Published online Mar 14, 2019. doi: 10.3748/wjg.v25.i10.1238
Peer-review started: November 12, 2018
First decision: December 12, 2019
Revised: January 30, 2019
Accepted: February 15, 2019
Article in press: February 16, 2019
Published online: March 14, 2019
Processing time: 126 Days and 8.9 Hours
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumor type in the gastrointestinal system. Presently, various classification systems to prognosticate GISTs have been proposed.
To evaluate the application value of four different risk stratification systems for GISTs.
Patients who were diagnosed with GISTs and underwent surgical resection at four hospitals from 1998 to 2015 were identified from a database. Risk of recurrence was stratified by the modified National Institute of Health (NIH) criteria, the Armed Forces Institute of Pathology (AFIP) criteria, the Memorial Sloan Kettering Cancer Center (MSKCC) prognostic nomogram, and the contour maps. Receiver operating characteristic (ROC) curves were established to compare the four abovementioned risk stratification systems based on the area under the curve (AUC).
A total of 1303 patients were included in the study. The mean age of the patients was 55.77 ± 13.70 yr; 52.3% of the patients were male. The mean follow-up period was 64.91 ± 35.79 mo. Approximately 67.0% the tumors were located in the stomach, and 59.5% were smaller than 5 cm; 67.3% of the patients had a mitotic count ≤ 5/50 high-power fields (HPFs). Thirty-four tumors ruptured before and during surgery. Univariate analysis demonstrated that tumor size > 5 cm (P < 0.05), mitotic count > 5/50 HPFs (P < 0.05), non-gastric location (P < 0.05), and tumor rupture (P < 0.05) were significantly associated with increased recurrence rates. According to the ROC curve, the AFIP criteria showed the largest AUC (0.754).
According to our data, the AFIP criteria were associated with a larger AUC than the NIH modified criteria, the MSKCC nomogram, and the contour maps, which might indicate that the AFIP criteria have better accuracy to support therapeutic decision-making for patients with GISTs.
Core tip: Our study evaluated the application value of four different risk stratification systems for gastrointestinal stromal tumors (GISTs). Patients who were diagnosed with GISTs and underwent surgical resection at four hospitals from 1998 to 2015 were identified from a database and were stratified by four different stratification systems. According to our data, the Armed Forces Institute of Pathology (AFIP) criteria were associated with a larger area under the curve than the National Institute of Health modified criteria, the Memorial Sloan Kettering Cancer Center nomogram, and the contour maps, which indicated that the AFIP criteria have better accuracy to support therapeutic decision-making for patients with GISTs.
- Citation: Chen T, Ye LY, Feng XY, Qiu HB, Zhang P, Luo YX, Yuan LY, Chen XH, Hu YF, Liu H, Li Y, Tao KX, Yu J, Li GX. Performance of risk stratification systems for gastrointestinal stromal tumors: A multicenter study. World J Gastroenterol 2019; 25(10): 1238-1247
- URL: https://www.wjgnet.com/1007-9327/full/v25/i10/1238.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i10.1238
Gastrointestinal stromal tumors (GISTs) are the most common type of mesenchymal tumor in the gastrointestinal (GI) system. They can occur anywhere in the human GI tract, including the stomach (60%-70%), small intestine (20%-30%), duodenum (4%-5%), rectum (4%-5%), colon (< 2%), and esophagus (< 1%)[1-2]. Their overall incidence has been estimated to be 10 to 20 per million, including incidental minimal tumors. What’s more, only 18% of these tumors were considered benign, whereas 35% were considered to have some malignant potential and 47% were of undetermined potential. A 42% recurrence rate with a median time to recurrence of 22 months was found in surgically resected tumors[3].
GISTs arise from interstitial cells of Cajal, are generally immunohistochemically positive for KIT (CD117), and contain KIT- or PDGFRA-activating mutations[4-7]. Until 2000, the treatment of GISTs was limited in radical surgery, as GISTs are resistant to chemo- and radiotherapy. In 2000, imatinib was first used in GISTs as a tyrosine kinase inhibitor (TKI). This significantly improved median overall survival from < 1 yr to > 5 yr nowadays[8]. Adjuvant therapy with imatinib benefits patients with a high risk of recurrence, with studies suggesting most benefit with at least 3 yr of therapy. TKI treatment was also recommended by the National Comprehensive Cancer Network (NCCN) in 2015, for GIST patients with a moderate or high risk of recurrence. In other words, patients in the low-risk group may not benefit from TKI treatment. Otherwise, overtreatment may bring them adverse effects and financial burden. Another important thing is the frequency of reexamination. For patients with a low risk to recur, computed tomography examination is recommended to be taken every 6 mo, lasting 5 yr. However, for patients with a median or high risk to recur, examination should be taken every 3 mo in the first 3 yr. Therefore, the accuracy of risk stratification is very important in the treatment of GISTs.
Fletcher published a consensus approach to diagnose GISTs based on tumor size, tumor site, and mitosis number. It was approved by the National Institutes of Health (NIH) in 2002 and was the first guide in risk stratification for GISTs[9]. Subsequently, the Armed Forces Institute of Pathology (AFIP) criteria were put forward by Miettinen and Losota[10] in 2006 according to the long-term follow-up results of 1684 patients. In 2008, the NIH system was modified to include both tumor location and rupture; these new criteria have been widely accepted around the world because they are easier to apply than the AFIP criteria[11]. In 2009, a prognostic nomogram was developed by the Memorial Sloan Kettering Cancer Center (MSKCC) to predict the risk of recurrence[12]. A novel risk stratification method was developed by Joensuu et al[13] in 2011, in which tumor size and mitosis count were treated as continuous non-linear variables. Although there are many grading methods available, clinicians are sometimes confused as to which one should be used to determine a patient's risk rating.
