Published online May 28, 2019. doi: 10.3748/wjg.v25.i20.2442
Peer-review started: February 27, 2019
First decision: March 27, 2019
Revised: March 30, 2019
Accepted: April 19, 2019
Article in press: April 20, 2019
Published online: May 28, 2019
Processing time: 91 Days and 10.9 Hours
Hepatocellular adenoma (HCA) is a rare benign liver tumour associated with the use of oral contraceptives or other steroid medications which occurs predominantly in young and middle-aged women. Unlike other benign liver tumours, an HCA may be complicated by bleeding and malignant transformation. HCAs have been divided into four subtypes based on molecular and pathological features: hepatocyte nuclear factor 1α-mutated HCA, inflammatory HCA, β-catenin-mutated HCA, and unclassified HCA. β-catenin-mutated HCA has the highest risk of haemorrhage or malignant transformation. In the latest upgrade of the guidelines regarding the management of benign liver tumours published in 2016 by the European Association for the Study of the Liver, magnetic resonance imaging (MRI) was recognized to be superior to all other imaging modalities in detecting HCAs and in being able to subtype HCAs up to 80%, with positive identification of 1α-mutated HCA or inflammatory HCA achievable with > 90% specificity. This review analyzed the imaging features of HCA using MRI with hepato-specific contrast agents, focusing on the limitations in the HCA characterization.
Core tip: In 2017, a group of French Researchers proposed a new classification of hepatocellular adenoma based on genomic analysis, identifying three additional subtypes. However, in this latest paper, the possible imaging features of the aforementioned new subtypes of hepatocellular adenoma were not reported. This review analyzed the imaging features of hepatocellular adenoma using magnetic resonance imaging with hepato-specific contrast agents, focusing on the limitations of the “old” imaging criteria proposed by the European Association for the Study of the Liver when applied to the new subtypes of adenoma.
- Citation: Renzulli M, Clemente A, Tovoli F, Cappabianca S, Bolondi L, Golfieri R. Hepatocellular adenoma: An unsolved diagnostic enigma. World J Gastroenterol 2019; 25(20): 2442-2449
- URL: https://www.wjgnet.com/1007-9327/full/v25/i20/2442.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i20.2442
Hepatocellular adenoma (HCA) is a benign hepatic tumour which occurs predominantly in young and middle-aged women who take oral contraceptives or other steroid medications[1,2]. The relationship between HCA and the use of oral contraceptives was first described by Bühler et al[3], and the incidence of HCA has been reported to be approximately 30 times greater in oral contraceptive users as compared to nonusers[1,4]. Moreover, HCA can also be found in patients with glycogen storage disease or metabolic syndrome and in men using anabolic steroids[5]. Spontaneous regression following the withdrawal of estrogens has also been described[3,4,6]. However, the exact role of estrogen in HCA remains elusive[7]. In non-cirrhotic patients, HCA represents the second most common benign lesion of hepatocellular origin after focal nodular hyperplasia (FNH) which in turn is the second most common liver benign lesion after hemangioma when considering benign lesions of all origins[8]. However, HCA is a rare tumour, approximately 10 times less common than FNH[8].
Although both FNH and HCA are benign lesions, they are managed quite differently[9] since, in contrast to FNH, HCA may involve complications, such as life-threatening bleeding and malignant degeneration[2,10]. In fact, FNH is conservatively managed in the majority of cases, and resection is not required. Conversely, HCA is commonly treated with surgical resection due to its serious clinical consequences, such as spontaneous haemorrhage or malignant transformation[10,11]. Therefore, a correct differential diagnosis between FNH and HCA is mandatory but, with state-of-the-art imaging, this diagnosis is relatively easy to reach by using magnetic resonance imaging (MRI) with a hepato-specific contrast agent, such as gadoxetic acid[12,13]. In particular, hyper-/iso-intensity in the hepatobiliary phase of gadoxetic acid MRI is characteristic and is a prevalent finding of FNH or FNH-like lesions[13,14].
