Published online Jan 7, 2019. doi: 10.3748/wjg.v25.i1.138
Peer-review started: October 15, 2018
First decision: November 7, 2018
Revised: December 5, 2018
Accepted: December 6, 2018
Article in press: December 6, 2018
Published online: January 7, 2019
Processing time: 93 Days and 18.9 Hours
To evaluate the clinical properties of three subpopulations of circulating tumor cells (CTCs) undergoing epithelial-mesenchymal transition (EMT) in pancreatic ductal adenocarcinoma (PDAC) patients.
We identified CTCs for expression of the epithelial cell marker cytokeratin or epithelial cell adhesion molecule (EpCAM) (E-CTC), the mesenchymal cell markers vimentin and twist (M-CTC), or both (E/M-CTC) using the CanPatrol system. Between July 2014 and July 2016, 107 patients with PDAC were enrolled for CTC evaluation. CTC enumeration and classification were correlated with patient clinicopathological features and outcomes.
CTCs were detected in 78.5% of PDAC patients. The number of total CTCs ranged from 0 to 26 across all 107 patients, with a median value of six. CTC status correlated with lymph node metastasis, TNM stage, distant metastasis, blood lymphocyte counts, and neutrophil-to-lymphocyte ratio (NLR). Kaplan-Meier survival analysis showed that patients with ≥ 6 total CTCs had significantly decreased overall survival and progression-free survival compared with patients with < 6 total CTCs. The presence of M-CTCs was positively correlated with TNM stage (P < 0.01) and distant metastasis (P < 0.01). Additionally, lymphocyte counts and NLR in patients without CTCs were significantly different from those in patients testing positive for each CTC subpopulation (P < 0.01).
Classifying CTCs by EMT markers helps to identify the more aggressive CTC subpopulations and provides useful evidence for determining a suitable clinical approach.
Core tip: In the present study, circulating tumor cell (CTC) enumeration and classification in pancreatic ductal adenocarcinoma (PDAC) patients were examined using the CanPatrol system. We explored the relationship between CTC status and survival and prognosis in 107 PDAC patients in China. CTC status was correlated with lymph node metastasis, TNM stage, distant metastasis, blood lymphocyte counts, neutrophil-to-lymphocyte ratio, and patient prognosis. Our findings demonstrate that CTCs show promise as a prognostic biomarker and provide useful evidence for determining an appropriate clinical approach for pancreatic adenocarcinoma patients.
- Citation: Zhao XH, Wang ZR, Chen CL, Di L, Bi ZF, Li ZH, Liu YM. Molecular detection of epithelial-mesenchymal transition markers in circulating tumor cells from pancreatic cancer patients: Potential role in clinical practice. World J Gastroenterol 2019; 25(1): 138-150
- URL: https://www.wjgnet.com/1007-9327/full/v25/i1/138.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i1.138
Pancreatic ductal adenocarcinoma (PDAC), which is derived from the ductal epithelium, is the most common histological subtype of pancreatic malignancy, accounting for 90% of all cases[1,2] . It ranks fourth in cancer-related mortality. Because of its late presentation and propensity to invade adjacent organs and metastasize, PDAC remains one of the most lethal solid malignancies[3,4]. Endoscopic ultrasound (EUS) is the most sensitive nonoperative imaging test for the detection of benign or malignant pancreatic lesions, while fine needle aspiration (FNA) is a minimally invasive sampling technique that has proved to be a safe and accurate method of tissue acquisition. It has been demonstrated that the operating characteristics of EUS-FNA of solid pancreatic masses are: sensitivity 95%, specificity 92%, positive predictive value 98%, and negative predictive value 80%. The overall accuracy of EUS-FNA is 94.1%. Thus, EUS-FNA is the current gold standard technique for tissue acquisition in patients with unresectable pancreatic cancer[3,4]. However, EUS-FNA is costly and inconvenient, and is correlated with risk of complications, including pancreatitis and bowel perforations[5]. Computed tomography (CT) and magnetic resonance imaging (MRI) are commonly used in tumor staging, but they show limited ability in detecting small-volume metastatic disease, leading to routine under-staging[6]. Thus, identification of a biomarker for early diagnosis and accurate staging at the time of disease presentation can better inform first-line therapy.
Circulating tumor cells (CTCs) are disseminated cancer cells that escape from the primary lesion or from metastatic foci and enter the bloodstream, exemplifying the switch from localized to systemic disease[7,8]. CTCs are thought to represent the intravasated tumor stage between the formation of an invasive cancer and its eventual distant metastasis[9,10]. CTCs have been examined in numerous cancer types, such as prostate, colorectal, breast, gastric, and lung cancers for guiding clinical management, evaluating curative efficacy, predicting prognosis, and monitoring tumor recurrence[11-14] . In PDAC, CTCs have also been explored. Initial studies have confirmed the presence of CTCs in patients with PDAC[15,16]. A study reported by Liu et al[17] classified cells as triploid, tetraploid, and multiploid CTCs based on chromosome 8 copy number, and found that both total CTC number and CTC subtype number are useful in PDAC diagnosis. Additionally, CTC positivity was also associated with poor tumor differentiation, shorter overall survival (OS), and increased metastasis in PDAC[18,19]. These data provide support for further exploration of CTC counts as indicators of PDAC progression. However, to date, CTCs are not as well established as a biomarker as compared with other solid cancers[17,20]. One reason may be the low sensitivity of previous technology in detecting CTCs from peripheral blood in PDAC.
