Published online Mar 7, 2018. doi: 10.3748/wjg.v24.i9.957
Peer-review started: December 29, 2017
First decision: January 17, 2018
Revised: February 1, 2018
Accepted: February 9, 2018
Article in press: February 9, 2018
Published online: March 7, 2018
Processing time: 66 Days and 22.8 Hours
Two-dimensional shear wave elastography (2D-SWE) is a rapid, simple and novel noninvasive method that has been proposed for assessing hepatic fibrosis in patients with chronic liver diseases (CLDs) based on measurements of liver stiffness. 2D-SWE can be performed easily at the bedside or in an outpatient clinic and yields immediate results with good reproducibility. Furthermore, 2D-SWE was an efficient method for evaluating liver fibrosis in small to moderately sized clinical trials. However, the quality criteria for the staging of liver fibrosis are not yet well defined. Liver fibrosis is the main pathological basis of liver stiffness and a key step in the progression from CLD to cirrhosis; thus, the management of CLD largely depends on the extent and progression of liver fibrosis. 2D-SWE appears to be an excellent tool for the early detection of cirrhosis and may have prognostic value in this context. Because 2D-SWE has high patient acceptance, it could be useful for monitoring fibrosis progression and regression in individual cases. However, multicenter data are needed to support its use. This study reviews the current status and future perspectives of 2D-SWE for assessments of liver fibrosis and discusses the technical advantages and limitations that impact its effective and rational clinical use.
Core tip: There has been considerable research in recent years dedicated to the development of noninvasive methods of chronic liver diseases (CLDs). These include novel elastography methods. In this review, we outline the current state and future perspectives of the commonly used two-dimensional shear wave elastography (2D-SWE) in CLDs. In particular, we discuss the applications and problems in chronic viral hepatitis, nonalcoholic fatty liver disease, alcoholic liver disease, liver transplantation, focal liver lesions, and autoimmune liver disease to synthesize existing evidence for the reader. This is the first full and complete review to assess various CLDs using 2D-SWE.
- Citation: Xie LT, Yan CH, Zhao QY, He MN, Jiang TA. Quantitative and noninvasive assessment of chronic liver diseases using two-dimensional shear wave elastography. World J Gastroenterol 2018; 24(9): 957-970
- URL: https://www.wjgnet.com/1007-9327/full/v24/i9/957.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i9.957
Liver fibrosis results from repetitive or sustained liver inflammation caused by chronic liver diseases (CLDs) and has serious long-term consequences in terms of patient morbidity and mortality due to the progression to cirrhosis. Patients with cirrhosis are at a higher risk of developing complications, including esophageal varices, ascites, liver failure and hepatocellular carcinoma (HCC), than healthy individuals[1,2]. Liver fibrosis is an important factor in the development of various CLDs and is mainly mediated by chronic liver injury, which leads to liver fibrosis characterized by increased extracellular matrix production by fibroblast-like cells and increased liver stiffness (LS)[3]. The precise assessment of the severity of liver fibrosis and reliable diagnosis of cirrhosis are vital steps in the management of CLDs, as they provide information that impacts therapeutic decisions[4,5].
Liver biopsy remains the reference standard for the staging of liver fibrosis, despite its limitations, including its invasivity and the pain experienced by patients, high cost, risk of bleeding and poor reproducibility, and contraindications, such as cases of massive ascites[6,7]. Furthermore, the accuracy of liver biopsy is influenced by several factors, including intra- and interobserver variability and sampling error[8,9]. Given these limitations, liver biopsy is not an ideal method for repeated assessments of disease progression as well.
The noninvasive assessment of liver fibrosis has recently become a research focus, leading to the introduction of new technologies. In particular, shear wave elastography (SWE), which is based on ultrasound (US) technology, has been widely applied and has gained acceptance. The latest guidelines on the clinical use of liver US elastography of the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB)[10] and the guidelines on the evaluation of liver disease severity and prognosis of the European Association for the Study of the Liver- Asociación Latinoamericana para el Estudio del Hígado state that two-dimension (2D)-SWE is a valid and promising technique for noninvasive staging of liver fibrosis in viral hepatitis and seems to be at least equivalent to transient elastography (TE) and point SWE (pSWE)/acoustic radiation force impulse (ARFI)[11].
Several elasticity imaging techniques have recently been developed for assessing the mechanical properties of liver tissues and staging the fibrosis level using different imaging modalities. In accordance with the Guidelines for the Basic Principles and Technology of EFSUMB[12], the available US-based elastography techniques include strain elastography (SE) and SWE; SWE can be further subdivided into three techniques - TE, pSWE and 2D-SWE - in addition to radiology-based magnetic resonance elastography (MRE).
Among these, TE is the most widely used elastography method. However, in a study of 13369 CLD patients over a 5-year period of using TE, unreliable results were obtained in 15.8% of cases[13]. The limitations of TE, such as the difficulty in measuring obese patients and patients with narrow intercostal spaces, the variations in operator experience, and the fact that it is not applicable in patients with ascites, were the primary factors contributing to these unreliable results. Importantly, the issue of obesity has been partially addressed by the introduction of specially designed XL probes that measure LS deeper than the standard M probes. It has also been reported that TE and ARFI elastography are inaccurate in the detection of early and intermediate fibrosis[14,15].
MRE can be used to quantitatively diagnose liver fibrosis with high accuracy and is mainly used for the diagnosis of advanced liver fibrosis and cirrhosis. The diagnostic accuracy of MRE increased when the liver fibrosis was getting serious, whereas the level of early fibrosis detected by MRE is inaccurate[16,17]. 2D-SWE is the latest elastography technology and can assess the elasticity of liver tissue easily and quickly, thus reflecting the degree of liver fibrosis. Table 1 demonstrates the comparison of currently available noninvasive methods in patients with CLD.
