Published online Mar 7, 2018. doi: 10.3748/wjg.v24.i9.1022
Peer-review started: December 23, 2017
First decision: January 17, 2018
Revised: January 30, 2018
Accepted: February 8, 2018
Article in press: February 8, 2018
Published online: March 7, 2018
Processing time: 71 Days and 19 Hours
To evaluate the prognostic value of the number of retrieved lymph nodes (LNs) and other prognostic factors for patients with distal cholangiocarcinomas, and to determine the optimal retrieved LNs cut-off number.
The Surveillance, Epidemiology and End Results database was used to screen for patients with distal cholangiocarcinoma. Patients with different numbers of retrieved LNs were divided into three groups by the X-tile program. X-tile from Yale University is a useful tool for outcome-based cut-point optimization. The Kaplan-Meier method and Cox regression analysis were utilized for survival analysis.
A total of 449 patients with distal cholangiocarcinoma met the inclusion criteria. The Kaplan-Meier survival analysis for all patients and for N1 patients revealed no significant differences among patients with different retrieved LN counts in terms of overall and cancer-specific survival. In patients with node-negative distal cholangiocarcinoma, patients with four to nine retrieved LNs had a significantly better overall (P = 0.026) and cancer-specific survival (P = 0.039) than others. In the subsequent multivariate analysis, the number of retrieved LNs was evaluated to be independently associated with survival. Additionally, patients with four to nine retrieved LNs had a significantly lower overall mortality risk [hazard ratio (HR) = 0.39; 95% confidence interval (CI): 0.20-0.74] and cancer cause-specific mortality risk (HR = 0.32; 95%CI: 0.15-0.66) than other patients. Additionally, stratified survival analyses showed persistently better overall and cancer-specific survival when retrieving four to nine LNs in patients with any T stage of tumor, a tumor between 20 and 50 mm in diameter, or a poorly differentiated or undifferentiated tumor, and in patients who were ≤ 70-years-old.
The number of retrieved LNs was an important independent prognostic factor for patients with node-negative distal cholangiocarcinoma. Additionally, patients with four to nine retrieved LNs had better overall and cancer-specific survival rates than others, but the reason and mechanism were unclear. This conclusion should be validated in future studies.
Core tip: The prognostic value of retrieved lymph node (LN) counts is still under debate for patients with distal cholangiocarcinomas. The aim of the present study was to evaluate the prognostic value of the number of retrieved lymph nodes and other prognostic factors for patients with distal cholangiocarcinomas and to determine the optimal retrieved LNs cut-off number. A total of 449 patients with distal cholangiocarcinoma were included in this study. The univariate and multivariate analyses revealed that the number of retrieved LNs was independently associated with survival. And, patients with four to nine retrieved LNs had a better overall and cancer-specific survival rate than others.
- Citation: Lin HP, Li SW, Liu Y, Zhou SJ. Prognostic value of lymph nodes count on survival of patients with distal cholangiocarcinomas. World J Gastroenterol 2018; 24(9): 1022-1034
- URL: https://www.wjgnet.com/1007-9327/full/v24/i9/1022.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i9.1022
Cholangiocarcinoma constitutes approximately 15% of hepatobiliary tumors and 3% of gastrointestinal tumors[1]. According to its anatomic location, cholangiocarcinoma is classified as intrahepatic, perihilar or distal malignancy. Distal cholangiocarcinoma comprise approximately 30% of all cholangiocarcinoma; it is a relatively uncommon disease. The only optimal treatment for distal cholangiocarcinoma is surgical resection, as a result of the insensitivity of cholangiocarcinoma to radiation and chemotherapy[2]. Additionally, complete tumor resection of distal cholangiocarcinoma always relies on pancreaticoduodenectomy, which is a complicated operation with high morbidity and mortality[3]. Hence, the postoperative prognosis of patients with distal cholangiocarcinoma has attracted great interest in several studies[4,5]. Lymph node (LN) status was determined to be a strong predictor for the prognosis of patients with distal cholangiocarcinoma[6]. Patients without LN metastasis had a better prognosis than those with LN involvement. Thus, an adequate number of retrieved LNs is vital to distinguish N0 patients from N1 ones. The appropriate cut-off of retrieved LNs counts should be determined.
Currently, the number of LNs that should be retrieved is still under debate. Several studies evaluated the prognostic value of retrieved LN counts and tried to determine the benchmark number of examined LNs[6-9]. Nevertheless, most of them were designed retrospectively with a small sample size, and cases that met their inclusion criteria comprised both perihilar and distal cholangiocarcinomas. The differences in biological and pathological features, as well as surgical strategies and prognoses, between perihilar and distal cholangiocarcinomas lead to a different influence of retrieving LN counts on survival.
The American Joint Committee on Cancer (AJCC) staging system suggested a different appropriate number of retrieved LNs for perihilar and distal cholangiocarcinomas. For distal cholangiocarcinoma, the number that AJCC suggested was 12. However, this suggestion lacks verification because the retrieved LN counts in most previous studies did not reach 12. Additionally, in the study of Kawai et al[10], patients with more than 12 retrieved LNs only had a moderately better survival rate than patients with a smaller number of retrieved LNs in a univariate analysis, not a multivariate analysis. A subgroup study of Kiriyama et al[11], using a cohort of N0 patients, found that patients with more than 10 retrieved LNs had a better survival. This subgroup analysis was based on a small sample size with a univariate analysis, and the cancers of the involved cases were all stages I and II. Therefore, the appropriate cut-off number of retrieved LNs is still unconfirmed.
Our study was performed to evaluate the interactions between the number of retrieved LNs and the prognosis of patients with distal cholangiocarcinoma; additionally, this study determined the appropriate retrieved LN cut-off number. To obtain a larger sample size, the Surveillance, Epidemiology and End Results (SEER) database was used for the selection of patients with at least one retrieved LN.
SEER is a public dataset that collects survival and incidence data of various types of cancers and covers more than 25% of the United States’ population. SEER data include tumor characteristics such as primary tumor site, TNM staging of tumor, tumor size, type of treatment and cause of death, and demographic characteristics such as race of patients, age of diagnosis, sex, etc. Our study used the latest 11 years’ data from SEER (from 2004-2014). We downloaded the data from SEER with SEER*Stat Software (version 8.3.4; https://seer.cancer.gov/seerstat/).
Our study was designed to be a retrospective study. The inclusion criteria were (1) patients greater than 20 years in age; (2) patients diagnosed with distal cholangiocarcinoma according to the term “006-BileDuctsDistal” of “CS SCHEMA v0204+”; (3) patients with histology code of 8010, 8020, 8070, 8140, 8144, 8160, 8162, 8163, 8260, 8480, 8490 or 8560; (4) patients with diagnoses that were not confirmed by a death certificate or autopsy; (5) patients with active follow-up; (6) patients from a time span of 2004 to 2014 according to the term “year of diagnosis”; (7) patients with only one tumor who had survived more than 1 mo; (8) patients without distant metastasis (the M0 patients); (9) patients who received intent surgery in terms of the combination of “Surg Prim Site” and “Reason no cancer-directed surgery”; (10) patients who did not receive preoperative radiotherapy according to the terms of “Radiation” and “Surg/Rad Seq”; and (11) patients with at least one retrieved LN according to the terms “Regional Nodes Examined”. Demographics of patients such as race, age at diagnosis and marital status, and tumor characteristics such as tumor size, laterality of tumor, grade and stage of tumor were all extracted for subsequent analysis. The terms “SEER cause-specific death classification” and “SEER other cause of death classification” were used to distinguish our two endpoints: all-cause mortality and cancer cause-specific mortality.