Unfortunately, the real unsolved problems of liver imaging still remain the differentiation between HCA and malignant entities[15], and the characterisation of the different molecular types of HCA[16,17]. In fact, the different types of HCA have a different risk of malignant degeneration and, therefore, a correct diagnosis of the HCA subtype could allow tailored therapy. This review analyses the imaging of HCA, focusing in particular on the problems which imaging encounters in the diagnosis of the different molecular subtypes of HCA when applied to the new classification proposed by the French Researchers in 2017[17].
In 2016, the Clinical Practice Guidelines regarding the management of benign liver tumours was published by the European Association for the Study of the Liver (EASL), with a section dedicated to HCA[16]. HCA was divided into four subtypes according to genomic analysis: Inflammatory HCA (I-HCA) accounting for 30% to 40% of HCAs, hepatocyte nuclear factor 1A mutated HCA (H-HCA) accounting for 40 to 55% of HCAs, β-catenin activated HCA (β-HCA) accounting for 10% to 20% of HCAs and unclassified HCA accounting for 5% to 10% of HCAs. β-HCA was additionally subdivided into two subgroups, each one constituting 50% of all β-HCAs: exon 3 β-catenin mutated and exon 7-8 β-catenin mutated. Finally, five molecular subtypes of HCA were recognizable.
The molecular classification of HCA has markedly contributed to the understanding of the oncogenic pathways involved in liver tumourigenesis. In fact, molecular subtyping is highly associated with the risk of malignant transformation into hepatocellular carcinoma (HCC). Among the different subgroups, β-HCAs exhibit the highest risk for malignancy, including those with dual β-catenin and inflammatory phenotypes[16].
In many cases, when analyzing the recommended management for a presumed HCA, a conservative approach is contemplated, for example in a female with a stable or reduced in size lesion < 5 cm after a 6-mo MRI follow-up. However, whether the risk of haemorrhage or malignant transformation attributed to β-catenin activation in HCAs is independent of the clinical risk factors identified (gender, size, rate of change) is presently unknown[16]. Therefore, a correct imaging diagnosis is necessary.
HCA is no longer a unique entity, and imaging features reflect the tumour subtypes. The EASL Guidelines have confirmed the superiority of MRI over all other imaging modalities in diagnosing HCAs due to its intrinsic properties in fat and vascular space detection, offering an up to 80% opportunity of subtyping HCAs with evidence level II-2 and grade of recommendation 1. On MRI, H-HCA is characterized by a diffuse and homogeneous signal dropout on opposed-phase T1-weighted sequence, due to the presence of marked steatosis[16]. I-HCA, pathologically characterized by telangiectatic traits, shows two typical features on MRI[16]: (1) A strong hyperintensity on T2-weighted images (as strong as the signal of the spleen), which may be either diffuse or as a rim-like band in the periphery of the lesion (the atoll sign), especially in gross adenomas; and (2) persistent enhancement in the delayed phase using extracellular MRI contrast agents. Unfortunately, there are no specific features on MRI for the remaining types of HCA, such as β-HCA and unclassified HCA. In summary, the positive identification of H-HCA or I-HCA is achievable on MRI with > 90% specificity. By contrast, the identification of β-HCA and its differentiation from unclassified HCA and HCC is not possible using any imaging techniques, with evidence level II-2 and grade of recommendation 1[16].
In 2017, one year after the release of the updated EASL Clinical Practice Guidelines on the management of benign liver tumours, an important study by French Authors was published proposing a new molecular classification of HCA[17]. They confirmed the five already known subtypes of HCA, but also discovered three new subtypes. The first two HCAs are mixed tumours in which I-HCA is mixed with β-HCA: one with the β-catenin exon 3 activated subtype and another with the exon 7,8 activated subtype. The third one is correlated with the activation of the sonic hedgehog pathway, sonic hedgehog HCA (shHCA).
Therefore, according to this new proposed classification, eight subtypes of HCA can be identified: the most common HCAs remain H-HCA (34%) and I-HCA (34%). The remaining 32% are divided into exon 3 β-catenin mutated HCA (7%), exon 7,8 β-catenin mutated HCA (3%), the two mixed forms of I-HCA with exon 3 β-HCA (6%) and exon 7,8 β-HCA (4%), the new entity of sh-HCA resulting from the unclassified type (4%), and finally unclassified HCA (7%).
