Published online May 14, 2019. doi: 10.3748/wjg.v25.i18.2217
Peer-review started: December 13, 2018
First decision: December 28, 2018
Revised: January 27, 2019
Accepted: January 28, 2019
Article in press: January 28, 2019
Published online: May 14, 2019
Processing time: 152 Days and 13.4 Hours
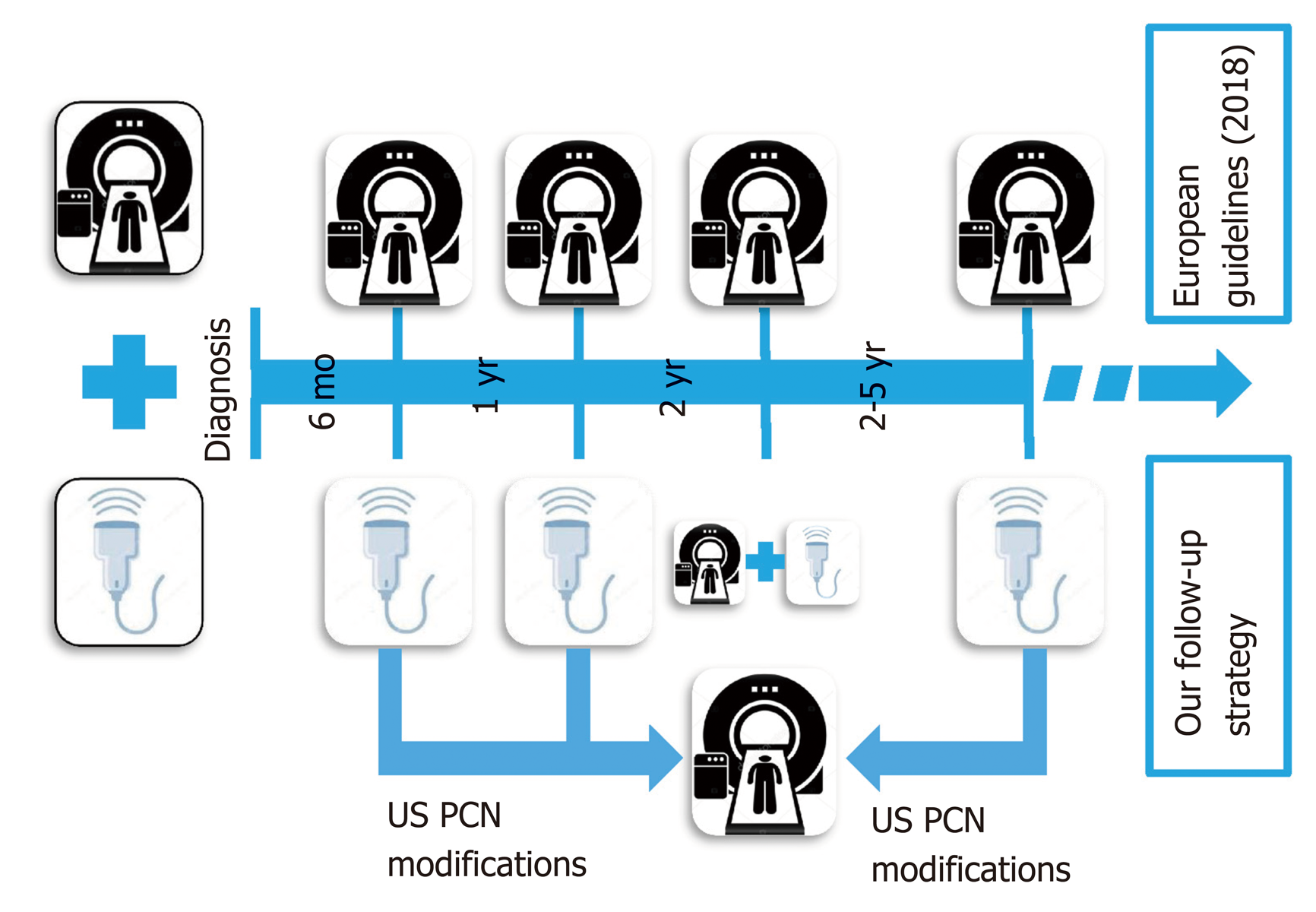
Patients with pancreatic cystic neoplasms (PCN), without surgical indication at the time of diagnosis according to current guidelines, require lifetime image-based surveillance follow-up. In these patients, the current European evidenced-based guidelines advise magnetic resonance imaging (MRI) scanning every 6 mo in the first year, then annually for the next five years, without reference to any role for trans-abdominal ultrasound (US). In this study, we report on our clinical experience of a follow-up strategy of image-based surveillance with US, and restricted use of MRI every two years and for urgent evaluation whenever suspicious changes are detected by US.
To report the results and cost-efficacy of a US-based surveillance follow-up for known PCNs, with restricted use of MRI.
We retrospectively evaluated the records of all the patients treated in our institution with non-surgical PCN who received follow-up abdominal US and restricted MRI from the time of diagnosis, between January 2012 and January 2017. After US diagnosis and MRI confirmation, all patients underwent US surveillance every 6 mo for the first year, and then annually. A MRI scan was routinely performed every 2 years, or at any stage for all suspicious US findings. In this communication, we reported the clinical results of this alternative follow-up, and the results of a comparative cost-analysis between our surveillance protocol (abdominal US and restricted MRI) and the same patient cohort that has been followed-up in strict accordance with the European guidelines recommended for an exclusive MRI-based surveillance protocol.
In the 5-year period, 200 patients entered the prescribed US-restricted MRI surveillance follow-up. Mean follow-up period was 25.1 ± 18.2 mo. Surgery was required in two patients (1%) because of the appearance of suspicious features at imaging (with complete concordance between the US scan and the on-demand MRI). During the follow-up, US revealed changes in PCN appearance in 28 patients (14%). These comprised main pancreatic duct dilatation (n = 1), increased size of the main cyst (n = 14) and increased number of PNC (n = 13). In all of these patients, MRI confirmed US findings, without adding more information. The bi-annual MRI identified evolution of the lesions not identified by US in only 11 patients with intraductal papillary mucinous neoplasms (5.5%), largely consisting of an increased number of very small PCN (P = 0.14). The overall mean cost of surveillance, based on a theoretical use of the European evidenced-based exclusive MRI surveillance in the same group of patients, would have been 1158.9 ± 798.6 € per patient, in contrast with a significantly lower cost of 366.4 ± 348.7 € (P < 0.0001) incurred by the US-restricted MRI surveillance used at our institution.
In patients with non-surgical PCN at the time of diagnosis, US surveillance could be a safe complementary approach to MRI, delaying and reducing the numbers of second level examinations and therefore reducing the costs.
