Published online Jan 14, 2019. doi: 10.3748/wjg.v25.i2.269
Peer-review started: October 16, 2018
First decision: November 22, 2018
Revised: December 3, 2018
Accepted: December 13, 2018
Article in press: December 13, 2018
Published online: January 14, 2019
Processing time: 91 Days and 7.1 Hours
Visceral hypersensitivity is considered to play a vital role in the pathogenesis of irritable bowel syndrome (IBS). Neurotrophins have drawn much attention in IBS recently. Brain-derived neurotrophic factor (BDNF) was found to mediate visceral hypersensitivity via facilitating sensory nerve growth in pre-clinical studies. We hypothesized that BDNF might play a role in the pathogenesis of diarrhea-predominant IBS (IBS-D).
To investigate BDNF levels in IBS-D patients and its role in IBS-D pathophysiology.
Thirty-one IBS-D patients meeting the Rome IV diagnostic criteria and 20 age- and sex-matched healthy controls were recruited. Clinical and psychological assessments were first conducted using standardized questionnaires. Visceral sensitivity to rectal distension was tested using a high-resolution manometry system. Colonoscopic examination was performed and four mucosal pinch biopsies were taken from the rectosigmoid junction. Mucosal BDNF expression and nerve fiber density were analyzed using immunohistochemistry. Mucosal BDNF mRNA levels were quantified by quantitative real-time polymerase chain reaction. Correlations between these parameters were examined.
The patients had a higher anxiety score [median (interquartile range), 6.0 (2.0-10.0) vs 3.0 (1.0-4.0), P = 0.003] and visceral sensitivity index score [54.0 (44.0-61.0) vs 21.0 (17.3-30.0), P < 0.001] than controls. The defecating sensation threshold [60.0 (44.0-80.0) vs 80.0 (61.0-100.0), P = 0.009], maximum tolerable threshold [103.0 (90.0-128.0) vs 182.0 (142.5-209.3), P < 0.001] and rectoanal inhibitory reflex threshold [30.0 (20.0-30.0) vs 30.0 (30.0-47.5), P = 0.032] were significantly lower in IBS-D patients. Intestinal mucosal BDNF protein [3.46E-2 (3.06E-2-4.44E-2) vs 3.07E-2 (2.91E-2-3.48E-2), P = 0.031] and mRNA [1.57 (1.31-2.61) vs 1.09 (0.74-1.42), P = 0.001] expression and nerve fiber density [4.12E-2 (3.07E-2-7.46E-2) vs 1.98E-2 (1.21E-2-4.25E-2), P = 0.002] were significantly elevated in the patients. Increased BDNF expression was positively correlated with abdominal pain and disease severity and negatively correlated with visceral sensitivity parameters.
Elevated mucosal BDNF may participate in the pathogenesis of IBS-D via facilitating mucosal nerve growth and increasing visceral sensitivity.
Core tip: This study comprehensively investigated the psychological and clinical characteristics and visceral sensitivity in patients with diarrhea-predominant irritable bowel syndrome (IBS-D) and further explored the levels of brain-derived neurotrophic factor (BDNF) protein and mRNA and nerve fiber density in the intestinal mucosa. It was demonstrated that increased mucosal BDNF expression was positively correlated with abdominal pain and disease severity and negatively correlated with visceral sensitivity parameters, which provides new evidence that BDNF may participate in the pathogenesis of IBS-D via facilitating mucosal nerve growth and increasing visceral sensitivity in IBS-D patients.
- Citation: Zhang Y, Qin G, Liu DR, Wang Y, Yao SK. Increased expression of brain-derived neurotrophic factor is correlated with visceral hypersensitivity in patients with diarrhea-predominant irritable bowel syndrome. World J Gastroenterol 2019; 25(2): 269-281
- URL: https://www.wjgnet.com/1007-9327/full/v25/i2/269.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i2.269
Irritable bowel syndrome (IBS) is a common functional bowel disorder, characterized by recurrent abdominal pain related to defecation and a change in bowel habits[1]. The mean prevalence of IBS among individual countries around the world ranges from 1.1% to 35.5%[2]. As a major subtype, diarrhea-predominant IBS (IBS-D) accounts for 39.0%-61.9% of all IBS cases[3,4].
The pathogenesis of IBS-D remains poorly understood, and as a result, there has not been an ideal approach to manage it. Early life stressors, food intolerance, enteric infection, altered brain-gut interaction, dysbiosis, increased intestinal permeability, increased gut mucosal immune activation and visceral hypersensitivity may play a role in the pathogenesis of IBS-D[5]. In recent years, neurotrophins have drawn much attention in IBS[6-8]. Brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family, affects the development and regeneration of the nervous system and has been known for its roles in many chronic pain conditions[9].
In animal studies, intraperitoneal injection of BDNF induced a significant decrease in the colonic reaction threshold[10,11]. Nerve growth is dependent upon neurotrophins including BDNF during development[12]. Based on the above findings, it could be hypothesized that BDNF may play a role in the pathogenesis of IBS-D.
There have been few studies reporting increased BDNF levels in IBS patients[8,10]. Nevertheless, the relationship between elevated BDNF levels and IBS symptom severity, psychological conditions, visceral sensitivity as well as mucosal nerve fiber density has not been clearly defined in IBS-D patients, which needs further investigation.
Therefore, the aim of this study was to measure intestinal BDNF levels in patients with IBS-D and to explore the association between mucosal BDNF expression and clinical features and experimental parameters above.
A total of 31 IBS-D patients and 20 age- and sex-matched healthy controls were recruited in the study. All patients were treated at the gastroenterological department of China-Japan Friendship Hospital from July 2016 to October 2017. Diagnosis was made according to Rome IV criteria. Controls were selected through public advertisement or from asymptomatic patients who underwent colonoscopy for colorectal cancer screening or polyposis follow-up receiving negative results. Subjects were excluded if they had organic diseases such as celiac disease, allergic diseases or psychiatric disorders screened by history-taking, physical examination and essential laboratory and imaging examinations, or if they had undergone major abdominal surgery. Female participants who were pregnant or lactating and those with dysmenorrhea or other painful gynecological disorders such as endometriosis were also excluded. Subjects were not allowed to receive antispasmodics, analgesics, prokinetics, antacids, antibiotics, nonsteroidal anti-inflammatory drugs, mast cell stabilizers, histamine antagonists or antidepressants within 2 wk, or corticosteroids and immunosuppressants within 6 mo.
