Published online May 28, 2019. doi: 10.3748/wjg.v25.i20.2503
Peer-review started: October 10, 2018
First decision: November 8, 2018
Revised: November 18, 2018
Accepted: December 27, 2018
Article in press: December 28, 2018
Published online: May 28, 2019
Processing time: 239 Days and 20.6 Hours
Shear wave speed has been widely applied to quantify a degree of liver fibrosis. However, there is no standardized procedure, which makes it difficult to utilize the speed universally.
To provide procedural standardization of shear wave speed measurement.
Point shear wave elastography (pSWE) was measured in 781 patients, and two-dimensional shear wave elastography (2dSWE) was measured on the same day in 18 cases. Regions-of-interest were placed at 12 sites, and the median and robust coefficient-of-variation (CVR) were calculated. A residual sum-of-square (Σdi2) was computed for bootstrap values of 1000 iterations in 18 cases with each assumption of 1 to 12 measurements. The proportion of the Σdi2 (%Σdi2) was calculated as the ratio of Σdi2 to pSWE after converting it based on the correlation between pSWE and 2dSWE.
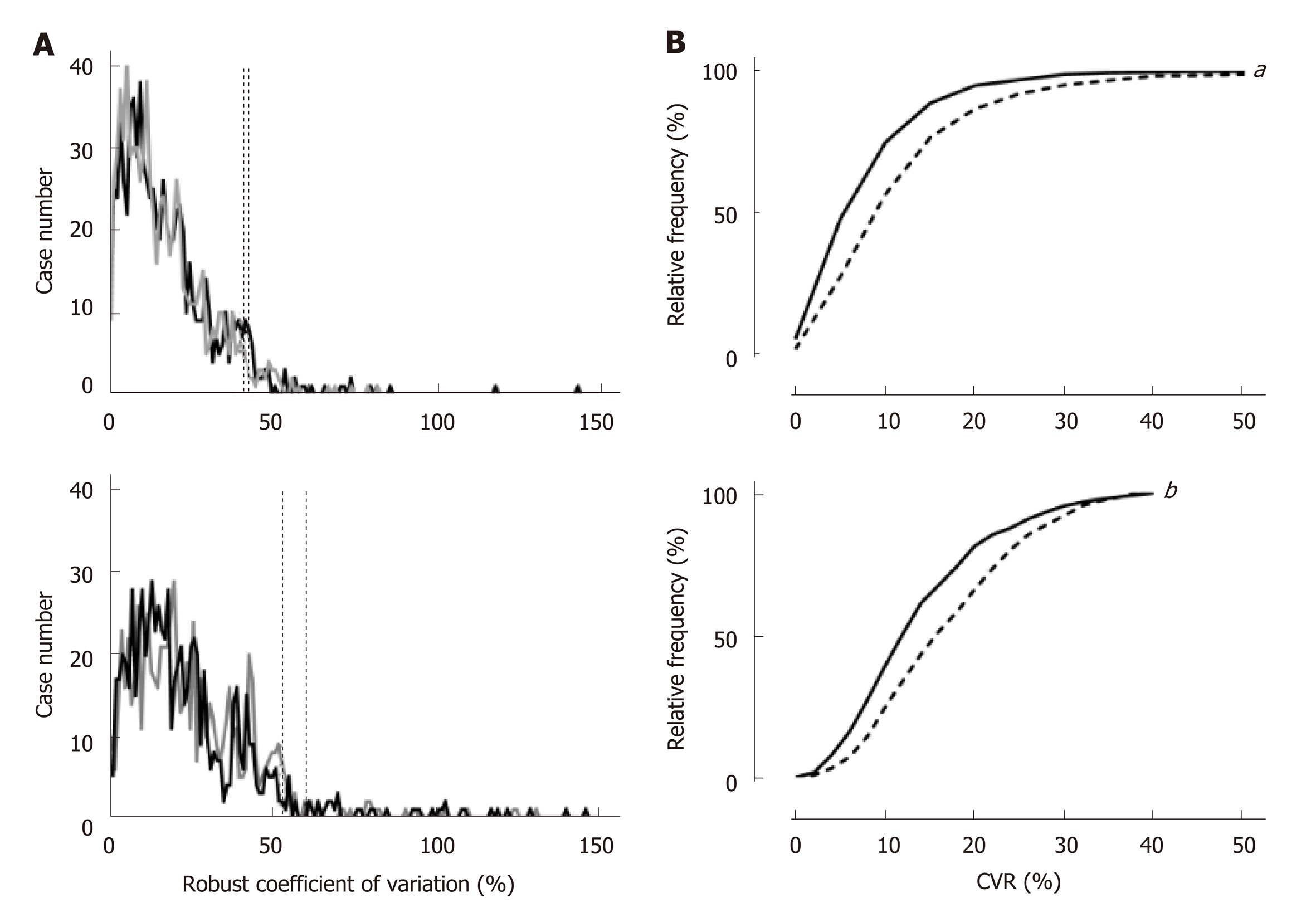
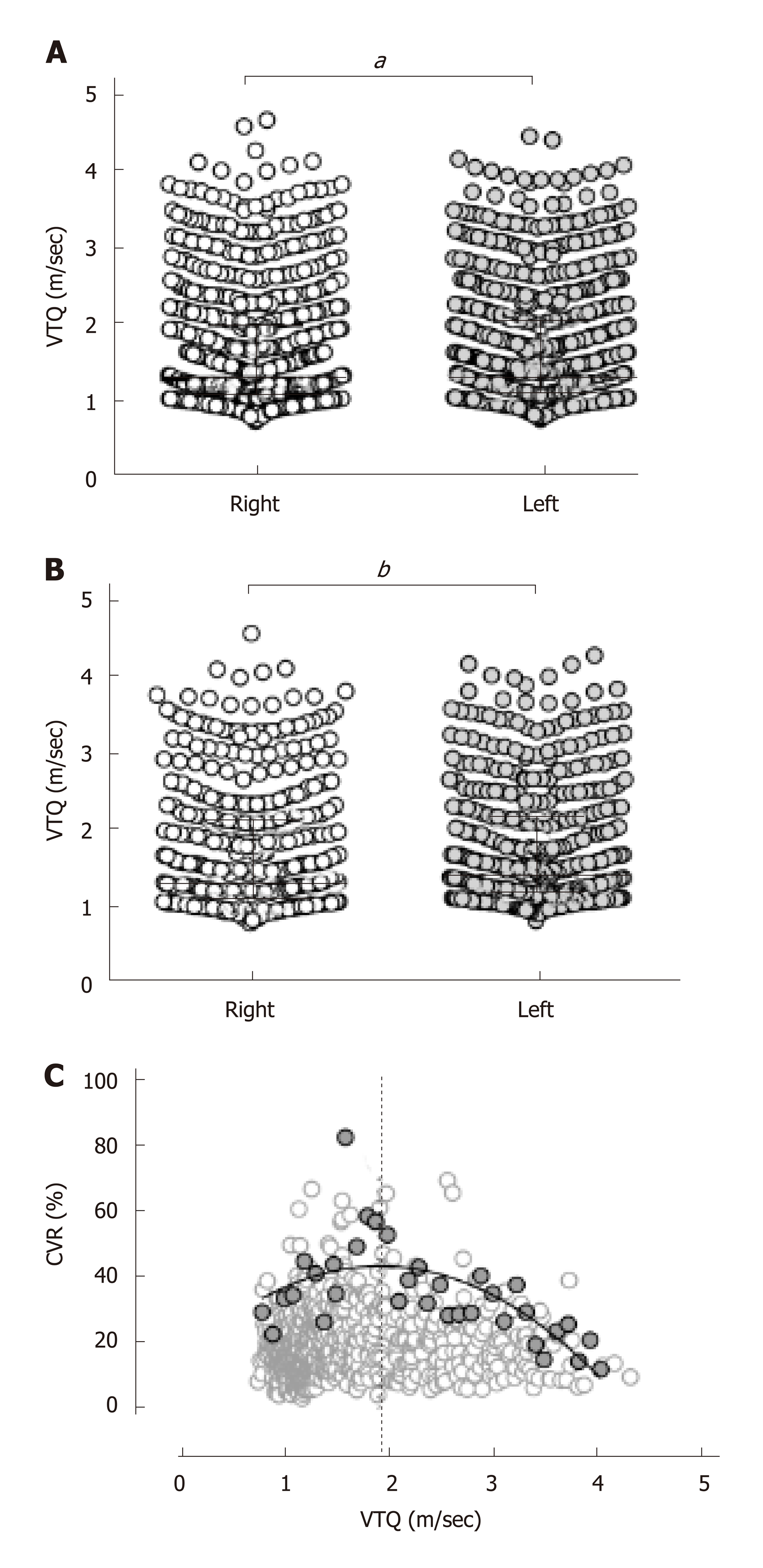
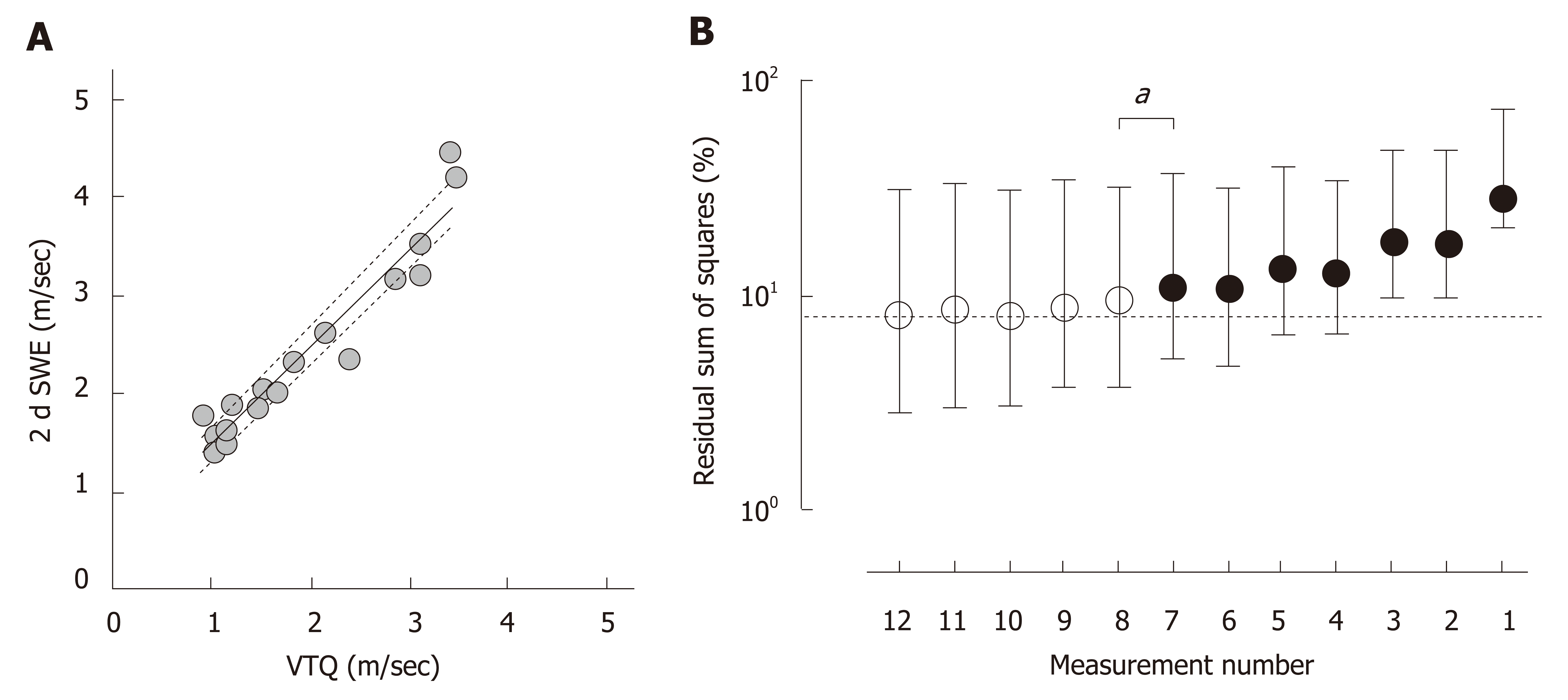
The CVR showed a significantly broader distribution in the left lobe (P < 0.0001), and the smallest CVR in the right anterior segment that covered 95% cases was 40.4%. pSWE was significantly higher in the left lobe than in the right lobe (1.63 ± 0.78 m/s vs 1.61 ± 0.78 m/s, P = 0.0004), and the difference between the lobes became further discrete when the subjects were limited to the cases with a CVR less than 40.4% in any segment (1.76 ± 0.80 m/s vs 1.70 ± 0.82 m/s, P < 0.0001). The highest values of the CVR in every 0.1 m/s interval were plotted in convex upward along pSWE and peaked at 1.93 m/s. pSWE and 2dSWE were significantly correlated (P < 0.0001, r = 0.95). In 216000 resamples from 18 cases, the %Σdi2 of 12 sites was 8.0% and gradually increased as the acquisition sites decreased to reach a significant difference with a %Σdi2 of 7 sites (P = 0.027).
These data suggest that shear wave speed should be measured at 8 or more sites of spreading in both lobes.