| Content | Methods | |||
| TE | pSWE | 2D-SWE | MRE | |
| Technical principle | TE was the first commercially available elastography method developed for measuring liver stiffness using a dedicated device that includes an amplitude modulation (A) mode image for organ localization | pSWE can be implemented on a common ultrasound diagnostic system. It uses a regular ultrasonic probe to emit a single impulse of acoustic radiation force and generates a shear wave to detect the shear wave propagation velocity | 2D-SWE is the combination of a radiation force applied to the tissues by focused ultrasonic beams and a very high frame rate US imaging sequence, which is able to capture the propagation of resulting the shear waves in real time | MRE enables the measurement of liver stiffness with an MRI-compatible generator; mechanical shear waves are delivered to the tissue and displayed as elastograms using phase-contrast image sequences |
| Reference point | ▪Young’s modulus (kPa) | ▪Shear wave speed (m/s) ▪Young’s modulus (kPa) | ▪Shear wave speed (m/s) ▪Young’s modulus (kPa) | ▪Shear wave speed (m/s) ▪Young’s modulus (kPa) |
| Selected example | ▪FibroScan (Echosens, France) | ▪VTQ using ARFI imaging (Siemens Healthcare, Germany) ▪ElastPQ (Philips Healthcare, Netherlands) ▪Shear Wave Measurement (Hitachi Aloka Medical, Japan) | ▪SWE (SuperSonic Imagine, France) ▪Virtual Touch IQ (Siemens Healthcare, Germany) ▪Logiq E9 (GE Healthcare, United Kingdom) ▪Aplio 500 (Toshiba Medical Systems, United Kingdom) | ▪MR Touch (GE Healthcare, United Kingdom) ▪MRE (Philips Healthcare, Netherlands; Siemens Healthcare, Germany) |
| Advantages | ▪Most widely used and validated technique ▪Quality criteria well defined ▪ User friendly, rapid, easy to measure at the bedside ▪Good reproducibility ▪Good performance for noninvasive assessments of liver fibrosis staging ▪Excellent diagnostic accuracy for excluding liver cirrhosis ▪Prognostic value in cirrhosis | ▪Can be performed using a regular US machine ▪ The ROI can be positioned under B-mode visualization ▪Higher applicability than TE (not limited by ascites or obesity) ▪pSWE is equal to the performance of TE for significant fibrosis and cirrhosis | ▪Can be performed using a regular US machine ▪ Simple and fast to use ▪ The ROI can be positioned under B-mode visualization ▪ A larger ROI than that of TE and pSWE ▪Good applicability (not limited by ascites or obesity) ▪Good stability and reproducibility ▪Generates a real-time quantitative map of liver tissue stiffness ▪Can avoid large vessels and the gallbladder ▪ High performance for cirrhosis | ▪Can be performed using a regular MRI machine ▪ Good stability and reproducibility ▪Scans the whole liver ▪Higher applicability than TE (not limited by ascites or obesity) ▪Excellent diagnostic accuracy for noninvasive staging of liver fibrosis and cirrhosis |
| Disadvantages | ▪Requires a special device and probe ▪ ROI size is rather small and cannot be chosen ▪Lack of applicability (limited by ascites, severe obesity) ▪No B-mode orientation ▪ Cannot avoid large vessels or the gallbladder ▪Unable to distinguish between intermediate stages of liver fibrosis | ▪ROI size is smaller than that of TE and cannot be modified ▪Quality criteria not yet well defined ▪Narrow range of values ▪Unable to distinguish between intermediate stages of liver fibrosis | ▪Quality criteria not well defined ▪ No further prospective studies published ▪ Many factors cause failed measurements in clinical practice ▪Unable to distinguish between intermediate stages of liver fibrosis | ▪Time-consuming ▪Even more costly than SWE and TE ▪ Failure can occur due to claustrophobia and iron overload ▪Affected by respiratory movement ▪ Hepatic MRE signal may be so low that waves cannot be adequately visualized with a gradient-echo based MRE sequence |
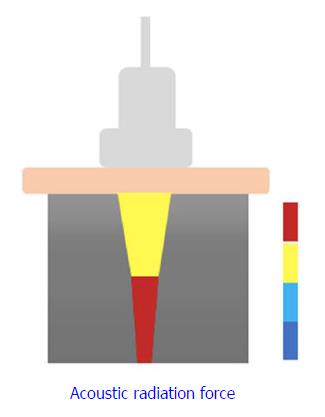
2D-SWE is a novel noninvasive method that has been proposed for assessing LS by measuring the velocity of elastic shear waves in the liver parenchyma[18]. In SWE, shear waves are created by US-generated pulses of an acoustic radiation force (Figure 1). The velocity of the shear wave is then estimated by a Doppler-like effect over a region of interest (ROI) and is related to the stiffness or elasticity of the medium[19]. This shear wave velocity can be used to calculate the tissue stiffness by the formula E = ρc2, where E is tissue elasticity (Young’s modulus, kPa), ρ is tissue density (kg/m3), and c is shear wave velocity (m/s)[20,21].
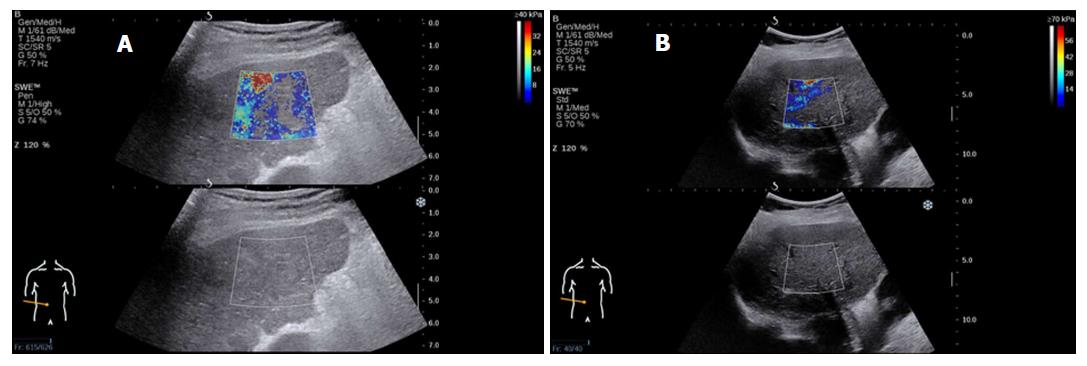
Elasticity is shown on a color-coded image displayed on a B-mode image. Then, with the ROI on the elasticity image, the mean, maximal and minimal LS values within the ROI can be visualized on a screen, along with the standard deviation (SD) of the measured elasticity[19,22]. The size and position of the ROI can be modified by the operator based on the specific goals of each liver assessment (Figure 2). In general, red areas indicate larger Young’s modulus of stiffer tissues, while blue areas indicate smaller Young’s modulus of softer tissues.
2D-SWE examination of the liver is performed by using convex US probes with integrated technological solutions allowing elasticity imaging and measurements, according to the World Federation for Ultrasound in Medicine and Biology[22,23] and the EFSUMB Guidelines for the Clinical Use of US Elastography[10,12], as well as the Society of Radiologists in Ultrasound Consensus Conference Statement regarding the assessment of liver fibrosis by elastography[24]. The proper examination procedure is described in Table 2, and the precautions and techniques of 2D-SWE in Table 3.
| Key points | Procedures |
| Adequate preparation | ▪ Fast and rest before the exam ▪ Perform in a supine position ▪ Train the patients on breathing |
| Accurate positioning | ▪ Scan the 6/7/8 intercostal spaces of the right liver ▪ Acquire stable and high-quality images ▪ Instruct the patient to hold the breath for 3-5 s |
| Stable measurement | ▪ Switch to SWE mode ▪ Freeze the image and adjust the position of the ROI ▪ Calculate the LS automatically ▪ Average the repeated measurement values |
| Key points | Precautions and techniques |
| Fasting and resting | ▪ Patients should fast for a minimum of 2 h and rest for a minimum of 10 min before undergoing liver stiffness measurement with SWE |
| Position | ▪ Measurement of liver stiffness by 2D-SWE should be performed in a supine position with the right arm maximally extended; this position ensures the best possible access for assessing the right liver lobe ▪ The transducer is placed in a right intercostal space to visualize the right liver lobe in B mode |
| Breathing train | ▪ Instruct the patient not to breathe in or breathe out deeply in order to eliminate unreliable measurements induced by breathing movements ▪ It has been suggested that a breath hold for a few seconds during quiet breathing may lead to the best results |
| Clear 2D-US images | ▪ Adequate B-mode liver image is a prerequisite for 2D-SWE measurements ▪ Must avoid the ribs, gas and other factors of routine ultrasound ▪ The appropriate pressure can be applied with the ultrasound probe to broaden the intercostal space and, thus, acquiring clear images. Contrary to the ordinary suggestion, this does not increase the liver’s stiffness, as the intervening tissues prevent distortion of the liver surface |
| Scale | ▪ Generally, the Young’s modulus scale should not be less than 30 kPa and preferably not higher than 150 kPa |
| Depth | ▪ Liver stiffness measured by 2D-SWE should be performed at least 10 mm under the liver capsule ▪ Measurements should not be performed too deep or too close, in order to avoid reverberation artifacts, insufficient penetration and acoustic shadow, as these factors will lead to incorrect results |
| Sampling frame | ▪ The sampling frame should be placed in a well-visualized area of the right liver lobe, free of large vessels, the gallbladder, the liver capsule, and any other hollow organs ▪ In addition, the sampling frame should be placed in the center of the image |
| ROI | ▪ For valid measurement quality of 2D-SWE, the ROI should be placed at a minimum of 1-2 cm and a maximum of 6 cm beneath the liver capsule ▪ The SWE acquisition is continued for 4-5 s once a stable SWE image is obtained ▪ The operator freezes the image, and the ROI should be placed in the most homogeneously colored area of the SWE ROI |
| Penetration mode | ▪ When measuring patients with thick subcutaneous fat, fatty liver or advanced cirrhosis, SWE can be adjusted to “Pen” mode to improve the measurement success rate ▪ In 2D-SWE, if the signal is weak or unstable, the penetration mode can be activated |
“Normal” LS values have recently been studied in healthy subjects who have undergone a professional check-up and who have no overt causes of liver disease, with normal liver enzymes. Various results for normal LS values are shown in Table 4.
