Published online Jul 28, 2019. doi: 10.3748/wjg.v25.i28.3808
Peer-review started: March 22, 2019
First decision: April 16, 2019
Revised: May 4, 2019
Accepted: July 5, 2019
Article in press: July 5, 2019
Published online: July 28, 2019
Processing time: 128 Days and 14.9 Hours
Magnetic resonance enterography (MRE) and wireless capsule endoscopy (WCE) are equally accepted modalities for noninvasive screening of small bowel involvement (SBI) in children with Crohn’s disease (CD) and indeterminate colitis (IC) albeit there is a paucity of data comparing the two and thereby guiding the clinician in selecting the ideal diagnostic approach. Therefore, the goal of this study is to provide additional evidence for capsule endoscopy role in the evaluation of established Crohn’s disease exacerbation compared to MRE in relation to Pediatric Crohn's Disease Activity Index (PCDAI), and histological indices.
To prospectively compare the findings of MRE and WCE and their agreement with PCDAI or histology in children with CD or IC.
Consecutive patients diagnosed with CD and IC were screened for inclusion. After informed consent, patient’s demographic and clinical data was abstracted. The current pediatric disease activity index (PCDAI) and endoscopic findings were included. Patients underwent MRE and WCE including preprocedural patency capsule within a maximum of 7 d of each other. Pathological presence of active small bowel disease in ileal and duodenal biopsies were collected if the endoscopy was performed within 2 mo of the WCE study. Patients who failed to pass the PC were excluded from the study. WCE was read by two different experienced gastroenterologists (Attard TM and Colombo JM) blinded to each other's findings and to the findings on MRE (Mardis NJ). Agreement between WCE reviewers, WCE and MRE findings and concordance between positive PCDAI and SBI based on MRE compared with WCE was computed.
Forty-five patients were included in the study, 18 withdrew and 27 (20 males and 20 CD), mean age (standard deviation) 13.46 (2.4) years, completed the study protocol. There were no instances of capsule retention. Concordance between gastroenterologist reviewers was excellent for the diagnosis of small intestinal CD with good correlation between the two Lewis scores (r = 0.875, P < 0.001). Concordance between WCE and MRE was poor (69%). In CD patients, when both MRE and WCE were compared using PCDAI > 10 as the standard reference reflecting active small intestinal CD, the sensitivity of MRE and WCE were 100% and 83% respectively and the specificity of MRE and WCE were 57.14% and 78.6%, respectively. If the histology in ileum or/and duodenum was used as the reference for active small bowel involvement, WCE had a higher specificity as compared to MRE (83.3% vs 50%). In patients with Crohn’s disease, those with a positive PCDAI (> 10) were more likely to have a positive WCE as compared to those with a negative PCDAI (83% vs 21%; P = 0.018).
We suggest that MRE and WCE have a complementary role in the assessment of SBI in CD. WCE detected SBI with a much higher specificity while MRE had a higher sensitivity.
Core tip: There are a number of prospective adult studies and few in pediatrics comparing magnetic resonance imaging (MRE) to wireless capsule endoscopy (WCE) in identifying small bowel (SB) Crohn’s disease (CD) that showed no significant difference in the diagnostic yield and accuracy of MRE and WCE in established non-stricturing crohns disease or suspected and established CD together. This study is the first prospective study in children with established inflammatory bowel disease in the United States assessing and comparing the roles of MRE and WCE in identifying SB disease involvement in relation to clinical and histological indices.
- Citation: Hijaz NM, Attard TM, Colombo JM, Mardis NJ, Friesen CA. Comparison of the use of wireless capsule endoscopy with magnetic resonance enterography in children with inflammatory bowel disease. World J Gastroenterol 2019; 25(28): 3808-3822
- URL: https://www.wjgnet.com/1007-9327/full/v25/i28/3808.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i28.3808
Crohn’s disease (CD) is a chronic inflammatory disorder primarily involving the gastrointestinal tract. Although any part of the gastrointestinal tract may be involved, proximal small intestinal involvement is more common in pediatric patients than in adult patients with a prevalence of up to 20% [1]. The effects of small intestinal involvement in CD are variable and may include obscure abdominal pain, nutritional sequelae resulting in growth delay, iron deficiency anemia, stricture formation, and potentially small bowel obstruction[1,2]. Proximal small bowel (SB) involvement in CD is associated with a more aggressive disease course and an increased need for surgery[3,4]. Therefore, accurate determination of SB involvement (SBI) in pediatric CD is crucial for optimal patient management[3,4].
Current clinical guidelines include suggested modalities to identify SBI and determine management plans[5]. Available options include small bowel series, computed tomography enterography (CTE), small bowel wireless capsule endoscopy (WCE), gadolinium enhanced magnetic resonance imaging (GAD MRI), and small bowel contrast enhanced ultrasound (US). The choice of modality is largely determined by available resources, radiation exposure risk, physician and institutional preferences. MRE and contrast enhanced US are radiation free, while other radiologic modalities entail a risk of radiation exposure[6]. WCE may entail a risk of capsule retention. The risk of capsule retention resulting in obstruction is increased in the context of stricturing or fistulizing disease in CD and has been estimated at 2.6%[7] but may be greatly mitigated by patency capsule screening[8]. Magnetic resonance enterography (MRE) and small intestine contrast ultrasound (SICUS) have diagnostic effectiveness comparable to other radiological modalities for evaluation of CD patients[1,2,9]. However, both studies have their own limitations. MRE is limited by expense, the availability of the requisite equipment and software, variable expertise in interpretation of the findings, and (potentially) the need for sedation in pediatric population. SICUS is similarly affected by being operator dependent with the requisite need of accumulated expertise and heightened need for cooperation during the study that can limit its use in pediatric populations[10].
Other diagnostic modalities have been evaluated in comparison to WCE in several pediatric and adult inflammatory bowel disease (IBD) studies. Table 1 summarizes the adult and pediatric studies comparing different modalities to WCE. The studies conducted in children with IBD were mostly retrospective and aimed at evaluating the role of MRE and WCE for detection of SB disease. They concluded that MRE and WCE were comparable with similar sensitivities[11]. Only three prospective studies (all European) in pediatric IBD have compared WCE and MRE modalities in identifying SB disease involvement. Two were studies in established CD[9,12] and one in suspected CD[13] and again, they suggested that the tests appear complementary for detection of active CD. The current study is the first prospective study in children with established IBD in the United States assessing the roles of MRE and WCE in identifying SB disease involvement in IBD. This study provides evidence for capsule endoscopy role in the evaluation of established disease exacerbation in patients with IBD in relation to MRE.