Core tip: Liver stiffness measurements play a key role in the management of chronic liver diseases; however, a standard procedure of liver stiffness measurements has not been established yet. This study provides the information for standardization of a measuring site and number of liver stiffness measurements from the statistical point of view.
- Citation: Yokoo T, Kanefuji T, Suda T, Nagayama I, Hoshi T, Abe S, Morita S, Kamimura H, Kamimura K, Tsuchiya A, Takamura M, Yagi K, Terai S. Rational arrangement of measuring shear wave speed in the liver. World J Gastroenterol 2019; 25(20): 2503-2513
- URL: https://www.wjgnet.com/1007-9327/full/v25/i20/2503.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i20.2503
Liver stiffness is reported to be a useful surrogate marker for the degree of fibrous accumulation in the liver[1-4], which is a good prognostic indicator for chronic liver diseases. For nonalcoholic fatty liver diseases (NAFLD), liver stiffness is almost exceptional noninvasive marker to diagnose and infer the pathophysiological state, as in the case of various markers for viral hepatitis such as HCV-RNA and anti-HBe. An ultrasound-based methodology is especially helpful when assessing NAFLD in a large target population. Unfortunately, however, ultrasound study has an inherent subjective nature, and liver stiffness measurement is not exceptional, either. While acoustic radiation force impulse technology makes it possible to induce constant tissue displacement, still there are many factors that cause substantial variabilities in shear wave speed (SWS) measurements[5-8], such as probing skill, placement of the region of interest (ROI), holding a breath or exhaling, and the number of measurements[9-13].
Currently, SWS measurement is recommended in the right lobe and is calculated as a mean or median value representative of a fibrous stage of the entire liver[14-16]. It was reported that 3 measurements are sufficient to calculate reliable values by placing 15 mm or larger acquisition circles in an ROI using supersonic shear imaging[17]. On the other hand, it is not recommended to convert SWS values measured using machines implementing different technologies from different companies and/or with different versions. To date, the reliability and accuracy of SWS were evaluated by referencing histological findings of liver biopsy specimens, which were obtained from the right lobe, or by referencing the liver stiffness, which was solely measured in the right lobe using transient