Published online Jan 14, 2019. doi: 10.3748/wjg.v25.i2.245
Peer-review started: September 27, 2018
First decision: October 23, 2018
Revised: November 19, 2018
Accepted: December 19, 2018
Article in press: December 19, 2018
Published online: January 14, 2019
Processing time: 110 Days and 5.4 Hours
To evaluate the effectiveness and safety of submucosal tunneling endoscopic resection (STER) and compare its outcomes in esophageal and cardial submucosal tumors (SMTs) of the muscularis propria (MP) layer.
From May 2012 to November 2017, 173 consecutive patients with upper gastrointestinal (GI) SMTs of the MP layer underwent STER. Overall, 165 patients were included, and 8 were excluded. The baseline characteristics of the patients and SMTs were recorded. The en bloc resection rate, complete resection rate, residual rate, and recurrence rate were calculated to evaluate the effectiveness of STER, and the complication rate was recorded to evaluate its safety. Effectiveness and safety outcomes were compared between esophageal and cardial SMTs.
One hundred and twelve men and 53 women with a mean age of 46.9 ± 10.8 years were included. The mean tumor size was 22.6 ± 13.6 mm. Eleven SMTs were located in the upper esophagus (6.7%), 49 in the middle esophagus (29.7%), 46 in the lower esophagus (27.9%), and 59 in the cardia (35.7%). Irregular lesions accounted for 48.5% of all lesions. STER achieved an en bloc resection rate of 78.7% (128/165) for GI SMTs with an overall complication rate of 21.2% (35/165). All complications resolved without intervention or were treated conservatively without the need for surgery. The en bloc resection rates of esophageal and cardial SMTs were 81.1% (86/106) and 72.1% (42/59), respectively (P = 0.142), and the complication rates were 19.8% (21/106) and 23.7% (14/59), respectively, (P = 0.555). The most common complications for esophageal SMTs were gas-related complications and fever, while mucosal injury was the most common for cardial SMTs.
STER is an effective and safe therapy for GI SMTs of the MP layer. Its effectiveness and safety are comparable between SMTs of the esophagus and cardia.
Core tip: Submucosal tunneling endoscopic resection (STER) was initially reported in 2012 for the resection of submucosal tumors (SMTs) originating from the muscularis propria. It has an advantage in maintaining the integrity of the mucosa. Several studies have demonstrated the effectiveness and safety of STER; however; few studies have enrolled large populations over 100 cases and compared the effectiveness and safety of STER for SMTs located in different locations. In this study, we aimed to further evaluate the effectiveness and safety of STER for gastrointestinal SMTs in a large population and compare the feasibility of STER for resection of esophageal and cardial SMTs.
- Citation: Du C, Chai NL, Ling-Hu EQ, Li ZJ, Li LS, Zou JL, Jiang L, Lu ZS, Meng JY, Tang P. Submucosal tunneling endoscopic resection: An effective and safe therapy for upper gastrointestinal submucosal tumors originating from the muscularis propria layer. World J Gastroenterol 2019; 25(2): 245-257
- URL: https://www.wjgnet.com/1007-9327/full/v25/i2/245.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i2.245
A submucosal tumor (SMT) is defined as a protuberance in the gastrointestinal tract with a normal mucosa-covered surface. SMTs are often incidentally detected on imaging. SMTs have a broad differential diagnosis, and most SMTs with a diameter less than 3 cm are believed to be benign leiomyomas[1]. However, a proportion of SMTs, such as gastrointestinal stromal tumors (GISTs), have malignant potential[2]. SMTs have a greater probability of malignancy when they originate from the muscularis propria (MP) layer, have a large diameter, or are mesenchymal neoplasms[3-7].
Thus, the accurate diagnosis of SMTs is of the greatest importance to guide further treatment. Without resection, it is difficult to obtain an accurate diagnosis of the subtypes of SMTs even by endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) and biopsy, which are regarded as the most reliable methods for obtaining a histological diagnosis[8-11]. Lifelong follow-up not only increases the financial burden and psychological stress to the patients but also delays the urgent diagnosis of malignancy and treatment[12-14].
Digestive endoscopic tunnel technique (DETT) was first reported by Ling-Hu et al[15,16] in 2009. In 2010, Inoue et al[17] reported peroral endoscopic myotomy (POEM) using DETT for the treatment of achalasia cardia (AC). Submucosal tunneling endoscopic resection (STER), which was inspired by DETT, was initially reported by Xu et al[18] in 2012 for the resection of SMTs originating from the MP layer. A tunnel between the mucosa and the MP layer is established, and the operation is performed within the tunnel. SMTs are resected while the mucosal covering was maintained. Although endoscopic submucosal excavation (ESE) and endoscopic full-thickness resection (EFR) have been reported to be effective and safe for the resection of SMTs located in the MP[19-23], they fail to maintain the integrity of the mucosa like the STER procedure. SMTs located in the cardia are considered more challenging and difficult to be resected by STER than those located in the esophagus. Several studies have demonstrated the effectiveness and safety of STER[12,14,24-26], however, few studies have enrolled large populations of more than 100 cases and compared the effectiveness and safety of STER for SMTs located in different locations[27,28]. In this retrospective study, we aimed to further evaluate the effectiveness and safety of STER for gastrointestinal (GI) SMTs originating from the MP layer in a large population and compare the feasibility of STER for resection of esophageal and cardial SMTs.