| Author /year / type | Country | Age group/ total No. | Patient population | Modalities compared to CE | Results |
| Albert 2005[14] | Germany | Adults/52 | Established and suspected CD | MRE | Diagnostic yield of WCE is superior to MRE ( +ve MRE 32/52 vs +ve WCE 25/27) |
| Golder 2006 prospective[15] | Germany | Adults/16 | Established CD | MRE | Diagnostic yield of WCE is similar to that of MRE ( +ve MRE 9/15 vs +ve WCE 11/15), but the WCE is superior in detecting proximal SB disease |
| Tillack 2008 prospective[16] | Germany | Adults/19 | Established CD | MRE | Diagnostic yield of WCE is similar to that of MRE (+ve MRE 18/19 vs +ve WCE 18/19) but the WCE is superior in detecting proximal SB disease |
| Dionisio 2010 prospective | Europe, Canada, Israel and United States | All ages/ 428 | Established and suspected CD | CTE and SBFT and MRE | Diagnostic yield of WCE is superior to that of CTE and SBR in suspected CD but it is similar to MRE in suspected and established CD |
| Metanalysis[17] | |||||
| Crook 2009 prospective[18] | Switzerland | Adults/5 | Suspected CD | MRE | Diagnostic yield of WCE is similar to that of MRE and complementary to each other |
| Bocker 2010 prospective[19] | Germany | Adults/21 | Established and suspected CD | MRE | Diagnostic yield of WCE is similar to that of MRE ( +ve MRE 6/21 vs +ve WCE 9/21) but the WCE is superior in detecting proximal SB disease |
| Jensen 2011 prospective[20] | Denmark | Adults/93 | Established and suspected CD | MRE | Diagnostic yield of WCE is similar to that of MRE( +ve MRE 24/80 vs +ve WCE 22/80) but the WCE is superior in detecting proximal SB disease |
| Wiarda 2011 prospective[21] | The Netherlands | Adults/38 | Established and suspected CD | MRE | Diagnostic yield of WCE is similar that of MRE ( +ve MRE 16/38 vs +ve WCE 6/25) |
| Kopylov 2015 prospective[22] | Israel | Adults/77 | Established CD | MRE | Diagnostic yield of WCE is similar to that of MRE ( +ve MRE 40/52 vs +ve WCE 42/52) but the WCE is superior in detecting proximal SB disease |
| Gonzalez Suarez 2017 retrospective[23] | Spain | Adults/47 | Established and suspected CD | MRE | WCE is superior to MRE in detection of small bowel lesions mainly proximal(+ve WCE 36/47 vs +ve MRE 21/47) |
| Di Nardo 2010 prospective[24] | Italy | Peds/117 | Established and suspected CD | MRI and SICUS | reclassifying indeterminate colitis (IC) into CD (60%), detection of CD lesions in known CD (41%) and establishing new diagnosis in suspected CD (50%) |
| Casciani 2011 prospective[13] | Italy | Peds/60 | suspected CD | MRE | Diagnostic yield of WCE is similar to that of MRE ( +ve MRE 19/37 vs +ve WCE 10/60) |
| Gralnek 2012 prospective[25] | Israel | Peds /18 | Established and suspected CD | No studies compared | |
| Kovanlikaya 2013[11] retrospective | United States | Peds/23 | Established and suspected CD | MRE | Sensitivity of MRE 75% was similar to WCE 77.8% |
| Aloi 2015 prospective[9] | Italy | Peds/25 | Established and suspected CD | MRE and SICUS | Diagnostic yield of WCE is similar to that of MRE and SICUS ( +ve MRE 15/25 vs +ve SICUS 16/25 vs +ve WCE 16/25) but the WCE is superior in detecting proximal SB disease |
| Oliva 2016 prospective[12] | Italy | Peds/38 | Established CD | MRE and SICUS | Diagnostic yield of WCE is similar to that of MRE and SICUS ( +ve MRE 19/38 vs +ve WCE 19/38 vs +ve SICUS 21/38) but the CCE is superior in detecting proximal SB disease |
The primary goals of this study are to prospectively compare the diagnostic yield, concordance rate, sensitivity and specificity between MRE and WCE findings and their agreement with the Pediatric Crohn's Disease Activity Index (PCDAI) or with histological small bowel involvement in children with known IBD; CD or IC. Secondary goals are to assess the performance of each of the modalities (MRE, WCE and PCDAI) in relation to each other in order predict the results of the compared tests and to assess the correlation between Lewis capsule endoscopy score and PCDAI.
This study was a prospective single blinded comparison study of a cohort of pediatric patients with established indeterminate colitis (IC) or CD at a tertiary referral pediatric IBD center. The diagnosis of CD was confirmed by using widely validated clinical, endoscopic, and histological criteria. The study was approved by the ethics committee of the hospital IRB #13080263 and written informed assent/consent was obtained from all children and their parents. Study participants were enrolled if they were 4-18 years of age inclusive with an established diagnosis of IC or CD and planned to have an MRE as part of standard of care. Patients were excluded if they had recent intestinal tract surgery, resection involving small bowel, gastrointestinal obstruction or ileus, swallowing disorders, esophageal stricture, nonsteroidal anti-inflammatory drugs or prokinetic medication use in the 4 wk prior to enrollment, inability to swallow the capsule, or if they had an electro-medical device or pacemaker. Demographic and clinical data were recorded including subject demographics, medical and surgical history, imaging results, initial disease presentation, and patient current clinical status which was used to calculate the PCDAI. The PCDAI score is considered positive (active disease) if ≥ 10 and negative (inactive disease) if < 10 (Table 2).
| All patients CD and IC n = 27 | CD n = 20 | |
| Age at diagnosis year | 13.46 (2.40) | 13.48 (2.02) |
| Male % | 74% | 75% |
| Medications ratio (%) | ||
| Biological alone or combination therapy | 12/27 (44.4%) | 11/20 (55%) |
| Immune modulators with no biologic combination | 8/27 (30%) | 5/20 (25%) |
| 5 ASA +- steroids | 4/27 (15%) | 3/20 (15%) |
| Steroids alone | 2/27 (7%) | 0/20 (0%) |
| Antibiotic alone | 1/27 (4%) | 1/20 (5%) |
| Phenotype% | ||
| Inflammatory | 93% | 93% |
| Stricturing | 7% | 7% |
| Duration of disease year | 1.7 (2.32) | 2.1 (2.57) |
| BMI percentile | 57 (32.9) | 58.18 (35.83) |
| PCDAI | 10.2 (12.5) | 9.8 (11.6) |
| SB transit time min | 233 (115.4) | 241(184.99) |
| Days between MRE and WCE days | 4.19 (1.88) | 4 (1.90) |
All patients swallowed a patency capsule (PC; size 11 mm × 26 mm) to assess small bowel patency. All patients with confirmed passage of PC in the first 40 h underwent WCE (Pillcam™ SB Capsule, Given Imaging Ltd, Israel 11 mm × 26 mm) within 1 wk of completion of MRE. Patients excluded from the study if they failed to swallow or pass the PC. WCE was read by two different experienced gastroenterologists, each with > 10 years of experience (Attard TM and Colombo JM) blinded to each other's findings and to the findings on MRE (Mardis NJ). The PCDAI was recorded from the most recent medical chart and laboratory data. Blood samples for hemoglobin and hematocrit, erythrocyte sedimentation rate, C-reactive protein (CRP), and albumin were collected within 7 d if these labs were not obtained in the last 2 wk prior to WCE.
MRE examinations were performed as a standard of care by using a whole body magnetic resonance imaging unit (Children’s Mercy Hospital, Kansas City, MO, United States) with an 8-channel abdominal phased-array coil. A benefiber dissolved in liquid with weight-based dosing was used as the intraluminal oral contrast agent. Intravenous contrast was administered to reduce SB peristalsis and to prolong luminal distention. Axial and coronal T1 weighted images with fat suppression were performed. When the distention quality was inadequate, images were reobtained 30 minutes after the ingestion of a more appropriate dose for age of fiber water solution. Axial T2, axial diffusion and coronal true cine images were obtained.
One radiologist retrospectively reviewed the MRE for all subjects to provide a consistent assessment of the extent of SB activity for each subject. Patients with a MRE score of >3 were considered to have positive MRE study[20]. The score was modified in this study to exclude counting colonic segment involvement in the overall radiological score (maximum score is 13). Evaluated findings included SB wall thickness (0-3 mm or 3-6 mm, > 6mm), SB wall enhancement after intravenous contrast media (none, mild or severe), mucosal and serosal enhancement suggestive of mesenteric fatty infiltration, strictures (defined as luminal narrowing to be less than 10 mm), increased mesenteric vascularity close to the inflamed bowel loop, mesenteric lymphadenopathy, the presence of fistula, stricture or abscess and the number of SB segments involved (duodenal, jejunal and ileal)[9]. MRE score used is provided in supplemental material.
