Published online Mar 28, 2019. doi: 10.3748/wjg.v25.i12.1513
Peer-review started: December 21, 2018
First decision: January 18, 2019
Revised: January 29, 2019
Accepted: February 22, 2019
Article in press: February 23, 2019
Published online: March 28, 2019
Processing time: 99 Days and 3.3 Hours
Nonalcoholic fatty liver disease (NAFLD) is currently the outstanding cause of chronic liver disease in children and adolescents, especially in overweight and obese groups. Liver biopsy is the reference standard to diagnose NAFLD but invasive, thus it is not the best choice in clinical diagnosis and follow-up. Magnetic resonance (MR) is widely used in clinical trials to noninvasively quantify liver fat content in adults and children in foreign countries. While currently, it is rarely used in Chinese children and adolescents. We postulated that quantifying hepatic steatosis by MR could be extended to children and adolescents in China.
To investigate the accuracy of MR imaging (MRI) in quantifying liver fat with MR spectroscopy (MRS) as a reference. A secondary goal was to assess the prevalence of NAFLD in overweight and obese Chinese children and adolescents.
There were 86 children and adolescents enrolled in this study, including 65 overweight and obese children and 21 healthy children. The participants underwent MRI and MRS. MRI and MRS were performed using multi-echo Dixon and HISTO sequences, respectively, to calculate hepatic proton density fat fraction (PDFF). Hepatic steatosis was diagnosed using MRS-PDFF > 5% as the threshold. Spearman’s analysis was used to evaluate the correlation between MRI and MRS. The agreement between these two methods was assessed by Bland-Altman analysis.
The MRI-PDFF in the MRS region of interest and the entire liver was 9.9% ± 10.3% with a range of 0.3%-39.9%, and 10.6% ± 9.4% with a range of 1.9%-38.9%, respectively. The MRS-PDFF was 9.1% ± 10.0%, with a range of 0.5%-37.8%. The incidence of hepatic steatosis detected by MRS-PDFF was 46.5% (40/86) of all participants, all of whom belonged to the overweight and obese group. Spearman’s analysis indicated an excellent correlation between multi-echo Dixon and MRS (r > 0.9, P < 0.01). Bland-Altman analysis also demonstrated a good agreement between these two methods.
Multi-echo Dixon shows an excellent correlation and agreement with MRS in quantifying liver fat content and could be a potential tool to detect hepatic steatosis in Chinese children and adolescents.
Core tip: Magnetic resonance spectroscopy (MRS) is thought to be the noninvasive gold standard in the quantification of hepatic steatosis. The present study investigated the accuracy of magnetic resonance imaging (MRI) in quantifying liver fat content in Chinese children and adolescents, with MRS as a reference. MRI and MRS were performed with multi-echo Dixon and HISTO sequences, respectively, to calculate hepatic proton density fat fraction. Multi-echo Dixon showed an excellent correlation and agreement with MRS in quantifying liver fat content, indicating that it could be a potential tool to detect hepatic steatosis in Chinese children and adolescents.
- Citation: Zhao YZ, Gan YG, Zhou JL, Liu JQ, Cao WG, Cheng SM, Bai DM, Wang MZ, Gao FQ, Zhou SM. Accuracy of multi-echo Dixon sequence in quantification of hepatic steatosis in Chinese children and adolescents. World J Gastroenterol 2019; 25(12): 1513-1523
- URL: https://www.wjgnet.com/1007-9327/full/v25/i12/1513.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i12.1513
Nonalcoholic fatty liver disease (NAFLD) is the outstanding cause of chronic liver disease in children, especially in overweight and obese groups. The characteristic manifestation of NAFLD is the deposit of fat within more than 5% of hepatocytes, that is, hepatic steatosis. Steatosis may develop into nonalcoholic steatohepatitis, cirrhosis, or hepatocellular carcinoma. NAFLD is also a risk factor for liver transplantation and chronic kidney disease[1-3]. A study by the World Health Organization indicated that, over the past four decades, the number of obese children and adolescents around the world has increased 10 times, and that this number reached 124 million in 2016[4]. A meta-analysis by Anderson et al[5] demonstrated that the prevalence of NAFLD in general children was 7.6%, while it was 34.2% in obese children. Researchers have found that lifestyle intervention and weight loss can reduce liver fat content[6,7]. Therefore, early diagnosis of NAFLD is very important.
Liver biopsy is the reference standard for diagnosing NAFLD, but it is invasive and sample limited, which may lead to misdiagnosis[8,9]. For children, it is difficult to tolerate the operation and the required sedation. Therefore, there is an urgent need for a noninvasive method to diagnose NAFLD in clinical work. Ultrasonography (US) is currently the primary imaging method to detect hepatic steatosis because it is cheap, does not require radiation exposure, and is easily available. However, US cannot quantify liver fat content and has a poor sensitivity and specificity for diagnosing mild steatosis. Based on the ratio of Hounsfield units of liver and kidney parenchyma, computed tomography (CT) can detect steatosis quickly and reproducibly. The major drawbacks of CT are radiation exposure and poor diagnostic accuracy for mild hepatic steatosis.