Comparing this new classification with the older one, the percentage of H-HCA is the same as that of I-HCA. Globally, the lesions with β-catenin mutated, in pure form or in mixed form, are the same. Finally, the percentage of shHCA which derives from the unclassified type plus unclassified HCA is the same. The same Authors of the new HCA classification also suggested the possibility of having different tumour subtypes even within the same liver as a consequence of different deregulated pathways[17]. In this latest paper[17], the possible imaging features of the aforementioned new subtypes of HCA were not reported. However, as a consequence of this new HCA classification, what are the implications for the imaging diagnosis?
When applying the imaging criteria of the different subtypes of HCA recognized in the current version of the EASL Guidelines for the management of benign liver lesions to the new HCA classification, many considerations should be made.
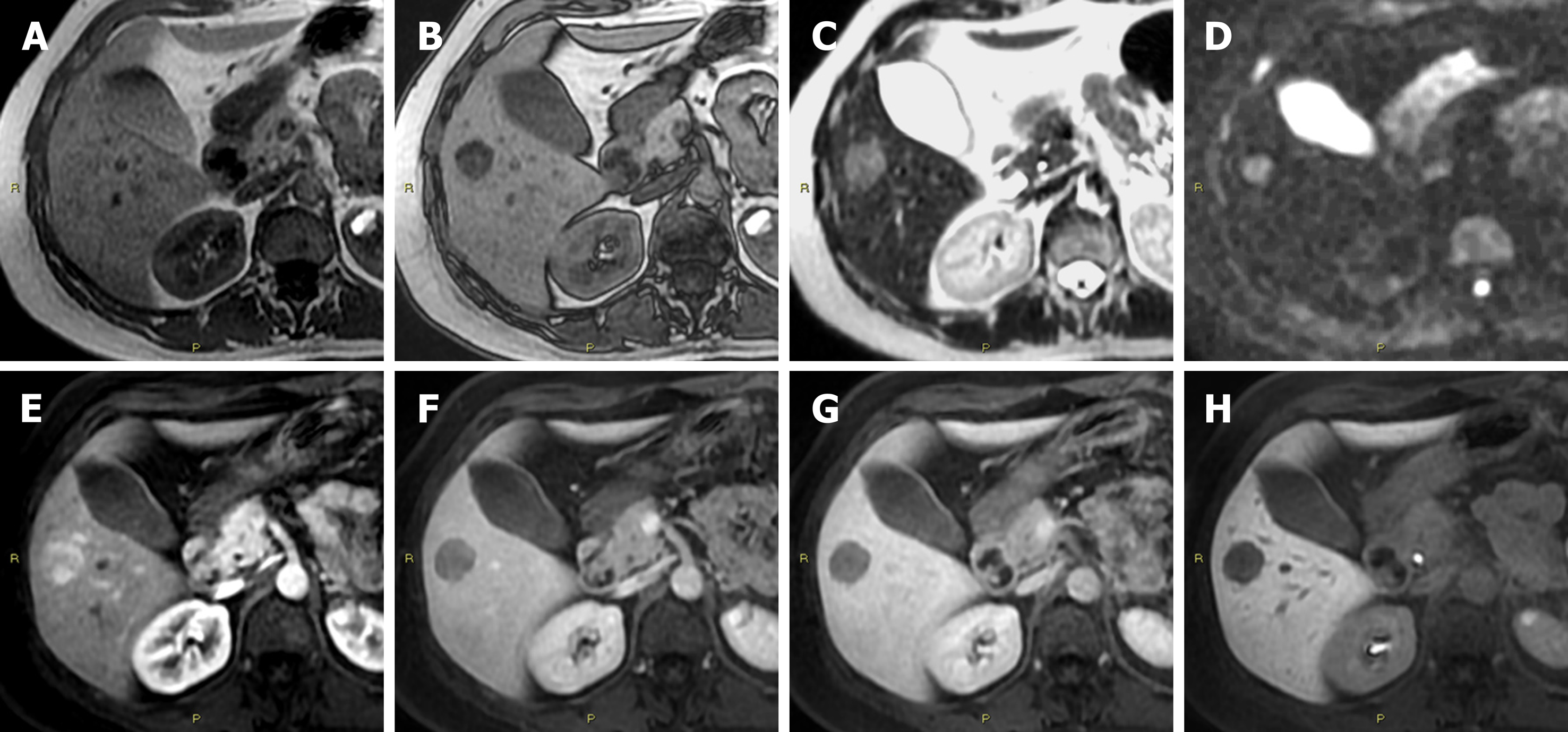
H-HCA can be identified by the presence of marked steatosis, resulting from diffuse and homogeneous signal dropout on chemical shift T1-weighted MRI sequence[16]. The percentage of this subtype of HCA is the same in the new proposed classification and, therefore, one-third of HCAs are able to be identified. However, in 2017, other French Authors from Bordeaux described an atypical form of H-HCA characterized by the absence of fatty components[18]. Moreover, the Authors declared that four other cases had been reported which were similar to their observation, either in the context of mixed liver adenomatosis or HNF1α-inactivated liver adenomatosis[18]. As such, these HCAs could be categorized as atypical or borderline between HCA and HCC; the Authors suggested additional studies for a more precise categorization[18]. Therefore, the