elastography. Because it is well known that pathological progression occurs heterogeneously in the liver, it is reasonable to assume that SWS reveals a higher correlation coefficient in the right lobe than in the left lobe if the referencing value is obtained from the right lobe. In addition, a larger acquisition circle must be effective to compensate for the variability of SWS and to reduce the number of measurements required to calculate a statistically reasonable mean or median value. On the other hand, a larger acquisition circle diminishes the possibility of evaluating the heterogeneity through pathological progression. Because repetitive histological evaluations at multiple sites are practically unacceptable, SWS measurements are a unique technology that enables hepatologists for the first time ever to repeatedly evaluate pathological alterations at multiple sites over the liver. However, the use of a large acquisition circle restricts our ability to evaluate pathological heterogeneity, and the inability to interconvert SWS measurements from variable instruments implementing different technologies substantially limits the inferences we can draw regarding liver pathophysiologies. Both of these issues are important drawbacks for the use of liver stiffness measurements in the study of liver diseases.
Fundamentally, if the same physical property is evaluated and each technology reveals reliable results, data conversion is reasonably possible among different technologies. In terms of SWS measurement utilizing acoustic radiation force impulse, it was reported that no statistically significant differences were found in SWS estimates among operators using the same or equivalent systems under the same conditions[18,19]. Therefore, it should be practically acceptable to convert SWS estimates between different technologies as long as a measurement condition is established to ensure reliable measurements with each technology. In this study, SWS was evaluated mainly regarding the point of dispersion over the liver by adopting small acquisition circles to clarify the significance of measurements not only in the right lobe but also in the left lobe and to define the number of measurements required for reliable measurements. The importance of legislative definitions for the area and number of acquisition sites in the liver is discussed.