Statistical analyses were conducted with SPSS (version 23.0; IBM Corp., Armonk, NY, United States). The demographic data of patients were compared by t tests (for continuous variables) and chi-square tests (for proportion variables). A P-value of < 0.05 was defined to be statistically significant. Patients with different numbers of retrieved LNs were divided into three groups.
The cut-off number of retrieved LNs for grouping was determined by the X-tile program (http://www.tissuearray.org/rimmlab/). X-tile from Yale University is a useful tool for outcome-based cut-point optimization. The strategies of the X-tile program for grouping included that it would try each number between the range of the retrieved LN counts as the cut-off; then, the χ2 score and P-value were calculated with this number as the cut-off[12,13]. Eventually, the number with a maximum χ2 score and a minimum P-value would be suggested to be the final cut-off.
The Kaplan-Meier method (univariate analysis) with log-rank tests and Cox regression analysis (multivariate analysis) were utilized for survival analysis. The overall survival and cancer-specific survival were compared between patients with the different categories of retrieved LNs counts. Then, we performed stratified survival analyses for the number of retrieved LNs, in terms of the confounders that were evaluated to be independently associated with survival in the multivariate analysis.
A total of 449 patients with distal cholangiocarcinoma (2004-2014) met the inclusion criteria for this research. The majority of them were white and male. The distributions of age and the diagnosis year were averaged. Nearly 70% of the patients were married. The size of the tumor was less than 50 mm in most patients. Patient and tumor characteristics are shown in Table 1.
| Variable | No. of patients, n (%) n = 449 |
| Race | |
| White | 321 (71.5) |
| Black | 35 (7.8) |
| Other | 93 (20.7) |
| Sex | |
| Male | 286 (63.7) |
| Female | 163 (36.3) |
| Age at diagnosis, in yr | |
| ≤ 60 | 128 (28.5) |
| 60-70 | 171 (38.1) |
| > 70 | 150 (33.4) |
| Marital status | |
| Married | 302 (67.3) |
| Divorced | 33 (7.3) |
| Separated or single | 58 (12.9) |
| Widowed | 40 (8.9) |
| Unknown | 16 (3.6) |
| Year of diagnosis | |
| 2004-2010 | 110 (24.5) |
| 2011-2012 | 160 (35.6) |
| 2013-2014 | 179 (39.9) |
| Tumor size, in mm | |
| ≤ 20 | 203 (45.2) |
| 20-50 | 191(42.5) |
| > 50 | 15 (3.3) |
| Unknown | 40 (8.9) |
| Grade | |
| Well differentiated | 54 (12.0) |
| Moderately differentiated | 204 (45.4) |
| Poorly differentiated | 158 (35.2) |
| Undifferentiated | 3 (0.7) |
| Unknown | 30 (6.7) |
| Stage | |
| IA | 44 (9.8) |
| IB | 60 (13.7) |
| IIA | 89 (19.8) |
| IIB | 161 (35.9) |
| III | 95 (21.2) |
| T stage | |
| T1 | 49 (10.9) |
| T2 | 85 (18.9) |
| T3 | 220 (49.0) |
| T4 | 95 (21.2) |
| pN stage | |
| pN0 | 226 (50.3) |
| pN1 | 223 (49.7) |
| Surgery type | |
| Local excision | 102 (22.7) |
| Extensive surgery | 347 (77.3) |
| Adjuvant radiotherapy | |
| No/Unknown radiotherapy | 298 (66.3) |
| Beam radiation | 151 (33.7) |
| Adjuvant chemotherapy | |
| No/Unknown chemotherapy | 204 (45.4) |
| Chemotherapy performed | 245 (54.6) |
| No. of LNs retrieved | |
| 1-10 | 196 (43.7) |
| 11-20 | 151 (33.6) |
| > 20 | 102 (22.7) |
The retrieved LN counts ranged from one to sixty-three. More than half of the patients had > 10 LNs retrieved, and 22.7% of patients had > 20 LNs retrieved. There were 226 N0 patients and 223 N1 patients. Most patients underwent extensive surgery and postoperative chemotherapy; the number of patients who received adjuvant radiotherapy was less.
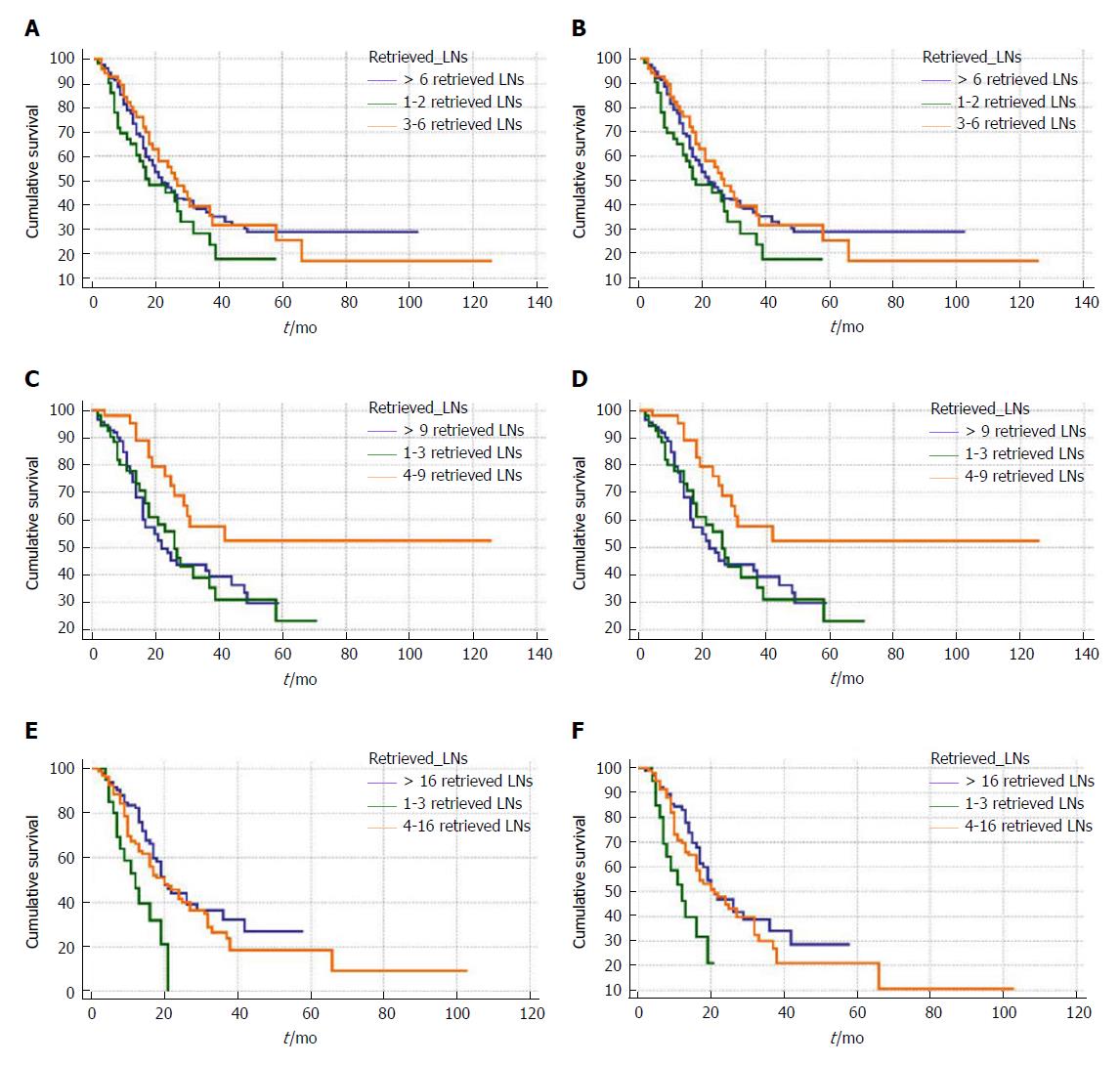
We divided patients with different numbers of retrieved LNs into three groups, by use of the X-tile program. Then, the retrieved LN count was converted from continuous variables into categorical variables to study its impact on survival. As shown in Figure 1, the cut-off numbers for grouping in all patients were 3 and 6, the cut-off numbers for N0 patients were 4 and 9, and the cut-off numbers for N1 patients were 4 and 16.
