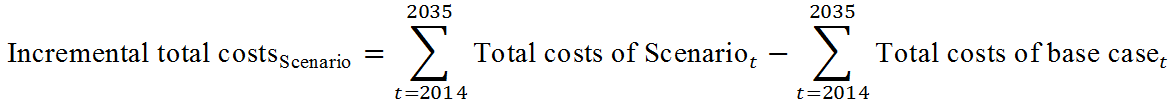Published online Mar 21, 2019. doi: 10.3748/wjg.v25.i11.1327
Peer-review started: October 16, 2018
First decision: December 5, 2018
Revised: February 20, 2019
Accepted: February 22, 2019
Article in press: February 22, 2019
Published online: March 21, 2019
Processing time: 155 Days and 13.1 Hours
Hepatitis C virus (HCV) is a leading cause of worldwide liver-related morbidity and mortality. The World Health Organization released an integrated strategy targeting HCV-elimination by 2030. This study aims to estimate the required interventions to achieve elimination using updated information for direct-acting antiviral (DAA) treatment coverage, to compute the total costs (including indirect/societal costs) of the strategy and to identify whether the elimination strategy is cost-effective/cost-saving in Greece.
To estimate the required interventions and subsequent costs to achieve HCV elimination in Greece.
A previously validated mathematical model was adapted to the Greek HCV-infected population to compare the outcomes of DAA treatment without the additional implementation of awareness or screening campaigns versus an HCV elimination strategy, which includes a sufficient number of treated patients. We estimated the total costs (direct and indirect costs), the disability-adjusted life years and the incremental cost-effectiveness ratio using two different price scenarios.
Without the implementation of awareness or screening campaigns, approximately 20000 patients would be diagnosed and treated with DAAs by 2030. This strategy would result in a 19.6% increase in HCV-related mortality in 2030 compared to 2015. To achieve the elimination goal, 90000 patients need to be treated by 2030. Under the elimination scenario, viremic cases would decrease by 78.8% in 2030 compared to 2015. The cumulative direct costs to eliminate the disease would range from 2.1-2.3 billion euros (€) by 2030, while the indirect costs would be €1.1 billion. The total elimination cost in Greece would range from €3.2-3.4 billion by 2030. The cost per averted disability-adjusted life year is estimated between €10100 and €13380, indicating that the elimination strategy is very cost-effective. Furthermore, HCV elimination strategy would save €560-895 million by 2035.
Without large screening programs, elimination of HCV cannot be achieved. The HCV elimination strategy is feasible and cost-saving despite the uncertainty of the future cost of DAAs in Greece.
Core tip: Elimination of hepatitis C virus (commonly known as HCV) cannot be achieved in Greece without the implementation of large awareness and screening programs, as treatment coverage will be suboptimal. To achieve the elimination goals, 90000 patients need to be treated by 2030. The overall cumulative cost of elimination would range from 3.2-3.4 billion euros by 2030. The HCV elimination strategy in Greece is feasible and cost-saving despite the uncertainty of the future cost of the direct-acting antivirals.
- Citation: Gountas I, Sypsa V, Papatheodoridis G, Souliotis K, Athanasakis K, Razavi H, Hatzakis A. Economic evaluation of the hepatitis C elimination strategy in Greece in the era of affordable direct-acting antivirals. World J Gastroenterol 2019; 25(11): 1327-1340
- URL: https://www.wjgnet.com/1007-9327/full/v25/i11/1327.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i11.1327
Hepatitis C virus (HCV) infection is a major public health problem; it affects 1% of the world population[1] and is one of the main causes of chronic liver disease-related death in the developed world[1,2]. It has been estimated that approximately 50%-85% of HCV cases developed chronic hepatitis. Many patients with chronic HCV (CHC) infection do not result in clinically apparent liver disease because it is generally a slow progressive infection. Five percent to 30% of chronically infected individuals developed cirrhosis over a span of 20 to 30 yr[3,4]. The recent introduction of direct-acting antivirals (DAAs) has the potential to change the future disease burden, as they achieve higher sustained virological response (SVR) rates, have fewer side effects and are simpler regimens compared to interferon (IFN)-based therapies[5,6]. Due to recent developments of antiviral treatments, the target of eliminating HCV by 2030 has become achievable[7]. Although the cost of providing DAAs has been extensively debated since their introduction, the cost and cost-effectiveness of implementing an HCV elimination strategy has recently been put on the agenda[8-12].
Greece has one of the highest prevalence rates of CHC infection in Europe, with approximately 33% of chronically infected patients in the advanced fibrosis stages (≥ F3)[13,14]. Additionally, it has an older infected population compared to other countries, meaning that the mean fibrosis progression is relatively rapid and the probability of HCV-related mortality or morbidity is high[15-17]. Therefore, although HCV prevalence in Greece has been decreasing since its peak in 2005, morbidity and mortality are forecasted to increase in the next years[14,18]. Moreover, the diagnostic rate is low (approximately 20%). Except for the significant HCV epidemic, Greece has faced a substantial financial crisis since 2008. Since then, the Greek economy has substantially shrunk; the gross domestic product (GDP) fell by 22%, about one-fifth of the aggregate production was lost and the public pharmaceutical expenditure was reduced by more than 50%[19].
Recently, a modeling study quantified the impact of IFN-free DAAs on HCV-related morbidity and mortality in Greece under the World Health Organization (WHO) Global Hepatitis Strategy[14]. This study showed that improved prevention strategies, large and effective screening programs and increased treatment coverage with DAAs were necessary to reach the goal of HCV elimination in Greece by 2030. To implement this strategy, it is vital to consider the cost of the proposed strategy, which poses significant financial challenges for the healthcare system.
The aims of this study are (1) to estimate the required interventions to achieve elimination using updated information for DAA treatment coverage, (2) to compute the total costs (including the indirect costs) of the strategy and to (3) identify whether the elimination strategy is cost-effective for Greece.
To estimate the current number of patients in the various disease stages and to project the future disease burden and the associated costs, we used an Excel-based disease progression model, which represents the natural history of CHC according to the METAVIR scoring system constructed by the Center for Disease Analysis (CO, United States)[20]. It has been used in several countries with country-specific data as input[18]. Appropriate input for Greece was obtained from the literature. Further details about the description of the model and Greek-specific epidemiological inputs have been previously published[14,18].
To examine the epidemiological and economic impact of the WHO Global Hepatitis Strategy, we created a scenario according to WHO recommendations and compared it with the current HCV management strategy in Greece, where patients are treated with INF-free DAA regimens with limited population coverage due to the low diagnostic rate and the lack of awareness or screening campaigns.
