Published online Feb 7, 2018. doi: 10.3748/wjg.v24.i5.641
Peer-review started: December 4, 2017
First decision: January 4, 2018
Revised: January 5, 2018
Accepted: January 15, 2018
Article in press: January 15, 2018
Published online: February 7, 2018
Processing time: 59 Days and 14.6 Hours
To assess magnetic resonance imaging (MRI) and faecal calprotectin to detect endoscopic postoperative recurrence in patients with Crohn’s disease (CD).
From two tertiary centers, all patients with CD who underwent ileocolonic resection were consecutively and prospectively included. All the patients underwent MRI and endoscopy within the first year after surgery or after the restoration of intestinal continuity [median = 6 mo (5.0-9.3)]. The stools were collected the day before the colonoscopy to evaluate faecal calprotectin level. Endoscopic postoperative recurrence (POR) was defined as Rutgeerts’ index ≥ i2b. The MRI was analyzed independently by two radiologists blinded from clinical data.
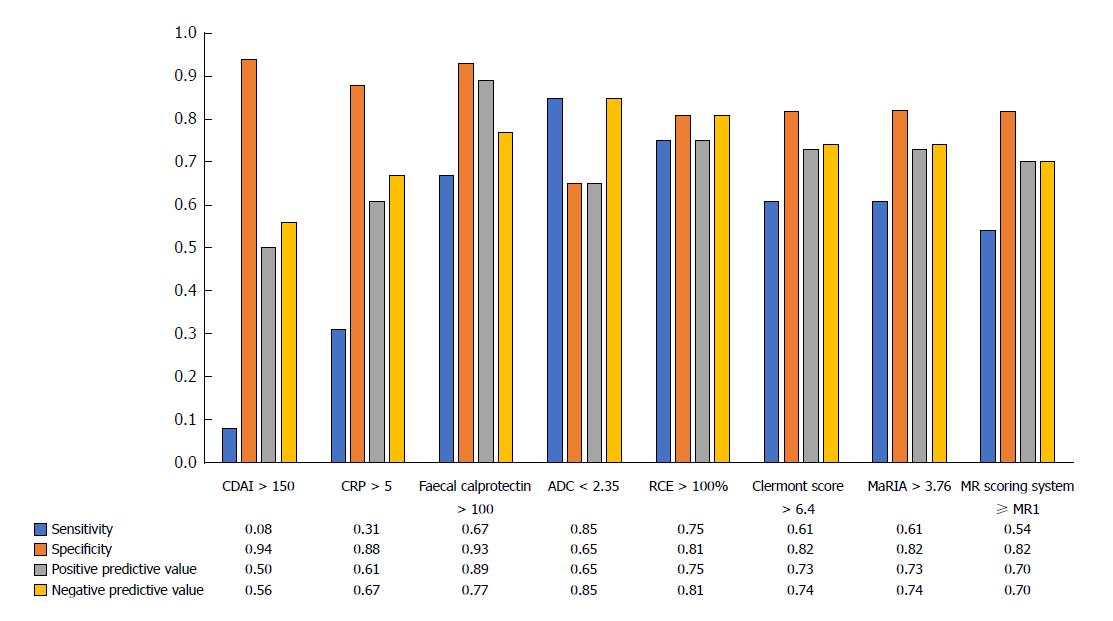
Apparent diffusion coefficient (ADC) was lower in patients with endoscopic POR compared to those with no recurrence (2.03 ± 0.32 vs 2.27 ± 0.38 × 10-3 mm²/s, P = 0.032). Clermont score (10.4 ± 5.8 vs 7.4 ± 4.5, P = 0.038) and relative contrast enhancement (RCE) (129.4% ± 62.8% vs 76.4% ± 32.6%, P = 0.007) were significantly associated with endoscopic POR contrary to the magnetic resonance index of activity (MaRIA) (7.3 ± 4.5 vs 4.8 ± 3.7; P = 0.15) and MR scoring system (P = 0.056). ADC < 2.35 × 10-3 mm²/s [sensitivity = 0.85, specificity = 0.65, positive predictive value (PPV) = 0.85, negative predictive value (NPV) = 0.65] and RCE > 100% (sensitivity = 0.75, specificity = 0.81, PPV = 0.75, NPV = 0.81) were the best cut-off values to identify endoscopic POR. Clermont score > 6.4 (sensitivity = 0.61, specificity = 0.82, PPV = 0.73, NPV = 0.74), MaRIA > 3.76 (sensitivity = 0.61, specificity = 0.82, PPV = 0.73, NPV = 0.74) and a MR scoring system ≥ MR1 (sensitivity = 0.54, specificity = 0.82, PPV = 0.70, and NPV = 0.70) demonstrated interesting performances to detect endoscopic POR. Faecal calprotectin values were significantly higher in patients with endoscopic POR (114 ± 54.5 μg/g vs 354.8 ± 432.5 μg/g; P = 0.0075). Faecal calprotectin > 100 μg/g demonstrated high performances to detect endoscopic POR (sensitivity = 0.67, specificity = 0.93, PPV = 0.89 and NPV = 0.77).
Faecal calprotectin and MRI are two reliable tools to detect endoscopic POR in patients with CD.
Core tip: Performing a colonoscopy within the first year after surgery is now recommended in the management of postoperative Crohn’s disease (CD) to decrease the risk of symptomatic recurrence. However, endoscopy is felt as a burdensome procedure by the patients highlighting the need for more convenient tools. In our prospective study from two referral centers, we showed that faecal calprotectin measurement and magnetic resonance imaging with Clermont score or magnetic resonance index of activity calculation are two reliable tools to detect early endoscopic postoperative recurrence in CD and could then be an alternative to colonoscopy.