The review board of Niigata University Medical and Dental Hospital approved the present study, which did not require informed consent because it was a retrospective study using medical records or noninvasive imaging examinations. Virtual touch quantification (VTQ) of pSWE was measured in 781 cases, which were referred to our ultrasound department for liver imaging study from April 2010 to March 2015 and consisted of various liver diseases as summarized in Table 1. Among these cases, 2dSWE was also measured in 18 cases on the same day.
| Median | Minimum | Maximum | Number of cases | ||
| Age (yr) | 61.1 | 0.7 | 91.8 | Female | 393 |
| BMI (kg/m2) | 23.2 | 11.4 | 47.8 | Male | 388 |
| AllVTQ (m/s) | 1.36 | 0.77 | 4.31 | HBV | 72 |
| RtVTQ (m/s) | 1.26 | 0.70 | 4.65 | HCV | 179 |
| LtVTQ (m/s) | 1.28 | 0.73 | 4.44 | ALD | 49 |
| AllCVR (%) | 24.4 | 4.2 | 270.5 | NAFLD | 230 |
| RtCVR (%) | 16.7 | 1.5 | 137.0 | CLD | 61 |
| LtCVR (%) | 34.0 | 2.7 | 496.1 | NCLD | 190 |
HBsAg and anti-HCV antibodies were detected by a chemiluminescence immunoassay using ARCHITECT HBsAg QT and ARCHITECT HCV (Abbott Japan Co. Ltd., Chiba, Japan), respectively. Routine blood biochemistry was measured in the clinical laboratories of our hospital, where a quality control of each test is regularly performed every day. NAFLD was diagnosed based on the criteria proposed by the Asia-Pacific Working Party on NAFLD[20]. In brief, each of the following requirements was met: (1) abnormal values of aspartate aminotransferase and/or alanine aminotransferase; (2) negative results for HBsAg, anti-HCV, anti-nuclear antibody, and anti-mitochondrial antibody; (3) no suspicious drug usage, alcohol abuse over 20 g/d, hereditary diseases or any other clinical manifestations causing liver cell damage; and (4) fatty liver as observed by abdominal US, which was defined by an increased echogenicity of the liver along with the presence of any two of the following three findings: liver-kidney contrast, vascular blurring, and deep-attenuation of echo-beam[21].
SWS evoked by acoustic radiation force impulse was measured as VTQ using an ACUSON S2000 ultrasound system (Siemens Healthcare, Eriangen, Germany) or as 2dSWE using an Aplio 500 (Canon Medical System Corporation, Tokyo, Japan). SWS was measured three times in each segment (posterior, anterior, medial, and lateral) while the patient, who fasted the previous night, was in the supine position with a transient breath hold at a neutral cycle followed by a 10-min or longer rest, and the median value, which is less affected by outliers, was calculated from twelve measurements per case as the representative value for the entire liver. The ROI was placed between 1 to 5 cm beneath the liver capsule. In the 2dSWE measurement, the ROI was set as a square approximately 30 mm x 30 mm in size, and 3 measurements were achieved in each ROI by placing an acquisition circle 2 mm in diameter after confirming a proper propagation of shear wave in the “wavefront” style display. Next, a robust counterpart to the standard deviation was calculated. First, the median absolute deviation was calculated as the median of the difference in the absolute values between each VTQ value and the median of 12 measurements; thereafter, a constant factor of 1.4826 was multiplied to adjust the resulting robust standard deviation to the equivalent of a normal population distribution. Finally, the CVR was calculated by dividing the robust standard deviation with the median and is expressed as a percentage.
To define the cut-off values of VTQ based on the referenced histological fibrous stages, the VTQ was evaluated in 98 other cases, of which 89 cases were suffering from various chronic liver diseases that require histological evaluation of the liver. Two expert pathologists independently evaluated liver biopsy specimens and assessed fibrous staging and inflammatory grading. The remaining 9 controls fulfilled all requirements for NASH diagnosis except for abnormal values of transaminases and histological abnormalities, which were not evaluated. Cases with chronic liver diseases consisted of 15, 23, 28, and 23 cases of the F1, F2, F3, and F4 fibrous stages, respectively. The area under the receiver operating characteristic curve to distinguish F0 - F1 from F2 or higher or F4 from the others was 84.4% (P < 0.0001) and 79.3% (P < 0.0001), respectively, and the defined cut-off values were 1.37 and 2.10 m/sec, respectively; these corresponded to a sensitivity and specificity of 78.4% and 82.8% and 73.9% and 75.0%, respectively, as shown in Supplementary Figure 1.
To compare the cumulative distributions of CVR between the liver lobes, the Kolmogorov-Smirnov test was performed. Wilcoxon matched-pairs signed rank test was employed to compare the VTQ values between the liver lobes. A peak of CVR in the distribution along the VTQ values was calculated by adopting a nonlinear regression model of a second order polynomial. A Spearman correlation coefficient was calculated to evaluate the degree of association between VTQ and 2dSWE. To prepare datasets of 2dSWE with the assumption of different numbers of measured sites from 1 to 12, a 1000 iteration of bootstrap resampling[22] was performed in each case using the 2dSWE values from 12 measurements. Σdi2 was calculated as the summation of the squares of the difference between the actual 2dSWE value and the calculated value from VTQ based on the linear regression model of least-squares between VTQ and 2dSWE. Σdi2 was converted to %Σdi2, which represents the percentage against the calculated value from VTQ. %Σdi2 was compared among the different numbers of acquisition sites in the liver using ANOVA with post hoc multiple comparisons. All analyses were conducted using GraphPad Prism 7 software (GraphPad Software Inc., La Jolla, United States), except for bootstrapping, which was performed with Microsoft Excel 2016 (Microsoft, Seattle, United States). A two-sided p-value less than 0.05 was considered statistically significant.