Base case: In the baseline scenario, patients are treated with IFN-free DAA regimens without the additional implementation of awareness or screening campaigns. This would lead to a gradual decrease in the available patients for treatment. Treatment is limited to fibrosis stage ≥ 2, which represents the 2017 national treatment guideline. Approximately 2,000 cases were treated in Greece in 2017. We assumed that this figure would be the same in 2018 but would gradually decrease to 1000 cases by 2020 and would then remain at this number. SVR rates were assumed to be 90% for genotype 1, 3 and 95% for genotype 2, 4[15] (Table 1).
| Scenario | Year | SVR | Treatment coverage/yr | Fibrosis stage | Diagnosed patients/yr |
| Base case | 2015-2016 | 90%-95% | ~1000 | ≥ F3 | 4000 |
| 2017-2019 | 95% | ~2000 | ≥ F2 | 3200 | |
| 2020-2021 | 95% | ~1300 | ≥ F2 | 2000 | |
| 2022-2023 | 95% | ~1000 | ≥ F2 | 1400 | |
| 2024-2035 | 95% | ~1000 | ≥ F2 | 1000 | |
| WHO Global Hepatitis Strategy | 2015-2016 | 90%-95% | ~1000 | ≥ F3 | 4000 |
| 2017-2019 | 95% | ~4700 | ≥ F2 | 4800 | |
| 2020-2021 | 95% | ~6800 | ≥ F0 | 6820 | |
| 2022-2023 | 95% | ~6800 | ≥ F0 | 6130 | |
| 2024-2035 | 95% | ~7000 | ≥ F0 | 6130 |
WHO Global Hepatitis Strategy: The WHO Global Hepatitis Strategy integrates both prevention and disease burden targets[7]. More specifically, the prevention target aims to reduce new infections by 90%, while the mortality target aims to reduce HCV mortality to 65% by 2030 compared to 2015. To achieve the WHO goals in Greece, the number of diagnosed and treated patients should gradually increase up to 7000 and 6800 patients per year, respectively. Initially, through 2018, patients with fibrosis stage ≥ F2 will be treated. After 2019, treatment coverage should be expanded to all patients (Table 1).
Regarding the economic portion of the model, we have computed the direct and the indirect/societal costs of HCV infection.
Direct and indirect/societal costs: Direct costs include the cost of antiviral treatment per treated patient per year, annual health care costs per patient, screening/diagnostic costs per patient, as well as laboratory costs per treated patient (Table 2)[21].
| Annual costs, € | |
| Lab costs for anti-HCV, RNA test, genotyping exam and liver biopsy/elastography | 350 |
| Screening cost per screen | 101 |
| Cost per diagnosed patient without antiviral treatment | |
| F0-F3 | 230 |
| Compensated cirrhosis, F4 | 1340 |
| Decompensated cirrhosis | 4460 |
| Hepatocellular carcinoma | 33000 |
| Liver transplantation | 1346002 |
| Liver transplant - subsequent years | 4600 |
| Antiviral treatment costs of DAAs | |
| 2015-2016 | 42000 |
| 2017-2023 | 13000 |
| 2024-2035 | 13000/85003 |
Data for healthcare costs were obtained through a database from Greek liver clinics. The annual cost for F0-F3 patients, for the third-party payer in Greece, without antiviral treatment, is 230 €. The costs of compensated cirrhosis (F4), decompensated cirrhosis and hepatocellular carcinoma (HCC) without the cost of antiviral treatments are 1340 €, 4460 €, and 33000 € per year, respectively. Liver transplant patients have a cost of 134630 € in the first year and 4640 € in subsequent years[22]. Lab costs (e.g., anti-HCV, RNA test, genotyping exam and liver biopsy/elastography) are 350 €, while the cost per anti-HCV screening is 10 €.
The average treatment cost per DAA-treated patient in Greece in 2016 was 42000 €[23]. Recently, price negotiations concerning the cost of DAAs were implemented, resulting in reduced treatment costs. The price per DAA regimen is confidential. However, the average cost per treatment can be calculated. According to the official press release of the Ministry of Health, a closed pharmaceutical budget of about 67.6 million has been committed to treat 5500 patients in the next 14 mo[24,25]. Dividing the budgeted money by the expected treated patients equates to a cost of treatment of about 12300 €. Furthermore, the Minister for Health stated[26,27] that the negotiation achieved a savings of 68% compared to the average pre-negotiated price (42000 € × 32% = 13400 €). Combining the above estimations, we assumed that the average cost of DAAs after negotiation can be estimated at 13000 € per treated patient.
Due to the considerable uncertainty of the future price evolution for DAAs, we considered two cost-scenarios. In the first scenario (conservative scenario), we assumed that the price of DAAs would remain constant through our study (until 2035), while in the second scenario (optimistic scenario), we assumed a further 35% price reduction after 2024. Under the base case, we assumed no price reduction of DAAs due to the limited number of treated patients (Table 2).
The indirect or societal cost was used to approximate loss of productivity due to HCV related disabilities and loss of life. The disability-adjusted life year (DALY) metric[28] was used to estimate the indirect costs of the disease. One DALY can be thought of as one lost year of "healthy" life. DALYs are computed by combining years of life lost (YLLs) and years lost due to disability (YLDs) and weighted by the severity of the disease[29]. Future direct and indirect costs were discounted at rate of 3%. The cost per DALY was estimated to be equal to the gross national income (GNI) per capita in 2016 (19000 €).
The cost-effectiveness of the elimination strategy was estimated using the incremental cost-effectiveness ratio (ICER), which have been compared to the GNI per capita of Greece in 2016. If ICER is lower than 1 GNI per capita, then the intervention is considered “highly cost effective”[30].
ICER = (Cost of examine strategy - cost of the base strategy)/|DALYs of examine strategy - DALYs of the base strategy|
We considered a strategy as “cost-saving” when the difference between all direct and indirect costs of the elimination scenario up to 2035 from those of the base case was positive.