Early and widespread metastasis remains a major challenge in the effective treatment of PDAC. Epithelial-mesenchymal transition (EMT), which is indispensable for PDAC metastasis, is a multi-step process involving many molecular and cellular changes, including the downregulation of epithelial proteins and the upregulation of mesenchymal proteins, endowing the cells with increased motility and invasiveness[21-23]. Recent studies have revealed that the EMT phenotype in CTCs may facilitate tumor metastasis. Characterizing the epithelial vs mesenchymal phenotypes of CTCs may be helpful in identifying the most aggressive CTC subpopulations and determining an appropriate therapy[24-26]. Recent work described a new technique called CanPatrol CTC enrichment, which evaluates CTC classification based on the EMT phenotype. Using this technique, CTCs are classified into three subpopulations based on the expression of epithelial (E-CTCs), biphenotypic epithelial/mesenchymal (E/M-CTCs), and mesenchymal markers (M-CTCs).
In the present study, CTCs from 107 PDAC patients were isolated and their EMT phenotype was characterized using the CanPatrol CTC enrichment technique. In addition, the relationships between clinicopathological parameters and the relative abundance of the three circulating EMT-CTC subpopulations were evaluated.
From July 2014 and July 2016, 107 patients with newly diagnosed PDAC at Sun Yat-sen Memorial Hospital of Sun Yat-sen University were enrolled in the study. Informed consent was obtained from all patients before sample collection. All patients selected met the following criteria: pathological diagnoses were clear and definite; and no preoperative chemotherapy or radiotherapy had been administered. Follow-up was carried out completely and OS was defined as the time interval between the date of surgery and the date of death or the end of follow-up. This retrospective study was conducted in compliance with the institutional policy to protect private patient information and was approved by the institutional review board of Sun Yat-sen Memorial Hospital.
CTC isolation was conducted using the CanPatrol CTC filtration system. For PDAC patients, 5-mL peripheral blood samples were collected in EDTA tubes by venipuncture and filtered with a calibrated membrane with 8-μm diameter pores. The filtration system included the membrane (Sur Exam, Guangzhou, China), a manifold vacuum plate with valve settings (SurExam, Guangzhou, China), an E-Z96 vacuum manifold (Omega, Norcrss, GA, United States), and a vacuum pump (Auto Science, Tianjin, China). Prior to filtration, red blood cell lysis was applied to remove erythrocytes, then PBS with 4% formaldehyde was used to resuspend the cells for 5 min. The cell suspension was transferred to a filtration tube and pumped with at least 0.08 MPa.
CTC subpopulations were identified using a multiplex RNA-ISH assay. Four epithelial biomarkers [epithelial cell adhesion molecule (EpCAM) and cytokeratin (CK) 8/18/19], two mesenchymal biomarkers (vimentin and twist), and a leukocyte biomarker, leukocyte common antigen (CD45), were applied to capture and characterize the CTCs. The hybridization assay was performed as previously described[22]. The assay was performed in a 24-well plate (Corning, NY, United States), and the cells on the membrane were treated with protease (Qiagen, Hilden, Germany) and subsequently subjected to serial hybridization reactions with capture probes that were specific for the intended examined genes (Supplementary Table 1). We applied 4′,6-diamindino-2-phenylindole (DAPI) to stain the nuclei, and the cells were analyzed with a fluorescence microscope.
| Group | Patients n (%) |
| Number of patients with no CTC | 23/107 (21.5) |
| Number of patients with ≥ 1 CTC | 84/107 (78.5) |
| Number of patients with ≥ 6 CTC | 55/107 (51.4) |
| Number of patients with E-CTCs | 65/107(60.7) |
| Number of patients with E/M-CTCs | 39/107 (36.4) |
| Number of patients with M-CTCs | 49/107 (45.8) |
| CTC dynamic range | |
| Total CTC | 0-26 |
| Median | 6 |
| E-CTC | 1-11 |
| E/M-CTC | 0-26 |
| M-CTC | 0-9 |
All statistical analyses were performed using SPSS Statistics 22.0 and GraphPad Prism 7.0. The results are presented as percentages for categorical variables. The Mann-Whitney U test was used to assess the differences between two groups because the data were not normally distributed, and the Kruskal-Wallis H test was used for multi-group analysis. Differences in patient survival were assessed using the Kaplan-Meier method and analyzed using the log-rank test in a univariate analysis. P-values less than 0.05 were considered statistically significant. Cox regression analyses were performed to assess the relative risk for each factor. A receiver operating characteristic (ROC) curve was established to evaluate the diagnostic value of CTCs. The area under the curve (AUC) was used to assess the predictive power. A two-tailed P-value of < 0.05 was considered statistically significant (aP < 0.05, bP < 0.01).