The present study aimed to compare the predictive accuracy of the modified HIN criteria, the AFIP criteria, the MSKCC nomogram, and the Joensuu’s contour maps. To the best of our knowledge, this study constitutes the first comparison of these four risk criteria based on multicenter data. Our aim was to elucidate which risk stratification system provides the best support for therapeutic decision-making.
We searched a database for patients who were diagnosed with GISTs by standard pathologic criteria at the Southern Medical University Nanfang Hospital, Sun Yat-sen University Cancer Center, Guangdong General Hospital, and Wuhan Union Hospital from January 1998 to December 2015. Patients who underwent complete resection with negative margins and no metastasis and did not undergo or did not completely undergo TKI therapy in a neoadjuvant or adjuvant setting were included in the study. Pregnant or breastfeeding women and patients with other serious diseases or with a history of malignancy were excluded. Patients with uncomplete data were also excluded.
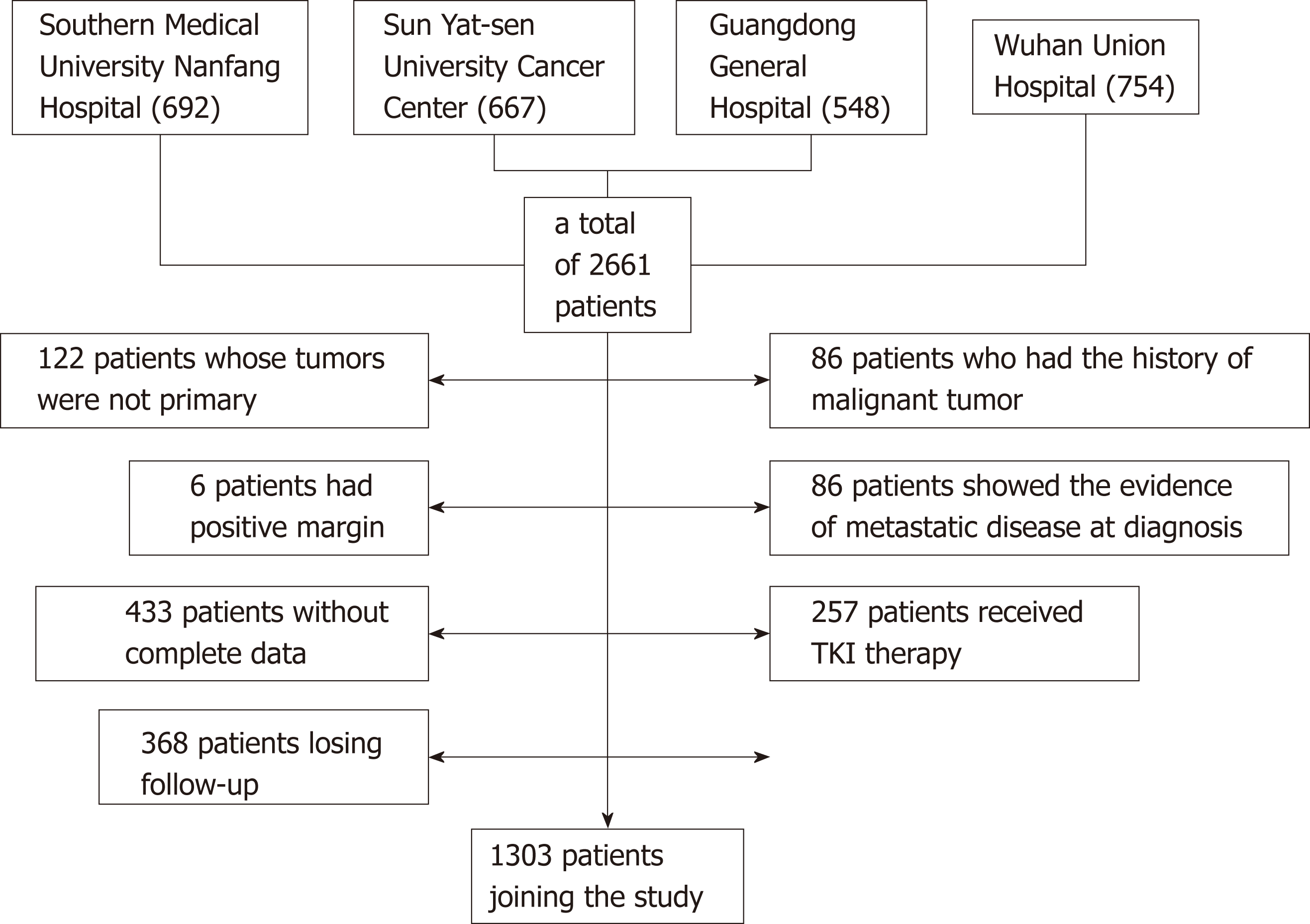
Between January 1998 and December 2015, a total of 2661 patients who were diagnosed with GISTs and underwent complete gross resection at Southern Medical University Nanfang Hospital (692), Sun Yat-sen University Cancer Center (667), Guangdong General Hospital (548), and Wuhan Union Hospital (754) were identified. Of these, 122 patients whose tumors were not primary and 86 patients who had a history of malignant tumor were excluded from the analysis. Six patients had positive margins, and 86 patients showed evidence of metastatic disease at diagnosis. Four hundred and thirty-three patients without complete data and 368 patients lost to follow-up were also excluded. Two hundred and fifty-seven patients regularly underwent TKI therapy in a neoadjuvant or adjuvant setting. Thus, a total of 1303 patients were included in the present study (Figure 1).