A frequency distribution of the CVR was discrete between the right and left lobes (Figures 1A upper and lower panels) and showed a significantly larger dispersion in the left lobe (Figure 1B upper panel, P < 0.0001). In the right anterior and posterior segments, 95% of cases were distributed within 40.4% and 42.1% of the CVR, respectively (Figure 1a upper panel), while the values that discriminated 95% of cases in the left medial and lateral segments were 60.4% and 52.8% of the CVR, respectively (Figure 1a lower panel). Because we hypothesized that the cases showing larger CVR had a significantly larger dispersion of VTQ in the left lobe, the CVR was compared between the lobes only for 439 cases in which the CVR in any lobe or segment was 40.4% or less. However, the cumulative frequency distribution curve revealed that the CVR was still significantly dispersed in the left lobe compared to all cases (Figure 1B, lower panel, P < 0.0001).
The VTQ values of 781 cases were 1.26 (interquartile range, 1.07–1.97) m/sec and 1.28 (1.08–2.02) m/s in the right and left lobes, respectively, and were significantly higher in the left lobe (Figure 2A, P = 0.0004). Because it was anticipated that the cases with a higher CVR exhibited a higher VTQ value in the left lobe, the VTQ value was compared only in 439 cases with a CVR of 40.4% or less. The comparison in the restricted cases, however, resulted in a greater significant difference in the VTQ values between the right and left lobes. The VTQ value of 1.39 (1.18–2.19) m/sec in the left lobe was significantly higher than that in the right lobe (1.31 (1.11–2.12) m/s) as shown in Figure 2B (P < 0.0001).
If artifacts such as cardiac pulsation are a main cause of the higher CVR when measuring VTQ, it is reasonable to assume that the higher CVR would be evenly distributed along the VTQ values. However, inconsistent with this assumption, the highest CVR at every 0.1 ± 0.02 m/s interval of the VTQ values from 0.81 to 4.03 m/s scattered in convex upward pattern along the VTQ values with a peak at 1.93 m/s, as observed in Figure 2C. As shown in Supplementary Figure 1, a concomitant evaluation of VTQ and histological fibrous stages revealed that 1.93 m/s of VTQ suggests F2-F3 fibrous stages.
Because the VTQ values are heterogeneous over the liver, as shown in Figure 2c, a representative VTQ value for the entire liver would vary depending on the number of measurements in the liver. To define the smallest number of measurements required that minimize Σdi2 from an ideal liver stiffness, SWS was measured by two different modalities of VTQ and 2dSWE on the same day in 18 cases. Because the speeds were highly correlated between the two types of measurements over a sufficient range in the clinic (Figure 3A, P < 0.0001, Spearman r = 0.953), the estimated value calculated from an actual measurement of the VTQ by means of the least-square method was presumed as an ideal value representing liver stiffness for the entire liver.
To enhance statistical confidence, a dataset of VTQ measured at 12 sites was produced by a 1000 iteration of bootstrapping in each case. Similar datasets were prepared for the assumption of measuring at 11 sites, 10 sites, and so on to 1 site in each case. In total, 216,000 VTQ values (1000 datasets x 1 to 12 measurements in the liver x 18 cases) were generated and processed to calculate %Σdi2. As shown in Figure 3b, the %Σdi2 for 12 measurements was 8.0% (2.8%–31.0%), and this value gradually increased as the number of sites measured decreased to reach a significant difference with %Σdi2 of measuring at 7 sites [11.0% (5.0%–36.8%), P = 0.027].
NAFLD is a pandemic throughout the world and among people at a productive age, which causes substantial social loss[23,24]. It is socially urgent to establish a system to manage NAFLD well not only as a liver disorder but also as a major target of metabolic syndrome. In this regard, a major drawback is necessity of liver histology for diagnosis of NAFLD[20]. To address the enormous number of NAFLD cases, which is estimated to compose more than 30% of the general population, a surrogate to measure liver fibrosis is required from a practical point of view. So far, liver stiffness is one of most promising alternatives for fibrous liver stage due to its noninvasiveness and liver specificity. Liver stiffness measurements are roughly classified into 3 groups with respect to the force evoking shear wave in the tissues and the method used to measure the speed of propagating wave. An ultrasound-based technology employing acoustic radiation force impulse[25-28] for tissue displacement is advantageous against other 2 groups, transient elastography[1,27,29-31] and magnetic resonance elastography[3,28,32-34], due to its versatility for the measurable area and popularity, especially in a primary care setting. The ultrasound-based technology is, however, a subjective examination, which requires a protocol to make the measurement clinically reliable.