Core tip: Considering the high incidence of pancreatic cystic neoplasms (PCN) in the general population and the low risk of malignant progression in these patients, health care providers need to consider cost-effective follow-up programs. Current guidelines advise only magnetic resonance imaging (MRI) surveillance for the routine follow-up of these patients. This image-based surveillance carries issues concerning accessibility and high costs. The present retrospective analysis enrolling 200 patients has demonstrated that a modified surveillance based on ultrasound and restricted use of MRI is both safe and significantly more cost-effective. However, this retrospective study requires confirmation by a prospective randomized controlled clinical trial comparing the two follow-up regimens in patients with non-surgical PCN.
- Citation: Morelli L, Guadagni S, Borrelli V, Pisano R, Di Franco G, Palmeri M, Furbetta N, Gambaccini D, Marchi S, Boraschi P, Bastiani L, Campatelli A, Mosca F, Di Candio G. Role of abdominal ultrasound for the surveillance follow-up of pancreatic cystic neoplasms: a cost-effective safe alternative to the routine use of magnetic resonance imaging. World J Gastroenterol 2019; 25(18): 2217-2228
- URL: https://www.wjgnet.com/1007-9327/full/v25/i18/2217.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i18.2217
The widespread use of high resolution imaging has resulted in a marked increase in the incidental detection of pancreatic cystic lesions, such that these lesions are encountered in 3% of abdominal computed tomography (CT) examinations and in up to 13%-19.6% of magnetic resonance imaging (MRI) scans[1-3]. The MRI prevalence data are in agreement with the previously reported autopsy studies demonstrating pancreatic cystic lesions in up to a quarter of cases[4]. Cystic pancreatic lesions are a heterogeneous group. The classification of pancreatic cyst neoplasms (PCNs) is based either on their neoplastic potential or their epithelial or mesenchymal origin.
The two most common PCN lesions are the intraductal papillary mucinous neoplasms (IPMN) and the mucinous cystic neoplasms (MCN). Both are benign, but with an established risk of malignant progression; whereas others are always benign, without any risk for malignant transformation, e.g., serous cystic neoplasm (SCN). More rarely, PCN are overtly malignant at the time of diagnosis (cystadenocar-cinomas). PCN not only have diverse histological and imaging appearances, but also differ in their clinical presentation, biological behavior, growth pattern, and risk of malignancy[5]. Accurate risk stratification and decisions regarding treatment and follow-up strategies necessitate precise lesion characterization and diagnosis. Several recommendations have been published on their pathology and management. The most relevant are the International Consensus Guidelines (2006[6], 2012[7] and 2017[8]), the guidelines of the American Gastroenterological Association (2015[9]), and the European study group on cystic tumors of the pancreas guidelines (2012[10] and 2018[11]). All these guidelines consider repeated MRI scans with similar frequency as the preferred surveillance tool in the follow-up strategy of non-surgical PCN. The European evidence-based guidelines on pancreatic cystic neoplasms consider the presence of jaundice, mural nodes (> 5 mm) and main duct dilatation ≥ 10 mm as “absolute indications” for surgery. The presence of main duct dilatation 5-9.9 mm, cyst growth rate > 5 mm/year, mural nodes < 5 mm, cystic diameter ≥ 40 mm, increased serum markers and new onset of diabetes or acute pancreatitis are relative indications for surgical intervention. In the presence of one or more of these features, surgical indication has to be balanced by the patients’ general condition, including co-morbid disorders.
Surgical intervention in the remaining “low risk” PCN (Wirsung diameter < 5 mm, cyst size < 40 mm, growth rate < 5 mm/years and no mural nodes) is not indicated at the time of diagnosis. Instead, accurate surveillance follow-up is recommended in the first instance. This consists of a MRI every 6 mo during the first year and then annually for the next 5 years, in order to detect the appearance of progressive changes, e.g., an increase in the size of the major lesion, main pancreatic duct dilatation or mural nodules. Although MRI is considered the gold standard imaging technique[12-14] to follow-up on these lesions, it has some issues, including limited access to this imaging modality and high costs[15,16]. In addition, MRI examinations are lengthy and can be uncomfortable for patients, particularly those who suffer from claustrophobia. Additionally, there are patients in whom MRI is contraindicated. Other imaging modalities used include endoscopic ultrasound (EUS) with or without fine needle aspiration (FNA), transabdominal ultrasound (US), contrast enhanced US (CEUS) and contrast enhanced-EUS (CH-EUS). EUS is recommended in the current guidelines as an adjunct to the other imaging modalities in the assessment of patients harboring PNC with features identified during the initial investigation or follow-up, which may indicate the need for surgical resection. Despite its accuracy, EUS-FNA is invasive and thus should be performed only when the results are expected to change clinical management. Although US and CEUS are included in the Italian consensus guidelines for the diagnostic work-up and follow-up of cystic pancreatic neoplasms[17], this recommendation is not included in the European Evidence Based Guidelines; although CH-EUS is considered for evaluation of mural nodules[11].
A pragmatic approach is needed, especially in public healthcare hospitals, in the clinical management of patients harboring relatively common benign lesions but a varying risk of malignant transformation. Although these PCN do not require surgery at the time of diagnosis, there is an evidence-based absolute need for expensive image-based long-term surveillance follow-up. Hence, cost considerations, with the emphasis on cost-efficacy and utility of long-term surveillance, is particularly essential in public healthcare systems. However, in the quest for cost containment, an alternative cheaper follow-up system for non-surgical PCN is only acceptable if it is safe and proven to be fit for purpose, i.e. if it does not miss malignant evolution of PCN. Recently, a few publications[18-20] have evaluated the role of US in monitoring PCN. Nevertheless, to date there has not been any reports of a safe alternative follow-up strategy based on US with limited MRI use outlined by the present study.
Records from all patients with PCN diagnosis without indications for surgery (absolute or relative), who were enrolled in our modified surveillance protocol between January 2012 and January 2017, were reviewed retrospectively. The patient cohort for this retrospective study was obtained from our institutional, prospectively-collected database and selected as patients with confirmed diagnosis of PCN without absolute or relative indications for surgery according to the current European evidence-based guidelines[11]. The diagnostic US criteria for suspect PNC were the identification of one or more partial or completely anechoic areas within the pancreatic parenchyma and/or dilation of Wirsung duct > 2 mm, in the absence of identifiable causes of obstruction. In the protocol, the US diagnosis was always confirmed with an MRI scan. Exclusion criteria were suspected or proven malignancy at the time of diagnosis (presence of solid vascularized tissue in the cyst, presence of nodal or distant metastasis at imaging, or positive histopathological findings)[18], PCN with absolute or relative surgical criteria[11], clear diagnosis of SCN, absence of diagnostic MRI scan, and follow-up period less than 10 mo. The last date of entrance into the US follow-up for the group of patients included in the study was January 1, 2017, with an end-point date of January 1, 2018 for the surveillance follow-up period.