We collected the demographic and clinicopathologic data of the included patients accurately. Tumor size and mitotic index were measured by the pathologists. Mitotic index was defined as the number of mitoses per 50 randomly selected microscopic high-power fields (HPFs). Tumor rupture included those ruptures before and during the surgery. Continuous variables are presented as the mean (standard deviation) and median (minimum, maximum). Categorical variables are presented as the frequency (percentage). Patients were classified using the modified NIH consensus criteria, the AFIP criteria, the MSKCC nomogram, and the Joensuu’s contour maps. The Wilcoxon-Mann-Whitney test and the Chi-squared/Fisher’s exact test were used to analyze continuous variables and categorical variables, respectively. Univariate analysis was performed for exploring the relationship between the above characteristics and tumor recurrence. A binary logistic regression model was used to calculate odds ratios (ORs).
Recurrence-free survival (RFS) was defined as the time from diagnosis to recurrence of the tumor after complete resection. Patients who were alive without recurrence at the time of data collection and those who died without recurrence were censored. Overall survival was calculated from the date of surgery or diagnosis to the date of death. RFS and overall survival between groups were compared using the Kaplan-Meier life-table method and a non-stratified Cox proportional hazards model or log-rank test. Receiver operating characteristic (ROC) curves were used to compare the accuracy of the risk stratification criteria. Both 2- and 5-year RFS rates were reported in the MSKCC nomogram. The areas under the curve (AUCs) of all the risk stratification systems were calculated. Comparisons between ROC curves were performed. Two-tailed P-values were reported and were considered to be statistically significant when P < 0.05.
