Published online Feb 7, 2018. doi: 10.3748/wjg.v24.i5.593
Peer-review started: August 27, 2017
First decision: September 12, 2017
Revised: September 26, 2017
Accepted: November 21, 2017
Article in press: November 21, 2017
Published online: February 7, 2018
Processing time: 157 Days and 16.1 Hours
To study the role of semaphorin 4D (Sema4D) expression promoted by tumor-associated macrophages (TAMs) in gastric carcinoma cells and its clinical significance in the invasion and metastasis of gastric carcinoma.
CD68 and Sema4D expression was analyzed in gastric carcinoma and adjacent normal tissues from 290 patients using the immunohistochemical streptavidin-peroxidase method, and their relationships with clinicopathological features were evaluated. Human M2 macrophages were induced in vitro and co-cultured in non-contact with gastric carcinoma SGC-7901 cells. Changes in the secretory Sema4D level in the SGC-7901 cell supernatant were measured using an enzyme-linked immunosorbent assay. The effects of TAMs on SGC-7901 cell invasion and migration were assessed with invasion and migration assays, respectively.
CD68 and Sema4D protein expression was significantly higher in gastric carcinoma tissues than in adjacent normal tissues (71.7% vs 33.8% and 74.5% vs 42.8%, respectively; P < 0.01). CD68 and Sema4D protein expression was significantly associated with histological differentiation, TNM stage, and lymph node metastasis (P < 0.05), and their expression levels were positively correlated with one another (r = 0.467, P < 0.01). In the in vitro experiment, secretory Sema4D protein expression was significantly increased in the supernatant of SGC-7901 cells co-cultured with TAMs compared with the blank control (1224.13 ± 29.43 vs 637.15 ± 33.84, P < 0.01). Cell invasion and metastasis were enhanced in the Transwell invasion and migration assays (P < 0.01).
TAMs promote the invasion and metastasis of gastric carcinoma cells possibly through upregulated secretory Sema4D protein expression. Combined detection of TAM markers, CD68 and Sema4D, in gastric carcinoma tissue shows potential to predict the trend of gastric carcinoma progression.
Core tip: This study explored the role and clinical significance of semaphorin 4D (Sema4D) expression promoted by tumor-associated macrophages (TAMs) in gastric carcinoma cells. By using immunohistochemical streptavidin-peroxidase method on tissue species and gastric carcinoma cells in non-contact co-culture with human M2 macrophages in vitro, we found that Sema4D protein expression was significantly higher in gastric carcinoma tissues than in adjacent normal tissues, and TAMs promoted the invasion and metastasis of gastric carcinoma cells possibly through upregulated Sema4D protein expression.
- Citation: Li H, Wang JS, Mu LJ, Shan KS, Li LP, Zhou YB. Promotion of Sema4D expression by tumor-associated macrophages: Significance in gastric carcinoma. World J Gastroenterol 2018; 24(5): 593-601
- URL: https://www.wjgnet.com/1007-9327/full/v24/i5/593.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i5.593
Gastric carcinoma is one of the most common malignancies. The incidence of gastric carcinoma currently ranks fourth in malignant tumors worldwide after only lung cancer, breast cancer, and colon cancer, and the mortality of gastric carcinoma is ranked second among cancers[1,2]. The tumor microenvironment plays a major role in the invasion and metastasis of gastric carcinoma. Tumor-associated macrophages (TAMs) are an important component of the tumor microenvironment. TAMs are recruited in the hypoxic microenvironment of various solid tumors and are involved in multiple steps of tumor progression[3,4]. Macrophages can be divided into two classes according to differences in the activation method, surface markers, and functions as follows: M1 macrophages with classic activation, and M2 macrophages with selective activation. Numerous TAMs are present in the inflammatory environment of tumor and are recognized as M2 macrophages[5]. CD68 is an important marker molecule of TAMs[6]. TAMs have been shown to enhance the infiltration and metastasis ability of tumor cells by expressing certain growth factors and cytokines[7]. TAMs also suppress immune responses in the microenvironment and promote tumor progression by facilitating tumor angiogenesis and lymphangiogenesis[8].
Semaphorin 4D (Sema4D) is an important member of the semaphorin subfamily and plays a major role in the nervous and immune systems. Sema4D is expressed at high levels in various tumor tissues, including head and neck squamous cell carcinoma, prostate cancer, and colon cancer, and its role in promoting tumor angiogenesis is becoming a hot research topic[9-12]. Sema4D is another important proangiogenic factor following vascular endothelial growth factor (VEGF)[13]. In the present study, we examined the expression of the TAM markers CD68 and Sema4D in gastric carcinoma and adjacent normal tissues using immunohistochemical assays and analyzed their clinical significance. We also evaluated Sema4D expression in gastric carcinoma cells and the changes in the cellular invasion and metastasis abilities through co-culture of TAMs and gastric carcinoma cells in vitro. We explored the effect of TAMs on Sema4D expression in gastric carcinoma tissues and its role in the development and progression of gastric carcinoma to provide a new theoretical reference for the prevention and treatment of this cancer.