| Ref. | Year | Country | Number | Mean age | Sex, female/male | Mean SWE, standard deviation, range | Remarks |
| Muller et al[19] | 2009 | France | 15 | NA | NA | 2.6-6.2 kPa | No data available regarding age or sex of normal subjects |
| Ferraioli et al[79] | 2012 | Italy | 42 | 34.8 | 13/29 | 4.92-5.39 kPa | |
| Sirli et al[82] | 2013 | Romania | 82 | 26 | 56/26 | 6.0 ± 1.4 kPa | Female: 5.7 ± 1.3 kPa |
| Male: 6.6 ± 1.5 kPa | |||||||
| BMI ≥ 25 kg/m²: 6.5 ± 1.5 kPa | |||||||
| BMI < 25 kg/m²: 5.8 ± 1.3 kPa | |||||||
| Hudson et al[83] | 2013 | Canada | 15 | 27 | 5/10 | 5.55 ± 0.74 kPa | |
| Wang et al[84] | 2014 | China | 30 | 36.1 ± 14.7 | 14/16 | 4.29 kPa | |
| Suh et al[73] | 2014 | South Korea | 196 | 29.2 ± 9.2 | 66/130 | 2.6-6.2 kPa | |
| Huang et al[85] | 2014 | China | 502 | 37.9 | 310/192 | 5.10 ± 1.02 kPa | Female: 5.45 ± 1.02 kPa |
| Male: 4.89 ± 0.96 kPa | |||||||
| Yoon et al[86] | 2014 | South Korea | 122 | NA | NA | 5.12 ± 1.46 kPa (session I) | No data available regarding age or sex of normal subjects |
| 4.95 ± 1.40 kPa (session II) | |||||||
| Leung et al[33] | 2013 | China | 171 | 40.6 ± 10.8 | 103/68 | 5.5 ± 0.7 kPa | Female: 5.7 ± 0.5 kPa |
| Male: 5.4 ± 0.7 kPa | |||||||
| Franchi-Abella et al[87] | 2016 | France | 51 | 0-15 | 26/25 | 6.53 ± 1.38 kPa | No significant differences were observed between male and female patients, right and left lobes, or different breathing conditions |
Viral hepatitis is a global public health issue, with approximately 248 million and 185 million people worldwide suffering from chronic hepatitis B (CHB) and hepatitis C (CHC), respectively[25-27]. Patients with advanced liver fibrosis from chronic viral hepatitis have a high risk of developing liver cancer and related complications, including portal hypertension (PH), esophageal and gastric variceal bleeding (EGVB), and liver failure. In these cases, prognosis and treatment are mainly affected by the degree of fibrosis, and the primary aim of treatment is controlling the progression of liver fibrosis[28,29]. The clinical practice guidelines for the management of CHB and CHC of the European Association for the Study of the Liver (EASL)[30,31], as well as the World Health Organization Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection[32], recommend elastography as a routine method for clinically evaluating liver fibrosis to avoid the need for liver biopsy in some patients.
Recent studies performed worldwide have focused on 2D-SWE for determining the role of viral hepatitis in liver fibrosis. In one study, 454 CHB patients were examined by 2D-SWE and TE[33]. The results showed that 2D-SWE provided a more accurate correlation of liver elasticity with liver fibrosis stage than TE, especially for the identification of significant fibrosis (≥ F2). Bavu et al[18] examined 113 CHC patients and found that SWE performed better diagnostically for early, intermediate and advanced predicted levels of fibrosis than TE. Ferraioli et al[20] used biopsy as a reference to assess the accuracy of SWE and TE in 121 CHC patients; they found that real-time SWE was more accurate than TE for assessing significant fibrosis (≥ F2). These results were strongly supported by three meta-analyses published by other authors[34-36]. In addition, a recent large-sample meta-analysis concluded that SWE was more accurate for advanced fibrosis (≥ F3) and cirrhosis (F4)[37]. What’s more, Grgurevic et al[38] evaluated the performance of real-time 2D-SWE for quantitatively assessing liver and spleen stiffness in patients with chronic viral hepatitis. They showed that real-time 2D-SWE can accurately identify various degrees of liver fibrosis and cirrhosis and demonstrated that liver and spleen stiffness continue to increase even after cirrhosis has developed. In fact, they noticed that spleen and liver stiffness tended to converge in more advanced stages of liver cirrhosis. This is an important study showing that 2D-SWE can be used to study the evolution of liver disease beyond cirrhosis.
As described above, SWE has high accuracy and specificity for assessing liver fibrosis due to chronic viral hepatitis, which makes it convenient for the real-time clinical monitoring of liver fibrosis. SWE technology is expected to replace biopsy, thereby eliminating the invasiveness and poor reproducibility of the latter technique. However, the quality criteria for the staging of liver fibrosis are not yet well defined. In addition, 2D-SWE has shown different diagnostic accuracies for liver fibrosis in patients with CHB and CHC. We suppose that this is probably due to the different etiologies of hepatitis B virus (HBV) and hepatitis C virus that lead to different tissue patterns and distributions of fibrosis development. Specifically, cirrhosis patients with HBV tend to produce larger regenerative nodules, which may result in different LS measurements if the ROI is placed in such an area. These findings suggest that assessments of the degree of liver fibrosis in different liver diseases cannot be based on the same diagnostic criteria, leading to the issue of how to establish diagnostic criteria for patients with multiple CLDs, e.g., HBV patients with nonalcoholic fatty liver disease (NAFLD).
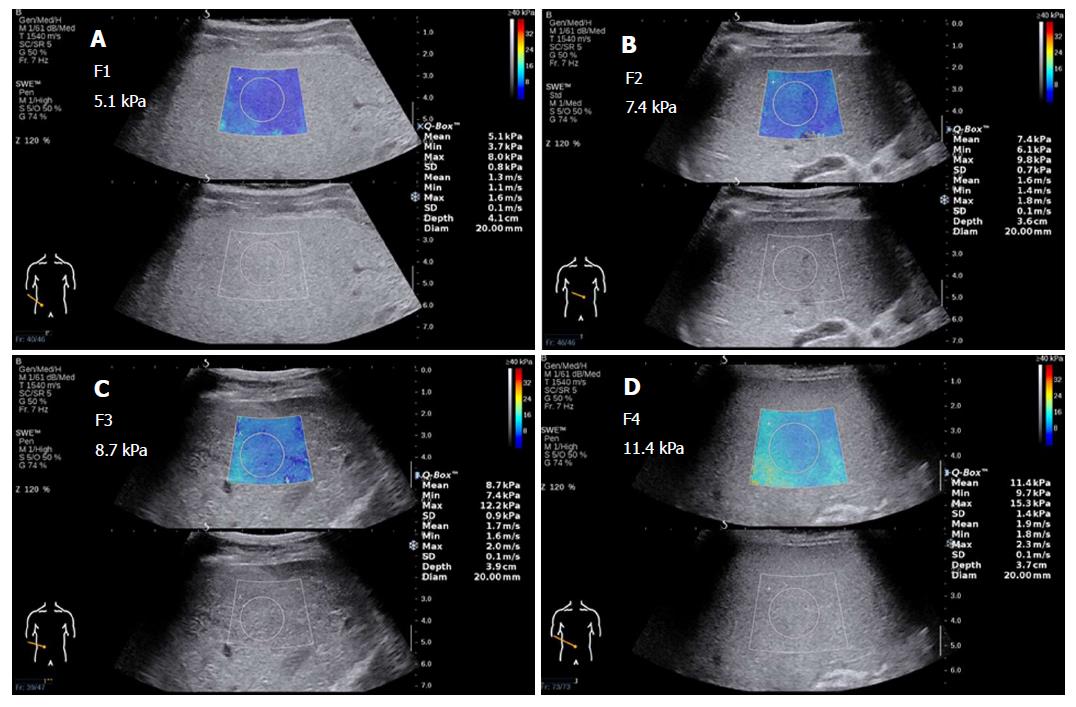
In the future, diagnosis and treatment in such situations will require personalization. The lack of specific criteria may also be due to the paucity of studies, small sample sizes, and diverse research areas; future developments will require more evidence-based medical data from large-scale, multicenter studies. Table 5 includes the cut-off values of LS assessed by 2D-SWE in various studies. Figure 3 shows 2D-SWE of liver fibrosis at METAVIR stages F1 through F4. Note that the LS gradually increases, and the color of the sampling frame gradually changes in the initial stages and incrementally increases in the later stages of fibrosis.