Table 1 shows the demographic and clinicopathologic data of the included population. The average age of the included patients was 55.77 ± 13.70 yr; 52.3% were male. The mean follow-up period was 64.91 ± 35.79 months. Approximately 67.0% of the tumors were located in the stomach, and 59.5% were smaller than 5 cm; 67.3% of patients had a mitotic count ≤ 5/50 HPFs. There were 34 tumors that ruptured, including those ruptures before and during surgery. According to the modified NIH criteria, 347 (26.6%) patients were in the very-low-risk group, while 400 (30.7%) were in the high-risk group. Recurrent disease was found in 107 (8%) patients; 77.6% of these patients were classified in a moderate- or high-risk group by the modified NIH criteria, while 71.0% were designated such by the AFIP criteria. A total of 159 persons died during our research. According to the contour map criteria, age (P = 0.118), gender (P = 0.339), or follow-up period (P = 0.067) among the different risk groups showed no difference. Neither age (P = 0.333) nor gender (P = 0.067) showed a difference between the recurrence group and the non-recurrence group. Univariate analysis demonstrated that tumor size > 5 cm [OR 4.694, 95% confidence interval (CI) (3.003, 7.337), P < 0.05], mitotic count > 5/50 HPFs [OR 3.286, 95%CI (2.193, 4.923), P < 0.05], non-gastric location [OR 4.200, 95%CI (2.774, 6.359), P < 0.05], and tumor rupture [OR 57.327, 95%CI (24.220, 135.685), P < 0.05] were significantly associated with increased recurrence rates.
| Overall (n = 1303) | Recurrence (107) | No recurrence (1196) | OR (95%CI) | P-value | |
| Gender | |||||
| Male | 681 (52.3) | 65 (60.7) | 616 (51.5) | ||
| Female | 622 (47.7) | 42 (39.3) | 580 (48.5) | 0.686 (0.458, 1.028) | 0.067 |
| Age (yr) | |||||
| Mean (SD) | 55.77 (13.696) | 55.76 (0.390) | 55.83 (1.538) | 1.000 (0.986, 1.015) | 0.276 |
| Tumor location | |||||
| Gastric | 873 (67.0) | 38 (35.5) | 835 (69.8) | ||
| Non-gastric | 430 (33.0) | 69 (64.5) | 361 (30.2) | 4.200 (2.774, 6.359) | < 0.05 |
| Follow-up period (mo) | |||||
| Mean (SD) | 64.91 (35.793) | 75.36 (4.608) | 63.98 (0.995) | 1.008 (1.003, 1.013) | < 0.05 |
| Tumor size | |||||