In recent years, magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS) have become accurate methods to quantify liver triglyceride concentration based on the difference in the resonant frequencies of water and fat[10]. MRS is thought to be the noninvasive gold standard in the quantification of hepatic steatosis. As early as 2005, Szczepaniak et al[11] used MRS as a reference to diagnose NAFLD in a large group of people. Then, some studies were performed using MRI to quantify liver fat content with MRS as reference and demonstrated equivalent accuracy to MRS[12-16]. In addition, further research validated the accuracy of MRI and MRS. Studies performed by Kukuk et al[17] and Kang et al[18] using multi-echo Dixon (ME Dixon), MRS, and liver biopsy indicated excellent correlations in the quantification of liver fat between these methods. Kang et al[18] also found that the correlation in the right lobe was higher than that in the left lobe. Satkunasingham et al[19] conducted a retrospective study on liver donor candidates with liver biopsy as a reference; the researchers demonstrated a significant correlation (r = 0.83) between ME Dixon and MRS (HISTO), and they showed that these two methods could obviate the need for liver biopsy when proton density fat fraction (PDFF) was < 5%. Furthermore, Bannas et al[20] performed MRI, MRS, liver biopsy, and biochemical triglyceride extraction on ex vivo human livers that were unsuitable for transplantation. These authors found that MRI showed a strong correlation with MRS, liver biopsy, and biochemical triglyceride extraction (r = 0.984, 0.850, and 0.871, respectively). Researchers in China also demonstrated that PDFF assessed by ME Dixon was highly correlated with MRS (HISTO) in adults[21]. Therefore, both MRI and MRS are accurate methods to quantify liver fat content, and liver biopsy may be obviated.
In recent pediatric studies, MRI also showed strong correlations with biopsy and MRS in the quantification of liver fat[22-25]. However, emerging confounder-corrected quantitative ME Dixon and HISTO methods have rarely been used in Chinese children and adolescents. The aim of the present study was to investigate the accuracy and agreement of ME Dixon in quantifying liver fat with MRS as a reference in Chinese children. A secondary goal was to assess the prevalence of NAFLD in overweight and obese children and adolescents.
The present study was approved by the Human Ethics Committee of Shenzhen Children’s Hospital. All parents were told about the aims and methods of the study and signed an informed consent form. Eighty-six participants were enrolled in the study from August 2017 to July 2018. All participants attended local middle school of Shenzhen, China. Among them, 65 participants were overweight and obese children [body mass index (BMI) above age- and gender-specific 85th/95th percentile], and 21 were age- and sex-matched healthy children. Their ages ranged from 9 to 17 years, with an average age of 13.6 years. The inclusion criteria of the overweight and obese children were no drinking history or alcohol consumption of less than 210 g per week for males, and less than 140 g per week for females, and BMI exceeding the classification criteria established by the Working Group on Obesity in China[26]. The inclusion criteria for the control group were that the BMI index and serum aminotransferase were normal. The exclusion criteria included type 1 diabetes, drug-induced hepatitis, hepatitis virus infection, hepatolenticular degeneration, chronic liver disease, or other chronic diseases that did harm to hepatic or renal function, alcohol consumption greater than the amounts mentioned above, contraindications of MRI including metallic implants, claustrophobia and so on. Age, sex, height, weight, BMI, and a brief survey of personal lifestyle habits and family medical history were recorded.
All patients underwent MRI scanning performed by an experienced technologist using a 3 Tesla MR unit (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany). Before scanning, a simple breath-hold training was performed by the participants. Coronal and transversal T2-weighted images were acquired to initially assess whether there were any imaging changes in the upper abdominal organs, especially the liver. MRI and MRS were conducted using ME Dixon and HISTO sequences, respectively, to calculate hepatic PDFF.
Multi-echo gradient echo sequences (ME Dixon; Siemens Healthcare, Erlangen, Germany) and online reconstruction (VIBE-Dixon; Siemens Healthcare, Erlangen, Germany) were performed with T2* correction[14,27]. A low flip angle (4°) was used to minimize the effects of T1 weighting[28,29]. In a 13 s breath-hold, six fractional echo magnitude images were acquired at 1.05, 2.46, 3.69, 4.92, 6.15, and 7.38 ms of echo times (TE). The repetition time (TR), section thickness, field of view, and voxel size were 9.00 ms, 3.5 mm, 450 mm, and 1.4 mm × 1.4 mm × 3.5 mm, respectively. The center of the liver, coil, and magnetic field were aligned before scanning. Screening Dixon (dual-echo VIBE-Dixon; Siemens Healthcare, Erlangen, Germany) and ME Dixon sequences were performed sequentially. The screening Dixon sequence was used to roughly and rapidly measure the liver fat fraction in patients. The TE, TR, field of view, flip angle, and section thickness of screening Dixon were 1.29 ms, 3.97 ms, 380 mm, 9° and 3 mm, respectively.
As a reference, we performed single-voxel MRS called HISTO with stimulated echo acquisition mode (STEAM). In addition, high-speed T2 correction was used to prevent over-evaluating PDFF, as the T2-decay of water and fat are different[28]. During a single 15 s breath hold, five STEAM spectra were generated at 12, 24, 36, 48, and 72 ms of TE. To minimize the effects of T1 weighting, a TR of 3000 ms was used. The flip angle was 90°, and a 15 mm × 15 mm × 15 mm region of interest (ROI) was placed on the largest surface of the right hepatic lobe (Couinaud segments V-VIII), avoiding major vessels and bile ducts.
MRI: The images were analyzed by an experienced MR physicist and reanalyzed by a deputy chief physician (all of them were unaware of the clinical/MRS data). Water images, fat images, goodness of fit images, MRI-PDFF maps and reports of screening and ME Dixon were acquired automatically. The results of the screening scan were defined as normal, and fat infiltration was defined according to a cut-off value of 5%. We evaluated the quality of the image on the goodness of fit images (goodness of fit = mean value/10, < 5% indicating that the images were acceptable). A 15-mm diameter circular ROI was colocalized with the MRS voxel on the corresponding PDFF map. The results of the screening Dixon, and PDFF values of the ROI and the entire liver were recorded.