A total of 290 patients with gastric carcinoma confirmed by a histopathological diagnosis from January to December 2012 with complete medical record data were collected from Shandong Provincial Hospital, the Affiliated Hospital of Qingdao University, and Weifang People’s Hospital in China. None of the patients received radiotherapy or chemotherapy prior to surgery or had a hereditary family medical history. The patients included 158 men and 132 women aged 30 to 81 years (median age, 55 years). Regarding the pathological type, 78 patients had well differentiated adenocarcinoma, 113 patients had moderately differentiated adenocarcinoma, and 99 patients had poorly differentiated adenocarcinoma. Based on the 7th edition of the International Union Against Cancer (UICC) tumor, node, metastasis (TNM) staging system, 105 patients had stage I/II disease, and 185 patients had stage III/IV disease. A total of 195 patients had lymph node metastasis, and 95 patients did not have lymph node metastasis. Normal adjacent tissue was collected at a 3-cm distance from the lesion as the control. Two pathologists determined the pathological type and tumor grade. In case of disagreement in the diagnosis, a third pathologist reviewed the case, and the final diagnosis was made through discussion.
Reagents and methods: The monoclonal rabbit anti-Sema4D antibody, anti-CD68 antibody, and streptavidin-peroxidase (SP) kit were purchased from Abcam, United States. All paraffin-embedded tissue blocks were cut into 4-μm thick continuous sections. Immunohistochemical staining using the SP method was performed following the kit instructions after positioning by hematoxylin and eosin staining. Briefly, the sections were subjected to conventional deparaffinization, 3% H2O2 inactivation of endogenous enzymes, antigen retrieval by heating, and blocking of non-specific staining with rabbit serum. The primary antibody (1:100 dilution), biotin-labeled secondary antibody, and enzyme-labeled streptavidin were successively added, and the sections were incubated at 37 °C for 25 min. After color development with 3,3-diaminobenzidine, the sections were slightly counterstained with hematoxylin, conventionally dehydrated, cleared with xylene, mounted with neutral resin, observed under an inverted microscope, and photographed. The primary antibody was substituted with phosphate-buffered saline (PBS) as a negative control. Known positive sections provided by the reagent company were used as positive controls. Each sample was re-stained once.
Result interpretation: The sections were reviewed according to the criteria of Birner et al[14]. The protein staining intensity and the percentage of positive cells in the total cell count were analyzed semi-quantitatively. The criteria for determining positive expression were as follows: (1) Positive expression was mainly shown as brownish-yellow or brown particles in the cell membrane, cytoplasm, or nucleus. The intensity of positive staining was scored as follows: cells without staining, 0 points; cells stained light yellow, 1 point; cells stained yellow, 2 points; and cells stained brown, 3 points; (2) Five different fields of view were selected at random under a high-resolution electron microscope to count the numbers of total cells and positive cells. Scores were recorded based on the percentage of positive cells as follows: <5% positive cells, 0 points; 5%-25% positive cells, 1 point; 26%-50% positive cells, 2 points; 51%-75% positive cells, 3 points; and > 75% positive cells, 4 points. The final score was the sum of the scores obtained using the above two criteria. A total score of 0-2 points was considered negative expression and 3-7 points was considered positive expression.
The gastric carcinoma SGC-7901 cell line and the human monocyte THP-1 cell line were purchased from the Shanghai Cell Bank, Chinese Academy of Sciences. The monoclonal rabbit anti-CD68 antibody and fluorescein (FITC)-labeled goat anti-rabbit secondary IgG were purchased from Abcam, United States. The Sema4D ELISA kit was purchased from BD, United States. Matrigel was purchased from Sigma, United States. Six-well Transwells with 0.4-μm and 8-μm pore polycarbonate membrane inserts were purchased from Corning, United States. Phorbol ester (PMA), interleukins (IL)-4 and -13, and Giemsa dye were purchased from Zsbio, Beijing, China. RPMI 1640 medium and fetal bovine serum (FBS) were purchased from Gibco, United States.
Conventional cell culture: Gastric carcinoma SGC-7901 cells and human THP-1 mononuclear cells were cultured in RPMI 1640 medium containing 10% deactivated FBS at 37 °C with 5% CO2 and saturated humidity. The cells were passaged every 2-3 d.
In vitro activation of human M2 macrophages: Suspended human THP-1 mononuclear cells were centrifuged and resuspended with RPMI 1640 medium containing 10% FBS. The cells were counted and then seeded at a density of 7.5 × 105 cells/well into 6-well culture plates. Human THP-1 macrophages were treated with 320 nmol/L of PMA for 6 h, followed by 20 ng/mL of IL-4 and 20 ng/mL of IL-13 for 18 h, for a total of 24 h in culture. Thereafter, the medium was aspirated using a pipette. The cells were washed with PBS three times and resuspended in serum-free RPMI 1640 medium.