In many cases, an average SWS was calculated from several measurements in the right lobe as a representative value for the entire liver[26]. It may be assumed that the right lobe measurement is a holdover from transient elastography, the first technology that gained popularity as a means to measure liver stiffness in the clinic and that can be applied solely to the right lobe. Alternatively, the measurements in the left lobe tend to be deemed inappropriate because of artifacts such as heart beat[35]. Consistent with this assumption, SWS was significantly dispersed in the left lobe, even when the subject cohort was limited to cases with a relatively smaller CVR (Figure 1). As reported in the literature, SWS was significantly higher in the left lobe (Figure 2A). This significant difference in the SWS between lobes was true in the limited cases with a relatively smaller CVR, suggesting that a higher SWS in the left lobe is not simply due to higher dispersion in the left lobe (Figure 2b). On the other hand, higher CVR values were unevenly spread along the VTQ and peaked at 1.93 m/s (Figure 2C). It is assumed that a higher CVR would be similar irrespective of the SWS if the higher dispersion of SWS in the left lobe is simply the result of artifacts. The distribution of the higher CVR values in a convex upward trend along the SWS strongly suggests that liver stiffness is relatively homogenous at the early stage of chronic liver diseases and gradually appears to become heterogeneous as the disease progresses toward F2 to F3 stages, after which the dispersion again gets smaller during the progression toward cirrhosis. It is reasonable to assume that histological complexity is highest in the middle of the clinical course from the beginning of chronic liver diseases to the completion of cirrhotic change. The above data suggest that a higher SWS and its CVR may not be rational reasons to argue the inappropriateness of SWS measurements in the left lobe. Given the noninvasive nature of SWS measurements, they should be taken in both lobes to clarify the pathophysiological differences among the segments, as suggested by a diverse progression/alleviation process based on the streamline theory[36].
To define the appropriate number of SWS measurements in the liver, we used a strategy to calculate the distance between the values of an actual measurement and the ideal SWS in each case. We hypothesized that if different methods of SWS measurements detected highly correlated values, the value deduced from the correlation would be an ideal SWS. Because two different methods of VTQ and 2dSWE measurements produced highly correlated median values when the measurement was performed at 12 sites throughout the liver, the use of 12 measurements is likely to be sufficient to define a representative value for the entire liver by tolerating fibrosis heterogeneity and suppressing deviation due to technical and instrumental limitations. On the other hand, as the acquisition sites decreased, the %Σdi2 of the 2dSWE value gradually increased from that in 12 measurements. Because a statistically significant difference of the %Σdi2 from that in 12 measurements first appeared when it was assumed that SWS was measured at 7 sites, it is suggested that SWS should be measured 8 or more sites in the liver.
Although it is challenging to regularly verify a unified specification of SWS mea-surements from both industrial and practical perspectives, establishing a standard SWS measuring condition and enforcing a regulation that would standardize SWS values, which ensure the conversion and enable the implication of liver pathogenesis, is paramount. This study strongly suggests that the SWS values measured in both lobes at 8 or more acquisition sites would provide values applicable for the conversion between different technologies. However, the results of this study are based on a small number of cases, in which SWS was measured by 2 different methods of VTQ and 2dSWE. The limited number of cases may have contributed to an inadequate assessment of the biological variability. To mitigate the effects of a small sample size on statistical judgement and to create hypothetical sample sets consisting of 1 to 12 measurements in the liver, bootstrap resampling was conducted to prepare 216000 samples in total. Because our results for an adequate number of measurements were deduced from hypothetical sample sets, they should be reconfirmed with data from a larger cohort, in which actual parallel measurements were preformed using multiple methods of SWS measurement.
In this report, we rationalized measuring SWS not only in the right lobe but also in the left lobe. In addition, we defined the smallest number of SWS measurements in the liver required to minimize the deviation of the SWS from an ideal value. The basic data presented in this report provide important information to develop a clinically reliable protocol for SWS measurement in the liver.
Although it is inevitable to measure shear wave elastography in the same manner among different institutions to utilize the elastography as a standard clinical property, so far there is no unified protocol for this technology.
A degree of liver fibrosis is the most reliable indicator for survival in chronic liver diseases. A standardization of the process to define shear wave elastography should make it valuable not only in a daily clinic but also in various clinical studies by surrogating liver fibrosis.
In this article, it is addressed to clarify from where and how many times shear wave elastography should be measured in the liver to calculate an elastography being representative for the entire liver.
Shear wave elastography was evaluated using two different technologies by placing a region-of-interest with a relatively small size at twelve points scattering throughout the liver to calculate not only a representative value for the entire liver but also a variability of the value throughout the liver. A residual sum-of-square was calculated as a distance from the correlation between the values obtained from two technologies. The limited number of cases was compensated by applying bootstrap values of 1000 iterations in each case.
Both median and distribution of shear wave elastography were significantly different between the right and left lobes. Even after excluding the cases showing the deviation larger than a certain level, the difference of median values was further discrete between lobes. The dispersion of the elastography in the liver was getting larger as the median value was increased toward 1.93 m/sec, then after that the dispersion was getting smaller as the median value was further increased. A residual sum-of-square was increased as the number of measurements in the liver was decreased from twelve points. A sum-of-square was appeared to be significantly larger than that of measurements at twelve sites, when the number of measurement points was decreased to seven.
The difference of shear wave elastography between lobes is not likely due to the difference of dispersion between lobes. The liver fibrosis seems to take place heterogeneously. The heterogeneity should be largest in the middle of the clinical course of chronic liver diseases toward cirrhosis. The variability of median shear wave elastography was increased as the measuring points were decreased.
Shear wave elastography should be measured in both lobes. Heterogeneity of shear wave elastography in the liver would reflect the severity of liver fibrosis. Shear wave elastography should be measured at more than 7 sites in the liver.
Shear wave elastography should be measured at more than seven sites in both lobes. Dispersion of shear wave elastography would provide another insight for the pathogenesis of chronic liver diseases. A recommendation of the number and sites for shear wave elastography measurements in the liver; more than seven points in the both lobes. Dispersion of shear wave elastography in the liver was increased as the lobular reorganization takes place and in turn decreased toward cirrhosis. The evaluation of shear wave elastography, a region-of-interest should be placed eight or more throughout the liver including both lobes. The heterogeneity of fiber accumulation in the liver peaks in the middle of the course of chronic liver diseases from normal to cirrhotic liver. To include the heterogeneity in the evaluation of liver fibrosis using shear wave elastography, the measurements should be performed at more than seven sites in both lobes.The standardization of the procedure in shear wave elastography measurements enables a large-scale multicenter study to achieve multiple evaluations of liver fibrosis in time and space, which leads to clarification of a novel pathogenesis, an efficacy of new drugs, and so on in chronic liver diseases.