| Mean (SD) | 5.14 (4.862) | 9.01 (0.663) | 4.80 (0.130) | 1.128 (1.092, 1.165) | < 0.05 |
| ≤ 5 cm | 775 (59.5) | 28 (26.2) | 747 (62.5) | ||
| > 5 cm | 528 (40.5) | 79 (73.8) | 449 (37.5) | 4.694 (3.003, 7.337) | < 0.05 |
| Mitotic index | |||||
| ≤ 5/50 HPFs | 877 (67.3) | 44 (41.1) | 833 (69.6) | ||
| > 5/50 HPFs | 426 (32.7) | 63 (58.9) | 363 (30.4) | 3.286 (2.193, 4.923) | < 0.05 |
| Tumor rupture | |||||
| Yes | 34 (2.6) | 27 (25.2) | 7 (0.6) | 57.327 (24.220, 135.685) | |
| No | 1269 (97.4) | 80 (74.8) | 1189 (99.4) | < 0.05 | |
| Modified NIH | |||||
| Very low risk | 347 (26.6) | 14 (13.10) | 333 (27.80) | 0.182 (0.101, 0.329) | < 0.05 |
| Low risk | 394 (30.2) | 10 (9.30) | 384 (32.10) | 0.113 (0.057, 0.222) | < 0.05 |
| Intermediate risk | 162 (12.4) | 8 (7.50) | 154 (12.90) | 0.225 (0.106, 0.478) | < 0.05 |
| High risk | 400 (30.7) | 75 (70.10) | 325 (27.20) | ||
| AFIP criteria | |||||
| Very low risk | 619 (47.5) | 15 (14.00) | 604 (50.50) | 0.081 (0.045, 0.146) | < 0.05 |
| Low risk | 250 (19.2) | 16 (15.00) | 234 (19.60) | 0.224 (0.125, 0.401) | < 0.05 |
| Intermediate risk | 173 (13.3) | 15 (14.00) | 158 (13.20) | 0.311 (0.170, 0.568) | < 0.05 |
| High risk | 261 (20.0) | 61 (57.00) | 200 (16.70) |
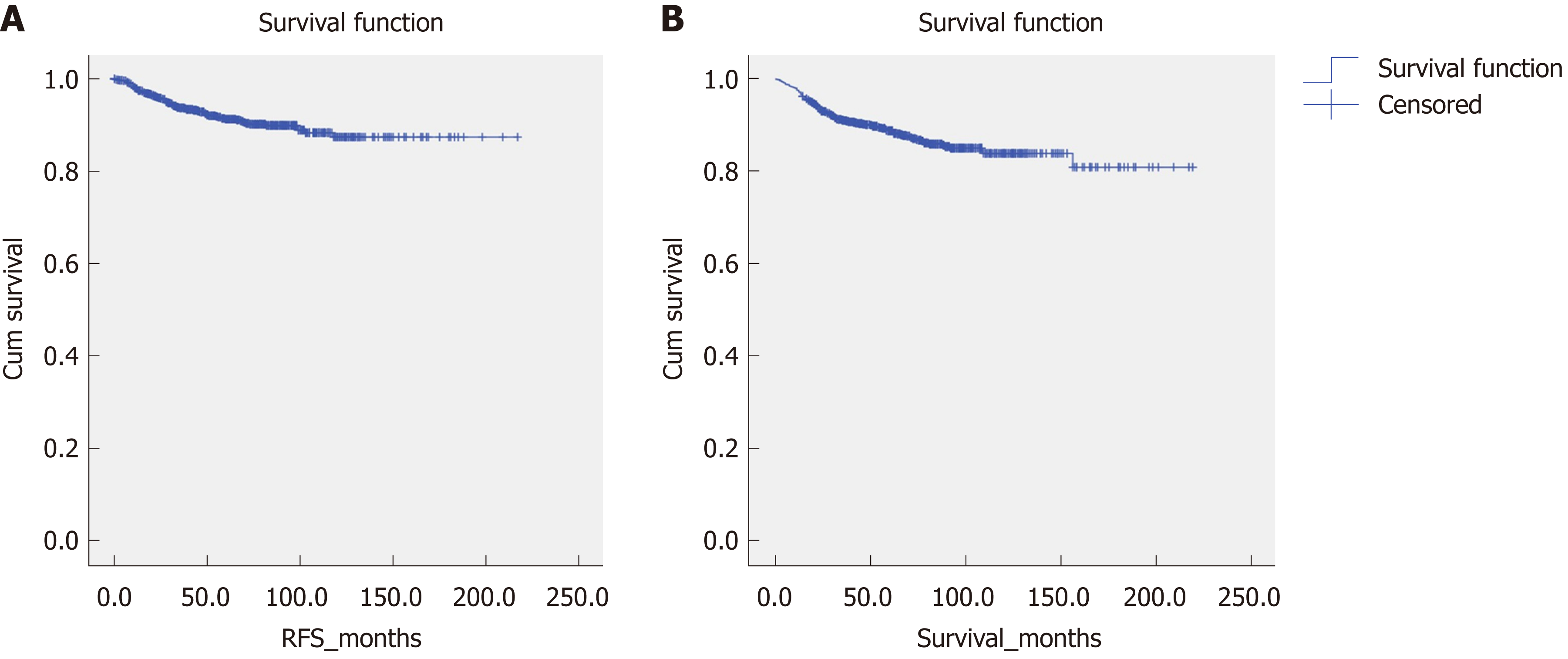
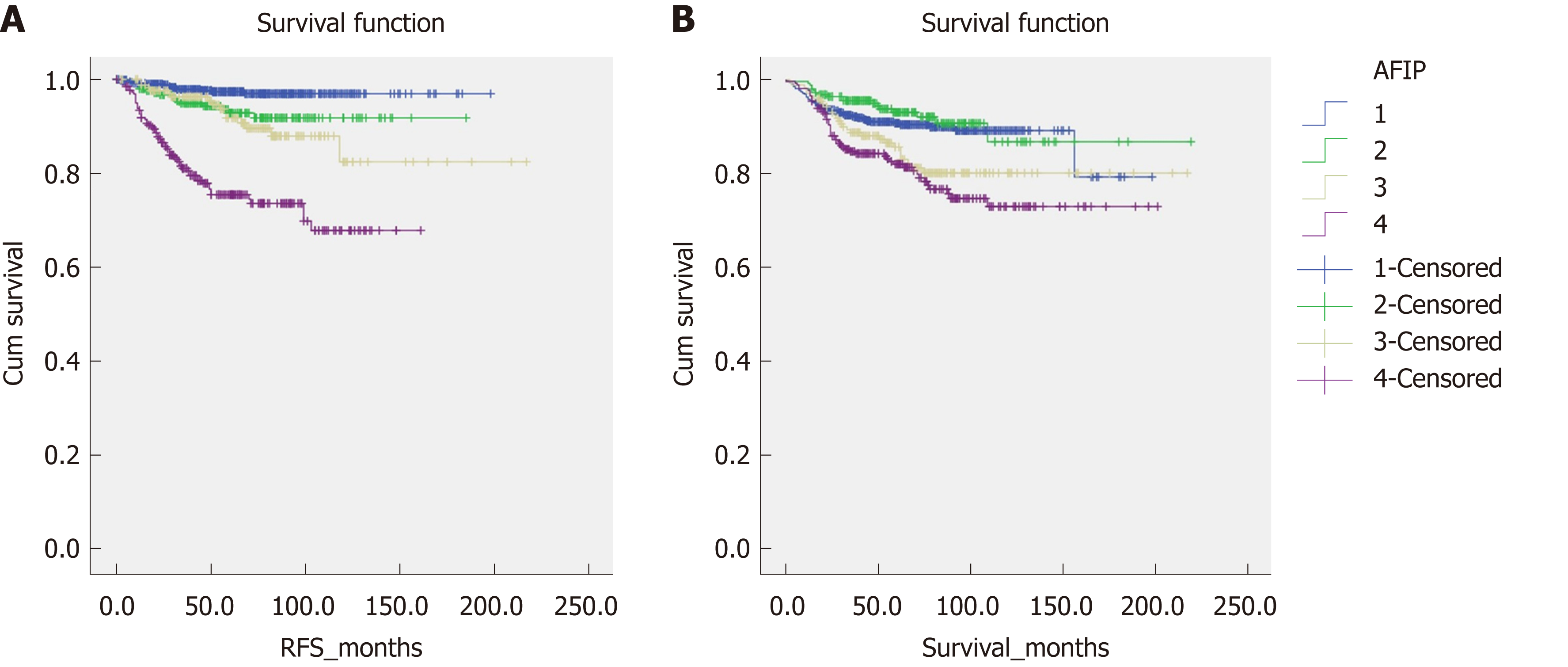
Figure 2 shows the overall survival and RFS for the entire cohort of patients. The mean overall survival was 188.28 (2.915) mo, while the RFS was 195.697 (2.234) mo. According to the AFIP criteria, the high-risk group showed the shortest RFS [122.212 (4.364) mo, P < 0.05] and overall survival [158.542 (5.193) months, P < 0.05] (Figure 3).
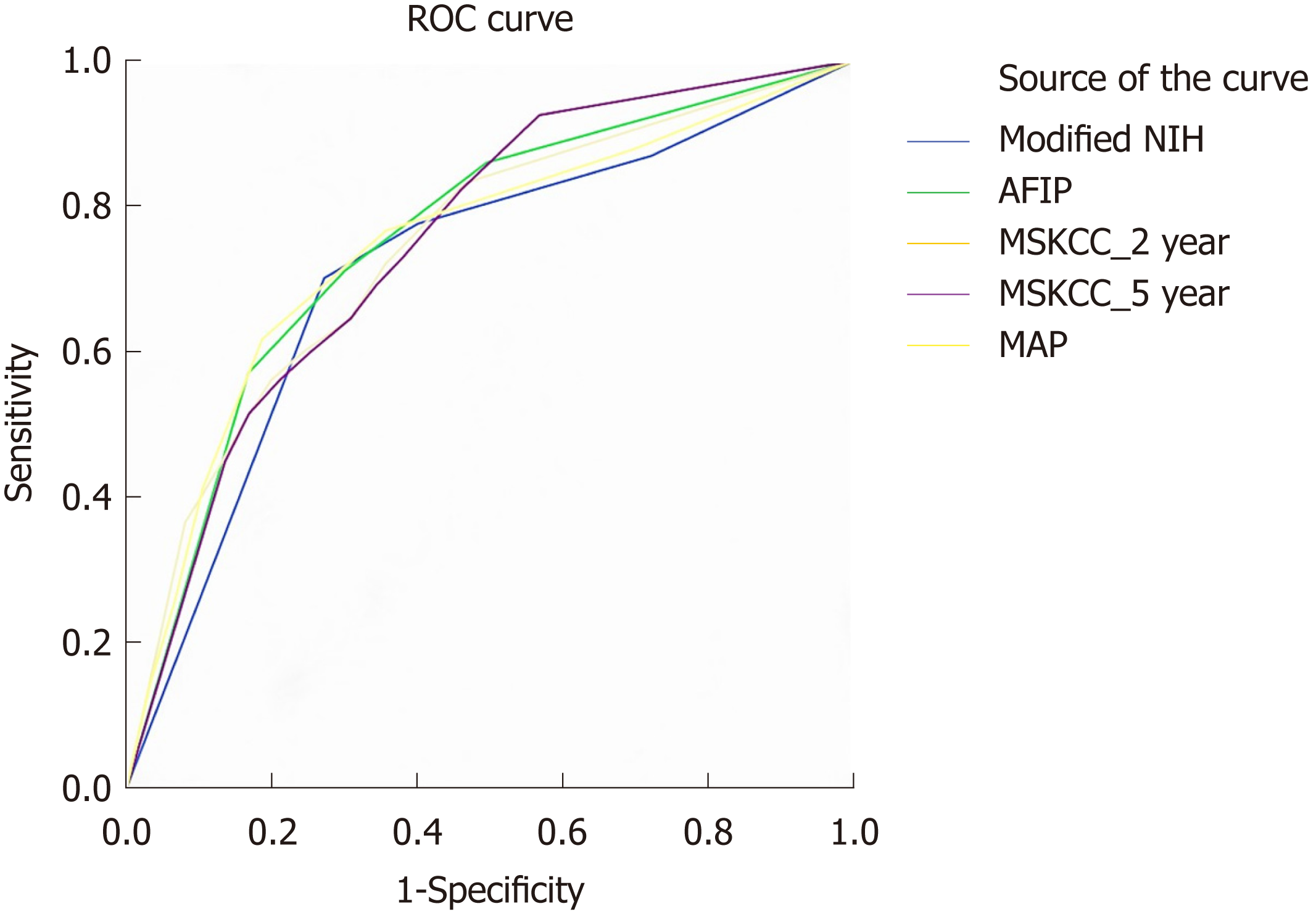
We performed ROC analysis to compare the accuracy of the above GIST risk stratification systems (Figure 4). Both the 2- and 5-year predicated probabilities of RFS were calculated in the MSKCC nomogram. The AUCs of modified NIH, AFIP, MSKCC (2-year), MSKCC (5-year), and contour map criteria were 0.726, 0.754, 0.725, 0.737, and 0.739, respectively. Pairwise comparisons of the ROC curves are shown in Table 2.