In the Kaplan-Meier survival analysis, no significant difference was observed among the three categories of retrieved LN counts for all and N1 patients with distal cholangiocarcinomas. For patients with node-negative distal cholangiocarcinomas, there was a significantly better overall and cancer-specific survival in patients with 4-9 retrieved LNs than in patients with 1-3 or > 9 retrieved LNs. Additionally, we compared overall and cancer-specific survival among each number of retrieved LNs. Because of space limitations, we could only put part of the results into the table (Table 2).
| Retrieved LNs counts | No. | 3-yr OS | 95%CI | 3-yr CSS | 95%CI |
| For all patients | |||||
| 1 | 26 | 29.51% | 10.90-51.07 | 29.51% | 10.90-51.07 |
| 3 | 23 | 35.33% | 13.50-58.23 | 43.64% | 18.10-66.88 |
| 5 | 18 | 46.55% | 16.40-72.36 | 51.20% | 17.95-77.03 |
| 7 | 22 | 63.31% | 35.24-81.81 | 63.31% | 35.24-81.84 |
| 9 | 16 | 57.29% | 27.94-78.40 | 66.20% | 32.37-86.00 |
| 11 | 16 | 37.09% | 11.27-63.72 | 40.46% | 12.19-67.77 |
| 13 | 11 | 28.41% | 4.52-59.96 | 28.41% | 4.52-59.96 |
| 15 | 13 | 16.46% | 0.90-50.11 | 16.46% | 0.90-50.11 |
| 17 | 15 | 15.80% | 0.82-49.22 | 17.01% | 0.84-51.90 |
| 19 | 9 | 34.29% | 4.81-68.55 | 34.29% | 4.81-68.55 |
| 21 | 12 | 47.62% | 19.35-71.52 | 47.62% | 19.35-71.52 |
| 23 | 5 | 33.86% | 5.73-66.35 | 33.86% | 5.73-66.35 |
| 25 | 15 | 37.50% | 9.37-66.61 | 37.50% | 9.37-66.61 |
| For N0 patients | |||||
| 1 | 17 | 40.38% | 14.15-65.68 | 40.38% | 14.15-65.68 |
| 3 | 18 | 43.65% | 17.20-67.68 | 47.01% | 18.51-71.34 |
| 5 | 11 | 30.00% | 1.23-71.92 | 37.50% | 1.10-80.80 |
| 7 | 16 | 76.15% | 42.67-91.65 | 76.15% | 42.67-91.65 |
| 9 | 5 | 66.67% | 5.41-94.52 | 66.67% | 5.41-94.52 |
| 11 | 9 | 50.79% | 15.67-78.07 | 59.26% | 18.59-84.95 |
| 13 | 4 | 37.50% | 1.10-80.80 | 37.50% | 1.10-80.80 |
| 15 | 6 | 26.67% | 0.97-68.61 | 26.67% | 0.97-68.61 |
| 17 | 4 | - | - | - | - |
| 19 | 2 | - | - | - | - |
| 21 | 6 | 55.56% | 7.34-87.61 | 55.56% | 7.34-87.61 |
| 23 | 1 | - | - | - | - |
| 25 | 7 | 42.00% | 7.01-75.34 | 52.50% | 8.42-84.55 |
| For N1 patients | |||||
| 1 | 9 | 14.29% | 0.71-46.49 | 14.29% | 0.71-46.49 |
| 3 | 5 | 33.33% | 0.90-77.41 | 33.33% | 0.90-77.41 |
| 5 | 7 | 53.57% | 13.20-82.50 | 53.57% | 13.20-82.50 |
| 7 | 6 | 55.56% | 7.34-87.61 | 55.56% | 7.34-87.61 |
| 9 | 11 | 45.00% | 13.88-72.41 | 58.33% | 18.02-84.41 |
| 11 | 7 | 25.00% | 1.23-64.59 | 25.00% | 1.23-64.59 |
| 13 | 7 | 41.67% | 5.60-76.65 | 41.67% | 5.60-76.65 |
| 15 | 7 | 35.71% | 1.41-77.98 | 35.71% | 1.41-77.98 |
| 17 | 11 | 17.05% | 0.84-51.92 | 18.94% | 0.87-55.82 |
| 19 | 7 | 26.67% | 0.97-68.61 | 26.67% | 0.97-68.61 |
| 21 | 6 | 43.64% | 11.29-72.96 | 43.64% | 11.29-72.96 |
| 23 | 4 | - | - | - | - |
| 25 | 8 | 28.57% | 4.11-61.15 | 28.57% | 4.11-61.15 |
There was a similar trend of survival rate as the retrieved LN count increased in all, N0 and N1 patients; patients with seven retrieved LNs had the best survival rate compared with the others. In N0 patients, patients with seven or nine retrieved LNs had a significantly higher survival rate compared with other patients; this result was confirmed in analysis regarding retrieved LN counts as categorical variables.