| Ref. | Etiologies | Year | Country | Patients, n | ≥ F1 (fibrosis) | ≥ F2 (significant fibrosis) | ≥ F3 (severe fibrosis) | F4 (cirrhosis) | ||||
| Cut-off, kPa | AUROC, % | Cut-off, kPa | AUROC, % | Cut-off, kPa | AUROC, % | Cut-off, kPa | AUROC, % | |||||
| Leung et al[33] | HBV | 2013 | China | 454 | 6.5 | 86 | 7.1 | 88 | 7.9 | 93 | 10.1 | 98 |
| Herrmann et al[37] | HBV | 2017 | Germany | 206 | NA | NA | 7.1 | 91 | 8.1 | 91 | 11.5 | 96 |
| Zeng et al[29] | HBV | 2014 | China | 303 | NA | NA | 7.2 | 92 | 9.7 | 95 | 11.7 | 95 |
| Bavu et al[18] | HCV | 2011 | France | 113 | NA | NA | 9.1 | 95 | 10.1 | 96 | 13.3 | 97 |
| Ferraioli et al[20] | HCV | 2012 | Italy | 121 | NA | NA | 7.1 | 92 | 8.7 | 98 | 10.4 | 98 |
| Grgurevic et al[38] | CVH | 2015 | Croatia | 123 | NA | NA | 8.1 | 99 | NA | NA | 10.8 | 95 |
| Cassinotto et al[46] | NAFLD | 2014 | France | 108 | NA | NA | 6.3 | 86 | 8.3 | 89 | 10.5 | 88 |
| Garcovich et al[47] | NAFLD | 2016 | Italy | 78 | 5.1 | 92 | 6.7 | 96 | NA | NA | NA | NA |
| Thiele et al[51] | ALD | 2016 | Denmark | 199 | NA | NA | 10.2 | 94 | NA | NA | 16.4 | 95 |
NAFLD is the most prevalent CLD, representing a spectrum ranging from simple fatty liver (SFL) to nonalcoholic steatohepatitis (NASH)[39,40]. NAFLD has become a global health problem, with a prevalence comparable to the rates of metabolic syndrome, obesity, type 2 diabetes mellitus and dyslipidemia[41,42]. The morbidity and mortality of patients with NAFLD are related to the development of NASH, which may progress to fibrosis and cirrhosis, even resulting in an increased risk of HCC[43]. A meta-analysis[44] demonstrated that even SFL may result in fibrosis development, and fibrosis development was observed in approximately 30% of patients with SFL, as well as in patients with NASH. Liver fibrosis, but no other histological features, has been demonstrated as the single most crucial histological feature associated with the risk of liver-related complications and death in patients with NAFLD[45]. Therefore, the most important issue for patients with NAFLD is recognizing and assessing the stage of liver fibrosis, which is possible by using US elastography.
Cassinotto et al[46] enrolled 291 patients with NAFLD and compared the performance of 2D-SWE, TE and Virtual Touch Quantification using liver biopsy as the reference method. They found that although obesity was related to an increased rate of failed LS measurements, these techniques, especially SWE, were valuable for diagnosing liver fibrosis in patients with NAFLD. The diagnostic performances of 2D-SWE for assessing liver fibrosis are as follows. For a sensitivity of at least 90% for diagnosing significant fibrosis (≥ F2), severe fibrosis (≥ F3) and cirrhosis (F4), the specificity is approximately 60%-70%; for a specificity of at least 90%, the sensitivity is approximately 50%-70%. 2D-SWE had areas under the receiver operating characteristic curves of 86%, 89% and 88% for the diagnosis of ≥ F2, ≥ F3 and F4, respectively. Furthermore, LS was mainly related to the fibrosis stage, and steatosis, inflammation and hepatocyte ballooning did not have significant influences on LS. Some NASH patients may have no fibrosis, and SWE may not be useful for the diagnosis of early-stage NASH without fibrosis. Hence, the next question became whether SWE can differentiate NASH from SFL, especially in the early stages of fibrosis. Probably not, and this is an area that relies on biochemical methods. Solutions to these problems will require multifaceted, prospective clinical studies to establish cut-off values for fibrosis staging.
In a study using SWE to identify the diagnostic accuracy of different degrees of fibrosis in 78 pediatric patients with biopsy-proven NASH[47], SWE was shown to be able to accurately evaluate significant liver fibrosis and, less efficiently, mild liver fibrosis in pediatric NAFLD patients. The study suggested that SWE can be used to assess liver fibrosis not only in adults but also in children and adolescents. However, few studies have focused on children; although fibrosis can be detected in children, the stage of fibrosis cannot yet be fully resolved, especially for fibrosis due to fatty liver. Consequently, the technique should be extended to all ages to further demonstrate the stability and reliability of the results.
Alcoholic liver disease (ALD) is the most common liver disease in the Western world. Chronic and excessive alcohol consumption is responsible for the progression of alcoholic steatosis, hepatitis, fibrosis and cirrhosis. Complete therapy requires abstention from alcohol. Moreover, liver transplantation (LT) remains a life-saving treatment for patients with advanced ALD[48,49]. The EASL published clinical practice guidelines for the management of ALD in 2012[50]. The guidelines noted that elastography is a notable method for the early diagnosis of alcoholic cirrhosis.
A recent prospective study of 199 alcohol-overusing individuals with varying degrees of alcoholic liver fibrosis evaluated two elastography methods for the diagnosis of alcoholic fibrosis and cirrhosis, using liver biopsy as a reference[51]. The study found that 2D-SWE has a high accuracy (area under the curve ≥ 0.92) for identifying subjects with significant fibrosis and cirrhosis, and the 2D-SWE cut-off values for optimal identification of significant fibrosis and cirrhosis were 10.2 kPa and 16.4 kPa. 2D-SWE was an extremely useful tool for diagnosing alcoholic fibrosis and, furthermore, was more appropriate for diagnosing early cirrhosis than for distinguishing between early and advanced cirrhosis. However, the threshold values that will allow the accurate diagnosis of cirrhosis by SWE and obviate the need for liver biopsy in adults with ALD remain to be determined.
Patients with advanced ALD ultimately experience life-threatening complications, such as PH and ascites. These may lead to EGVB, which is among the most important causes of high mortality in patients with cirrhosis[52]. Therefore, assessing the severity of PH during follow-up is essential in patients with cirrhosis. The hepatic venous pressure gradient (HVPG) has been used to assess PH, but it is rarely used in clinical practice because of its invasiveness. A recent study found that LS measured by SWE is an independent predictor of the presence of HCC and esophageal and gastric varices in patients with CLD[53]. Another study demonstrated that SWE, as a promising tool to diagnose PH, has outstanding diagnostic accuracy, with a specificity and sensitivity ranging around 80%, and showed it to be superior to TE[54]; however, more than 30% of the clinically significant PH patients could not be conclusively diagnosed or ruled disease-free because their SWE values were between the cut-offs. Several recent studies have reported that LS measured by SWE in connection with HVPG is important for predicting clinically significant PH in patients with cirrhosis[55,56]. Thus, although 2D-SWE has exceptional clinical value for assessing HCC patients with PH and EGVB, it still cannot replace digestive endoscopy[52].
LT is the only therapy for many patients who eventually progress to end-stage liver disease. However, LT is associated with a high rate of complications, which are difficult to identify in the early stages. One study reported a right lobe liver transplant recipient who experienced anastomotic stenosis of the right hepatic vein. SWE was quantitatively used to evaluate stiffness. The results suggested that SWE may be a noninvasive tool for assessing alterations in LS secondary to hepatic venous congestion after LT[57]. Another study showed that SWE may be useful during follow-up after LT. Liver rejection or hepatitis can be predicted at > 4 wk based on liver grafts that are stiffer than normal[58]. In a more recent study, SWE was successfully utilized to monitor the therapeutic effects of direct-acting antivirals in hepatitis C recurrence after LT; the median LS values decreased dramatically after treatment (P < 0.001). The study suggested that SWE is a valid diagnostic tool to follow-up hepatitis C patients undergoing LT[59].