| Difference (95%CI) | P-value | |
| AFIP-MAP | 0.3485 | 0.7275 |
| AFIP-MSKCC_2 yr | -0.2933 | 0.7693 |
| AFIP-MSKCC_5 yr | 0.5597 | 0.5757 |
| AFIP-Modified NIH | 4.2594 | < 0.05 |
| MAP-MSKCC_2 yr | -0.4296 | 0.6675 |
| MAP-MSKCC_5 yr | 0.3559 | 0.7219 |
| MAP-Modified NIH | 1.7202 | 0.0854 |
| MSKCC_2 yr-MSKCC_5 yr | 0.7895 | 0.4298 |
| MSKCC_2 yr-Modified NIH | 1.2829 | 0.1995 |
| MSKCC_5 yr-Modified NIH | 0.5711 | 0.5679 |
Proper stratification is important to determine whether a patient should undergo TKI therapy or whether frequent review is necessary. Tumor size > 5 cm, mitotic count > 5/50 HPFs, non-gastric location, and tumor rupture were significantly associated with increased recurrence rates in our study. Jumniensuk et al[14] found that metastasis happened in 27.7% of GIST patients, which mostly occurred within 2 yr. They also found that metastasis correlated with tumor size > 10 cm (P = 0.023) and mitotic count > 5/5 mm2 (P = 0.000). In the study of Supsamutchai et al[15], they demonstrated that there were significant differences between mitotic index or tumor size and the risk of recurrence or metastasis (P = 0.036). Our data demonstrated that tumor location was also an important factor affecting recurrence. According to common dogma, intestinal GISTs are associated with a worse prognosis compared with gastric GISTs[16]. Emory et al[17] showed that overall survival was best for those patients with tumors confined to the esophagus and worst for those whose tumors originating in the small bowel (P = 0.00109). Tumors located in the fundus or at the gastroesophageal junction were associated with recurrence (P < 0.001)[18]. However, none of the currently available prognostic criteria take tumor site inside the stomach into account when calculating the risk of recurrence of GISTs. According to our study, tumor rupture is another factor that should be considered, which is consistent with the study of Rutkowski et al[19]. Hohenberger et al[20] showed that 15 patients with a GIST rupturing into the abdominal cavity recurred in 16 (94%) patients without adjuvant treatment. The AFIP criteria, which demonstrated the largest AUC in our study, cover all these prognostic factors.
Upon pairwise comparison of the ROC curves, the AUC of the AFIP criteria was greater than that of the modified NIH criteria (P < 0.05), although the other pairwise comparisons were not significantly different. This result is consistent with the recommendation made by the NCCN on GISTs in 2017, which also concluded that the AFIP criteria have advantages over the modified NIH criteria based on a number of studies. The study by Goh et al also illustrated that the AFIP risk criteria performed best among the three systems (NIH, modified NIH, and AFIP) for primary localized GISTs[21]. However, in the study by Belfiori et al[22], the MSKCC nomogram seemed to perform better than the NIH, modified NIH, and AFIP criteria in their sample and was suggested for use in clinical practice to predict the risk of recurrence. However, this study only covered 37 GISTs and observed 9 (24%) recurrences with a median follow-up period of 65 mo, which was shorter than the follow-up period in our study. The study by Chok et al[23] reached the same conclusion. It is hard to explain the exact reasons why the AFIP criteria better predicted recurrence compared to the other risk classification systems in our included patients. However, the AFIP criteria are based on a population of 1684 patients, which is much larger than those corresponding to the other prognostic classification systems, and this difference may support the more objective nature of the AFIP criteria. In addition, the AFIP system draws a wider prognostic divergence between tumors located in the gastric region and the non-gastric region. For example, a tumor smaller than 2 cm with a mitotic count between 5 and 10 per 50 HPFs in a non-gastric location would be classified in the intermediate-risk group by the modified NIH criteria, whereas the AFIP criteria would classify such a tumor in the high-risk group. In contrast, for the nomogram criteria, the risk levels depend on whether the tumor location is colorectal or intestinal. Although intestinal GISTs show a worse prognosis than colorectal tumors, the low proportion of intestinal GISTs in our study limited this predictive impact. Moreover, the nomogram method tends to overestimate the probability of recurrence in low-risk tumors, as a result of the fact that its performance tends to be poorer in study cohorts with a high proportion of low-risk tumors as our data. With regard to the contour maps, these emphasize tumors outside of the GI tract and those which have ruptured. In clinical work, it is rare to encounter tumors outside of the GI tract and those that rupture. Moreover, the reported frequency of rupture in GISTs varies greatly, from 2% to 22%[19,24]. In our multicenter data, the frequency of rupture was 2.6%. However, contour maps might benefit for the individual prognosis estimation because the tumor size and mitotic count are integrated as continuous variables, especially when the tumor size or mitotic count is close to the cutoff values of modified NIH or AFIP criteria based on the categories. Nevertheless, further studies should focus on more rigorous analysis of the accuracy of the nomogram method and contour maps.