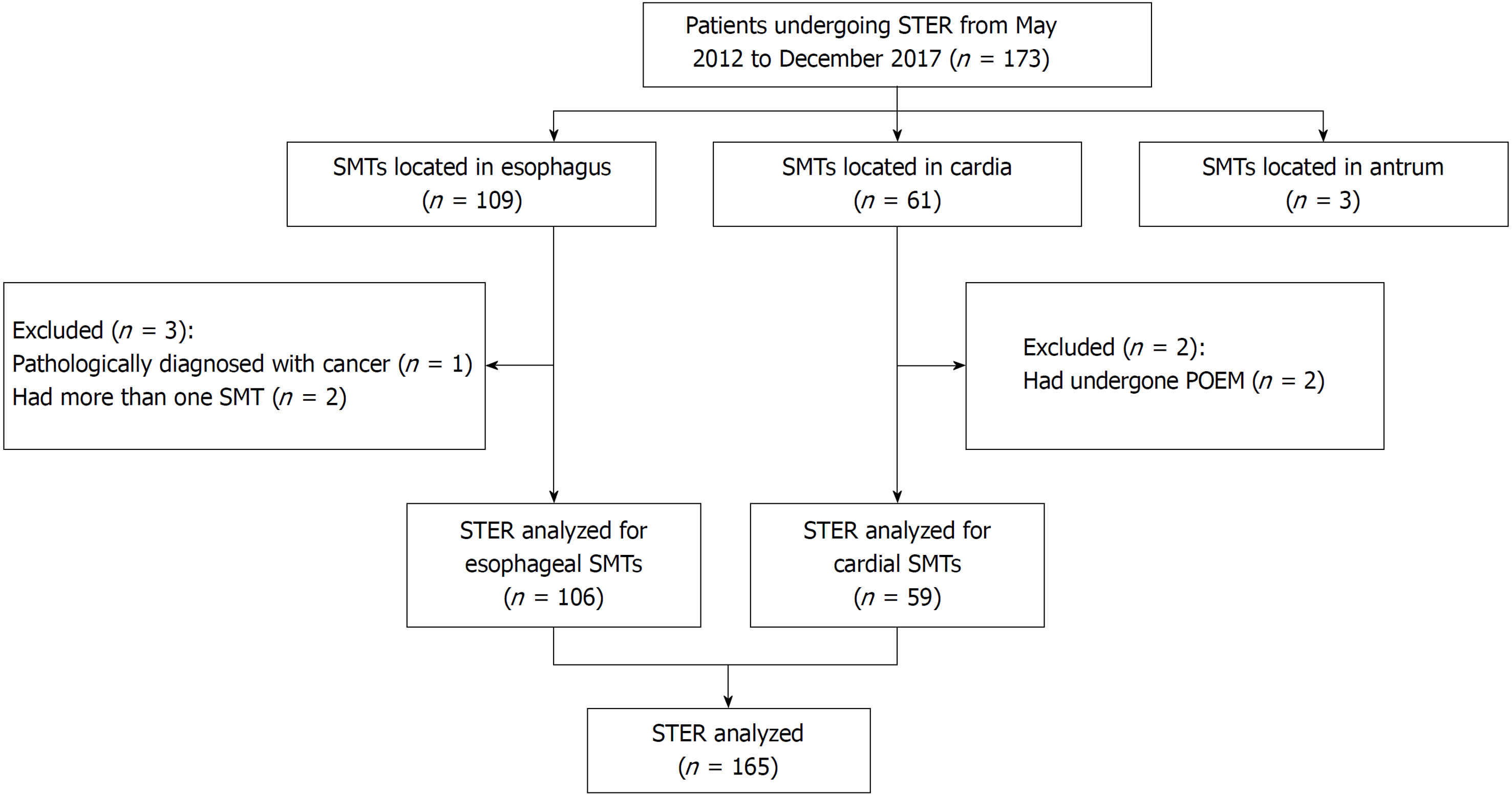
From May 2012 to November 2017, at our center, STER was performed on 173 consecutive patients diagnosed with GI SMTs originating from the MP layer. In all, 165 patients were included, and 8 were excluded (Figure 1). We excluded three patients with gastric antrum SMTs who underwent STER due to the small number of patients and because STER is less commonly performed in the antrum and requires further evaluation. The bent anatomical orientation of the stomach makes it challenging to perform STER because of the difficulty in establishing a submucosal tunnel. One patient diagnosed with cancer was excluded because the disease was not indicative of STER. Two patients with more than one SMT were excluded to eliminate the intervention for results. Two patients with AC and cardial SMTs underwent POEM and STER simultaneously and were excluded for the difficulty in the evaluation of the outcomes of STER.
In our study, patients with SMTs were considered eligible for STER if the following criteria were met: (1) SMTs were covered with the intact mucosa; (2) SMTs originated from the MP layer as confirmed by CT and/or endoscopic ultrasound (EUS); (3) SMTs had a transverse diameter of no more than 35.0 mm (≤ 35.0 mm); (4) patients were older than 18 years old; (5) patients had no signs of metastasis or invasion outside the digestive tract; (6) SMTs had no high-risk features of malignancy as assessed by EUS; and (7) patients signed an informed consent form. The exclusion criteria were as follows: (1) SMTs that were located less than 3-5 cm from the esophageal inlet; (2) SMTs that had signs of metastasis and/or invasion outside the digestive tract; (3) SMTs that were considered to be at high risk from surgery, such as those with an abundant blood supply; (4) patients who were pregnant; and (5) patients with coagulopathies (international normalized ratio > 1.5 and/or a platelet count < 50000). SMTs located in the upper esophagus at a distance less than 3-5 cm could not be resected by STER because of insufficient room to produce a tunnel.
Preoperative contrast-enhanced mediastinal CT and EUS (Prosound F75, Aloka, Tokyo, Japan and GF-UCT260, Olympus, Tokyo, Japan) were recommended for patients with suspected SMTs to evaluate the size, location, shape, and depth of the tumors and to rule out invasion outside the digestive tract and metastasis. All patients were fasted for 8 h before the procedures. STER was performed by experts with POEM experience in more than 100 cases.
