Published online Jul 21, 2019. doi: 10.3748/wjg.v25.i27.3649
Peer-review started: March 18, 2019
First decision: April 11, 2019
Revised: April 18, 2019
Accepted: June 7, 2019
Article in press: June 8, 2019
Published online: July 21, 2019
Processing time: 123 Days and 12.3 Hours
Hepatocellular carcinoma (HCC) has been revealed as the second most common cause of cancer-related deaths worldwide. The introduction of cell-based immunotherapy, including dendritic cells (DCs) and cytokine-induced killer cells (CIKs), has brought HCC patients an effective benefit. However, the efficacy and necessity of cellular immunotherapy after different interventional therapy remains to be further explored.
To investigate the efficacy of cellular immunotherapy, involving DCs and CIKs, combined with different conventional treatments of HCC.
We performed a literature search on PubMed and Web of Science up to February 15, 2019. Long-term efficacy (overall survival and recurrence) and short-term adverse effects were investigated to assess the effectiveness of immunotherapy with DCs and/or CIKs. Review Manager 5.3 was used to perform the analysis.
A total of 22 studies involving 3756 patients selected by eligibility inclusion criteria were forwarded for meta-analysis. Combined with the conventional clinical treatment, immunotherapy with DCs and/or CIKs was demonstrated to significantly improve overall survival at 6 mo [risk ratio (RR) = 1.07; 95% confidence interval (CI): 1.01-1.13, P = 0.02], 1 year (RR = 1.12; 95%CI: 1.07-1.17, P < 0.00001), 3 years (RR = 1.23; 95%CI: 1.15-1.31, P < 0.00001) and 5 years (RR = 1.26; 95%CI: 1.15-1.37, P < 0.00001). Recurrence rate was significantly reduced by cellular immunotherapy at 6 mo (RR = 0.50; 95%CI: 0.36-0.69, P < 0.0001) and 1 year (RR = 0.82; 95%CI: 0.75-0.89, P < 0.00001). Adverse effect assessment addressed that immunotherapy with DCs and/or CIKs was accepted as a safe, feasible treatment.
Combination immunotherapy with DCs, CIKs and DC/CIK with various routine treatments for HCC was evidently suggested to improve patients’ prognosis by increasing overall survival and reducing cancer recurrence.
Core tip: Hepatocellular carcinoma has been revealed as the second most common cause of cancer-related deaths worldwide. Though several analyses have supported the effective benefit of cellular immunotherapy when combined with specific hepatocellular carcinoma treatment, the efficacy and necessity of cellular immunotherapy after different interventional therapy remains to be interrogated. Our study suggested that the combination of immunotherapy with dendritic cells, cytokine-induced killer cells and a combination of the two with various routine hepatocellular carcinoma treatments could evidently improve patients’ prognosis by increasing the overall survival and reduce the recurrence of the malignancy.
- Citation: Cao J, Kong FH, Liu X, Wang XB. Immunotherapy with dendritic cells and cytokine-induced killer cells for hepatocellular carcinoma: A meta-analysis. World J Gastroenterol 2019; 25(27): 3649-3663
- URL: https://www.wjgnet.com/1007-9327/full/v25/i27/3649.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i27.3649
Liver cancer, approximately 75% of which is ascribed to hepatocellular carcinoma (HCC), has been revealed as the second most common cause of cancer-related deaths worldwide with around 745000 deaths annually[1]. Both the incidence and mortality are rising worldwide. Cirrhosis, hepatitis B virus and hepatitis C virus are commonly regarded as the main risk factors for HCC.
Curative treatment is achieved by surgical resection, orthotopic liver trans-plantation, transarterial chemoembolization (TACE) and local percutaneous tumor ablation. However, these curative approaches are only accessible to limited numbers of patients, as most are diagnosed at advanced stages with unresectable tumors. Although much progress has been made with promising phase II trial results, drug development has been disappointing with many failures in phase III trials[2]; hence, few drugs have been approved so far by the United States Food and Drug Administra-tion. A multitarget tyrosine kinase inhibitor, sorafenib, the only approved systemic therapy, achieves an increased survival of only up to 3 mo[3,4].
Immunotherapy was introduced into the field as the ability to escape from immunological surveillance, which forms the basis for tumor progression. The underlying mechanism comprises of defective antigen presentation, dysfunction of effector T cells, cytokine disarray and alterations in immune checkpoints[5]. While conventional chemotherapy exerts its effect by directly reducing tumor volume morphologically, immunotherapy works in an indirect way and takes longer to induce an effective immune response. However, it provides a more durable antitumor effect. Immune-based approaches include cytokines, vaccines, adoptive cell therapy [based on peripheral blood mononuclear cells or dendritic cells (DCs)][6-8], tumor-antibody-based immunotherapy[9] and immune checkpoint inhibitors. Recombinant interferon-α was the first immunotherapeutic agent introduced into the field, although, even with its features of immunostimulation and antiangiogenesis, it failed to show a significant effect in clinical trials of patients with HCC[10,11].
Vaccine strategies are carried out on different anti-cancer platforms, including RNA-, peptide- and protein-based vaccines, whole-tumor-cell vaccine, and most widely, DC-based vaccines. DC-based vaccines are adapted more for solid malignancies, including melanoma, renal cancer and prostate cancer, as well as HCC[12,13]. As mature DCs prime T cells and boost memory T cells, induction of DC maturation by Toll-like receptor ligand or cytokines is often applied clinically. Cross-presentation, the process of DCs presenting captured antigen to CD8+ cells via major histocompatibility complex class I, is regarded as a critical step in the efficient induction of antitumor cytotoxic response[12]. An important subtype of DCs was identified in 2010 on account of its high capability in cross-presentation with high expression of CD141 and Toll-like receptor 3. These CD141+ DCs yielded high amounts of type I interferon under stimulation with polyinsionic-polycytidylic acid, which leads to a vigorous T helper 1 cell response; hence the effective anticancer response[14]. Therefore, polyinsionic-polycytidylic acid and its derivatives are adopted as adjuvants for DC-based vaccines.
