Published online Jul 21, 2019. doi: 10.3748/wjg.v25.i27.3634
Peer-review started: March 20, 2019
First decision: March 27, 2019
Revised: May 3, 2019
Accepted: May 31, 2019
Article in press: June 1, 2019
Published online: July 21, 2019
Processing time: 122 Days and 9.5 Hours
Acute liver failure (ALF) has a high mortality varying from 80% to 85% with rapid progress in multi-organ system failure. Bioartificial liver (BAL) support systems have the potential to provide temporary support to bridge patients with ALF to liver transplantation or spontaneous recovery. In the past decades, several BAL support systems have been conducted in clinical trials. More recently, concerns have been raised on the renovation of high-quality cell sources and configuration of BAL support systems to provide more benefits to ALF models in preclinical experiments.
To investigate the characteristics of studies about BAL support systems for ALF, and to evaluate their effects on mortality.
Eligible clinical trials and preclinical experiments on large animals were identified on Cochrane Library, PubMed, and EMbase up to March 6, 2019. Two reviewers independently extracted the necessary information, including key BAL indicators, survival and indicating outcomes, and adverse events during treatment. Descriptive analysis was used to identify the characteristics of the included studies, and a meta-analysis including only randomized controlled trial (RCT) studies was done to calculate the overall effect of BAL on mortality among humans and large animals, respectively.
Of the 30 selected studies, 18 were clinical trials and 12 were preclinical experiments. The meta-analysis result suggested that BAL might reduce mortality in ALF in large animals, probably due to the recent improvement of BAL, including the type, cell source, cell mass, and bioreactor, but seemed ineffective for humans [BAL vs control: relative risk (95% confidence interval), 0.27 (0.12-0.62) for animals and 0.72 (0.48-1.08) for humans]. Liver and renal functions, hematologic and coagulative parameters, encephalopathy index, and neurological indicators seemed to improve after BAL, with neither meaningful adverse events nor porcine endogenous retrovirus infection.
BAL may reduce the mortality of ALF by bridging the gap between preclinical experiments and clinical trials. Clinical trials using improved BAL must be designed scientifically and conducted in the future to provide evidence for transformation.
Core tip: This systematic review and meta-analysis included a large number of studies about clinical trials and preclinical experiments of bioartificial liver (BAL) support systems for treating patients and large animal models with acute liver failure. We summarized the characteristics of studies, BAL, and outcomes in all the studies and compared the pooled effect by meta-analysis including only randomized controlled trial studies regarding mortality after BAL among humans and large animals, respectively.
- Citation: He YT, Qi YN, Zhang BQ, Li JB, Bao J. Bioartificial liver support systems for acute liver failure: A systematic review and meta-analysis of the clinical and preclinical literature. World J Gastroenterol 2019; 25(27): 3634-3648
- URL: https://www.wjgnet.com/1007-9327/full/v25/i27/3634.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i27.3634
Acute liver failure (ALF) is characterized by an acute episode of liver dysfunction in individuals without underlying chronic liver diseases, sometimes causing a rapid onset of encephalopathy and coagulopathy followed by multiorgan system failure. Patients with ALF have a high mortality ranging from 80% to 85%, approaching 90% among those with severe fulminant hepatic failure (FHF)[1]. The most effective treatment method for patients with ALF is liver transplantation, as it has increased the 5-year survival rate by 75%[2]. Although some patients might recover spontaneously, many would die during waiting for a compatible donor because of aggressive deterioration of liver function or development of cerebral edema, intracranial hypertension, and even irreversible brain damage. Thus, a liver support system must be developed to maintain a viable status of these patients prior to the transplantation.
During the past decades, several artificial devices for removing toxins from patients’ blood through filtration and adsorption have improved clinical status in some cases. However, a meta-analysis of six randomized controlled trials (RCTs) concluded that artificial liver support systems might not reduce the mortality in ALF[3]. Moreover, the newly developed bioartificial liver (BAL) support systems that incorporate a hepatoma cell line or primary hepatocytes into a bioreactor when processing blood or plasma proved meaningful for prolonging the survival time of ALF animals in preclinical trials. Several types have been applied for the treatment of patients with ALF in phase I studies or controlled clinical trials, and improved neurological status and liver and renal functions, thus bridging to transplantation or spontaneous recovery[4-7]. However, the survival outcome and adverse effects of such alternative methods are controversial[3,8].
In addition, the BAL has various types with different cell sources, cell mass, and culture methods, as well as architectural design such as the bioreactor, scaffold, and separation, which might be associated with the effect and safety of the BAL in treating ALF[9]. Furthermore, the BAL has been modified and renovated in preclinical experiments on large animals but has not been used in clinical trials[10-12], which caused a significant gap between clinical and preclinical studies.
One objective of our study was to investigate the characteristics of studies about BAL for ALF in both clinical trials and recent preclinical experiments on large animals. In particular, we looked at key indicators of the BAL, survival outcome, and adverse events regarding the treatment. Another objective was to evaluate the pooled effect of the BAL on mortality by conducting a meta-analysis of randomized controlled studies stratified among patients with ALF and large animals.
This study was constructed following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. The protocol has been registered in PROSPERO, an international prospective register of systematic reviews (Registration number: CRD42019133215).
We included studies about any of the BAL for ALF, including all clinical trials, case reports, and RCTs in patients with ALF and preclinical experiments in large animals (monkeys, pigs, and dogs) published in the past 10 years. The language was limited to English.
The exclusion criteria were as follows: (1) Not focusing on the outcome of BAL, or the ALF group could not be separated from the other study populations such as patients with acute-on-chronic liver failure; (2) Duplicates of previous publications; (3) Based on incomplete data; and (4) Reviews, meta-analyses, comments, guidelines, letters, editorial articles, and project or conference summaries. If more than one study by the same author using the same data was published, either the study with the largest sample size or the most recently published study was included.
By using a searching strategy and filter that combined keywords or subjects about BAL and ALF, which had been pre-tested and improved repeatedly, we searched the Cochrane Library, PubMed, and EMbase to identify eligible articles till March 6, 2019 according to the inclusion and exclusion criteria, by setting the following key elements (Patients: ALF; Intervention: BAL; Comparison: None; Outcome: including but not limited to mortality, bridging time, liver and renal function; keywords used for literature search are shown in Supplementary-material). Literature about preclinical experiments in large animals was limited to papers published in the past 10 years. The references used in the eligible articles were also reviewed to examine other potential sources.