MRS: After scanning, the system automatically went into postprocessing using the prototype software package (Siemens Healthcare, Erlangen, Germany). Peak amplitudes of water and fat at each TE were calculated with an exponential least-squares fitting algorithm. MRS-PDFF was estimated automatically based on the ratio of areas under fat peaks to the sum of areas under water and fat peaks. The spectral maps, water and fat peak curves, and PDFF values were recorded.
Statistical analyses were performed using SPSS 22.0 software. Subjects’ PDFF values are summarized as the mean ± standard deviation. Differences in MRI-PDFF and MRS-PDFF were evaluated using the Wilcoxon matched-pairs signed-rank test. Spearman’s analysis was used to evaluate the correlation between MRI and MRS. The agreement between these two methods was assessed by Bland-Altman analysis. According to the liver classification results of MRS-PDFF, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated to assess the diagnostic accuracy of MRI-PDFF. To assess the accuracy and find the optimal threshold, a receiver operating characteristics (ROC) curve was generated. A P-value < 0.05 indicated statistical significance.
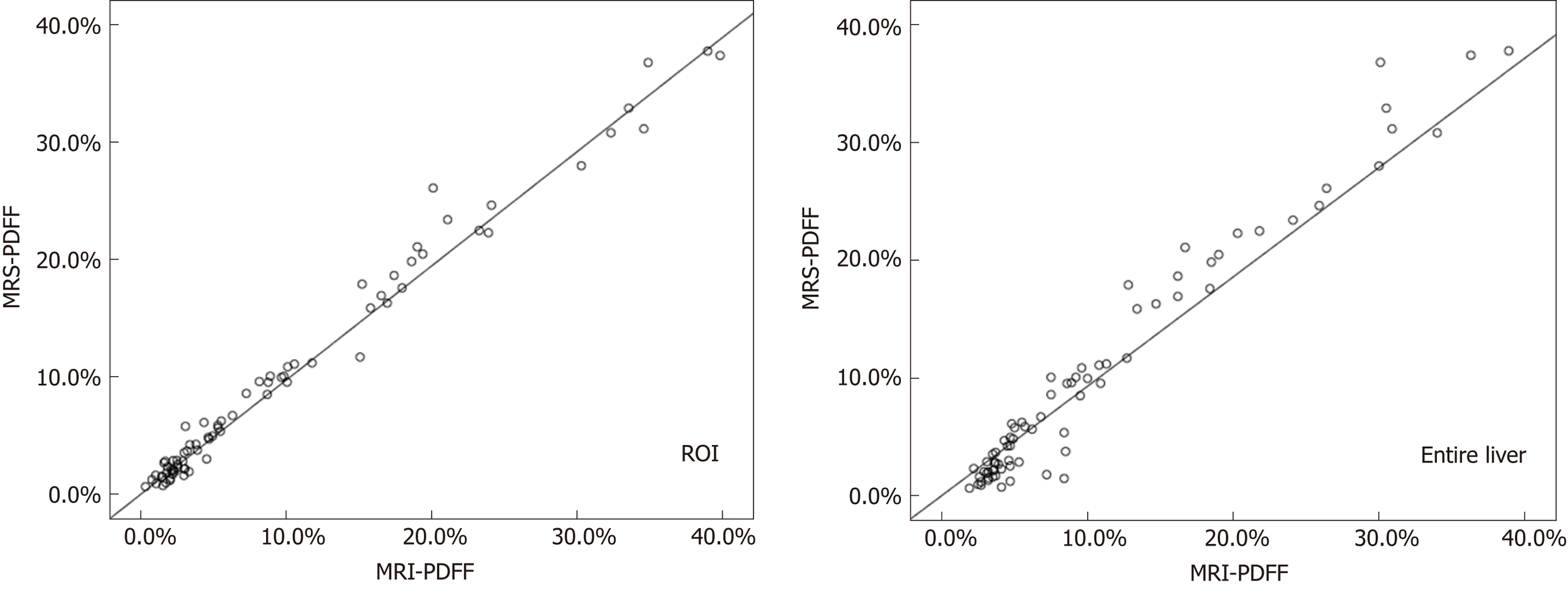
Participants were children and adolescents (mean age 13.6 ± 1.9 years, range 9-17 years) with a mean BMI of 26.3 ± 5.3 kg/m2 (range 16.6-38.0 kg/m2). The characteristics of the participants are shown in Table 1. The MRI-PDFF in the MRS ROI and the entire liver was 9.9% ± 10.3% with a range of 0.3%-39.9%, and 10.6% ± 9.4% with a range of 1.9%-38.9%, respectively. The MRS-PDFF was 9.1% ± 10.0%, with a range of 0.5%-37.8%. The MRS-PDFF and MRI-PDFF measured with ROI corresponding to the MRS voxel were not significantly different (P = 0.384). However, the MRS-PDFF was significantly lower than the MRI-PDFF measured with the entire liver (P = 0.038). Four groups of typical MRI-PDFF maps and matching MRS with varying percentages of PDFF values are presented in Figure 1.