All subjects were informed of all the details of the study and gave written informed consent before participation. The study was approved by the Ethics Committee of China-Japan Friendship Hospital (No. 2015-33) and was conducted in accordance with the Declaration of Helsinki.
A total of 62 patients with IBS-D symptoms were referred to the gastroenterological department. Fourteen refused to participate and 10 were excluded in the next series of screenings (1 for severe mental disorder, 2 for colon mucosal inflammation, 4 for a history of abdominal surgery, 1 for lactose intolerance and 2 for poor compliance). The remaining 38 participants underwent colonoscopy and 7 refused to undergo biopsy. As a result, 31 patients and 20 controls were included in this study.
After enrollment, the clinical and psychological states were first assessed using validated questionnaires. Then, visceral sensitivity was assessed. The next day, subjects underwent colonoscopy after a standard bowel preparation with polyethylene glycol electrolytes power (Fortrans, BEAUFOUR IPSEN Industrie, Dreux, France), and four mucosal pinch biopsies were taken from the rectosigmoid junction. Two specimens were immediately fixed in 10% formalin for at least 72 h, embedded in paraffin and sectioned (4 μm) for routine haematoxylin and eosin staining and immunohistochemistry. Two specimens were immediately immersed in storage reagent (RNA-Be-Locker A; Sangon, Shanghai, China) and stored at -80 °C for quantitative real-time polymerase chain reaction (qRT-PCR).
Questionnaires: Disease severity was assessed using the IBS Symptom Severity Scale (IBS-SSS)[13], including five items (severity and frequency of abdominal pain, abdominal bloating, bowel habit dissatisfaction and overall interference with quality of life). Scores for each item were summed to obtain a total score ranging from 0 to 500 and were then classified as mild (75-174), moderate (175-299) or severe (300-500).
The severity of abdominal pain during the prior 2 wk was measured using a validated questionnaire and was graded 0-4 according to its impact on usual activities: 0, absent; 1, mild (not influencing usual activities); 2, relevant (diverting from, but not urging modification of, usual activities); 3, severe (influencing usual activities markedly enough to urge modifications); and 4, extremely severe (precluding daily activities)[14].
The visceral sensitivity index (VSI), consisting of 15 items, was used to measure visceral sensitivity and gastrointestinal-specific anxiety[15]. The VSI was “reverse scored” as described in a previous study[15] with each item’s score ranging from 0 (no gastrointestinal-specific anxiety or visceral hypersensitivity) to 5 (severe gastrointestinal-specific anxiety and visceral hypersensitivity).
Psychological states were assessed using the validated Hospital Anxiety and Depression Scale (HADS) consisting of 14 items (7 anxiety and 7 depression items), with each score ranging from 0 to 3[16].
Visceral sensitivity test: The visceral sensitivity test was performed by the same investigator at the Gastroenterology Kinetic Laboratory during the same time period (13:00-16:00). A Manoscan 360™ high-resolution manometry system (Model A100) (Sierra Scientific Instruments, Los Angeles, CA, United States), ManoScan™ high-resolution anorectal catheter (ANN1522) (Given Imaging, Los Angeles, CA, United States) and Manoview™ AR Analysis Software 2.1 (Sierra Scientific Instruments, Los Angeles, CA, United States) were used. First, all subjects were prepared with a glycerin enema and placed in the left lateral decubitus position. Then, the catheter with a latex balloon at the tip was inserted into the rectum following a digital rectal examination. Three minutes were given for the subjects to adapt to the catheter. Next, the balloon was manually inflated with 10 mL of air within 2 s and was deflated within 3-5 s. Twenty, 30, 40 and 50 mL of air were inflated and deflated as mentioned above, sequentially. The operation interval was 30 s. If the internal anal sphincter relaxed (a reduction of internal anal sphincter pressure from baseline of at least 10 mmHg with duration of 5 s) when the balloon was inflated, the rectoanal inhibitory reflex (RAIR) was present[17,18]. The lowest balloon volume evoking RAIR was RAIR threshold. Then, the balloon was manually inflated with air at a speed of 10 mL/5 s. During inflation, subjects were asked to report their feelings of initial perception, defecation urge and discomfort/pain that could not be tolerated. The balloon volumes were recorded as the first sensation threshold, defecating sensation threshold and maximum tolerable threshold, respectively.
Histology and immunohistochemistry: Paraffin sections were processed for haematoxylin and eosin staining and immunohistochemistry. For the latter, following deparaffinization, antigen repairing, endogenous peroxidase inhibition and nonspecific antigen blocking, the sections were incubated with primary antibodies [rabbit monoclonal anti-BDNF antibody, 1:100; mouse monoclonal anti-protein gene product (PGP) 9.5, 1:2000; Abcam, Cambridge, United Kingdom] overnight at 4 °C. Following thorough washing with PBS, slides were incubated at room temperature for 1 h with horseradish-peroxidase-conjugated anti-mouse-rabbit secondary antibody (1/200, Zhongshan Gold Bridge, Beijing, China) and then visualized using diaminobenzidine. Finally, slides were counterstained with hematoxylin and viewed under a light microscope.
For each section, five nonoverlapping fields at 400 × magnification were randomly selected and scanned under a Nikon Eclipse 80i microscope (Nikon Instruments Co., Ltd., Shanghai, China). Photographs were taken with a Nikon DS-Ri1 camera and then exported via the Nikon NIS-Elements imaging software. Images were analyzed with Image-Pro Plus 6.0 software (Media Cybernetics, Bethesda, MD, United States). PGP 9.5-immunoreactive area per square millimeter of mucosa was used to measure the mucosal nerve fiber density and the mean optical density (MOD) of mucosal staining area was used for measuring the expression of BDNF. All sections were inspected independently by two blinded observers, and the mean values of the readings were used for final analysis.