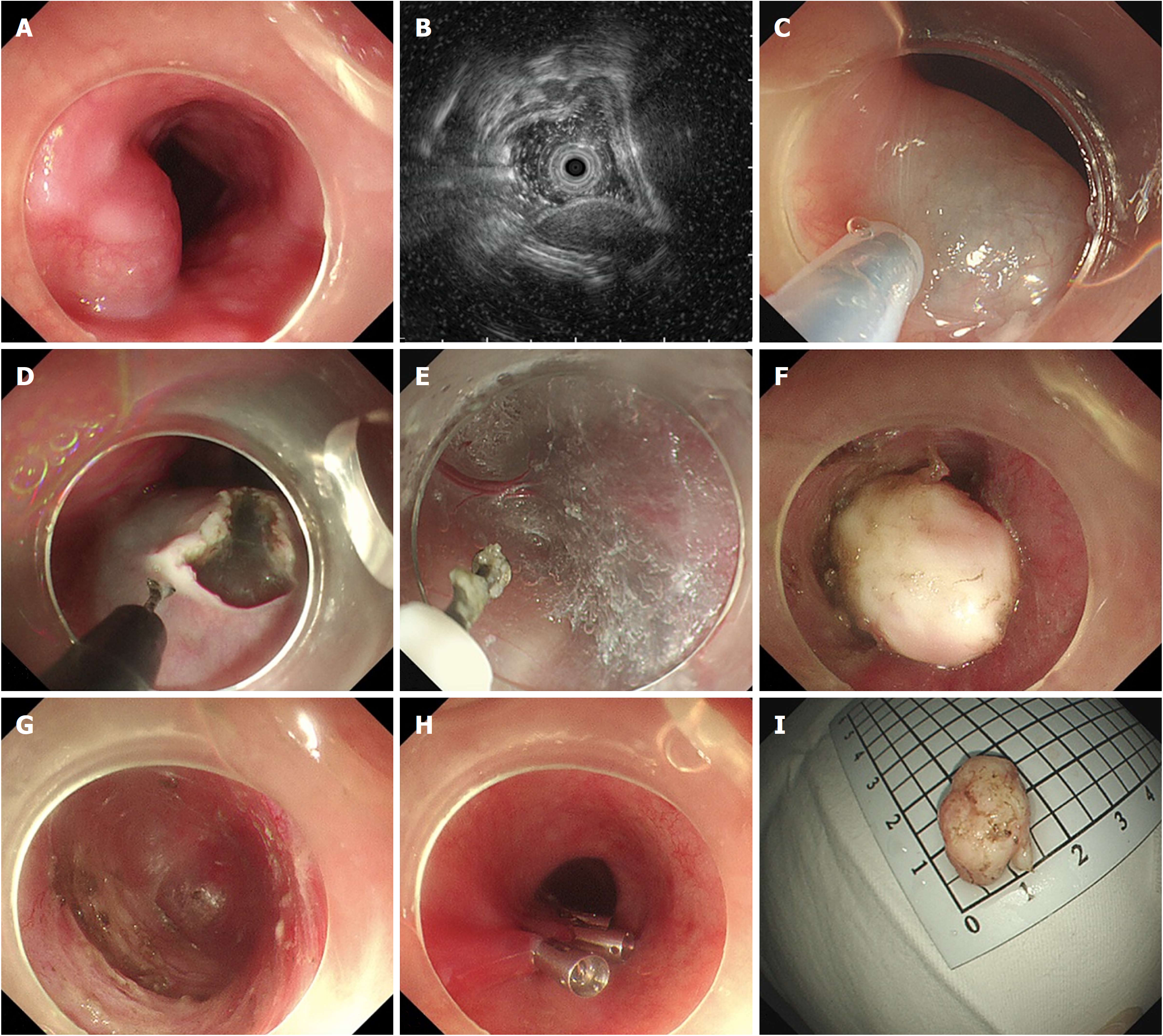
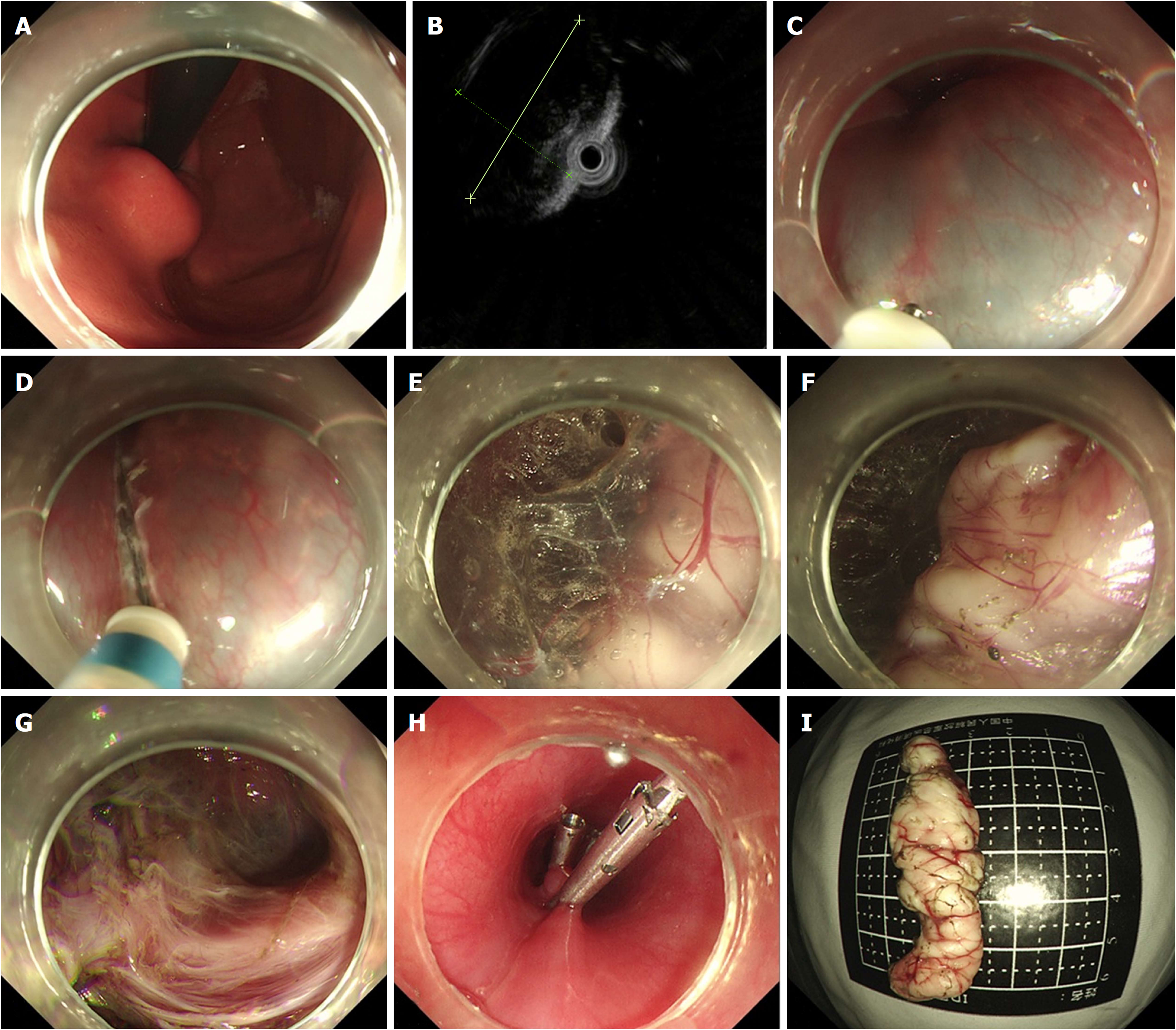
STER was performed on patients under general anesthesia with a single-accessory channel endoscope (GIF Q260J/GIF Q290, Olympus) equipped with a transparent cap (D-201-11802, Olympus). A carbon dioxide (CO2) insufflator (UCR, Olympus) was used to achieve CO2 insufflation. A high-frequency generator (VIO 200D, ERBE, Tübingen, Germany) and an argon plasma coagulation unit (APC300, ERBE) were used during the procedures. The STER procedures were primarily performed after endoscopic evaluation as follows (Figures 2 and 3): (1) Several milliliters of a mixture solution (100 mL saline + 2 mL indigo carmine + 1 mL epinephrine) were injected 3 cm to 5 cm proximal to the SMT with an injection needle (NM-4L-1, Olympus); (2) a longitudinal mucosal incision, transverse incision, or inverted T incision was made with a triangular knife (KD-640L, Olympus) as the tunnel entrance; (3) a tunnel was created between the mucosal and MP layers with the triangular knife and the tunnel ended at 1 cm to 2 cm distal to the SMT; (4) an insulation-tip knife (KD611L, IT2, Olympus), a triangular knife, or a snare (ASM-1-S or ASJ-1-S, Cook, Limerick, Ireland) was used to resect the SMT after it was completely exposed; and (5) the incision was closed with clips (HX-610-135, Olympus) after examination of the tunnel.