The capsule images were independently interpreted by two gastroenterologists with > 10 years of experience in capsule studies. To optimize the visualization of the jejunum and ileum of the CE, after an overnight fast, patients ingested Polyethylene Glycol 3350 PEG before they swallowed the capsule (PEG doses adjusted based on age: 34 g in 480 mL clear liquid if age of the subject was < 5 year, 51 g in 720 mL if 5-10 years, 68 g in 960 mL if >10 years). The CE used in this study was the PillCam™ SB video capsule (Given Imaging, Medtronics Ltd, Yokneam, Israel). It measures 11 mm × 26 mm and it weighs less than 4 g. This capsule was ingested orally in all patients except for one patient who was scheduled to have endoscopy on the same day, so the capsule was deployed by esophagogastroduodenoscopy. Capsule retention is defined as a failure of the passage of the capsule from the gastrointestinal tract for ≥ 2 wk[20]. The examination was incomplete if the capsule did not reach the cecum by the end of the study. Images were considered as negative (or inactive) if no abnormalities were seen and as positive (or active) if clear abnormalities of the SB mucosa (ulcerations > 3, erosions, polyps, vascular lesions, and bleeding lesions were seen). White lesions within a crater with surrounding erythema were considered ulcers, whereas small superficial white lesions, even with surrounding erythema, were considered erosions[24]. If no abnormalities or non-specific findings (such as erythematous spots or mucosal damage) were seen, the examination was considered non-specific or normal. All capsule readers were blinded to each other’s findings or radiological MRE images but were aware of the patient’s medical history and laboratory testing. In addition, evaluators used the capsule endoscopy data collection form including the Lewis scoring system that is automatically calculated and included in the RAPID™ software[26]. The Lewis score is a WCE ranking of inflammatory activity into three levels based on erythema, stenosis, edema and erosions in small intestinal tertiles: (1) No disease or clinically insignificant disease (LS < 135); (2) Mild disease (135 ≤ LS ≤ 790); and (3) Moderate or severe disease (LS > 790). Any WCE with Lewis score more than 135 is considered positive[26].
A subgroup of 15 of the 27 patients had pathology specimens available for review within 2 mo of the WCE study [mean 3.9 wk, standard deviation (SD) = 2.58]. Pathology specimens from the terminal ileum and duodenum were evaluated as they are considered the accepted reference standard to determine active CD in the SB.
Histology findings were considered positive if the subject had final impression of chronic active ileitis or duodenitis or if there was a description of at least one of the chronic changes (architectural changes, increase in lamina propria mononuclear cells and lamina propria PMNs) together with at least one of activity histology findings (epithelial damage, intraepithelial PMNs in surface epithelium, cryptitis, crypt abscess, erosions/ulcers, or granulomas) in either ileal or duodenal biopsies. This is based on the histological remission definition proposed by a systemic review with absence of neutrophils in crypt and lamina propria, basal and lamina propria plasma cells and eosinophils[27] and the in the diagnosis guidelines for CD[2]. The histology grading used is provided in the supplemental material.