BDNF gene expression analysis by qRT-PCR: Gene expression of BDNF in the colonic mucosa was analyzed using qRT-PCR. Total RNA was isolated with TRIzol Reagent (Invitrogen Life Technologies, Waltham, Massachusetts, United States) according to the manufacturer’s instruction. Then, 2 μg of RNA was used to perform reverse transcription with the RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, MA, United States). Next, real-time PCR was performed in the StepOnePlus Real Time PCR System (Applied Biosystems, Waltham, MA, United States) using the FastStart Universal SYBR Green Master Rox kit (Roche, Shanghai, China). Finally, BDNF mRNA expression was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression. The following primers were used for PCR amplification: forward, GGAAGCTTGTCATCAATGGAAATC and reverse, TGATGACCCTTTTGGCTCCC for GAPDH; forward, CTTGACATCATTGGCTGACACTT and reverse, GACTACTGAGCATCACCCTGGAC for BDNF. All samples were amplified in triplicate using the following conditions: 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and then 60 °C for 60 se. The specificity of PCR products was verified by dissociation curve analysis. The results were first normalized to GAPDH expression using the 2-∆∆Ct method[19]. BDNF mRNA level of each sample is expressed as a fold-change relative to the average level of healthy controls.
Statistical analyses were performed using SPSS software, version 24.0 (SPSS Inc, Chicago, IL, United States). Continuous data were first tested for normal distribution using the Kolmogorov-Smirnov test and are presented as mean ± standard deviation (SD) if normally distributed, or median [interquartile range (IQR)] if not. Comparisons between groups were performed using independent samples t-test for normally distributed data with homogeneous variances, or nonparametric Mann-Whitney U-test, otherwise. The χ2 test or Fisher’s exact test was used to analyze qualitative data. Correlations between BDNF and other parameters were explored using Pearson’s correlation analysis for normally distributed data, or Spearman’s correlation analysis for nonnormally distributed data or ranked data. P-values were two-sided and differences were considered significant at P < 0.05. In correlation analysis, multiple comparisons were adjusted using Bonferroni correction, and the corrected significance level was 0.0071 (0.05/7) in the patients and 0.01 (0.05/5) in all subjects according to the number of correlations analyzed. Statistical charts were generated with GraphPad Prism 5.0 software (GraphPad Software Inc, La Jolla, CA, United States).