Math 1
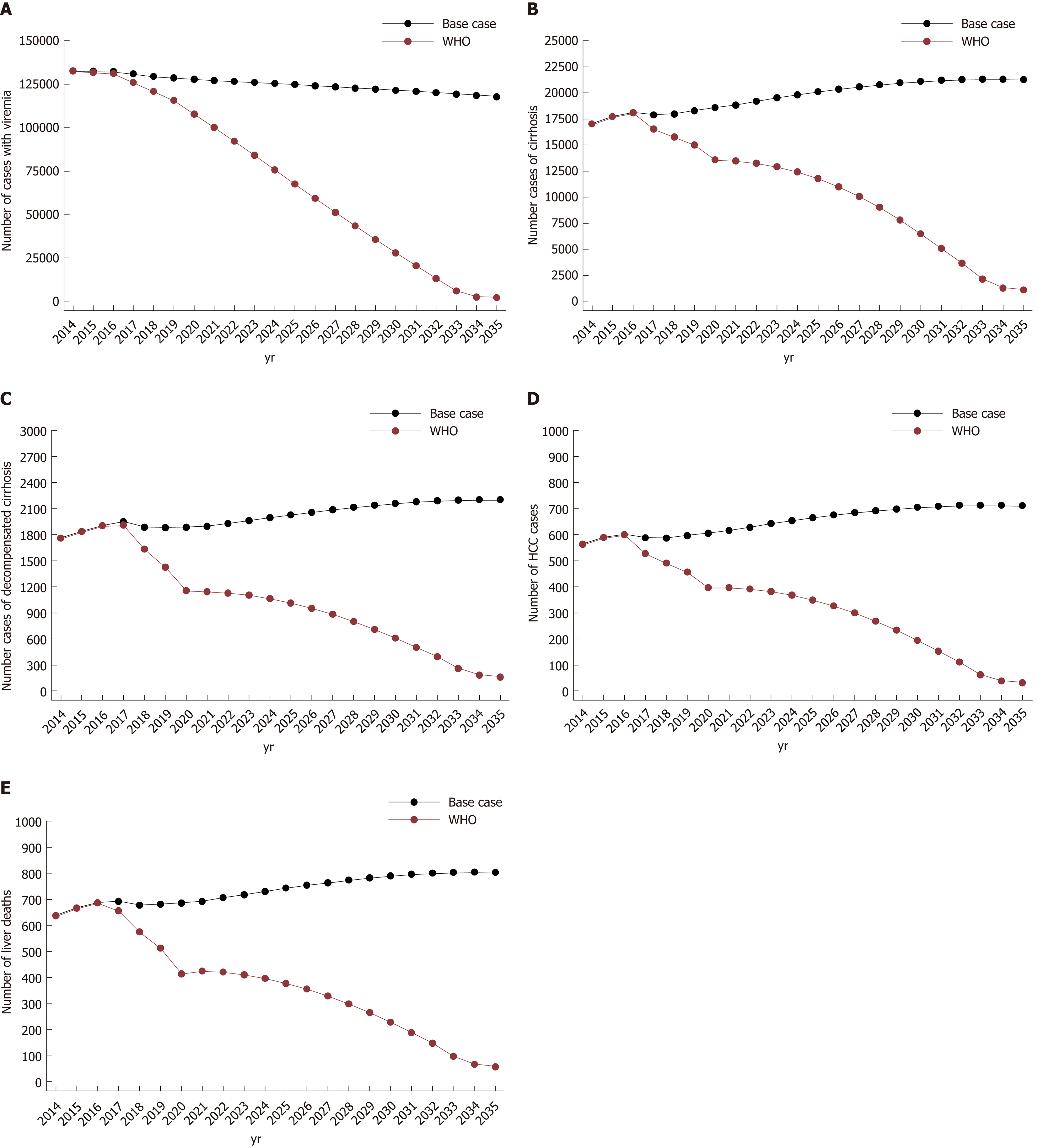
Under the base case scenario, the model predicted a continuous decline in the number of viremic cases in Greece through 2035 (Table 3, Figure 1A-E). The viremic population would decrease by 8.3% and 11.1% in 2030 and 2035 compared to 2015, respectively. However, unlike viremic cases, HCV complications are anticipated to increase over the same time period.
| 2015, Base n | 2020, n (% change compared to 2015) | 2030, n (% change compared to 2015) | 2035, n (% change compared to base case in 2015) | |
| Base case | ||||
| Total infected | 132500 | 127940 (-3.4) | 121460 (-8.3) | 117750 (-11.1) |
| Compensated cirrhosis | 17700 | 18584 (+4.9) | 21100 (+19.2) | 21280 (+20.2) |
| Decompensated cirrhosis | 1830 | 1885 (+3.0) | 2160 (+18.3) | 2200 (+20.2) |
| HCC | 590 | 605 (+2.5) | 705 (+19.4) | 710 (+20.3) |
| Liver related deaths | 660 | 686 (+3.9) | 790 (+19.6) | 805 (+21.9) |
| WHO Global Hepatitis Strategy | ||||
| Total infected | 107910 (-18.5) | 28000 (-78.8) | 2100 (-98.4) | |
| Compensated cirrhosis | 13584 (-23.2) | 6480 (-63.3) | 1084 (-93.9) | |
| Compensated cirrhosis | 1155 (-36.9) | 610 (-66.7) | 160 (-91.2) | |
| HCC | 395 (-33.0) | 195 (-66.9) | 30 (-94.9) | |
| Liver related deaths | 415 (-37.2) | 226 (-65.7) | 57 (-91.4) | |
The number of patients with compensated cirrhosis is anticipated to be 21100 cases by 2030 (19.2% higher than 2015). In 2035, the number of compensated cirrhosis cases is expected to be 21280 (20.2% higher than 2015). Similarly, the number of decompensated cirrhosis cases would increased to 2160 (18.3% higher than 2015) and 2200 (20.2% higher than 2015) cases in 2030 and 2035, respectively. Regarding HCC cases, the model projected an increase of 19.4% (705 cases) and 20.3% (710 cases) in 2030 and 2035 compared to 2015, respectively. Concerning liver related deaths, the model projects an increase to 790 (19.6% higher than 2015) and 805 (21.9% higher than 2015) in 2030 and 2035, respectively (Table 3, Figure 1A-E). Under the base case, the model estimates that about 20000 patients would be diagnosed and treated with DAAs by 2030.
Under the Global Hepatitis Strategy, significant declines would be observed in HCV morbidity and mortality. More specifically, individuals with compensated and decompensated cirrhosis are expected to decrease by 63.3%, 66.7% in 2030 and 93.9%, 91.2% in 2035, respectively, compared to the corresponding number of cases of compensated and decompensated cirrhosis in 2015. Similarly, HCC cases are anticipated to decrease by 66.9% (195 cases) and 94.9% (30 cases) in 2030 and 2035 compared to 2015, respectively. Liver related deaths are projected to decrease by 65.7% (226 deaths) in 2030 and 91.4% (57 deaths) in 2035 compared to 2015, respectively. Finally, the number of viremic cases would decrease by 78.8% (28000 cases) and 98.4% (2100 cases) in 2030 and 2035 compared to the number of viremic cases in 2015, respectively (Table 3, Figure 1A-E). To achieve the elimination goals, 90000 patients need to be treated by 2030.