From this study, we observed that SWE aids in the clinical diagnosis and detection of complications after LT, which represents great progress in SWE technology from diagnosis to monitoring. However, few studies have examined elastography in LT. This study represents the first step, although there is great potential for future research opportunities. Moreover, US elastography should be applied more for monitoring and predicting the complications of LT, particularly for assessing disease progression and prognosis.
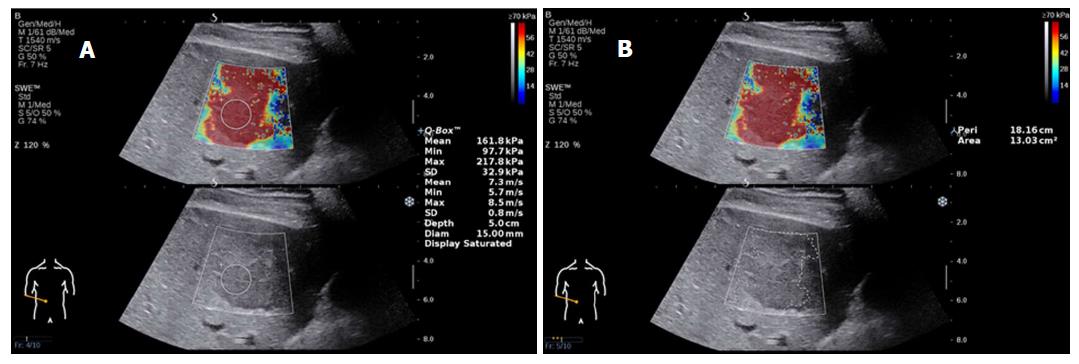
Focal liver lesions (FLLs) represent novel lesions for the field of US elastography and include those found in hemangioma, HCC, focal nodular hyperplasia (FNH), metastatic cancer and adenoma. Several studies have reported that 2D-SWE is a useful method for distinguishing FLLs, although it does not completely distinguish benign from malignant lesions[60-64]. The stiffness of lesions in liver malignancies is significantly higher than that of the liver parenchyma and is also higher than that of benign lesions[60,61] (Figure 4 shows HCC images of 2D-SWE). In malignant tumors, the hardness values of metastases were significantly higher than those found in HCC and cholangiocarcinoma[62]. In benign lesions, 2D-SWE has been regarded as a practical tool for distinguishing FNH from adenoma[63].
A recent study demonstrated the correct differentiation between benign and malignant FLLs by using real-time 2D-SWE in 96.1% of patients (area under the curve = 0.98)[64]. They used three elastography parameters, the mean stiffness of the FLL, the ratio between the minimal and maximum lesion stiffness, and the ratio between the stiffness of the FLL and the surrounding liver parenchyma to construct a new Liver Elastography Malignancy Prediction score based on a regression analysis. A simpler approach considering only mean lesion stiffness in a dichotomized fashion could be used to diagnose or rule-out malignancy with cut-off values of 14 kPa and 32.5 kPa, respectively.
Nonetheless, conclusions based on the current literature remain unclear. When 2D US and Doppler US suggest that a lesion is a benign tumor but elastography suggests malignancy, the lesion cannot therefore be diagnosed as malignant. Several technical limitations still exist. In situations in which the lesion is smaller than the ROI or located deep or near the heart, the shear wave propagation and imaging stability will be affected. Furthermore, lesion areas with necrotic liquefaction or calcification are not representative of the true lesion stiffness, which often leads to misdiagnosis.
Autoimmune liver disease (AILDs) are intricate disorders resulting from the effects of multiple genes in association with environmental factors. The three major AILDs are primary biliary cirrhosis, primary sclerosing cholangitis and autoimmune hepatitis[65]. As they progress, these diseases may ultimately lead to chronic liver damage, liver fibrosis and cirrhosis. Zeng et al[66] used liver biopsy as the reference standard for determining the diagnostic accuracy of 2D-SWE for noninvasively assessing liver fibrosis in patients with AILD. Their results indicated that the optimal cut-off values for significant fibrosis (≥ F2), severe fibrosis (≥ F3) and cirrhosis (F4) were 9.7 kPa, 13.2 kPa and 16.3 kPa, respectively. Moreover, the sensitivity and specificity ranged from 75% to 87% for all fibrosis stages. As these data show, SWE has high cut-off values in patients with AILD. The critical cut-off value was obviously higher than that for CHB/CHC. Therefore, different diseases have different thresholds, representing different developmental processes. AILD is relatively rare, and the current study was small; thus, further confirmation is necessary.
These results of LT, FLLs and AILD based on SWE cannot yet be clinically translated, as the research published to date is insufficient to definitively establish diagnostic criteria. However, elastography has shown promise for use in many research and development opportunities and future applications.
Although there is evidence that 2D-SWE could become a crucial tool in end-stage liver disease, additional research and development are necessary. Additional work should focus on the role of SWE not only in cross-sectional diagnosis but also in longitudinal studies considering disease progression, regression and clinical outcomes[67]. Priority should be given to multicenter, large-scale verification research. Indeed, the longitudinal monitoring of fibrosis in patients with CLD may be a highly practical application for SWE. Preliminary research has revealed a remarkable decrease in LS values in patients with chronic viral hepatitis after continued antiviral treatment[68,69]. Given its high patient acceptance and good accessibility, SWE may also become a convenient tool for identifying patients with liver disease and a promising avenue for future research. However, several issues warrant further consideration.
1. Although alternative techniques, such as pSWE/ARFI or 2D-SWE, seem to overcome the limitations of TE, their quality criteria for the staging of liver fibrosis are not yet well defined.
a. It remains unclear whether the shear wave Young’s modulus or shear wave velocity is more representative.
b. It remains unknown whether multiple measurements in one location are necessary when satisfactory measures of stiffness are obtained. Or, whether measurements in more than one location are needed.
c. There is currently no agreement on objective quality criteria regarding what constitutes a valid measurement and what is an invalid measurement.
2. It remains unknown whether a simple semi-quantitative fibrosis score can adequately reflect the complexity of the pathophysiological process.
1. In the era of promotion of precision medicine, can 2D-SWE accurately guide clinical work to aid in the design of an antiviral therapy for hepatitis B patients?
2. Can 2D-SWE be a reliable measurement of the prognosis of liver fibrosis?
3. Does a greater Young’s modulus indicate more serious disease progression? Does a reduced Young’s modulus indicate disease remission or effective treatment?
4. How do we diagnose the mixed types of liver disease?
5. In adult patients with NAFLD, what SWE LS cut-off value allows us to accurately diagnose the presence of cirrhosis and eliminate the need for liver biopsy?
6. In compensated cirrhosis of adult CLD, what 2D-SWE liver stiffness cut-off value allows us to avoid the need for gastroscopy?
7. Focal lesions can lead to erroneous results. How should these be resolved?
1. During follow-up monitoring, are there any definite cut-off values to weigh the progression, regression and outcome of liver disease?
2. What is a minimal time span for measuring LS over time? How often should measurements be performed?
3. How should elastography be used to predict the risk for HCC in patients with cirrhosis, regardless of etiology?
Here, we will address the first clinical issue. On the basis of the current study, we believe that SWE can guide clinical work to help develop a reasonable treatment strategy for patients with CLDs by measuring the degree of LS, which reflects the degree of liver fibrosis, and by monitoring the progress of the disease in real time. According to the EASL guidelines, it is of particular significance to identify patients with cirrhosis, as their treatment regimen and posttreatment surveillance must be adapted[27]. The latest report[70] from a European, 10-center, cohort study of 1951 adult Caucasian CHB patients found that HCC incidence distinctly dropped after the first 5 years of therapy, particularly in those with compensated cirrhosis. Furthermore, multivariable analysis showed that a LS ≥ 12 kPa at year 5 was related to greater HCC development after year 5, which likely represents valuable indirect markers of the severity of liver disease. Consequently, we believe that SWE can reflect liver fibrosis and guide therapy, thus decreasing the risk of HCC and remarkably improving the prognosis. This represents a major advancement in the treatment of CLD.
2D-SWE is known to be a multifactorial technique that factors in the anatomy of the liver, the physical characteristics and the underlying illness of the patient, the experience of the examiner, and differences in equipment. The following details include the factors associated with SWE reliability and ways to improve its performance in liver applications.