After US diagnosis and MRI confirmation, all scheduled patients were followed-up with a non-conventional surveillance protocol that was used in our Unit since 2012. It consisted of a US scan every 6 mo for the first year and then, in patients with stable disease, annually from the second to the fifth year. A planned MRI was performed routinely every two years for stable disease, or at any time when suspicious changes were observed on US. Abdominal US was always performed just before the planned routine MRI in the second year of follow-up (Figure 1). The reasons for reducing the imaging intervals and advancing the MRI were dilatation of the main duct > 50%, increased size of the cyst ≥ 2 mm from previous examinations, or development of new lesions (Figure 2). The development of new PCN is diagnosed by conventional US as a new anechoic area into the pancreatic parenchyma. Stable disease was defined as PNC without detectable changes between two subsequent follow-up images.
Retrieved data included baseline patient characteristics, Wirsung caliber (the widest portion regardless of location), PCN size (largest diameter), connection of the cyst to the ductal system, and numbers and locations at the time of diagnosis. The duration of the follow-up period, Wirsung caliber, PCN modifications discovered during surveillance with subsequent performance of US as a follow-up examination, and MRI were also evaluated. Finally, the number and cost of US and MRI performed for each patient were obtained.
Costs of a single examination were expressed in Euros and obtained from our regional rates as follows: 60 € for US and 480 € for MRI, with an extra 254 € for a contrast-enhanced study. The total number of US and MRI scans (both routine and urgent) for each patient included in the study, together with the total number of examinations, were obtained. In the same way, the overall number of MRI alone, which would have been performed if the same patient group had undergone the standard guidance-approved MRI only surveillance[11], were calculated. According to the guidelines, a short MRI protocol without the administration of contrast provides equivalent information to a longer contrast enhanced MRI protocol for the surveillance of PCN. As a consequence, the MRI exam in the ‘virtual’ control group was estimated as 480 € and scheduled, as suggested, every 6 mo. This enabled the calculation of the theoretical overall cost of the control group.
These data were used in the cost-analysis comparison between the US-MRI restricted surveillance-based follow-up strategy and surveillance had the same group of patients been subjected to the evidenced-based existing guidelines surveillance with MRI alone. Sensitivity, negative predictive value, and the accuracy of US with respect to MRI in the follow-up were evaluated. Specifically, the sensitivity, negative predictive value and accuracy refer to the ability of US to detect changes in PNC, with respect to the gold standard MRI at two years. The diagnostic criteria evaluated for this analysis are the same for both US and MRI, which include a detected increase in the number of PCN (detection of new anechoic areas not identified in previous examination; increased of size PCN > 2 mm; increase of Wirsung caliber > 50%). In this series, no patients developed mural nodules or PCN wall thickness. Hence, these were not included in the analysis.
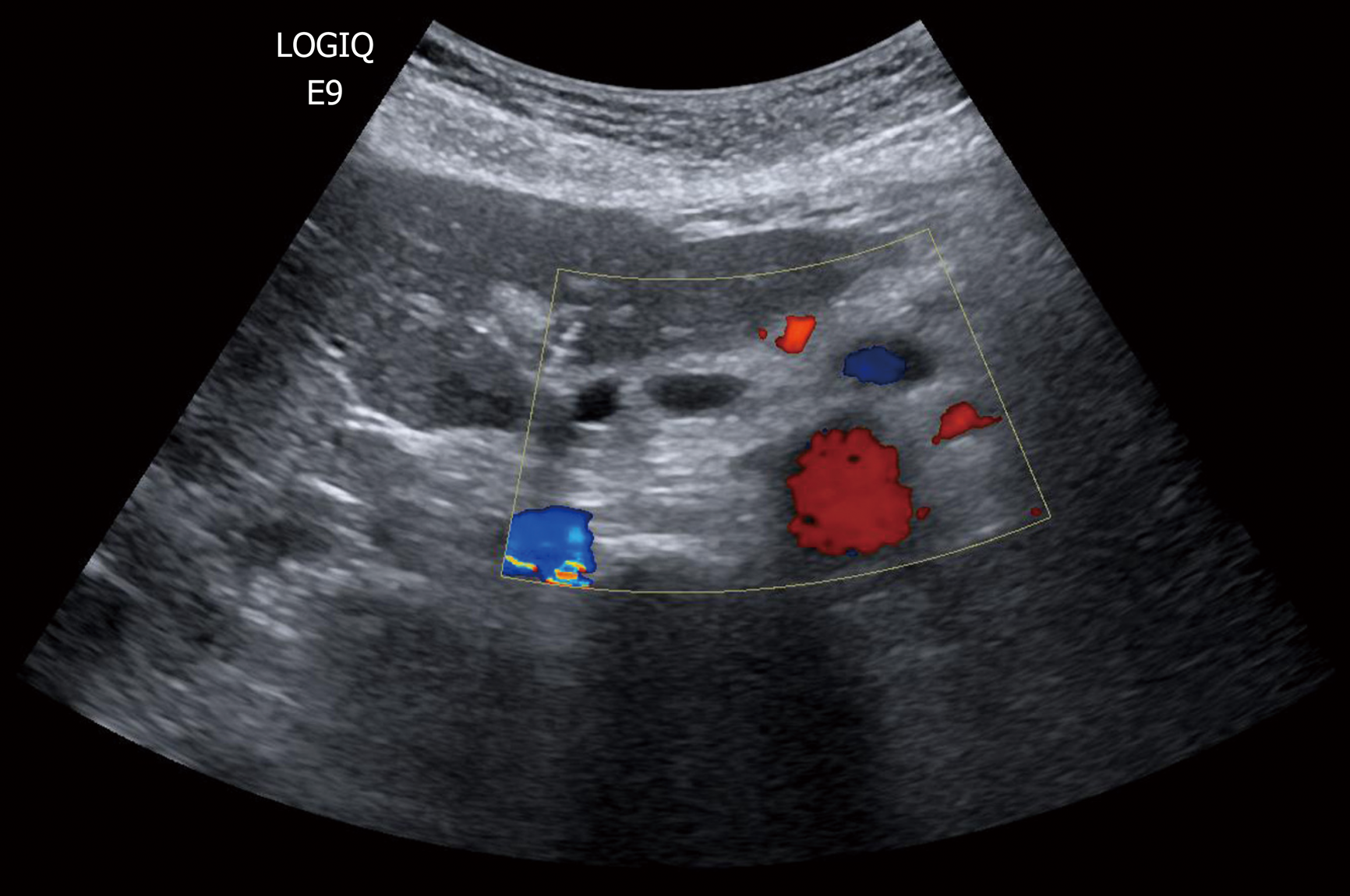
B-mode US was performed in all patients at our department by surgeons with expertise in US examinations and with over 25 years of experience in pancreatic surgery and imaging. All patients signed an informed consent to authorize the scientific use of the collected data. A GE Logiq 9 (GE Healthcare, Milwaukee, WI, United States) with a probe frequency of 3.5-5.0 MHz was utilized. Patients were scanned supine or in other positions with grey scale imaging and Color-Doppler. Harmonic imaging was routinely used to reduce artefact and increase the signal-to-noise ratio. The position, size, boundaries, and contents of all PCN, as well as the diameter of the pancreatic duct, if visible, were recorded. During the examinations, previous US or MRI images, including those performed at the time of diagnosis, were available using Resolution and then Suit Estensa PACS (Esaote Spa, Genova, Italy). They were used as correlative images to identify known PNC and evaluate their modifications. CEUS was only used occasionally in a few selected patients with relative surgical indications, which had shown at the time of the diagnosis septa or cystic wall thickness by B-mode US. Hence, it was not considered in the present analysis.