| Variable | n (%) or mean ± SD | Minimum | Maximum |
| Sex (male) | 63 (73.3) | ||
| Age (yr) | 13.6 ± 1.9 | 9 | 17 |
| Weight (kg) | 72.5 ± 20.0 | 37.0 | 115.8 |
| Height (m) | 164.7 ± 10.7 | 133.0 | 190.0 |
| BMI (kg/m2) | 26.3 ± 5.3 | 16.6 | 38.0 |
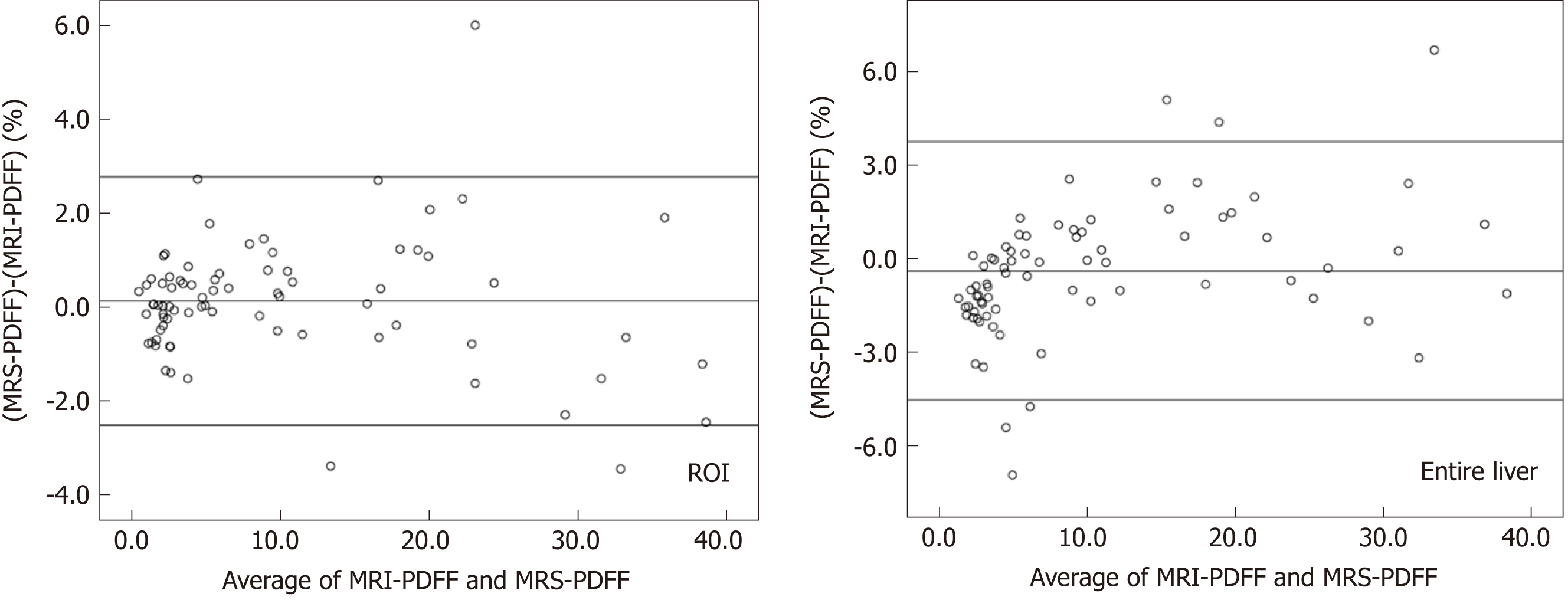
MRI-PDFF and MRS-PDFF values of all subjects were assessed by Spearman’s analysis. The results indicated an excellent correlation between MRI and MRS (r = 0.973, P < 0.01 when MRI-PDFF was measured with ROI corresponding to the MRS voxel; r = 0.929, P < 0.01 when MRI-PDFF was measured with the entire liver) (Figure 2). Bland-Altman analysis demonstrated a good agreement between these two methods with few outliers [bias 0.126%, 95% confidence interval (CI) (-2.513, 2.765), when MRI-PDFF was measured with ROI corresponding to the MRS voxel; bias -0.395%, 95%CI (-4.543, 3.753), when MRI-PDFF was measured with the entire liver] (Figure 3).
Hepatic steatosis was detected based on MRS-PDFF > 5% in 46.5% (40/86) of all participants and in 61.5% (40/65) of the overweight and obese group. With MRS-PDFF as a reference, the sensitivity, specificity, PPV, and NPV of MRI-PDFF were 95%, 100%, 100%, and 94.9%, respectively, based on the ROI at the same location as MRS, and they were 97.5%, 88.6%, 90.7%, and 96.9%, respectively, when covering the entire liver. For screening Dixon, 10 participants were diagnosed with steatosis, while the MRI-PDFF and MRS-PDFF values were lower than 5%.