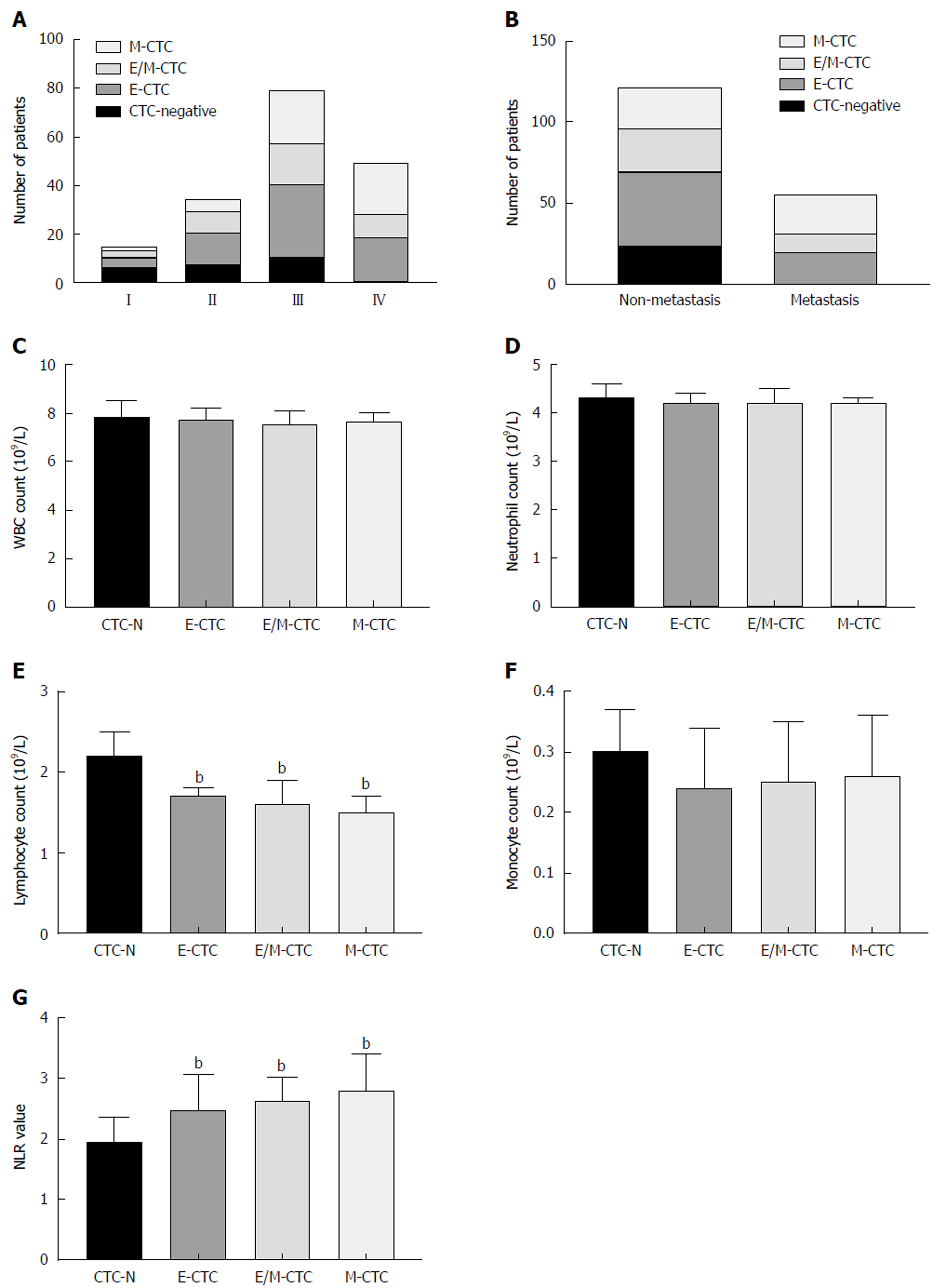
CTCs from 107 PDAC patients were identified using the CanPatrol system. As shown in Figure 1, epithelial CTCs showed red fluorescence (Figure 1A), while mesenchymal CTCs exhibited green fluorescence (Figure 1B), corresponding to their specific genes (i.e., EpCAM or CK, and vimentin and twist), respectively. Additionally, a third hybrid population of CTCs expressing both epithelial- and mesenchymal-specific genes was also observed (Figure 1C). Of the total population, 84 (78.5%) patients had positive CTC counts at baseline. The number of total CTCs ranged from 0 to 26 across all 107 patients, with a median value of six. Overall, ≥6 total CTC(s) were present in 51.4% of samples. The positive rates of epithelial, hybrid, and mesenchymal CTCs were 60.7%, 36.4%, and 45.8%, respectively (Table 1).
As shown in Table 2, 107 PDAC patients were included in the analysis. The presence of CTCs was positively correlated with TNM stage (P < 0.001), lymph node metastasis (P = 0.016), and distant metastasis (P < 0.001) (Figure 2A-C). The other clinicopathological features showed no statistical relationship with CTC status. Univariate analysis of OS revealed that lymph node metastasis (P = 0.007), TNM stage (P < 0.001), distant metastasis (P < 0.001), and ≥ 6 total CTCs (P < 0.001) were prognostic indicators. Multivariate analysis revealed that lymph node metastasis (P = 0.011), TNM stage (P < 0.001), distant metastasis (P < 0.001), and ≥ 6 total CTCs (P < 0.001) were all independent prognostic indicators for OS of patients with PDAC (Table 3). We constructed a ROC curve analysis (Figure 2D) to distinguish metastatic PDAC patients from those without metastasis; the area under the ROC curve (AUROC) was 0.8 (P < 0.0001) with an optimal cut-off point of 7 (sensitivity = 0.893, specificity = 0.633). For comparison, CA-199 at the optimum cut-off of 884 U/mL distinguished metastatic PDAC patients from those without metastasis, with a sensitivity of 0.536, specificity of 0.785, and AUROC of 0.637. Kaplan-Meier survival analysis showed that patients with ≥ 6 total CTCs had significantly decreased OS and progression-free survival (PFS) as compared with patients with < 6 total CTCs (OS: median 11 mo vs 18 mo; HR = 0.504; 95%CI: 0.330-0.768; P < 0.001. PFS: median 8 mo vs 13 mo; HR = 0.520; 95%CI: 0.342-0.791) (Figure 2E and F).