Descriptive data was expressed as the mean [± standard deviation (SD)] for the continuous variables. Categorical data were expressed as frequencies and per-centages. A Chi square with the Fisher correction was used to evaluate the differences for categorical variables when appropriate. Statistical significance is expressed as P < 0.05
For each of the 2 methods evaluated (MRE and WCE), sensitivity, specificity, negative predictive value, positive predictive value, and accuracy were determined by the available PCDAI and histological findings from the terminal ileum and duodenum. The Fisher exact test was used to evaluate the performance of each method in relation to another. Exact binomial 95% confidence intervals were also reported. The sample size of 34 children was estimated as having an 80% power to detect 23% difference in IBD small intestinal MRE findings and WCE detection rate. This size was estimated based on our previous retrospective study[24].
The Pearson correlation coefficient was utilized to assess agreement between Lewis capsule endoscopy score and PCDAI. All P values were 2 sided with statistical significance evaluated as statistical significance P < 0.05. All analyses were performed in SPSS Version 19.0 (SPSS Inc., Chicago, IL, United States).
Forty-five subjects with the diagnosis of CD or IC were enrolled. Twenty-seven patients completed all of the procedures of the study, 20 with CD (74%) and 7 with IC (26%). Eighteen patients were excluded because of inability to swallow PC (4/18), failure to pass PC (3/18) or failure to follow or complete study procedures (3/18), screen failure (1/18) or elective withdrawal from study (7/18).
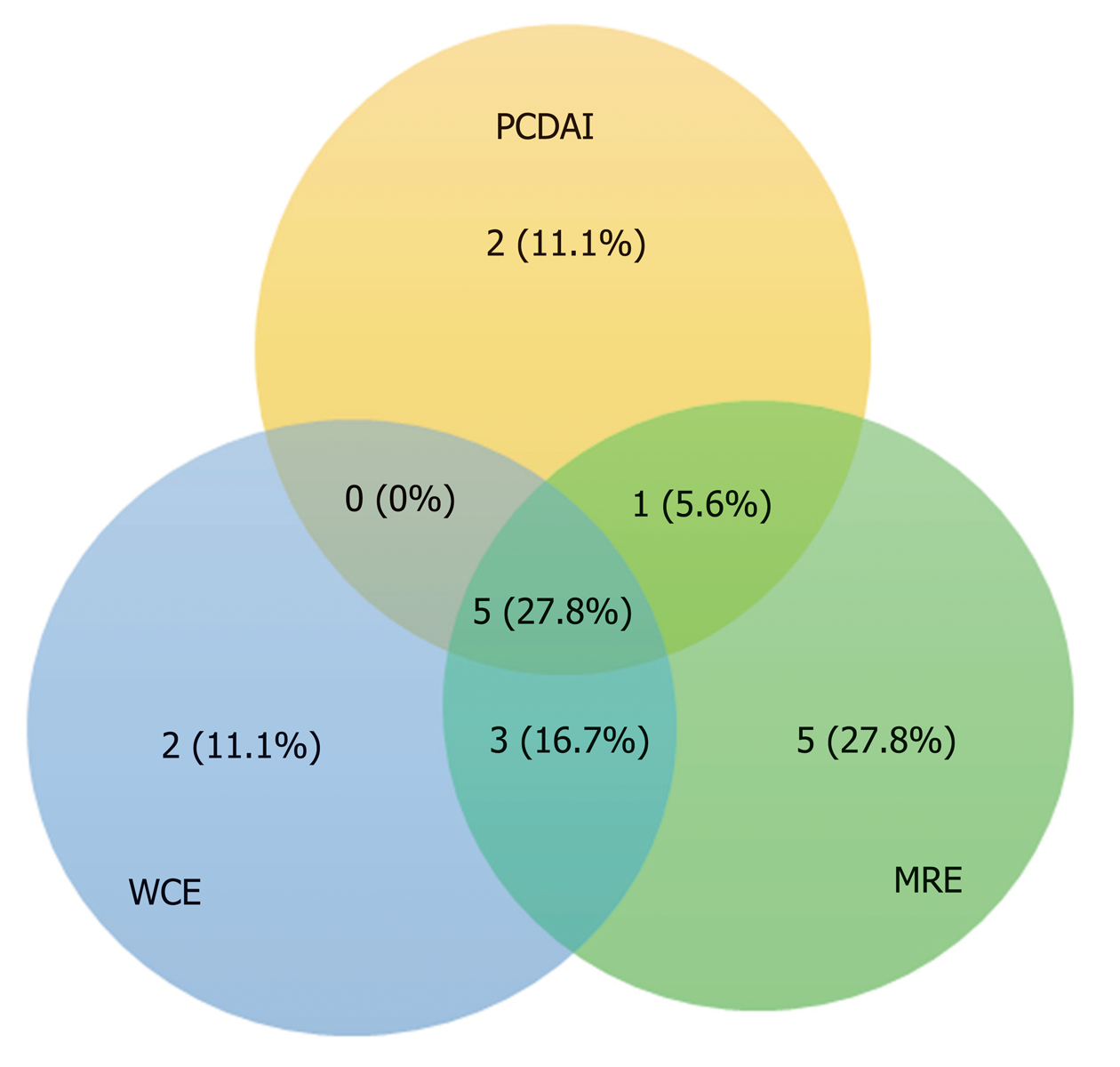
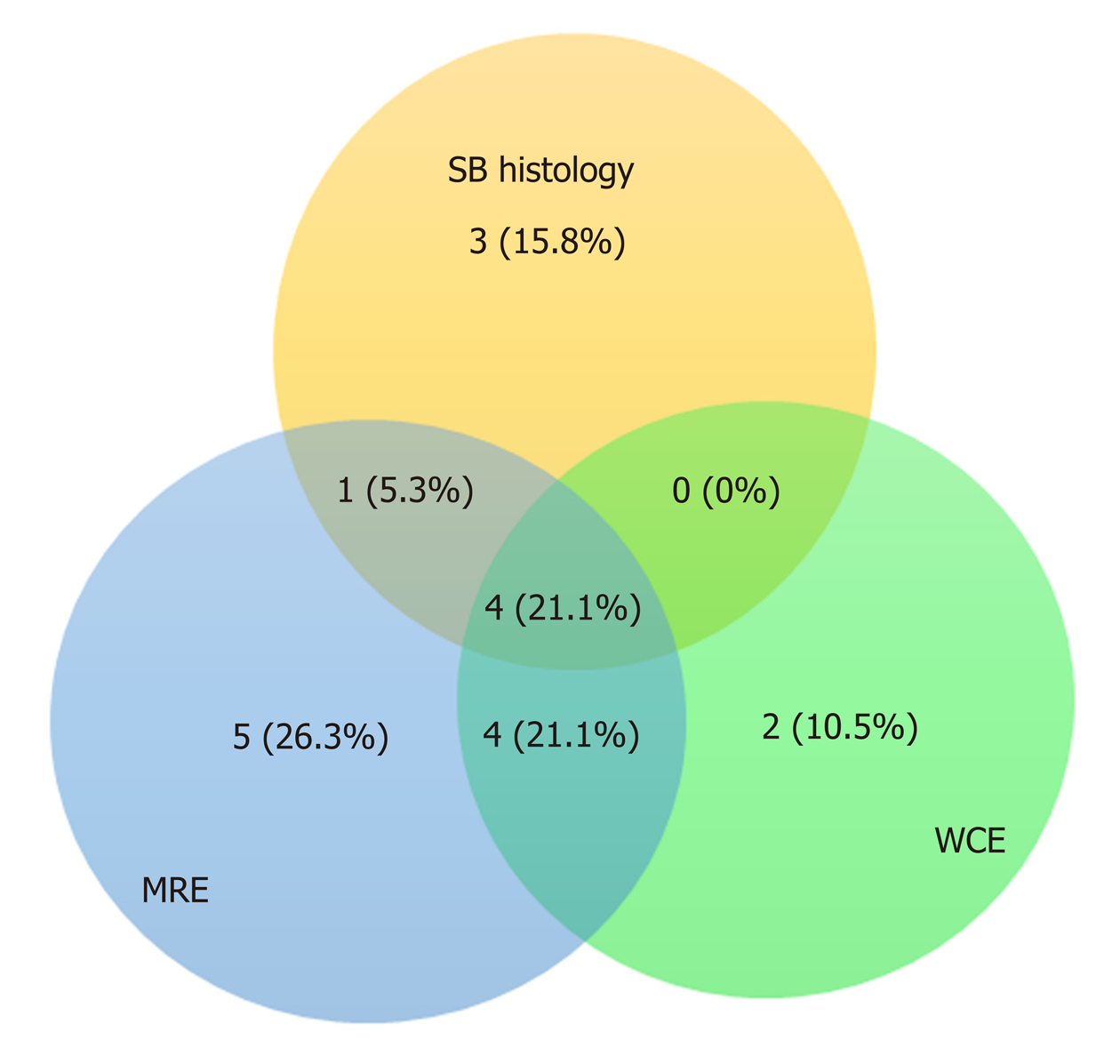
Concordance between gastroenterologist reviewers for the diagnosis of small intestinal CD was excellent with strong correlation between the two Lewis score (r = 0.875, P < 0.001). The studies were incomplete in 3 patients. Two of these demonstrated active CD and one was negative. The patient with a negative incomplete study was excluded. There were no capsule retentions in any of the studies. All capsules passed within 2 wk of the WCE and no surgical interventions were needed. The mean small intestinal transit time was comparable (260.2 min, 218.2 min, P = NS) for WCE positive and negative studies respectively. The Pearson correlation coefficient between average Lewis score between both reviewers and PCDAI is very poor (r = 0.12, P = NS). Agreement rates for positive WCE, MRE, and PCDAI for the total subject group is shown in Figure 1. Agreement rates for positive WCE, MRE, and SB Histology for the 14 patients in which the histology was available are shown in Figure 2.
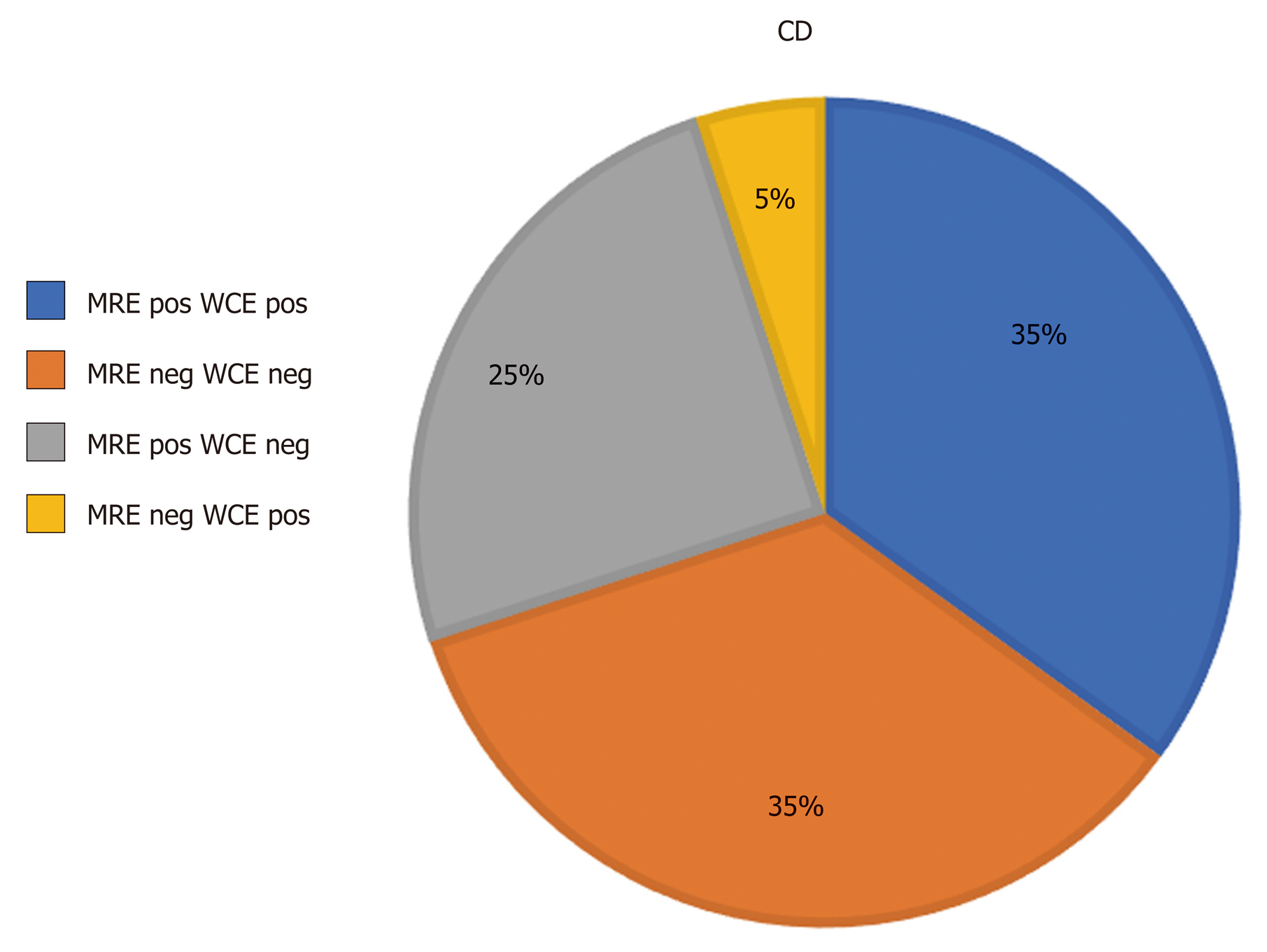
The concordance rate between WCE and MRE was poor (69%) in collectively matched positive and matched negative subjects. The concordance rate between MRE and WCE is shown in Figure 3 in all subject patients (CD and IC) and in Figure 4 in CD only patients.