Adoptive cell therapy is commonly performed with cytokine-induced killer cells (CIKs), tumor-infiltrating lymphocytes and genetically modified T cells. Among these, CIKs have been used in more clinical trials. CIKs, consisting of NKG2Dhigh T cells, activated natural killer cells and natural killer T cells[15], are generated ex vivo from peripheral blood mononuclear cells and stimulated with cytokines and antibodies targeting CD3. CIKs harbor high capacity of proliferation and have a cytolytic effect against cancer cells. Although several analyses have supported the beneficial effect of cellular immunotherapy when combined with specific HCC treatment[16,17], the efficacy and necessity of cellular immunotherapy after different interventional therapy remains to be interrogated. In this study, a systematic review and meta-analysis was performed to investigate the efficacy of cellular immunotherapy, involving DCs, CIKs and DC/CIK combination therapy, combined with different treatments of HCC.
We conducted a widespread literature search on PubMed and Web of Science Core Collection. Articles up to February 15, 2019 were filtered out by key words including hepatocellular OR liver AND cancer OR tumor OR tumour OR carcinoma OR neoplasm AND immunotherapy OR immune checkpoint OR immunotherapeutic. Study selection was conducted by two independent investigators. Discrepancies were resolved by discussion and consensus. Full text was retrieved for further decision if the abstract was insufficient to support the inclusion criteria. Three reviewers independently evaluated studies for eligibility.
Randomized controlled trials (RCTs) and controlled trials were taken into considera-tion, but trials with only safety data, animal studies and in vitro studies were excluded. Studies that met the following criteria were included: (1) Clinical trials on immunotherapy for HCC patients with full text and available data in English; and (2) Clinical trials providing survival data [disease-free survival, progression-free survival, and overall survival (OS)] and adverse effects.
The following information was extracted for each article: last name of first author, year of publication, phase of clinical trial, number of enrolled subjects, treatment arms, number of patients in control and conventional groups, OS, recurrence rate and adverse effects. The quality assessment was performed with Cochrane Collaboration’s tool for RCT trails and MINORS[18] for non-RCT cohort studies.
We conducted all the analysis with Review Manager 5.3 (Cochrane Collaboration). Risk ratio (RR) was calculated to assess the effect of interventions. Results were presented with 95% confidence interval (CI). We performed Cochrane’s Q statistic (χ2) to assess the heterogeneity of the trials and the I2 statistic for inconsistency. P < 0.05 or I2 > 50% was considered an invalid assumption of homogeneity. A random-effects model was applied for clinical trials of significant heterogeneity, otherwise a fixed-effects model was applied. Assessment of potential publication bias was evaluated by funnel plot, where two-tailed P < 0.05 was considered statistically significant.
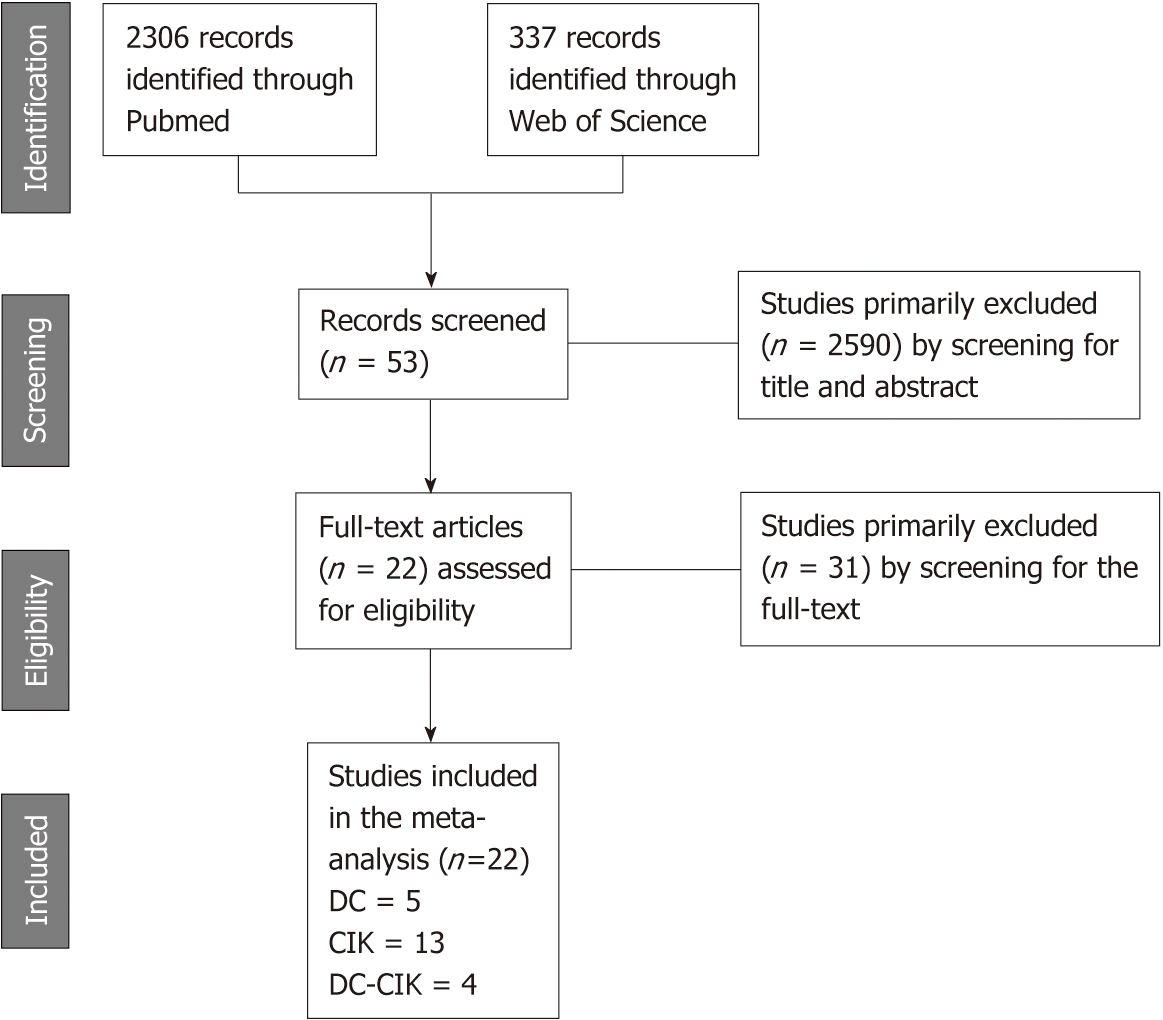
A total of 2643 citations were identified from the primary literature search. Among them, 2621 studies were excluded for the following reasons: overlapping studies, reviews, no access to full-text in English, letters, and individual case studies. According to the inclusion criteria, 22 studies (Figure 1) including 3756 patients were adopted in the meta-analysis. Five of the included studies involving 390 patients were aligned for analyzing the effect of mono-immunotherapy with DC-based vaccines. A total of 3211 patients involved in 13 trials were assigned for comparative analysis of mono-immunotherapy with CIKs. The other four trials with 155 patients focused on the effect of combined approach with DC vaccines and CIKs (Table 1). Most of those trials evaluated the effect of immunotherapy based on interventions for primary tumor ablation by surgical resection, radiofrequency ablation and cryoablation. Five studies[19,20,24,26,27] were conducted with patients receiving TACE treatment. A few studies assigned patients with chemotherapy[32] or only supportive treatment[21] as the control group.