| Total CTC-negative patients (%) | Total CTC-positive patients (%) | P-value | |
| Age, yr (median: 63, IQR: 52-76) | |||
| < 60 | 8/23 (34.8) | 32/84 (38.1) | 0.769 |
| ≥ 60 | 15/23 (65.2) | 52/84 (61.9) | |
| Gender | |||
| Male | 16/23 (69.6) | 47/84 (56.0) | 0.313 |
| Female | 7/23 (30.4) | 37/84 (44.0) | |
| Ethnic group | |||
| Han ethnic group | 14/23 (60.9) | 59/84 (70.2) | 0.346 |
| Zhuang ethnic group | 5/23 (21.7) | 16/84 (19.1) | |
| Li ethnic group | 4/23 (17.4) | 9/84 (10.7) | |
| Differentiation | |||
| Well | 3/23 (13.0) | 7/84 (8.3) | 0.435 |
| Moderate | 13/23 (56.5) | 54/84 (64.3) | |
| Poor | 7/23 (30.5) | 23/84 (27.4) | |
| TNM stage | |||
| I | 6/23 (26.1) | 4/84 (4.8) | < 0.001 |
| II | 7/23 (30.4) | 13/84 (15.5) | |
| III | 10/23 (43.5) | 39/84 (46.4) | |
| IV | 0/23 (0) | 28/84 (33.3) | |
| Lymph node metastasis | |||
| Negative | 13/23 (56.5) | 34/84 (40.5) | 0.023 |
| Positive | 10/23 (43.5) | 50/84 (59.5) | |
| Neural invasion | |||
| Negative | 10/23 (43.5) | 29/84 (34.5) | 0.247 |
| Positive | 13/23 (56.5) | 55/84 (65.5) | |
| Distant metastasis | |||
| Negative | 23/23 (100) | 56/84 (66.7) | < 0.001 |
| Positive | 0/23 (0) | 28/84 (33.3) | |
| Predictor | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P-value | HR | 95%CI | P-value | |
| Age, < 60 vs ≥ 60 | 0.818 | 0.798-1.017 | 0.792 | |||
| Gender (female vs male) | 0.928 | 0.781-1.130 | 0.952 | |||
| Differentiation (well vs moderate vs poor) | 1.045 | 0.873-1.186 | 0.134 | |||
| Ethnic group (Han/Zhuang/Li) | 0.917 | 0.834-1.048 | 0.866 | |||
| TNM stage (I/II vs III/IV) | 1.452 | 1.215-1.475 | < 0.001 | 1.148 | 1.031-1.268 | 0.011 |
| Lymph node metastasis (negative vs positive) | 1.287 | 1.048-1.364 | 0.007 | 1.198 | 1.036-1.380 | 0.017 |
| Neural invasion (negative vs positive) | 1.008 | 0.781-1.155 | 0.949 | |||
| Distant metastasis (negative vs positive) | 1.426 | 1.236-1.710 | < 0.001 | 1.521 | 1.256-1.887 | < 0.001 |
| CTC, < 6 vs ≥ 6 | 1.849 | 1.717-2.238 | < 0.001 | 1.851 | 1.637-2.173 | < 0.001 |
We quantified the immune cells in the peripheral blood of patients with PDAC and analyzed the associations between immune cell parameters and the presence of CTCs (Figure 2G and H). There were no significant differences between the CTC-negative and CTC-positive patient groups with respect to total white blood cell (WBC), neutrophil, or monocyte counts. However, there was a significant difference in lymphocyte count and the neutrophil-to-lymphocyte ratio (NLR) between the CTC-positive and CTC-negative patient groups (P < 0.001).
The associations between the presence of CTC subpopulations of each EMT phenotype and clinicopathological features were analyzed. Table 4 shows that E-CTCs were present in 65 (60.7%) patients, while E/M-CTCs and M-CTCs were present in 39 (36.4%) and 49 (45.8%) patients, respectively. The presence of M-CTCs was positively correlated with TNM stage (P < 0.01) and distant metastasis (P < 0.01). As shown in Figure 3A and B, M-CTCs were present in significantly more patients with distant metastasis than in those without. Regarding TNM stage, all patients with stage IV PDAC were CTC-positive and M-CTCs were more common in patients with stages III and IV disease.