The demographics, clinical characteristics and psychological assessments of IBS-D patients and controls are presented in Table 1. There were 31 IBS-D patients (22 males and 9 females; median age 33.0 years, IQR: 29.0-42.0) and 20 healthy controls (13 males and 7 females; media age 28.0 years, IQR: 26.0-36.0) participating in this study. There were no statistically significant differences in terms of age, gender, or body mass index between the groups. The average IBS-SSS score was 274.0 (SD = 29.8) and the median duration of disease was 5.0 years (IQR: 2.0-10.0) in the IBS-D group. The median pain intensity for IBS-D patients was mild. Scores of VSI (IBS-D: median 54.0, IQR: 44.0-61.0; control: 21.0, 17.3-30.0, P < 0.01) and anxiety (IBS-D: median 6.0, IQR: 2.0-10.0; control: 3.0, 1.0-4.0, P = 0.003) were significantly elevated in IBS-D patients. The depression score tended to increase in the IBS-D group but the difference failed to reach the significant level (P = 0.055).
| Feature | IBS-D patients | Controls | P-value |
| N | 31 | 20 | NA |
| Age (yr) | 33.0 (29.0-42.0) | 28.0 (26.0-36.0) | 0.056 |
| Gender (male:female) | 22:09 | 13:07 | 0.654 |
| Body mass index (kg/m2) | 24.2 ± 4.3 | 22.2 ± 2.6 | 0.095 |
| Duration of disease (yr) | 5.0 (2.0-10.0) | NA | NA |
| IBS-SSS | 274.2 ± 29.8 | NA | NA |
| Severity of abdominal pain | 1.0 (1.0-2.0) | NA | NA |
| VSI score | 54.0 (44.0-61.0)a | 21.0 (17.3-30.0) | < 0.001 |
| HADS anxiety score | 6.0 (2.0-10.0)a | 3.0 (1.0-4.0) | 0.003 |
| HADS depression score | 4.0 (1.0-8.0) | 2.5 (1.3-4.0) | 0.055 |
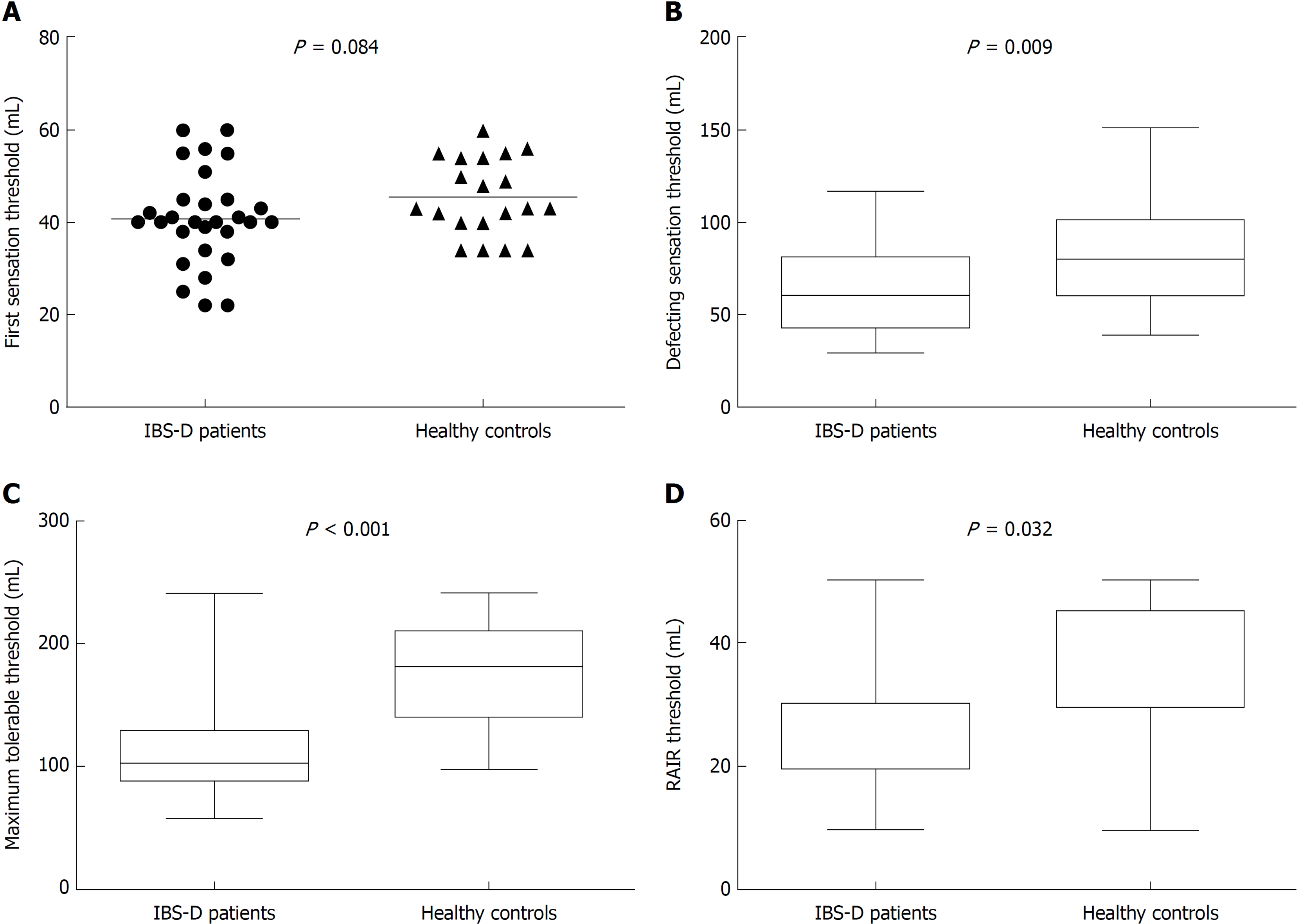
We detected the four parameters for assessing visceral sensitivity (the first sensation threshold, defecating sensation threshold, maximum tolerable threshold and RAIR threshold) (Figure 1A-D). The first sensation threshold had the tendency to decrease in patients, but failed to reach the level of significance (P = 0.084). The defecating sensation threshold was significantly lower in patients than in controls [60.0 mL (44.0-80.0) vs 80.0 (61.0-100.0) mL, P = 0.009]. The maximum tolerance threshold in IBS-D patients was 103.0 (90.0-128.0) mL, significantly lower than the 182.0 (142.5-209.3) mL in controls (P < 0.001). The RAIR threshold in IBS-D patients was also significantly lower [30.0 (20.0-30.0) mL] compared with that of controls [30.0 (30.0-47.5) mL] (P = 0.032).
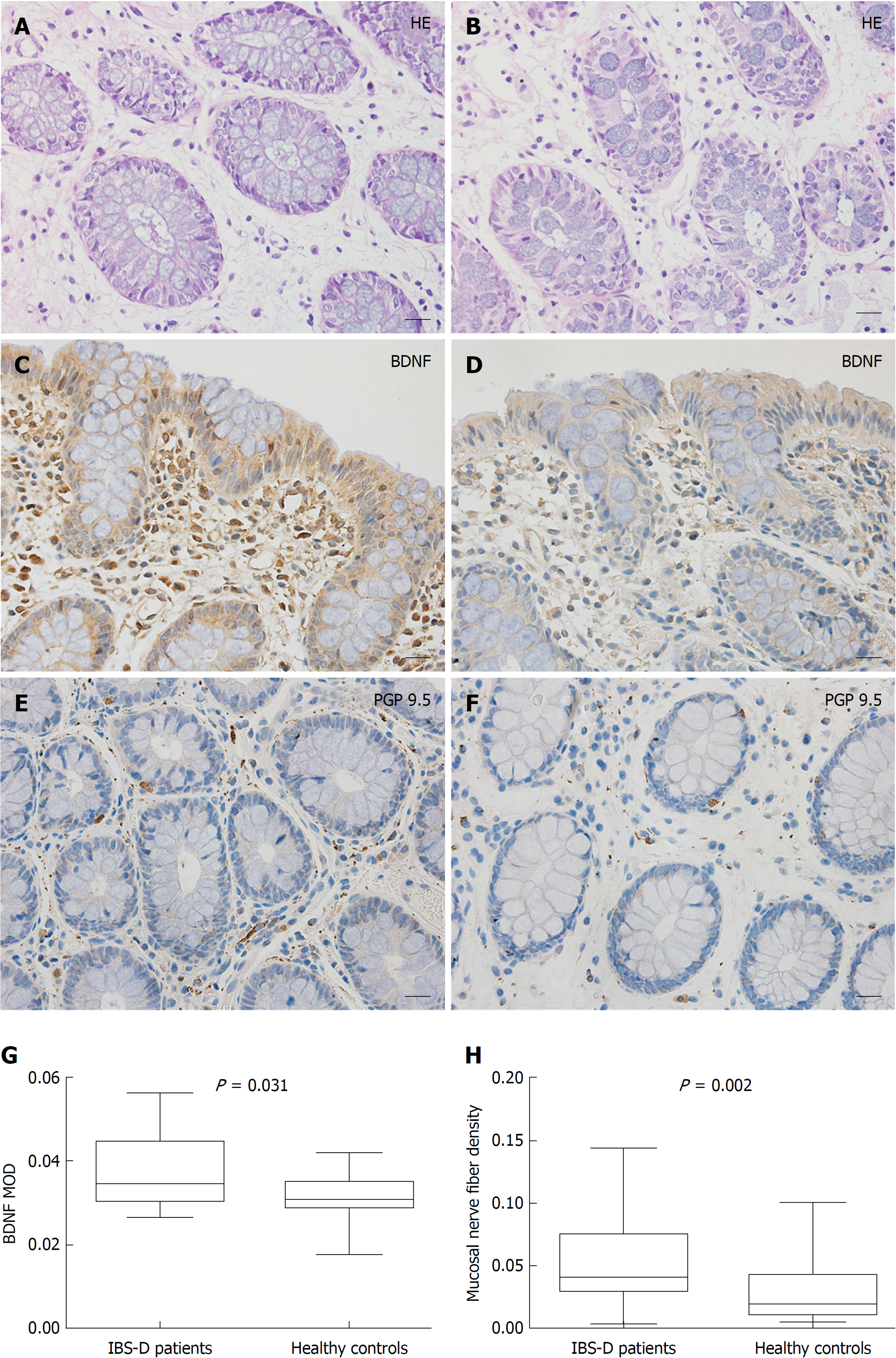
Hematoxylin and eosin staining of all colonic mucosa specimens (×400 magnification) exhibited normal histology and morphology, without evidence of inflammatory cell infiltration, cellular edema or parasites (Figure 2A and B). Representative photomicrographs of the immunoreactivity of BDNF and PGP 9.5 in the mucosa of IBS-D patients and healthy controls are shown in Figure 2C-F (×400 magnification). The level of BDNF in mucosal biopsies was significantly higher in IBS-D patients than in controls [median (IQR), 3.46E-2, (3.06E-2-4.44E-2) vs 3.07E-2 (2.91E-2-3.48E-2), P = 0.031] (Figure 2G). PGP 9.5-immunoreactive area per square millimeter of mucosa in IBS-D patients was also greater than in control subjects [4.12E-2 (3.07E-2-7.46E-2) vs 1.98E-2 (1.21E-2-4.25E-2), P = 0.002] (Figure 2H).