| Author (year) | Country | Control | Immuno-therapy interven-tions | Patients | Child-Pugh score A/B/C | Immune cell regimens | Course | ||
| Control | Trial | Control | Trial | ||||||
| Nakamoto (2007)[19] | Japan | TACE | TACE + DC | 11 | 10 | 4/7/0 | 8/2/0 | 5 × 106 | 1 |
| Nakamoto (2011)[20] | Japan | TACE | TACE + DC | 22 | 13 | NR | 5/8/0 | 5 × 106 | 1 |
| El Ansary (2013)[21] | Egypt | Supportive treatment | DC | 15 | 15 | 0/14/1 | 0/15/0 | 20 × 106 | 1 |
| Sun (2015)[22] | China | Resection + FOLFOX6 | Resection + DC | 80 | 80 | NR | NR | 8.9 × 109 | 6 |
| Lee (2017)[23] | South Korea | Resection/RFA/PEI | Resection/RFA/PEI + DC | 75 | 69 | NR | NR | 3 × 107 | 6 |
| Weng (2007)[24] | China | TACE + RFA | TACE + RFA + CIK | 40 | 45 | 33/7/0 | 36/9/0 | 1.0-1.5 × 1010 | 8/9 |
| Dong (2008)[25] | China | Resection | Resection + CIK | 43 | 84 | 34/9/0 | 68/16/0 | 1.0-2.0 × 1010 | 3/5 |
| Hao (2010)[26] | China | TACE | TACE + RFA + CIK | 74 | 72 | 66/8/0 | 65/7/0 | 1.0-5.0 × 1010 | 4 |
| Pan (2010)[27] | China | TACE + RFA | TACE + RFA + CIK | 39 | 42 | NR | NR | 1.0 × 1010 | 4 |
| Pan (2013)[28] | China | Resection | Resection + CIK | 206 | 204 | 206/0/0 | 204/0/0 | 1.0-1.5 × 1010 | 4 |
| Lee (2015)[29] | South Korea | Resection/RFA/PEI | Resection/RFA/PEI + CIK | 112 | 114 | 112/0/0 | 114/0/0 | NR | 16 |
| Pan (2015)[30] | China | Resection | Resection + CIK | 520 | 511 | NR | NR | 1.0-1.5 × 1010 | 4 |
| Chen (2016)[31] | China | Resection | Resection + CIK | 118 | 231 | 205/12/0 | 222/9/0 | 1.0-1.5 × 1010 | 4 |
| Li (2016)[32] | China | Oxaliplatin + Capecitabine | CIK | 37 | 37 | 33(A + B)/4(C) | 27(A + B)/10(C) | NR | NR |
| Chang (2018)[33] | China | Resection | Resection + CIK | 145 | 145 | 145/0/0 | 145/0/0 | NR | NR |
| Lee (2018)[34] | South Korea | Resection/RFA/PEI | Resection/RFA/PEI + CIK | 112 | 114 | 112/0/0 | 114/0/0 | 6.4 × 109 | 16 |
| Cui (2014)[35] | China | RFA | RFA + NK/γδT/CIK | 32 | 30 | 14/18/0 | 18/12/0 | 1.2-2.0 × 109 | 8 |
| Qian (2016)[36] | China | RFA | RFA + NK/γδT/CIK | 31 | 73 | NR | NR | 1.0-2.0 × 109 | 8 |
| Qiu (2011)[37] | China | Resection | Resection + DC/CIK | 9 | 9 | NR | NR | 2.0-20.0 × 109 | NR |
| Niu (2013)[38] | China | Cryoablation | Cytotherapy + DC/CIK | 12 | 21 | NR | NR | 6.0-10.0 × 109 | NR |
| Shimizu (2014)[39] | Japan | Resection | Resection + DC/CIK | 40 | 35 | 44/8/0 | 34/8/0 | NR | NR |
| Yu (2015)[40] | China | Microwave ablation | Microwave ablation + DC/CIK | 15 | 14 | 15/0/0 | 13/1/0 | NR | NR |
Compared with the patients in the control group, regardless of original treatment arm, patients receiving monotherapy with DC-based vaccine had a higher 1-year OS (RR = 1.16; 95%CI: 1.03-1.30, P = 0.01), although there was no significant improvement in OS at 6 mo or 3 years. Monotherapy with CIKs demonstrated significant improvement in OS at 6 mo (RR = 1.09; 95%CI: 1.03-1.16, P = 0.005), 1 year (RR = 1.11; 95%CI: 1.06-1.16, P < 0.00001), 3 years (RR = 1.23; 95%CI: 1.15-1.31, P < 0.00001) and 5 years (RR = 1.25; 95%CI: 1.14-1.36, P < 0.00001). Unsurprisingly, combined therapy with DCs and CIKs also improved OS at 1 year (RR = 3.8; 95%CI: 1.29-11.22, P = 0.02) and 5 years (RR = 1.45; 95%CI: 0.99-2.12, P = 0.05). Taken together, immunotherapy based on DCs and/or CIKs significantly increased OS at 6 mo (RR = 1.07; 95%CI: 1.01-1.13, P = 0.02), 1 year (RR = 1.12; 95%CI: 1.07-1.17, P < 0.00001), 3 years (RR = 1.23; 95%CI: 1.15-1.31, P < 0.00001) and 5 years (RR = 1.26; 95%CI: 1.15-1.37, P < 0.00001) (Figure 2).