presence of signal drop-out on T1-weighted sequences does not allow identifying all the possible forms of H-HCA (Figure 1) and the absence of signal drop-out on T1-weighted sequences does not allow excluding the H-HCA diagnosis. Finally, the diagnostic performance of this MRI sign cited in the guidelines is drastically decreased.
Furthermore, many of the same Authors who proposed the new classification of HCA[17] also published an interesting paper in the same year concerning an atypical type of HCA[19]. In this paper, they primarily endorsed the importance of the correct differentiation between the new HCA subtypes due to the different risks of evolution into HCC[19]. Furthermore, they described an interesting case in which a hepatic lesion showed both the atoll sign and the signal dropout on chemical shift imaging, resembling an H-HCA with an inflammatory component. What was it? It was a new entity, an I-HCA containing fat which introduces a new consideration; even when a fat containing hepatic lesion is detected on imaging, it could contain an inflammatory component. Therefore, two limitations in the imaging diagnosis of H-HCAs can be summarised: (1) The possibility of having an H-HCA without evident fatty components; and (2) the fatty components can be identified without excluding the simultaneous presence of inflammatory components. As a consequence, even H-HCAs which make up 34% of HCAs probably cannot be considered entirely diagnosable.
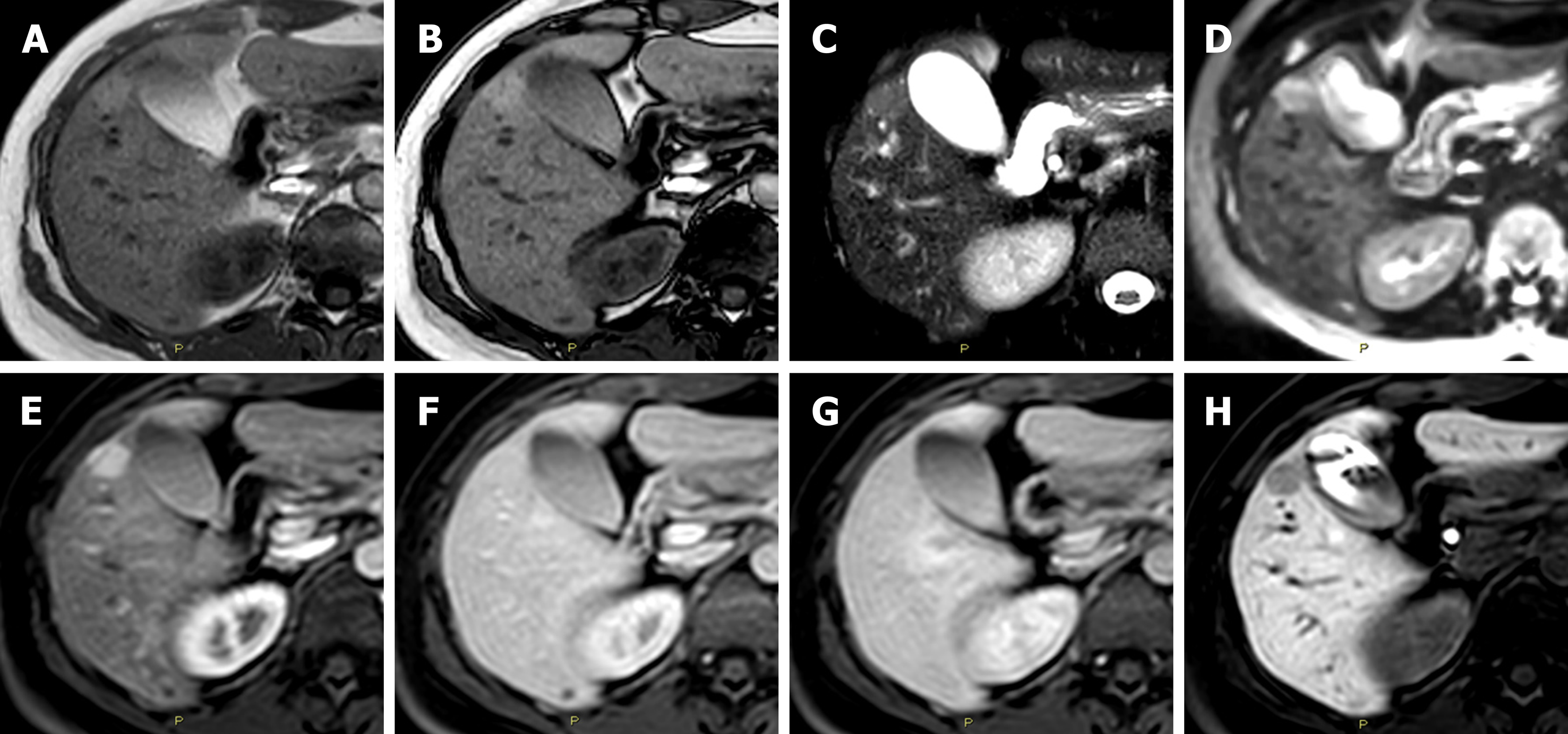
According to the current EASL Clinical Practice Guidelines, I-HCAs are recognizable on imaging by two important MRI features: Strong hyperintensity on T2-weighted images and persistent enhancement on delayed phase images using extracellular MR contrast agents[16]. Instead, as reported by the EASL Guidelines, the two subtypes of β-HCA