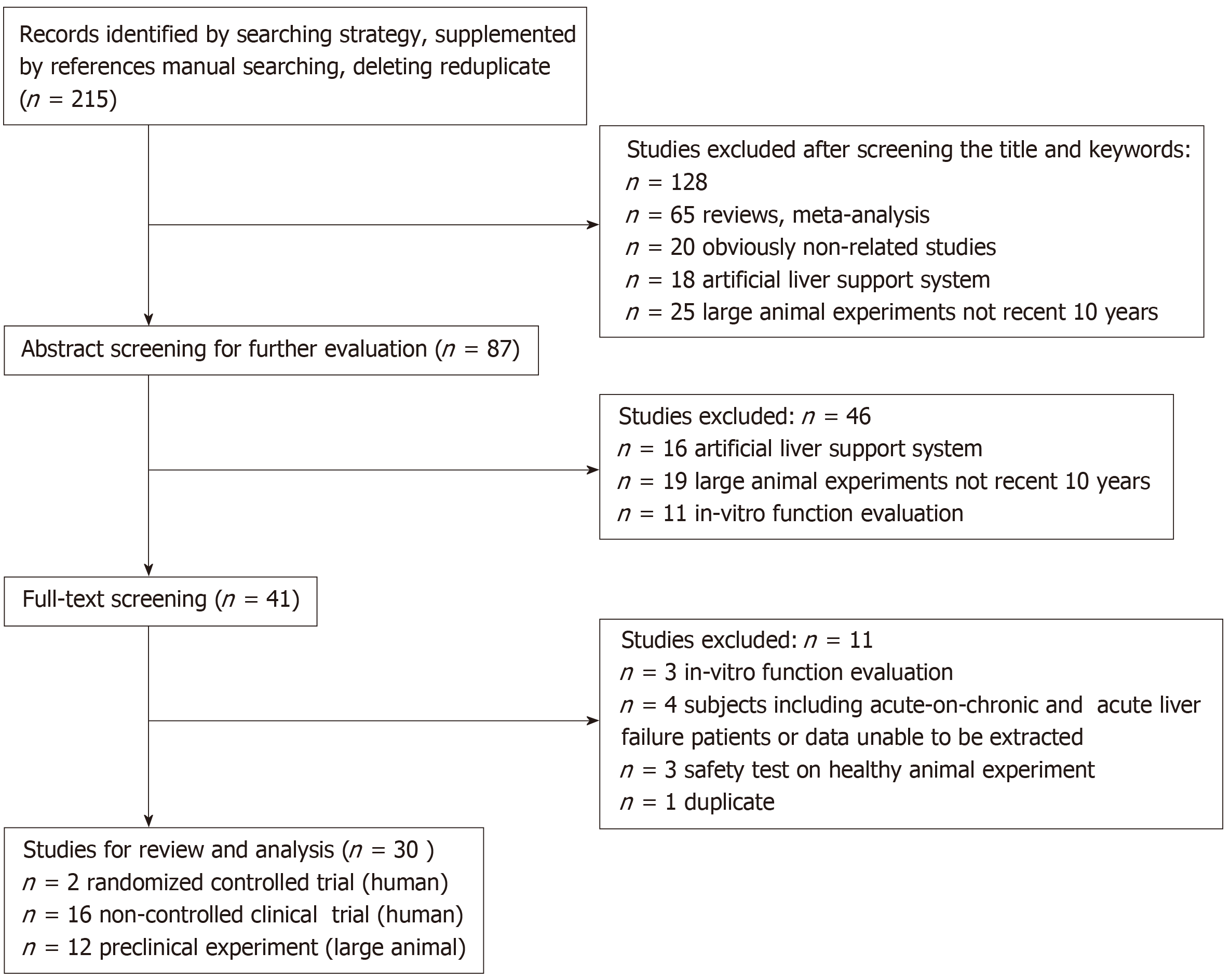
Teams of paired reviewers who were trained and knowledgeable about the study screened the literature independently. We screened the title and keywords first and excluded unqualified studies according to the predefined criteria. Then, we read the abstracts and full texts carefully to further exclude unqualified literature. The decision to exclude studies was determined by two reviewers. Inconsistent results were resolved either by discussion or decided by a third reviewer. Finally, the remaining studies were enrolled to be reviewed and analyzed. The flow chart of the study selection is shown in Figure 1.
We extracted the following information from each eligible article: (1) basic information of the included studies, including the publication year, title of the article, journal along with impact factor in 2018, country of the first author, study setting, study type (clinical trial, case report, or preclinical experiment), and data sources (full text and abstract); (2) detailed information of clinical trials in humans, including the type of BAL [e.g., HepatAssist, extracorporeal liver assist device (ELAD), academic medical center (AMC)-BAL, modular extracorporeal liver support (MELS), novel bioartificial liver support system (BLSS), radial-flow bioreactor (RFB)-BAL, and hybrid bioartificial liver (HBAL)], whether hybrid or not, cell sources (e.g., porcine hepatocytes, C3A cells, or primary human hepatocytes), cell mass, sample size, ALF subtype [fulminant hepatic failure (FHF) or primary nonfunction (PNF)], age and sex of subjects, disease etiology [e.g., PNF, viral, indeterminate, autoimmune, Acetaminophen (AO), and ischemic], BAL treatment time, outcomes (i.e., bridging time, orthotopic liver transplantation, death events, and recovery), follow-up time, stable or unstable hemodynamics, effects on liver and renal functions, hematologic and coagulative parameters, encephalopathy index, neurological status, adverse events during treatment (e.g., transitory hypotension), and porcine endogenous retrovirus (PERV) test result; (3) data of preclinical experiment on large animals, including animal species (e.g., pig, canine, and monkey), number of animals, sex (female and male), weight (kg), inducer of acute liver failure (e.g., D-galactosamine, hepatic artery ligation, surgical ligation of all blood flow to the liver, α-amanitin, and lipopolysaccharide), type of BAL (e.g., FBBAL, HBAL, HBALSS, SRBAL, hiHep-BAL, and UCLBAL), cell sources (e.g., alginate-chitosan encapsulated primary porcine hepatocytes, co-cultured pig hepatocytes, bone marrow mesenchymal stem cells, human hepatic CL-1 cells grown in microgravity culture, HiHeps, and three-dimensional HepG2-cell spheroids), cell mass, bioreactor (e.g., choanoid fluidized bed bioreactor, multi-layer flat-plate bioreactor anionic resin adsorption column, fluidized-bed bioreactor, perfusion bioreactor, spheroid reservoir, and packed-bed bioreactor), treatment time, survival time or rate at a specific time point, other effects such as ammonia level, and PERV test result.
For data extraction and scoring, paired reviewers conducted the survey independently on the basis of the literature database and recorded the necessary information. The results of the paired reviewers were cross-checked, and disagreements were resolved by discussion or decided by a third reviewer.
We conducted description analyses of the study characteristics of clinical trials and preclinical experiments on large animals by using the absolute numbers and percentages of the qualitative variables, and mean [standard deviation (SD)] or median (percentile) of the quantitative data. The bias risk of the included RCT studies was assessed according to the Cochrane assessment method for RCTs, while risk of non-RCT studies was assessed according to the Newcastle-Ottawa Quality Assessment Scale for cohort studies.
By selecting clinical RCTs and preclinical RCT experiments with survival outcome (death event) recorded at a specific time, we combined the effect of selected studies on the relative ratio (RR) scale by performing a meta-analysis with a random effect model and assigning weights according to the estimated variance. The heterogeneity of the included studies was also tested, with Q-test significance (P < 0.05) or I2 > 50% indicating that heterogeneity existed between studies. The overall RR and 95% confidence interval (CI) were calculated. We also conducted sensitivity analyses to examine the impact of using alternative effect measures (odds ratio vs relative ratio), pooling methods [Peto vs Mantel-Hanszel (M-H)], and statistical models (fixed- vs random-effects). Begg’s funnel plot was also used to evaluate the publication bias.
All the statistical analyses were performed with SPSS 23.0 (IBM Corp. Armonk, NY, United States) and Review Manager 5.3 (RevMan, the Cochrane Collaboration, Oxford, England).
We identified 215 studies preliminarily, and then excluded 67 unrelated or ineligible articles, 34 articles about artificial liver support system, 14 articles about in vitro function evaluation, 1 duplicate article, 4 articles with unavailable data, and 65 reviews. We finally included 30 articles in the analyses, of which 18 were human clinical trials[6,7,13-28] (only 2 were RCT studies[6,7]) and 12 were preclinical experiments in large animals[5,10-12,29-36].