- Citation: Baillet P, Cadiot G, Goutte M, Goutorbe F, Brixi H, Hoeffel C, Allimant C, Reymond M, Obritin-Guilhen H, Magnin B, Bommelaer G, Pereira B, Hordonneau C, Buisson A. Faecal calprotectin and magnetic resonance imaging in detecting Crohn’s disease endoscopic postoperative recurrence. World J Gastroenterol 2018; 24(5): 641-650
- URL: https://www.wjgnet.com/1007-9327/full/v24/i5/641.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i5.641
Crohn’s disease (CD) is a chronic, progressive and disabling inflammatory disorder, which can highly impacts the patients’ quality of life[1-3]. The natural course of CD can lead to bowel damages such as strictures or fistulas requiring surgical management[1-3]. Despite an increased use of biologics, surgery is still warranted in half of the patients within ten years after diagnosis[1]. As intestinal resection is not curative, postoperative recurrence (POR) remains a key issue in the management of patients with CD. Up to 75% of the patients experienced endoscopic POR within the first year after surgery in referral centers[4]. More than 25 years ago, Rutgeerts and colleagues demonstrated that the postoperative course of CD is very heterogeneous[5]. They proposed a stratification of the patients according to the early endoscopic findings within the first year after the surgery, namely the Rutgeerts’ index, to predict the risk of clinical postoperative recurrence[5]. Recently, the postoperative Crohn’s endoscopic recurrence (POCER) trial confirmed previous retrospective data suggesting that an endoscopy-based strategy with a therapeutic step-up according to the Rutgeerts’ index decreased the risk of clinical POR in CD patients[6-10]. Even though the best threshold to define endoscopic POR using this index is still debated[11-13], performing an endoscopy is now recommended for all the patients with CD within the first year after intestinal resection[14]. However, colonoscopy remains a burdensome procedure for the patients owing to the bowel cleansing, the general anesthesia and the risk of complications[15,16] highlighting the need to develop more convenient tools.
In this context, magnetic resonance imaging (MRI) is more accepted than endoscopy and has shown a reliable accuracy to detect endoscopic activity in CD patients[17-21]. The magnetic resonance index of activity (MaRIA)[17,18,22] and the Clermont score[20,21,23-25] are the two main MRI scores that have been validated compared to endoscopy in CD. These two scores demonstrated high performances to grade CD severity and to evaluate mucosal healing[19,26]. However, the MaRIA and the Clermont score have not been investigated so far in the early postoperative course of patients with CD. Only one Austrian group has hitherto proposed an index, so-called the MR scoring system, to detect endoscopic POR. The authors observed promising results compared to the Rutgeerts’ index in predicting the risk of clinical POR in patients with CD[27,28].
Another alternative could be the use of faecal calprotectin measurement to predict the risk of clinical POR. In the last decade, faecal calprotectin demonstrated very reliable performances to diagnose CD, to assess disease activity and to predict clinical relapse[29-40]. Recently, a few works reported convincing results on the use of faecal calprotectin in the early postoperative phase in CD patients[41-44].
In the present study, we aimed to assess the performances of MRI and faecal calprotectin to detect endoscopic POR within the first year following surgery in patients with CD.
The study was performed in accordance with the Declaration of Helsinki, Good Clinical Practice and applicable regulatory requirements. Informed consent was obtained from each patient included in the study. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) as reflected in a priori approval by the institution’s human research committee. The study was approved by local Ethics Committee (#2014/CE 42).
All the patients with CD older than 18 years-old, who underwent a CD-related ileocolonic resection, were consecutively enrolled in this prospective study. They were included from two tertiary centers between January 2014 and December 2016. An endoscopic evaluation was performed for each patient within the first year after surgery (or after restoration of intestinal continuity for the patients with temporary ostomy). The anastomosis was reached by the colonoscope for all the patients. Patients’ demographics and clinical activity were collected the same day. We used the Crohn’s disease activity index (CDAI)[45] to grade disease activity. Blood samples were taken prior to the endoscopy to measure high sensitive serum C-reactive protein (CRP) level by immuno-nephelometric method (Vista, Siemens, Berlin, Germany). Stools were collected in the morning the day before the endoscopy. All the patients were also evaluated by magnetic resonance imaging with diffusion-weighted sequences. Colonoscopy and MRI had to be performed within one month. The choice of medication to prevent postoperative recurrence was free and depended on the physician’s decision.
Stools were collected in the morning the day before the endoscopy to reduce intra-individual variation, and immediately stored at 4 °C. The bowel cleansing was started in all patients after stool collection. Patients were instructed to transport the stool samples in a dedicated container at 4 °C. Faecal samples were immediately transferred, upon patient arrival, to the local Biochemistry Laboratory. Stool cultures were performed on all inflammatory bowel disease (IBD) samples to exclude gastrointestinal infection. Calprotectin was measured using quantitative immunochromatographic test Quantum Blue High Range (Bühlmann Laboratories AG, Schönenbuch, Switzerland), according to the manufacturer’s instructions. All the biochemistry tests were done by individuals blinded from clinical, endoscopic and radiological data.
After bowel cleansing, endoscopy was performed under anaesthesia with propofol (PROPOFOL DAKOTA PHARM; Sanofi-Aventis, Paris, France). All colonoscopies were performed by experienced IBD endoscopists in each center using column video colonoscopy (QFC L 140; Olympus, Tokyo, Japan). The endoscopists were blinded from biochemistry and MRI data. The endoscopic lesions were graded using the Rutgeerts’ index[5] as routinely used in the two IBD units during the postoperative phase. We defined endoscopic POR as Rutgeerts’ index ≥ i2b. However, we performed also sensitivity analyses using different cut-off values such as Rutgeerts’ index ≥ i2 or ≥ i3.
On the day of MRI, patients had to have been fasting for at least four h before the examination. An oral ingestion of 500 mL to 1000 mL of PEG (Fortrans®, Ipsen Pharma, Paris, France) was used to achieve an adequate intestinal distension.The MRI imaging examinations with no bowel cleansing the day before the examination and with no colonic distension (no enema) were performed as previously described[20,21,23,24] with a 1.5 Tesla GE Optima MR 450w (General Electric HealthCare, Fairfield, CT) in Clermont-Ferrand, France, and with a 1.5 Tesla Avanto MRC1 (Siemens, Erlangen, Germany) in Reims, France.