Besides the improved OS of patients, reduced tumor recurrence rate was achieved in most trials. DC-based vaccination alone addressed a declining shift in 1-year recurrence (RR = 0.64; 95%CI: 0.44-1.93, P = 0.02). Immunotherapy with CIKs showed similar effects on recurrence at 6 mo (RR = 0.45; 95%CI: 0.30-0.66, P < 0.0001), 1 year (RR = 0.83; 95%CI: 0.76-0.91, P < 0.0001) and 1.5 years (RR = 0.39; 95%CI: 0.18-0.85, P = 0.02). The data on recurrence with DC/CIK combined therapy were unfortunately not available. The retardation effect of recurrence was observed at 6 mo (RR = 0.50; 95%CI: 0.36-0.69, P < 0.0001) and 1 year (RR = 0.82; 95%CI: 0.75-0.89, P < 0.00001) (Figure 3).
None of the studies reported immunotherapy-related hospital mortality. Only 23 patients in four studies[23,29,32,36] were reported with grade III or IV adverse events. Fever was addressed as the most common event after immunotherapy. Other adverse effects included shivering, vomiting, fatigue, abdominal pain and leukopenia. The overall adverse event rate was higher in immunotherapy groups, but symptomatic remissions were declared within 24 h. In brief, immunotherapy was accepted as a safe, feasible treatment, though with an increased incidence of adverse events.
Seven studies included in this systematic review were RCTs, including two with DC-based vaccine, three with mono-immunotherapy with CIKs and two with combination immunotherapies. Cochrane Collaboration’s tool was used to assess the quality of the RCTs. All these RCTs were verified with high quality (Table 2), and 15 non-RCTs were validated as high quality by MINORS (Table 3).
| Author (year) | Selection | Performance | Detection | Attrition | Reporting | Other |
| Bias | Bias | Bias | Bias | Bias | Bias | |
| El Ansary (2013) | Unclear | High | Unclear | Low | Low | Low |
| Sun (2015) | Unclear | High | Unclear | Low | Low | Low |
| Dong (2008) | Unclear | High | Unclear | Low | Low | Low |
| Lee (2015) | Unclear | High | Unclear | Low | Low | Low |
| Lee (2018) | Unclear | High | Unclear | Low | Low | Low |
| Qiu (2011) | Unclear | High | Unclear | Low | Low | Low |
| Yu (2015) | Unclear | High | Unclear | Low | Low | Low |
| Author (year) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| Nakamoto (2007) | 2 | 2 | 2 | 0 | 0 | 1 | 2 | 0 | 2 | 2 | 2 | 2 |
| Nakamoto (2011) | 2 | 2 | 2 | 2 | 0 | 1 | 2 | 0 | 2 | 2 | 2 | 2 |
| Lee (2017) | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 2 | 2 | 2 |
| Weng (2007) | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 2 | 2 |
| Hao (2010) | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Pan (2010) | 2 | 2 | 2 | 2 | 0 | 1 | 2 | 0 | 2 | 2 | 2 | 2 |
| Pan (2013) | 2 | 2 | 2 | 2 | 0 | 1 | 2 | 0 | 2 | 2 | 2 | 2 |
| Pan (2015) | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 2 | 2 |
| Chen (2016) | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 2 | 2 |
| Li (2016) | 2 | 2 | 2 | 2 | 0 | 1 | 2 | 2 | 2 | 2 | 2 | 2 |
| Chang (2018) | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Cui (2014) | 2 | 2 | 2 | 0 | 0 | 1 | 2 | 0 | 2 | 2 | 2 | 2 |