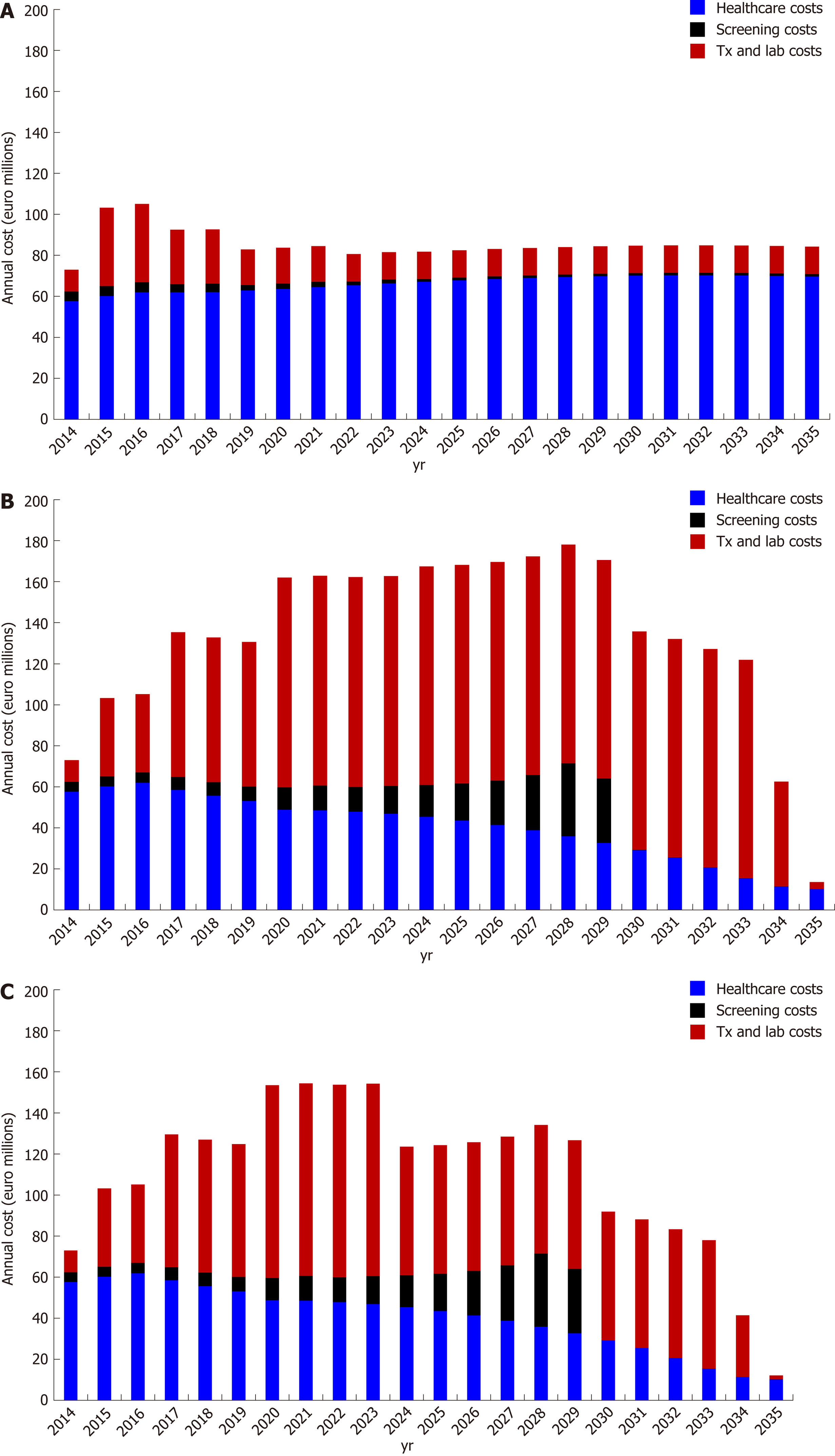
Annual direct costs: Under the base case scenario, the annual direct costs of HCV in 2016 are €105 million. Without the additional implementation of awareness or screening campaigns, the number of available patients for treatment would drop, leading to a corresponding decrease in the cost attributed to antiviral therapies. Specifically, the annual direct costs would decline from €105 to €83 million by 2019 and remain at this level through 2035 (Figures 2 and 3).
Regarding the WHO Global Hepatitis Strategy, the model predicts a steep upward trend in direct costs until 2023 and 2028 for the optimistic and the conservative price reduction scenario, respectively, followed by a significant decline through 2035. Compared to the base case, the annual direct cost of the elimination scenario would be higher until 2032 and 2034 under the optimistic and the conservative price reduction scenario, respectively (Figures 2 and 3).
The cost distribution is significantly different between the two strategies. In the base case, where treatment coverage is relatively low, the majority of the cost is attributed to healthcare costs. In contrast, under the HCV elimination scenario, the dominant costs would be for antiviral treatments and laboratory costs. Under the HCV elimination strategy (irrespective of price reduction scenarios), screening costs would represent a significant share of direct costs between 2026 and 2029 due to the fact that it would become more difficult to diagnose HCV infections due to the low prevalence (Figure 3).
Annual indirect/societal costs: Concerning indirect/societal costs, the WHO strategy is expected to result in substantial savings throughout the length of the study compared to the base case. More specifically, the indirect costs of WHO strategy would be €16, €89, and €95 million lower compared to the base case in 2016, 2030 and 2035, respectively. The above results arise from the significant reduction of HCV related end-stage liver disease, as well as the subsequent aversion of disability and premature deaths (Figure 2).
Overall annual cost (total direct and indirect costs): The annual overall cost of base case would have a slight decrease by 2035 due to the limited cost of antiviral treatment (limited available patients for treatment). Compared to the base case, the elimination strategy would be more expensive in the first phase, but it would become less costly by 2024 and 2028 under the optimistic or the conservative price reduction scenario, respectively.