The results of survival analysis for all patients in the present study were similar to previous studies. As shown in Figure 1 and Table 3, retrieved LN counts were not associated with survival in all patients (P = 0.233). Factors such as tumor size and T and N stages that were significant in univariate analysis were entered into a multivariate model. N stage was shown to be independently associated with overall survival [hazard ratio (HR) = 1.40; 95% confidence interval (CI): 1.05-1.86]. In terms of cancer-specific survival, T stage [(HR = 1.45; 95%CI: 1.02-2.07)] was shown to be an independent risk factor of survival, along with N stage (HR = 1.42; 95%CI: 1.05-1.92).
| Variables | Overall survival | Cancer-specific survival | ||||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||
| 3-yr OS | χ2 | P value | HR (95%CI) | P value | 3-yr OS | χ2 | P value | HR (95%CI) | P value | |
| Race | 2.22 | 0.329 | 1.95 | 0.377 | ||||||
| White | 33.3% | 36.1% | ||||||||
| Black | 34.8% | 36.9% | ||||||||
| Other | 38.9% | 42.1% | ||||||||
| Sex | 0.44 | 0.507 | 0.54 | 0.463 | ||||||
| Male | 35.3% | 38.3% | ||||||||
| Female | 37.7% | 40.9% | ||||||||
| Age at diagnosis, in yr | 1.89 | 0.387 | 0.48 | 0.783 | ||||||
| ≤ 60 | 31.2% | 35.8% | ||||||||
| 60-70 | 39.1% | 41.4% | ||||||||
| > 70 | 30.7% | 36.8% | ||||||||
| Marital status | 5.02 | 0.285 | 5.98 | 0.201 | ||||||
| Married | 37.3% | 39.6% | ||||||||
| Divorced | - | - | ||||||||
| Separated or single | 31.4% | 35.3% | ||||||||
| Widowed | 34.1% | - | ||||||||
| Unknown | - | |||||||||
| Year of diagnosis | 1.82 | 0.402 | 3.25 | 0.197 | ||||||
| 2004-2010 | 37.3% | 41.4% | ||||||||
| 2011-2012 | 30.9% | 33.2% | ||||||||
| 2013-2014 | - | - | ||||||||
| Tumor size, in mm | 8.04 | 0.045 | 0.230 | 8.76 | 0.032 | 0.249 | ||||
| ≤ 20 | 40.8% | Reference | 44.0% | Reference | ||||||
| 20-50 | 29.9% | 1.25 (0.94-1.67) | 33.2% | 1.23 (0.91-1.68) | ||||||
| > 50 | 0.0% | 1.67 (0.86-3.23) | 0.0% | 1.76 (0.91-3.43) | ||||||
| Unknown | 37.7% | 0.95 (0.57-1.58) | 46.3% | 0.96 (0.56-1.65) | ||||||
| Grade | 6.77 | 0.079 | 6.92 | 0.074 | ||||||
| Well differentiated | 48.6% | 52.4% | ||||||||
| Moderately differentiated | 32.6% | 38.9% | ||||||||
| Poorly or undifferentiated | 30.3% | 32.6% | ||||||||
| Unknown | 43.2% | 43.2% | ||||||||
| T stage | 5.57 | 0.018 | 0.270 | 11.16 | < 0.001 | 0.037 | ||||
| T1/T2 | 44.3% | Reference | 51.6% | Reference | ||||||
| T3/T4 | 29.6% | 1.19 (0.87-1.64) | 32.6% | 1.45 (1.02-2.07) | ||||||
| N stage | 10.64 | 0.001 | 0.020 | 12.27 | < 0.001 | 0.022 | ||||
| N0 | 44.5% | Reference | 47.4% | Reference | ||||||
| N1 | 26.9% | 1.40 (1.05-1.86) | 30.3% | 1.42 (1.05-1.92) | ||||||
| Surgery type | 1.20 | 0.272 | 0.73 | 0.393 | ||||||
| Local excision | 44.0% | 45.3% | ||||||||
| Extensive surgery | 34.4% | 37.9% | ||||||||
| Adjuvant radiotherapy | 0.16 | 0.683 | 0.79 | 0.372 | ||||||
| No/Unknown radiotherapy | 39.9% | 44.3% | ||||||||
| Beam radiation | 29.5% | 30.5% | ||||||||
| Adjuvant chemotherapy | 1.10 | 0.293 | 2.92 | 0.087 | ||||||
| No/Unknown chemotherapy | 41.2% | 47.5% | ||||||||
| Chemotherapy performed | 32.2% | 33.3% | ||||||||
| No. of LNs retrieved | 2.91 | 0.233 | 3.90 | 0.141 | ||||||
| 1-2 | 23.6% | 29.9% | ||||||||
| 3-6 | 35.6% | 39.4% | ||||||||
| > 6 | 36.0% | 39.0% | ||||||||
For N0 patients, univariate analysis showed that retrieved LN counts, age at diagnosis, and grade of tumor were associated with overall survival. After those factors were entered into multivariate analysis, retrieved LN counts and grade of tumor were determined to be independent risk factors of overall survival. Patients with four to nine retrieved LNs had a significantly lower all-cause mortality risk than other patients (HR = 0.39; 95%CI: 0.20-0.74). In terms of cancer-specific survival, tumor size, grade of tumor, T stage and retrieved LN counts were evaluated to be associated with survival in both univariate and multivariate analyses. There was a significant decrease in terms of cancer cause-specific mortality risk (HR = 0.32; 95%CI: 0.15-0.66) for patients with four to nine retrieved LNs (Table 4).