have no characteristic features on MRI and cannot be differentiated from HCC. βI-HCA represents a mixed form of I-HCA with a β-catenin component and, therefore, this new entity was not able to be diagnosed using MRI due to the absence of specific features for the β-catenin component. Probably, these mixed lesions demonstrate the MRI pattern of I-HCA. This aspect presents a huge diagnostic problem: how is it possible to correctly diagnose an I-HCA and, therefore, to exclude the possibility that it is not a βI-HCA (Figure 2)? As a consequence, when considering the sum of the rates of these three types of HCA (I-HCA – β-HCA - βI-HCA, 54%), they probably cannot be considered diagnosable.
According to the current EASL Clinical Practice Guidelines, unclassified HCAs have no characteristic features on MRI and cannot be differentiated from HCC[16]. Consequently, sh-HCA, previously considered an unclassified form, is not diagnosable by imaging.
The guidelines commonly used in current clinical practice regarding the management of benign liver tumours, such as HCAs, were recently published in 2016 by EASL[16]. These guidelines have pointed out the superiority of MRI as compared to all other imaging modalities in diagnosing HCA and its opportunity of subtyping HCAs up to 80%. In particular, the differential diagnosis between H-HCA and I-HCA is possible using MRI, having a sensitivity of 87%-91% and a specificity of 89%-100% in diagnosing H-HCAs and a sensitivity of 85%-88% and a specificity of 88%-100% for I-HCA diagnosis. By contrast, the EASL guidelines stated that the identification of β-HCA and its differentiation from unclassified HCA and HCC is not possible by any imaging technique. As H-HCAs and I-HCAs make up a total of 95% of all HCAs, the current knowledge regarding MRI should allow diagnosing the vast majority of HCAs.