Among the 30 articles, 94.4% (17/18) were clinical trials published before 2005, with only 1 phase I clinical trial published in 2018 (only abstract available). Studies about preclinical experiments in large animals in the recent 10 years accounted for 40% of the articles (12/30). The median (P25-P75) impact factor (IF) of the journals was 4.04 (2.60-9.20), with 8 articles having an IF < 3 and 5 having an IF > 10. Approximately two-thirds of the studies were conducted by authors in the United States (11/30) and China (8/30). One RCT study was done in England and published in Hepatology in 1996[7] while the other one was performed in 11 United States and 9 European sites by United States researchers and published in Annals of Surgery in 2004[6]. More than one-third (12/30) of the studies were about hybrid support systems (e.g., HBAL, MELS, HBALSS, and HepatAssist), distributed in 9 clinical trial studies and 3 preclinical experimental studies. The available data that we analyzed were mostly from full texts (29/30), and 1 abstract of clinical trial was also included considering the limited number of eligible studies in humans and the availability of valuable information in the abstract (as shown in Supplemental Table 1).
| ID | Publication year | Type of trial | BAL system | Subject | |||||||
| Type | Hybrid | Cell | Mass | Sample No. | Subtype | Age | Sex | Disease etiology | |||
| 1 | 1994 | Non-controlled trial | HepatAssist | Yes | Porcine hepatocytes | 6 × 109 | 7 | FHF 6 PNF 1 | 10-58 | 4M:3F | PNF 1 ; Virus 1; AO 2; Indeterminate 3 |
| 2 | 1997 | Non-controlled trial | HepatAssist | Yes | Porcine hepatocytes | 5 × 109 | 21 | FHF 18 | 36.1 ± 3.4 | 7M:11F | Virus 4; Indeterminate 8; AO 4; Ischemic 2 |
| PNF 3 | 48.3 ± 11.2 | 1M:2F | Virus 1; Indeterminate 1; Autoimmune 1 | ||||||||
| 3 | 2010 | Non-controlled trial | HepatAssist | Yes | Porcine hepatocytes | 5 × 109 | 18 | FHF 15 | 10-56 | 10M;5F | Indeterminate 7; Virus 3; AO 4; Ischemic 1 |
| PNF 3 | 26-58 | 1M:2F | Indeterminate 1; Virus 1; Autoimmune 1; | ||||||||
| 4 | 2002 | Non-controlled trial | HepatAssist | Yes | Porcine hepatocytes | 5 × 109 | 10 | FHF 10 | 31 | 4M:6F | Indeterminate 6; Virus 2; Wilson 1; Pyrazinamide 1 |
| 5 | 1994 | Non-controlled trial | ELAD | No | C3A cells | 200 g | 11 | FHF 10 PNF 1 | 38.5 ± 18.1 | 5M:6F | Idiopathic 1; Virus 3; AO 2; INH 1; FIAU 3; Anhepatic 1 |
| 6 | 2002 | Non-controlled trial | ELAD | No | C3A cells | 300-400g | 5 | FHF 5 | 22.2 ± 9.4 | 2M:3F | asparaginase toxicity 1; Indeterminate 3; Autoimmune 1 |
| 7 | 2002 | Phase I trial | AMC-BAL | No | Porcine hepatocytes | 11.9 × 109 | 7 | FHF 7 | 34.3 ± 15.2 | 2M:5F | Virus 4; AFLP 1; Indeterminate 2 |
| 8 | 2003 | Case report | AMC-BAL | No | Porcine hepatocytes | 10 × 109 | 1 | FHF | 35 | F | Virus 1 |
| 9 | 1996 | Non-controlled trial | NR | No | Porcine hepatocytes | 5 × 109 | 12 | FHF 11 PNF 1 | 37.2 ± 15.5 | 6M:6F | Indeterminate 4; AO 2; Virus 3; Ischemic Failure 1; Autoimmune 1; PNF 1 |
| 10 | 2003 | Phase I trial | MELS | Yes | Porcine hepatocytes; non-parenchymal cells | (1.8-4.4) × 1010 | 8 | FHF 8 | 34.3 | 1M:7F | Rug-related 2; Virus 3; Indeterminate 3 |
| 11 | 2003 | Case report | MELS | Yes | Human hepatocytes | 470 g | 1 | PNF | 26 | F | Intoxication with amanita phalloides |
| 12 | 2002 | Case report | BLSS | No | Porcine hepatocytes | 70-100 g | 1 | FHF | 41 | F | Indeterminate 1 |
| 13 | 2002 | Non-controlled trial | RFB-BAL | No | Porcine hepatocytes | 200-230 g | 7 | FHF 4 PNF 3 | 37.4 ± 18.4 | 5M:2F | Virus 3; PNF 3; Liver trauma 1 |
| 14 | 2003 | Non-controlled trial | HBAL | Yes | Porcine hepatocytes | 1.0 × 109 | 12 | FHF 12 | 41.8 ± 13.0 | 9M:3F | Virus 12 |
| 15 | 1999 | Non-controlled trial | HepatAssis | Yes | Porcine hepatocytes | 5 × 109 | 8 | FHF 8 | 33.4 ± 11.0 | 1M:7F | AO 8 |
| 161 | 2018 | Phase 1/2a Trial | LifeLiver BAL | NR | Porcine hepatocytes spheroids | NR | 8 | FHF 8 | NR | NR | NR |
| 17 | 1996 | Pilot-Controlled Trial | ELAD | No | C3A cells | 400 g | Group-1 17 | FHF 17 | 30 | 6M:3F vs 3M:5F2 | AO 5, Virus 3, ATB 5 vs AO 6, Virus 12; ATB 1 |
| Group-2 7 | FHF 7 | 0M:3F vs 3M:1F2 | AO 3 vs AO 3, Virus 12 | ||||||||
| 18 | 2004 | RCT | HepatAssist | Yes | Porcine hepatocytes | 7 × 109 | 171 | FHF 147 PNF 24 | 37 | 26M:60F vs 25M:60F2 | Known causes 83, Indeterminate 64 |
From the 18 clinical trials, 332 patients with ALF were included, with 295 cases of FHF subtypes and 37 cases of PNF subtypes. The mean age was 35.4 years, and females accounted for 69.4% of the patients according to the reported data. Most of the disease etiologies were indeterminate (103 cases), viruses (45 cases), and AO (33 cases). The types of BAL included HepatAssist (6 cases), ELAD (3 cases), AMC-BAL (2 cases), MELS (2 cases), BLSS (1 case), RFB-BAL (1 case), HBAL (1 case), Lifeliver (1 case), and BAL (1 case), and in one case the BAL type was unclear; of these 9 were hybrids and 8 were non-hybrids. Most of the cells were sourced from porcine hepatocytes (14/18), followed by C3A cells (3/18) and primary human hepatocytes (1/18). The mean cell mass was approximately 9 × 109 (Table 1).
The mean treatment time was around 25 h. Among the 317 reported patients, 201 received orthotopic liver transplant (OLT) and 57 recovered without OLT. The mean survival rate in the OLT group was 93%, with the follow-up period ranging from 7 days to 62 months. Hemodynamics were stable in all patients. These results show that BAL improved liver and renal functions in all the patients, except 3 with PNF[6,14,15], in terms of the different biochemical parameters, with decreased ammonia, bilirubin, and transaminase levels in 12, 12, and 10 studies, respectively. Other meaningful indicators were reduced, including lactate ALB, BUN, and creatinine levels. By examining prothrombin time (PT), international normalized ratio, or other parameters, 9 studies found improvements in hematological and coagulative status. Ten of 12 reported studies showed an improvement in encephalopathy index. Thirteen of 16 reported studies showed improvements in neurological indicators, showing decreased intracranial pressure (ICP) and increased cerebral perfusion pressure, Glasgow coma score, and comprehensive level of consciousness score. In 3 studies on ALF subtypes, the encephalopathy index and neurological indicators showed improvements in patients with FHF but not in patients with PNF[6,14,15]. However, one case report of a patient with PNF showed improvement in the neurological status with a change in coma stage from IV to I after BAL treatment[22] (Table 2).