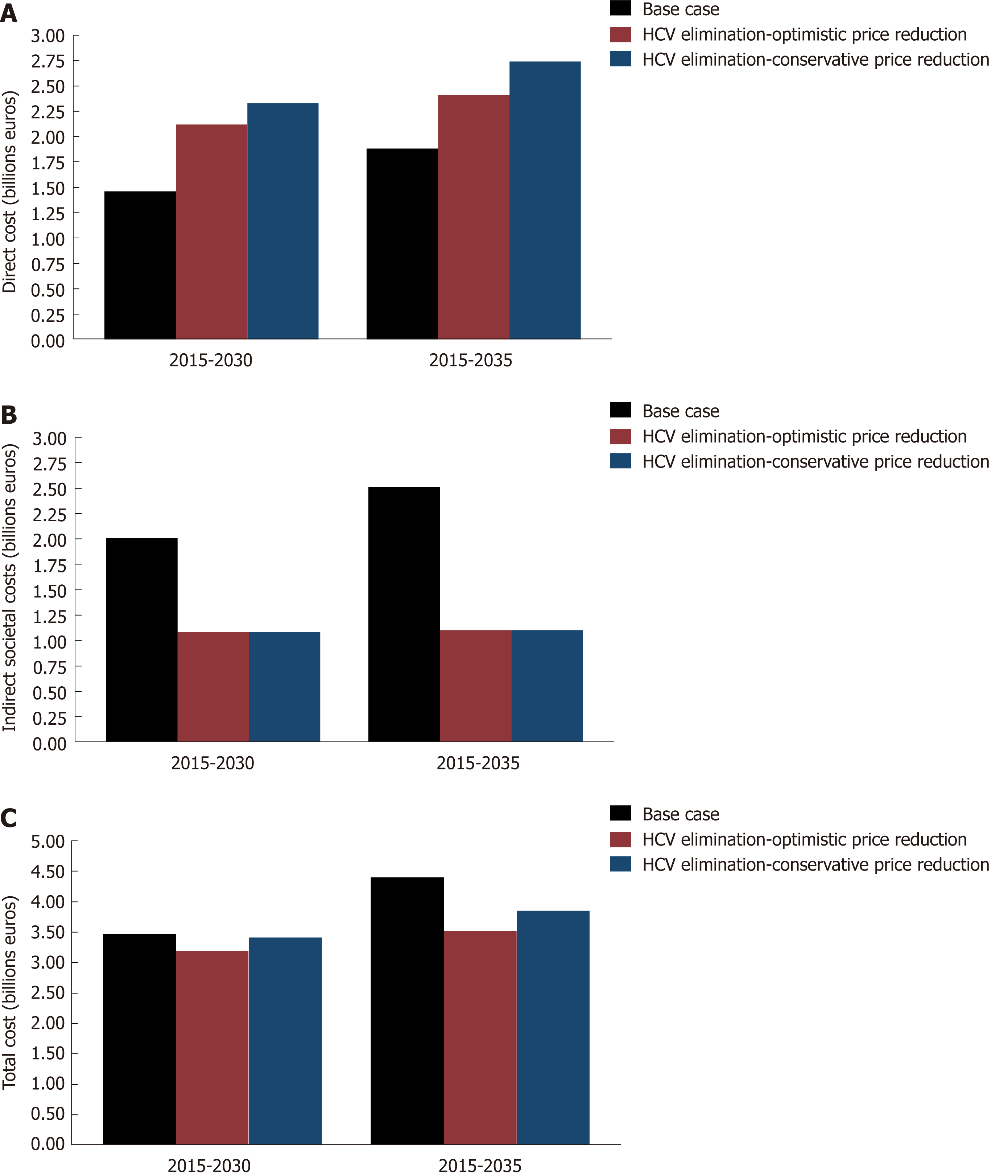
It was estimated that to achieve elimination, an investment (direct costs) of €2.12 or 2.33 billion should be made by 2030 under the optimistic or the conservative price reduction scenario, respectively. It is also important that at the same time, about €1.1 billion would be lost (indirect costs). Summing up the above, the overall cumulative cost of HCV elimination would be range between €3.2 and 3.4 billion by 2030. The corresponding costs by 2035 would vary between €3.5 and 3.8 billion (Figure 4).
The ICER computes that the cost per averted DALY by 2030 would be €10100 and €13380 under the optimistic or the conservative price reduction scenario, respectively. Similarly, the cost per averted DALYs by 2035 would be €5100 and €8300. In all scenarios, the elimination strategy was always a very cost-effective strategy (Table 4).
| Base case | HCV elimination strategy | |||||||
| Optimistic | Conservative | |||||||
| Years | Direct cost (billion euros) | DALYS | Direct cost (billion euros) | DALYS | ICER (compared to base case) | Direct cost (billion euros) | DALYS | ICER (compared to base case) |
| 2015-2030 | 1.46 | 145.920 | 2.12 | 80.920 | 10.100 € | 2.33 | 80.920 | 13.400 € |
| 2015-2035 | 1.88 | 187.470 | 2.41 | 84.250 | 5.100 € | 2.74 | 84.250 | 8.300 € |
The HCV elimination strategy appears to be a cost-saving strategy, as €895 million and €560 million would be saved by 2035 under the optimistic or the conservative price reduction scenario, respectively (Figures 2D and 4).
The analysis shows that while overall HCV prevalence in Greece will decline, disease burden related to HCV and associated costs will continue to grow in the era of DAAs due to failure to diagnose and treat sufficient numbers of patients. Similar patterns have been observed in the IFN era[13,16,31-33].
Our analysis highlights that HCV management without effective awareness and screening campaigns would be an expensive and ineffective health policy strategy. The reason is that few patients would be diagnosed and treated, leaving a significant proportion of patients to progress to more advanced stages of the disease. Thus, the additional budget gained from lower HCV awareness campaigns or low treatment coverage would be paid in the next years in healthcare costs to treat the new cases of compensated or decompensated cirrhosis and HCC. On the contrary, the HCV elimination strategy is a cost saving intervention (savings by 2035 varies from €560-890 million), as it eliminates the high cost of HCV attributed to related end-stage liver disease or premature death. It is important to note that the HCV elimination strategy is an upfront investment, as a significant amount of money should be spent in the beginning in order to save money later. For example, in Greece, the direct costs need to be increased by 142% in 2020 compared to 2016.
Although the cumulative direct costs of the elimination strategy are costlier than the base scenario (Table 4), when we take also into account the indirect costs caused by the disease, this relation changes and the elimination scenario become cheaper, as HCV is a disease with high indirect costs (Figure 2D). In line with other studies, our results have shown that the indirect cost of HCV is a significant component of the elimination strategy, almost comparable to the corresponding direct cost of the disease[9,10,20,33,34], as the vast majority of HCV-infected persons are of working age and HCV-infected patients are more likely to incur absenteeism (lost hours of work) and presenteeism (decreased productivity while at work)[35,36].
The HCV elimination strategy is a cost-saving investment in the case of Greece (i.e., improved life expectancy and reduced costs at the same time). More specifically, this investment would save €560-895 million by 2035, making it a very rewarding public health investment.