Patients underwent MRI on a superconductive 1.5T system (Signa HDx; GE Healthcare) using a twelve-channel phased-array body coil for both excitation and signal reception. Immediately before starting the examination, scopolamine methyl-bromide (20 mg; Buscopan®, Boehringer Ingelheim, Italy) was administered intramuscularly to avoid peristaltic artefacts. The standard imaging protocol included, first, T1-weighted breath-hold SPGR in-phase and out-of-phase axial sequences (with and/or without fat suppression), and T2-weighted axial sequences (both breath-hold, single-shot fast spin-echo and respiratory-triggered, fat-suppressed fast spin-echo) of the upper abdomen. Next, MRCP was performed by respiratory-triggered, three-dimensional, heavy T2-weighted fast spin-echo (3D FRFSE) sequence and breath-hold, thick-slab, single-shot FSE T2-weighted sequences performed in the coronal and oblique-coronal projections. Diffusion-weighted MR imaging of the pancreatic region was performed using an axial respiratory-triggered spin-echo echo-planar sequence with multiple b values (300, 500, 700, 1000 s/mm²) in all diffusion directions. If there were some doubts on the pre-contrast study, a three-dimensional fat-suppressed Liver Acquisition with Volumetric Acceleration (LAVA) sequence was obtained in the axial and sometimes coronal plane before and after intravenous injection of Gadolium-based contrast agents. Post-contrast graphic images were obtained in the arterial, portal-venous and delayed (between 3 and 5 min) phases. Acquisition time for the whole examination ranges from 30 to 35 min. The size of the voxels and therefore the spatial resolution depends on matrix size, the field-of-view (FOV), and slice thickness. Moreover, higher magnetic field allows improved resolution. In our study, the MR cholangiography matrix was 256 × 160, the slice thickness/spacing was 2.4/-1.2 mm, and the FOV was about 40. By applying these parameters, the 1.5 T MR device allowed evaluation of variation in the dimension of 2 mm. The spatial resolution of the 1.5 T MR commercial device has been reported in the literature by Arizono et al[21] as 1.1 × 1.0 mm (inplane resolution) and 0.84 mm (minimum slice resolution).
SPSS version 21.0 (IBM Corp., Armonk, NY, United States) and STATA version 13 (STATA Corp., College Station, TX, United States) software were employed for statistical evaluation. Continuous variables are reported as mean ± standard deviation (SD) and compared using Student’s t-test. Variables with a non-normal distribution are expressed as median and compared using the Wilcoxon Test. To determine the competency of US policy in the surveillance period relative to the gold standard MRI, a receiver operating characteristic curve (ROC) test was performed with a calculation of sensitivity, negative predictive value, accuracy and area under the curve (AUC). P values less than 0.05 were considered statistically significant.
Two hundred patients harboring 261 PCN were followed-up with the US-Restricted MRI surveillance program described above. At diagnosis, 140 patients (74.5%) had a single PNC and 51 patients had multiple PNC (25.5%), the multiple PNC being referred to as IMPNs. The median number of cysts was two (range 2-5). The study group comprised 138 (69%) females and 62 (31%) males with a mean age of 67 ± 14 years. At the time of diagnosis, the median Wirsung diameter was 2.6 (range 1.8-4.5 mm) and the mean cystic diameter was 16 ± 13 mm, with 97 (37%) measuring less than 10 mm. Most lesions were located in the pancreatic head (106 PCN, 40% of total). Connections to the ductal system and MRI diagnosis of IPMN was documented in 148 (74% of total patients). The mean follow-up period was 25.1 ± 18.2 mo. Surgery was required in two patients (1%) because of the appearance of suspicious features on the surveillance scans (with complete concordance between the US and the on-demand MRI scans). In the first patient, a 35 mm lesion located in the tail was detected by the 1-year US scan, which confirmed a rapid increase in cyst diameter reaching 42 mm. After MRI, EUS was also performed. However, due to the distal localization of the lesion and poor acoustic window, needle aspiration was not performed. This female patient was treated by laparoscopic distal pancreatectomy. Pathological examination of the excised specimen confirmed a cystic neuroendocrine tumor. For the second patient with a PCN located in the pancreatic head, follow-up imaging showed a progressive Wirsung dilatation that was accompanied by a rising serum Ca 19.9 level and, hence, had clear-cut indications for surgery. The final histologic diagnosis of the excised lesion was confirmed as a mucinous carcinoma arising from IPMN and staged T2N0M0. Data summarized in Table 1.
| Characteristics | |
| Number of patients | 200 |
| Baseline total PCN | 261 |
| Multiple PNC patients, n (%) | 51 (25) |
| Sex ratio, M:F | 62:138 |
| Age in yr, mean ± SD | 67 ± 14 |
| Main duct diameter in mm, median (range) | 2.6 (1.8-4.0) |
| Cystic size in mm, mean ± SD | 16 ± 13 |
| PNC < 10 mm, n (%) | 97 (37) |
| Location, uncinate process, n (%) | 73 (28) |
| Location, pancreatic head, n (%) | 106 (40) |
| Location, pancreatic body, n (%) | 54 (21) |
| Location, pancreatic tail, n (%) | 28 (11) |
| Radiologically suspected IPMN, n (%) | 148 (74) |
| Mean follow-up, mo (± SD) | 25.1 (± 18.2) |
| Surgery during follow-up, n (%) | 2 (1) |
In 28 patients (14%), US showed non-surgical changes in PCN during surveillance, consisting of pancreatic duct dilatation in one case (from 2 to 3 mm) and an increase in diameter of the main cyst in 14 cases (median increased = 2.5 mm; range 2-5 mm). One female 88-year-old patient with PCN in the head of the pancreas that enlarged by 5 mm in one year was treated conservatively because of significant ischemic heart disease and diminished cardiac function. The lesion remained stable during a follow-up of 28 mo. In the remaining 13 patients, US discovered new cystic lesions during image-based surveillance. All these patients underwent MRI, which confirmed the US findings.
In 11 patients (5.5% of total), the routine 2 years MRI identified evolution of the lesions not detected at the same time US (P = 0.14), but mainly related to an increased number of PCN (6 cases; 54%). In all these cases, the new PCN detected by MRI were located in the uncinate and tail of the pancreas. In five cases (46%), the routine MRI demonstrated a median PCN enlargement of 3 mm (range 3-4 mm) not detected by US. However, all these patients had PCN diameters < 15 mm and an MRI every 6 mo would not have altered the clinical management of these patients. In the present study, the follow-up surveillance program did not identify the development of mural nodules or thickness of the wall either by US or MRI.