| ID | Treating time | Bridgingtime | OLT /total | SR of OLT | SR of no OLT | Recovery without OLT | Follow-up time | Hemodynamics | Liver and renal function | Hematologic and coagulation | Encephalopathy index | Neurologic | Adverse events | PERV test | |
| 1 | 1.6 × 6 h | 24 h | 7/7 | 100% | NA | NA | NR | Stable | Decreased: ammonia; transaminases | Decreased: Fibrinogen | Improved | Decreased: ICP Increased: CPP | None | NR | |
| 2 | 2.1 × 6 h | 45.3 h | 16/18 | 100% | 50% | 1 | NR | Stable | Decreased: ammonia; ALB; transaminases; bilirubin; BUN; creatinine Increased: glucose | Not improved | Improved | Decreased: ICP; Increased: CPP; GCS; CLOCS | Transient hypotension | NR | |
| 1.6 × 6 h | 83.0 h | 3/3 | 100% | NA | NA | NR | Stable | Decreased: transaminases; bilirubin; ALB Increased: glucose | Not improved | Not improved | Not improved | ||||
| 3 | 1.8 × 6 h | 38.6 h | 14/15 | 100% | 100% | 1 | 2-62 m | Stable | Decreased: ammonia; bilirubin; transaminases; ALB; BUN; creatinine; Increased: Glucose | Not improved | Improved | Decreased: ICP; Increased: CLOCS | None | Negative (5 y) | |
| 1.7 × 6 h | 21 h-8 d | 3/3 | 100% | NA | NA | 2-62 m | Stable | Not improved | Not improved | Improved | Not improved | ||||
| 4 | 1.9 × 6 h | 46 h | 10/10 | 80% | NA | NA | 24.5m | Stable | Decreased: bilirubin; transaminases | Decreased: Platelets; Fibrinogen | Improved | Increased: GCS; CLOCS | Bleeding; transitory hypotension | Negative | |
| 5 | 52.6 h | 40.8 h | 4/11 | 100% | 14% | 1 | 10 d | Stable | Improved | NR | Improved | Improved | Transitory hypotension | NA | |
| 6 | 50.8 h | 50.8 h | 5/5 | 80% | NA | NA | 30 d | Stable | Improved | NR | Not improved | Not improved | None | NA | |
| 7 | 18.9 h | NR | 6/7 | 100% | 100% | 1 | 18 m | Stable | Decreased: bilirubin; ammonia | NR | NR | Improved | Transitory hypotension | Negative -18 m | |
| 8 | 35 h | 35 h | 1/1 | 100% | NA | NA | 15 m | Stable | Decreased: bilirubin; ammonia; lactate | Not improved | NR | Improved | NR | Negative 12 m | |
| 9 | 1.7 × 6 h | 39.3 h | 12/12 | 100% | NR | NR | NR | Stable | Decreased: bilirubin; ammonia; transaminases; Increased: glucose | Not improved | Improved | Decreased: ICP; Increased: CPP; CLOCS | None | NR | |
| 10 | 27.3 h | NR | 8/8 | 100% | NR | NR | 3y | Stable | Decreased: bilirubin; ALB | Decreased: platelets | Improved | NA | Decreased body temperature | Negative 3 y | |
| 11 | 79 h | 79 h | 1/1 | 100% | NR | NR | 1y | Stable | Decreased: lactate; BUN; creatinine | Improved | Improved | Improved | Decreased body temperature | NA | |
| 12 | 12 h | 7 d | NA1 | NA1 | NA1 | NA1 | 7d | Stable | Decreased: lactate; ammonia; transaminases | Improved | NR | Not improved | Hypoglycemia | NR | |
| 13 | 11.7 h | 11.7 h | 6/7 | 83% | 0% | 0 | NA | Stable | Decreased: bilirubin; transaminase; ammonia; lactate; urea | Improved | NR | Improved | None | Negative 180 d | |
| 14 | 1.1 × 6 h | NR | NA2 | NA2 | NR | NR | 1 m | Stable | Decreased: bilirubin; transaminase; ammonia | Improved | NR | Improved | None | NR | |
| 15 | 2.9 × 6 h | NR | 3/8 | 100% | 100% | 5 | NR | Stable | Decreased: ammonia; bilirubin; transaminases; BUN; creatinine | Improved | Improved | Decreased: ICP Increased: CLOCS | None | NR | |
| 16 | NR | NR | 2/6 | 100% | 75% | 3 | NR | NR | Decreased: ammonia | Not improved | NR | Improved | Coagulopathy pneumonia, sepsis, and diseased progression | Negative | |
| 17 | 72 (3-168) h | NR | 2/9 vs 2/83 | 50% vs 50%3 | 6/7 vs 5/63 | 6 vs 53 | NR | Stable | Decreased ammonia | Improved | NR | NR | Tachypnoeic; tachycardic; pyrexial; disseminated intravascular coagulation | NA | |
| 1/3 vs 1/43 | 100% vs 100%3 | 0% vs 0%3 | 0 vs 03 | ||||||||||||
| 18 | 17.4 h | 5 d vs 3 d | 45/85 vs 49/863 | 89% vs 80%3 | 50% vs 38%3 | 20 vs 143 | 30 d | Stable | Decreased bilirubin | Not improved | NR | Not improved | None | Negative 12 m | |
During BAL treatment, 9 studies reported adverse events such as transient hypotension, decreased body temperature, tachycardia, pyrexia, and hypoglycemia, which had no clinical significance and resolved in all. PERV test results in 8 reported studies were all negative (Table 2).
Of 12 studies that performed preclinical experiments with pigs (8 studies, 160 animals), monkeys (2 studies, 45 animals), and canines (2 studies, 40 animals), the proportions of male and female animals were 45.3% (111/245) and 30.2% (74/245), respectively. Inducers included D-galactosamine in 7 studies (145 animals)[5,12,29-33], surgical operation in 3 studies (53 animals)[11,34,35], 85% hepatectomy in 1 study (18 animals)[36], and α-amanitin and lipopolysaccharide in 1 study (30 animals)[10] (Table 3).
| ID | Year | Animal | BAL system | Outcomes | Test for PERV | |||||||||
| Species | No | Sex | Weight(kg) | Inducer | Type | Cell | Mass | Bioreactor | Treatment time | Survival | Other | |||
| 19 | 2011 | Pig | 30 | M | 10-15 | D-galactosamine | FBBAL | Alginate-chitosan encapsulated primary porcine hepatocytes | 5 × 109 | Choanoid fluidized bed bioreactor | 6 h | Survival time: BAL 72.9 ± 4.72 h Sham BAL 54.6 ± 4.09 h Control 54.8 ± 3.98 h | Decreased: lactate; glucose | NR |