There are a number of limitations that could impact the outcomes of the study. First, patients who achieved SVR were not tracked, so all reinfection cases in the model were managed as naïve ones. Second, there is an assumption that new infections would remain stable at the 2015 levels. Thus, the model does not account for treatment as prevention, and its predictions would be more conservative. However, the strategy was cost effective despite the use of conservative assumptions, since the probability of reaching cost-effectiveness would be increased in the dynamic approach from the inclusion of the prevention of secondary cases. Third, the model assumed that new therapies, guidelines or treatment strategies are adopted immediately - a fact that may not be the case in real life settings. Fourth, although extrahepatic manifestations of HCV represent a significant part of direct cost[37,38], they were not considered in this analysis. Fifth, possible reductions in the future cost of screening tests were not considered.
In conclusion, our results support that elimination of HCV cannot be achieved without the implementation of large awareness and screening programs, as treatment coverage will be suboptimal. Nevertheless, HCV elimination is a cost-saving strategy, irrespective the uncertainty of the future cost of DAAs in Greece.
Hepatitis C virus (HCV) infection is a major global public health problem. Greece has one of the highest rates of chronic HCV (CHC) infection in Europe, and approximately 33% of the chronically infected patients are at advanced fibrosis stages (≥ F3).
Greece faces a substantial economic crisis, which has resulted in more than 50% cut off in the public pharmaceutical expenditure. Therefore, it is important that every proposed healthcare intervention be accompanied by a cost-effectiveness analysis.
The main objectives of the study are (1) to estimate the required interventions to achieve elimination using updated information for direct-acting antiviral treatment coverage, (2) to compute the total costs (including the indirect/societal costs) of the strategy, and (3) to identify whether the elimination strategy is cost-effective/cost-saving in Greece.
To project the future burden of disease and to estimate subsequent future costs, we used a previously validated, Excel-based disease progression model constructed by the Center for Disease Analysis. This model simulates the progression of HCV-infected persons through the various stages of the disease, according to the METAVIR scoring system, with appropriate transition probabilities between stages.
Progression was simulated by multiplying the total number of cases at a particular stage of disease by the appropriate progression rate to the next stage. Newly infected patients can enter the model at any year, progress through the disease stages based on progression rates, and exit the model on: (1) spontaneous clearance of HCV; (2) achieving sustained virological response rates; and (3) death (all-cause or HCV-related). Thirty-six cohorts every 5 yr of age and gender were used through 84 yr of age. Individuals older than 85 were treated as one cohort. Each year, one-fifth of the population in each age group, except for 85 and older, was moved to the next age cohort to simulate aging after taking into consideration mortality. Treated patients with sustained virological response rates were considered cured, and they had the same risk of hepatocellular carcinoma and similar mortality as the general population.
The analysis showed that while overall HCV prevalence in Greece would decline, disease burden related to HCV and associated costs would continue to grow. To achieve the elimination targets, 90000 patients need to be treated between 2015-2030. It was estimated that the investment (direct costs) of the intervention would range from €2.1-2.3 billion by 2030, while about €1.1 billion would be lost due to premature deaths or decreased productivity (indirect costs). The overall cumulative cost of HCV elimination in Greece would range from €3.2 and 3.4 billion by 2030. The model showed that the cost per averted disability-adjusted life years by 2030 would be between €8330-€13380. Furthermore, the HCV elimination strategy is cost-saving, as €560-€895 million would be saved by 2035.
Our study highlighted that without the implementation of large awareness or screening programs, HCV elimination cannot be achieved, due to suboptimal treatment coverage. To eliminate the disease, significant public health reforms should be implemented (e.g., enhance harm reduction programs, implement case-finding, linkage to care interventions). Although the elimination of HCV is a costly investment, our analysis showed that it is also a cost-saving intervention, irrespective of the uncertainty of the future direct-acting antiviral cost in Greece, as the proposed strategy reduces the disease morbidity and mortality and restores productivity of the HCV-infected population.
Elimination of HCV is a demanding public health intervention, which poses significant challenges in the Greek health care system. Nevertheless, our analysis highlighted that HCV elimination is a cost-saving intervention.
Angelos Hatzakis was supported by unrestricted grants from Gilead and MSD.
| 1. | Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1493] [Cited by in RCA: 1514] [Article Influence: 168.2] [Reference Citation Analysis (0)] |
| 2. | Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM. Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9500] [Cited by in RCA: 9740] [Article Influence: 695.7] [Reference Citation Analysis (0)] |
| 3. | Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513-521, 521.e1-521.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 666] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 4. | Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48:418-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 629] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 5. | Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, Weiland O, Aguilar H, Xiong J, Pilot-Matias T, DaSilva-Tillmann B, Larsen L, Podsadecki T, Bernstein B. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 659] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 6. | Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, Lawitz E, Lok AS, Hinestrosa F, Thuluvath PJ, Schwartz H, Nelson DR, Everson GT, Eley T, Wind-Rotolo M, Huang SP, Gao M, Hernandez D, McPhee F, Sherman D, Hindes R, Symonds W, Pasquinelli C, Grasela DM; AI444040 Study Group. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370:211-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 914] [Article Influence: 76.2] [Reference Citation Analysis (1)] |