| Qian (2016) | 2 | 2 | 2 | 1 | 0 | 2 | 0 | 0 | 2 | 2 | 2 | 2 |
| Niu (2013) | 2 | 2 | 2 | 0 | 0 | 2 | 2 | 0 | 2 | 2 | 2 | 2 |
| Shimizu (2014) | 2 | 2 | 2 | 0 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
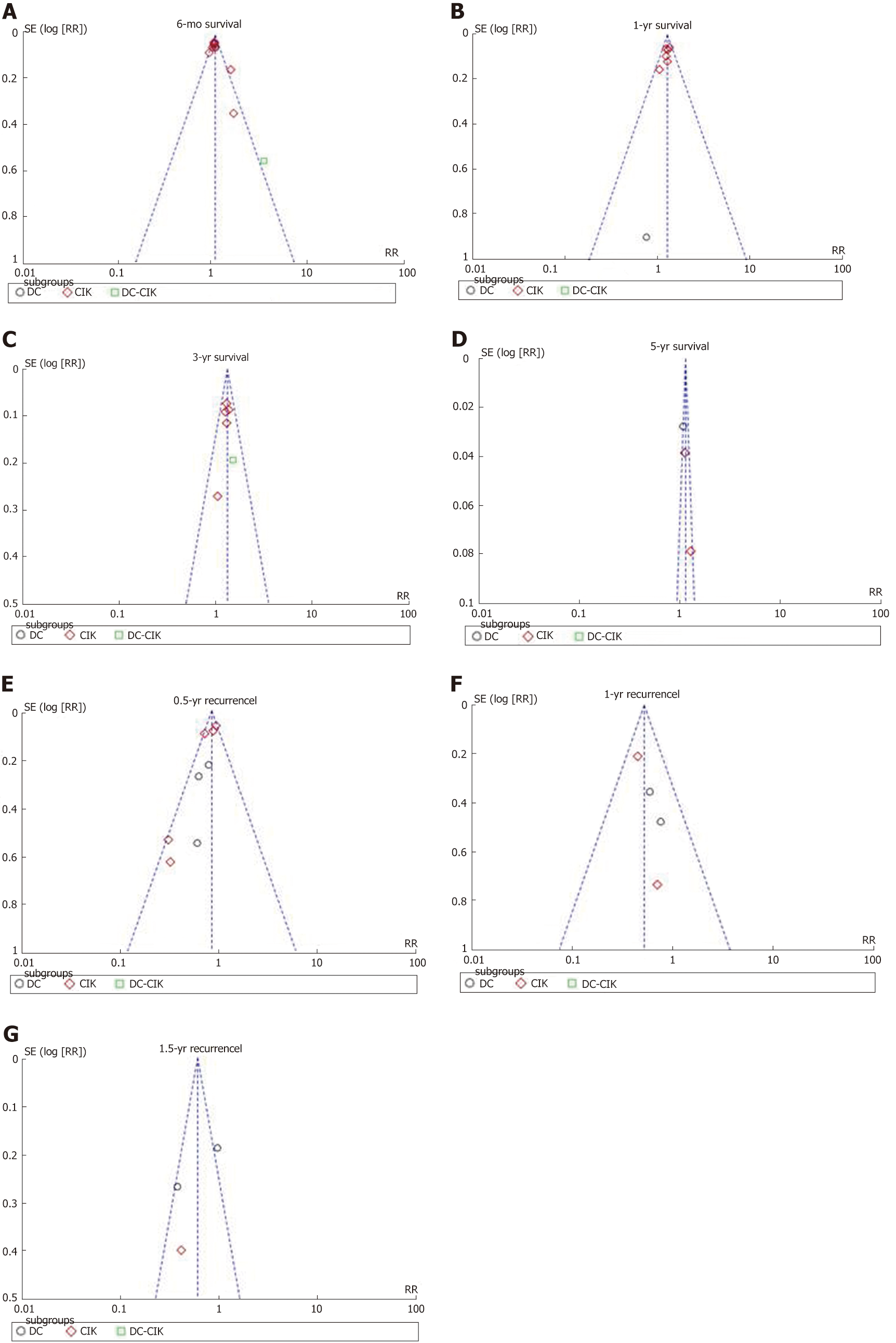
Funnel plots and Egger’s regression test were applied to OS and recurrence rate in order to guarantee the potency of this meta-analysis (Figure 4). Symmetrical distribution of individual studies indicated no evident publication bias.
Although surgical resection, transplantation, local tumor ablation and TACE are well accepted with proven survival benefit[41], these clinical treatments of HCC have their limited scope of application according to the tumor progression of individual patients. Besides, tumor recurrence can occur within five years even with curative interven-tions[42]. Unlike some other solid tumors, no effective neoadjuvant or adjuvant therapy has been validated to reduce the recurrence risk so far[41]. The introduction of immunotherapy, however, brought new hope in this regard. In this meta-analysis, we found that immunotherapy could increase OS and decrease recurrence rate in HCC.
We comprehensively analyzed 22 individual studies with 3756 HCC patients in this meta-analysis and illustrated a positive prognostic efficacy of immunotherapy with DC-based vaccine and/or CIK-based adoptive therapy. Our results demonstrated that an extended OS (6 mo, 1, 3 and 5 years) was achieved with the aforesaid immuno-therapy based on different HCC interventional therapies. Similar benefits in OS were described in another meta-analysis[43] of adjuvant adoptive immunotherapy, including CIKs, lymphokine-activated killer cells and lymphocytes in HCC patients after surgery. In that study, a significant reduction in mortality and recurrence was observed at 1, 2 and 3 years but not 5 years[43]. However, compared to the slight increase in OS in our study, another systematic review demonstrated a more dramatic shift in OS in TACE-treated HCC patients[16]. The smaller increase in OS in our analysis could have been ascribed to the heterogeneity of the patients included in the controlled trials. Yet, a significant benefit in OS was confirmed regardless of subgroup composition, and more intriguingly, our analysis indicated that short-term recurrence was intensely reduced with immunotherapy independent of the heterogeneity. The clinical benefit of the combined immunotherapy with DCs and CIKs has been demonstrated in many clinical studies[17,44]. This combined approach was reported to have greater antitumor activity in vitro than CIK treatment alone[45], while our analysis failed to find sufficient clinical data to support it.
One RCT[22] and one non-RCT cohort study[32] included in this meta-analysis were based on conventional chemotherapy as the control group. Cellular immunotherapy also resulted in improved outcomes, implying a critical role for immunotherapy in patients who have lost their chance for tumor ablation. The combination of chemotherapy and immunotherapy has accumulated positive efficacy in a number of unresectable malignancies[46-48].