Histology was available for fifteen patients within 2 mo of the WCE study (mean 3.9 wk and SD = 2.58) and 8 of them demonstrated active CD histology in the ileum and one in the duodenum. For one of the patients who has diagnosis of IC with positive histology, the WCE interpretation was discrepant between reviewers and this patient was dropped from the analysis leaving 14 patients analyzed in the histology comparison and 26 total patients. In CD patients, when both MRE and WCE were compared using PCDAI > 10 as the standard reference reflecting active small intestinal CD, the sensitivity of MRE and WCE were 100% and 83% respectively and the specificity of MRE and WCE were 57.14% and 78.6%, respectively. If the histology in ileum or/and duodenum was used as the reference for active small bowel involvement, WCE had a higher specificity as compared to MRE (83.3% vs 50%). See Table 3.
| Reference standard is PCDAI > 10 indicate active CD | Reference standard is histology in ileum and duodenum | |||||||
| CD only patients (n = 20) | Histology available samples only (n = 14) | |||||||
| MRE | WCE | MRE | WCE | |||||
| Value | 95%CI | Value | 95%CI | Value | 95%CI | Value | 95%CI | |
| SEN | 100% | 54.07% to 100% | 83.3% | 35.88% to 99.58% | 62.50% | 24.49% to 91.48% | 50.00% | 15.70% to 84.30% |
| SP | 57.14% | 28.86% to 82.34% | 78.6% | 49.20% to 95.34% | 50.00 % | 11.81% to 88.19% | 83.33 % | 35.88% to 99.58% |
| PPV | 50% | 35.32% to 64.68% | 62.5% | 36.49% to 82.86% | 62.50% | 38.87% to 81.37% | 80.00% | 36.99% to 96.46% |
| NNP | 100% | 91.7% | 64.29% to 98.53% | 50.00 % | 23.14% to 76.86% | 55.56 % | 36.43% to 73.17% | |
| Accuracy | 70% | 45.72 to 88.11% | 80.0% | 56.34% to 94.27% | 57.14% | 28.86-82.34% | 64.29% | 35.14% to 87.24% |
When all IBD patients were taken collectively, there was no statistically significant relationship between the performance of either MRE or WCE with PCDAI or with each other. However, in patients with CD, those with a positive PCDAI (> 10) were more likely to have a positive WCE as compared to those with a negative PCDAI (83% vs 21%; P = 0.018). There was no significant difference in the frequency of a positive MRE comparing those with and without a positive PCDAI. See Table 4.
| Studies compared | All patients (n = 26) | CD only (n = 20) |
| MRE and WCE | P = 0.428 | P = 0.373 |
| MRE and PCDAI | P = 0.395 | P = 0.325 |
| WCE and PCDAI | P =0.1892 | P = 0.0181 |
There are several modalities available to screen for small intestinal involvement in IBD[1]. However, there is no consensus on a gold standard and it remains controversial whether one of the available examinations is adequate for assessment of SB Crohn’s alone or if it should be used in conjunction with other investigative modalities.
There are several prospective adult studies comparing MRE to WCE in identifying SB Crohn’s which conclude that there is no significant difference in the diagnostic yield and accuracy of MRE and WCE in established non-stricturing CD[15,16,28] or suspected and established CD together[19,20,21]. However, proximal small bowel lesions were more often detected using WCE rather than MRE[16,19,20,21]. Moreover, other prospective studies have shown superiority for WCE[14,23].
The published pediatric studies are far more limited especially ones utilizing MRE as radiological modalities[9,12,13] and they have evaluated heterogeneous groups of IBD patients[9,13,24]. Because there is no consensus on the best screening tool for SB in CD, most of the previous studies evaluated the performance of WCE or imaging studies as the measure of diagnostic yield. It is noteworthy that this approach is suboptimal and simply suggests that a test can detect abnormalities rather than confirming its significance.
Our study is one of the first prospective studies in the United States to compare clinical, radiological and histological measures to WCE in assessing SB activity in pediatric IBD specifically CD and indeterminate colitis. The primary focus was on established Crohn’s disease and did not include heterogeneous populations with suspected IBD[9,24]. Our study demonstrates excellent inter-observer agreement in the interpretation of WCE, suggesting WCE is highly reproducible.
Because of the absence of a standard criteria for confirming proximal SB CD activity that is feasible and less invasive in children, this study used two different references to compare MRE with WCE. The first was the PCDAI as a global clinical standard for overall disease activity and the second was pathological findings in the ileum and duodenum as histological standards for SBI. We have used PCDAI because the evidence suggested its moderate correlation with pediatric CD activity and endoscopic scores[29,30]. PCDAI < 10 is the standard definition of inactive CD that is used in clinical trials for clinical response to medical therapies[29,30]. Pediatric onset CD runs a more aggressive active disease course, including more extensive disease location, more upper GI involvement and increased need for more aggressive medical therapy, in pediatric studies[31-33]. This is also replicated in adult studies; proximal small bowel involvement should be considered as high risk in terms of CD-related surgery[34-36]. In particular L4 (proximal SB not including TI) disease phenotype was associated with stricturing disease, and significantly increased risk for multiple surgeries[37,38]. Pediatric phenotypes of CD at the time of diagnosis showed 50.9%were affected by CD proximal to the terminal ileum in United Kingdom[39]. In Europe, isolated ileal disease (L1) is reported to be 16% in CD children, or proximal to terminal ileal (L4) in 24% and esophagogastroduodenal (EGD) involvement in 30%[33]. If pediatric CD mostly runs an aggressive and extensive course involving small bowel either in more than half of children, then using PCDAI can arguably be justified to reflect active small bowel disease. However, this is still a limitation in this study because it does not exclude the possibility of bowel disease activity overall and it is not validated to accurately reflect SBI compared to other invasive reliable standards.
Pediatric prospective studies used ileocolonoscopy as the reference standard for identifying active CD in the terminal. Moreover, a consensus reference standard was used to determine active CD in the proximal bowel[9,12]. This consensus is basically made up of clinical expert opinion reviewing the results of available images, labs and capsule endoscopy to decide jejunal and duodenal activity.
The current study showed near similar results for both references. However, there was relatively poor agreement between WCE and MRE in sensitivity or specificity. We found a higher sensitivity for MRE as compared to WCE with both standards. While WCE was more specific than MRE in detecting SB disease, the two modalities were comparable in test accuracy.
Our findings are consistent with previously reported pediatric studies which suggest that MRE and WCE are comparable in accuracy for detecting SB disease[9,13]. In contrast, Oliva and colleagues, in a study of established CD in children, demonstrated slightly better accuracy of colon capsule endoscopy including SB images than MRE and SICUS. [12] Our results are consistent with a recent systemic review by Giles revealing a pooled sensitivity and specificity for MRE for detecting active SB CD of 84% and 97%, respectively, with endoscopy as the reference test[39]. However, the specificity of MRE is much lower in our study at 50%-57%, likely attributed to a smaller sample size.