STER procedures for SMTs located in the cardia were more challenging due to the need to create a tunnel from the esophagus, through the lower esophageal sphincter (LES), to the cardia. It was difficult to identify the direction of tunnel. Methylene blue or indigo carmine can be used to locate the tumor and guide the direction of the tunnel after endoscopic evaluation[29].
Patients were fasted for 2-3 d, followed by a liquid diet for 3 d, then they gradually returned to a normal diet within 2 wk after the STER procedures. Oral proton pump inhibitor (PPI) therapy was used for 4 wk, following 3 d of intravenous PPI treatment. Intravenous antibiotics were administered from the day STER was performed and were stopped after 2-3 d if no signs of infection were observed. Patients were closely monitored for any complications, such as subcutaneous emphysema, mediastinal emphysema, pneumothorax, pneumoperitoneum, fever, chest pain, abdominal pain, hematemesis, and hematochezia. If a patient suffered from severe chest and/or abdominal pain, a chest/abdominal X-ray or CT was performed.
Gastroscopy and/or EUS were recommended at 1, 3, 6, and 12 mo after the operation and then annually thereafter. For patients who were diagnosed with GISTs, an additional contrast-enhanced CT scan was recommended every 3-6 mo for approximately 5 years.
The en bloc resection rate, complete resection rate, residual rate, and recurrence rate were calculated to evaluate the effectiveness of STER, while the complication rate was recorded to evaluate its safety.
Complete resection was defined as removal of the tumor en bloc with tumor free-lateral and basal margins upon pathologic examination. Residual tumor was defined as redetection of an SMT within 1.0 cm around the primary resected SMT less than 6 mo after STER, while recurrence was defined as redetection of an SMT within 1.0 cm around the primary resected lesion more than 6 mo after STER. Fever was diagnosed if the axillary temperature was > 38 °C. Tumor size was determined according to the longest diameter measured on the resected specimen. If the tumor was removed by piecemeal resection, it was reconstructed to evaluate its size. Accurate specimen size was not available for residual SMTs, and therefore, the size of those tumors was determined by EUS evaluation. Operative time was regarded as the period between submucosal injection and endoscope withdrawal, while the hospital time began on the day of surgery. Subcutaneous emphysema, mediastinal emphysema, pneumothorax, and pneumoperitoneum were regarded as gas-related complications.
The analyses were performed with SPSS 22.0 software (IBM Corp, Armonk, NY, United States). Parametric data, including the tumor size, operative time, hospital time, tunnel length, number of clips, medical cost, and follow-up period, are expressed as the mean ± standard deviation (SD) or median with the range and were assessed by Student’s t-test or a nonparametric test. Nonparametric variables, such as sex, location, shape, en bloc resection rate, and complete resection rate, are expressed as proportions and were assessed by the χ2 test or Fisher’s exact test. A P-value (two-tailed) < 0.05 was considered statistically significant.
From May 2012 to December 2017, 173 patients with GI SMTs originating from the MP layer underwent STER. After 8 patients were excluded, 165 patients were retrospectively enrolled, including 112 men and 53 women, with a mean age of 46.9 ± 10.8 years. The median size of tumor sample was 20.0 (range, 5.0-80.0 mm). Of the 165 lesions, 11 (6.7%) were located in the upper esophagus, 49 (29.7%) in the middle esophagus, 46 (27.9%) in the lower esophagus, and 59 (35.7%) in the cardia. Irregular lesions accounted for 48.5% of all lesions. Pathological diagnosis revealed that there were 157 (95.2%) leiomyomas, 3 (1.8%) GISTs, 3 (1.8%) lipomas, 1 (0.6%) schwannoma, and 1 (0.6%) fibrous tumor. The sizes of each of these three GISTs were 8.0 mm × 8.0 mm, 24.0 mm × 11.0 mm, and 25.2 mm × 13.2 mm, while their mitotic rates were no more than 5/50 high-power fields. The detailed characteristics of the patients and SMTs are summarized in Table 1.
| Outcome | Result (n = 165) |
| Age, mean ± SD (yr) | 46.9 (10.8) |
| Sex | |
| Men | 112 (67.9) |
| Women | 53 (32.1) |
| Tumor size, median (range, mm) | 20.0 (5.0-80.0) |
| Tumor location | |
| Upper esophagus | 11 (6.7) |
| Middle esophagus | 49 (29.7) |
| Lower esophagus | 46 (27.9) |
| Cardia | 59 (35.7) |
| Tumor shape | |
| Regular | 85 (51.5) |
| Irregular | 80 (48.5) |
| Pathological diagnosis | |
| Leiomyomas | 157 (95.2) |
| GISTs | 3 (1.8) |
| Lipomas | 3 (1.8) |
| Schwannomas | 1 (0.6) |
| Fibrous tumor | 1 (0.6) |
En bloc resection was achieved in 128 of the 165 lesions treated, for which the the en bloc resection rate was 78.7%. The complete resection rate was 78.7%. Four SMTs were not resected completely due to their large size, deep invasion, and/or proximity to the aortic arch, which resulted in a residual rate of 2.4% (4/165). No recurrence was noted during follow-up. The median operative time was 46 min (range 10-221 min). The median hospital time was 7 d (range 4-18 d). The median length of the tunnel was 7 cm (range 5-14 cm). The median number of clips was 5 (range 3-22). Patients spent a median of 4957.72 USD (range 3160.63-12882.63 USD). The effectiveness outcomes are shown in Table 2.
| Characteristic | Result (n = 165) |
| En bloc resection | 128 (77.6) |
| Complete resection | 128 (77.6) |
| Residual | 4 (2.4) |
| Recurrence | 0 |
| Operative time, median (range, min) | 46 (10-221) |
| Hospital time, median (range, d) | 7 (4-18) |
| Length of tunnel, median (range, cm) | 7 (5-14) |
| Clips, median (range) | 5 (3-22) |
| Cost, median (range, USD) | 4957.72 (3160.63-12882.63) |
All 35 patients experienced intraoperative and postoperative complications at a rate of 21.2% (35/165) (Table 3). The most common complications were fever (13/165), mucosal injury (12/165), and gas-related complications (10/165). No severe complications occurred, and all complications resolved without intervention or were treated conservatively without the need for surgery. One case of a large perforation of the MP layer occurred in a large lesion located in the cardia that deeply invaded the MP layer and adhered to the serosa. Clips were used to close the perforation after the resection of the mucosa near the perforation. The integrity of the mucosa was not maintained in that case.