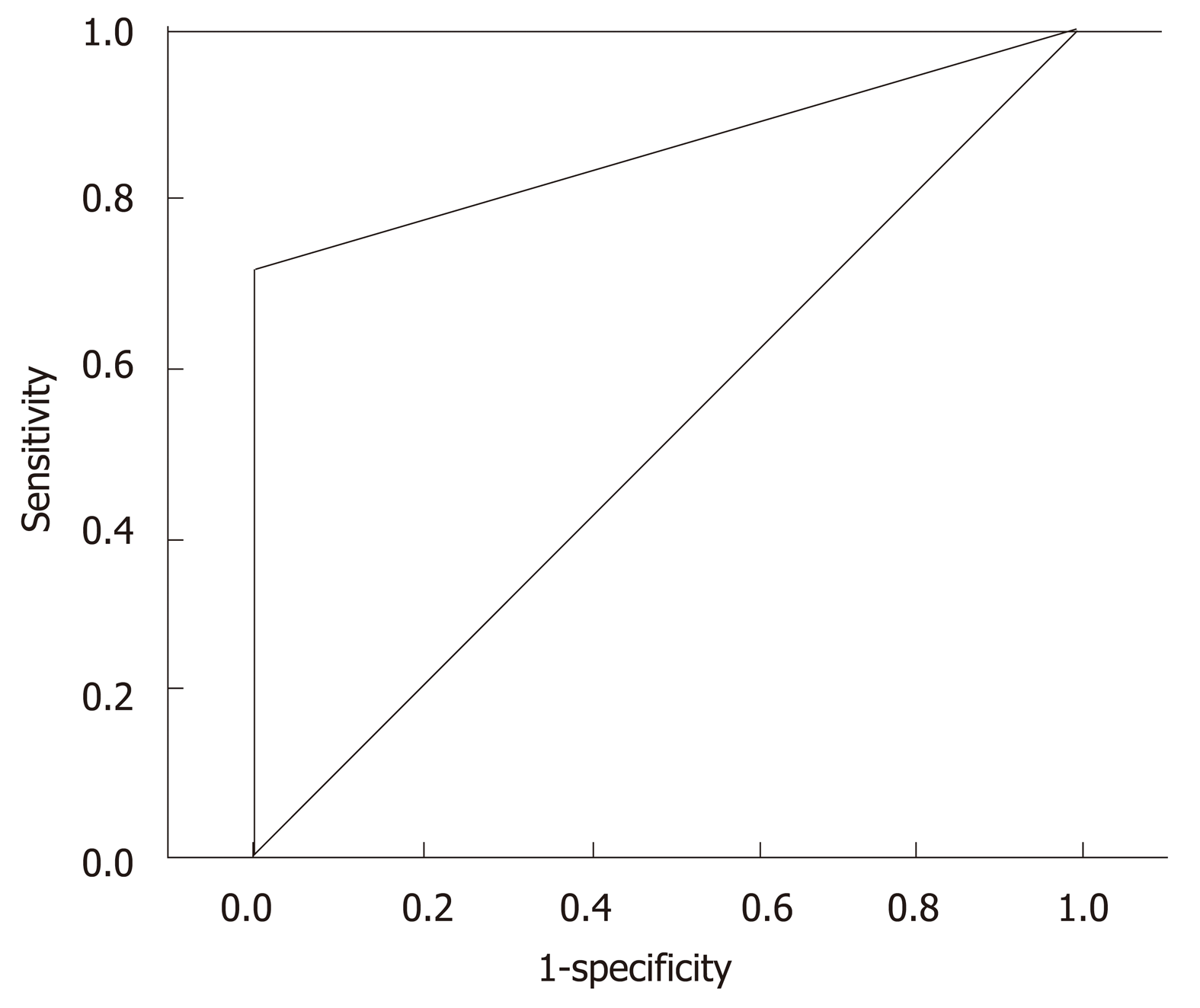
Considering the MRI as the gold standard, US used in PCN surveillance showed a sensitivity of 72%, negative predictive value of 94%, accuracy of 95% and AUC of a ROC curve of 86% (confidence interval 77%-94%, P < 0.001) (Figure 3).
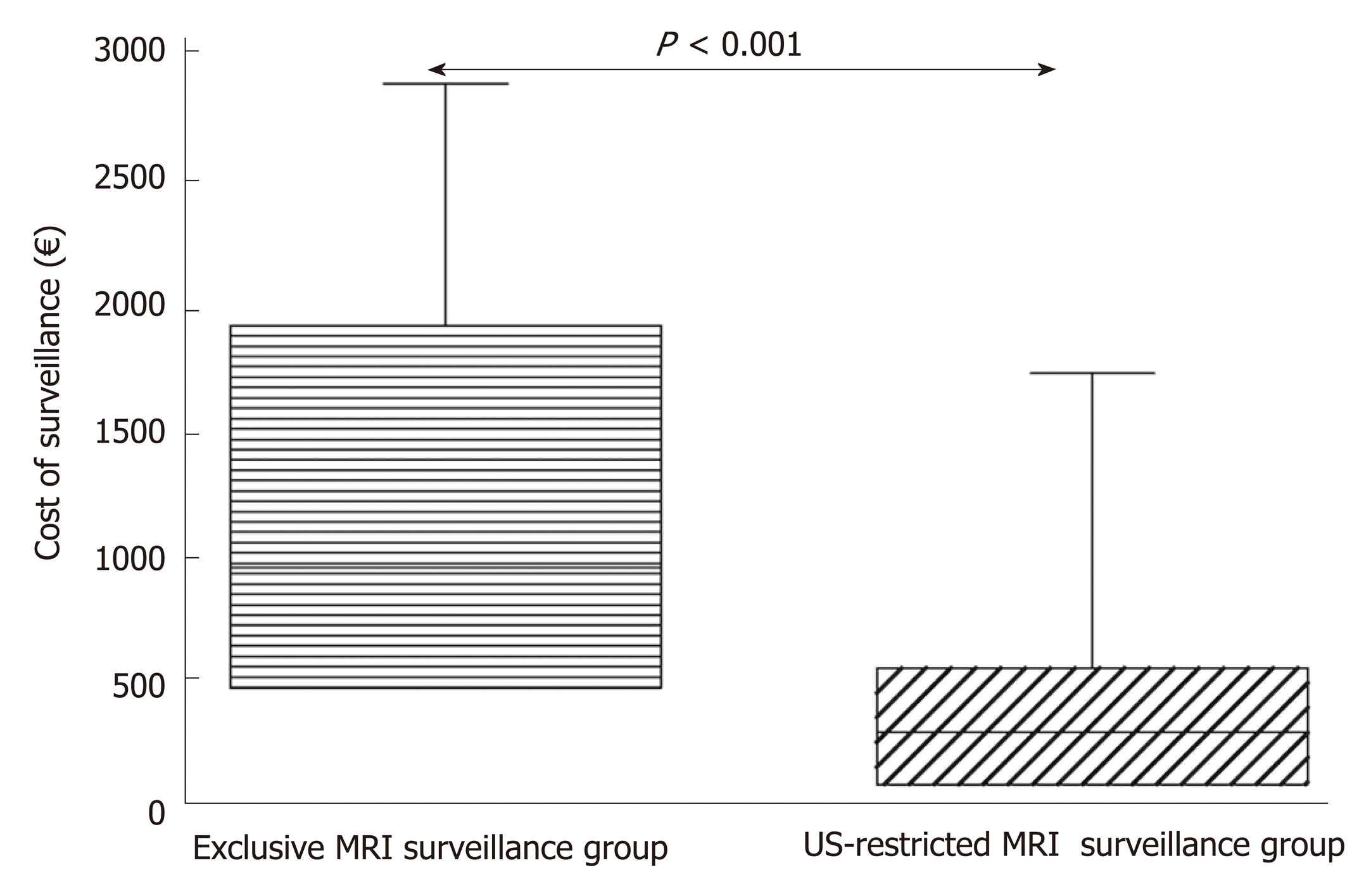
The mean cost of surveillance for each patient according to the proposed US-restricted MRI surveillance follow-up strategy was 366.4 ± 348.7 €. Had we used the surveillance recommended by the European evidenced-based guidelines with MRI in the same group of patients, the costs incurred would have been 1158.9 ± 798.6 € (P < 0.0001), i.e. nearly trebled (Figure 4).