A number of other factors have recently been shown to be associated with the prognosis of stromal tumors, from the genetic level to the protein level[25-29]. In 2016, Feng et al found that the parameters in peripheral blood cells such as high neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio were associated with a poor prognosis among GISTs and thus may constitute a convenient, reproducible, and inexpensive approach to predict the prognosis of these tumors[30]. These factors may have the opportunity to be added to the prognosis evaluation systems for stromal tumors in the future, which requires further studies.
There is no denying that there are still some deficiencies in our research. First, our study was a retrospective study, and prospective studies are needed to verify our conclusions. Second, the follow-up time was relatively short and a large number of people were lost to follow-up. All of these factors might result in bias during the analysis. We are establishing better follow-up systems and diagnostic methods, hoping to enlarge our sample size. We firmly believe that more accurate data can be obtained in the future.
In summary, our results demonstrate that the AFIP criteria performed better than the NIH modified criteria, the MSKCC nomogram, and the contour maps in Chinese patients and may therefore be preferred to use in clinical practice to predict the risk of recurrence for GISTs in the Chinese population.
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumor type in the gastrointestinal (GI) system. Presently, various classification systems to prognosticate GISTs have been proposed.
It is unknown which classification system is more accurate when predicting the prognosis of patient with GISTs. This study will help doctors decide when considering the frequency of reexamination and whether to take a tyrosine kinase inhibitor.
The aim of this study was to evaluate the application value of four different risk stratification systems for GISTs.
Patients who were diagnosed with GISTs and underwent surgical resection at four hospitals from 1998 to 2015 were identified from a database. Risk of recurrence was stratified by the modified National Institute of Health (NIH) criteria, the Armed Forces Institute of Pathology (AFIP) criteria, the Memorial Sloan Kettering Cancer Center (MSKCC) prognostic nomogram, and the contour maps. Receiver operating characteristic (ROC) curves were established to compare the four abovementioned risk stratification systems based on the area under the curve (AUC).
According to the ROC curve, the AFIP criteria showed the largest AUC (0.754).
According to our data, the AFIP criteria were associated with a larger AUC than the NIH modified criteria, the MSKCC nomogram, and the contour maps, which might indicate that the AFIP criteria have better accuracy to support therapeutic decision-making for patients with GISTs.
The study evaluated the application value of four different risk stratification systems for GISTs and found that the AFIP criteria have better accuracy in clinical application. Due to the imperfection of China's follow-up system and the particularity of its medical system, there may be some bias in this data. In the future, we will improve the follow-up mechanism to ensure the accuracy of data, and prospective studies may bring more accurate results.
| 1. | Heinrich MC, Corless CL, Blanke CD, Demetri GD, Joensuu H, Roberts PJ, Eisenberg BL, von Mehren M, Fletcher CD, Sandau K, McDougall K, Ou WB, Chen CJ, Fletcher JA. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol. 2006;24:4764-4774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 624] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 2. | Dematteo RP, Gold JS, Saran L, Gönen M, Liau KH, Maki RG, Singer S, Besmer P, Brennan MF, Antonescu CR. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer. 2008;112:608-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 350] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 3. | Machado-Aranda D, Malamet M, Chang YJ, Jacobs MJ, Ferguson L, Silapaswan S, Goriel Y, Kolachalam R, Mittal VK. Prevalence and management of gastrointestinal stromal tumors. Am Surg. 2009;75:55-60. [PubMed] |
| 4. | Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A, Demetri GD, Fletcher CD, Fletcher JA. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1712] [Cited by in RCA: 1743] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 5. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3215] [Cited by in RCA: 3150] [Article Influence: 112.5] [Reference Citation Analysis (2)] |
| 6. | Hirota S, Nishida T, Isozaki K, Taniguchi M, Nakamura J, Okazaki T, Kitamura Y. Gain-of-function mutation at the extracellular domain of KIT in gastrointestinal stromal tumours. J Pathol. 2001;193:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Lux ML, Rubin BP, Biase TL, Chen CJ, Maclure T, Demetri G, Xiao S, Singer S, Fletcher CD, Fletcher JA. KIT extracellular and kinase domain mutations in gastrointestinal stromal tumors. Am J Pathol. 2000;156:791-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 467] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 8. | Langenberg SMCH, Reyners AKL, Wymenga ANM, Sieling GCM, Veldhoven CMM, van Herpen CML, Prins JB, van der Graaf WTA. Caregivers of patients receiving long-term treatment with a tyrosine kinase inhibitor (TKI) for gastrointestinal stromal tumour (GIST): A cross-sectional assessment of their distress and burden. Acta Oncol. 2018;1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2231] [Cited by in RCA: 2170] [Article Influence: 90.4] [Reference Citation Analysis (1)] |
| 10. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: Pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1339] [Article Influence: 70.5] [Reference Citation Analysis (33)] |