Recently, the introduction of immune checkpoint inhibitors has led to a clinical breakthrough in cancer treatment[49]. Six immune checkpoint inhibitors for HCC treatment have been approved by the Food and Drug Administration, and even one (nivolumab) has been introduced as a second-line treatment[50,51] due to its promising effect on immune checkpoints inhibitors in HCC patients. Although some clinical data have presented a good safety profile, disease control and time to progression[50-52], clinical data concerning comparative trials with immune checkpoints in HCC patients are still insufficient for analysis.
A bias could have been raised due to the low number of available trials and the low number of included patients in the DC-based vaccine group and DC/CIK combina-tion group, which comprised five trials with 390 patients and four trials with 155 patients, respectively. The quality of this meta-analysis was still assured as we have shown no evidence of publication bias.
There were a few limitations to this systematic review and meta-analysis. First, although we included 22 trials in the analysis, the DC and DC/CIK groups comprised only five and four trials, respectively. Most of the trials were conducted in East Asian countries (China, Japan and South Korea); hence, they had less-sufficient statistical power due to the lack of multinational or multiracial clinical data. Second, heter-ogeneity was observed between the included studies. Factors, including stage of malignancy, different surgical method, number of immunotherapy fusion cycles, and duration of immunotherapy in different clinical centers could have contributed to the heterogeneity.
In conclusion, we demonstrated that the application of immunotherapy with DCs, CIKs and DCs/CIK in addition to various routine HCC treatments could evidently improve patients’ prognosis by increasing OS and reducing recurrence. The efficacy of novel immune checkpoint inhibitors based on clinical trials should be assessed in further studies when sufficient data are available. Due to the diverse mechanism behind the immune evasion of tumor cells, approaches combining different immunotherapies might lead to an appealing strategy to treat HCC.
Hepatocellular carcinoma (HCC) has been revealed as the second most common cause of cancer-related deaths worldwide. The introduction of cell-based immunotherapy, including dendritic cells (DCs) and cytokine-induced killer cells (CIKs), has brought HCC patients an effective benefit. However, the efficacy and necessity of cellular immunotherapy after different interventional therapy remain to be further explored.
Only patients with early to intermediate stage of HCC can benefit from curable interventions. Unfortunately, tumor recurrence within 5 years occurs even with curable treatment. As the introduction of immunotherapy has brought beneficial effects to HCC treatment, better strategies with combined interventions would help to improve the outcomes of HCC patients.
A systematic review and meta-analysis were performed in this study to investigate the efficacy of cellular immunotherapy, involving DCs, CIKs and DC/CIK combination therapy combined with different treatments of HCC.
A literature search was performed on PubMed and Web of Science up to February 15, 2019. Long-term efficacy (overall survival and recurrence) and short-term adverse effects were investigated to assess the effectiveness of immunotherapy with DCs and/or CIKs. Review Manager 5.3 was used to perform the analysis.
A total of 22 studies involving 3756 patients selected by eligibility inclusion criteria were forwarded for meta-analysis. Combined with the conventional clinical treatment, immunotherapy with DCs and/or CIKs was demonstrated to significantly improve overall survival at 6 mo, 1 year, 3 years and 5 years. Recurrence rate was significantly reduced by cellular immunotherapy at 6 mo and 1 year. Adverse effect assessment addressed that immunotherapy with DCs and/or CIKs was accepted as a safe, feasible treatment.
Combination immunotherapy with DCs, CIKs and DC/CIK with various routine treatments for HCC was evidently suggested to improve patients’ prognosis by increasing overall survival and reducing cancer recurrence.
This meta-analysis indicated that the combination of conventional therapy and the intervention of immunotherapy with DCs, CIK and DC/CIK could pave the way for a promising approach for HCC treatment. Further assessment of the efficacy of novel immune checkpoint inhibitors based on clinical trials will help us to better identify immunotherapy strategies to treat HCC.
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20718] [Article Influence: 1883.5] [Reference Citation Analysis (23)] |
| 2. | Greten TF, Sangro B. Targets for immunotherapy of liver cancer. J Hepatol. 2017;pii:S0168-8278(17)32287-0. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 3. | European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4562] [Article Influence: 325.9] [Reference Citation Analysis (4)] |
| 4. | Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G; RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2160] [Cited by in RCA: 2836] [Article Influence: 315.1] [Reference Citation Analysis (1)] |