| Complication | Number of patients |
| Gas-related complications | 6 |
| Moderate fever | 7 |
| Mucosal injury | 10 |
| Chest or abdominal pain | 4 |
| Gas-related complications and mucosal injury | 1 |
| Gas-related complications and moderate fever | 3 |
| Moderate fever and chest pain | 1 |
| Moderate fever and pleural effusion | 1 |
| Moderate fever and mucosal injury | 1 |
| Big perforation of the MP layer | 1 |
| Total | 35 |
The patients were divided into two groups (esophagus group and cardia group) based on the location of the lesions. When the esophagus group was compared with the cardia group in terms of baseline characteristics (Table 4), SMTs located in the cardia appeared to be larger and were more likely to have an irregular shape than those located in the esophagus. No significant differences were observed in age or sex between patients in the two groups.
| Characteristic | Esophagus group (n = 106) | Cardia group (n = 59) | P-value |
| Age, mean ± SD (yr) | 45.7 (10.5) | 48.2 (10.9) | 0.053 |
| Sex | 0.159 | ||
| Men | 76 | 36 | |
| Women | 30 | 23 | |
| Tumor size, median (range, mm) | 16.5 (5.0-55.0) | 25.0 (6.0-80.0) | 0.005a |
| Tumor shape | 0.000a | ||
| Regular | 66 | 19 | |
| Irregular | 40 | 40 | |
| Pathological diagnosis | NA | ||
| Leiomyomas | 102 | 55 | |
| GISTs | 1 | 2 | |
| Lipomas | 1 | 2 | |
| Schwannomas | 1 | 0 | |
| Fibrous tumor | 1 | 0 |
En bloc resection was achieved in 86 (81.1%) patients in the esophagus group and 42 (72.1%) in the cardia group, and the difference was not statistically significant (P = 0.142). Two residual tumors were noted in both groups, but no recurrence was noted during follow-up. The comparison of effectiveness outcomes between the two groups is shown in Table 5. No significant differences were seen in operative time, hospital time, number of clips, or medical cost between the esophagus and cardia groups.
| Characteristic | Esophagus group (n = 106) | Cardia group (n = 59) | P-value |
| En bloc resection | 86 (81.1) | 42 (71.2) | 0.142 |
| Complete resection | 86 (81.1) | 42 (71.2) | 0.142 |
| Residual | 2 (1.9) | 2 (3.4) | 0.941 |
| Recurrence | 0 (0) | 0 (0) | NA |
| Operative time, median (range, min) | 46 (12-169) | 50 (10-221) | 0.232 |
| Hospital time, median (range, d) | 7 (4-18) | 7 (4-16) | 0.261 |
| Clips; median (range) | 5 (3-22) | 5 (3-16) | 0.980 |
| Cost; median (range, USD) | 4974.48 (3160.63-12882.63) | 4926.60 (3276.43-8718.35) | 0.333 |
Procedure-related complications occurred in 21 (19.8%) patients in the esophagus group and 14 (23.7%) patients in the cardia group (Table 6). Difference in the complication rates between the two groups was not statistically significant (esophagus, 19.8%; cardia, 23.7%; P = 0.555). The most common complications in the esophagus group were gas-related complications (8/106) and fever (9/106), while the mucosal injury (9/59) was the most common complication in the cardia group.
| Complication | Esophagus group (n = 106) | Cardia group (n = 59) |
| Gas-related complications | 5 | 1 |
| Moderate fever | 5 | 2 |
| Mucosal injury | 3 | 7 |
| Chest or abdominal pain | 3 | 1 |
| Gas-related complications and mucosal injury | 1 | 0 |
| Gas-related complications and moderate fever | 2 | 1 |
| Moderate fever and chest pain | 1 | 0 |
| Moderate fever and pleural effusion | 1 | 0 |
| Moderate fever and mucosal injury | 0 | 1 |
| Big perforation of the MP layer | 0 | 1 |
| Total | 21 | 14 |
SMTs have a broad differential diagnosis and are mainly divided into leiomyomas, GISTs, fibrous tumors, and schwannomas. With the development of imaging techniques, the detection rate of SMTs has been increasing and the incidence of SMTs has been reported to be 3%[7,30]. SMTs are covered by intact mucosa, which increases the difficulty of EUS-FNA and biopsy, especially when the tumors originate in the MP layer. Considering the limited diagnostic value and the challenge of preoperative tissue collection especially when SMTs are easily resected and the accuracy of biopsy seems low[7,11,18,31-34], preoperative EUS-FNA was not performed in our study. Although benign leiomyomas are the most common SMTs in the esophagus, GISTs with malignant potential are the second most common SMTs. Treatment of SMTs in the esophagus is also important. Surgical resection and endoscopic resection are two methods used to resect SMTs. However, surgical resection, regardless of whether open surgery or video-assisted thoracoscopic surgery (VATS) is performed, seems to be more invasive than endoscopic resection[35-38]. STER is regarded as the optimum method for resecting SMTs originating from the MP layer based on its advantages of a high en bloc resection rate and the ability to maintain the integrity of the mucosa[29,39-41]. The creation of a tunnel not only maintains the integrity of the mucosa, but also decreases the likelihood of perforation, postoperative infection, fistula, and stricture[12]. Although several studies have reported results of STER for GI SMTs[18,29,40,42-49], few studies have enrolled a large population. Thus, the results are less convincing and further studies are necessary[27,28]. This study was designed to further evaluate the effectiveness and safety of STER for GI SMTs and to compare the outcomes of STER between esophageal and cardial SMTs.
In our current study, STER achieved an en bloc resection rate of 78.7% (128/165) for GI SMTs with an overall complication rate of 21.2% (35/165). Only four SMTs were not completely resected, which resulted in a residual rate of 2.4% (4/165), however, no recurrence was noted during follow-up. Large size, deep invasion, and/or proximity to the aortic arch were risk factors for residual tumors. STER was not indicated for SMTs with a transverse diameter larger than 35.0 mm because the inner diameter of the tunnel was approximately 3.5 cm, however, the upper limit of the longest tumor diameter remains unknown. A 7-cm SMT was successfully resected by Chen et al[28] However, larger size is associated with a high risk of malignancy and may result in loss of endoscopic visualization. The en bloc resection rates of STER for esophageal SMTs and cardial SMTs were 81.1% (86/106) and 72.1% (42/59), respectively. The complication rates of STER for esophageal SMTs and cardial SMTs were19.8% (21/106) and 23.7% (14/59), respectively. No significant differences were observed in en bloc resection or complication rates between those two locations. The most common complications that occurred in the esophagus were gas-related complications and fever, while mucosal injury was the most common complication in the cardia. No severe complications that required surgical treatment or led to death occurred in our study.