Immunofluorescence for identification of cells: The medium was aspirated from the 6-well plates used for TAM culture. The cells were fixed with 4% paraformaldehyde at room temperature, clarified with 0.3% Triton X-100, and blocked at room temperature with 1% bovine serum albumin. Subsequently, the cells were incubated with an anti-CD68 monoclonal antibody (1:100 dilution) at 4 °C overnight, followed by incubation with a FITC-labeled secondary antibody at room temperature in the dark. After nuclear staining, the cells were mounted and observed. The primary antibody was substituted with PBS as the negative control.
Invasion assay: The upper compartment of a six-well Transwell chamber was coated with 200 μL of diluted matrigel (matrigel:PBS = 1:9) and incubated in a 37 °C incubator for 30 min. Gastric carcinoma SGC-7901 cells were seeded at a density of 2 × 105 cells/well into the upper compartment of the Transwell chamber (8-µm pore size) and cultured in serum-free RPMI 1640 medium. Tumor-associated M2 macrophages were implanted into the lower compartment as an experimental group, and serum-free RPMI 1640 medium was used as a blank control group. The cells were cultured at 37 °C in a 5% CO2 incubator for 18 h and then fixed and stained. Three replicate wells were set for each group, and the experiment was repeated three times. The cells were continuously counted in five fields of view selected at random under high magnification (200 ×), and the mean number was calculated.
Migration assay: The Transwell did not need to be coated with matrigel. The remaining procedures were identical to those used in the invasion assay.
ELISA assay: Gastric carcinoma SGC-7901 cells were seeded into the lower compartment of a 6-well Transwell chamber (0.4-μm pore size) at a density of 2 × 105 cells/well. Tumor-associated M2 macrophages were implanted into the upper compartment as an experimental group, and serum-free RPMI 1640 medium was used as a blank control group. Three replicate wells were set up for each group. The cells were cultured at 37 °C in a 5% CO2 incubator for 18 h and then fixed and stained. At the end of the culture period, the upper compartment was removed. The SGC-7901 cell supernatant was discarded, and the cells were washed three times with PBS, followed by the addition of fresh medium. The cell supernatants of the two groups were collected 18 h later, centrifuged at 1000 g/min for 15 min, and frozen at -80 °C. The supernatant was thawed in a 37 °C water bath 30 min prior to the ELISA. The procedure followed the instructions of the Sema4D ELISA kit.
Data were analyzed using SPSS 18.0 software. Clinical data were analyzed using the χ2 test or Fisher’s exact probability test with a four-fold table, and correlations were tested by Spearman’s rank correlation analysis. Count data, including cell counts in the invasion and migration assays and the Sema4D concentration in the cell supernatant estimated by ELISA, were analyzed using an independent sample t-test. Differences were considered significant at P < 0.05.
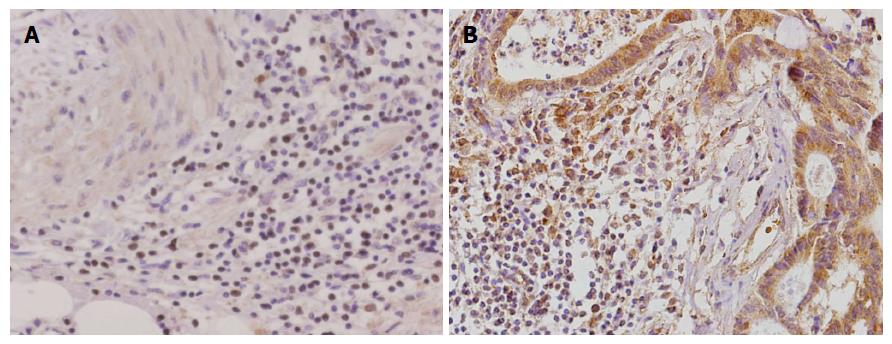
Immunohistochemical staining showed that CD68 was mainly concentrated in the interstitium of gastric carcinoma tissues. CD68 mainly infiltrated interstitial cells in gastric carcinoma tissues, which was stained as a brown color. Sema4D protein expression was mainly localized in the cytoplasm and nuclei of gastric carcinoma cells and the cytoplasm of tumor interstitial cells (mainly TAMs), which stained a brown color. CD68 and Sema4D expression was significantly higher in gastric carcinoma tissues than in adjacent normal tissues [71.7% (208/290) vs 33.8% (98/290), χ2 = 83.703; 74.5% (216/290) vs 42.8% (124/290), χ2 = 60.161; P < 0.01 for both, Figure 1].