However, considering the new proposed classification of HCAs and its clinical implications[17] and, according to the considerations expressed in the previous section, none of the HCA subtypes could be correctly identifiable. Even for the same H-HCAs and I-HCAs, which could previously be diagnosed with high reliability, MRI does no longer provides the same diagnostic accuracy. Therefore, while molecular typing is evolving towards a more specific characterization of HCAs, the imaging reliability in the detection of the various molecular subtypes is not proceeding in the same direction. In fact, in 2016, it was possible to diagnose two out of the four types of HCA which together represented the vast majority of HCAs and, after only one year, it was not possible to obtain a certain diagnosis in the vast majority of HCAs according to the more recent classification.
For this reason, the imaging diagnosis of HCA has become a sticking point: what should the imaging approach for diagnosing HCA be in current clinical practice? As is well known, HCA is a rare benign lesion; however, atypical, indeterminate lesions detected by other imaging techniques such as ultrasound or computed tomography are rather frequent in clinical practice and, in the opinion of the Authors, the first step in these cases remains a correct differential diagnosis between benign and malignant liver lesions. For example, in a non-cirrhotic liver, strong enhancement on the arterial phase images and hyper- or iso-intensity in the hepatobiliary phase images of gadoxetic acid MRI is helpful in diagnosing FNH and differentiating it from other lesions such as HCA, with a sensitivity and specificity of 83.8% and 98.5%, respectively[20]. Unlike FNH, HCAs appear hypointense in the hepatobiliary phase due to the reduction in the OATP1 or similar membrane carrier expressions, such as in the de-differentiation process of regenerative nodules in a cirrhotic liver, permitting an imaging diagnosis with a sensitivity and specificity of 83.7% and 100%, respectively[11,21-24]. The combination of low signal intensity in the hepatobiliary phase with routine MRI features and risk factors of liver disease, could substantially improve the diagnosis of HCAs[24]. In addition, MRI performed with gadoxetic acid was demonstrated to be the most cost-effective strategy for differentiating FNH from HCA in patients with incidentally detected focal liver lesions in a non-cirrhotic liver[25,26].
However, in cases in which there is a diagnosis of HCA, is it possible to reach a correct diagnosis regarding the type of HCA according to the most recent classification[17]? Consequent to the previously expressed considerations, the answer is no. Therefore, what should the diagnostic approach be, in current clinical practice, for a lesion suspected of being an HCA? The Authors believe that the signal dropout on opposed T1-weighted images on MRI can be pathognomonic of H-HCA only when the entire lesion shows homogeneous steatosis. Moreover, when using this imaging features it is not possible to diagnose all H-HCA as HCAs without steatosis[18]. Therefore, a good percentage of but not all H-HCAs which globally represent only 30% of all HCAs are able to be diagnosed.
For the remaining non-diagnosable H-HCAs and for the remaining types of HCA for which there are no specific imaging features, the only diagnostic strategy currently available is a biopsy[16]. However, the same Authors of the new classification of HCAs[16] pointed out the intra-tumoural heterogeneity of HCAs. For these reasons, how many biopsy would be needed for a single lesion? Furthermore, the same Authors described the inter-tumoural heterogeneity of HCAs, characterized by the presence of different molecular subtypes within the same liver[17]. How many lesions is it necessary to biopsy in order to reach a diagnosis? Therefore, it is evident that even biopsy does not represent the solution for managing the new HCA subtypes.
Surely, the size of a presumed HCA is an important imaging feature because it is usually correlated with a high risk of haemorrhage, especially for exophytic lesions, irrespective of the molecular HCA subtype. In particular, an HCA greater than 5 cm or increasing in size (> 20% in 6 mo) should be considered for resection or curative treatment[16]. Patient gender has an importance influence on lesion management since a significantly higher incidence of malignant transformation is reported in men, regardless of the lesion size[27]. However, the EASL Guidelines have reported that “Whether the risk of haemorrhage or malignant transformation attributed to β-HCA is independent of the identified clinical risk factors (sex, size, rate of change) is presently unknown”. This is still another open question!
What is the future scenario of HCA imaging? Some Authors have recently compared the behaviour regarding diffusion-weighted images of MRI among the different HCA types. They have revealed different apparent diffusion coefficient values among the HCA types, the lowest being for β-HCA, and they therefore found that the apparent diffusion coefficient value is a potential marker of malignant transformation[28]. Obviously, future studies are needed to validate or to redefine these data permitting an imaging diagnosis for almost all HCA types with a high rate of malignant degeneration.