| Variable | Overall survival | Cancer-specific survival | ||||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||
| 3-yr OS | χ2 | P value | HR (95%CI) | P value | 3-yr OS | χ2 | P value | HR (95%CI) | P value | |
| Race | 0.82 | 0.663 | 0.13 | 0.939 | ||||||
| White | 44.3% | 46.8% | ||||||||
| Black | 49.4% | 49.4% | ||||||||
| Other | 40.4% | 44.0% | ||||||||
| Sex | 1.32 | 0.250 | 0.77 | 0.377 | ||||||
| Male | 39.5% | 46.0% | ||||||||
| Female | 47.0% | 49.8% | ||||||||
| Age at diagnosis, in yr | 3.85 | 0.049 | 0.056 | 2.73 | 0.097 | |||||
| ≤ 70 | 49.8% | Reference | 51.8% | |||||||
| > 70 | 31.4% | 1.51 (0.98-2.33) | 35.7% | |||||||
| Marital status | 6.30 | 0.177 | 6.52 | 0.163 | ||||||
| Married | 42.9% | 48.5% | ||||||||
| Divorced | - | - | ||||||||
| Separated or single | 31.8% | 39.6% | ||||||||
| Widowed | 46.9% | 46.9% | ||||||||
| Unknown | - | - | ||||||||
| Year of diagnosis | 3.41 | 0.181 | 4.05 | 0.131 | ||||||
| 2004-2010 | 50.0% | 52.1% | ||||||||
| 2011-2012 | 36.5% | 41.4% | ||||||||
| 2013-2014 | 48.8% | 54.0% | ||||||||
| Tumor size, in mm | 7.47 | 0.058 | 9.11 | 0.027 | 0.036 | |||||
| ≤ 20 | 47.3% | 49.6% | Reference | |||||||
| 20-50 | 36.9% | 40.0% | 1.57 (0.98-2.53) | |||||||
| > 50 | 0.0% | 0.0% | 3.08 (1.16-8.21) | |||||||
| Unknown | 46.8% | 60.8% | 0.72 (0.30-1.71) | |||||||
| Grade | 9.65 | 0.021 | 0.014 | 12.56 | 0.005 | 0.003 | ||||
| Well differentiated | 56.8% | Reference | 63.3% | Reference | ||||||
| Moderately differentiated | 43.5% | 1.59 (0.84-2.99) | 51.8% | 1.44 (0.70-2.97) | ||||||
| Poorly or undifferentiated | 26.0% | 2.35 (1.24-4.46) | 30.5% | 2.90 (1.43-5.88) | ||||||
| Unknown | 57.0% | 0.76 (0.27-2.16) | 57.0% | 1.14 (0.37-3.50) | ||||||
| T stage | 1.35 | 0.244 | 4.63 | 0.031 | 0.030 | |||||
| T1/T2 | 48.7% | 58.4% | Reference | |||||||
| T3/T4 | 37.1% | 38.7% | 1.69 (1.05-2.71) | |||||||
| Surgery type | 0.10 | 0.748 | 0.23 | 0.631 | ||||||
| Local excision | 45.7% | 48.2% | ||||||||
| Extensive surgery | 44.1% | 47.0% | ||||||||
| Adjuvant radiotherapy | 1.64 | 0.199 | 1.53 | 0.215 | ||||||
| No/Unknown radiotherapy | 48.2% | 53.2% | ||||||||
| Beam radiation | 35.5% | 37.2% | ||||||||
| Adjuvant chemotherapy | 1.31 | 0.251 | 1.48 | 0.222 | ||||||
| No/Unknown chemotherapy | 46.3% | 53.8% | ||||||||
| Chemotherapy performed | 38.5% | 42.1% | ||||||||
| No. of LNs retrieved | 7.24 | 0.026 | 0.013 | 6.47 | 0.039 | 0.008 | ||||
| 1-3 | 35.1% | Reference | 40.9% | Reference | ||||||
| 4-9 | 52.3% | 0.39 (0.20-0.74) | 60.0% | 0.32 (0.15-0.66) | ||||||
| > 9 | 39.3% | 0.85 (0.53-1.36) | 44.9% | 0.62 (0.36-1.07) | ||||||
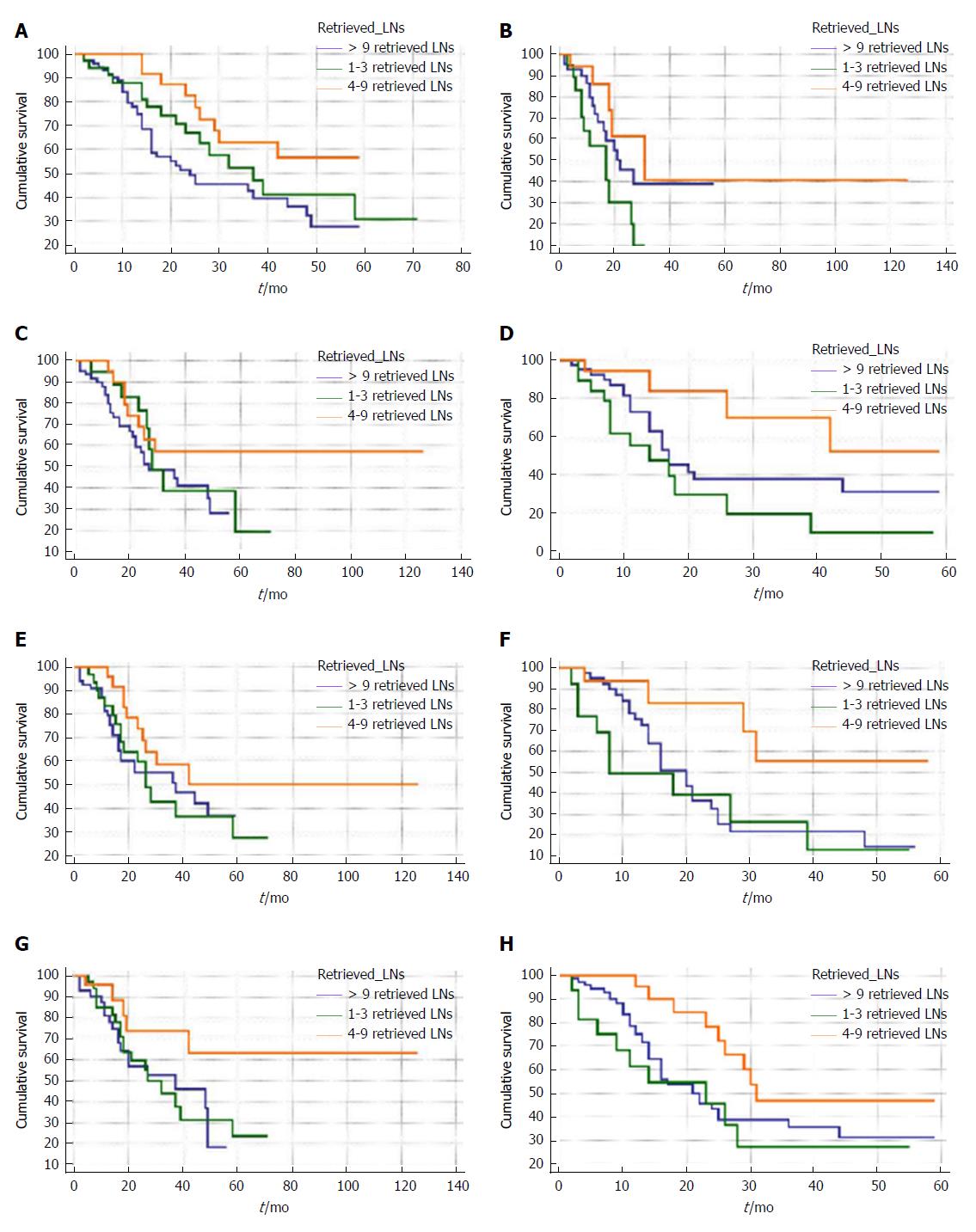
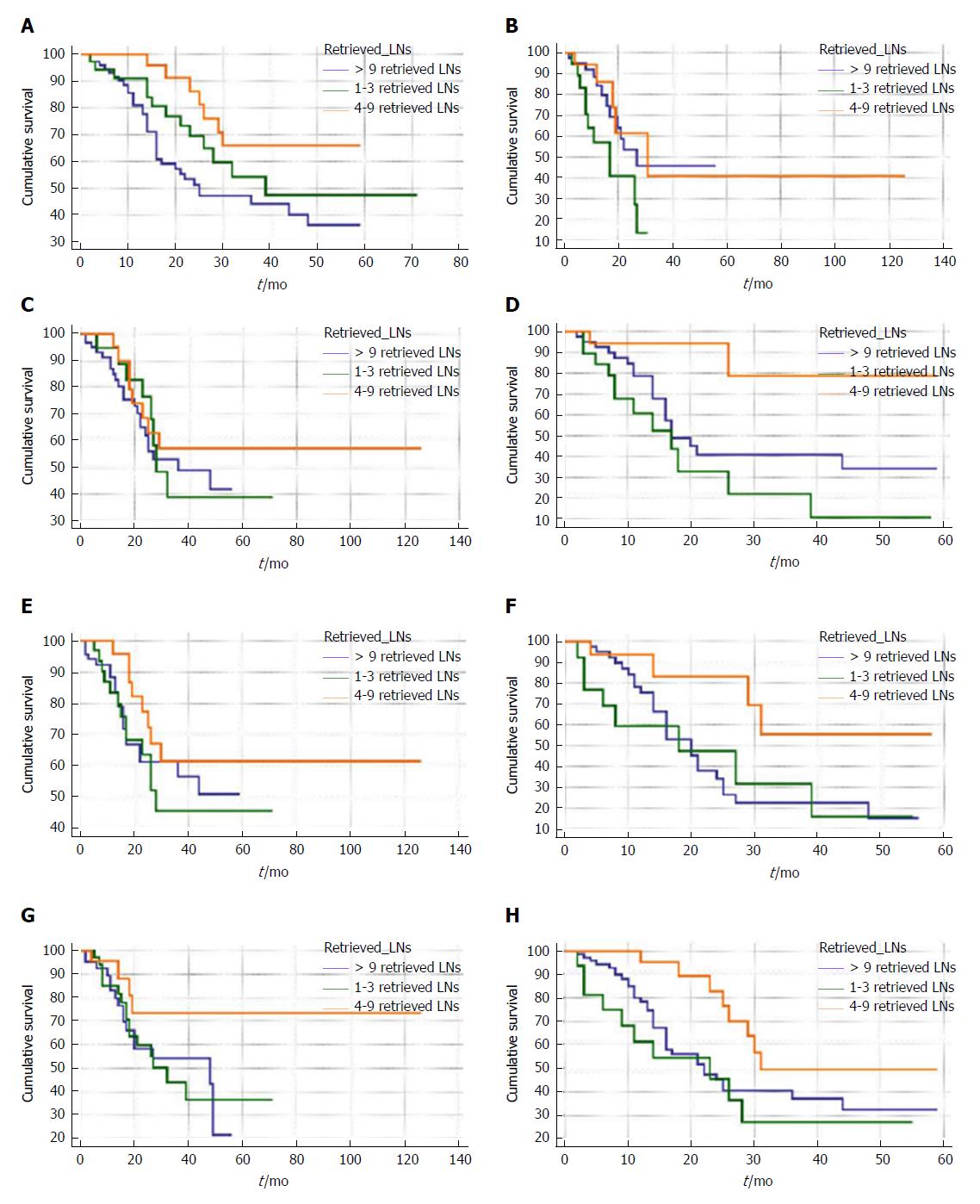
To further study the interactions between retrieved LN counts and prognoses of patients with node-negative distal cholangiocarcinoma, we performed survival analysis stratified by size, grade, T stage of tumor and age of patients. For patients ≤ 70-years-old, retrieving four to nine LNs resulted in a significantly better survival rate than retrieving one to three LNs in terms of overall survival (Figure 2A) and cancer-specific survival (Figure 3A). Additionally, no significant difference between patients with one to three retrieved LNs and > 9 retrieved LNs in terms of overall and cancer-specific survival was observed.