| 20 | 2012 | Canine | 32 | NR | 10-15 | D-galactosamine | HBAL | Co-cultured porcine hepatocytes and bone marrow mesenchymal stem cells | 1x 1010 | Multi-layer flat-plate bioreactor + anionic resin adsorption column | 3 h | 7 day survival: HBAL 7/8 BAL 5/8 NBAL 4/8 Control 3/8 | Decreased: transaminases; LDH; ammonia; bilirubin; PT Increased: ALB | Negative (7 d) |
| 21 | 2013 | Pig | 13 | F | 25-30 | Hepaticartery ligation | NA | Alginate-encapsulated HepG2cell-spheroids | 5 × 1010 | Fluidised-bed bioreactor | 7 h | Survival time: BAL 10.5 ± 20.7 h SBAL 8.6 ± 21.4 h | Decreased: ICP; ammonia; Increased: bilirubin; acidosis | NR |
| 22 | 2014 | Cynomolgus monkey | 15 | M | 6.5-7.0 | D-galactosamine | HBALSS | Human hepatic CL-1 cells grown in microgravity culture | 4 × 109 | Perfusion bioreactor | 6 h | Survival time: 128 h BAL 5/10 Control 0/5 | Decreased: bilirubin; TBA; BUN; Cr; ammonia; Fischer indices Increased: ALB | NR |
| 23 | 2015 | Canine | 8 | NR | 10-13 | D-galactosamine | HBAL | Co-cultured porcine hepatocytes and bone marrow mesenchymal cells | 1 × 1010 | Multi-layer flat-plate bioreactor + anionic resin adsorption column | 6 h | 1 year survival: 7/8 | Decreased: transaminases; PT; bilirubin; LDH; ammonia; Increased: ALB | Negative (1 year) |
| 24 | 2015 | Pig | 18 | F | 45 | D-galactosamine | SRBAL | Porcine hepatocytes spheroids | 200 g | Spheroid reservoir | 24 h | Survival 90 h (%) ST 0/6, 0% ST + Non-cell 1/6,17% ST + SRBAL 5/6, 83% | Decreased: ammonia; ICP; brain water content | NR |
| 25 | 2016 | Pig | 21 | M | 10-15 | D-galactosamine | FBBAL | Alginate-chitosan encapsulated porcine hepatocytes | 5 × 109 | Choanoid fluidized bed bioreactor | 6h | Survival time: FBBAL 70.4 ± 11.5 h Sham FBBAL 51.6 ± 7.9 h Control 49.3 ± 6.6 h | Decreased: PCs; LPCs; FAs; SM; Increased: CBAs | NR |
| 26 | 2016 | Pig | 20 | NR | 15-25 | D-galactosamine | HiHep-BAL | HiHeps | 3 × 109 | Multi-layer radial-flow bioreactor | 3h | Survival 7 d: hiHep-BAL 7/8 Empty-BAL 1/6 No-BAL 0/6 | Decreased: transaminase ammonia; bilirubin; PT | NR |
| 27 | 2017 | Pig | 25 | F | 30-35 | Surgical ligation of all blood flow to the liver | UCLBAL | 3-dimensional (3D) HepG2-cell spheroids | 7.3 × 1010 | Fuidised bed bioreactor | 6h | Survival time: Control-BAL 7.04 ± 1.9h, n = 15 Cell-BAL 8.21 ± 2.3 h, n = 13 | Decreased: PT; INR; ICP; ammonia | NR |
| 28 | 2017 | Pig | 15 | M | 45-55 | Complete hepatic inflow devascularization | NA | Ca-alginate-immobilized hepatocyte spheroids | 2 × 1010 | Packed-bed bioreactor | 12h | Median survival time: Control 21 h n = 5 BAL 28.5 h n = 5 Cell-free BAL 21 h n = 5 | Decreased: ammonia; creatinine ICP; BP Increased: urine | NR |
| 29 | 2018 | Rhesus monkey | 30 | M | 10-20 | α-amanitin and lipopolysaccharide | SRBAL | Pig hepatocyte-HUVEC organoids | 2.6 × 1010 | Spheroid reservoir | 6 h | Median survival time: SRBAL 12 h 336 h n = 6 SRBAL 24 h 248 h n = 6 SRBAL 36 h 132 h n = 6 Sham no-cell SRBAL 12 h 90 h n = 6 Control 60.5 h n = 6 | Decreased: ammonia; bilirubin Increased: albumin | Negative (6 h) |
| 30 | 2019 | Pig | 18 | F | 20-30 | 85% hepatectomy | SRBAL | Porcine hepatocyte spheroids | 200g | Spheroid reservoir | 24 h | Survival rate at 90 h: SMT 0/6 SMT plus no-cell SRBAL 0/6 SMT plus SRBAL (200 g) 5/6 | Decreased: ammonia; ICP; INR Increased: volume regeneration | NR |
The types of BAL included SRBAL (n = 3), HBAL (n = 2), FBBAL (n = 1), UCLBAL (n = 1), hiHep-BAL (n = 1), HBALSS (n = 1), FBBAL (n = 1), and BAL (n = 1), using cells from porcine hepatocyte (9/12), human hepatic CL-1 cells or HepG2 cells (2/12), and HiHeps (1/12), which were all cultured using modified three-dimensional methods such as spheroids, organoid, alginate-chitosan encapsulated, and even microgravity culture, with corresponding bioreactors such as a spheroid reservoir. The mean cell mass was 2 × 1010, and the treatment time ranged from 3 to 24 h (mean: 9 h; Table 3).
Compared with the control group, the survival outcomes (median survival time or survival rate) were better in the BAL group, and the biochemical metabolic function showed improvement, especially decreased ammonia levels (10/12), bilirubin levels (6/12), ICP (5/12), and PT (4/12). All the PERV test results were negative in reported studies (Table 3).
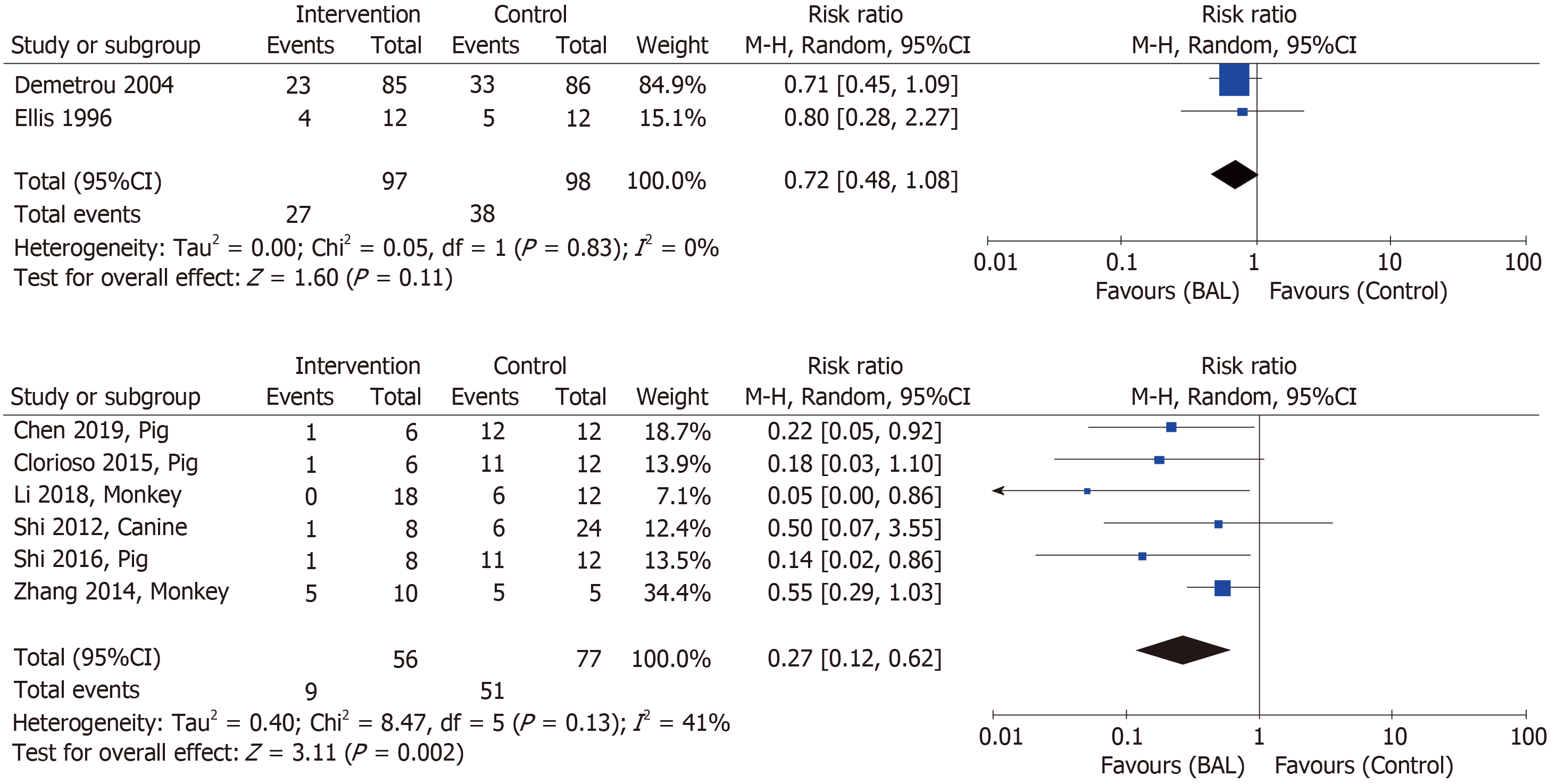
As shown in Figure 2, the overall effect of the BAL on mortality in the patients with ALF[6,7] was insignificant [BAL 97 vs control 98: RR (95%CI), 0.72 (0.48-1.08)]. However, the meta-analysis of the preclinical experiments of large animals[10,12,29,30,32,36] indicated a significant effect of the BAL [BAL 56 vs control 77: RR (95%CI), 0.27 (0.12-0.62)]. The test of heterogeneity showed no significant difference between included studies, with I2 being 0% and 41% for human clinical studies and pre-clinical experimental studies, respectively.