Positive staining for CD68 and Sema4D was correlated with histological differentiation type, TNM stage, and lymph node metastasis (P < 0.05), but not with age, gender, or tumor size (Table 1, P > 0.05).
| Clinicopathological feature | CD68-positive | Sema4D-positive | ||||
| Positive rate | χ2 | P value | Positive rate | χ2 | P value | |
| Age (yr) | 0.761 | 0.383 | 3.538 | 0.060 | ||
| ≤ 50 | 103/149 (69.0) | 104/149 (70.0) | ||||
| > 50 | 104/141 (73.8) | 112/141 (79.4) | ||||
| Gender | 0.524 | 0.469 | 2.194 | 0.139 | ||
| Male | 105/151 (69.5) | 113/151 (77.2) | ||||
| Female | 102/139 (73.4) | 114/139 (71.2) | ||||
| Histological differentiation type | 18.511 | 0.000 | 30.6961 | 0.0001 | ||
| Well differentiated adenocarcinoma | 41/78 (52.5) | 40/78 (51.3) | ||||
| Moderately differentiated adenocarcinoma | 88/113 (87.9) | 96/113 (85.0) | ||||
| Poorly differentiated adenocarcinoma | 78/99 (78.8) | 80/99 (80.8) | ||||
| Tumor diameter (cm) | 3.120 | 0.077 | 1.189 | 0.276 | ||
| < 5 | 116/153 (75.8) | 118/153 (77.1) | ||||
| ≥ 5 | 91/137 (66.4) | 98/137 (71.5) | ||||
| TNM stage | 6.142 | 0.013 | 20.639 | 0.000 | ||
| I/II | 58/105 (55.2) | 60/105 (57.1) | ||||
| III/IV | 149/185 (80.5) | 1.000 | 156/185 (84.3) | |||
| Lymph node metastasis | 7.375 | 0.007 | 6.319 | 0.012 | ||
| Yes | 149/195 (76.4) | 154/195 (80.0) | ||||
| No | 58/95 (61.1) | 62/95 (65.3) | ||||
Sema4D expression was positive in 183 of the 207 gastric carcinoma tissues with positive CD68 expression (88.4%, 183/237). Of the 83 gastric carcinoma tissues with negative CD68 expression, Sema4dD showed positive expression in only 33 (39.8%, 33/83) cases. Spearman’s correlation analysis revealed a positive correlation between CD68 and Sema4D expression in gastric carcinoma tissues (r = 0.467, P < 0.01, Table 2).
| Sema4D | |||
| Group | n | Negative | Positive |
| CD68 | |||
| Negative | 83 | 50 | 33 |
| Positive | 207 | 24 | 183 |
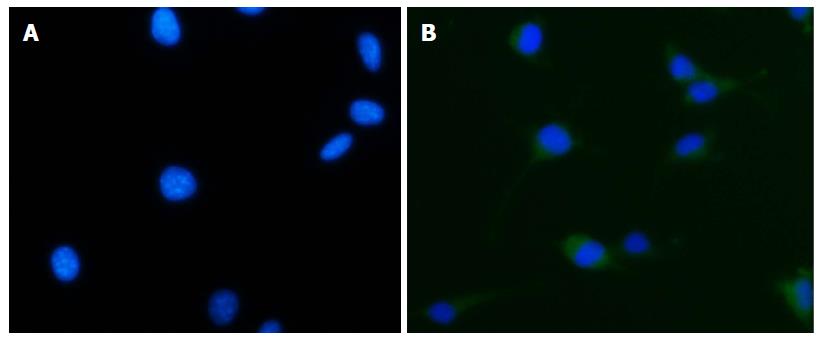
Suspended human THP-1 mononuclear cells were treated with PMA, IL-4, and IL-13, followed by adherent growth. CD68 was expressed in the cytoplasm of macrophages, and positive material in the cytoplasm emitted green fluorescence after staining (Figure 2). When the primary antibody was substituted with PBS, the cytoplasm showed a negative result (Figure 2).