Subsequently, the above results were confirmed in multivariate survival analyses after adjusting for all confounders (Table 5). As shown in Figures 2 and 3 and Table 5, similar results were found for patients with any T stage of tumor, tumor size between 20 and 50 mm, and tumors that were poorly defined or undifferentiated. The prognostic effect of retrieved LN counts was not present when analyses were limited to well or moderately differentiated tumors, tumors ≤ 20 mm, and patients greater than 70 years in age.
| Variable | 4-9 retrieved LNs (1-3 retrieved LNs as the reference) | > 9 retrieved LNs (1-3 retrieved LNs as the reference) | ||||||
| Overall survival | Cancer-specific survival | Overall survival | Cancer-specific survival | |||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Age, in yr | ||||||||
| ≤ 70 | 0.33 (0.13-0.86) | 0.023 | 0.34 (0.13-0.89) | 0.030 | 1.02 (0.49-2.14) | 0.940 | 0.93 (0.46-1.88) | 0.848 |
| > 70 | 0.32 (0.11-0.98) | 0.047 | 0.33 (0.11-1.04) | 0.059 | 0.63 (0.26-1.49) | 0.294 | 0.40 (1.55-1.04) | 0.059 |
| Tumor size, in mm | ||||||||
| ≤ 20 | 0.55 (0.21-1.46) | 0.232 | 0.61 (0.22-1.68) | 0.340 | 1.34 (0.60-2.97) | 0.472 | 0.96 (0.41-2.23) | 0.921 |
| 20-50 | 0.22 (0.07-0.69) | 0.010 | 0.12 (0.03-0.55) | 0.007 | 0.54 (0.24-1.21) | 0.134 | 0.48 (0.21-1.12) | 0.090 |
| > 50 | - | - | - | - | - | - | - | - |
| Grade | ||||||||
| Well or moderately differentiated | 0.56 (0.24-1.30) | 0.180 | 0.47 (0.18-1.20) | 0.114 | 0.99 (0.49-2.01) | 0.994 | 0.74 (0.34-1.62) | 0.454 |
| Poorly or undifferentiated | 0.23 (0.07-0.79) | 0.020 | 0.26 (0.07-0.89) | 0.032 | 0.69 (0.31-1.58) | 0.389 | 0.75 (0.32-1.78) | 0.514 |
| T stage | ||||||||
| T1/T2 | 0.30 (0.11-0.85) | 0.024 | 0.30 (0.09-0.94) | 0.039 | 0.79 (0.39-1.59) | 0.501 | 0.76 (0.36-1.59) | 0.467 |
| T3/T4 | 0.34 (0.13-0.85) | 0.022 | 0.31 (0.12-0.79) | 0.016 | 0.66 (0.31-1.41) | 0.285 | 0.65 (0.30-1.39) | 0.267 |
Nodal status is a well-studied indicator for the prognosis of patients with distal cholangiocarcinoma. In addition to the stage of LNs, the prognostic value of positive node counts and lymph node ratios has been evaluated in several studies[6,10,11]. While the prognostic value of the number of retrieved LNs is still under debate, the optimal cut-off number of retrieved LNs is also unconfirmed. Several studies of other diseases revealed the difference in retrieved LNs’ influence on survival between N0 and N1 patients[8,14,15]. Nevertheless, for N0 patients, more LNs retrieved significantly improved survival. Therefore, studies of distal cholangiocarcinoma on retrieved LN counts should be performed in a cohort of N0 patients for whom the prognostic value of retrieved LN counts has never been systematically studied.