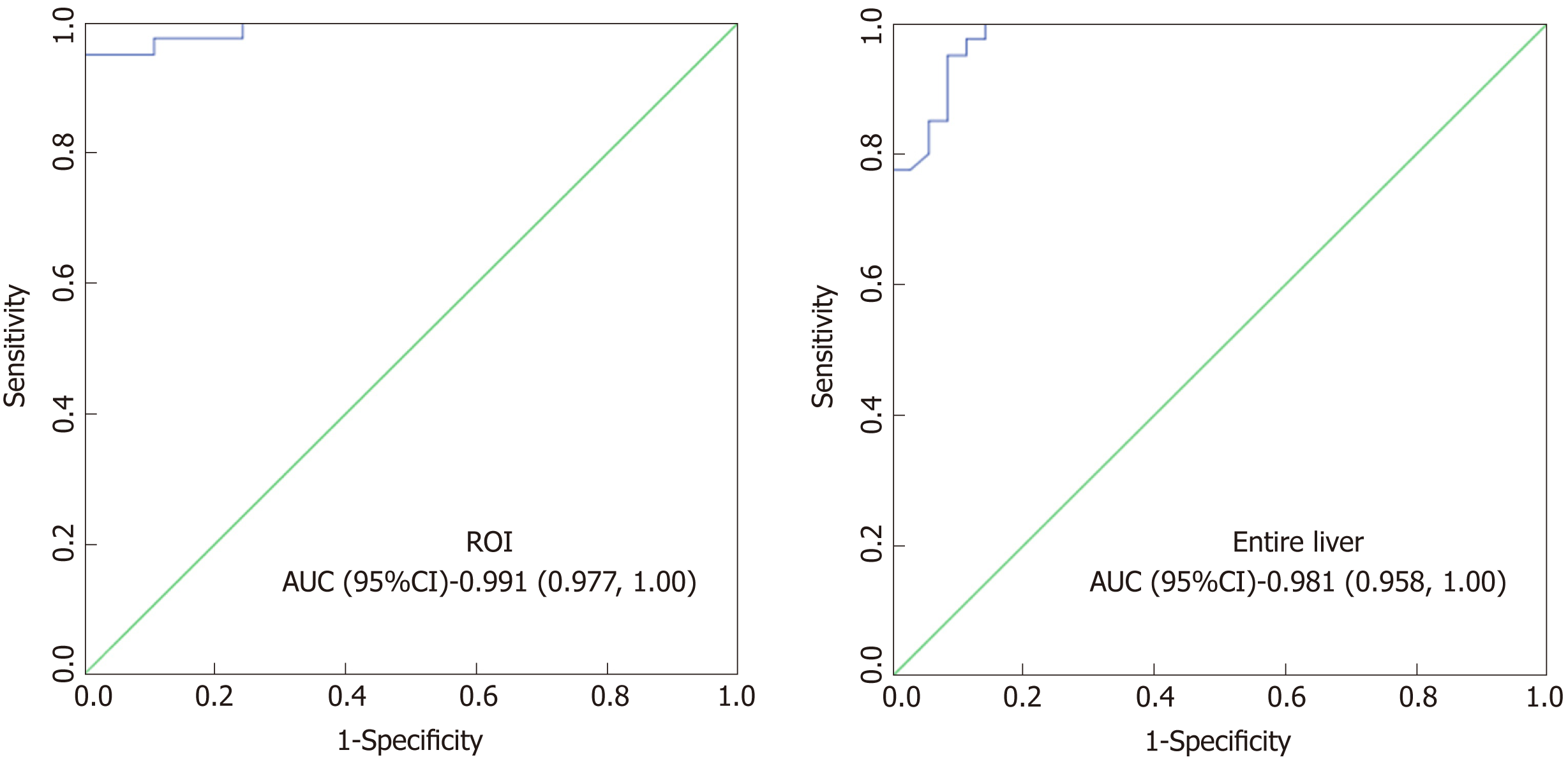
An ROC analysis was conducted to evaluate the diagnostic efficiency of MRI-PDFF for hepatic steatosis, with MRS-PDFF as a reference (Figure 4). MRI-PDFF was demonstrated to be a good predictor of steatosis with an area under the curve (AUC) of 0.991 (95%CI 0.977-1.00) based on an ROI corresponding to the MRS voxel, and an AUC of 0.981 (95%CI 0.958-1.00) based on the entire liver. According to the Youden index, the optimal MRI-PDFF threshold to detect hepatic steatosis was 5.1%, with a sensitivity and specificity of 95% and 100%, respectively, when MRI-PDFF values were measured with an ROI corresponding to the MRS voxel. Analogously, the optimal threshold for detecting hepatic steatosis was 5.4%, with a sensitivity and specificity of 95% and 91.4%, respectively, when MRI-PDFF values were measured with the entire liver.
In this observational study of Chinese children and adolescents, 3T MRI with an ME Dixon sequence accurately quantified liver fat content, with MRS (HISTO sequences) as a reference. This study indicates that quantifying hepatic steatosis by MRI in adults could be extended to children and adolescents[16,30]. These data demonstrate the feasibility and potential clinical utility of MRI in quantifying steatosis in children. In the present study, the prevalence of NAFLD was 46.5% among all subjects and 61.5% in overweight and obese children. The prevalence in our study is higher than 34.2%, a value reported in a meta-analysis by Anderson et al[5]. This may be due to differences in age, sex, race, region, and BMI. Additionally, studies with different diagnostic methods were enrolled in the meta-analysis. As steatosis, especially NAFLD, is closely related to atherosclerosis, chronic kidney disease, and changes in myocardial function[2,31,32], the present results are meaningful for the early diagnosis and prevention of NAFLD.
PDFF measured by ME Dixon and MRS had been validated as an accurate assessment of liver fat content that demonstrates an excellent correlation with liver biopsy[18]. In our study, we observed a strong correlation between MRI-PDFF and MRS-PDFF. Bland-Altman analysis also indicated a good agreement between these two methods. These findings are consistent with those of previous studies[16,30], indicating that the Dixon-based technique could be a potentially useful tool to quantify liver fat content for whole liver coverage.
With MRS-PDFF > 5% differentiating hepatic steatosis from normal fat fractions in our study, MRI achieved a good sensitivity and specificity in quantifying liver fat content. Moreover, MRI showed good potential as a tool for the early detection of hepatic steatosis. With MRS-PDFF > 5% as the cut-off value, the optimal thresholds for MRI-PDFF were 5.1% and 5.4%, when MRI-PDFF was measured with ROI and the entire liver, respectively. Research by Rehm et al[12] suggested that MRI-PDFF thresholds should be 3.0% and 3.5% for detecting steatosis with two metabolic syndrome criteria, which is in agreement with studies by Di Martino et al[25] and Nasr et al[33]. A lack of laboratory markers and liver biopsies are the main reason for the difference between previous studies and ours. Hence, further studies are needed to validate the optimal threshold of MRI-PDFF.