STER was effective not only for esophageal SMTs but also for cardial SMTs, and had a high en bloc resection rate. En bloc resection rate demonstrated in this study was slightly lower than those in previous studies which ranged from 83.3% to 100%[24]. We speculated that a snare was used to quickly resect the lesion after the majority part of the SMT was exposed in some cases, which led to a high incidence of piecemeal resection[12,26]. Although STER for cardial SMTs was more challenging than that for esophageal SMTs, their en bloc resection rates were comparable even though cardial SMTs were larger and more irregular than esophageal SMTs. We speculate that there may be two reasons that explain these findings. First, the therapeutic outcomes of STER in different locations, including the esophagus, cardia, stomach, and rectum have been reported to be good, and therefore, the difference between these two locations might be too small to show any difference. In our study, the en bloc resection rate in the esophagus group was higher than that of the cardia group, however, the difference was not significant. Second, the cardia group only contained 59 patients, which is relatively small. The operative time was also comparable. The creation of a tunnel during STER for cardial SMTs was more difficult than for esophageal SMTs and thus required more time. However, SMT exposure as well as the resection and incision closure accounted for the majority of the operative time, thus, no significant difference was observed between the two groups with respect to operative time.
The STER-related complication rates mainly range from 5% to 25% with no reported deaths, and most of the complications reported are mild[14], which is in accordance with our results. A meta-analysis involving 12 studies including 397 patients and 430 lesions showed that the pooled complication rate of STER for GI SMTs was 21.5% (95%CI: 13.2-33.1%)[25]. Gas-related complications are regarded as the most common complication related to STER[28,35,45,46,48]. In our study, gas-related complications were the most common complications of STER for GI SMTs, with a complication rate of 9.7% (16/165). Gas-related complications were also the most common complications for esophageal SMTs, with a rate of 19.8% (19/106), which is consistent with previous studies. The pooled prevalence of gas-related symptoms was 14.8% (95%CI: 10.5%-20.5%) for subcutaneous emphysema and pneumomediastinum, 6.1% (95%CI: 4.0%-9.0%) for pneumothorax, and 6.8% (95%CI: 4.7%-9.6%) for pneumoperitoneum, which were demonstrated in another meta-analysis[50].
The most common complications in patients with esophageal and cardial tumors were different in our study. The reasons for this were as follows: (1) The absence of a serous membrane in the esophagus makes it easier for gas to diffuse into the subcutis, mediastinum, thorax, and abdomen, thus, gas-related complications were more prevalent in the esophagus group; (2) the MP layer is thicker in the cardia than in the esophagus, which decreases the likelihood and amount of gas effusion; and (3) the anatomic structure of the cardia makes the direction of the tunnel difficult to identify and the tunnel difficult to establish, therefore, the mucosa was at higher risk of injury when the tunnel was created.
The present study has are several limitations. First, it was designed as a single-center, retrospective study. Second, the accuracy of the origin of the SMTs from the MP layer was not considered in this study due to the difficulty of retrospective evaluation based on EUS images. Third, the number of patients in the cardia group was small. Fourth, no control groups were included. Thus, randomized controlled studies involving a large population are warranted to evaluate the long-term outcome of STER compared with that of other treatments, such as ESE and EFR, for SMTs originating from the MP layer.
In conclusion, STER is an effective and safe method for the resection of upper GI SMTs with an overall en bloc resection rate of 77.6% and a complication rate of 21.2%. Gas-related symptoms and fever are the most common complications in patients with esophageal SMTs, while submucosal injury is the most common complication in patients with cardial SMTs. The effectiveness and safety of STER for tumors in the esophagus and cardia are comparable.
Submucosal tumors (SMTs) have a greater possibility of malignancy when they originate from the muscularis propria (MP) layer, have a large diameter, or are mesenchymal neoplasms. Without resection, it is difficult to obtain an accurate diagnosis of the subtypes of SMTs even by endoscopic ultrasound-guided fine needle aspiration and biopsy, which are regarded as the most reliable methods. Submucosal tunneling endoscopic resection (STER), which was inspired by digestive endoscopic tunnel technique, was reported for the resection of SMTs originating from the MP layer with the advantage to maintain the integrity of the mucosa in 2012. As a minimally invasive produce, STER acts an important role in the treatment of SMTs.
Few studies describing STER for SMTs located in the MP layer have enrolled large populations of greater than 100 cases. Studies enrolled large samples are needed. Although STER procedures for SMTs located in the cardia were regarded to be more challenging due to the need to create a tunnel from the esophagus, through the lower esophageal sphincter, to the cardia, no studies comparing the effectiveness and safety of STER for SMTs located in different locations have been performed.
In this retrospective study, we further evaluated the effectiveness and safety of STER for gastrointestinal (GI) SMTs originating from the MP layer in a large population and compared the feasibility of STER for resection of esophageal and cardial SMTs.
From May 2012 to November 2017, 173 consecutive patients with upper GI SMTs of the MP layer underwent STER. Overall, 165 patients were included, and 8 were excluded. The en bloc resection rate, complete resection rate, residual rate, and recurrence rate were calculated to evaluate the effectiveness of STER, and the complication rate was recorded to evaluate its safety. Effectiveness and safety outcomes of STER were compared between esophageal and cardial SMTs.
En bloc resection was achieved in 128 of the 165 lesions treated with an en bloc resection rate of 78.7%. Four SMTs were not resected completely owing to large size, deep invasion, and/or proximity to the aortic arch, leading to a residual rate of 2.4% (4/165). No recurrence was noted during follow-up. The complete resection rate was 78.7%. Thirty-five patients had intraoperative or postoperative complications, with a rate of 21.2% (35/165). The most common complications were fever (13/165), mucosal injury (12/165), and gas-related complications (10/165). No severe complications occurred. En bloc resection was achieved in 86 (81.1%) patients in the esophagus group and 42 (72.1%) in the cardia group, and there was no significant difference between them (P = 0.142). There was no significant difference in the complication rate between the two groups (esophagus, 19.8%; cardia, 23.7%; P = 0.555). The most common complications in the esophagus group were gas-related complications (8/106) and fever (9/106), while mucosal injury (9/59) was the most common complication in the cardia group. However, the accurate origin from the MP layer of the SMTs was not taken into consideration in this study and the number of patients in the cardia group was small.