Our study screened 449 patients with distal cholangiocarcinoma in a population-based database; a total of 226 patients with node-negative distal cholangiocarcinoma were among them. Retrieved LN counts did not show its prognostic value in the whole cohort and N1 patients. However, in patients with node-negative distal cholangiocarcinoma, patients with four to nine retrieved LNs were determined to have a significantly better prognosis than patients with ≤ 3 retrieved LNs in terms of overall and cancer-specific survivals. Additionally, tumor size, grade and T stage of tumor were evaluated to be independent risk factors of cancer-specific survival. Therefore, retrieving at least four LNs would be optimal for patients with node-negative distal cholangiocarcinoma.
More retrieved LNs could promote the accuracy of LNs staging to avoid the under-staging effect, thus to improve survival of patients with distal cholangiocarcinoma. Studies on cholangiocarcinoma demonstrated there were micrometastases in approximately 5% of LNs which were diagnosed as negative nodes[16]. The more LNs that were resected and retrieved meant less micrometastases were left, therefore the survival of patients with more retrieved LNs counts could be improved. Additionally, more retrieved LNs represented adequate surgical, pathological and institutional care[17]. What’s more, anatomic studies determined that more resected LNs could improve the underlying tumor-host interactions and reset the immunological balance to improve survival[18].
The AJCC system suggested the optimal number of retrieved LNs of patients with distal cholangiocarcinoma should be 12. Whereas study of Sasaki et al[6] demonstrated there was no difference between patients with ≥ 12 retrieved LNs and < 12 retrieved LNs in terms of overall survival. A subgroup analysis in the study of Kiriyama et al[11] revealed that patients with stage I or II tumors who had more than 10 LNs retrieved had a better survival rate than others. While 83.2% of patients in their study retrieved more than 12 LNs, the number of patients with < 10 retrieved LNs was very small (n = 22), and their results were based on a univariate analysis. Therefore, selection bias should be kept in mind when interpreting their results. The fact that retrieving more than 10 or 12 LNs is an indicator of better prognosis is still disputable.
The present study denoted that retrieving more than nine LNs did not indicate a better prognosis in patients with node-negative distal cholangiocarcinoma, but an increase in terms of all-cause mortality risk and cancer cause-specific mortality risk was observed compared with retrieving four to nine LNs. For patients with distal cholangiocarcinoma, retrieving too many LNs did not obtain better outcomes. This result was contrary to the prevailing dogma that a better prognosis was always associated with higher retrieved LN counts.
There were several hypotheses for the reason why more retrieved LNs represented a worse prognosis. Necrosis represented an aggressive biology of tumor and a decreased survival rate; it had a close association with LN hyperplasia. LN hyperplasia always resulted in increases in the size and number of detectable LNs; therefore, more retrieved LNs (detectable LNs) were related to a worse prognosis[19-21]. The other hypothesis was that there might be a difference at the molecular level between tumors with more and less detectable LNs. Tumors with more detectable LNs, i.e. more retrieved LNs, might belong to another subset of distal cholangiocarcinoma that acts biologically more aggressively[19]. Additionally, routine histologic techniques for retrieving LNs may ignore the micrometastases, leading to the under-staging of LNs. Hence, without the application of immunohistochemical techniques that was determined to increase the detection rate of micrometastases, more retrieved LNs could not promote the accuracy of LN staging or improve patient survival. And, we wanted to know if more retrieved LNs reflected extended lymphadenectomy that may result in increasing postoperative complications. However, because of the limitation of the SEER database, we could not compare the background data in the > 9 retrieved LNs group with that in four to nine retrieved LN groups.
There were several limitations in our study. First, although a population-based database was utilized to screen patients, the total number of patients involved in our study was still not large enough compared with congener studies for other diseases. Second, information for adjuvant chemotherapy and radiotherapy in survival analysis did not contain the details of the protocols, and the SEER database did not provide data. Third, disease-free survival could not be calculated because of the lack of information about local recurrence in the SEER. Fourth, patients who received preoperative radiation were excluded. There might be patients who received radiation in some other centers that were not recorded in the SEER, so the down-staging effect of radiation could not be entirely ruled out. Fifth, the number of lymph nodes retrieved may depend upon the type of surgical procedures. And, the lymph nodes distant from the lesion (for example, nodes from Whipple’s procedure) may not have the same predicting value as these from local or limited resection specimen. However, the detailed operation methods were unknown because of the limitation of the SEER, i.e. we could not know how many patients underwent pancreatoduodenectomy or segmental bile duct resection. Sixth, the number of lymph nodes retrieved may rely on lymph node dissection skill in each individual institution (grossing by resident vs practicing pathologists or pathologist assistant). But, the SEER database only provided the information of the region where the patients were from, and the classes of hospital were unknown. Therefore, such institution bias should be taken into consideration when interpreting our results. Seventh, we could only compare survival among the groups of patients with four to nine lymph nodes and others based upon pathological stage and not clinical stage due to the limitation of SEER (the SEER database only provided the information of pathological stage for patients with resectable distal cholangiocarcinomas). Eighth, the AJCC staging system utilized in the present study was the 6th edition, which was not the most commonly used one nowadays (due to limitations of SEER). Finally, we could not get the data referring to the surgical margin status in SEER; surgical margin status was an important prognostic factor in patients with resected distal cholangiocarcinoma.
In conclusion, the number of retrieved LNs did not show its prognostic value in the whole group of patients (a mixture of N0 and N1 patients) and N1 patients. However, the number of retrieved LNs was an independent prognostic factor of overall and cancer-specific survival for patients with node-negative distal cholangiocarcinoma. Patients with four to nine retrieved LNs had better overall and cancer-specific survival rates than others, but the reason and mechanism were unclear. The future studies should consider more operation- and adjuvant therapy-related parameters into their analysis to evaluate our results.
Lymph node (LN) status was determined to be a strong predictor for the prognosis of patients with distal cholangiocarcinoma. However, the prognostic value of the retrieved LNs counts in distal cholangiocarcinoma is still under debate.
The benchmark number of retrieved LNs has been determined in many gastrointestinal carcinomas, in addition to the distal cholangiocarcinomas. Previous studies regarding the retrieved LNs counts in distal cholangiocarcinomas were limited by their small sample size, and the patients in those studies comprised both perihilar and distal cholangiocarcinomas. The present study tried to determine the interactions between the retrieved LNs counts and the prognosis in patients with only distal cholangiocarcinomas, and a population-based database was used for patients’ selection that provided a sufficient sample size.
We aimed to evaluate the prognostic value of the number of retrieved LNs for patients with distal cholangiocarcinomas and to determine the optimal retrieved LNs cut-off number.