While awaiting novelties regarding the imaging diagnosis of the new types of HCA, it is necessary to draw up precise and newer guidelines with respect to the EASL guidelines in order to establish the correct and univocal management of the new HCA entities to be adopted into current clinical practice.
| 1. | Rooks JB, Ory HW, Ishak KG, Strauss LT, Greenspan JR, Hill AP, Tyler CW. Epidemiology of hepatocellular adenoma. The role of oral contraceptive use. JAMA. 1979;242:644-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 289] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 2. | Hussain SM, van den Bos IC, Dwarkasing RS, Kuiper JW, den Hollander J. Hepatocellular adenoma: Findings at state-of-the-art magnetic resonance imaging, ultrasound, computed tomography and pathologic analysis. Eur Radiol. 2006;16:1873-1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Bühler H, Pirovino M, Akobiantz A, Altorfer J, Weitzel M, Maranta E, Schmid M. Regression of liver cell adenoma. A follow-up study of three consecutive patients after discontinuation of oral contraceptive use. Gastroenterology. 1982;82:775-782. [PubMed] |
| 4. | Reddy KR, Schiff ER. Approach to a liver mass. Semin Liver Dis. 1993;13:423-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 43] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Shanbhogue AK, Prasad SR, Takahashi N, Vikram R, Sahani DV. Recent advances in cytogenetics and molecular biology of adult hepatocellular tumors: Implications for imaging and management. Radiology. 2011;258:673-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Steinbrecher UP, Lisbona R, Huang SN, Mishkin S. Complete regression of hepatocellular adenoma after withdrawal of oral contraceptives. Dig Dis Sci. 1981;26:1045-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 56] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Thomeer MG, E Bröker ME, de Lussanet Q, Biermann K, Dwarkasing RS, de Man R, Ijzermans JN, de Vries M. Genotype-phenotype correlations in hepatocellular adenoma: An update of MRI findings. Diagn Interv Radiol. 2014;20:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 8. | Karhunen PJ. Benign hepatic tumours and tumour like conditions in men. J Clin Pathol. 1986;39:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 218] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Cherqui D, Rahmouni A, Charlotte F, Boulahdour H, Métreau JM, Meignan M, Fagniez PL, Zafrani ES, Mathieu D, Dhumeaux D. Management of focal nodular hyperplasia and hepatocellular adenoma in young women: A series of 41 patients with clinical, radiological, and pathological correlations. Hepatology. 1995;22:1674-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 232] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 10. | van Aalten SM, de Man RA, IJzermans JN, Terkivatan T. Systematic review of haemorrhage and rupture of hepatocellular adenomas. Br J Surg. 2012;99:911-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | Grazioli L, Bondioni MP, Haradome H, Motosugi U, Tinti R, Frittoli B, Gambarini S, Donato F, Colagrande S. Hepatocellular adenoma and focal nodular hyperplasia: Value of gadoxetic acid-enhanced MR imaging in differential diagnosis. Radiology. 2012;262:520-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 12. | McInnes MD, Hibbert RM, Inácio JR, Schieda N. Focal Nodular Hyperplasia and Hepatocellular Adenoma: Accuracy of Gadoxetic Acid-enhanced MR Imaging--A Systematic Review. Radiology. 2015;277:413-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Suh CH, Kim KW, Kim GY, Shin YM, Kim PN, Park SH. The diagnostic value of Gd-EOB-DTPA-MRI for the diagnosis of focal nodular hyperplasia: A systematic review and meta-analysis. Eur Radiol. 2015;25:950-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Renzulli M, Lucidi V, Mosconi C, Quarneti C, Giampalma E, Golfieri R. Large regenerative nodules in a patient with Budd-Chiari syndrome after TIPS positioning while on the liver transplantation list diagnosed by Gd-EOB-DTPA MRI. Hepatobiliary Pancreat Dis Int. 2011;10:439-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Cherqui D, Mathieu D, Zafrani ES, Dhumeaux D. [Focal nodular hyperplasia and hepatocellular adenoma in women. Current data]. Gastroenterol Clin Biol. 1997;21:929-935. [PubMed] |