However, the cost of our proposed US-restricted MRI-based surveillance could be higher than the above cost of 366 ± 349 €, since it would be influenced by the number of patients with PCN requiring urgent MRI. This is because of changes documented by surveillance imaging (total = 30 in present cohort) and/or the need for a contrast-based MRI scanning contrast phase (n = 5 in present study). The overall costs of our proposed follow-up strategy still remain significantly lower than the exclusive MRI-based surveillance, i.e. 907.2 ± 382.9 € vs 1511.6 ± 790.4 € respectively, P < 0.05).
The crucial objective in the clinical management of patients harboring PCN is the early identification of those at high risk of malignant degeneration[22]. To this effect, MRI is considered the gold standard imaging modality[13] in all the published guidelines[8,11], both in the diagnostic workup and in the subsequent follow-up. MRI is useful for establishing the diagnosis and presence of any connection between PCN and the ductal system, the baseline cystic diameter and other features. Likewise, the follow-up imaging-based surveillance used has to be capable of the risk features/changes predictive of neoplastic evolution[23-25]. Despite its proven efficacy, MRI has certain issues, including contrast-related side effects, claustrophobia, limited accessibility and high costs. EUS is helpful in resolving PCN with suspicious features, but on its own exhibits modest useful diagnostic performance for these lesions. However, when combined with fine-needle aspiration (FNA), the diagnostic yield and accuracy of EUS are increased significantly. Nevertheless, because of the invasive nature of FNA, this combination should be reserved in selected PNC cases with suspicious features on the MRI that suggest a need for surgery. Otherwise, because of its invasive nature, EUS with FNA is not suitable or recommended as a surveillance follow-up modality[11].
In the management of patients with PCN who are largely asymptomatic and often young, within the context of increasing costs of secondary and tertiary healthcare in both public and private hospitals, cost considerations cannot be ignored. The clinical management of patients is essentially based on image-based surveillance follow-up, and we need an imaging protocol that is both fit for purpose and affordable. In this context, US is an imaging modality worthy of consideration as an alternative imaging modality in the follow-up of PCN. US can be used to evaluate most patients by assessing pancreatic duct caliber, diameter of the wall, and the internal aspect of pancreatic cysts[19]. US scanning for the detection and evaluation of PCN has been analyzed in a few reports, with the most important being the report by Sun et al[19]. This seminal publication is based on a study involving 57 patients who underwent blinded US on the same day that each had an MRI. The authors demonstrate that an abdominal US for established PNC provides visualization and accurate measurement of many PCN of the cyst size, location, and other lesion characteristics. The conclusion from this study was that US was a valid adjunct of MRI in monitoring patients harboring PCN diagnosed by MRI. In another recent report, Jeon et al[20] reported that the detection rate and utility of US is significantly improved by repeat imaging if the initial diagnostic US image is available for use as a reference map; thereby confirming the increased usefulness of US in PCN surveillance. Apart from the patients’ stature, the cyst location, availability of initial (baseline) images that affect US successful evaluation, and different PCN changes are not detected equally by US. In line with these studies, we have shown that during follow-up, Wirsung duct caliber and cyst diameter are the factors that are well-visualized with US scan, whereas the development of new cysts and small mural nodes are detected less successfully. When detectable, the US features indicative of the development of new cysts and small mural nodes include the appearance of new anechoic areas in the pancreatic parenchyma and the appearance of a solid iso/iper-echoic component inside the anechoic cystic area[26].
Although dilatation of pancreatic duct caliber was clearly detected by US, the growth rate of PCN was missed in five cases and detected by MRI. However, all these missed lesions that measured smaller than 15 mm did not affect management. The development of new cyst(s) in other part of the pancreas was the main limitation of US surveillance, largely due to the inability for complete US exploration of the gland. However, the clinical importance of detecting small new PCN remains debatable. Since the clinical records of the patients included in the present study contained no data on mural nodes, the ability of US to detect these changes was not evaluated. Nevertheless, our view is that with conventional US, the difficulty of distinguishing genuine mural nodes from mucin plugs is substantial. CEUS can be useful to enhance the ability of conventional US to image and detect changes in the inner wall of pancreatic cysts[27]. In some reports[20], CEUS alone has not been found to be useful or reliable in documenting suspicious abnormalities that warrant change in management. In addition, these studies consider MRI to be better for detecting malignant transformation of IPMN, such that a detected intra-cystic solid mural node inevitably requires a second level MRI exam, thus limiting the utility of CEUS. The use of US as part of the surveillance protocol in the follow-up of patients with PCN has certain advantages: ease of performance by fully trained ultrasonographists, fast, widespread availability, and low cost.
The main potential risk of delaying the MRI imaging routine could be to miss early detection of worrisome features, however this has never been found in this study. Furthermore, even in this instance, the additional risk appears to be very low. In fact, relative indications for surgery according to European evidence-based guidelines[11] are not an expression of degeneration but only of increased risk, which is estimated in about 5.7% in patients with one relative indication for surgery[28].
Although CEUS has been proven to be more sensitive than US, it is a more complex procedure to be performed, requires venous access and the availability of the contras media, and is more time-consuming and costly (about double with respect to a conventional US). Furthermore, CEUS is not as panoramic as the MRI, because the various phases must be focused only on a precise target instead of on the whole gland.