Each examination was interpreted independently by two radiologists: one experienced IBD radiologist (CH) and one junior (PB)[20,21,23,24] who were blinded from endoscopy and biochemistry data. The analyses were focused on the perianastomotic area. The following characteristics were collected: oedema, ulcers, bowel wall thickening (mm), adjacent enlarged lymph nodes (> 8 mm in shortest diameter), comb sign, penetrating complications (fistula, abscess, phlegmon), and Relative Contrast Enhancement (RCE)[17]. For quantitative assessment, the apparent diffusion coefficient (ADC) was calculated on the ADC map independently in separate sessions by the two radiologists in the peri-anastomotic area or in the area of highest signal intensity in the bowel wall. As previously published, the definition of this area was based on the judgment of the radiologist [20,21,23-25]. MR scoring system was defined as MR0 (no abnormal features), MR1 (minimal mucosal changes), MR2 (diffuse aphtoid ileitis, moderate recurrence), and MR3 (severe recurrence with trans- and extramural changes)[27,28]. The Clermont score[20,21,23-25] was calculated using the following formula: 1.646 × bowel thickness - 1.321 × ADC + 5.613 × oedema + 8.306 × ulcers + 5.039. The MaRIA was calculated using the following formula for each of the five segments: MaRIA = 1.5 × wall thickening (mm) + 0.02 × RCE + 5 × edema + 10 × ulceration[17,18,22].
Study data were collected and managed using REDCap electronic data capture tools hosted at Clermont-Ferrand University Hospital[46]. REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources.
Statistical analyses were performed using Stata software (version 13, StataCorp, College Station, United States). The tests were two-sided, with a type I error set at α = 0.05. Baseline characteristics were presented as mean ± SD or median (interquartile range) according to statistical distribution (assumption of normality assessed using the Shapiro-Wilk test) for continuous data and as the number of patients and associated percentages for categorical parameters. Comparisons of patients’ characteristics between the independent groups (i.e., Rutgeerts’ index) were performed using chi-squared or Fisher’s exact tests for categorical variables, and Student’s t-test or Mann-Whitney test if assumptions of t-test were not met (1) normality, and (2) assumption of homoscedasticity studied using Fisher-Snedecor test) for quantitative parameters. Receiver operating characteristic (ROC) curves were used to determine the best biomarker to predict Rutgeerts’ index. The best thresholds were determined according to biological relevance and to usually recommended indices reported in literature (Youden, Liu and efficiency). Sensitivity (se), specificity (spe) and negative (NPV) and positive predictive values (PPV) were presented with 95% confidence intervals. Concordance has been studied using kappa coefficient and accuracy for categorical parameters. Kappa values were studied in relation to usual recommendations: < 0.2 (negligible), 0.2-0.4 (low/weak consistency), 0.4-0.6 (moderate agreement), 0.6-0.8 (substantial/good agreement) and > 0.8 (excellent agreement). For quantitative parameters, the concordance was studied using correlation coefficient, Lin’s concordance coefficient and Bland and Altman graph[47].
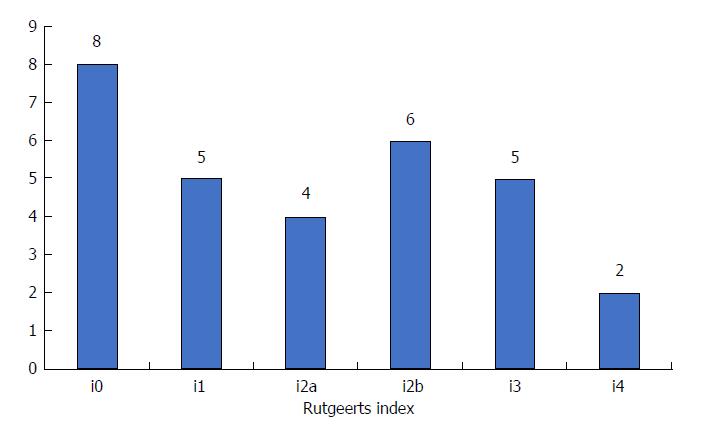
Overall, 30 CD patients were enrolled in this study. The main characteristics of these patients are provided in Table 1. Among them, half of the patients (15/30) were female and 7 (23.3%) were active smokers. Mean age and mean disease duration at the time of inclusion were 34.9 ± 14.1 years and 9.0 ± 9.5 years, respectively. Fifteen patients presented with pure ileal involvement (L1 according to Montreal classification), only one patient (3.3%) had pure colonic location (L2 according to Montreal classification) and 14 patients had ileocolonic CD (46.7%). Six patients experienced perianal lesions (21.4%). In the current study, the patients were treated with no medication (20.0%), 5-ASA (6.7%), thiopurines (56.7%), anti-TNF agents (20.0%) or several of these drugs for preventing endoscopic POR. The distribution of the endoscopic findings according to the Rutgeerts’ index within the first year of surgery is provided in Figure 1.