| Epithelia CTC (%) | Hybrid CTC (%) | Mesenchymal CTC (%) | ||||||||
| Positive | Negative | P-value | Positive | Negative | P-value | Positive | Negative | P-value | ||
| Age (yr) | < 60 | 24/65 | 16/42 | > 0.9 | 12/39 | 28/68 | 0.18 | 21/49 | 19/58 | 0.19 |
| (36.9) | (38.1) | (30.8) | (41.2) | (42.9) | (32.8) | |||||
| ≥ 60 | 41/65 | 26/42 | 27/39 | 40/68 | 28/49 | 39/58 | ||||
| (63.1) | (61.9) | (69.2) | (58.8) | (57.1) | (67.2) | |||||
| Gender | Male | 37/65 | 26/42 | 0.56 | 24/39 | 39/68 | 0.56 | 28/49 | 35/58 | 0.77 |
| (56.9) | (61.9) | (61.5) | (57.4) | (57.1) | (60.3) | |||||
| Female | 28/65 | 16/42 | 15/39 | 29/68 | 21/49 | 23/58 | ||||
| (43.1) | (38.1) | (38.5) | (42.6) | (42.9) | (39.7) | |||||
| Differentiation | Well | 6/65 | 4/42 | 0.21 | 4/39 | 6/68 | 0.97 | 5/49 | 5/58 | 0.84 |
| (9.2) | (9.5) | (10.3) | (8.8) | (8.1) | (9.3) | |||||
| Moderate | 38/65 | 29/42 | 24/39 | 43/68 | 31/49 | 36/58 | ||||
| (58.5) | (69.1) | (61.5) | (63.2) | (64.9) | (60.5) | |||||
| Poor | 21/65 | 9/42 | 11/39 | 19/68 | 13/49 | 17/58 | ||||
| (32.3) | (21.4) | (28.2) | (28) | (27) | (30.2) | |||||
| TNM stage | I | 4/65 | 6/42 | 0.29 | 3/39 | 7/68 | 0.59 | 1/49 | 9/58 | < 0.01 |
| (6.1) | (14.3) | (7.7) | (10.3) | (2) | (15.5) | |||||
| II | 13/65 | 7/42 | 9/39 | 11/68 | 5/49 | 15/58 | ||||
| (20) | (16.7) | (23.1) | (16.2) | (10.2) | (25.9) | |||||
| III | 30/65 | 19/42 | 17/39 | 32/68 | 22/49 | 27/58 | ||||
| (46.2) | (45.2) | (43.6) | (47.1) | (44.9) | (46.6) | |||||
| IV | 18/65 | 10/42 | 10/39 | 18/68 | 21/49 | 7/58 | ||||
| (27.7) | (23.8) | (25.6) | (26.4) | (42.9) | (12) | |||||
| Lymph node metastasis | Negative | 25/65 | 22/42 | 0.08 | 18/39 | 29/68 | 0.78 | 24/49(49.0) | 23/58 | 0.26 |
| (38.5) | (52.4) | (46.2) | (42.6) | (39.7) | ||||||
| Positive | 40/65 | 20/42 | 21/39 | 39/68 | 25/49 | 35/58 | ||||
| (61.5) | (47.6) | (53.8) | (57.4) | (51) | (60.3) | |||||
| Neural invasion | Negative | 22/65 | 17/42 | 0.38 | 18/39 | 21/68 | 0.78 | 17/49 | 22/58 | 0.77 |
| (33.8) | (40.5) | (46.2) | (42.6) | (34.7) | (37.9) | |||||
| Positive | 43/65 | 25/42 | 21/39 | 39/68 | 32/49 | 36/58 | ||||
| (66.2) | (59.5) | (53.8) | (57.4) | (65.3) | (62.1) | |||||
| Distant metastasis | Negative | 46/65 | 33/42 | 0.25 | 27/39 | 52/68 | 0.26 | 25/49 | 54/58 | < 0.01 |
| (70.8) | (78.6) | (69.2) | (76.5) | (51) | (93.1) | |||||
| Positive | 19/65 | 9/42 | 12/39 | 16/68 | 24/49 | 4/58 | ||||
| (29.2) | (21.4) | (30.8) | (23.5) | (49) | (6.9) | |||||
| Ethnic group | Li ethnic group | 8/65 | 5/42 | 0.98 | 5/39 | 8/68 | 0.52 | 5/49 | 8/58 | 0.24 |
| (12.3) | (11.9) | (12.8) | (11.8) | (10.2) | (13.8) | |||||
| Han ethnic group | 44/65 | 29/42 | 25/39 | 48/68 | 32/49 | 41/58 | ||||
| (67.7) | (69.1) | (64.1) | (70.6) | (65.3) | (70.7) | |||||
| Zhuang ethnic group | 13/65 | 8/42 | 9/39 | 12/68 | 12/49 | 9/58 | ||||
| (20) | (19) | (23.1) | (17.6) | (24.5) | (15.5) | |||||
We analyzed the correlation of different CTC subpopulations with the EMT phenotype and WBC count. There were no significant differences between different subpopulations with respect to total WBC, neutrophil, or monocyte counts (Figure 3C, D, and F). Figure 3E and G demonstrates that patients with any CTC subpopulations had significantly lower lymphocyte counts and higher NLRs than patients without CTCs (P < 0.001).
PDAC remains one of the most devastating diseases because of its late presentation and resistance to systemic therapy. Cross-sectional imaging alone often results in under-staging of PDAC patients[23,24,27]. Many studies have indicated that a “surgery first” paradigm for borderline resectable, and even early-stage, patients does not lead to a survival benefit and may in fact impair survival because of high recurrence and metastasis rates[27]. Therefore, there is an urgent need for novel biomarkers to complement cross-sectional imaging, especially for the identification of patients likely to benefit from non-surgical treatments first.
In recent years, the detection and characterization of CTCs have received tremendous attention because of the minimally invasive approaches applied to obtain sequential blood specimens from cancer patients and their potential clinical implications. It is anticipated that quantification and molecular subtyping of CTCs could be adopted for monitoring tumor burden and metastasis of PDAC. To date, published CTC data for PDAC mostly rely on small patient cohorts at different disease stages and use various CTC techniques, showing contradictory results. A large cohort of 154 patients had discovered CTCs using RT-PCR for CK20, obtaining a CTC rate of 34%. This study demonstrated that CTCs had a significantly negative prognostic impact on patient survival[28]. In 2013, CTC detection rates and prognostic value were studied in a prospective cohort of locally advanced pancreatic carcinoma patients. This study used the Cellsearch technique to detect CTCs and demonstrated that CTC positivity was associated with poor tumor differentiation and shorter OS[19]. Another study used the microfluidic Nano Velcro assay and identified CTCs in 78% of 126 PDAC patients. The most significant finding of their study was the predictive ability of CTCs for occult metastatic disease in the preoperative setting[29]. Additionally, a recent study also detected CTCs through immunofluorescence staining for CK 19 or EpCAM in portal vein blood from PDAC patients. Their results demonstrated that CTC counts in portal vein blood were highly associated with intrahepatic metastases and poorer prognosis[30]. In our study, a novel technology applying a combination of epithelial and mesenchymal markers was used to isolate CTCs in peripheral blood from 107 patients with PDAC. We detected CTCs in 84 out of 107 (78.5%) patients. Our results demonstrated that positive CTC status was significantly correlated with lymph node metastasis, distant metastasis, and late TNM stage. Meanwhile, CTCs were associated with a significantly higher risk of death independent of other clinical risk factors.