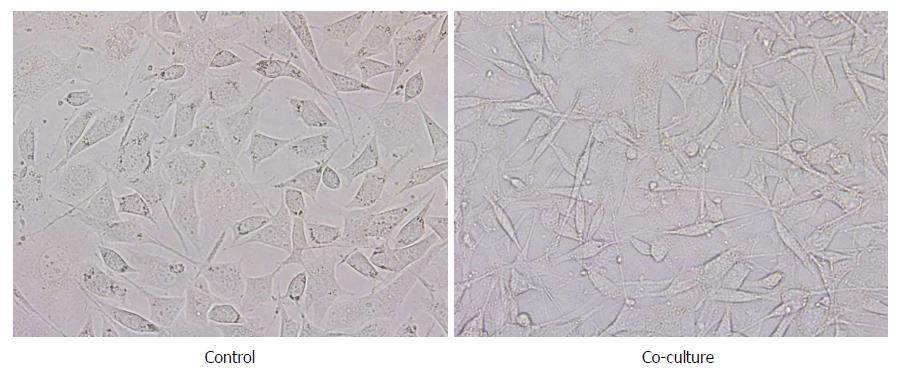
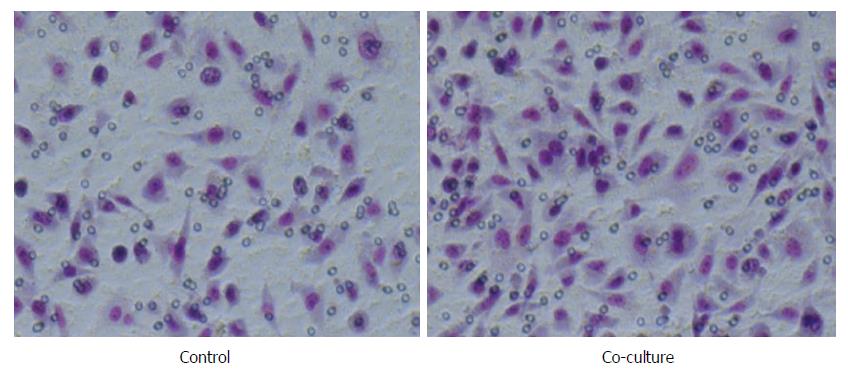
After co-culture with TAMs, gastric carcinoma SGC-7901 cells showed great morphological changes. The cells in the blank control group showed an epithelial cell structure that was cubic, with a blunt edge and a compact arrangement, and the cell confluence was high. Gastric carcinoma SGC-7901 cells in the co-culture group presented a narrow, long, interstitial cell-like shape that was long spindle, with pseudopodium elongation, loose cell arrangement, and even individual cell migration, and the confluence between the cells decreased (Figure 3).
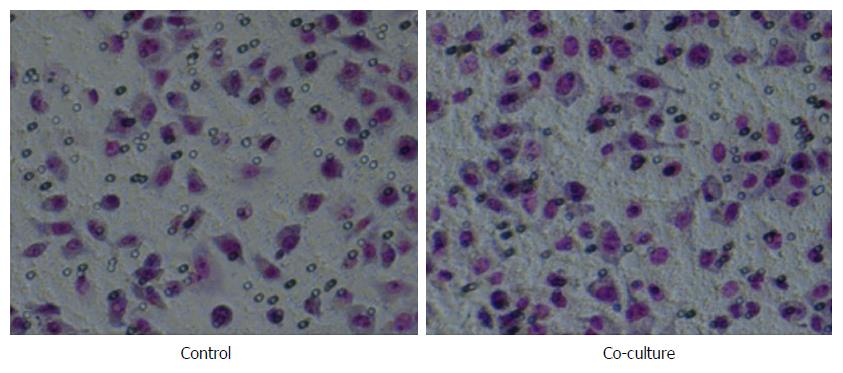
Migration assay: The number of migrating cells in the co-culture group was significantly higher than that in the control group (111.80 ± 11.82 vs 71.27 ± 6.44, P < 0.01, Figure 4).
Invasion assay: The numbers of invasive SGC-7901 cells that passed through the matrigel were 120.40 ± 8.10 in the co-culture group and 76.67 ± 8.63 in the control group; the difference between the two groups was significant (P < 0.01, Figure 5). Thus, TAMs enhanced the in vitro invasion and metastasis abilities of the SGC-7901 cells.
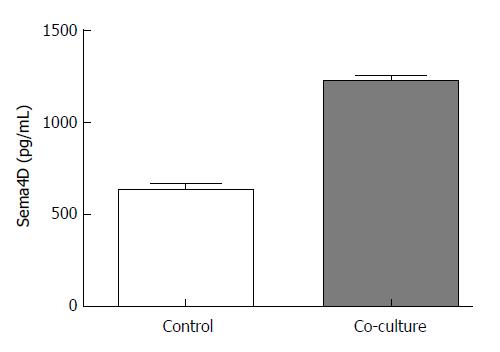
At the end of co-culture, we measured the expression of the secretory Sema4D protein in the SGC-7901 cell supernatants of the control and co-culture groups using the Sema4D ELISA kit. The Sema4D level in the blank control group was 637.15 ± 33.84 pg/mL, and the level in the co-culture group was 1224.13 ± 29.43 pg/mL; the difference between the two groups was significant (P < 0.01, Figure 6).
Distant metastasis and recurrence are the leading causes of death in patients with gastric carcinoma and are critical factors that affect the clinical efficacy and prognosis[15]. Gastric carcinoma is an immunogenic tumor, and the immune microenvironment of the tumor interstitium plays a major role in the development and progression of gastric carcinoma[16]. A dynamic balance exists between normal cells and their surrounding microenvironment, both of which jointly act to regulate cell activity, proliferation, differentiation, apoptosis, and the secretion and expression of related cytokines[17]. Once this balance is broken, the normal microenvironment changes to allow the malignant transformation of the cells. With the malignant growth of tumor, tumor cells continue to invade outward to fetch nutrients, and the surrounding microenvironment gradually evolves into a special environment with tissue hypoxia, acidosis, numerous growth factors, and proteolysis[18]. This microenvironment and the tumor cells mutually stimulate each other, which plays an important role in tumor invasion, migration, and metastasis[19-21].