MRE was found to be a sensitive and specific test with a decent diagnostic yield in a systemic review published in 2013[40]. The higher sensitivity of MRE may be attributed to the low threshold being used in MRI scoring systems in few studies, the inclusion of colonic activity in some of the studies or localization of SB segments based on anatomic sectioning of the images[16,20]. Detection of proximal small bowel inflammation in CD by MRE is challenging. Newer suggested scoring systems such as MRI global score MEGS provide potential accurate evaluation of the SB and strongly correlates with inflammation detected with fecal calprotectin and with pan-intestinal inflammatory activity[41] but it is very time consuming and cumbersome limiting practicality[42-44]. Moreover, terminal ileum MRI index of Activity (MaRIA) score has been developed but it did not address perfectly the activity of the proximal SB disease[42].
Our study has uniquely modified the score reported by Jensen and discounted colonic involvement to accurately focus on scoring only small bowel findings in term of enhancement, thickening, vascular, lymphatic or fatty mesenteric changes or presence of SB complications (abscess, fistula, stricture) with same cut off > 3 to robust SB MRI score. Whether this modified score has a clinical significance is yet to be validated. This certainly suggests the need for standardizing MRI scoring, especially in children. Until a validated score is universally accepted, the possibility of MRE false positive results and the possibility of an overestimated positive yield MRE should be taken into consideration.
In the current study, the specificity of WCE was higher than that of MRE (83% vs 50%) which contrasts with what has been reported in an established CD population (94% in WCE vs 89% in MRE)[12]. Specificities of both WCE and MRE in the current study were lower than that reported in pediatric patients with suspected or established CD populations[12,13] and disagreed with Aloi et al[9] who found MRE to be more specific than WCE (89% vs 72%, respectively). Our results differed with other published studies likely because of the heterogeneity of populations used in their analysis and possibly to our small sample size. Therefore, WCE may be suggested as a unique confirmatory test in the assessment of mucosal disease activity. WCE has been suggested as a secondary test if MRE is inconclusive[13]. Published expert recommendations state that a negative capsule endoscopy in CD likely excludes the presence of small bowel disease[45].
We were able to make comparison of the performance of pairs of tools (WCE, MRE and PCDAI) with each other in patients with IBD (CD and IC) overall, and in patients with CD only. The performance of one test was not able to predict the results of the other test when WCE was compared to MRE or when MRE was compared toPCDAI. However, in patients with CD, those with a positive PCDAI (> 10) were more likely to have a positive WCE as compared to those with a negative PCDAI (P = 0.018). See Table 4. This suggests that active disease defined with higher PCDAI score, will increase the predictive ability of WCE to be positive and it supports the use of PCDAI routinely in the assessment of SBI along with radiologic or endoscopic modalities.
This current study is limited by lack of an established reference or gold standard that can be used to compare modalities that may result in a confirmation bias. We therefore had to adopt several surrogate indices to determine if either diagnostic modality correlated with SB disease. Additionally, the current study only partially controls for timing of histology which might impact treatment measures that in turn could impact study results from MRE, SBC or both. It also lacks the evaluation of jejunal histology that can be affected in up to 20% of IBD patients. It is however explained by the assumption that histological changes may lag longer than endoscopic findings and microscopic inflammation persists in 25%-37% of cases of endoscopically quiescent CD[27]. Finally, each subject acted as its own control as there was no use of control group population.
Future studies should continue to integrate the use of WCE, low risk imaging modalities and clinical parameters in defining of SBI in children with CD. It will be useful to integrate a composite of these modalities in a practical validated scoring measure that identify SBI in the least invasive approach.
Our study supports the use of the radiation free, less invasive and generally tolerated imaging modalities of WCE and MRE with each having a favorable role in the assessment of SBI in children with established CD. Although the unique ability of the capsule to detect mucosal changes, and similar unique ability of MRE to detect mural changes, there is still need for a standardized scoring system to describe the specificity of these findings. WCE more accurately detected small bowel disease with a much higher specificity while MRE had a higher sensitivity in pediatric IBD. Patients with active CD (PCDAI > 10) were more likely to have a positive WCE as compared to those with a negative PCDAI. Despite the disagreement between the two modalities, accuracy was comparable between MRE and WCE suggesting that they may have a complementary role in the assessment of small bowel disease.
Magnetic resonance enterography (MRE) and wireless capsule endoscopy (WCE) are equally accepted modalities for noninvasive screening of small bowel involvement (SBI) in children with Crohn’s disease (CD) and indeterminate colitis (IC) and there is a paucity of data comparing the two in children. Thereby guiding the clinician in selecting the ideal diagnostic approach. Many prospective adult studies and few in pediatrics comparing MRE to WCE in identifying small bowel (SB) CD showed no significant difference in the diagnostic yield and accuracy of MRE and WCE in established non-stricturing CD or suspected and established CD together. The current study is the first prospective study in children with established IBD in the United States assessing the roles of MRE and WCE in identifying SB disease involvement in IBD. This study provides evidence for capsule endoscopy role whether it is superior or complementary in the evaluation of established disease exacerbation in patients with IBD in relation to MRE thereby guiding the clinician in selecting the ideal diagnostic approach.
Therefore, the goal of this study is to provide additional evidence and guidance for capsule endoscopy role in the evaluation of established CD exacerbation compared to MRE into relation Pediatric Crohn's Disease Activity Index (PCDAI), and histological indices.
The primary goals of this study are to prospectively compare the diagnostic yield, concordance rate, sensitivity and specificity between MRE and WCE findings and their agreement with the PCDAI or with histological small bowel involvement in children with known IBD; CD or IC. Secondary goals are to assess the performance of each of the modalities (MRE, WCE and PCDAI) in relation to each other in order to predict the results of the compared tests and to assess the correlation between Lewis capsule endoscopy score and PCDAI.
Consecutive patients diagnosed with CD and IC were screened for inclusion. After informed consent patient’s demographic and clinical data was abstracted. The current pediatric disease activity index (PCDAI) and endoscopic findings were included. Patients underwent MRE and WCE including preprocedural patency capsule within a maximum of 7 d of each other. Pathological presence of active small bowel disease in ileal and duodenal biopsies were collected if the endoscopy was performed within 2 mo of the WCE study. Patients who failed to pass the PC were excluded from the study. WCE was read by two different experienced gastroenterologists (Attard TM and Colombo JM) blinded to each other's findings and to the findings on MRE (Mardis NJ). Agreement between WCE reviewers, WCE and MRE findings and concordance between positive PCDAI and SBI based on MRE compared with WCE was computed.
In CD patients, when both MRE and WCE were compared using PCDAI > 10 as the standard reference reflecting active small intestinal CD, the sensitivity of MRE is higher than WCE but specificity of MRE were lower than WCE. If the histology in ileum or/and duodenum was used as the reference for active small bowel involvement which is usually the most reliable reported standard, WCE had a higher specificity as compared to MRE (83.3% vs 50%). Concordance between WCE and MRE was poor (69%) whether both agreed positively or negatively. While WCE was more specific than MRE in detecting SB disease, the two modalities were comparable in test accuracy. Specificities of both WCE and MRE in the current study were lower than that reported in pediatric patients with suspected or established CD populations. An active disease defined with higher PCDAI score > 10, will increase the predictive ability of WCE to be positive and it supports the use of PCDAI routinely in the assessment of SBI along with radiologic or endoscopic modalities. The argument remains to be elucidated on what is the gold standard that best identify the SBI in patients with IBD.