The Surveillance, Epidemiology and End Results (SEER) database was used to screen for patients with distal cholangiocarcinoma. The retrieved LNs counts were transformed from continuous variables to categorical variables, and the cut-off was defined by the X-tile program. The overall and cancer-specific survival was compared between the different categories of retrieved LNs counts by the means of the Kaplan-Meier method and Cox regression analysis. Then, we performed stratified analyses by the clinical factors that were evaluated to be independently associated with survival in the Cox regression analysis, among patients within the different LNs groups.
A total of 449 patients with distal cholangiocarcinoma were included in the present study. The Kaplan-Meier survival analysis for all patients and for N1 patients revealed no significant differences among patients with different retrieved LN counts in terms of overall and cancer-specific survivals. In patients with node-negative distal cholangiocarcinoma, patients with four to nine retrieved LNs had a significantly better overall (P = 0.026) and cancer-specific (P = 0.039) survival than others. In the subsequent multivariate analysis, the number of retrieved LNs was evaluated to be independently associated with survival. Additionally, patients with four to nine retrieved LNs had a significantly lower overall mortality risk (hazard ratio (HR): 0.39; 95% confidence interval (CI): 0.20-0.74) and cancer cause-specific mortality risk (HR: 0.32; 95%CI: 0.15-0.66) than other patients. Additionally, stratified survival analyses showed persistent better overall and cancer-specific survival when retrieving four to nine LNs in patients with any T stage of tumor, a tumor between 20 and 50 mm in diameter, or a poorly differentiated or undifferentiated tumor and in patients who were ≤ 70-years-old.
The number of retrieved LNs was an important independent prognostic factor for patients with node-negative distal cholangiocarcinoma. Additionally, patients with four to nine retrieved LNs had a better overall and cancer-specific survival rate than patients with less than four or more than nine retrieved LNs.
Although our study revealed retrieving four to nine LNs in patients with node-negative distal cholangiocarcinoma had better overall and cancer-specific survival rates than others, the reason and mechanism for that were unclear. The future studies should consider more operation- and adjuvant therapy-related parameters into their analysis to evaluate our results.
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8406] [Cited by in RCA: 8988] [Article Influence: 642.0] [Reference Citation Analysis (3)] |
| 2. | Komaya K, Ebata T, Shirai K, Ohira S, Morofuji N, Akutagawa A, Yamaguchi R, Nagino M; Nagoya Surgical Oncology Group. Recurrence after resection with curative intent for distal cholangiocarcinoma. Br J Surg. 2017;104:426-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 3. | Gouma DJ, van Geenen RC, van Gulik TM, de Haan RJ, de Wit LT, Busch OR, Obertop H. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg. 2000;232:786-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 656] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 4. | Murakami Y, Uemura K, Hayashidani Y, Sudo T, Hashimoto Y, Ohge H, Sueda T. Prognostic significance of lymph node metastasis and surgical margin status for distal cholangiocarcinoma. J Surg Oncol. 2007;95:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Murakami Y, Uemura K, Sudo T, Hashimoto Y, Nakashima A, Kondo N, Sakabe R, Ohge H, Sueda T. Prognostic factors after surgical resection for intrahepatic, hilar, and distal cholangiocarcinoma. Ann Surg Oncol. 2011;18:651-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 6. | Oshiro Y, Sasaki R, Kobayashi A, Murata S, Fukunaga K, Kondo T, Oda T, Ohkohchi N. Prognostic relevance of the lymph node ratio in surgical patients with extrahepatic cholangiocarcinoma. Eur J Surg Oncol. 2011;37:60-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Schwarz RE, Smith DD. Lymph node dissection impact on staging and survival of extrahepatic cholangiocarcinomas, based on U.S. population data. J Gastrointest Surg. 2007;11:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Ito K, Ito H, Allen PJ, Gonen M, Klimstra D, D’Angelica MI, Fong Y, DeMatteo RP, Brennan MF, Blumgart LH. Adequate lymph node assessment for extrahepatic bile duct adenocarcinoma. Ann Surg. 2010;251:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 9. | Pomianowska E, Westgaard A, Mathisen Ø, Clausen OP, Gladhaug IP. Prognostic relevance of number and ratio of metastatic lymph nodes in resected pancreatic, ampullary, and distal bile duct carcinomas. Ann Surg Oncol. 2013;20:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Kawai M, Tani M, Kobayashi Y, Tsuji T, Tabuse K, Horiuchi T, Oka M, Yamaguchi K, Sakata Y, Shimomura T. The ratio between metastatic and examined lymph nodes is an independent prognostic factor for patients with resectable middle and distal bile duct carcinoma. Am J Surg. 2010;199:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 11. | Kiriyama M, Ebata T, Aoba T, Kaneoka Y, Arai T, Shimizu Y, Nagino M; Nagoya Surgical Oncology Group. Prognostic impact of lymph node metastasis in distal cholangiocarcinoma. Br J Surg. 2015;102:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 12. | Xiao J, Ye ZS, Wei SH, Zeng Y, Lin ZM, Wang Y, Teng WH, Chen LC. Prognostic significance of pretreatment serum carcinoembryonic antigen levels in gastric cancer with pathological lymph node-negative: A large sample single-center retrospective study. World J Gastroenterol. 2017;23:8562-8569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Qureshi YA, Sarker SJ, Walker RC, Hughes SF. Proximal Resection Margin in Ivor-Lewis Oesophagectomy for Cancer. Ann Surg Oncol. 2017;24:569-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Bagante F, Tran T, Spolverato G, Ruzzenente A, Buttner S, Ethun CG, Groot Koerkamp B, Conci S, Idrees K, Isom CA. Perihilar Cholangiocarcinoma: Number of Nodes Examined and Optimal Lymph Node Prognostic Scheme. J Am Coll Surg. 2016;222:750-759.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Mao K, Liu J, Sun J, Zhang J, Chen J, Pawlik TM, Jacobs LK, Xiao Z, Wang J. Patterns and prognostic value of lymph node dissection for resected perihilar cholangiocarcinoma. J Gastroenterol Hepatol. 2016;31:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Mantel HT, Wiggers JK, Verheij J, Doff JJ, Sieders E, van Gulik TM, Gouw AS, Porte RJ. Lymph Node Micrometastases are Associated with Worse Survival in Patients with Otherwise Node-Negative Hilar Cholangiocarcinoma. Ann Surg Oncol. 2015;22 Suppl 3:S1107-S1115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Johnson PM, Porter GA, Ricciardi R, Baxter NN. Increasing negative lymph node count is independently associated with improved long-term survival in stage IIIB and IIIC colon cancer. J Clin Oncol. 2006;24:3570-3575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 288] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 18. | Zheng WF, Ji TT, Lin Y, Li RZ. The prognostic value of lymph nodes count on survival of patients with node-negative gastric cancer. Oncotarget. 2016;7:43680-43688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Mersin H, Yildirim E, Bulut H, Berberoğlu U. The prognostic significance of total lymph node number in patients with axillary lymph node-negative breast cancer. Eur J Surg Oncol. 2003;29:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Antonini F, Nah YW, Xu RL S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Huang Y