotype 4 infection: results from a randomised phase III study in Egypt. Gut. 2019;68:721-728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 157. | Ahmed OA, Kaisar HH, Badawi R, Hawash N, Samir H, Shabana SS, Fouad MHA, Rizk FH, Khodeir SA, Abd-Elsalam S. Efficacy and safety of sofosbuvir-ledipasvir for treatment of a cohort of Egyptian patients with chronic hepatitis C genotype 4 infection. Infect Drug Resist. 2018;11:295-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 158. | Crespo J, Calleja JL, Fernández I, Sacristan B, Ruiz-Antorán B, Ampuero J, Hernández-Conde M, García-Samaniego J, Gea F, Buti M, Cabezas J, Lens S, Morillas RM, Salcines JR, Pascasio JM, Turnes J, Sáez-Royuela F, Arenas J, Rincón D, Prieto M, Jorquera F, Sanchez Ruano JJ, Navascués CA, Molina E, Moya AG, Moreno-Planas JM; Spanish Group for the Study of the Use of Direct-acting Drugs Hepatitis C Collaborating Group. Real-World Effectiveness and Safety of Oral Combination Antiviral Therapy for Hepatitis C Virus Genotype 4 Infection. Clin Gastroenterol Hepatol. 2017;15:945-949.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 159. | Sanai FM, Altraif IH, Alswat K, AlZanbagi A, Babatin MA, AlMousa A, Almutairi NH, Aljawad MS, Alghamdi AS, Aljumah AA, Alalwan AM, Al-Hamoudi WK, Assiri AM, Dahlan Y, Alsahafi A, Alothmani HS, AlSaleemi MS, Mousa WA, Albenmousa A, Awny A, Albiladi H, Abdo AA, AlGhamdi H. Real life efficacy of ledipasvir/sofosbuvir in hepatitis C genotype 4-infected patients with advanced liver fibrosis and decompensated cirrhosis. J Infect. 2018;76:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 160. | Babatin MA, AlGhamdi AS, Assiri AM, AlBiladi H, AlOthmani HS, Mogharbel MH, Mahallawi W, Asselah T, Sanai FM. Treatment efficacy of ledipasvir/sofosbuvir for 8 weeks in non-cirrhotic chronic hepatitis C genotype 4 patients. Saudi J Gastroenterol. 2019;25:55-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 161. | Ahmed OA, Safwat E, Khalifa MO, Elshafie AI, Fouad MHA, Salama MM, Naguib GG, Eltabbakh MM, Sherief AF, Abd-Elsalam S. Sofosbuvir Plus Daclatasvir in Treatment of Chronic Hepatitis C Genotype 4 Infection in a Cohort of Egyptian Patients: An Experiment the Size of Egyptian Village. Int J Hepatol. 2018;2018:9616234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 162. | Abdel-Aziz AM, Ibrahim MA, El-Sheikh AA, Kamel MY, Zenhom NM, Abdel-Raheim S, Abdelhaleem H. Effect of Sofosbuvir Plus Daclatasvir in Hepatitis C Virus Genotype-4 Patients: Promising Effect on Liver Fibrosis. J Clin Exp Hepatol. 2018;8:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 163. | Abdel-Moneim A, Aboud A, Abdel-Gabaar M, Zanaty MI, Ramadan M. Efficacy and safety of sofosbuvir plus daclatasvir with or without ribavirin: large real-life results of patients with chronic hepatitis C genotype 4. Hepatol Int. 2018;12:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 164. | Shiha G, Soliman R, ElBasiony M, Hassan AA, Mikhail NNH. Sofosbuvir plus Daclatasvir with or without ribavirin for treatment of chronic HCV genotype 4 patients: real-life experience. Hepatol Int. 2018;12:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 165. | Abergel A, Asselah T, Metivier S, Kersey K, Jiang D, Mo H, Pang PS, Samuel D, Loustaud-Ratti V. Ledipasvir-sofosbuvir in patients with hepatitis C virus genotype 5 infection: an open-label, multicentre, single-arm, phase 2 study. Lancet Infect Dis. 2016;16:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 166. | Nguyen MH, Trinh H, Do S, Nguyen T, Nguyen P, Henry L. Open Label Study of 8 vs. 12 Weeks of Ledipasvir/Sofosbuvir in Genotype 6 Treatment Naïve or Experienced Patients. Am J Gastroenterol. 2017;112:1824-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 167. | Mettikanont P, Bunchorntavakul C, Reddy KR. Systematic review: epidemiology and response to direct-acting antiviral therapy in genotype 6 chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2019;49:492-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 168. | Wu DB, Jiang W, Wang YH, Chen B, Wang ML, Tao YC, Chen EQ, Tang H. Safety and efficacy of sofosbuvir-based direct-acting antiviral regimens for hepatitis C virus genotype 6 in Southwest China: Real-world experience of a retrospective study. J Viral Hepat. 2019;26:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 169. | Xue Y, Zhang LX, Wang L, Li T, Qu YD, Liu F. Efficacy and safety of sofosbuvir and daclatasvir in treatment of kidney transplantation recipients with hepatitis C virus infection. World J Gastroenterol. 2017;23:5969-5976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 170. | Dienstag JL, Ghany MG, Morgan TR, Di Bisceglie AM, Bonkovsky HL, Kim HY, Seeff LB, Szabo G, Wright EC, Sterling RK, Everson GT, Lindsay KL, Lee WM, Lok AS, Morishima C, Stoddard AM, Everhart JE; HALT-C Trial Group. A prospective study of the rate of progression in compensated, histologically advanced chronic hepatitis C. Hepatology. 2011;54:396-405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Cheungpasitporn W, Farshadpour F, Goral V, Rezaee-Zavareh MS, Tabll AA, Yang SS S-Editor: Ma RY L-Editor: Filipodia E-Editor: Ma YJ