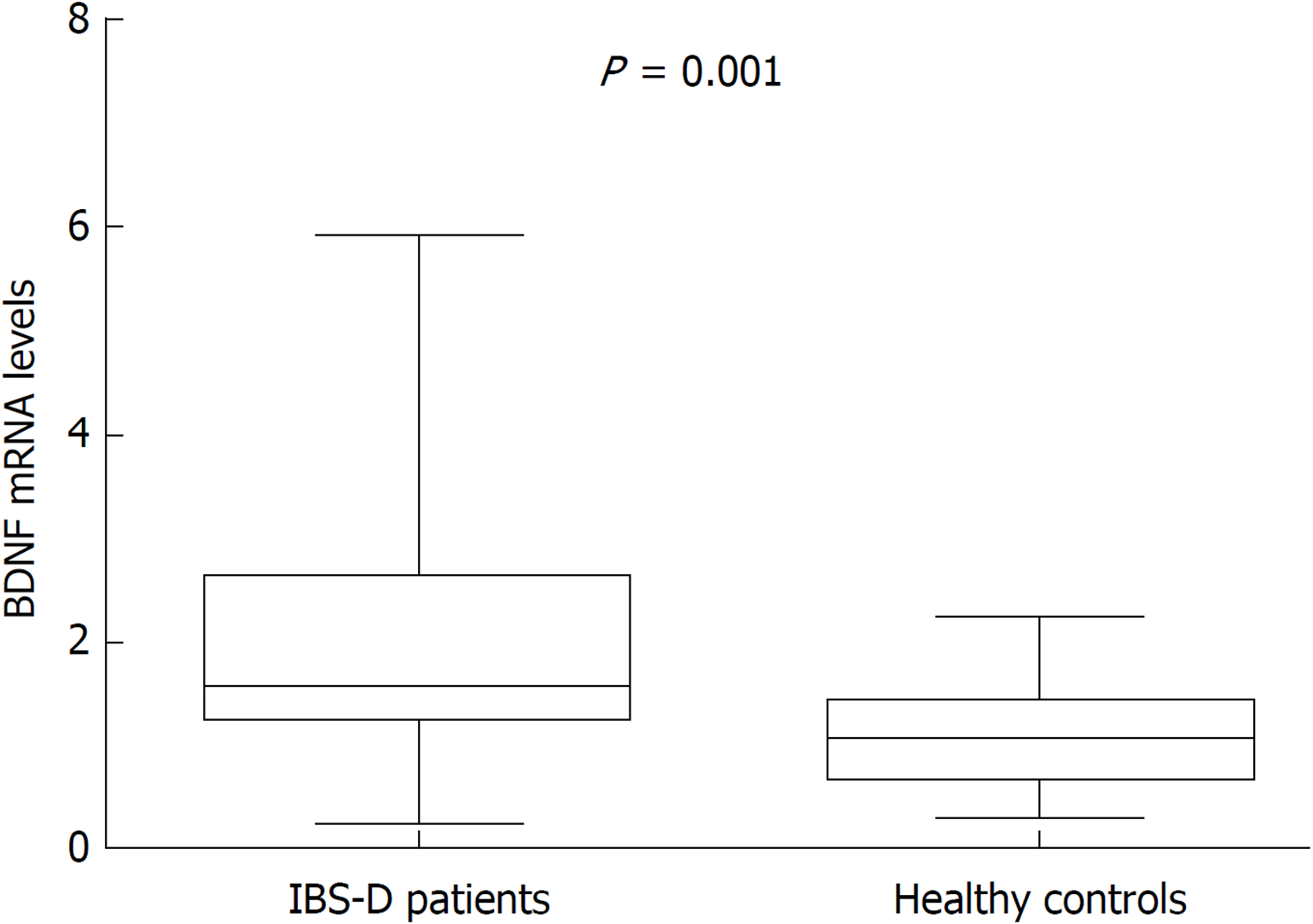
BDNF mRNA expression in colonic mucosa specimens was quantified by qRT-PCR, and the mean value in healthy controls was used as a reference, set at 1. The relative BDNF mRNA level was significantly higher in IBS-D patients (median = 1.57; IQR: 1.31-2.61) than in controls (median = 1.09; IQR: 0.74-1.42; P = 0.001) (Figure 3).
In IBS-D patients, BDNF protein expression was positively correlated with severity of abdominal pain and IBS-SSS score, and negatively correlated with the first sensation threshold and RAIR threshold (adjusted P < 0.0071). However, the association between BDNF protein expression and IBS-SSS score lost significance after Bonferroni correction (Table 2). In all subjects, a significantly negative correlation was found between mucosal BDNF levels and the first sensation threshold, maximum tolerable threshold and RAIR threshold (adjusted P < 0.01) (Table 3). We found no correlations between BDNF levels and other characteristics (P > 0.05).
| BDNF expression | |
| Clinical characteristic | |
| Severity of abdominal pain | 0.565 (0.001)a |
| IBS-SSS | 0.393 (0.029)b |
| Visceral sensitivity | |
| First sensation threshold | -0.562 (0.001)a |
| Defecating sensation threshold | -0.015 (0.938) |
| Maximum tolerable threshold | -0.290 (0.113) |
| RAIR threshold | -0.508 (0.004)a |
| Mucosal nerve fiber density | -0.058 (0.756) |
This study comprehensively investigated the psychological and clinical characteristics and visceral sensitivity in IBS-D patients and further explored the possible roles of BDNF in the pathogenesis of IBS-D. Levels of both BDNF protein and mRNA were significantly higher in the intestinal mucosa of IBS-D patients. The elevated BDNF levels were significantly correlated with pain severity, IBS-SSS score and visceral sensitivity. Also, BDNF may have an effect on the plasticity of intestinal sensory neurons in IBS-D patients.