In addition to the procedural standardization in shear wave elastography measurements, an industrial standardization of this technology is required for the direct comparison among data that were obtained using machines from different companies and/or implementing a different version of this technology. In parallel with the establishment of a standard procedure in shear wave elastography measurements, a phantom to calibrate an accuracy of shear wave elastography should be explored. Because the significance of shear wave elastography should be determined from the point of clinical outcome, it should be conducted to measure shear wave elastography according to a standard procedure and follow to see the impact of the value on progression/alleviation of the diseases.
The authors are grateful for the technicians who performed the SWS measurements and developed the questionnaires about daily energy intake and physical activities.
| 1. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, Beaugrand M, Palau R. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 1957] [Article Influence: 85.1] [Reference Citation Analysis (8)] |
| 2. | Sarvazyan AP, Rudenko OV, Swanson SD, Fowlkes JB, Emelianov SY. Shear wave elasticity imaging: a new ultrasonic technology of medical diagnostics. Ultrasound Med Biol. 1998;24:1419-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1271] [Cited by in RCA: 943] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 3. | Aguirre DA, Behling CA, Alpert E, Hassanein TI, Sirlin CB. Liver fibrosis: noninvasive diagnosis with double contrast material-enhanced MR imaging. Radiology. 2006;239:425-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 143] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Osaki A, Kubota T, Suda T, Igarashi M, Nagasaki K, Tsuchiya A, Yano M, Tamura Y, Takamura M, Kawai H, Yamagiwa S, Kikuchi T, Nomoto M, Aoyagi Y. Shear wave velocity is a useful marker for managing nonalcoholic steatohepatitis. World J Gastroenterol. 2010;16:2918-2925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, Cantisani V, Correas JM, D'Onofrio M, Drakonaki EE, Fink M, Friedrich-Rust M, Gilja OH, Havre RF, Jenssen C, Klauser AS, Ohlinger R, Saftoiu A, Schaefer F, Sporea I, Piscaglia F. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013;34:169-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 793] [Article Influence: 61.0] [Reference Citation Analysis (2)] |
| 6. | Ferraioli G, Filice C, Castera L, Choi BI, Sporea I, Wilson SR, Cosgrove D, Dietrich CF, Amy D, Bamber JC, Barr R, Chou YH, Ding H, Farrokh A, Friedrich-Rust M, Hall TJ, Nakashima K, Nightingale KR, Palmeri ML, Schafer F, Shiina T, Suzuki S, Kudo M. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 3: liver. Ultrasound Med Biol. 2015;41:1161-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 502] [Article Influence: 45.6] [Reference Citation Analysis (1)] |
| 7. | Sporea I, Sirli RL, Deleanu A, Popescu A, Focsa M, Danila M, Tudora A. Acoustic radiation force impulse elastography as compared to transient elastography and liver biopsy in patients with chronic hepatopathies. Ultraschall Med. 2011;32 Suppl 1:S46-S52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Barr RG, Ferraioli G, Palmeri ML, Goodman ZD, Garcia-Tsao G, Rubin J, Garra B, Myers RP, Wilson SR, Rubens D, Levine D. Elastography Assessment of Liver Fibrosis: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2015;276:845-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 451] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 9. | Wang CZ, Zheng J, Huang ZP, Xiao Y, Song D, Zeng J, Zheng HR, Zheng RQ. Influence of measurement depth on the stiffness assessment of healthy liver with real-time shear wave elastography. Ultrasound Med Biol. 2014;40:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Liao LY, Kuo KL, Chiang HS, Lin CZ, Lin YP, Lin CL. Acoustic radiation force impulse elastography of the liver in healthy patients: test location, reference range and influence of gender and body mass index. Ultrasound Med Biol. 2015;41:698-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Goertz RS, Zopf Y, Jugl V, Heide R, Janson C, Strobel D, Bernatik T, Haendl T. Measurement of liver elasticity with acoustic radiation force impulse (ARFI) technology: an alternative noninvasive method for staging liver fibrosis in viral hepatitis. Ultraschall Med. 2010;31:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 12. | Goertz RS, Egger C, Neurath MF, Strobel D. Impact of food intake, ultrasound transducer, breathing maneuvers and body position on acoustic radiation force impulse (ARFI) elastometry of the liver. Ultraschall Med. 2012;33:380-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Zhuang Y, Ding H, Zhang Y, Sun H, Xu C, Wang W. Two-dimensional Shear-Wave Elastography Performance in the Noninvasive Evaluation of Liver Fibrosis in Patients with Chronic Hepatitis B: Comparison with Serum Fibrosis Indexes. Radiology. 2017;283:873-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 14. | Horster S, Mandel P, Zachoval R, Clevert DA. Comparing acoustic radiation force impulse imaging to transient elastography to assess liver stiffness in healthy volunteers with and without valsalva manoeuvre. Clin Hemorheol Microcirc. 2010;46:159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Karlas T, Pfrepper C, Wiegand J, Wittekind C, Neuschulz M, Mössner J, Berg T, Tröltzsch M, Keim V. Acoustic radiation force impulse imaging (ARFI) for non-invasive detection of liver fibrosis: examination standards and evaluation of interlobe differences in healthy subjects and chronic liver disease. Scand J Gastroenterol. 2011;46:1458-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (3)] |
| 16. | Samir AE, Dhyani M, Vij A, Bhan AK, Halpern EF, Méndez-Navarro J, Corey KE, Chung RT. Shear-wave elastography for the estimation of liver fibrosis in chronic liver disease: determining accuracy and ideal site for measurement. Radiology. 2015;274:888-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (1)] |