For these reasons, we think that CEUS is a good diagnostic technique in selected cases, particularly when we have to study a precise finding of a B mode US, as an alternative or complimentary study to MRI. However, it is not a good technique for a routine follow-up.
The results of the present study indicate the potential benefit of including US scanning with restricted MRI, as outlined in our hospital protocol, for surveillance of patients with PCN. The study has confirmed that restricting MRI imaging to patients with progressive PCN modifications identified by abdominal US can reduce hospital costs without incurring missing patients who develop changes that require prompt surgical intervention or overt malignant transformation. Several studies[29-31] have confirmed the utility and importance of a cheaper imaging alternative to the MRI protocol. In our institution, the MRI protocol includes diffusion-weighted imaging for a more reliable definition of suspicious PCN elements, together with the routine use of contrast-enhanced sequences. The routine MRI sequences differ substantially from those reported by Pozzi-Mucelli et al[30], and our costs seem lower despite the comprehensive protocol. In Italy, several factors influence the imaging workflow, and these include examination time. US is the most acceptable imaging modality in this setting because it is quick, widely accessible and low cost. This is confirmed by the Italian Consensus guideline for diagnostic work-up and follow-up of PCN[17]. This may be different in other countries where a short MRI is preferred.
The primary goal of any surveillance program is to reduce the frequency of high-level tests exemplified by MRI without compromising patient safety. The shortcomings of US are related to the patients’ acoustic window and the undeniable fact that US is operator-dependent. Hence, the successful outcome of the present study may be related to the expertise of the surgeon US operators involved. For this reason, we believe that this program should be followed only in tertiary care hospitals by a dedicated team with specialist expertise in managing pancreatico-biliary disorders. Even so, there can be no doubt that patients/PNC locations with poor acoustic window cannot be safely followed by US. In our opinion, the standard exclusive MRI surveillance is needed for the follow-up of these patients.
We acknowledge that the study has some limitations. The first is its retrospective nature, which prevented the inclusion of patients with PCN who could not be assessed by US because of a poor acoustic window, thereby increasing the risk of selection bias. However, in the literature, cases with poor acoustic windows precluding US assessment are not common and range from 2%-12% of cases[32]. The retrospective nature of the study may also influence extrapolated cost estimations based on the same cohort of patients undergoing surveillance by exclusive MRI surveillance. Another limitation of the study is the short follow-up, as this may inflate the performance of US since many PCNs remained unchanged during the study period. Clearly, the two surveillance regimens for patients with PCN (MRI-based surveillance (current gold standard) vs our proposed US-restricted MRI surveillance protocols) need to be evaluated and confirmed by a prospective RCT with both clinical and health economic endpoints.
In conclusion, in patients with good US window, and with PCN without absolute or relative surgical criteria, abdominal US performed by an expert physician could be a safe complementary approach to MRI. This would delay and reduce the numbers of second-level examinations and therefore reduce the cost of surveillance. Considering the growing pressure for the allocation of healthcare resources, US is an inexpensive option for follow-up of a large number of PCN patients in a protracted period. However, the proposed abdominal US-restricted MRI surveillance protocol needs to be evaluated and confirmed by a prospective RCT against the currently recommended MRI-based surveillance, with the RCT having both clinical and health economic endpoints.
The current international guidelines only consider magnetic resonance imaging (MRI) for the follow-up of patients with pancreatic cystic neoplasms (PCN). Given the great number of patients with PCN that have to be followed-up due to the inherent risk of malignant progression, the use of abdominal ultrasound (US) might be a quick, easily accessible and cost-saving imaging modality. Recent publications have evaluated the role of US in monitoring PCN, but none have proposed a safe alternative follow-up surveillance based on US with restricted MRI use.
We performed this study in order to evaluate the safety and cost-efficacy of US as a diagnostic tool to simplify the follow-up of selected patients with low risk pancreatic cystic neoplasms.
The objectives of this study were: (1) to evaluate the safety of the use of US in the surveillance of patients with good acoustic window and low-risk pancreatic cystic neoplasms; and (2) to propose an alternative follow-up protocol that reduces the cost with respect to the cost incurred by current international guidelines.
We retrospectively evaluated the safety and costs of a follow-up surveillance for patients with low-risk PCN, performed with 6 monthly abdominal US for the first year, and then annually and with recourse to MRI scans performed every 2 years, or for confirmation of suspicious US findings.
Between January 2012 and January 2017, we followed 200 patients with a specific protocol that included abdominal US scans for pancreatic cystic neoplasms. During a follow-up period of 25.1 ± 18.2 mo, MRI identified evolution of the lesions not detected by US in only 11 patients (5.5%). However, MRI every 6 mo would not have changed patient management in any case. The mean cost of surveillance for each patient based on theoretical application MRI surveillance (recommended by international guidelines) within the group of patients included in the study would have incurred costs of 1158.9 ± 798.6 €, compared to the surveillance costs incurred by the proposed US-restricted MRI protocol of 366.4 ± 348.7 € (P < 0.0001).
Abdominal US seems to provide a cost-effective surveillance that reduces the frequency of MRI scans without affecting patient outcome. This is important in reducing the financial burden on hospital healthcare, aside from reducing the examination time and MRI-related issues and side effects. For patients with PCN, we have proposed a follow-up surveillance that includes abdominal US, and demonstrated that it is safe and complementary to MRI. In addition, it effectively delays and reduces the number of MRI scans, thereby reducing the cost of surveillance.
The results of the present study need to be confirmed by a comparative prospective randomized trial with both clinical (long-term patient outcome safety) and health economic primary endpoints.
The authors thank Prof. Sir Alfred Cuschieri for constructive criticism and for providing language editing.