For demographics, a relatively higher proportion of male patients (gender ratio, 22:9) were recruited in this study. Compared with males, females were more predisposed to exhibit the constipation-predominant subtype, while IBS-D subtype was less common in women[20,21]. What’s more, we excluded women with dysmenorrhea and other painful gynecological disorders, women who were pregnant or lactating and women with a history of cesarean section. Furthermore, males often had more trust in this study than females. In contrast to males, more females refused to participate in this study.
Psychological disorders have been found in IBS patients previously and are considered to be implicated in the pathogenesis of IBS[22-25]. Accordingly, in the present study, we also found that anxiety score measured on HADS questionnaire was higher in IBS-D patients than in controls. The VSI score, which is commonly used to measure gastrointestinal symptom-specific anxiety, was significantly higher in IBS-D patients (P < 0.001). However, the difference of depression score between the two groups was not significant, which implied that anxiety was a more prominent problem in IBS-D patients compared with depression. As a result, psychological health, especially anxiety, should be paid more attention during the management of IBS-D.
Visceral hypersensitivity is thought to play a vital role in the pathophysiology of IBS and could account for IBS symptoms, especially abdominal pain[26-28]. A few authors suggested that this could be considered as a biological marker of IBS[18]. Previous studies reported enhanced visceral sensitivity in IBS patients[7,29-31]. In agreement with these studies, we detected that defecating sensation threshold and maximum tolerable threshold were significantly lower in IBS-D patients than in controls. Furthermore, the lowest volume required to evoke RAIR was significantly lower in the IBS-D group, suggesting the sensitization of rectal sensation and the reflex responses. Nevertheless, there were some limitations in the methods of measuring visceral sensitivity. First, the first sensation threshold, defecating sensation threshold and maximum tolerable threshold were determined on the basis of the subjects’ subjective feelings, which may have been influenced by the subjects’ personal characteristics, including emotion, cognition and attention[32]. By contrast, the lowest balloon volume evoking RAIR may be more objective. Second, compared with measuring with a barostat, the balloon was inflated with a syringe manually. Volume parameters, rather than both pressure parameters and volume parameters measured by a barostat, may not fully reflect the biomechanical properties of the rectum[33]. Despite these limitations, volume thresholds have been used as a valid assessment of visceral sensitivity in several studies[34-36]. The mechanisms may involve various levels of the nervous system, including the sensitization of the primary visceral afferent fibers at the end-organ level, hyperexcitability of dorsal horn neurons and increased perception of the intestinal stimuli in the brain[37,38]. Besides, neuromediators (such as substance P, calcitonin gene-related peptide and serotonin), mucosal inflammation and the intestinal microbiota also have effects on visceral hypersensitivity[37,39].
Similar to other neurotrophins, BDNF was originally known for its role in neuronal growth and survival[9,12] and thought to be involved in many chronic pain conditions including chronic pancreatitis and colitis[40,41]. Intestinal epithelial cells were reported to be the source of BDNF in pigs, mice and humans[8,42-44]. In accordance with these studies, we also found that BDNF immunoreactions were mainly located in epithelial cells and the lamina propria. In the present study, the expression of both the protein and mRNA of BDNF was significantly higher in the intestinal mucosa of IBS-D patients, consistent with the findings of another study[8].
Animal studies demonstrated that BDNF facilitated the growth of mucosal nerve fibers and visceral hypersensitivity[11]. Based on these findings, we sought to clarify whether there were similar mechanisms in IBS-D patients. The plexuses of the enteric nervous system are located in the muscle, the submucosa and the lamina propria of the mucosa of humans[45]. As mucosal pinch biopsy contains mucosa and part of submucosa, we only examined the nerve fibers in the lamina propria of the colonic mucosa. Mucosal nerve fiber density as shown by PGP 9.5 was significantly higher in IBS-D patients than in controls. The intestinal nerve fiber hypertrophy coupled with increased expression of neurotrophins also existed in patients with inflammatory diseases[46]. In animals, BDNF+/- mice had significantly lower colonic mucosal nerve fiber density than BDNF+/+ mice[8]. Based on these findings, the increased mucosal nerve fiber density of IBS-D patients in our study was possibly attributed to the upregulation of BDNF.
To clarify the role of BDNF in the pathogenesis of IBS-D, we conducted correlation analysis between BDNF expression and relevant factors (abdominal pain severity, IBS-SSS score, visceral sensitivity parameters and mucosal nerve fiber density). In IBS-D patients, both abdominal pain severity and IBS-SSS scores had positive correlations with BDNF levels, although the significance of the correlation between IBS-SSS scores and BDNF expression was lost after Bonferroni correction. Notably, the correlation coefficient for BDNF and IBS-SSS score was lower than that for BDNF and abdominal pain severity, possibly because the IBS-SSS score could be influenced by the other four factors in addition to abdominal pain. With respect to visceral sensitivity, the first sensation threshold, maximum tolerable threshold and RAIR threshold were negatively correlated with BDNF expression in IBS-D patients or in all subjects even after Bonferroni correction, suggesting that BDNF may play a role in visceral hypersensitivity in IBS-D patients. Nevertheless, significant correlations should be interpreted carefully because correlations do not constitute causal associations.
There were several limitations in this study. First, among the four subtypes of IBS, only IBS-D patients were recruited and the findings may not be generalized to other IBS subtypes. Second, due to limited time and conditions, the sample size was relatively small, possibly impacting the reliability of the conclusion. Third, we did not further distinguish between postinfectious IBS and non-postinfectious IBS, considering the small sample size. Nevertheless, these two subgroups may have different pathogeneses and need to be separately studied in the future. Finally, we cannot draw causal inferences in this cross-sectional study, and conclusions need to be further verified by well-designed large-sample clinical studies.
In conclusion, our study provides new evidence that BDNF may participate in the pathogenesis of IBS-D via facilitating mucosal nerve growth and increasing visceral sensitivity. These preliminary findings may provide clues to understanding the complicated IBS-D pathophysiology and developing more effective therapeutic methods.
Visceral hypersensitivity was considered to be involved in the pathogenesis of irritable bowel syndrome (IBS). Pre-clinical studies have shown that brain-derived neurotrophic factor (BDNF) mediates visceral hypersensitivity via facilitating sensory nerve growth. There have been few studies reporting the intestinal BDNF levels in diarrhea-predominant IBS (IBS-D) patients. And the relationship between BDNF and IBS symptom severity, psychological conditions, visceral sensitivity as well as mucosal nerve fiber density has not been investigated in IBS-D. It could be hypothesized that BDNF may play a role in the pathogenesis of diarrhea-predominant IBS-D.