Our study supports the use of the radiation free, less invasive and generally tolerated imaging modalities of WCE and MRE with each having a favorable role in the assessment of SBI in children with established CD. Although the unique ability of the capsule to detect mucosal changes and similar unique ability of MRE to detect mural changes, there is still need for a standardized scoring system to describe the specificity of these findings. WCE more accurately detected small bowel disease with a much higher specificity while MRE had a higher sensitivity in pediatric IBD. Patients with active CD (PCDAI > 10) were more likely to have a positive WCE as compared to those with a negative PCDAI. Despite the disagreement between the two modalities, accuracy was comparable between MRE and WCE suggesting that they may have a complementary role in the assessment of small bowel disease.
Future studies should continue to integrate the use of WCE, low risk imaging modalities and clinical parameters in defining the best standard to identify SBI in children with CD. It will be useful to integrate a composite of these modalities in a practical validated scoring measure that identify SBI in the least invasive approach.
| 1. | Cuffari C, Dubinsky M, Darbari A, Sena L, Baldassano R. Crohn's jejunoileitis: the pediatrician's perspective on diagnosis and management. Inflamm Bowel Dis. 2005;11:696-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition; Colitis Foundation of America, Bousvaros A, Antonioli DA, Colletti RB, Dubinsky MC, Glickman JN, Gold BD, Griffiths AM, Jevon GP, Higuchi LM, Hyams JS, Kirschner BS, Kugathasan S, Baldassano RN, Russo PA. Differentiating ulcerative colitis from Crohn disease in children and young adults: report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn's and Colitis Foundation of America. J Pediatr Gastroenterol Nutr. 2007;44:653-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 354] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 3. | Flamant M, Trang C, Maillard O, Sacher-Huvelin S, Le Rhun M, Galmiche JP, Bourreille A. The prevalence and outcome of jejunal lesions visualized by small bowel capsule endoscopy in Crohn's disease. Inflamm Bowel Dis. 2013;19:1390-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Kopylov U, Nemeth A, Koulaouzidis A, Makins R, Wild G, Afif W, Bitton A, Johansson GW, Bessissow T, Eliakim R, Toth E, Seidman EG. Small bowel capsule endoscopy in the management of established Crohn's disease: clinical impact, safety, and correlation with inflammatory biomarkers. Inflamm Bowel Dis. 2015;21:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 5. | Ladas SD, Triantafyllou K, Spada C, Riccioni ME, Rey JF, Niv Y, Delvaux M, de Franchis R, Costamagna G; ESGE Clinical Guidelines Committee. European Society of Gastrointestinal Endoscopy (ESGE): recommendations (2009) on clinical use of video capsule endoscopy to investigate small-bowel, esophageal and colonic diseases. Endoscopy. 2010;42:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 173] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 6. | Levi Z, Fraser E, Krongrad R, Hazazi R, benjaminov O, meyerovitch J, Tal OB, Choen A, Niv Y, Fraser G. Factors associated with radiation exposure in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2009;30:1128-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Liao Z, Gao R, Xu C, Li ZS. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc. 2010;71:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 484] [Article Influence: 30.3] [Reference Citation Analysis (1)] |
| 8. | Herrerias JM, Leighton JA, Costamagna G, Infantolino A, Eliakim R, Fischer D, Rubin DT, Manten HD, Scapa E, Morgan DR, Bergwerk AJ, Koslowsky B, Adler SN. Agile patency system eliminates risk of capsule retention in patients with known intestinal strictures who undergo capsule endoscopy. Gastrointest Endosc. 2008;67:902-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Aloi M, Di Nardo G, Romano G, Casciani E, Civitelli F, Oliva S, Viola F, Maccioni F, Gualdi G, Cucchiara S. Magnetic resonance enterography, small-intestine contrast US, and capsule endoscopy to evaluate the small bowel in pediatric Crohn's disease: a prospective, blinded, comparison study. Gastrointest Endosc. 2015;81:420-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Pallotta N, Tomei E, Viscido A, Calabrese E, Marcheggiano A, Caprilli R, Corazziari E. Small intestine contrast ultrasonography: an alternative to radiology in the assessment of small bowel disease. Inflamm Bowel Dis. 2005;11:146-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Kovanlikaya A, Watson E, Hayward J, Beneck D, Sockolow R, Solomon A, Christos P, Brill PW. Magnetic resonance enterography and wireless capsule endoscopy in the evaluation of patients with inflammatory bowel disease. Clin Imaging. 2013;37:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Oliva S, Cucchiara S, Civitelli F, Casciani E, Di Nardo G, Hassan C, Papoff P, Cohen SA. Colon capsule endoscopy compared with other modalities in the evaluation of pediatric Crohn's disease of the small bowel and colon. Gastrointest Endosc. 2016;83:975-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Casciani E, Masselli G, Di Nardo G, Polettini E, Bertini L, Oliva S, Floriani I, Cucchiara S, Gualdi G. MR enterography versus capsule endoscopy in paediatric patients with suspected Crohn's disease. Eur Radiol. 2011;21:823-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Albert JG, Martiny F, Krummenerl A, Stock K, Lesske J, Göbel CM, Lotterer E, Nietsch HH, Behrmann C, Fleig WE. Diagnosis of small bowel Crohn's disease: a prospective comparison of capsule endoscopy with magnetic resonance imaging and fluoroscopic enteroclysis. Gut. 2005;54:1721-1727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 195] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Gölder SK, Schreyer AG, Endlicher E, Feuerbach S, Schölmerich J, Kullmann F, Seitz J, Rogler G, Herfarth H. Comparison of capsule endoscopy and magnetic resonance (MR) enteroclysis in suspected small bowel disease. Int J Colorectal Dis. 2006;21:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Tillack C, Seiderer J, Brand S, Göke B, Reiser MF, Schaefer C, Diepolder H, Ochsenkühn T, Herrmann KA. Correlation of magnetic resonance enteroclysis (MRE) and wireless capsule endoscopy (CE) in the diagnosis of small bowel lesions in Crohn's disease. Inflamm Bowel Dis. 2008;14:1219-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Dionisio PM, Gurudu SR, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn's disease: a meta-analysis. Am J Gastroenterol. 2010;105:1240-8; quiz 1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 287] [Article Influence: 17.9] [Reference Citation Analysis (36)] |
| 18. | Crook DW, Knuesel PR, Froehlich JM, Eigenmann F, Unterweger M, Beer HJ, Kubik-Huch RA. Comparison of magnetic resonance enterography and video capsule endoscopy in evaluating small bowel disease. Eur J Gastroenterol Hepatol. 2009;21:54-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Böcker U, Dinter D, Litterer C, Hummel F, Knebel P, Franke A, Weiss C, Singer MV, Löhr JM. Comparison of magnetic resonance imaging and video capsule enteroscopy in diagnosing small-bowel pathology: localization-dependent diagnostic yield. Scand J Gastroenterol. 2010;45:490-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Jensen MD, Nathan T, Rafaelsen SR, Kjeldsen J. Diagnostic accuracy of capsule endoscopy for small bowel Crohn's disease is superior to that of MR enterography or CT enterography. Clin Gastroenterol Hepatol. 2011;9:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (36)] |
| 21. | Wiarda BM, Mensink PB, Heine DG, Stolk M, Dees J, Hazenberg H, Stoker J, van der Woude CJ, Kuipers EJ. Small bowel Crohn's disease: MR enteroclysis and capsule endoscopy compared to balloon-assisted enteroscopy. Abdom Imaging. 2012;37:397-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Kopylov U, Yablecovitch D, Lahat A, Neuman S, Levhar N, Greener T, Klang E, Rozendorn N, Amitai MM, Ben-Horin S, Eliakim R. Detection of Small Bowel Mucosal Healing and Deep Remission in Patients With Known Small Bowel Crohn's Disease Using Biomarkers, Capsule Endoscopy, and Imaging. Am J Gastroenterol. 2015;110:1316-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (36)] |