Compared with MRS, there were several advantages to using multi-echo MRI techniques in the quantification of hepatic steatosis in our study. First, MRI can quantify liver fat content of the entire liver in addition to ROI, thereby avoiding sampling errors when liver fat is inhomogeneously distributed. Second, there is no spatial error, as in-phase and opposed-phase images are acquired at the same time. Third, MRI is widely used, while the availability of MRS is limited. Although a previous study demonstrated that the best method to measure MRI-PDFF was calculating the average value of ROI in nine Couinaud segments[34], the MRI-PDFF of this study was measured with ROI only at the MRS location and the entire liver. A segmented measurement could be considered in future studies.
An advantage of the present study is the enrollment of relatively healthy children and adolescents for MRI and MRS. A limitation is that we did not perform liver biopsy. In previous MRI studies, known or suspected liver disease patients were enrolled. Therefore, liver biopsy was conducted for further diagnosis[17,18]. The aim of our study was to evaluate the accuracy of MRI in the quantification of hepatic steatosis in Chinese children and adolescents. Another goal was to assess the prevalence of NAFLD in the pediatric population. It is unethical to subject relatively healthy participants to liver biopsy evaluation. Furthermore, MRS could not acquire images during the breath-hold time, and a physicist was needed to conduct complex post-processing with specific analysis software[16,35]. However, the HISTO sequence we performed in the present study allowed rapid and simple processing for image acquisition in a single 15 s breath hold[27,36]. In addition, both MRI and MRS assess liver triglyceride content by the relative volume of fat protons. Therefore, MRI-PDFF and MRS-PDFF can be directly compared with each other, while liver biopsy evaluates the number of cells with intracellular fat but not volume. Therefore, we chose MRS rather than histologic examination as a reference.
The second limitation is that MRI segmentation in screening Dixon is sometimes not accurate. Muscles, major vessels, gallbladder, stomach, or kidneys may be included. Moreover, we cannot correct this manually, which may affect the accuracy of fat content measurements of the entire liver. This is one of the reasons why MRI-PDFF of ROI and the entire liver are different in our study. Another reason is sampling errors of ROI when hepatic fat infiltration is heterogeneous. This phenomenon may also occur in single-voxel MRS measurements.
The third limitation is the lack of serum metabolic markers. Studies have found that NAFLD is associated with serum lipid, liver enzyme, glucose, and insulin levels[12,37]. However, we did not collect blood samples from the participants to assess these risk factors. Further studies are needed to evaluate the correlation of these risk factors and liver fat content.
In conclusion, the present study demonstrates the accuracy of ME Dixon in the quantification of hepatic steatosis in Chinese children and adolescents, which showed an excellent correlation and agreement with MRS. The high prevalence of NAFLD in overweight and obese children is worthy of our attention.
Nonalcoholic fatty liver disease (NAFLD) is a common cause of chronic liver disease in children and adolescents. Many methods are used to diagnose NAFLD, including liver biopsy, ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI), and magnetic resonance spectroscopy (MRS). Liver biopsy is the gold standard to diagnose NAFLD but invasive, which is not the best choice in clinical use. Hence, screening for a noninvasive and accurate method to diagnose NAFLD in Chinese children and adolescents is of great importance.
MR is widely used in clinical trials to detect hepatic steatosis. Compared with US and CT, MR can provide more accurate and reliable diagnosis information. Besides, it can be used in early diagnosis and follow-up of NAFLD. Unfortunately, limited by samples and software device, it is rarely used in Chinese children and adolescents.
To investigate the accuracy and agreement between MRI and MRS in estimation of hepatic proton density fat fraction in Chinese children and adolescents, which will be helpful to early diagnosis and follow-up of NAFLD in China.
Eighty-six children and adolescents were enrolled in this study (mean age 13.6 ± 1.9 years, range 9-17 years) from Shenzhen, China. They underwent MRI and MRS scans with a 3 Tesla MR unit (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany). MRI and MRS were performed with multi-echo Dixon (ME Dixon) and HISTO sequences, respectively, to calculate hepatic proton density fat fraction (PDFF). Hepatic steatosis was defined as MRS-PDFF > 5%. The correlation and agreement between ME Dixon and MRS were assessed via spearman’s analysis and Bland-Altman analysis, respectively. According to the liver classification results of MRS-PDFF, the sensitivity, specificity, positive predictive value, and negative predictive value were calculated to assess the diagnostic accuracy of MRI-PDFF.
PDFF of all participants were calculated via MRI and MRS. With MRS as a reference, MRI exhibited high accuracy (r > 0.9, P < 0.01) and consistency in estimation of liver fat content. More importantly, MRI can quantify hepatic steatosis not only in region of interest but also the entire liver. Due to ethical restrictions, liver biopsy was not performed on our relatively healthy participants. Further study is needed to validate the accuracy of MRI and MRS in the quantification of hepatic steatosis in Chinese children and adolescents, with histology as a reference.
According to our study, MRI (ME Dixon) appears to detect hepatic steatosis in Chinese children and adolescents successfully and accurately, with MRS (HISTO sequences) as a reference. MRI (ME Dixon) may be an ideal tool for early diagnosis and follow-up of NAFLD in China.