| 5. | Tsuchiya N, Sawada Y, Endo I, Uemura Y, Nakatsura T. Potentiality of immunotherapy against hepatocellular carcinoma. World J Gastroenterol. 2015;21:10314-10326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Iwashita Y, Tahara K, Goto S, Sasaki A, Kai S, Seike M, Chen CL, Kawano K, Kitano S. A phase I study of autologous dendritic cell-based immunotherapy for patients with unresectable primary liver cancer. Cancer Immunol Immunother. 2003;52:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Lee WC, Wang HC, Hung CF, Huang PF, Lia CR, Chen MF. Vaccination of advanced hepatocellular carcinoma patients with tumor lysate-pulsed dendritic cells: a clinical trial. J Immunother. 2005;28:496-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 142] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y, Kakizoe T. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 658] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 9. | Zhu AX, Stuart K, Blaszkowsky LS, Muzikansky A, Reitberg DP, Clark JW, Enzinger PC, Bhargava P, Meyerhardt JA, Horgan K, Fuchs CS, Ryan DP. Phase 2 study of cetuximab in patients with advanced hepatocellular carcinoma. Cancer. 2007;110:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 182] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 10. | Llovet JM, Sala M, Castells L, Suarez Y, Vilana R, Bianchi L, Ayuso C, Vargas V, Rodés J, Bruix J. Randomized controlled trial of interferon treatment for advanced hepatocellular carcinoma. Hepatology. 2000;31:54-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 181] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Chen LT, Chen MF, Li LA, Lee PH, Jeng LB, Lin DY, Wu CC, Mok KT, Chen CL, Lee WC, Chau GY, Chen YS, Lui WY, Hsiao CF, Whang-Peng J, Chen PJ; Disease Committee of Adjuvant Therapy for Postoperative Hepatocellular Carcinoma, Taiwan Cooperative Oncology Group, National Health Research Institutes, Zhunan, Taiwan. Long-term results of a randomized, observation-controlled, phase III trial of adjuvant interferon Alfa-2b in hepatocellular carcinoma after curative resection. Ann Surg. 2012;255:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Radford KJ, Tullett KM, Lahoud MH. Dendritic cells and cancer immunotherapy. Curr Opin Immunol. 2014;27:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | Quinn DI, Shore ND, Egawa S, Gerritsen WR, Fizazi K. Immunotherapy for castration-resistant prostate cancer: Progress and new paradigms. Urol Oncol. 2015;33:245-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Bachem A, Güttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, Salama A, Movassaghi K, Opitz C, Mages HW, Henn V, Kloetzel PM, Gurka S, Kroczek RA. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 571] [Cited by in RCA: 665] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 15. | Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 464] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 16. | Ding M, Wang Y, Chi J, Wang T, Tang X, Cui D, Qian Q, Zhai B. Is Adjuvant Cellular Immunotherapy Essential after TACE-Predominant Minimally-Invasive Treatment for Hepatocellular Carcinoma? A Systematic Meta-Analysis of Studies Including 1774 Patients. PLoS One. 2016;11:e0168798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | He G, Zheng C, Huo H, Zhang H, Zhu Z, Li J, Zhang H. TACE combined with dendritic cells and cytokine-induced killer cells in the treatment of hepatocellular carcinoma: A meta-analysis. Int Immunopharmacol. 2016;40:436-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3743] [Cited by in RCA: 6133] [Article Influence: 266.7] [Reference Citation Analysis (0)] |
| 19. | Nakamoto Y, Mizukoshi E, Tsuji H, Sakai Y, Kitahara M, Arai K, Yamashita T, Yokoyama K, Mukaida N, Matsushima K, Matsui O, Kaneko S. Combined therapy of transcatheter hepatic arterial embolization with intratumoral dendritic cell infusion for hepatocellular carcinoma: clinical safety. Clin Exp Immunol. 2007;147:296-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 20. | Nakamoto Y, Mizukoshi E, Kitahara M, Arihara F, Sakai Y, Kakinoki K, Fujita Y, Marukawa Y, Arai K, Yamashita T, Mukaida N, Matsushima K, Matsui O, Kaneko S. Prolonged recurrence-free survival following OK432-stimulated dendritic cell transfer into hepatocellular carcinoma during transarterial embolization. Clin Exp Immunol. 2011;163:165-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | El Ansary M, Mogawer S, Elhamid SA, Alwakil S, Aboelkasem F, Sabaawy HE, Abdelhalim O. Immunotherapy by autologous dendritic cell vaccine in patients with advanced HCC. J Cancer Res Clin Oncol. 2013;139:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Sun TY, Yan W, Yang CM, Zhang LF, Tang HL, Chen Y, Hu HX, Wei X. Clinical research on dendritic cell vaccines to prevent postoperative recurrence and metastasis of liver cancer. Genet Mol Res. 2015;14:16222-16232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Lee JH, Tak WY, Lee Y, Heo MK, Song JS, Kim HY, Park SY, Bae SH, Lee JH, Heo J, Kim KH, Bae YS, Kim YJ. Adjuvant immunotherapy with autologous dendritic cells for hepatocellular carcinoma, randomized phase II study. Oncoimmunology. 2017;6:e1328335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Weng DS, Zhou J, Zhou QM, Zhao M, Wang QJ, Huang LX, Li YQ, Chen SP, Wu PH, Xia JC. Minimally invasive treatment combined with cytokine-induced killer cells therapy lower the short-term recurrence rates of hepatocellular carcinomas. J Immunother. 2008;31:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Hui D, Qiang L, Jian W, Ti Z, Da-Lu K. A randomized, controlled trial of postoperative adjuvant cytokine-induced killer cells immunotherapy after radical resection of hepatocellular carcinoma. Dig Liver Dis. 2009;41:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 26. | Hao MZ, Lin HL, Chen Q, Ye YB, Chen QZ, Chen MS. Efficacy of transcatheter arterial chemoembolization combined with cytokine-induced killer cell therapy on hepatocellular carcinoma: a comparative study. Chin J Cancer. 2010;29:172-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Pan CC, Huang ZL, Li W, Zhao M, Zhou QM, Xia JC, Wu PH. Serum alpha-fetoprotein measurement in predicting clinical outcome related to autologous cytokine-induced killer cells in patients with hepatocellular carcinoma undergone minimally invasive therapy. Chin J Cancer. 2010;29:596-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Pan K, Li YQ, Wang W, Xu L, Zhang YJ, Zheng HX, Zhao JJ, Qiu HJ, Weng DS, Li JJ, Wang QJ, Huang LX, He J, Chen SP, Ke ML, Wu PH, Chen MS, Li SP, Xia JC, Zeng YX. The efficacy of cytokine-induced killer cell infusion as an adjuvant therapy for postoperative hepatocellular carcinoma patients. Ann Surg Oncol. 2013;20:4305-4311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 29. | Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW, Yoon JH. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383-1391.