Sensitivity analyses of pooled results using an alternative effect measure (M-H OR = 0.61, 95%CI: 0.33-1.11 for humans; OR = 0.05, 0.01-0.17 for animals), pooling method (Peto fixed effects OR = 0.61, 95%CI: 0.34-1.11 for humans; OR = 0.07, 0.03-0.15 for animals), and statistical model (fixed effects M-H RR = 0.72, 95%CI: 0.48-1.08 for humans; RR = 0.24, 0.13-0.44 for animals) showed similar findings.
As shown in Supplemental Figure, we observed publication bias and found none in the clinical trials, and little in the preclinical experiment studies. However, it remains unclear because of the small number of included studies.
By bias assessment, all the included RCT studies were regarded as low or middle-risk overall, with 4 to 6 of 7 items listed by the Cochrane assessment standards for RCT assessed as low risk (Supplemental Table 2). For non-RCT studies, all the clinical trials among humans scored 5 or 6 (total score: 9), indicating a low or middle risk, while those of pre-clinical experiments scored a little higher, with most scoring 7 or 8 (Supplemental Table 3).
By conducting a systematic review of 18 clinical trials and 12 preclinical experiment in large animals, including a meta-analysis of selected studies, we suggest that the BAL might reduce mortality from ALF in large animals, but not in humans [BAL vs control: RR (95%CI), 0.27 (0.12-0.62) for animals and 0.72 (0.48-1.08) for humans], with no heterogeneity observed between included studies. Compared with the preclinical experiments, most of the clinical trials were conducted more than 10 years ago. Moreover, the BAL used in large animals has undergone an obvious improvement regarding the type, cell source, cell mass, and bioreactor. All the studies showed improvements in liver and renal functions, hematologic and coagulative parameters, encephalopathy index, and neurological indicators after the treatment with BAL, with neither significant adverse events nor PERV infection.
At present, whether the BAL is able to reduce mortality in the ALF population remains controversial. For example, a meta-analysis performed in 2011[8] indicated that BAL appeared to affect mortality in patients with ALF, while another three meta-analyses of clinical controlled trials conducted by Liu et al[37], Kjaergard et al[3], and Zheng et al[38] demonstrated that the use of the BAL was not associated with the improvement of survival outcome among patients with ALF, which is consistent with our study.
The quality of the BAL is the most important indicator that affects the outcome and adverse events of BAL treatment for ALF, which might support the effect difference between clinical trials and preclinical experiments. As reported in our study, cell source, culture mode, cell mass, and the bioreactor of the BAL were different between the two types of study.
There are currently four main sources of cells for the BAL and their pros and cons are as follows: (1) Human primary hepatocytes are the most suitable cells but are limited by low availability due to a shortage of donor organs[38]; (2) Immortalized human hepatoblastoma cell lines (HepG2/C3A/hepatic CL-1) are sufficiently expanded but are considered to have less metabolic functions than primary hepatocytes[39]; (3) Human-induced hepatocytes (hiHep) were reported to have a potential for metabolic detoxification[12], but it is difficult to meet the demands on a clinical scale because of the cost and complexity of hiHep; and (4) Porcine hepatocytes are the main cells used in the BAL and have similar function with human hepatocytes, are readily available, and are low-cost. Although no PERV infection has been found in 42 patients with long-term immunosuppression and 13 healthcare workers after a follow-up of 5-8 years by a new highly sensitive and specific quantitative real-time polymerase chain reaction assay[40,41], xenozoonosis and the potential risks of PERV infection after treatment remain a concern.
In addition, primary hepatocytes easily lose their function in vitro during long-term monolayer culture[42]. In the preclinical experiments, to maintain and improve the viability and metabolic functions of hepatocytes, cells were cultured in a three-dimensional environment to simulate microgravity to form spheroids[30,32,36] or organoids[10], and alginate-chitosan encapsulated spheroids[5,11,33]. Hepatocytes were also co-cultured with bone marrow mesenchymal cells and human umbilical vein endothelial cells to maintain the function of porcine hepatocytes by providing cell-to-cell interactions[10,29,31,43]. In addition, to adapt to the changes of the cells, bioreactors were modified as spheroid reservoirs and multi-layer radial-flow bioreactors to provide a suitable environment for hepatocytes to survive and maintain their cell functions.
Adequate liver cell mass is another crucial indicator for evaluating the BAL, and the innovation requires a higher number of hepatocytes and enhanced function during long-term culture[44]. It has been widely suggested that approximately 30% of the total liver volume is required for survival and that 10-40 billion liver cells without loss of function would be required for BAL treatment[45,46]. Therefore, the low functionality and availability of cells for the clinical scale mass of all the BAL might explain the insignificant effect based on the meta-analysis of the two controlled clinical trials in comparison with the preclinical experiments.
Furthermore, in the preclinical experiments, the subjects in each study were the homogeneous ALF models, but in the clinical trials, the etiologies of ALF varied and were complex, which might have led to different effects of the BAL. In comparison to the patients with PNF, the patients with FHF showed a strikingly different effect of the BAL treatment in a randomized multicenter controlled trial[6] and two non-controlled clinical trials[14,15]. Only unremarkable metabolic effects were observed in the patients with PNF, without amelioration of the neurological state and survival benefit after BAL treatment, whereas an improvement in neurological state and benefit were observed in the patients with FHF, even though one case report on PNF showed a great improvement in neurological state with a change in coma stage from IV to I after treatment with MELS[22].
Our study has several strengths. First, by using a systematic searching strategy and selection procedures, we included all the clinical trials of BAL for ALF and preclinical experiments on large animals in the recent decade, which might represent a current overview of research in this domain, making our study probably the first review to provide evidence for future research. Second, we calculated the combined effect of BAL for ALF by performing a meta-analysis of RCT studies stratified according to clinical trial and preclinical experiment, making the effects comparable between the two study types; meanwhile, the bias of all the included studies was assessed as low to middle-risk and the publication bias was subtle. Finally, we created a detailed checklist of all the potential information associated with the outcome of BAL. Two independent reviewers conducted data extraction, ensuring quality data and allowing for examination of the gap between preclinical experiments and clinical trials.
Our study has two main limitations. One is that the number of RCT clinical trials included was limited to meta-analyses even if many studies have been conducted on ALF and other liver diseases such as acute-on-chronic liver failure. Nevertheless, our data could be usable. Thus, the overall effect of BAL for ALF in humans was desirable but must be verified in the future. Another limitation is that it was not necessary or proper for us to use a meta-regression for controlling covariates, because there existed no heterogeneity between included studies and the limited number of included studies did not meet the requirement of the precondition for regression. Thus, we could not provide further evidence for future research and practice.
Based on the results of our study, we suggest the following for future clinical trials, preclinical experiments, and transformations. First, alternative cells or methods for acquiring high-quality liver cells in vitro must be identified to achieve clinical-scale goals. Second, the effects of the subgroups, patients with PNF or FHF, and patients with different etiologies should be determined and examined in clinical trials in the future. Finally, the advanced BAL, which proved to have a significant benefit on the survival outcome of the large-animal ALF model, should undergo clinical transformation as much as possible.
Acute liver failure (ALF) has a high mortality varying from 80% to 85% with rapid progress in multi-organ system failure. Bioartificial liver (BAL) support systems have a potential effect to provide temporary support to bridge patients with acute liver failure to liver transplantation or spontaneous recovery. In the past decades, several BAL support systems have been conducted in clinical trials, but remained verified. More recently, concerns have been raised on the renovation of high-quality cell sources and configuration of BAL support systems to provide more benefits to ALF models in preclinical experiments.