The liver is located in the upper abdomen, near the heart, lungs and gastrointestinal tract, and is particularly affected by respiratory movements as well as the heartbeat and gas in the gastrointestinal tract[71]. Measurements of the left liver lobe yield markedly higher values and are more variable than the right lobe; thus, LSM in the left liver lobe should be avoided whenever possible[72]. During the examination, the patient needs to cooperate by holding their breath (neither in the expiratory phase nor in the aspirated phase) to stabilize the 2D image measurement because stable 2D imaging is the basis of successful results[24].
Previous studies have reported that SWE measurements are not affected by a high body mass index, ascites or fatty liver[20,73], while in clinical practice, high measurement failure rates are due to obesity or ascites causing poor penetration[4]. Obese patients often have a fatty liver with a thick layer of subcutaneous fat and echo attenuation throughout the liver, which is not conducive to clear 2D imaging. In some very thin patients with a narrow intercostal space, especially patients who have entered the end-stage of liver cirrhosis, the right liver has been severely reduced to the extent that it affects elasticity measurements[33].
Using current methods, according to the patient’s liver, the depth should be dropped, and the image should be appropriately enlarged while the areas of the sampling frame and ROI are adjusted to the shrinking liver. In addition, some factors influence the LS measured by elastography, leading to an overestimation of liver fibrosis[71,74-78]. Such factors include excessive alcohol intake, elevated central venous pressure, cardiac failure, intra- or extrahepatic cholestasis, alanine aminotransferase flare in acute or chronic hepatitis, and histological necroinflammation activity.
LS measurements by 2D-SWE should be made by an expert operator. It has been reported that experienced examiners have higher reproducibility of measurements than novice examiners[79,80]. It is advised that at least 50 supervised 2D-SWE measurements should be performed by beginners in order to acquire reliable results[79]. This issue therefore necessitates strict prejob training for operators to reduce the error caused by lack of experience and to improve the stability of clinical implementations (Figure 5 shows an analysis of failed measurements).
There may be significant variation in the frequency of acoustic radiation forces used across manufacturers, although variations can even be observed across different equipment from the same manufacturer or due to different settings applied using the same equipment. Large differences among the measurements provided by different settings create obstacles to the clinical application of SWE that need be addressed in the future[81]. In summary, these techniques need to be performed using a standardized protocol with critically interpreted results, taking confounding factors into account.
In conclusion, 2D-SWE appears to be a valid, simple, rapid and reproducible method for the noninvasive assessment of liver fibrosis, with advantages including its low cost and widespread availability. As liver biopsies cannot be performed frequently, SWE can be used to regularly monitor liver fibrosis over the long term. SWE is a promising technology with potential clinical applications, including accurate quantification and therapeutic monitoring. We believe that with the popularity of US elastography, the noninvasive assessment of liver fibrosis will be further promoted. Most importantly, in the future, SWE may become a routine method of screening for patients with CLDs. Finally, large-scale, multicenter, multifield clinical studies are needed to explore the applications of SWE, as there may be variability in terms of demographics, disease and liver cancer incidence predictions. We are hopeful that SWE will continue to evolve and attain a utility equal to that of Doppler as a new mode of US imaging.
| 1. | Singh S, Muir AJ, Dieterich DT, Falck-Ytter YT. American Gastroenterological Association Institute Technical Review on the Role of Elastography in Chronic Liver Diseases. Gastroenterology. 2017;152:1544-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 218] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 2. | GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5495] [Cited by in RCA: 5354] [Article Influence: 486.7] [Reference Citation Analysis (0)] |
| 3. | Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut. 2015;64:830-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 722] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 4. | Friedrich-Rust M, Poynard T, Castera L. Critical comparison of elastography methods to assess chronic liver disease. Nat Rev Gastroenterol Hepatol. 2016;13:402-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 191] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 5. | Stasi C, Milani S. Non-invasive assessment of liver fibrosis: Between prediction/prevention of outcomes and cost-effectiveness. World J Gastroenterol. 2016;22:1711-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Sharma S, Khalili K, Nguyen GC. Non-invasive diagnosis of advanced fibrosis and cirrhosis. World J Gastroenterol. 2014;20:16820-16830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 125] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (1)] |
| 7. | Seeff LB, Everson GT, Morgan TR, Curto TM, Lee WM, Ghany MG, Shiffman ML, Fontana RJ, Di Bisceglie AM, Bonkovsky HL. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol. 2010;8:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 343] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 8. | Schiavon Lde L, Narciso-Schiavon JL, de Carvalho-Filho RJ. Non-invasive diagnosis of liver fibrosis in chronic hepatitis C. World J Gastroenterol. 2014;20:2854-2866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Cassinotto C, Lapuyade B, Mouries A, Hiriart JB, Vergniol J, Gaye D, Castain C, Le Bail B, Chermak F, Foucher J. Non-invasive assessment of liver fibrosis with impulse elastography: comparison of Supersonic Shear Imaging with ARFI and FibroScan®. J Hepatol. 2014;61:550-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 251] [Article Influence: 20.9] [Reference Citation Analysis (1)] |
| 10. | Dietrich CF, Bamber J, Berzigotti A, Bota S, Cantisani V, Castera L, Cosgrove D, Ferraioli G, Friedrich-Rust M, Gilja OH. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med. 2017;38:e16-e47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 606] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 11. | European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1177] [Cited by in RCA: 1379] [Article Influence: 125.4] [Reference Citation Analysis (1)] |
| 12. | Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, Cantisani V, Correas JM, D’Onofrio M, Drakonaki EE. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013;34:169-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 793] [Article Influence: 61.0] [Reference Citation Analysis (2)] |
| 13. | Castéra L, Foucher J, Bernard PH, Carvalho F, Allaix D, Merrouche W, Couzigou P, de Lédinghen V. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51:828-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 426] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 14. | Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1091] [Article Influence: 60.6] [Reference Citation Analysis (1)] |
| 15. | Haque M, Robinson C, Owen D, Yoshida EM, Harris A. Comparison of acoustic radiation force impulse imaging (ARFI) to liver biopsy histologic scores in the evaluation of chronic liver disease: A pilot study. Ann Hepatol. 2010;9:289-293. [PubMed] |
| 16. | Singh S, Venkatesh SK, Wang Z, Miller FH, Motosugi U, Low RN, Hassanein T, Asbach P, Godfrey EM, Yin M. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol. 2015;13:440-451.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 437] [Article Influence: 39.7] [Reference Citation Analysis (1)] |
| 17. | Huwart L, Sempoux C, Vicaut E, Salameh N, Annet L, Danse E, Peeters F, ter Beek LC, Rahier J, Sinkus R. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 545] [Article Influence: 30.3] [Reference Citation Analysis (2)] |
| 18. | Bavu E, Gennisson JL, Couade M, Bercoff J, Mallet V, Fink M, Badel A, Vallet-Pichard A, Nalpas B, Tanter M. Noninvasive in vivo liver fibrosis evaluation using supersonic shear imaging: a clinical study on 113 hepatitis C virus patients. Ultrasound Med Biol. 2011;37:1361-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 297] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 19. | Muller M, Gennisson JL, Deffieux T, Tanter M, Fink M. Quantitative viscoelasticity mapping of human liver using supersonic shear imaging: preliminary in vivo feasibility study. Ultrasound Med Biol. 2009;35:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 283] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 20. | Ferraioli G, Tinelli C, Dal Bello B, Zicchetti M, Filice G, Filice C; Liver Fibrosis Study Group. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology. 2012;56:2125-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 517] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 21. | Poynard T, Munteanu M, Luckina E, Perazzo H, Ngo Y, Royer L, Fedchuk L, Sattonnet F, Pais R, Lebray P. Liver fibrosis evaluation using real-time shear wave elastography: applicability and diagnostic performance using methods without a gold standard. J Hepatol. 2013;58:928-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (2)] |