| 7. | WHO. Global Health Sector Strategy on viral hepatitis, 2016-2021. 2015;https://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/. |
| 8. | Aggarwal R, Chen Q, Goel A, Seguy N, Pendse R, Ayer T, Chhatwal J. Cost-effectiveness of hepatitis C treatment using generic direct-acting antivirals available in India. PLoS One. 2017;12:e0176503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Chahal HS, Marseille EA, Tice JA, Pearson SD, Ollendorf DA, Fox RK, Kahn JG. Cost-effectiveness of Early Treatment of Hepatitis C Virus Genotype 1 by Stage of Liver Fibrosis in a US Treatment-Naive Population. JAMA Intern Med. 2016;176:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Younossi ZM, Tanaka A, Eguchi Y, Henry L, Beckerman R, Mizokami M. Treatment of hepatitis C virus leads to economic gains related to reduction in cases of hepatocellular carcinoma and decompensated cirrhosis in Japan. J Viral Hepat. 2018;25:945-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | He T, Lopez-Olivo MA, Hur C, Chhatwal J. Systematic review: cost-effectiveness of direct-acting antivirals for treatment of hepatitis C genotypes 2-6. Aliment Pharmacol Ther. 2017;46:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Samur S, Kues B, Ayer T, Roberts MS, Kanwal F, Hur C, Donnell DMS, Chung RT, Chhatwal J. Cost Effectiveness of Pre- vs Post-Liver Transplant Hepatitis C Treatment With Direct-Acting Antivirals. Clin Gastroenterol Hepatol. 2018;16:115-122.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Saraswat V, Norris S, de Knegt RJ, Sanchez Avila JF, Sonderup M, Zuckerman E, Arkkila P, Stedman C, Acharya S, Aho I, Anand AC. Andersson MI, Arendt V, Baatarkhuu O, Barclay K, Ben-Ari Z, Bergin C, Bessone F, Blach S, Blokhina N, Brunton CR, Choudhuri G, Chulanov V, Cisneros L, Croes EA, Dahgwahdorj YA, Dalgard O, Daruich JR, Dashdorj NR, Davaadorj D, de Vree M, Estes C, Flisiak R, Gadano AC, Gane E, Halota W, Hatzakis A, Henderson C, Hoffmann P, Hornell J, Houlihan D, Hrusovsky S, Jarčuška P, Kershenobich D, Kostrzewska K, Kristian P, Leshno M, Lurie Y, Mahomed A, Mamonova N, Mendez-Sanchez N, Mossong J, Nurmukhametova E, Nymadawa P, Oltman M, Oyunbileg J, Oyunsuren Ts, Papatheodoridis G, Pimenov N, Prabdial-Sing N, Prins M, Puri P, Radke S, Rakhmanova A, Razavi H, Razavi-Shearer K, Reesink HW, Ridruejo E, Safadi R, Sagalova O, Sanduijav R, Schréter I, Seguin-Devaux C, Shah SR, Shestakova I, Shevaldin A, Shibolet O, Sokolov S, Souliotis K, Spearman CW, Staub T, Strebkova EA, Struck D, Tomasiewicz K, Undram L, van der Meer AJ, van Santen D, Veldhuijzen I, Villamil FG, Willemse S, Zuure FR, Silva MO, Sypsa V, Gower E. Historical epidemiology of hepatitis C virus (HCV) in select countries - volume 2. J Viral Hepat. 2015;22 Suppl 1:6-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Gountas I, Sypsa V, Papatheodoridis G, Souliotis G, Razavi H, Hatzakis A. Is elimination of HCV possible in a country with low diagnostic rate and moderate HCV prevalence?: The case of Greece. J Gastroenterol Hepatol. 2017;32:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Gane E, Kershenobich D, Seguin-Devaux C, Kristian P, Aho I, Dalgard O, Shestakova I, Nymadawa P, Blach S, Acharya S, Anand AC, Andersson MI. Arendt V, Arkkila P, Baatarkhuu O, Barclay K, Ben-Ari Z, Bergin C, Bessone F, Blokhina N, Brunton CR, Choudhuri G, Chulanov V, Cisneros L, Croes EA, Dahgwahdorj YA, Daruich JR, Dashdorj NR, Davaadorj D, de Knegt RJ, de Vree M, Gadano AC, Gower E, Halota W, Hatzakis A, Henderson C, Hoffmann P, Hornell J, Houlihan D, Hrusovsky S, Jarčuška P, Kostrzewska K, Leshno M, Lurie Y, Mahomed A, Mamonova N, Mendez-Sanchez N, Mossong J, Norris S, Nurmukhametova E, Oltman M, Oyunbileg J, Oyunsuren Ts, Papatheodoridis G, Pimenov N, Prins M, Puri P, Radke S, Rakhmanova A, Razavi H, Razavi-Shearer K, Reesink HW, Ridruejo E, Safadi R, Sagalova O, Sanchez Avila JF, Sanduijav R, Saraswat V, Schréter I, Shah SR, Shevaldin A, Shibolet O, Silva MO, Sokolov S, Sonderup M, Souliotis K, Spearman CW, Staub T, Stedman C, Strebkova EA, Struck D, Sypsa V, Tomasiewicz K, Undram L, van der Meer AJ, van Santen D, Veldhuijzen I, Villamil FG, Willemse S, Zuckerman E, Zuure FR, Prabdial-Sing N, Flisiak R, Estes C. Strategies to manage hepatitis C virus (HCV) infection disease burden - volume 2. J Viral Hepat. 2015;22 Suppl 1:46-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Wedemeyer H, Duberg AS, Buti M, Rosenberg WM, Frankova S, Esmat G, Örmeci N, Van Vlierberghe H, Gschwantler M, Akarca U, Aleman S, Balık I. Berg T, Bihl F, Bilodeau M, Blasco AJ, Brandão Mello CE, Bruggmann P, Calinas F, Calleja JL, Cheinquer H, Christensen PB, Clausen M, Coelho HS, Cornberg M, Cramp ME, Dore GJ, Doss W, El-Sayed MH, Ergör G, Estes C, Falconer K, Félix J, Ferraz ML, Ferreira PR, García-Samaniego J, Gerstoft J, Giria JA, Gonçales FL Jr, Guimarães Pessôa M, Hézode C, Hindman SJ, Hofer H, Husa P, Idilman R, Kåberg M, Kaita KD, Kautz A, Kaymakoglu S, Krajden M, Krarup H, Laleman W, Lavanchy D, Lázaro P, Marinho RT, Marotta P, Mauss S, Mendes Correa MC, Moreno C, Müllhaupt B, Myers RP, Nemecek V, Øvrehus AL, Parkes J, Peltekian KM, Ramji A, Razavi H, Reis N, Roberts SK, Roudot-Thoraval F, Ryder SD, Sarmento-Castro R, Sarrazin C, Semela D, Sherman M, Shiha GE, Sperl J, Stärkel P, Stauber RE, Thompson AJ, Urbanek P, Van Damme P, van Thiel I, Vandijck D, Vogel W, Waked I, Weis N, Wiegand J, Yosry A, Zekry A, Negro F, Sievert W, Gower E. Strategies to manage hepatitis C virus (HCV) disease burden. J Viral Hepat. 2014;21 Suppl 1:60-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 17. | Gountas I, Sypsa V, Delladetsima I, Tassopoulos N, Papatheodoridis G, Hatzakis A. The Impact of Age on Fibrosis Progression in Chronic Hepatitis C Patients. EASL Barcelona. 2016;64:S459-S460. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Hatzakis A, Chulanov V, Gadano AC, Bergin C, Ben-Ari Z, Mossong J, Schréter I, Baatarkhuu O, Acharya S, Aho I, Anand AC, Andersson MI. Arendt V, Arkkila P, Barclay K, Bessone F, Blach S, Blokhina N, Brunton CR, Choudhuri G, Cisneros L, Croes EA, Dahgwahdorj YA, Dalgard O, Daruich JR, Dashdorj NR, Davaadorj D, de Knegt RJ, de Vree M, Estes C, Flisiak R, Gane E, Gower E, Halota W, Henderson C, Hoffmann P, Hornell J, Houlihan D, Hrusovsky S, Jarčuška P, Kershenobich D, Kostrzewska K, Kristian P, Leshno M, Lurie Y, Mahomed A, Mamonova N, Mendez-Sanchez N, Norris S, Nurmukhametova E, Nymadawa P, Oltman M, Oyunbileg J, Oyunsuren Ts, Papatheodoridis G, Pimenov N, Prabdial-Sing N, Prins M, Radke S, Rakhmanova A, Razavi-Shearer K, Reesink HW, Ridruejo E, Safadi R, Sagalova O, Sanchez Avila JF, Sanduijav R, Saraswat V, Seguin-Devaux C, Shah SR, Shestakova I, Shevaldin A, Shibolet O, Silva MO, Sokolov S, Sonderup M, Souliotis K, Spearman CW, Staub T, Stedman C, Strebkova EA, Struck D, Sypsa V, Tomasiewicz K, Undram L, van der Meer AJ, van Santen D, Veldhuijzen I, Villamil FG, Willemse S, Zuckerman E, Zuure FR, Puri P, Razavi H. The present and future disease burden of hepatitis C virus (HCV) infections with today's treatment paradigm - volume 2. J Viral Hepat. 2015;22 Suppl 1:26-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 19. | Souliotis K, Papageorgiou M, Politi A, Frangos N, Tountas Y. Estimating the Fiscal Effects of Public Pharmaceutical Expenditure Reduction in Greece. Front Public Health. 2015;3:203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Razavi H, Elkhoury AC, Elbasha E, Estes C, Pasini K, Poynard T, Kumar R. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology. 2013;57:2164-2170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 363] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 21. | Athanasakis K, Arzoumanidou D, Petrakis I, Karampli E, Theodoropoulou T, Retsa MP, Kyriopoulos J. A Cost-Of-Illness Analysis of Hepatitis C in Greece. Value in Health. 2013;16:A496. [DOI] [Full Text] |
| 22. | Chounta A, Ellinas C, Tzanetakou V, Pliarhopoulou F, Mplani V, Oikonomou A, Leventogiannis K, Giamarellos-Bourboulis EJ. Serum soluble urokinase plasminogen activator receptor as a screening test for the early diagnosis of hepatocellular carcinoma. Liver Int. 2015;35:601-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Papatheodoridis G. The use of the new antivirals for hepatitis C in the Greek clinical practice - Cost of the therapeutic intervention. 2016;. |
| 24. | Newspaper of the government of the hellenic republic (in Greek). In: Health Mo 2017. . |
| 25. | Available from: http://healthmag.gr/. Συμφωνία - σταθμός για την πρόσβαση των ασθενών με ηπατίτιδα C σε καινοτόμες θεραπείες υψηλού κόστους. |
| 26. | Available from: http://healthmag.gr/. Έκπτωση 68% για την ηπατίτιδα C - Καλύπτονται πενταπλάσιοι ασθενείς. |
| 27. | Available from: https://virus.com.gr/. Ο κλειστός προϋπολογισμός της Ηπατίτιδας. |
| 28. | Murray CJ, Lopez AD. Evidence-based health policy--lessons from the Global Burden of Disease Study. Science. 1996;274:740-743. [PubMed] |
| 29. | WHO. Health statistics and information systems, 2016. Available from: https://www.who.int/healthinfo/en/. |
| 30. | Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93:118-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 478] [Cited by in RCA: 631] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 31. | Razavi H, Waked I, Sarrazin C, Myers RP, Idilman R, Calinas F, Vogel W, Mendes Correa MC, Hézode C, Lázaro P, Akarca U, Aleman S, Balık I, Berg T. Bihl F, Bilodeau M, Blasco AJ, Brandão Mello CE, Bruggmann P, Buti M, Calleja JL, Cheinquer H, Christensen PB, Clausen M, Coelho HS, Cramp ME, Dore GJ, Doss W, Duberg AS, El-Sayed MH, Ergör G, Esmat G, Falconer K, Félix J, Ferraz ML, Ferreira PR, Frankova S, García-Samaniego J, Gerstoft J, Giria JA, Gonçales FL Jr, Gower E, Gschwantler M, Guimarães Pessôa M, Hindman SJ, Hofer H, Husa P, Kåberg M, Kaita KD, Kautz A, Kaymakoglu S, Krajden M, Krarup H, Laleman W, Lavanchy D, Marinho RT, Marotta P, Mauss S, Moreno C, Murphy K, Negro F, Nemecek V, Örmeci N, Øvrehus AL, Parkes J, Pasini K, Peltekian KM, Ramji A, Reis N, Roberts SK, Rosenberg WM, Roudot-Thoraval F, Ryder SD, Sarmento-Castro R, Semela D, Sherman M, Shiha GE, Sievert W, Sperl J, Stärkel P, Stauber RE, Thompson AJ, Urbanek P, Van Damme P, van Thiel I, Van Vlierberghe H, Vandijck D, Wedemeyer H, Weis N, Wiegand J, Yosry A, Zekry A, Cornberg M, Müllhaupt B, Estes C. The present and future disease burden of hepatitis C virus (HCV) infection with today's treatment paradigm. J Viral Hepat. 2014;21 Suppl 1:34-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 295] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 32. | El Khoury AC, Klimack WK, Wallace C, Razavi H. Economic burden of hepatitis C-associated diseases in the United States. J Viral Hepat. 2012;19:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Estes C, Abdel-Kareem M, Abdel-Razek W, Abdel-Sameea E, Abuzeid M, Gomaa A, Osman W, Razavi H, Zaghla H, Waked I. Economic burden of hepatitis C in Egypt: the future impact of highly effective therapies. Aliment Pharmacol Ther. 2015;42:696-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 34. | Younossi ZM, Park H, Dieterich D, Saab S, Ahmed A, Gordon SC. Assessment of cost of innovation versus the value of health gains associated with treatment of chronic hepatitis C in the United States: The quality-adjusted cost of care. Medicine (Baltimore). 2016;95:e5048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Younossi ZM, Stepanova M, Henry L, Younossi I, Weinstein A, Nader F, Hunt S. Association of work productivity with clinical and patient-reported factors in patients infected with hepatitis C virus. J Viral Hepat. 2016;23:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Younossi I, Weinstein A, Stepanova M, Hunt S, Younossi ZM. Mental and Emotional Impairment in Patients With Hepatitis C is Related to Lower Work Productivity. Psychosomatics. 2016;57:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Cacoub P, Vautier M, Desbois AC, Saadoun D, Younossi Z. Direct medical costs associated with the extrahepatic manifestations of hepatitis C virus infection in France. Aliment Pharmacol Ther. 2018;47:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic Manifestations of Hepatitis C: A Meta-analysis of Prevalence, Quality of Life, and Economic Burden. Gastroenterology. 2016;150:1599-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 307] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P- Reviewer: El-Shabrawi MHF, Gencdal G S- Editor: Ma RY L- Editor: Filipodia E- Editor: Huang Y