A systematic review and meta-analysis of the existing literature on the use of BAL among humans and large animals with ALF could help bridge the gap between preclinical experiments and clinical trials regarding the effect of BAL for treating acute liver failure.
To investigate the characteristics of studies about BAL for ALF, and to evaluate their effects on mortality.
Eligible clinical trials and preclinical experiments on large animals were identified on Cochrane Library, PubMed, and EMbase up to March 6, 2019. Two reviewers independently extracted the necessary information, including the key BAL indicators, survival and indicating outcomes, and adverse events during treatment. Descriptive analysis was used to identify the characteristics of the included studies, and a meta-analysis by including only RCT studies was performed to combine the overall effect of BAL on mortality among humans and large animal, respectively.
Of 30 selected studies, 18 were clinical trials and 12 were preclinical experiments. The meta-analysis results suggested that BAL might reduce the mortality of ALF in large animals, probably due to the recent improvement of BAL, including the type, cell source, cell mass, and bioreactor, but seemed ineffective for humans. Liver and renal functions, hematologic and coagulative parameters, encephalopathy index, and neurological indicators seemed to improve after BAL, with neither meaningful adverse events nor porcine endogenous retrovirus infection.
BAL may reduce the mortality of ALF by bridging the gap between preclinical experiments and clinical trials. Clinical trials using improved BAL must be designed scientifically and conducted in the future to provide evidence for transformation.
Our study could provide some suggestions for future clinical trials, preclinical experiments, and transformations. First, alternative cells or methods for acquiring high-quality liver cells in vitro must be identified to achieve clinical-scale goals. Second, the effects of the subgroups, patients with PNF or FHF, and patients with different etiologies should be determined and examined in clinical trials in the future. Finally, the advanced BAL, which proved to have a significant benefit on the survival outcome of the large-animal ALF model, should undergo clinical transformation as much as possible.
| 1. | Bernuau J, Rueff B, Benhamou JP. Fulminant and subfulminant liver failure: definitions and causes. Semin Liver Dis. 1986;6:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 402] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 2. | Germani G, Theocharidou E, Adam R, Karam V, Wendon J, O'Grady J, Burra P, Senzolo M, Mirza D, Castaing D, Klempnauer J, Pollard S, Paul A, Belghiti J, Tsochatzis E, Burroughs AK. Liver transplantation for acute liver failure in Europe: outcomes over 20 years from the ELTR database. J Hepatol. 2012;57:288-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 199] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 3. | Kjaergard LL, Liu J, Als-Nielsen B, Gluud C. Artificial and bioartificial support systems for acute and acute-on-chronic liver failure: a systematic review. JAMA. 2003;289:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 242] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 4. | Pless G. Bioartificial liver support systems. Methods Mol Biol. 2010;640:511-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Lv G, Zhao L, Zhang A, Du W, Chen Y, Yu C, Pan X, Zhang Y, Song T, Xu J, Chen Y, Li L. Bioartificial liver system based on choanoid fluidized bed bioreactor improve the survival time of fulminant hepatic failure pigs. Biotechnol Bioeng. 2011;108:2229-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Demetriou AA. Brown RS Jr, Busuttil RW, Fair J, McGuire BM, Rosenthal P, Am Esch JS 2nd, Lerut J, Nyberg SL, Salizzoni M, Fagan EA, de Hemptinne B, Broelsch CE, Muraca M, Salmeron JM, Rabkin JM, Metselaar HJ, Pratt D, De La Mata M, McChesney LP, Everson GT, Lavin PT, Stevens AC, Pitkin Z, Solomon BA. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg. 2004;239:660-7; discussion 667-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 411] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 7. | Ellis AJ, Hughes RD, Wendon JA, Dunne J, Langley PG, Kelly JH, Gislason GT, Sussman NL, Williams R. Pilot-controlled trial of the extracorporeal liver assist device in acute liver failure. Hepatology. 1996;24:1446-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 326] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 8. | Stutchfield BM, Simpson K, Wigmore SJ. Systematic review and meta-analysis of survival following extracorporeal liver support. Br J Surg. 2011;98:623-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | van de Kerkhove MP, Hoekstra R, Chamuleau RA, van Gulik TM. Clinical application of bioartificial liver support systems. Ann Surg. 2004;240:216-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 123] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Li Y, Wu Q, Wang Y, Weng C, He Y, Gao M, Yang G, Li L, Chen F, Shi Y, Amiot BP, Nyberg SL, Bao J, Bu H. Novel spheroid reservoir bioartificial liver improves survival of nonhuman primates in a toxin-induced model of acute liver failure. Theranostics. 2018;8:5562-5574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Selden C, Spearman CW, Kahn D, Miller M, Figaji A, Erro E, Bundy J, Massie I, Chalmers SA, Arendse H, Gautier A, Sharratt P, Fuller B, Hodgson H. Evaluation of encapsulated liver cell spheroids in a fluidised-bed bioartificial liver for treatment of ischaemic acute liver failure in pigs in a translational setting. PLoS One. 2013;8:e82312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Shi XL, Gao Y, Yan Y, Ma H, Sun L, Huang P, Ni X, Zhang L, Zhao X, Ren H, Hu D, Zhou Y, Tian F, Ji Y, Cheng X, Pan G, Ding YT, Hui L. Improved survival of porcine acute liver failure by a bioartificial liver device implanted with induced human functional hepatocytes. Cell Res. 2016;26:206-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 13. | Rozga J, Podesta L, LePage E, Morsiani E, Moscioni AD, Hoffman A, Sher L, Villamil F, Woolf G, McGrath M. A bioartificial liver to treat severe acute liver failure. Ann Surg. 1994;219:538-544; discussion 544-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 156] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Watanabe FD, Mullon CJ, Hewitt WR, Arkadopoulos N, Kahaku E, Eguchi S, Khalili T, Arnaout W, Shackleton CR, Rozga J, Solomon B, Demetriou AA. Clinical experience with a bioartificial liver in the treatment of severe liver failure. A phase I clinical trial. Ann Surg. 1997;225:484-91; discussion 491-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 320] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 15. | Chen SC, Mullon C, Kahaku E, Watanabe F, Hewitt W, Eguchi S, Middleton Y, Arkadopoulos N, Rozga J, Solomon B, Demetriou AA. Treatment of severe liver failure with a bioartificial liver. Ann N Y Acad Sci. 1997;831:350-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 49] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Samuel D, Ichai P, Feray C, Saliba F, Azoulay D, Arulnaden JL, Debat P, Gigou M, Adam R, Bismuth A, Castaing D, Bismuth H. Neurological improvement during bioartificial liver sessions in patients with acute liver failure awaiting transplantation. Transplantation. 2002;73:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Sussman NL, Gislason GT, Conlin CA, Kelly JH. The Hepatix extracorporeal liver assist device: initial clinical experience. Artif Organs. 1994;18:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 193] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Millis JM, Cronin DC, Johnson R, Conjeevaram H, Conlin C, Trevino S, Maguire P. Initial experience with the modified extracorporeal liver-assist device for patients with fulminant hepatic failure: system modifications and clinical impact. Transplantation. 2002;74:1735-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 19. | van de Kerkhove MP, Di Florio E, Scuderi V, Mancini A, Belli A, Bracco A, Dauri M, Tisone G, Di Nicuolo G, Amoroso P, Spadari A, Lombardi G, Hoekstra R, Calise F, Chamuleau RA. Phase I clinical trial with the AMC-bioartificial liver. Int J Artif Organs. 