| Patients’ characteristics | Value |
| Age at diagnosis, (yr), mean ± SD | 34.9 ± 14.1 |
| Disease duration, (yr), mean ± SD | 9.0 ± 9.5 |
| Female gender | 15 (50.0) |
| Active smokers | 7 (23.3) |
| Montreal classification | |
| CD location | |
| L1 | 15 (50.0) |
| L2 | 1 (3.3) |
| L3 | 14 (46.7) |
| CD behaviour | |
| B1 | 1 (3.3) |
| B2 | 16 (53.3) |
| B3 | 13(43.3) |
| Perianal lesions | 7 (23.3) |
| Therapy to prevent endoscopic POR1 | |
| None | 6 (20.0) |
| 5-ASA | 2 (6.7) |
| Thiopurines | 17 (56.7) |
| Anti-TNF agent | 6 (20.0) |
| CDAI, median (interquartile range) | 78 (26-86) |
| CRP, median (interquartile range) | 2.9 (2.9-3.9) |
| Faecal Calprotectin (μg/g), median (IQR) | 100 (100-136) |
The median interval between surgery and endoscopy was 6 mo (5.0-9.3) The median interval between MRI and endoscopy was 14 d (6.5-31). We compared all the MRI parameters in patients with or without endoscopic POR. We did not observe any ulceration among the 30 MRI. The bowel wall was not significantly thickened in the patients with endoscopic POR (3.59 ± 1.69 mm vs 2.83 ± 1.55 mm, P = 0.26). The detection of oedema was not significantly associated with the occurrence of endoscopic POR (38.5% vs 11.8%, P = 0.19). The value of ADC was lower in the patients with endoscopic POR compared to those with no recurrence (2.03 ± 0.32 vs 2.27 ± 0.38 × 10-3 mm²/s, P = 0.032). The Clermont score (10.4 ± 5.8 vs 7.4 ± 4.5, P = 0.038) and the RCE (129.4% ± 62.8% vs 76.4% ± 32.6%, P = 0.007) were increased in the patients with endoscopic POR. In contrast, MaRIA 7.3 ± 4.5 vs 4.8 ± 3.7; P = 0.15) was not significantly higher in the patients with endoscopic POR. The proportion of patients with endoscopic POR was 30.0%, 71.4% and 66.7% in the patients with MR scoring system MR0, MR1 and MR2, respectively (P = 0.11). However, the proportion of patients with endoscopic POR seemed to be higher in the patients with MR scoring system MR1 and MR2 vs MR0 (53.8% vs 17.6%, P = 0.056).
Using ROC curves, we determined that a value of ADC < 2.35 × 10-3 mm²/s [Se = 0.85 (0.38-0.86), Spe = 0.65 (0.55-0.98), PPV = 0.85 (0.55-0.98), NPV = 0.65 (0.38-0.86)] and a value of RCE > 100% [Se = 0.75 (0.46-0.91), Spe = 0.81 (0.56-0.91), PPV = 0.75 (0.46-0.91), NPV = 0.81 (0.56-0.91)] were the best cut-off values to identify endoscopic POR (Figure 2). We showed also that a Clermont score > 6.4 [Se = 0.61 (0.35-0.82), Spe = 0.82 (0.58-0.94), PPV = 0.73 (0.47-0.95), NPV = 0.74 (0.46-0.95)] and a MaRIA > 3.76 [Se = 0.61 (0.35-0.82), Spe = 0.82 (0.58-0.94), PPV = 0.73 (0.46-0.98), NPV = 0.74 (0.46-0.96)] demonstrated interesting performances to detect endoscopic POR (Figure 2). In addition, a MR scoring system ≥ MR1 showed the following performances to detect endoscopic POR: Se = 0.54 (0.29-0.77), Spe = 0.82 (0.58-0.94), PPV = 0.70 (0.42-0.98), and NPV = 0.70 (0.50-0.90) (Figure 2).
The CDAI was not significantly higher in patients with endoscopic POR [median values = 80 (51-86) vs 62 (10-85); P = 0.17] and the performances of CDAI > 150 to detect endoscopic POR were: Se = 0.08 (0.02-0.36), Spe = 0.94 (0.70-1.00), PPV 0.50 (0.01-0.99) and NPV = 0.56 (0.35-0.75) (Figure 2).
The CRP value was not significantly higher in patients with endoscopic POR ( 6.2 ± 7.8 vs 3.2 ± 1.6; P = 0.43). Using a ROC curve, we observed the following performances of CRP level above 5 g/L: Se = 0.31 (0.09-0.61), Spe = 0.88 (0.62-0.98), PPV = 0.67 (0.22-0.96), NPV = 0.61 (0.39-0.80).
Faecal calprotectin values were significantly higher in patients with endoscopic POR (354.8 ± 432.5 μg/g vs 114 ± 54.5 μg/g; P = 0.0075) (Figure 2). Using a ROC curve, we found that the cut-off value of faecal calprotectin > 100 μg/g demonstrated high performances to detect endoscopic POR [Se = 0.67 (0.39-0.86), Spe = 0.93 (0.66-1.00), PPV = 0.89 (0.68-1.00) and NPV = 0.77 (0.56-0.97) and accuracy 0.80 (0.66-0.96)] (Figure 2). The exclusive measure of faecal calprotectin level in the postoperative setting would have been able to adequately stratify patients as having no sign of endoscopic POR in most of the patients and therefore might allow in our cohort avoiding 13 colonoscopies i.e. 43.3% of the total number (13 true negative patients). However, it would have missed 4 false negative patients (13.3%).
We did not observe any improvement of performances to detect endoscopic POR with concomitant or successive use of MRI and faecal calprotectin (data not shown).
We performed the same investigations in using different cut-off value of Rutgeerts’ index ≥ i2 or ≥ i3. These results are detailed in Supplementary Tables 1 and 2.
The inter-observer agreement between the two radiologists was 96.7% for the MR scoring system with a κ-value of 0.933 ± 0.139. We observed substantial concordances (Lin’s concordance coefficient with 95% confidence interval) between the two readers regarding bowel thickness [0.97 (0.94-0.99)], ADC [0.71 (0.53-0.89)], RCE [0.73 (0.55-0.91)], Clermont score [0.990 (0.98-1.00)], and MaRIA [0.99 (0.98-1.00)]. We calculated the median relative variation of ADC [6.5% (5.1-11.0)], RCE [15.0% (8.8-25.5)], Clermont score [9.5% (4.5-9.5)], and MaRIA [9.6% (5.3-13.6)]. The inter-reader agreement was 96.7% using a cut-off value of MaRIA of 3.76 (κ = 0.92 ± 0.18) and 93.3% using a threshold of Clemont score above 6.4 (κ = 0.86 ± 0.18) (no significant difference between the two scores).