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 401] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 30. | Pan QZ, Wang QJ, Dan JQ, Pan K, Li YQ, Zhang YJ, Zhao JJ, Weng DS, Tang Y, Huang LX, He J, Chen SP, Ke ML, Chen MS, Wicha MS, Chang AE, Zeng YX, Li Q, Xia JC. A nomogram for predicting the benefit of adjuvant cytokine-induced killer cell immunotherapy in patients with hepatocellular carcinoma. Sci Rep. 2015;5:9202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Chen CL, Pan QZ, Zhao JJ, Wang Y, Li YQ, Wang QJ, Pan K, Weng DS, Jiang SS, Tang Y, Zhang XF, Zhang HX, Zhou ZQ, Zeng YX, Xia JC. PD-L1 expression as a predictive biomarker for cytokine-induced killer cell immunotherapy in patients with hepatocellular carcinoma. Oncoimmunology. 2016;5:e1176653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 32. | Li W, Wang Y, Kellner DB, Zhao L, Xu L, Gao Q. Efficacy of RetroNectin-activated cytokine-induced killer cell therapy in the treatment of advanced hepatocelluar carcinoma. Oncol Lett. 2016;12:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Chang B, Shen L, Wang K, Jin J, Huang T, Chen Q, Li W, Wu P. High number of PD-1 positive intratumoural lymphocytes predicts survival benefit of cytokine-induced killer cells for hepatocellular carcinoma patients. Liver Int. 2018;38:1449-1458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW, Yoon JH. Sustained efficacy of adjuvant immunotherapy with cytokine-induced killer cells for hepatocellular carcinoma: an extended 5-year follow-up. Cancer Immunol Immunother. 2019;68:23-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 35. | Cui J, Wang N, Zhao H, Jin H, Wang G, Niu C, Terunuma H, He H, Li W. Combination of radiofrequency ablation and sequential cellular immunotherapy improves progression-free survival for patients with hepatocellular carcinoma. Int J Cancer. 2014;134:342-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 36. | Qian L, Wang N, Tian H, Jin H, Zhao H, Niu C, He H, Ge T, Han W, Hu J, Li D, Han F, Xu J, Ding X, Chen J, Li W, Cui J. Dual Effects of Cellular Immunotherapy in Inhibition of Virus Replication and Prolongation of Survival in HCV-Positive Hepatocellular Carcinoma Patients. J Immunol Res. 2016;2016:6837241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Qiu Y, Xu MB, Yun MM, Wang YZ, Zhang RM, Meng XK, Ou-Yang XH, Yun S. Hepatocellular carcinoma-specific immunotherapy with synthesized α1,3- galactosyl epitope-pulsed dendritic cells and cytokine-induced killer cells. World J Gastroenterol. 2011;17:5260-5266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Niu LZ, Li JL, Zeng JY, Mu F, Liao MT, Yao F, Li L, Liu CY, Chen JB, Zuo JS, Xu KC. Combination treatment with comprehensive cryoablation and immunotherapy in metastatic hepatocellular cancer. World J Gastroenterol. 2013;19:3473-3480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Shimizu K, Kotera Y, Aruga A, Takeshita N, Katagiri S, Ariizumi S, Takahashi Y, Yoshitoshi K, Takasaki K, Yamamoto M. Postoperative dendritic cell vaccine plus activated T-cell transfer improves the survival of patients with invasive hepatocellular carcinoma. Hum Vaccin Immunother. 2014;10:970-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 40. | Yu MA, Liang P, Yu XL, Han ZY, Dong XJ, Wang YU, Cheng C, Li X. Multiple courses of immunotherapy with different immune cell types for patients with hepatocellular carcinoma after microwave ablation. Exp Ther Med. 2015;10:1460-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4359] [Article Influence: 544.9] [Reference Citation Analysis (6)] |
| 42. | Hasegawa K, Kokudo N, Makuuchi M, Izumi N, Ichida T, Kudo M, Ku Y, Sakamoto M, Nakashima O, Matsui O, Matsuyama Y. Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol. 2013;58:724-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 305] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 43. | Yuan BH, Li RH, Yuan WP, Yang T, Tong TJ, Peng NF, Li LQ, Zhong JH. Harms and benefits of adoptive immunotherapy for postoperative hepatocellular carcinoma: an updated review. Oncotarget. 2017;8:18537-18549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Li XD, Xu B, Wu J, Ji M, Xu BH, Jiang JT, Wu CP. Review of Chinese clinical trials on CIK cell treatment for malignancies. Clin Transl Oncol. 2012;14:102-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Pan Y, Tao Q, Wang H, Xiong S, Zhang R, Chen T, Tao L, Zhai Z. Dendritic cells decreased the concomitant expanded Tregs and Tregs related IL-35 in cytokine-induced killer cells and increased their cytotoxicity against leukemia cells. PLoS One. 2014;9:e93591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Rizvi NA, Peters S. Immunotherapy for Unresectable Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:1986-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 47. | Simon A, Kourie HR, Kerger J. Is there still a role for cytotoxic chemotherapy after targeted therapy and immunotherapy in metastatic melanoma? A case report and literature review. Chin J Cancer. 2017;36:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 48. | Yang Y, Lin H, Zhao L, Song Y, Gao Q. Combination of sorafenib and cytokine-induced killer cells in metastatic renal cell carcinoma: a potential regimen. Immunotherapy. 2017;9:629-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | McNutt M. Cancer immunotherapy. Science. 2013;342:1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 50. | Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M, Uppala A, Korangy F, Kleiner DE, Figg WD, Venzon D, Steinberg SM, Venkatesan AM, Krishnasamy V, Abi-Jaoudeh N, Levy E, Wood BJ, Greten TF. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 661] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 51. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3449] [Article Influence: 383.2] [Reference Citation Analysis (2)] |
| 52. | Sangro B, Gomez-Martin C, de la Mata M, Iñarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E, Alfaro C, Sarobe P, Lasarte JJ, Pérez-Gracia JL, Melero I, Prieto J. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 772] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jiang YF, Tashiro H S-Editor: Ma RY L-Editor: Filipodia E-Editor: Liu JH