| 22. | Shiina T, Nightingale KR, Palmeri ML, Hall TJ, Bamber JC, Barr RG, Castera L, Choi BI, Chou YH, Cosgrove D. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med Biol. 2015;41:1126-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 680] [Article Influence: 61.8] [Reference Citation Analysis (5)] |
| 23. | Ferraioli G, Filice C, Castera L, Choi BI, Sporea I, Wilson SR, Cosgrove D, Dietrich CF, Amy D, Bamber JC. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 3: liver. Ultrasound Med Biol. 2015;41:1161-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 502] [Article Influence: 45.6] [Reference Citation Analysis (1)] |
| 24. | Barr RG, Ferraioli G, Palmeri ML, Goodman ZD, Garcia-Tsao G, Rubin J, Garra B, Myers RP, Wilson SR, Rubens D. Elastography Assessment of Liver Fibrosis: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2015;276:845-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 451] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 25. | Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 2051] [Article Influence: 186.5] [Reference Citation Analysis (4)] |
| 26. | Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016;22:7824-7840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 514] [Cited by in RCA: 585] [Article Influence: 58.5] [Reference Citation Analysis (16)] |
| 27. | European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu.; European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 4009] [Article Influence: 445.4] [Reference Citation Analysis (1)] |
| 28. | Rockey DC. Noninvasive assessment of liver fibrosis and portal hypertension with transient elastography. Gastroenterology. 2008;134:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Zeng J, Liu GJ, Huang ZP, Zheng J, Wu T, Zheng RQ, Lu MD. Diagnostic accuracy of two-dimensional shear wave elastography for the non-invasive staging of hepatic fibrosis in chronic hepatitis B: a cohort study with internal validation. Eur Radiol. 2014;24:2572-2581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 30. | European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2323] [Cited by in RCA: 2411] [Article Influence: 172.2] [Reference Citation Analysis (1)] |
| 31. | European Association for Study of Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60:392-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 658] [Article Influence: 54.8] [Reference Citation Analysis (2)] |
| 32. | WHO Guidelines Approved by the Guidelines Review Committee. Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection. Geneva: World Health Organization Copyright (c) World Health Organization 2015 2015; . |
| 33. | Leung VY, Shen J, Wong VW, Abrigo J, Wong GL, Chim AM, Chu SH, Chan AW, Choi PC, Ahuja AT. Quantitative elastography of liver fibrosis and spleen stiffness in chronic hepatitis B carriers: comparison of shear-wave elastography and transient elastography with liver biopsy correlation. Radiology. 2013;269:910-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 237] [Article Influence: 18.2] [Reference Citation Analysis (1)] |
| 34. | Feng JC, Li J, Wu XW, Peng XY. Diagnostic Accuracy of SuperSonic Shear Imaging for Staging of Liver Fibrosis: A Meta-analysis. J Ultrasound Med. 2016;35:329-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Li C, Zhang C, Li J, Huo H, Song D. Diagnostic Accuracy of Real-Time Shear Wave Elastography for Staging of Liver Fibrosis: A Meta-Analysis. Med Sci Monit. 2016;22:1349-1359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Jiang T, Tian G, Zhao Q, Kong D, Cheng C, Zhong L, Li L. Diagnostic Accuracy of 2D-Shear Wave Elastography for Liver Fibrosis Severity: A Meta-Analysis. PLoS One. 2016;11:e0157219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Herrmann E, de Lédinghen V, Cassinotto C, Chu WC, Leung VY, Ferraioli G, Filice C, Castera L, Vilgrain V, Ronot M. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: An individual patient data-based meta-analysis. Hepatology. 2018;67:260-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 361] [Article Influence: 45.1] [Reference Citation Analysis (2)] |
| 38. | Grgurevic I, Puljiz Z, Brnic D, Bokun T, Heinzl R, Lukic A, Luksic B, Kujundzic M, Brkljacic B. Liver and spleen stiffness and their ratio assessed by real-time two dimensional-shear wave elastography in patients with liver fibrosis and cirrhosis due to chronic viral hepatitis. Eur Radiol. 2015;25:3214-3221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 39. | Haga Y, Kanda T, Sasaki R, Nakamura M, Nakamoto S, Yokosuka O. Nonalcoholic fatty liver disease and hepatic cirrhosis: Comparison with viral hepatitis-associated steatosis. World J Gastroenterol. 2015;21:12989-12995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 40. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7921] [Article Influence: 792.1] [Reference Citation Analysis (8)] |
| 41. | Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1522] [Cited by in RCA: 1637] [Article Influence: 109.1] [Reference Citation Analysis (1)] |
| 42. | Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, Ratziu V, McCullough A. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54:344-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 593] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 43. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2325] [Article Influence: 155.0] [Reference Citation Analysis (1)] |
| 44. | Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643-54.e1-9; quiz e39-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 932] [Cited by in RCA: 1301] [Article Influence: 118.3] [Reference Citation Analysis (1)] |
| 45. | Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389-97.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2304] [Cited by in RCA: 2326] [Article Influence: 211.5] [Reference Citation Analysis (2)] |
| 46. | Cassinotto C, Boursier J, de Lédinghen V, Lebigot J, Lapuyade B, Cales P, Hiriart JB, Michalak S, Bail BL, Cartier V. Liver stiffness in nonalcoholic fatty liver disease: A comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63:1817-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 401] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 47. | Garcovich M, Veraldi S, Di Stasio E, Zocco MA, Monti L, Tomà P, Pompili M, Gasbarrini A, Nobili V. Liver Stiffness in Pediatric Patients with Fatty Liver Disease: Diagnostic Accuracy and Reproducibility of Shear-Wave Elastography. Radiology. 2017;283:820-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 48. | Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol. 2013;59:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 550] [Article Influence: 42.3] [Reference Citation Analysis (1)] |
| 49. | Mueller S, Seitz HK, Rausch V. Non-invasive diagnosis of alcoholic liver disease. World J Gastroenterol. 2014;20:14626-14641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 99] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (2)] |
| 50. | European Association for the Study of Liver. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol. 2012;57:399-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 464] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 51. | Thiele M, Detlefsen S, Sevelsted Møller L, Madsen BS, Fuglsang Hansen J, Fialla AD, Trebicka J, Krag A. Transient and 2-Dimensional Shear-Wave Elastography Provide Comparable Assessment of Alcoholic Liver Fibrosis and Cirrhosis. Gastroenterology. 2016;150:123-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 170] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 52. | Tripathi D, Stanley AJ, Hayes PC, Patch D, Millson C, Mehrzad H, Austin A, Ferguson JW, Olliff SP, Hudson M. U.K. guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut. 2015;64:1680-1704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 454] [Cited by in RCA: 426] [Article Influence: 38.7] [Reference Citation Analysis (2)] |