| 16. | European Association for the Study of the Liver (EASL). EASL Clinical Practice Guidelines on the management of benign liver tumours. J Hepatol. 2016;65:386-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 355] [Article Influence: 35.5] [Reference Citation Analysis (2)] |
| 17. | Nault JC, Couchy G, Balabaud C, Morcrette G, Caruso S, Blanc JF, Bacq Y, Calderaro J, Paradis V, Ramos J, Scoazec JY, Gnemmi V, Sturm N, Guettier C, Fabre M, Savier E, Chiche L, Labrune P, Selves J, Wendum D, Pilati C, Laurent A, De Muret A, Le Bail B, Rebouissou S, Imbeaud S; GENTHEP Investigators, Bioulac-Sage P, Letouzé E, Zucman-Rossi J. Molecular Classification of Hepatocellular Adenoma Associates With Risk Factors, Bleeding, and Malignant Transformation. Gastroenterology. 2017;152:880-894.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 287] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 18. | Mohkam K, Darnis B, Cazauran JB, Rode A, Manichon AF, Ducerf C, Bancel B, Mabrut JY. Polymorphic multiple hepatocellular adenoma including a non-steatotic HNF1α-inactivated variant. Hepatobiliary Pancreat Dis Int. 2017;16:552-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 19. | Nault JC, Paradis V, Cherqui D, Vilgrain V, Zucman-Rossi J. Molecular classification of hepatocellular adenoma in clinical practice. J Hepatol. 2017;67:1074-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 20. | Ba-Ssalamah A, Antunes C, Feier D, Bastati N, Hodge JC, Stift J, Cipriano MA, Wrba F, Trauner M, Herold CJ, Caseiro-Alves F. Morphologic and Molecular Features of Hepatocellular Adenoma with Gadoxetic Acid-enhanced MR Imaging. Radiology. 2015;277:104-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 21. | Goodwin MD, Dobson JE, Sirlin CB, Lim BG, Stella DL. Diagnostic challenges and pitfalls in MR imaging with hepatocyte-specific contrast agents. Radiographics. 2011;31:1547-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Vasuri F, Golfieri R, Fiorentino M, Capizzi E, Renzulli M, Pinna AD, Grigioni WF, D'Errico-Grigioni A. OATP 1B1/1B3 expression in hepatocellular carcinomas treated with orthotopic liver transplantation. Virchows Arch. 2011;459:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Golfieri R, Garzillo G, Ascanio S, Renzulli M. Focal lesions in the cirrhotic liver: Their pivotal role in gadoxetic acid-enhanced MRI and recognition by the Western guidelines. Dig Dis. 2014;32:696-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Guo Y, Li W, Xie Z, Zhang Y, Fang Y, Cai W, Hong G. Diagnostic Value of Gd-EOB-DTPA-MRI for Hepatocellular Adenoma: A Meta-Analysis. J Cancer. 2017;8:1301-1310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Suh CH, Kim KW, Park SH, Shin S, Ahn J, Pyo J, Shinagare AB, Krajewski KM, Ramaiya NH. A cost-effectiveness analysis of the diagnostic strategies for differentiating focal nodular hyperplasia from hepatocellular adenoma. Eur Radiol. 2018;28:214-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Guo Y, Li W, Cai W, Zhang Y, Fang Y, Hong G. Diagnostic Value of Gadoxetic Acid-Enhanced MR Imaging to Distinguish HCA and Its Subtype from FNH: A Systematic Review. Int J Med Sci. 2017;14:668-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Farges O, Ferreira N, Dokmak S, Belghiti J, Bedossa P, Paradis V. Changing trends in malignant transformation of hepatocellular adenoma. Gut. 2011;60:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 205] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 28. | Wang H, Yang C, Rao S, Ji Y, Han J, Sheng R, Zeng M. MR imaging of hepatocellular adenomas on genotype-phenotype classification: A report from China. Eur J Radiol. 2018;100:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P-Reviewer: Farshadpour F, Gencdal G S-Editor: Yan JP L-Editor: A E-Editor: Zhang YL