Although millions of disseminated tumor cells are shed from primary lesions into the peripheral blood during metastasis, they very rarely survive to form new lesions[31]. A recent study demonstrated that CTC status is influenced both by the type of primary tumor and by the number of immune cells in the bloodstream[32]. To study this further, we evaluated the correlation between CTCs and immune cell counts. The inflammatory response is correlated with the progression of various tumors, including lung, colorectal, and pancreatic cancers[33-36]. Chronic pancreatitis is one of the risk factors for pancreatic cancer[33-36]. Some studies demonstrated that a high NLR, which is a circulating systemic inflammation marker, is a poor prognostic factor in PDAC[37]. Our results demonstrated that lymphocyte count was reduced while NLR was significantly increased in the CTC-positive group compared with the negative group. Furthermore, there was a negative correlation between CTCs and lymphocytes and a positive correlation between CTCs and NLR. These results indicated that lymphocytes may have an important role in the clearance of CTCs.
In previous studies, CTCs were detected by examining the expression of epithelial-specific markers, such as EpCAM and CK. Thus far, the CellSearch system is the only Food and Drug Administration-approved CTC enumeration assay, which defines a CTC according to its size, positivity for EpCAM and CK, and negative CD45 expression[38]. However, multiple studies have reported that phenotypic alterations, such as the upregulation of mesenchymal markers and the loss of epithelial markers, are common in CTCs in general, suggesting that methods that rely on the expression of epithelial markers most likely overlook CTCs undergoing EMT[39,40]. Several studies exploring the EMT phenomenon of CTCs have showed that mesenchymal CTCs were associated with tumor progression and therapy resistance in some cancer types[41-43] . However, to date, there is no research describing the EMT phenomenon in CTCs in PDAC. In the present study, we detected and characterized CTCs from patients with PDAC using the cell size- and phenotype-based CanPatrol technique, which has been reported to detect CTCs with high efficiency. We identified CTCs with different EMT phenotypes in 84 patients. Furthermore, it was discovered that M-CTCs were most common in patients with advanced cancer. However, circulating tumor microemboli were not detected in the present study. Our results support the notion of a role for EMT in tumor metastasis and indicate that the role of M-CTCs might be more essential than the other subpopulations in terms of the risk of disease progression. Moreover, we also observed that M-CTCs were negatively correlated with lymphocyte counts and that patients who were positive for M-CTC had significantly lower circulating lymphocyte counts than the CTC-negative group or the group lacking M-CTCs.
There is no doubt that liquid biopsy is superior to conventional methods for dynamically monitoring cancer status, and the detection of CTCs is likely to gain popularity in the clinic. To our knowledge, here we report for the first time the prognostic utility of CTCs, as detected by the CanPatrol CTC enrichment technique, in patients with PDAC. Our data support the potential clinical value of PDAC CTCs. Both total CTC number and CTC EMT phenotype may act as potential biomarkers for PDAC prognosis. However, our study suffered from a small sample size, and the results should be interpreted with caution. Large well-designed clinical trials are required to elucidate the potential value of EMT markers in CTCs.
Circulating tumor cells (CTCs) have been demonstrated to be a prognostic indicator in numerous cancers. However, in pancreatic ductal adenocarcinoma (PDAC), CTCs remain to be studied. Here, we report for the first time the prognostic utility of CTCs, as detected by CanPatrol CTC enrichment technique, in patients with PDAC. Our data support the potential clinical value of PDAC CTCs. Both total CTC number and CTC epithelial-to-mesenchymal transition (EMT) phenotype may act as potential biomarkers for PDAC prognosis.
In the present study, we explored the relationships between clinicopathological parameters and the relative abundance of three circulating EMT-CTC subpopulations. We found that CTC status correlated with lymph node metastasis, TNM stage, distant metastasis, blood lymphocyte counts, and the neutrophil-to-lymphocyte ratio (NLR). Kaplan-Meier survival analysis showed that patients with ≥ 6 total CTCs had significantly decreased OS and PFS compared to patients with < 6 total CTCs. The presence of M-CTCs was positively correlated with TNM stage and distant metastasis. Additionally, lymphocyte counts and NLR in patients without CTCs were significantly different from those in patients testing positive for each CTC subpopulation. Our data support the potential clinical value of PDAC CTCs. Furthermore, our data also provide support for further large well-designed clinical trials to explore CTC counts as indicators of PDAC progression.
The objective of this research was to explore the relationships between clinicopathological parameters and the relative abundance of the three circulating EMT-CTC subpopulations in PDAC. This research demonstrated that positive CTC status was significantly correlated with lymph node metastasis, distant metastasis, late TNM stage, and poor patient prognosis. Meanwhile, M-CTCs were most common in patients with advanced cancer. These results demonstrated that classifying CTCs by EMT markers helps to identify the more aggressive CTC subpopulations and provides useful evidence for determining a suitable clinical approach.
This research utilized the cell size- and phenotype-based CanPatrol CTC filtration system to isolate CTCs. CTC subpopulations were identified using a multiplex RNA-ISH assay. Four epithelial biomarkers (epithelial cell adhesion molecule and cytokeratin 8/18/19), two mesenchymal biomarkers (vimentin and twist), and a leukocyte biomarker, CD45, were applied to capture and characterize the CTCs.