| 23. | González-Suárez B, Rodriguez S, Ricart E, Ordás I, Rimola J, Díaz-González Á, Romero C, de Miguel CR, Jáuregui A, Araujo IK, Ramirez A, Gallego M, Fernández-Esparrach G, Ginés Á, Sendino O, Llach J, Panés J. Comparison of Capsule Endoscopy and Magnetic Resonance Enterography for the Assessment of Small Bowel Lesions in Crohn's Disease. Inflamm Bowel Dis. 2018;24:775-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 24. | Di Nardo G, Oliva S, Ferrari F, Riccioni ME, Staiano A, Lombardi G, Costamagna G, Cucchiara S, Stronati L. Usefulness of wireless capsule endoscopy in paediatric inflammatory bowel disease. Dig Liver Dis. 2011;43:220-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Gralnek IM, Cohen SA, Ephrath H, Napier A, Gobin T, Sherrod O, Lewis J. Small bowel capsule endoscopy impacts diagnosis and management of pediatric inflammatory bowel disease: a prospective study. Dig Dis Sci. 2012;57:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Gralnek IM, Defranchis R, Seidman E, Leighton JA, Legnani P, Lewis BS. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther. 2008;27:146-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 332] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 27. | Bryant RV, Winer S, Travis SP, Riddell RH. Systematic review: histological remission in inflammatory bowel disease. Is 'complete' remission the new treatment paradigm? An IOIBD initiative. J Crohns Colitis. 2014;8:1582-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 246] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 28. | Kopylov U, Yung DE, Engel T, Vijayan S, Har-Noy O, Katz L, Oliva S, Avni T, Battat R, Eliakim R, Ben-Horin S, Koulaouzidis A. Diagnostic yield of capsule endoscopy versus magnetic resonance enterography and small bowel contrast ultrasound in the evaluation of small bowel Crohn's disease: Systematic review and meta-analysis. Dig Liver Dis. 2017;49:854-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 29. | Hyams J, Markowitz J, Otley A, Rosh J, Mack D, Bousvaros A, Kugathasan S, Pfefferkorn M, Tolia V, Evans J, Treem W, Wyllie R, Rothbaum R, del Rosario J, Katz A, Mezoff A, Oliva-Hemker M, Lerer T, Griffiths A; Pediatric Inflammatory Bowel Disease Collaborative Research Group. Evaluation of the pediatric crohn disease activity index: a prospective multicenter experience. J Pediatr Gastroenterol Nutr. 2005;41:416-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 260] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 30. | Turner D, Levine A, Walters TD, Focht G, Otley A, López VN, Koletzko S, Baldassano R, Mack D, Hyams J, Griffiths AM. Which PCDAI Version Best Reflects Intestinal Inflammation in Pediatric Crohn Disease? J Pediatr Gastroenterol Nutr. 2017;64:254-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 31. | Abraham BP, Mehta S, El-Serag HB. Natural history of pediatric-onset inflammatory bowel disease: a systematic review. J Clin Gastroenterol. 2012;46:581-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 32. | Pigneur B, Seksik P, Viola S, Viala J, Beaugerie L, Girardet JP, Ruemmele FM, Cosnes J. Natural history of Crohn's disease: comparison between childhood- and adult-onset disease. Inflamm Bowel Dis. 2010;16:953-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 227] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 33. | de Bie CI, Paerregaard A, Kolacek S, Ruemmele FM, Koletzko S, Fell JM, Escher JC, EUROKIDS Porto IBD Working Group of ESPGHAN. Disease phenotype at diagnosis in pediatric Crohn's disease: 5-year analyses of the EUROKIDS Registry. Inflamm Bowel Dis. 2013;19:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 34. | Kim OZ, Han DS, Park CH, Eun CS, Kim YS, Kim YH, Cheon JH, Ye BD, Kim JS. The Clinical Characteristics and Prognosis of Crohn's Disease in Korean Patients Showing Proximal Small Bowel Involvement: Results from the CONNECT Study. Gut Liver. 2018;12:67-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Freeman HJ. Natural history and long-term clinical course of Crohn's disease. World J Gastroenterol. 2014;20:31-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 101] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (5)] |
| 36. | Keh C, Shatari T, Yamamoto T, Menon A, Clark MA, Keighley MR. Jejunal Crohn's disease is associated with a higher postoperative recurrence rate than ileocaecal Crohn's disease. Colorectal Dis. 2005;7:366-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Mao R, Tang RH, Qiu Y, Chen BL, Guo J, Zhang SH, Li XH, Feng R, He Y, Li ZP, Zeng ZR, Eliakim R, Ben-Horin S, Chen MH. Different clinical outcomes in Crohn's disease patients with esophagogastroduodenal, jejunal, and proximal ileal disease involvement: is L4 truly a single phenotype? Therap Adv Gastroenterol. 2018;11:1756284818777938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Lazarev M, Huang C, Bitton A, Cho JH, Duerr RH, McGovern DP, Proctor DD, Regueiro M, Rioux JD, Schumm PP, Taylor KD, Silverberg MS, Steinhart AH, Hutfless S, Brant SR. Relationship between proximal Crohn's disease location and disease behavior and surgery: a cross-sectional study of the IBD Genetics Consortium. Am J Gastroenterol. 2013;108:106-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 39. | Van Limbergen J, Russell RK, Drummond HE, Aldhous MC, Round NK, Nimmo ER, Smith L, Gillett PM, McGrogan P, Weaver LT, Bisset WM, Mahdi G, Arnott ID, Satsangi J, Wilson DC. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology. 2008;135:1114-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 759] [Cited by in RCA: 725] [Article Influence: 40.3] [Reference Citation Analysis (1)] |
| 40. | Giles E, Barclay AR, Chippington S, Wilson DC. Systematic review: MRI enterography for assessment of small bowel involvement in paediatric Crohn's disease. Aliment Pharmacol Ther. 2013;37:1121-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Klang E, Amitai MM, Lahat A, Yablecovitch D, Avidan B, Neuman S, Levhar N, Rozendorn N, Weiss B, Ben-Horin S, Eliakim R, Kopylov U, Israeli IBD research Nucleus [IIRN). Capsule Endoscopy Validation of the Magnetic Enterography Global Score in Patients with Established Crohn's Disease. J Crohns Colitis. 2018;12:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Klang E, Kopylov U, Eliakim R, Rozendorn N, Yablecovitch D, Lahat A, Ben-Horin S, Amitai MM. Diffusion-weighted imaging in quiescent Crohn's disease: correlation with inflammatory biomarkers and video capsule endoscopy. Clin Radiol. 2017;72:798.e7-798.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Kopylov U, Yung DE, Engel T, Avni T, Battat R, Ben-Horin S, Plevris JN, Eliakim R, Koulaouzidis A. Fecal calprotectin for the prediction of small-bowel Crohn's disease by capsule endoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2016;28:1137-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 44. | Kopylov U, Klang E, Yablecovitch D, Lahat A, Avidan B, Neuman S, Levhar N, Greener T, Rozendorn N, Beytelman A, Yanai H, Dotan I, Chowers Y, Weiss B, Ben-Horin S, Amitai MM, Eliakim R; Israeli IBD research Nucleus (IIRN). Magnetic resonance enterography versus capsule endoscopy activity indices for quantification of small bowel inflammation in Crohn's disease. Therap Adv Gastroenterol. 2016;9:655-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 45. | Mergener K, Ponchon T, Gralnek I, Pennazio M, Gay G, Selby W, Seidman EG, Cellier C, Murray J, de Franchis R, Rösch T, Lewis BS. Literature review and recommendations for clinical application of small-bowel capsule endoscopy, based on a panel discussion by international experts. Consensus statements for small-bowel capsule endoscopy, 2006/2007. Endoscopy. 2007;39:895-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Hosoe N, Rath T S-Editor: Ma RY L-Editor: A E-Editor: Zhang YL