| 1. | Chang YR, Park JK, Jang JY, Kwon W, Yoon JH, Kim SW. Incidental pancreatic cystic neoplasms in an asymptomatic healthy population of 21,745 individuals: Large-scale, single-center cohort study. Medicine (Baltimore). 2016;95:e5535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 2. | Zhang XM, Mitchell DG, Dohke M, Holland GA, Parker L. Pancreatic cysts: depiction on single-shot fast spin-echo MR images. Radiology. 2002;223:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 281] [Article Influence: 11.7] [Reference Citation Analysis (1)] |
| 3. | Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 446] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 4. | Farrell JJ. Prevalence, Diagnosis and Management of Pancreatic Cystic Neoplasms: Current Status and Future Directions. Gut Liver. 2015;9:571-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 5. | Jana T, Shroff J, Bhutani MS. Pancreatic cystic neoplasms: Review of current knowledge, diagnostic challenges, and management options. J Carcinog. 2015;14:3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S; International Association of Pancreatology. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1539] [Cited by in RCA: 1457] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 7. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K; International Association of Pancreatology. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1646] [Article Influence: 117.6] [Reference Citation Analysis (0)] |
| 8. | Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 1237] [Article Influence: 137.4] [Reference Citation Analysis (1)] |
| 9. | Vege SS, Ziring B, Jain R, Moayyedi P; Clinical Guidelines Committee; American Gastroenterology Association. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819-22; quize12-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 808] [Article Influence: 73.5] [Reference Citation Analysis (1)] |
| 10. | Del Chiaro M, Verbeke C, Salvia R, Klöppel G, Werner J, McKay C, Friess H, Manfredi R, Van Cutsem E, Löhr M, Segersvärd R; European Study Group on Cystic Tumours of the Pancreas. European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis. 2013;45:703-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 337] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 11. | European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1144] [Cited by in RCA: 976] [Article Influence: 122.0] [Reference Citation Analysis (1)] |
| 12. | Kim YC, Choi JY, Chung YE, Bang S, Kim MJ, Park MS, Kim KW. Comparison of MRI and endoscopic ultrasound in the characterization of pancreatic cystic lesions. AJR Am J Roentgenol. 2010;195:947-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Pinho DF, Rofsky NM, Pedrosa I. Incidental pancreatic cysts: role of magnetic resonance imaging. Top Magn Reson Imaging. 2014;23:117-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Megibow AJ, Baker ME, Morgan DE, Kamel IR, Sahani DV, Newman E, Brugge WR, Berland LL, Pandharipande PV. Management of Incidental Pancreatic Cysts: A White Paper of the ACR Incidental Findings Committee. J Am Coll Radiol. 2017;14:911-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 234] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 15. | Budde C, Beyer G, Kühn JP, Lerch MM, Mayerle J. The Clinical and Socio-Economic Relevance of Increased IPMN Detection Rates and Management Choices. Viszeralmedizin. 2015;31:47-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Chiang AL, Lee LS. Clinical approach to incidental pancreatic cysts. World J Gastroenterol. 2016;22:1236-1245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Italian Association of Hospital Gastroenterologists and Endoscopists, Italian Association for the Study of the Pancreas, Cystic Pancreatic Neoplasm Study Group; Buscarini E, Pezzilli R, Cannizzaro R, De Angelis C, Gion M, Morana G, Zamboni G, Arcidiacono P, Balzano G, Barresi L, Basso D, Bocus P, Calculli L, Capurso G, Canzonieri V, Casadei R, Crippa S, D'Onofrio M, Frulloni L, Fusaroli P, Manfredi G, Pacchioni D, Pasquali C, Rocca R, Ventrucci M, Venturini S, Villanacci V, Zerbi A, Falconi M. Italian consensus guidelines for the diagnostic work-up and follow-up of cystic pancreatic neoplasms. Dig Liver Dis. 2014;46:479-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | D'Onofrio M, Barbi E, Dietrich CF, Kitano M, Numata K, Sofuni A, Principe F, Gallotti A, Zamboni GA, Mucelli RP. Pancreatic multicenter ultrasound study (PAMUS). Eur J Radiol. 2012;81:630-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Sun MRM, Strickland CD, Tamjeedi B, Brook A, Mortele KJ, Brook OR, Kane RA, Siewert B. Utility of transabdominal ultrasound for surveillance of known pancreatic cystic lesions: prospective evaluation with MRI as reference standard. Abdom Radiol (NY). 2018;43:1180-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Jeon JH, Kim JH, Joo I, Lee S, Choi SY, Han JK. Transabdominal Ultrasound Detection of Pancreatic Cysts Incidentally Detected at CT, MRI, or Endoscopic Ultrasound. AJR Am J Roentgenol. 2018;210:518-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Arizono S, Isoda H, Maetani YS, Hirokawa Y, Shimada K, Nakamoto Y, Shibata T, Togashi K. High spatial resolution 3D MR cholangiography with high sampling efficiency technique (SPACE): comparison of 3T vs. 1.5T. Eur J Radiol. 2010;73:114-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Yu MH, Lee JY, Kim JH, Han JK, Choi BI. Value of near-isovoxel ultrasound for evaluation of ductal communications with pancreatic cystic lesions: correlation with magnetic resonance cholangiopancreatography. Ultrasound Med Biol. 2013;39:2279-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Pergolini I, Sahora K, Ferrone CR, Morales-Oyarvide V, Wolpin BM, Mucci LA, Brugge WR, Mino-Kenudson M, Patino M, Sahani DV, Warshaw AL, Lillemoe KD, Fernández-Del Castillo C. Long-term Risk of Pancreatic Malignancy in Patients With Branch Duct Intraductal Papillary Mucinous Neoplasm in a Referral Center. Gastroenterology. 2017;153:1284-1294.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 190] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 24. | Lekkerkerker SJ, Besselink MG, Busch OR, Dijk F, Engelbrecht MR, Rauws EA, Fockens P, van Hooft JE. Long-term follow-up of neoplastic pancreatic cysts without high-risk stigmata: how often do we change treatment strategy because of malignant transformation? Scand J Gastroenterol. 2016;51:1138-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Dewhurst CE, Mortele KJ. Cystic tumors of the pancreas: imaging and management. Radiol Clin North Am. 2012;50:467-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Fujita M, Itoi T, Ikeuchi N, Sofuni A, Tsuchiya T, Ishii K, Kamada K, Umeda J, Tanaka R, Tonozuka R, Honjo M, Mukai S, Moriyasu F. Effectiveness of contrast-enhanced endoscopic ultrasound for detecting mural nodules in intraductal papillary mucinous neoplasm of the pancreas and for making therapeutic decisions. Endosc Ultrasound. 2016;5:377-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Beyer-Enke SA, Hocke M, Ignee A, Braden B, Dietrich CF. Contrast enhanced transabdominal ultrasound in the characterisation of pancreatic lesions with cystic appearance. JOP. 2010;11:427-433. [PubMed] |
| 28. | Pérez-Cuadrado-Robles E, Uribarri-González L, Borbath I, Vila JJ, López-López S, Deprez PH. Risk of advanced lesions in patients with branch-duct IPMN and relative indications for surgery according to European evidence-based guidelines. Dig Liver Dis. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Macari M, Lee T, Kim S, Jacobs S, Megibow AJ, Hajdu C, Babb J. Is gadolinium necessary for MRI follow-up evaluation of cystic lesions in the pancreas? Preliminary results. AJR Am J Roentgenol. 2009;192:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Nougaret S, Reinhold C, Chong J, Escal L, Mercier G, Fabre JM, Guiu B, Molinari N. Incidental pancreatic cysts: natural history and diagnostic accuracy of a limited serial pancreatic cyst MRI protocol. Eur Radiol. 2014;24:1020-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Pozzi-Mucelli RM, Rinta-Kiikka I, Wünsche K, Laukkarinen J, Labori KJ, Ånonsen K, Verbeke C, Del Chiaro M, Kartalis N. Pancreatic MRI for the surveillance of cystic neoplasms: comparison of a short with a comprehensive imaging protocol. Eur Radiol. 2017;27:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P-Reviewer: de Moura DTH, Dietrich CF S-Editor: Ma RY L-Editor: Filipodia E-Editor: Zhang YL