This research indicated that the presence of CTCs was significantly associated with PDAC poor prognosis. Moreover, M-CTCs were most common in patients with advanced cancer. These results demonstrate that CTCs are promising biomarker for PDAC prognosis and identification of EMT markers in CTCs provide more information on tumor progression.
In the present study, a novel technology called CanPatrol CTC filtration system applying a combination of epithelial and mesenchymal markers was used to detect CTCs in peripheral blood from 107 patients with PDAC. We found that CTC positivity was correlated with clinicopathologic variables and outcomes. Meanwhile, the presence M-CTCs was associated with advanced stage and distant metastasis. These results demonstrate that CTC enumeration and classification show promise as a prognostic biomarker and may provide useful evidence for determining a suitable clinical approach.
This research supports the potential clinical value of PDAC CTCs. Both total CTC number and CTC EMT phenotype may act as potential biomarkers for PDAC prognosis. However, our study suffered from a small sample size, and the results should be interpreted with caution. Large well-designed clinical trials are required to elucidate the potential value of EMT markers in CTCs.
| 1. | Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2078] [Cited by in RCA: 2228] [Article Influence: 139.3] [Reference Citation Analysis (2)] |
| 2. | Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, Hruban RH. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63:318-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 686] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 3. | Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1397] [Cited by in RCA: 1790] [Article Influence: 179.0] [Reference Citation Analysis (1)] |
| 4. | Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11065] [Cited by in RCA: 12229] [Article Influence: 1358.8] [Reference Citation Analysis (3)] |
| 5. | Al-Haddad M, Eloubeidi MA. Interventional EUS for the diagnosis and treatment of locally advanced pancreatic cancer. JOP. 2010;11:1-7. [PubMed] |
| 6. | Khorana AA, Mangu PB, Katz MHG. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update Summary. J Oncol Pract. 2017;13:388-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Al-Hawary M. Role of Imaging in Diagnosing and Staging Pancreatic Cancer. J Natl Compr Canc Netw. 2016;14:678-680. [PubMed] |
| 8. | Nagrath S, Jack RM, Sahai V, Simeone DM. Opportunities and Challenges for Pancreatic Circulating Tumor Cells. Gastroenterology. 2016;151:412-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Alix-Panabières C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer. 2014;14:623-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 917] [Cited by in RCA: 1033] [Article Influence: 86.1] [Reference Citation Analysis (1)] |
| 10. | Fan JL, Yang YF, Yuan CH, Chen H, Wang FB. Circulating Tumor Cells for Predicting the Prognostic of Patients with Hepatocellular Carcinoma: A Meta Analysis. Cell Physiol Biochem. 2015;37:629-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM, Doyle GV, Matera J, Allard WJ, Miller MC, Fritsche HA, Hortobagyi GN, Terstappen LW. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005;23:1420-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 848] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 12. | Danila DC, Heller G, Gignac GA, Gonzalez-Espinoza R, Anand A, Tanaka E, Lilja H, Schwartz L, Larson S, Fleisher M, Scher HI. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13:7053-7058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 516] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 13. | Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse MA, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen LW, Meropol NJ. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann Oncol. 2009;20:1223-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 392] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 14. | Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV, Terstappen LW. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12:4218-4224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 765] [Cited by in RCA: 785] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 15. | Khoja L, Backen A, Sloane R, Menasce L, Ryder D, Krebs M, Board R, Clack G, Hughes A, Blackhall F, Valle JW, Dive C. A pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br J Cancer. 2012;106:508-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 212] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 16. | Kurihara T, Itoi T, Sofuni A, Itokawa F, Tsuchiya T, Tsuji S, Ishii K, Ikeuchi N, Tsuchida A, Kasuya K, Kawai T, Sakai Y, Moriyasu F. Detection of circulating tumor cells in patients with pancreatic cancer: a preliminary result. J Hepatobiliary Pancreat Surg. 2008;15:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Liu H, Sun B, Wang S, Liu C, Lu Y, Li D, Liu X. Circulating Tumor Cells as a Biomarker in Pancreatic Ductal Adenocarcinoma. Cell Physiol Biochem. 2017;42:373-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | de Albuquerque A, Kubisch I, Breier G, Stamminger G, Fersis N, Eichler A, Kaul S, Stölzel U. Multimarker gene analysis of circulating tumor cells in pancreatic cancer patients: a feasibility study. Oncology. 2012;82:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Bidard FC, Huguet F, Louvet C, Mineur L, Bouché O, Chibaudel B, Artru P, Desseigne F, Bachet JB, Mathiot C, Pierga JY, Hammel P. Circulating tumor cells in locally advanced pancreatic adenocarcinoma: the ancillary CirCe 07 study to the LAP 07 trial. Ann Oncol. 2013;24:2057-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 20. | Court CM, Ankeny JS, Sho S, Winograd P, Hou S, Song M, Wainberg ZA, Girgis MD, Graeber TG, Agopian VG, Tseng HR, Tomlinson JS. Circulating Tumor Cells Predict Occult Metastatic Disease and Prognosis in Pancreatic Cancer. Ann Surg Oncol. 2018;25:1000-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 21. | Whittle MC, Hingorani SR. Disconnect between EMT and metastasis in pancreas cancer. Oncotarget. 2015;6:30445-30446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Kang Y, Pantel K. Tumor cell dissemination: emerging biological insights from animal models and cancer patients. Cancer Cell. 2013;23:573-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 328] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 23. | Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6805] [Cited by in RCA: 7906] [Article Influence: 465.1] [Reference Citation Analysis (1)] |