The main topics of this study included assessing clinical and psychological features and visceral sensitivity, examining BDNF expression and nerve fibres in IBS-D and healthy controls, performing correlation analyses between these parameters, and clarifying whether there were similar mechanisms to that of pre-clinical studies in IBS-D patients. The findings proposed a mechanism by which BDNF may participate in the pathophysiology of IBS-D and may provide new clues for the management of IBS-D.
The present study aimed to measure BDNF expression in the intestinal mucosa of IBS-D patients and to analyze the relationship of BDNF with the clinical and psychological features, visceral sensitivity and nerve fiber density in these patients.
Participants were evaluated for clinical and psychological characteristics using validated questionnaires [IBS Symptom Severity Scale (IBS-SSS), visceral sensitivity index, Hospital Anxiety and Depression Scale (HADS) anxiety Scale and HADS depression Scale]. They also underwent colonoscopy and mucosal biopsy. Visceral sensitivity was tested with a Manoscan 360™ high-resolution manometry system. Mucosal BDNF expression and localization were measured by immunohistochemistry. Mucosal BDNF mRNA levels were quantified using quantitative real-time polymerase chain reaction. Correlation analyses between these parameters were performed. Statistical analyses were performed using SPSS, version 24.0 (SPSS Inc, Chicago, IL, United States).
It was found that IBS-D patients had significantly increased anxiety symptoms and visceral sensitivity, and their mucosal expression of BDNF protein and mRNA and nerve fibers density were significantly elevated. BDNF expression was positively correlated with abdominal pain severity and IBS-SSS score and negatively correlated with visceral sensitivity parameters.
The present study showed evidence that BDNF levels were increased in the intestinal mucosa of IBS-D patients and might be involved in the pathophysiology of IBS-D via facilitating the growth of the nerve fibers in the intestinal mucosa. It also provided some potential clues for more effective treatments in these patients. The authors believe that this study not only contributes to the understanding of the pathophysiology of IBS, but also offers valuable reference for future research and therapies.
This study preliminarily explored the possible role of BDNF involved in the pathogenesis of IBS-D. Future studies should focus on the following aspects. First, the present study only recruited IBS-D patients and the findings could not be generalized to other subtypes. As a result, future research should investigate the possible role of BDNF in the pathophysiology of the other three IBS subtypes. Second, the pathophysiology of post-infectious (PI) and non-PI IBS are different and the two subtypes should be studied separately in the future. Finally, a cause and effect inference could not be made in our study. Therefore, additional well-designed clinical studies on the effect of BDNF on nerve growth, visceral sensitivity and abdominal pain are needed.
We thank Dr. Du S and Dr. Zhang M for enrollment of participants, and Dr. Gao C, Dr. Liu J and Dr. Liu F for assistance of colonoscopy examinations.
| 1. | Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel Disorders. Gastroenterology. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1781] [Cited by in RCA: 2027] [Article Influence: 202.7] [Reference Citation Analysis (4)] |
| 2. | Sperber AD, Dumitrascu D, Fukudo S, Gerson C, Ghoshal UC, Gwee KA, Hungin APS, Kang JY, Minhu C, Schmulson M, Bolotin A, Friger M, Freud T, Whitehead W. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome Foundation working team literature review. Gut. 2017;66:1075-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 374] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 3. | Ersryd A, Posserud I, Abrahamsson H, Simrén M. Subtyping the irritable bowel syndrome by predominant bowel habit: Rome II versus Rome III. Aliment Pharmacol Ther. 2007;26:953-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Bai T, Xia J, Jiang Y, Cao H, Zhao Y, Zhang L, Wang H, Song J, Hou X. Comparison of the Rome IV and Rome III criteria for IBS diagnosis: A cross-sectional survey. J Gastroenterol Hepatol. 2017;32:1018-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, Mayer EA, Niesler B, Quigley EM, Rajilić-Stojanović M, Schemann M, Schwille-Kiuntke J, Simren M, Zipfel S, Spiller RC. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:16014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 692] [Article Influence: 69.2] [Reference Citation Analysis (1)] |
| 6. | Dothel G, Barbaro MR, Boudin H, Vasina V, Cremon C, Gargano L, Bellacosa L, De Giorgio R, Le Berre-Scoul C, Aubert P, Neunlist M, De Ponti F, Stanghellini V, Barbara G. Nerve fiber outgrowth is increased in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 2015;148:1002-1011.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 7. | Xu XJ, Zhang YL, Liu L, Pan L, Yao SK. Increased expression of nerve growth factor correlates with visceral hypersensitivity and impaired gut barrier function in diarrhoea-predominant irritable bowel syndrome: a preliminary explorative study. Aliment Pharmacol Ther. 2017;45:100-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Yu YB, Zuo XL, Zhao QJ, Chen FX, Yang J, Dong YY, Wang P, Li YQ. Brain-derived neurotrophic factor contributes to abdominal pain in irritable bowel syndrome. Gut. 2012;61:685-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 9. | Obata K, Noguchi K. BDNF in sensory neurons and chronic pain. Neurosci Res. 2006;55:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 186] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Wang P, Du C, Chen FX, Li CQ, Yu YB, Han T, Akhtar S, Zuo XL, Tan XD, Li YQ. BDNF contributes to IBS-like colonic hypersensitivity via activating the enteroglia-nerve unit. Sci Rep. 2016;6:20320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (3)] |
| 11. | Delafoy L, Gelot A, Ardid D, Eschalier A, Bertrand C, Doherty AM, Diop L. Interactive involvement of brain derived neurotrophic factor, nerve growth factor, and calcitonin gene related peptide in colonic hypersensitivity in the rat. Gut. 2006;55:940-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Tucker KL, Meyer M, Barde YA. Neurotrophins are required for nerve growth during development. Nat Neurosci. 2001;4:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 419] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 13. | Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 1294] [Article Influence: 44.6] [Reference Citation Analysis (1)] |
| 14. | Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, Collins SM, Corinaldesi R. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1004] [Cited by in RCA: 1045] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 15. | Labus JS, Bolus R, Chang L, Wiklund I, Naesdal J, Mayer EA, Naliboff BD. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol Ther. 2004;20:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 346] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 16. | Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6329] [Cited by in RCA: 7465] [Article Influence: 311.0] [Reference Citation Analysis (0)] |