2002;25:950-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Van De Kerkhove MP, Di Florio E, Scuderi V, Mancini A, Belli A, Bracco A, Scala D, Scala S, Zeuli L, Di Nicuolo G, Amoroso P, Calise F, Chamuleau RAFM. Bridging a Patient with Acute Liver Failure to Liver Transplantation by the AMC-Bioartificial Liver. Cell Transplant. 2003;12:563-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Chen SC, Hewitt WR, Watanabe FD, Eguchi S, Kahaku E, Middleton Y, Rozga J, Demetriou AA. Clinical experience with a porcine hepatocyte-based liver support system. Int J Artif Organs. 1996;19:664-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Sauer IM, Zeilinger K, Pless G, Kardassis D, Theruvath T, Pascher A, Goetz M, Neuhaus P, Gerlach JC. Extracorporeal liver support based on primary human liver cells and albumin dialysis--treatment of a patient with primary graft non-function. J Hepatol. 2003;39:649-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Mazariegos GV. Patzer JF 2nd, Lopez RC, Giraldo M, Devera ME, Grogan TA, Zhu Y, Fulmer ML, Amiot BP, Kramer DJ. First clinical use of a novel bioartificial liver support system (BLSS). Am J Transplant. 2002;2:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Morsiani E, Pazzi P, Puviani AC, Brogli M, Valieri L, Gorini P, Scoletta P, Marangoni E, Ragazzi R, Azzena G, Frazzoli E, Di Luca D, Cassai E, Lombardi G, Cavallari A, Faenza S, Pasetto A, Girardis M, Jovine E, Pinna AD. Early experiences with a porcine hepatocyte-based bioartificial liver in acute hepatic failure patients. Int J Artif Organs. 2002;25:192-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Ding YT, Qiu YD, Chen Z, Xu QX, Zhang HY, Tang Q, Yu DC. The development of a new bioartificial liver and its application in 12 acute liver failure patients. World J Gastroenterol. 2003;9:829-832. [PubMed] [DOI] [Full Text] |
| 26. | Detry O, Arkadopoulos N, Ting P, Kahaku E, Watanabe FD, Rozga J, Demetriou AA. Clinical use of a bioartificial liver in the treatment of acetaminophen-induced fulminant hepatic failure. Am Surg. 1999;65:934-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 27. | Lee S, Lee J-H, Lee D-H, Park H-J, Kim Y-A, Park MN, Noh J-K, Jung JG, Lee JE, Yang MS. Phase 1/2a Trial of a Bioartificial Liver Support System (LifeLiver) for Acute Liver Failure Patients. Transplantation. 2018;102:S123. |
| 28. | Sauer IM, Kardassis D, Zeillinger K, Pascher A, Gruenwald A, Pless G, Irgang M, Kraemer M, Puhl G, Frank J, Müller AR, Steinmüller T, Denner J, Neuhaus P, Gerlach JC. Clinical extracorporeal hybrid liver support--phase I study with primary porcine liver cells. Xenotransplantation. 2003;10:460-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 127] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Shi XL, Zhang Y, Chu XH, Han B, Gu JY, Xiao JQ, Tan JJ, Gu ZZ, Ren HZ, Yuan XW, Ding YT. Evaluation of a novel hybrid bioartificial liver based on a multi-layer flat-plate bioreactor. World J Gastroenterol. 2012;18:3752-3760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 30. | Zhang Z, Zhao YC, Cheng Y, Jian GD, Pan MX, Gao Y. Hybrid bioartificial liver support in cynomolgus monkeys with D-galactosamine-induced acute liver failure. World J Gastroenterol. 2014;20:17399-17406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 31. | Han B, Shi XL, Zhang Y, Gu ZZ, Yuan XW, Ren HZ, Qiu Y, Ding YT. No transmission of porcine endogenous retrovirus in an acute liver failure model treated by a novel hybrid bioartificial liver containing porcine hepatocytes. Hepatobiliary Pancreat Dis Int. 2015;14:492-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Glorioso JM, Mao SA, Rodysill B, Mounajjed T, Kremers WK, Elgilani F, Hickey RD, Haugaa H, Rose CF, Amiot B, Nyberg SL. Pivotal preclinical trial of the spheroid reservoir bioartificial liver. J Hepatol. 2015;63:388-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Zhou P, Shao L, Zhao L, Lv G, Pan X, Zhang A, Li J, Zhou N, Chen D, Li L. Efficacy of Fluidized Bed Bioartificial Liver in Treating Fulminant Hepatic Failure in Pigs: A Metabolomics Study. Sci Rep. 2016;6:26070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Selden C, Bundy J, Erro E, Puschmann E, Miller M, Kahn D, Hodgson H, Fuller B, Gonzalez-Molina J, Le Lay A, Gibbons S, Chalmers S, Modi S, Thomas A, Kilbride P, Isaacs A, Ginsburg R, Ilsley H, Thomson D, Chinnery G, Mankahla N, Loo L, Spearman CW. A clinical-scale BioArtificial Liver, developed for GMP, improved clinical parameters of liver function in porcine liver failure. Sci Rep. 2017;7:14518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 35. | Lee JH, Lee DH, Lee S, Kwon CHD, Ryu JN, Noh JK, Jang IK, Park HJ, Yoon HH, Park JK, Kim YJ, Kim SK, Lee SK. Functional Evaluation of a Bioartificial Liver Support System Using Immobilized Hepatocyte Spheroids in a Porcine Model of Acute Liver Failure. Sci Rep. 2017;7:3804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Chen HS, Joo DJ, Shaheen M, Li Y, Wang Y, Yang J, Nicolas CT, Predmore K, Amiot B, Michalak G, Mounajjed T, Fidler J, Kremers WK, Nyberg SL. Randomized Trial of Spheroid Reservoir Bioartificial Liver in Porcine Model of Posthepatectomy Liver Failure. Hepatology. 2019;69:329-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 37. | Liu JP, Gluud LL, Als-Nielsen B, Gluud C. Artificial and bioartificial support systems for liver failure. Cochrane Database Syst Rev. 2004;CD003628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Zheng Z, Li X, Li Z, Ma X. Artificial and bioartificial liver support systems for acute and acute-on-chronic hepatic failure: A meta-analysis and meta-regression. Exp Ther Med. 2013;6:929-936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Nyberg SL, Remmel RP, Mann HJ, Peshwa MV, Hu WS, Cerra FB. Primary hepatocytes outperform Hep G2 cells as the source of biotransformation functions in a bioartificial liver. Ann Surg. 1994;220:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Pitkin Z, Mullon C. Evidence of absence of porcine endogenous retrovirus (PERV) infection in patients treated with a bioartificial liver support system. Artif Organs. 1999;23:829-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 105] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | Di Nicuolo G, D'Alessandro A, Andria B, Scuderi V, Scognamiglio M, Tammaro A, Mancini A, Cozzolino S, Di Florio E, Bracco A, Calise F, Chamuleau RA. Long-term absence of porcine endogenous retrovirus infection in chronically immunosuppressed patients after treatment with the porcine cell-based Academic Medical Center bioartificial liver. Xenotransplantation. 2010;17:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Nicolas CT, Hickey RD, Chen HS, Mao SA, Lopera Higuita M, Wang Y, Nyberg SL. Concise Review: Liver Regenerative Medicine: From Hepatocyte Transplantation to Bioartificial Livers and Bioengineered Grafts. Stem Cells. 2017;35:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 43. | Brophy CM, Luebke-Wheeler JL, Amiot BP, Khan H, Remmel RP, Rinaldo P, Nyberg SL. Rat hepatocyte spheroids formed by rocked technique maintain differentiated hepatocyte gene expression and function. Hepatology. 2009;49:578-586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 44. | Allen JW, Hassanein T, Bhatia SN. Advances in bioartificial liver devices. Hepatology. 2001;34:447-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 218] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 45. | Bianconi E, Piovesan A, Facchin F, Beraudi A, Casadei R, Frabetti F, Vitale L, Pelleri MC, Tassani S, Piva F, Perez-Amodio S, Strippoli P, Canaider S. An estimation of the number of cells in the human body. Ann Hum Biol. 2013;40:463-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 582] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 46. | DeMatteo RP, Fong Y, Blumgart LH. Surgical treatment of malignant liver tumours. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:557-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kayaalp C, Milovanovic T, Nacif LS, Williams R S-Editor: Ma RY L-Editor: Wang TQ E-Editor: Wu YXJ