In this prospective study, we showed, for the first time in the same cohort that faecal calprotectin and MRI are two reliable alternative tools to detect endoscopic POR with CD. In addition, MRI parameters including Clermont score, MaRIA and MR scoring system demonstrated substantial inter-reader agreement.
As surgery is not curative in CD and the rate of endoscopic POR can reach more than 75% within the first year after the surgery in some referral centers[4], the prevention of POR remains a major concern for the IBD physicians. Recently, the data retrieved from the POCER trial confirmed the positive impact of a tailored therapeutic management based on the early endoscopic findings (within the first year following the intestinal resection) compared to a monitoring based on clinical activity[6]. A lower rate of endoscopic POR at 18 mo was observed in the active care arm compared to the control group (49% vs 67%, P = 0.03)[6]. Then, performing an endoscopic evaluation within the first week following the surgery (or the restoration of intestinal continuity) is recommended in patients with CD. However, the colonoscopy is felt as a burdensome procedure by most of the patients leading some of them to deny performing this examination. This observation highlights the need to develop more convenient tools for these patients. A nationwide survey including 916 IBD patients recently reported that stools collection for faecal biomarkers and MRI were considered as more acceptable than colonoscopy by CD patients[15]. Accordingly, investigating the performances of faecal calprotectin and MRI to detect endoscopic POR seemed highly relevant.
The early endoscopic evaluation is performed using the Rutgeerts’ index[5]. The usual definition of endoscopic POR is a Rutgeerts’ index ≥ i2 as the likelihood of reappearance of symptoms in the five years following the surgery was 40% in i2-patients and more than 75% in i3- or i4-patients compared to less than 15% in patients with Rutgeerts’ index ≤ i1[5]. However, the debate is currently growing regarding this definition owing to the heterogeneity of the i2-subgroup encompassing several conditions such as more than five aphthous lesions with normal mucosa between the lesions, or skip areas of larger lesions or ulcers up to 1 cm confined to ileocolonic anastomosis. The characterization of the lesions confined to the anastomosis is a problem in daily practice as it is sometimes difficult to exclude alternative diagnosis such as post-surgical or ischemic consequences. Then, the i2-group is now divided into two subgroups i.e. i2a (lesions confined to the anastomosis) and i2b (more than 5 aphthous lesions with normal mucosa between the lesions, or skip areas of larger lesions). Two teams attempted recently to show a different course of the disease between these two subgroups (i2a vs i2b) but failed to do so[11,12]. Unfortunately, this question should be very difficult to figure out as a step-up therapeutic strategy is performed in almost all the patients with endoscopic lesions classified as i2a and i2b. Consequently, investigating the natural history of this subgroup of patients will be probably no longer possible. Although it is still matter of debate, we decided to define endoscopic POR as a Rutgeerts’ index ≥ i2b but we performed a sensitivity analysis using other thresholds including Rutgeerts’ index ≥ i2.
To date, only one team studied the potential role of MRI to replace early endoscopic evaluation in CD patients who underwent ileocolonic resection[27,28]. They arbitrarily created the MR scoring system dedicated to the postoperative phase as: MR0 (no abnormal features), MR1 (minimal mucosal changes), MR2 (diffuse aphtoid ileitis, moderate recurrence), and MR3 (severe recurrence with trans- and extramural changes)[28]. The authors observed a good interobserver agreement between the MR scoring system and the Rutgeerts’ index (agreement rate = 77.8% and κ = 0.67). AMR scoring system ≥ MR2 showed a trend to be predictive of a higher risk of clinical POR (P = 0.09)[27]. In our cohort, the MR scoring system demonstrated a clear trend to be able to detect the occurrence of endoscopic POR (53.8% vs 17.6%, P = 0.056). However, we did not find the same threshold (≥ MR1 rather than ≥ MR2). It could be partly explained by the very high level of severe endoscopic POR in the Austrian cohort i.e. 5 patients with i3 (16.7%) and 14 patients with i4 (46.7%) contrary to ours: 5 patients with i3 (16.7%) and 2 patients with i4 (6.7%). In our study, we investigated, for the first time, the potential role of each individual MRI item to detect endoscopic POR. We observed that the bowel thickness was not a reliable parameter to distinguish patients with or without endoscopic POR, which is not surprising, as a mildly thickened bowel wall can be seen in CD patients after surgery even in the absence of endoscopic recurrence. We also observed that the presence of oedema was not significantly different between the two groups (38.5% vs 11.8%, P = 0.19). Finally, we did not observe any severe lesions on MRI such as ulcerations, fistula or stenosis, which is in line with the early stage of the disease within the first year following the surgery.
The MaRIA[17-19,22] and the Clermont score[19-21,23,24,48] are the two available MRI scores validated against endoscopy to assess ileocolonic activity in patients with CD. We investigated the performances of these two validated scores to detect endoscopic POR. We observed substantial positive (0.73 for both) and negative predictive values (0.74 for both) of these two scores to identify POR. The cut-off values were lower than those usually used to detect inflammatory activity in CD[18,21]. It is consistent with the fact that the early postoperative lesions are mostly mild and limited to the mucosa. The most sensitive MRI features to detect these minimal shifts are the quantitative parameters assessing the degree of inflammation such as the RCE (for the injected sequences) and the ADC (for the diffusion-weighted sequences), which showed substantial accuracy to detect endoscopic POR in our cohort. However, the impact of these quantitative parameters among the calculation of the MaRIA and the Clermont score remains limited. Even though RCE and ADC demonstrated high inter-reader agreement[49], these items could be two equipment-dependent metric values, which could be difficult to use alone despite their substantial performances to detect endoscopic POR.