| 17. | Xu X, Pasricha PJ, Sallam HS, Ma L, Chen JD. Clinical significance of quantitative assessment of rectoanal inhibitory reflex (RAIR) in patients with constipation. J Clin Gastroenterol. 2008;42:692-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109:40-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 681] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 19. | Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16283] [Cited by in RCA: 19930] [Article Influence: 1107.2] [Reference Citation Analysis (0)] |
| 20. | Lovell RM, Ford AC. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:991-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 302] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 21. | Yang J, Fox M, Cong Y, Chu H, Zheng X, Long Y, Fried M, Dai N. Lactose intolerance in irritable bowel syndrome patients with diarrhoea: the roles of anxiety, activation of the innate mucosal immune system and visceral sensitivity. Aliment Pharmacol Ther. 2014;39:302-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Eriksson EM, Andrén KI, Eriksson HT, Kurlberg GK. Irritable bowel syndrome subtypes differ in body awareness, psychological symptoms and biochemical stress markers. World J Gastroenterol. 2008;14:4889-4896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Liu L, Xiao QF, Zhang YL, Yao SK. A cross-sectional study of irritable bowel syndrome in nurses in China: prevalence and associated psychological and lifestyle factors. J Zhejiang Univ Sci B. 2014;15:590-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Singh P, Agnihotri A, Pathak MK, Shirazi A, Tiwari RP, Sreenivas V, Sagar R, Makharia GK. Psychiatric, somatic and other functional gastrointestinal disorders in patients with irritable bowel syndrome at a tertiary care center. J Neurogastroenterol Motil. 2012;18:324-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (3)] |
| 25. | Liu DR, Xu XJ, Yao SK. Increased intestinal mucosal leptin levels in patients with diarrhea-predominant irritable bowel syndrome. World J Gastroenterol. 2018;24:46-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Zhou Q, Verne GN. New insights into visceral hypersensitivity--clinical implications in IBS. Nat Rev Gastroenterol Hepatol. 2011;8:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 27. | Kanazawa M, Hongo M, Fukudo S. Visceral hypersensitivity in irritable bowel syndrome. J Gastroenterol Hepatol. 2011;26 Suppl 3:119-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 28. | Simrén M, Törnblom H, Palsson OS, van Tilburg MAL, Van Oudenhove L, Tack J, Whitehead WE. Visceral hypersensitivity is associated with GI symptom severity in functional GI disorders: consistent findings from five different patient cohorts. Gut. 2018;67:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 180] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 29. | Liu L, Liu BN, Chen S, Wang M, Liu Y, Zhang YL, Yao SK. Visceral and somatic hypersensitivity, autonomic cardiovascular dysfunction and low-grade inflammation in a subset of irritable bowel syndrome patients. J Zhejiang Univ Sci B. 2014;15:907-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Bouin M, Plourde V, Boivin M, Riberdy M, Lupien F, Laganière M, Verrier P, Poitras P. Rectal distention testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology. 2002;122:1771-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 345] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 31. | Kanazawa M, Palsson OS, Thiwan SI, Turner MJ, van Tilburg MA, Gangarosa LM, Chitkara DK, Fukudo S, Drossman DA, Whitehead WE. Contributions of pain sensitivity and colonic motility to IBS symptom severity and predominant bowel habits. Am J Gastroenterol. 2008;103:2550-2561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 32. | Whitehead WE, Palsson OS. Is rectal pain sensitivity a biological marker for irritable bowel syndrome: psychological influences on pain perception. Gastroenterology. 1998;115:1263-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 199] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 33. | Whitehead WE, Delvaux M. Standardization of barostat procedures for testing smooth muscle tone and sensory thresholds in the gastrointestinal tract. The Working Team of Glaxo-Wellcome Research, UK. Dig Dis Sci. 1997;42:223-241. [PubMed] |
| 34. | Klooker TK, Kuiken SD, Lei A, Boeckxstaens GE. Effect of long-term treatment with octreotide on rectal sensitivity in patients with non-constipated irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:605-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Sabate JM, Veyrac M, Mion F, Siproudhis L, Ducrotte P, Zerbib F, Grimaud JC, Dapoigny M, Dyard F, Coffin B. Relationship between rectal sensitivity, symptoms intensity and quality of life in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:484-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Zhu Y, Zheng X, Cong Y, Chu H, Fried M, Dai N, Fox M. Bloating and distention in irritable bowel syndrome: the role of gas production and visceral sensation after lactose ingestion in a population with lactase deficiency. Am J Gastroenterol. 2013;108:1516-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 37. | Camilleri M, Boeckxstaens G. Dietary and pharmacological treatment of abdominal pain in IBS. Gut. 2017;66:966-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 38. | Delgado-Aros S, Camilleri M. Visceral hypersensitivity. J Clin Gastroenterol. 2005;39:S194-203; discussion S210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Delvaux M. Role of visceral sensitivity in the pathophysiology of irritable bowel syndrome. Gut. 2002;51 Suppl 1:i67-i71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 40. | Xia C, Shen S, Hashmi F, Qiao LY. Colitis-induced bladder afferent neuronal activation is regulated by BDNF through PLCγ pathway. Exp Neurol. 2016;285:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Hughes MS, Shenoy M, Liu L, Colak T, Mehta K, Pasricha PJ. Brain-derived neurotrophic factor is upregulated in rats with chronic pancreatitis and mediates pain behavior. Pancreas. 2011;40:551-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Yu YB, Zhao DY, Qi QQ, Long X, Li X, Chen FX, Zuo XL. BDNF modulates intestinal barrier integrity through regulating the expression of tight junction proteins. Neurogastroenterol Motil. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Boesmans W, Gomes P, Janssens J, Tack J, Vanden Berghe P. Brain-derived neurotrophic factor amplifies neurotransmitter responses and promotes synaptic communication in the enteric nervous system. Gut. 2008;57:314-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 44. | Hoehner JC, Wester T, Påhlman S, Olsen L. Localization of neurotrophins and their high-affinity receptors during human enteric nervous system development. Gastroenterology. 1996;110:756-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Goyal RK, Hirano I. The enteric nervous system. N Engl J Med. 1996;334:1106-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 477] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 46. | Geboes K, Collins S. Structural abnormalities of the nervous system in Crohn’s disease and ulcerative colitis. Neurogastroenterol Motil. 1998;10:189-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 171] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P- Reviewer: Iovino P, O’Malley D S- Editor: Ma RY L- Editor: Wang TQ E- Editor: Yin SY