The tumor microenvironment is a chronic inflammatory environment composed of inflammatory cells, endothelial cells, fibroblasts, and extracellular matrix[22]. Macrophages are an essential component of the innate immune system as well as a class of important cells involved in the chronic inflammatory response. Macrophages in vivo can acquire specific phenotypes from the local environment and show different functions, including phagocytosis, antigen presentation, antibacterial activity, cytotoxicity, tissue remodeling, and secretory functions[23]. TAMs are important inflammatory cells in the tumor microenvironment that are formed by monocytes in the peripheral circulating blood that migrate to the tumor microenvironment to proliferate and differentiate under the action of tumor-derived cytokines and chemokines[3]. TAMs infiltrate the interstitium of many malignancies and interact with tumor cells. TAMs also secrete growth factors and various cytokines, including matrix metalloproteinase (MMP)-2, MMP-9, VEGF, and TNF-α, to enhance the invasion and metastasis abilities of tumor cells, facilitate angiogenesis and lymphangiogenesis, and induce the immunosuppressive state of the microenvironment, ultimately promoting tumor development and progression[24]. TAMs are closely related to tumor development, progression, and prognosis[25]. CD68 is a cytoplasmic protein which is the most reliable TAMs marker[6]. In the present study, TAMs extensively infiltrated the interstitial gastric carcinoma tissues, and significantly higher expression was observed in gastric carcinoma tissues than in adjacent normal tissues. Patients with lymph node metastasis presented higher expression than those without. A lower histological differentiation level was associated with a higher positive rate of CD68 expression. No significant correlation was found with other clinicopathological features, such as patient age or tumor size. These findings suggest that TAMs are closely associated with the prognosis of gastric carcinoma.
Sema4D, also known as CD100, is an important member of the class IV semaphorin subfamily that was originally identified as a semaphorin affecting neurodevelopment[26]. Further studies found that SEMA4D could bind to the receptors cluster of differentiation 72 and Plexin B and thus play an important role in the nervous system, immune system, thrombosis, and tumor neovascularization[27]. Sema4D is another important proangiogenic factor after VEGF and is highly expressed in various tumor tissues, including head and neck squamous cell carcinoma, prostate cancer, colon cancer, breast cancer, and lung cancer[28]. Sema4D expression level is positively correlated with tumor progression level, metastasis, and resistance to radiotherapy and chemotherapy; it plays a major role in tumor development, progression, adhesion, metastasis, and invasive growth[29]. Sema4D plays a critical role in tumor angiogenesis and vascular maturation and can increase the tumorigenicity of tumor cells. In the Sema4D-deficient microenvironment, the tumorigenicity of tumor cells and their invasion and metastasis abilities are seriously weakened[30]. In the present study, we found that Sema4D was highly expressed in gastric carcinoma tissues and was closely associated with histological differentiation type, TNM stage, and lymph node metastasis. A significant positive correlation was found between positive CD68 and Sema4D expression in gastric carcinoma tissues by Spearman’s correlation analysis. This finding indicates that TAMs in gastric carcinoma tissues have a synergistic relationship with Sema4D production in gastric carcinoma, which may be an important factor promoting the invasion and metastasis of gastric carcinoma.
Based on the clinical research results, we conducted Transwell non-contact co-culture assays with TAMs and gastric cancer SGC-7901 cells. SGC-7901 cells showed significant morphological changes, with the cell body changing from the cubic shape with a blunt edge into a long spindle; meanwhile, the pseudopodium increased to varying degrees and became slender, the cell confluence declined, and individual cell migration was observed. The epithelial cell characteristics were lost, and more interstitial cell characteristics were observed, suggesting that the epithelial-mesenchymal transition (EMT) occurred to a certain degree. The EMT is the first step by which tumor cells isolated from the primary lesion achieve metastatic invasion and thus is a critical step[31]. The EMT promotes the loss of epithelial cell polarity, reduces cell adhesion, and enhances cell motility; these biological changes lead to damage to the polarity and tight junctions of the cells. Additionally, the EMT alters the cytoskeleton remodeling process, thereby enhancing tumor cell invasiveness and contributing to the acquisition of the ability to form distant metastases[31,32]. The invasion and migration assays showed that SGC-7901 cells in the co-culture group had significantly enhanced invasion and migration abilities compared to the cells in the control group. This result suggested that intercellular adhesion was reduced and that the infiltration and invasion abilities were enhanced after the morphological changes of SGC-7901 cells.
TAMs and Sema4D are highly expressed in many tumors and are associated with tumor development, progression, angiogenesis, invasion, and metastasis. Research on their functions and related mechanisms makes progress every day. Combined detection of CD68 and Sema4D proteins shows potential for predicting the progression trend of gastric carcinoma and determining the patient prognosis. Continuous in-depth study of their mechanisms is bound to inject new vitality into the prevention and treatment of tumors.