| 24. | Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, Concannon KF, Donaldson MC, Sequist LV, Brachtel E, Sgroi D, Baselga J, Ramaswamy S, Toner M, Haber DA, Maheswaran S. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1963] [Cited by in RCA: 1973] [Article Influence: 151.8] [Reference Citation Analysis (0)] |
| 25. | Okabe H, Ishimoto T, Mima K, Nakagawa S, Hayashi H, Kuroki H, Imai K, Nitta H, Saito S, Hashimoto D, Chikamoto A, Ishiko T, Watanabe M, Nagano O, Beppu T, Saya H, Baba H. CD44s signals the acquisition of the mesenchymal phenotype required for anchorage-independent cell survival in hepatocellular carcinoma. Br J Cancer. 2014;110:958-966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Satelli A, Brownlee Z, Mitra A, Meng QH, Li S. Circulating tumor cell enumeration with a combination of epithelial cell adhesion molecule- and cell-surface vimentin-based methods for monitoring breast cancer therapeutic response. Clin Chem. 2015;61:259-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 27. | Riediger H, Keck T, Wellner U, zur Hausen A, Adam U, Hopt UT, Makowiec F. The lymph node ratio is the strongest prognostic factor after resection of pancreatic cancer. J Gastrointest Surg. 2009;13:1337-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 286] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 28. | Soeth E, Grigoleit U, Moellmann B, Röder C, Schniewind B, Kremer B, Kalthoff H, Vogel I. Detection of tumor cell dissemination in pancreatic ductal carcinoma patients by CK 20 RT-PCR indicates poor survival. J Cancer Res Clin Oncol. 2005;131:669-676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3360] [Cited by in RCA: 3448] [Article Influence: 156.7] [Reference Citation Analysis (0)] |
| 30. | Liu X, Li C, Li J, Yu T, Zhou G, Cheng J, Li G, Zhou Y, Lou W, Wang X, Gong G, Liu L, Chen Y. Detection of CTCs in portal vein was associated with intrahepatic metastases and prognosis in patients with advanced pancreatic cancer. J Cancer. 2018;9:2038-2045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 31. | Wan L, Pantel K, Kang Y. Tumor metastasis: moving new biological insights into the clinic. Nat Med. 2013;19:1450-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 615] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 32. | Chen F, Wang S, Fang Y, Zheng L, Zhi X, Cheng B, Chen Y, Zhang C, Shi D, Song H, Cai C, Zhou P, Xiong B. Feasibility of a novel one-stop ISET device to capture CTCs and its clinical application. Oncotarget. 2017;8:3029-3041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Momi N, Kaur S, Krishn SR, Batra SK. Discovering the route from inflammation to pancreatic cancer. Minerva Gastroenterol Dietol. 2012;58:283-297. [PubMed] |
| 34. | Abu-Remaileh M, Bender S, Raddatz G, Ansari I, Cohen D, Gutekunst J, Musch T, Linhart H, Breiling A, Pikarsky E, Bergman Y, Lyko F. Chronic inflammation induces a novel epigenetic program that is conserved in intestinal adenomas and in colorectal cancer. Cancer Res. 2015;75:2120-2130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Rivas-Fuentes S, Salgado-Aguayo A, Pertuz Belloso S, Gorocica Rosete P, Alvarado-Vásquez N, Aquino-Jarquin G. Role of Chemokines in Non-Small Cell Lung Cancer: Angiogenesis and Inflammation. J Cancer. 2015;6:938-952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 36. | Becker AE, Hernandez YG, Frucht H, Lucas AL. Pancreatic ductal adenocarcinoma: risk factors, screening, and early detection. World J Gastroenterol. 2014;20:11182-11198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 200] [Cited by in RCA: 237] [Article Influence: 19.8] [Reference Citation Analysis (6)] |
| 37. | Stotz M, Gerger A, Eisner F, Szkandera J, Loibner H, Ress AL, Kornprat P, AlZoughbi W, Seggewies FS, Lackner C, Stojakovic T, Samonigg H, Hoefler G, Pichler M. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109:416-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 315] [Cited by in RCA: 407] [Article Influence: 31.3] [Reference Citation Analysis (1)] |
| 38. | Esmaeilsabzali H, Beischlag TV, Cox ME, Parameswaran AM, Park EJ. Detection and isolation of circulating tumor cells: principles and methods. Biotechnol Adv. 2013;31:1063-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 39. | Friedlander TW, Premasekharan G, Paris PL. Looking back, to the future of circulating tumor cells. Pharmacol Ther. 2014;142:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 40. | Harouaka R, Kang Z, Zheng SY, Cao L. Circulating tumor cells: advances in isolation and analysis, and challenges for clinical applications. Pharmacol Ther. 2014;141:209-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 41. | Satelli A, Mitra A, Brownlee Z, Xia X, Bellister S, Overman MJ, Kopetz S, Ellis LM, Meng QH, Li S. Epithelial-mesenchymal transitioned circulating tumor cells capture for detecting tumor progression. Clin Cancer Res. 2015;21:899-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 194] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 42. | Mego M, Cierna Z, Janega P, Karaba M, Minarik G, Benca J, Sedlácková T, Sieberova G, Gronesova P, Manasova D, Pindak D, Sufliarsky J, Danihel L, Reuben JM, Mardiak J. Relationship between circulating tumor cells and epithelial to mesenchymal transition in early breast cancer. BMC Cancer. 2015;15:533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Liu H, Zhang X, Li J, Sun B, Qian H, Yin Z. The biological and clinical importance of epithelial-mesenchymal transition in circulating tumor cells. J Cancer Res Clin Oncol. 2015;141:189-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P- Reviewer: Gali-Muhtasib H, Lim SC, Peparini N, Tanabe S S- Editor: Ma RY L- Editor: Wang TQ E- Editor: Huang Y