STER is an effective and safe therapy for GI SMTs of the MP layer with an en bloc resection rate of 78.7% and a complication rate of 21.2%. No recurrence was observed during follow-up, even after piecemeal resection. Although STER for cardial SMTs was more challenging than that of esophageal SMTs, their en bloc resection rates were comparable even though cardial SMTs were larger and more irregular than esophageal SMTs. The most common complications in the esophagus group were gas-related complications and fever, while mucosal injury was the most common complication in the cardia group.
Although piecemeal resection may do not influence long-term outcomes, it affects pathological evaluation. Therefore, en bloc resection should be maintained. Randomized controlled studies involving a large population are warranted to evaluate the long-term outcome of STER compared with other treatments for SMTs originating from the MP layer, such as endoscopic submucosal excavation and endoscopic full-thickness resection.
| 1. | Ponsaing LG, Kiss K, Hansen MB. Classification of submucosal tumors in the gastrointestinal tract. World J Gastroenterol. 2007;13:3311-3315. [PubMed] |
| 2. | Lee IL, Lin PY, Tung SY, Shen CH, Wei KL, Wu CS. Endoscopic submucosal dissection for the treatment of intraluminal gastric subepithelial tumors originating from the muscularis propria layer. Endoscopy. 2006;38:1024-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 153] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 3. | Otani Y, Furukawa T, Yoshida M, Saikawa Y, Wada N, Ueda M, Kubota T, Mukai M, Kameyama K, Sugino Y, Kumai K, Kitajima M. Operative indications for relatively small (2-5 cm) gastrointestinal stromal tumor of the stomach based on analysis of 60 operated cases. Surgery. 2006;139:484-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 4. | Gill KR, Camellini L, Conigliaro R, Sassatelli R, Azzolini F, Messerotti A, Woodward TA, Wallace MB, Jamil LH, Raimondo M. The natural history of upper gastrointestinal subepithelial tumors: a multicenter endoscopic ultrasound survey. J Clin Gastroenterol. 2009;43:723-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Connolly EM, Gaffney E, Reynolds JV. Gastrointestinal stromal tumours. Br J Surg. 2003;90:1178-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 206] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Polkowski M, Butruk E. Submucosal lesions. Gastrointest Endosc Clin N Am. 2005;15:33-54, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (2)] |
| 7. | Nishida T, Kawai N, Yamaguchi S, Nishida Y. Submucosal tumors: comprehensive guide for the diagnosis and therapy of gastrointestinal submucosal tumors. Dig Endosc. 2013;25:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 197] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 8. | Hoda KM, Rodriguez SA, Faigel DO. EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc. 2009;69:1218-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 9. | El Chafic AH, Loren D, Siddiqui A, Mounzer R, Cosgrove N, Kowalski T. Comparison of FNA and fine-needle biopsy for EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc. 2017;86:510-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Polkowski M, Bergman JJ. Endoscopic ultrasonography-guided biopsy for submucosal tumors: needless needling? Endoscopy. 2010;42:324-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF, Schuetze S, Sundar HM, Trent JC, Wayne JD. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8 Suppl 2:S1-S41; quiz S42-S44. [PubMed] |
| 12. | Du C, Ma L, Chai N, Gao Y, Niu X, Zhai Y, Li Z, Meng J, Tang P, Linghu E. Factors affecting the effectiveness and safety of submucosal tunneling endoscopic resection for esophageal submucosal tumors originating from the muscularis propria layer. Surg Endosc. 2018;32:1255-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Kim GH. Endoscopic resection of subepithelial tumors. Clin Endosc. 2012;45:240-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Du C, Linghu E. Submucosal Tunneling Endoscopic Resection for the Treatment of Gastrointestinal Submucosal Tumors Originating from the Muscularis Propria Layer. J Gastrointest Surg. 2017;21:2100-2109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Linghu EQ. Endoscopic resection for gastrointestinal pre-cancerous lesion and early cancer. Electronic Image Press of the Chinese Medical Association. 2009;. |
| 16. | Endoscopy CSoD. Consensus on Digestive Endoscopic Tunnel Technique. Zhonghua Weichang Neijing Dianzi Zazhi (Eletronic Edition). 2017;4:145-158. |
| 17. | Inoue H, Minami H, Kobayashi Y, Sato Y, Kaga M, Suzuki M, Satodate H, Odaka N, Itoh H, Kudo S. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1168] [Cited by in RCA: 1284] [Article Influence: 80.3] [Reference Citation Analysis (1)] |
| 18. | Xu MD, Cai MY, Zhou PH, Qin XY, Zhong YS, Chen WF, Hu JW, Zhang YQ, Ma LL, Qin WZ, Yao LQ. Submucosal tunneling endoscopic resection: a new technique for treating upper GI submucosal tumors originating from the muscularis propria layer (with videos). Gastrointest Endosc. 2012;75:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 249] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 19. | Reinehr R. [Endoscopic submucosal excavation (ESE) is a safe and useful technique for endoscopic removal of submucosal tumors of the stomach and the esophagus in selected cases]. Z Gastroenterol. 2015;53:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (33)] |
| 20. | Stavropoulos SN, Modayil R, Friedel D, Brathwaite CE. Endoscopic full-thickness resection for GI stromal tumors. Gastrointest Endosc. 2014;80:334-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Tan Y, Tang X, Guo T, Peng D, Tang Y, Duan T, Wang X, Lv L, Huo J, Liu D. Comparison between submucosal tunneling endoscopic resection and endoscopic full-thickness resection for gastric stromal tumors originating from the muscularis propria layer. Surg Endosc. 2017;31:3376-3382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 22. | Ye LP, Zhang Y, Wang CY, He SQ, Feng XJ, Zhang JS, Ding JX. Endoscopic submucosal enucleation for gastric submucosal tumors originated from muscularis propria layer: clinical analysis of 116 case. Zhonghua Wei Chang Wai Ke Za Zhi. 2012;15:1175-1177. [PubMed] |
| 23. | Guo H, Sheng JQ, Wang HH, Jin P, Zhao XJ, Li N, Wang X, Li AQ, Yu DL, Xie H, Wang XW, Tang S. The diagnosis and treatment of gastrointestinal submucosal tumor under endoscopy. Weichangbingxue He Ganbingxue Zazhi. 2013;22:872-876. |