Gastric carcinoma is one of the most common malignancies and its mortality is ranked second among cancers. The tumor microenvironment plays a major role in the invasion and metastasis of gastric carcinoma. Tumor-associated macrophages (TAMs) are an important component of the tumor microenvironment. TAMs have been shown to enhance the infiltration and metastasis ability of tumor cells by expressing certain growth factors and cytokines. TAMs also suppress immune responses in the microenvironment and promote tumor progression by facilitating tumor angiogenesis and lymphangiogenesis.
Semaphorin 4D (Sema4D) is an important member of the semaphorin subfamily and plays a major role in the nervous and immune systems. Sema4D is expressed at high levels in various tumor tissues, including head and neck squamous cell carcinoma, prostate cancer, and colon cancer, and its role in promoting tumor angiogenesis is becoming a hot research topic. Sema4D is another important proangiogenic factor following vascular endothelial growth factor.
The expression of Sema4D in gastric cancer and its relationship with TAMs have not been explored. If TAMs can promote Sema4D expression and influence the characters of gastric cancer cells, this will be greatly helpful to inject new vitality into the prevention and treatment of gastric cancer.
In the present study, we explored the effect of TAMs on Sema4D expression in gastric carcinoma tissues and its role in the development and progression of gastric carcinoma to provide a new theoretical reference for the prevention and treatment of this cancer.
By using the immunohistochemical method, the expression of the TAM markers CD68 and Sema4D in gastric carcinoma and adjacent normal tissues from 290 patients and their relationships with clinical significance were analyzed. In vitro, changes of the secretory Sema4D level in SGC-7901 cells were measured using ELISA, and the effect of TAMs on SGC-7901 cell invasion and migration was assessed with invasion and migration assays, respectively.
CD68 and Sema4D protein expression was significantly higher in gastric carcinoma tissues than in adjacent normal tissues. CD68 and Sema4D protein expression was significantly associated with histological differentiation type, TNM stage, and lymph node metastasis, and their expression levels were positively correlated with one another. In the in vitro experiment, secretory Sema4D protein expression was significantly increased in the supernatant of SGC-7901 cells co-cultured with TAMs compared with the blank control. Cell invasion and metastasis were enhanced in the Transwell invasion and migration assays. However, the mechanism by which TAMs promote gastric cancer cells to express Sema4D is not clearly now, so we should explore the signal pathway and other mechanism in the next step to make this more clearly.
In our study we found that TAMs promoted the invasion and metastasis of gastric carcinoma cells possibly through upregulated secretory Sema4D protein expression. Combined detection of TAM markers, CD68 and Sema4D, in gastric carcinoma tissue shows potential to predict the trend of gastric carcinoma progression. TAMs and Sema4D are highly expressed in many tumors and are associated with tumor development, progression, angiogenesis, invasion, and metastasis. Research on their functions and related mechanisms makes progress every day. Combined detection of CD68 and Sema4D proteins shows potential for predicting the progression trend of gastric carcinoma and determining the patient prognosis. Continuous in-depth study of their mechanisms is bound to inject new vitality into the prevention and treatment of tumors.
By this study, we can learn that TAMs and Sema4D are highly expressed in gastric carcinoma and are associated with tumor development, progression, angiogenesis, invasion, and metastasis. Combined detection of CD68 and Sema4D proteins shows potential for predicting the progression trend of gastric carcinoma and determining the patient prognosis. Research on their functions and related mechanisms will make great progress in anti-tumor field. Continuous in-depth study of their mechanisms is bound to inject new vitality into the prevention and treatment of tumors.