| 17. | Sporea I, Grădinaru-Taşcău O, Bota S, Popescu A, Şirli R, Jurchiş A, Popescu M, Dănilă M. How many measurements are needed for liver stiffness assessment by 2D-Shear Wave Elastography (2D-SWE) and which value should be used: the mean or median? Med Ultrason. 2013;15:268-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Hall TJ, Milkowski A, Garra B, Carson P. RSNA/QIBA: shear wave speed as a biomarker for liver fibrosis staging. 2013 IEEE International Ultrasonics Symposium. . [DOI] [Full Text] |
| 19. | Palmeri M, Nightingale K, Fielding S. RSNA/QIBA: ultrasound shear wave speed Phase II phantom study in viscoelastic media. 2015 IEEE International Ultrasonics Symposium. . [DOI] [Full Text] |
| 20. | Farrell GC, Chitturi S, Lau GK, Sollano JD, Asia-Pacific Working Party on NAFLD. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: executive summary. J Gastroenterol Hepatol. 2007;22:775-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 432] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 21. | Yajima Y, Ohta K, Narui T, Abe R, Suzuki H, Ohtsuki M. Ultrasonographical diagnosis of fatty liver: significance of the liver-kidney contrast. Tohoku J Exp Med. 1983;139:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 126] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Lunneborg CE. Random assignment of available cases: bootstrap standard errors and confidence intervals. Psychol Methods. 2001;6:402-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | El-Zayadi AR. Hepatic steatosis: a benign disease or a silent killer. World J Gastroenterol. 2008;14:4120-4126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 24. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7925] [Article Influence: 792.5] [Reference Citation Analysis (8)] |
| 25. | Ye XP, Ran HT, Cheng J, Zhu YF, Zhang DZ, Zhang P, Zheng YY. Liver and spleen stiffness measured by acoustic radiation force impulse elastography for noninvasive assessment of liver fibrosis and esophageal varices in patients with chronic hepatitis B. J Ultrasound Med. 2012;31:1245-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Friedrich-Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-Braticevici C, Strobel D, Takahashi H, Yoneda M, Suda T, Zeuzem S, Herrmann E. Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat. 2012;19:e212-e219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 364] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 27. | Cassinotto C, Boursier J, de Lédinghen V, Lebigot J, Lapuyade B, Cales P, Hiriart JB, Michalak S, Bail BL, Cartier V, Mouries A, Oberti F, Fouchard-Hubert I, Vergniol J, Aubé C. Liver stiffness in nonalcoholic fatty liver disease: A comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63:1817-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 401] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 28. | Cui J, Heba E, Hernandez C, Haufe W, Hooker J, Andre MP, Valasek MA, Aryafar H, Sirlin CB, Loomba R. Magnetic resonance elastography is superior to acoustic radiation force impulse for the Diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: A prospective study. Hepatology. 2016;63:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 157] [Article Influence: 15.7] [Reference Citation Analysis (1)] |
| 29. | Degos F, Perez P, Roche B, Mahmoudi A, Asselineau J, Voitot H, Bedossa P; FIBROSTIC study group. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol. 2010;53:1013-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 340] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 30. | Loong TC, Wei JL, Leung JC, Wong GL, Shu SS, Chim AM, Chan AW, Choi PC, Tse YK, Chan HL, Wong VW. Application of the combined FibroMeter vibration-controlled transient elastography algorithm in Chinese patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2017;32:1363-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Wong VW, Vergniol J, Wong GL, Foucher J, Chan AW, Chermak F, Choi PC, Merrouche W, Chu SH, Pesque S, Chan HL, de Lédinghen V. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2012;107:1862-1871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 268] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 32. | Shi Y, Xia F, Li QJ, Li JH, Yu B, Li Y, An H, Glaser KJ, Tao S, Ehman RL, Guo QY. Magnetic Resonance Elastography for the Evaluation of Liver Fibrosis in Chronic Hepatitis B and C by Using Both Gradient-Recalled Echo and Spin-Echo Echo Planar Imaging: A Prospective Study. Am J Gastroenterol. 2016;111:823-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 33. | Dyvorne HA, Jajamovich GH, Bane O, Fiel MI, Chou H, Schiano TD, Dieterich D, Babb JS, Friedman SL, Taouli B. Prospective comparison of magnetic resonance imaging to transient elastography and serum markers for liver fibrosis detection. Liver Int. 2016;36:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Chen J, Yin M, Talwalkar JA, Oudry J, Glaser KJ, Smyrk TC, Miette V, Sandrin L, Ehman RL. Diagnostic Performance of MR Elastography and Vibration-controlled Transient Elastography in the Detection of Hepatic Fibrosis in Patients with Severe to Morbid Obesity. Radiology. 2017;283:418-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 137] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 35. | Toshima T, Shirabe K, Takeishi K, Motomura T, Mano Y, Uchiyama H, Yoshizumi T, Soejima Y, Taketomi A, Maehara Y. New method for assessing liver fibrosis based on acoustic radiation force impulse: a special reference to the difference between right and left liver. J Gastroenterol. 2011;46:705-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 36. | Kashiwagi T, Kamada T, Abe H. Dynamic studies on the portal hemodynamics of scintiphotosplenoportography. Streamline flow in the human portal vein. Gastroenterology. 1975;69:1292-1296. [PubMed] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): A
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P-Reviewer: Abenavoli L, Ciccone MM S-Editor: Gong ZM L-Editor: A E-Editor: Zhang YL