This study describes a noninvasive tool for diagnosing NAFLD in Chinese children and adolescents. This method could be widely used in detecting hepatic steatosis in China, especially for those who have risk factors of NAFLD. Of course, liver biopsy and large sample sizes are needed in further study.
The authors thank all staff for excellent work and the clinical screening facility at Radiology Department, Shenzhen Children’s Hospital, Shenzhen, China. All MR scans were performed using a 3 Tesla MR unit.
| 1. | Losurdo G, Castellaneta A, Rendina M, Carparelli S, Leandro G, Di Leo A. Systematic review with meta-analysis: De novo non-alcoholic fatty liver disease in liver-transplanted patients. Aliment Pharmacol Ther. 2018;47:704-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 2. | Targher G, Byrne CD. Non-alcoholic fatty liver disease: An emerging driving force in chronic kidney disease. Nat Rev Nephrol. 2017;13:297-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 240] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 3. | Mann JP, Valenti L, Scorletti E, Byrne CD, Nobili V. Nonalcoholic Fatty Liver Disease in Children. Semin Liver Dis. 2018;38:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 4. | NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627-2642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5072] [Cited by in RCA: 4754] [Article Influence: 528.2] [Reference Citation Analysis (2)] |
| 5. | Anderson EL, Howe LD, Jones HE, Higgins JP, Lawlor DA, Fraser A. The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. PLoS One. 2015;10:e0140908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 459] [Cited by in RCA: 691] [Article Influence: 62.8] [Reference Citation Analysis (1)] |
| 6. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 5233] [Article Influence: 654.1] [Reference Citation Analysis (9)] |
| 7. | Medrano M, Cadenas-Sanchez C, Álvarez-Bueno C, Cavero-Redondo I, Ruiz JR, Ortega FB, Labayen I. Evidence-Based Exercise Recommendations to Reduce Hepatic Fat Content in Youth- a Systematic Review and Meta-Analysis. Prog Cardiovasc Dis. 2018;61:222-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | El-Badry AM, Breitenstein S, Jochum W, Washington K, Paradis V, Rubbia-Brandt L, Puhan MA, Slankamenac K, Graf R, Clavien PA. Assessment of hepatic steatosis by expert pathologists: The end of a gold standard. Ann Surg. 2009;250:691-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 243] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 9. | Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T; LIDO Study Group. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 1592] [Article Influence: 75.8] [Reference Citation Analysis (1)] |
| 10. | Reeder SB, Hu HH, Sirlin CB. Proton density fat-fraction: A standardized MR-based biomarker of tissue fat concentration. J Magn Reson Imaging. 2012;36:1011-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 391] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 11. | Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: Prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462-E468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1119] [Cited by in RCA: 1205] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 12. | Rehm JL, Wolfgram PM, Hernando D, Eickhoff JC, Allen DB, Reeder SB. Proton density fat-fraction is an accurate biomarker of hepatic steatosis in adolescent girls and young women. Eur Radiol. 2015;25:2921-2930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Yokoo T, Serai SD, Pirasteh A, Bashir MR, Hamilton G, Hernando D, Hu HH, Hetterich H, Kühn JP, Kukuk GM, Loomba R, Middleton MS, Obuchowski NA, Song JS, Tang A, Wu X, Reeder SB, Sirlin CB; RSNA-QIBA PDFF Biomarker Committee. Linearity, Bias, and Precision of Hepatic Proton Density Fat Fraction Measurements by Using MR Imaging: A Meta-Analysis. Radiology. 2018;286:486-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 262] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 14. | Yokoo T, Shiehmorteza M, Hamilton G, Wolfson T, Schroeder ME, Middleton MS, Bydder M, Gamst AC, Kono Y, Kuo A, Patton HM, Horgan S, Lavine JE, Schwimmer JB, Sirlin CB. Estimation of hepatic proton-density fat fraction by using MR imaging at 3.0 T. Radiology. 2011;258:749-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 247] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 15. | Tyagi A, Yeganeh O, Levin Y, Hooker JC, Hamilton GC, Wolfson T, Gamst A, Zand AK, Heba E, Loomba R, Schwimmer J, Middleton MS, Sirlin CB. Intra- and inter-examination repeatability of magnetic resonance spectroscopy, magnitude-based MRI, and complex-based MRI for estimation of hepatic proton density fat fraction in overweight and obese children and adults. Abdom Imaging. 2015;40:3070-3077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Hetterich H, Bayerl C, Peters A, Heier M, Linkohr B, Meisinger C, Auweter S, Kannengießer SA, Kramer H, Ertl-Wagner B, Bamberg F. Feasibility of a three-step magnetic resonance imaging approach for the assessment of hepatic steatosis in an asymptomatic study population. Eur Radiol. 2016;26:1895-1904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Kukuk GM, Hittatiya K, Sprinkart AM, Eggers H, Gieseke J, Block W, Moeller P, Willinek WA, Spengler U, Trebicka J, Fischer HP, Schild HH, Träber F. Comparison between modified Dixon MRI techniques, MR spectroscopic relaxometry, and different histologic quantification methods in the assessment of hepatic steatosis. Eur Radiol. 2015;25:2869-2879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 18. | Kang BK, Kim M, Song SY, Jun DW, Jang K. Feasibility of modified Dixon MRI techniques for hepatic fat quantification in hepatic disorders: Validation with MRS and histology. Br J Radiol. 