| 1. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13326] [Article Influence: 1332.6] [Reference Citation Analysis (4)] |
| 2. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21466] [Article Influence: 1951.5] [Reference Citation Analysis (6)] |
| 3. | Sawa-Wejksza K, Kandefer-Szerszeń M. Tumor-Associated Macrophages as Target for Antitumor Therapy. Arch Immunol Ther Exp (Warsz). 2017; ; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 4. | Wu H, Xu JB, He YL, Peng JJ, Zhang XH, Chen CQ, Li W, Cai SR. Tumor-associated macrophages promote angiogenesis and lymphangiogenesis of gastric cancer. J Surg Oncol. 2012;106:462-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 5. | Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1056] [Cited by in RCA: 1140] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 6. | Shabo I, Svanvik J. Expression of macrophage antigens by tumor cells. Adv Exp Med Biol. 2011;714:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Yang L, Zhang Y. Tumor-associated macrophages, potential targets for cancer treatment. Biomark Res. 2017;5:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Zhu J, Zhi Q, Zhou BP, Tao M, Liu J, Li W. The Role of Tumor Associated Macrophages in the Tumor Microenvironment: Mechanism and Functions. Anticancer Agents Med Chem. 2016;16:1133-1141. [PubMed] |
| 9. | Wu M, Li J, Gao Q, Ye F. The role of Sema4D/CD100 as a therapeutic target for tumor microenvironments and for autoimmune, neuroimmune and bone diseases. Expert Opin Ther Targets. 2016;20:885-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Takada H, Ibaragi S, Eguchi T, Okui T, Obata K, Masui M, Morisawa A, Takabatake K, Kawai H, Yoshioka N. Semaphorin 4D promotes bone invasion in head and neck squamous cell carcinoma. Int J Oncol. 2017;51:625-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Zhou H, Kann MG, Mallory EK, Yang YH, Bugshan A, Binmadi NO, Basile JR. Recruitment of Tiam1 to Semaphorin 4D Activates Rac and Enhances Proliferation, Invasion, and Metastasis in Oral Squamous Cell Carcinoma. Neoplasia. 2017;19:65-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Cao J, Zhang C, Chen T, Tian R, Sun S, Yu X, Xiao C, Wang G, Liu Y, Fu M. Plexin-B1 and semaphorin 4D cooperate to promote cutaneous squamous cell carcinoma cell proliferation, migration and invasion. J Dermatol Sci. 2015;79:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Zhou H, Yang YH, Basile JR. Characterization of the Effects of Semaphorin 4D Signaling on Angiogenesis. Methods Mol Biol. 2017;1493:429-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Birner P, Schindl M, Obermair A, Plank C, Breitenecker G, Oberhuber G. Overexpression of hypoxia-inducible factor 1alpha is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Res. 2000;60:4693-4696. [PubMed] |
| 15. | Ajani JA, Lee J, Sano T, Janjigian YY, Fan D, Song S. Gastric adenocarcinoma. Nat Rev Dis Primers. 2017;3:17036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 432] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 16. | Venerito M, Link A, Rokkas T, Malfertheiner P. Gastric cancer - clinical and epidemiological aspects. Helicobacter. 2016;21 Suppl 1:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 17. | Sung SY, Chung LW. Prostate tumor-stroma interaction: molecular mechanisms and opportunities for therapeutic targeting. Differentiation. 2002;70:506-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Nyberg P, Salo T, Kalluri R. Tumor microenvironment and angiogenesis. Front Biosci. 2008;13:6537-6553. [PubMed] |
| 19. | Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453-461. [PubMed] |
| 20. | Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549-555. [PubMed] |
| 21. | van Netten JP, Ashmead BJ, Parker RL, Thornton IG, Fletcher C, Cavers D, Coy P, Brigden ML. Macrophage-tumor cell associations: a factor in metastasis of breast cancer? J Leukoc Biol. 1993;54:360-362. [PubMed] |
| 22. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10123] [Cited by in RCA: 11515] [Article Influence: 479.8] [Reference Citation Analysis (2)] |
| 23. | Sica A, Allavena P, Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267:204-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 443] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 24. | Siveen KS, Kuttan G. Role of macrophages in tumour progression. Immunol Lett. 2009;123:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 277] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 25. | Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2416] [Cited by in RCA: 2596] [Article Influence: 118.0] [Reference Citation Analysis (0)] |
| 26. | Hall KT, Boumsell L, Schultze JL, Boussiotis VA, Dorfman DM, Cardoso AA, Bensussan A, Nadler LM, Freeman GJ. Human CD100, a novel leukocyte semaphorin that promotes B-cell aggregation and differentiation. Proc Natl Acad Sci USA. 1996;93:11780-11785. [PubMed] |
| 27. | Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer. 2008;8:632-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 308] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 28. | Muratori C, Tamagnone L. Semaphorin signals tweaking the tumor microenvironment. Adv Cancer Res. 2012;114:59-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Ch’ng ES, Kumanogoh A. Roles of Sema4D and Plexin-B1 in tumor progression. Mol Cancer. 2010;9:251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 30. | Sierra JR, Corso S, Caione L, Cepero V, Conrotto P, Cignetti A, Piacibello W, Kumanogoh A, Kikutani H, Comoglio PM. Tumor angiogenesis and progression are enhanced by Sema4D produced by tumor-associated macrophages. J Exp Med. 2008;205:1673-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 220] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 31. | Beach JR, Hussey GS, Miller TE, Chaudhury A, Patel P, Monslow J, Zheng Q, Keri RA, Reizes O, Bresnick AR. Myosin II isoform switching mediates invasiveness after TGF-β-induced epithelial-mesenchymal transition. Proc Natl Acad Sci USA. 2011;108:17991-17996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 32. | Cardiff RD. Epithelial to Mesenchymal Transition Tumors: Fallacious or Snail’s Pace? Clin Cancer Res. 2005;11:8534-8537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Dar NA, Kazuya S, Snyder J S- Editor: Chen K L- Editor: Wang TQ E- Editor: Huang Y