Faecal calprotectin is hitherto the most effective faecal biomarker to assess endoscopic activity in patients with CD[29-40]. Recently, a French prospective study and a post-hoc analysis of the POCER trial reported that a level of faecal calprotectin > 100 μg/g was the best threshold to detect endoscopic POR (Rutgeerts’ index ≥ i2) with high negative predictive value between 91 and 93%[42,43]. Then, the authors calculated that they could avoid from 30% to 47% of unnecessary colonoscopies[42,43]. In our cohort, we also identified a level of faecal calprotectin > 100 μg/g as the best cut-off value to show an endoscopic POR (Rutgeerts’ index ≥ i2b). Contrary to the previous study, we observed a lower negative predictive value (77%) but a higher positive predictive value (89%). The lower level of NPV is consistent with the data from a secondary analysis of the TOPPIC trial[50] (88 patients) reporting a NPV of 75% even though they observed in the same time a low PPV (58%). Concerning the high PPV, it could be partly explained by the different assays used across the studies and should be confirmed in other larger independent cohorts. In our cohort, the use of faecal calprotectin would have avoided 43.3% of unnecessary colonoscopy (13 true negative patients/30) but would have missed 13.3% of recurrences (4 false negative patients /30). However, we did not observe any severe endoscopic POR (i3 or i4) among these 4 patients. Finally, we confirmed that the CDAI and the CRP level are not accurate enough to monitor patients with CD within the first months after the surgery.
In the same cohort, we investigated the performances of faecal calprotectin and MRI. Our study was not powered to directly compare these two potential alternatives to colonoscopy and then we did not observe any significant difference between these two tools. However, we observed numerically a mild trend favoring faecal calprotectin. Of course, this result has to be confirmed in independent larger cohorts before drawing any conclusion.
The main strengths of our study included its prospective design with concomitant evaluation of faecal biomarker and MRI. In addition, we investigated the potential impact of each MRI parameter including the two most validated scores i.e., the MaRIA and the Clermont score. The main limitation of this study is the sample size even though our sample size calculation was based on the two prior Austrian studies[27,28] and then remains one of the larger published so far on the role of MRI in the postoperative CD.
Faecal calprotectin and MRI are two reliable options to detect endoscopic POR within the first year after ileocolonic resection in patients with CD and could be used as a more convenient tool than colonoscopy.
Surgical resection is unfortunately not curative in Crohn’s disease (CD), and postoperative recurrence (POR) remains a crucial issue in these patients. Performing an endoscopy within the first year after surgery is recommended in clinical practice. However, colonoscopy remains a burdensome procedure for the patients highlighting the need to develop more convenient tools. In this context, MRI and faecal calprotectin are more accepted than endoscopy and have shown a reliable accuracy to detect endoscopic activity in patients with CD. MRI scores such as the magnetic resonance index of activity (MaRIA) and the Clermont score and faecal calprotectin could then be used as an alternative to detect endoscopic POR but their performances remains poorly investigated.
Developping more convenient tools to detect early postoperative recurrence, is a key point in patients with Crohn’s disease.
In this study, we assessed the performances of MRI and faecal calprotectin to detect endoscopic postoperative recurrence in patients with Crohn’s disease.
It was a multicentre prospective observational study.
ADC < 2.35 × 10-3 mm²/s (sensitivity = 0.85, specificity = 0.65, positive predictive value (PPV) = 0.85, negative predictive value (NPV) = 0.65) and RCE > 100% (sensitivity = 0.75, specificity = 0.81, PPV = 0.75, NPV = 0.81) were the best cut-off values to identify endoscopic POR. Clermont score > 6.4 (sensitivity = 0.61, specificity = 0.82, PPV = 0.73, NPV = 0.74), MaRIA > 3.76 (sensitivity = 0.61, specificity = 0.82, PPV = 0.73, NPV = 0.74) and a MR scoring system ≥ MR1 (sensitivity = 0.54, specificity = 0.82, PPV = 0.70, and NPV = 0.70) demonstrated interesting performances to detect endoscopic POR. Faecal calprotectin > 100 μg/g demonstrated high performances to detect endoscopic POR (sensitivity = 0.67, specificity = 0.93, PPV = 0.89 and NPV = 0.77).
Faecal calprotectin and MRI are two reliable options to detect endoscopic POR within the first year after ileocolonic resection in patients with CD and could be used as a more convenient tool than colonoscopy.
Additional studies from independent cohorts should be conducted to confirm these data.
| 1. | Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 793] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 2. | Peyrin-Biroulet L, Cieza A, Sandborn WJ, Coenen M, Chowers Y, Hibi T, Kostanjsek N, Stucki G, Colombel JF; International Programme to Develop New Indexes for Crohn’s Disease (IPNIC) group. Development of the first disability index for inflammatory bowel disease based on the international classification of functioning, disability and health. Gut. 2012;61:241-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 279] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 3. | Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1601] [Article Influence: 106.7] [Reference Citation Analysis (3)] |
| 4. | Buisson A, Chevaux JB, Allen PB, Bommelaer G, Peyrin-Biroulet L. Review article: the natural history of postoperative Crohn’s disease recurrence. Aliment Pharmacol Ther. 2012;35:625-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 282] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 5. | Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990;99:956-963. [PubMed] |