2018;91:20170378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Satkunasingham J, Nik HH, Fischer S, Menezes R, Selzner N, Cattral M, Grant D, Jhaveri K. Can negligible hepatic steatosis determined by magnetic resonance imaging-proton density fat fraction obviate the need for liver biopsy in potential liver donors? Liver Transpl. 2018;24:470-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Bannas P, Kramer H, Hernando D, Agni R, Cunningham AM, Mandal R, Motosugi U, Sharma SD, Munoz del Rio A, Fernandez L, Reeder SB. Quantitative magnetic resonance imaging of hepatic steatosis: Validation in ex vivo human livers. Hepatology. 2015;62:1444-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 21. | Li SJ, Wang Z, Chen LG, Fu CX, Lu JP. 3.0T MRI multi-echo Dixon technique in quantitative analysis of liver fat in petients with nonalcoholic fatty liver disease. Di-er Junyi Daxue Xuebao. 2016;37:1088-1094. [DOI] [Full Text] |
| 22. | Deng J, Fishbein MH, Rigsby CK, Zhang G, Schoeneman SE, Donaldson JS. Quantitative MRI for hepatic fat fraction and T2* measurement in pediatric patients with non-alcoholic fatty liver disease. Pediatr Radiol. 2014;44:1379-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Pacifico L, Martino MD, Catalano C, Panebianco V, Bezzi M, Anania C, Chiesa C. T1-weighted dual-echo MRI for fat quantification in pediatric nonalcoholic fatty liver disease. World J Gastroenterol. 2011;17:3012-3019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Schwimmer JB, Middleton MS, Behling C, Newton KP, Awai HI, Paiz MN, Lam J, Hooker JC, Hamilton G, Fontanesi J, Sirlin CB. Magnetic resonance imaging and liver histology as biomarkers of hepatic steatosis in children with nonalcoholic fatty liver disease. Hepatology. 2015;61:1887-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 25. | Di Martino M, Pacifico L, Bezzi M, Di Miscio R, Sacconi B, Chiesa C, Catalano C. Comparison of magnetic resonance spectroscopy, proton density fat fraction and histological analysis in the quantification of liver steatosis in children and adolescents. World J Gastroenterol. 2016;22:8812-8819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 26. | Group of China Obesity Task Force; Ji CY. Body mass index reference norm for screening overweight and obesity in Chinese children and adolescents. Zhonghua Liuxingbingxue Zazhi. 2004;25:97-102. |
| 27. | Min J, Park HS, Kim YJ, Yu MH, Jung SI, Jeon HJ. Estimation of hepatic fat fraction using modified Dixon magnetic resonance imaging techniques: Effect of liver cirrhosis. Clin Imaging. 2018;51:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Bydder M, Yokoo T, Hamilton G, Middleton MS, Chavez AD, Schwimmer JB, Lavine JE, Sirlin CB. Relaxation effects in the quantification of fat using gradient echo imaging. Magn Reson Imaging. 2008;26:347-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 351] [Cited by in RCA: 346] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 29. | Liu CY, McKenzie CA, Yu H, Brittain JH, Reeder SB. Fat quantification with IDEAL gradient echo imaging: Correction of bias from T(1) and noise. Magn Reson Med. 2007;58:354-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 414] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 30. | Heba ER, Desai A, Zand KA, Hamilton G, Wolfson T, Schlein AN, Gamst A, Loomba R, Sirlin CB, Middleton MS. Accuracy and the effect of possible subject-based confounders of magnitude-based MRI for estimating hepatic proton density fat fraction in adults, using MR spectroscopy as reference. J Magn Reson Imaging. 2016;43:398-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol. 2016;65:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1049] [Cited by in RCA: 1058] [Article Influence: 105.8] [Reference Citation Analysis (0)] |
| 32. | Ozturk K, Uygun A, Guler AK, Demirci H, Ozdemir C, Cakir M, Sakin YS, Turker T, Sari S, Demirbas S, Karslıoğlu Y, Saglam M. Nonalcoholic fatty liver disease is an independent risk factor for atherosclerosis in young adult men. Atherosclerosis. 2015;240:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | Nasr P, Forsgren MF, Ignatova S, Dahlström N, Cedersund G, Leinhard OD, Norén B, Ekstedt M, Lundberg P, Kechagias S. Using a 3% Proton Density Fat Fraction as a Cut-Off Value Increases Sensitivity of Detection of Hepatic Steatosis, Based on Results From Histopathology Analysis. Gastroenterology. 2017;153:53-55.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 34. | Campo CA, Hernando D, Schubert T, Bookwalter CA, Pay AJV, Reeder SB. Standardized Approach for ROI-Based Measurements of Proton Density Fat Fraction and R2* in the Liver. AJR Am J Roentgenol. 2017;209:592-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 35. | Cassidy FH, Yokoo T, Aganovic L, Hanna RF, Bydder M, Middleton MS, Hamilton G, Chavez AD, Schwimmer JB, Sirlin CB. Fatty liver disease: MR imaging techniques for the detection and quantification of liver steatosis. Radiographics. 2009;29:231-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 213] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 36. | Pineda N, Sharma P, Xu Q, Hu X, Vos M, Martin DR. Measurement of hepatic lipid: High-speed T2-corrected multiecho acquisition at 1H MR spectroscopy--a rapid and accurate technique. Radiology. 2009;252:568-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 37. | Benetolo PO, Fernandes MIM, Ciampo IRLD, Elias-Junior J, Sawamura R. Evaluation of nonalcoholic fatty liver disease using magnetic resonance in obese children and adolescents. J Pediatr (Rio J). 2019;95:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fouad YM, Gregorio BM, Sugimura H S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Song H