| 11. | Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 898] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 12. | Gold JS, Gönen M, Gutiérrez A, Broto JM, García-del-Muro X, Smyrk TC, Maki RG, Singer S, Brennan MF, Antonescu CR, Donohue JH, DeMatteo RP. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: A retrospective analysis. Lancet Oncol. 2009;10:1045-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 374] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 13. | Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C, Bordoni A, Magnusson MK, Linke Z, Sufliarsky J, Federico M, Jonasson JG, Dei Tos AP, Rutkowski P. Risk of recurrence of gastrointestinal stromal tumour after surgery: An analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 690] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 14. | Jumniensuk C, Charoenpitakchai M. Gastrointestinal stromal tumor: Clinicopathological characteristics and pathologic prognostic analysis. World J Surg Oncol. 2018;16:231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Supsamutchai C, Wilasrusmee C, Hiranyatheb P, Jirasiritham J, Rakchob T, Choikrua P. A cohort study of prognostic factors associated with recurrence or metastasis of gastrointestinal stromal tumor (GIST) of stomach. Ann Med Surg (Lond). 2018;35:1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Dematteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD, Blackstein ME, Blanke CD, von Mehren M, Brennan MF, Patel S, McCarter MD, Polikoff JA, Tan BR, Owzar K; American College of Surgeons Oncology Group (ACOSOG) Intergroup Adjuvant GIST Study Team. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: A randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097-1104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1075] [Cited by in RCA: 983] [Article Influence: 57.8] [Reference Citation Analysis (1)] |
| 17. | Emory TS, Sobin LH, Lukes L, Lee DH, O'Leary TJ. Prognosis of gastrointestinal smooth-muscle (stromal) tumors: Dependence on anatomic site. Am J Surg Pathol. 1999;23:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 409] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 18. | Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: A clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 872] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 19. | Rutkowski P, Nowecki ZI, Michej W, Debiec-Rychter M, Woźniak A, Limon J, Siedlecki J, Grzesiakowska U, Kakol M, Osuch C, Polkowski M, Głuszek S, Zurawski Z, Ruka W. Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Ann Surg Oncol. 2007;14:2018-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 188] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 20. | Hohenberger P, Ronellenfitsch U, Oladeji O, Pink D, Ströbel P, Wardelmann E, Reichardt P. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumour. Br J Surg. 2010;97:1854-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 21. | Goh BK, Chow PK, Yap WM, Kesavan SM, Song IC, Paul PG, Ooi BS, Chung YF, Wong WK. Which is the optimal risk stratification system for surgically treated localized primary GIST? Comparison of three contemporary prognostic criteria in 171 tumors and a proposal for a modified Armed Forces Institute of Pathology risk criteria. Ann Surg Oncol. 2008;15:2153-2163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Belfiori G, Sartelli M, Cardinali L, Tranà C, Bracci R, Gesuita R, Marmorale C. Risk stratification systems for surgically treated localized primary Gastrointestinal Stromal Tumors (GIST). Review of literature and comparison of the three prognostic criteria: MSKCC Nomogramm, NIH-Fletcher and AFIP-Miettinen. Ann Ital Chir. 2015;86:219-227. [PubMed] |
| 23. | Chok AY, Goh BK, Koh YX, Lye WK, Allen JC, Quek R, Teo MC, Chow PK, Ong HS, Chung AY, Wong WK. Validation of the MSKCC Gastrointestinal Stromal Tumor Nomogram and Comparison with Other Prognostication Systems: Single-Institution Experience with 289 Patients. Ann Surg Oncol. 2015;22:3597-3605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Yanagimoto Y, Takahashi T, Muguruma K, Toyokawa T, Kusanagi H, Omori T, Masuzawa T, Tanaka K, Hirota S, Nishida T. Re-appraisal of risk classifications for primary gastrointestinal stromal tumors (GISTs) after complete resection: Indications for adjuvant therapy. Gastric Cancer. 2015;18:426-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Miyamoto H, Kunisaki C, Otsuka Y, Takahashi M, Takagawa R, Misuta K, Kameda K, Makino H, Matsuda G, Yamaguchi N, Kamiya N, Murakami T, Morita S, Akiyama H, Endo I. Macroscopic type is a prognostic factor for recurrence-free survival after resection of gastric GIST. Anticancer Res. 2014;34:4267-4273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Bednarski BK, Araujo DM, Yi M, Torres KE, Lazar A, Trent JC, Cormier JN, Pisters PW, Lev DC, Pollock RE, Feig BW, Hunt KK. Analysis of prognostic factors impacting oncologic outcomes after neoadjuvant tyrosine kinase inhibitor therapy for gastrointestinal stromal tumors. Ann Surg Oncol. 2014;21:2499-2505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Martin-Broto J, Gutierrez A, Garcia-del-Muro X, Lopez-Guerrero JA, Martinez-Trufero J, de Sande LM, Lainez N, Maurel J, De Juan A, Losa F, Andres R, Casado A, Tejido PG, Blanco R, Carles J, Bellmunt J, Gomez-España A, Ramos R, Martinez-Serra J, Llombart-Bosch A, Poveda A. Prognostic time dependence of deletions affecting codons 557 and/or 558 of KIT gene for relapse-free survival (RFS) in localized GIST: A Spanish Group for Sarcoma Research (GEIS) Study. Ann Oncol. 2010;21:1552-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Qi Y, Zhao W, Wang Z, Li T, Meng X. Tumor sites and microscopic indicators are independent prognosis predictors of gastrointestinal stromal tumors. Tohoku J Exp Med. 2014;233:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Li CF, Liu TT, Chuang IC, Chen YY, Fang FM, Chan TC, Li WS, Huang HY. PLCB4 copy gain and PLCß4 overexpression in primary gastrointestinal stromal tumors: Integrative characterization of a lipid-catabolizing enzyme associated with worse disease-free survival. Oncotarget. 2017;8:19997-20010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Feng F, Tian Y, Liu S, Zheng G, Liu Z, Xu G, Guo M, Lian X, Fan D, Zhang H. Combination of PLR, MLR, MWR, and Tumor Size Could Significantly Increase the Prognostic Value for Gastrointestinal Stromal Tumors. Medicine (Baltimore). 2016;95:e3248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P- Reviewer: Aurello P, Milone M, Tomažič A, Ueda H S- Editor: Yan JP L- Editor: Wang TQ E- Editor: Yin SY