| 6. | De Cruz P, Kamm MA, Hamilton AL, Ritchie KJ, Krejany EO, Gorelik A, Liew D, Prideaux L, Lawrance IC, Andrews JM. Crohn’s disease management after intestinal resection: a randomised trial. Lancet. 2015;385:1406-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 467] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 7. | De Cruz P, Bernardi MP, Kamm MA, Allen PB, Prideaux L, Williams J, Johnston MJ, Keck J, Brouwer R, Heriot A. Postoperative recurrence of Crohn’s disease: impact of endoscopic monitoring and treatment step-up. Colorectal Dis. 2013;15:187-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Baudry C, Pariente B, Lourenço N, Simon M, Chirica M, Cattan P, Munoz-Bongrand N, Gornet JM, Allez M. Tailored treatment according to early post-surgery colonoscopy reduces clinical recurrence in Crohn’s disease: a retrospective study. Dig Liver Dis. 2014;46:887-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Boucher AL, Pereira B, Decousus S, Goutte M, Goutorbe F, Dubois A, Gagniere J, Borderon C, Joubert J, Pezet D. Endoscopy-based management decreases the risk of postoperative recurrences in Crohn’s disease. World J Gastroenterol. 2016;22:5068-5078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Bordeianou L, Stein SL, Ho VP, Dursun A, Sands BE, Korzenik JR, Hodin RA. Immediate versus tailored prophylaxis to prevent symptomatic recurrences after surgery for ileocecal Crohn’s disease? Surgery. 2011;149:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Bayart P, Duveau N, Nachury M, Zerbib P, Gerard R, Branche J, Maunoury V, Wils P, Boruchowicz A, Boualit M. Ileal or Anastomotic Location of Lesions Does Not Impact Rate of Postoperative Recurrence in Crohn’s Disease Patients Classified i2 on the Rutgeerts Score. Dig Dis Sci. 2016;61:2986-2992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Rivière P, Vermeire S, Van Assche GA, Rutgeerts P, van Overstraeten A de B, D’Hoore A, Ferrante M. The Modified Postoperative Endoscopic Recurrence Score for Crohn’s Disease: Does it Really Make a Difference in Predicting Clinical Recurrence? Gastroenterology. 2017;152:S376. [DOI] [Full Text] |
| 13. | Lemmens B, de Buck van Overstraeten A, Arijs I, Sagaert X, Van Assche G, Vermeire S, Tertychnyy A, Geboes K, Wolthuis A, D’Hoore A. Submucosal Plexitis as a Predictive Factor for Postoperative Endoscopic Recurrence in Patients with Crohn’s Disease Undergoing a Resection with Ileocolonic Anastomosis: Results from a Prospective Single-centre Study. J Crohns Colitis. 2017;11:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Dignass A, Lindsay JO, Sturm A, Windsor A, Colombel JF, Allez M, D’Haens G, D’Hoore A, Mantzaris G, Novacek G. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6:991-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 708] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 15. | Buisson A, Gonzalez F, Poullenot F, Nancey S, Sollellis E, Fumery M, Pariente B, Flamant M, Trang-Poisson C, Bonnaud G. Comparative Acceptability and Perceived Clinical Utility of Monitoring Tools: A Nationwide Survey of Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017;23:1425-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 189] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 16. | Buisson A, Chevaux JB, Hudziak H, Bresler L, Bigard MA, Peyrin-Biroulet L. Colonoscopic perforations in inflammatory bowel disease: a retrospective study in a French referral centre. Dig Liver Dis. 2013;45:569-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Rimola J, Rodriguez S, García-Bosch O, Ordás I, Ayala E, Aceituno M, Pellisé M, Ayuso C, Ricart E, Donoso L. Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut. 2009;58:1113-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 527] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 18. | Rimola J, Ordás I, Rodriguez S, García-Bosch O, Aceituno M, Llach J, Ayuso C, Ricart E, Panés J. Magnetic resonance imaging for evaluation of Crohn’s disease: validation of parameters of severity and quantitative index of activity. Inflamm Bowel Dis. 2011;17:1759-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 404] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 19. | Rimola J, Alvarez-Cofiño A, Pérez-Jeldres T, Ayuso C, Alfaro I, Rodríguez S, Ricart E, Ordás I, Panés J. Comparison of three magnetic resonance enterography indices for grading activity in Crohn’s disease. J Gastroenterol. 2017;52:585-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 20. | Hordonneau C, Buisson A, Scanzi J, Goutorbe F, Pereira B, Borderon C, Da Ines D, Montoriol PF, Garcier JM, Boyer L. Diffusion-weighted magnetic resonance imaging in ileocolonic Crohn’s disease: validation of quantitative index of activity. Am J Gastroenterol. 2014;109:89-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 175] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 21. | Buisson A, Hordonneau C, Goutte M, Boyer L, Pereira B, Bommelaer G. Diffusion-weighted magnetic resonance imaging is effective to detect ileocolonic ulcerations in Crohn’s disease. Aliment Pharmacol Ther. 2015;42:452-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Ordás I, Rimola J, Rodríguez S, Paredes JM, Martínez-Pérez MJ, Blanc E, Arévalo JA, Aduna M, Andreu M, Radosevic A. Accuracy of magnetic resonance enterography in assessing response to therapy and mucosal healing in patients with Crohn’s disease. Gastroenterology. 2014;146:374-382.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 298] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 23. | Buisson A, Joubert A, Montoriol PF, Da Ines D, Hordonneau C, Pereira B, Garcier JM, Bommelaer G, Petitcolin V. Diffusion-weighted magnetic resonance imaging for detecting and assessing ileal inflammation in Crohn’s disease. Aliment Pharmacol Ther. 2013;37:537-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 24. | Buisson A, Hordonneau C, Goutte M, Scanzi J, Goutorbe F, Klotz T, Boyer L, Pereira B, Bommelaer G. Diffusion-weighted magnetic resonance enterocolonography in predicting remission after anti-TNF induction therapy in Crohn’s disease. Dig Liver Dis. 2016;48:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Caruso A, D’Incà R, Scarpa M, Manfrin P, Rudatis M, Pozza A, Angriman I, Buda A, Sturniolo GC, Lacognata C. Diffusion-weighted magnetic resonance for assessing ileal Crohn’s disease activity. Inflamm Bowel Dis. 2014;20:1575-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Buisson A, Pereira B, Goutte M, Reymond M, Allimant C, Bommelaer G, Hordonneau C. Magnetic Resonance Index of Activity (MaRIA) and Clermont Score are Two MRI Indices Which are Highly and Equally Effective in Detecting Mucosal Healing in Crohn’s Disease. Gastroenterology. 2017;152:S210. [DOI] [Full Text] |