| 53. | Kasai Y, Moriyasu F, Saito K, Hara T, Kobayashi Y, Nakamura I, Sugimoto K. Value of shear wave elastography for predicting hepatocellular carcinoma and esophagogastric varices in patients with chronic liver disease. J Med Ultrason (2001). 2015;42:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 54. | Jansen C, Bogs C, Verlinden W, Thiele M, Möller P, Görtzen J, Lehmann J, Praktiknjo M, Chang J, Krag A. Algorithm to rule out clinically significant portal hypertension combining Shear-wave elastography of liver and spleen: a prospective multicentre study. Gut. 2016;65:1057-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 55. | Procopet B, Berzigotti A, Abraldes JG, Turon F, Hernandez-Gea V, García-Pagán JC, Bosch J. Real-time shear-wave elastography: applicability, reliability and accuracy for clinically significant portal hypertension. J Hepatol. 2015;62:1068-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 161] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 56. | Maruyama H, Kobayashi K, Kiyono S, Sekimoto T, Kanda T, Yokosuka O. Two-dimensional shear wave elastography with propagation-based reliability assessment for grading hepatic fibrosis and portal hypertension. J Hepatobiliary Pancreat Sci. 2016;23:595-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 57. | Wang HK, Lai YC, Tseng HS, Lee RC, Loong CC, Lin NC, Chou YH, Chiou HJ, Chang CY. Hepatic venous congestion after living donor liver transplantation: quantitative assessment of liver stiffness using shear wave elastography--a case report. Transplant Proc. 2012;44:814-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 58. | Yoon JH, Lee JY, Woo HS, Yu MH, Lee ES, Joo I, Lee KB, Yi NJ, Lee YJ, Han JK. Shear wave elastography in the evaluation of rejection or recurrent hepatitis after liver transplantation. Eur Radiol. 2013;23:1729-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 59. | Korda D, Lenard ZM, Gerlei Z, Jakab Z, Haboub-Sandil A, Wagner L, Varga M, Cseprekal O, Marton A, Horvathy D. Shear-wave elastography for the assessment of liver fibrosis in liver transplant recipients treated for hepatitis C virus recurrence. Eur J Gastroenterol Hepatol. 2018;30:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 60. | Conti CB, Cavalcoli F, Fraquelli M, Conte D, Massironi S. Ultrasound elastographic techniques in focal liver lesions. World J Gastroenterol. 2016;22:2647-2656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 61. | Park HS, Kim YJ, Yu MH, Jung SI, Jeon HJ. Shear Wave Elastography of Focal Liver Lesion: Intraobserver Reproducibility and Elasticity Characterization. Ultrasound Q. 2015;31:262-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 62. | Lu Q, Ling W, Lu C, Li J, Ma L, Quan J, He D, Liu J, Yang J, Wen T. Hepatocellular carcinoma: stiffness value and ratio to discriminate malignant from benign focal liver lesions. Radiology. 2015;275:880-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 63. | Brunel T, Guibal A, Boularan C, Ducerf C, Mabrut JY, Bancel B, Boussel L, Rode A. Focal nodular hyperplasia and hepatocellular adenoma: The value of shear wave elastography for differential diagnosis. Eur J Radiol. 2015;84:2059-2064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 64. | Grgurevic I, Bokun T, Salkic NN, Brkljacic B, Vukelić-Markovic M, Stoos-Veic T, Aralica G, Rakic M, Filipec-Kanizaj T, Berzigotti A. Liver elastography malignancy prediction score for noninvasive characterization of focal liver lesions. Liver Int. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 65. | Carbone M, Neuberger JM. Autoimmune liver disease, autoimmunity and liver transplantation. J Hepatol. 2014;60:210-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (3)] |
| 66. | Zeng J, Huang ZP, Zheng J, Wu T, Zheng RQ. Non-invasive assessment of liver fibrosis using two-dimensional shear wave elastography in patients with autoimmune liver diseases. World J Gastroenterol. 2017;23:4839-4846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Grgurević I, Bokun T, Mustapić S, Trkulja V, Heinzl R, Banić M, Puljiz Ž, Lukšić B, Kujundžić M. Real-time two-dimensional shear wave ultrasound elastography of the liver is a reliable predictor of clinical outcomes and the presence of esophageal varices in patients with compensated liver cirrhosis. Croat Med J. 2015;56:470-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 68. | Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Aguilar Schall R. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1410] [Article Influence: 108.5] [Reference Citation Analysis (1)] |
| 69. | Hartl J, Denzer U, Ehlken H, Zenouzi R, Peiseler M, Sebode M, Hübener S, Pannicke N, Weiler-Normann C, Quaas A. Transient elastography in autoimmune hepatitis: Timing determines the impact of inflammation and fibrosis. J Hepatol. 2016;65:769-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 70. | Papatheodoridis GV, Idilman R, Dalekos GN, Buti M, Chi H, van Boemmel F, Calleja JL, Sypsa V, Goulis J, Manolakopoulos S. The risk of hepatocellular carcinoma decreases after the first 5 years of entecavir or tenofovir in Caucasians with chronic hepatitis B. Hepatology. 2017;66:1444-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 234] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 71. | Cosgrove D, Piscaglia F, Bamber J, Bojunga J, Correas JM, Gilja OH, Klauser AS, Sporea I, Calliada F, Cantisani V. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med. 2013;34:238-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 640] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 72. | Karlas T, Pfrepper C, Wiegand J, Wittekind C, Neuschulz M, Mössner J, Berg T, Tröltzsch M, Keim V. Acoustic radiation force impulse imaging (ARFI) for non-invasive detection of liver fibrosis: examination standards and evaluation of interlobe differences in healthy subjects and chronic liver disease. Scand J Gastroenterol. 2011;46:1458-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (3)] |
| 73. | Suh CH, Kim SY, Kim KW, Lim YS, Lee SJ, Lee MG, Lee J, Lee SG, Yu E. Determination of normal hepatic elasticity by using real-time shear-wave elastography. Radiology. 2014;271:895-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 74. | Mueller S, Millonig G, Sarovska L, Friedrich S, Reimann FM, Pritsch M, Eisele S, Stickel F, Longerich T, Schirmacher P. Increased liver stiffness in alcoholic liver disease: differentiating fibrosis from steatohepatitis. World J Gastroenterol. 2010;16:966-972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 160] [Cited by in RCA: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 75. | Millonig G, Friedrich S, Adolf S, Fonouni H, Golriz M, Mehrabi A, Stiefel P, Pöschl G, Büchler MW, Seitz HK. Liver stiffness is directly influenced by central venous pressure. J Hepatol. 2010;52:206-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 411] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 76. | Millonig G, Reimann FM, Friedrich S, Fonouni H, Mehrabi A, Büchler MW, Seitz HK, Mueller S. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 471] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 77. | Arena U, Vizzutti F, Corti G, Ambu S, Stasi C, Bresci S, Moscarella S, Boddi V, Petrarca A, Laffi G. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 581] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 78. | Fraquelli M, Rigamonti C, Casazza G, Donato MF, Ronchi G, Conte D, Rumi M, Lampertico P, Colombo M. Etiology-related determinants of liver stiffness values in chronic viral hepatitis B or C. J Hepatol. 2011;54:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 79. | Ferraioli G, Tinelli C, Zicchetti M, Above E, Poma G, Di Gregorio M, Filice C. Reproducibility of real-time shear wave elastography in the evaluation of liver elasticity. Eur J Radiol. 2012;81:3102-3106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 198] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 80. | Zhuang Y, Ding H, Zhang Y, Sun H, Xu C, Wang W. Two-dimensional Shear-Wave Elastography Performance in the Noninvasive Evaluation of Liver Fibrosis in Patients with Chronic Hepatitis B: Comparison with Serum Fibrosis Indexes. Radiology. 2017;283:873-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 81. | Piscaglia F, Salvatore V, Mulazzani L, Cantisani V, Colecchia A, Di Donato R, Felicani C, Ferrarini A, Gamal N, Grasso V. Differences in liver stiffness values obtained with new ultrasound elastography machines and Fibroscan: A comparative study. Dig Liver Dis. 2017;49:802-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 82. | Sirli R, Bota S, Sporea I, Jurchis A, Popescu A, Gradinaru-Tascău O, Szilaski M. Liver stiffness measurements by means of supersonic shear imaging in patients without known liver pathology. Ultrasound Med Biol. 2013;39:1362-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 83. | Hudson JM, Milot L, Parry C, Williams R, Burns PN. Inter- and intra-operator reliability and repeatability of shear wave elastography in the liver: a study in healthy volunteers. Ultrasound Med Biol. 2013;39:950-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 84. | Wang CZ, Zheng J, Huang ZP, Xiao Y, Song D, Zeng J, Zheng HR, Zheng RQ. Influence of measurement depth on the stiffness assessment of healthy liver with real-time shear wave elastography. Ultrasound Med Biol. 2014;40:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 85. | Huang Z, Zheng J, Zeng J, Wang X, Wu T, Zheng R. Normal liver stiffness in healthy adults assessed by real-time shear wave elastography and factors that influence this method. Ultrasound Med Biol. 2014;40:2549-2555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 86. | Yoon JH, Lee JM, Han JK, Choi BI. Shear wave elastography for liver stiffness measurement in clinical sonographic examinations: evaluation of intraobserver reproducibility, technical failure, and unreliable stiffness measurements. J Ultrasound Med. 2014;33:437-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 87. | Franchi-Abella S, Corno L, Gonzales E, Antoni G, Fabre M, Ducot B, Pariente D, Gennisson JL, Tanter M, Corréas JM. Feasibility and Diagnostic Accuracy of Supersonic Shear-Wave Elastography for the Assessment of Liver Stiffness and Liver Fibrosis in Children: A Pilot Study of 96 Patients. Radiology. 2016;278:554-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Grgurevic I, Tahiri M, Tantau M S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Huang Y