| 24. | Liu BR, Song JT. Submucosal Tunneling Endoscopic Resection (STER) and Other Novel Applications of Submucosal Tunneling in Humans. Gastrointest Endosc Clin N Am. 2016;26:271-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Song S, Wang X, Zhang S, Li Y, Zhang X, Chu X. Efficacy and complications of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors and exploration for influencing factors. Z Gastroenterol. 2018;56:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Li Z, Gao Y, Chai N, Xiong Y, Ma L, Zhang W, Du C, Linghu E. Effect of submucosal tunneling endoscopic resection for submucosal tumors at esophagogastric junction and risk factors for failure of en bloc resection. Surg Endosc. 2018;32:1326-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Chen T, Zhou PH, Chu Y, Zhang YQ, Chen WF, Ji Y, Yao LQ, Xu MD. Long-term Outcomes of Submucosal Tunneling Endoscopic Resection for Upper Gastrointestinal Submucosal Tumors. Ann Surg. 2017;265:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 28. | Chen T, Zhang C, Yao LQ, Zhou PH, Zhong YS, Zhang YQ, Chen WF, Li QL, Cai MY, Chu Y, Xu MD. Management of the complications of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors. Endoscopy. 2016;48:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Mao XL, Ye LP, Zheng HH, Zhou XB, Zhu LH, Zhang Y. Submucosal tunneling endoscopic resection using methylene-blue guidance for cardial subepithelial tumors originating from the muscularis propria layer. Dis Esophagus. 2017;30:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc. 1991;5:20-23. [PubMed] |
| 31. | American Gastroenterological Association Institute. American Gastroenterological Association Institute medical position statement on the management of gastric subepithelial masses. Gastroenterology. 2006;130:2215-2216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Levy MJ, Jondal ML, Clain J, Wiersema MJ. Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc. 2003;57:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 172] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 33. | Williams DB, Sahai AV, Aabakken L, Penman ID, van Velse A, Webb J, Wilson M, Hoffman BJ, Hawes RH. Endoscopic ultrasound guided fine needle aspiration biopsy: a large single centre experience. Gut. 1999;44:720-726. [PubMed] |
| 34. | Cantor MJ, Davila RE, Faigel DO. Yield of tissue sampling for subepithelial lesions evaluated by EUS: a comparison between forceps biopsies and endoscopic submucosal resection. Gastrointest Endosc. 2006;64:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 35. | Tan Y, Lv L, Duan T, Zhou J, Peng D, Tang Y, Liu D. Comparison between submucosal tunneling endoscopic resection and video-assisted thoracoscopic surgery for large esophageal leiomyoma originating from the muscularis propria layer. Surg Endosc. 2016;30:3121-3127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Li QY, Meng Y, Xu YY, Zhang Q, Cai JQ, Zheng HX, Qing HT, Huang SL, Han ZL, Li AM, Huang Y, Zhang YL, Zhi FC, Cai RJ, Li Y, Gong W, Liu SD. Comparison of endoscopic submucosal tunneling dissection and thoracoscopic enucleation for the treatment of esophageal submucosal tumors. Gastrointest Endosc. 2017;86:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 37. | Meng FS, Zhang ZH, Hong YY, Li DJ, Lin JQ, Chen X, Ji F. Comparison of endoscopic submucosal dissection and surgery for the treatment of gastric submucosal tumors originating from the muscularis propria layer: a single-center study (with video). Surg Endosc. 2016;30:5099-5107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 38. | Chen T, Lin ZW, Zhang YQ, Chen WF, Zhong YS, Wang Q, Yao LQ, Zhou PH, Xu MD. Submucosal Tunneling Endoscopic Resection vs Thoracoscopic Enucleation for Large Submucosal Tumors in the Esophagus and the Esophagogastric Junction. J Am Coll Surg. 2017;225:806-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 39. | Lu J, Jiao T, Zheng M, Lu X. Endoscopic resection of submucosal tumors in muscularis propria: the choice between direct excavation and tunneling resection. Surg Endosc. 2014;28:3401-3407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Zhou DJ, Dai ZB, Wells MM, Yu DL, Zhang J, Zhang L. Submucosal tunneling and endoscopic resection of submucosal tumors at the esophagogastric junction. World J Gastroenterol. 2015;21:578-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 41. | Duan TY, Tan YY, Wang XH, Lv L, Liu DL. A comparison of submucosal tunneling endoscopic resection and endoscopic full-thickness resection for gastric fundus submucosal tumors. Rev Esp Enferm Dig. 2018;110:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Liu BR, Song JT, Kong LJ, Pei FH, Wang XH, Du YJ. Tunneling endoscopic muscularis dissection for subepithelial tumors originating from the muscularis propria of the esophagus and gastric cardia. Surg Endosc. 2013;27:4354-4359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 43. | Li B, Liu J, Lu Y, Hao J, Liu H, Jiang J, Jiang Y, Qin C, Xu H. Submucosal tunneling endoscopic resection for tumors of the esophagogastric junction. Minim Invasive Ther Allied Technol. 2016;25:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Yang XZ, Dai WJ, Wang HG, Wang Q, Sun SH, Zhou JF, Ma G, Zhang J. Submucosal tunneling endoscopic resection for esophageal submucosal tumors. Shijie Huaren Xiaohua Zazhi. 2014;22:5310. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Li QL, Chen WF, Zhang C, Hu JW, Zhou PH, Zhang YQ, Zhong YS, Yao LQ, Xu MD. Clinical impact of submucosal tunneling endoscopic resection for the treatment of gastric submucosal tumors originating from the muscularis propria layer (with video). Surg Endosc. 2015;29:3640-3646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 46. | Wang H, Tan Y, Zhou Y, Wang Y, Li C, Zhou J, Duan T, Zhang J, Liu D. Submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors originating from the muscularis propria layer. Eur J Gastroenterol Hepatol. 2015;27:776-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 47. | Ye LP, Zhang Y, Mao XL, Zhu LH, Zhou X, Chen JY. Submucosal tunneling endoscopic resection for small upper gastrointestinal subepithelial tumors originating from the muscularis propria layer. Surg Endosc. 2014;28:524-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 48. | Zhang C, Hu JW, Chen T, Zhou PH, Zhong YS, Zhang YQ, Chen WF, Li QL, Yao LQ, Xu MD. Submucosal tunneling endoscopic resection for upper gastrointestinal multiple submucosal tumors originating from the muscular propria layer: a feasibility study. Indian J Cancer. 2015;51 Suppl 2:e52-e55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 49. | Wang XY, Xu MD, Yao LQ, Zhou PH, Pleskow D, Li QL, Zhang YQ, Chen WF, Zhong YS. Submucosal tunneling endoscopic resection for submucosal tumors of the esophagogastric junction originating from the muscularis propria layer: a feasibility study (with videos). Surg Endosc. 2014;28:1971-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 50. | Lv XH, Wang CH, Xie Y. Efficacy and safety of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors: a systematic review and meta-analysis. Surg Endosc. 2017;31:49-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P- Reviewer: Harada H, Ishida T, Kobara H, Yamamoto K S- Editor: Wang XJ L- Editor: Wang TQ E- Editor: Yin SY