| 27. | Koilakou S, Sailer J, Peloschek P, Ferlitsch A, Vogelsang H, Miehsler W, Fletcher J, Turetschek K, Schima W, Reinisch W. Endoscopy and MR enteroclysis: equivalent tools in predicting clinical recurrence in patients with Crohn’s disease after ileocolic resection. Inflamm Bowel Dis. 2010;16:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 28. | Sailer J, Peloschek P, Reinisch W, Vogelsang H, Turetschek K, Schima W. Anastomotic recurrence of Crohn’s disease after ileocolic resection: comparison of MR enteroclysis with endoscopy. Eur Radiol. 2008;18:2512-2521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 29. | Tibble J, Teahon K, Thjodleifsson B, Roseth A, Sigthorsson G, Bridger S, Foster R, Sherwood R, Fagerhol M, Bjarnason I. A simple method for assessing intestinal inflammation in Crohn’s disease. Gut. 2000;47:506-513. [PubMed] |
| 30. | af Björkesten CG, Nieminen U, Turunen U, Arkkila P, Sipponen T, Färkkilä M. Surrogate markers and clinical indices, alone or combined, as indicators for endoscopic remission in anti-TNF-treated luminal Crohn’s disease. Scand J Gastroenterol. 2012;47:528-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 31. | D’Haens G, Ferrante M, Vermeire S, Baert F, Noman M, Moortgat L, Geens P, Iwens D, Aerden I, Van Assche G. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2218-2224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 643] [Article Influence: 45.9] [Reference Citation Analysis (2)] |
| 32. | D’Incà R, Dal Pont E, Di Leo V, Ferronato A, Fries W, Vettorato MG, Martines D, Sturniolo GC. Calprotectin and lactoferrin in the assessment of intestinal inflammation and organic disease. Int J Colorectal Dis. 2007;22:429-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 33. | Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008;103:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 448] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 34. | Nancey S, Boschetti G, Moussata D, Cotte E, Peyras J, Cuerq C, Haybrard J, Charlois AL, Mialon A, Chauvenet M. Neopterin is a novel reliable fecal marker as accurate as calprotectin for predicting endoscopic disease activity in patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2013;19:1043-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 35. | Schoepfer AM, Beglinger C, Straumann A, Trummler M, Vavricka SR, Bruegger LE, Seibold F. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn’s disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol. 2010;105:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 430] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 36. | Sipponen T, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008;14:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 371] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 37. | Goutorbe F, Goutte M, Minet-Quinard R, Boucher AL, Pereira B, Bommelaer G, Buisson A. Endoscopic Factors Influencing Fecal Calprotectin Value in Crohn’s Disease. J Crohns Colitis. 2015;9:1113-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Buisson A, Vazeille E, Minet-Quinard R, Goutte M, Bouvier D, Goutorbe F, Pereira B, Barnich N, Bommelaer G. Faecal chitinase 3-like 1 is a reliable marker as accurate as faecal calprotectin in detecting endoscopic activity in adult patients with inflammatory bowel diseases. Aliment Pharmacol Ther. 2016;43:1069-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 39. | Costa F, Mumolo MG, Ceccarelli L, Bellini M, Romano MR, Sterpi C, Ricchiuti A, Marchi S, Bottai M. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut. 2005;54:364-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 426] [Article Influence: 20.3] [Reference Citation Analysis (1)] |
| 40. | Molander P, Färkkilä M, Ristimäki A, Salminen K, Kemppainen H, Blomster T, Koskela R, Jussila A, Rautiainen H, Nissinen M. Does fecal calprotectin predict short-term relapse after stopping TNFα-blocking agents in inflammatory bowel disease patients in deep remission? J Crohns Colitis. 2015;9:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 41. | Sorrentino D, Terrosu G, Paviotti A, Geraci M, Avellini C, Zoli G, Fries W, Danese S, Occhipinti P, Croatto T. Early diagnosis and treatment of postoperative endoscopic recurrence of Crohn’s disease: partial benefit by infliximab--a pilot study. Dig Dis Sci. 2012;57:1341-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 42. | Boschetti G, Laidet M, Moussata D, Stefanescu C, Roblin X, Phelip G, Cotte E, Passot G, Francois Y, Drai J. Levels of Fecal Calprotectin Are Associated With the Severity of Postoperative Endoscopic Recurrence in Asymptomatic Patients With Crohn’s Disease. Am J Gastroenterol. 2015;110:865-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 43. | Wright EK, Kamm MA, De Cruz P, Hamilton AL, Ritchie KJ, Krejany EO, Leach S, Gorelik A, Liew D, Prideaux L. Measurement of fecal calprotectin improves monitoring and detection of recurrence of Crohn’s disease after surgery. Gastroenterology. 2015;148:938-947.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 232] [Article Influence: 21.1] [Reference Citation Analysis (1)] |
| 44. | Lobatón T, López-García A, Rodríguez-Moranta F, Ruiz A, Rodríguez L, Guardiola J. A new rapid test for fecal calprotectin predicts endoscopic remission and postoperative recurrence in Crohn’s disease. J Crohns Colitis. 2013;7:e641-e651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (2)] |
| 45. | Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439-444. [PubMed] |
| 46. | Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38562] [Cited by in RCA: 40259] [Article Influence: 2368.2] [Reference Citation Analysis (0)] |
| 47. | Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307-310. [PubMed] |
| 48. | Buisson A, Pereira B, Goutte M, Reymond M, Allimant C, Obritin-Guilhen H, Bommelaer G, Hordonneau C. Magnetic resonance index of activity (MaRIA) and Clermont score are highly and equally effective MRI indices in detecting mucosal healing in Crohn’s disease. Dig Liver Dis. 2017;49:1211-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 49. | Buisson A, Hordonneau C, Goutte M, Bommelaer G. What score should be used for evaluation of Crohn’s disease severity using magnetic resonance imaging? J Gastroenterol. 2017;52:652-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 50. | Mowat C, Arnott I, Cahill A, Smith M, Ahmad T, Subramanian S, Travis S, Morris J, Hamlin J, Dhar A. Mercaptopurine versus placebo to prevent recurrence of Crohn’s disease after surgical resection (TOPPIC): a multicentre, double-blind, randomised controlled trial. Lancet Gastroenterol Hepatol. 2016;1:273-282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Eder P S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y